Abstract
Purpose
Herpes zoster (HZ) is an acute viral eruption caused by the reactivation of varicella zoster virus (VZV), a herpes virus causing chicken pox in children. We aimed to report a 3-month neglected case of acute herpes zoster-induced third nerve palsy and optic neuritis, followed by a late-onset keratouveitis in an immunocompetent young adult.
Observations
A 36-year old immunocompetent Egyptian male patient presented with 3-month complaints of blurred vision and drooping of his left upper eyelid that appeared 4 days after a herpetic rash. He had been diagnosed with herpes zoster ophthalmicus (HZO) of the left eye. However, he had not received any systemic antiviral treatment. The patient had an abnormal head posture with post-eruptive scars on the left forehead and the nose tip. Examination revealed weakness of elevation and adduction, partial ptosis, and mid-dilated non-reactive pupil in the left eye. A relative afferent pupillary defect (RAPD) was present in the affected eye. His blood sugar and blood pressure were within normal limits. Contrast magnetic resonance imaging (MRI) showed no space-occupying lesion. However, there were enhancement and enlargement of the left optic nerve on T1-weighted images, denoting optic neuritis. A diagnosis of acute left third nerve palsy with pupil involvement and optic neuritis secondary to HZO was made. Despite late treatment with oral acyclovir and prednisolone, the patient recovered. One and a half months later, he developed a late-onset keratouveitis about 8 months after the rash onset. After the resolution of the episode, oral acyclovir was continued at a prophylactic dose (400 mg BID).
Conclusions and importance
HZ is a rare cause of third nerve palsy with pupil involvement and optic neuritis. Oral acyclovir and steroids were effective in the delayed treatment in this case. Abnormal optic nerve enhancement on MRI 3 months after the appearance of vesicular rash may suggest chronic HZ activity. Concurrent optic neuritis and third cranial nerve palsy in the absence of other signs of orbital apex syndrome can be seen in cases of HZO. Regular follow-up of patients with HZ is important for detecting recurrence and initiating prompt treatment.
Keywords: Varicella zoster virus, Third nerve palsy, Herpes zoster ophthalmoplegia, Retrobulbar optic neuritis, Keratouveitis, Zoster chronicity/recurrence
1. Introduction
Herpes zoster (HZ) is an acute viral eruption caused by the reactivation of varicella zoster virus (VZV), a herpes virus causing chicken pox in children. This is probably due to alteration of the host cellular immunity. Aging and immunosuppression are the main risk factors for viral reactivation. Other risk factors include personal and family history1,2 of HZ, depression, sleep disturbance,2 race/ethnicity, and comorbidities.3
Although HZ is largely thought to be a disease of the elderly, it also occurs in young adults. Human immunodeficiency virus infection (HIV),4 immunosuppression, and smoking5 are associated with the young age onset of herpes zoster ophthalmicus (HZO). HZO occurs when reactivation takes place in the trigeminal ganglion. The usual ocular findings in HZO are conjunctivitis, keratitis, uveitis, and secondary glaucoma. Ocular nerve palsies and optic neuritis rarely occur either as a sequelae or early in the course of the disease.6 There is growing evidence that HZ is followed by chronic or recurrent HZ infection causing various ocular and non-ocular complications. These complications include eye disease, post herpetic neuralgia (PHN), varicella zoster (VZ) vasculopathy, and stroke.7
We report a unique case of a neglected HZ diagnosis featured an unusual concurrent third cranial nerve palsy and optic neuritis. It also showed abnormal T1-enhancement of the optic nerve on MRI 3 months after the herpetic rash onset. In contrast with most cases of HZ in immunocompetent adults with neuro-ophthalmic sequelae, this case was successful with oral treatment alone.
2. Case presentation
A 36-year old Egyptian male patient presented to our institution, with 3-month complaints of drooping of his left upper eyelid and blurred vision in his left eye. He had been diagnosed with HZO of the left eye and was previously determined negative for HIV and hepatitis B (HBV) and C (HCV) infections at another institution. However, he had not received any systemic antiviral agents. He reported that 3 months prior to presentation, he had experienced severe unilateral pain in the left side of the forehead. The pain lasted 10 days and was associated with malaise and generalized muscle aches before vesicular eruptions appeared. The rash had occupied the left side of the forehead, the left upper eyelid, and the nose tip. Four days later, he noted drooping of his left upper eyelid. One week after ptosis onset, he noted reduced vision and pain in his left eye. His past medical history was negative for hypertension, diabetes, and renal problems. However, he was a heavy smoker. He was treated with only topical medications (acyclovir 3% eye ointment, prednisolone acetate 1% eye drops, and a lubricant eye gel) for the last 3 months. He was experiencing PHN and was given gabapentin tablets.
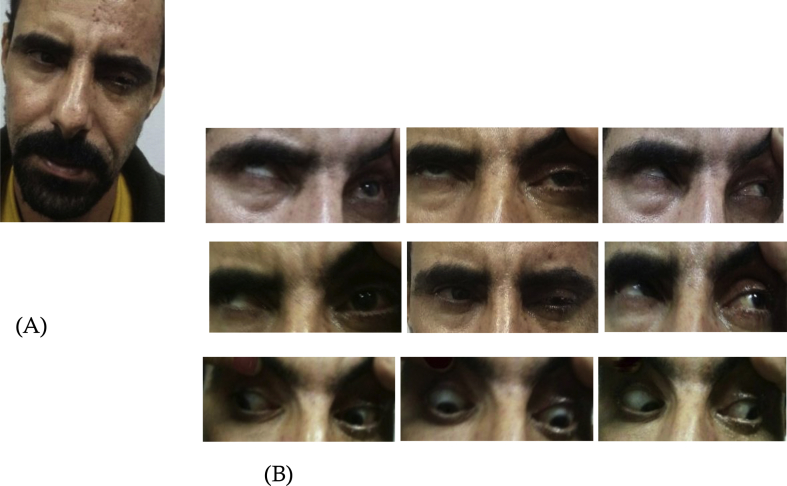
On examination, the patient had an abnormal head posture (ipsilateral head tilt and chin up) (Fig. 1A), with post-eruptive scars on the left side of the forehead and the nose tip belonging to the V1 dermatome (Fig. 1A). There was partial ptosis of the left upper eyelid (Fig. 1A). His visual acuity was 20/20 OD and 20/200 OS. The best corrected visual acuity remained unchanged at 20/200 OS. During extraocular motility testing, restricted elevation and weakness of adduction (OS) were observed (Fig. 1B). He had a left mid-dilated, non-reactive pupil. A relative afferent pupillary defect was present in the left eye as the right pupil dilated during the consensual light response (reverse RAPD). Slit-lamp examination revealed mild conjunctival congestion and clear cornea (OS). The anterior chamber was quiet. Fundus examination showed normal disc and macula. The right eye was normal.
Fig. 1.
Herpes zoster-induced partial third cranial nerve palsy of the left eye.
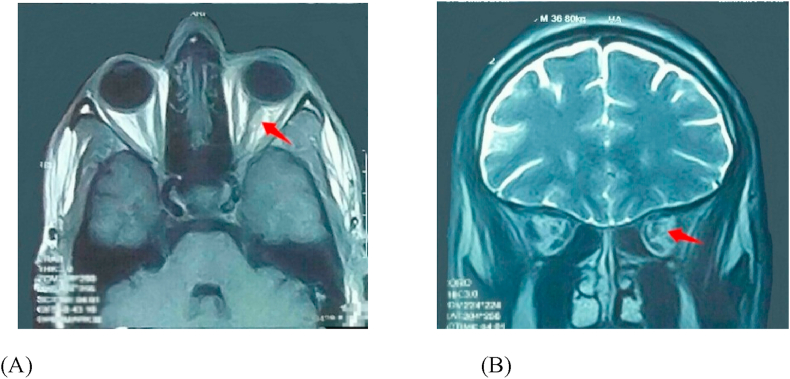
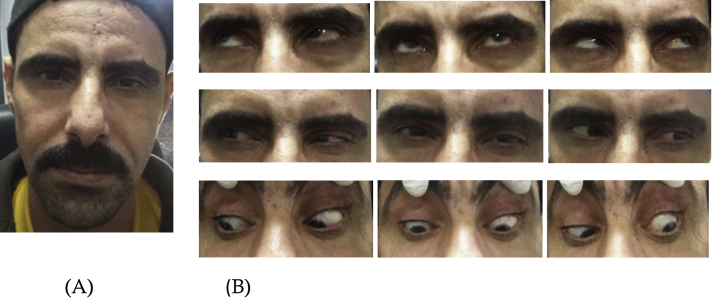
His CBC, CD3, and CD4 white blood cell counts, as well as blood sugar level, and blood pressure were within normal limits. CRP and ANCA were within normal range with a mild elevation of ESR. Gadolinium-enhanced magnetic resonance imaging (MRI) study of the brain was performed to exclude other causes of third nerve palsy. No space-occupying lesion was found. However, left optic nerve enhancement and enlargement in the orbital region on T1-weighted images were revealed (Fig. 2A and B). Based on these findings, the patient was diagnosed with acute partial left third nerve palsy with pupil involvement and retrobulbar optic neuritis secondary to HZO. A treatment course consisting of oral acyclovir 800 mg 5 times daily and oral prednisolone tablets (60 mg/day) was started. Nine days after initiating the treatment, the abnormal head posture and ptosis markedly improved with full recovery of extraocular motility (Fig. 3B). One month later, his vision improved to 20/60 (OS). We continued to monitor his blood pressure and blood sugar levels (for steroid therapy), and kidney function tests were performed (for antiviral medication) during treatment. Oral prednisolone was tapered at 60, 50, 40, 30, 20, 10 and 5 mg/day per week over 2.5 months. Oral acyclovir was continued at the same dose (800 mg 5 times daily) and stopped after 2.5 months, along with the steroids. He continued to demonstrate a mild degree of ptosis at the conclusion of treatment (OS). The abnormal head posture disappeared completely. He had residual pupil dilation (OS). His left visual acuity was 20/40.
Fig. 2.
Herpes zoster-induced retrobulbar optic neuritis of the left eye.
Fig. 3.
Recovery of HZ-induced third nerve palsy of the left eye 9 days after treatment. The arrangement of figures in the manuscript.
Six weeks after the treatment was terminated, the patient returned with redness and reduced visual acuity in the left eye. His visual acuity was 20/200 OS. The slit-lamp examination was remarkable for conjunctival injection and diffuse superficial keratopathy. There were scattered pigmented keratic precipitates on the corneal endothelium (OS). The anterior chamber had numerous inflammatory cells (grade 3+) with flare (grade 3+). The intraocular pressure was 12 mmHg (OS). Fundus examination was unremarkable (OS). A diagnosis of late-onset HZ keratouveitis was made 8 months after the herpetic rash onset. Oral acyclovir 400 mg 5 times daily was started. Topical treatment consisted of acyclovir 3% eye ointment 5 times/day and steroid eye drops (prednisolone acetate 1%) 6 times/day. Recovery was achieved after 2 months. The left eye visual acuity improved to 20/60, with left corneal haze. The anterior chamber was quiet (OS). The intraocular pressure was 11 mmHg (OS). After the resolution of this recurrent episode, oral acyclovir was continued at a prophylactic dose (400 mg BID).
3. Discussion
Herpes zoster ophthalmicus (HZO) occurs with reactivation of latent varicella zoster virus (VZV) in the trigeminal ganglion, probably due to impaired cell-mediated immunity caused by several risk factors such as aging, immunosuppression, and HIV in young adults. Therefore, the occurrence of HZO at a young age is thought to be associated with an underlying HIV infection.2,4 It was found that in adults younger than 40 years, the incidence of HZO is 15 times higher in those with HIV compared to those without.8 It is important to test young patients with HZ for HIV infection. In contrast with most reported cases of young zoster patients in whom HZO was the presenting sign of HIV,9,10 the patient in the present case was negative for HIV. The patient's tests for HBV and HCV were negative. The normal values for C-reactive protein (CRP) and anti-neutrophil cytoplasmic antibodies (ANCA) excluded other inflammatory conditions and autoimmune diseases in this patient. His CD3 and CD4 white blood cell counts were within normal range, indicating immunocompetency. Nevertheless, immunocompetent young adults can also develop HZO.11 However, in patients without HIV, the disease course is localized, less severe, and more responsive to therapy when compared with young adults with HIV.11 The outcomes of the delayed treatment in this patient were as good as those achieved in similar cases treated early. Smoking has been identified as a risk factor for HZ reactivation, with the onset being earlier by 11.5 years.5 The patient in the present case was a heavy smoker, which could have been the risk factor for HZ reactivation.
The mechanism of multiple cranial nerve affection in HZO is unclear. Theories of contiguous spread of inflammation from the trigeminal nerve to the cavernous sinus or the superior orbital fissure,12 occlusive vasculitis,13 and demyelination14 have been postulated. In the present case, the trigeminal (V), oculomotor (III), and optic (II) nerves were simultaneously affected.
Immediate central nervous system imaging is indicated for all third nerve palsies whether pupil involving or pupil sparing to rule out a slowly compressive lesion or an aneurysm. One possible exception is a patient with complete third nerve palsy and complete sparing of the pupil.15 Despite the typical history of HZO, this patient had left partial third nerve palsy with pupil involvement that necessitated neuroimaging. The involvement of cranial nerves controlling extraocular muscles in the course of HZ is rare, attributing to 1.1–2.9% incidence rate.16 The oculomotor nerve is the most commonly affected nerve. The pupil may or may not be involved. HZO-induced ophthalmoplegia is more likely to manifest 2 weeks17,18 up to 2 months19 after the rash onset. Acute ophthalmoplegia appearing within the first days of disease presentation is rare.20,21 It was reported that ptosis and ophthalmoplegia caused by HZ appeared to be self-limiting. However, in some patients, residual ptosis and/or limited ocular motility can last indefinitely.22 Three months after the onset, the patient's ophthalmoplegia and ptosis had not improved.
Optic neuritis is a rare sequelae of HZO. It may present either in the anterior or retrobulbar form with variable visual outcomes.23, 24, 25 In the retrospective study by Gupta et al.,11 the main cause of moderate to severe visual loss at presentation in young, immunocompetent zoster patients was optic neuritis, which was easily reversible with an early course of pulse intravenous steroids and aggressive antiviral therapy. In contrast, the present patient was treated late and only with oral steroids and acyclovir, and good visual recovery was achieved. An unusual finding in this patient was the concurrence of optic neuritis and partial third nerve palsy in the absence of other signs of orbital apex syndrome. Harthan and Borgman17 reported a case of left partial third cranial nerve palsy with pupillary involvement secondary to HZO, in which APD was present in the left eye. It was an accurately described case of HZ third cranial nerve palsy and optic neuritis similar to ours, but the diagnosis was not emphasized. In this report, we highlighted the possibility of optic nerve involvement with third cranial nerve palsy in the absence of other signs of orbital apex syndrome in cases of HZO. Kupersmith et al.26 showed that optic nerves affected by acute optic neuritis demonstrated abnormal enhancement on contrast MRI. Abnormal contrast enhancement of the optic nerve is a sensitive (94%) finding in patients with acute optic neuritis; it is absent in unaffected or previously affected optic nerves.26 Although this patient had a contrast MRI scan 3 months after the onset of his ocular complaints, the optic nerve showed the sensitive sign of the disease activity. This indicates persistent VZV virulence/activity for more than 3 months from the herpetic onset in a chronic course. It also demonstrates the importance of treatment even if it was as late as in our case.
This patient also suffered from PHN. This is another rarely reported complication of HZO in young immunocompetent individuals.11,27,28
Acyclovir remains the drug of choice in the treatment of HZO. In view of the immune response mechanism, corticosteroids were added. Acyclovir is usually given intravenously to treat cranial nerve palsy secondary to HZO. This patient recovered with oral acyclovir treatment. The recovery of our patient from both HZ-induced third nerve palsy with pupil involvement and optic neuritis may indicate the efficacy of acyclovir and steroid treatment, and the reversible pathological nature of the inflammation rather than the ischemic occlusive vasculitis.
The long-term clinical course of HZO is not fully understood.29 Recent data suggest that some patients experienced a chronic or recurrent disease course.7 Studies showed that VZV DNA was detected in late corneal lesion as well as in the aqueous humour of patients with chronic or recurrent iritis.30,31 The suggested causes of HZ chronicity and recurrence are viral replication and infection and/or the host immune response to the virus.29 Ocular hypertension, uveitis, and active eye disease >90 days after the initial presentation (chronic course) were shown to be the ocular risk factors.29 A retrospective study found that administering acyclovir 400 mg orally twice daily or valacyclovir 500 mg for at least 1 year decreased HZO recurrences by 35% and HSV recurrences by 39%.32
In the current case, after the resolution of a late-onset keratouveitis, anti-suppressive treatment was started. Follow-up visits were scheduled every 3 months. Because of poor patient compliance with the treatment regimen, he experienced a recurrent attack of HZ epithelial keratitis, which resolved after 1 month of treatment with topical acyclovir 3% ointment, albeit with more residual corneal opacity. The patient continued the prophylactic use of oral acyclovir. The last follow-up visit was 2 months before this report was written. No recurrence was detected as long as the patient adhered to the prophylactic regimen. Further study to evaluate the risks and benefits of the oral prophylaxis after 1 year is recommended.
4. Conclusions
The recovery of a 3-month neglected case of acute HZ-induced partial third nerve palsy and optic neuritis is considered to be clinically unique. Oral acyclovir and steroids were effective in the delayed treatment in this case. The presence of abnormal enhancement of the optic nerve on MRI 3 months after the vesicular rash onset may suggest chronic HZ activity. Optic neuritis could co-exist with third cranial nerve palsy in the absence of other signs of orbital apex syndrome in the setting of HZO. A late-onset keratouveitis occurred 8 months after the rash onset, denoting recurrence. In the present case, the HZO course demonstrated chronicity and recurrence. Therefore, regular follow-up of patients with herpes zoster is important for the detection of recurrence and initiation of prompt treatment, thereby preventing potential visual impairments.
Patient consent
Informed and signed consent was obtained from the patient for publication of this case and any accompanying images. A copy of the written consent is available for review by the Editor of this journal.
Funding
None.
Authorship
The author attests that she meets the current ICMJE criteria for Authorship.
Author declaration
[Instructions: Please check all applicable boxes and provide additional information as requested.]
The nature of potential conflict of interest is described below
No conflict of interest exists.
We wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Intellectual property
We confirm that we have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing we confirm that we have followed the regulations of our institutions concerning intellectual property.
Research ethics
We further confirm that any aspect of the work covered in this manuscript that has involved human patients has been conducted with the ethical approval of all relevant bodies and that such approvals are acknowledged within the manuscript.
IRB approval was obtained (required for studies and series of 3 or more cases).
Written consent to publish potentially identifying information, such as details or the case and photographs, was obtained from the patient(s) or their legal guardian(s).
Authorship
The International Committee of Medical Journal Editors (ICMJE) recommends that authorship be based on the following four criteria:
-
1.
Substantial contributions to the conception or design of the work; or the acquisition, analysis, or interpretation of data for the work; AND
-
2.
Drafting the work or revising it critically for important intellectual content; AND
-
3.
Final approval of the version to be published; AND
-
4.
Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
All those designated as authors should meet all four criteria for authorship, and all who meet the four criteria should be identified as authors. For more information on authorship, please see http://www.icmje.org/recommendations/browse/roles-and-responsibilities/defining-the-role-of-authors-and-contributors.html#two.
All listed authors meet the ICMJE criteria. We attest that all authors contributed significantly to the creation of this manuscript, each having fulfilled criteria as established by the ICMJE.
One or more listed authors do(es) not meet the ICMJE criteria.
We believe these individuals should be listed as authors because:
[Please elaborate below]
We confirm that the manuscript has been read and approved by all named authors
We confirm that the order of authors listed in the manuscript has been approved by all named authors.
The institutional Review Board approval statement
The current study was approved by the institutional review board of The Research Institute of Ophthalmology, Egypt. The study adhered strictly to the tenets of the Declaration of Helsinki (2013 Revision).
Declaration of competing interest
The author has no conflict of interest to declare.
Acknowledgement
We would like to thank “Editage” (www.editage.com) for English language editing.
References
- 1.Yawn B.P., Wollan P.C., Kurland M.J. Herpes zoster recurrences more frequent than previously reported. Mayo Clin Proc. 2011;86(2):88–93. doi: 10.4065/mcp.2010.0618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marin M., Harpaz R., Zhang J., Wollan P.C., Bialek S.R., Yawn B.P. Risk factors for herpes zoster among adults. Open Forum Infect Dis. 2016;3(3):ofw119. doi: 10.1093/ofid/ofw119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kawai K., Yawn B.P. Risk factors for herpes zoster: a systemic review and meta-analysis. Mayo Clin Proc. 2017;92(12):1806–18021. doi: 10.1016/j.mayocp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 4.Hodge W.G., Seiff S.R., Margolis T.P. Ocular opportunistic infection incidences among patients who are HIV positive compared to patients who are HIV negative. Ophthalmology. 1998;105(5):895–900. doi: 10.1016/S0161-6420(98)95033-3. [DOI] [PubMed] [Google Scholar]
- 5.Chan A.Y., Conrady C.D., Ding K., Dvorak J.D., Stone D.U. Factors associated with age of onset of herpes zoster ophthalmicus. Cornea. 2015;34(5):535–540. doi: 10.1097/ICO.0000000000000362. [DOI] [PubMed] [Google Scholar]
- 6.Miller N.R., Newman N.J. fifth ed. Lippincott Williams & Wilkins; Philadelphia: 1998. Walsh & Hoyt's Clinical Neuro-Ophthalmology; pp. 5043–5046. [Google Scholar]
- 7.Cohen E.J. Management and prevention of herpes zoster ocular disease. Cornea. 2015;34(suppl 10):S3–S8. doi: 10.1097/ICO.0000000000000503. [DOI] [PubMed] [Google Scholar]
- 8.Buchbinder S.P., Katz M.H., Hessol N.A. Herpes zoster and human immunodeficiency virus infection. J Infect Dis. 1992;166(5):1153–1156. doi: 10.1093/infdis/166.5.1153. [DOI] [PubMed] [Google Scholar]
- 9.Ubani U. Herpes-zoster virus ophthalmicus as presenting sign of HIV disease. J Optom. 2011;4(4):117–121. [Google Scholar]
- 10.Lai S.W., Lin C.L., Liao K.F., Chen W.C. Herpes zoster could be an early manifestation of undiagnosed human immunodeficiency virus infection. J Formos Med Assoc. 2016;115(5):372–376. doi: 10.1016/j.jfma.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Gupta N., Sachdev R., Sinha R., Titiyal J.S., Tandon R. Herpes Zoster ophthalmicus: diseases spectrum in young adults. Middle East Afr J Ophthalmol. 2011;18(2):178–182. doi: 10.4103/0974-9233.80710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Edrgton A.E. Herpes zoster ophthalmicus: report of cases and a review of literature. Arch Ophthalmol. 1945;34(1):40–62. [Google Scholar]
- 13.Naumann G., Gass J.D., Font R.L. Histopathology of herpes zoster ophthalmicus. Am J Ophthalmol. 1968;65(4):533–541. doi: 10.1016/0002-9394(68)93869-5. [DOI] [PubMed] [Google Scholar]
- 14.Carroll W.M., Mastaglia F.L. Optic neuropathy and ophthalmoplegia in herpes zoster oticus. Neurology. 1979;29(5):726–729. doi: 10.1212/wnl.29.5.726. [DOI] [PubMed] [Google Scholar]
- 15.Bagheri N., Wajda B.N. 7 th ed. Lippincott Williams & Wilkins; Philadelphia: 2017. The Wills Eye Manual: Office and Emergency Room Diagnosis and Treatment of Eye Disease; pp. 238–240. [Google Scholar]
- 16.Im M., Kim B.J., Seo Y.J., Park J.K., Lee J.H. Complete ophthalmoplegia after herpes zoster. Clin Exp Dermatol. 2007;32:162–164. doi: 10.1111/j.1365-2230.2006.02296.x. [DOI] [PubMed] [Google Scholar]
- 17.Harthan J.S., Borgman C.J. Herpes zoster ophthalmicus-induced oculomotor nerve palsy. J Optom. 2013;6(1):60–65. [Google Scholar]
- 18.Shaker H., Rai V. Oculomotor nerve palsy after herpes zoster ophthalmicus: a case report and review of literature. Neurology. 2016;86(16):297. [Google Scholar]
- 19.Chang-Godinich A., Lee A.G., Brazis P.W., Liesegang T.J., Jones D.B. Complete ophthalmoplegia after zoster ophthalmicus. J Neuro Ophthalmol. 1997;17(4):262–265. [PubMed] [Google Scholar]
- 20.Hakim W., Sherman R., Rezk T., Pannu K. An acute case of herpes zoster ophthalmicus with ophthalmoplegia. Case Rep Ophthalmol Med. 2012;2012:953910. doi: 10.1155/2012/953910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suraida A.R., Evelyn-Tai L.M., Madhusudhan, Thavaratnam L.K. Report of a child with acute herpes zoster ophthalmicus induced partial third nerve palsy. J Acute Dis. 2015;4(2):162–164. [Google Scholar]
- 22.Drolet M., Brisson M., Levin M.J. A prospective study of the herpes zoster severity of illness. Clin J Pain. 2010;26(8):656–666. doi: 10.1097/AJP.0b013e3181eef686. [DOI] [PubMed] [Google Scholar]
- 23.Wang A.G., Liu J.H., Hsu W.M., Lee A.F., Yen M.Y. Optic neuritis in herpes zoster ophthalmicus. Jpn J Ophthalmol. 2000;44(5):550–554. doi: 10.1016/s0021-5155(00)00204-5. [DOI] [PubMed] [Google Scholar]
- 24.Bourke R.D., Pyle J. Herpes zoster ophthalmicus and the orbital apex syndrome. Aust N Z J Ophthalmol. 1994;22(1):77–80. doi: 10.1111/j.1442-9071.1994.tb01701.x. [DOI] [PubMed] [Google Scholar]
- 25.Menon V., Kumar G., Tandon R. Optic neuropathy secondary to herpes zoster ophthalmicus. Indian J Ophthalmol. 1995;43(2):78–79. [PubMed] [Google Scholar]
- 26.Kupersmith M.J., Alban T., Zeiffer B., Lefton D. Contrast-enhanced MRI in acute optic neuritis: relationship to visual performance. Brain. 2002;125(Pt 4):812–822. doi: 10.1093/brain/awf087. [DOI] [PubMed] [Google Scholar]
- 27.Gialloreti L.E., Merito M., Pezzotti P. Epidemiology and economic burden of herpes zoster and post-herpetic neuralgia in Italy: a retrospective, population-based study. BMC Infect Dis. 2010;10:230. doi: 10.1186/1471-2334-10-230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Imafuku S., Matsuki T., Mizukami A. Burden of herpes zoster in the Japanese population with immunocompromised/chronic disease conditions: results from a cohort study claims database from 2005-2014. Dermatol Ther [Heidelb] 2019;9(1):117–133. doi: 10.1007/s13555-018-0268-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tran K.D., Falcone M.M., Choi D.S. Epidemiology of herpes zoster ophthalmicus: recurrence and chronicity. Ophthalmology. 2016;123(7):1469–1475. doi: 10.1016/j.ophtha.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wenkel H., Rummelt C., Rummelt V. Detection of varicella zoster virus DNA and viral antigen in human cornea after herpes zoster ophthalmicus. Cornea. 1993;12(2):131–137. doi: 10.1097/00003226-199303000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Kido S., Sugita S., Horie S. Association of varicella zoster virus load in the aqueous humour with clinical manifestations of anterior uveitis in herpes zoster ophthalmicus and zoster sine herpete. Br J Ophthalmol. 2008;92(4):505–508. doi: 10.1136/bjo.2007.125773. [DOI] [PubMed] [Google Scholar]
- 32.Miserocchi E., Fogliato G., Bianchi I., Bandello F., Modorati G. Clinical features of ocular herpetic infection in an Italian referral Center. Cornea. 2014;33(6):565–570. doi: 10.1097/ICO.0000000000000129. [DOI] [PubMed] [Google Scholar]