Abstract
Several studies have implicated codeine use in the aetiopathogenesis of male infertility. The purpose of this study was to investigate the role of HER2, Ki67, oestrogen and p53/Bcl-2 signaling pathways and the possible outcome of codeine cessation on codeine-induced reproductive toxicity. Thirty adult male Wistar rats of comparable ages and weights were randomly allocated into 5 groups. The control animals received distilled water per os (p.o), while animals in the low-dose (LDC) and high dose (HDC) codeine-treated groups received 2 and 5 mg/kg/day of codeine respectively p.o for 6 weeks. The animals in the low-dose codeine recovery (LDC-R) and high-dose codeine recovery (HDC-R) groups received treatment as LDC and HDC respectively followed by another drug-free six weeks, recovery period. Cessation of codeine exposure led to a partial reversal of codeine-induced poor sperm quality, reduced litter size and weight, increased oxidative testicular injury, testicular apoptosis, and testicular DNA damage caused by codeine administration. Codeine-induced gonado-spermotoxicity was associated with a reduction of circulatory testosterone, suppression of testicular HER2, Ki67, and Bcl-2 expression, down-regulation of oestrogen signaling, and upregulation of testicular caspase 3 activities and p53 signaling pathway. Conclusion: Upregulation of oestrogen signaling associated with enhanced testicular HER2 and Ki67 expression during the recovery period is seemingly beneficial in protecting against codeine-related testicular injury and infertility.
Keywords: Opioid, Oxidative stress, p53, Bcl-2, HER2, Ki67, Biological sciences, Chemistry, Environmental science, Health sciences, Veterinary medicine
Opioid; Oxidative stress; p53; Bcl-2; HER2; Ki67; Biological sciences; Chemistry; Environmental science; Health sciences; Veterinary medicine
1. Introduction
Increasing evidence is implicating various drugs in the aetiopathogenesis of infertility. Drug-induced infertility may be reversible or irreversible [1]. These drugs include those that are used for medical and recreational purposes [2] such as alcohol, marijuana, cannabis and opioids. Global reports showed codeine as the most commonly abused opioid [3]. Although codeine enhances locomotor activities associated with sexual performance, it has been reported to impair copulatory efficiency and fertility [4]. Following oral codeine exposure in rabbits, generation of reactive oxygen species and associated redox imbalance triggered a rise in caspase 3 activities and results in structural and functional impairment in the testes with subsequent gonadal apoptosis [5, 6]. Also, codeine has been observed to induce sperm DNA damage and results in reduced sperm quality [7].
A strong link has been identified between p53, a sequence-specific transcription factor, and Bcl-2. p53 has been reported to activate cell cycle check-points, while Bcl-2 inhibits apoptosis [8]. p53 mediates apoptosis in response to various cellular stress [9, 10] through its tumor suppressive function [11] and its ability to function as a transcription factor [12, 13, 14, 15]. Primarily, it triggers apoptosis by regulating Bcl-2 expression [11]. Stress-activated p53 induces Bax transcription, which overwhelms anti-apoptotic Bcl-2 [16,17]. Furthermore, p53 could directly repress Bcl-2 expression [11]. Similarly, in response to oxidative stress, activation of caspase 3 defaults to an intrinsic apoptotic pathway [18].
The essential role of erb type-1 tyrosine kinase receptors such as c-erbB2, commonly referred to as HER2, in male germ cell development has been established. HER2 mediates Sertoli and Leydig cell proliferation and differentiation [19]. Studies have shown that HER2 is important in mitosis and meiotic entry of germ cells, production of sperm cells and steroids by mediating epidermal growth factor-growth factor (EGF-GF) signaling [20]. HER2-mediated Sertoli and Leydig cell functions influence oestrogen production. Before puberty, Sertoli cells serve as the primary source of oestrogen, while Leydig cells take over this responsibility in adulthood via the activity of aromatase [21, 22]. Besides the regulation of Sertoli and Leydig cell proliferation, growth and function, oestrogen influences the apoptosis of germ cells and acrosome biogenesis [23, 24, 25]. Oestrogen regulates spermiogenesis via oestrogen receptor-α (ERα), while it regulates spermatocyte apoptosis and spermiation via ERβ [26].
Human and animal studies have implicated several drugs, including drugs that are commonly abused such as codeine, in male infertility [27]. Although, our previous studies reported that codeine impaired male fertility, testicular and sperm integrity with their functions [4, 5, 7] via oxidative damage and caspase 3-mediated apoptosis, there are still some shortcomings. The previous studies did not provide information on the effect of codeine cessation and other apoptotic pathways. Therefore, the present study aimed at evaluating whether or not codeine-induced reproductive toxicity is irreversible. It also investigated the role of HER2, Ki67, oestrogen and p53/Bcl-2 signaling pathways on codeine-induced gonadotoxicity.
2. Materials and methods
2.1. Animals
Thirty male rats (150–180g) of the Wistar strain, obtained from the Ladoke Akintola University of Technology (LAUTECH) Animal house, were housed in a well-ventilated room with light and dark cycle of 12 h and under standard laboratory conditions. Animals had unrestricted access to rat chow and water. The experiments were carried out in compliance with the guidelines of the Ministry of Health, Oyo state, and Guide for the Care and Use of Laboratory Animals outlined by the National Academy of Science (NAS), as published by the National Institute of Health. Experimental procedures were approved by the Ministry of Health, Oyo state (AD13/479/1396).
2.2. Drugs and chemicals
Codeine was a donation of the National Drug Law Enforcement Agency (NDLEA), Nigeria. All reagents used, except otherwise stated, were of analytical grade and obtained from Sigma Chemical Co, USA.
2.3. Experimental design
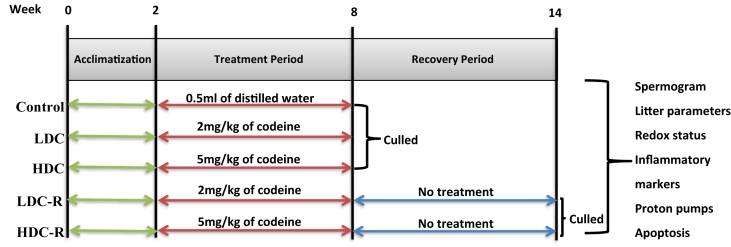
Rats were randomly assigned to 5 groups (n = 6/group) after two weeks of acclimatization. The control group received distilled water per os, low-dose (LDC) and high dose (HDC) codeine-treated groups received 2 and 5 mg/kg of codeine respectively per os for six weeks, while the low-dose recovery (LDC-R) and high-dose codeine recovery (HDC-R) groups also received 2 and 5 mg/kg of codeine respectively per os for six weeks which was immediately followed by another drug-free 6 weeks, recovery period (Figure 1). Although spermatogenesis takes about 50–57 days in rats, the present study did not target a particular phase of the spermatogenic cycle; it rather focused on the toxic effect of codeine on the testes and testicular functions, hence codeine was administered for six weeks [28].
Figure 1.
Experimental protocol chart.
The dose of 2 mg/kg used in this study is the human equivalent dose recommended using the formula:
| Human Equivalent Dose (in mg/kg) = Animal dose (in mg/kg) X Animal Km/Human Km |
where the adult human Km is 37 and rat km is 6 [29].
The rationale of the high dose selection in this study was based on our previous study, using the rat equivalent doses of the rabbit doses previously used [4, 5, 7].
Five days prior to culling, male rats were cohabitated with untreated females (1:1), previously confirmed by a combination of visual assessment and vaginal smear to be in estrus [30], for a maximum of two nights. Mating was established by the presence of sperm in the vaginal smear. The litter (offspring) size and weight of the damns were determined and recorded.
At the end of the experiment, rats were euthanized with intracardiac blood withdrawal under ketamine-xylazine anaesthesia [31], and the testes were removed. The caudal epididymis of each animal was excised for sperm analysis. Half of the left testis of each animal was put in formaldehyde solution for immunohistochemistry, while the other half was put in phosphate buffer solution (PBS) and stored at -20 °C for biochemical analyses. The right testis of each rat was placed in lyses buffer containing 5mM Tris–HCl, pH 8.0, 20mM EDTA, and 0.5% Triton X-100 for assessment of caspase 3 activity and DNA fragmentation. Testosterone and oestrogen levels were measured in serum samples by Enzyme-Linked ImmunoSorbent Assay (ELISA) following the manufacturer's guidelines (Monobind Inc., USA).
2.4. Assessment of caudal epididymal sperm and litter parameters
For sperm analysis, about 1mm-long incision was made on the caudal epididymis to liberate the sperm content into a clean petri dish containing 2ml normal saline solution [7]. A drop of the sperm suspension was placed on a clean pre-warmed slide mixed with 2 drops of 2.9% sodium citrate and, covered with a coverslip. Motility was assessed under a microscope at x 10 magnification. For sperm count, a drop of sperm suspension in formo-saline was transferred to each chamber of the Improved Neubauer Haemocytometer and counted under a light microscope at x 100 magnification. For sperm viability, a mixture of Eosin and Nigrosin stains was added to the drop of sperm suspension on a glass slide. A thin smear was made, air-dried, and examined under a light microscope at x 40 magnification to obtain the ratio of live/dead sperm. For sperm morphology, a smear of the sperm suspension was made, fixed with alcohol, air-dried, and stained with Methylene blue, and then observed under a light microscope to assess various sperm defects.
Pups delivered by the untreated damns were counted and weighed to determine the litter size and weight, respectively.
2.5. Extraction of testicular mitochondrial fraction and measurement of oxidative stress and inflammatory markers
Testes were homogenized in PBS and centrifuged at 10, 000 rpm for 15 min at 4 °C to obtain the supernatant (mitochondrial fraction) for biochemical assay. Markers of oxidative stress were determined spectrophotometrically. Ferrous Oxidation-Xylenol Assay with Triphenylphosphine was employed to assess the production of hydrogen peroxide as previously described [5, 32], while malondialdehyde (MDA) levels were identified as a product of lipid peroxidation by measuring thiobarbituric acid reactive substances (TBARS) [5, 33]. The testicular concentrations of Advanced Glycation Endproducts (AGE), a product of oxidative protein denaturation, was determined by ELISA method using standard kits according to the manufacturer's guideline (Elabscience Biotechnology Co Ltd, USA), while reduced glutathione (GSH) levels were determined by spectrophotometric methods using Ellman's procedure [5, 34]. Testicular activities of endogenous enzymatic antioxidants such as superoxide dismutase (SOD), catalase, glutathione peroxidase (GPx), and glutathione–S-transferase (GST) were assayed using established spectrophotometric methods as reported by Fridovich and Misra [35], Euler et al. [36], Rotruck et al. [37], and Habig et al. [38] respectively.
Testicular myeloperoxidase (MPO) activity was determined to quantify the accumulation of polymorphonuclear leukocytes. This assay is based on hydrogen peroxide-dependent oxidation of guaiacol [39]. Testicular levels of nitric oxide (NO) were assessed using the Griess reaction [40]. The concentrations of testicular TNF-α and IL-1β were determined by ELISA method using standard kits according to the manufacturer's guideline (Elabscience Biotechnology Co Ltd, USA).
2.6. Measurement of the activities of testicular proton pumps
Testicular Na+-K+-ATPase and Ca2+-ATPase activities were spectrophotometrically determined as previously reported [41]. Two (2mL) of the standard reagent was added to the homogenate and left to stand for 30 min at room temperature for colour change development. Afterwards, the absorbance was read at 820mm using a spectrophotometer. The principle is based on the hydrolysis of adenosine triphosphate (ATP) in the presence of appropriate cations to release inorganic phosphate. The amount of inorganic phosphate released is measured using the ammonium molybdate-ascorbic acid system. Concentrated sulphuric acid oxidizes ammonium molybdate acid and gives a yellow colour with inorganic phosphate. Ascorbic acid reduces the molybdic acid to give a characteristic blue colour. The intensity of the colour is proportional to the concentration of the inorganic phosphate liberated into the reaction medium.
2.7. Immunohistochemistry analyses for Ki67, HER2, p53 and Bcl-2 expression
Formalin-fixed and paraffin-embedded testicular tissues were sectioned at 4 μm for immunohistochemistry. Immunohistochemical procedures were performed using Thermo Fischer kit (Thermo Fischer Scientific Inc., USA) and appropriate antibodies; anti-mouse Ki67 monoclonal for Ki67 expression (1:200), anti-mouse HER2 monoclonal for HER2 expression (1:100), anti-mouse p53 monoclonal for p53 expression (1:100), and anti-mouse Bcl-2 monoclonal for Bcl-2 expression (1:200) (Thermo Fischer Scientific Inc., USA). Shortly, after de-paraffinization and rehydration of sections, the antigen was retrieved using preheated citrate buffer and allowed to cool for 30 min. The slides were cleaned with Kim wipes, section areas marked with a hydrophobic pen, and slides were then arranged in a humidified chamber. The slides were incubated for 10 min following blockade of endogenous peroxidase activity using hydrogen peroxide. The slides were rinsed with PBS once, and ultra V protein block was applied and incubated for 10 min. Afterwards, the slides were rinsed with PBS twice, and the corresponding primary antibodies (Ki67, HER2, p53, and Bcl-2) were applied. The slides were then incubated for 45 min, rinsed twice with PBS, and the primary antibody amplifier (secondary antibody) was applied. The slides were further incubated for 25 min, rinsed with PBS twice, and HRP polymer was added. The cycle of incubation for 25 min and rinsing twice with PBS was repeated. Sections were incubated for 5 min in diaminobenzidine (DAB) substrate, rinsed with PBS twice, counterstained with Haematoxylin, and rinsed with distilled water. Blueing solution was applied to the sections and rinsed, dehydrated, clear, and mounted for qualitative examination. For quantification, digital photographs obtained were imported unto the Image J software with specific plugins. Immunoratio pseudo images were produced and the percentage of DAB stained nuclear area evaluated as previously established by Tuominen et al. [42]. Briefly, a microscope image, an optional blankfield correction image, and thresholding adjustment parameters were received as an input, and background subtraction was carried out using the Rolling ball algorithm. The Colour Deconvolution plugin was used to separate the stains into two eight-bit component images: diaminobenzidine (DAB) and hematoxylin (H). The components were processed with a mean filter and binarized using adaptive IsoData thresholding. Component specific threshold adjustments were applied and processed with a median filter to smooth the thresholding result. Nucleus segmentation was performed on both components by using the Watershed algorithm and small particles were discarded based on their sizes. For the H component, thin (fibroblastic) cells were identified and discarded using non-round particle removal. The H and DAB components are overlaid on the source image, and then the percentage of DAB-stained nuclear area out of the total nuclear area (the labeling index) was calculated. The results were normalized with the control group.
2.8. Measurement of testicular 8-hydroxydeoxyguanosine (8OHdG), caspase 3 activity and DNA fragmentation
Testicular oxidative DNA damage was evaluated by measuring the tissue concentrations of 8OHdG method using ELISA kits according to the manufacturer's guideline (Elabscience Biotechnology Co Ltd, USA). Similarly, testicular caspase 3 activities were determined as a marker of apoptosis using ELISA kits (Elabscience Biotechnology Co Ltd, USA) as previously documented [5, 7]. Testicular DNA fragmentation index was determined spectrophotometrically using diphenylamine (DPA) methods as previously reported [5, 43].
2.9. Statistical analysis
Statistical analysis was carried out by the use of GraphPad Prism 5.0 (GraphPad Software, San Diego, USA). One-way ANOVA and Tukey's posthoc test was used for data analysis. Data are expressed as means ± SD. p < 0.05 was considered statistically significant.
3. Results
3.1. Sperm and litter parameters
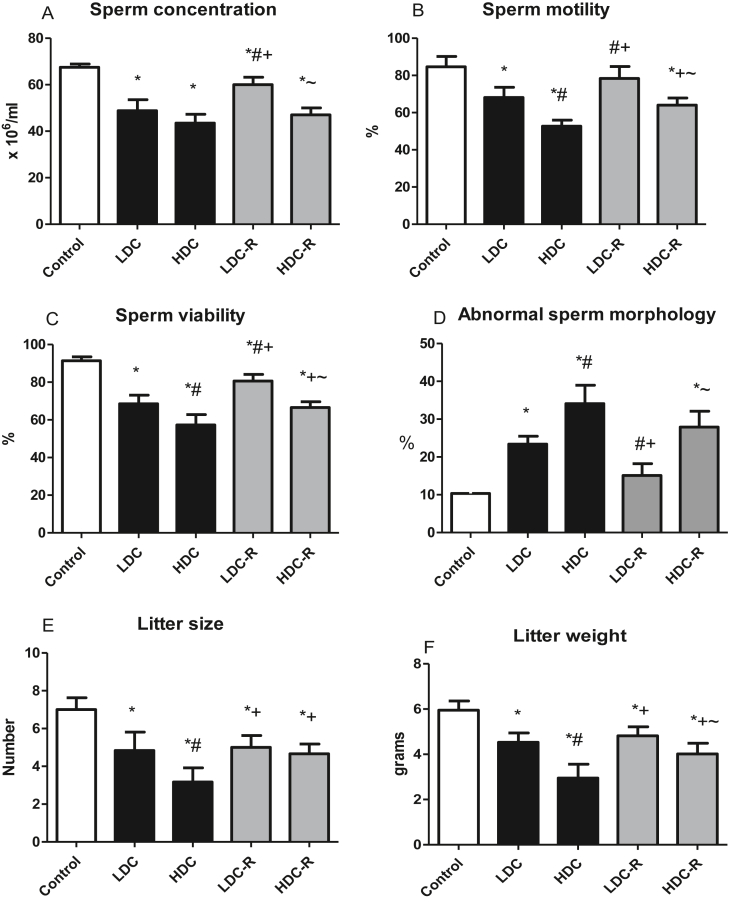
Codeine, at low and high doses, significantly reduced sperm count, motility, viability, and increased abnormal morphology. The differences observed were dose-dependent except for sperm count which was similar between the low- and high-dose groups. Caseation of codeine administration for six weeks to allow recovery significantly improved sperm quality, although the sperm quality was not completely restored. Similarly, the litter size and weight were significantly lower in codeine-treated rats in a dose-dependent fashion; codeine caseation led to a significantly higher litter size and weight (Figure 2).
Figure 2.
Effect of low-dose codeine (LDC) and high-dose codeine (HDC) and their cessation during the recovery period (-R) on sperm count (a), sperm motility (b), sperm viability (c), normal sperm morphology (d), litter size (e), and litter weight (f) in wistar rats. Values are expressed as mean ± SD of 6 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. ∗p < 0.05 vs control, #p < 0.05 vs LDC, + p < 0.05 vs HDC, ~ p < 0.05 vs LDC-R.
3.2. Oxidative markers
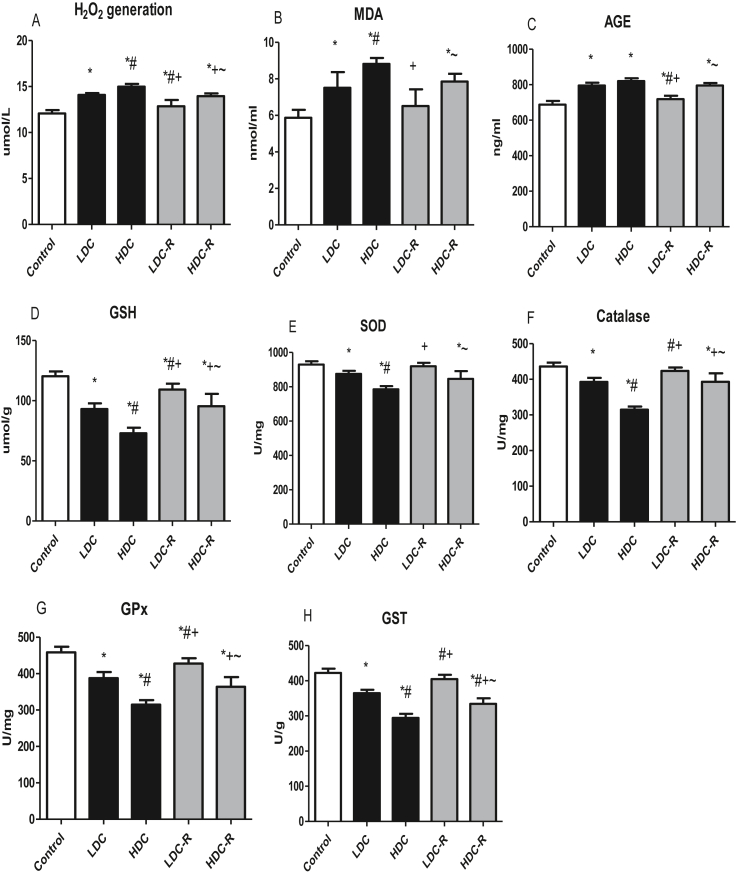
Treatment with codeine impaired the activities of testicular enzymatic antioxidants and led to increased generation of hydrogen peroxide when compared with the control group. The treatment also caused enhanced production of oxidative stress markers assessed by MDA, AGE, and GSH. Nevertheless, these alterations were significantly improved following codeine cessation (Figure 3).
Figure 3.
Effect of low-dose codeine (LDC) and high-dose codeine (HDC) and their cessation during the recovery period (-R) on testicular H2O2 generation (a), MDA (b), AGE (c), GSH (d), SOD (e), catalase (f), GPx (g), and GST (h) in wistar rats. Values are expressed as mean ± SD of 6 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. ∗p < 0.05 vs control, #p < 0.05 vs LDC, + p < 0.05 vs HDC, ~ p < 0.05 vs LDC-R.
3.3. Inflammatory markers and proton pumps
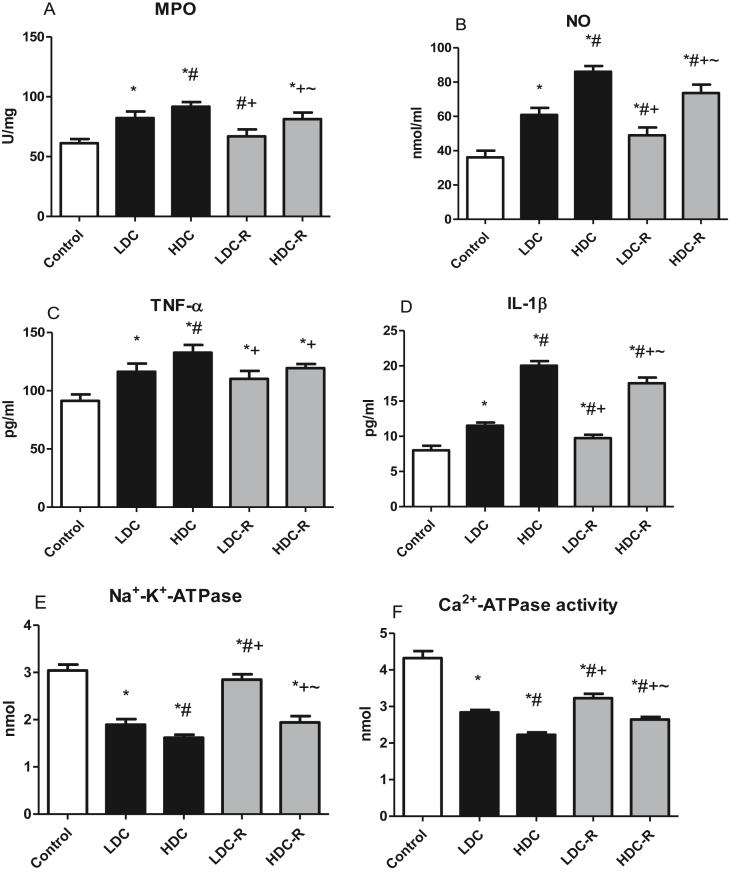
Testicular MPO activity, used as a marker of neutrophil infiltration to the testes, was significantly raised in the codeine-treated groups when compared to the control group. NO, TNF-α, and IL-1β concentrations in the rat testes were elevated considerably in the codeine-treated groups, suggesting elevated inflammatory response. However, these elevations were abolished significantly in the recovery groups (Figure 4). Testicular Na+-K+-ATPase and Ca2+-ATPase activities demonstrated significant depression as compared to the control group indicating codeine-induced impairment of the proton pumps. Nonetheless, drug cessation improved the activities of these proton pumps (Figure 4).
Figure 4.
Effect of low-dose codeine (LDC) and high-dose codeine (HDC) and their cessation during the recovery period (-R) on testicular MPO (a), NO (b), TNF-α (c), IL-1β (d), Na+-K+-ATPase (e), and Ca2+-ATPase (f) in wistar rats. Values are expressed as mean ± SD of 6 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. ∗p < 0.05 vs control, #p < 0.05 vs LDC, + p < 0.05 vs HDC, ~ p < 0.05 vs LDC-R.
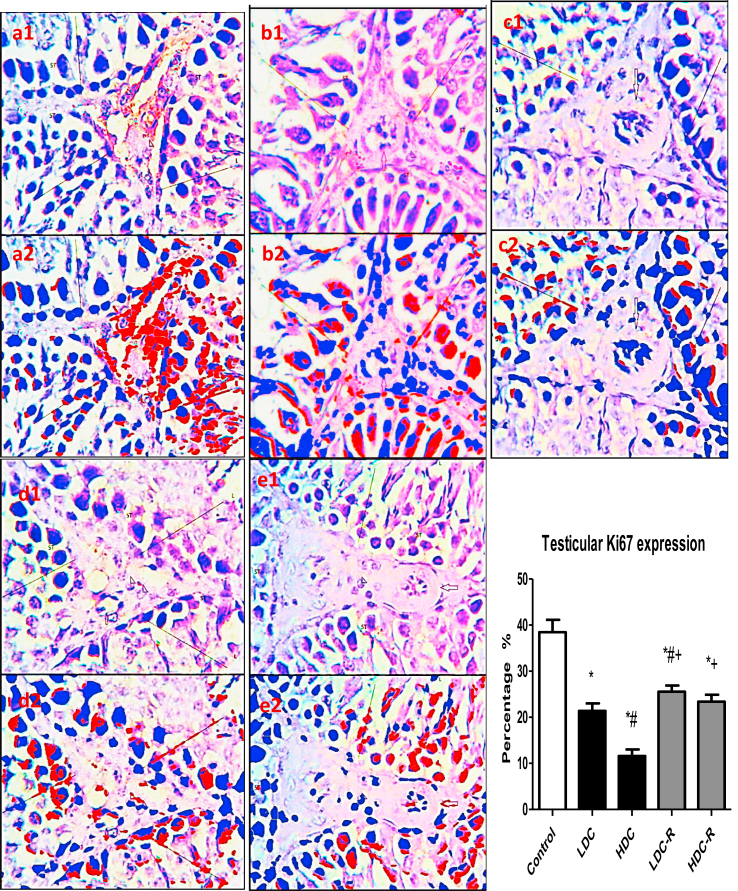
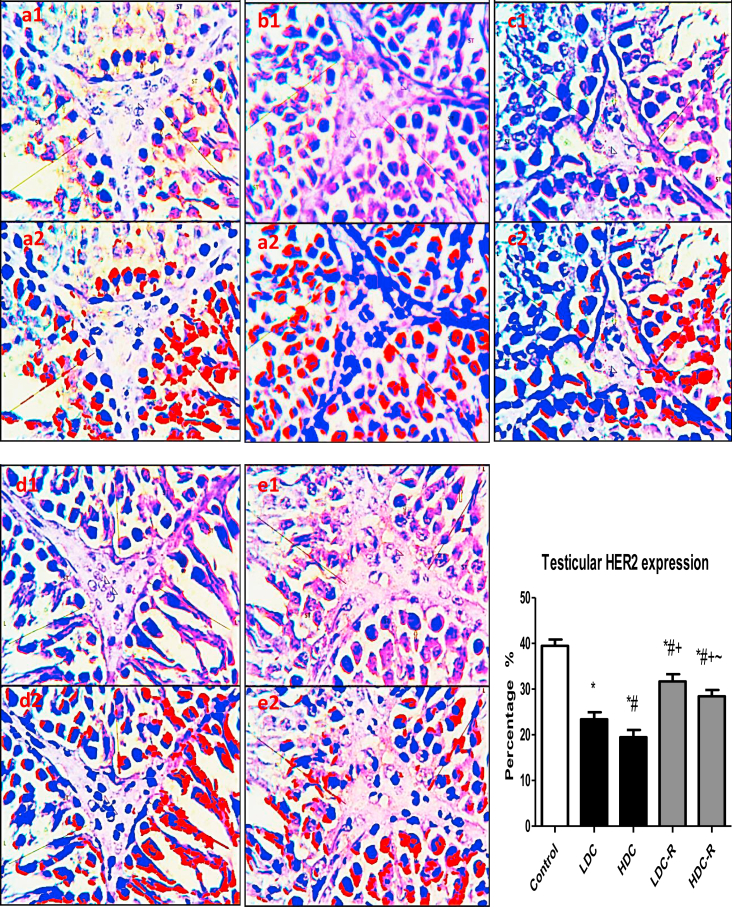
3.4. Testicular Ki67 and HER2 expression
Immunohistochemistry analysis revealed that the administration of codeine to Wistar rats significantly reduced the expression of nuclear-associated antigen Ki67 (Figure 5), a marker of cell proliferation, indicating that codeine impairs germ cell proliferation, especially the actively growing and rapidly dividing spermatogonia and primary spermatocytes. Likewise, codeine treatment led to a significant reduction in testicular expression of HER2 (Figure 6), a mediator of EGF-GF signaling which is essential for Sertoli and Leydig cells growth, indicating repression of the proliferation and differentiation of Sertoli and Leydig cells. These aberrations were significantly, though not completely, attenuated by codeine cessation.
Figure 5.
Effect of low-dose codeine (LDC) and high-dose codeine (HDC) and their cessation during the recovery period (-R) on testicular Ki67 expression in the negative control (a), LDC (b), HDC (c), LDC-R (d), and HDC-R (E)groups in wistar rats. Values are expressed as mean ± SD of 6 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. ∗p < 0.05 vs control, #p < 0.05 vs LDC, + p < 0.05 vs HDC, ~ p < 0.05 vs LDC-R. 1: original image; 2: Immunoratio pseudo image. Spermatogenic cells (LINE) of the seminiferous tubules (ST) and leydig cells (arrow head).
Figure 6.
Effect of low-dose codeine (LDC) and high-dose codeine (HDC) and their cessation during the recovery period (-R) on testicular HER2 expression in the negative control (a), LDC (b), HDC (c), LDC-R (d), and HDC-R (E)groups in wistar rats. Values are expressed as mean ± SD of 6 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. ∗p < 0.05 vs control, #p < 0.05 vs LDC, + p < 0.05 vs HDC, ~ p < 0.05 vs LDC-R. 1: original image; 2: Immunoratio pseudo image. Spermatogenic cells (LINE) of the seminiferous tubules (ST) and leydig cells (arrow head).
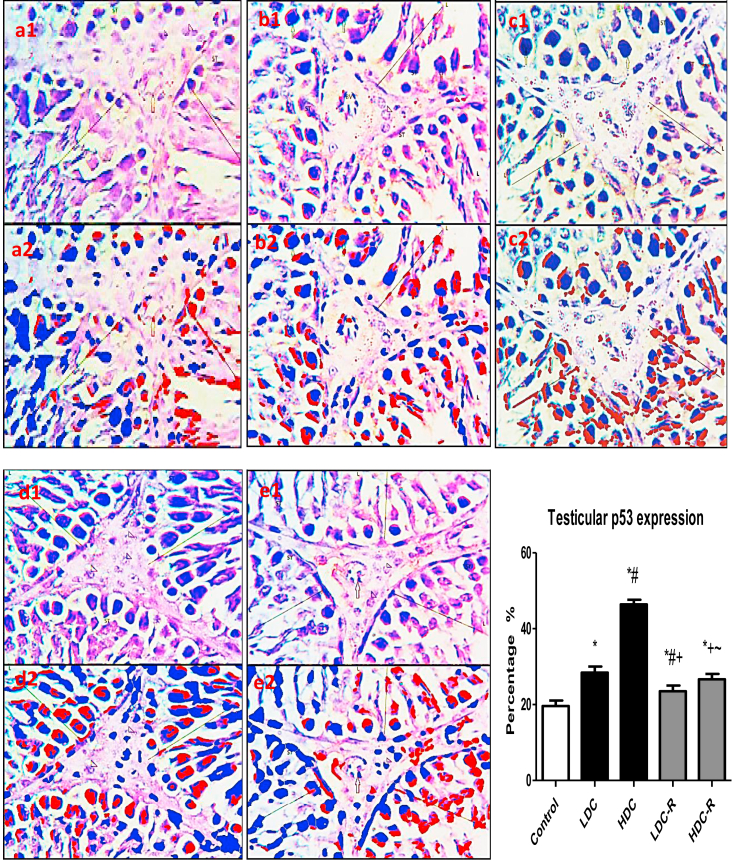
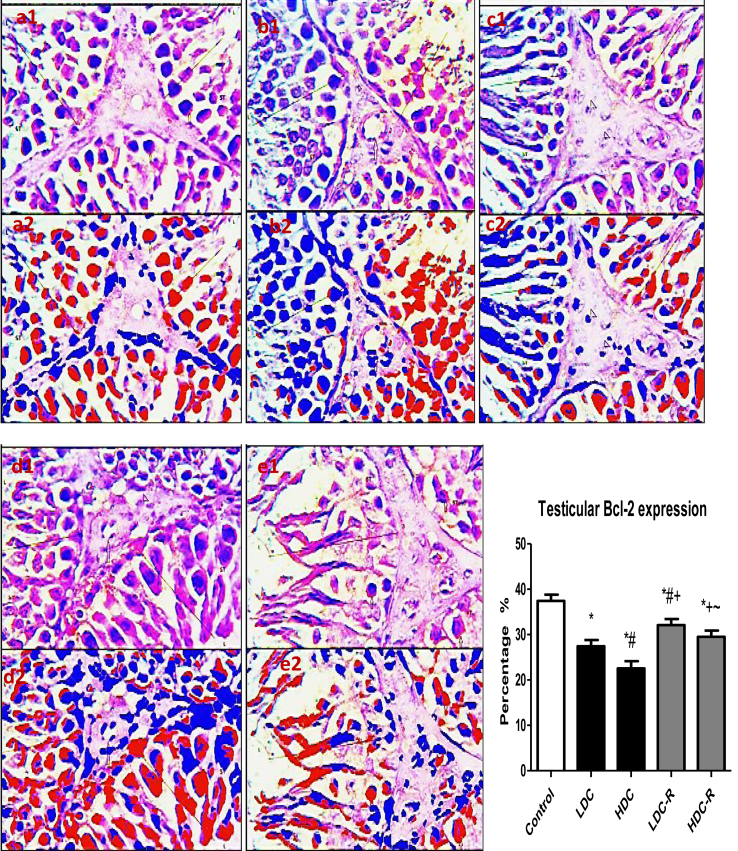
3.5. Testicular apoptosis and DNA damage
As an indicator for apoptosis, testicular p53 was significantly elevated in the codeine-treated groups with respect to the control group, while codeine cessation abrogated p53 expression in rat testes (Figure 7). In parallel, caspase 3 activities in the testes of rats treated with codeine significant increased. However, caspase 3 activities were suppressed significantly following recovery. On the other hand, testicular expression of Bcl-2, an anti-apoptotic marker, was significantly decreased in codeine-treated animals when compared with the control animals. Codeine cessation at the recovery phase up-regulated Bcl-2 expression in the testes (Figure 8).
Figure 7.
Effect of low-dose codeine (LDC) and high-dose codeine (HDC) and their cessation during the recovery period (-R) on testicular p53 expression in the negative control (a), LDC (b), HDC (c), LDC-R (d), and HDC-R (E)groups in wistar rats. Values are expressed as mean ± SD of 6 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. ∗p < 0.05 vs control, #p < 0.05 vs LDC, + p < 0.05 vs HDC, ~ p < 0.05 vs LDC-R. 1: original image; 2: Immunoratio pseudo image. Spermatogenic cells (LINE) of the seminiferous tubules (ST) and leydig cells (arrow head).
Figure 8.
Effect of low-dose codeine (LDC) and high-dose codeine (HDC) and their cessation during the recovery period (-R) on testicular Bcl-2 expression in the negative control (a), LDC (b), HDC (c), LDC-R (d), and HDC-R (E)groups in wistar rats. Values are expressed as mean ± SD of 6 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. ∗p < 0.05 vs control, #p < 0.05 vs LDC, + p < 0.05 vs HDC, ~ p < 0.05 vs LDC-R. 1: original image; 2: Immunoratio pseudo image. Spermatogenic cells (LINE) of the seminiferous tubules (ST) and leydig cells (arrow head).
Treatment with codeine comparably increased testicular levels of 8OHdG, a marker of oxidative DNA damage, and testicular DNA fragmentation when compared with the control. The DNA damage observed in codeine-treated animals was significantly abolished following codeine cessation (Table 1). Similarly, serum testosterone and oestrogen concentrations were significantly diminished following codeine treatment when compared with the control groups; yet, codeine cessation for six weeks significantly restored these hormones to near normal (Table 2).
Table 1.
Effect of codeine and its cessation during the recovery period on testicular genotoxicity, apoptosis, and DNA fragmentation.
| Control | LDC | HDC | LDC-R | HDC-R | |
|---|---|---|---|---|---|
| 8OHdG (ng/ml) | 7.39 ± 0.41 | 10.02 ± 0.75∗ | 12.48 ± 0.53∗# | 8.88 ± 0.69∗#+ | 11.29 ± 0.67∗#+~ |
| Caspase 3 activity (ng/mg) | 0.92 ± 0.14 | 2.20 ± 0.18∗ | 3.88 ± 0.19∗# | 1.99 ± 0.132∗+ | 2.98 ± 0.18∗#+~ |
| Testicular DFI (%) | 10.28 ± 1.34 | 22.27 ± 1.18∗ | 30.58 ± 1.40∗# | 20.16 ± 1.09∗+ | 28.50 ± 1.31∗#~ |
LDC: low dose codeine, HDC: high dose codeine, –R: recovery period, DFI: DNA fragmentation index. Values are expressed as mean ± SD of 6 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. ∗p < 0.05 vs control, #p < 0.05 vs LDC, + p < 0.05 vs HDC, ~ p < 0.05 vs LDC-R.
Table 2.
Effect of codeine and its cessation during the recovery period on serum testosterone and oestrogen.
| Control | LDC | HDC | LDC-R | HDC-R | |
|---|---|---|---|---|---|
| Testosterone (ng/ml) | 8.40 ± 0.74 | 6.69 ± 0.57∗ | 5.14 ± 0.49∗# | 7.46 ± 1.15+ | 6.46 ± 0.35∗+ |
| Oestrogen (ng/ml) | 9.33 ± 0.76 | 8.17 ± 0.95∗ | 7.17 ± 0.41∗ | 8.94 ± 0.35+ | 8.29 ± 0.49+ |
LDC: low dose codeine, HDC: high dose codeine, –R: recovery period. Values are expressed as mean ± SD of 6 rats per group. Data were analyzed by one-way ANOVA followed by Tukey's post hoc test. ∗p < 0.05 vs control, #p < 0.05 vs LDC, + p < 0.05 vs HDC.
4. Discussion
The key finding of this study is that codeine induces gonadotoxicity and poor sperm quality. The data collected reveal that codeine administration led to reduced litter size and weight, oxidative DNA damage, hyper-inflammatory response, proton pump dysfunction, and suppression of Ki67 and HER2 expression. Besides, the study demonstrates that redox imbalance-driven gonadotoxicity induced by codeine is accompanied by suppression of oestrogen concentration, as well as upregulation of caspase 3 activities and p53/Bcl-2 signaling pathway. However, the negative effect exerted by codeine on male reproductive function is partially reversible (Figure 7).
Accumulating evidence elucidates the role of oxidative stress on sperm quality and reproductive function [5, 7, 44, 45, 46]. The present study demonstrates that codeine exposure triggered the generation of free radicals that are associated with the development of oxidative stress [45], testicular damage [5], and poor sperm quality [7]. Although the testes and sperm contain enzymatic antioxidants such as SOD, catalase and glutathione system [47, 48, 49], as well as non-enzymatic antioxidants like ascorbate and α-tocopherol [50, 51], the high polyunsaturated fatty acid makes the testes-sperm complex prone to oxidative damage [5, 7, 52, 53, 54]. Our present findings do not only align with our previous reports [5, 7], but also provide an extension that codeine-exaggerated free radical generation and suppression of antioxidant is accompanied by impairment of testicular proton pumps revealed by decline testicular Na+-K+-ATPase and Ca2+-ATPase activities. This infers that codeine administration promotes testicular proton pump dysfunction and disrupts redox balance that may likely predispose to testicular damage and poor sperm quality. These alterations were restored by codeine cessation. Although it has been established that spermatogenesis in rats takes about 50 days and an extra 7 days for sperm release [28], the administration of codeine for 6 weeks in the present study did not particularly focus on the effect of codeine on spermatogenesis, but on sperm parameters and testicular integrity and functions. Our data could imply that with continuous codeine exposure, sperm cells produced from subsequent cycles of spermatogenesis would be adversely affected by codeine-driven redox imbalance.
Also, our present study that codeine-induced elevated neutrophil recruitment evident by enhanced testicular MPO activity accompanied by activation of pro-inflammatory cytokines reiterates earlier observations. The elevated testicular levels of NO, TNF-α, and IL-1β are a reflection of pro-inflammatory response; however, this was abolished by codeine withdrawal. Studies have revealed that the hyper-inflammatory process within the male reproductive system reduces fertility potentials [5, 7, 55]. Mobilization of immune cells to inflammatory sites causes the production of reactive oxygen intermediates and cytokines via activation of neutrophils and macrophages [56]. Consistently, the results of this study showed elevated testicular levels of NO, TNF-α, and IL-1β in oxidative stress-induced gonado-spermotoxcity following codeine exposure. This aligns with previous studies that revealed that inflammatory cytokines impair reproductive function via enhanced NO production [57, 58].
In a previous study, we demonstrated that codeine-enhanced copulatory locomotor activity was associated with a declined fertility index [4]. Besides, the present data show that chronic codeine administration further led to a significant reduction in litter size and weight. Moreover, codeine-induced reduced litter size and weight were reversed by its cessation. Although there is a lack of data on the effect of paternal codeine exposure on offsprings, previous studies on nicotine revealed reduced average weight of progenies following nicotine exposure [59]. This is in dissonance with studies on 3,4-methylenedioxymethamphetamine (ecstasy) [60]. The present study revealed that codeine does not only trigger oxido-inflammatory damage to the gonads and sperm; it also exerts a negative effect on the quality of offsprings produced likely via epigenetic modification.
In addition to androgen suppression established in our previous studies [4, 5], the present study shows that codeine administration leads to a decline in serum levels of oestrogen. This is possibly via downregulation of HER2 expression. The role of HER2 in male fertility has been demonstrated. Codeine-induced oestrogen suppression is secondary to, at least partly, HER2 down-regulation observed in this study. Codeine exposure impaired HER2-mediated Sertoli and Leydig cell proliferation and differentiation [19] and Ki67 expression, a marker of proliferation. This was possibly accompanied by a decline in aromatase activity [21, 22] and resultant low oestrogen production. Besides, HER2 also mediates spermiogenesis [20]. Hence, the down-regulation of HER2 contributes to the poor sperm quality seen following codeine treatment.
The decline in circulatory oestrogen has multiple effects; it further impairs Sertoli and Leydig cell growth leading to a vicious cycle, deregulates spermiogenesis leading to poor sperm quality, and induces apoptosis [23, 24, 25, 26]. Thus, the role of oestrogen in testicular function and male fertility [61] cannot be over-emphasized. The modulatory effects of oestrogen in regulating testicular proliferation and apoptosis have been linked to ER-mediated genomic and rapid non-genomic activities through the membrane receptors [62]. In parallel with the oxidative testicular injury, codeine resulted in increased testicular caspase 3 activity and expression of p53. This was observed to be associated with down-regulation of Bcl-2. The current data is in consonance with our previous study that elucidated that codeine triggered caspase 3 activity [5, 7]. It also demonstrates the role of p53/Bcl-2 in codeine-induced apoptosis. The present study revealed that oestrogen-dependent testicular apoptosis is reversible following codeine withdrawal.
Interestingly, our results suggest that codeine induces testicular apoptosis via several linking pathways. The raised levels of pro-inflammatory cytokines following codeine administration as well as oxidative injury explain the testicular apoptosis observed [58]. More so, codeine-induced testicular apoptosis might be attributed to impaired HER2-regulated oestrogen production. These findings were found to be associated with oxidative DNA damage and DNA fragmentation of the testes. This implies that codeine promotes the oxidation of guanines to generate 8OHdG which labilizes the glycosyl bond that attaches the guanines to adjacent ribose unit, resulting in loss of guanine and generation of abasic sites [45]. This destabilizes the DNA backbone and leads to induction of localized strand breaks.
Despite the adverse effects of codeine on testicular function, its cessation appears to partially restore oestrogen signaling, at least partly, thus enhancing testicular HER2, Ki67, and Bcl-2 expression, and down-regulating p53 pathway resulting in the maintenance of male fertility. A novel finding in the present study is the up-regulation of HER-2 and oestrogen signaling following codeine cessation with possible Sertoli and Leydig cell regeneration. This aligns with the few data available in the literature that reported possible Sertoli and Leydig cell regeneration after maturation. Although Sertoli cells have been known to be quiescent after sexual maturity, Martínez-Hernández [63] observed increased Sertoli cell proliferation and restoration after a short photoperiod-induced apoptosis in Syrian hamster (Mesocricetus auratus). Our findings that codeine cessation up-regulates HER-2 and oestrogen signaling is at least partly responsible for the enhanced sperm quality and suppressed apoptosis observed following withdrawal. It might also suggest that not all Sertoli cells are quiescent after sexual maturity.
5. Conclusion
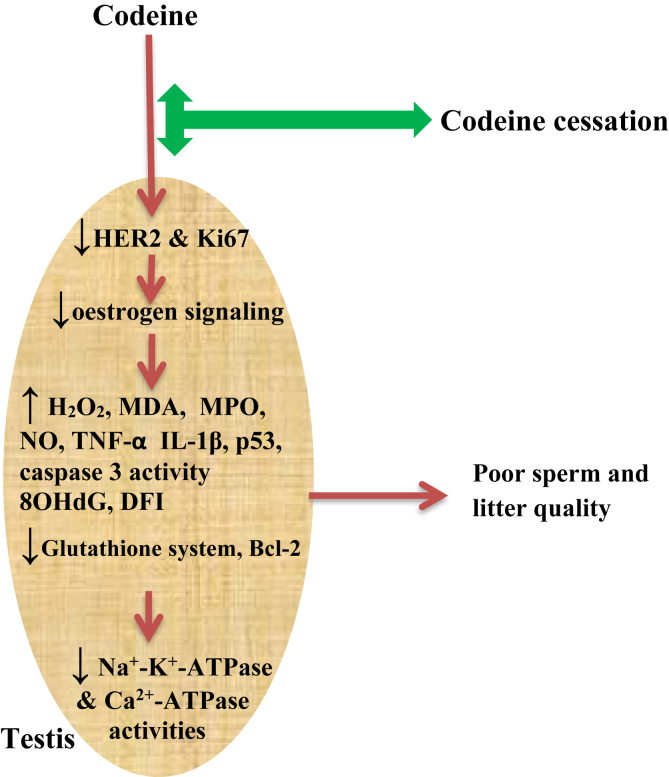
Our present study is in full agreement with our previous documentation and also extends our knowledge on the mechanisms associated with reproductive toxicity induced by codeine. The results of our current study revealed that codeine exposure does not only predispose to possible male infertility via oxidative damage and caspase 3-dependent apoptosis, it also led to the suppression of testicular HER2, Ki67, and Bcl-2 expression, as well as down-regulation of oestrogen signaling, and upregulation of caspase 3 activity and p53 signaling. Codeine cessation promoted the partial and dose-dependent reversal of the poor sperm quality, reduced litter size and weight, increased oxidative testicular injury, apoptosis, and DNA damage caused by codeine administration (Figure 9).
Figure 9.
Graphical abstract of the effects of codeine and codeine cessation in gonado-spermotoxicity.
Declarations
Author contribution statement
R.E. Akhigbe: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data; Wrote the paper.
A. Ajayi: Conceived and designed the experiments; Performed the experiments; Analyzed and interpreted the data; Contributed reagents, materials, analysis tools or data.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of interests statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
The authors are thankful to the National Law Drug Enforcement Agency (NDLEA), Nigeria, for the donation of codeine used in the study. We also thank Mr Aremu Goke (Department of Histopathology, Obafemi Awolowo University Teaching Hospital Complex, Ile Ife), and Hamed Moses (Buntai Medical and Research Laboratories, Osogbo) for the support rendered in the laboratory analysis.
References
- 1.Ding J., Shang X., Zhang Z., Jing H., Shao J., Fei Q., Rayburn E.R., Li H. FDA-approved medications that impair human spermatogenesis. Oncotarget. 2017;8(6):10714–10725. doi: 10.18632/oncotarget.12956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cummins T., Miller S. The effects of drug abuse on sexual functioning. In: Levine S.B., editor. Handbook of Clinical Sexuality for Mental Health Professionals. Brunner-Routledge; New York: 2003. pp. 443–456. [Google Scholar]
- 3.INCB, International Narcotics Control Board. Narcotic Drugs Estimated World Requirements for 2012. Vienna.
- 4.Ajayi A.F., Akhigbe R.E. Assessment of sexual behaviour and fertility indices in male rabbits following chronic codeine use. Andrology. 2020;8:509–515. doi: 10.1111/andr.12717. [DOI] [PubMed] [Google Scholar]
- 5.Akhigbe R., Ajayi A. Testicular toxicity following chronic codeine administration is via oxidative DNA damage and up-regulation of NO/TNF-α and caspase 3 activities. PloS One. 2020;15(3) doi: 10.1371/journal.pone.0224052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.National Toxicology Program NTP toxicology and carcinogenesis studies of codeine (CAS No. 76-57-3) in F344 rats and B6C3F1 Mice (Feed studies) Natl. Toxicol. Program Tech. Rep. Ser. Actions. 1996;455:1–275. [PubMed] [Google Scholar]
- 7.Ajayi A.F., Akhigbe R.E. Codeine-induced sperm DNA damage is mediated predominantly by oxidative stress rather than apoptosis. Redox Rep. 2020;25(1):33–40. doi: 10.1080/13510002.2020.1752003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Korsmeyer S.J. Bcl-2 initiates a new category of oncogenes: regulators of cell death. Blood. 1992;80:879–886. [PubMed: 1498330] [PubMed] [Google Scholar]
- 9.Kastan M.B., Onyekwere O., Sidransky D., Vogelstein B., Craig R.W. Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 1991;51:6304–6311. [PubMed: 1933891] [PubMed] [Google Scholar]
- 10.Kastan M.B., Zhan Q., el-Deiry W.S., Carrier F., Jacks T., Walsh W.V., Plunkett B.S., Vogelstein B., Fornace A.J. A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in ataxia-telangiectasia. Cell. 1992;71:587–597. doi: 10.1016/0092-8674(92)90593-2. [PubMed: 1423616] [DOI] [PubMed] [Google Scholar]
- 11.Hemann M.T., Lowe S.W. The p53–Bcl-2 connection. Cell Death Differ. 2006;13(8):1256–1259. doi: 10.1038/sj.cdd.4401962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Attardi L.D., Lowe S.W., Brugarolas J., Jacks T. Transcriptional activation by p53, but not induction of the p21 gene, is essential for oncogene-mediated apoptosis. EMBO J. 1996;15:3693–3701. [PubMed: 8758936] [PMC free article] [PubMed] [Google Scholar]
- 13.Jimenez G.S., Nister M., Stommel J.M., Beeche M., Barcarse E.A., Zhang X.Q., O'Gorman S., Wahl G.M. A transactivation-deficient mouse model provides insights into Trp 53 regulation and function. Nat. Genet. 2000;26:37–43. doi: 10.1038/79152. [PubMed: 10973245] [DOI] [PubMed] [Google Scholar]
- 14.Haupt Y., Rowan S., Shaulian E., Vousden K.H., Oren M. Induction of apoptosis in HeLa cells by trans-activation-deficient p53. Genes Dev. 1995;9:2170–2183. doi: 10.1101/gad.9.17.2170. [PubMed: 7657168] [DOI] [PubMed] [Google Scholar]
- 15.Caelles C., Helmberg A., Karin M. p53-dependent apoptosis in the absence of transcriptional activation of p53-target genes. Nature. 1994;370:220–223. doi: 10.1038/370220a0. [PubMed: 8028670] [DOI] [PubMed] [Google Scholar]
- 16.McCurrach M.E., Connor T.M., Knudson C.M., Korsmeyer S.J., Lowe S.W. Bax-deficiency promotes drug resistance and oncogenic transformation by attenuating p53-dependent apoptosis. Proc. Natl. Acad. Sci. U.S.A. 1997;94:2345–2349. doi: 10.1073/pnas.94.6.2345. [PubMed: 9122197] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin C., Knudson C.M., Korsmeyer S.J., Van Dyke T. Bax suppresses tumorigenesis and stimulates apoptosis in vivo. Nature. 1997;385:637–640. doi: 10.1038/385637a0. [PubMed: 9024662] [DOI] [PubMed] [Google Scholar]
- 18.Aitken R.J., Koppers A.J. Apoptosis and DNA damage in human spermatozoa. Asian J. Androl. 2011;13:36–42. doi: 10.1038/aja.2010.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quenby S.M., Gazvani M.R., Brazeau C., Neilson J., Lewis-Jones D.I., Vince G. Oncogenes and tumour suppressor genes in first trimester human fetal gonadal development. Mol. Hum. Reprod. 1999;5:737–741. doi: 10.1093/molehr/5.8.737. [DOI] [PubMed] [Google Scholar]
- 20.Shin I., Kim H.J., Nah W.H., Park H.J., Gye M.C., Park H.Y. Expression of activated HER2 in human Testes. Fertil. Steril. 2011;95:2725–2728. doi: 10.1016/j.fertnstert.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Rommerts F.F. And cellular localization of rat testicular aromatase activity. J. Reprod. Fertil. 1982;65(2):281–288. doi: 10.1530/jrf.0.0650281. [DOI] [PubMed] [Google Scholar]
- 22.van der Molen H.J. Testicular oestrogens. J. Endocrinol. 1981;89(Suppl):33P–46P. [PubMed] [Google Scholar]
- 23.O'Donnell L. Estrogen and spermatogenesis. Endocr. Rev. 2001;22(3):289–318. doi: 10.1210/edrv.22.3.0431. [DOI] [PubMed] [Google Scholar]
- 24.Revelli A., Massobrio M., Tesarik J. Nongenomic actions of steroid hormones in reproductive tissues. Endocr. Rev. 1998;19(1):3–17. doi: 10.1210/edrv.19.1.0322. [DOI] [PubMed] [Google Scholar]
- 25.Li H. Regulation of rat testis gonocyte proliferation by platelet-derived growth factor and estradiol: identification of signaling mechanisms involved. Endocrinology. 1997;138(3):1289–1298. doi: 10.1210/endo.138.3.5021. [DOI] [PubMed] [Google Scholar]
- 26.Walczak-Jedrzejowska R. During seminiferous tubule maturation testosterone and synergistic action of FSH with estradiol support germ cell survival while estradiol alone has pro-apoptotic effect. Folia Histochem. Cytobiol. 2007;45(Suppl 1):S59–64. [PubMed] [Google Scholar]
- 27.Ajayi A.F., Akhigbe R.E. The physiology of male reproduction: impact of drugs and their abuse on male fertility. Andrologia. 2020 doi: 10.1111/and.13672. [DOI] [PubMed] [Google Scholar]
- 28.Adler I. Comparison of the duration of spermatogenesis between male rodents and humans. Mutat. Res. 1996;352:169–172. doi: 10.1016/0027-5107(95)00223-5. [DOI] [PubMed] [Google Scholar]
- 29.Reagan-Shaw S., Nihal M., Ahmad N. Dose translation from animal to human studies revisited. Faseb. J. 2007;22:659–661. doi: 10.1096/fj.07-9574LSF. [DOI] [PubMed] [Google Scholar]
- 30.Ajayi A.F., Akhigbe R.E. Staging of the estrous cycle and induction of estrus in experimental rodents: an update. Fertil. Res. Pract. 2020;6:5. doi: 10.1186/s40738-020-00074-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ajayi A.F., Aniviye B.O., Kehinde B.D., Akintola A.O. Age-related changes in the expression of heat shock protein 70 and 90 on the gastric mucosa during gastric ulcer healing. UK J.o Pharmaceut. Biosci. 2018;6(4):1–10. [Google Scholar]
- 32.Nouroozzadeh J., Tajaddinisarmadi J., Wolff S.P. Measurement of plasma hydroperoxide concentrations by the ferrous oxidation-xylenol assay in conjunction with triphenylphosphine. Anal. Biochem. 1994;220(2):403–409. doi: 10.1006/abio.1994.1357. [DOI] [PubMed] [Google Scholar]
- 33.Adegunlola J.G., Afolabi O.K., Akhigbe R.E., Adegunlola G.A., Adewumi O.M., Oyeyipo I.P. Lipid peroxidation in brain tissue following administration of low and high doses of arsenite and L-ascorbate in Wistar strain rats. Toxicol. Int. 2012;19:47–50. doi: 10.4103/0971-6580.94516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beutler E., Duron O., Kelly B.M. Improved method for the determination of blood glutathione. J. Lab. Clin. Med. 1963;61:882–888. [PubMed] [Google Scholar]
- 35.Fridovich I., Misra H.P. The role of superoxide anion in the autooxidation of epinephrine and a simple assay for superoxide dismutase. J. Biol. Chem. 1972;247:3170–3175. [PubMed] [Google Scholar]
- 36.Euler H.V., Josephson K. Uber katalase. I European. J. Org. Chem. 1972;452:158–181. [Google Scholar]
- 37.Rotruck J.T., Pope A.L., Ganther H.E., Swanson A.B., Hafeman D.G., Hoekstra W.G. Selenium: biochemical role as a component of glutathione peroxidase. Science. 1973;179(4073):588–590. doi: 10.1126/science.179.4073.588. [DOI] [PubMed] [Google Scholar]
- 38.Habig W.H., Pabst M.J., Jakoby W.B. Glutathione-S-transferases. The first enzymatic step in mercapturic acid formation. J. Biol. Chem. 1974;249(22):7130–7139. [PubMed] [Google Scholar]
- 39.Desser R.K., Himmelhoch S.R., Evans W.H., Januska M., Mage M., Shelton E. Guinea pig heterophil and eosinophil peroxidase. Arch. Biochem. Biophys. 1972;148:452–465. doi: 10.1016/0003-9861(72)90164-6. [DOI] [PubMed] [Google Scholar]
- 40.Ridnour L.A., Sim J.E., Hayward M.A., Wink D.A., Martin S.M., Buettner G.R. A spectrophotometric method for the direct detection and quantitation of nitric oxide, nitrite, and nitrate in cell culture media. Anal. Biochem. 2000;281:223–229. doi: 10.1006/abio.2000.4583. [DOI] [PubMed] [Google Scholar]
- 41.Juel C., Nordsborg N.B., Bangsbo J. Exercise-induced increase in maximal in vitro Na,K-TPase activity in human skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2013;304:1161–1165. doi: 10.1152/ajpregu.00591.2012. [DOI] [PubMed] [Google Scholar]
- 42.Tuominen V.J., Ruotoistenmäki S., Viitanen A., Jumppanen M., Isola J. ImmunoRatio: a publicly available web application for quantitative image analysis of estrogen receptor (ER), progesterone receptor (PR), and Ki-67. Breast Cancer Res. 2010;12:R56. doi: 10.1186/bcr2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Perandones C.E., Illera V.A., Peckham D., Stunz L.L., Ashman R.F. Regulation of apoptosis in vitro in mature murine spleen T cells. J. Immunol. 1993;151(7):3521–3529. [PubMed] [Google Scholar]
- 44.Agarwal A., Nallella K.P., Allamaneni S.S.R., Said T.M. Role of antioxidants in treatment of male infertility: an overview of the literature. Reprod. Biomed. Online. 2004;(8):616–627. doi: 10.1016/s1472-6483(10)61641-0. [DOI] [PubMed] [Google Scholar]
- 45.Aitken R.J., Jones K.T., Robertson S.A. Reactive oxygen species and sperm function-in sickness and in Health. J. Androl. 2012;33:1096–1106. doi: 10.2164/jandrol.112.016535. [DOI] [PubMed] [Google Scholar]
- 46.Wagner H., Cheng J.W., Ko E.Y. Role of reactive oxygen species in male infertility: an updated review of literature. Arab J. Urol. 2018;16:35–43. doi: 10.1016/j.aju.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chaudiere J., Wilhelmsen E.C., Tappel A.L. Mechanism of selenium-glutathione peroxidase and its inhibition by mercaptocarboxylic acids and other mercaptans. J. Biol. Chem. 1984;259:1043–1050. [PubMed] [Google Scholar]
- 48.Jeulin C., Soufir J.C., Weber P., Laval-Martin D., Calvayrac R. Catalase activity in human spermatozoa and seminal plasma. Gamete Res. 1989;24:185–196. doi: 10.1002/mrd.1120240206. [DOI] [PubMed] [Google Scholar]
- 49.Alvarez J.G., Touchstone J.C., Blasco L., Storey B.T. Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa: superoxide dismutase as major enzyme protectant against oxygen toxicity. J. Androl. 1987;8:336–348. doi: 10.1002/j.1939-4640.1987.tb00973.x. [DOI] [PubMed] [Google Scholar]
- 50.Aitken R.J., Clarkson J.S. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J. Androl. 1988;9:367–376. doi: 10.1002/j.1939-4640.1988.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 51.Fraga G.G., Motchnik P.A., Shigenaga M.K., Helbrock J.H., Jacob R.A., Ames B. Ascorbic acid protects against endogenous oxidative DNA damage in human sperm. Proc. Natl. Acad. Sci. U.S.A. 1991;88:11003–11006. doi: 10.1073/pnas.88.24.11003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Makker K., Agarwal A., Sharma R. Oxidative stress & male infertility. Indian J. Med. Res. 2009;129:357–367. [PubMed] [Google Scholar]
- 53.Aitken R.J. Free radicals, lipid peroxidation and sperm function. Reprod. Fertil. Dev. 1995;7:659–668. doi: 10.1071/rd9950659. [DOI] [PubMed] [Google Scholar]
- 54.Kodama H., Kuribayashi Y., Gagnon C. Effect of sperm lipid peroxidation on fertilization. J. Androl. 1996;17:151–157. [PubMed] [Google Scholar]
- 55.Chyra-Jach D., Kaletka Z., Dobrakowski M., Machoń-Grecka A., Kasperczyk S., Birkner E., Kasperczyk A. The associations between infertility and antioxidants, proinflammatory cytokines, and chemokines. Oxidative Medicine and Cellular Longevity. 2018;2018 doi: 10.1155/2018/8354747. Article ID 8354747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Frączek M., Sanocka D., Kamieniczna M., Kurpisz M. Proinflammatory cytokines as an intermediate factor enhancing lipid sperm membrane peroxidation in in vitro conditions. J. Androl. 2008;29(1):85–92. doi: 10.2164/jandrol.107.003319. [DOI] [PubMed] [Google Scholar]
- 57.Lampiao F., du Plessis S.S. TNF-α and IL-6 affect human sperm function by elevating nitric oxide production. Reprod. Biomed. Online. 2008;17(5):628–631. doi: 10.1016/s1472-6483(10)60309-4. [DOI] [PubMed] [Google Scholar]
- 58.Perdichizzi A., Nicoletti F., Vignera S., Barone N., D’agata R., Vicari E., Calogero A.E. Effects of tumour necrosis factor-α on human sperm motility and apoptosis. J. Clin. Immunol. 2007;27(2):152–162. doi: 10.1007/s10875-007-9071-5. [DOI] [PubMed] [Google Scholar]
- 59.Zhang Q., Pei L.-g., Liu M., Lv F., Chen G., Wang H. Reduced testicular steroidogenesis in rat offspring by prenatal nicotine exposure: epigenetic programming and heritability via nAChR/HDAC4. Food Chem. Toxicol. 2020 doi: 10.1016/j.fct.2019.111057. [DOI] [PubMed] [Google Scholar]
- 60.Barenysa M., Gomez-Catalana J., Campsb L., Teixidoa E., de Lapuenteb J., Gonzalez-Linaresb J., Serretb J., Borrasb M., Rodamilansa M., Llobeta J.M. MDMA (ecstasy) delays pubertal development and alters sperm quality after developmental exposure in the rat. Toxicol. Lett. 2010;197:135–142. doi: 10.1016/j.toxlet.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 61.Carreau S. Estrogens and male reproduction: a new concept. Braz. J. Med. Biol. Res. 2007;40(6):761–768. doi: 10.1590/s0100-879x2007000600003. [DOI] [PubMed] [Google Scholar]
- 62.Chimento A. Role of estrogen receptors and G protein-coupled estrogen receptor in the regulation of hypothalamus-pituitary–testis axis and spermatogenesis. Front. Endocrinol. 2014;5:1. doi: 10.3389/fendo.2014.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Martinez-Hernandez J., Seco-Rovira V., Beltran-Frutos E., Ferrer C., Serrano-Sanchez M.I., Pastor L.M. Proliferation, apoptosis and number of sertoli cells in the Syrian hamster during recrudescence after exposure to short photoperiod. Biol. Reprod. 2020;102(3):588–597. doi: 10.1093/biolre/ioz198. [DOI] [PubMed] [Google Scholar]