Abstract
Simple Summary
Evidence indicates that recurrence risk after colon cancer today is less than it was when trials performed decades ago showed that adjuvant chemotherapy reduces the risk and prolong disease-free and overall survival. After rectal cancer surgery, local recurrence rates have decreased but it is unclear if systemic recurrences have. After a systematic review of available literature reporting recurrence risks after curative colorectal cancer surgery we report that the risks are lower today than they were in the past and that this risk reduction is not solely ascribed to the use of adjuvant therapy. Adjuvant therapy always means overtreatment of many patients, already cured by the surgery. Fewer recurrences mean that progress in the care of these patients has happened but also that the present guidelines giving recommendations based upon old data must be adjusted. The relative gains from adding chemotherapy are not altered, but the absolute number of patients gaining is less.
Abstract
Adjuvant chemotherapy aims at eradicating tumour cells sometimes present after radical surgery for a colorectal cancer (CRC) and thereby diminish the recurrence rate and prolong time to recurrence (TTR). Remaining tumour cells will lead to recurrent disease that is usually fatal. Adjuvant therapy is administered based upon the estimated recurrence risk, which in turn defines the need for this treatment. This systematic overview aims at describing whether the need has decreased since trials showing that adjuvant chemotherapy provides benefits in colon cancer were performed decades ago. Thanks to other improvements than the administration of adjuvant chemotherapy, such as better staging, improved surgery, the use of radiotherapy and more careful pathology, recurrence risks have decreased. Methodological difficulties including intertrial comparisons decades apart and the present selective use of adjuvant therapy prevent an accurate estimate of the magnitude of the decreased need. Furthermore, most trials do not report recurrence rates or TTR, only disease-free and overall survival (DFS/OS). Fewer colon cancer patients, particularly in stage II but also in stage III, today display a sufficient need for adjuvant treatment considering the burden of treatment, especially when oxaliplatin is added. In rectal cancer, neo-adjuvant treatment will be increasingly used, diminishing the need for adjuvant treatment.
Keywords: colorectal cancer, colon cancer, rectal cancer, chemotherapy, adjuvant treatment, recurrence risk, systematic overview
1. Introduction
Colorectal cancer (CRC) is the fourth most common cancer worldwide and the number two cause of cancer death [1]. In early stages (stages I–III), constituting 75–80% of newly diagnosed cases, adjuvant chemotherapy is often administered since it may kill sub-clinical disease and, thereby, decrease the risk of recurrence and improve survival. After colon cancer surgery, it is routine therapy in stage III and in stage II with risk factors for recurrence [2,3,4,5,6], whereas it is less established in rectal cancer, particularly if pre-operative radiotherapy/chemoradiotherapy (RT/CRT) has been administered. The randomized rectal cancer trials have not unequivocally shown enough benefit [7,8]. Despite this, some guidelines recommend the same treatment as in colon cancer [9]. Increased use of adjuvant chemotherapy is one reason for the improved overall survival (OS) for CRC patients observed in cancer registries [10]. Other reasons are linked to screening/earlier detection, improved general health allowing preoperative treatments and surgery in more patients and improved pre- and postoperative care [11,12,13,14,15,16,17,18].
Evidence indicates that the recurrence risks after CRC surgery have decreased due to improvements in care other than that provided by adjuvant therapy [19,20]. Better staging with computed tomography (CT) and magnetic resonance imaging (MRI) of the thorax, abdomen and pelvis will detect smaller distant metastases, resulting in fewer recurrences in those operated [14]. Improved surgical techniques, both in rectal cancer [21], and later also in colon cancer [14,22,23], further reduce recurrence risks. Improved examination of the surgical specimen does not per se reduce recurrence risks, but results in fewer recurrences in each pathological stage, i.e., stage migration [24,25]. An international congress in 1990 recommended that at least 12 nodes should be investigated to properly define the TN-stage [26]. With time, the quality of the pathological examinations, including reporting of the number of investigated nodes, has improved [27]. High numbers of investigated nodes correlate with better outcome in both stage II and III [28,29,30].
In an analysis of the Adjuvant Colon Cancer Endpoints (ACCENT) collaborative group database, recurrence risks were several percentage points lower in patients enrolled since 2000 compared with those enrolled earlier and shown in a nomogram based upon trial data, Adjuvant! Online [31]. It was stated that “the last 15 years have produced an overall improvement in patient outcomes due to a number of different factors including optimization of surgery”, and that “many calculators (of recurrence risk) reflect older practice”. Similar results were seen in another ACCENT study [32], noting signs of stage migration in stage II but not in stage III, with improved outcomes in newer-era trials (initiated patient inclusion between 1995–2000 vs 1978–1993). They “called into question historical data related to the benefit of FU-based adjuvant therapy in such (stage II) patients”. In a third ACCENT database analysis [33], patients did better in the trials including patients between 2004–2009 than in those including patients between 1998–2003, but this was probably explained by better possibilities of tolerating oxaliplatin-containing chemotherapy than reflecting decreased recurrence risks as in the two previous analyses. In 2008, The Memorial Sloan Kettering Cancer Center (MSKCC, www.mskcc.org) published a nomogram describing the risk of recurrence and survival in colon cancer stages I–III based upon patients operated between 1990–2000 [34]. Although validated by others [35,36,37], lower recurrence risks were seen in an update in patients operated between 2007–2014 [38]. In a recent secondary analysis of an adjuvant trial exploring the potential benefit of adding bevacizumab to chemotherapy, the AVANT trial, excellent results were seen suggesting that “the definition of high-risk stage II needs to be revisited” [39].
This review aims at describing the present recurrence rates in patients radically operated for a primary non-metastatic CRC, i.e., that they have cancer cells that will develop into metastases provided the patient lives long enough and does not receive adjuvant treatment. This question is important for many patients and their doctors approximately one month after surgery has taken place. In stage II without any risk factors, adjuvant treatment is not given, but one must ask whether the recurrence risk is sufficiently high to merit treatment if one or two risk factors are present, when guidelines recommend therapy [4,9,40]? Do all stage III patients have such a high risk that an oxaliplatin combination is motivated? When is the risk of recurrence in stage III so high that six months of oxaliplatin is motivated? The elderly and patients with co-morbidities may need better knowledge to weigh the increased risks against the benefits involved [41].
Due to the lack of firm data in literature, a second aim of this review is to describe stage-specific recurrence risks in a Swedish CRC population between 2010 and 2018 where validation of whether patients in stage I–III are recurrence-free or not has been carried out.
2. Methodological Considerations
2.1. Time-To Event Endpoints
In adjuvant trials, the gains have been recorded as improved OS, being the ultimate goal of these interventions, or as disease-free survival (DFS), balancing gains (fewer recurrences) with significant losses (any deaths and secondary malignancies) [42,43]. However, adjuvant chemotherapy cannot improve OS or DFS without reducing the recurrence risk. Thus, recurrence risk (or freedom from recurrence, FFR) or improved time to recurrence (TTR) is probably the most direct measure of the effect of adjuvant chemotherapy. However, this is rarely recorded in trials and has been used as a primary endpoint in only one trial [44]. TTR ought also to be the most informative endpoint in tumour marker studies but is again rarely used. Although neither TTR nor FFR are ideal methods of estimating the risk of remaining sub-clinical tumour cells, they are more adequate than other time-to-event outcomes. However, even if all endpoints including deaths (even toxic deaths) tend to overestimate “this need”, all endpoints not including deaths may underestimate the risk if the death occurs before the event, i.e., the recurrence. This competing risk is most pronounced in elderly patients but not in the average trial patient where median age lies between 62–65 years. However, most recurrences come early or within the first 3–4 years whereas short-term survival even for very old persons who have undergone major CRC surgery is clearly longer [41]. Toxic deaths are fortunately rare and, in most studies, less than one per cent [45,46]. In the estimation of TTR, all deaths except death from the same cancer, here CRC, are censored [42,43], making TTR a better estimate than FFR estimated from the crude number of recurrences after a specific time. Most of the older adjuvant trials published, besides OS, only report crude recurrence rates. This is also the case in several more recent studies of surveillance strategies or surgical techniques (to be described below) Recurrence-free survival (RFS) also includes all deaths, but not secondary other malignancies [42,43]. With time, secondary malignancies included in DFS but not in RFS increasingly contribute to the number of events in an aged CRC population [47]. The relative importance of secondary malignancies will also increase when recurrences become fewer [48]. Although endpoints have been clearly defined, they have not always been used properly, hampering any literature evaluation.
2.2. Representativity
Patients included in clinical trials are not representative of the background population [49,50,51]. Population-based registries seldom include recurrence data, and if they do, they are unreliable, underestimating the risks [52,53]. For example, in two US health care databases, only 7% recurrences were registered in a mixed population of CRC patients operated between 1995–2014 [54]. Lack of registration may also reflect difficulties involved with adequate follow-up. In a large prospective cohort of 15,096 colon cancer patients with stage I + II from 346 German hospitals, only 68% of the patients had a satisfactory follow-up [55]. In the study, 5% developed a local relapse and 10% developed distant metastases, numbers that apparently are too low considering that recurrence risks are generally at least twice as high. OS is properly recorded in registries but overestimate the need for adjuvant therapy since many deaths in individuals with a median age of 70 years (62 in the trials) can never be prevented by adjuvant chemotherapy.
2.3. Selective Delivery of Adjuvant Therapy
Since adjuvant chemotherapy is today routine therapy for many patients with colon cancer [4,40], the recurrence risks in patient cohorts collected during the past decades being lower than if this had not been administered. This will falsely decrease the need. Furthermore, patients not treated with adjuvant therapy are selected, often to a poorer alternative with poorer OS and DFS than patients selected for treatment [56]. Whether recurrence risks (or TTR) are also biased is not known. The situation is slightly different in rectal cancer where there is less evidence of favourable effects, and adjuvant therapy is not always given [2,57,58]. In rectal cancer, two recent randomized trials have included a postoperatively non-treated group providing up-to-date recurrence rates after preoperative treatment [59,60]. In colon cancer, a surgery alone group has since the 1990s only been included in one Japanese study including stage II patients [48].
Recent trials on surveillance strategies or comparisons of different surgical techniques are a source of information, but if recent, they are performed at hospitals providing adjuvant chemotherapy for sub-groups. Multiple prognostic studies are published yearly, providing outcome data according to different tumour or patient characteristics but they practically only report OS or DFS.
The methodological difficulties in estimating the recurrence risk in populations where interventions other than providing adjuvant chemotherapy (chiefly staging and surgery) have improved the outcomes were noted in an attempt made five years ago to produce a systematic overview of the recurrence risks reported in modern series. In the study, 25 out of 2596 randomized or observational studies published after January 2005 and enrolling patients after January 1995 provided reasonable information about the quality of the care and disease outcome [19]. Since TTR or FFR were seldom reported, conclusions were based on DFS or, occasionally RFS.
In our ambition to produce an updated systematic overview of the recurrence risks (or FFR/TTR), we relied on previous systematic overviews in identifying relevant studies. If needed, we made an update, using the same search criteria as in the overviews, as briefly described in Table 1, Table 2, Table 3, Table 4, Table 5 and Table 6.
Table 1.
Time to recurrence rate/freedom from recurrence and recurrence-free and disease-free survival in adjuvant predominantly colon cancer trials with a control group where systemic chemotherapy was given in the experimental group(s).
| Trial/Reference | Inclusion Years | Number Control Pts/Total Number Pts | Number of Control Patients in Stage I/II/III | Colon/Rectum | Proportion Receiving ACT | Follow-Up Time (Years) |
FFR/TTR | RFS/EFS | DFS | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| VASOG [61] | 1973–1979 | 318/645 | 0/182/136 | NR, majority colon cancer | 0 | 5 | 70% | |||
| Hafström et al. [62] | 1976–1978 | 205/421 | 0/98/75 | 141/64 | 0 | II: 75% III: 45% |
||||
| NCCTG [63] | ?-1985 | 135/401 | 0/49/86 | 127/8 | 0 | 5 | All: 44% II: 59%, III: 35%, |
|||
| Dahl et al. [64] | 1993–1996 | 206/412 | 0/126/80 | 146/65 | 0 | 5 | All: 63% III: 37% |
|||
| QUASAR uncertain [46] | 1994–2003 | 1143/2291 | 0/1073/70 | 100/0 | 0 | 5 | 75% | Stage II at 10 years projected TTR 71% | ||
| O’Connell et al. [65] | 1988–1989 | 151/309 | 0/27/124 | 124/27 | 0 | 5 | 58% | |||
| Francini et al. [66] | 1985–1990 | 118/239 | 0/60/58 | 100/0 | 0 | 5 | 41% | |||
| Moertel et al. [67] | 1984–1987 | 315/1296 | 0/?/315 | 100/0 | 0 | 3.5 | All: 47% II: 67% | |||
| Moertel et al. [68] | 1984–1987 | 0/0/929 | 100/0 | 0 | 5 | 45% | At 8 years projected FFR 40% | |||
| GIVIO-SITAC 01 [69] | 1989-? | 446/869 | 0/228/218 | 100/0 | 0 | 5 | 54% | |||
| Taal et al. [70] | 1990–1996 | 575/1029 | 0/235/280 | 280/235 | 0 | 5 | All: 55% III: 46% |
49% | 76% of the recurrences were distant | |
| SWOG, Panettiere et al. [71] | 1977–1988 | 94/317 | NR | 80/14 | 0 | 7 | 44% | 44% | ||
| Windle et al. [72] | NR–1970 s | 45/141 | NR | 26/19 | 0 | 5 | 48% | |||
| IMPACT-1 [73] | 1982-? | 757/1493 | 0/423/334 | 100/0 | 0 | 3 | All: 62% II: 76% III: 44% |
FFCD, GIVIO, NCIC-CGT trials | ||
| NSABP-C-01–05, Wilkinson et al. [74] | 1977–1994 | 693/2966 | Stage II, 51% | 100/0 | 0 | 5 | II: 77%, III: 52% | 60% | 50% | Pooled data from 5 trials. FFR at 10 years stage II 73%, stage III 44%. Less than half <12 nodes |
| IMPACT-2 [75] | 1982-? | 509/1116 | 0/509/0 | 100/0 | 0 | 5 | 73% | FFCD, GIVIO, NCIC-CGT, NCCTG Intergroup trials | ||
| Matsuda et al. [48] | 2006–2010 | 997/1982 | 0/997/0 | 100/0 | 0 | 5 | 87% | 85% | 78% | 78% 12+ nodes, median 19. The worse DFS than RFS (and FFR) is mainly caused by secondary malignancies |
| Li and Ross [76] | 1960–1965 | 84/213 | 53/41 | NR | 0 | 5 | II: 59%, III: 24% | Historical controls |
These trials were identified in a meta-analysis/systematic overview [77] and only one further study using a surgery only group was identified [48]. The key publications for all trials were scrutinized to find information of recurrence rates (or TTR) and not only DFS or OS as presented in the overview. Abbreviations: DFS = disease-free survival, OS = overall survival, RFS = recurrence-free survival, EFS = event-free survival, TTR = time to recurrence, FFR = freedom from recurrence (100-crude recurrence rate in % as provided in the articles), ACT = adjuvant chemotherapy, NR or ? = not reported. In the individual trials, RFS/DFS is only presented if FFR/TTR was not available.
Table 2.
Time to recurrence rate/freedom from recurrence and disease-free survival in adjuvant colorectal cancer trials with a control group where regional chemotherapy or miscellaneous treatments were administered in the experimental group(s).
| Trial/Reference | Inclusion Dates | Number Control Pts/Total Number Pts | Number of Patients in Stage I/II/III | Colon/ Rectum |
Proportion Receiving ACT | Follow-Up Time (Years) | FFR/TTR | DFS | Comments |
|---|---|---|---|---|---|---|---|---|---|
| SAKK et al. [78] | 1981–1987 | 266/533 | 0/157/79 | 161/92 | 0 | 5 | 55% | All: 48% II: 63% III: 29% |
|
| Scheithauer et al. [79] | 1998–1990 | 60/121 | 0/31/29 | 60/0 | 0 | 4.5 | 58% | Intraperitoneal and intravenous | |
| Vaillant et al. [80] | 1986–1991 | 134/267 | 0/77/57 | 134/0 | 0 | 5 | 69% | All: 62% II: 69% |
|
| Rougier et al. [81] | 1987–1993 | 619/1235 | 113/262/217 | 367/232 | 0 | 5 | 73% | 65% | |
| Wolmark et al./NSABP C02 [82] | 1984–1988 | 459/901 | 114/202/140 | 459/0 | 0 | 4 | 64% | ||
| AXIS [83] | 1989–1987 | 1792/3583 | 186/707/514 | 1018/774 | 0 | 5 | 55% | DFS colon 57%, rectum 51%, if curative resection 64% |
|
| EORTC-GITCCG [84] | 1983–1987 | 79/235 | 6/41/23 | 72/0 | 0 | 9 | 60% | 48% | |
| Lawrence et al. [85] | 1973–1975 | 101/203 | ?/64/37 | 62/39 | 0 | 5 | 51% | Stage I, II not separated | |
| Wereldsma et al. [86] | 1981–1984 | 102/372 | NR | 58/44 | 0 | 3.7 | 58% | Rotterdam trial, only OS data | |
| Irvin et al. [87] | NR | 65/128 | 5/29/33 | 38/29 | 0 | 5 | 66% | Only liver metastases reported | |
| Hanna et al. [88] | 1980-? | 159/233 | NR | NR | 0 | 5 | 68% | Vaccination | |
| Hanna et al. [88] | 1980-? | 217/324 | NR | NR | 0 | 5 | 62% | Vaccination, unclear reporting | |
| Riethmuller et al. [89] | 1985–1990 | 76/189 | 0/0/76 | 100/0 | 0 | 5 | 42% | 38% | Treatment with 17-1 A antibody |
| CALGB 9581 [90] | 1997–2002 | 873/1738 | 0/873/0 | 100/0 | 0 | 7 | 83 (81–85)% | 74 (72–76)% | Surgery +/− 17-1 A antibody. Note the difference in TTR (designated disease-specific DFS) and DFS at 7 years |
These trials were identified in two meta-analysis/systematic overviews [77,91] and no further studies using a surgery only group were identified when the same search strategies were used as in [77,91]. The key publications for all trials were scrutinized to find information of recurrence rates and not only DFS or OS as presented in the meta-analyses. Abbreviations: DFS = disease-free survival, OS = overall survival, TTR = time to recurrence, FFR = freedom from recurrence (100-recurrence rate in % as provided in the articles), ACT = adjuvant chemotherapy, NR or ? = not reported.
Table 3.
Time to recurrence rate/freedom from recurrence and recurrence-free or disease-free survival in adjuvant trials in rectal cancer with a surgery alone group and where systemic chemotherapy was provided in the experimental group.
| Trial/Reference | Inclusion Dates | Number Control Pts/Total Number Pts | Number of Patients in Stage I/II/III | Proportion Receiving ACT | Preoperative Treatment |
Follow-Up Time (Years) |
TTR/FFR | RFS/DFS | Comments |
|---|---|---|---|---|---|---|---|---|---|
| GITSG [92] | 1975–1980 | 62/227 | 0/21/37 | 0 | None | 5 | II: 67% III: 32% |
Before TME | |
| NSABP R01 [93] | 1977–1986 | 179/555 | 0/67/109 | 0 | None | 5 | 29% | Before TME, DM risks given in [94] | |
| Gunderson et al., 5 US trials pooled [94] | 1977–1986 | 179/3791 | 0/67/109 | 0 | None | 5 | II: ~60% III: ~40% |
Present DM rates of the NSABP-trial, 40% pT1–2 N+, 60% pT3 N1, 34% pT3 N0, 59% pT3 N2 | |
| QUASAR uncertain [46] | 1994–2003 | 474/948 | 0/407/67 | 0 | 6% neo-RT (8% adj-RT) |
5 | 68% | Before TME, projected 5 year | |
| EORTC 22921 [95] | 1993–2003 | 505/1011 | NR | 0 | RT or CRT | 5 | 65% | 52% | 90% T3, 10% T4. 34% DM overall |
| Gerard et al., FFCD [96] | 1993–2003 | 0/742 | 87% T3, 13% T4 | 70% | RT or CRT | 5 | 57% | Before TME, LR 17% RT vs 8% CRT, adjuvant chemo planned both groups | |
| PROCTOR/SCRIPT [59] | 2000–2013 | 221/437 | 0/32/189 | 0 | 5 × 5 or CRT | 5 | 60% | Systemic recurrence 39%, local 8% | |
| Chronicle [60] | 2004–2008 | 59/113 | 31/28 | 0 | CRT | 3 | 73% | ||
| Stockholm III [97] | 1998–2013 | 920 | NR | About 15% | 5 × 5 direct or delayed surgery, RT 2 × 25 | 5 | projected 79% | 65% | Intermediate risk tumors. ACT only recorded in patients included from 2007. ypTN I/II/III/IV/X = 271/250/278/25/11 |
| Bujko et al., Polish I trial [98,99] | 1999–2002 | 316 | 170/113 | NR | 5 × 5 direct surgery or CRT | 4 | 67% | 57% | Locally advanced, low-lying |
| Polish II trial [100] | 2008–2014 | 254/515 | NR | 39% | CRT | 8 | 67% | 41% | TNT= FF-DM |
| RAPIDO [101] | 2011–2016 | 452/920 | 42% | CRT | 3 | 73% | 70% | Locally advanced, ugly tumours, TNT provided in experimental group, RFS/DFS = DrTF, TTR/FFR = FF-DM | |
| PRODIGE 23 [102] | 2012–2017 | 230/461 | 69% | CRT | 3 | 72% | 69% | TNT in experimental group, RFS/DFS = FF-DM | |
| Valentini et al., Five European trials pooled [103] | 1993–2003 | 1209/2795 | 1879/833 | 56% | RT/CRT | 5 | Distant all 69%, ypN0 79%, ypN1–2 48%, local all 87% | Created a nomogram. ACT limited effect. Few events after 5 to 10 years (distant all from 69% to 66%) | |
| Bregoum et al. [7] | 1992–2013 | 598/1196 | 207/391 | 0 | 5 × 5 or CRT | 5 | 63% | Meta-analysis 4 trials, TME, FF-DM |
The old trials were identified in one meta-analysis/systematic overview [77] and the more recent ones in three overviews/meta-analysis [7,8,103] and four further studies where the recurrence risk could be described after preoperative RT [97] or CRT [98,99,100,101] were identified. The key publications for all trials were scrutinized to find information of recurrence rates and not only DFS or OS as mostly presented in the overviews. Abbreviations: DFS = disease-free survival, OS = overall survival, RFS = recurrence-free survival, EFS = event-free survival, TTR = time to recurrence, FFR = freedom from recurrence (100-recurrence rate in % as provided in the articles), TME = total mesorectal excision, DM = distant metastasis, RT = radiotherapy, CRT = chemoradiotherapy to 46–50 Gy, 5 × 5 = 5 times 5 Gy radiotherapy in one week, FF-DM = freedom from distant metastasis, DrTF = disease-related treatment failure, ACT = adjuvant chemotherapy, TNT total neoadjuvant treatment evaluated in the experimental arm.
Table 4.
Time to recurrence rate/freedom from recurrence and disease-free survival in studies comparing different follow-up routines in colorectal cancer.
| Trial/Reference | Inclusion Years | Total Number of Pts | Number of Patients in Stage I/II/III | Colon/Rectum | Proportion Receiving ACT |
Follow-Up Time (Years) | TTR/FFR | DFS | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Kjeldsen et al. [104] | 1983–1994 | 597 | 138/293/166 | 313/284 | 0 | 5 | 68% | Recurrence risk 13% stage I, 20% stage II, 48% stage III, slightly higher in rectum than in colon | |
| Ohlsson et al. [105] | NR | 107 | 0 | 5 | 67% | Limited information provided | |||
| Mäkelä et al. [106] | 1988–1990 | 106 | 28/48/30 | 75/31 | 0 | 5 | 59% | Recurrence risk 36% stage I, 38% stage II, 50% stage III | |
| Secco et al. [107] | 1988–1996 | 358 | ?/201/137 | 0/358 | 0 | 5 | 45% | Did not separate stage I + II | |
| Schoemaker et al. [108] | 1984–1990 | 325 | 71/153/101 | 238/87 | 0 | 5 | 67% | Median number of nodes = 7 | |
| Rodriguez-Moranta et al. [109] | 1988–2001 | 259 | 0/157/102 | 194/65 | 100% | 4 | 73% | ||
| GILDA [110] | 1998–2006 | 1228 | 0/617/611 | 933/295 | 85% | 5 | 80% | 75–82% | DFS about 73% at 8 years |
| COLOFOL [111] | 2006–2010 | 2555 | 0/1352/1203 | 1671/884 | 47% | 5 | 78% | NR | 5-year cancer-specific survival stage II 93%, stage III 84% |
| FACS [112,113,114] | 2003–2009 | 1202 | 254/553/354 | 843/359 | 41% | 4.4 | 83% | Recurrence risk 16% colon, 24% rectum, 9% stage I, 16% stage II, 27% stage III |
The trials were included in a systematic review published in 2015 [115]. Three trials [116,117,118] did not provide any meaningful recurrence data. Using the same search criteria, one additional study [111] was found. Abbreviations: TTR/FFR: time to tumour recurrence/freedom from recurrence (100-recurrence rate in % as provided in the articles), DFS = disease-free survival, ACT= adjuvant chemotherapy, NR or ? = not reported.
Table 5.
Time to recurrence rate/freedom from recurrence and disease-free survival in studies comparing open vs laparoscopic surgery or studies where patients were operated with a circumferential mesocolic resection (CME).
| Trial/Reference | Inclusion Years | Total Number of Pts | Number of Patients in Stage I/II/III | Colon/Rectum | Proportion Receiving ACT |
Follow-Up Time (Years) | TTR/FFR | DFS | Comments |
|---|---|---|---|---|---|---|---|---|---|
| Cochrane et al. [119] | NR | 3346 | NR | 2518/828 | NR | NR | 86–94% | Meta-analysis 7 trials, short follow-up, many likely adjuvant/neo-adjuvant treatment | |
| Liang et al [120] | 1997–2006 | 2474 | NR | NR | NR | At least 2 | 85% | Meta-analysis 10 trials, local recurrence 5%, distant 11% | |
| Ng et al. [121] | NR | 169,236 | NR | NR | NR | NR | 86% | Meta-analysis 73 trials, 6 RCTs | |
| Lacy et al. [122] | 1993–1998 | 219 | 45/90/73 | 100/0 | 58% | 7.5 | 77% | Majority locoregional recurrences | |
| Leung et al. [123] | 1993–2002 | 403 | 59/145/133 | 0/100 | 21% (II: 12%, III: 55%) |
5 | 80% | 77% | Distant metastasis in 17% |
| Tan et al. [124] | 2005–2009 | 633 | 119/166/246 | 0/100 | 34% | 5 | 63% | 65% | No RCT, median 14 lgll, 5% preop RT/CT |
| CLASSIC [125] | 1996–2002 | 794 | 132/281/288 | 413/381 | 28% | 3 | Distant 85%, local 92% | 67% | C: 12%, R: 17%, LR C: 7%, R:10% |
| Liang et al. [126] | 2000–2004 | 286 | 0/132/137 | 100/0 | NR | 3 | II: 85% III: 76% |
Additionally, 4% had recurrences after 3 years | |
| ACOSOG Z6051 [127] | 2008–2013 | 242 | 2/99/141 | 0/100 | 46% | 4 | 84% | preoperative CRT 86% | |
| COLOR II [128] | 2004–2010 | 1044 | 338/271/358 | 0/100 | NR | 3 | 80% | 73% | preop RT/CRT 60% |
| ROLARR, Jayne et al. [129,130] | 2011–2014 | 471 | 132/296/175 | NR | 47% | 3 | 85% | 76% | Robotic vs conv laparoscopy, 46% preoperative treatment |
| Storli et al. [131,132] | 2007–2010 | 251 | 60/117/74 | 100/0 | NR | 3 | 87% | 77% | CME, 83% 12+ nodes, TTR stage I 95%, stage II 93%, stage III 70% |
| Shin et al. [133] | 2006–2009 | 168 | 0/87/81 | 100/0 | 54% | 5 | 92% | 88% | CME, 94% 12+ nodes, RR 5% stage II, 12% stage III |
Multiple meta-analyses have been identified exploring various aspects of the outcomes after open vs laparoscopic surgery, whether performed conventionally or more lately as robotic surgery [120,121,134,135,136,137]. The far majority have only reported short-term outcomes. The studies included above are the largest trials reporting reasonably long follow-up times and risk of recurrence. No additional trials were identified. Abbreviations: TTR/FFR: time to tumor recurrence/freedom from recurrence (100-recurrence rate in % as provided in the articles), DFS = disease-free survival, RR: recurrence risk, CME = circumferential mesocolic excision, RCT = randomized clinical trial, NR = not reported, RT = radiotherapy, CRT = chemoradiotherapy, ACT = adjuvant chemotherapy, LR = local recurrence, C = colon cancer, R = rectal cancer, lgll = lymph nodes.
Table 6.
Time to recurrence rate/freedom from recurrence, recurrence- and disease-free survival in surgical or population-based series of colon or rectal cancer.
| Trial/First Author | Inclusion Dates | Total Number Pts | Number of Patients in Stage I/II/III | Colon/ Rectum |
Proportion Receiving ACT |
Follow-Up Time (Years) | TTR/FFR | RFS/ EFS |
DFS | Comments |
|---|---|---|---|---|---|---|---|---|---|---|
| Konishi et al., MSKCC early [38] | 1990–2000 | 1320 | 421/520/379 | 100/0 | II: 14% III: 85% |
5 | II: 81% III: 64% |
61% 12+ nodes, created a nomogram | ||
| Konishi et al., MSKCC late [38] | 2007–2014 | 1095 | 286/425/384 | 100/0 | II: 25%, III: 89% |
5 | II: 89%; III: 72% | 85% | 97% 12+ nodes, created a new nomogram because of better results | |
| Collins et al. [35] | 2000–2005 | 134 | 19/90/25 | 100/0 | 46% | 5 | 73% | 48% | Validated the early MSK nomogram, projected | |
| Kazem et al. [37] | 1998–2003 | 138 | 0/10/128 | 100/0 | 30% | 5 | 73% | Validated the early MSK nomogram | ||
| Merkel and Erlangen [138] | 1981–1997 | 305 | 0/305/0 | 100/0 | 0 | 5 | 85% | Well documented quality of the surgery. A small high-risk group identified | ||
| Touchefeu et al. [139] | 2003–2009 | 195 | 0/195/0 | 100/0 | 17% | 3 | 89% | 83% | 93% 12+ nodes, DFS projected | |
| Yamanaka et al. [140] | 2000–2005 | 1487 | 0/1010/564 | 100/0 | 0 | 5 | All 83%, II: 89%, III: 74% | 12 hospitals | ||
| Lavanchy et al. [141] | 2002–2013 | 475 | 94/118/98 | 334/141 | 29% | 5 | 88% | Unclear if RFS or DFS, 165 pts stage IV | ||
| Wanis et al. [142] | 2006–2015 | 1180 | 233/503/444 | 100/0 | 31% | 5 | 84% | 83% | If emergency surgery TTR:71% | |
| Tsikitis and Mayo [143] | 1995–2007 | 1136 | 0/871/265 | 100/0 | II: 20%, III: 72% | 5 | II: 90%, III: 70% |
Mean 17 nodes sampled. Intensive follow-up | ||
| Amri et al., MGH [144] | 2004–2011 | 313 | 0/313/0 | 100/0 | 0 | 5 | 88% | TTR 7% 0–1 risk factor (CEA, high grade, PNI, EMVI, n = 124), 18% if 2–3 risk factors, (n = 50), 25% if all 4 (8 patients), 90% 12+ nodes | ||
| Gertler et al. [145] | 1982–2006 | 492 | 0/492/0 | 100/0 | 0 | 10 | 84% | 85% had RFS 87%, 15% RFS 75%, 83% 12+ nodes. Most patients operated after 1996 | ||
| Kumar et al. [146] | 1999–2008 | 1697 | 0/1697/0 | 100/0 | Low risk: 12%, High risk: 29% |
3 | High-risk group (n = 1286), RFS 79 vs 80% if AC/no AC, Low risk-group (n = 411), 84% RFS if AC, 93% if no AC. No overall data presented | |||
| Tersteeg et al. [147] | 2011–2016 | 407 | 286/121 | 0/100 | NR | 2 | proj 78% | 2% LR, present early results after changed guidelines for RT/CRT | ||
| Ruppert et al., OCUM [148] | 2007–2016 | 545 | 122/125/298 | 0/100 | 5 | 81% | 72% | 41% (n = 174) preop CRT. 5-year DM 19%, 3% LR, 97% had mesorectal plane excision | ||
| Rasanen et al. [149] | 2005–2011 | 481 | 116/129/167 | 0/100 | 42% | 5 | 74% | About half had preop CRT. DM at 5 years in 18% stage O-II, 30% stage III | ||
| Tan et al. [150] | 1999–2007 | 326 | 71/106/149 | 0/100 | 34% | 10 | All: 70% | LR: 8%, DM 22% (42% if ACT, 12% if no ACT), 99% of recurrences within 5 years | ||
| Ishihara et al. [151] | 1997–2006 | 5664 | 2877/2787 | 100/0 | 38% | 5 | All: 83% II: 90% III: 76% |
83% | 22 hospitals, right-sided 84%, left-sided 81% | |
| Chapuis et al. [23] | 1995–2010 | 363 | 0/0/363 | 100/0 | 56% | 5 | All: 65% | CME, competing risk analysis, no difference if ACT or not | ||
| Mroczkowski et al. [55] | 2000–2004 | 15,096 | 5451/9645/8616 | 100/0 | NR | 5 | 90% | 80% 12+ nodes, only 68% adequate follow-up, questioning the recurrence data | ||
| Poulsen et al. [152] | 2009–2010 | 1633 | 524/553/502 | 0/100 | NR | 5 | 89% | LR 4%, 11% systemic recurrences, 54 pts stage IV, 479 (29%) had preop CRT | ||
| Osterman et al. [153] | 2007–2012 | 14,325 | 2,730/6,314/5,201 | 100/0 | II: 12%, III: 61% | 5 | All: 84% II: 89% III: 71% |
Stage II 0–1 risk factor (pT4, <12 nodes, high grade, emergency surgery, vessel/nerve infiltration.) no ACT 90%, 2+ risk factors 78%. Stage III 0 risk factor 78%. 82% 12+ nodes | ||
| Glimelius et al. [16] | 1995–2012 | 28,962 | NR | 0/100 | NR, limited | 5 | 80% | LR down to 4% in both countries from higher values in Norway, DM decreased from 22% to 18% during the time period in both countries | ||
| Uppsala, Sweden (present article) | 2010–2017 | 1212 | 172/381/410 | 806/406 | II: 19%, III: 62% | 5 | 83% | TTR 84% colon, 83% rectum, for further details, see Table 7. |
The same search strategies as used in a previous similar systematic overview [19] evaluating “modern” recurrence risks is colon cancer patients were utilized for this overview. We did not exclude articles that did not present stage-specific results. Totally 25,588 articles were identified, of which the above contained relevant information. In the previous overview, it was reported that patients operated between 1995–2008 and not treated with adjuvant chemotherapy had a DFS (TTR was not reported adequately) of 81% in stage II (n = 2250) and 49% in stage III (n = 312). If adjuvant chemotherapy was provided, DFS was 79% vs 64%. Few of the 37 evaluated studies reported the quality of the care [19]. Abbreviations: TTR/FFR = time to tumour recurrence/freedom from recurrence (100-recurrence rate in % as provided in the articles), RFS/EFS = recurrence-free/event-free survival, DFS = disease-free survival, LR = local recurrence, DM = distant metastasis, RR = recurrence risk, ACT = adjuvant chemotherapy.
3. Recurrences Risks in the Control Group of Randomized, Chiefly Colon Cancer Trials
In the “old” colon cancer trials with a surgery alone group, using OS and DFS (occasionally RFS or EFS (event-free survival) or crude recurrence rates) as endpoints, DFS in the surgery alone group after 4–5 years ranged from 44 to 62% for stages II + III together (See [77] for references to the 31 trials contributing data and Table 1 and Table 2 for the trials reporting recurrence rates/TTR). When reported separately, the corresponding figures in stage II ranged from 59 to 77% and from 35 to 44% in stage III. In a review, the EORTC group reported that 31–59% of the surgically operated patients would have a recurrence within 5 years [154], in line with the DFS reported in the trials. In the QUASAR trial [46], which mainly included stage II patients, recurrences were reported in 22%, the actuarial risk at 5 years was25%. In the recent Japanese SACURA trial [48], which only included stage II patients, recurrences were seen in 13% of the patients in the surgery alone group. Thus, recurrence risks in the order of 40–50% for stage II + III together (over 55% for stage III and about 25–30% for stage II) were seen in patients operated during the 1970–1990s. With a median age of about 62 years and a comparative short follow-up (4–5 years), most events included in DFS/RFS/EFS were recurrences and, thus, not other deaths or secondary malignancies. However, in the SACURA trial [48], where only 13% of the patients experienced a recurrence, 8% (about 40% of all events) developed a secondary malignancy, greatly influencing DFS but not RFS (Table 1).
The antibody 17-1 A was explored in a randomized study in colon cancer stage III with favourable results [89] and followed by two multicentre trials, one comparing surgery alone with surgery followed by treatment with the antibody [90,155]. The trial was negative allowing the outcome of a large group of operated stage II patients to be assessed [90]. At 7 years, the “disease-specific” DFS (i.e., TTR) was 83%, the traditional DFS was 74% (Table 2).
4. Recurrences Risks in the Control Group of Randomized Rectal Cancer Trials
A few randomized studies with a surgery alone group, none of which showed any significant gain from adding postoperative chemotherapy individually or collectively, allows an estimate of recurrence risks after different preoperative treatments and variable quality of the surgery, i.e., before or after the introduction of the TME procedure. In the old US trials comparing surgery alone with postoperative therapy, the risk of distant metastasis after 5 years was 33–40% in stage II and 60–70% in stage III [92,94,156], studies that resulted in adjuvant therapy becoming routine treatment at least in the US. In 4 more recent European studies [46,59,60,95] where the patients had received either preoperative short-course RT or CRT, distant recurrences were seen in 30–40% of the patients in the observation group.Five major European trials were included in an analysis with the aim of predicting the risk of the local and systemic relapse risk after CRT, i.e., to identify patients who may benefit the most from postoperative chemotherapy [103]. Thirty-one % of the patients had a distant recurrence after 5 years. This risk was marginally influenced by adjuvant chemotherapy. In yet another meta-analysis [7] based upon individual patient data from four trials, systemic recurrences were seen in 37% of patients in the control group after 5 years. In patients treated with preoperative CRT, it was 35%.
The risk of recurrence is also evaluable in trials comparing different radiation schedules or comparing conventional CRT with radiation (5×5 Gy or CRT) combined with neo-adjuvant chemotherapy (Table 3).
5. Recurrence Risk in Studies Exploring Different Follow-Up Routines, Comparing Open vs Laparoscopic Surgery or Using the Circumferential Mesocolic Resection (CME) Technique
Randomized or observational trials comparing different follow-up routines provide information about recurrence risks but often lack other relevant information. In three large studies including patients operated between 1998–2010 and administered adjuvant chemotherapy according to guidelines, the 4.5–5-year recurrence rates varied between 17 and 22% [110,111,112,113,114]. In six older trials, including patients operated during the 1980–90s, in which adjuvant chemotherapy was not given (except in one trial) and the quality of surgery not reported, but was probably representative of the time period, recurrences were seen more often (TTR/FFR 45–73%) in a mixture of stage I-III patients with colon and rectal cancers [104,105,106,107,108,109].
Multiple studies have since the early 1990s explored surgical techniques, some randomized comparing open with laparoscopic, conventional laparoscopic with robotic and conventional resection with more extended mesocolic resection. Multiple meta-analyses have been performed, occasionally exploring long-term outcomes including recurrence rates [119,120,121,134,135,136,157]. In the trials, approximately 20% or less have had a recurrence after up to 5 years in a mixture of stage I–III [119,125,126,127,128].
In multiple studies exploring the CME-technique [158,159], resulting in an oncologically superior specimen [160,161] versus standard colon cancer surgery, distant recurrence rates were reported in 2 studies, being about 10% after 3–4.5 years [131,132,133].
6. Recurrence Risks in Prognostic Studies
Numerous studies have explored the relevance of tumour-related or other factors for recurrence risks in primary CRC. It is beyond the scope of this work to review them. Furthermore, recurrence risks are seldom reported; most used parameters are OS/DFS. The outcomes are often not presented for the entire group and most data are hazard/odds ratios illustrating the differences between marker expression. Absolute recurrence risks are seldom given. The characteristics of the patients are often not well described, and they are often “convenience samples” why the representativeness is questionable.
Microsatellite instability (MSI) indicates a low risk of recurrence in primary colon cancer, at least in stage II (DFS/RFS HR 0.59 in a meta-analysis of 39 studies including 12,110 patients [162]). The absolute risk of recurrence, important for this review, was not reported. The importance of MMR-status in stage III is more unclear. In the adjuvant PETACC-8 trial, recurrences were seen in 26% of the patients operated between 2005 and 2009, 19% in MSI patients and 27% in microsatellite stable (MSS) patients (p = 0.02) [163]. “The need” is thus higher in patients with MSS tumours than in those approximately 15% having MSI tumours [164]. The consensus molecular subtypes (CMS) have also emerged as potentially important prognosticators with 5-year RFS rates of about 75% in CMS 1–3 and 60% in CMS 4 in 1785 patients in stages I–III from different sources, where 73% received adjuvant chemotherapy [165].
7. Recurrence Risk in Hospital- and Population-Based Series
7.1. Predominantly Colon Cancer
The MSKCC results from two time periods described above illustrate the changes seen with time [38]. In hospital-based series, recurrence rates in the order of 10–20% (/FFR/TTR rates 80–90% after 5 years) have been reported in patients operated about 10–20 years ago [55,138,140,141,142,143,144,151]. In most studies, some patients received adjuvant therapy, but several hospitals gave no adjuvant therapy [138,140,144,145] (Table 6). Similarly low recurrence risks were reported in two series of patients operated during the 1980–1990s; those centres presented information indicating that the surgical quality was “of a higher level” than at non-specialized centres [138,143]. Several studies found that in stage II, the recurrence risk at 5 years was very low in most patients whereas a minor fraction (about 10–15%) had a higher recurrence risk, motivating adjuvant chemotherapy. This has also been the conclusion of numerous prognostic studies (data not presented since chiefly only DFS/OS have been reported).
Fewer studies have reported the prognostic heterogeneity in stage III. In a German study, 1453 stage III CRC patients operated between 1978–1997, three groups with markedly different OS (no recurrence data provided) could be identified, 5-year OS was about 80% in 10% of the patients (pT1,2 N1), about 60% in 50% of the patients (p > T3,4 N1 or pT1,2 N2) and about 30% in the remaining 40% (pT3,4 N2) [166]. Others have also defined similar sub-grouping of stage III, including NCCN (stage IIIA–C) [4], without stating that a subgroup of stage III might not need adjuvant therapy.
Apparently fewer recurrences than historically reported after colon cancer surgery were seen in the entire Swedish population of 14,325 radically operated patients between 2007–2012 [153]. After a minimum follow-up of 5 years, 16% have had a recurrence, 11% in stage II and 29% in stage III (Table 6). When grouped according to UICC-stage, the number of risk factors (pT4, less than 12 nodes, vessel/nerve infiltration, high grade and emergency surgery) and whether adjuvant treatment was initiated (12% in stage II and 61% stage III) or not, stage II patients with 0–1 risk factor not treated with adjuvant therapy had 10% risk of recurrence, if 2 or more risk factors (19% of stage II) the risk rose to 22%. In patients where adjuvant treatment was given, marginally higher recurrence risks were seen. In stage III without risk factors, the recurrence risk was 22% without adjuvant treatment and 14% if it was initiated.
We further substantiated the apparently low recurrence risks in radically operated colon cancer patients between 2010–2015 in one Swedish region (n = 416). In the region, a prospective biobank initiative [167] was running, minimizing the risk of under-reporting of patients and recurrences. The results of the prospectively collected Uppsala region material were representative of the national material [153,168].
7.2. Rectal Cancer
In the entire Swedish and Norwegian populations of rectal cancer between 1995–2012, amounting to 29,000 Swedish and 15,500 Norwegian patients [16], preoperative RT was delivered to 61% of resected patients in Sweden versus 24%, chiefly CRT, in Norway. During the time period, local recurrence rates decreased to about 4% at 5 years from significantly higher values in Norway, whereas distant metastatic rates were identical and decreased from slightly above to slightly below 20%. Post-operative CRT was administered to some patients in Norway but not in Sweden. Adjuvant chemotherapy was rarely given in Norway and to a limited number of patients in Sweden with no change during the years.
In a hospital-based series from Finland [149], about half of the 481 rectal cancer patients received preoperative RT/CRT and 42% were administered adjuvant chemotherapy. Overall, 26% of the patients had a recurrence, local in 8% and distal in 23% (9% had both local and distant). The same quality assurance measures have not taken place in Finland as in Sweden, Norway, Denmark [101,169] and The Netherlands [147] where local recurrence rates are only a few per cent. In Denmark, only 11% of the patients developed distant metastases.
In a Chinese series of 185 rectal cancer patients curatively operated without neo-adjuvant therapy between 2006–2014 [170], 120 belonged to a high-risk group according to MRI. In these patients, distant metastases were seen in 42% and local recurrences in 10% despite postoperative CRT to 84 patients.
In recent population-based materials of rectal cancer, less than 20% of the patients develop distant metastases during follow-up, this being responsible for the majority of deaths [16,97,101,152]. In recent trials, distant recurrence rates are usually higher (30–40%), even if pre-treated with CRT, but the trials have only included patients with high-risk criteria [101,102,103]. It is, thus, more difficult to assess whether and how much distant recurrence risks have decreased in rectal cancer when compared with patients treated for colon cancer.
8. Recurrence Risks in Sweden during the Past Decade after Validation of Recurrence Data
8.1. Validation of Data
Several quality assurance programmes have been implemented in Sweden during the past decades, firstly in rectal cancer and subsequently in colon cancer [171,172,173,174]. All information is collected in the national Swedish Colorectal Cancer Registry (SCRCR), having complete coverage [172,175] and where reporting of all recurrences is mandatory once they have been diagnosed. A request is sent to the treating hospitals after 3 and 5 years. Thus, most recurrences have in all probability been recorded in the registry. According to the national care programme from 2008, updated in 2016, staging using preferably CT should be performed of the primary tumour (and MRI for rectal tumours), liver and lungs, although ultrasonography of the liver and plain chest X-ray were permitted previously. During follow-up, clinical investigation, CT of the liver and lungs and CEA are mandatory after 1 and 3 years.
The SCRCR contains demographics, staging and primary treatments with a high level of accuracy [176], but the accuracy of the M-stage at diagnosis and recurrence data has not been validated.
In the Uppsala region (375,000 inhabitants in 2018), 1707 patients were between 2010–2018 diagnosed with 1736 CRCs. The medical records of the 398 patients (23%) with registered metastases at diagnosis (265 colon cancers, 133 rectal cancers), the 201 patients in stage I–III with registered recurrences until 23 January 2020 (134, 16%, colon, 69, 13%, rectum) and the 1108 patients without a registered recurrence were re-examined.
Evidence of metastatic disease in patients registered as M1 at diagnosis was seen in all but 10 (3.6%) colon cancer patients and five (3.7%) rectal cancer patients. Reasons were chiefly misclassified liver or lung lesions, either confirmed by histology after removal of the lesion, spontaneous disappearance of the lesion or a prolonged period (at least 2 years) of lack of progression. The opposite, namely that patients had synchronous disease diagnosed at the latest at surgery, but not registered, was even more uncommon (seven (0.8%) colon cancers and one (1.4%) rectal cancer). Of the 201 recurrences, six (4.5%) colon cancer cases and one (0.3%) rectal cancer case were wrongly registered (in four cases a new colon cancer, in two cases another malignancy and in one case a haemangioma).
Nine (seven colon cancer, two rectal cancer) non-registered recurrences were diagnosed more than 3 months before data retrieval, representing under-reporting. Thus, of 722 colon cancer patients without a recurrence, seven (1%) patients had a non-registered recurrence. The corresponding figure in rectal cancer was 0.5% (2/386). It can be concluded that the number of mistakes in the register is small concerning the registration of both synchronous and metachronous metastases.
In a corrected transcript from the register performed after the completion of the validation study, 1734 cancers were diagnosed in the 1706 patients (one originally diagnosed patient without an invasive carcinoma was removed) with a primary diagnosis of CRC between 2010–2018. Twelve patients had two cancers, three had three synchronous cancers and in 10 patients a metachronous cancer developed. In the case of synchronous cancers, the least advanced was removed and in case of metachronous, the last diagnosed was removed prior to analysis for the evaluation of recurrence risks.
Few patients with colon cancer received neo-adjuvant chemotherapy (mainly those with a locally advanced non-resectable or difficult-to-resect tumour) whereas more patients received this in rectal cancer (locally advanced tumours at high risk of recurrence and included in the RAPIDO [177] or LARCT-US (ClinicalTrials.gov Identifier: NCT03729687) trials; these patients are not of interest when evaluating the need for adjuvant treatment and were excluded from the evaluations.
8.2. Stage-Stratified Risk of Recurrence
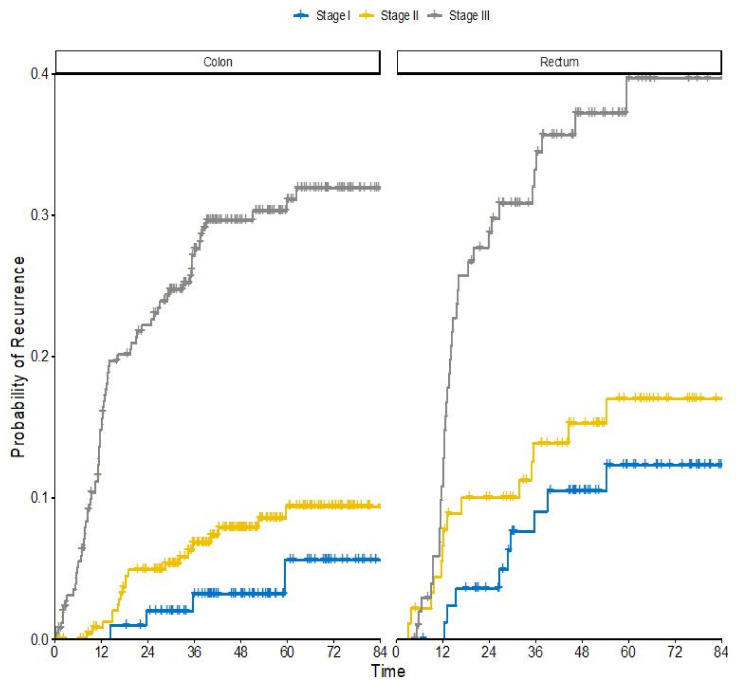
Of 1212 patients with a radically operated CRC diagnosed between 2010–2018, 200 (17%) patients have had a recurrence, 16% in colon cancer and 17% in rectal cancer. In the 971 patients between 2010–2017 (ensuring a minimum follow-up of 2 years), similar recurrence numbers were seen (overall 17%, colon 16%, rectum 19%). Patient characteristics for these patients are shown in Table S1. Recurrence risks according to stage, number of risk factors and adjuvant treatment are shown in Table 7. They are for rectal cancer patients shown according to pre-treatment regimen, pathological or clinical stage, and adjuvant treatment or not. The actuarial risk at 5 years in all stages together is 17% for colon cancer and 21% for rectal cancer (Figure 1). In stages II and III, the risks are 10% and 31% in colon cancer, and 17% and 40% in rectal cancer.
Table 7.
Recurrence rates at 3 and 5 years after radical surgery in a Swedish population-based patient cohort diagnosed between 2010–2017 according to whether adjuvant therapy was initiated or not.
| Colon Cancer | Stage | Risk Factors | No Adjuvant Treatment | Adjuvant Treatment | ||||
| Recurrence Rate | Recurrence Rate | |||||||
| n | 3 Year | 5 Year | n | 3 Year | 5 Year | |||
| Stage II | 0–1 | 182 | 3% | 6% | 27 | 12% | 18% | |
| ≥2 | 25 | 23% | 23% | 23 | 14% | 14% | ||
| Stage III | 0 | 28 | 22% | 22% | 44 | 9% | 12% | |
| 1 | 30 | 36% | 36% | 42 | 15% | 15% | ||
| ≥2 | 42 | 38% | 55% | 75 | 39% | 43% | ||
| Rectal Cancer | Primary Treatment | Stage | No Adjuvant Treatment | Adjuvant Treatment | ||||
| pStage | Recurrence Rate | Recurrence Rate | ||||||
| n | 3 Year | 5 Year | n | 3 Year | 5 Year | |||
| Direct surgery or scRT without delay to surgery | I | 46 | 9% | 12% | 0 | - | - | |
| II | 42 | 5% | 5% | 1 | 0% | 0% | ||
| III | 18 | 47% | 55% | 35 | 23% | 28% | ||
| cStage | ||||||||
| scRT with delay or scRT and chemotherapy or CRT | I | 4 | 0% | 50% | 0 | - | - | |
| II | 10 | 10% | 10% | 2 | 50% | - | ||
| III | 108 | 22% | 28% | 32 | 16% | 16% | ||
| before surgery | - | - | - | - | - | - | - | |
| - | - | - | - | - | - | - | ||
Abbreviations: scRT= short-course radiotherapy. CRT= chemoradiotherapy, n= number of patients. pStage= pathological stage, cStage, clinical stage using pelvic MRI. For risk factors, see Table S1.
Figure 1.
Colorectal cancer recurrence risks by tumor location and stage. Kaplan Meier event plot split by diagnosis and stage. Outcome is recurrence after radical surgery in a Swedish population-based cohort diagnosed between 2010–2017 with minimum 2 years follow-up.
Altogether, 627 patients had metastases, either synchronous (n = 427, 67%) or metachronous (n = 200, 33%). The median OS from the diagnosis of metastatic/recurrent disease was 15.6 months, i.e., considerably shorter than presently reported from clinical trials/hospital-based series [2,178], reflecting the unselected nature of the population. OS was considerably longer for those who primarily received anti-tumour treatment, mostly chemotherapy, occasionally surgery or radiotherapy (median 20.8 months) compared with those who did not (median 3.4 months).
9. Correlations between Time of Inclusion and Rates of Time-To-Recurrence (TTR)/Freedom from Recurrence (FFR)
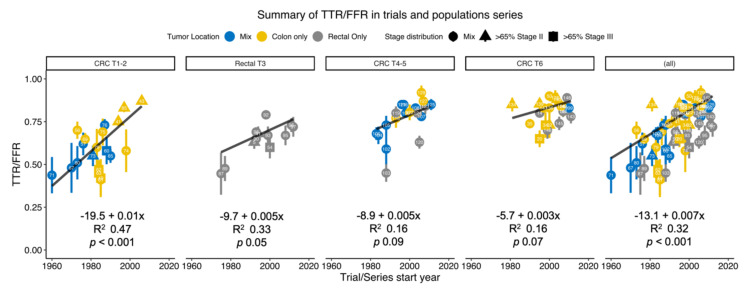
Rates of TTR/FFR in the different trials shown in Table 1, Table 2, Table 3, Table 4, Table 5, Table 6 and Table 7 according to the start of patient inclusion are shown in Figure 2 separately for the adjuvant trials with a untreated control group (Table 1 and Table 2 for predominantly colon cancer and Table 3 for rectal cancer), for the surveillance and surgical technique studies (Table 4 and Table 5), the hospital- and population-based series (Table 6 including the Swedish data presented in Table 7) and for all studies together. Although the studies within each group are very heterogeneous, significant correlations were found. In all studies together, the improvement was 0.7% (p < 0.001) in linear regression adjusted for case numbers. When further adjusted for tumour location, stage distribution, the improvement was 0.54% (p < 0.001). The figure legend describes the position of individual trials with respect to inclusion times and stage distributions. Details of the extent of adjuvant therapy provided are shown in the tables.
Figure 2.
Time to recurrence (TTR) or freedom from (crude) recurrence (FFR) in surgically operated nonmetastatic colorectal cancer patients according to when the first patient was operated. Improvements with time were seen when all trials were included (right panel) and in the different types of trials as presented in the six Tables. The numbers in each filled point refer to the reference number as provided in the tables and reference list. Individual points are coloured by distribution of colon vs rectal cancer and shapes represents stage mix in each study. Linear regression for the trend, weighted by number of cases in the studies, are presented for each panel and for the total with the equation, R2 and p-value presented for each panel. In the left panel (CRC T1–2), TTR/FFR are from the untreated control group in randomized (chiefly) colon cancer trials. The best results are seen in a recent Japanese study in stage II [48], in a trial [90] including only low-risk stage II patients, and in [46], mainly including patients with stage II where the doctor was uncertain about the benefit of adjuvant therapy. In the second left panel (rectal T3) a marked improvement is seen from the two older US trials [92,94] reporting improved results after adjuvant chemotherapy/chemoradiotherapy. No apparent improvement has been observed since then. However, the trial with the best results [97] was initiated at an early stage but included most patients between 2008–2011 and included “intermediate risk” tumours as opposed to “locally advanced tumours” in most of the other trials (although most of the tumours anyhow belonged to an intermediate risk group). One of the most recent trials [177] included only patients at high risk for relapse. Also [100] included only high-risk patients. In these trials preoperative chemoradiotherapy was given to all and adjuvant chemotherapy to some. Two of the older trials [95,96] had worse results despite including less advanced (most cT3 and not cT4) tumours. In the middle chart (CRC T4–5), being a systematic review of all randomized surveillance and laparoscopic trials, a clear improvement with time is seen. However, adjuvant therapy was provided to more patients in the recent trials than in the older trials, explaining some of the improvement. The two studies with the best results used the circumferential mesorectal technique, CME, potentially explaining few recurrences. In the second chart to the right (CRC T6), including recent patient series, the results are apparently better than in the older trials. Few recurrences were seen in an early trial from Erlangen [138], where the surgical quality was “at a high level”. No adjuvant therapy was provided, further emphasizing the good results. Besides this trial, there still appears to be an improvement with time, but the studies are heterogenous and many factors may lie behind this improvement.
10. Discussion
This overview has raised a practically important question for many doctors/patients worldwide but, for methodological reasons, this question is impossible to give a precise answer to. Even if an accurate estimate of the magnitude of improvement cannot be given and may not even be needed, it is evident that the risk of recurrence after radical CRC surgery and, thus, the need for adjuvant chemotherapy, is less today than in the past. This should influence how patients are informed and whether and which adjuvant therapy is indicated. General guidelines by international organizations, such as NCCN and ESMO [4,9,40] do, in our opinion, not properly consider this continuous development, that most probably has not yet come to an end. Only few studies, including one from MSKCC have recently pointed to this dilemma [34]. This is positive news for many patients since fewer will experience a recurrence, but if not corrected will result in overtreatment of more patients than was the case in the past.
It is extremely difficult to initiate a new generation of randomized adjuvant trials, at least in colon cancer, having a surgery only group. It is likewise similarly difficult in rectal cancer; the negative experience of trying to run such trials by several collaborative groups during recent years speaks against any success [59,60]. Even if those recent randomized trials did not show sufficient gains, adjuvant chemotherapy is frequently given according to principles in colon cancer, further emphasizing the difficulties [4,9,40,179]. Thus, further analyses of the results of unselected populations are needed; this is, however, for obvious reasons hampered by the selective provision of adjuvant chemotherapy. Several measures could, however, be taken to improve the relevance of population-based studies, one of which is the reporting of recurrences.
In order to overcome the obstacles of poor tumour recurrence registration in population registers, a US study stated that if intense support is given to such registries, the recurrences can be properly captured and more generalizable results be obtained [180]. In Sweden, it is the duty of individual hospitals to feed the quality registries with proper and complete data, but the registries are voluntary and thus rely on whether the departments can create enough resources to complete data. However, the incentive for all departments to have their own data as complete as possible is strong since anonymized key data per hospital is officially released yearly with rankings. Furthermore, the quality registries are excellent resources for research [181]. Many hospitals participate in research projects, and none wants to belong to a group having incomplete and poor data. Our extensive validation in one region in Sweden, detecting extremely few mistakes, tells that the Swedish Registry is characterized by a high degree of validity.
Since follow-up routines in Sweden are not intensive, it is possible that a patient dying of another cause may have developed a recurrence, if death had not intervened. There is thus, always a possibility that the number of patients with subclinical disease, requiring adjuvant therapy, is larger than the number with detected recurrences, but the extent of this is probably small. Even if adjuvant chemotherapy was selectively provided according to guidelines, this does not prevent conclusions to be made, at least not in stage II where the use was limited. Additionally, since much evidence tells us that recurrence risks are independent of age, many elderly patients not treated may provide relevant information. Intercurrent deaths are common among the elderly, but most recurrences come early or within a few years and the survival prospects of older adults having survived major surgery are longer.
11. What Were the Risk of Recurrence a Few Decades Ago and What Are They Presently If Adjuvant Treatment Is Not Given?
11.1. Colon Cancer
In the randomized trials comparing surgery alone with surgery and adjuvant chemotherapy, TTR/FFR varied between 41 and 60% and the RFS/DFS between 44 and 67% in stages II + III together in the surgery alone group after about 5 years. In stage II, RFS/DFS varied between 59 and 79% and in stage III between 29 and 44% (since median age was 62 years and follow-up comparatively short, most events were caused by recurrences). Further, a few more recurrences will occur with a longer follow-up, indicating that subclinical disease after surgery performed during the 1970–90s was present in about 40–50% of stage II + III patients (Figure 2), in 30–35% of stage II patients and in 60–70% in stage III, whether included in a clinical trial or not. In a few trials exploring experimental treatments during the same time period, recurrence risks were 30–40% overall, 60% in stage III but only 10% in one trial including only low-risk stage II patients (pT4 bN0-cases were excluded) randomized between placebo or an ineffective treatment [90], The need for adjuvant chemotherapy was, thus, previously quite substantial although it was possible to identify groups with limited need.
The two retrospective subgroup analyses of patients included in the ACCENT database, revealing better results in more recent compared with the older trials that could be ascribed to fewer recurrences, did not quantify the magnitude of the difference [31,32]. When an old nomogram (by MSKCC) was validated, 7–8 percentage points fewer recurrences in both stage II and III were seen (Table 6) [33].
In our overview of trials comparing two different surveillance strategies, the recurrence risks varied according to when the patients were operated. In 5 trials including patients between 1983–1996, recurrence risks after 5 years ranged from 31 to 55% [105,106,107,108]. It is likely that no patients received adjuvant chemotherapy in those trials. In a trial including patients between 1997 and 2001, the recurrence risk was 27% at 4 years, however, it was stated that all patients should have been administered adjuvant treatment, this possibly being the reason for the slightly better results. In the most recent trials [110,111,112,113,114], the recurrence rates have varied been between 17 and 20%, but adjuvant chemotherapy was selectively provided. In recent trials comparing surgical details, recurrence risks have been even lower or overall, about 15%, however, with a shorter follow-up. The improvements with time in these “surgical” trials can, thus, be the result of more recurrence-reducing adjuvant chemotherapy but also that recurrences have become less frequent. However, a few hospital-based series, where no adjuvant treatment was provided and where statements about high quality in the care were made, similarly report recurrence rates in the order of 10–15% for stage II patients [140,144,145,146,166]. This was also the case in the surgery alone group in the recent randomized Japanese trial [48]. With further support from the Swedish population-based data ([153] and further presented here after validation of the register data, Table 7), it is reasonable to conclude that recurrence rates overall are in the order of 15–20%, about 10–12% for stage II (without adjuvant chemotherapy) and about 25–30% for stage III. The estimate for stage III is uncertain due to the paucity of studies and that adjuvant chemotherapy has been administered to many patients.
11.2. Rectal Cancer
Without doubt, the risk of local failure has decreased during the past several decades down to less than about 5% at dedicated hospitals and in national populations [2,16,147,148,173,182]. Better staging, better surgery and appropriate use of pre-operative treatments are responsible. In the early trials, systemic recurrences were if anything a few percent higher in rectal cancer than in colon cancer, or overall, up to 50%. Similar to the situation in colon cancer, these risks have decreased, but remain around 30–40% in patients with “locally advanced rectal cancer” included in the radiotherapy trials. In populations and in the most recent generation of radiotherapy trials, they remain around 20% in patients with intermediate risk tumours (bad group) and around 30% if locally advanced (bad/ugly group) despite being treated with pre-operative CRT (about 50 Gy with a fluoropyrimidine), indicative of the present need for adjuvant therapy on this level. In these patients, multiple trials have not shown any clear beneficial effect of adjuvant chemotherapy. During ASCO 2020, two randomized trials reported fewer systemic recurrences using neo-adjuvant chemotherapy [101,102]. Most patients with locally advanced tumours may thus in the future receive total neoadjuvant therapy (TNT), although some patients will probably have a need for additional post-operative treatment, like those where staging MRI misinterpreted the findings and where the response to the TNT was poor. The chances of beneficial effects from conventional chemotherapy will probably be minimal in the latter group.
11.3. Methodological Considerations
Any summary of heterogeneous data collected during different time periods, as provided in Figure 2, can be subject to bias. Despite the great heterogeneity, particularly with provision of adjuvant therapy in the recent series, the strong correlations including the visually strong impression are probably true but overestimated. We have considered several potential errors in the legend to Figure 2. The strong correlation between response rates to first line chemotherapy and liver resection rates in metastatic CRC confined to the liver, reported 15 years ago, identified an important correlation, although it was exaggerated (Figure 1 in [183]).
12. Conclusions
Interstudy comparisons, particularly if the trials were performed decades apart, cannot allow firm conclusions, but most evidence tells us that recurrence risks are substantially lower today than they were when the trials showing the benefit from adjuvant chemotherapy were performed, at least in the case of colon cancer. It is, thus, no longer appropriate to treat patients based upon old data as still recommended in most guidelines. It is not possible to give an exact figure of how large the improvement is, although the numbers seen in the Swedish material (see Table 7), where not a single patient or recurrence was missed, together with the recent surveillance studies (Table 4), the studies exploring surgical techniques (Table 5) and several surgical and population-based series (Table 6) give a good indication of what can be achieved at centres/in countries having an interest in the quality of the CRC care. Many patients with stage II that previously were at sufficient risk of recurrence (1 risk factor) probably have such a limited recurrence risk (<10%) that adjuvant treatment is not motivated. Nevertheless, some (maybe 20%) stage II patients (presence of the high-risk factors pT4 or <12 lymph nodes or 2 or more other risk factors) [3] still may have a sufficiently high risk (about 15–20%) to motivate additional treatment, although not necessarily with oxaliplatin. Conversely, some (maybe 20–25%) stage III patients have such a low recurrence risk (about 20%) that the addition of oxaliplatin can be questioned. It should be noted that not only the recurrence risk is important when deciding whether treatment should be recommended or not but also patient related factors and, above all and not reviewed here, the likelihood that the treatment will eradicate tumour cells.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/11/3308/s1, Table S1: Demographics of non-metastatic patients in a population-based Swedish Colorectal Cancer cohort diagnosed between 2010–2017.
Author Contributions
Conceptualization, E.O. (Erik Osterman) and B.G.; methodology, E.O. (Erik Osterman) and B.G; data collection, E.O. (Erik Osterman), K.H., I.I., T.S., E.O. (Emerik Osterlund) and B.G.; formal analysis, E.O. (Erik Osterman), K.H. and B.G.; writing—original draft preparation, review and editing, B.G. All authors contributed critical feedback to shape the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Swedish Cancer Society, grant number 190382.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bray F., Ferlay J., Soerjomataram I., Siegel R.L., Torre L.A., Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.Schmoll H.J., Van Cutsem E., Stein A., Valentini V., Glimelius B., Haustermans K., Nordlinger B., van de Velde C.J., Balmana J., Regula J., et al. ESMO Consensus Guidelines for management of patients with colon and rectal cancer. a personalized approach to clinical decision making. Ann. Oncol. 2012;23:2479–2516. doi: 10.1093/annonc/mds236. [DOI] [PubMed] [Google Scholar]
- 3.Argiles G., Tabernero J., Labianca R., Hochhauser D., Salazar R., Iveson T., Laurent-Puig P., Quirke P., Yoshino T., Taieb J., et al. Localised colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2020 doi: 10.1016/j.annonc.2020.06.022. In press. [DOI] [PubMed] [Google Scholar]
- 4.NCCN Guidelines Colon Cancer, Version 3. [(accessed on 23 May 2020)];2020 Available online: www.NCCN.org.
- 5.Taieb J., Gallois C. Adjuvant Chemotherapy for Stage III Colon Cancer. Cancers (Basel) 2020;12:2679. doi: 10.3390/cancers12092679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rebuzzi S.E., Pesola G., Martelli V., Sobrero A.F. Adjuvant Chemotherapy for Stage II Colon Cancer. Cancers (Basel) 2020;12:2584. doi: 10.3390/cancers12092584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breugom A.J., Swets M., Bosset J.F., Collette L., Sainato A., Cionini L., Glynne-Jones R., Counsell N., Bastiaannet E., van den Broek C.B., et al. Adjuvant chemotherapy after preoperative (chemo)radiotherapy and surgery for patients with rectal cancer: A systematic review and meta-analysis of individual patient data. Lancet Oncol. 2015;16:200–207. doi: 10.1016/S1470-2045(14)71199-4. [DOI] [PubMed] [Google Scholar]
- 8.Bujko K., Glimelius B., Valentini V., Michalski W., Spalek M. Postoperative chemotherapy in patients with rectal cancer receiving preoperative radio(chemo)therapy: A meta-analysis of randomized trials comparing surgery +/- a fluoropyrimidine and surgery + a fluoropyrimidine +/- oxaliplatin. Eur. J. Surg. Oncol. 2015;41:713–723. doi: 10.1016/j.ejso.2015.03.233. [DOI] [PubMed] [Google Scholar]
- 9.NCCN Guidelines Rectal Cancer, Version 6. [(accessed on 10 September 2020)]; Available online: www.NCCN.org.
- 10.Van Steenbergen L.N., Rutten H.J., Creemers G.J., Pruijt J.F., Coebergh J.W., Lemmens V.E. Large age and hospital-dependent variation in administration of adjuvant chemotherapy for stage III colon cancer in southern Netherlands. Ann. Oncol. 2010;21:1273–1278. doi: 10.1093/annonc/mdp482. [DOI] [PubMed] [Google Scholar]
- 11.Den Dulk M., Krijnen P., Marijnen C.A., Rutten H.J., van de Poll-Franse L.V., Putter H., Meershoek-Klein Kranenbarg E., Jansen-Landheer M.L., Coebergh J.W., van de Velde C.J. Improved overall survival for patients with rectal cancer since 1990: the effects of TME surgery and pre-operative radiotherapy. Eur. J. Cancer. 2008;44:1710–1716. doi: 10.1016/j.ejca.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 12.Elferink M.A., van Steenbergen L.N., Krijnen P., Lemmens V.E., Rutten H.J., Marijnen C.A., Nagtegaal I.D., Karim-Kos H.E., de Vries E., Siesling S., et al. Marked improvements in survival of patients with rectal cancer in the Netherlands following changes in therapy, 1989–2006. Eur. J. Cancer. 2010;46:1421–1429. doi: 10.1016/j.ejca.2010.01.025. [DOI] [PubMed] [Google Scholar]
- 13.Brenner H., Kloor M., Pox C.P. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 14.Iversen L.H., Green A., Ingeholm P., Osterlind K., Gogenur I. Improved survival of colorectal cancer in Denmark during 2001–2012 - The efforts of several national initiatives. Acta Oncol. 2016;55(Suppl. S2):10–23. doi: 10.3109/0284186X.2015.1131331. [DOI] [PubMed] [Google Scholar]
- 15.Favoriti P., Carbone G., Greco M., Pirozzi F., Pirozzi R.E., Corcione F. Worldwide burden of colorectal cancer: a review. Updates Surg. 2016;68:7–11. doi: 10.1007/s13304-016-0359-y. [DOI] [PubMed] [Google Scholar]
- 16.Glimelius B., Myklebust T.Å., Lundqvist K., Wibe A., Guren M.G. Two countries - two treatment strategies for rectal cancer. Radiother. Oncol. 2016;121:357–363. doi: 10.1016/j.radonc.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 17.Arnold M., Sierra M.S., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683–691. doi: 10.1136/gutjnl-2015-310912. [DOI] [PubMed] [Google Scholar]
- 18.Benitez Majano S., Di Girolamo C., Rachet B., Maringe C., Guren M.G., Glimelius B., Iversen L.H., Schnell E.A., Lundqvist K., Christensen J., et al. Surgical treatment and survival from colorectal cancer in Denmark, England, Norway, and Sweden: a population-based study. Lancet Oncol. 2019;20:74–87. doi: 10.1016/S1470-2045(18)30646-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bockelman C., Engelmann B.E., Kaprio T., Hansen T.F., Glimelius B. Risk of recurrence in patients with colon cancer stage II and III: A systematic review and meta-analysis of recent literature. Acta Oncol. 2015;54:5–16. doi: 10.3109/0284186X.2014.975839. [DOI] [PubMed] [Google Scholar]
- 20.Pahlman L.A., Hohenberger W.M., Matzel K., Sugihara K., Quirke P., Glimelius B. Should the benefit of adjuvant chemotherapy in colon cancer be re-evaluated? J. Clin. Oncol. 2016;34:1297–1299. doi: 10.1200/JCO.2015.65.3048. [DOI] [PubMed] [Google Scholar]
- 21.Heald R.J., Ryall R.D.H. Recurrence and survival after total mesorectal excision for rectal cancer. Lancet. 1986;28:1479–1482. doi: 10.1016/S0140-6736(86)91510-2. [DOI] [PubMed] [Google Scholar]
- 22.Bokey L., Chapuis P.H., Chan C., Stewart P., Rickard M.J., Keshava A., Dent O.F. Long-term results following an anatomically based surgical technique for resection of colon cancer: a comparison with results from complete mesocolic excision. Colorectal Dis. 2016;18:676–683. doi: 10.1111/codi.13159. [DOI] [PubMed] [Google Scholar]
- 23.Chapuis P.H., Bokey E., Chan C., Keshava A., Rickard M., Stewart P., Young C.J., Dent O.F. Recurrence and cancer-specific death after adjuvant chemotherapy for Stage III colon cancer. Colorectal Dis. 2019;21:164–173. doi: 10.1111/codi.14434. [DOI] [PubMed] [Google Scholar]
- 24.Feinstein A.R., Sosin D.M., Wells C.K. The Will Rogers phenomenon. Stage migration and new diagnostic techniques as a source of misleading statistics for survival in cancer. N. Engl. J. Med. 1985;312:1604–1608. doi: 10.1056/NEJM198506203122504. [DOI] [PubMed] [Google Scholar]
- 25.Shahrier M., Ahnen D.J. Colorectal cancer survival in Europe: The Will Rogers phenomenon revisited. Gut. 2000;47:463–464. doi: 10.1136/gut.47.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fielding L.P., Arsenault P.A., Chapuis P.H., Dent O., Gathright B., Hardcastle J.D., Hermanek P., Jass J.R., Newland R.C. Clinicopathological staging for colorectal cancer: an International Documentation System (IDS) and an International Comprehensive Anatomical Terminology (ICAT) J. Gastroenterol. Hepatol. 1991;6:325–344. doi: 10.1111/j.1440-1746.1991.tb00867.x. [DOI] [PubMed] [Google Scholar]
- 27.Resch A., Langner C. Lymph node staging in colorectal cancer: old controversies and recent advances. World J. Gastroenterol. 2013;19:8515–8526. doi: 10.3748/wjg.v19.i46.8515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jestin P., Pahlman L., Glimelius B., Gunnarsson U. Cancer staging and survival in colon cancer is dependent on the quality of the pathologists’ specimen examination. Eur. J. Cancer. 2005;41:2071–2078. doi: 10.1016/j.ejca.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 29.Lykke J., Roikjaer O., Jess P., Danish Colorectal Cancer Group The relation between lymph node status and survival in Stage I-III colon cancer: results from a prospective nationwide cohort study. Colorectal Dis. 2013;15:559–565. doi: 10.1111/codi.12059. [DOI] [PubMed] [Google Scholar]
- 30.Del Paggio J.C., Peng Y., Wei X., Nanji S., MacDonald P.H., Krishnan Nair C., Booth C.M. Population-based study to re-evaluate optimal lymph node yield in colonic cancer. Br. J. Surg. 2017;104:1087–1096. doi: 10.1002/bjs.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Papamichael D., Renfro L.A., Matthaiou C., Yothers G., Saltz L., Guthrie K.A., Van Cutsem E., Schmoll H.J., Labianca R., Andre T., et al. Validity of Adjuvant! Online in older patients with stage III colon cancer based on 2967 patients from the ACCENT database. J. Geriatr. Oncol. 2016;7:422–429. doi: 10.1016/j.jgo.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shi Q., Andre T., Grothey A., Yothers G., Hamilton S.R., Bot B.M., Haller D.G., Van Cutsem E., Twelves C., Benedetti J.K., et al. Comparison of outcomes after fluorouracil-based adjuvant therapy for stages II and III colon cancer between 1978 to 1995 and 1996 to 2007: evidence of stage migration from the ACCENT database. J. Clin. Oncol. 2013;31:3656–3663. doi: 10.1200/JCO.2013.49.4344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salem M.E., Yin J., Goldberg R.M., Pederson L.D., Wolmark n., Alberts S.R., Taieb J., Marshall J.L., Lonardi S., Yoshino T., et al. Evaluation of the change of outcomes over a 10-year period in patients with stage III colon cancer: pooled analysis of 6501 patients treated with fluorouracil, leucovorin, and oxaliplatin in the ACCENT database. Ann. Oncol. 2020;31:480–486. doi: 10.1016/j.annonc.2019.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weiser M.R., Landmann R.G., Kattan M.W., Gonen M., Shia J., Chou J., Paty P.B., Guillem J.G., Temple L.K., Schrag D., et al. Individualized prediction of colon cancer recurrence using a nomogram. J. Clin. Oncol. 2008;26:380–385. doi: 10.1200/JCO.2007.14.1291. [DOI] [PubMed] [Google Scholar]
- 35.Collins I.M., Kelleher F., Stuart C., Collins M., Kennedy J. Clinical decision aids in colon cancer: A comparison of two predictive nomograms. Clin. Colorectal Cancer. 2012;11:138–142. doi: 10.1016/j.clcc.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 36.Liu M., Qu H., Bu Z., Chen D., Jiang B., Cui M., Xing J., Yang H., Wang Z., Di J., et al. Validation of the Memorial Sloan-Kettering Cancer Center Nomogram to Predict Overall Survival After Curative Colectomy in a Chinese Colon Cancer Population. Ann. Surg. Oncol. 2015;22:3881–3887. doi: 10.1245/s10434-015-4495-2. [DOI] [PubMed] [Google Scholar]
- 37.Kazem M.A., Khan A.U., Selvasekar C.R. Validation of nomogram for disease free survival for colon cancer in UK population: A prospective cohort study. Int. J. Surg. 2016;27:58–65. doi: 10.1016/j.ijsu.2015.12.069. [DOI] [PubMed] [Google Scholar]
- 38.Konishi T., Shimada Y., Hsu M., Wei I.H., Pappou E., Smith J.J., Nash G.M., Guillem J.G., Paty P.B., Garcia-Aguilar J., et al. Contemporary Validation of a Nomogram Predicting Colon Cancer Recurrence, Revealing All-Stage Improved Outcomes. JNCI Cancer Spectr. 2019;3:pkz015. doi: 10.1093/jncics/pkz015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chibaudel B., Henriques J., Rakez M., Brenner B., Kim T.W., Martinez-Villacampa M., Gallego-Plazas J., Cervantes A., Shim K., Jonker D., et al. Association of Bevacizumab Plus Oxaliplatin-Based Chemotherapy With Disease-Free Survival and Overall Survival in Patients With Stage II Colon Cancer: A Secondary Analysis of the AVANT Trial. JAMA Netw. Open. 2020;3:e2020425. doi: 10.1001/jamanetworkopen.2020.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Labianca R., Nordlinger B., Beretta G.D., Mosconi S., Mandala M., Cervantes A., Arnold D., ESMO Guidelines Working Group Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24(Suppl. S6):vi64–vi72. doi: 10.1093/annonc/mdt354. [DOI] [PubMed] [Google Scholar]
- 41.Glimelius B., Osterman E. Adjuvant Chemotherapy in Elderly Colorectal Cancer Patients. Cancers (Basel) 2020;12:2289. doi: 10.3390/cancers12082289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Punt C.J., Buyse M., Kohne C.H., Hohenberger P., Labianca R., Schmoll H.J., Pahlman L., Sobrero A., Douillard J.Y. Endpoints in adjuvant treatment trials: a systematic review of the literature in colon cancer and proposed definitions for future trials. J. Natl. Cancer Inst. 2007;99:998–1003. doi: 10.1093/jnci/djm024. [DOI] [PubMed] [Google Scholar]
- 43.Cohen R., Vernerey D., Bellera C., Meurisse A., Henriques J., Paoletti X., Rousseau B., Alberts S., Aparicio T., Boukovinas I., et al. Guidelines for time-to-event end-point definitions in adjuvant randomised trials for patients with localised colon cancer: Results of the DATECAN initiative. Eur. J. Cancer. 2020;130:63–71. doi: 10.1016/j.ejca.2020.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Alberts S.R., Sargent D.J., Nair S., Mahoney M.R., Mooney M., Thibodeau S.N., Smyrk T.C., Sinicrope F.A., Chan E., Gill S., et al. Effect of oxaliplatin, fluorouracil, and leucovorin with or without cetuximab on survival among patients with resected stage III colon cancer: a randomized trial. JAMA. 2012;307:1383–1393. doi: 10.1001/jama.2012.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andre T., Boni C., Mounedji-Boudiaf L., Navarro M., Tabernero J., Hickish T., Topham C., Zaninelli M., Clingan P., Bridgewater J., et al. Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N. Engl. J. Med. 2004;350:2343–2351. doi: 10.1056/NEJMoa032709. [DOI] [PubMed] [Google Scholar]
- 46.Quasar Collaborative Group. Gray R., Barnwell J., McConkey C., Hills R.K., Williams N.S., Kerr D.J. Adjuvant chemotherapy versus observation in patients with colorectal cancer: A randomised study. Lancet. 2007;370:2020–2029. doi: 10.1016/S0140-6736(07)61866-2. [DOI] [PubMed] [Google Scholar]
- 47.Birgisson H., Wallin U., Holmberg L., Glimelius B. Survival endpoints in colorectal cancer and the effect of second primary other cancer on disease free survival. BMC Cancer. 2011;11:438. doi: 10.1186/1471-2407-11-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Matsuda C., Ishiguro M., Teramukai S., Kajiwara Y., Fujii S., Kinugasa Y., Nakamoto Y., Kotake M., Sakamoto Y., Kurachi K., et al. A randomised-controlled trial of 1-year adjuvant chemotherapy with oral tegafur-uracil versus surgery alone in stage II colon cancer: SACURA trial. Eur. J. Cancer. 2018;96:54–63. doi: 10.1016/j.ejca.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 49.Sorbye H., Pfeiffer P., Cavalli-Bjorkman N., Qvortrup C., Holsen M.H., Wentzel-Larsen T., Glimelius B. Clinical trial enrollment, patient characteristics, and survival differences in prospectively registered metastatic colorectal cancer patients. Cancer. 2009;115:4679–4687. doi: 10.1002/cncr.24527. [DOI] [PubMed] [Google Scholar]
- 50.Ludmir E.B., Mainwaring W., Lin T.A., Miller A.B., Jethanandani A., Espinoza A.F., Mandel J.J., Lin S.H., Smith B.D., Smith G.L., et al. Factors Associated With Age Disparities Among Cancer Clinical Trial Participants. JAMA Oncol. 1001 doi: 10.1001/jamaoncol.2019.2055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hovdenak Jakobsen I., Juul T., Thaysen H.V., Johansen C., Laurberg S. Differences in baseline characteristics and 1-year psychological factors between participants and non-participants in the randomized, controlled trial regarding patient-led follow-up after rectal cancer (FURCA) Acta Oncol. 2019;58:627–633. doi: 10.1080/0284186X.2019.1581948. [DOI] [PubMed] [Google Scholar]
- 52.In H., Bilimoria K.Y., Stewart A.K., Wroblewski K.E., Posner M.C., Talamonti M.S., Winchester D.P. Cancer recurrence: an important but missing variable in national cancer registries. Ann. Surg. Oncol. 2014;21:1520–1529. doi: 10.1245/s10434-014-3516-x. [DOI] [PubMed] [Google Scholar]
- 53.In H., Simon C.A., Phillips J.L., Posner M.C., Ko C.Y., Winchester D.P. The quest for population-level cancer recurrence data; current deficiencies and targets for improvement. J. Surg. Oncol. 2015;111:657–662. doi: 10.1002/jso.23883. [DOI] [PubMed] [Google Scholar]
- 54.Chubak J., Yu O., Ziebell R.A., Bowles E.J.A., Sterrett A.T., Fujii M.M., Boggs J.M., Burnett-Hartman A.N., Boudreau D.M., Chen L., et al. Risk of colon cancer recurrence in relation to diabetes. Cancer Causes Control. 2018;29:1093–1103. doi: 10.1007/s10552-018-1083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mroczkowski P., Schmidt U., Sahm M., Gastinger I., Lippert H., Kube R. Prognostic factors assessed for 15,096 patients with colon cancer in stages I and II. World J. Surg. 2012;36:1693–1698. doi: 10.1007/s00268-012-1531-2. [DOI] [PubMed] [Google Scholar]
- 56.Sanoff H.K., Carpenter W.R., Martin C.F., Sargent D.J., Meyerhardt J.A., Sturmer T., Fine J.P., Weeks J., Niland J., Kahn K.L., et al. Comparative effectiveness of oxaliplatin vs non-oxaliplatin-containing adjuvant chemotherapy for stage III colon cancer. J. Natl. Cancer Inst. 2012;104:211–227. doi: 10.1093/jnci/djr524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Glynne-Jones R., Wyrwicz L., Tiret E., Brown G., Rodel C., Cervantes A., Arnold D., Committee E.G. Corrections to: Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2018;29:IV263. doi: 10.1093/annonc/mdy161. [DOI] [PubMed] [Google Scholar]
- 58.Glimelius B., Tiret E., Cervantes A., Arnold D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2013;24(Suppl. S6):vi81–vi88. doi: 10.1093/annonc/mdt240. [DOI] [PubMed] [Google Scholar]
- 59.Breugom A.J., van Gijn W., Muller E.W., Berglund A., van den Broek C.B., Fokstuen T., Gelderblom H., Kapiteijn E., Leer J.W., Marijnen C.A., et al. Adjuvant chemotherapy for rectal cancer patients treated with preoperative (chemo)radiotherapy and total mesorectal excision: a Dutch Colorectal Cancer Group (DCCG) randomized phase III trial. Ann. Oncol. 2015;26:696–701. doi: 10.1093/annonc/mdu560. [DOI] [PubMed] [Google Scholar]
- 60.Glynne-Jones R., Counsell N., Quirke P., Mortensen N., Maraveyas A., Meadows H.M., Ledermann J., Sebag-Montefiore D. Chronicle: results of a randomised phase III trial in locally advanced rectal cancer after neoadjuvant chemoradiation randomising postoperative adjuvant capecitabine plus oxaliplatin (XELOX) versus control. Ann. Oncol. 2014;25:1356–1362. doi: 10.1093/annonc/mdu147. [DOI] [PubMed] [Google Scholar]
- 61.Higgins G.A., Jr., Amadeo J.H., McElhinney J., McCaughan J.J., Keehn R.J. Efficacy of prolonged intermittent therapy with combined 5-fluorouracil and methyl-CCNU following resection for carcinoma of the large bowel. A Veterans Administration Surgical Oncology Group report. Cancer. 1984;53:1–8. doi: 10.1002/1097-0142(19840101)53:1<1::AID-CNCR2820530102>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 62.Hafstrom L., Rudenstam C.M., Domellof L. A randomized trial of oral 5-fluorouracil versus placebo as adjuvant therapy in colorectal cancer Dukes’ B and C: results after 5 years observation time. Br. J. Surg. 1985;72:138–141. doi: 10.1002/bjs.1800720223. [DOI] [PubMed] [Google Scholar]
- 63.Laurie J.A., Moertel C.G., Fleming T.R., Wieand H., Leigh J.E., Rubin J., McCormack G.W., Gerstner J.B., Krook J.E., Malliard J., et al. Surgical adjuvant therapy of large-bowel carcinoma: An evaluation of levamisole and the combination of levamisole and fluorouracil. J. Clin. Oncol. 1989;7:1447–1456. doi: 10.1200/JCO.1989.7.10.1447. [DOI] [PubMed] [Google Scholar]
- 64.Dahl O., Fluge O., Carlsen E., Wiig J.N., Myrvold H.E., Vonen B., Podhorny N., Bjerkeset O., Eide T.J., Halvorsen T.B., et al. Final results of a randomised phase III study on adjuvant chemotherapy with 5 FU and levamisol in colon and rectum cancer stage II and III by the Norwegian Gastrointestinal Cancer Group. Acta Oncol. 2009;48:368–376. doi: 10.1080/02841860902755244. [DOI] [PubMed] [Google Scholar]
- 65.O’Connell M.J., Mailliard J.A., Kahn M.J., Macdonald J.S., Haller D.G., Mayer R.J., Wieand H.S. Controlled trial of fluorouracil and low-dose leucovorin given for 6 months as postoperative adjuvant therapy for colon cancer. J. Clin. Oncol. 1997;15:246–250. doi: 10.1200/JCO.1997.15.1.246. [DOI] [PubMed] [Google Scholar]
- 66.Francini G., Petrioli R., Lorenzini L., Mancini S., Armenio S., Tanzini G., Marsili S., Aquino A., Marzocca G., Civitelli S., et al. Folinic acid and 5-fluorouracil as adjuvant chemotherapy in colon cancer. Gastroenterology. 1994;106:899–906. doi: 10.1016/0016-5085(94)90748-X. [DOI] [PubMed] [Google Scholar]
- 67.Moertel C.G., Fleming T.R., Macdonald J.S., Haller G., Laurie J.A., Goodman P.J., Ungerleider J.S., Emerson W.A., Tormey D.C., Glick J.H., et al. Levamisole and fluorouracil for adjuvant therapy of resected colon carcinoma. N. Engl. J. Med. 1990;322:352–358. doi: 10.1056/NEJM199002083220602. [DOI] [PubMed] [Google Scholar]
- 68.Moertel C.G., Fleming C.G., Macdonald J.S., Haller D.G., Laurie J.A., Tangen C.M., Ungerledier J.S., Emerson W.A., Tormey D.C., Glick J.H., et al. Fluorouracil plus levamisole as effective adjuvant therapy after resection of stage III colon cancer. A final report. Ann. Intern. Med. 1995;122:321–326. doi: 10.7326/0003-4819-122-5-199503010-00001. [DOI] [PubMed] [Google Scholar]
- 69.Zaniboni A., Labianca R., Marsoni S., Torri V., Mosconi P., Grilli R., Apolone G., Cifani S., Tinazzi A. GIVIO-SITAC 01: A randomized trial of adjuvant 5-fluorouracil and folinic acid administered to patients with colon carcinoma--long term results and evaluation of the indicators of health-related quality of life. Gruppo Italiano Valutazione Interventi in Oncologia. Studio Italiano Terapia Adiuvante Colon. Cancer. 1998;82:2135–2144. doi: 10.1002/(sici)1097-0142(19980601)82:113.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 70.Taal B.G., Van Tinteren H., Zoetmulder F.A. Adjuvant 5 FU plus levamisole in colonic or rectal cancer: improved survival in stage II and III. Br. J. Cancer. 2001;85:1437–1443. doi: 10.1054/bjoc.2001.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Panettiere F.J., Goodman P.J., Costanzi J.J., Cruz A.B., Jr., Vaitkevicius V.K., McCracken J.D., Brownlee R.W., Laufman L., Stephens R.L., Bonnet J. Adjuvant therapy in large bowel adenocarcinoma: long-term results of a Southwest Oncology Group Study. J. Clin. Oncol. 1988;6:947–954. doi: 10.1200/JCO.1988.6.6.947. [DOI] [PubMed] [Google Scholar]
- 72.Windle R., Bell P.R., Shaw D. Five year results of a randomized trial of adjuvant 5-fluorouracil and levamisole in colorectal cancer. Br. J. Surg. 1987;74:569–572. doi: 10.1002/bjs.1800740707. [DOI] [PubMed] [Google Scholar]
- 73.Efficacy of adjuvant fluorouracil and folinic acid in colon cancer. International Multicentre Pooled Analysis of Colon Cancer Trials (IMPACT) investigators. Lancet. 1995;345:939–944. [PubMed] [Google Scholar]
- 74.Wilkinson N.W., Yothers G., Lopa S., Costantino J.P., Petrelli N.J., Wolmark N. Long-term survival results of surgery alone versus surgery plus 5-fluorouracil and leucovorin for stage II and stage III colon cancer: pooled analysis of NSABP C-01 through C-05. A baseline from which to compare modern adjuvant trials. Ann. Surg. Oncol. 2010;17:959–966. doi: 10.1245/s10434-009-0881-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Efficacy of adjuvant fluorouracil and folinic acid in B2 colon cancer. International Multicentre Pooled Analysis of B2 Colon Cancer Trials (IMPACT B2) Investigators. J. Clin. Oncol. 1999;17:1356–1363. [PubMed] [Google Scholar]
- 76.Li M.C., Ross S.T. Chemoprophylaxis for patients with colorectal cancer. Prospective study with five-year follow-up. JAMA. 1976;235:2825–2828. doi: 10.1001/jama.1976.03260520019015. [DOI] [PubMed] [Google Scholar]
- 77.Figueredo A., Charette M.L., Maroun J., Brouwers M.C., Zuraw L. Adjuvant Therapy for Stage II Colon Cancer: A Systematic Review From the Cancer Care Ontario Program in Evidence-Based Care’s Gastrointestinal Cancer Disease Site Group. J. Clin. Oncol. 2004;22:3395–3407. doi: 10.1200/JCO.2004.03.087. [DOI] [PubMed] [Google Scholar]
- 78.Swiss Group for Clinical Cancer Research (SAKK) Long-term results of single course of adjuvant intraportal chemotherapy for colorectal cancer. Lancet. 1995;345:349–353. doi: 10.1016/S0140-6736(95)90340-2. [DOI] [PubMed] [Google Scholar]
- 79.Scheithauer W., Kornek G., Rosen H., Sebesta C., Marcell A., Kwasny W., Karall M., Depisch D. Combined intraperitoneal plus intravenous chemotherapy after curative resection for colonic adenocarcinoma. Eur. J. Cancer. 1995;31 A:1981–1986. doi: 10.1016/0959-8049(95)00426-2. [DOI] [PubMed] [Google Scholar]
- 80.Vaillant J.C., Nordlinger B., Deuffic S., Arnaud J.P., Pelissier E., Favre J.P., Jaeck D., Fourtanier G., Grandjean J.P., Marre P., et al. Adjuvant intraperitoneal 5-fluorouracil in high-risk colon cancer: A multicenter phase III trial. Ann. Surg. 2000;231:449–456. doi: 10.1097/00000658-200004000-00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Rougier P., Sahmoud T., Nitti D., Curran D., Doci R., De Waele B., Nakajima T., Rauschecker H., Labianca R., Pector J.C., et al. Adjuvant portal-vein infusion of fluorouracil and heparin in colorectal cancer: a randomised trial. European Organisation for Research and Treatment of Cancer Gastrointestinal Tract Cancer Cooperative Group, the Gruppo Interdisciplinare Valutazione Interventi in Oncologia, and the Japanese Foundation for Cancer Research. Lancet. 1998;351:1677–1681. doi: 10.1016/s0140-6736(97)08169-5. [DOI] [PubMed] [Google Scholar]
- 82.Wolmark N., Rockette H., Wickerham D.L., Fisher B., Redmond C., Fisher E.R., Potvin M., Davies R.J., Jones J., Robidoux A. Adjuvant therapy of Dukes’ A, B and C adenocarcinoma of the colon with portal-vein fluorouracil hepatic infusion: preliminary results of National Surgical Adjuvant Breast and Bowel Project Protocol C-02. Prior annotation incorrect: see comments. J. Clin. Oncol. 1990;8:1466–1475. doi: 10.1200/JCO.1990.8.9.1466. [DOI] [PubMed] [Google Scholar]
- 83.James R.D., Donaldson D., Gray R., Northover J.M., Stenning S.P., Taylor I. Randomized clinical trial of adjuvant radiotherapy and 5-fluorouracil infusion in colorectal cancer (AXIS) Br. J. Surg. 2003;90:1200–1212. doi: 10.1002/bjs.4266. [DOI] [PubMed] [Google Scholar]
- 84.Nitti D., Wils J., Sahmoud T., Curran D., Couvreur M.L., Lise M., Rauschecker H., dos Santos J.G., Stremmel W., Roelofsen F. Final results of a phase III clinical trial on adjuvant intraportal infusion with heparin and 5-fluorouracil (5-FU) in resectable colon cancer (EORTC GITCCG 1983–1987). European Organization for Research and Treatment of Cancer. Gastrointestinal Tract Cancer Cooperative Group. Eur. J. Cancer. 1997;33:1209–1215. doi: 10.1016/s0959-8049(97)00052-x. [DOI] [PubMed] [Google Scholar]
- 85.Lawrence W., Jr., Terz J.J., Horsley J.S., 3rd, Brown P.W., Romero C. Chemotherapy as an adjuvant to surgery for colorectal cancer. A follow-up report. Arch. Surg. 1978;113:164–168. doi: 10.1001/archsurg.1978.01370140054011. [DOI] [PubMed] [Google Scholar]
- 86.Wereldsma J.C., Bruggink E.D., Meijer W.S., Roukema J.A., van Putten W.L. Adjuvant portal liver infusion in colorectal cancer with 5-fluorouracil/heparin versus urokinase versus control. Results of a prospective randomized clinical trial (colorectal adenocarcinoma trial I) Cancer. 1990;65:425–432. doi: 10.1002/1097-0142(19900201)65:3<425::AID-CNCR2820650309>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 87.Irvin T., Vowles K.D., Golby M.G. Fluorouracil in chemoprophylaxis of colorectal cancer. Results of a controlled clinical trial. Dis. Colon. Rectum. 1986;29:704–706. doi: 10.1007/BF02555313. [DOI] [PubMed] [Google Scholar]
- 88.Hanna M.G., Jr., Hoover H.C., Jr., Vermorken J.B., Harris J.E., Pinedo H.M. Adjuvant active specific immunotherapy of stage II and stage III colon cancer with an autologous tumor cell vaccine: first randomized phase III trials show promise. Vaccine. 2001;19:2576–2582. doi: 10.1016/S0264-410X(00)00485-0. [DOI] [PubMed] [Google Scholar]
- 89.Riethmüller G., Schnedier-Gädicke E., Schlimok G., Schmiegel W., Raab R., Höffken K., Gruber R., Pichlmaier H., Hirche H., Pichlmayr R., et al. Randomised trial of monoclonal antibody for adjuvant therpay of resected Dukes’ C colorectal carcinoma. Lancet. 1994;343:1177–1183. doi: 10.1016/s0140-6736(94)92398-1. [DOI] [PubMed] [Google Scholar]
- 90.Niedzwiecki D., Bertagnolli M.M., Warren R.S., Compton C.C., Kemeny N.E., Benson A.B., 3rd, Eckhardt S.G., Alberts S., Porjosh G.N., Kerr D.J., et al. Documenting the natural history of patients with resected stage II adenocarcinoma of the colon after random assignment to adjuvant treatment with edrecolomab or observation: results from CALGB 9581. J. Clin. Oncol. 2011;29:3146–3152. doi: 10.1200/JCO.2010.32.5357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liver Infusion Meta-analysis Group Portal vein chemotherapy for colorectal cancer: a meta-analysis of 4000 patients in 10 studies. J. Natl. Cancer Inst. 1997;89:497–505. doi: 10.1093/jnci/89.7.497. [DOI] [PubMed] [Google Scholar]
- 92.Gastrointestinal Tumor Study Group Prolongation of the disease-free interval in surgically treated rectal carcinoma. New Engl. J. Med. 1985;312:1465–1472. doi: 10.1056/NEJM198506063122301. [DOI] [PubMed] [Google Scholar]
- 93.Fisher B., Wolmark N., Rockette H., Redmond C., Deutsch M., Wickerman D.L., Fisher E.R., Caplan R., Jones J., Lerner H., et al. Postoperative adjuvant chemotherapy or radiation therapy for rectal cancer: Results from NSABP Protocol R-01. J. Natl. Cancer Inst. 1988;80:21–29. doi: 10.1093/jnci/80.1.21. [DOI] [PubMed] [Google Scholar]
- 94.Gunderson L.L., Sargent D.J., Tepper J.E., Wolmark N., O’Connell M.J., Begovic M., Allmer C., Colangelo L., Smalley S.R., Haller D.G., et al. Impact of T and n stage and treatment on survival and relapse in adjuvant rectal cancer: a pooled analysis. J. Clin. Oncol. 2004;22:1785–1796. doi: 10.1200/JCO.2004.08.173. [DOI] [PubMed] [Google Scholar]
- 95.Bosset J.F., Calais G., Mineur L., Maingon P., Stojanovic-Rundic S., Bensadoun R.J., Bardet E., Beny A., Ollier J.C., Bolla M., et al. Fluorouracil-based adjuvant chemotherapy after preoperative chemoradiotherapy in rectal cancer: long-term results of the EORTC 22921 randomised study. Lancet Oncol. 2014;15:184–190. doi: 10.1016/S1470-2045(13)70599-0. [DOI] [PubMed] [Google Scholar]
- 96.Gerard J.P., Conroy T., Bonnetain F., Bouche O., Chapet O., Closon-Dejardin M.T., Untereiner M., Leduc B., Francois E., Maurel J., et al. Preoperative radiotherapy with or without concurrent fluorouracil and leucovorin in T3–4 rectal cancers: results of FFCD 9203. J. Clin. Oncol. 2006;24:4620–4625. doi: 10.1200/JCO.2006.06.7629. [DOI] [PubMed] [Google Scholar]
- 97.Erlandsson J., Holm T., Pettersson D., Berglund Å., Cedermark B., Radu C., Johansson H., Machado M., Hjern F., Hallböök O. The Stockholm III Trial on optimal fractionation of preoperative radiotherapy and timing to surgery for rectal cancer - a randomised controlled trial. Lancet Oncol. 2017;18:336–346. doi: 10.1016/S1470-2045(17)30086-4. [DOI] [PubMed] [Google Scholar]
- 98.Bujko K., Nowacki M., Nasierowska-Guttmejer A., Michalski W., Benek M., Pudelko M., Kryj M., Oledzki J., Szmeja J., Shuszniak J., et al. Sphincter preservation following reoperative radiotherapy for rectal cancer: Report of a randomised trial comparing short-term radiotherapy vs. conventionally fractionated radiochemotherapy. Radiother. Oncol. 2004;72:15–24. doi: 10.1016/j.radonc.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 99.Bujko K., Nowacki M.P., Nasierowska-Guttmejer A., Michalski W., Bebenek M., Kryj M., Oledzki J., Szmeja J., Kladny J., Pietrzak L. Long-term results of a randomised trial comparing preoperative short-course radiotherapy vs preoperative conventionally fractionated chemoradiation for rectal cancer. Br. J. Surg. 2006;93:1215–1223. doi: 10.1002/bjs.5506. [DOI] [PubMed] [Google Scholar]
- 100.Cisel B., Pietrzak L., Michalski W., Wyrwicz L., Rutkowski A., Kosakowska E., Cencelewicz A., Spalek M., Polkowski W., Jankiewicz M., et al. Long-course preoperative chemoradiation vs. 5 x 5 Gy and consolidation chemotherapy for clinical T4 and fixed clinical T3 rectal cancer: Long-term results of the randomized Polish II study. Ann. Oncol. 2019;30:1298–1303. doi: 10.1093/annonc/mdz186. [DOI] [PubMed] [Google Scholar]
- 101.Bahadoer R., Dijkstra E., van Etten B., Marijnen C., Putter H., Meershoek-Klein Kranenbarg E., Roodvoets A., Nagtegaal I., Beets-Tan R., Blomqvist L., et al. Short-course radiotherapy followed by chemotherapy before total mesorectal excision (TME) in locally advanced rectal cancer – the randomized RAPIDO trial. Lancet Oncol. 2020 In press. [Google Scholar]
- 102.Conroy T., Lamfichekh N., Etienne P.-L., Rio E., Francois E., Mesgouez-Nebout N., Vendrely V., Artignan X., Bouché O., Gargot D., et al. Total neoadjuvant therapy with mFOLFIRINOX versus preoperative chemoradiation in patients with locally advanced rectal cancer: Final results of PRODIGE 23 phase III trial, a UNICANCER GI trial. Proc. Am. Soc. Clin. Oncol. 2020 doi: 10.1200/JCO.2020.38.15_suppl.4007. Abstr 4007. [DOI] [Google Scholar]
- 103.Valentini V., van Stiphout R.G., Lammering G., Gambacorta M.A., Barba M.C., Bebenek M., Bonnetain F., Bosset J.F., Bujko K., Cionini L., et al. Nomograms for predicting local recurrence, distant metastases, and overall survival for patients with locally advanced rectal cancer on the basis of European randomized clinical trials. J. Clin. Oncol. 2011;29:3163–3172. doi: 10.1200/JCO.2010.33.1595. [DOI] [PubMed] [Google Scholar]
- 104.Kjeldsen B.J., Kronborg O., Fenger C., Jorgensen O.D. The pattern of recurrent colorectal cancer in a prospective randomised study and the characteristics of diagnostic tests. Int. J. Colorectal Dis. 1997;12:329–334. doi: 10.1007/s003840050118. [DOI] [PubMed] [Google Scholar]
- 105.Ohlsson B., Breland U., Ekberg H., Graffner H., Tranberg K.-G. Follow-up after curative surgery for colorectal carcinoma. Randomised comparison with no follow-up. Dis. Colon. Rectum. 1995;38:619–626. doi: 10.1007/BF02054122. [DOI] [PubMed] [Google Scholar]
- 106.Makela J.T., Laitinen S.O., Kairaluoma M.I. Five-year follow-up after radical surgery for colorectal cancer. Results of a prospective randomized trial. Arch. Surg. 1995;130:1062–1067. doi: 10.1001/archsurg.1995.01430100040009. [DOI] [PubMed] [Google Scholar]
- 107.Secco G.B., Fardelli R., Gianquinto D., Bonfante P., Baldi E., Ravera G., Derchi L., Ferraris R. Efficacy and cost of risk-adapted follow-up in patients after colorectal cancer surgery: a prospective, randomized and controlled trial. Eur. J. Surg. Oncol. 2002;28:418–423. doi: 10.1053/ejso.2001.1250. [DOI] [PubMed] [Google Scholar]
- 108.Schoemaker D., Black R., Giles L., Toouli J. Yearly colonscopy, liver CT, and chest radiography do not influence 5-year survival of colorectal cancer patients. Gastroenterology. 1998;114:7–14. doi: 10.1016/S0016-5085(98)70626-2. [DOI] [PubMed] [Google Scholar]
- 109.Rodriguez-Moranta F., Salo J., Arcusa A., Boadas J., Pinol V., Bessa X., Batiste-Alentorn E., Lacy A.M., Delgado S., Maurel J., et al. Postoperative surveillance in patients with colorectal cancer who have undergone curative resection: a prospective, multicenter, randomized, controlled trial. J. Clin. Oncol. 2006;24:386–393. doi: 10.1200/JCO.2005.02.0826. [DOI] [PubMed] [Google Scholar]
- 110.Rosati G., Ambrosini G., Barni S., Andreoni B., Corradini G., Luchena G., Daniele B., Gaion F., Oliverio G., Duro M., et al. A randomized trial of intensive versus minimal surveillance of patients with resected Dukes B2-C colorectal carcinoma. Ann. Oncol. 2016;27:274–280. doi: 10.1093/annonc/mdv541. [DOI] [PubMed] [Google Scholar]
- 111.Wille-Jorgensen P., Syk I., Smedh K., Laurberg S., Nielsen D.T., Petersen S.H., Renehan A.G., Horvath-Puho E., Pahlman L., Sorensen H.T., et al. Effect of More vs Less Frequent Follow-up Testing on Overall and Colorectal Cancer-Specific Mortality in Patients With Stage II or III Colorectal Cancer: The COLOFOL Randomized Clinical Trial. JAMA. 2018;319:2095–2103. doi: 10.1001/jama.2018.5623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Primrose J.N., Perera R., Gray A., Rose P., Fuller A., Corkhill A., George S., Mant D., Investigators F.T. Effect of 3 to 5 years of scheduled CEA and CT follow-up to detect recurrence of colorectal cancer: the FACS randomized clinical trial. JAMA. 2014;311:263–270. doi: 10.1001/jama.2013.285718. [DOI] [PubMed] [Google Scholar]
- 113.Pugh S.A., Shinkins B., Fuller A., Mellor J., Mant D., Primrose J.N. Site and Stage of Colorectal Cancer Influence the Likelihood and Distribution of Disease Recurrence and Postrecurrence Survival: Data From the FACS Randomized Controlled Trial. Ann. Surg. 2016;263:1143–1147. doi: 10.1097/SLA.0000000000001351. [DOI] [PubMed] [Google Scholar]
- 114.Shinkins B., Primrose J.N., Pugh S.A., Nicholson B.D., Perera R., James T., Mant D. Serum carcinoembryonic antigen trends for diagnosing colorectal cancer recurrence in the FACS randomized clinical trial. Br. J. Surg. 2018;105:658–662. doi: 10.1002/bjs.10819. [DOI] [PubMed] [Google Scholar]
- 115.Pita-Fernandez S., Alhayek-Ai M., Gonzalez-Martin C., Lopez-Calvino B., Seoane-Pillado T., Pertega-Diaz S. Intensive follow-up strategies improve outcomes in nonmetastatic colorectal cancer patients after curative surgery: a systematic review and meta-analysis. Ann Oncol. 2015;26:644–656. doi: 10.1093/annonc/mdu543. [DOI] [PubMed] [Google Scholar]
- 116.Wang T., Cui Y., Huang W.S., Deng Y.H., Gong W., Li C.J., Wang J.P. The role of postoperative colonoscopic surveillance after radical surgery for colorectal cancer: A prospective, randomized clinical study. Gastrointest Endosc. 2009;69:609–615. doi: 10.1016/j.gie.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 117.Wattchow D.A., Weller D.P., Esterman A., Pilotto L.S., McGorm K., Hammett Z., Platell C., Silagy C. General practice vs surgical-based follow-up for patients with colon cancer: randomised controlled trial. Br. J. Cancer. 2006;94:1116–1121. doi: 10.1038/sj.bjc.6603052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Pietra N., Sarli L., Costi R., Ouchemi C., Grattarola M., Peracchia A. Role of follow-up in management of local recurrences of colorectal cancer: a prospective, randomized study. Dis. Colon. Rectum. 1998;41:1127–1133. doi: 10.1007/BF02239434. [DOI] [PubMed] [Google Scholar]
- 119.Kuhry E., Schwenk W.F., Gaupset R., Romild U., Bonjer H.J. Long-term results of laparoscopic colorectal cancer resection. Cochrane Database Syst. Rev. 2008:CD003432. doi: 10.1002/14651858.CD003432.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Liang Y., Li G., Chen P., Yu J. Laparoscopic versus open colorectal resection for cancer: a meta-analysis of results of randomized controlled trials on recurrence. Eur. J. Surg. Oncol. 2008;34:1217–1224. doi: 10.1016/j.ejso.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 121.Ng K.T., Tsia A.K.V., Chong V.Y.L. Robotic Versus Conventional Laparoscopic Surgery for Colorectal Cancer: A Systematic Review and Meta-Analysis with Trial Sequential Analysis. World J. Surg. 2019;43:1146–1161. doi: 10.1007/s00268-018-04896-7. [DOI] [PubMed] [Google Scholar]
- 122.Lacy A.M., Delgado S., Castells A., Prins H.A., Arroyo V., Ibarzabal A., Pique J.M. The long-term results of a randomized clinical trial of laparoscopy-assisted versus open surgery for colon cancer. Ann. Surg. 2008;248:1–7. doi: 10.1097/SLA.0b013e31816a9d65. [DOI] [PubMed] [Google Scholar]
- 123.Leung K.L., Kwok S.P., Lam S.C., Lee J.F., Yiu R.Y., Ng S.S., Lai P.B., Lau W.Y. Laparoscopic resection of rectosigmoid carcinoma: prospective randomised trial. Lancet. 2004;363:1187–1192. doi: 10.1016/S0140-6736(04)15947-3. [DOI] [PubMed] [Google Scholar]
- 124.Tan W.J., Chew M.H., Dharmawan A.R., Singh M., Acharyya S., Loi C.T., Tang C.L. Critical appraisal of laparoscopic vs open rectal cancer surgery. World J. Gastrointest. Surg. 2016;8:452–460. doi: 10.4240/wjgs.v8.i6.452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jayne D.G., Guillou P.J., Thorpe H., Quirke P., Copeland J., Smith A.M., Heath R.M., Brown J.M., Group U.M.C.T. Randomized trial of laparoscopic-assisted resection of colorectal carcinoma: 3-year results of the UK MRC CLASICC Trial Group. J. Clin. Oncol. 2007;25:3061–3068. doi: 10.1200/JCO.2006.09.7758. [DOI] [PubMed] [Google Scholar]
- 126.Liang J.T., Huang K.C., Lai H.S., Lee P.H., Jeng Y.M. Oncologic results of laparoscopic versus conventional open surgery for stage II or III left-sided colon cancers: a randomized controlled trial. Ann. Surg. Oncol. 2007;14:109–117. doi: 10.1245/s10434-006-9135-4. [DOI] [PubMed] [Google Scholar]
- 127.Fleshman J., Branda M.E., Sargent D.J., Boller A.M., George V.V., Abbas M.A., Peters W.R., Jr., Maun D.C., Chang G.J., Herline A., et al. Disease-free Survival and Local Recurrence for Laparoscopic Resection Compared With Open Resection of Stage II to III Rectal Cancer: Follow-up Results of the ACOSOG Z6051 Randomized Controlled Trial. Ann. Surg. 2019;269:589–595. doi: 10.1097/SLA.0000000000003002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Bonjer H.J., Deijen C.L., Abis G.A., Cuesta M.A., van der Pas M.H., de Lange-de Klerk E.S., Lacy A.M., Bemelman W.A., Andersson J., Angenete E., et al. A randomized trial of laparoscopic versus open surgery for rectal cancer. N. Engl. J. Med. 2015;372:1324–1332. doi: 10.1056/NEJMoa1414882. [DOI] [PubMed] [Google Scholar]
- 129.Jayne D., Pigazzi A., Marshall H., Croft J., Corrigan N., Copeland J., Quirke P., West N., Rautio T., Thomassen N., et al. Effect of Robotic-Assisted vs Conventional Laparoscopic Surgery on Risk of Conversion to Open Laparotomy Among Patients Undergoing Resection for Rectal Cancer: The ROLARR Randomized Clinical Trial. JAMA. 2017;318:1569–1580. doi: 10.1001/jama.2017.7219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Jayne D., Pigazzi A., Marshall H., Croft J., Corrigan N., Copeland J., Quirke P., West N., Edlin R., Hulme C., et al. Robotic-assisted surgery compared with laparoscopic resection surgery for rectal cancer: The ROLARR RCT. Effic. Mech. Eval. 2019;6 doi: 10.3310/eme06100. [DOI] [PubMed] [Google Scholar]
- 131.Storli K.E., Sondenaa K., Furnes B., Eide G.E. Outcome after introduction of complete mesocolic excision for colon cancer is similar for open and laparoscopic surgical treatments. Dig. Surg. 2013;30:317–327. doi: 10.1159/000354580. [DOI] [PubMed] [Google Scholar]
- 132.Storli K.E., Eide G.E. Laparoscopic Complete Mesocolic Excision versus Open Complete Mesocolic Excision for Transverse Colon Cancer: Long-Term Survival Results of a Prospective Single Centre Non-Randomized Study. Dig. Surg. 2016;33:114–120. doi: 10.1159/000442716. [DOI] [PubMed] [Google Scholar]
- 133.Shin J.W., Amar A.H., Kim S.H., Kwak J.M., Baek S.J., Cho J.S., Kim J. Complete mesocolic excision with D3 lymph node dissection in laparoscopic colectomy for stages II and III colon cancer: long-term oncologic outcomes in 168 patients. Tech. Coloproctol. 2014;18:795–803. doi: 10.1007/s10151-014-1134-z. [DOI] [PubMed] [Google Scholar]
- 134.Karanikolic A., Golubovic I., Radojkovic M., Pavlovic M., Sokolovic D., Kovacevic P. Comparison of recurrence patterns of colorectal cancer in laparoscopic and open surgery groups of patients: A meta-analysis. J. BUON. 2018;23:302–311. [PubMed] [Google Scholar]
- 135.Negoi I., Hostiuc S., Negoi R.I., Beuran M. Laparoscopic vs open complete mesocolic excision with central vascular ligation for colon cancer: A systematic review and meta-analysis. World J. Gastrointest. Oncol. 2017;9:475–491. doi: 10.4251/wjgo.v9.i12.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Prete F.P., Pezzolla A., Prete F., Testini M., Marzaioli R., Patriti A., Jimenez-Rodriguez R.M., Gurrado A., Strippoli G.F.M. Robotic Versus Laparoscopic Minimally Invasive Surgery for Rectal Cancer: A Systematic Review and Meta-analysis of Randomized Controlled Trials. Ann. Surg. 2018;267:1034–1046. doi: 10.1097/SLA.0000000000002523. [DOI] [PubMed] [Google Scholar]
- 137.Song J.L., Wu H., Yang J.Y. Laparoscopic donor right hepatectomy in a donor with type III portal vein anomaly: A case report. Medicine (Baltimore) 2019;98:e16736. doi: 10.1097/MD.0000000000016736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Merkel S., Wein A., Gunther K., Papadopoulos T., Hohenberger W., Hermanek P. High-risk groups of patients with Stage II colon carcinoma. Cancer. 2001;92:1435–1443. doi: 10.1002/1097-0142(20010915)92:6<1435::AID-CNCR1467>3.0.CO;2-N. [DOI] [PubMed] [Google Scholar]
- 139.Touchefeu Y., Provost-Dewitte M., Lecomte T., Morel A., Valo I., Mosnier J.F., Bossard C., Eugene J., Duchalais E., Chetritt J., et al. Clinical, histological, and molecular risk factors for cancer recurrence in patients with stage II colon cancer. Eur. J. Gastroenterol. Hepatol. 2016;28:1394–1399. doi: 10.1097/MEG.0000000000000725. [DOI] [PubMed] [Google Scholar]
- 140.Yamanaka T., Oki E., Yamazaki K., Yamaguchi K., Muro K., Uetake H., Sato T., Nishina T., Ikeda M., Kato T., et al. 12-Gene Recurrence Score Assay Stratifies the Recurrence Risk in Stage II/III Colon Cancer With Surgery Alone: The SUNRISE Study. J. Clin. Oncol. 2016;34:2906–2913. doi: 10.1200/JCO.2016.67.0414. [DOI] [PubMed] [Google Scholar]
- 141.Lavanchy J.L., Vaisnora L., Haltmeier T., Zlobec I., Brugger L.E., Candinas D., Schnuriger B. Oncologic long-term outcomes of emergency versus elective resection for colorectal cancer. Int. J. Colorectal Dis. 2019;34:2091–2099. doi: 10.1007/s00384-019-03426-8. [DOI] [PubMed] [Google Scholar]
- 142.Wanis K.N., Ott M., Van Koughnett J.A.M., Colquhoun P., Brackstone M. Long-term oncological outcomes following emergency resection of colon cancer. Int. J. Colorectal Dis. 2018;33:1525–1532. doi: 10.1007/s00384-018-3109-4. [DOI] [PubMed] [Google Scholar]
- 143.Tsikitis V.L., Larson D.W., Huebner M., Lohse C.M., Thompson P.A. A. Predictors of recurrence free survival for patients with stage II and III colon cancer. BMC Cancer. 2014;14:336. doi: 10.1186/1471-2407-14-336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Amri R., England J., Bordeianou L.G., Berger D.L. Risk Stratification in Patients with Stage II Colon Cancer. Ann. Surg. Oncol. 2016;23:3907–3914. doi: 10.1245/s10434-016-5387-9. [DOI] [PubMed] [Google Scholar]
- 145.Gertler R., Rosenberg R., Schuster T., Friess H. Defining a high-risk subgroup with colon cancer stages I and II for possible adjuvant therapy. Eur. J. Cancer. 2009;45:2992–2999. doi: 10.1016/j.ejca.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 146.Kumar A., Kennecke H.F., Renouf D.J., Lim H.J., Gill S., Woods R., Speers C., Cheung W.Y. Adjuvant chemotherapy use and outcomes of patients with high-risk versus low-risk stage II colon cancer. Cancer. 2015;121:527–534. doi: 10.1002/cncr.29072. [DOI] [PubMed] [Google Scholar]
- 147.Tersteeg J.J.C., van Esch L.M., Gobardhan P.D., Kint P.A.M., Rozema T., Crolla R., Schreinemakers J.M.J. Early local recurrence and one-year mortality of rectal cancer after restricting the neoadjuvant therapy regime. Eur. J. Surg. Oncol. 2019;45:597–605. doi: 10.1016/j.ejso.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 148.Ruppert R., Junginger T., Ptok H., Strassburg J., Maurer C.A., Brosi p., Sauer J., Baral J., Kreis M., Wollschlaeger D., et al. Oncological outcome after MRI-based selection for neoadjuvant chemoradiotherapy in the OCUM Rectal Cancer Trial. Br. J. Surg. 2018;105:1519–1529. doi: 10.1002/bjs.10879. [DOI] [PubMed] [Google Scholar]
- 149.Rasanen M., Carpelan-Holmstrom M., Mustonen H., Renkonen-Sinisalo L., Lepisto A. Pattern of rectal cancer recurrence after curative surgery. Int. J. Colorectal Dis. 2015;30:775–785. doi: 10.1007/s00384-015-2182-1. [DOI] [PubMed] [Google Scholar]
- 150.Tan W.J., Tan H.J., Dorajoo S.R., Foo F.J., Tang C.L., Chew M.H. Rectal Cancer Surveillance-Recurrence Patterns and Survival Outcomes from a Cohort Followed up Beyond 10 Years. J. Gastrointest. Cancer. 2018;49:422–428. doi: 10.1007/s12029-017-9984-z. [DOI] [PubMed] [Google Scholar]
- 151.Ishihara S., Murono K., Sasaki K., Yasuda K., Otani K., Nishikawa T., Tanaka T., Kiyomatsu T., Kawai K., Hata K., et al. Impact of Primary Tumor Location on Postoperative Recurrence and Subsequent Prognosis in Nonmetastatic Colon Cancers: A Multicenter Retrospective Study Using a Propensity Score Analysis. Ann. Surg. 2018;267:917–921. doi: 10.1097/SLA.0000000000002206. [DOI] [PubMed] [Google Scholar]
- 152.Poulsen L.O., Yilmaz M.K., Ljungmann K., Jespersen N., Wille-Jorgensen p., Petersen L.n., Falkmer U.G. Local recurrence rate in a national Danish patient cohort after curative treatment for rectal cancer. Acta Oncol. 2018;57:1639–1645. doi: 10.1080/0284186X.2018.1497299. [DOI] [PubMed] [Google Scholar]
- 153.Osterman E., Glimelius B. Recurrence Risk After Up-to-Date Colon Cancer Staging, Surgery, and Pathology: Analysis of the Entire Swedish Population. Dis. Colon. Rectum. 2018;61:1016–1025. doi: 10.1097/DCR.0000000000001158. [DOI] [PubMed] [Google Scholar]
- 154.Lise M., Gerard A., Nitti D., Zane D., Buyse M., Duez N., Arnaud J.P., Metzger U. Adjuvant therapy for colorectal cancer. The EORTC experience and a review of the literature. Dis. Colon. Rectum. 1987;30:847–854. doi: 10.1007/BF02555422. [DOI] [PubMed] [Google Scholar]
- 155.Hartung G., Hofheinz R.D., Dencausse Y., Sturm J., Kopp-Schneider A., Dietrich G., Fackler-Schwalbe I., Bornbusch D., Gonnermann M., Wojatschek C., et al. Adjuvant therapy with edrecolomab versus observation in stage II colon cancer: a multicenter randomized phase III study. Onkologie. 2005;28:347–350. doi: 10.1159/000084595. [DOI] [PubMed] [Google Scholar]
- 156.Fisher B., Costantino J.P., Wickerham D.L., Redmond C.K., Kavanah M., Cronin W.M., Vogel V., Robidoux A., Dimitrov n., Atkins J., et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project p-1 Study. J. Natl. Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 157.Song X.J., Liu Z.L., Zeng R., Ye W., Liu C.W. A meta-analysis of laparoscopic surgery versus conventional open surgery in the treatment of colorectal cancer. Medicine (Baltimore) 2019;98:e15347. doi: 10.1097/MD.0000000000015347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Gouvas N., Agalianos C., Papaparaskeva K., Perrakis A., Hohenberger W., Xynos E. Surgery along the embryological planes for colon cancer: a systematic review of complete mesocolic excision. Int. J. Colorectal Dis. 2016;31:1577–1594. doi: 10.1007/s00384-016-2626-2. [DOI] [PubMed] [Google Scholar]
- 159.Zhao B., Lv W., Mei D., Luo R., Bao S., Huang B., Lin J. Comparison of short-term surgical outcome between 3 D and 2 D laparoscopy surgery for gastrointestinal cancer: a systematic review and meta-analysis. Langenbecks Arch. Surg. 2020;405:1–12. doi: 10.1007/s00423-020-01853-8. [DOI] [PubMed] [Google Scholar]
- 160.West N.P., Hohenberger W., Weber K., Perrakis A., Finan P.J., Quirke P. Complete mesocolic excision with central vascular ligation produces an oncologically superior specimen compared with standard surgery for carcinoma of the colon. J. Clin. Oncol. 2010;28:272–278. doi: 10.1200/JCO.2009.24.1448. [DOI] [PubMed] [Google Scholar]
- 161.Kim N.K., Kim Y.W., Han Y.D., Cho M.S., Hur H., Min B.S., Lee K.Y. Complete mesocolic excision and central vascular ligation for colon cancer: Principle, anatomy, surgical technique, and outcomes. Surg. Oncol. 2016;25:252–262. doi: 10.1016/j.suronc.2016.05.009. [DOI] [PubMed] [Google Scholar]
- 162.Petrelli F., Ghidini M., Cabiddu M., Pezzica E., Corti D., Turati L., Costanzo A., Varricchio A., Ghidini A., Barni S., et al. Microsatellite Instability and Survival in Stage II Colorectal Cancer: A Systematic Review and Meta-analysis. Anticancer Res. 2019;39:6431–6441. doi: 10.21873/anticanres.13857. [DOI] [PubMed] [Google Scholar]
- 163.Bruzzi M., Auclin E., Lo Dico R., Voron T., Karoui M., Espin E., Cianchi F., Weitz J., Buggenhout A., Malafosse R., et al. Influence of Molecular Status on Recurrence Site in Patients Treated for a Stage III Colon Cancer: a Post Hoc Analysis of the PETACC-8 Trial. Ann. Surg. Oncol. 2019;26:3561–3567. doi: 10.1245/s10434-019-07513-6. [DOI] [PubMed] [Google Scholar]
- 164.Mukherji R., Marshall J.L., Seeber A. Genomic Alterations and Their Implications on Survival in Nonmetastatic Colorectal Cancer: Status Quo and Future Perspectives. Cancers (Basel) 2020;12:2001. doi: 10.3390/cancers12082001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Guinney J., Dienstmann R., Wang X., de Reynies A., Schlicker A., Soneson C., Marisa L., Roepman P., Nyamundanda G., Angelino P., et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Merkel S., Mansmann U., Papadopoulos T., Wittekind C., Hohenberger W., Hermanek P. The prognostic inhomogeneity of colorectal carcinomas Stage III: a proposal for subdivision of Stage III. Cancer. 2001;92:2754–2759. doi: 10.1002/1097-0142(20011201)92:11<2754::AID-CNCR10083>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 167.Glimelius B., Melin B., Enblad G., Alafuzoff I., Beskow A., Ahlstrom H., Bill-Axelson A., Birgisson H., Bjor O., Edqvist P.H., et al. U-CAN: a prospective longitudinal collection of biomaterials and clinical information from adult cancer patients in Sweden. Acta Oncol. 2018;57:187–194. doi: 10.1080/0284186X.2017.1337926. [DOI] [PubMed] [Google Scholar]
- 168.Osterman E., Mezheyeuski A., Sjoblom T., Glimelius B. Beyond the NCCN Risk Factors in Colon Cancer: An Evaluation in a Swedish Population-Based Cohort. Ann. Surg. Oncol. 2020;27:1036–1945. doi: 10.1245/s10434-019-08148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Erlandsson J., Martling A. Short-course radiotherapy with delayed surgery for rectal cancer - Authors’ reply. Lancet Oncol. 2017;18:e295. doi: 10.1016/S1470-2045(17)30323-6. [DOI] [PubMed] [Google Scholar]
- 170.Jia X.X., Wang Y., Cheng J., Yao X., Yin M.J., Zhou J., Ye Y.J. Low- Versus High-Risk Rectal Cancer Based on MRI Features: Outcomes in Patients Treated Without Neoadjuvant Chemoradiotherapy. AJR Am. J. Roentgenol. 2018;211:327–334. doi: 10.2214/AJR.17.18980. [DOI] [PubMed] [Google Scholar]
- 171.Martling A.L., Holm T., Rutqvist L.E., Moran B.J., Heald R.J., Cedemark B. Effect of a surgical training programme on outcome of rectal cancer in the County of Stockholm. Lancet. 2000;356:93–96. doi: 10.1016/S0140-6736(00)02469-7. [DOI] [PubMed] [Google Scholar]
- 172.Kodeda K., Nathanaelsson L., Jung B., Olsson H., Jestin P., Sjovall A., Glimelius B., Pahlman L., Syk I. Population-based data from the Swedish Colon Cancer Registry. Br. J. Surg. 2013;100:1100–1107. doi: 10.1002/bjs.9166. [DOI] [PubMed] [Google Scholar]
- 173.Kodeda K., Johansson R., Zar N., Birgisson H., Dahlberg M., Skullman S., Lindmark G., Glimelius B., Pahlman L., Martling A. Time trends, improvements and national auditing of rectal cancer management over an 18-year period. Colorectal Dis. 2015;17:O168–O179. doi: 10.1111/codi.13060. [DOI] [PubMed] [Google Scholar]
- 174.Bernhoff R., Martling A., Sjovall A., Granath F., Hohenberger W., Holm T. Improved survival after an educational project on colon cancer management in the county of Stockholm - A population based cohort study. Eur. J. Surg. Oncol. 2015;41:1479–1484. doi: 10.1016/j.ejso.2015.07.019. [DOI] [PubMed] [Google Scholar]
- 175.Påhlman L., Bohe M., Cedermark B., Dahlberg M., Lindmark G., Sjodahl R., Ojerskog B., Damber L., Johansson R. The Swedish rectal cancer registry. Br. J. Surg. 2007;94:1285–1292. doi: 10.1002/bjs.5679. [DOI] [PubMed] [Google Scholar]
- 176.Moberger P., Skoldberg F., Birgisson H. Evaluation of the Swedish Colorectal Cancer Registry: an overview of completeness, timeliness, comparability and validity. Acta Oncol. 2018;57:1611–1621. doi: 10.1080/0284186X.2018.1529425. [DOI] [PubMed] [Google Scholar]
- 177.Nilsson P.J., van Etten B., Hospers G.A., Pahlman L., van de Velde C.J., Beets-Tan R.G., Blomqvist L., Beukema J.C., Kapiteijn E., Marijnen C.A., et al. Short-course radiotherapy followed by neo-adjuvant chemotherapy in locally advanced rectal cancer--the RAPIDO trial. BMC Cancer. 2013;13:279. doi: 10.1186/1471-2407-13-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Van Cutsem E., Cervantes A., Adam R., Sobrero A., Van Krieken J.H., Aderka D., Aranda Aguilar E., Bardelli A., Benson A., Bodoky G., et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann. Oncol. 2016;27:1386–1422. doi: 10.1093/annonc/mdw235. [DOI] [PubMed] [Google Scholar]
- 179.Glynne-Jones R., Wyrwicz L., Tiret E., Brown G., Rödel C., Cervantes A., Arnold D. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann. Oncol. 2017;28:iv22–iv40. doi: 10.1093/annonc/mdx224. [DOI] [PubMed] [Google Scholar]
- 180.Thompson T.D., Pollack L.A., Johnson C.J., Wu X.C., Rees J.R., Hsieh M.C., Rycroft R., Culp M., Wilson R., Wu M., et al. Breast and colorectal cancer recurrence and progression captured by five U.S. population-based registries: Findings from National Program of Cancer Registries patient-centered outcome research. Cancer Epidemiol. 2020;64:101653. doi: 10.1016/j.canep.2019.101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Lysholm J., Lindahl B. Strong development of research based on national quality registries in Sweden. Ups. J. Med. Sci. 2019;124:9–11. doi: 10.1080/03009734.2018.1520761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Kodeda K., Holmberg E., Steineck G., Nordgren S. Regional differences in local recurrence rates after rectal cancer surgery. Colorectal Dis. 2010;12:e206–e215. doi: 10.1111/j.1463-1318.2009.02137.x. [DOI] [PubMed] [Google Scholar]
- 183.Folprecht G., Grothey A., Alberts S., Raab H.R., Kohne C.H. Neoadjuvant treatment of unresectable colorectal liver metastases: correlation between tumour response and resection rates. Ann. Oncol. 2005;16:1311–1319. doi: 10.1093/annonc/mdi246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.