Abstract
Haemonchus contortus is a critical parasite of goats and sheep. Infection by this blood-feeding gastrointestinal nematode (GIN) parasite has significant health consequences, especially in lambs and kids. The parasite has developed resistance to virtually all known classes of small molecule anthelmintics used to treat it, giving rise in some areas to multidrug resistant parasites that are very difficult to control. Thus, new anthelmintics are urgently needed. Bacillus thuringiensis (Bt) crystal protein 5B (Cry5B), a naturally occurring protein made by a bacterium widely and safely used around the world as a bioinsecticide, represents a new non-small molecule modality for treating GINs. Cry5B has demonstrated anthelmintic activities against parasites of monogastric animals, including some related to those that infect humans, but has not yet been studied in a ruminant. Here we show that H. contortus adults are susceptible to Cry5B protein in vitro. Cry5B produced in its natural form as a spore-crystal lysate against H. contortus infections in goats had no significant efficacy. However, a new Active Pharmaceutical Ingredient (API) paraprobiotic form of Cry5B called IBaCC (Inactivated Bacterium with Cytosolic Crystals), in which Cry5B crystals are encapsulated in dead Bt cell wall ghosts, showed excellent efficacy in vitro against larval stages of H. contortus and relative protein stability in bovine rumen fluid. When given to sheep experimentally infected with H. contortus as three 60 mg/kg doses, Cry5B IBaCC resulted in significant reductions in fecal egg counts (90%) and parasite burdens (72%), with a very high impact on female parasites (96% reduction). These data indicate that Cry5B IBaCC is a potent new treatment tool for small ruminants in the battle against H. contortus.
Keywords: Haemonchus contortus, Anthelmintic, Bacillus thuringiensis crystal Protein Cry5B, Paraprobiotic, Inactivated bacterium with cytosolic crystal (IBaCC), Sheep
Graphical abstract
1. Introduction
There are many species of gastrointestinal nematodes (GINs) that parasitize small ruminants, such as sheep and goats. The main GIN group of concern for producers and veterinarians is the trichostrongyles, common nematode parasites that cause major production loss and disease. They possess a direct life cycle, where the adults in the host produce eggs that are shed in the feces. The eggs develop to third stage larvae, or L3, in the feces and then circulate into the ambient environment. The infective L3 stage (L3i) is ingested by the host and develops into an egg-laying adult in the gastrointestinal tract of the animal (Scott and Sutherland, 2009). One species stands out as particularly pathogenic due to the fact it feeds on blood. This parasite, Haemonchus contortus, is an abomasal species that can cause severe anemia, hypoproteinemia, weight loss, lethargy, rough hair coats, poor wool/milk/meat production, and death in heavily infected individuals (Zajac, 2006; Gilleard, 2013). Lambs and kids are at the greatest risk of developing disease due to their immature immune response and high rate of infection from environmental contamination due to peri-parturient egg production by infected ewes and does (Getachew et al., 2007).
Until recently, this parasite was controlled almost exclusively with anthelmintics. Unfortunately, the wide and frequent use of these drugs has created selection pressure that favors individual worms resistant to these anthelmintics (Kaplan, 2004a). The offspring of the adult parasites that survive treatment make up greater and greater portions of the worm population as more treatments are administered, leading to a resistant population and treatment failures (Jackson and Coop, 2000). Resistance in small ruminant GINs has been reported to all widely available anthelmintics, including multidrug resistance (Kaplan, 2004b; Wolstenholme et al., 2004; Howell et al., 2008; Kaplan and Vidyashankar, 2012). For each major class of anthelmintic, resistance was reported within a decade of the drug becoming commercially available (Kaplan, 2004b); very recently resistance was seen within a few short years of introduction of monepantel, which belongs to one of the newest classes of anthelmintics for H. contortus control (Mederos et al., 2014; Van den Brom et al., 2015). Thus, anthelmintics involving new mechanisms of action are needed.
We have shown that the nematode-intoxicating crystal (Cry) protein Cry5B made by the soil bacterium Bacillus thuringiensis (Bt) has broad in vivo anthelmintic properties against GINs in monogastric animals (mice, hamsters, dogs, and pigs (Cappello et al., 2006; Hu et al., 2010a; Urban et al., 2013; Hu et al., 2018b)). Furthermore, Bt Cry proteins related to Cry5B are used safely, globally, and extensively as the number one biological insecticide agent in the world, encompassing ~75% of the bioinsecticide market and expressed in nearly 100 million hectares of transgenic crops (Karthickumar and Balasubramanian, 2017; Xiao and Wu, 2019). The main insecticidal components of Bt are three-domain Cry proteins, named aptly for their propensity to form large crystals (Schnepf et al., 1998; de Maagd et al., 2001). Three-domain Cry proteins are pore-forming proteins that specifically bind to and disrupt the integrity of the invertebrate gut (Griffitts and Aroian, 2005). There are a few reports of using Bt against H. contortus, for example, in vitro studies using a Bt strain expressing Cry5B and a larvicidal study using Cry11Aa (Kotze et al., 2005; de Lara et al., 2016); however, in all cases there were caveats (e.g., the strain was not deconvolved to show specific Cry activity or the doses used were very high).
Here, for the first time, we test the efficacy of Cry5B specifically against H. contortus. Cry5B was tested in vitro in a larval development assay and then against adult parasites from an H. contortus strain resistant to three classes of anthelmintics (triple anthelmintic resistant). Following these in vitro studies, recombinant Cry5B spore-crystal lysates were tested against experimental H. contortus infections in goats. Based on the results of these studies, a new Active Pharmaceutical Ingredient containing Cry5B called Inactivated Bacterium with Cytosolic Crystal (IBaCC, a paraprobiotic) was developed and used to treat sheep experimentally infected with H. contortus. Here, we present the results of these studies, showing that Cry5B has excellent potential to positively impact sheep health and productivity by targeting H. contortus infections and reproduction.
2. Materials and methods
2.1. Animal approvals
All protocols in the study were approved by the Virginia Tech Institutional Biosafety Committee (IBC, Protocol #17–006) and the Institutional Animal Care and Use Committee (IACUC, Protocol #18–135, #18–141) and USDA Beltsville IACUC #12–025 and IBC #271.
2.2. H. contortus in vitro assays
Parasite eggs were isolated from infected sheep stool as described (Mes et al., 2007). Egg-to-larval assays (larval development assays) were carried out as described as for C. elegans and cyathostome eggs (Hu et al., 2010b, 2018a). Briefly, parasite eggs were placed in S Medium in a 96-well format supplemented with Escherichia coliOP50 and incubated at 25 °C for seven days. The total number that developed to the L3i stage were counted. Feces from sheep experimentally mono-infected with H. contortus were shipped overnight from Virginia Tech to the Aroian Lab for these studies. Cry5B IBaCC was prepared as described (Li et al., 2020).
Adult H. contortus (Isolate Hc/2004A originally provided by Dr. Ray Kaplan under an MTA between the University of Georgia and USDA/ARS) was isolated from French Alpine goats. This strain is resistant to 3 major classes of anthelmintics 1) benzimidazole (fenbendazole, albendazole, others), 2) imidazothiazole/tetrahydropyrimidine (levamisole, morantel, others) and 3) avermectin (ivermectin, doramectin, others), and slightly resistant to moxidectin. Three goats each at approximately three months of age were orally inoculated with a single dose of 5000 H. contortus L3i and maintained for an additional 50 days (primary infection). At 50 days post inoculation (dpi), the goats were euthanized and adult worms were screened from the abomasal contents then panned using a glass bowl and fine forceps to remove the worms. Tubes containing about 20–30 adult worms were placed in 50 ml of RPMI-1640 media with antibiotics after washing by sedimentation at least 10 times in sterile media and shipped overnight to the Aroian laboratory. In vitro assays were carried out as described for hookworms using the same medium, same temperature, and same CO2 concentration with the addition of 25 mM HEPES pH 7.2 (Cappello et al., 2006; Hu et al., 2012, 2013a) in a 24-well plate with volume of 500 μL, four adult H. contortus per well, and two wells per dose. Cry5B was purified from spore crystal lysates as described (Griffitts et al., 2001). Adult parasites were scored once per day as live/dead as described (dead = no motility even after repeated touching); (Hu et al., 2013a; Urban et al., 2013).
2.3. In vivo goat study
Male Sire (Boer) x Dam (Kiko x Savanna x Boer) goats 19–28 weeks of age were purchased locally before relocating to a pasture at the Beltsville Agricultural Research Center in early spring; the pasture had been free of livestock for the previous three years. Goats on pasture were supplemented ad libitum with orchard grass hay. The goats had been treated with moxidectin by the producer 26 days before shipment and received a CDT vaccine at 26 and 5 days prior to arrival in Beltsville. Initial egg counts from feces were evaluated as described (Gasbarre et al., 2015). Upon arrival in Beltsville, the goats showed strongyle eggs at <50 eggs per gram of feces or epg in 7 of 24 goats (the remainder had no strongyle eggs), Moniezia proglottids in 7 of 24, Strongyloides eggs in 10 of 24 goats, Trichuris eggs in 1 of 24 goats and coccidia oocysts in all samples. The average initial body weight was 27.3 kg (22–34 kg range). All goats were given a subcutaneous injection of 200 mg of ceftiofur hydrochloride upon arrival in Beltsville.
Haemonchus contortus L3i were obtained from sheep infected at Virginia Tech (see below for details) and sent overnight as a suspension to Beltsville. The goats were each subsequently given an oral inoculation of 5000 H. contortus L3i per animal two weeks after arrival and placement on pasture. Weights and fecal samples were collected after three weeks to evaluate the acquisition of parasite infection and changes in weight. The average weight at that time was 26.8 kg (19–33 kg range) with an average strongyle epg of 5843 (8–21,249 range). Full fecal egg counts of all relevant time points are given in Fig. S1. Two weeks later, goats were relocated to an open-aired concrete-surfaced enclosure and randomly assigned to three groups of eight designated as 1) untreated, 2) Cry5B-SCL-treated twice (40 mg/kg body weight once per day one day apart) and 3) moxidectin-treated (single dose 0.2 mg/kg body weight), with groups 2 and 3 inoculated by oral gavage. Cry5B SCL was prepared as described (Marroquin et al., 2000; Hu et al., 2010a). The goats were fed orchard grass hay ad libitum and a supplement of pelleted grain concentrate while on the enclosure.
For oral dosing, animals were manually restrained, and the anthelmintic suspensions were given with a 50 ml syringe and ball-tipped inoculating needle. Animals were observed daily for any adverse reactions; none were seen throughout the course of the study. The necropsy of the goats was conducted over three days between eight and 10 days after moxidectin treatment and after the initial treatment with a two-dose regimen of Cry5B-SCL based on the logistics of availability of facilities and personnel. After euthanasia, the contents of the abomasum and small intestine, a rectal fecal sample, and tissue from the large intestine were collected. Fecal egg counts (Gasbarre et al., 2015) were determined from feces taken from the rectum at necropsy. The abomasum and small intestine contents were evaluated for parasite burden using a modified agar gel method originally used to detect luminal parasites of swine (Slotved et al., 1996; Urban et al., 2013). Very few parasites were found in the contents from the small intestine. Aliquots of samples of abomasum contents collected from the gel after a 3-h incubation in normal saline at 37C were examined for worms using a dissecting microscope and gridded Petri dish. All worms present from the abomasum contents were identified as adult H. contortus based on morphologic characteristics.
2.4. In vitro rumen fluid stability study
Cry5B crystals were isolated from Cry5B IBaCC (Li et al., 2020) and incubated in duplicate in cow rumen fluid for 12, 24, or 48 h as described (Goeser, 2008; Goeser et al., 2009; Goeser and Combs, 2009). Incubations were carried out courtesy of Zinpro Corporation. To determine any percent degradation of Cry5B, the amount of full length Cry5B was quantitated by SDS PAGE relative to BSA standards and compared to the total amount used in the in vitro assay.
2.5. In vivo sheep study
Dorset, Suffolk, and Dorset/Suffolk cross lambs aged 7–8 months were used for this study. The six female and six castrated male lambs had been previously pastured but were maintained in housing for the duration of the study to prevent additional natural H. contortus exposure. All animals were orally administered albendazole (7.5 mg/kg), ivermectin (0.2 mg/kg), and levamisole (8.0 mg/kg) sequentially to remove existing strongylid infection. Sheep were orally inoculated with 10,000 H. contortus L3i six weeks prior to beginning IBaCC treatment. The H. contortus L3i used for infection were obtained from feces of a sheep with a monoinfection of H. contortus. Fecal samples were collected once weekly to determine fecal egg counts (FEC) for each animal. To determine FEC, fecal samples were collected rectally from the sheep and analyzed using the Mini-FLOTAC® test (University of Naples Federico II, Naples, Italy) with a detection limit of 5 eggs per gram of feces (EPG).
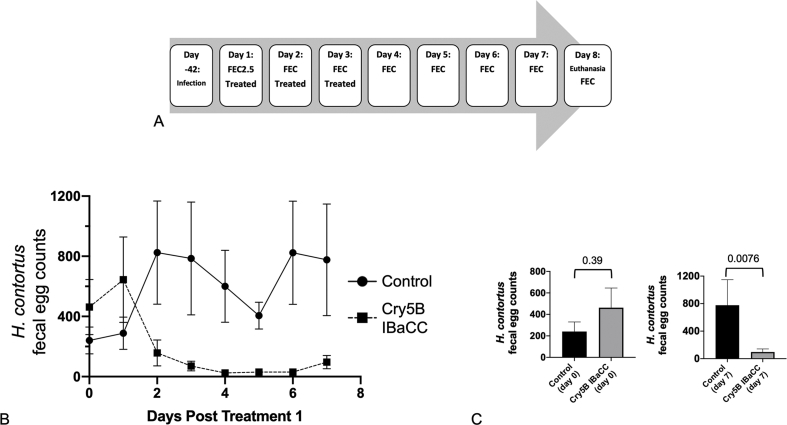
The day before treatment began, sheep were weighed to determine the treatment dose required for each animal and divided into experimental groups balanced for mean FEC with an equal number of males and females in each group. Mean FEC was determined using the previous three weeks FEC data for each sheep. Each animal in the treatment group received a suspension of 60 mg/kg of Cry5B IBaCC in water, administered orally by syringe. Animals in the control group received 200 mL of water orally. Treatments were carried out at approximately the same time of day on three consecutive days (see Fig. 5A). FEC were determined daily. Fecal samples on Days 1–3 were collected before treatment was given. On Day 8, the sheep were humanely euthanized. A 20% aliquot of abomasal contents was collected from each animal. An equal volume of 10% formalin was used to fix the worms. One control animal's abomasal contents were unfortunately lost during collection. The number of worms in the entire 20% aliquot of abomasal contents from each animal was counted using a dissecting microscope and gridded Petri dish. All worms present were identified as adult Haemonchus contortus based on morphologic characteristics.
Fig. 5.
Experimental design and FECs of curative sheep study with IBaCC (n = 6 sheep/group). (A) Experimental design of sheep study. (B) Fecal egg counts (FECs) over time (eggs per gram of feces) relative to the day of first treatment for control (water) and treated (IBaCC) groups (six sheep per group). FECs were always determined before treatment on any given day. The difference between fecal egg counts between control and treated groups based on two-way analysis of variance (P = 0.0009) was significant. (C) Comparison of starting and ending FECs for both groups.
2.6. Statistics
All graphs and analyses were generated using GraphPad Prism v. 8. The comparisons between multiple groups in the goat study were carried out using one-tailed Dunnett's multiple comparison test, comparing to control. The comparison of FEC in the sheep study (Fig. 5B) was carried out using two-way analysis of variance (mixed-effects analysis Time x Treatment, as there was one data point missing for one sheep). All comparisons between two groups were carried out using one-tailed Mann-Whitney test with the assumption that treatment would reduce the infection, except for starting FEC for the sheep study (two-tailed Mann-Whitney to test for equality at the beginning).
3. Results
3.1. Cry5B is active against H. contortus adults in vitro
We first determined if Cry5B on its own was active against H. contortus. Adult parasites from a triple anthelmintic-resistant strain of H. contortus maintained at the USDA were isolated from French-Alpine goats and set up for testing an in vitro dose-response using Cry5B purified from spore-crystal lysates (SCLs) (Griffitts et al., 2001, Griffitts et al., 2005; Cappello et al., 2006). We found that purified Cry5B intoxicates triple anthelmintic-resistant adult H. contortus (Fig. 1).
Fig. 1.
Effects of purified Cry5B on H. contortus adults. Triple anthelmintic-resistant H. contortus adults were incubated in vitro with varying concentrations of purified Cry5B and scored for live or dead (no movement even after touch). Intoxication in this scoring scheme is seen at 100 and 1000 μg/mL. All other doses overlapped completely with HEPES control.
3.2. Cry5B spore crystal lysate was not effective against H. contortus in goats
We next tested whether Cry5B spore crystal lysates could be used against an experimental H. contortus infection in goats. SCLs are formed when Bt sporulates, producing crystal(s) in the cytosol of the mother cell. The cell subsequently lyses and releases the crystal(s) and the spore. SCLs are the form of Bt Cry proteins used in topical applications worldwide as insecticides (Roh et al., 2007; Koch et al., 2015). Goats experimentally infected with H. contortus larvae were treated with either nothing (placebo, negative control), Cry5B SCL (40 mg/kg) given twice one day apart per os, or a single dose of moxidectin (0.2 mg/kg; positive control). Moxidectin treatment resulted in a statistically significant drop in parasite burdens and fecal egg counts (FEC), while Cry5B SCL treatment did not, although both parasite burdens and FEC for Cry5B treated goats were slightly lower than control (see Fig. 2).
Fig. 2.
Cry5B SCL treatment of H. contortus infected goats (n = 8 per group). (A) Abomasal H. contortus burdens of goats untreated (control), treated with Cry5B in SCL, or treated with moxidectin (positive control). Moxidectin treatment resulted in a 62% reduction relative to control. (B) Corresponding fecal egg counts. Moxidectin treatment resulted in an 84% reduction relative to control. Bar represents the average burden. Numbers shown are P values relative to control.
3.3. The paraprobiotic Cry5B IBaCC is effective against H. contortus larvae and crystals are stable in rumen fluid
We recently developed a new Active Pharmaceutical Ingredient (API) containing Cry5B called IBaCC for inactivated bacterium with cytosolic crystal (Fig. 3A (Li et al., 2020)). In this form, Cry5B is expressed in a sporulation defective Bt strain under control of a non-sporulating promoter, giving rise to production of a Cry5B crystal inside a vegetative Bt cell. This vegetative Bt is then inactivated (killed) with food-grade plant essential oil (monoterpenoid), giving rise to a dead bacterial cell wall ghost, harboring within its peptidoglycan shell a Cry5B crystal that is fully bioactive. Such a dead probiotic is known as a paraprobiotic (Taverniti and Guglielmetti, 2011). An appropriate negative control for IBaCC is IBa (inactivated bacterium). IBa is identical to IBaCC in all ways except that the plasmid harboring the Cry5B gene has no insert (empty vector). The parent Bt strain, plasmid backbone, and all processing steps are otherwise identical for IBa and IBaCC.
Fig. 3.
IBaCC. (A) Photomicrograph of IBaCC showing dead Bt cells and encapsulated crystals (dark inclusions). Scale bar is 5 μM. (B) Efficacy of Cry5B IBaCC against H. contortus egg-to-larval development showing number of eggs that developed to the L3i stage over seven days in various concentrations of Cry5B IBaCC (average of three independent experiments). Each concentration of IBaCC had a corresponding concentration of IBa (empty vector control bacteria containing no Cry5B) normalized for OD600. Error bars here and elsewhere represent standard error of the mean.
Although we were no longer able to access H. contortus adults for Cry5B efficacy testing, we were able to obtain fecal samples from H. contortus-infected sheep to evaluate larval development in vitro in the presence/absence of Cry5B IBaCC. From these fecal samples, we isolated parasite eggs and carried out egg to larval development assays in a 96 well format culture system over the course of 7 days as described (Hu et al., 2018a). Three independent experiments were carried out, looking to see how many H. contortus eggs developed to the infective L3 stage (L3i) in 7 days at 28 °C. As shown (Fig. 3B), H. contortus larval development is exquisitely sensitive to Cry5B IBaCC, showing near complete (98%) inhibition at 56 ng/mL. These results were confirmed with an independent batch of IBaCC, showing complete (100%) inhibition of L3i development at 37.5 ng/mL (Fig. S2).
These results demonstrated that IBaCC was potent against H. contortus larvae. Since the goat study failed to show an effect of Cry5B on H. contortus, we were concerned that Cry5B crystals might not be stable during passage through the rumen. To determine if Cry5B from IBaCC might be stable in a ruminant, Cry5B crystals isolated from Cry5B IBaCC were incubated in cow rumen fluid in vitro for 12, 24, and 48 h. No appreciable degradation was seen at 12 and 24 h (Fig. 4). At 48 h, approximately 40% of the Cry5B was degraded, indicating that Cry5B crystals in IBaCC would be stable up to 24 h in the rumen after ingestion and passage to the abomasum.
Fig. 4.
Stability of Cry5B IBaCC crystals in cow rumen fluid. Sodium Dodecyl Sulfate–Polyacrylamide Gel Electrophoresis (SDS-PAGE) of Cry5B IBaCC crystals after incubation for indicated number of hours in cow rumen fluid. Arrow points to full-length Cry5B protein. Although the input Cry5B was not run on the gel, relative to the amount of Cry5B put into the rumen fluid, there is no significant degradation of Cry5B at 12 and 24 h (see methods).
3.4. Cry5B IBaCC is effective against H. contortus infestations in sheep
Based on Cry5B IBaCC efficacy against H. contortus larvae and on stability studies with Cry5B IBaCC crystals in rumen fluid, we tested the efficacy of Cry5B IBaCC against H. contortus infections in sheep (Fig. 5A). Twelve sheep were experimentally inoculated with H. contortus L3i. Fecal samples were taken and pre-treatment FEC were determined on the day of treatment with IBaCC. Six sheep were given water (placebo control) and six sheep were given 60 mg/kg Cry5B in IBaCC daily for three consecutive days. Fecal egg counts were determined daily (on days when treatment occurred, fecal samples were taken before treatment). The sheep were euthanized seven days after the first treatment to determine the worm burden in the abomasum.
Sharp reductions in FEC were seen within two days after treatment that was statistically significant (Fig. 5B). Although starting FEC were similar in both control and treated groups, by the end of the study they were statistically much lower in the treated group (Fig. 5C). Between 88% and 96% reductions in FEC were seen relative to untreated controls starting three days after the first treatment.
By the end of the study, parasite burdens in Cry5B IBaCC treated sheep were significantly reduced 72% relative to infected but untreated control sheep (Fig. 6A). The larger reduction in FEC relative to reduced adult worm burden was likely due to a 96% reduction in female worms versus a 60% reduction in male worms (both statistically significant; Fig. 6B and C).
Fig. 6.
H. contortus abomasal parasite burdens in curative sheep study with IBaCC. (A) Total parasite burdens in control vs IBaCC treated sheep. (B) Male parasite burdens in control vs IBaCC treated sheep. (C). Female parasite burdens in control vs IBaCC treated sheep. Data from one sheep in the control group was not collected (see Materials and Methods).
4. Discussion
New therapies against H. contortus in small ruminants are desperately needed due to the highly pathogenic nature of the parasite and its propensity for developing anthelmintic drug resistance to small molecules. Here, we have shown in vitro that Cry5B protein intoxicates adult H. contortus parasites and is very potent against H. contortus larval development (complete inhibition ~50 ng/mL). We have presented data showing for the first time that a Bt Cry protein, Cry5B, when produced as part of the paraprobiotic IBaCC (crystal encapsulated within the shell of an inactivated bacterium), significantly reduced H. contortus infection in sheep. Three 60 mg/kg doses of Cry5B in IBaCC cleared >70% of the adult parasites and 96% of the female parasites, which led to a comparable reduction in parasite FEC. Although three doses were given, further testing may show that 1–2 doses can achieve a biologically significant effect that reduces infection transmission. Further dosing studies to determine the minimal effective dose and development of formulations to promote delivery to the abomasum and posterior GI tract are warranted.
Our data show that Cry5B crystals from IBaCC will survive the rumen and that H. contortus adults can ingest Cry5B since they are intoxicated in vitro. How Cry5B is ingested by H. contortus in the abomasum is an area for investigation but there is precedent for a blood feeder accessing Cry5B in vivo with hookworms (Kalkofen, 1970; Cappello et al., 2006; Hu et al., 2012, 2013b, 2018a, 2018b; Wu et al., 2015; Li et al., 2020).
Although Cry5B IBaCC was effective in H. contortus-infected sheep, we found that Cry5B delivered as part of Bt spore crystal lysates (SCLs) had no significant impact on H. contortus infections in goats. The reasons for these disparate results are yet unclear but could include: 1) differences between goats and sheep; 2) differences in dosing (2 × 40 mg/kg versus 3 × 60 mg/kg); and/or 3) differences between spore crystal lysates and IBaCC and the ability of IBaCC and/or Cry5B crystals in IBaCC to better survive rumen conditions before entering the abomasum to intoxicate H. contortus.
The mechanism of action of Cry5B has been studied extensively in C. elegans and to some extent in hookworms and Ascaris, where the mechanism of action was shown to be conserved with C. elegans (Cappello et al., 2006; Hu et al., 2012; Urban et al., 2013). Cry5B, which is a three-domain Cry protein, needs to be ingested by the target nematode to act, following which it binds to invertebrate-specific glycosphingolipids on nematode intestinal cells and forms pores, resulting in intoxication, dysfunction, and death as has been demonstrated in insects targeted by three-domain Cry proteins (Griffitts et al., 2001, Griffitts et al., 2003, Griffitts et al., 2005; Huffman et al., 2004; Griffitts and Aroian, 2005; Barrows et al., 2007; Bischof et al., 2008; Kao et al., 2011; Los et al., 2011; Hui et al., 2012). The mechanism of action against H. contortus is likely the same and can be subjected to more study in the future (e.g., investigation of Cry5B binding to H. contortus intestinal glycosphingolipds).
Other studies have examined the impact of Bt and Cry proteins on H. contortus. One group showed that a Cry5B-containing strain of Bt was toxic to H. contortus adults and larvae (Kotze et al., 2005). Although this study did not confirm that the anthelmintic activity was due to Cry5B since other Cry proteins were also produced by this strain, the level of toxicity against larvae and adults in vitro was remarkably similar to that shown here (Fig. 1, Fig. 3), suggesting that Cry5B was likely the active component in those studies. In other studies, either the toxicity seen (e.g., against larvae) was 2-3 orders of magnitude less effective than shown here, or the doses used were not indicated, or the efficacy could not be attributed to Cry protein (e.g., activity was seen against non-feeding egg stages or activity seen when soluble fractions were injected by intraperitoneal or intramuscular routes) (Lopez-Arellano et al., 2002; Lopez et al., 2006; Linares et al., 2008; Vázquez-Pineda et al., 2010; Sinott et al., 2012; de Lara et al., 2016; Beena et al., 2019).
One interesting finding here was the higher sensitivity of female versus male H. contortus in vivo. Since Cry proteins need to be ingested to intoxicate, this may mean that the females are feeding more than males or their feeding behavior is more conducive towards ingesting Cry proteins. The significant difference in size between female and male worms are consistent with the former. Alternatively, the metabolic requirements of egg-producing females may make them more sensitive to intoxication.
Development of anthelmintic resistance in H. contortus is an important consideration. In this regard, Cry5B may have some advantages. Forward genetic screens for resistance showed that it is 3X more difficult for C. elegans to develop resistance to Cry5B than to benzimidazoles and nicotinic acetylcholine receptor (nAChR) agonists (Hu et al., 2010b). Furthermore, Cry5B worked synergistically with nAChR agonists and showed mutual hyper-susceptibility (e.g., nematodes resistant to nAChR agonists are hyper-susceptible to Cry5B) (Hu et al., 2010b, 2018a). To our knowledge, no other anthelmintic combinations showed both these properties. These data predict that exposure of GI parasites to Cry5B in combination with anthelmintics like pyrantel, tribendimidine, and levamisole would limit the induction of resistance (Hu et al., 2010b, 2018a). Finally, it has been shown that pyramiding (combinations of) insecticidal Cry proteins can provide significant protection against the development of resistance by insects, even under high selective pressure (Roush, 1998; Shelton et al., 2000; Stewart et al., 2001; Cao et al., 2002; Tabashnik et al., 2003; Zhao et al., 2003; Bates et al., 2005; Yang et al., 2011). Thus, Cry5B could eventually be combined with other Cry proteins to impede the development of parasite resistance by GIN parasites of livestock and humans.
Our data indicated that Cry5B IBaCC has significant potential to augment current control strategies against H. contortus infections in sheep and to overcome parasite resistance to drugs currently used to control this important parasite of ruminants.
Declarations of interest
None.
Acknowledgements
The support of Veterinary Services at the Beltsville Agricultural Research Center through Dr. Craig Storozuk and Research Animal Services through George Bowman, Roxane Macdonald, Andy Covell, and Jason Evans is appreciated, as is the technical support for isolation of H. contortus adults by Deborah Hebert and the procurement and management of goats by Donald Carbaugh. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture (USDA). The USDA is an equal opportunity provider and employer.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijpddr.2020.11.004.
Funding
This project was supported by (1) the National Institutes of Health/National Institute of Allergy and Infectious Diseases grants R01AI056189 and R01AI50866 to R.V.A., (2) Agriculture and Food Research Initiative Competitive Grantno. 2015–11323 from the USDA National Institute of Food and Agriculture to R.V.A.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
Fecal egg counts over time for the goat study. Shown are the average and standard error for the goats in each group taken upon arrival (Arrival), three weeks after infection with H. contortus larvae and two weeks before treatment (After infection), and then at necropsy (After treatment). The last data point was taken more than three weeks after the second data point, which explains the rise in fecal egg counts in the untreated group.
Efficacy of Cry5B IBaCC against H. contortus egg-to-larval development using independent IBaCC sample (average of three independent experiments).
References
- Barrows B.D., Haslam S.M., Bischof L.J., Morris H.R., Dell A., Aroian R.V. Resistance to Bacillus thuringiensis toxin in Caenorhabditis elegans from loss of fucose. J. Biol. Chem. 2007;282:3302–3311. doi: 10.1074/jbc.M606621200. [DOI] [PubMed] [Google Scholar]
- Bates S.L., Zhao J.-Z., Roush R.T., Shelton A.M. Insect resistance management in GM crops: past, present and future. Nat. Biotechnol. 2005;23:57–62. doi: 10.1038/nbt1056. [DOI] [PubMed] [Google Scholar]
- Beena V., Ramnath V., Girija D., Karthiayini K., Sreekumar K.P., Lakshmanan B., Radhika R. Bacillus thuringiensis strains from Western Ghats of India possess nematocidal property against Haemonchus contortus larvae of goats. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof L.J., Kao C.-Y., Los F.C.O., Gonzalez M.R., Shen Z., Briggs S.P., van der Goot F.G., Aroian R.V. Activation of the unfolded protein response is required for defenses against bacterial pore-forming toxin in vivo. PLoS Pathog. 2008;4 doi: 10.1371/journal.ppat.1000176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao J., Zhao J.-Z., Tang D., Shelton M., Earle D. Broccoli plants with pyramided cry1Ac and cry1C Bt genes control diamondback moths resistant to Cry1A and Cry1C proteins. Theor. Appl. Genet. 2002;105:258–264. doi: 10.1007/s00122-002-0942-0. [DOI] [PubMed] [Google Scholar]
- Cappello M., Bungiro R.D., Harrison L.M., Bischof L.J., Griffitts J.S., Barrows B.D., Aroian R.V. A purified Bacillus thuringiensis crystal protein with therapeutic activity against the hookworm parasite Ancylostoma ceylanicum. Proc. Natl. Acad. Sci. U. S. A. 2006;103:15154–15159. doi: 10.1073/pnas.0607002103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lara A.P., Lorenzon L.B., Vianna A.M., Santos F.D.S., Pinto L.S., Aires Berne M.E., Leite F.P.L. Larvicidal activity of Bacillus thuringiensis var. israelensis Cry11Aa toxin against Haemonchus contortus. Parasitology. 2016;143:1665–1671. doi: 10.1017/S0031182016001451. [DOI] [PubMed] [Google Scholar]
- Gasbarre L.C., Ballweber L.R., Stromberg B.E., Dargatz D.A., Rodriguez J.M., Kopral C.A., Zarlenga D.S. Effectiveness of current anthelmintic treatment programs on reducing fecal egg counts in United States cow-calf operations. Can. J. Vet. Res. 2015;79:296–302. [PMC free article] [PubMed] [Google Scholar]
- Getachew T., Dorchies P., Jacquiet P. Trends and challenges in the effective and sustainable control of Haemonchus contortus infection in sheep. Review. Parasite. 2007;14:3–14. doi: 10.1051/parasite/2007141003. [DOI] [PubMed] [Google Scholar]
- Gilleard J.S. Haemonchus contortus as a paradigm and model to study anthelmintic drug resistance. Parasitology. 2013;140:1506–1522. doi: 10.1017/S0031182013001145. [DOI] [PubMed] [Google Scholar]
- Goeser J.P. University of Wisconsin--Madison; 2008. Improvement of Rumen in Vitro NDF Digestibility Techniques and Data Analysis. [Google Scholar]
- Goeser J.P., Combs D.K. An alternative method to assess 24-h ruminal in vitro neutral detergent fiber digestibility. J. Dairy Sci. 2009;92:3833–3841. doi: 10.3168/jds.2008-1136. [DOI] [PubMed] [Google Scholar]
- Goeser J.P., Hoffman P.C., Combs D.K. Modification of a rumen fluid priming technique for measuring in vitro neutral detergent fiber digestibility. J. Dairy Sci. 2009;92:3842–3848. doi: 10.3168/jds.2008-1745. [DOI] [PubMed] [Google Scholar]
- Griffitts J.S., Aroian R.V. Many roads to resistance: how invertebrates adapt to Bt toxins. Bioessays. 2005;27:614–624. doi: 10.1002/bies.20239. [DOI] [PubMed] [Google Scholar]
- Griffitts J.S., Haslam S.M., Yang T., Garczynski S.F., Mulloy B., Morris H., Cremer P.S., Dell A., Adang M.J., Aroian R.V. Glycolipids as receptors for Bacillus thuringiensis crystal toxin. Science. 2005;307:922–925. doi: 10.1126/science.1104444. [DOI] [PubMed] [Google Scholar]
- Griffitts J.S., Huffman D.L., Whitacre J.L., Barrows B.D., Marroquin L.D., Müller R., Brown J.R., Hennet T., Esko J.D., Aroian R.V. Resistance to a bacterial toxin is mediated by removal of a conserved glycosylation pathway required for toxin-host interactions. J. Biol. Chem. 2003;278:45594–45602. doi: 10.1074/jbc.M308142200. [DOI] [PubMed] [Google Scholar]
- Griffitts J.S., Whitacre J.L., Stevens D.E., Aroian R.V. Bt toxin resistance from loss of a putative carbohydrate-modifying enzyme. Science. 2001;293:860–864. doi: 10.1126/science.1062441. [DOI] [PubMed] [Google Scholar]
- Howell S.B., Burke J.M., Miller J.E., Terrill T.H., Valencia E., Williams M.J., Williamson L.H., Zajac A.M., Kaplan R.M. Prevalence of anthelmintic resistance on sheep and goat farms in the southeastern United States. J. Am. Vet. Med. Assoc. 2008;233:1913–1919. doi: 10.2460/javma.233.12.1913. [DOI] [PubMed] [Google Scholar]
- Huffman D.L., Abrami L., Sasik R., Corbeil J., van der Goot F.G., Aroian R.V. Mitogen-activated protein kinase pathways defend against bacterial pore-forming toxins. Proc. Natl. Acad. Sci. U. S. A. 2004;101:10995–11000. doi: 10.1073/pnas.0404073101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui F., Scheib U., Hu Y., Sommer R.J., Aroian R.V., Ghosh P. Structure and glycolipid binding properties of the nematicidal protein Cry5B. Biochemistry. 2012;51:9911–9921. doi: 10.1021/bi301386q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Ellis B.L., Yiu Y.Y., Miller M.M., Urban J.F., Shi L.Z., Aroian R.V. An extensive comparison of the effect of anthelmintic classes on diverse nematodes. PloS One. 2013;8 doi: 10.1371/journal.pone.0070702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Georghiou S.B., Kelleher A.J., Aroian R.V. Bacillus thuringiensis Cry5B protein is highly efficacious as a single-dose therapy against an intestinal roundworm infection in mice. PLoS Neglected Trop. Dis. 2010;4:e614. doi: 10.1371/journal.pntd.0000614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Miller M.M., Derman A.I., Ellis B.L., Monnerat R.G., Pogliano J., Aroian R.V. Bacillus subtilis strain engineered for treatment of soil-transmitted helminth diseases. Appl. Environ. Microbiol. 2013;79:5527–5532. doi: 10.1128/AEM.01854-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Miller M., Zhang B., Nguyen T.-T., Nielsen M.K., Aroian R.V. In vivo and in vitro studies of Cry5B and nicotinic acetylcholine receptor agonist anthelmintics reveal a powerful and unique combination therapy against intestinal nematode parasites. PLoS Neglected Trop. Dis. 2018;12 doi: 10.1371/journal.pntd.0006506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Nguyen T.-T., Lee A.C.Y., Urban J.F., Jr., Miller M.M., Zhan B., Koch D.J., Noon J.B., Abraham A., Fujiwara R.T., Bowman D.D., Ostroff G.R., Aroian R.V. Bacillus thuringiensis Cry5B protein as a new pan-hookworm cure. Int. J. Parasitol. Drugs Drug Resist. 2018;8:287–294. doi: 10.1016/j.ijpddr.2018.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Platzer E.G., Bellier A., Aroian R.V. Discovery of a highly synergistic anthelmintic combination that shows mutual hypersusceptibility. Proc. Natl. Acad. Sci. U. S. A. 2010;107:5955–5960. doi: 10.1073/pnas.0912327107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Y., Zhan B., Keegan B., Yiu Y.Y., Miller M.M., Jones K., Aroian R.V. Mechanistic and single-dose in vivo therapeutic studies of Cry5B anthelmintic action against hookworms. PLoS Neglected Trop. Dis. 2012;6 doi: 10.1371/journal.pntd.0001900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson F., Coop R.L. The development of anthelmintic resistance in sheep nematodes. Parasitology. 2000;120(Supp. l):S95–S107. doi: 10.1017/s0031182099005740. [DOI] [PubMed] [Google Scholar]
- Kalkofen U.P. Attachment and feeding behavior of Ancylostoma caninum. Z. Parasitenkd. 1970;33:339–354. doi: 10.1007/BF00331470. [DOI] [PubMed] [Google Scholar]
- Kao C.-Y., Los F.C.O., Huffman D.L., Wachi S., Kloft N., Husmann M., Karabrahimi V., Schwartz J.-L., Bellier A., Ha C., Sagong Y., Fan H., Ghosh P., Hsieh M., Hsu C.-S., Chen L., Aroian R.V. Global functional analyses of cellular responses to pore-forming toxins. PLoS Pathog. 2011;7 doi: 10.1371/journal.ppat.1001314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M. Drug resistance in nematodes of veterinary importance: a status report. Trends Parasitol. 2004;20:477–481. doi: 10.1016/j.pt.2004.08.001. [DOI] [PubMed] [Google Scholar]
- Kaplan R.M., Vidyashankar A.N. An inconvenient truth: global worming and anthelmintic resistance. Vet. Parasitol. 2012;186:70–78. doi: 10.1016/j.vetpar.2011.11.048. [DOI] [PubMed] [Google Scholar]
- Karthickumar P., Balasubramanian P. Sustainable Utilization of Natural Resources. CRC Press; 2017. Biofertilizers and biopesticides: a holistic approach for sustainable agriculture; pp. 269–298. [Google Scholar]
- Koch M.S., Ward J.M., Levine S.L., Baum J.A., Vicini J.L., Hammond B.G. The food and environmental safety of Bt crops. Front. Plant Sci. 2015;6:283. doi: 10.3389/fpls.2015.00283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotze A.C., O'Grady J., Gough J.M., Pearson R., Bagnall N.H., Kemp D.H., Akhurst R.J. Toxicity of Bacillus thuringiensis to parasitic and free-living life-stages of nematode parasites of livestock. Int. J. Parasitol. 2005;35:1013–1022. doi: 10.1016/j.ijpara.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Li H., Abraham A., Gazzola D., Hu Y., Beamer G., Flanagan K., Soto E., Rus F., Mirza Z., Draper A., Vakalapudi S., Stockman C., Bain P., Urban J.F., Ostroff G.R., Aroian R.V. Recombinant paraprobiotics as a new paradigm for treating gastrointestinal nematode parasites of humans. 2020. [DOI] [PMC free article] [PubMed]
- Linares I.H., Arellano M.E.L., de Gives P.M., Hernández E.L., de la Parra A.B. Lethal activity of two Bacillus thuringiensis strains against Haemonchus contortus histotropic larvae. Ann. N. Y. Acad. Sci. 2008;1149:164–166. doi: 10.1196/annals.1428.077. [DOI] [PubMed] [Google Scholar]
- Lopez-Arellano M.E., Flores-Crespo J., de Gives P.M., de la Parra A.B., Herrera-Rodríguez D., Liébano-Hernández E., Vázquez-Prats V.M., Várgas-Urióstegui P. In vitro lethal activity of Bacillus thuringiensis toxins against Haemonchus contortus eggs and infective larvae. Int. J. Nematol. 2002;12:66–72. [Google Scholar]
- Lopez M.E., Flores J., Mendoza P., Vazquez V., Liebano E., Bravo A., Herrera D., Godines E., Vargas P., Zamudio F. Use of Bacillus thuringiensis toxin as an alternative method of control against Haemonchus contortus. Ann. N. Y. Acad. Sci. 2006;1081:347–354. doi: 10.1196/annals.1373.049. [DOI] [PubMed] [Google Scholar]
- Los F.C.O., Kao C.-Y., Smitham J., McDonald K.L., Ha C., Peixoto C.A., Aroian R.V. RAB-5- and RAB-11-dependent vesicle-trafficking pathways are required for plasma membrane repair after attack by bacterial pore-forming toxin. Cell Host Microbe. 2011;9:147–157. doi: 10.1016/j.chom.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marroquin L.D., Elyassnia D., Griffitts J.S., Feitelson J.S., Aroian R.V. Bacillus thuringiensis (Bt) toxin susceptibility and isolation of resistance mutants in the nematode Caenorhabditis elegans. Genetics. 2000;155:1693–1699. doi: 10.1093/genetics/155.4.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mederos A.E., Ramos Z., Banchero G.E. First report of monepantel Haemonchus contortus resistance on sheep farms in Uruguay. Parasites Vectors. 2014;7:598. doi: 10.1186/s13071-014-0598-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mes T.H.M., Eysker M., Ploeger H.W. A simple, robust and semi-automated parasite egg isolation protocol. Nat. Protoc. 2007;2:486–489. doi: 10.1038/nprot.2007.56. [DOI] [PubMed] [Google Scholar]
- Roh J.Y., Choi J.Y., Li M.S., Jin B.R., Je Y.H. Bacillus thuringiensis as a specific, safe, and effective tool for insect pest control. J. Microbiol. Biotechnol. 2007;17:547–559. [PubMed] [Google Scholar]
- Roush R.T. Two–toxin strategies for management of insecticidal transgenic crops: can pyramiding succeed where pesticide mixtures have not? Philos. Trans. R. Soc. Lond. B Biol. Sci. 1998;353:1777–1786. [Google Scholar]
- Scott I., Sutherland I. John Wiley & Sons; 2009. Gastrointestinal Nematodes of Sheep and Cattle: Biology and Control. [Google Scholar]
- Shelton A.M., Tang J.D., Roush R.T., Metz T.D., Earle E.D. Field tests on managing resistance to Bt-engineered plants. Nat. Biotechnol. 2000;18:339–342. doi: 10.1038/73804. [DOI] [PubMed] [Google Scholar]
- Sinott M.C., Cunha Filho N.A., Castro L.L.D., Lorenzon L.B., Pinto N.B., Capella G.A., Leite F.P.L. Bacillus spp. toxicity against Haemonchus contortus larvae in sheep fecal cultures. Exp. Parasitol. 2012;132:103–108. doi: 10.1016/j.exppara.2012.05.015. [DOI] [PubMed] [Google Scholar]
- Slotved H.C., Barnes E.H., Bjørn H., Christensen C.M., Eriksen L., Roepstorff A., Nansen P. Recovery of Oesophagostomum dentatum from pigs by isolation of parasites migrating from large intestinal contents embedded in agar-gel. Vet. Parasitol. 1996;63:237–245. doi: 10.1016/0304-4017(95)00916-7. [DOI] [PubMed] [Google Scholar]
- Stewart S.D., Adamczyk J.J., Jr., Knighten K.S., Davis F.M. Impact of Bt cottons expressing one or two insecticidal proteins of Bacillus thuringiensis Berliner on growth and survival of noctuid (Lepidoptera) larvae. J. Econ. Entomol. 2001;94:752–760. doi: 10.1603/0022-0493-94.3.752. [DOI] [PubMed] [Google Scholar]
- Tabashnik B.E., Carrière Y., Dennehy T.J., Morin S., Sisterson M.S., Roush R.T., Shelton A.M., Zhao J.-Z. Insect resistance to transgenic Bt crops: lessons from the laboratory and field. J. Econ. Entomol. 2003;96:1031–1038. doi: 10.1603/0022-0493-96.4.1031. [DOI] [PubMed] [Google Scholar]
- Taverniti V., Guglielmetti S. The immunomodulatory properties of probiotic microorganisms beyond their viability (ghost probiotics: proposal of paraprobiotic concept) Genes Nutr. 2011;6:261–274. doi: 10.1007/s12263-011-0218-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urban J.F., Jr., Hu Y., Miller M.M., Scheib U., Yiu Y.Y., Aroian R.V. Bacillus thuringiensis-derived Cry5B has potent anthelmintic activity against Ascaris suum. PLoS Neglected Trop. Dis. 2013;7:e2263. doi: 10.1371/journal.pntd.0002263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van den Brom R., Moll L., Kappert C., Vellema P. Haemonchus contortus resistance to monepantel in sheep. Vet. Parasitol. 2015;209:278–280. doi: 10.1016/j.vetpar.2015.02.026. [DOI] [PubMed] [Google Scholar]
- Vázquez-Pineda A., Yáñez-Pérez G.N., López-Arellano M.E., Mendoza-de-Gives P., Liébano-Hernández E., Bravo-de-la-Parra A. Biochemical characterization of two purified proteins of the IB-16 Bacillus thuringiensis strains and their toxicity against the sheep nematode Haemonchus contortus in vitro. Transbound. Emerg. Dis. 2010;57:111–114. doi: 10.1111/j.1865-1682.2010.01124.x. [DOI] [PubMed] [Google Scholar]
- Wolstenholme A.J., Fairweather I., Prichard R., von Samson-Himmelstjerna G., Sangster N.C. Drug resistance in veterinary helminths. Trends Parasitol. 2004;20:469–476. doi: 10.1016/j.pt.2004.07.010. [DOI] [PubMed] [Google Scholar]
- Wu C.-C., Hu Y., Miller M., Aroian R.V., Sailor M.J. Protection and delivery of anthelmintic protein Cry5B to nematodes using mesoporous silicon particles. ACS Nano. 2015;9:6158–6167. doi: 10.1021/acsnano.5b01426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao Y., Wu K. Philosophical Transactions of the Royal Society B: Biological Sciences; 2019. Recent progress on the interaction between insects and Bacillus thuringiensis crops. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Chen H., Tang W., Hua H., Lin Y. Development and characterisation of transgenic rice expressing two Bacillus thuringiensis genes. Pest Manag. Sci. 2011;67:414–422. doi: 10.1002/ps.2079. [DOI] [PubMed] [Google Scholar]
- Zajac A.M. Gastrointestinal nematodes of small ruminants: life cycle, anthelmintics, and diagnosis. Vet. Clin. North Am. Food Anim. Pract. 2006;22:529–541. doi: 10.1016/j.cvfa.2006.07.006. [DOI] [PubMed] [Google Scholar]
- Zhao J.-Z., Cao J., Li Y., Collins H.L., Roush R.T., Earle E.D., Shelton A.M. Transgenic plants expressing two Bacillus thuringiensis toxins delay insect resistance evolution. Nat. Biotechnol. 2003;21:1493–1497. doi: 10.1038/nbt907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fecal egg counts over time for the goat study. Shown are the average and standard error for the goats in each group taken upon arrival (Arrival), three weeks after infection with H. contortus larvae and two weeks before treatment (After infection), and then at necropsy (After treatment). The last data point was taken more than three weeks after the second data point, which explains the rise in fecal egg counts in the untreated group.
Efficacy of Cry5B IBaCC against H. contortus egg-to-larval development using independent IBaCC sample (average of three independent experiments).