Highlights
-
•
Vitamin D deficiency is common in patients with bone tumours.
-
•
Potential association between pre-diagnostic vitamin D status and tumour malignancy in patients with bone tumours.
-
•
25(OH)D status should routinely be assessed and monitored in patients with bone tumours.
Keywords: Bone tumour, Vitamin D, Hypovitaminosis D, Vitamin D deficiency, Malignancy, Tumour malignancy
Abstract
Vitamin D deficiency is a global health concern that is estimated to afflict over one billion people globally. The major role of vitamin D is that of a regulator of calcium and phosphate metabolism, thus, being essential for proper bone mineralisation. Concomitantly, vitamin D is known to exert numerous extra-skeletal actions. For example, it has become evident that vitamin D has direct anti-proliferative, pro-differentiation and pro-apoptotic actions on cancer cells. Hence, vitamin D deficiency has been associated with increased cancer risk and worse prognosis in several malignancies. We have recently demonstrated that vitamin D deficiency promotes secondary cancer growth in bone. These findings were partly attributable to an increase in bone remodelling but also through direct effects of vitamin D on cancer cells. To date, very little is known about vitamin D status of patients with bone tumours in general. Thus, the objective of this study was to assess vitamin D status of patients with diverse bone tumours. Moreover, the aim was to elucidate whether or not there is an association between pre-diagnostic vitamin D status and tumour malignancy in patients with bone tumours.
In a multi-center analysis, 25(OH)D, PTH and calcium levels of 225 patients that presented with various bone tumours between 2017 and 2018 were assessed. Collectively, 76% of all patients had insufficient vitamin D levels with a total mean 25(OH)D level of 21.43 ng/ml (53.58 nmol/L). In particular, 52% (117/225) of patients were identified as vitamin D deficient and further 24% of patients (55/225) were vitamin D insufficient. Notably, patients diagnosed with malignant bone tumours had significantly lower 25(OH)D levels than patients diagnosed with benign bone tumours [19.3 vs. 22.75 ng/ml (48.25 vs. 56.86 nmol/L); p = 0.04).
In conclusion, we found a widespread and distressing rate of vitamin D deficiency and insufficiency in patients with bone tumours. However, especially for patients with bone tumours sufficient vitamin D levels seem to be of great importance. Thus, we believe that 25(OH)D status should routinely be monitored in these patients. Collectively, there should be an increased awareness for physicians to assess and if necessary correct vitamin D status of patients with bone tumours in general or of those at great risk of developing bone tumours.
1. Introduction
Bone tumours are a heterogeneous group of neoplasms with wide-ranging radiological and histological appearances [1], [2]. In general, benign bone tumours are distinguished from malignant tumours, while the latter can be further classified into primary and secondary bone tumours ie. metastases. Bone metastases are frequent and seen in many malignancies, but preferentially occur in patients with breast, prostate, lung, thyroid, kidney and bladder cancers. In contrast to bone metastases, the aetiology of many primary malignant bone tumours is largely unknown. The ability of cancer cells to survive within the bone microenvironment is a conditio sine qua non for tumour cells to expand and thrive in bone [3]. For this reason, the bone microenvironment plays a pivotal role in the growth of bone tumours. Under physiological conditions, resident osteoclasts and osteoblasts continuously remove and replace bone in a well-orchestrated manner [4]. This process of bone remodelling is a life-long and susceptible continuum aiming to break down old or mechanically unnecessary bone and replacing the removed tissue by an equal amount of newly formed bone. The presence of cancer cells within this complex bone microenvironment greatly perturbs this balance. Upon manifestation in bone, cancer cells are known to parasite and control the local environment by exploiting resident osteoblast and osteoclasts to foster their own prosperity [5], [6], [7]. Hence, alterations within the bone microenvironment have been linked to an increased skeletal susceptibility to cancer metastases [5]. Vitamin D is of utmost importance to maintain a balanced and physiological metabolism of both minerals and bone structure within the bone micronenvironment [8], [9]. Therefore, it is not surprising that vitamin D deficiency has been reported to accelerate local tumour growth in bone [10], [11]. Consequently, numerous randomised controlled trials and most observational studies suggested that vitamin D plays a role in reducing cancer mortality [12].
In addition, vitamin D deficiency has been linked to an increased risk of developing cancer by regulating the expression of tumour-related genes [13], [14], [15], [16]. Further, vitamin D deficiency is associated with a higher incidence of skeletal-related events and poorer prognosis in patients with breast and prostate cancer [17], [18].
Notably, it has become evident that the effects of vitamin D on tumour growth are partly attributable to an increase in bone remodelling but are also mediated through direct effects of vitamin D on cancer cells. In particular, it has been demonstrated that vitamin D directly regulates cell proliferation, differentiation and apoptosis in numerous tissues, including malignant tumours [15], [19], [20], [21], [22], [23], [24], [25]. These direct effects of vitamin D are mediated through binding of the biologically active metabolite1,25-dihydroxyvitamin D (1,25D) to the vitamin D receptor (VDR). The VDR is widely expressed in most cell types including many cancers and several studies have reported a negative correlation between VDR expression and tumour malignancy [26].
Collectively, there is mounting evidence that vitamin D signalling plays a pivotal role in bone cancer growth. Thus, the assessment of vitamin D status may be useful in the clinical monitoring of patients with bone tumours. With scant exceptions, however, data from clinical studies do not provide robust information on vitamin D status in patients with bone tumours. For this reason, the purpose of this study was to assess pre-diagnostic serum vitamin D status of patients with diverse bone tumours in a multi-center analysis. Moreover, the aim was to elucidate whether pre-diagnostic vitamin D status of patients with malignant bone tumours is different to those with benign bone tumours.
2. Patients and methods
Pre-diagnostic serum 25(OH)D, PTH and calcium levels of 225 patients that presented with bone tumours were measured between January 2017 and December 2018 in three different Orthopaedic Departments of University Hospitals in Germany. Patients were either referred to the Department of Orthopaedics, Koenig-Ludwig-Haus, University of Wuerzburg, Germany (49.76°N latitude, N = 101), the Department of Orthopaedics, Klinikum rechts der Isar, TU Munich, Germany (48.14°N latitude, N = 108), or the Department of Orthopaedic Surgery, Pius-Hospital, Carl-von-Ossietzky-University, Oldenburg, Germany (53.14°N latitude, N = 16). Diagnostic assessment consisted of clinical examination, review of patient history, radiographic imaging (X-ray, MRI and/or CT scans) and histopathological evaluation of the obtained tissues. Further, cases were discussed and diagnosis was confirmed within an interdisciplinary tumour board. Collectively, all patients diagnosed with any type of benign or malignant bone tumour ie. primary or secondary were included in the study. Patients were excluded from the study if any other concomitant diagnose that could potentially impact on vitamin D status such as e.g. general bone metabolism disorders, hypo-/hypercalcaemia or hypo-/hyperparathyroidism was previously known. Additionally, patients that reported of a routine intake of vitamin D supplements or drugs specifically affecting bone health such as e.g. PTH analogues, bisphosphonates or RANKL inhibitors were excluded.
Blood samples were collected at the first day of consultation. Each orthopaedic department utilised standardised serum measurements; the hospital laboratory at Wuerzburg University used the cobas® 25-Hydroxyvitamin D Assay (Vitamin D Total) and the Elecsys PTH (1–84) assay for cobas® e 411 Analyzer (Roche Diagnostics, Mannheim, Germany). The laboratory at Munich used the Liaison XL analyzer (DiaSorin, Saluggia, Italy) while the Elecsys® Vitamin D total II was employed for 25(OH)D measurements at Oldenburg laboratory. Both laboratories at Munich and Oldenburg used the cobas® PTH STAT for cobas® e411 Analyzer (Roche Diagnostics, Mannheim, Germany) for PTH measurements. All laboratory results were collected using a retrospective chart review.
The study was conducted in accordance with the guidelines of the local Committee of Medical Ethics and in accordance with the World Medical Association Declaration of Helsinki. Approval for the collection of all blood samples and testing for serum 25(OH)D, PTH and calcium levels was obtained from every patient (Ethics number 38/17-MK).
At present, there is still no international consensus on a specific serum 25(OH)D level that is considered as ‘insufficient’ [27]. For data analysis and interpretation, we complied with the guidelines of the endocrine society defining vitamin D deficiency as a 25(OH)D level of less than 20 ng/ml (50 nmol/L) and vitamin D insufficiency as a 25(OH)D level between 20 and 29 ng/ml (50–72.5 nmol/L) [28]. Accordingly, serum 25(OH)D levels greater than or equal to 30 ng/ml (75 nmol/L) were considered to be vitamin D sufficient [28]. All patients with valid 25(OH)D levels were included in statistical analysis and grouped according to age, sex, season, tumour type and tumour malignancy. Serum vitamin D, PTH and calcium levels were compared between cohorts using Student’s t test for independent samples and one-way ANOVA for multiple comparisons. Multivariate analysis for subgroups was conducted using ANCOVA. Level of statistical significance was set a p < 0.05.
3. Results
Altogether, 225 patients that presented to either of the orthopaedic departments with a bone tumour of unknown origin/identity were enrolled in this study (n = 101 for Wuerzburg, n = 108 for Munich and 16 for Oldenburg). Hereof, 109 patients (48%) were female and 116 (52%) male with a cumulative mean age of 44.9 years. In total, 142 patients presented with benign bone tumours (63%) whereas 83 patients presented with bone tumours that were identified as malignant bone tumours (37%). Collectively, we found a wide variety of benign and malignant (primary and secondary) bone tumours. Bone metastasis were the most frequent malignant bone tumours accounting for 31 (37%) patients followed by chondrosarcoma (N = 16; 19%) and osteosarcoma (n = 15; 18%). In patients with benign bone tumours, osteochondroma (n = 30; 21%) was the most common tumour followed by chondroma (n = 20; 14%) and bone cysts (n = 18; 13%). The five most frequent benign and malignant tumours of patients surveyed with corresponding mean 25(OH)D levels are presented in (Table 1).
Table 1.
Distribution of the five most frequent malignant and benign tumours with corresponding mean serum 25(OH)D level.
| Malignant Bone Tumours |
Benign Bone Tumours |
||||
|---|---|---|---|---|---|
| Tumour Type | Number of Patients | Mean 25(OH)D | Tumour Type | Number of Patients | Mean 25(OH)D |
| Bone Metastases | 31 | 23.9 | Osteochondroma/Exostosis | 30 | 23.7 |
| Chondrosarcoma | 16 | 16.9 | Chondroma | 20 | 25.3 |
| Osteosarcoma | 15 | 16.0 | Bone Cyst | 18 | 21.1 |
| Lymphoma | 5 | 12.8 | Giant-cell tumor of bone | 16 | 22.0 |
| Pleomorph. Undiff. sarcoma | 3 | 19.3 | Fibrous Dysplasia | 9 | 19.3 |
Serum 25(OH)D measurements were normally distributed with the lowest measured level of 3 ng/ml (7.5 nmol/L) and the highest level of 69 ng/ml (172.5 nmol/L). Markedly, 76% of all bone tumour patients (172/225) presented with low vitamin D levels with a total mean 25(OH)D level of 21.43 ng/ml (53.58 nmol/L). In particular, 52% of patients (117/225) were vitamin D deficient, further 24% (55/225) vitamin D insufficient and merely 24% of patients (53/225) had sufficient vitamin D levels greater than or equal to 30 ng/ml (75 nmol/L) (Table 2, Table 3).
Table 2.
Overview of vitamin D deficiency, insufficiency and sufficiency among patients with malignant bone tumours compared to patients with benign bone tumours.
| Patients | Malignant Bone Tumours | Benign Bone Tumours | Overall |
|---|---|---|---|
| Vitamin D sufficient (≥30 ng/ml) | 16.9% | 27.5% | 23.6% |
| Vitamin D insufficient (20–29 ng/ml) | 22.9% | 25.3% | 24.4% |
| Vitamin D deficient (<20 ng/ml) | 60.2% | 47.2% | 52% |
Table 3.
Patient characteristics.
| Location |
|||||
|---|---|---|---|---|---|
| Wuerzburg | Munich | Oldenburg | Overall | ||
| Sex | Female | 47 | 52 | 10 | 109 |
| Male | 54 | 56 | 6 | 116 | |
| Age | Mean | 51.07 | 37.8 | 54.2 | 44.94 |
| Range | 15–84 | 8–88 | 19–83 | 8–88 | |
| No. of Tumours | Malignant | 34 | 38 | 11 | 83 |
| Benign | 67 | 70 | 5 | 142 | |
| Vit D | Mean | 20.59 | 22.55 | 18.91 | 21.43 |
| Median | 17 | 21 | 13.55 | 19 | |
| PTH | Mean | 42.86 | 36 | 35.53 | 38.09 |
| Median | 37.1 | 32 | 31 | 34.2 | |
| Calcium | Mean | 2.41 | 2.40 | 2.41 | 2.41 |
| Median | 2.4 | 2.42 | 2.44 | 2.41 | |
| Number/Percentage of Patients Vit D | Sufficient | 24/24% | 26/24% | 3/19% | 53/24% |
| Insuffcient | 20/20% | 33/31% | 2/13% | 55/24% | |
| Deficient | 57/56% | 49/45% | 11/68% | 117/52% | |
| Patients per Season/Mean Vit D per Season | Spring | 41/16.17 | 33/20.58 | 3/11.1 | 77/17.86 |
| Summer | 17/31.53 | 36/26.16 | 4/23.1 | 57/27.54 | |
| Autumn | 21/24.57 | 22/23.45 | 4/24.78 | 47/24.06 | |
| Winter | 22/16.59 | 17/17.55 | 5/16.56 | 44/16.95 | |
Most notably, 83% of patients that were diagnosed with a malignant bone tumour had insufficient vitamin D levels. The highest proportion of patients (60%) were identified to be vitamin D deficient, while in 23% of patients vitamin D insufficiency was diagnosed. Hence, only 17% of patients with malignant bone tumours had sufficient vitamin D levels. Conversely, 73% of the 142 patients that presented with benign bone tumours, had low vitamin D levels with 47% being vitamin D deficient and 25% being vitamin D insufficient. Statistical analyses of 25(OH)D levels identified significant differences between patients with malignant bone tumours (mean 19.3 ± 1.347 SEM) compared to patients with benign bone tumours (22.75 ± 1.005 SEM) (p = 0.04) (Fig. 1). Comparison of 25(OH)D levels of the five most frequent malignant and benign tumours of the survey did not show any significant differences (Table 2).
Fig. 1.
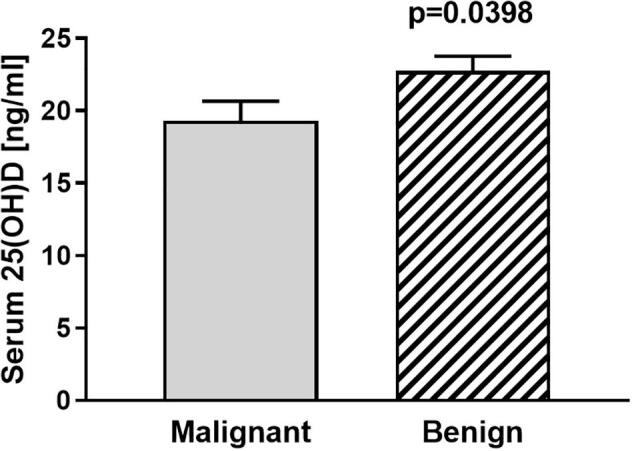
Serum vitamin D [25(OH)D] levels of patients with benign bone tumours (n = 142) compared to patients with malignant (n = 82) bone tumours (n = 225).
To identify any differences in mean serum vitamin D levels depending on the mean sunshine hours, we subdivided the cohort into 4 groups (spring = March-May; summer = June-August; autumn = September-November and winter = December-February). As expected, vitamin D levels were generally lower in winter and spring compared to summer and autumn in which daily sunshine hours are elevated (Fig. 2).
Fig. 2.
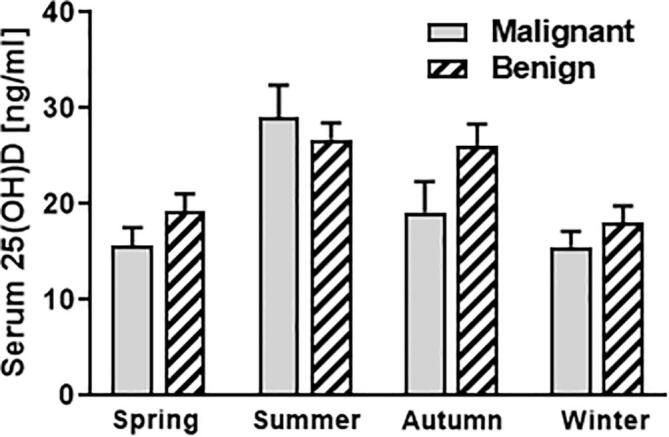
Serum vitamin D [25(OH)D] levels of patients with benign compared to malignant bone tumours in correlation to the season [n = 77 for spring (March-May); n = 57 for summer (June-August); n = 47 for autumn (September-November); n = 44 for winter (December-February)].
Further subdivision of the cohorts for gender did not show any significant differences between serum 25(OH)D levels of females with malignant tumours [mean 19.71 ng/ml (49.28 nmol/L)] compared to males with malignant tumours [18.90 ng/ml (47.25 nmol/L)] or females with benign tumours [22.94 ng/ml; (57.35 nmol/L)] compared to males with benign tumours [22.45 ng/ml; (56.13 nmol/L)]. However, there were significant differences between 25(OH)D levels of patients with primary malignant tumours [mean 16.56 ng/ml; (41.4 nmol/L)] compared to patients with bone metastases [mean 23.9 ng/ml; (59.75 nmol/L)] (p = 0.008) (Fig. 3). Due to the heterogeneity of tumours and small samples sizes, we omit statistical tests of significance between all tumour entities.
Fig. 3.
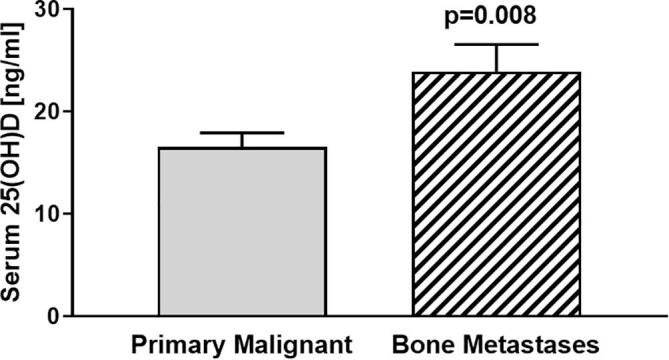
Comparing serum vitamin D [25(OH)D] levels of patients with primary malignant bone tumours [mean 16.56 ng/ml (41.4 nmol/L); n = 51] to patients with bone metastases [mean 23.9 ng/ml (59.75 nmol/L); n = 31] revealed significant differences between groups (p = 0.008).
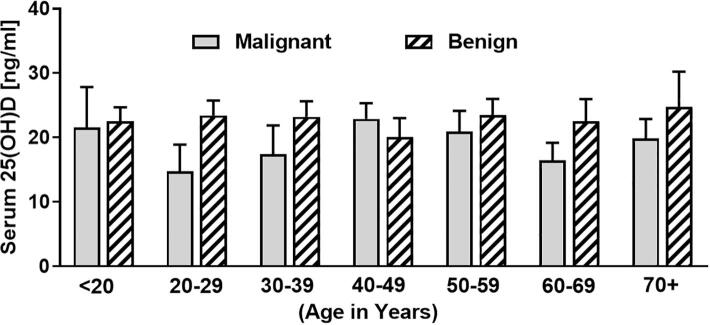
The cohort was further grouped according to age into either under 20 years, 20–29, 30–39, 40–49, 50–59, 60–69, and over 70 years old. We could not identify any statistical differences in serum 25(OH)D status between groups. However, mean serum 25(OH)D levels of patients with malignant bone tumours were lower than in patients with benign tumours in all groups except for the 40–49 year olds (Fig. 4).
Fig. 4.
Serum vitamin D [25(OH)D] levels of patients with malignant compared to benign bone tumours grouped according to age (age < 20 years n = 4 for malignant, n = 27 for benign; 20–29 years n = 5 for malignant and n = 26 for benign; 30–39 years n = 7 for malignant and n = 27 for benign; 40–49 years n = 9 for malignant and n = 19 for benign; 50–59 years n = 20 for malignant and n = 23 for benign; 60–69 years n = 17 for malignant and n = 13 for benign; 70 + years n = 21 for malignant and n = 7 for benign).
Most malignant bone tumours were found in the group of 70+ olds whereas most benign tumours were identified in the group of under 20 and 30–39-year-olds.
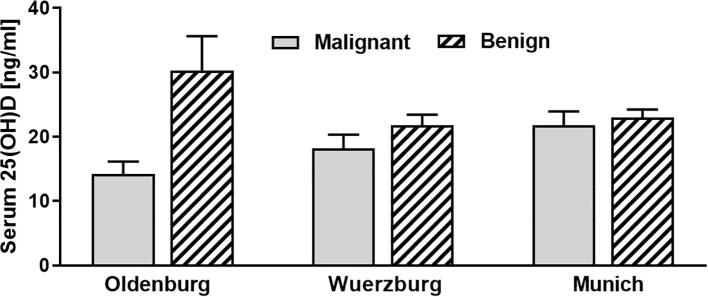
Analysis of 25(OH)D levels comparing the three different locations (Wuerzburg, Munich and Oldenburg) did not show any significant differences (Fig. 5). In addition, we could not detect any significant differences in overall mean PTH (mean 38.09 ng/l; range 5.5 ng/l to 220.6 ng/l; n = 210) or calcium (mean 2.41 mmol/l; range 1.94 to 3.57 mmol/l; n = 223) levels when comparing for tumour malignancy, sex, age or location (Table 3).
Fig. 5.
Serum vitamin D [25(OH)D] levels of patients with malignant bone tumours (Wuerzburg n = 34; Munich n = 38; Oldenburg n = 11) compared to benign bone tumours (Wuerzburg n = 67; Munich n = 70; Oldenburg n = 5) grouped according to location.
4. Discussion
In this multi-center analysis, we identified a widespread and distressing rate of vitamin D deficiency and insufficiency in patients with diverse bone tumours. It is estimated that vitamin D deficiency and insufficiency afflict more than one billion children and adults globally [29]. In the past decades, research on vitamin D has been particularly popular which has led to a plethora of clinical and pre-clinical studies focusing on potential health-beneficial effects of vitamin D [4], [9], [27], [30]. Hence, numerous studies have demonstrated that the impact of vitamin D goes far beyond its classical function of regulating bone remodelling. For example, it has been demonstrated that vitamin D effects cell proliferation, differentiation and apoptosis in many different organs and tissues [8], [29], [31], [32]. Further, it is known to mediate neuromuscular functions, affect immune and inflammatory responses and to influence the onset and progression of multiple diseases including cancer [4], [15], [30]. Moreover, several studies that investigated circulating 25(OH)D concentrations in cancer patients reported an inverse correlation between 25(OH)D levels and cancer risk, tumour progression and mortality [15], [33], [34]. In bone oncology, it has recently been demonstrated that vitamin D deficiency enhances secondary cancer growth in bone. These findings were partly explained through indirect effects of vitamin D deficiency as a result of increased bone remodelling. However, this observation was also attributable to direct effects of vitamin D on cancer cells which are mostly mediated through binding of 1,25D to the VDR [4], [10], [11], [35]. Notably, we have recently demonstrated that the VDR itself, and independent of its ligand has a critical function in controlling cancer cell behaviour in bone and cancer metastasis to bone [36], [37].
Collectively, previous findings provide an experimental rationale for the clinical observation that accelerated bone turnover due to vitamin D deficiency is associated with cancer progression in bone. Moreover, mounting evidence suggests that the VDR itself plays a pivotal role in cancer progression and metastasis.
In many cancers secondary position of tumour cells in bone occurs with astonishing frequency [1]. Metastatic spread to bone depends on a complex cascade of actions that eventually results in cancer cell engraftment and progression within the bone microenvironment [3], [4], [38]. The presence of cancer cells within the bone microenvironment greatly perturbs the balance of bone turnover mostly resulting in accelerated bone resorption by activating osteoclasts. Importantly, this does not only provide space for the tumour to expand into, but also causes the release of growth factors embedded in the bone matrix. This in turn enhances further tumour growth resulting in the previously described vicious cycle [4], [39]. Vitamin D deficiency causes alterations within the bone microenvironment increasing local cancer growth and potentially promoting the susceptibility to the onset of primary bone cancers.
It is therefore not surprising that vitamin D deficiency is associated with a higher incidence of skeletal-related events and poorer prognosis in patients with breast and prostate cancer - both cancers with a high propensity to metastasise to bone [17], [18]. For this reason, avoiding vitamin D deficiency is pivotal for patients with bone metastasis and for patients that are at high risk of developing bone metastasis. Interestingly however, very little has been reported on vitamin D status in patients with bone metastases. We have previously demonstrated that there is a high prevalence of vitamin D deficiency in patients with bone metastasis [5], [40]. In the current study, mean serum vitamin D status of patients with bone metastasis was significantly higher than vitamin D status of patients with primary malignant bone tumours. Further, it was somewhat surprising and unexpected that mean serum vitamin D status of patients with bone metastases was relatively high compared to our previously conducted study [23.9 ng/ml (59.75 nmol/L) compared to 13.9 ng/ml (34.75 nmol/L)] [5]. A possible explanation for this discrepancy might be attributable to the fact that four patients with bone metastases in the current study showed inexplicable high vitamin D serum levels above 50 ng/ml (125 nmol/L) [one of them as high as 69 ng/ml (172.5 nmol/L)]. We assume that these patients might have previously taken any vitamin D supplementation, however, being unaware or unable to recall doing so. Hence, we believe that effective vitamin D status of this patient cohort might in fact be lower than currently reported.
Comparing vitamin D levels between the different locations, distinct differences were observed at Oldenburg (Fig. 5). These results are likely to be related to the fact that most patients at Oldenburg presented with malignant bone tumours and in winter months (Table 3).
In contrast to bone metastasis, the pathogenesis and aetiology of many primary malignant bone tumours remains obscure. It appears that several factors may contribute to the onset of diverse primary malignant bone tumours. For example, exposure to several chemical agents, viruses and radiation have been identified as potent inducers of osteosarcoma [41]. Interestingly, osteosarcoma is known to predominantly occur in adolescents, thus coinciding to appear in a period of rapid skeletal growth [42]. Moreover, osteosarcoma seems to arise in areas of pre-existing Paget’s disease. These observations clearly indicate that a transformation of the bone microenvironment may play a role in the carcinogenesis of osteosarcoma [43]. Hence, it is likely that endocrine changes such as vitamin D deficiency that likewise result in alterations of the bone microenvironment impact on primary bone cancer growth. Besides these indirect ‘environmental’ factors induced by vitamin D deficiency, the VDR has recently been reported to be highly expressed in human osteosarcoma [44], [45]. Moreover, Thompson et al. have demonstrated that osteosarcoma cells respond to vitamin D treatment by undergoing differentiation and apoptosis [45]. In the current multi-centre study, we found a high prevalence of vitamin D deficiency and insufficiency in patients with osteosarcoma (Table 1). Considering the direct and indirect effects of vitamin D on osteosarcoma, it may be suggested that vitamin D should routinely be assessed in patients with osteosarcoma. Concomitantly, we found low vitamin D levels in patients with chondrosarcoma (Table 1). Although there is a lack of data on vitamin D status in patients with chondrosarcoma, it may be assumed that alterations of the bone microenvironment due to vitamin D deficiency similarly promote local cancer growth. Moreover, we observed particularly low vitamin D levels in patients with lymphoma (Table 1). Although only five cases were recorded in this study, this observation might be of value as it has been demonstrated that low vitamin D status is associated with increased tumour stage and worse clinical outcomes of patients with lymphoma [46], [47].
The most striking result to emerge from the current study is that mean vitamin D levels of patients with malignant bone tumours were significantly lower compared to patients with benign bone tumours. In 2008 the International Agency for Research on Cancer (IARC) released a report on ‘Vitamin D in Cancer’ [48], [49]. In a systematic review they concluded that there is a preventative role of vitamin D in colorectal cancer [48], [49]. More recent reports on vitamin D and cancer risk support a role of higher 25(OH)D concentrations in reducing risk of breast and colorectal cancer incidence and mortality [50]. In addition, the secondary analysis from the VITAL clinical trial indicated significant reductions from 2.000 IU per day of vitamin D3 supplementation in all-cancer incidence and mortality rates for selected subgroups [50]. However, an association between low vitamin D levels and bone cancer risk has so far not been described.
Together, it is likey that vitamin D signaling impacts on tumour growth in most, if not all, bone tumours. These effects are possibly attributable to indirect effects of vitamin D deficiency on the bone micronenvironment but also to direct effects of vitamin D on cancer cells.
We are aware that this investigation has several limitations. At first, the shown data represents patient data of a geographical localisation between 48.14 and 53.14°N latitude. As such, patient data can only be compared to serum 25(OH)D levels of patients living in comparable latitudes. Moreover, we did not perform cross-calibrations between the different methods/assays used at the reported institutes. For this reason, there might be slight differences between measurements comparing the diverse locations. Furthermore, this is a pure observational study and no causal relationship between vitamin D status and tumour malignancy can be drawn. Comparing vitamin D levels of patients in the current study with malignant bone tumours to vitamin D status of patients living in similar latitudes, apparent differences can be found. For example, in an overall pooled estimate, irrespective of age group, ethnic mix, and latitude of study populations, it has been shown that 40% of the 55,844 European individuals had serum 25(OH)D concentrations <20 ng/ml (50 nmol/L) [51]. In contrast, 60% of patients with malignant bone tumours of the current study were identified to be vitamin D deficient. However, comparing data from the current study to vitamin D status of the general population in Germany, no clear differences can be found [52], [53]. Further, we have previously conducted a single-center analysis measuring vitamin D status of 1119 patients that consecutively admitted to an orthopaedic surgery department of a university hospital in Germany [54]. Although vitamin D levels of patients with malignant bone tumours in the current study [mean 19.3 ng/ml (48.25 nmol/L)] are lower than vitamin D levels of patients in the last-mentioned study [mean 20.57 ng/ml (51.43 nmol/L)], no apparent differences in vitamin D status of patients with bone tumours compared to orthopaedic patients in general can be found.
Moreover, most patients tested in this study were lighter skinned-toned. Thus, vitamin D status among darker-skinned patients might be underrepresented in this study. Lastly, no significant changes in mean PTH or calcium levels between cohorts were observed. For this reason, it is debatable whether or not vitamin D deficiency and insufficiency actually caused alterations within the bone microenvironment of patients. Consequently, the described indirect effects of vitamin D deficiency on local bone tumour growth might be neglectable.
5. Conclusion
We identified a concerning rate of vitamin D deficiency and insufficiency in patients with diverse bone tumours. Most notably, vitamin D levels of patients with malignant bone tumours were significantly lower compared to patients with benign bone tumours. It is undisputable that adequate vitamin D levels are of utmost importance for patients with bone tumours and for cancer patients that are at high risk of developing bone metastasis. Especially, it should be borne in mind that vitamin D supplementation is simple and safe. For this reason, there should be an increased awareness for physicians to assess and if necessary correct vitamin D status of patients with bone tumours.
Financial support
This publication was supported by the OpenAccess Publication Fund of the University of Wuerzburg.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Guise T.A., Mundy G.R. Cancer and bone. Endocr. Rev. 1998;19(1):18–54. doi: 10.1210/edrv.19.1.0323. [DOI] [PubMed] [Google Scholar]
- 2.Flanagan A.M., Lindsay D. A diagnostic approach to bone tumours. Pathology. 2017;49(7):675–687. doi: 10.1016/j.pathol.2017.08.003. [DOI] [PubMed] [Google Scholar]
- 3.Zheng Y.u., Zhou H., Dunstan C.R., Sutherland R.L., Seibel M.J. The role of the bone microenvironment in skeletal metastasis. J. Bone Oncol. 2013;2(1):47–57. doi: 10.1016/j.jbo.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Horas K., Holzapfel B.M., Jakob F., Kurth A.A., Maier G. The role of vitamin D and the vitamin D receptor in bone oncology. Osteologie. 2018;27(03):129–134. [Google Scholar]
- 5.Horas K., Maier G., Jakob F., Maus U., Kurth A., Jakuscheit A., Rudert M., Holzapfel B.M. High prevalence of vitamin D deficiency in patients with bone tumors. Cancer Invest. 2017;35(8):562–568. doi: 10.1080/07357907.2017.1351985. [DOI] [PubMed] [Google Scholar]
- 6.Mundy G.R. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat. Rev. Cancer. 2002;2(8):584–593. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 7.Coleman R.E. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat. Rev. 2001;27(3):165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 8.Christakos S., Dhawan P., Verstuyf A., Verlinden L., Carmeliet G. Vitamin D: metabolism, molecular mechanism of action, and pleiotropic effects. Physiol. Rev. 2016;96(1):365–408. doi: 10.1152/physrev.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holick M.F. Humana Press; 2010. Vitamin D: Physiology, Molecular Biology, and Clinical Applications. [Google Scholar]
- 10.Zheng Y.u., Zhou H., Ooi L.L., Snir A.D., Dunstan C.R., Seibel M.J. Vitamin D deficiency promotes prostate cancer growth in bone: vitamin D and prostate cancer growth in bone. Prostate. 2011;71(9):1012–1021. doi: 10.1002/pros.21316. [DOI] [PubMed] [Google Scholar]
- 11.Ooi L.L., Zhou H., Kalak R., Zheng Y., Conigrave A.D., Seibel M.J., Dunstan C.R. Vitamin D deficiency promotes human breast cancer growth in a murine model of bone metastasis. Cancer Res. 2010;70(5):1835–1844. doi: 10.1158/0008-5472.CAN-09-3194. [DOI] [PubMed] [Google Scholar]
- 12.Kim H., Giovannucci E. Vitamin D status and cancer incidence, survival, and mortality. Adv. Exp. Med. Biol. 2020;1268:39–52. doi: 10.1007/978-3-030-46227-7_3. [DOI] [PubMed] [Google Scholar]
- 13.Giovannucci E., Liu Y., Rimm E.B., Hollis B.W., Fuchs C.S., Stampfer M.J., Willett W.C. Prospective study of predictors of vitamin D status and cancer incidence and mortality in men. J. Natl. Cancer Inst. 2006;98(7):451–459. doi: 10.1093/jnci/djj101. [DOI] [PubMed] [Google Scholar]
- 14.Garland C.F., Garland F.C., Gorham E.D., Lipkin M., Newmark H., Mohr S.B., Holick M.F. The role of vitamin D in cancer prevention. Am. J. Public Health. 2006;96(2):252–261. doi: 10.2105/AJPH.2004.045260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feldman D., Krishnan A.V., Swami S., Giovannucci E., Feldman B.J. The role of vitamin D in reducing cancer risk and progression. Nat. Rev. Cancer. 2014;14(5):342–357. doi: 10.1038/nrc3691. [DOI] [PubMed] [Google Scholar]
- 16.Tagliabue E., Raimondi S., Gandini S. Vitamin D, cancer risk, and mortality. Adv. Food Nutr. Res. 2015;75:1–52. doi: 10.1016/bs.afnr.2015.06.003. [DOI] [PubMed] [Google Scholar]
- 17.Coleman R.E., Major P., Lipton A., Brown J.E., Lee K.A., Smith M., Saad F., Zheng M., Hei Y.J., Seaman J., Cook R. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J. Clin. Oncol. 2005;23(22):4925–4935. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 18.Churilla T.M., Brereton H.D., Klem M., Peters C.A. Vitamin D deficiency is widespread in cancer patients and correlates with advanced stage disease: a community oncology experience. Nutr. Cancer. 2012;64(4):521–525. doi: 10.1080/01635581.2012.661515. [DOI] [PubMed] [Google Scholar]
- 19.Peng X., Hawthorne M., Vaishnav A., St-Arnaud R., Mehta R.G. 25-Hydroxyvitamin D3 is a natural chemopreventive agent against carcinogen induced precancerous lesions in mouse mammary gland organ culture. Breast Cancer Res. Treat. 2009;113(1):31–41. doi: 10.1007/s10549-008-9900-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend K., Banwell C.M., Guy M., Colston K.W., Mansi J.L., Stewart P.M., Campbell M.J., Hewison M. Autocrine metabolism of vitamin D in normal and malignant breast tissue. Clin. Cancer Res. 2005;11(9):3579–3586. doi: 10.1158/1078-0432.CCR-04-2359. [DOI] [PubMed] [Google Scholar]
- 21.Cross H.S., Lipkin M., Kallay E. Nutrients regulate the colonic vitamin D system in mice: relevance for human colon malignancy. J. Nutr. 2006;136(3):561–564. doi: 10.1093/jn/136.3.561. [DOI] [PubMed] [Google Scholar]
- 22.Pendas-Franco N., Gonzalez-Sancho J.M., Suarez Y., Aguilera O., Steinmeyer A., Gamallo C., Berciano M.T., Lafarga M., Munoz A. Vitamin D regulates the phenotype of human breast cancer cells. Differentiation. 2007;75(3):193–207. doi: 10.1111/j.1432-0436.2006.00131.x. [DOI] [PubMed] [Google Scholar]
- 23.Rohan J.N., Weigel N.L. 1Alpha,25-dihydroxyvitamin D3 reduces c-Myc expression, inhibiting proliferation and causing G1 accumulation in C4–2 prostate cancer cells. Endocrinology. 2009;150(5):2046–2054. doi: 10.1210/en.2008-1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ebert R., Schutze N., Adamski J., Jakob F. Vitamin D signaling is modulated on multiple levels in health and disease. Mol. Cell. Endocrinol. 2006;248(1–2):149–159. doi: 10.1016/j.mce.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 25.Klotz B., Mentrup B., Regensburger M., Zeck S., Schneidereit J., Schupp N., Linden C., Merz C., Ebert R., Jakob F. 1,25-dihydroxyvitamin D3 treatment delays cellular aging in human mesenchymal stem cells while maintaining their multipotent capacity. PLoS ONE. 2012;7(1) doi: 10.1371/journal.pone.0029959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jeon S.M., Shin E.A. Exploring vitamin D metabolism and function in cancer. Exp. Mol. Med. 2018;50(4):20. doi: 10.1038/s12276-018-0038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holick M.F. Vitamin D deficiency. New Engl. J. Med. 2007;357(3):266–281. doi: 10.1056/NEJMra070553. [DOI] [PubMed] [Google Scholar]
- 28.Holick M.F., Binkley N.C., Bischoff-Ferrari H.A., Gordon C.M., Hanley D.A., Heaney R.P., Murad M.H., Weaver C.M. Evaluation, treatment, and prevention of vitamin D deficiency: an Endocrine Society clinical practice guideline. J. Clin. Endocrinol. Metabol. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385. [DOI] [PubMed] [Google Scholar]
- 29.Holick M.F. The vitamin D deficiency pandemic: approaches for diagnosis, treatment and prevention. Rev. Endocrine Metabol. Disord. 2017;18(2):153–165. doi: 10.1007/s11154-017-9424-1. [DOI] [PubMed] [Google Scholar]
- 30.Harrison S.R., Li D., Jeffery L.E., Raza K., Hewison M. Vitamin D, autoimmune disease and rheumatoid arthritis. Calcif. Tissue Int. 2020;106(1):58–75. doi: 10.1007/s00223-019-00577-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang T.J. Vitamin D and cardiovascular disease. Annu. Rev. Med. 2016;67:261–272. doi: 10.1146/annurev-med-051214-025146. [DOI] [PubMed] [Google Scholar]
- 32.Charoenngam N., Shirvani A., Holick M.F. Vitamin D for skeletal and non-skeletal health: What we should know. J. Clin. Orthop. Trauma. 2019;10(6):1082–1093. doi: 10.1016/j.jcot.2019.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McDonnell S.L., Baggerly C., French C.B., Baggerly L.L., Garland C.F., Gorham E.D., Lappe J.M., Heaney R.P. Serum 25-hydroxyvitamin D Concentrations >/=40 ng/ml are associated with >65% lower cancer risk: pooled analysis of randomized trial and prospective cohort study. PLoS ONE. 2016;11(4) doi: 10.1371/journal.pone.0152441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van der Rhee H., Coebergh J.W., de Vries E. Sunlight, vitamin D and the prevention of cancer: a systematic review of epidemiological studies. Eur. J. Cancer Prevent. 2009;18(6):458–475. doi: 10.1097/CEJ.0b013e32832f9bb1. [DOI] [PubMed] [Google Scholar]
- 35.Campbell M.J., Trump D.L. Vitamin D receptor signaling and cancer. Endocrinol. Metab. Clin. North Am. 2017;46(4):1009–1038. doi: 10.1016/j.ecl.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Horas K., Zheng Y., Fong-Yee C., Macfarlane E., Manibo J., Chen Y., Qiao J., Gao M., Haydar N., McDonald M.M., Croucher P.I., Zhou H., Seibel M.J. Loss of the vitamin D receptor in human breast cancer cells promotes epithelial to mesenchymal cell transition and skeletal colonization. J. Bone Mineral Res. 2019;34(9):1721–1732. doi: 10.1002/jbmr.3744. [DOI] [PubMed] [Google Scholar]
- 37.Zheng Y., Trivedi T., Lin R.C., Fong-Yee C., Nolte R., Manibo J., Chen Y., Hossain M., Horas K., Dunstan C., Zhou H., Seibel M.J. Loss of the vitamin D receptor in human breast and prostate cancers strongly induces cell apoptosis through downregulation of Wnt/beta-catenin signaling. Bone Res. 2017;5:17023. doi: 10.1038/boneres.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Horas K., Zheng Y., Zhou H., Seibel M.J. Animal models for breast cancer metastasis to bone: opportunities and limitations. Cancer Invest. 2015:1–10. doi: 10.3109/07357907.2015.1065500. [DOI] [PubMed] [Google Scholar]
- 39.Mundy G.R. Mechanisms of bone metastasis. Cancer. 1997;80(8 Suppl):1546–1556. doi: 10.1002/(sici)1097-0142(19971015)80:8+<1546::aid-cncr4>3.3.co;2-r. [DOI] [PubMed] [Google Scholar]
- 40.Maier G.S., Horas K., Kurth A.A., Lazovic D., Seeger J.B., Maus U. Prevalence of vitamin D deficiency in patients with bone metastases and multiple myeloma. Anticancer Res. 2015;35(11):6281–6285. [PubMed] [Google Scholar]
- 41.Fuchs B., Pritchard D.J. Etiology of osteosarcoma. Clin. Orthop. Related Res. 2002;397:40–52. doi: 10.1097/00003086-200204000-00007. [DOI] [PubMed] [Google Scholar]
- 42.Weaver C.M. Adolescence: the period of dramatic bone growth. Endocrine. 2002;17(1):43–48. doi: 10.1385/ENDO:17:1:43. [DOI] [PubMed] [Google Scholar]
- 43.Brennecke P., Arlt M.J., Muff R., Campanile C., Gvozdenovic A., Husmann K., Holzwarth N., Cameroni E., Ehrensperger F., Thelen M., Born W., Fuchs B. Expression of the chemokine receptor CXCR7 in CXCR4-expressing human 143B osteosarcoma cells enhances lung metastasis of intratibial xenografts in SCID mice. PLoS ONE. 2013;8(9) doi: 10.1371/journal.pone.0074045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gallagher R., Keighley J., Tancabelic J., Garimella R., Pinson D., Templeton K., Tawfik O. Clinicopathologic correlation of vitamin D receptor expression with retinoid X receptor and MIB-1 expression in primary and metastatic osteosarcoma. Ann. Diagn. Pathol. 2012;16(5):323–329. doi: 10.1016/j.anndiagpath.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 45.Thompson L., Wang S., Tawfik O., Templeton K., Tancabelic J., Pinson D., Anderson H.C., Keighley J., Garimella R. Effect of 25-hydroxyvitamin D3 and 1 α,25 dihydroxyvitamin D3 on differentiation and apoptosis of human osteosarcoma cell lines. J. Orthop. Res. 2012;30(5):831–844. doi: 10.1002/jor.21585. [DOI] [PubMed] [Google Scholar]
- 46.Chen P., Cao Y., Duan X., Li J., Zhao W., Wang H. Bioavailable 25(OH)D level is associated with clinical outcomes of patients with diffuse large B-cell lymphoma: an exploratory study. Clin. Nutr. 2020 doi: 10.1016/j.clnu.2020.04.040. [DOI] [PubMed] [Google Scholar]
- 47.Ng A.C., Kumar S.K., Rajkumar S.V., Drake M.T. Impact of vitamin D deficiency on the clinical presentation and prognosis of patients with newly diagnosed multiple myeloma. Am. J. Hematol. 2009;84(7):397–400. doi: 10.1002/ajh.21412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant W.B. A critical review of Vitamin D and Cancer: a report of the IARC working group. Dermato-endocrinology. 2009;1(1):25–33. doi: 10.4161/derm.1.1.7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.I.A.f.R.o.C. (IARC), Vitamin D and Cancer, IARC Working Group Report Lyon, France, 2008.
- 50.Grant W.B. Review of recent advances in understanding the role of vitamin D in reducing cancer risk: breast, colorectal, prostate, and overall cancer. Anticancer Res. 2020;40(1):491–499. doi: 10.21873/anticanres.13977. [DOI] [PubMed] [Google Scholar]
- 51.Cashman K.D., Dowling K.G., Škrabáková Z., Gonzalez-Gross M., Valtueña J., De Henauw S., Moreno L., Damsgaard C.T., Michaelsen K.F., Mølgaard C., Jorde R., Grimnes G., Moschonis G., Mavrogianni C., Manios Y., Thamm M., Mensink G.B., Rabenberg M., Busch M.A., Cox L., Meadows S., Goldberg G., Prentice A., Dekker J.M., Nijpels G., Pilz S., Swart K.M., van Schoor N.M., Lips P., Eiriksdottir G., Gudnason V., Cotch M.F., Koskinen S., Lamberg-Allardt C., Durazo-Arvizu R.A., Sempos C.T., Kiely M. Vitamin D deficiency in Europe: pandemic? Am. J. Clin. Nutr. 2016;103(4):1033–1044. doi: 10.3945/ajcn.115.120873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zittermann A. The estimated benefits of vitamin D for Germany. Mol. Nutr. Food Res. 2010;54(8):1164–1171. doi: 10.1002/mnfr.200900494. [DOI] [PubMed] [Google Scholar]
- 53.Hintzpeter B., Mensink G.B., Thierfelder W., Muller M.J., Scheidt-Nave C. Vitamin D status and health correlates among German adults. Eur. J. Clin. Nutr. 2008;62(9):1079–1089. doi: 10.1038/sj.ejcn.1602825. [DOI] [PubMed] [Google Scholar]
- 54.Maier G.S., Jakob P., Horas K., Roth K.E., Kurth A.A., Maus U. Vitamin D deficiency in orthopaedic patients: a single center analysis. Acta Orthop. Belg. 2013;79(5):587–591. [PubMed] [Google Scholar]