Abstract
Purpose
To describe the clinical and pathologic characteristics of a case of retinal vasculitis and vitritis following brolucizumab administration and subsequent ranibizumab treatment.
Observations
A 76-year old Caucasian woman experienced pain, decreased vision and floaters one week after receiving her third monthly intravitreal brolucizumab injection in the right eye for exudative age-related macular degeneration. Examination was significant for 0.5+ anterior chamber cells, vitritis, mild peripheral vascular sheathing, and decreased vision from 20/70 to 20/200. She was started on topical 1% prednisolone acetate with improvement in her examination. She was switched to ranibizumab one month after her last brolucizumab injection of the right eye. Three weeks after her ranibizumab injection, she noticed photophobia, pain and decreased vision. Examination revealed worsening uveitis, vitritis, vascular sheathing, and decreased vision to count fingers. Despite starting on 0.05% difluprednate drops every 2 hours and oral high-dose methylprednisolone, the patient did not have any significant improvement in her symptoms or examination. She underwent pars plana vitrectomy and vitreous biopsy with intravitreal triamcinolone injection to the right eye. Vitreous biopsy and culture ruled out infectious endophthalmitis, and further cytopathologic analysis revealed chronic inflammatory infiltrate.
Conclusion and importance
Treatment with brolucizumab can result in intraocular inflammation and retinal vasculitis likely due to a delayed hypersensitivity reaction to the drug, supported by cytopathologic analysis of a vitreous sample. We demonstrate a case where retreatment with an alternative anti-VEGF agent resulted in worsening vision and vasculitis.
Keywords: Brolucizumab, Retinal vasculitis, Intraocular inflammation, Cytopathology, Hypersensitivity reaction, Age-related macular degeneration
1. Introduction
Brolucizumab intravitreal anti-vascular endothelial growth factor (VEGF) therapy is the latest agent in the treatment of exudative age-related macular degeneration available since October 2019. It is a humanized, single-chain antibody fragment that inhibits VEGF-A, with a molecular mass of 26 kDa as opposed to 114 kDa for aflibercept and 48 kDa for ranibizumab.1, 2, 3, 4 The molar equivalent of 6.0 mg of brolucizumab is 12 times that of 2.0 mg of aflibercept, and 22 times that of 0.5mg of ranibizumab.1, 2, 3, 4, 5 The HAWK and HARRIER phase 3 clinical trials comparing brolucizumab with aflibercept found brolucizumab to be non-inferior to aflibercept in terms of best corrected visual acuity at week 48 of the trial.6 In addition, a promising feature of brolucizumab was that half of the study patients sustained 12-week injection intervals as opposed to 8-week intervals with aflibercept.6
However, an extended 96-week safety outcomes report from HAWK and HARRIER revealed a 4.4% rate of intraocular inflammation associated with brolucizumab, with 6 patients developing an occlusive retinal vasculitis.7 Post-marketing reports estimated the incidence of retinal occlusive vasculitis to be around 1–3 in 10,000 vials.7,8 Recently, two case reports and one case series published a variety of presentations for brolucizumab-related intraocular inflammation and occlusive vasculitis, including arterial sheathing, retinal whitening, plaque deposition, venous phlebitis, perivascular hemorrhages, optic disc swelling, and others.9, 10, 11 The American Society of Retinal Specialists (ASRS) also released their Research and Safety in Therapeutics (ReST) committee report on 26 eyes with retinal vasculitis after treatment with brolucizumab.12 To date, although multiple hypotheses have been made, the mechanism of action for intraocular inflammation and vasculitis due to brolucizumab has not been elucidated.
The current guidelines by ASRS recommend treating intraocular inflammation with aggressive steroids, and to avoid treating with anti-VEGF until inflammation has resolved.12 We present a case of an elderly female patient with complications post-brolucizumab injections, retreated with ranibizumab.
2. Case report
76-year old Caucasian woman with a history of exudative age-related macular degeneration in both eyes presented with persistent subretinal fluid and a large pigment epithelial detachment in the right eye despite receiving regular intravitreal treatments with multiple anti-VEGF agents including bevacizumab, aflibercept, ranibizumab. She was switched to brolucizumab in the right eye and one month later had an improvement in her visual acuity from 20/100 to 20/80. She received two addition intravitreal injections of brolucizumab to the right eye four weeks apart. One week after her 3rd brolucizumab injection, she returned to the clinic complaining of pain, ocular aches, floaters and decreased vision. Visual acuity in the right eye had dropped from 20/70 at her last visit to 20/200. Examination revealed 0.5+ anterior chamber cells and significant vitreous debris. Fundus examination of the right eye had arterial plaques, mild vascular sheathing and boxcarring temporally (Fig. 1A). The patient was started on topical 1% prednisolone acetate with improvement in pain, inflammation and vascular sheathing over the next week.
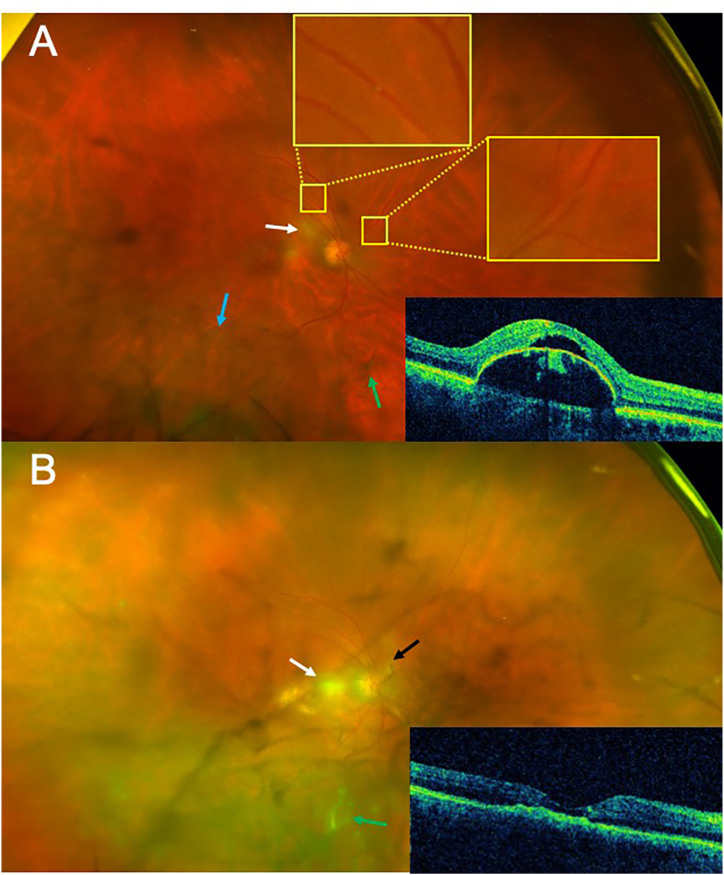
Fig. 1.
(A) Fundus photograph of the right eye one week after the 3rd injection of brolucizumab. Near the disc there are areas of retinal whitening (white arrow). Plaques with associated sheathing are present in the superotemporal artery (yellow boxes). In addition, in the periphery there is boxcarring of the vessels (blue arrow). Multiple vitreous debris was also seen (green arrows). Optical coherence tomography insert demonstrates a large pigment epithelial detachment with subretinal fluid. (B) Fundus photography of the right eye 21 days after treatment with ranibizumab. Fundus has significant debris and haze (green arrow), worsening retinal whitening (white arrow), vascular sheathing nasal to disc (black arrow), and persistent boxcarring of the vessels. Optical coherence tomography insert reveals a collapsed pigment epithelial detachment with retinal atrophy and resolution of the subretinal fluid. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Given the inflammatory response following brolucizumab, the patient was switched to ranibizumab which was administered to the right eye four weeks following her previous brolucizumab injection. Three weeks later, the patient awoke with intense light sensitivity, pain, and decreased vision in the right eye. Examination revealed a drop in her vision to count fingers at 3 feet, fine keratic precipitates, and 2+ anterior chamber cells. Posterior examination demonstrated significant debris and haze with an occlusive vasculitis (Fig. 1B). The pigment epithelial detachment had collapsed and the subretinal fluid had resolved (Fig. 1B). She was started on 0.05% difluprednate drops every 2 hours while awake and oral high-dose methylprednisolone pulse therapy. Our patient returned 3 days later with only mild improvement in her symptoms and persistent vasculitis. She underwent pars plana vitrectomy to remove the inflammatory mediators the following day with vitreous biopsy and intravitreal triamcinolone injection (Fig. 2A and B).
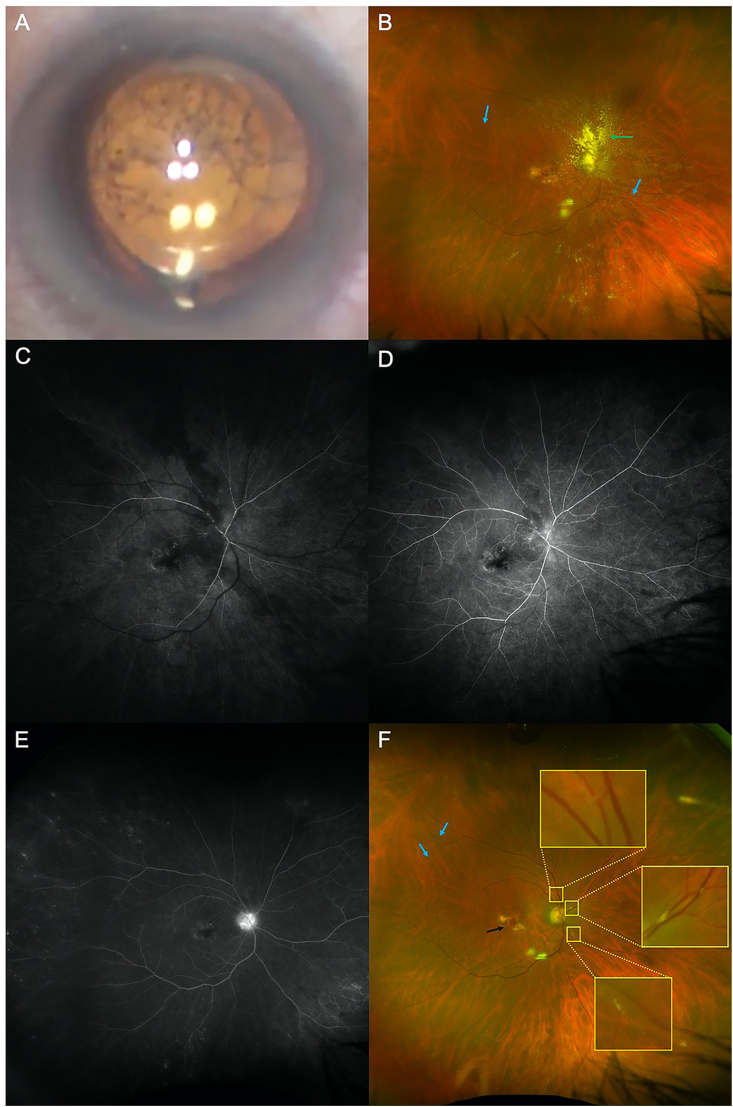
Fig. 2.
(A) Pre-operative image reveals vitreous debris. (B) Post-operative image from day 1 status post pars plana vitrectomy, vitreous biopsy and intravitreal triamcinolone of the right eye (green arrow). Persistent sheathing, plaques and boxcarring of the vessels (blue arrows) is present (C) Early fluorescein angiography had delayed perfusion of both veins and arteries. Mid- and late-phase fluorescein angiography showed vessel boxcarring (D) and late disc leakage (E). (F) Fundus photograph two weeks status post pars plana vitrectomy demonstrates new plaques (yellow boxes), persistent boxcarring (blue arrows), macular hemorrhage (black arrow) and resolution of vitritis. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fluorescein angiography performed on post-operative day one revealed delayed vessel perfusion, boxcarring of the vessels, and late optic disc hyperfluorescence (Fig. 2C–E). Two weeks later, the vision was 20/400 with mild, persistent vascular changes (Fig. 2F). One month later, the retina remained stable with vision improving to 20/200; she was re-injected with ranibizumab without inflammation.
3. Histopathology
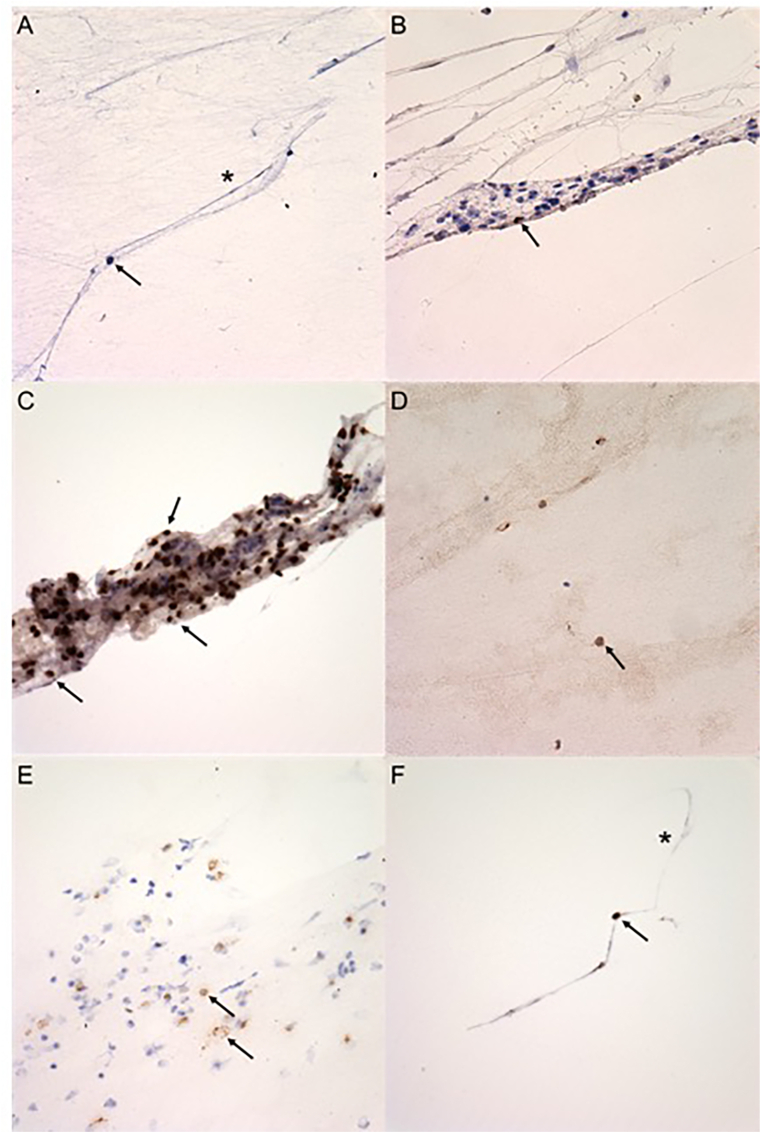
The specimen consisted of approximately 1.5 mL of clear fluid. Examination of 12 cytospin prepared slides of the vitreous aspirate were reviewed. Examination disclosed a chronic inflammatory cell infiltrate composed of lymphocytes, histiocytes, and vitreous strands. Rare CD20 positive B cells were present (Fig. 3B), and the majority of the lymphocytes were CD3 positive T cells (Fig. 3C). Some CD4 and CD8 positive helper and cytotoxic T cells were present (Fig. 3D and E). A mild amount of CD68 positive histiocytes were present (Fig. 3F). No organisms were identified on gram, Grocott's methenamine silver (GMS), and acid-fast bacilli (AFB) staining.
Fig. 3.
Cytopathology of the vitreous biopsy: (A) Cytospin vitreous sample demonstrates lymphocytes (black arrow) and vitreous strands (asterisk) (PAP, original magnification ×400). (B) Rare CD20 positive B-cells are present (black arrow) (CD20, original magnification ×400). (C) Many CD3 positive T-cells are present (black arrows) (CD3, original magnification ×400). (D) Few CD4 positive T-helper cells are present (black arrow) (CD4, original magnification ×400). (E) A moderate number of CD8 positive T-cytotoxic cells are present (black arrows) (CD8, original magnification ×400). (F) CD68 positive histiocytes (black arrow) are present with foci of vitreous strands (asterisk) (CD68, original magnification ×400).
4. Discussion
Retinal occlusive vasculitis with intraocular inflammation has been a devastating adverse event for brolucizumab, leading to blinding visual outcomes for many patients. Although intraocular inflammation has been seen with other anti-VEGF medications,13, 14, 15 severe vision loss due to retinal occlusive vasculitis has not been reported. This case report is a unique scenario where our patient was treated with three injections of brolucizumab, resulting in retinal vasculitis and intraocular inflammation one week after the third injection; after improvement with topical steroids, approximately one month later she was retreated with ranibizumab resulting in a delayed onset of vitritis, occlusive vasculitis, disc edema and worsening vision. We were able to analyze the vitreous as a surrogate for retinal vasculature in an attempt to better understand the pathophysiology of this inflammatory process.
Brolucizumab may be more immunogenic than other anti-VEGF agents by virtue of its relative small size and consequent ability to unfold which exposes epitopes that may not be recognized by the immune system.16 Alternatively during the post-translational modification process of protein fragments like brolucizumab, structural changes such as cleavage and cross-linking of the protein may result in the creation of new protein epitopes.17 These new protein structures could lead to formation of aggregates, which can significantly enhance immunogenicity.17
Possible mechanisms for brolucizumab-related intraocular inflammation are infection and direct cytotoxicity of the drug or vehicle to the ocular tissues. There was no evidence of infection on gram stain, Grocott's methenamine silver (GMS) stain, and acid-fast bacilli (AFB) stain. Toxicity can often cause intraocular inflammation, as seen in conditions such as toxic anterior segment syndrome (TASS) or toxic posterior segment syndrome.18 TASS causes cellular and extracellular damage postulated to be from detergents, metallic compounds, sterilization techniques, bacterial endotoxins, ophthalmic viscosurgical devices, and irrigation solutions.19, 20, 21 TASS is associated with an increase in granulocytes, including a high neutrophil/lymphocyte ratio.19,22 In addition, toxicity from medications can also cause a sterile intraocular inflammation. An example is retinal toxicity due to intravitreal gentamicin seen in the primate model, with retinal infarction thought to be due to granulocytic plugging of the capillary bed.23,24 Typically, TASS and other toxic reactions occur within 12–48 hours from exposure, whereas in the present case there was a week between the last injection and clinical presentation. While the presence of granulocytes may indicate a toxic reaction, neutrophils, basophils and eosinophils were not seen in the vitreous sample from our patient making a toxic reaction less likely.
Inflammatory mechanisms with delayed onset include type III and IV hypersensitivities as defined by Gell and Coombs.25 Type III hypersensitivity is often associated with systemic autoimmune conditions involving vasculitis, and this has been suspected as the mechanism for intraocular inflammation due to brolucizumab.26,27 Type III hypersensitivity reactions involve non-clearing complement that bind to excess antigen, leading to an immune complex formation and eventual inflammatory response.26,27 This type of immune mechanism is primary humoral i.e. antibody dependent. Immunohistochemical markers that might be expected in a type III hypersensitivity reaction include plasma cells, kappa chain, lambda chain and IgG, which were rarely present in the evaluated vitreous sample. Among findings favoring type III hypersensitivity are frequent demonstration of anti-drug antibodies in the Hawk and Harrier trials, delayed onset retinal vasculitis, and some clinical overlap with hemorrhagic occlusive retinal vasculitis which is also postulated to involve type III hypersensitivity.28
Type IV hypersensitivity requires CD4+ T cells, which sense an initial exposure to an antigen (such as brolucizumab) through antigen presenting cells displaying the antigen peptide on MHC II molecules.26,29,30 This type of mechanism is termed cellular mechanism, because is completely dependent on T lymphocytes. This process involves sensitization of the immune system in preparation for a second exposure. Upon re-exposure, the antigen peptide is displayed by antigen presenting cells once again. The antigen-specific T-cells generated during the initial exposure recognize the peptide on MHC II and release a variety of cytokines including interferon gamma that stimulates histiocytes and other cells to react to the antigen.26,29,30 The vitreous sample stained positively for CD3, CD4, and CD8 cells indicating the presence of T cells, (including helper and cytotoxic T cells) (Fig. 3C–E) as well as CD68, a marker of histiocytes (Fig. 3F). Although the vitreous sample was located adjacent to the presumed focus of the retinal inflammatory process, the cellular composition of the infiltrate favors a type IV hypersensitivity reaction. Of the recognized types of hypersensitivities, type IV is the most delayed in onset and requires repeat exposure to antigen. The ASRS ReST committee report stated that 56% of patients developed an occlusive retinal vasculitis after the 2nd or 3rd brolucizumab injection.12 In eyes treated with brolucizumab, retinal vasculitis can be delayed as long as 8 weeks after an injection, with an average time of 25.5 days from the most recent brolucizumab injection.11,12 Without better pathologic data and/or an animal model it will be impossible to determine definitively whether type III, type IV hypersensitivity, or some combination of both mechanisms are implicated in brolucizumab induced retinal vasculitis. The current case demonstrates that both T cell and B cells were present, potentially implicating both humoral and cellular mechanisms.
In our patient, after the 3rd injection of brolucizumab there was a mild episode of inflammation and retinal sheathing; presumably, the initial exposure was from the previous brolucizumab injections. After injecting ranibizumab, retinal occlusive vasculitis, worsening intraocular inflammation and severe vision loss occurred. The rationale for using ranibizumab was that previous reports from the ASRS ReST committee already documented cases after rechallenge with aflibercept without worsening inflammation, and recommended re-treatment with anti-VEGF agents after inflammation resolved.12 In this patient, apparent vascular inflammation had subsided at the time of re-treatment and she had also previously responded more favorably to ranibizumab. One possibility is that during an inflammatory event when an immune response is directed to proteins such as brolucizumab, the body upregulates its immunogenicity to other similar proteins with decreased specificity. Since both brolucizumab and ranibizumab are humanized antibody sequences produced in Escherichia coli,2,3 ranibizumab may have some cross-reactivity and antigenic similarity to brolucizumab, thus becoming a target through an already primed immune system. Another possibility is that the adverse effects of the brolucizumab hypersensitivity was delayed and its full effects were only seen coincidentally after injecting ranibizumab.
Although majority of retinal occlusive vasculitis occurs after multiple injections of brolucizumab, 44% can develop occlusive retinal vasculitis an average of 26 days after the 1st injection according to the ASRS ReST committee report.12 This may be due to the fact that the presence of pre-existing local serum antibodies to brolucizumab have been noted to be higher than other anti-VEGF agents. Specifically, 36–52% of treatment naïve patients were found to have anti-brolucizumab antibodies.3 Pre-treatment serum antibodies to brolucizumab have been shown to correlate with intraocular inflammation in clinical trials, and may also play a role in developing intraocular inflammation sooner especially for patients treated for the first-time.11
The ASRS recommends not to inject brolucizumab or any other anti-VEGF agent until inflammation has resolved.12 In our case, the intraocular inflammation after brolucizumab had improved with topical steroids, and retreatment with ranibizumab one month after brolucizumab still resulted in worsening inflammation with vision loss. While the ASRS recommends treatment of intraocular inflammation with aggressive steroids, intraocular inflammation and retinal occlusive vasculitis in our patient persisted without improvement despite topical and oral steroid treatments. A case by Haug et al. also demonstrated that even after treatment with intravitreal dexamethasone, retinal occlusive vasculitis progressed several weeks later.10 Prompt treatment with aggressive corticosteroid treatment may be helpful but should be continued for several weeks until there is significant clearance of the drug from the vitreous. Vitrectomy may expedite this clearance but may only be helpful early on, prior to evidence of vascular occlusion. Anti-VEGF retreatment should also be withheld until drug has been adequately cleared from the vitreous or at least until all inflammation has resolved.
5. Conclusion
We report a case of an elderly woman with exudative macular degeneration who had brolucizumab-related intraocular inflammation and mild retinal vasculitis to the right eye, which improved on topical prednisolone. When retreated with ranibizumab 28 days later, she developed severe vitritis with worsening occlusive retinal vasculitis. She failed topical difluprednate and oral steroids, and was taken for pars plana vitrectomy and vitreous biopsy. Cytopathology of the vitreous sample ruled out a toxic reaction and favored an immune response, specifically a delayed-type hypersensitivity reaction either a humoral or cellular immune process over a toxic or infectious cause. Identifying symptoms and signs of this immunogenic response early and prompt corticosteroid treatment is essential, as retinal arterial vascular occlusions can ensue with profound, irrecoverable vision loss.
Patient consent
Consent to publish this case report has been obtained from the patient.
Financial disclosure and conflicts of interest
Marc C Peden is an investigator for Allergan, Alkeus, Apellis, Genentech, Kodiak and Novartis. He is a consultant and speaker for Genentech.
Ivan J. Suñer is an investigator for Allergan, Alkeus, Apellis, Genentech, Kodiak, Novartis and Regeneron. He is a consultant for Allergan, Genentech, Novartis, and Regeneron. He is also a speaker for Novartis and Regeneron.
Thomas A. Albini is a consultant for Allegro Allergan, Genentech, Ophthalmics, Beaver Visitec, Clearside Biomedical Inc, Eyepoint Pharmaceuticals, Inc, Janssen Biotech Inc, Notal Vision, Novartis.
The following authors have no financial disclosures: PGI, NP, SRD.
Funding support
Florida Lions Eye Bank.
Authorship
All authors attest that they meet the current ICMJE criteria for Authorship.
Acknowledgements
None.
References
- 1.Dugel P.U., Jaffe G.J., Sallstig P. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology. 2017;124(9):1296–1304. doi: 10.1016/j.ophtha.2017.03.057. [DOI] [PubMed] [Google Scholar]
- 2.European Medicines Agency Assessment Report EMA/CHMP/406682/2019 Committee for medicinal products for human use (CHMP) https://www.ema.europa.eu/en/documents/variation-report/lucentis-h-c-715-ii-0074-g-epar-assessment-report-variation_en.pdf Accessed.
- 3.European Medicines Agency Assessment Report EMA/23630/2020 Committee for medicinal products for human use (CHMP) https://www.ema.europa.eu/en/documents/assessment-report/beovu-epar-public- assessment-report_en.pdf Accessed.
- 4.European Medicines Agency Assessment Report EMA/646256/2012 Committee for medicinal products for human use (CHMP) https://www.ema.europa.eu/en/documents/assessment-report/eylea-epar-public-assessment-report_en.pdf Accessed.
- 5.Tietz J.S.G., Konrad K. Affinity and potency of RTH258 (ESBA1008), a novel inhibitor of vascular endothelial growth factor alpha for the treatment of retinal disorders. Invest Ophthalmol Vis Sci. 2015;56:1501. [Google Scholar]
- 6.Dugel P.U., Koh A., Ogura Y. HAWK and HARRIER: phase 3, multicenter, randomized, double-masked trials of brolucizumab for neovascular age-related macular degeneration. Ophthalmology. 2020;127(1):72–84. doi: 10.1016/j.ophtha.2019.04.017. [DOI] [PubMed] [Google Scholar]
- 7.Dugel PU HJ, Srivastava SK, Kaiser PK. Expanded 96-week safety outcomes from HAWK and HARRIER. Presented At the 43nd Annual Macula Society Meeting. 19‒22 February 2020. Rancho Bernardo, San Diego, CA, USA.
- 8.Novartis. Novartis Provides Update on Use and Safety of Beovu (Brolucizumab). April vol. 28, 2020.
- 9.Jain A., Chea S., Matsumiya W. Severe vision loss secondary to retinal arteriolar occlusions after multiple intravitreal brolucizumab administrations. Am J Ophthalmol Case Rep. 2020;18:100687. doi: 10.1016/j.ajoc.2020.100687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Haug S.J., Hien D.L., Uludag G. Retinal arterial occlusive vasculitis following intravitreal brolucizumab administration. Am J Ophthalmol Case Rep. 2020;18:100680. doi: 10.1016/j.ajoc.2020.100680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baumal C.R., Spaide R.F., Vajzovic L. Retinal vasculitis and intraocular inflammation after intravitreal injection of brolucizumab. Ophthalmology. 2020;127(10):1345–1359. doi: 10.1016/j.ophtha.2020.04.017. [DOI] [PubMed] [Google Scholar]
- 12.Research and Safety Therapeutics committee of the American society of retina Specialists. Occlusive retinal vasculitis following intravitreal brolucizumab. https://wwwasrsorg/clinical/clinical-updates/2976/New-nbsp-ReST-Committee- Report-Summarizes-Analysis-of-Reported-Cases-of-Inflamma Accessed.
- 13.Kitchens J.W., Do D.V., Boyer D.S. Comprehensive review of ocular and systemic safety events with intravitreal aflibercept injection in randomized controlled trials. Ophthalmology. 2016;123(7):1511–1520. doi: 10.1016/j.ophtha.2016.02.046. [DOI] [PubMed] [Google Scholar]
- 14.Ladas I.D., Karagiannis D.A., Rouvas A.A., Kotsolis A.I., Liotsou A., Vergados I. Safety of repeat intravitreal injections of bevacizumab versus ranibizumab: our experience after 2,000 injections. Retina. 2009;29(3):313–318. doi: 10.1097/IAE.0b013e31819a5f98. [DOI] [PubMed] [Google Scholar]
- 15.Goldberg R.A., Shah C.P., Wiegand T.W., Heier J.S. Noninfectious inflammation after intravitreal injection of aflibercept: clinical characteristics and visual outcomes. Am J Ophthalmol. 2014;158(4):733–737. doi: 10.1016/j.ajo.2014.06.019. e731. [DOI] [PubMed] [Google Scholar]
- 16.Walters B.T., Mayne L., Hinshaw J.R., Sosnick T.R., Englander S.W. Folding of a large protein at high structural resolution. Proc Natl Acad Sci U S A. 2013;110(47):18898–18903. doi: 10.1073/pnas.1319482110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuriakose A., Chirmule N., Nair P. Immunogenicity of biotherapeutics: causes and association with posttranslational modifications. J Immunol Res. 2016;2016:1298473. doi: 10.1155/2016/1298473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hernandez-Bogantes E., Navas A., Naranjo A. Toxic anterior segment syndrome: a review. Surv Ophthalmol. 2019;64(4):463–476. doi: 10.1016/j.survophthal.2019.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Yazgan S., Celik U., Ayar O. The role of patient's systemic characteristics and plateletcrit in developing toxic anterior segment syndrome after uneventful phaco surgery: a case-control study. Int Ophthalmol. 2018;38(1):43–52. doi: 10.1007/s10792-016-0418-x. [DOI] [PubMed] [Google Scholar]
- 20.Jun E.J., Chung S.K. Toxic anterior segment syndrome after cataract surgery. J Cataract Refract Surg. 2010;36(2):344–346. doi: 10.1016/j.jcrs.2009.07.052. [DOI] [PubMed] [Google Scholar]
- 21.Maier P., Birnbaum F., Böhringer D., Reinhard T. Toxic anterior segment syndrome following penetrating keratoplasty. Arch Ophthalmol. 2008;126(12):1677–1681. doi: 10.1001/archopht.126.12.1677. [DOI] [PubMed] [Google Scholar]
- 22.Shouchane-Blum K., Dotan A., Bahar I. The evolution of toxic anterior segment syndrome. Curr Opin Ophthalmol. 2019;30(1):50–55. doi: 10.1097/ICU.0000000000000540. [DOI] [PubMed] [Google Scholar]
- 23.Conway B.P., Tabatabay C.A., Campochiaro P.A., D'Amico D.J., Hanninen L.A., Kenyon K.R. Gentamicin toxicity in the primate retina. Arch Ophthalmol. 1989;107(1):107–112. doi: 10.1001/archopht.1989.01070010109037. [DOI] [PubMed] [Google Scholar]
- 24.Brown G.C., Eagle R.C., Shakin E.P., Gruber M., Arbizio V.V. Retinal toxicity of intravitreal gentamicin. Arch Ophthalmol. 1990;108(12):1740–1744. doi: 10.1001/archopht.1990.01070140094037. [DOI] [PubMed] [Google Scholar]
- 25.Gell P.G.H., Coombs R.R.A. The classification of allergic reactions underlying disease. In: Coombs R.R.A., Gells P.G.H., editors. Clinical Aspects of Immunology. Blackwell; Oxford: 1963. [Google Scholar]
- 26.Kumar V. Vol eighth ed. Elsevier; Philadelphia: 2010. Robbins and Cotran Pathologic Mechanisms of Disease. [Google Scholar]
- 27.Hypersensitivity: Immune complex mediated (type III). In: eLS.
- 28.Witkin A.J., Chang D.F., Jumper J.M. Vancomycin-associated hemorrhagic occlusive retinal vasculitis: clinical characteristics of 36 eyes. Ophthalmology. 2017;124(5):583–595. doi: 10.1016/j.ophtha.2016.11.042. [DOI] [PubMed] [Google Scholar]
- 29.Pichler W.J. Delayed drug hypersensitivity reactions. Ann Intern Med. 2003;139(8):683–693. doi: 10.7326/0003-4819-139-8-200310210-00012. [DOI] [PubMed] [Google Scholar]
- 30.Kobayashi K., Kaneda K., Kasama T. Immunopathogenesis of delayed-type hypersensitivity. Microsc Res Tech. 2001;53(4):241–245. doi: 10.1002/jemt.1090. [DOI] [PubMed] [Google Scholar]