Summary
Membrane-type 1 matrix metalloproteinase (MT1-MMP, MMP-14), a transmembrane proteinase with a short cytoplasmic tail, is a major effector of extracellular matrix remodeling. Genetic silencing of MT1-MMP in mouse (Mmp14−/−) and man causes dwarfism, osteopenia, arthritis, and lipodystrophy, abnormalities ascribed to defective collagen turnover. We have previously shown non-proteolytic functions of MT1-MMP mediated by its cytoplasmic tail, where the unique tyrosine (Y573) controls intracellular signaling. The Y573D mutation blocks TIMP-2/MT1-MMP-induced Erk1/2 and Akt signaling without affecting proteolytic activity. Here, we report that a mouse with the MT1-MMP Y573D mutation (Mmp14Y573D/Y573D) shows abnormalities similar to but also different from those of Mmp14−/− mice. Skeletal stem cells (SSC) of Mmp14Y573D/Y573D mice show defective differentiation consistent with the mouse phenotype, which is rescued by wild-type SSC transplant. These results provide the first in vivo demonstration that MT1-MMP modulates bone, cartilage, and fat homeostasis by controlling SSC differentiation through a mechanism independent of proteolysis.
Subject Areas: Pathophysiology, Cell Biology
Graphical Abstract

Highlights
-
•
The proteinase MT1-MMP controls signaling by its non-proteolytic cytoplasmic tail
-
•
The MT1-MMP cytoplasmic tail regulates skeletal stem cell differentiation
-
•
MT1-MMP modulates bone, joint cartilage, and fat homeostasis by its cytoplasmic tail
Pathophysiology; Cell Biology
Introduction
Membrane-type 1 matrix metalloproteinase (MT1-MMP, or MMP-14), the product of the gene MMP14, is a cell membrane–bound proteinase with an extracellular catalytic site and a 20-amino acid cytoplasmic tail (Itoh, 2015; Sato et al., 1994). It degrades a variety of extracellular matrix (ECM) components and is expressed by a wide array of normal and tumor cells. Notably, MT1-MMP is the only MMP whose genetic deficiency in the mouse results in severe phenotypes and early death. These features have implicated MT1-MMP as an important component of the proteolytic mechanisms of physiological and pathological processes including bone, cartilage, and adipose tissue homeostasis, as well as tumor invasion, angiogenesis, and metastasis. The analysis of the phenotype of mice genetically deficient in MT1-MMP (Mmp14−/−) has shown key roles of this proteinase in the postnatal development and growth of cartilage, bone, and adipose tissue. Mmp14 deficiency in the mouse results in dwarfism, severe osteopenia, generalized arthritis, and lipodystrophy, among other abnormalities (Chun et al., 2006; Chun and Inoue, 2014; Holmbeck et al., 1999; Zhou et al., 2000). In humans, a mutation of MMP14 causes multicentric osteolysis and arthritis disease, or Winchester syndrome, which recapitulates much of the phenotype of the Mmp14−/− mouse (Evans et al., 2012). Conditional Mmp14 knockout in uncommitted skeletal stem cells (SSCs, also referred to as mesenchymal stem cells), the common progenitors of osteoblasts, chondrocytes, and adipocytes, recapitulates the skeletal phenotype of the global Mmp14 knockout mouse (Tang et al., 2013). Conversely, unlike global Mmp14 deficiency, conditional Mmp14 knockout in SSC results in thickening of articular cartilage and increased bone marrow (BM)–associated fat but decreased subcutaneous fat, which derives from distinct progenitor cells (Chun et al., 2006; Gupta et al., 2012; Tran et al., 2012). In light of the fundamental role of MT1-MMP in ECM degradation, it has been proposed that the phenotypes of Mmp14−/− mice result from defective collagen turnover (Chun et al., 2006; Chun and Inoue, 2014; Holmbeck et al., 1999; Zhou et al., 2000).
However, a number of in vitro studies have provided evidence for a variety of non-proteolytic roles of MT1-MMP. We have previously shown that the MT1-MMP cytoplasmic tail activates Ras-ERK1/2 and Ras-AKT signaling by a non-proteolytic mechanism that controls cell proliferation, migration, and apoptosis in vitro, as well as tumor growth in vivo (D'Alessio et al., 2008; Valacca et al., 2015). Signaling is activated in a dose- and time-dependent manner by MT1-MMP binding of low nanomolar concentrations of tissue inhibitor of metalloproteinases-2 (TIMP-2), a physiological protein inhibitor of MT1-MMP. Signaling activation is also mediated by mutant TIMP-2 lacking MMP inhibitory activity (Ala + TIMP-2), as well as by mutant MT1-MMP devoid of proteolytic activity (MT1-MMP E240A), showing that the signaling mechanism is proteolysis-independent. We also showed that MT1-MMP signaling requires the unique tyrosine (Y573) in the cytoplasmic tail, which is phosphorylated by Src and LIM1 kinases (Lagoutte et al., 2016; Nyalendo et al., 2007), and that Y573 substitution with aspartic acid (Y573D), a negatively charged amino acid like phosphotyrosine, abrogates MT1-MMP–mediated activation of Ras-ERK1/2 (D'Alessio et al., 2008). Y573 is required for activation of the small GTPase Rac1 (Gonzalo et al., 2010), controls macrophage migration and infiltration at sites of inflammation (Gonzalo et al., 2010; Sakamoto and Seiki, 2009), as well as MT1-MMP interaction with Src and focal adhesion kinase (FAK) (Wang and McNiven, 2012).
Based on these observations, we generated a mutant mouse with the Y573D substitution in the cytoplasmic tail of MT1-MMP (MT1-MMP Y573D). Here, we report that this mouse shows abnormalities in postnatal bone, cartilage, and adipose tissue development and growth that partly recapitulate the phenotype of the MMP14−/− mouse, but also significant dissimilarities that indicate the relevance of the balance between MT1-MMP proteolytic activity and proteolysis-independent signaling. These phenotypes derive from dysregulation of BM-SSC differentiation, and are rescued by wild-type (wt) BM transplant.
Results
Generation and Macroscopic Characterization of MT1-MMP Y573D Mice
Mice heterozygous (Mmp14Y573D/wt) or homozygous (Mmp14Y573D/Y573D) for the MT1-MMP Y573D mutation, generated as described in Transparent Methods (Figures 1A and 1B), were macroscopically indistinguishable from Mmp14wt/wt mice at birth. However, after the first 2 months, Mmp14Y573D/wt and Mmp14Y573D/Y573D mice showed a slightly lower growth rate than their Mmp14wt/wt littermates (Figure 1C). In mice older than 3 months, the weight of Mmp14Y573D/Y573D male and female mice was 15% lower than that of age- and sex-matched Mmp14wt/wt littermates (Figure 1D). Adult Mmp14Y573D/Y573D mice showed no macroscopic morphological alterations. Both male and female Mmp14Y573D/Y573D mice were fertile, bred, and lived up to >2 years of age similarly to Mmp14wt/wt mice. Thus, the macroscopic phenotype of Mmp14Y573D/Y573D mice did not show the dramatic abnormalities of Mmp14−/− mice, which are up to 75% smaller than their wt littermate, have striking skeletal defects and a shortened life span (Table 1).
Figure 1.
Generation and Macroscopic Characterization of the MT1-MMP Y573D Mouse
(A) Schematic representation of the targeting vector and targeted locus. To construct the targeting vector the genomic sequence of mouse MT1-MMP containing exons 2 to 10 was PCR-amplified from W4 embryonic stem (ES) cells' DNA. A floxed neomycin resistance cassette (neo) was inserted into intron 9, adjacent to the 5′ end of exon 10, which encodes Y573, and a hsv-thymidine kinase cassette (hsvtk) was placed at the 5′ end of the construct. The TAC codon for Y573 was mutated into GAC by PCR. The construct was electroporated into W4 ES cells and colonies of recombinant cells were selected as described in Transparent Methods. The red X indicates the Y573D substitution; B: BamHI; C: ClaI; E: EcoRI; N: NotI; S: SalI; X: XhoI.
(B) Polymerase chain reaction (PCR) genotyping. The mice were genotyped by PCR using primers flanking the loxP sites, which afford identification of the three genotypes. The sequence of the primers is reported in Transparent Methods. The size of the amplicons is shown in base pairs (bp) on the left.
(C) Growth curve of Mmp14wt/wt (▲), Mmp14wt/Y573D (▼) and (Mmp14Y573D/Y573D (•) littermates.
(D) Body mass of 5- to 7-month-old Mmp14wt/wt (wt/wt; n = 14) and Mmp14Y573D/Y573D (YD/YD; n = 15) mice of both sexes (mean ± S.D.: 33.54 ± 1.44 g vs. 28.5 ± 1.15 g, respectively). ∗: p ≤ 0.001 by two-tailed Student's t-test.
Table 1.
Comparison of the Phenotypes of MT1-MMP Mutant Mice
| Genotype | Global Knockout | SSC Dermo-1 | Cartoona | Y573D |
|---|---|---|---|---|
| Life span | 2-3 months | 4-5 months | 3-6 weeks | Normal (2 years) |
| Generalized fibrosis of soft tissues | +++ | Unknown | Unknown | No fibrosis |
| Skeleton | Dwarfism Craniofacial abnormalities: domed skull and short snout Osteopenia |
Dwarfism Craniofacial abnormalities: domed skull and short snout Osteopenia |
Dwarfism craniofacial abnormalities: domed skull and short snout Osteopenia | Decreased cortical bone but increased trabecular bone |
| Joints | Generalized arthritis with degeneration of articular cartilage | Increased cartilage thickness | Increased cartilage thickness | Decreased cartilage thickness Osteoarthritis-like lesions |
| Adipose tissue | Generalized lipodystrophy Normal brown fat |
Increased bone marrow fat Decreased subcutaneous fat Brown fat unknown |
Generalized lipodystrophy Brown fat unknown |
Generalized lipodystrophy Brown fat hypertrophy |
| Inflammation | Increase in inflammatory cytokines | Unknown | Unknown | Decrease of inflammatory cytokines |
| Hematopoiesis | Pancytopenia | Unknown | Unknown | Normal levels of circulating blood cells |
| SSC differentiation in 2D culture | Unknown | Decreased osteogenesis Increased adipogenesis and chondrogenesis |
Decreased osteogenesis Increased adipogenesis (both in 3D collagen culture) |
Increased osteogenesis Decreased adipogenesis and chondrogenesis |
a The Cartoon mouse harbors a S466P point mutation in the MT1-MMP hemopexin domain that generates a misfolded, temperature-sensitive mutant that is retained in the endoplasmic reticulum (Sakr et al., 2018).
The Y573D Mutation Does Not Affect the Proteolytic Activity of MT1-MMP
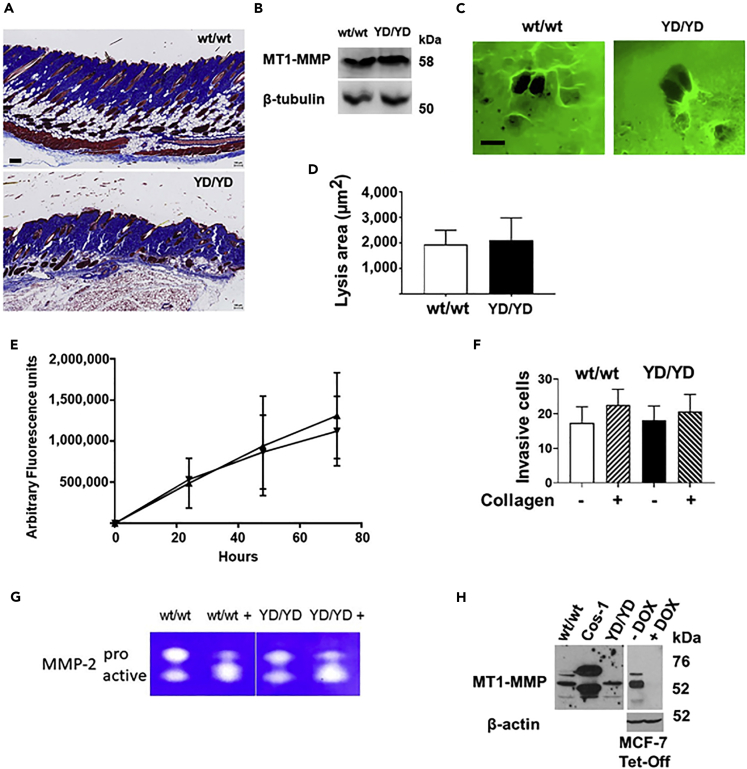
A major consequence of the genetic deficiency of MT1-MMP collagenolytic activity in Mmp14−/− mice is the progressive development of fibrosis of soft tissues, including fibrosis of the dermis and hair follicles, with subsequent hair loss (Holmbeck et al., 1999). Mmp14Y573D/Y573D mice up to 2 years of age showed no hair loss, and histological analysis of their skin showed no fibrosis of the dermis or hair follicles (Figure 2A), indicating that the collagenolytic activity of MT1-MMP Y573D in vivo is comparable to that of wt MT1-MMP. Consistent with this finding, primary fibroblasts from 3-weeks old Mmp14Y573D/Y573D and Mmp14wt/wt mice, which had comparable levels of MT1-MMP (Figure 2B), showed a similar capacity to degrade collagen, invade 3D collagen and activate proMMP-2 (Figures 2C–2G). Because mutations of the cytoplasmic tail can affect MT1-MMP sorting to, and recycling from the cell membrane (Uekita et al., 2001), we also characterized the cell membrane-associated levels of MT1-MMP in vivo by cell surface biotinylation of primary fibroblasts and by flow cytometric analysis of circulating white blood monocytes from Mmp14Y573D/Y573D and Mmp14wt/wt mice. Consistent with our analysis of the cell-associated collagenolytic activity in vitro, the results (Figures 2H and S1A) showed that cells of both genotypes had comparable levels of cell membrane-associated MT1-MMP, indicating that the Y573D mutation has minimal or no detectable effect on cell membrane expression and activity of MT1-MMP in vitro and in vivo.
Figure 2.
The Y573D Mutation Does Not Affect the Proteolytic Activity of MT1-MMP
(A) Representative histological sections of the skin of 3-months old, male Mmp14wt/wt (wt/wt; top panel) and Mmp14Y573D/Y573D mice (YD/YD; bottom panel). Trichrome staining; scale bar, 100 μm.
(B) Western blotting analysis of MT1-MMP expression in primary fibroblasts from Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D mice (YD/YD). β–tubulin is shown in the lower panel as a loading control.
(C) Representative images of primary fibroblasts from Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D mice (YD/YD) grown atop Alexa Fluor 488-labeled collagen fibrils for 72 hr. Areas of collagen degradation appear as black holes in the fluorescent collagen layer. Scale bar, 100 μm.
(D) Lysis areas (wt/wt n = 60; YD/YD n = 76) were measured by Photoshop. Mean ± S.D is shown. This experiment was repeated three times with comparable results.
(E) Confluent primary fibroblasts from Mmp14wt/wt (▲) and Mmp14Y573D/Y573D mice (▼) were grown atop Alexa Fluor 488-labeled collagen fibrils for 72 hr, and the fluorescence released into the culture supernatant was measured at the indicated times as described in Transparent Methods. Mean ± S.D. of two independent experiments is shown for each time point.
(F) Analysis of cell migration and collagen invasion by primary fibroblasts from Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D mice (YD/YD) on 8-μm pore membranes coated (+) or non-coated (−) with type I collagen as described in Transparent Methods. Shown in the ordinate is the mean ± S.D. of the number of migrated cells per 20X microscopic field. This experiment was repeated twice with comparable results.
(G) Gelatin zymographic analysis of proMMP-2 activation in the serum-free conditioned medium of primary cultures of Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) mouse fibroblasts incubated with exogenous human recombinant proMMP-2 for 16 hr. Before zymography the conditioned medium was incubated in the absence or presence (+) of APMA (1 mM in DMSO) for 1.5 hr at 37°C as a positive control for proMMP-2 activation. This experiment was repeated three times with comparable results.
(H) Cell surface biotinylation of MT1-MMP. Left panel: cell membrane-associated MT1-MMP in primary fibroblasts from Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) mice analyzed as described in Transparent Methods. COS-1 cells transiently transfected with MT1-MMP cDNA and surface biotinylated are shown as a control for the biotinylation procedure. Right panel: Western blotting analysis of extracts of human MCF-7 breast carcinoma cells stably expressing MT1-MMP under control by a Tet-Off promoter (D'Alessio et al., 2008), grown in the absence (−) or presence (+) of doxycycline (DOX; 1 μg/mL). This blot is shown as a control for the specificity of the antibody used for MT1-MMP immunoprecipitation after cell surface biotinylation. β-actin is shown as a loading control. This experiment was done twice with comparable results.
See also Figure S1.
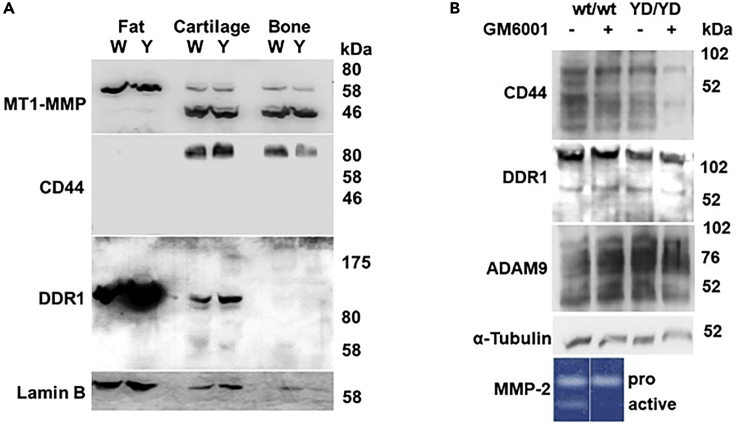
In addition to degrading collagen and other ECM components, MT1-MMP cleaves a variety of cell membrane proteins including the collagen receptors CD44 and discoidin domain receptor 1 (DDR1), as well as the metalloproteinase ADAM9 (Chan et al., 2012; Fu et al., 2013; Kajita et al., 2001). Fat, cartilage, and bone (Figure 3A), the tissues affected by the MT1-MMP Y573D mutation (as described under the following subheading), as well as other tissues (Figure S1B), showed minor variations in the levels of MT1-MMP expression in mice of the two genotypes. In fat MT1-MMP was expressed almost exclusively in the proenzyme form (62 kDa), whereas in cartilage and bone the 42-44 kDa autocatalytic degradation product was prevalent, indicating high levels of proteolytic activity (Rozanov et al., 2001).
Figure 3.
The Y573D Mutation Does Not Affect MT1-MMP Cleavage of CD44, DDR1, or ADAM9
(A) Western blotting analysis of MT1-MMP, CD44, and DDR1 expression and degradation in abdominal fat, joint cartilage, and bone (femur) from 3-month-old Mmp14wt/wt (W) and Mmp14Y573D/Y573D (Y) male mice. Tissues from three littermates per genotype were pooled for protein extraction. Lamin B is shown as a loading control. A representative result of multiple experiments is shown.
(B) Western blotting analysis of CD44, DDR1, and ADAM9 in cell extracts of primary fibroblasts from Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) mice grown in the presence (+) or absence (−) of GM6001 (50 μM) for 24 hr. α-tubulin is shown as a loading control. A representative result of multiple experiments is shown. MT1-MMP expression is shown in Figure 2B. Bottom panel: gelatin zymography analysis of proMMP-2 activation is shown as a control for GM6001 inhibition of MT1-MMP. The cells were grown for 16 hr in serum-free medium supplemented with human recombinant proMMP-2 without (−) or with (+) GM6001 (50 μM), and the conditioned medium was analyzed by gelatin zymography.
See also Figure S1.
We could not detect CD44 in the fat of our mice (Figure 3A), and quantitative polymerase chain reaction (qPCR) analysis showed extremely low levels of CD44 mRNA in both Mmp14Y573D/Y573D and Mmp14wt/wt mice (Ct values 32.1 and 37.9, respectively). However, the 80 kDa native form of CD44 was present in the cartilage and bone of Mmp14Y573D/Y573D and Mmp14wt/wt mice in comparable amounts, and no degradation products were detected in mice of either genotype. DDR1 could not be detected in the bone of Mmp14Y573D/Y573D or Mmp14wt/wt mice (Figure 3A), a result consistent with qPCR analysis (Ct values 31.89 and 31.76, respectively). Conversely, DDR1 was detected in fat and cartilage, but not in bone, extracts as a ∼120 kDa form. Comparable levels of a ∼62–65 kDa form consistent with a degradation product resulting from MT1-MMP proteolysis (Fu et al., 2013) were present in cartilage, whereas higher levels of ∼120 kDa DDR1 was expressed in Mmp14Y573D/Y573D than in Mmp14wt/wt fat. No other degradation products were detected, showing that wt MT1-MMP and MT1-MMP Y573D have comparable capacity to cleave CD44 and DDR1 in vivo.
To corroborate these findings, we analyzed CD44, DDR1, and ADAM9 in primary fibroblasts from adult Mmp14Y573D/Y573D and Mmp14wt/wt mice (Figure 3B). Western blotting analysis of cell extracts showed comparable levels of native, 80 kDa CD44 and lower molecular weight immunoreactive bands. Mmp14Y573D/Y573D and Mmp14wt/wt cell extracts showed similar levels of native, ∼ 120 kDa DDR1 and ∼62–65 kDa bands consistent with the degradation product resulting from MT1-MMP proteolysis (Fu et al., 2013), as well as ∼ 90 kDa ADAM9 (Figure 3B) and ∼40–60 kDa immunoreactive bands consistent with ADAM9 degradation products generated by MT1-MMP (Chan, et al., 2012). However, similar CD44 and DDR1 bands were observed in extracts of cells grown in the presence or absence of the MMP inhibitor GM6001 (Ilomastat), indicating that they either represent nonspecific bands or degradation products generated by non-MMP–mediated cleavage. Therefore, these results showed that the Y573D mutation does not affect MT1-MMP cleavage of CD44, DDR1 or ADAM9 in vitro or in vivo.
MT1-MMP Y573D Mice Show Structural and Gene Expression Abnormalities in Bone, Articular Cartilage and Adipose Tissue
A striking phenotype of Mmp14−/− mice is the dramatically decreased length of long bones, with severe osteopenia and arthritis (Holmbeck et al., 1999; Zhou et al., 2000). At 5 months of age, the femurs of Mmp14Y573D/Y573D mice were only ∼5% shorter than those of Mmp14wt/wt mice (14.81 ± 0.1519 mm vs. 15.61 ± 0.0619 mm; n = 10; p = 0.0001). However, cortical bone thickness at the femur mid-diaphysis was markedly decreased (20-25%) in Mmp14Y573D/Y573D vs. Mmp14wt/wt mice (Figure 4A), a finding consistent with the osteopenia of the Mmp14−/− mouse (Holmbeck et al., 1999; Zhou et al., 2000). Conversely, unlike Mmp14−/− mice, which have reduced trabecular bone, and in the homozygous but not in the heterozygous state (Holmbeck et al., 1999; Zhou et al., 2000), femurs and tibias showed significantly increased (40-50%) trabecular bone in both Mmp14Y573D/Y573D and Mmp14Y573D/wt mice relative to age- and sex-matched Mmp14wt/wt mice (Figures 4B–4D). This effect was accompanied by increased TRAP-positive osteoclasts (Figure S2), indicating enhanced bone remodeling, a feature also observed in Mmp14−/− mice (Holmbeck et al., 1999).
Figure 4.
MT1-MMP Y573D Mice Show Decreased Cortical Bone and Increased Trabecular Bone
(A and B) MicroCT analysis of cortical (A) and trabecular bone (B) from Mmp14wt/wt (wt/wt; n = 3) and Mmp14Y573D/Y573D (YD/YD; n = 3) 3-month-old, male mice. Upper panels; 3D reconstruction; lower panels: quantitative analysis. The histograms show mean ± S.D. (A) BV/TV: bone volume/tissue volume; ∗: p = 0.0067; Cs. Th.: cortical thickness; ∗∗p = 0.0009; M. Ar.: bone marrow area; ∗∗∗p = 0.0524. (B) BV: bone volume; †: p = 0.0115; BV/TV: bone volume/tissue volume; #: p = 0.0366; Tb. Th.: trabecular thickness; Ӿ: p = 0.0290. All p values by two-tailed Student's t-test.
(C) Representative sections of the femurs of Mmp14wt/wt (wt/wt), Mmp14Y573D/wt (wt/YD) and Mmp14Y573D/Y573D (YD/YD) 2-month-old, male mice. H&E staining; scale bar, 1 mm.
(D) Osteomeasure analysis of trabecular bone in femurs (blue bars) and tibiae (red bars) of the mice shown in panel (C) Mean ± s.d. of 5 mice/group is shown. BV/TV: bone volume/total volume; ∗: p = 0.0329; ∗∗: p = 0.0012; #: p = 0.0489. Tb. N.: number of trabecule; ∗: p = 0.0425; ∗∗: p = 0.0021; #: p = 0.0018. Tb. Th.: trabecular thickness; ∗: p = 0.0478; ∗∗: p = 0.0018; #: p = 0.0089. Tb. Sp.: space between trabecule; ∗: p = 0.0014; ∗∗: p = 0.0019; #: p = 0.0197 (sample vs corresponding wt control). All p values by two-tailed Student's t-test.
(E) RNA-seq analysis of differential gene expression in bone from Mmp14Y573D/Y573Dvs. Mmp14wt/wt mice. Volcano plot representing genes with a significant (p ≤ 0.05) fold change higher than 2 (red dots) or lower than - 2 (green dots).
(F) GO analysis of pathways overrepresented in the bone of Mmp14Y573D/Y573Dvs. Mmp14wt/wt 3-months-old male mice.
See also Figure S2.
Gene expression profiling of bone from Mmp14Y573D/Y573D mice (Figure 4E) showed 76 genes significantly (p ≤ 0.05) up- or downregulated relative to Mmp14wt/wt littermates. A subset of 17 of these transcripts were upregulated 2-fold or more, and 26 transcripts were downregulated 2-fold or more. Consistent with the morphometric analyses, gene ontology analysis showed highly significant enrichment for biological processes related to osteoblast differentiation, bone remodeling, ossification, and bone growth (Figure 4F).
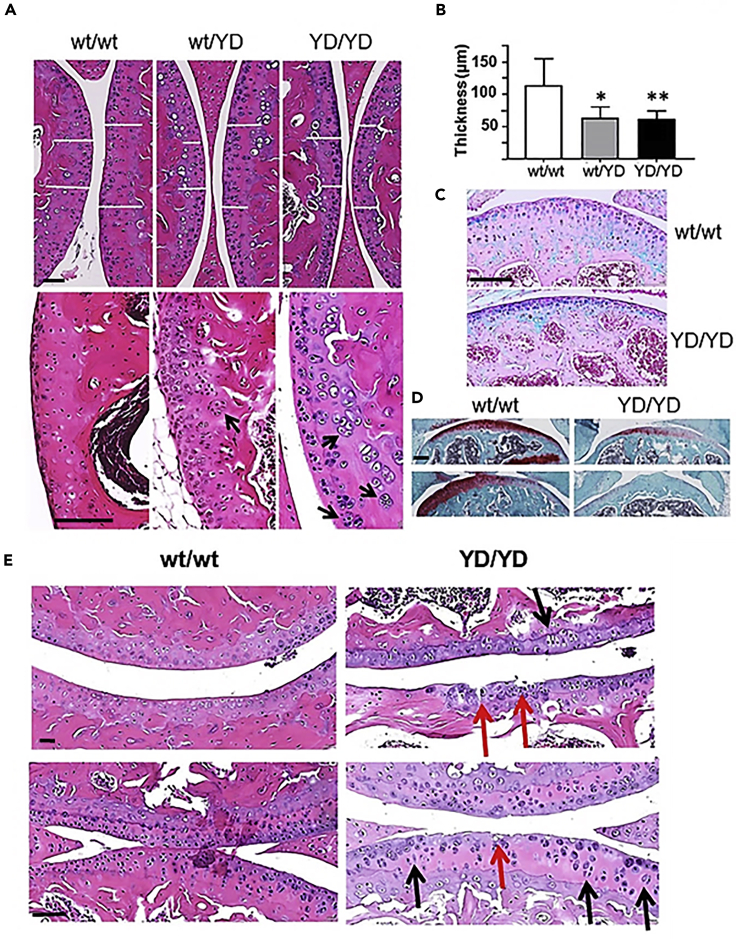
The knee joints of 2-month-old mice showed several abnormalities. Both Mmp14Y573D/wt and Mmp14Y573D/Y573D mice displayed marked thinning of articular cartilage (Figure 5A top panels, and B) with loss of proteoglycans (Figures 5C and 5D), clustering and cloning of chondrocytes (Figure 5A lower panels), classic histologic features of the articular cartilage degeneration associated with human osteoarthritis (OA) and surgical models of OA in mice. The knee cartilage of 2-year-old mice also showed fissures, chondrocyte clustering and cloning, abnormalities typical of aging-associated cartilage degeneration that were not observed in age- and sex-matched Mmp14wt/wt mice (Figure 5E).
Figure 5.
MT1-MMP Y573D Mice Show Reduced Thickness and Structural Abnormalities of Articular Cartilage
Histological and histochemical analyses of knee joint cartilage from Mmp14wt/wt (wt/wt), Mmp14Y573D/wt (wt/YD), and Mmp14Y573D/Y573D (YD/YD) 3-month-old male mice.
(A) H&E staining; black scale bars in top and bottom panels, 100 μm. White bars indicate the thickness of the cartilage at comparable locations in the joint. Arrows: chondrocyte cloning.
(B) Thickness of articular cartilage measured by Photoshop as shown in panel A (top panels) on multiple sections of knee joints of 3 mice per genotype. Mean ± S.D. is shown. ∗: p = 0.0004; ∗∗: p = 0.0001 by two-tailed Student's t-test.
(C and D) (C) Alcian blue staining, and (D) Safranin O staining of sections of knee joints of 2-month-old male mice with the indicated genotypes. Scale bars in C and D, 100 μm.
(E) Histological sections of the shoulder (upper panels) and knee joints (lower panels) of 2-year old Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) male mice. Red arrows point to cartilage erosion and fissures, and black arrows to chondrocytes oriented orthogonally to the cartilage surface, typical signs of cartilage degeneration. H&E staining; scale bars in top and bottom panels, 100 μm.
RNA-Seq analysis of the transcriptome of cartilage from Mmp14Y573D/Y573D mice (Figure 6A) showed significant dysregulation of the expression of 1,549 genes relative to Mmp14wt/wt mice (p ≤ 0.05). Of these genes, 694 were upregulated 2-fold or more, and 92 were downregulated 2-fold or more. Gene ontology analysis (Figure 6B) showed highly significant enrichment for biological processes related to ECM homeostasis, cartilage development, and chondrocyte differentiation. As these biological processes are strongly dysregulated in human OA, we analyzed the transcriptome of Mmp14Y573D/Y573D cartilage for expression of genes involved in human OA (Figure 6C). Gene expression profiling of human OA cartilage has revealed 1,423 genes significantly (p ≤ 0.05) up- or downregulated relative to normal cartilage, 111 of which are strongly up- or downregulated (≥2-fold or ≤ 2-fold, respectively) (Geyer et al., 2009; Karlsson et al., 2010). We found that 48 of these 111 OA-associated genes are also strongly regulated in Mmp14Y573D/Y573D mouse cartilage, including all the genes for collagens and other ECM proteins upregulated in human OA, ECM-degrading proteinases, as well as genes involved in cell metabolism (Figure 6D). Thus, consistent with its histological features, Mmp14Y573D/Y573D joint cartilage showed significant gene expression similarity to human OA.
Figure 6.
The Gene Expression Profile of the Articular Cartilage of Mmp14Y573D/Y573D Mice Shows Similarity to that of Human OA
(A) RNA-seq analysis of differential gene expression in articular cartilage from Mmp14Y573D/Y573Dvs. Mmp14wt/wt 3-month-old male mice. Volcano plot representing genes with a significant (p ≤ 0.05) fold change higher than 2 (red dots) or lower than - 2 (green dots).
(B) GO analysis of pathways overrepresented in the articular cartilage of Mmp14Y573D/Y573Dvs. Mmp14wt/wt 3-month-old male mice.
(C) Venn diagram of differentially expressed genes in articular cartilage from Mmp14Y573D/Y573Dvs. Mmp14wt/wt 3-month-old male mice, and human OA cartilage vs. normal knee joint cartilage (Geyer et al., 2009; Karlsson et al., 2010).
(D) Gene families upregulated ≥ 2-fold (p ≤ 0.05) in knee joint cartilage from both Mmp14Y573D/Y573D 3-month-old male mice and OA patients.
Sections of the long bones of adult Mmp14Y573D/wt and Mmp14Y573D/Y573D mice also showed marked decrease in BM-associated fat relative to Mmp14wt/wt littermates (Figures 7A and 7B). A similar reduction was apparent in all other fat pads, as evidenced by ∼ 50% decrease in body adiposity by DEXA analysis and in the gonadal fat of Mmp14Y573D/Y573D mice relative to age- and sex-matched Mmp14wt/wt littermates (Figures 7C and 7D). This effect was observed in mice of both sexes and ages ranging 3 months to 2 years (Figures S3 and S4). Histological analysis of abdominal and subcutaneous WAT from Mmp14Y573D/Y573D mice revealed marked decrease in the size of adipocytes (Figures 8A and 8C). These findings are consistent with the lipodystrophy of Mmp14−/− mice (Chun et al., 2006, 2010). In contrast, the brown adipose tissue (BAT) of Mmp14Y573D/Y573D mice showed pronounced adipocyte hypertrophy, with decreased expression of the characteristic marker of BAT, uncoupling protein-1 (UCP-1; Figures 8B and S5).
Figure 7.
MT1-MMP Y573D Mice Show Decreased White Adipose Tissue
(A and B) Sections of the tibias of Mmp14wt/wt (wt/wt), Mmp14wt/Y573D (wt/YD) and Mmp14Y573D/Y573D (YD/YD) 4-month (A) or 2-year-old male mice (B). H&E staining; scale bar, 1 mm.
(C) Dexa scanning analysis of Mmp14wt/wt (wt/wt; n = 27) and Mmp14Y573D/Y573D (YD/YD; n = 30) male and female, 7-month-old mice. Mean ± S.D. is shown. ∗: p = 0.0018, ∗∗: p = 0.0295, #: p = 0.0177, †: p = 0.0230 by two-tailed Student's t-test. Statistically significant percent differences between the two genotypes are indicated in the closed bars.
(D) Weight of abdominal fat normalized to total body weight of 2-, 4- and 7-month-old, Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) male and female mice; p = 0.0001.
See also Figures S3 and S4.
Figure 8.
MT1-MMP Y573D Mice Have Hypotrophic White Fat and Hypertrophic Brown Fat
(A) H&E stained sections of periepididymal (left panels) and subcutaneous fat (right panels) of Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) 4-month-old male mice. Scale bar, 100 μm.
(B) Sections of infrascapular brown fat from Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) 4-month-old male mice, immunostained with antibody to UCP-1. Scale bars in left and right panels, 100 μm.
(C) Adipocyte size of periepididymal fat from Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) 4-month-old male mice. The area of individual adipocytes was measured by Photoshop using multiple sections (5 mice/genotype) of periepididymal fat similar to those shown in A (left panels). Mean ± S.D. is shown. ∗p = 0.0003 by two-tailed Student's t-test.
(D) RNA-seq analysis of differential gene expression white adipose tissue from Mmp14Y573D/Y573Dvs. Mmp14wt/wt 3-month-old male mice. Volcano plot representing genes with a significant (p ≤ 0.05) fold change higher than 2 (red dots) or lower than - 2 (green dots).
(E) GO analysis of pathways overrepresented in white adipose tissue of Mmp14Y573D/Y573Dvs. Mmp14wt/wt mice.
See also Figures S3–S7.
The transcriptome of abdominal WAT from Mmp14Y573D/Y573D mice (Figure 8D) showed significant (p ≤ 0.05) up- or downregulation of the expression of 151 genes, relative to Mmp14wt/wt mice. Gene ontology analysis (Figure 8E) identified highly significant enrichment for biological processes including control of small-molecule synthesis, lipid and fatty acid metabolism, response to corticosteroids, and ECM homeostasis. Some of these pathways are also overrepresented in Mmp14+/− mice on a high-fat diet (Chun et al., 2010). Moreover, similar to Mmp14+/− mice, Mmp14Y573D/Y573D mice were protected from body weight gain induced by high-fat diet (Figure S6A).
In addition to reduced WAT, Mmp14Y573D/Y573D mice showed strongly decreased fasting levels of plasma insulin relative to age- and sex-matched Mmp14wt/wt littermates, with normoglycemia and normal food consumption (Figure S6B). Mmp14Y573D/Y573D and Mmp14wt/wt mice also had comparable levels of adrenocorticotropic hormone, which controls adipose tissue metabolism, and leptin, a hormone secreted predominantly by adipose tissue. Conversely, Mmp14Y573D/Y573D mice had extremely low serum levels of the inflammatory cytokines interleukin-6 (IL-6), monocyte chemoattractant protein-1/chemokine (C-C motif) ligand 2 (MCP-1/CCL2), and IL-10 relative to age- and sex-matched Mmp14wt/wt mice (Figure S7).
MT1-MMP Y573D Expression Alters SSC Erk1/2 and Akt Signaling, Proliferation and Apoptosis, and Skews Differentiation from Chondro- and Adipogenesis toward Osteogenesis
Our previous in vitro studies have shown than the Y573D mutation abrogates activation of ERK1/2 and AKT signaling induced by TIMP-2 binding to MT1-MMP (D'Alessio et al., 2008). However, Y573 substitution with D (a negatively charged amino acid) might also mimic phosphotyrosine and result in constitutive activation of other signaling pathways, including FAK, whose activation is downregulated in SSC from conditional MT1-MMP knockout mice (Tang et al., 2013). Therefore, we characterized adipose tissue, articular cartilage and bone from Mmp14wt/wt and Mmp14Y573D/Y573D mice for activation of Erk1/2, Akt and Fak. The results (Figure 9A) showed no significant differences in activation of these signaling pathways between the two genotypes. However, consistent with our previous studies (D'Alessio et al., 2008; Valacca et al., 2015), exogenous TIMP-2 strongly upregulated Erk1/2 and Akt activation in primary fibroblasts from Mmp14wt/wt mice but had no such effect in Mmp14Y573D/Y573D cells. Conversely, the levels of active Fak were not increased by exogenous TIMP-2 in either Mmp14Y573D/Y573D or Mmp14wt/wt fibroblasts (Figure 9B).
Figure 9.
Western Blotting Analysis of Intracellular Signaling in Tissues and Cells from Mmp14wt/wt and Mmp14Y573D/Y573D Mice
(A) ERK1/2, Akt, and FAK signaling in perigonadal adipose tissue (FAT), knee joint cartilage (CART), and femurs (BONE) of Mmp14wt/wt (W) and Mmp14Y573D/Y573D (Y) 4-month-old male mice.
(B and C) Analysis of ERK1/2, Akt and FAK activation (p-ERK1/2, p-Akt and p-FAK) in primary fibroblasts (B) or BM-derived SSC (C) from Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) 2-month-old male mice, incubated with human recombinant TIMP-2 (100 ng/mL) or an equivalent volume of control vehicle for 15 min. Total ERK1/2, Akt and FAK protein is shown as a loading control. Equal amounts of cell extract protein were run in two separate gels and transferred onto separate membranes, one of which was probed with antibodies to the phosphorylated forms and the other to the total forms of ERK1/2, Akt and FAK. These experiments were performed two to three times with similar results.
The observation that the phenotype of Mmp14Y573D/Y573D mice involves abnormalities of bone, cartilage, and adipose tissue indicated that the MT1-MMP Y573D mutation might affect the differentiation of SSC, the common progenitor cells of these tissues. Therefore, we isolated SSC from the BM of Mmp14wt/wt and Mmp14Y573D/Y573D littermates and characterized them in vitro for Erk1/2, Akt, and Fak signaling. The results (Figure 9C) showed no significant differences in the basal levels of active Erk1/2, Akt, or Fak; however, exogenous TIMP-2 activated Erk1/2 and Akt in Mmp14wt/wt but not in Mmp14Y573D/Y573D SSC. Conversely, TIMP-2 did not activate Fak in cells of either genotype.
Mmp14Y573D/Y573D SSC contained fewer colony-forming units-fibroblasts (CFU-F) than Mmp14wt/wt SSC (1.5x10−6 vs. 4.5x10−6, respectively), formed much smaller colonies (Figure 10A), and showed remarkably lower proliferation and higher apoptosis (Figures 10B and 10C). RNA-Seq analysis (Figure 10D) showed 486 genes significantly dysregulated (p ≤ 0.05) in Mmp14Y573D/Y573D vs. Mmp14wt/wt SSC. Gene ontology analysis (Figure 10E) revealed highly significant enrichment of genes involved in DNA synthesis, cell cycle regulation and response to DNA damage, indicating dysregulation of cell proliferation and survival, cell functions controlled by Erk1/2 and Akt signaling.
Figure 10.
MT1-MMP Y573D Expression Impairs SSC Proliferation and Survival and Skews Differentiation toward Osteogenesis
(A) CFU-F of Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) SSC; Giemsa staining. This experiment was repeated twice with similar results.
(B) MTS assay of cells grown for 48 hr. Mean ± S.D. is shown; ∗p = 0.0001 by two-tailed Student's t-test. This experiment was repeated three times with similar results.
(C) Percentage of apoptotic nuclei in DAPI-stained cells (298–320 nuclei). Mean ± S.D. is shown; ∗p = 0.001 by two-tailed Student's t test. This experiment was repeated twice with similar results.
(D) RNA-seq analysis of differential gene expression in articular cartilage from Mmp14Y573D/Y573Dvs. Mmp14wt/wt mice. Volcano plot representing genes with a significant (p ≤ 0.05) fold change higher than 2 (red dots) or lower than - 2 (green dots).
(E) GO analysis of pathways overrepresented in BM-derived SSC from Mmp14wt/wt (wt/wt) and Mmp14Y573D/Y573D (YD/YD) 3-month-old male mice.
(F) qPCR analysis of osteoblast (top), chondrocyte (middle) and adipocyte markers (bottom panel) in BM-derived SSC from Mmp14wt/wt (open bars) and Mmp14Y573D/Y573D (solid bars) 4-month-old littermates, after 17 days in osteoblast or chondrocyte or adipocyte differentiation medium. Shown are mean ± S.D. of data normalized to geomean of GAPDH and HPRT1 for the three cell types. ∗: p ≤ 0.05 by two-tailed Student's t-test. This experiment was repeated twice with similar results.
(G) Differentiation of C3H10T1/2 cells stably transfected with wt or MT1-MMP Y573D cDNA. Top panel, osteogenesis: Alizarin red staining of cells after 21 days in osteoblast differentiation medium. Middle panel, chondrogenesis: Alcian blue staining of cell pellets after 21 days in chondrocyte differentiation medium. Scale bar, 500 μm. Bottom panel, adipogenesis: oil red O staining of cells grown in adipocyte differentiation medium for 7 days. Scale bar, 500 μm. This experiment was repeated three times with similar results.
We then induced BM-derived SSC from Mmp14Y573D/Y573D and Mmp14wt/wt littermates to differentiate in vitro into the osteoblast, chondrocyte, and adipocyte lineages. qPCR analysis of the expression of lineage-specific markers showed a dramatic increase in osteogenesis (Figure 10F, top panel), and marked decrease in chondrocyte and adipocyte differentiation (Figure 10F, middle and bottom panels, respectively) in Mmp14Y573D/Y573D vs. Mmp14wt/wt SSC. The expression levels of wt MT1-MMP and MT1-MMP Y573D did not change during osteoblast differentiation, and on day 17 comparable levels of MT1-MMP mRNA were expressed by Mmp14wt/wt and Mmp14Y573D/Y573D SSC (Figure 10F; top panel). Conversely, in agreement with previous reports (Y. Tang et al., 2013), MT1-MMP expression decreased by ∼ 80% during chondrocyte differentiation in both Mmp14wt/wt and Mmp14Y573D/Y573D SSC, and on day 17 Mmp14Y573D/Y573D cells expressed significantly lower MT1-MMP levels than Mmp14wt/wt SSC (Figure 10F, middle panel).
To confirm that the differences in BM-SSC differentiation between Mmp14wt/wt and Mmp14Y573D/Y573D mice are mediated by the MT1-MMP Y573D mutation, we transfected wt MT1-MMP or MT1-MMP Y573D cDNA into mouse C3H10T1/2 cells, which are functionally similar to SSC (Tang et al., 2004), and analyzed their capacity to differentiate into osteoblasts, chondrocytes and adipocytes (Figure 10G) by lineage-specific staining. Consistent with the results obtained with primary BM-derived SSC, C3H10T1/2 cells transfected with MT1-MMP Y573D showed markedly increased osteogenesis, and decreased chondro- and adipogenesis relative to wt MT1-MMP transfectants (Figure 10G). Therefore, in both BM-derived SSC and C3H10T1/2 cells MT1-MMP Y573D expression skewed differentiation toward the osteogenic lineage.
The Bone and Fat Phenotypes of Mmp14Y573D/Y573D Mice Are Rescued by wt BM Transplant
These results indicated that the bone, cartilage, and fat phenotypes of Mmp14Y573D/Y573D mice result from a defect in SSC. Therefore, we hypothesized that these phenotypes could be rescued by transplantation of Mmp14wt/wt BM; and, vice versa, transplantation of Mmp14Y573D/Y573D BM could transfer the phenotype to Mmp14wt/wt mice. We therefore transplanted 3- and 5-month-old mice with BM from mice of the same age and the opposite genotype or, as controls, from mice of the same age and genotype, and analyzed their phenotypes two months later.
We characterized the trabecular and cortical bone of the femurs of the transplanted mice by microCT analysis (Figure 11). The results showed that transplantation of Mmp14wt/wt BM had no effect on the cortical bone of Mmp14Y573D/Y573D mice transplanted at 3 months or 5 months of age (Figures 11A and S8); however, it completely rescued their trabecular bone phenotype (Figures 11B and S8). Conversely, transplantation of Mmp14Y573D/Y573D BM did not transfer the trabecular or cortical bone phenotype to Mmp14wt/wt mice.
Figure 11.
wt BM Transplant Rescues the Trabecular Bone Phenotype of MT1-MMP Y573D Mice
(A) MicroCT 3D reconstruction (upper panels) and quantitative measurements (lower panels) of cortical bone of Mmp14wt/wt male mice transplanted with BM from age- and sex-matched Mmp14Y573D/Y573D mice (YD►wt) or Mmp14Y573D/Y573 male mice transplanted with BM from age- and sex-matched Mmp14wt/wt mice (wt►YD). Mice transplanted with BM of the same genotype (wt►wt and YD►YD) were used as controls. The mice (n = 9–11/group) were transplanted at 3 months of age and analyzed 2 months later. BV/TV: bone volume normalized to total tissue volume; Cs. Th.: cortical thickness; Ma. Ar.: bone marrow area. The YD►YD samples show ∼20% reduced cortical bone relative to wt ► wt samples, as expected (Figure 1A); wt ► YD or YD► wt BM transplant does not change the phenotype of the recipient. Mean ± S.D. is shown. ∗: p = 0.0003; ∗∗: p = 0.0021; ‡: p = 0.0038 by two-tailed Student's t-test.
(B) MicroCT 3D reconstruction (upper panels) and quantitative measurements (lower panels) of trabecular bone of the mice described in (A). The YD►YD samples show 35-40% increase in trabecular bone relative to wt ► wt samples, as expected (Figure 1B); Mmp14wt/wt BM transplant lowers the amount of trabecular bone in Mmp14Y573D/Y573D mice to a level similar to that of the wt►wt controls (dotted line). Conversely, BM transplant from Mmp14Y573D/Y573D mice does not transfer the phenotype to Mmp14wt/wt mice. BV/TV: bone volume normalized to total tissue volume; Tb. N. trabecular number/mm; Tb. Th.: trabecular thickness. Mean ± S.D. is shown. ∗: p = 0.047; ∗∗: p = 0.0150; ‡: p = 0.0124; Ӿ: p = 0.0397; ¥: p = 0.0117 by two-tailed Student's t-test.
See also Figures S8 and S9.
We also found that BM transplant induced no significant changes in articular cartilage thickness relative to the original phenotype of the transplant recipients in this short-term study (data not shown).
Similarly to the bone phenotype, Mmp14wt/wt BM transplant completely rescued the decrease in fat mass of Mmp14Y573D/Y573D mice. Mmp14Y573D/Y573D BM did not transfer the adipose tissue phenotype to Mmp14wt/wt mice (Figure 12A). However, consistent with the existence of BM-derived adipocyte precursors able to colonize peripheral WAT and BAT (Crossno et al., 2006), histological analysis of WAT and BAT from transplanted mice (Figures 12B and 12C) showed mixtures of normal and hypotrophic white adipocytes in both Mmp14wt/wt mice transplanted with Mmp14Y573D/Y573D BM and Mmp14Y573D/Y573D mice transplanted with Mmp14wt/wt BM. Conversely, BM recipients acquired the BAT phenotype of donor mice (Figure 12C).
Figure 12.
BM Transplant Partially Transfers the White and Brown Fat Phenotypes to Recipient Mice
(A) wt BM Transplant Rescues the Adipose Tissue Phenotype of MT1-MMP Y573D Mice. Weight of visceral fat normalized to total weight of the mice (n = 9–11/group) described in Figure 8, transplanted at 3 or 5 months of age and analyzed 2 months later. The YD►YD samples show ∼50% less fat than age-matched wt►wt controls, as expected (Figure 4). Mmp14wt/wt BM transplant increases the amount of adipose tissue in Mmp14Y573D/Y573D mice to a level similar to that of the wt►wt controls (dotted line). Conversely, BM transplant from Mmp14Y573D/Y573D mice does not reduce the fat mass in Mmp14wt/wt mice. Mean ± S.D. is shown. ∗: p = 0.006; ‡: p = 0.008; Ӿ: p = 0.003; ¥: p = 0.001 by two-tailed Student's t-test.
(B and C) WAT and BAT of BM-transplanted mice show a mixture of donor and recipient phenotypes. Histological sections of periepididymal WAT (B) and infrascapular BAT (C) of the mice described in Figure 8. H&E staining; scale bars in B and C, 100 μm. The YD►YD samples show hypotrophic white adipocytes (A) and hypertrophic brown adipocytes, as expected (Figures 5B and 5C); wt ► YD or YD► wt BM transplant results in a mixture of normal and hypotrophic white adipocytes (A), and in recipient mice acquiring the BAT phenotype of the donors.
See also Figures S8 and S9.
The analysis of BM engraftment, described in Transparent Methods, showed virtually complete replacement of the recipient's BM with the donors' BM (Figure S9). The circulating blood cells of Mmp14Y573D/Y573D mice displayed no significant abnormalities and numbers comparable to those of Mmp14wt/wt mice, showing that the MT1-MMP Y573D mutation does not affect hematopoietic cells. Therefore, these results showed that the bone and fat phenotype of transplanted Mmp14Y573D/Y573D mice resulted from the transfer of SSC and not from other BM cells.
Discussion
MT1-MMP plays an important role in the postnatal development of a variety of tissues including bone, cartilage, and fat. The striking abnormalities of Mmp14−/− mice – dwarfism, severe osteopenia, generalized arthritis, fibrosis, and lipodystrophy – have been ascribed to impaired collagen turnover, which also plays a fundamental role in SSC differentiation and affects adipocyte development (Chun et al., 2006; Chun and Inoue, 2014; Holmbeck et al., 1999; Zhou et al., 2000). The data presented in this paper show that, in addition to its proteolytic activity, MT1-MMP contributes to bone, cartilage, and adipose tissue homeostasis through a proteolysis-independent mechanism mediated by its cytoplasmic domain. This conclusion is based on the following observations.
We have previously shown that MT1-MMP activates ERK1/2 and Akt signaling upon binding of physiological concentrations of TIMP-2 (D'Alessio et al., 2008; Valacca et al., 2015). Signaling is activated by mutant MT1-MMP devoid of proteolytic activity (MT1-MMP E240A) or TIMP-2 lacking MMP inhibitory activity (Ala + TIMP-2) and is blocked by the Y573D substitution in the MT1-MMP cytoplasmic tail (D'Alessio et al., 2008; Figure 2G). Here we showed that the Y573 mutation does not significantly alter the collagenolytic activity of MT1-MMP or its cleavage of cell membrane proteins in vivo and in vitro (Figures 2 and 3). It should also be noted that changes in the levels of membrane proteins cleaved by MT1-MMP result in mouse phenotypes different from those of Mmp14Y573D/Y573 mice. CD44−/− or ADAM9−/− mice have no phenotype (Protin et al., 1999; Weskamp et al., 2002), whereas the genetic deficiency of FGFR2 (cleaved by ADAM9) or Notch1 results in embryonic lethality, and Notch1 haploinsufficiency causes virtually no phenotype (Conlon et al., 1995; Xu et al., 1998). DDR1 deficiency in the mouse results in female infertility and defective lactation, which we did not observe in Mmp14−/− mice; conversely, DDR1 haploinsufficiency causes no phenotype (Vogel et al., 2001). Therefore, consistent with our previous findings (D'Alessio et al., 2008; Valacca et al., 2015), our present data show that MT1-MMP activation of Erk1/2 and Akt signaling is independent of its proteolytic activity and mediated by its cytoplasmic domain.
To investigate the physiological significance of our in vitro findings, we generated a mouse in which MT1-MMP activation of Erk1/2 and Akt is abrogated by the Y573D mutation. The data presented in this paper show that mice bearing this mutation present phenotypes that partially recapitulate those caused by Mmp14 deficiency, as well as significantly different phenotypes. The phenotypes of Mmp14Y573D/Y573 mice appear to be milder than those of Mmp14−/− mice, indicating that both the proteolytic and signaling functions of MT1-MMP are required for normal postnatal development and homeostasis. However, while comparing the phenotypes of Mmp14Y573D/Y573 and Mmp14−/− mice (Table 1) provides information about the relative contribution of the proteolytic and non-proteolytic functions of MT1-MMP to postnatal development and tissue homeostasis, significant limitations must be considered. Mmp14−/− mice die within 2–3 months of age (Holmbeck & al., 1999), whereas Mmp14Y573D/Y573 mice have a normal life span, and their phenotypes become apparent in animals older than 2 months. The global deficiency of Mmp14 causes severe runting and wasting that ultimately lead to death. It is possible that the dramatic abnormalities of some tissues including cartilage and fat result from indirect effects. For instance, the generalized inflammatory state of Mmp14−/− mice (Shimizu-Hirota et al., 2012), as opposed to the decreased inflammatory state of Mmp14Y573D/Y573D- mice, may be the cause of, or contribute to, their severe arthritis. Indeed, conditional Mmp14 knockout in uncommitted SSC (Table 1) results in increased thickness of the articular cartilage in 3-month-old mice (Y. Tang et al., 2013), in contrast with the reduced thickness and degeneration of Mmp14−/− and Mmp14Y573D/Y573D cartilage (Holmbeck and al., 1999).
Furthermore, the relative contribution of MT1-MMP proteolytic and signaling functions to postnatal development and homeostasis may vary at different stages of postnatal life. The lethality of Mmp14 deficiency limits our understanding of the role of MT1-MMP to relatively early stages of postnatal development; in contrast, the normal life span of Mmp14Y573D/Y573 mice affords studying the role of MT1-MMP in adult animals. Our finding that the phenotype of the Mmp14Y573D/Y573 mouse becomes apparent in adult mice indicates that the proteolytic and the signaling function of MT1-MMP have predominant roles at different stages of postnatal development. The severe phenotype and precocious mortality of the Mmp14−/− mouse shows that MT1-MMP proteolytic activity has a fundamental role at early stages. Conversely, silencing MT1-MMP signaling is compatible with normal development but results in altered tissue homeostasis in the adult animal.
Our analysis of the articular cartilage of Mmp14Y573D/Y573D mice showed signs of tissue degeneration similar to but milder than that of Mmp14−/− mice. However, whereas Mmp14−/− mice have severe, acute arthritis (Holmbeck and al., 1999), the articular cartilage of Mmp14Y573D/Y573D mice shows histological signs and gene expression profile comparable to human OA, a chronic degenerative condition. The striking similarity of the gene expression profile of the articular cartilage of Mmp14Y573D/Y573D mice to that of human OA raises an interesting point about the role of MT1-MMP in articular cartilage homeostasis and the pathogenesis of OA. MT1-MMP expression decreases during chondrocyte differentiation, and in differentiated chondrocytes is ∼80% lower than in undifferentiated SSC (Tang et al., 2013). Consistent with this finding, Mmp14 deficiency in uncommitted SSC results in increased differentiation into chondrocytes and thickening of articular cartilage (Tang et al., 2013), suggesting that MT1-MMP expression contrasts normal chondrocyte development and articular cartilage homeostasis. Indeed, MT1-MMP is upregulated in OA cartilage relative to normal cartilage (Dreier et al., 2004; Kevorkian et al., 2004; Tchetina et al., 2005), indicating that MT1-MMP expression must be strictly controlled for normal cartilage homeostasis. In Mmp14Y573D/Y573D SSC-derived chondrocytes MT1-MMP expression is lower than in Mmp14wt/wt mice (Figure 10F); however, their cartilage is thinner and presents signs of degeneration, showing that altering MT1-MMP signaling in SSC has a pathological effect even in the presence of low levels of MT1-MMP proteolytic activity. Thus, MT1-MMP signaling is required for articular cartilage homeostasis.
Our phenotypic analysis of Mmp14Y573D/Y573D mice showed significant abnormalities in WAT, consistent with the phenotype of Mmp14−/− mice and of mice with conditional Mmp14 knockout in uncommitted SSC (Tang et al., 2013) (Table 1). However, some differences are noteworthy. In contrast to WAT hypotrophy, we surprisingly found BAT hypertrophy in Mmp14Y573D/Y573 mice, a finding at variance with the normal BAT of Mmp14−/− mice (Chun et al., 2006). Similarly, BM-associated WAT is decreased in Mmp14Y573D/Y573 mice but increased in mice with conditional knockout of Mmp14 in uncommitted SSC (Tang et al., 2013) (unfortunately, the severe wasting and early death of Mmp14−/− mice preclude a reliable assessment of BM-associated fat, which typically develops with aging). However, while the SSC of conditional Mmp14−/− mice show increased adipocyte differentiation in vitro (Tang et al., 2013), the SSC of Mmp14Y573D/Y573D mice show decreased adipocyte differentiation. MT1-MMP is a fundamental effector of adipocyte growth through collagen degradation, a process required for adipocyte increase in size (Chun et al., 2006; Chun and Inoue, 2014). However, Mmp14−/− mice have multiple, severe developmental and metabolic defects that can affect adipose tissue development indirectly. Our data show that in vivo MT1-MMP contributes to both WAT and BAT homeostasis by a proteolysis-independent mechanism mediated by its cytoplasmic tail. Several studies have shown the involvement of MT1-MMP in adipose tissue homeostasis (Chun et al., 2006; Feinberg et al., 2016; Fenech et al., 2015a, 2015b), and genetic associations between MT1-MMP and obesity in humans have been reported (Chun et al., 2010). MT1-MMP has also been proposed to control metabolic balance (Mori et al., 2016), a function that could explain the WAT and BAT abnormalities of MT1-MMP Y573D mice. Understanding the proteolysis-independent mechanism of MT1-MMP control of adipose tissue homeostasis can therefore have significant clinical and pharmacological implications.
The cortical bone of Mmp14Y573D/Y573 mice shows a significant decrease in thickness, a phenotype similar to – if milder than – that caused by Mmp14 deficiency. In contrast, the increased trabecular bone of Mmp14Y573D/Y573 mice contrasts with the severe osteopenia of Mmp14−/− mice and mice with Mmp14 knockout in uncommitted SSC (Holmbeck & al., 1999; Tang et al., 2013). This discrepancy between the phenotypic effects of the MT1-MMP Y573D mutation and Mmp14 deficiency suggests that the proteolytic and signaling functions of MT1-MMP can have opposing roles in bone physiology, and that a balance between the two functions is required for tissue homeostasis. Deletion of the gene abrogates both functions, whereas the MT1-MMP Y573D mutation only affects signaling, altering the balance between ECM proteolysis and intracellular signaling. Moreover, it should be noted again that the bone phenotype of the global or conditional Mmp14 knockout in SSC can only be observed within the first two-three months of age, whereas the bone abnormalities of Mmp14Y573D/Y573 mice become apparent in animals older than two months. It is possible that the proteolytic and signaling functions of MT1-MMP play different roles in bone modeling (postnatal development) and remodeling (adult life), respectively.
Consistent with their respective phenotypes, the BM-SSC of Mmp14Y573D/Y573D mice show increased osteoblast differentiation and decreased chondrocyte and adipocyte differentiation. Our finding that the bone and adipose tissue phenotypes can be rescued by Mmp14wt/wt BM transplant shows that the in vivo effects of the MT1-MMP Y573D mutation result from dysregulation of SSC differentiation. Several considerations can explain the failure of Mmp14Y573D/Y573D BM to transfer the mutant phenotypes to Mmp14wt/wt mice, as well as the incapacity of Mmp14wt/wt BM to rescue the cartilage phenotype of Mmp14Y573D/Y573D mice. Mmp14Y573D/Y573D SSC have significantly reduced proliferation and increased apoptosis relative to Mmp14wt/wt SSC (Figures 10A–10C). We examined the phenotypes of the transplanted mice 2 months after the transplant. While hematopoietic cells from Mmp14Y573D/Y573D mice were able to efficiently repopulate the BM of wt mice – indeed, we found no peripheral blood abnormalities in Mmp14Y573D/Y573D mice – Mmp14Y573D/Y573D SSC might have required a longer time than wt cells for the phenotypic effects to become apparent. Similarly, BM transplant did not affect the cortical bone phenotype of Mmp14Y573D/Y573D mice. Cortical bone has a much slower turnover than trabecular bone (Clarke, 2008); therefore a longer time is required for its homeostasis to be altered. The failure of our BM transplant experiments to affect the articular cartilage phenotype is consistent with the absence of vascularization of this tissue. Indeed, no attempts at treating joint cartilage diseases by systemic stem cell administration have thus far been effective (Jevotovsky et al., 2018).
MT1-MMP is constitutively expressed in SSC and its levels are differentially modulated during osteogenic vs. chondrogenic/adipogenic differentiation (Y. Tang et al., 2013). The MT1-MMP Y573D mutation and Mmp14 deficiency have opposing effects on SSC differentiation in vitro (Table 1). Mmp14 knockout in uncommitted SSC has no effect on their differentiation in 2D culture; however, it causes decreased osteogenesis and increased chondro- and adipogenesis in 3D collagen gel (Y. Tang et al., 2013). In contrast, in 2D culture MT1-MMP Y573D expression upregulates SSC differentiation into osteoblasts and downregulates chondrocyte and adipocyte differentiation (Figures 10F and 10G). While the in vitro differentiation of Mmp14Y573D/Y573D SSC and SSC with conditional Mmp14 knockout is consistent with the phenotypes of the respective mice, both mutations fail to fully recapitulate the bone, cartilage and fat abnormalities of the global Mmp14−/− mouse, which has osteopenia, lipodystrophy and arthritis (Table 1). These discrepancies indicate that MT1-MMP controls SSC differentiation by both proteolytic and non-proteolytic mechanisms, and the balance of these two functions is required for normal differentiation. MT1-MMP-mediated ECM degradation modulates mechanosignaling that controls gene expression during SSC differentiation into osteoblasts, and conditional Mmp14 deficiency in SSC blocks osteogenesis and causes severe osteopenia (Tang et al., 2013). Normal SSC differentiation requires the concerted action of ECM remodeling and intracellular signaling, cell functions modulated by the extracellular environment. In vitro, in the presence of abundant ECM – such as in 3D collagen gel culture (Tang et al., 2013) – the proteolytic activity of MT1-MMP is indispensable. Conversely, in the presence of relatively low amounts of ECM – such as in 2D culture – the role of intracellular signaling becomes prevalent. In vivo, cell differentiation in the stem cell niche, tissue/organ development and remodeling have different proteolytic and signaling requirements, which are spatially and temporally modulated.
The relative contribution of proteolysis and signaling to MT1-MMP function is also coordinated by extracellular ligands that can inhibit extracellular MT1-MMP proteolytic activity and activate intracellular signaling. This hypothesis is supported by our finding that TIMP-2 binding to MT1-MMP activates ERK1/2 and Akt signaling (D'Alessio et al., 2008; Valacca et al., 2015), as well as by the observation that in the mouse embryo MT1-MMP is temporally and spatially co-expressed with TIMP-2 in the developing skeleton (Apte et al., 1997; Kinoh et al., 1996). TIMP-2−/− mice do not display the severe phenotype of Mmp14−/− mice (Wang et al., 2000). We speculate that in these mice signaling can be activated by MT1-MMP binding of TIMP-3 or TIMP-4, as well as a variety of extracellular and transmembrane proteins, including integrins and CD44, that physiologically interact with the MT1-MMP ectodomains (Mori et al., 2002; Zhao et al., 2004).
Thus, the loss of signaling function caused by the Y573D substitution (D'Alessio et al., 2008; Valacca et al., 2015) (Figure 2E) can be at the basis of the defects in SSC differentiation and the consequent phenotypes of the Mmp14Y573D/Y573D mouse. Studies by other groups, as well as our own (unpublished), have shown that, although ERK1/2 signaling is important for osteoblast differentiation (Miraoui et al., 2009; Wu et al., 2015; Xiao et al., 2002), chronic inhibition of ERK1/2 activation results in increased SSC differentiation into osteoblasts (Ge et al., 2007; Higuchi et al., 2002; Nakayama et al., 2003; Schindeler and Little, 2006; Zhang et al., 2012); conversely, inhibition of PI3K/Akt signaling blocks adipo- and chondrogenesis (J. E. Kim and Chen, 2004; Lee et al., 2015; Li and Dong, 2016; Yang et al., 2011). Similarly, the genetic deficiency of Akt (Akt1 and Akt2 double knockout) or ERK1/2 in the mouse results in decreased adiposity and impaired adipogenesis. Conversely, Akt deficiency and conditional knockout of ERK1/2 in osteoprogenitors causes impaired bone development and severe osteopenia (Bost et al., 2005; Cho et al., 2001; Kim et al., 2019; Peng et al., 2003).
The molecular mechanism that relays signaling from the Y573 residue of the MT1-MMP cytoplasmic tail to the Ras-ERK1/2 and Ras-Akt pathways (D'Alessio et al., 2008; Valacca et al., 2015), remains to be investigated. The adaptor protein p130Cas, which binds to the cytoplasmic tail of MT1-MMP by a mechanism involving Y573 (Gonzalo et al., 2010; Wang and McNiven, 2012), recruits Src and/or FAK, which activate Ras (Bunda et al., 2014; Schlaepfer and Hunter, 1997). We speculate that TIMP-2 binding to MT1-MMP triggers the assembly of the p130Cas/Src/FAK complex at the MT1-MMP tail, and that this effect is abrogated by the Y573D substitution, which thus prevents the downstream activation of ERK1/2 and AKT signaling. In addition, Y573 could control intracellular signaling by modulating cytoplasmic tail interactions with a variety of transmembrane or membrane-bound proteins including caveolin-1 (Annabi et al., 2001; Galvez et al., 2002, 2004; Labrecque et al., 2004) and β1 integrins or growth factor receptors (Langlois et al., 2007). The cytoplasmic tail of MT1-MMP also controls energy production by activating hypoxia-inducible factor-1 (HIF-1) by a non-proteolytic mechanism (Sakamoto and Seiki, 2010).
In conclusion, our findings provide the first in vivo evidence for an important role of MT1-MMP–mediated, proteolysis-independent signaling in postnatal development and tissue homeostasis. Understanding the relative contribution of the proteolytic and signaling functions of MT1-MMP to the control of metabolic processes that affect a variety of tissues and organs will require the development of additional genetically engineered mouse models, as well as the molecular dissection of extracellular and intracellular components of the MT1-MMP signaling mechanism. The knowledge obtained from these studies can increase our understanding of the pathogenesis of important diseases that affect bone, cartilage, and adipose tissue homeostasis, such as osteopenia, osteoarthritis, and obesity, and potentially direct the design of pharmacological tools for their treatment.
Limitations of the Study
Our results show that the Y573D mutation in the MT1-MMP cytoplasmic tail abrogates TIMP-2–induced activation of Erk1/2 and Akt in SSC, suggesting that this effect is responsible for the defects in SSC differentiation and the development of the MT1-MMP Y573D mouse phenotypes. However, this mutation may also block or activate other signaling pathways. Future studies are warranted to understand the molecular mechanism by which the Y573 residue of the MT1-MMP cytoplasmic tail activates intracellular signaling that controls tissue homeostasis.
Resource Availability
Lead Contact
Paolo Mignatti, NYU School of Medicine, Department of Medicine, Division of Rheumatology, 301 East 17th Street, Suite 1612A, New York, NY 10003, USA. E-mail: mignap01@nyumc.org.
Materials Availability
The Mmp14Y573D/Y573D mouse is available upon request to the lead contact.
Data and Code Availability
The RNA-seq raw data are available at https://datadryad.org/stash/dataset/doi:10.5061/dryad.s4mw6m950.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by the US National Institutes of Health (NIH) grants R01 CA136715, R01CA136715-05S1 and R21 AG033735 (to P.M.) and in part by R21 AR069240-01A1 (to S.A.). We gratefully acknowledge the precious collaboration of the Rodent Genetic Engineering, High-Throughput Biology Laboratory, Experimental Pathology Research Laboratory, and Genome Technology Center of NYU School of Medicine, which are partially supported by NIH grant P30CA016087 to the Laura and Isaac Perlmutter Cancer Center and the Shared Instrumentation Grant S10 OD021747, and the MicroCT Core of NYU College of Dentistry, supported by NIH grant S10 OD010751 to Dr. Nicola C. Partridge.
Authors Contribution
Conceptualization, P.M.; Methodology, P.M., M.A., X.Z., B.B.D., S.Y. and S.A.; Validation, P.M., M.A.; Formal Analysis: P.M., M.A. and C.L.; Investigation, M.A., C.L., X.Z., T.H, C.A., C.V., S.Z., S.M., E.V., Q.Y., V.K. and S.Y.; Data Curation, P.M.; Writing – Original Draft, P.M.; Writing – Review & Editing, P.M., M.A., S.Y. and S.A.; Visualization, P.M., M.A. and S.A.; Supervision, P.M. and M.A.; Project Administration, P.M.; Funding Acquisition, P.M. and S.A.
Declaration of Interests
The authors declare no competing interests.
Published: December 18, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101789.
Supplemental Information
References
- Annabi B., Lachambre M., Bousquet-Gagnon N., Page M., Gingras D., Beliveau R. Localization of membrane-type 1 matrix metalloproteinase in caveolae membrane domains. Biochem. J. 2001;353(Pt 3):547–553. doi: 10.1042/0264-6021:3530547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Apte S.S., Fukai N., Beier D.R., Olsen B.R. The matrix metalloproteinase-14 (MMP-14) gene is structurally distinct from other MMP genes and is co-expressed with the TIMP-2 gene during mouse embryogenesis. J. Biol. Chem. 1997;272(41):25511–25517. doi: 10.1074/jbc.272.41.25511. [DOI] [PubMed] [Google Scholar]
- Bost F., Aouadi M., Caron L., Even P., Belmonte N., Prot M., Dani C., Hofman P., Pages G., Pouyssegur J. The extracellular signal-regulated kinase isoform ERK1 is specifically required for in vitro and in vivo adipogenesis. Diabetes. 2005;54:402–411. doi: 10.2337/diabetes.54.2.402. [DOI] [PubMed] [Google Scholar]
- Bunda S., Heir P., Srikumar T., Cook J.D., Burrell K., Kano Y., Lee J.E., Zadeh G., Raught B., Ohh M. Src promotes GTPase activity of Ras via tyrosine 32 phosphorylation. Proc. Natl. Acad. Sci. U S A. 2014;111:E3785–E3794. doi: 10.1073/pnas.1406559111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan K.M., Wong H.L., Jin G., Liu B., Cao R., Cao Y., Lehti K., Tryggvason K., Zhou Z. MT1-MMP inactivates ADAM9 to regulate FGFR2 signaling and calvarial osteogenesis. Dev. Cell. 2012;22(6):1176–1190. doi: 10.1016/j.devcel.2012.04.014. [DOI] [PubMed] [Google Scholar]
- Cho H., Thorvaldsen J.L., Chu Q., Feng F., Birnbaum M.J. Akt1/PKBalpha is required for normal growth but dispensable for maintenance of glucose homeostasis in mice. J. Biol. Chem. 2001;276:38349–38352. doi: 10.1074/jbc.C100462200. [DOI] [PubMed] [Google Scholar]
- Chun T.H., Hotary K.B., Sabeh F., Saltiel A.R., Allen E.D., Weiss S.J. A pericellular collagenase directs the 3-dimensional development of white adipose tissue. Cell. 2006;125:577–591. doi: 10.1016/j.cell.2006.02.050. [DOI] [PubMed] [Google Scholar]
- Chun T.H., Inoue M. 3-D adipocyte differentiation and peri-adipocyte collagen turnover. Methods Enzymol. 2014;538:15–34. doi: 10.1016/B978-0-12-800280-3.00002-5. [DOI] [PubMed] [Google Scholar]
- Chun T.H., Inoue M., Morisaki H., Yamanaka I., Miyamoto Y., Okamura T., Sato-Kusubata K., Weiss S.J. Genetic link between obesity and MMP14-dependent adipogenic collagen turnover. Diabetes. 2010;59:2484–2494. doi: 10.2337/db10-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008;3(Suppl 3):S131–S139. doi: 10.2215/CJN.04151206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conlon R.A., Reaume A.G., Rossant J. Notch1 is required for the coordinate segmentation of somites. Development. 1995;121(5):1533–1545. doi: 10.1242/dev.121.5.1533. [DOI] [PubMed] [Google Scholar]
- Crossno J.T., Jr., Majka S.M., Grazia T., Gill R.G., Klemm D.J. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells. J. Clin. Invest. 2006;116:3220–3228. doi: 10.1172/JCI28510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Alessio S., Ferrari G., Cinnante K., Scheerer W., Galloway A.C., Roses D.F., Rozanov D.V., Remacle A.G., Oh E.S., Shiryaev S.A. Tissue inhibitor of metalloproteinases-2 binding to membrane-type 1 matrix metalloproteinase induces MAPK activation and cell growth by a non-proteolytic mechanism. J. Biol. Chem. 2008;283:87–99. doi: 10.1074/jbc.M705492200. [DOI] [PubMed] [Google Scholar]
- Dreier R., Grassel S., Fuchs S., Schaumburger J., Bruckner P. Pro-MMP-9 is a specific macrophage product and is activated by osteoarthritic chondrocytes via MMP-3 or a MT1-MMP/MMP-13 cascade. Exp. Cell Res. 2004;297(2):303–312. doi: 10.1016/j.yexcr.2004.02.027. [DOI] [PubMed] [Google Scholar]
- Evans B.R., Mosig R.A., Lobl M., Martignetti C.R., Camacho C., Grum-Tokars V., Glucksman M.J., Martignetti J.A. Mutation of membrane type-1 metalloproteinase, MT1-MMP, causes the multicentric osteolysis and arthritis disease Winchester syndrome. Am. J. Hum. Genet. 2012;91(3):572–576. doi: 10.1016/j.ajhg.2012.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinberg T.Y., Rowe R.G., Saunders T.L., Weiss S.J. Functional roles of MMP14 and MMP15 in early postnatal mammary gland development. Development. 2016;143(21):3956–3968. doi: 10.1242/dev.136259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenech M., Gavrilovic J., Malcolm P., Toms A., Turner J. The role of metalloproteinases and their tissue inhibitors in adipose tissue remodelling and whole-body lipid distribution: a cross-sectional clinical study. Lancet. 2015;385:S36. doi: 10.1016/S0140-6736(15)60351-8. [DOI] [PubMed] [Google Scholar]
- Fenech M., Gavrilovic J., Turner J. Effect of tissue inhibitor of metalloproteinases 3 on DLK1 shedding in cultured human pre-adipocytes and implications for adipose tissue remodelling. Lancet. 2015;385:S35. doi: 10.1016/S0140-6736(15)60350-6. [DOI] [PubMed] [Google Scholar]
- Fu H.L., Sohail A., Valiathan R.R., Wasinski B.D., Kumarasiri M., Mahasenan K.V., Bernardo M.M., Tokmina-Roszyk D., Fields G.B., Mobashery S., Fridman R. Shedding of discoidin domain receptor 1 by membrane-type matrix metalloproteinases. J. Biol. Chem. 2013;288:12114–12129. doi: 10.1074/jbc.M112.409599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez B.G., Matias-Roman S., Yanez-Mo M., Sanchez-Madrid F., Arroyo A.G. ECM regulates MT1-MMP localization with beta1 or alphavbeta3 integrins at distinct cell compartments modulating its internalization and activity on human endothelial cells. J. Cell Biol. 2002;159:509–521. doi: 10.1083/jcb.200205026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvez B.G., Matias-Roman S., Yanez-Mo M., Vicente-Manzanares M., Sanchez-Madrid F., Arroyo A.G. Caveolae are a novel pathway for membrane-type 1 matrix metalloproteinase traffic in human endothelial cells. Mol. Biol. Cell. 2004;15(2):678–687. doi: 10.1091/mbc.E03-07-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge C., Xiao G., Jiang D., Franceschi R.T. Critical role of the extracellular signal-regulated kinase-MAPK pathway in osteoblast differentiation and skeletal development. J. Cell Biol. 2007;176:709–718. doi: 10.1083/jcb.200610046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geyer M., Grassel S., Straub R.H., Schett G., Dinser R., Grifka J., Gay S., Neumann E., Muller-Ladner U. Differential transcriptome analysis of intraarticular lesional vs intact cartilage reveals new candidate genes in osteoarthritis pathophysiology. Osteoarthritis Cartilage. 2009;17(3):328–335. doi: 10.1016/j.joca.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Gonzalo P., Guadamillas M.C., Hernandez-Riquer M.V., Pollan A., Grande-Garcia A., Bartolome R.A., Vasanji A., Ambrogio C., Chiarle R., Teixido J. MT1-MMP is required for myeloid cell fusion via regulation of Rac1 signaling. Dev. Cell. 2010;18(1):77–89. doi: 10.1016/j.devcel.2009.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta R.K., Mepani R.J., Kleiner S., Lo J.C., Khandekar M.J., Cohen P., Frontini A., Bhowmick D.C., Ye L., Cinti S., Spiegelman B.M. Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab. 2012;15(2):230–239. doi: 10.1016/j.cmet.2012.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi C., Myoui A., Hashimoto N., Kuriyama K., Yoshioka K., Yoshikawa H., Itoh K. Continuous inhibition of MAPK signaling promotes the early osteoblastic differentiation and mineralization of the extracellular matrix. J. Bone Miner. Res. 2002;17(10):1785–1794. doi: 10.1359/jbmr.2002.17.10.1785. [DOI] [PubMed] [Google Scholar]
- Holmbeck K., al e. MT1-MMP-deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Holmbeck K., Bianco P., Caterina J., Yamada S., Kromer M., Kuznetsov S.A., Mankani M., Gehron Robey P., Robin Poole A., Pidoux I. MT1-MMP-Deficient mice develop dwarfism, osteopenia, arthritis, and connective tissue disease due to inadequate collagen turnover. Cell. 1999;99:81–92. doi: 10.1016/s0092-8674(00)80064-1. [DOI] [PubMed] [Google Scholar]
- Itoh Y. Membrane-type matrix metalloproteinases: their functions and regulations. Matrix Biol. 2015;44-46:207–223. doi: 10.1016/j.matbio.2015.03.004. [DOI] [PubMed] [Google Scholar]
- Jevotovsky D.S., Alfonso A.R., Einhorn T.A., Chiu E.S. Osteoarthritis and stem cell therapy in humans: a systematic review. Osteoarthritis Cartilage. 2018;26(6):711–729. doi: 10.1016/j.joca.2018.02.906. [DOI] [PubMed] [Google Scholar]
- Kajita M., Itoh Y., Chiba T., Mori H., Okada A., Kinoh H., Seiki M. Membrane-type 1 matrix metalloproteinase cleaves CD44 and promotes cell migration. J. Cell Biol. 2001;153:893–904. doi: 10.1083/jcb.153.5.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlsson C., Dehne T., Lindahl A., Brittberg M., Pruss A., Sittinger M., Ringe J. Genome-wide expression profiling reveals new candidate genes associated with osteoarthritis. Osteoarthritis Cartilage. 2010;18(4):581–592. doi: 10.1016/j.joca.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Kevorkian L., Young D.A., Darrah C., Donell S.T., Shepstone L., Porter S., Brockbank S.M., Edwards D.R., Parker A.E., Clark I.M. Expression profiling of metalloproteinases and their inhibitors in cartilage. Arthritis Rheum. 2004;50:131–141. doi: 10.1002/art.11433. [DOI] [PubMed] [Google Scholar]
- Kim J.E., Chen J. Regulation of peroxisome proliferator-activated receptor-gamma activity by mammalian target of rapamycin and amino acids in adipogenesis. Diabetes. 2004;53:2748–2756. doi: 10.2337/diabetes.53.11.2748. [DOI] [PubMed] [Google Scholar]
- Kim J.M., Yang Y.S., Park K.H., Oh H., Greenblatt M.B., Shim J.H. The ERK MAPK pathway is essential for skeletal development and homeostasis. Int. J. Mol. Sci. 2019;20(8):1803. doi: 10.3390/ijms20081803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoh H., Sato H., Tsunezuka Y., Takino T., Kawashima A., Okada Y., Seiki M. MT-MMP, the cell surface activator of proMMP-2 (pro-gelatinase A), is expressed with its substrate in mouse tissue during embryogenesis. J. Cell Sci. 1996;109(Pt 5):953–959. doi: 10.1242/jcs.109.5.953. [DOI] [PubMed] [Google Scholar]
- Labrecque L., Nyalendo C., Langlois S., Durocher Y., Roghi C., Murphy G., Gingras D., Beliveau R. Src-mediated tyrosine phosphorylation of caveolin-1 induces its association with membrane type 1 matrix metalloproteinase. J. Biol. Chem. 2004;279:52132–52140. doi: 10.1074/jbc.M409617200. [DOI] [PubMed] [Google Scholar]
- Lagoutte E., Villeneuve C., Lafanechere L., Wells C.M., Jones G.E., Chavrier P., Rosse C. LIMK regulates tumor-cell invasion and matrix degradation through tyrosine phosphorylation of MT1-MMP. Sci. Rep. 2016;6:24925. doi: 10.1038/srep24925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois S., Nyalendo C., Di Tomasso G., Labrecque L., Roghi C., Murphy G., Gingras D., Beliveau R. Membrane-type 1 matrix metalloproteinase stimulates cell migration through epidermal growth factor receptor transactivation. Mol. Cancer Res. 2007;5:569–583. doi: 10.1158/1541-7786.MCR-06-0267. [DOI] [PubMed] [Google Scholar]
- Lee N., Kim I., Park S., Han D., Ha S., Kwon M., Kim J., Byun S.H., Oh W., Jeon H.B. Creatine inhibits adipogenesis by downregulating insulin-induced activation of the phosphatidylinositol 3-kinase signaling pathway. Stem Cells Dev. 2015;24(8):983–994. doi: 10.1089/scd.2014.0130. [DOI] [PubMed] [Google Scholar]
- Li J., Dong S. The signaling pathways involved in chondrocyte differentiation and hypertrophic differentiation. Stem Cells Int. 2016;2016:2470351. doi: 10.1155/2016/2470351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miraoui H., Oudina K., Petite H., Tanimoto Y., Moriyama K., Marie P.J. Fibroblast growth factor receptor 2 promotes osteogenic differentiation in mesenchymal cells via ERK1/2 and protein kinase C signaling. J. Biol. Chem. 2009;284:4897–4904. doi: 10.1074/jbc.M805432200. [DOI] [PubMed] [Google Scholar]
- Mori H., Bhat R., Bruni-Cardoso A., Chen E.I., Jorgens D.M., Coutinho K., Louie K., Bowen B.B., Inman J.L., Tecca V. New insight into the role of MMP14 in metabolic balance. PeerJ. 2016;4:e2142. doi: 10.7717/peerj.2142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H., Tomari T., Koshikawa N., Kajita M., Itoh Y., Sato H., Tojo H., Yana I., Seiki M. CD44 directs membrane-type 1 matrix metalloproteinase to lamellipodia by associating with its hemopexin-like domain. Embo J. 2002;21(15):3949–3959. doi: 10.1093/emboj/cdf411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K., Tamura Y., Suzawa M., Harada S., Fukumoto S., Kato M., Miyazono K., Rodan G.A., Takeuchi Y., Fujita T. Receptor tyrosine kinases inhibit bone morphogenetic protein-Smad responsive promoter activity and differentiation of murine MC3T3-E1 osteoblast-like cells. J. Bone Miner. Res. 2003;18(5):827–835. doi: 10.1359/jbmr.2003.18.5.827. [DOI] [PubMed] [Google Scholar]
- Nyalendo C., Michaud M., Beaulieu E., Roghi C., Murphy G., Gingras D., Beliveau R. Src-dependent phosphorylation of membrane type I matrix metalloproteinase on cytoplasmic tyrosine 573: role in endothelial and tumor cell migration. J. Biol. Chem. 2007;282:15690–15699. doi: 10.1074/jbc.M608045200. [DOI] [PubMed] [Google Scholar]
- Peng X.D., Xu P.Z., Chen M.L., Hahn-Windgassen A., Skeen J., Jacobs J., Sundararajan D., Chen W.S., Crawford S.E., Coleman K.G., Hay N. Dwarfism, impaired skin development, skeletal muscle atrophy, delayed bone development, and impeded adipogenesis in mice lacking Akt1 and Akt2. Genes Dev. 2003;17(11):1352–1365. doi: 10.1101/gad.1089403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Protin U., Schweighoffer T., Jochum W., Hilberg F. CD44-deficient mice develop normally with changes in subpopulations and recirculation of lymphocyte subsets. J. Immunol. 1999;163:4917–4923. [PubMed] [Google Scholar]
- Rozanov D.V., Deryugina E.I., Ratnikov B.I., Monosov E.Z., Marchenko G.N., Quigley J.P., Strongin A.Y. Mutation analysis of membrane type-1 matrix metalloproteinase (MT1-MMP). The role of the cytoplasmic tail Cys(574), the active site Glu(240), and furin cleavage motifs in oligomerization, processing, and self-proteolysis of MT1-MMP expressed in breast carcinoma cells. J. Biol. Chem. 2001;276:25705–25714. doi: 10.1074/jbc.M007921200. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Seiki M. Cytoplasmic tail of MT1-MMP regulates macrophage motility independently from its protease activity. Genes Cells. 2009;14(5):617–626. doi: 10.1111/j.1365-2443.2009.01293.x. [DOI] [PubMed] [Google Scholar]
- Sakamoto T., Seiki M. A membrane protease regulates energy production in macrophages by activating hypoxia-inducible factor-1 via a non-proteolytic mechanism. J. Biol. Chem. 2010;285:29951–29964. doi: 10.1074/jbc.M110.132704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakr M., Li X.Y., Sabeh F., Feinberg T.Y., Tesmer J.J.G., Tang Y., Weiss S.J. Tracking the Cartoon mouse phenotype: hemopexin domain-dependent regulation of MT1-MMP pericellular collagenolytic activity. J. Biol. Chem. 2018;293:8113–8127. doi: 10.1074/jbc.RA117.001503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato H., Takino T., Okada Y., Cao J., Shinagawa A., Yamamoto E., Seiki M. A matrix metalloproteinase expressed on the surface of invasive tumour cells [see comments] Nature. 1994;370:61–65. doi: 10.1038/370061a0. [DOI] [PubMed] [Google Scholar]
- Schindeler A., Little D.G. Ras-MAPK signaling in osteogenic differentiation: friend or foe? J. Bone Miner. Res. 2006;21(9):1331–1338. doi: 10.1359/jbmr.060603. [DOI] [PubMed] [Google Scholar]
- Schlaepfer D.D., Hunter T. Focal adhesion kinase overexpression enhances ras-dependent integrin signaling to ERK2/mitogen-activated protein kinase through interactions with and activation of c-Src. J. Biol. Chem. 1997;272:13189–13195. doi: 10.1074/jbc.272.20.13189. [DOI] [PubMed] [Google Scholar]
- Shimizu-Hirota R., Xiong W., Baxter B.T., Kunkel S.L., Maillard I., Chen X.W., Sabeh F., Liu R., Li X.Y., Weiss S.J. MT1-MMP regulates the PI3Kdelta.Mi-2/NuRD-dependent control of macrophage immune function. Genes Dev. 2012;26(4):395–413. doi: 10.1101/gad.178749.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Q.Q., Otto T.C., Lane M.D. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc. Natl. Acad. Sci. U S A. 2004;101:9607–9611. doi: 10.1073/pnas.0403100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang Y., Rowe R.G., Botvinick E.L., Kurup A., Putnam A.J., Seiki M., Weaver V.M., Keller E.T., Goldstein S., Dai J. MT1-MMP-dependent control of skeletal stem cell commitment via a beta1-integrin/YAP/TAZ signaling axis. Dev. Cell. 2013;25(4):402–416. doi: 10.1016/j.devcel.2013.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchetina E.V., Squires G., Poole A.R. Increased type II collagen degradation and very early focal cartilage degeneration is associated with upregulation of chondrocyte differentiation related genes in early human articular cartilage lesions. J. Rheumatol. 2005;32:876–886. [PubMed] [Google Scholar]
- Tran K.V., Gealekman O., Frontini A., Zingaretti M.C., Morroni M., Giordano A., Smorlesi A., Perugini J., De Matteis R., Sbarbati A. The vascular endothelium of the adipose tissue gives rise to both white and brown fat cells. Cell Metab. 2012;15(2):222–229. doi: 10.1016/j.cmet.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uekita T., Itoh Y., Yana I., Ohno H., Seiki M. Cytoplasmic tail-dependent internalization of membrane-type 1 matrix metalloproteinase is important for its invasion-promoting activity. J. Cell Biol. 2001;155:1345–1356. doi: 10.1083/jcb.200108112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valacca C., Tassone E., Mignatti P. TIMP-2 interaction with MT1-MMP activates the AKT pathway and protects tumor cells from apoptosis. PLoS One. 2015;10:e0136797. doi: 10.1371/journal.pone.0136797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel W.F., Aszodi A., Alves F., Pawson T. Discoidin domain receptor 1 tyrosine kinase has an essential role in mammary gland development. Mol. Cell Biol. 2001;21(8):2906–2917. doi: 10.1128/MCB.21.8.2906-2917.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., McNiven M.A. Invasive matrix degradation at focal adhesions occurs via protease recruitment by a FAK-p130Cas complex. J. Cell Biol. 2012;196:375–385. doi: 10.1083/jcb.201105153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Juttermann R., Soloway P.D. TIMP-2 is required for efficient activation of proMMP-2 in vivo. J. Biol. Chem. 2000;275:26411–26415. doi: 10.1074/jbc.M001270200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weskamp G., Cai H., Brodie T.A., Higashyama S., Manova K., Ludwig T., Blobel C.P. Mice lacking the metalloprotease-disintegrin MDC9 (ADAM9) have no evident major abnormalities during development or adult life. Mol. Cell Biol. 2002;22(5):1537–1544. doi: 10.1128/mcb.22.5.1537-1544.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y., Zhou J., Li Y., Zhou Y., Cui Y., Yang G., Hong Y. Rap1A regulates osteoblastic differentiation via the ERK and p38 mediated signaling. PLoS One. 2015;10:e0143777. doi: 10.1371/journal.pone.0143777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao G., Jiang D., Gopalakrishnan R., Franceschi R.T. Fibroblast growth factor 2 induction of the osteocalcin gene requires MAPK activity and phosphorylation of the osteoblast transcription factor, Cbfa1/Runx2. J. Biol. Chem. 2002;277:36181–36187. doi: 10.1074/jbc.M206057200. [DOI] [PubMed] [Google Scholar]
- Xu X., Weinstein M., Li C., Naski M., Cohen R.I., Ornitz D.M., Leder P., Deng C. Fibroblast growth factor receptor 2 (FGFR2)-mediated reciprocal regulation loop between FGF8 and FGF10 is essential for limb induction. Development. 1998;125(4):753–765. doi: 10.1242/dev.125.4.753. [DOI] [PubMed] [Google Scholar]
- Yang H.J., Xia Y.Y., Wang L., Liu R., Goh K.J., Ju P.J., Feng Z.W. A novel role for neural cell adhesion molecule in modulating insulin signaling and adipocyte differentiation of mouse mesenchymal stem cells. J. Cell Sci. 2011;124(Pt 15):2552–2560. doi: 10.1242/jcs.085340. [DOI] [PubMed] [Google Scholar]
- Zhang M.Y., Ranch D., Pereira R.C., Armbrecht H.J., Portale A.A., Perwad F. Chronic inhibition of ERK1/2 signaling improves disordered bone and mineral metabolism in hypophosphatemic (Hyp) mice. Endocrinology. 2012;153(4):1806–1816. doi: 10.1210/en.2011-1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H., Bernardo M.M., Osenkowski P., Sohail A., Pei D., Nagase H., Kashiwagi M., Soloway P.D., DeClerck Y.A., Fridman R. Differential inhibition of membrane type 3 (MT3)-matrix metalloproteinase (MMP) and MT1-MMP by tissue inhibitor of metalloproteinase (TIMP)-2 and TIMP-3 rgulates pro-MMP-2 activation. J. Biol. Chem. 2004;279:8592–8601. doi: 10.1074/jbc.M308708200. [DOI] [PubMed] [Google Scholar]
- Zhou Z., Apte S.S., Soininen R., Cao R., Baaklini G.Y., Rauser R.W., Wang J., Cao Y., Tryggvason K. Impaired endochondral ossification and angiogenesis in mice deficient in membrane-type matrix metalloproteinase I. Proc. Natl. Acad. Sci. U S A. 2000;97:4052–4057. doi: 10.1073/pnas.060037197. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA-seq raw data are available at https://datadryad.org/stash/dataset/doi:10.5061/dryad.s4mw6m950.