Abstract
Introduction:
We assessed the impact of open or minimally-invasive partial cystectomy on surgical margin status in a nationwide hospital-based cohort.
Materials and Methods:
Patients who underwent partial cystectomy from 2010 to 2014 were identified in the National Cancer Data Base. The primary outcome was surgical margin status. A multivariable logistic regression model was fitted to identify patient, hospital, and surgical factors associated with positive surgical margins (PSMs).
Results:
Partial cystectomy was performed in 1,118 patients via open (n = 715, 64%), laparoscopic (n = 209, 19%), and robotic (n = 194, 17%) approaches. Overall, 220 (19.7%) patients had PSMs. The PSM rate by surgical approach was 19.6% for open, 18.2% for laparoscopic, and 21.6% for robotic (P = 0.678). Compared to open partial cystectomy, the laparoscopic (aOR 1.06, 95%CI 0.70–1.60, P = 0.782), and robotic (aOR 1.28, 95%CI 0.85–1.91, P = 0.235) approaches were not significantly different in terms of PSM rate. There were higher odds of PSMs in non-Hispanic blacks (aOR 1.93, 95%CI 1.09–3.39, P = 0.023) compared to non-Hispanic whites, and in patients with muscle invasive bladder cancer (aOR 3.28, 95%CI 2.00–5.37, P < 0.001) or tumor size ≥ 3 cm (aOR 1.67, 95%CI 1.21–2.30, P = 0.002). Tumors in a dome/urachal location had lower odds of a PSM compared to tumors in a nondome/urachal location (aOR 0.67, 95%CI 0.47–0.94, P = 0.022).
Conclusions:
Our results suggest that partial cystectomy using a laparoscopic or robotic-assisted approach is not associated with an increased risk of PSMs compared to open partial cystectomy
Keywords: Urinary bladder neoplasm, Urologic surgical procedures, Cystectomy, Margins of excision, Outcome and process assessment (health care)
1. Introduction
Approximately 81,190 new cases of bladder cancer will be diagnosed in the United States in 2018 [1]. Utilization of partial cystectomy for the treatment of bladder cancer has been decreasing due to increasingly stringent selection criteria [2,3]. Currently, clinical practice guidelines recommend partial cystectomy for solitary tumors amenable to resection and without concomitant carcinoma in situ [4,5].
While open partial cystectomy (OPC) represents the traditional surgical approach for management of appropriately selected bladder tumors, advances in technology have led to the introduction of laparoscopic and robotic-assisted techniques. These minimally-invasive approaches have increasingly become preferred by both patients and urologic surgeons. To our knowledge, no comparative studies evaluating perioperative outcomes and oncologic efficacy among OPC, laparoscopic partial cystectomy (LPC), and robotic-assisted LPC (RPC) have been published.
The goal of partial cystectomy is complete removal of the tumor with negative surgical margins regardless of the surgical approach. The positive surgical margin (PSM) rate has been reported to range between 9% and 14.8% for OPC [6–8] and between 0% and 3.6% for RPC [9–11]. There have been no reported cases of PSMs for LPC [12–15]. The use of case series and high volume, single institution studies limits the generalizability of these results. However, neither a comparative study nor a population-based cohort study has been reported to date to evaluate the likelihood of PSMs among different surgical approaches for partial cystectomy.
Robotic-assisted techniques will continue to be increasingly utilized for the management of bladder cancer, including partial cystectomy [16,17]. One major difference between robotic-assisted surgical techniques compared to open or laparoscopic surgery is the absence of haptic feedback. Therefore, it is essential to evaluate whether PSMs for RPC are comparable to open and laparoscopic techniques. We assessed the relationship between PSMs and surgical approach in a nationwide hospital-based cohort of patients undergoing partial cystectomy. We also identified other patient and hospital characteristics associated with PSMs for partial cystectomy.
2. Material and methods
2.1. Data source
We utilized the National Cancer Data Base (NCDB) to examine the surgical margin status in patients undergoing partial cystectomy. The NCDB, established in 1989, is a nationwide, facility-based, comprehensive clinical surveillance resource oncology data set that captures 70% of all newly diagnosed malignancies annually in the United States. It is a collaborative joint project of the American Cancer Society and the American College of Surgeons Commission on Cancer, which has executed a Business Associate Agreement that includes a data use agreement with each of its accredited hospitals.
2.2. Study population
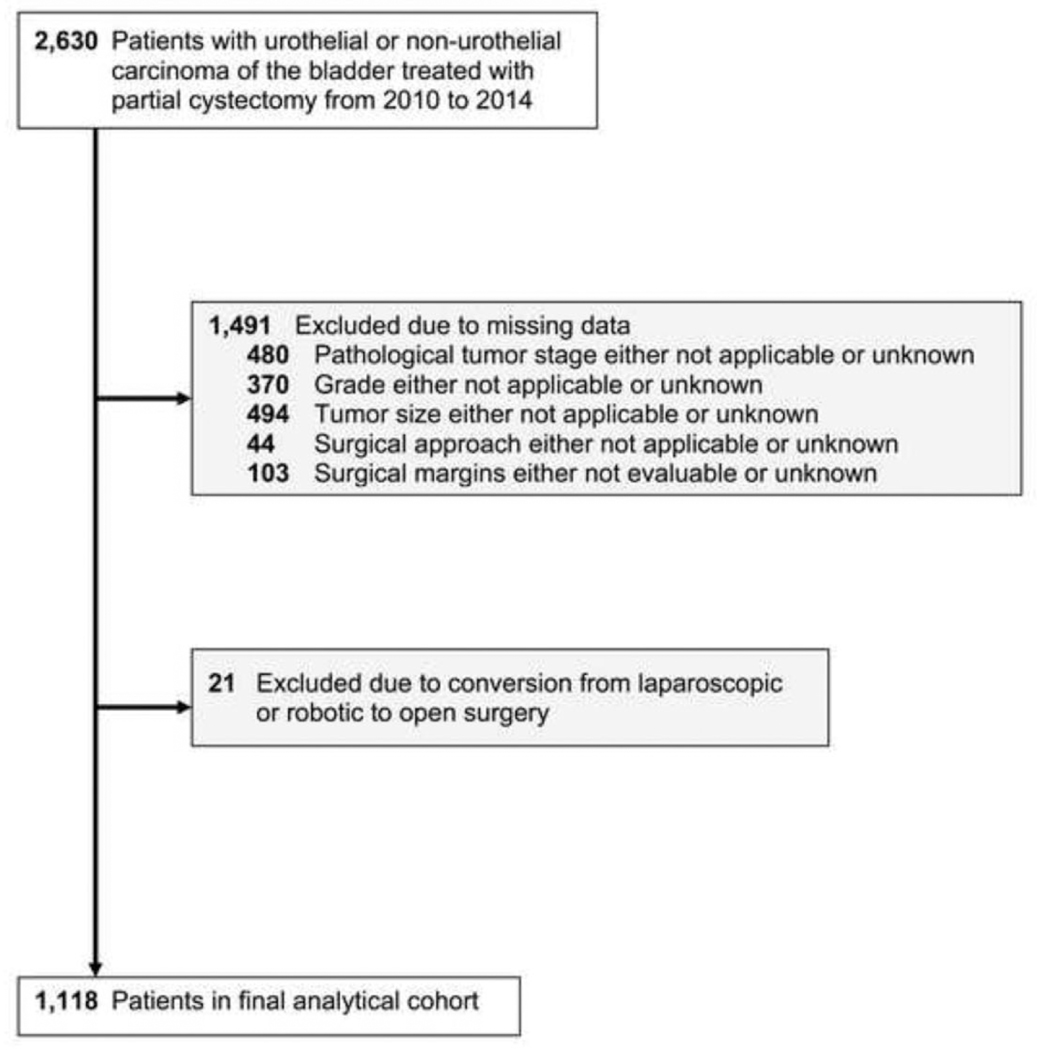
Fig. 1 shows the flow diagram of the selection process for our final analytical cohort. Initially 2,630 patients aged 18 years or older with urothelial or nonurothelial carcinoma (morphological codes 8031, 8082, 8120, 8122, 8130, 8131, 8041, 8051, 8052, 8070, 8140, 8240, 8261, 8720, 8830, 8890, 8900, 9120, and 9180) of the bladder (topographical code C67) [18], who underwent partial cystectomy (surgical code 30) [19] from 2010 to 2014, inclusive, were identified. We limited our study population from 2010 to 2014 because codes for all 3 surgical approaches were only available for these years. We excluded 480 patients for whom the AJCC 2010 pathologic T stage was coded as either not applicable or unknown, 370 patients for whom grade was coded as either not applicable or unknown, and 494 patients for whom tumor size was coded as either not applicable or unknown. We then excluded 44 patients for whom the surgical approach was coded as either not applicable or unknown, as well as 103 patients in whom the surgical margins were coded as either not evaluable or unknown. We also excluded 21 patients in whom surgery began using either a laparoscopic or robotic-assisted approach and was converted to an open approach. After applying all inclusions and exclusions, our final analytical cohort included 1,118 patients.
Fig. 1.
Flow diagram of selection process for final analytical cohort.
2.3. Study covariables and outcome
Age at diagnosis, gender, race, ethnicity, Charlson—Deyo comorbidity score, facility type, AJCC 2010 clinical and pathologic T stage, grade, histology, tumor location, tumor size, and surgical approach were selected for each patient in the NCBD. Topographical codes C67.0 (trigone of bladder), C67.2 (lateral wall of bladder), C67.3 (anterior wall of bladder), C67.4 (posterior wall of bladder), C67.5 (bladder neck), C67.6 (ureteric orifice), C67.8 (overlapping lesion of bladder), and C67.9 (bladder, not otherwise specified) were combined and reclassified as nonurachal. Facility type codes I (community cancer program), 2 (comprehensive community cancer program), 4 (integrated network cancer program), and 9 (other or unknown types of cancer programs) were combined and reclassified as nonacademic/research. Grade codes I (well-differentiated) and 2 (moderately differentiated; intermediate differentiation) were reclassified as low grade and intermediate grade, respectively; grade codes 3 (poorly differentiated) and 4 (undifferentiated; anaplastic) were combined and reclassified as high grade.
The primary clinical outcome was surgical margin status and was classified as either positive or negative. Surgical margin status in the NCDB was coded as it appeared on the final pathology report. Surgical margin codes I (residual tumor, not otherwise specified), 2 (microscopic residual tumor), and 3 (macroscopic residual tumor) were used to define PSMs.
2.4. Statistical analysis
An exploratory data analysis was initially conducted. Frequencies and percentages were tabulated for categorical variables, while means and standard deviations for continuous variables were calculated across each sociodemographic variable and type of surgical approach (OPC, LPC, RPC). The chi-square test of association was used to determine differences among categorical variables, while the I-way ANOVA was utilized to compare means across the 3 surgical groups for continuous variables. Trends in the use of OPC, LPC, and RPC were assessed from 2010 to 2014 using the Cochran —Armitage test. A multivariable stepwise logistic regression model was fitted to evaluate differences in surgical margin status (positive vs. negative) across various patient and hospital characteristics. Adjusted odds ratios and corresponding 95% confidence intervals (95% CIs) were calculated. All statistical analyses were done with 5% significance level (α = 0.05) and included 2-sided hypothesis testing procedures. Data management and statistical analysis were done using SAS version 9.4 for Windows (SAS Institute Inc., Cary, NC).
3. Results
Our final analytical cohort included a total of 1,118 patients, of which 715 (64%) underwent OPC, 209 (19%) underwent LPC, and 194 (17%) underwent RPC. Overall, the average age at diagnosis was 71.0 years (standard deviation = 12.0) and did not differ significantly among the open (71.2 ± 12.0), laparoscopic (70.6 ± 11.7), and robotic-assisted (69.3 ± 12.1) approaches (P = 0.160).
Demographic, clinical, and pathological characteristics are listed in Table 1. Overall, the rate of PSMs was 19.7% (220 cases). There was no significant difference in the rate of PSMs between patients who underwent OPC (19.6%), LPC (18.2%), and RPC (21.6%) (P = 0.678). Supplementary Table 1 shows the rate of PSMs for urachal adenocarcinoma and urothelial carcinoma. Final pathologic outcomes of patients with clinical stage Ta, Tis, or Tl before partial cystectomy are shown in Supplementary Table 2.
Table 1.
Demographic, clinical, and pathological characteristics of patients in the National Cancer Data Base who underwent partial cystectomy from 2010 to 2014
| All | OPC | LPC | RPC | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | (%) | n | (%) | n | (%) | n | (%) | P value | |
| Overall | 1118 | (100.0) | 715 | (64.0) | 209 | (18.7) | 194 | (17.4) | |
| Age (in y) | 0.274 | ||||||||
| <65 | 305 | (27.3) | 188 | (61.6) | 55 | (18.0) | 62 | (20.3) | |
| ≥65 | 813 | (72.7) | 527 | (64.8) | 154 | (18.9) | 132 | (16.2) | |
| Sex | 0.245 | ||||||||
| Male | 819 | (73.3) | 520 | (63.5) | 148 | (18.1) | 151 | (18.4) | |
| Female | 299 | (26.7) | 195 | (65.2) | 61 | (20.4) | 43 | (14.4) | |
| Race/Ethnicity | 0.226 | ||||||||
| Non-Hispanic white | 944 | (84.4) | 611 | (64.7) | 177 | (18.8) | 156 | (16.5) | |
| Non-Hispanic black | 71 | (6.4) | 48 | (67.6) | 9 | (12.7) | 14 | (19.7) | |
| Hispanic | 31 | (2.8) | 19 | (61.3) | 7 | (22.6) | 5 | (16.1) | |
| Other | 72 | (6.4) | 37 | (51.4) | 16 | (22.2) | 19 | (26.4) | |
| Chari son-Deyo Score | 0.099 | ||||||||
| 0 | 774 | (69.2) | 494 | (63.8) | 138 | (17.8) | 142 | (18.4) | |
| 1 | 255 | (22.8) | 172 | (67.5) | 46 | (18.0) | 37 | (14.5) | |
| 2+ | 89 | (8.0) | 49 | (55.1) | 25 | (28.1) | 15 | (16.9) | |
| Facility type | <0.001 | ||||||||
| Nonacademic/research | 744 | (66.5) | 487 | (65.5) | 150 | (20.2) | 107 | (14.4) | |
| Academic/research | 374 | (33.5) | 228 | (61.0) | 59 | (15.8) | 87 | (23.3) | |
| Clinical tumor stage | 0.443 | ||||||||
| Unknown | 446 | (39.9) | 281 | (63.0) | 80 | (17.9) | 85 | (19.1) | |
| Nonmuscle invasive | 319 | (28.5) | 197 | (61.8) | 65 | (20.4) | 57 | (17.9) | |
| Muscle invasive | 353 | (31.6) | 237 | (67.1) | 64 | (18.1) | 52 | (14.7) | |
| Pathologic tumor stage | 0.074 | ||||||||
| Nonmuscle invasive | 274 | (24.5) | 165 | (60.2) | 64 | (23.4) | 45 | (16.4) | |
| Muscle invasive | 844 | (75.5) | 550 | (65.2) | 145 | (17.2) | 149 | (17.7) | |
| Grade | <0.001 | ||||||||
| Low | 57 | (5.1) | 26 | (45.6) | 23 | (40.4) | 8 | (14.0) | |
| Intermediate | 161 | (14.4) | 97 | (60.3) | 32 | (19.9) | 32 | (19.9) | |
| High | 900 | (80.5) | 592 | (65.8) | 154 | (17.1) | 154 | (17.1) | |
| Histology | 0.014 | ||||||||
| Nonurothelial | 202 | (18.1) | 125 | (61.9) | 29 | (14.4) | 48 | (23.8) | |
| Urothelial | 916 | (81.9) | 590 | (64.4) | 180 | (19.7) | 146 | (15.9) | |
| Primary site | 0.797 | ||||||||
| Nondome/urachal | 770 | (68.9) | 489 | (63.5) | 148 | (19.2) | 133 | (17.3) | |
| Dome/urachal | 348 | (31.1) | 226 | (64.9) | 61 | (17.5) | 61 | (17.5) | |
| Tumor size (in cm) | 0.051 | ||||||||
| ≥3 | 481 | (43.0) | 290 | (60.3) | 94 | (19.5) | 97 | (20.2) | |
| >3 | 637 | (57.0) | 425 | (66.7) | 115 | (18.1) | 97 | (15.2) | |
| Surgical margins status | 0.678 | ||||||||
| Positive | 220 | (19.7) | 140 | (19.6) | 38 | (18.2) | 42 | (21.6) | |
| Negative | 898 | (80.3) | 575 | (80.4) | 171 | (81.8) | 152 | (78.4) | |
LPC = laparoscopic partial cystectomy; OPC = Open partial cystectomy; RPC = robotic-assisted laparoscopic partial cystectomy.
Table 2.
Multivariable logistic regression model of positive surgical margins by patient and hospital characteristics
| Feature (Referent) | aOR (95% Cl) | P value |
|---|---|---|
| Age (< 65 y) | ||
| ≥ 65 y | 1.03 (0.72–1.47) | 0.880 |
| Sex (Male) | ||
| Female | 1.10 (0.78–1.55) | 0.575 |
| Race/ethnicity (Non-Hispanic white) | ||
| Non-Hispanic black | 1.93 (1.09–3.39) | 0.023 |
| Hispanic | 0.62 (0.22–1.76) | 0.367 |
| Other | 0.95 (0.50–1.83) | 0.880 |
| Chari son score (0) | ||
| 1 | 1.13 (0.79–1.62) | 0.505 |
| ≥2 | 0.84 (0.46–1.52) | 0.563 |
| Academic/Research program (Nonacademic) | ||
| Academic | 0.75 (0.54–1.05) | 0.091 |
| AJCC pathological T (Nonmuscle invasive) | ||
| Muscle invasive | 3.28 (2.00–5.37) | <0.001 |
| Grade (Low) | ||
| Intermediate | 0.84 (0.29–2.41) | 0.740 |
| High | 1.67 (0.63–4.44) | 0.306 |
| Histology (Urothelial) | ||
| Nonurothelial | 1.26(0.79–2.01) | 0.338 |
| Primary site (Nondome/urachal) | ||
| Dome/urachal | 0.67 (0.47–0.94) | 0.022 |
| Tumor size (< 3 cm) | ||
| ≥ 3 cm | 1.67(1.21–2.30) | 0.002 |
| Surgical approach at this facility (Open) | ||
| Laparoscopic | 1.06 (0.70–1.60) | 0.782 |
| Robotic | 1.28(0.85–1.91) | 0.235 |
Adjusted odds ratio (aOR), 95% confidence interval (95% CI).
Several patient and hospital covariables were independently associated with PSMs on multivariable analysis (Table 2). There were higher odds of PSMs in non-Hispanic blacks (aOR 1.93, 95%CI 1.09–3.39, P = 0.023) when compared to non-Hispanic whites, and in those patients with muscle invasive disease (aOR 3.28, 95%CI 2.00–5.37, P < 0.001) or tumor size ≥ 3 cm (aOR 1.67, 95%CI 1.21–2.30, P = 0.002). Tumors in a dome/urachal location had lower odds of PSMs than those tumors in nondome/urachal locations (aOR 0.67, 95%CI 0.47–0.94, P = 0.022). There was, however, no statistically significant association between the odds of PSMs and surgical approach (P > 0.05). Supplementary Table 3 shows the rate of PSMs for the tumor factors found to be predictive of surgical margin status. The rate of utilization of adjuvant systemic therapy in patients with PSMs is shown in Supplementary Table 4.
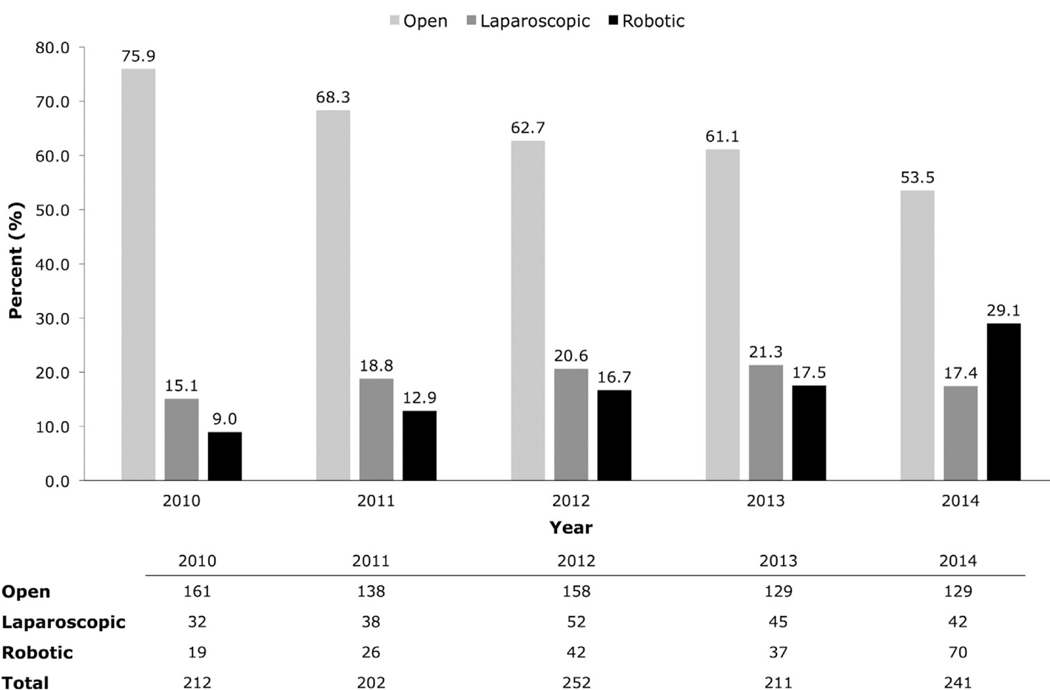
Temporal trends are shown in Fig. 2.From 2010 to 2014, the use of OPC significantly declined from 75.9% (161 cases) to 53.5% (129 cases) (P < 0.001), whereas the use of RPC significantly increased from 9.0% (19 cases) to 29.1% (70 cases) (P < 0.001). The use of LPC remained relatively constant over time (15.1% in 2010–17.4% in 2014; P = 0.431). Overall, the utilization of partial cystectomy ranged from 202 to 252 cases per year during this 5-year time period.
Fig. 2.
Yearly trend of surgical approach for partial cystectomy from 2010–2014.
4. Discussion
In the current study, we did not find a significant difference in the rate of PSMs between OPC, LPC, and RPC using hospital-level data from the NCDB. To our knowledge, our study is the first to assess surgical margin status by different surgical approaches for partial cystectomy. Our results suggest that tumor location (dome/urachal location vs. nondome/urachal locations) and disease biology, rather than surgical approach, are more relevant to surgical margin status.
In contrast to our study, case series have generally reported lower overall rates of PSMs for OPC, LPC, and RPC. The highest reported rates of PSMs for OPC and RPC have been 14.8% and 3.6%, respectively [8,9]. There have been no reports in the literature of PSMs for LPC. Among our cohort of patients, 19.6%, 18.2%, and 21.6% of patients had PSMs after OPC, LPC, and RPC, respectively. This discrepancy may be attributable to our larger sample size from a national hospital-based cohort, as compared to the previously published single-institutional cohort studies. In addition, it is likely that individual surgeon volume and experience play an important role in margin status. Unfortunately, the NCDB lacks unique surgeon identifiers, which precludes analysis of surgeon volume or experience. Nevertheless, the high frequency of PSMs reported herein should give pause to the use of bladder-sparing surgical procedures, especially when the rate of PSMs has been reported to be less than 5% for radical cystectomy [20].
The impact of PSMs on oncologic outcomes remains controversial. Shao et al. recently reported the outcomes of 27 partial cystectomies and found PSMs to be a significant predictor of local recurrence [8]. However, Holzbeierlein et al. reported the outcomes of 58 partial cystectomies and did not find surgical margin status to be a significant predictor of either local recurrence or distant metastasis [6]. Other studies have shown that PSMs are associated with inferior survival. Herr et al. reported their experience with 50 partial cystectomies for urachal carcinoma and found surgical margin status to be a significant predictor of cancer-free survival [7]. Bazzi et al. reported their experience with 36 partial cystectomies after neoadjuvant chemotherapy and found PSMs to be associated with worse recurrence-free and overall survival [21]. Our study only included cases of histologically confirmed cancer and further analysis of oncologic efficacy is needed after longer term survival data on this cohort of patients become available in the NCDB.
Racial differences in tumor biology may contribute to the increased risk of PSMs in African-American patients when compared to Caucasian patients. Using gene expression profiling, Kardos et al. found that African-American patients were more likely to develop the basal molecular subtype of urothelial carcinoma, which is known to exhibit more aggressive biology and be associated with worse overall survival [22,23]. Host genetic factors related to race have also been shown to influence tumor biology in prostate and breast cancer. Fowler et al. reported that African-American men have a greater propensity than Caucasian men for developing less differentiated and more aggressive prostate cancer [24]. Stark et al. reported a significantly greater prevalence of triple-negative breast cancer in African-American patients when compared to Caucasian patients [25]. Nongenetic factors may also contribute to the difference in risk of PSMs between African-American and Caucasian patients. Bach et al. reported that African-American patients were more likely to have been seen by primary care physicians without board certification and without access to high-quality diagnostic imaging and subspecialists [26]. Addressing these health disparities in bladder cancer remains a high priority and areas for future research should include attempts to better characterize the effect of race on tumor biology.
Our study covered a period when RPC was largely adopted into clinical practice. We found that the use of RPC increased from 9.0% in 2010 to 29.1% in 2014, along with a corresponding decrease in the use of OPC. We also found that RPC was more often performed on tumors < 3 cm (20.2%) than on tumors ≥ 3 cm (15.2%), while OPC was more often performed on tumors ≥ 3 cm (66.7%) than on tumors < 3 cm (60.3%); however, this association between tumor size and surgical approach was not statistically significant (P = 0.051). Nonetheless, this represents a potential bias toward selection of smaller tumors for minimally-invasive surgery.
The utilization of laparoscopy and robotic-assisted surgery for partial cystectomy has been intertwined with recent reports of refinements in surgical technique with the benefit of improved tumor identification and more complete resection. Kim et al. reported their experience with cystoscopic tattooing in 10 cases of LPC and found no cases of PSMs [27]. Hockenberry et al. discussed their technique of using near-infrared fluorescence without contrast agents in 3 cases of RPC [28]. Sood et al. reported the outcomes of 7 RPC using intraoperative frozen section assessment with a robotic ultrasound probe for tumor localization and found no cases of PSMs [29]. We acknowledge that the use of recent innovations in intraoperative tumor localization may represent an important tool in decreasing the incidence of PSMs after partial cystectomy. However, their use is currently not captured in the NCDB and further studies are warranted.
Our study has other limitations not previously mentioned above. One limitation is the retrospective cohort design, along with biases inherent in any analysis based on administrative databases. Another is the fact that the NCDB does not quantify the surgical margin; as a result, we could not analyze the impact of a wider resection of normal bladder tissue on the rate of PSMs. Of course, the lack of information regarding why partial cystectomy was chosen and whether there was local recurrence in the retained bladder limits the usefulness of our study in providing guidelines for prospective treatment of patients. Additional limitations include the fact that information on location of PSMs, presence of concomitant carcinoma-in-situ, and use of frozen sections is not coded for in the NCDB.
Nonetheless, our study identifies what is to our knowledge a previously underappreciated finding that partial cystectomy using the laparoscopic or robotic-assisted approach does not carry a higher risk of PSM compared to OPC. Data on surgical approach are only available up to 2014, and further analysis is warranted after data from later years are made available.
5. Conclusions
There was no statistically significant difference in the rate of PSMs between OPC, LPC, and RPC. Our results suggest that partial cystectomy using the laparoscopic or robotic-assisted approach does not carry a higher risk of PSMs and that tumor location and biology, rather than surgical approach, may be the important determinants of PSMs.
Supplementary Material
Acknowledgments
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary materials
Supplementary material associated with this article can be found in the online version at https://doi.org/10.1016/j.urolonc.2019.07.018.
References
- [1].Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- [2].Hollenbeck BK, Taub DA, Dunn RL, Wei JT. Quality of care: partial cystectomy for bladder cancer—a case of inappropriate use? J Urol 2005;174: 1050–4; discussion 4. [DOI] [PubMed] [Google Scholar]
- [3].Faiena I, Dombrovskiy V, Koprowski C, Singer EA, Jang TL, Weiss RE. Performance of partial cystectomy in the United States from 2001 to 2010: trends and comparative outcomes. Can J Urol 2014;21:7520–7. [PMC free article] [PubMed] [Google Scholar]
- [4].Chang SS, Bochner BH, Chou R, Dreicer RAM, Lerner SP, et al. Treatment of non-metastatic muscle-invasive bladder cancer: AUA/ASCO/ASTRO/SUO guideline. J Urol 2017;198:552–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].NCC Network. Bladder cancer: NCCN Clinical Practice Guideline in Oncology (NCN GuidelineTM) Version 2.2017. National Comprehensive Cancer Network; 2017. [Google Scholar]
- [6].Holzbeierlein JM, Lopez-Corona E, Bochner BH, Herr HW, Donat SM, Russo P, et al. Partial cystectomy: a contemporary review of the Memorial Sloan-Kettering Cancer Center experience and recommendations for patient selection. J Urol 2004;172:878–81. [DOI] [PubMed] [Google Scholar]
- [7].Herr HW, Bochner BH, Sharp D, Daibagni G, Reuter VE. Urachal carcinoma: contemporary surgical outcomes. J Urol 2007;178:74–8; discussion 8. [DOI] [PubMed] [Google Scholar]
- [8].Shao IH, Chang YH, Yu Kj, Lin PH, Liu cy, Chuang CK, et al. Outcomes and prognostic factors of simple partial cystectomy for local ized bladder urothelial cell carcinoma. Kaohsiung J Med Sci 2016;32: 191–5. [DOI] [PubMed] [Google Scholar]
- [9].Golombos DM, O’Malley P, Lewicki P, Stone BV, Scherr DS. Robot-assisted partial cystectomy: perioperative outcomes and early oncological efficacy. BJU Int 2017; 119:128–34. [DOI] [PubMed] [Google Scholar]
- [10].Allaparthi S, Ramanathan R, Balaji KC. Robotic partial cystectomy for bladder cancer: a single-institutional pilot study. J Endourol 2010; 24:223–7. [DOI] [PubMed] [Google Scholar]
- [11].James K, Vasdev N, Mohan-S G, Lane T, Adshead JM. Robotic partial cystectomy for primary urachal adenocarcinoma of the urinary bladder. Curr Urol 2014:8:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Mariano MB, Tefilli MV. Laparoscopic partial cystectomy in bladder cancer – initial experience. Int Braz J Urol 2004; 30:192–8. [DOI] [PubMed] [Google Scholar]
- [13].Wadhwa P, Kolla SB, Hemal AK. Laparoscopic en bloc partial cystectomy with bilateral pelvic lymphadenectomy for urachal adenocarcinoma. Urology 2006;67:837–43. [DOI] [PubMed] [Google Scholar]
- [14].Colombo JR, Desai M, Canes D, Frota R, Haber G-P, Moinzadeh A, et al. Laparoscopic partial cystectomy for urachal and bladder Cancer. Clinics 2008; 63:731–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Thyavihally YB, Tongaonkar HB. Outcomes after laparoscopic partial cystectomy and bilateral pelvic lymph adenectomy in urachal adenocarcinoma of bladder. J Urol 2008; 179:240.18001789 [Google Scholar]
- [16].Cole AP, Trinh QD, Sood A, Menon M. The rise of robotic surgery in the new millennium. J Urol 2017;197:S213–s5. [DOI] [PubMed] [Google Scholar]
- [17].Knoedler J, Frank I. Organ-sparing surgery in urology: partial cystectomy. Curr Opin Urol 2015;25:111–5. [DOI] [PubMed] [Google Scholar]
- [18].Percy C, Shanmugaratnam K, Whelan S, Parkin DM, Jack A, Fritz A, et al. International Classification of Diseases for Oncology (ICD-O). 3rd ed. Geneva: World Health Organization; 2001. [Google Scholar]
- [19].Phillips JL, Stewart AK. Facility Oncology Registry Data Standards (FORDS): revised for 2016. American College of Surgeons; 2002. [Google Scholar]
- [20].Dotan ZA, Kavanagh K, Yossepowitch O, Kaag M, Olgac S, Donat M, et al. Positive surgical margins in soft tissue following radical cystectomy for bladder cancer and cancer specific survival. J Urol 2007;178:2308–12; discussion 13. [DOI] [PubMed] [Google Scholar]
- [21].Bazzi WM, Kopp RP, Donahue TF, Bernstein M, Russo P, Bochner BH, et al. Partial cystectomy after neoadjuvant chemotherapy: memorial sloan kettering cancer center contemporary experience. Int Sch Res Notices 2014;2014:702653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Kardos J, Melquist JJ, Chism DD, Choi W, Cockerill K, Paluri RK, et al. Evaluation of basal and luminal subtypes of urothelial carcinoma in African American and non-African American patients. J Clin Oncol 2015;33:305. [Google Scholar]
- [23].Prout GR, Wesley MN, Greenberg RS, Chen VW, Brown CC, Miller AW, et al. Bladder cancer: race differences in extent of disease at diagnosis. Cancer 2000;89: 1349–58. [DOI] [PubMed] [Google Scholar]
- [24].Fowler JE, Bigler SA, Bowman G, Kilambi NK. Race and cause specific survival with prostate cancer: influence of clinical stage, Gleason score, age and treatment. J Urol 2000; 163:137–42. [DOI] [PubMed] [Google Scholar]
- [25].Stark A, Kleer CG, Martin I, Awuah B, Nsiah-Asare A, Takyi V, et al. African ancestry and higher prevalence of triple-negative breast cancer: findings from an international study. Cancer 2010;116: 4926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Bach PB, Pham HH, Schrag D, Tate RC, Hargraves JL. Primary care physicians who treat blacks and whites. N Engl J Med 2004;351: 575–84. [DOI] [PubMed] [Google Scholar]
- [27].Kim BK, song MH, Yang HJ, Kim DS, Lee NK, Jeon YS. Use of cystoscopic tattooing in laparoscopic partial cystectomy. Korean J Urol 2012;53:401–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Hockenberry MS, Smith ZL, Mucksavage P. A novel use of near-infrared fluorescence imaging during robotic surgery without contrast agents. J Endourol 2014;28:509–12. [DOI] [PubMed] [Google Scholar]
- [29].Sood A, Klett DE, Abdollah F, Sammon JD, Pucheril D, Menon M, et al. Robot-assisted partial cystectomy with intraoperative frozen section examination: evolution and evaluation of a novel technique. Investig Clin Urol 2016;57:221–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.