Abstract
Purpose
Despite triple antiemetic therapy use for breast cancer patients receiving emetogenic chemotherapy, nausea remains a clinical challenge. We evaluated adding olanzapine (5 mg) to triple therapy on nausea control in patients at high personal risk of chemotherapy-induced nausea and vomiting (CINV).
Methods
This multi-centre, placebo-controlled, double-blind trial randomized breast cancer patients scheduled to receive neo/adjuvant chemotherapy with anthracycline-cyclophosphamide or platinum-based chemotherapy to olanzapine (5 mg, days 1–4) or placebo. Primary endpoint was frequency of self-reported significant nausea, repeated for all cycles of chemotherapy. Secondary endpoints included: duration of nausea, overall total control of CINV, Health Related Quality of Life (HRQoL) using FLIE questionnaire, use of rescue mediation and treatment-related adverse events.
Results
218 eligible patients were randomised to placebo (105) or olanzapine (113). From days 0–5 following each cycle of chemotherapy, 41.3% (95%CI: 36.1–46.7%) of patients in the placebo group reported significant nausea compared to 27.7% (95%CI: 23.2–32.4%) in the olanzapine group (p = 0.001). Across all cycles of chemotherapy, patients receiving olanzapine experienced a statistically significant improvement in HRQoL (p < 0.001). Grade 1/2 sedation was the most commonly side effect reported at 40.8% in the placebo group vs. 54.1% with olanzapine (p < 0.001).
Conclusion
In patients at high personal risk of CINV, the addition of olanzapine 5 mg daily to standard antiemetic therapy significantly improves the control of nausea, HRQoL, with no unexpected toxicities.
Keywords: Breast cancer, Chemotherapy-induced nausea and vomiting, Risk model, Olanzapine
Highlights
-
•
Double-blind trial evaluated the addition of olanzapine to triple therapy in patients at high personal risk of CINV.
-
•
Adding 5 mg olanzapine was associated with significantly improved nausea control with no unexpected toxicities.
-
•
Olanzapine plus triple therapy should be considered standard of care for breast cancer patients at high risk of CINV.
Background
Despite multiple practice-based guidelines [[1], [2], [3]] recommending the use of “triple drug antiemetic therapy” (i.e. neurokinin 1 (NK1) receptor antagonist, 5HT3 receptor antagonist and dexamethasone) for patients receiving anthracycline and cyclophosphamide chemotherapy for breast cancer [4,5], nausea remains a common complication [6,7]. Approximately 70% of breast cancer patients still have uncontrolled chemotherapy-induced nausea and vomiting (CINV) and nausea in particular, even after “optimal guideline-directed” antiemetic therapy [1,[7], [8], [9], [10], [11]]. Regardless, most CINV trials continue to use vomiting-related endpoints as their primary study outcome [7,[12], [13], [14]]. In addition, despite significant variability in individual patient risk and the availability of validated models that can prospectively identify patents at high personal risk of CINV [[15], [16], [17], [18], [19]], most studies continue to use type of chemotherapy regimen as their defining factor for CINV risk.
Olanzapine targets dopaminergic, serotonergic, adrenergic, histaminergic, and muscarinic receptors. In combination with triple therapy olanzapine has significant anti-nausea effects at both the 10 mg [11,20] and 5 mg (days 1–4) doses [21,22]. Evidence-based practice guidelines recommend its use both upfront and in patients with uncontrolled nausea and vomiting [3,[23], [24], [25], [26]]. However, there has been limited incorporation of olanzapine into broader clinical practice [27,28]. This likely reflects concerns around its sedative and extra pyramidal effects [29].
Given the availability of validated risk models to prospectively identify patients at high personal risk of uncontrolled CINV [1,19,[30], [31], [32]] and the reported efficacy of olanzapine 5 mg, the current trial was designed to help clinicians prescribe antiemetics in a more personalized and evidence-based manner.
Patients and methods
Chemotherapy naïve, newly diagnosed breast cancer patients scheduled to receive neo/adjuvant chemotherapy with anthracycline-cyclophosphamide or platinum-based chemotherapy were enrolled. The main inclusion criteria included the ability to provide written informed consent and to complete all study-related diaries and questionnaires. Regulatory approval was obtained through Health Canada and ethics approval was through the Ontario Cancer Research Ethics Board (OCREB). The trial was registered with clinicaltrials.gov (NCT02861859).
Personal risk of emesis
Patients who were eligible and gave informed consent had their personal CINV risk score calculated using the Personalized Risk Model [17,19,32,33]. Patients with acute (i.e. first 24 h after chemotherapy) risk scores of ≥7 and/or delayed (i.e. 1–5 days after chemotherapy) score of ˃16 are classified as being high risk [4,5,19]. Patients at high risk were enrolled into the current study. Patients were prescribed their antiemetics several days before chemotherapy administration. On the morning prior to each cycle of chemotherapy, the actual amount of sleep the night before, the use of rescue antiemetics during the prior chemotherapy cycle and patient’s expectation to become nauseous were collected.
Study design, treatments and randomization
This was a double-blind, placebo-controlled, multi-centre, randomized trial. Eligible patients were randomized 1:1 to: Standard of care: aprepitant (125 mg PO OD day 1, 80 mg OD days 2–3), ondansetron (8 mg PO, BID on Day 1), dexamethasone (12 mg IV ×1 before chemotherapy and 4 mg PO BID days 2–3) and an identical olanzapine placebo (PO OD days 1–4), or to triple therapy but with olanzapine (5 mg PO OD days 1–4). The choice of rescue medication was left to the treating physician. If the patient had poorly controlled CINV and the physician wished to prescribe olanzapine for a subsequent cycle, then the patients subsequent CINV data was not included in the analysis. CINV outcomes were measured during each cycle of chemotherapy over the entire course of treatment with standardized patient diaries. Eligible and consenting patients were randomized using a web-based randomization system (developed by the Ottawa Methods Centre). A permuted variable block design with block sizes of four and six was used. Patients were stratified by study site, and chemotherapy regimen (i.e. FEC vs. AC vs. platinum). Patients, physicians and study coordinators were blinded to the treatment for the duration of the study.
Study hypothesis
In breast cancer patients receiving anthracycline-cyclophosphamide or platinum-based chemotherapy regimens at high personal risk for CINV, we hypothesized that the addition of olanzapine (5 mg) to standard antiemetic therapy would significantly reduce the prevalence of nausea over multiple cycles of chemotherapy.
Data collection
Patient diaries had been piloted previously [17,19,32,33] and included: Functional Living Index-Emesis (FLIE) questionnaires, Likert scores for nausea and vomiting and sections for additional study-related information. The FLIE is a validated patient-reported measure of the impact of CINV on daily life [34,35] with higher scores indicating better control of CINV and improved HRQoL [36]. The FLIE questionnaire also contains a self-rated nausea score where patients mark their self-rated symptoms as a vertical line through a 100 mm visual analogue scale (VAS). With the FLIE, the VAS scores are evaluated as follows: 0–5 mm (“no nausea”), 6–25 mm (“no significant nausea”), and >25 mm (“significant nausea”). The FLIE indexes were completed on days 1, 2 and 6 of each cycle.
The diary contained sections for patients to record nausea and/or vomiting episodes and their duration. For each episode of nausea, the patient rated both their nausea score (0 = none, 1 = able to eat, 2 = oral intake significantly decreased, 3 = requiring IV fluids) and its severity (1 = none, 2 = mild, 2 = moderate, 4 = severe). Similarly, a Likert Scale was used to record both the vomiting score (0 = none, 1 = 1 episode in 24 h, 2 = 2–5 episodes, 3= >6 episodes in 24 h or need for IV fluids, 4 = requiring hospitalization) and severity (1 = none, 2 = mild, 3 = moderate, 4 = severe). Use of rescue medications (type and timing) were also recorded. Prior to the next cycle of chemotherapy, patients were asked to rate their satisfaction with their overall control of nausea and vomiting on a 4-point scale (Excellent, Satisfactory, Poor, Terrible). Patients were contacted on days 2 and 6 by the study coordinator to assess the adverse events and to remind the patient to complete the questionnaires.
The NCI-CTCAE Version 4.02 was used to evaluate the side effects secondary to adding olanzapine to standard antiemetic therapy during the Day 2 and 6 telephone calls and at the post chemotherapy clinic visit [37]. Particular attention was given to symptoms of sedation and extrapyramidal drug effects.
Study endpoints
The primary endpoint was the frequency of self-reported significant nausea (defined at ≥ 26 mm on a 0–100 mm visual analog scale and/or moderate nausea on the 4 point Likert Scale) at any time, repeated over all cycles of chemotherapy. Secondary endpoints included complete cycle response (defined as no nausea, no vomiting and no use of rescue medications), control of acute and delayed nausea and vomiting, need for rescue medication, duration of nausea, HRQoL and treatment-related adverse events.
Sample size and statistical considerations
The prevalence of significant nausea from 0 to 120 h following each of 3–6 cycles of chemotherapy in patients deemed to be at “high personal emetic risk” is approximately 70% with standard aprepitant-based antiemetic therapy [16]. If the addition of olanzapine reduces the absolute risk of significant nausea by 12.5% (to 57.5%), this was deemed as being clinically significant by our group of practicing oncologists. With a target sample size of 100 per arm the study had an 80% power to detect an odds ratio (OR) of 0.52 (risk reduction in favour of the experimental group) in a design with 3 repeated measurements, with an alpha = 0.05. Given an anticipated 10% patient attrition rate, the final targeted sample size was 220 patients in this randomized study.
Patient demographics, clinical and treatment characteristics were presented descriptively as mean, medians or proportions for each intervention group. Nausea control between groups over all cycles of chemotherapy was compared using a main-effects generalized estimating equations (GEE) model, with an adjustment for clustering on the patient. Repeated measures mixed models were used to compare HRQoL between groups at day 2 and 6 following chemotherapy, over the full course of treatment. All efficacy endpoints were tested using the Hochberg step-up test procedure, which controls the overall level of significance at the 2-tailed, 0.05 level and minimizes the risk of a type I error [38]. The data were analyzed based on the principle of intention to treat. Due the effect of multiple statistical analyses on the threshold for statistical significance of the primary endpoint, no statistical comparisons of toxicities was planned. Any missing values in the efficacy outcome variables were treated as missing at random and included in the analysis. All of the statistical analyses were performed using Stata, V14.0 (Stata Corp., College Station, Texas, USA).
Results
From Dec 2016 to June 2019, 229 patients were randomised, however 11 were excluded from the analysis for protocol violations or withdrawal of consent (The participant flow diagram is presented in Fig. 1). The remaining 218 patients received the allocated intervention 105/218 (48%) to placebo and 113/218 (52%) to olanzapine. The patient and treatment characteristics in Table 1. Overall, 346 and 383 cycles of chemotherapy were received in the placebo or olanzapine groups respectively. Of patients in the control and olanzapine arms, totals of 76.2% and 82.3% (OR = 1.14, 95%CI 0.52 to 2.58) completed all their planned cycles of chemotherapy (median = 3 in both groups), respectively). The number of patients stopping chemotherapy early due to poor nausea control was 13 (12.4%) in the control arm and 4 with olanzapine (3.5%) (odds ratio [OR] 0.30 [95%CI: 0.007 to 1.04]).
Fig. 1.
CONSORT diagram.
Table 1.
Characteristics of patients and treatments in the olanzapine and control groups.
| Characteristic | Control Group (n = 105) |
Olanzapine Group (n = 113) |
|---|---|---|
| Mean age (range) | 52 (23–88) | 50 (23–74) |
| Mean weight in kg (range) | 77 (36–138) | 78 (39–127) |
| Breast cancer Stage | ||
| I | 7.7% | 8.0% |
| II | 57.7% | 54.0% |
| III | 34.6% | 36.3% |
| Concomitant medical conditionsa | 96.1% | 98.2% |
| History of motion sickness | 42.9% | 41.4% |
| History of morning sickness (if applicable) | 63.8% | 58.4% |
| History of alcohol intake | ||
| Less than 1 drink/day | 41.9% | 47.8% |
| More than 1 drink/day | 16.2% | 15.0% |
| None | 41.9% | 37.2% |
| Planned chemotherapy | ||
| AC x 4 | 51.4% | 53.1% |
| FEC x 3 | 39.0% | 37.2% |
| TCH x 6 | 9.5% | 9.7% |
| Median number of cycles (range) | 3 (1–6) | 3 (1–6) |
| Acute CINV risk score at enrollment (range)b | 8 (5–12) | 8 (5–12) |
| Delayed CINV risk score enrollment (range)c | 20 (11–37) | 20 (10–50) |
| Number of delivered cycles of chemotherapy | ||
| One | 12.4% | 6.2% |
| Two | 4.8% | 2.6% |
| Three | 38.1% | 41.6% |
| Four | 38.1% | 44.2% |
| ≥ Five | 6.7% | 5.3% |
| Total cycles delivered | 346 | 383 |
| Completed studyd | 76.2% | 82.3% |
Abbreviations: A = doxorubicin, C = cyclophosphamide, F = 5-fluorouracil, E = epirubicin, H = trastuzumab, T = docetaxel, CINV = chemotherapy induced nausea and vomiting.
Cardiovarcular disease, diabetes, gastrointestinal, musculoskeletal, thyroid, other.
From the acute risk model developed by Dranitaris et al. (2009). Patients with a risk score of 8 had an acute N&V risk of approximately 24%.
From the delayed risk model developed by Petrella et al. (2009). Patients with a risk score of 20 had an acute N&V risk of approximately 40%.
13 and 4 patients in the placebo and olanzapine groups quit the study because of poor nausea control.
Over all cycles of chemotherapy, patients in both groups received comparable prescribed antiemetics (Supplemental Table 1). Approximately 10% more patients in the placebo group self-medicated to alleviate CINV at home (Table 2).
Table 2.
Outcomes data at 24 h and days 2–5 following chemotherapy.
| Outcomes | Control Group (n = 346) |
Olanzapine Group (n = 383) |
|---|---|---|
| Overall nausea control from day 0 to 51 | ||
| Significant nausea | 41.3% | 27.7% |
| None or mild nausea | 56.1% | 72.1% |
| Missing | 2.6% | 0.03% |
| Overall vomiting control from days 0 to 52 | ||
| Yes | 7.5% | 4.2% |
| No | 89.3% | 95.8% |
| Missing | 3.2% | 0.03% |
| Acute nausea3 | ||
| Significant nausea | 28.3% | 19.6% |
| None or mild nausea | 69.4% | 80.4% |
| Missing | 2.3% | 0.0% |
| Acute vomiting4 | ||
| Yes | 3.2% | 2.1% |
| No | 94.5% | 97.9% |
| Missing | 2.3% | 0.0% |
| Delayed nausea5 | ||
| Significant nausea | 32.4% | 21.9% |
| None or mild nausea | 64.4% | 77.3% |
| Missing | 3.2% | 0.8% |
| Delayed vomiting6 | ||
| Yes | 5.2% | 3.6% |
| No | 91.6% | 96.1% |
| Missing | 3.2% | 0.03% |
| Mean duration of nausea (hours; 95%CI)7 | 23.5 (17.4–29.6) | 10.0 (7.1–12.9) |
| Patients requiring rescue medication over all cycles of chemotherapy8 | 40.0% | 32.1% |
| Complete cycle response from day 0 to day 59 | 32.4% | 41.8% |
1P = 0.001, 2P = 0.066, 3P = 0.011, 4P = 0.36, 5P = 0.006, 6P = 0.32, 7P < 0.001, 8P = 0.041, 9P = 0.03. Based on the Hochberg step-up test procedure, the threshold for statistical significance was P < 0.005.
Primary study endpoint
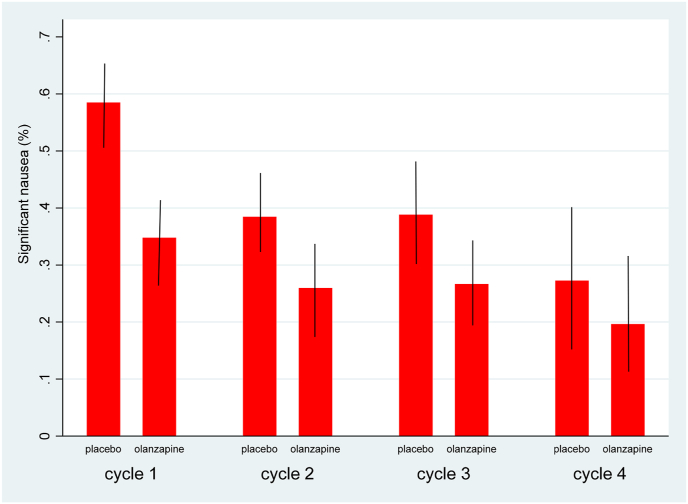
Patients randomized to the placebo group reported more self-reported significant nausea than patients in the olanzapine group (41.3% vs. 27.7%; p = 0.001) (Table 2 and Fig. 2). Significant nausea was more prevalence in the first cycle of chemotherapy, as was the magnitude of the difference between the olanzapine and placebo groups of the study. Over all 4 cycles of chemotherapy, the overall effect of olanzapine on nausea control reached the threshold of statistical significance. Overall vomiting control from day 0 to day 5 over all cycles of chemotherapy showed that even though the proportion was numerically lower in the olanzapine group (4.2% vs. 7.5%; P = 0.066), the difference did not reach statistical significance (Table 2).
Fig. 2.
Overall nausea control over four cycles of chemotherapy with 95% CI (odds ratio = 0.46 [0.30 to 0.72]; P = 0.001). Based on the Hochberg step-up test procedure, the threshold for statistical significance was P < 0.005.
Secondary study endpoints
The occurrence of significant nausea and vomiting was then examined within the first 24 h (acute phase) and from days 2–5 (delayed phase) post-chemotherapy. Both acute (28.3% vs. 19.6%: OR = 1.62, 95%CI: 1.13 to 2.32, P = 0.008) and delayed nausea (32.4% vs. 21.9%: OR = 1.66, 95% CI: 1.18 to 2.33, P = 0.006) were reduced in the olanzapine compared to the placebo groups respectively. The frequency of acute and delayed vomiting was also numerically lower in the olanzapine group, but the difference was not statistically significant (Table 2). Patients who developed nausea from day 0–5, over all cycles of chemotherapy, in the control group were affected for a longer duration of time compared to the olanzapine group (median duration = 23.5 vs. 10 h; P < 0.001) (Table 2). Fewer patients in the placebo group had complete cycle response (i.e. no nausea, no vomiting and no rescue medication) from day 0 to day 5 (32.4% vs. 41.8%; P = 0.03). Over the first three cycles of chemotherapy, complete cycle response rates were numerically higher in the olanzapine groups, but the overall effect did not meet the threshold for statistical significance (Supplemental Fig. 1).
Health Related Quality of Life
Over four cycles of chemotherapy, patients in the olanzapine group had better HRQoL. The significant improvement in nausea in the olanzapine group was reflected in the FLIE index, with significantly higher scores on days 2 (P < 0.001) and 6 (P = 0.001), throughout the entire course of chemotherapy. The greatest magnitude of HRQoL benefit was after the first cycle of chemotherapy (Supplemental Fig. 2). FLIE scores for vomiting control were comparable between groups over the 4 cycles of treatment (Supplemental Fig. 3) and did not reach statistical significance on either day 2 (P = 0.26) or 6 (P = 0.21).
Adverse events
The most common side effects reported in both groups were sedation, fatigue and insomnia, with the majority being of grade 1 or 2 severity (Table 3). In addition, the only events that were of higher magnitude in the olanzapine group (i.e. ≥4%) were grades 1/2 sedation (54.1% vs. 40.8%), extrapyramidal symptoms (17.8% vs. 13.3%) and increased appetite (12.8% vs. 6.6%). Overall, with 20.3% of patients required dose reductions to 2.5 mg dosing and 6.2% discontinued drug due to sedation (Table 3).
Table 3.
Adverse events by cycle of chemotherapy reported between groups.
| Adverse Events | Control Group (n = 346) |
Olanzapine Group (n = 383) |
|---|---|---|
| Sedation | ||
| Grade 1 | 26.6% | 35.8% |
| Grade 2 | 14.2% | 18.3% |
| Grade 3/4 | 0.0% | <1.0% |
| Fatigue | ||
| Grade 1 | 49.1% | 47.2% |
| Grade 2 | 27.4% | 25.6% |
| Grade 3/4 | 0.0% | <1.0% |
| Insomnia | ||
| Grade 1 | 22.5% | 22.4% |
| Grade 2 | 9.5% | 4.2% |
| Grade 3/4 | 0.0% | 0.0% |
| Gait disturbance | ||
| Grade 1 | <1.0% | <1.0% |
| Grade 2 | 0.0% | 0.0% |
| Grade 3/4 | 0.0% | 0.0% |
| Extrapyramidal symptoms | ||
| Grade 1 | 13.3% | 16.2% |
| Grade 2 | <1.0% | 1.6% |
| Grade 3/4 | 0.0% | 0.0% |
| Arm muscle movements | 1.1% | 2.6% |
| Eye twitching | 2.8% | 3.4% |
| Leg muscle movements | <1.0% | 0.0% |
| Restless legs | 2.0% | <1.0% |
| Restlessness | 3.1% | 4.1% |
| Other | 3.4% | 6.7% |
| Increased appetite | 6.6% | 12.8% |
| Dose reductions of study druga | 23 of 105 patients (12.4%) | 23 of 113 patients (20.3%) |
| Drug discontinuation | 0 of 105 patients (0%) | 7 of 113 patients (6.2%)b |
The dose reductions were for several reasons such as dizziness, drowsiness, extrapyramidal symptoms, fatigue, headache, increased appetite.
All of the drug discontinuations were due to sedation.
Discussion
In this randomized, double-blind, placebo-controlled, phase 3 trial of breast cancer patients at high personal risk of CINV, the use of olanzapine 5 mg daily in addition to standard triple antiemetic therapy was associated with a significant reduction in the frequency and duration of nausea, with a modest increase in sedation and extrapyramidal symptoms. The findings of this trial are particularly important to both patients and health care providers. Not only does this study confirm the substantial anti-nausea benefits of olanzapine seen in previous studies at both the 10 mg (PO OD, days 1–4) [11,20] and 5 mg [21,22] doses, it has done so in a population of breast cancer patients identified at high personal risk of emesis through the use of a validated risk-assessment model. Furthermore, this trial used nausea control as the primary endpoint as opposed to complete vomiting control. In addition, the magnitude of benefit in nausea control translated into reduced use of rescue medications, improved HRQoL and more patients completing all their chemotherapy.
The high proportion of patients identified at high personal risk of CINV and the low number in the low personal risk cohort (Fig. 1) is consistent with previous studies [17,19,32,33]. The findings from the current study also revealed that the greatest benefit from olanzapine in high risk patients would be in the first cycle of chemotherapy. Even with the greater use of rescue medications in the placebo group these patients continued to have worse nausea scores throughout the study. This would suggest that for patients at high personal risk of CINV, then olanzapine should be added to standard antiemetic therapy at cycle 1.
There are a number of study limitations that need to be acknowledged. The study did not compare olanzapine 5 mg to the 10 mg dose. However, a recent meta-analysis of 10 randomized trials reported comparable efficacy, but lower side effects with the 5 mg dose [39]. The study enrolled a homogenous sample of breast cancer patients primarily receiving AC or FEC adjuvant chemotherapy. Therefore, the efficacy of olanzapine may not be fully generalizable to all solid tumour patients receiving platinum-based chemotherapy. However, a recent placebo controlled randomized trial in solid tumour patients receiving cisplatin (≥50 mg/m2) reported an 11% improvement in complete response rates with olanzapine 5 mg [40].
In conclusion, the current trial demonstrated that olanzapine 5 mg reduced the frequency and duration of clinically significant nausea in high risk patients. More trials focusing on nausea and incorporating personalized risk factors (via a mathematical model) are needed so we can provide personalized care to our patients and avoid overprescribing. From the current trial, we have demonstrated that this can be achieved, and a lower dose of an effective agent can be reserved for high risk patients.
Contributors
MC, GD, AR, DF, BH, LV, and JH designed the study and prepared the protocol. MC, MS, SS, TN, AR, MM, TH, SM, OF, VK, LV and JH collected the data. MC acted as principal investigator, MS and LV coordinated data entry and GD did the statistical analysis. MC, GD, MS and LV had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. MC, GD, MS and LV wrote the manuscript. All authors were involved in the critical review of the manuscript and approved the final version.
Funding
The study investigators would like to express their gratitude to; The Canadian Cancer Society for funding this clinical trial (2016 CBCF grant competition, M. Clemons) and to the Canadian Cancer Clinical Trials Network (3CTN) for funding and operational support to conduct multicenter academic clinical trials at cancer centres across Canada.
Compliance with ethical standards
Disclosure of Conflicts of Interests: GD reports payment for statistical services from the Ottawa Hospital Research Institute (funded by the Canadian Cancer Society - CBCF study grant) during the conduct of this study. TN reports personal fees (honoraria) from ARIAD, Takeda and Boehringer-Ingelheim, outside the submitted work. MM reports personal fees (honorarium) from Novartis, outside the submitted work. TH reports personal fees (honoraria) from Ipsen, Apobiologix, Celgene, and Genomic Health, outside the submitted work. BH reports consulting fees from Cornerstone Research, outside the submitted work. All other authors declare no competing interests.
Research Involving Human Participants: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and the Ontario Cancer Research Ethics Board and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent: Informed consent was obtained from all trial participants included in the study.
Acknowledgements
We would like to thank the participating patients and their families as well as the oncologists who enrolled patients into the study (Drs Clemons (137), Hilton (26), Sehdev (13), Ng (10), Hsu (8), McGee (7), Srikanthan (5), Song (3), Segal (3), Gertler (3), Dent (1) and Awan (1) (Ottawa Hospital Cancer Centre); Robinson (9), Mates (9), Wasson (4), Vera Badillo (3), Ethier (2) (Kingston General Hospital); Drs Forbes (3), Chandhoke (2), Shim (2), Zalewski (1) Freedman (1) (R.S. McLaughlin Durham Regional Cancer Centre in Oshawa); Drs Kumar (1), Solow (1) Babak (1), Hajra (1) (Markham Stouffville Hospital). We are grateful for the independent Data Safety Monitoring Board (DSMB) (Drs D Rayson, T Asmis and G Nicholas) for their oversight.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.11.002.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Roila F., Herrstedt J., Aapro M., Gralla R.J., Einhorn L.H., Ballatori E., Bria E., Clark-Snow R.A., Espersen B.T., Feyer P., Grunberg S.M., Hesketh P.J., Jordan K., Kris M.G., Maranzano E., Molassiotis A., Morrow G., Olver I., Rapoport B.L., Rittenberg C., Saito M., Tonato M., Warr D. Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol. 2010;21(Suppl 5):v232–243. doi: 10.1093/annonc/mdq194. [DOI] [PubMed] [Google Scholar]
- 2.Basch E., Prestrud A.A., Hesketh P.J., Kris M.G., Feyer P.C., Somerfield M.R., Chesney M., Clark-Snow R.A., Flaherty A.M., Freundlich B., Morrow G., Rao K.V., Schwartz R.N., Lyman G.H. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2011;29(31):4189–4198. doi: 10.1200/jco.2010.34.4614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.War D., Pater J., Trip K., Antiemetic Working Group . Cancer Care Ontario; 2013. Clinical evidence for recommendations. Antiemetic Report. [Google Scholar]
- 4.ASCO oncology guidelines, clinical tools and resources. Clinical update on antiemetics, clinical practice guideline. 2011. http://www.asco.org/sites/www.asco.org/files/antiemetics_guideline_update_slides_92611_correction_may_2014.pdf
- 5.NCCN clinical practice guidelines. 2014. http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf
- 6.Booth C.M., Clemons M., Dranitsaris G., Joy A., Young S., Callaghan W., Trudeau M., Petrella T. Chemotherapy-induced nausea and vomiting in breast cancer patients: a prospective observational study. J Support Oncol. 2007;5(8):374–380. [PubMed] [Google Scholar]
- 7.Ng T.L., Hutton B., Clemons M. Chemotherapy-induced nausea and vomiting: time for more emphasis on nausea? Oncol. 2015;20(6):576–583. doi: 10.1634/theoncologist.2014-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Warr D.G., Hesketh P.J., Gralla R.J., Muss H.B., Herrstedt J., Eisenberg P.D., Raftopoulos H., Grunberg S.M., Gabriel M., Rodgers A., Bohidar N., Klinger G., Hustad C.M., Horgan K.J., Skobieranda F. Efficacy and tolerability of aprepitant for the prevention of chemotherapy-induced nausea and vomiting in patients with breast cancer after moderately emetogenic chemotherapy. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2005;23(12):2822–2830. doi: 10.1200/jco.2005.09.050. [DOI] [PubMed] [Google Scholar]
- 9.Yeo W., Mo F.K., Suen J.J., Ho W.M., Chan S.L., Lau W., Koh J., Yeung W.K., Kwan W.H., Lee K.K., Mok T.S., Poon A.N., Lam K.C., Hui E.K., Zee B. A randomized study of aprepitant, ondansetron and dexamethasone for chemotherapy-induced nausea and vomiting in Chinese breast cancer patients receiving moderately emetogenic chemotherapy. Breast Canc Res Treat. 2009;113(3):529–535. doi: 10.1007/s10549-008-9957-9. [DOI] [PubMed] [Google Scholar]
- 10.Ng T.L., Clemons M., Hutton B., Dranistaris G. Aprepitant versus dexamethasone to prevent delayed emesis after chemotherapy. J Clin Oncol : official journal of the American Society of Clinical Oncology. 2014;32(20):2184–2185. doi: 10.1200/jco.2014.55.3503. [DOI] [PubMed] [Google Scholar]
- 11.Navari R.M., Qin R., Ruddy K.J., Liu H., Powell S.F., Bajaj M., Dietrich L.L., Lafky J.M., Loprinzi C.L. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting (CINV) in patients receiving highly emetogenic chemotherapy (HEC): alliance A221301, a randomized, double-blind, placebo-controlled trial. American Society of Clinical Oncology. 2015 [Google Scholar]
- 12.Ng T., Mazzarello S., Wang Z., Hutton B., Dranitsaris G., Vandermeer L., Smith S., Clemons M. Choice of study endpoint significantly impacts the results of breast cancer trials evaluating chemotherapy-induced nausea and vomiting. Breast Canc Res Treat. 2016;155(2):337–344. doi: 10.1007/s10549-015-3669-8. [DOI] [PubMed] [Google Scholar]
- 13.Hutton B., Clemons M., Mazzarello S., Kuchuk I., Skidmore B., Ng T. Identifying an optimal antiemetic regimen for patients receiving anthracycline and cyclophosphamide-based chemotherapy for breast cancer--an inspection of the evidence base informing clinical decision-making. Canc Treat Rev. 2015;41(10):951–959. doi: 10.1016/j.ctrv.2015.09.007. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez Torres C., Mazzarello S., Ng T., Dranitsaris G., Hutton B., Smith S., Munro A., Jacobs C., Clemons M. Defining optimal control of chemotherapy-induced nausea and vomiting-based on patients’ experience. Support Care Canc: official journal of the multinational association of supportive care in cancer. 2015;23(11):3341–3359. doi: 10.1007/s00520-015-2801-y. [DOI] [PubMed] [Google Scholar]
- 15.Dranitsaris G., Molassiotis A., Clemons M., Roeland E., Schwartzberg L., Dielenseger P., Jordan K., Young A., Aapro M. The development of a prediction tool to identify cancer patients at high risk for chemotherapy-induced nausea and vomiting. Ann Oncol. 2017;28(6):1260–1267. doi: 10.1093/annonc/mdx100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clemons M., Bouganim N., Smith S., Mazzarello S., Vandermeer L., Segal R., Dent S., Gertler S., Song X., Wheatley-Price P., Dranitsaris G. Risk model-guided antiemetic prophylaxis vs physician’s choice in patients receiving chemotherapy for early-stage breast cancer: a randomized clinical trial. JAMA oncology. 2016;2(2):225–231. doi: 10.1001/jamaoncol.2015.3730. [DOI] [PubMed] [Google Scholar]
- 17.Dranitsaris G., Mazzarello S., Smith S., Vandermeer L., Bouganim N., Clemons M. Measuring the impact of guideline-based antiemetic therapy on nausea and vomiting control in breast cancer patients with multiple risk factors. Support Care Canc: official journal of the multinational association of supportive care in cancer. 2016;24(4):1563–1569. doi: 10.1007/s00520-015-2944-x. [DOI] [PubMed] [Google Scholar]
- 18.Dranitsaris G., Clemons M. Risk prediction models for chemotherapy-induced nausea and vomiting: almost ready for prime time? Support Care Canc: official journal of the multinational association of supportive care in cancer. 2014;22(4):863–864. doi: 10.1007/s00520-014-2134-2. [DOI] [PubMed] [Google Scholar]
- 19.Bouganim N., Dranitsaris G., Hopkins S., Vandermeer L., Godbout L., Dent S., Wheatley-Price P., Milano C., Clemons M. Prospective validation of risk prediction indexes for acute and delayed chemotherapy-induced nausea and vomiting. Curr Oncol. 2012;19(6):e414–421. doi: 10.3747/co.19.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Navari R.M., Nagy C.K., Gray S.E. The use of olanzapine versus metoclopramide for the treatment of breakthrough chemotherapy-induced nausea and vomiting in patients receiving highly emetogenic chemotherapy. Support Care Canc: official journal of the multinational association of supportive care in cancer. 2013;21(6):1655–1663. doi: 10.1007/s00520-012-1710-6. [DOI] [PubMed] [Google Scholar]
- 21.Mizukami N., Yamauchi M., Koike K., Watanabe A., Ichihara K., Masumori N., Yamakage M. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting in patients receiving highly or moderately emetogenic chemotherapy: a randomized, double-blind, placebo-controlled study. J Pain Symptom Manag. 2014;47(3):542–550. doi: 10.1016/j.jpainsymman.2013.05.003. [DOI] [PubMed] [Google Scholar]
- 22.Hashimoto H., Abe M., Yanai T., Yamaguchi T., Zenda S., Uchitomi Y., Fukuda H., Mori M., Iwasa S., Yamamoto N., Ohe Y. Study protocol for J-SUPPORT 1604 (J-FORCE): a randomized, double blind, placebo-controlled Phase III study evaluating olanzapine (5 mg) plus standard triple antiemetic therapy for prevention of chemotherapy induced nausea and vomiting in patients receiving cisplatin-based highly emetogenic chemotherapy. Jpn J Clin Oncol. 2018;48(10):950–952. doi: 10.1093/jjco/hyy114. [DOI] [PubMed] [Google Scholar]
- 23.Bc Cancer Agency Guidelines for prevention and treatment of chemotherapy-induced nausea and vomiting in adults. 2018. http://www.bccancer.bc.ca/chemotherapy-protocols-site/Documents/Supportive%20Care/SCNAUSEA_Protocol.pdf
- 24.National Comprehensive Cancer Network CCN clinical practice guidelines in oncology (NCCN guidelines) antiemesis. 2015. https://www.nccn.org/store/login/login.aspx?ReturnURL=http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf
- 25.Hesketh P.J., Kris M.G., Basch E., Bohlke K., Barbour S.Y., Clark-Snow R.A., Danso M.A., Dennis K., Dupuis L.L., Dusetzina S.B., Eng C., Feyer P.C., Jordan K., Noonan K., Sparacio D., Somerfield M.R., Lyman G.H. Antiemetics: American society of clinical oncology clinical practice guideline update. J Clin Oncol. 2017;35(28):3240–3261. doi: 10.1200/jco.2017.74.4789. [DOI] [PubMed] [Google Scholar]
- 26.Navari R.M., Qin R., Ruddy K.J., Liu H., Powell S.F., Bajaj M., Dietrich L., Biggs D., Lafky J.M., Loprinzi C.L. Olanzapine for the prevention of chemotherapy-induced nausea and vomiting. N Engl J Med. 2016;375(2):134–142. doi: 10.1056/NEJMoa1515725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patil V., Noronha V., Joshi A., Parikh P., Bhattacharjee A., Chakraborty S., Jandyal S., Muddu V., Ramaswamy A., Babu K.G., Lokeshwar N., Hingmire S., Ghadyalpatil N., Banavali S., Prabhash K. Survey of implementation of antiemetic prescription standards in Indian oncology practices and its adherence to the American society of clinical oncology antiemetic clinical guideline. Journal of global oncology. 2017;3(4):346–359. doi: 10.1200/jgo.2016.006023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jordan K., Gralla R., Jahn F., Molassiotis A. International antiemetic guidelines on chemotherapy induced nausea and vomiting (CINV): content and implementation in daily routine practice. Eur J Pharmacol. 2014;722:197–202. doi: 10.1016/j.ejphar.2013.09.073. [DOI] [PubMed] [Google Scholar]
- 29.Eli Lilly Canada Inc Product monograph: zyprexa (olanzapine) 2013. http://www.lilly.ca/en/pdf/product-monograph/29_zyprexa-pm_26feb2013.pdf
- 30.Dranitsaris G., Joy A., Young S., Clemons M., Callaghan W., Petrella T. Identifying patients at high risk for nausea and vomiting after chemotherapy: the development of a practical prediction tool. I. Acute nausea and vomiting. J Support Oncol. 2009;7(4):W1–W8. [Google Scholar]
- 31.Petrella T., Clemons M., Joy A., Young S., Callaghan W., Dranitsaris G. Identifying patients at high risk for nausea and vomiting after chemotherapy: the development of a practical validated prediction tool. II. Delayed nausea and vomiting. J Support Oncol. 2009;7(4):W9–W16. [Google Scholar]
- 32.Dranitsaris G., Bouganim N., Milano C., Vandermeer L., Dent S., Wheatley-Price P., Laporte J., Oxborough K.A., Clemons M. Prospective validation of a prediction tool for identifying patients at high risk for chemotherapy-induced nausea and vomiting. J Support Oncol. 2013;11(1):14–21. doi: 10.1016/j.suponc.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 33.Clemons M., Bouganim N., Smith S., Dranitsaris G. A randomized trial comparing risk model guided antiemetic prophylaxis to physician’s choice in patients receiving chemotherapy for early stage breast cancer. JAMA oncology. 2016;2:225–231. doi: 10.1001/jamaoncol.2015.3730. [DOI] [PubMed] [Google Scholar]
- 34.Martin A., Pearson J., Cai B., Elmer M., Horgan K., Lindley C. Validation of a 5-day recall version of the functional living index-emesis (FLIE) quality-of-life questionnaire for chemotherapy-induced emesis. Qual Life Res. 2000 296-296. [Google Scholar]
- 35.Decker G.M., DeMeyer E.S., Kisko D.L. Measuring the maintenance of daily life activities using the functional living index-emesis (FLIE) in patients receiving moderately emetogenic chemotherapy. J Support Oncol. 2006;4(1):35–41. 52. [PubMed] [Google Scholar]
- 36.Martin A.R., Pearson J.D., Cai B., Elmer M., Horgan K., Lindley C. Assessing the impact of chemotherapy-induced nausea and vomiting on patients’ daily lives: a modified version of the Functional Living Index-Emesis (FLIE) with 5-day recall. Support Care Canc: official journal of the multinational association of supportive care in cancer. 2003;11(8):522–527. doi: 10.1007/s00520-003-0482-4. [DOI] [PubMed] [Google Scholar]
- 37.Health UDo, Services H. Common terminology criteria for adverse events (CTCAE) version 4.0. National Institutes of Health, National Cancer Institute. 2009;4(3) [Google Scholar]
- 38.Hochberg Y. A sharper Bonferroni procedure for multiple tests of significance. Biometrika. 1988;75(4):800–802. [Google Scholar]
- 39.Yang T., Liu Q., Lu M., Ma L., Zhou Y., Cui Y. Efficacy of olanzapine for the prophylaxis of chemotherapy-induced nausea and vomiting: a meta-analysis. Br J Clin Pharmacol. 2017;83(7):1369–1379. doi: 10.1111/bcp.13242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hashimoto H., Abe M., Tokuyama O., Mizutani H., Uchitomi Y., Yamaguchi T., Hoshina Y., Sakata Y., Takahashi T.Y., Nakashima K., Nakao M., Takei D., Zenda S., Mizukami K., Iwasa S., Sakurai M., Yamamoto N., Ohe Y. Olanzapine 5 mg plus standard antiemetic therapy for the prevention of chemotherapy-induced nausea and vomiting (J-FORCE): a multicentre, randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(2):242–249. doi: 10.1016/s1470-2045(19)30678-3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.