Abstract
Background
The experience of older women during breast cancer treatment is insufficiently described by quantitative studies. This study aimed to systematically review qualitative data describing factors that influence older women’s (≥65 years old) experience with breast cancer treatment.
Methods
A systematic review was performed in accordance with Preferred Reporting Items for Systematic Review and Meta-analysis Protocols (PRISMA) principles. MEDLINE, CINAHL, PsycINFO, and EMBASE were searched (inception – 2020). Quality assessment of essential item reporting was performed using the Standards for Reporting Qualitative Research (SRQR) criteria. Common ideas were coded, thematically organized, and synthesized within a theoretical framework.
Results
Of 7,773 studies identified, twelve were included. The median SRQR score was 13.4 (range 11.3–15.9) (maximum score: 21). Data synthesis revealed that older women experienced breast cancer as a journey with challenges during each phase. During diagnosis, they delayed seeking medical help despite symptoms. Age and experience gave them perspective on the impact of their diagnosis. During decision-making, preconceptions and personal values determined choices. In the treatment phase, women experienced medical and social barriers to care. During the post-treatment phase, many experienced treatment adverse effects, but could move on or compartmentalize as coping mechanisms.
Conclusion
Older women with breast cancer have unique challenges specific to each phase of their treatment journey. Older women may benefit from proactive treatment discussions with health care providers to address their specific needs, individualize care, and assist with cancer care navigation.
Keywords: Aged, Breast neoplasms, Decision making, Clinical decision-making
Highlights
-
•
Older women have challenges specific to each phase of their treatment journey.
-
•
Older women experience barriers to care and depended on their social network.
-
•
Older women could conceptually move on or compartmentalize as coping mechanisms.
-
•
Physicians should be aware of the unique features of each treatment phase.
1. Introduction
Over 40% of breast cancers are diagnosed in older women [1]. Given that increasing age is a risk factor for breast cancer and advances in health care have improved life expectancy, an increase in the number of older women with breast cancer is expected. Older women with breast cancer have features that make their presentation unique. Although older women tend to present with tumors that are biologically favorable (e.g.: low-grade and hormone receptor positive), they tend to be larger in size and node-positive [2,3]. While some older women are highly functional and independent, other women have multiple medical comorbidities and progressive decline in baseline physiologic and cognitive function [[4], [5], [6], [7]]. As such, balancing patient and tumor-related factors with comprehensive breast cancer care requires an individualized approach.
Older women receive different breast cancer treatments compared to younger women because they have different tumor biology, physiology, social dynamics, and priorities [[8], [9], [10]]. Given that their tumors are frequently low-grade and hormone receptor positive, there is a greater tendency to offer non-surgical treatment [e.g.: primary endocrine therapy (PET)] or to omit sentinel lymph node biopsy or adjuvant radiation therapy. Competing mortality risk from diseases other than breast cancer increases with age. Treatment options for breast cancer have to be adjusted to mitigate the physiological changes imposed by the higher number of comorbidities and physiological decline [11]. Consequently, older women may not benefit from treatment as strongly as younger women [4]. Their treatment experiences are also different due to the varying social support systems and lack of independence. These features may hinder postoperative care, compliance with medications and appointments, and visiting treatment centers. Additionally, older women have different priorities than young women. For example, they may be less willing to sacrifice quality of life for survival prolongation [12]. Considerable variation in rates of treatment have been reported amongst older women with breast cancer. In a study of 17,129 older women with operable estrogen receptor-positive breast cancer across two regional cancer registries from the United Kingdom, considerable variation in rates of surgery at both hospital and clinician level were reported. This study highlighted variation in selection criteria for older women for operative treatment of early breast cancer, indicating that some patients may be undertreated or overtreated. These data stressed the urgent need for evidence-based guidelines for treatment selection criteria in older women with breast cancer.
Insight into how older women with breast cancer perceive diagnosis and treatment leads to better care [7]. Quantitative studies cannot comprehensively describe what older women experience during every aspect of breast cancer treatment. Several studies have reported how surgery and chemotherapy can have successful outcomes in select older women [8,9]. However, the trade-offs for side effect and quality of life have not necessarily been an area of focus. Several systematic reviews using qualitative research have described patient experience, but have only focused on particular aspects of treatment and decision-making [[13], [14], [15]]. For instance, a previous systematic review by our group solely described factors that influenced the decision-making process, leaving behind what older women experience as they are diagnosed and during the post-treatment phase of their care [13]. Both Puts et al. [14] and Tariman et al. [15] included other types of cancers, which limited their ability to analyze aspects specific to breast cancer including overall favorable prognosis and treatments such as breast reconstruction. Therefore, there is a need for a systematic review that addresses all aspects of breast cancer treatment including diagnosis, decision-making, treatment, and post-treatment specifically in older women. Comprehensive understanding of patient experience during all aspects of breast cancer treatment is relevant to individualize discussions with patients, enhance treatment adherence, tailor educational strategies, reduce misinformation, and improve outcomes and patient satisfaction. This study aimed to systematically review qualitative data describing factors that influence older women’s experience with breast cancer treatment.
2. Methods
2.1. Search strategy
A comprehensive search strategy was designed in consultation with a health sciences information specialist. This systematic review was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement for reporting systematic reviews [16]. A search using keywords and index terms was performed across four databases (MEDLINE, CINAHL, PsycINFO, EMBASE) from inception to July 1, 2020 (Supplement 1). The references from included articles and relevant existing reviews were screened to include additional studies. An updated search was performed prior to submission for publication.
Studies with the following characteristics were included: (1) female patients aged ≥ 65 years old with a first-time diagnosis of invasive breast cancer (stage I–III) who were considering or completed surgery, chemotherapy, radiation therapy, hormone therapy, and/or breast reconstruction; (2) data describing patient-reported factors that influenced treatment experience; (3) primary qualitative methodology or mixed methods studies that reported qualitative results; and (4) studies published in English peer-reviewed journals. Study exclusion criteria were as follows: (1) studies lacking information about patients’ age, breast cancer stage, prior breast cancer history, and patients’ treatment experience; (2) studies exploring patients’ experiences with alternative medicine, palliative care, or those who refused treatment altogether; and (3) non-peer reviewed publications including editorials, reviews, protocols, guidelines, and non-indexed journals. If a study involved other age groups or other cancer stages (in situ or metastatic), the study was included, provided it contained a subgroup analysis for the population of interest.
A chronological age cut-off of ≥65 years old was chosen as the definition of “older” in accordance with the American Society of Clinical Oncology (ASCO) recommendation to perform geriatric assessment in oncology patients in this age group [17]. Patient experience was defined as patient-reported data describing all interactions that influence patient perceptions across the continuum of health care.
2.2. Study selection
Three reviewers (FAA, YZ, and ME) were involved in study selection. Each reviewer independently screened the titles and abstracts. After removing duplications, the full texts of potential studies were independently screened by all reviewers. If a final decision could not be made from the title and abstract, the full text was analyzed. If two manuscripts represented the same study cohort, the most comprehensive text was used in order to minimize data duplication. Discrepancies regarding inclusion or exclusion of any study were discussed amongst all authors.
2.3. Data extraction
Two reviewers (YZ and FAA) extracted data from the included studies. Abstraction of the following data was performed: study characteristics (country and year), study methodology, participant and tumor characteristics, and patient treatment experience data using direct quotes. Summative thematic analyses developed by the original studies were not recorded because one of the goals of this systematic review was to perform an independent inductive thematic analysis. If any aspect of the study design was unclear, the authors were contacted.
2.4. Quality assessment of essential item reporting
Quality assessment of essential item reporting was performed by three reviewers (FAA, YZ, and ME) using the Standards for Reporting Qualitative Research (SRQR) [18]. The SRQR scoring system consists of 21 items and sub-items developed through a rigorous peer review process. Studies were graded based on the number of these essential items that were reported. For each sub-item, answer categories were ‘yes’ (if they met the criteria), ‘no’ (if they did not meet the criteria), or ‘unclear’ (if it was unclear whether they met the criteria). Each of the 21 quality items was scored on the presence and quality of sub-item criteria. Each item was given an individual score of up to 1.0. Scores were summed with a maximum score of 21 points. Scores were averaged over all three reviewers. Study quality was rated as ‘high’ (score = 16–21), ‘medium’ (score = 11–15), or ‘low’ (score = < 11). Significant discrepancies regarding quality assessment were resolved through discussion between the authors. The median and range of SRQR scores were reported. Manuscripts were not excluded based on quality assessment.
2.5. Data synthesis and analysis
Three authors (FAA, YZ, and NLH) iteratively analyzed the studies and discussed the similarities and differences. Higher-level thematic analysis was performed as described by Thomas and Harden [20]. Thematic analysis allows the researcher to extract recurrent themes from study findings and transparently develop a framework of understanding from the text of the primary studies [19]. In keeping with qualitative research principles outlined by Bearman and Dawson, thematic analysis was chosen as the qualitative synthesis methodology because it provides narrative value through building a collective understanding of the data regarding a particular issue or phenomenon, not by establishing definitive causal links [20]. The goal of this thematic analysis is to summarize the collective conclusions of the qualitative or mixed-method studies and establish patterns within the data through examination of data without a priori frameworks [20]. Thematic analysis involves three stages: (1) free line-by-line coding; (2) grouping of the codes into descriptive themes; and (3) the formation of inter-related conceptual ideas. Analysis negotiation was performed by three authors (FAA, YZ, and NLH) iteratively analyzing the studies and discussing the similarities and differences between the data. Discrepancies in themes amongst the authors were discussed until an agreement was achieved.
3. Results
3.1. Study selection
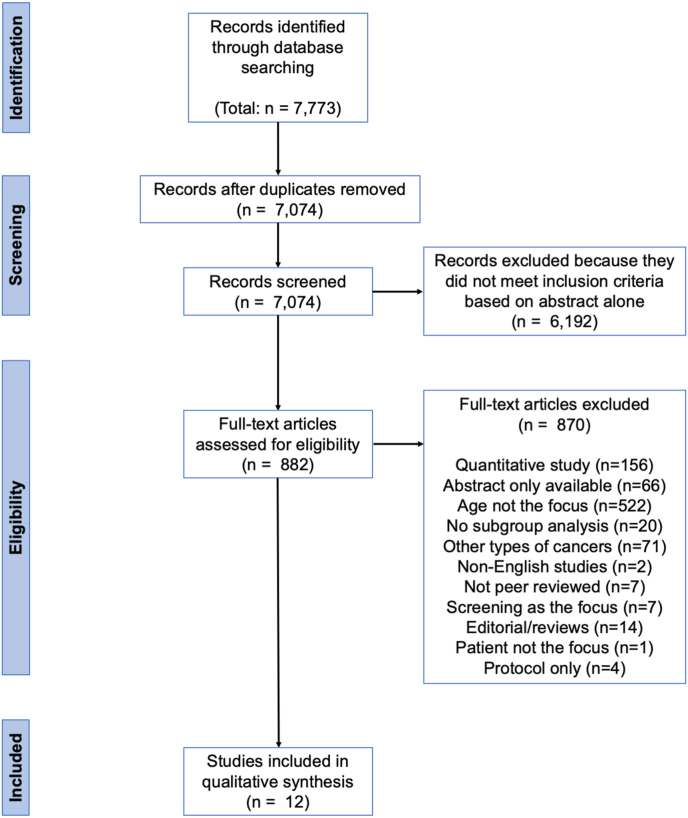
Fig. 1 depicts the PRISMA flowchart. A total of 7,773 studies were identified through the database search. Cross-referencing and a search of the grey literature did not yield additional studies. After removing duplicates, 7,074 articles were evaluated. After an initial review, 6,192 studies were excluded because they did not meet inclusion criteria based on title and abstract alone. A total of 882 manuscripts were reviewed in full of which 870 were excluded. Twelve studies were included in the final analysis [[21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32]]. Study characteristics are summarized in Table 1.
Fig. 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram of the systematic review.
Table 1.
Study characteristics.
| First Author [Reference] | Year of Publication | Country | Study aim | Sample Size | Age (y) Mean or median (range) |
Data Collection | Data Analysis | Average SRQR Score |
|---|---|---|---|---|---|---|---|---|
| Burton et al [21] | 2015 | UK | To determine the information needs and preferences for this age group of women relating to the choice between surgery and PET. | 33 | 82 (median) (75–95) |
Individual interview | Framework analysis | 12.8 |
| Ciambrone et al [22] | 2006 | USA | To identify factors associated with older women’s breast cancer primary therapy decision making processes, surveillance decision-making, and adherence. | 30 | 77 (mean) (67–90) | Individual interview | Thematic analysis | 11.3 |
| Fenlon et al [23] | 2013 | UK | To explore older women’s experience of living with breast cancer and their information and support needs and preferences. | 28 | >70 (N·S.) | Focus group and individual interview | Thematic analysis | 13.5 |
| Harder et al [24] | 2013 | UK | To explore the attitudes to adjuvant chemotherapy of the women who were offered chemotherapy, their experiences with information provision, and factors that influenced their treatment choice. | 58 | >70 (N·S.) | Individual interview | Thematic analysis | 14.0 |
| Husain et al [25] | 2008 | UK | To explore what older women with breast cancer feel are the most important factors in making their treatment choice and identify factors and attitudes that influenced treatment choice. | 21 | 83 (mean) (76–91) | In-depth interview | Framework analysis | 14.5 |
| Kreling et al [26] | 2006 | USA | To understand factors involved in older women’s use or non-use of indicated adjuvant non-hormonal chemotherapy. | 34 | >65 (N·S.) | Focus group | Thematic analysis | 14.5 |
| Loerzel et al [27] | 2012 | USA | To investigate the post-treatment concerns of older, early-stage breast cancer survivors. | 50 | 72.1 (mean) (65–83) | Individual interview | Thematic analysis | 13.5 |
|
Lu et al [28] |
2010 | Taiwan | To explore the experiences of older Taiwanese women in facing a new diagnosis of breast cancer. | 14 | 73 (mean) (65–91) | Individual interview | Thematic analysis | 13.3 |
| Morgan et al [29] | 2015 | UK | To explore the interaction between HCPs and older patients in the DM process, as well as the concordance between HCP and patient views regarding the process of DM about treatment of operable breast cancer. | 33 | >70 (75–94) | Individual interview | Framework analysis | 13.0 |
| Pieters et al [30] | 2011 | USA | To describe the experiences of women aged 70 years and older who recently completed treatment for early stage primary breast cancer. | 18 | 76 (mean) (70–94) | Individual interview | Constructivist grounded theory | 15.9 |
| Schonberg et al [31] | 2014 | USA | To explore older women’s perspectives and views of treatment decision making and intentions to accept breast cancer treatment. | 70 | Unspecified (65–74) | Individual interview | Framework analysis | 13.3 |
| Wong et al [32] | 2011 | Canada | To investigate the information needs of women 70 years and older with early-stage breast cancer in relation to adjuvant treatment post lumpectomy. | 16 | 76 (median) (70–84) |
Focus group and individual interview | Not specified | 13.0 |
Abbreviations: DM, decision making; HCP, health care provider; N·S., not specified; PET, primary endocrine therapy; SRQR, Standards for Reporting Qualitative Research; UK, United Kingdom; USA, United States of America; y, years old.
The studies were performed in the United Kingdom (n = 5), the United States of America (n = 5), Canada (n = 1), and Taiwan (n = 1). Data on patients’ level of education, socioeconomic background, religious belief, or marital status were not provided. Study designs included qualitative research (n = 11) and mixed methods with a qualitative component (n = 1).
3.2. Quality of essential item reporting
The quality of esential item reporting was variable across the included studies (Table 1). The overall quality of the reporting of essential items was medium as the median SRQR total score was 13.4 (range 11.3–15.9).
3.3. Older women’s experience with breast cancer
Synthesized findings with dominant themes and inter-relationships between themes are described in the subsequent sections. The overall framework of patients’ experience with breast cancer is that of a longitudinal journey. As older women enter different phases of breast cancer treatment, they perceive experiences specific to each phase. Table 2 summarizes patient-reported factors that influence their journey along with selected participant quotations. Interaction of these factors throughout the patients’ cancer journey is illustrated in Fig. 2.
Table 2.
Patient-reported factors influencing the experience of older women with breast cancer.
| Themes |
Illustrative Quote [Reference] |
Contributing Studies |
|---|---|---|
| Diagnosis phase | ||
| Age and misinformation impacts breast cancer diagnosis | “It was a discovery that, you know, old girls like us get it! (laughs) It was quite a new one to me.” [23] “You think well you’ve reached this age, which I never expected to, and I cannot believe I’m 78 now, and I just, and I’m very pleased I’m here but. I never thought I would reach this age, so I haven’t got much to lose like a younger person have I?” [23] “Well I am too old, 91 to go to a big operation like that.” [29] |
[23,25,28,29, 31] |
| Decision-making phase | ||
| Age and life experiences informed preconceptions and decision-making | “Oh you have no idea how horrible chemo was for my friend, I don’t think you should go that way, you’re going to be absolutely miserable. She added, This poor woman with all her metastases, the chemotherapy did not work and she died a miserable death.” [26] | [24,26,28] |
| Quality of life as a goal | “At 77, I’m happy to have lived this long. I said I wanted one last Christmas with my family. I rejected chemotherapy because I’m happy with my quality of life. I said why should I have misery? Chemo would make me sick and lose my hair. I don’t want to be a guinea pig for modern medicine. My mother died of medication. I’m not sure it would have done me any good.” [26] | [26,29] |
| Fear of hurting others | “I am thankful for the mastectomy, I am caring for 2 grandchildren.” [31] | [21,25,28,31] |
| Patient-centered decision-making | “… I’d already made my mind up because I knew it was cancer … —you know in my own mind and made my mind up that I was having the breast taken off.” [21] | [21,22,24,29] |
| Treatment phase | ||
| Dependence on social network for access to care | “I live on my own, sons live away, not very close if I needed them.” [26] “The transportation was a nightmare (radiation).” [31] |
[23,24,26,27,28,30,31] |
| Post-treatment phase | ||
| Treatment-related physical and emotional adverse effects | “I am experiencing hot flashes and not sleeping well from AI.” [31] “I’m nervous about breast cancer coming back.” [31] “It really is grotesque when you get undressed, well I don’t do it now, I don’t look; it looks absolutely grotesque.” [24] |
[23,27,31] |
Abbreviations: AI, aromatase inhibitors.
Fig. 2.
Diagram of the thematic analysis showing how older women view breast cancer as a journey.
3.4. Patient experience during the breast cancer diagnosis phase
Diagnosis covers the time from when a patient undergoes investigation of a breast complaint (clinical and/or radiological) to histopathological confirmation of breast cancer diagnosis. A dominant theme for this phase was that older women delayed seeking medical help when they had a breast symptom. In one study, women took 6 months to 2 years to visit the doctor after they noticed a breast abnormality [28]. The delay was attributed to the lack of pain and knowledge regarding breast cancer as well as the fear of requiring medical treatment. In contrast, in a study from the United Kingdom, some women immediately visited their physician upon discovering a breast mass, while others only mentioned symptoms when visiting a general practitioner for other illnesses [21]. The delay in presentation occurred because some patients were less concerned regarding their symptoms while others waited until their domestic responsibilities, such as being caretaker to a relative, changed [21].
Older women frequently described how their age and experience gave them perspective on the impact of a breast cancer diagnosis. Some women indicated they were less emotionally affected by the diagnosis because of their age [31]. In two studies, women described themselves as “not surprised” due to experiences of acquaintances of “similar age” [21,25]. An acknowledgement of having already lived a long life allowed some older women to pragmatically accept their diagnosis [25]. Four studies described how some women expressed shock, anxiety, and self-blame with the diagnosis [21,25,28,31]. Husain et al. described how these women particularly feared dying, being left disfigured, not knowing what to do, and leaving others behind [25].
3.5. Patient experience during the decision-making phase
Decision-making describes the phase during which patients met with health care providers (HCPs), occasionally in the presence of their support system, to discuss their diagnosis, treatment options, and decisions. Several studies described how women’s preconceptions of breast cancer and treatment was informed by other people’s experience. The experience of friends and family was the most commonly cited source of preconceptions [24,28,30]. Some women thought that breast cancer was lethal because they saw other family members die of cancer [28]. Others were fearful of treatment side effects, mutilation, and recurrence based on memories they had of friends and family who were treated for cancer decades ago [30]. These preconceptions, along with age and life experiences, informed the decision-making of older women with breast cancer. Age was cited by older women as a reason to avoid surgery both as primary treatment for the cancer and reconstruction [29,31]. Patients were concerned that surgery would impact their independence [21,28]. Other women thought surgery caused cancer to spread [30].
Decision-making was based on particular values expressed by patients. Older women valued quality of life over quantity [29]. Life expectancy, impact on functional status, duration of treatment, and low likelihood of survival advantage were factors that contributed to women refusing chemotherapy and radiation therapy [22,24,31]. For some women, being older gave them less fear about being diagnosed with breast cancer and more strength and ability to cope with treatment [26]. Fear of death after dealing with family members with cancer was also cited as an important motivator to undergo chemotherapy [22,26]. Women who were in good health and who had a support network of friends and family were also more likely to undergo treatment [24].
Decision-making preference amongst older women with breast cancer varied between two different viewpoints. In one viewpoint, women wanted ownership of their treatment decision-making[21,22,24,29]. In a study assessing the decision-making experiences of women choosing between PET and surgery, most women felt they had made the final decision and were pleased with their involvement in the process [21]. Women who favoured patient-centered decision-making saw themselves as making the final decision and were more likely to have already made up their mind prior to their consultation [[22], [29],29] The other viewpoint was that of women strongly influenced by recommendations from their HCPs. Participants described relying heavily on the advice and opinion of HCPs for treatment decision-making [21,22,25,29]. The rationale of this viewpoint was explored in one study, with women reporting they felt that treatment should be left to doctors who had specialist knowledge of breast cancer [21]. Some older women described how recommendations and reassurance from their oncologists increased the likelihood of them choosing chemotherapy [24,26]. Even women who independently made their own decisions reported wanting their HCPs approving their decision [21]. In some instances, older women felt they had not been given treatment options and were told what to do [26]. For example, in one study from the United Kingdom, women who underwent a mastectomy used a prosthesis rather breast reconstruction [23]. These women reported they would have liked to discuss breast reconstruction options but felt that the lack of this discussion with their surgeons was due to their age [23].
During the decision-making process, patients receive an influx of information, which they need to use to understand their options and choose what is best for them. HCPs were identified as the primary source of information encountered by patients after their diagnosis [21,23,26]. Older women preferred information from their physicians, with specialist nurses also identified as trusted and useful [21,23]. Four studies reported how older women preferred written information over electronic [21,23,24,32]. Electronic media was viewed as a barrier because older women lacked access to a computer or the Internet, did not trust the Internet, and were overwhelmed by the large volume of available information [21,23,32]. Older women reported preferring simplified, condensed information that addressed their individual needs [21,23,24,26]. Information that was described as helpful included specifics about treatment options, technical aspects of each treatment, and impact on physical function and self-care [21,23,24,26]. Kreling et al. described how targeted information helped dispel negative expectations of chemotherapy and acted as a promoter of chemotherapy use amongst older women [26]. Another approach is the development of decision support interventions (DESI) as reported by Lifford et al. After developing a prototype DESI to help guide older women choose between primary endocrine therapy or surgery with endocrine therapy, participants found that this tool contributed to improved wording and illustrations to address misunderstandings. Although most participants considered the DESIs helpful, they continued to acknowledge the importance of complementary discussions with HCPs.
3.6. Patient experience during the breast cancer treatment phase
The treatment phase comprises the experiences during the time older women received any type of care for their breast cancer. The dominant theme in this phase was that older women experienced unique barriers to treatment which were either rooted in physiological or psycho-social factors. The most common physiologic barrier was the fact older women had pre-existing medical conditions, which influenced which treatments they could tolerate [30]. In other scenarios, older women had medical conditions which precluded surgery altogether [21,23]. Several psycho-social factors influenced older women’s breast cancer treatment experience. Older women perceived they had barriers due to transportation availability. Friends and family were perceived as essential to the treatment experience of older women. In one study, older women indicated that treatment was conducted smoothly because of their family’s aid in transportation [28]. For older women who were reliant on others to travel to appointments this caused psychological distress as they saw themselves as a burden to their support system [30]. Another social barrier experienced by older women was the fact some also played the role of caretaker. Multiple appointments were particularly challenging to women who were caretakers of frail partners. Women who cared for ill family members during a significant portion of their treatment phase ignored symptoms and minimized health problems [27]. In contrast, some older women stated their family members were the reasons they wanted to live and therefore continue with treatment such as chemotherapy [27]. In one study, older women accepted treatment in order to be able to continue taking care of their husbands [28].
Older women reported concerns about the impact treatments would have on their health and social responsibilities. In one study older women were concerned about metastasis, being a burden to the family, mental and physical discomfort during medical treatment, and the possibility of death [28]. Pre-existing stressors such as family, housing, and financial problems negatively impacted their treatment experience [27]. One study in the United States found unique concerns amongst Hispanic women undergoing chemotherapy [26]. Hispanic women frequently reported being worried about the effects chemotherapy could have on their health and how they would keep their employment. Many women in this study also had financial concerns about paying for treatment, as they did not qualify for insurance [26].
The concept of how treatments would affect cosmesis and body image was not prominently described in the included studies. Only two studies reported data on older women’s experience with cosmesis. In one study few of the older women underwent breast reconstruction [23]. Although many older women in this study did not want breast reconstruction, there was a perception for a few that this treatment had not been offered or made available to them because of their age. Certainly, body image is important for older women. For some older women, the main concern after surgery was disfigurement [25]. In this study some women felt less of a woman because they had lost their breast. For these women cosmetic disfigurement was the worst part of their experience with breast cancer treatment. This finding was regardless of whether they were widowed or still married.
3.7. Patient experience during the post-treatment phase
The post-treatment phase involves the experiences of older women after they finished breast cancer treatment. Older women experienced numerous treatment-related physical and emotional adverse effects. Physical effects included decreased shoulder mobility, fatigue, pain, sleep disturbance, lymphedema, and cognitive changes [23,27,31]. Limited shoulder ability and lymphedema were particularly challenging to older women as these changes restricted their ability to partake in daily activities and worsened with their caretaking responsibilities [23,27]. Emotional challenges included negative effects on body image, worsening relationship quality, anxiety about recurrence, and concerns over how other aspects of life would be affected [27,31]. Breast cancer changed how women viewed their own bodies and, for some, how their partners viewed their bodies [23,31]. Some women reported a clear moment in which their relationships worsened while other women reported a change or discontinuation of sexual relationships with their partner [23,27].
To manage these adverse effects, older women used positive coping mechanisms. Women coped with their breast cancer by compartmentalizing the experience and attributing their symptoms to existing chronic conditions instead [27]. In two studies women indicated these other chronic health problems were more stressful than their breast cancer [27,31]. In some instances, women expected to live with their new symptoms and believed these problems were just another part of life [27]. Breast cancer was perceived by some women as a chronic health problem as opposed to an episodic one. Women felt that the most important outcome was the ability to carry on with life as if they did before their diagnosis [25,28,31].
4. Discussion
This study is the first systematic review of qualitative data to comprehensively describe the treatment experiences of older women with breast cancer. Older women experienced unique barriers to care and adverse effects from treatment that were compounded by their age, pre-existing medical conditions, and preconceptions of breast cancer. During the diagnosis phase, both age and preconceptions limited treatment discussion and influenced decision-making. During the treatment phase, women experienced barriers to care and depended on their support system to access care. Barriers to treatment, including medical comorbidities, dependency on others, and caregiving responsibilities for a frail spouse, reflect the unique social and physical circumstances of this age group. During the post-treatment phase, older women experienced treatment adverse effects, but could compartmentalize their issues, equating ongoing breast cancer effects with other manageable chronic illnesses. Consequently, older women were capable of coping with their symptoms and had a desire to live life as they had before.
Breast cancer as a journey for older women has unique experiences and expectations. While the diagnosis of breast cancer is the common thread binding all of these women together, is it clear that the longitudinal experience is unique to each individual. Throughout the diagnosis, decision-making, treatment, and post-treatment phases, older women demonstrate an immense sense of perspective and pragmatism. For example, this pragmatism is reflected in the delay to diagnosis experienced by women due to a lack of symptoms. Additionally, this is reflected in their approach during decision-making due to competing sentiments of balancing comorbidities and caretaking of frail partners along with the overwhelming desire to “carry-on” with life beyond the diagnosis. While older women often do not face the life practicalities related to ongoing job demands, caring for a young family, finding a partner, and fertility [33], their unique competing priorities are often integrated with a woman’s perceived life expectancy as physicians make recommendations for cancer treatment [15]. These recommendations may contribute to an increased age-dependent omission of surgery and adjuvant treatments [7]. As a result, older women viewed breast cancer practically as another facet of life to be managed.
A breast cancer diagnosis presents an adversity that calls on a patient’s intrinsic strength factors. One of these strength factors is resilience. When faced with the adversity of breast cancer, some older women restored balance to their lives with a sense that they did the work of managing cancer with self-efficacy and autonomy [34]. Regaining balance required tenacity, pragmatism, and dedication to do the work that is required to treat the cancer and move on with life [34]. Recognizing these themes and giving them importance during discussions with older women is essential to improve their breast cancer treatment experience. This would counterbalance the data that suggests that older women are often poorly prepared to participate in breast cancer treatment decisions [35].
Older women experience breast cancer in a different way than younger women, as they are in a different stage of life. Age alone has a significant impact on their treatment choices, as noted in several studies included in this systematic review. Older women with breast cancer may decline certain treatments for different reasons. Some older women believe they have a limited life span while others do not want to prolong their life either because of comorbidities or the perception of “having nothing left to do” [36]. These beliefs are what some refer to as a “sense of completeness that life has run its course” [37]. When older women with breast cancer view themselves at the end of their lives, they question the survival impact of treatments [36]. Another unique feature of older women is that they have various social dynamics. Some older women are fully independent and have a good social support system while others depend on friends and family or lack social support altogether. Women who have social support may want treatment because they fear their death may affect their family and friends [38,39], while others who are independent may desire to maintain their independence by avoiding any treatment [40].
Older women generally have other comorbidities and may have experienced other medical problems in the past, which changes their outlook on current diseases. Comorbidities are an important consideration when deciding on breast cancer treatment as they can preclude some treatments, increase the treatment complexity, or impede return to baseline function [41]. Interestingly, age-specific fear of being hospitalised or of undergoing surgery was not a common theme throughout the included studies. Husain et al. hypothesized it is possible that any recommendations by HCPs to have surgery may have been enough reassurance for these women. Additionally, several of these older women may have already experienced hospital care, including surgeries, and this may have left a positive impression on them. Physiologic factors did not appear to be the most important factor influencing older women’s treatment choice. However, this may be explained by the selection bias in the included studies, as women with significant medical comorbidities may have been excluded from participation or elected not to participate.
Several elements that are unique to older women with breast cancer include socioeconomic, marital, and retirement status [5,42]. These characteristics give some older women complete independence and sense of happiness with their quality of life. As such, some older women may desire to maintain independence and quality of life over quantity of life when deciding on treatment. Alternatively, given some older women are at the advanced stage of their life span, those who decline treatment may express they have a limited number of years left and do not want to prolong their life either because of comorbidities or the thought they “having nothing left to do”. When older women view themselves at the end of their lives, they may question the significance of treatment due to competing mortality risk.
This systematic review highlights the need for older women with breast cancer to be treated as individuals and not just uniformly as a single age group. HCPs should be aware of the important role they play as the most trusted source of medical information [43,44]. Discussion should focus on how treatments may affect existing comorbidities and the patient’s daily activities and social obligations. HCPs should remain mindful of their own inherent age-related biases, and instead try to tailor treatment to the individual as much as possible. This is particularly well illustrated in the case of breast reconstruction. The thought that body image is not important to older women is false, as noted in this systematic review. Preserving an ideal body image is important to some older women with breast cancer. Additionally, large database studies have confirmed the overall safety of different types of breast reconstruction amongst older women with breast cancer [45,46].
HCPs should be mindful of how their preference, personal values, and beliefs affect health care usage. The conviction by HCPs regarding minimal benefits of treatment is a well-documented reason for omitting treatments in older women with breast cancer [7]. As older patients are a heterogeneous population, the contribution of implicit bias to health care disparities amongst older women may be reduced if HCPs acknowledged their susceptibility to such bias and deliberately practice bias-reducing techniques. [47]. One bias-reducing technique is individuating, a conscious effort to focus on specific information about an individual to make it more prominent during the decision-making process. Specifically, individuated patient information prevents HCPs from filling in partial information with stereotype-based assumptions. Another strategy to reduce implicit bias is perspective-taking, in which there is a conscious attempt to envision the patient’s viewpoint. In addition to behavioral strategies aimed at individual HCPs, the contribution of implicit age bias to health care disparities may be reduced on a population level by further developing data that allows evidence-based decision making. The inclusion of a geriatric assessment (GA) may help in tailoring treatment as supported by organizations such as ASCO and the International Society of Geriatric Oncology (SIGO) [17,48,49]. A GA allows HCPs to assess domains including comorbidity, functional status, physical performance, and cognitive status [50]. The goal of a GA is to develop an integrated and individualized treatment by helping to estimate risks for adverse outcomes and to identify nononcologic problems that may be amenable to intervention [51]. As noted in this systematic review, some older women have pre-existing medical conditions and depend on family members to travel to appointments, both of which can influence the decision-making process [21,23,30]. Therefore, a GA may be a useful tool in these particular circumstances in which unique characteristics need to be identified so that a patient-centered plan can be developed. Institutional support services should be maximally utilized to address the social concerns of older women. A nurse navigator is especially important for coordinating health care services and providing details regarding practicalities of care [52,53]. Outreach services to older patients should be implemented to facilitate transportation to hospitals.
This review should be interpreted bearing in mind its limitations. The results are limited by the methodological quality and study design of the included studies. Unfortunately, all the studies omitted the interviewer topic guide, which makes it unclear if factors that were not reported were not important for the interviewers to ask or if patients did not talk about them despite being prompted. This review represents an additional level of interpretation of events. The thematic analysis performedis our interpretation of the findings presented by other researchers who themselves have interpreted the data generated by study participants, while the participants themselves are recounting their own interpretation of events. Another limitation of this review derives from the search strategy. Additional information could have been synthesized had non-indexed journals been included. Studies describing the experiences of patients who used alternative medicine, palliative care, or refused treatment altogether could have also provided more information on the overall experience. Lastly, the results presented in this systematic review do not reflect the experience of all women with breast cancer as the studies were predominantly performed in Western, English-speaking countries. Although data on education level and socioeconomical status were not provided on an individual level, the fact that the studies were primarily from developed nations allows one to hypothesize these patients have access to robust health care systems. Therefore, these data should be interpreted with caution as they may not apply to all breast cancer populations.
5. Conclusion
Older women with breast cancer have unique challenges specific to each phase of their breast cancer treatment. Advanced age, pre-existing co-morbidities, and social responsibilities influenced their experiences throughout different phases of their journey. Older women may benefit from more proactive treatment discussions with HCPs to address their specific needs. Implementing tailored outreach services may be useful in guiding older women through each step of their cancer journey. Future efforts should assess the long-term effects, durability, and patient satisfaction of these changes. Additionally, studies with a wider representation of women of different socioeconomic, educational, racial, and religious backgrounds are needed to fully understand the scope of older women with breast cancer. Only then can a comprehensive support system be developed to help older women navigate this difficult time in their lives.
Author contributions
FAA participated in the conception and design, data collection, analysis and interpretation of data, manuscript writing, and approval of final article. YZ participated in the conception and design, data collection, analysis and interpretation of data, manuscript writing, and approval of final article. ME participated in the conception and design, data collection, analysis and interpretation of data, manuscript writing, and approval of final article. NLH parcipated in the conception and design, data collection, analysis and interpretation of data, manuscript writing, and approval of final article. All authors have approved the final version of this manuscript.
Funding
This research did not receive any grant from funding agencies in the public, commercial, or not-for-profit sectors.
Declaration of competing interest
None.
Acknowledgments
The authors would like to thank Mr. Henry Lam (Sunnybrook Library Services, Sunnybrook Health Sciences Center, Toronto, ON, Canada) for assisting in the literature search.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.breast.2020.11.009.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.National Cancer Institute Surveillance, epidemiology, and end results program. https://seer.cancer.gov/statfacts/html/breast.html#incidence-mortality
- 2.Angarita F.A., Chesney T., Elser C., Mulligan A.M., McCready D.R., Escallon J. Treatment patterns of elderly breast cancer patients at two Canadian cancer centres. Eur J Surg Oncol. 2015;41:625–634. doi: 10.1016/j.ejso.2015.01.028. https://doi: 10.1016/j.ejso.2015.01.028 [DOI] [PubMed] [Google Scholar]
- 3.Lodi M., Scheer L., Reix N., Heitz D., Carin A.J., Thiebaut N. Breast cancer in elderly women and altered clinico-pathological characteristics: a systematic review. Breast Canc Res Treat. 2017;166:657–668. doi: 10.1007/s10549-017-4448-5. https://doi: 10.1007/s10549-017-4448-5 [DOI] [PubMed] [Google Scholar]
- 4.Hurria A., Muss H. Special issues in older women with breast cancer. Adv Exp Med Biol. 2015;862:23–37. doi: 10.1007/978-3-319-16366-6_3. https://doi: 10.1007/978-3-319-16366-6_3 [DOI] [PubMed] [Google Scholar]
- 5.Kiderlen M., de Glas N.A., Bastiaannet E., van de Water W., de Craen A.J., Guicherit O.R. Impact of comorbidity on outcome of older breast cancer patients: a FOCUS cohort study. Breast Canc Res Treat. 2014;145:185–192. doi: 10.1007/s10549-014-2917-7. https://doi:10.1007/s10549-014-2917-7 [DOI] [PubMed] [Google Scholar]
- 6.Inouye S.K., Studenski S., Tinetti M.E., Kuchel G.A. Geriatric syndromes: clinical, research and policy implications of a core geriatric concept. J Am Geriatr Soc. 2007;55:780–791. doi: 10.1111/j.1532-5415.2007.01156.x. https://doi:10.1111/j.1532-5415.2007.01156.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martinez-Ramos D., Simon-Monterde L., Queralt-Martin R., Suelves-Piqueres C., Menor-Duran P., Escrig-Sos J. Breast cancer in octogenarian. Are we doing our best? A population-registry based study. Breast. 2018;38:81–85. doi: 10.1016/j.breast.2017.12.007. https://doi: 10.1016/j.breast.2017.12.007 [DOI] [PubMed] [Google Scholar]
- 8.Eaker S., Dickman P.W., Bergkvist L., Holmberg L. Uppsala/Orebro Breast Cancer Group. Differences in management of older women influence breast cancer survival: results from a population-based database in Sweden. PLoS Med. 2006;3(3):e25. doi: 10.1371/journal.pmed.0030025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schonberg M.A., Marcantonio E.R., Li D., Silliman R.A., Ngo L., McCarthy E.P. Breast cancer among the oldest old: tumor characteristics, treatment choices, and survival. J Clin Oncol. 2010;28(12):2038–2045. doi: 10.1200/JCO.2009.25.9796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bastiaannet E., Portielje J.E., van de Velde C.J. Lack of survival gain for elderly women with breast cancer. Oncol. 2011;16(4):415–423. doi: 10.1634/theoncologist.2010-0234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Glas N.A., Kiderlen M., Vandenbroucke J.P., de Craen A.J., Portielje J.E., van de Velde C.J. Performing survival analyses in the presence of competing risks: a clinical example in older breast cancer patients. J Natl Cancer Inst. 2015;108 doi: 10.1093/jnci/djv366. https://doi: 10.1093/jnci/djv366 pii: djv366. [DOI] [PubMed] [Google Scholar]
- 12.Yellen S.B., Cella D.F., Leslie W.T. Age and clinical decision making in oncology patients. J Natl Cancer Inst. 1994;186:1766–1770. doi: 10.1093/jnci/86.23.1766. https://DOI:10.1093/jnci/86.23.1766 [DOI] [PubMed] [Google Scholar]
- 13.Angarita F.A., Elmi M., Zhang Y., Look Hong N.J. Patient-reported factors influencing the treatment decision-making process of older women with non-metastatic breast cancer: a systematic review of qualitative evidence. Breast Canc Res Treat. 2018;171(3):545–564. doi: 10.1007/s10549-018-4865-0. https://doi:10.1007/s10549-018-4865-0 [DOI] [PubMed] [Google Scholar]
- 14.Puts M.T., Tapscott B., Fitch M., Howell D., Monette J., Wan-Chow-Wah D. A systematic review of factors influencing older adults’ decision to accept or decline cancer treatment. Canc Treat Rev. 2015;41:197–215. doi: 10.1016/j.ctrv.2014.12.010. https://doi: 10.1016/j.ctrv.2014.12.010 [DOI] [PubMed] [Google Scholar]
- 15.Tariman J.D., Berry D.L., Cochrane B., Doorensbos A., Schepp K.G. Physician, patient, and contextual factors affecting treatment decisions in older adults with cancer and models of decision making: a literature review. Oncol Nurs Forum. 2012;39:E70–E83. doi: 10.1188/12.ONF.E70-E83. https://doi: 10.1188/12.ONF.E70-E83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liberati A., Altman D.G., Tetzlaff J., Mulrow C., Gøtzsche P.C., Ioannidis J.P. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. Clin Epidemiol. 2009;62:e1–34. doi: 10.1016/j.jclinepi.2009.06.006. https://doi: 10.1016/j.jclinepi.2009.06.006 [DOI] [PubMed] [Google Scholar]
- 17.Mohile S.G., Dale W., Somerfield M.R., Hurria A. Practical assessment and management of vulnerabilities in older patients receiving chemotherapy: ASCO guideline for geriatric oncology summary. J Oncol Pract. 2018;14(7):442–446. doi: 10.1200/JOP.18.00180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.O’Brien B.C., Harris I.B., Beckman T.J., Reed A., Cook D.A. Standards for reporting qualitative research: a synthesis of recommendations. Acad Med. 2014;89:1245–1251. doi: 10.1097/ACM.0000000000000388. https://doi: 10.1097/ACM.0000000000000388 [DOI] [PubMed] [Google Scholar]
- 19.Thomas J., Harden A. Methods for the thematic synthesis of qualitative research in systematic reviews. BMC Med Res Methodol. 2008;8:45. doi: 10.1186/1471-2288-8-45. https://doi: 10.1186/1471-2288-8-45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bearman M., Dawson P. Qualitative synthesis and systematic review in health professions education. Med Educ. 2013;47(3):252-260. doi: 10.1111/medu.12092. [DOI] [PubMed] [Google Scholar]
- 21.Burton M., Collins K.A., Lifford K.J., Brain K., Wyld L., Caldon L. The information and decision support needs of older women (>75 yrs) facing treatment choices for breast cancer: a qualitative study. Psycho Oncol. 2015;24:878–884. doi: 10.1002/pon.3735. https://doi:10.1002/pon.3735 [DOI] [PubMed] [Google Scholar]
- 22.Ciambrone D. Treatment decision-making among older women with breast cancer. J Women Aging. 2006;18:31–47. doi: 10.1300/J074v18n04_04. https://DOI: 10.1300/J074v18n04_04 [DOI] [PubMed] [Google Scholar]
- 23.Fenlon D., Franland J., Foster C.L., Brooks C., Coleman P., Payne S. Living into old age with the consequences of breast cancer. Eur J Oncol Nurs. 2013;17:311–316. doi: 10.1016/j.ejon.2012.08.004. https://doi: 10.1016/j.ejon.2012.08.004 [DOI] [PubMed] [Google Scholar]
- 24.Harder H., Ballinger R., Langridge C., Ring A., Fallowfield L.J. Adjuvant chemotherapy in elderly women with breast cancer: patients’ perspectives on information giving and decision making. Psycho Oncol. 2013;22:2729–2735. doi: 10.1002/pon.3338. https://doi:10.1002/pon.3338 [DOI] [PubMed] [Google Scholar]
- 25.Husain L.S., Collins K., Reed M., Wyld L. Choices in cancer treatment: a qualitative study of the older women’s (>70 years) perspective. Psycho Oncol. 2008;17:410–416. doi: 10.1002/pon.1242. https://DOI:10.1002/pon.1242 [DOI] [PubMed] [Google Scholar]
- 26.Kreling B., Figeriredo M.I., Sheppard V.L., Mandelblatt J.S. A qualitative study of factors affecting chemotherapy use in older women with breast cancer: barriers, promoters, and implications for intervention. Psycho Oncol. 2006;15:1065–1076. doi: 10.1002/pon.1042. https://DOI:10.1002/pon.1042 [DOI] [PubMed] [Google Scholar]
- 27.Loerzel V.W., Aroian K. Posttreatment concerns of older women with early-stage breast cancer. Canc Nurs. 2012;35:83–88. doi: 10.1097/NCC.0b013e31821a3843. https://doi: 10.1097/NCC.0b013e31821a3843 [DOI] [PubMed] [Google Scholar]
- 28.Lu M.H., Lin H.R., Lee M.D. The experiences among older Taiwanese women facing a new diagnosis of breast cancer. Canc Nurs. 2010;33:398–405. doi: 10.1097/NCC.0b013e3181d72c45. https://doi: 10.1097/NCC.0b013e3181d72c45 [DOI] [PubMed] [Google Scholar]
- 29.Morgan J.L., Burton M., Collins K., Lifford K.J., Robinson T.G., Cheung K.L. The balance of clinician and patient input into treatment decision-making in older women with operable breast cancer. Psycho Oncol. 2015;24:1761–1766. doi: 10.1002/pon.3853. https://doi: 10.1002/pon.3853 [DOI] [PubMed] [Google Scholar]
- 30.Pieters H.C., Heilemann M.V., Grant M., Maly R.C. Older women’s reflections on accessing care across their breast cancer trajectory: navigating beyond the triple barriers. Oncol Nurs Forum. 2011;38:175–184. doi: 10.1188/11.ONF.175-184. https://doi: 10.1188/11.ONF.175-184 [DOI] [PubMed] [Google Scholar]
- 31.Schonberg M.A., Birdwell R.L., Bychkovsky B.L., Hintz L., Fein-Zachary V., Wertheimer M.D. Older women’s experience with breast cancer treatment decisions. Breast Canc Res Treat. 2014;145:211–223. doi: 10.1007/s10549-014-2921-y. https://doi: 10.1007/s10549-014-2921-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wong J.J., D’Alimonte L., Angus J., Paszat L., Soren B., Szumacher E. What do older patients with early breast cancer want to know while undergoing adjuvant radiotherapy? J Canc Educ. 2011;26:254–261. doi: 10.1007/s13187-010-0188-5. https://doi: 10.1007/s13187-010-0188-5 [DOI] [PubMed] [Google Scholar]
- 33.Van Ee B., Smits C., Honkoop A., Kamper A., Slaets J., Hagedoorn M. Open wounds and healed scars: a qualitative study of elderly women’s experiences with breast cancer. Canc Nurs. 2019;42:190–197. doi: 10.1097/NCC.0000000000000575. https://doi: 10.1097/NCC.0000000000000575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pieters H.C. I’m still here": resilience among older survivors of breast cancer. Canc Nurs. 2016;39(1):E20–E28. doi: 10.1097/NCC.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 35.Soulos P.R., Yu J.B., Roberts K.B. Assessing the impact of a cooperative group trial on breast cancer care in the medicare population. J Clin Oncol. 2012;30(14):1601–1607. doi: 10.1200/JCO.2011.39.4890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sowerbutts A.M., Griffiths J., Todd C., Lavelle K. Why are older women not having surgery for breast cancer? A qualitative study. Psycho Oncol. 2015;24:1036–1042. doi: 10.1002/pon.3764. https://doi: 10.1002/pon.3764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morrison R. Patients’ sense of completion. BMJ. 1994;308:1722. doi: 10.1136/bmj.308.6945.1722. https://DOI:10.1136/bmj.308.6945.1722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chouliara Z., Miller M., Stott D., Molassiotis A., Twelves C., Kearney N. Older people with cancer: perceptions and feelings about information, decision-making and treatment--a pilot study. Eur J Oncol Nurs. 2004;8:257–261. doi: 10.1016/j.ejon.2003.12.010. https://doi:10.1016/j.ejon.2003.12.010 [DOI] [PubMed] [Google Scholar]
- 39.Shonberg M.A., Silliman R.A., McCarthy E.P., Marcantonio E.R. Factors noted to affect breast cancer treatment decisions of women aged 80 and older. J Am Geriatr Soc. 2012;60:538–544. doi: 10.1111/j.1532-5415.2011.03820.x. https://doi: 10.1111/j.1532-5415.2011.03820.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Overcash J.A. Using narrative research to understand the quality of life of older women with breast cancer. Oncol Nurs Forum. 2004;31:1153–1159. doi: 10.1188/04.ONF.1153-1159. https://doi:10.1188/04.ONF.1153-1159 [DOI] [PubMed] [Google Scholar]
- 41.Salisbury C. Multimorbidity: redesigning health care for people who use it. Lancet. 2012;380:7–9. doi: 10.1016/S0140-6736(12)60482-6. https://doi: 10.1016/S0140-6736(12)60482-6 [DOI] [PubMed] [Google Scholar]
- 42.Osborne C., Ostir G.V., Du X., Peek M.K., Goodwin J.S. The influence of marital status on the stage at diagnosis, treatment, and survival of older women with breast cancer. Breast Canc Res Treat. 2005;93:41–47. doi: 10.1007/s10549-005-3702-4. https://10.1007/s10549-005-3702-4 [DOI] [PubMed] [Google Scholar]
- 43.Bailey K. The nurse’s role in promoting breast awareness. Nurs Stand. 2000;14(30):34–36. doi: 10.7748/ns2000.04.14.30.34.c2811. [DOI] [PubMed] [Google Scholar]
- 44.Jenkins V.A., Fallowfield L.J., Poole K. Are members of multidisciplinary teams in breast cancer aware of each other’s informational roles? Qual Health Care. 2001;10(2):70–75. doi: 10.1136/qhc.10.2.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Angarita F.A., Dossa F., Hermann N., McCready D.R., Cil T.D. Does timing of alloplastic breast reconstruction in older women impact immediate postoperative complications? An analysis of the American College of Surgeons National Surgical Quality Improvement Program. ACS NSQIP) database. Breast. 2019 Dec;48:58–64. doi: 10.1016/j.breast.2019.09.001. [DOI] [PubMed] [Google Scholar]
- 46.Angarita F.A., Dossa F., Zuckerman J., McCready D.R., Cil T.D. Is immediate breast reconstruction safe in women over 70? An analysis of the National Surgical Quality Improvement Program (NSQIP) database. Breast Canc Res Treat. 2019 Aug;177(1):215–224. doi: 10.1007/s10549-019-05273-1. [DOI] [PubMed] [Google Scholar]
- 47.Chapman E.N., Kaatz A., Carnes M. Physicians and implicit bias: how doctors may unwittingly perpetuate health care disparities. J Gen Intern Med. 2013;28:1504–1510. doi: 10.1007/s11606-013-2441-1. https://doi:10.1007/s11606-013-2441-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wildiers H., Heeren P., Puts M. International Society of Geriatric Oncology consensus on geriatric assessment in older patients with cancer. J Clin Oncol. 2014;32(24):2595–2603. doi: 10.1200/JCO.2013.54.8347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Decoster L., Van Puyvelde K., Mohile S. Screening tools for multidimensional health problems warranting a geriatric assessment in older cancer patients: an update on SIOG recommendations. Ann Oncol. 2015;26(2):288–300. doi: 10.1093/annonc/mdu210. [DOI] [PubMed] [Google Scholar]
- 50.Grube B.J. Barriers to diagnosis and treatment of breast cancer in the older woman. J Am Coll Surg. 2006;202(3):495–508. doi: 10.1016/j.jamcollsurg.2005.11.013. [DOI] [PubMed] [Google Scholar]
- 51.Ruiz M., Reske T., Cefalu C., Estrada J. Management of elderly and frail elderly cancer patients: the importance of comprehensive geriatrics assessment and the need for guidelines. Am J Med Sci. 2013;346:66–69. doi: 10.1097/MAJ.0b013e31826d59aa. https://doi: 10.1097/MAJ.0b013e31826d59aa [DOI] [PubMed] [Google Scholar]
- 52.Walker R., Szanton S.L., Wenzel J. Working toward normalcy post-treatment: a qualitative study of older adult breast and prostate cancer survivors. Oncol Nurs Forum. 2015;42(6):E358–E367. doi: 10.1188/15.ONF.E358-E367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Basu M., Linebarger J., Gabram S.G., Patterson S.G., Amin M., Ward K.C. The effect of nurse navigation on timeliness of breast cancer care at an academic comprehensive cancer center. Cancer. 2013;119(14):2524–2531. doi: 10.1002/cncr.28024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.