Abstract
Background
It is not clear whether clinical practice guidelines (CPGs) and consensus statements (CSs) are adequately promoting shared decision making (SDM).
Objective
To evaluate the recommendations about SDM in CPGs and CSs concerning breast cancer (BC) treatment.
Search strategy
Following protocol registration (Prospero no.: CRD42018106643), CPGs and CSs on BC treatment were identified, without language restrictions, through systematic search of bibliographic databases (MEDLINE, EMBASE, Web of Science, Scopus, CDSR) and online sources (12 guideline databases and 51 professional society websites) from January 2010 to December 2019.
Inclusion criteria
CPGs and CSs on BC treatment were selected whether published in a journal or in an online document.
Data extraction and synthesis
A 31‐item SDM quality assessment tool was developed and used to extract data in duplicate.
Main results
There were 167 relevant CPGs (139) and CSs (28); SDM was reported in only 40% of the studies. SDM was reported more often in recent publications after 2015 (42/101 (41.6 %) vs 46/66 (69.7 %), P = .0003) but less often in medical journal publications (44/101 (43.5 %) vs 17/66 (25.7 %), P = .009). In CPGs and CSs with SDM, only 8/66 (12%) met one‐fifth (6 of 31) of the quality items; only 14/66 (8%) provided clear and precise SDM recommendations.
Discussion and conclusions
SDM descriptions and recommendations in CPGs and CSs concerning BC treatment need improvement. SDM was more frequently reported in CPGs and CSs in recent years, but surprisingly it was less often covered in medical journals, a feature that needs attention.
Keywords: breast cancer, breast cancer treatment, clinical practice guidelines, consensus, shared decision making
1. INTRODUCTION
Breast cancer (BC) is the most common cancer in women, with 2.1 million new cases each year (25% of all female cancers), and it also causes the greatest number (about 670000 in 2018, 15%) of cancer‐related deaths among women 1 , 2 . Mortality and morbidity from BC have decreased in recent years thanks to early diagnosis and the combination of new treatments in a growing array of different strategies 3 , 4 . The best BC treatment must be personalized 4 , 5 , and choosing the ideal approach requires a high degree of specialization, scientific‐technical updating, multidisciplinary coordination and patient participation 6 , 7 , 8 , 9 .
This participation in shared decision making (SDM) is considered a keystone in the achievement of sustainable high‐quality cancer care, and it becomes especially important when separate treatment options with overall similar potential can yield very different results depending on patients' preferences 9 , 10 . In developed countries, SDM is a legal obligation 11 , 12 , 13 , and it has been shown to increase the satisfaction of the patient 9 , improve cost‐effectiveness 9 and reduce malpractice lawsuit 14 . It is claimed to be a keystone to guarantee good quality cancer care 9 , and it is highly recommended by medical associations 15 , 16 , 17 .
The implementation of SDM has persistent barriers 18 , 19 , 20 , 21 , 22 , and it is still poor 23 , 24 . Many authors have proposed strategies for promotion and practical application of SDM 10 , 21 , 25 , 26 , 27 , 28 . A three‐step model introducing choice, describing options and exploring preferences has been suggested 10 . Another proposal involves encouraging patients to make their own care goals that clinicians translate into treatment plans 21 , 25 . Option Grids and other decision aids are thought to make the SDM process easier 26 , 27 . Measuring SDM as a quality indicator and reimbursing professionals that actually use SDM have been floated as another idea involving incentivization 28 .
This important subject should be adequately covered in clinical practice guidelines (CPGs) and consensus statements (CSs), especially in those that are published in a medical journal. The aim of this systematic review was to evaluate the characteristics of CPGs and CSs with SDM compared to those without, to develop an SDM quality assessment tool and to collate the specific information and recommendations about SDM concerning BC treatment in women.
2. METHODS
This systematic review was carried out following protocol registration (Prospero No: CRD42018106643) and using a prospective protocol developed based on recommended methods for literature searches and assessment of guidelines. During the course of the work, no SDM assessment tool was identified in the literature, so we developed such a tool for data extraction in our work. It was reported according to the preferred reporting items for systematic reviews and meta‐analyses (PRISMA) 29 , 30 (see Appendix 1).
2.1. Data sources and searches
A systematic search combining MeSH terms "shared decision making", "clinical practice guidelines", "guidelines", "consensus", “breast cancer”, “breast cancer treatment” and including word variants was conducted using MEDLINE covering the period January 2010 to December 2019, without language restrictions. We further searched online databases (EMBASE, Web of Science, Scopus, CDSR, etc.), 12 guideline‐specific databases and 51 websites of relevant professional societies (see Appendix ). For completeness, we searched on the World Wide Web and the bibliographies of known relevant publications to identify additional studies of relevance to the review.
2.2. Study selection and data extraction
We included CPGs and CSs about BC management, produced by governmental agencies or national and international professional organizations and societies. We excluded CPGs and CSs about screening and diagnosis, obsolete guidelines replaced by updates from the same organization, and CPG and CSs for education and information purpose only.
Two reviewers (MMC and IMMN) independently considered the potential eligibility of each of the titles and abstracts from the citations and requested full‐text versions. Working independently, reviewers assessed the full text to confirm eligibility. Disagreements were resolved by consensus or arbitration by a third reviewer (MMD). Duplicate articles were identified and removed. Where multiple versions of a CPG or CS were retrieved, the most recent version was reviewed. Data were extracted from selected CPGs and CSs in duplicate, independently. The intraclass correlation coefficient (ICC) was used to assess consistency between reviewers in data extraction, and the reliability level was excellent >0.90 31 . Authoritative guidance 32 on systematic review methods recommends inter‐reviewer reliability assessment that is designed to compare measurements obtained by two or more reviewers extracting data from the same papers.
2.3. Guideline quality assessment and data extraction
We conducted a search to identify a quality assessment tool for SDM. No relevant tools were identified, so we constructed one using consensus to create a checklist from a long list of items identified in the literature searches. The quality of CPGs and CSs for SDM to manage patients with BC was independently evaluated by two different reviewers (MMC and IMMN) using a piloted data extraction form. Disagreements between the two authors (MMC and IMMN) over the risk of bias for particular studies were solved by group discussion involving an arbitrator (MMD) who took the final decision.
2.4. Data synthesis
Two authors (MMC and IMMN) synthesized the data extracted to summarize key information within using a piloted data extraction form concerning characteristics of CPGs and CSs with the SDM information and recommendations contained within them. Rate data were compared using chi‐square test to examine whether CPGs and CSs with SDM were different to those without SDM.
3. RESULTS
3.1. Study selection
Of the 4116 potential citations identified, a total of 167 documents (139 CPGs 33 , 34 , 35 , 36 , 37 , 38 , 39 , 40 , 41 , 42 , 43 , 44 , 45 , 46 , 47 , 48 , 49 , 50 , 51 , 52 , 53 , 54 , 55 , 56 , 57 , 58 , 59 , 60 , 61 , 62 , 63 , 64 , 65 , 66 , 67 , 68 , 69 , 70 , 71 , 72 , 73 , 74 , 75 , 76 , 77 , 78 , 79 , 80 , 81 , 82 , 83 , 84 , 85 , 86 , 87 , 88 , 89 , 90 , 91 , 92 , 93 , 94 , 95 , 96 , 97 , 98 , 99 , 100 , 101 , 102 , 103 , 104 , 105 , 106 , 107 , 108 , 109 , 110 , 111 , 112 , 113 , 114 , 115 , 116 , 117 , 118 , 119 , 120 , 121 , 122 , 123 , 124 , 125 , 126 , 127 , 128 , 129 , 130 , 131 , 132 , 133 , 134 , 135 , 136 , 137 , 138 , 139 , 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 , 149 , 150 , 151 , 152 , 153 , 154 , 155 , 156 , 157 , 158 , 159 , 160 , 161 , 162 , 163 , 164 , 165 , 166 , 167 , 168 , 169 , 170 , 171 and 28 CSs 172 , 173 , 174 , 175 , 176 , 177 , 178 , 179 , 180 , 181 , 182 , 183 , 184 , 185 , 186 , 187 , 188 , 189 , 190 , 191 , 192 , 193 , 194 , 195 , 196 , 197 , 198 , 199 ) were identified for final evaluation (Figure 1). ICC for reviewer agreement was 0.97.
Figure 1.
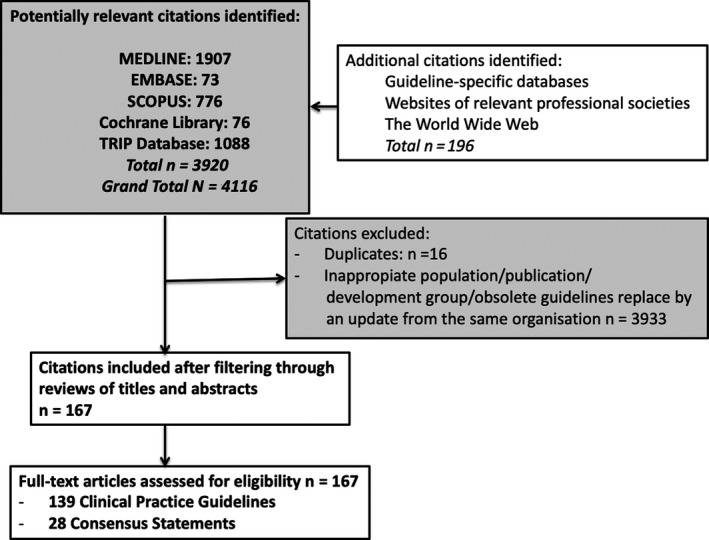
Flow diagram for study selection of CPGs and CSs
3.2. Development of a quality assessment tool
Individual quality items were scattered across a number of tools for guidelines assessment 200 , 201 . A long list of items was compiled and presented to a group of four BC and SDM specialists in a consensus meeting. This process including several revisions and iterations which led to a 31‐item checklist grouped into thirteen domains (see Appendix ). Of these, 68% (n = 21) were identified from the AGREE 201 and 48% (n = 15) from the RIGHT 200 tools. Only 13% (n = 4) of these items did not appear in any of these two tools. However, the expert consensus advised their inclusion after examining other literature in the bibliography of interest about SDM 9 , 21 , 24 , 25 , 27 . The consensus meeting following approval of the 31‐item checklist recommended that each item be examined for compliance. The greater the percentage of items complied with, the greater the quality for SDM in the CPG or CS assessed. The consensus meeting did not recommend the construction of a formal score or a cut point for defining quality.
3.3. Study characteristics
The distribution by countries of CPGs and CSs that speak about SDM was irregular (Figure 1). Europe stood out with a total of 25 CPGs and CSs (38%). North America developed 29 (44%) CPGs and CSs (USA: 19 and Canada: 10). South America released six (9%) CPGs and CSs (Colombia, Venezuela, Mexico, Peru and two from Costa Rica). Asia also carried out three (5%) CPGs and CSs (Japan, India and Malaysia). Oceania has developed also three (5%)CPGs and CSs: two from Australia and one from New Zealand. The basic characteristics of the CPGs and CSs including organization, country and year of release are summarized in Table 1. The duration since last update of each CPGs or CSs varied. Some AGO 46 , 48 , 49 , 59 , all the NCCN 149 , 150 , 151 , 152 , 153 and one of the AHS 89 CPGs, and ESMO 178 and the Mexican CS 173 were the most recently updated (highlighted in Table 2). Overall, the last update of the CPGs and CSs with SDM was more recent than that of those without SDM (mean 45 months (range: 3‐115) vs 52 months (range: 3‐116), P < .001). In this comparison, 9% (n = 15/167) did not specify the month of updated but only the year. SDM was reported more often in recent CPGs and CSs published after 2015 (42/101 (42.0%) vs 46/66 (69.7%), P =.0003) but less often in CPGs and CSs published in medical journal (44/101 (43.5%) vs 17/66 (25.7%), P = .009) (Table 3).
Table 1.
Description of the CPGs and CSs (n = 167) selected for the systematic review on the quality of reporting concerning SDM in BC treatment
| Abbreviated name | Entity | Country | Year | ||
|---|---|---|---|---|---|
| Name of the CPG | |||||
| 1 | Guidelines on the diagnosis and treatment of breast cancer (2011 edition) 32 | Chinese BC CPG 32 | CMH | China | 2012 |
| 2 | Chinese guidelines for diagnosis and treatment of breast cancer 2018 33 | Chinese BC diagnosis treatment 33 | NHCPRC | China | 2018 |
| 3 | The Japanese Breast Cancer Society Clinical Practice Guideline for radiation treatment of breast cancer, 2015 edition 34 | Japanese RT BC CPG 34 | JBCS | Japan | 2015 |
| 4 | The Japanese Breast Cancer Society Clinical Practice Guideline for systemic treatment of breast cancer, 2015 edition 35 | Japanese systemic BC CPG 35 | JBCS | Japan | 2015 |
| 5 | 2013 clinical practice guidelines (The Japanese Breast Cancer Society): history, policy and mission 36 | Japanese treatment BC CPG 36 | JBCS | Japan | 2014 |
| 6 | Singapore Cancer Network (SCAN) Guidelines for Adjuvant Trastuzumab Use in Early Stage HER2 Positive Breast Cancer 37 | SCAN early BC 37 | SCAN | Singapore | 2015 |
| 7 | Singapore Cancer Network (SCAN) Guidelines for Bisphosphonate Use in the Adjuvant Breast Cancer Setting 38 | SCAN adjuvant BC treatment 38 | SCAN | Singapore | 2015 |
| 8 | Breast cancer in women: diagnosis, treatment and follow‐up 39 | KCE BC CPG 39 | KCE | Belgium | 2015 |
| 9 | Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up 40 | ESMO BC 2019 40 | ESMO | Europe | 2019 |
| 10 | International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO) 41 | ESO MBC 41 | ESO | Europe | 2013 |
| 11 | The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer 42 | EUSOMA 2012 42 | EUSOMA | Europe | 2012 |
| 12 | AGO Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2019 43 | AGO early BC 43 | AGO | Germany | 2019 |
| 13 | Lesions of Uncertain Malignant Potential (B3) (ADH, LIN, FEA, Papilloma, Radial Scar) 44 | AGO uncertain lesions 44 | AGO | Germany | 2019 |
| 14 | Ductal Carcinoma in Situ (DCIS) 45 | AGO DCIS 45 | AGO | Germany | 2019 |
| 15 | Breast Cancer Surgery Oncological Aspects 46 | AGO oncological 46 | AGO | Germany | 2019 |
| 16 | Oncoplastic and Reconstructive Surgery 47 | AGO oncoplastic 47 | AGO | Germany | 2019 |
| 17 | Adjuvant Endocrine Therapy in Pre‐ and Postmenopausal Patients 48 | AGO adjuvant endocrine 48 | AGO | Germany | 2019 |
| 18 | Adjuvant Cytotoxic and Targeted Therapy 49 | AGO cytotoxic 49 | AGO | Germany | 2019 |
| 19 | Neoadjuvant (Primary) Systemic Therapy 50 | AGO neoadjuvant 50 | AGO | Germany | 2019 |
| 20 | Adjuvant Radiotherapy 51 | AGO RT 51 | AGO | Germany | 2019 |
| 21 | Therapy Side Effects 52 | AGO side effects 52 | AGO | Germany | 2019 |
| 22 | Supportive Care 53 | AGO supportive care 53 | AGO | Germany | 2019 |
| 23 | Breast Cancer: Specific Situations 54 | AGO‐specific situations 54 | AGO | Germany | 2019 |
| 24 | Breast Cancer Follow‐Up 55 | AGO follow‐up 55 | AGO | Germany | 2019 |
| 25 | Loco‐Regional Recurrence 56 | AGO recurrence 56 | AGO | Germany | 2019 |
| 26 | Endocrine and “Targeted” Therapy in Metastatic Breast Cancer 57 | AGO endocrine MBC 57 | AGO | Germany | 2019 |
| 27 | Chemotherapy With or Without Targeted Drugs* in Metastatic Breast Cancer 58 | AGO CT MBC 58 | AGO | Germany | 2019 |
| 28 | Osteooncology and Bone Health 59 | AGO osteooncology 59 | AGO | Germany | 2019 |
| 29 | Specific Sites of Metastases 60 | AGO‐specific MBC 60 | AGO | Germany | 2019 |
| 30 | CNS Metastases in Breast Cancer 61 | AGO CNS MBC 61 | AGO | Germany | 2019 |
| 31 | Complementary Therapy Survivorship 62 | AGO survivorship 62 | AGO | Germany | 2019 |
| 32 | Diagnosis and Treatment of Patients with Primary and Metastatic Breast Cancer 63 | AGO primary MBC 63 | AGO | Germany | 2018 |
| 33 | AGO Recommendations for the Diagnosis and Treatment of Patients with Advanced and Metastatic Breast Cancer: Update 2018 64 | AGO advanced MBC 64 | AGO | Germany | 2018 |
| 34 | DEGRO practical guidelines for radiotherapy of breast cancer VI: therapy of locoregional breast cancer recurrences 65 |
DEGRO BC recurrences 65 |
2014 | ||
| 35 | DEGRO practical guidelines: radiotherapy of breast cancer I. Radiotherapy following breast conserving therapy for invasive breast cancer. 66 | DEGRO RT conserving BC 66 | DEGRO | Germany | 2013 |
| 36 | DEGRO practical guidelines for radiotherapy of breast cancer IV. Radiotherapy following mastectomy for invasive breast cancer 67 | DEGRO RT mastectomy BC 67 | DEGRO | Germany | 2014 |
| 37 | DEGRO practical guidelines: radiotherapy of breast cancer III—radiotherapy of the lymphatic pathways 68 | DEGRO RT lymphatic 68 | DEGRO | Germany | 2014 |
| 38 | Diagnosis, staging and treatment of patients with breast cancer. National Clinical Guideline No. 7 69 | NCCP 69 | NCCP | Ireland | 2015 |
| 39 | Breast cancer 70 | Richtlijnendatabase BC 70 | Richtlijnen | Netherlands | 2018 |
| 40 | Dutch breast reconstruction guideline 71 | Dutch BCR 71 | DPRS | Netherlands | 2017 |
| 41 | Breast Cancer 72 | IKNL BC 72 | IKNL | Netherlands | 2012 |
| 42 | Cáncer de mama/ Breast Cancer 73 | Fisterra BC 73 | Fisterra | Spain | 2017 |
| 43 | SEOM clinical guidelines in early‐stage breast cancer 74 | SEOM early stage 74 | SEOM | Spain | 2018 |
| 44 | SEOM clinical guidelines in advanced and recurrent breast cancer 75 | SEOM advanced BC 75 | SEOM | Spain | 2018 |
| 45 | SEOM clinical guidelines in metastatic breast cancer 76 | SEOM MBC 76 | SEOM | Spain | 2015 |
| 46 | SEOM clinical guidelines in Hereditary Breast and ovarian cancer 77 | SEOM hereditary BC 77 | SEOM | Spain | 2015 |
| 47 | Abemaciclib with fulvestrant for treating hormone receptor‐positive, HER2‐negative advanced breast cancer after endocrine the therapy 78 | NICE abemaciclib 78 | NICE | UK | 2019 |
| 48 | Ribociclib with fulvestrant for treating hormone receptor‐positive, HER2‐negative advanced breast cancer 79 | NICE ribociclib 79 | NICE | UK | 2019 |
| 49 | Early and locally advanced breast cancer: diagnosis and management 80 | NICE early and advanced BC 80 | NICE | UK | 2018 |
| 50 | Breast cancer 81 | NICE BC 81 | NICE | UK | 2011 |
| 51 | Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer 82 | NICE familial BC 82 | NICE | UK | 2013 |
| 52 | Breast reconstruction using lipomodelling after breast cancer treatment 83 | NICE lipomodelling 83 | NICE | UK | 2012 |
| 53 | Gene expression profiling and expanded immunohistochemistry tests for guiding adjuvant chemotherapy decisions in early breast cancer management: MammaPrint, Oncotype DDX,X, IHC4 and Mammostrat 84 | NICE gene expression 84 | NICE | UK | 2013 |
| 54 | Pertuzumab for the neoadjuvant treatment of HER2‐positive breast cancer 85 | NICE pertuzumab BC 85 | NICE | UK | 2016 |
| 55 | Intraoperative tests (RD‐100i OSNA system and Metasin test) for detecting sentinel lymph node metastases in breast cancer 86 | NICE sentinel lymph 86 | NICE | UK | 2013 |
| 56 | Breast reconstruction following prophylactic or therapeutic mastectomy for breast cancer 87 | AHS reconstruction BC 87 | AHS | Canada | 2017 |
| 57 | Adjuvant systemic therapy for early stage (lymph node negative and lymph node positive) breast cancer 88 | AHS early BC 88 | AHS | Canada | 2018 |
| 58 | Optimal use of taxanes in metastatic breast cancer (MBC) 89 | AHS MBC 89 | AHS | Canada | 2013 |
| 59 | Adjuvant radiation therapy for invasive breast cancer 90 | AHS RT invasive 90 | AHS | Canada | 2015 |
| 60 | Adjuvant radiation therapy for ductal carcinoma in situ 91 | AHS RT DCI 91 | AHS | Canada | 2015 |
| 61 | Neo‐adjuvant (pre‐operative) therapy for breast cancer ‐ general considerations 92 | AHS neo‐adjuvant 92 | AHS | Canada | 2014 |
| 62 | The Role of Trastuzumab in Adjuvant and Neoadjuvant Therapy in Women with HER2/neu‐overexpressing Breast Cancer 93 |
CCO trastuzumab Her2 + BC 93 |
CCO | Canada | 2011 |
| 63 | Surgical management of early‐stage invasive breast cancer 94 | CCO surgical management BC 94 | CCO | Canada | 2015 |
| 64 | Breast irradiation in women with early stage invasive breast cancer following breast conserving surgery 95 | CCO RT 95 | CCO | Canada | 2016 |
| 65 | The role of the taxanes in the management of metastatic breast cancer 96 | CCO taxane MBC 96 | CCO | Canada | 2011 |
| 66 | Vinorelbine in stage IV breast cancer 97 | CCO vinorelbine 97 | CCO | Canada | 2012 |
| 67 | The role of aromatase inhibitors in the treatment of postmenopausal women with metastatic breast cancer 98 | CCO aromatase inhibitor MBC 98 | CCO | Canada | 2012 |
| 68 | Epirubicin, as a single agent or in combination, for metastatic breast cancer 99 | CCO epirubicin MBC 99 | CCO | Canada | 2011 |
| 69 | Adjuvant taxane therapy for women with early‐stage, invasive breast cancer 100 | CCO taxane adjuvant therapy BC 100 | CCO | Canada | 2011 |
| 70 | Adjuvant systemic therapy for node‐negative breast cancer 101 | CCO sQT for node‐negative BC 101 | CCO | Canada | 2011 |
| 71 | Adjuvant ovarian ablation in the treatment of premenopausal women with early stage invasive breast cancer 102 | CCO ovarian ablation early stage 102 | CCO | Canada | 2010 |
| 72 | The role of gemcitabine in the management of metastatic breast cancer 103 | CCO gemcitabine 103 | CCO | Canada | 2011 |
| 73 | The role of trastuzumab (herceptin) in the treatment of women with Her2/neu‐overexpressing metastatic breast cancer 104 | CCO trastuzumab MBC 104 | CCO | Canada | 2010 |
| 74 | Capecitabine in stage IV breast cancer 105 | CCO capecitabine 105 | CCO | Canada | 2011 |
| 75 | The role of her2/neu in systemic and radiation therapy for women with breast cancer 106 | CCO her2/neu and RT treatment 106 | CCO | Canada | 2012 |
| 76 | Locoregional therapy of locally advanced breast cancer (LABC) 107 | CCO LABC 107 | CCO | Canada | 2014 |
| 77 | The role of taxanes in neoadjuvant chemotherapy for women with non‐metastatic breast cancer 108 | CCO taxane neoadjuvant therapy 108 | CCO | Canada | 2011 |
| 78 | Optimal systemic therapy for early female breast cancer 109 | CCO early BC 109 | CCO | Canada | 2014 |
| 79 | Use of adjuvant bisphosphonates and other bone‐modifying agents in breast cancer 110 | CCO bone‐modifying agent BC 110 | CCO | Canada | 2016 |
| 80 | The Role of Aromatase Inhibitors in Adjuvant Therapy for Postmenopausal Women with Hormone Receptor‐positive Breast Cancer 111 |
CCO aromatase inhibitors HR + 111 |
CCO | Canada | 2012 |
| 81 | Margin width in breast conservation Surgery 112 | ABS margin width BC 112 | ABS | UK | 2015 |
| 82 | Antibiotic prophylaxis in breast surgery 113 | ABS AB prophylaxis 113 | ABS | UK | 2015 |
| 83 | Management of The malignant axilla In early breast cancer 114 | ABS axila BC 114 | ABS | UK | 2015 |
| 84 | Breast operation note Documentation 115 | ABS BC 115 | ABS | UK | 2015 |
| 85 | Update on optimal duration of adjuvant antihormonal therapy 116 | ABS antihormonal therapy 116 | ABS | UK | 2015 |
| 86 | Oncoplastic breast reconstruction 117 | ABS/BAPRAS oncoplastic 117 | ABS, BAPRAS | UK | 2012 |
| 87 | Acellular dermal matrix (ADM) assisted breast reconstruction procedures 118 | ABS/BAPRAS ADM 118 | ABS, BAPRAS | UK | 2012 |
| 88 | Breast Cancer Clinical Quality Performance Indicators 119 | SCT quality indicators 119 | SCT | UK | 2016 |
| 89 | Treatment of primary breast cancer 120 | SIGN 120 | SIGN | UK | 2013 |
| 90 | Lipomodelling Guidelines for Breast Surgery 121 | JGBSA lipomodelling 121 | JGBSA | UK | 2012 |
| 91 | Performance and Practice Guidelines for the Use of Neoadjuvant Systemic Therapy in the Management of Breast Cancer 122 | ASBS NaQT BC 122 | ASBS | USA | 2017 |
| 92 | Performance and Practice Guidelines for Mastectomy 123 | ASBS mastectomy 123 | ASBS | USA | 2014 |
| 93 | Performance and Practice Guidelines for Breast‐Conserving Surgery/Partial Mastectomy 124 | ASBS breast conserving 124 | ASBS | USA | 2014 |
| 94 | Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients 125 | ASBS ALD 125 | ASBS | USA | 2014 |
| 95 | Performance and Practice Guidelines for Sentinel Lymph Node Biopsy in Breast Cancer Patients 126 | ASBS SLND 126 | ASBS | USA | 2014 |
| 96 | Evidence‐Based Clinical Practice Guideline: Autologous Breast Reconstruction with DIEP or Pedicled TRAM Abdominal Flaps 127 | ASPS DIEP and TRAM 127 | ASPS | USA | 2017 |
| 97 | Use of Endocrine Therapy for Breast Cancer Risk Reduction: ASCO Clinical Practice Guideline Update 128 | ASCO endocrine therapy risk BC 128 | ASCO | USA | 2019 |
| 98 | Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update 129 | ASCO postmastectomy RT 129 | ASCO | USA | 2017 |
| 99 | Breast Cancer Surveillance Guidelines 130 | ASCO surveillance 130 | ASCO | USA | 2013 |
| 100 | Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Clinical Practice Guideline Focused Update 131 | ASCO treatment for early BC 131 | ASCO | USA | 2018 |
| 101 | Systemic Therapy for Patients With Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer: ASCO Clinical Practice Guideline Update 132 | ASCO systemic therapy EGR2 BC 132 | ASCO | USA | 2018 |
| 102 | Recommendations on Disease Management for Patients With Advanced Human Epidermal Growth Factor Receptor 2–Positive Breast Cancer and Brain Metastases: ASCO Clinical Practice Guideline Update 133 | ASCO EGRF2 MBC 133 | ASCO | USA | 2018 |
| 103 | Integrative Therapies During and After Breast Cancer Treatment: ASCO Endorsement of the SIO Clinical Practice Guideline 134 | ASCO BC treatment 134 | ASCO | USA | 2018 |
| 104 | Chemotherapy and Targeted Therapy for Women With Human Epidermal Growth Factor Receptor 2–Negative (or unknown) Advanced Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline 135 | ASCO EGFR2 advanced BC 135 | ASCO | USA | 2014 |
| 105 | Role of Bone‐Modifying Agents in Metastatic Breast Cancer: An American Society of Clinical Oncology–Cancer Care Ontario Focused Guideline Update 136 | ASCO bone‐modifying agent MBC 136 | ASCO | USA | 2017 |
| 106 | Recommendations for Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Update 137 | ASCO EGFR2 recommendations 137 | ASCO | USA | 2013 |
| 107 | Breast Cancer Follow‐Up and Management After Primary Treatment: American Society of Clinical Oncology Clinical Practice Guideline Update 138 | ASCO follow‐up/management BC 138 | ASCO | USA | 2013 |
| 108 | Adjuvant Endocrine Therapy for Women With Hormone Receptor–Positive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Update on Ovarian Suppression 139 | ASCO ovarian suppression BC 139 | ASCO | USA | 2016 |
| 109 | Role of Patient and Disease Factors in Adjuvant Systemic Therapy Decision Making for Early‐Stage, Operable Breast Cancer: American Society of Clinical Oncology Endorsement of Cancer Care Ontario Guideline Recommendations 140 | ASCO factors in early BC 140 | ASCO | USA | 2016 |
| 110 | Use of Adjuvant Bisphosphonates and Other Bone‐Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline 141 | ASCO use bone‐modifying agents BC 141 | ASCO | USA | 2017 |
| 111 | Use of Biomarkers to Guide Decisions on Adjuvant Systemic Therapy for Women With Early‐Stage Invasive Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline Focused Update 142 | ASCO biomarkers in early BC 142 | ASCO | USA | 2017 |
| 112 | Use of Biomarkers to Guide Decisions on Systemic Therapy for Women With Metastatic Breast Cancer: American Society of Clinical Oncology Clinical Practice Guideline 143 | ASCO biomarkers in MBC 143 | ASCO | USA | 2019 |
| 113 | American Society of Clinical Oncology Endorsement of the Cancer Care Ontario Practice Guideline on Adjuvant Ovarian Ablation in the Treatment of Premenopausal Women With Early‐Stage Invasive Breast Cancer 144 | ASCO ovarian ablation BC 144 | ASCO | USA | 2011 |
| 114 | American Society of Clinical Oncology/College of American Pathologists Guideline Recommendations for Immunohistochemical Testing of Estrogen and Progesterone Receptors in Breast Cancer 145 | ASCO hormonal BC 145 | ASCO | USA | 2010 |
| 115 | Use of Pharmacologic Interventions for Breast Cancer Risk Reduction: American Society of Clinical Oncology Clinical Practice Guideline 146 | ASCO risk reduction BC 146 | ASCO | USA | 2013 |
| 116 | Endocrine Therapy for Hormone Receptor–Positive Metastatic Breast Cancer: American Society of Clinical Oncology Guideline 147 | ASCO endocrine BC 147 | ASCO | USA | 2016 |
| 117 | Invasive Breast Cancer. Basic resources. Version 1.2019 148 | NCCN invasive BC basic 148 | NCCN | USA | 2019 |
| 118 | Invasive Breast Cancer. Core resources. Version 1.2019 149 | NCCN invasive BC core 149 | NCCN | USA | 2019 |
| 119 | Invasive Breast Cancer. Enhanced resources. Version 1.2019 150 | NCCN invasive BC enhanced 150 | NCCN | USA | 2019 |
| 120 | Breast Cancer. NCCN Evidence Blocks. Version 1.2019 151 | NCCN evidence block BC 151 | NCCN | USA | 2019 |
| 121 | Breast Cancer. Version 3.2019 152 | NCCN BC 152 | NCCN | USA | 2019 |
| 122 | Management of Breast Cancer (2nd Edition) 153 | MHM BC 153 | MHM | Malaysia | 2010 |
| 123 | Influencing best practice in breast cancer 154 | Australia BC 154 | AG | Australia | 2016 |
| 124 | Recommendations for staging and managing the axilla 155 | CA axilla 155 | CA | Australia | 2011 |
| 125 | Recommendations for use of hypofractionated radiotherapy for early operable breast cancer 156 | CA RT 156 | CA | Australia | 2011 |
| 126 | Recommendations for use of Bisphosphonates 157 | CA bisphosphonates 157 | CA | Australia | 2011 |
| 127 | Recommendations for the management of early breast cancer in women with an identified BRCA1 or BRCA2 gene mutation or at high risk of a gene mutation 158 | CA management BC 158 | CA |
Australia |
2014 |
| 128 | Guía de Práctica Clínica AUGE Cáncer de Mama 159 | GPC Chile 159 | MSC | Chile | 2015 |
| 129 | Guía de práctica clínica (GPC) para la detección temprana, tratamiento integral, seguimiento y rehabilitación del cáncer de mama 160 | GPC Colombia 160 | INC | Colombia | 2017 |
| 130 | Guía de Práctica Clínica del Tratamiento para el Cáncer de Mama 161 | GPC Costa Rica 161 | IHCAI | Costa Rica | 2011 |
| 131 | Guía de Práctica Clínica para el Tratamiento del Cáncer de Mama 162 | GPC Perú 162 | DDSS | Perú | 2017 |
| 132 | Guía para el Cáncer de Mama en Venezuela 163 | GPC Venezuela 163 | SAV | Venezuela | 2015 |
| 133 | Management of Early Breast Cancer 164 | New Zealand BC 164 | MHNZ | New Zealand | 2014 |
| 134 | The Screening, Diagnosis, Treatment, and Follow‐Up of Breast Cancer 165 | Würzburg BC 165 | UHW | Germany | 2018 |
| 135 | Breast cancer brain metastases: a review of the literature and a current multidisciplinary management guideline 166 | FESEO brain MBC 166 | FESEO | Spain | 2013 |
| 136 | Cirugía de la Mama 167 | AEC BC 167 | AEC | Spain | 2017 |
| 137 | NCA Breast Cancer Clinical Guidelines 168 | NCA BC 168 | NCA | UK | 2019 |
| 138 | Breast Cancer: Management and Follow‐Up 169 | BCMA management and follow‐up 169 | BCMA | Canada | 2013 |
| 139 | Clinical Guidelines for the Management of Breast Cancer 170 | WMCA BC 170 | WMCA | UK | 2016 |
| Name of the CS | |||||
| 140 | Consenso costarricense sobre prevención, diagnóstico y tratamiento del cáncer mamario 171 | CS Costa Rica 171 | CMCCR | Costa Rica | 2016 |
| 141 | Consenso Mexicano sobre diagnóstico y tratamiento del cáncer mamario 172 | GPC México 172 | SSM | México | 2019 |
| 142 | National consensus in China on diagnosis and treatment of patients with advanced breast cancer 173 | Chinese BC CS 173 | CECM | China | 2015 |
| 143 | Practical consensus recommendations for hormone receptor‐positive Her2‐negative advanced or metastatic breast cancer 174 | Indian ICON CS 174 | ICON | India | 2013 |
| 144 | Indian Solutions for Indian Problems—Association of Breast Surgeons of India (ABSI) Practical Consensus Statement, Recommendations, and Guidelines for the Treatment of Breast Cancer in India 175 | Indian ABSI CS 175 | ABSI | India | 2017 |
| 145 | Consensus document for management of breast cancer 176 | Indian ICMR CS 176 | ICMR | India | 2016 |
| 146 | 4th ESO–ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) 177 | ABC4 177 | ESMO | Europe | 2018 |
| 147 | St. Gallen/Vienna 2019: A Brief Summary of the Consensus Discussion about Escalation and De‐Escalation of Primary Breast Cancer Treatment 178 | St. Gallen 2019 178 | St. Gallen | Europe | 2019 |
| 148 | ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer 179 | ESTRO RT BC 179 | ESTRO | Europe | 2014 |
| 149 | Second international consensus guidelines for breast cancer in young women (BCY2) 180 | BCY2 180 | ESO | Europe | 2016 |
| 150 | Guidelines for diagnostics and treatment of aromatase inhibitor‐induced bone loss in women with breast cancer A consensus of Lithuanian medical oncologists, radiation oncologists, endocrinologists, and family medicine physicians 181 | LOEGP 181 | LOEGP | Lithuania | 2014 |
| 151 | Biomarkers in breast cancer: A consensus statement by the Spanish Society of Medical Oncology and the Spanish Society of Pathology 182 | SEOM and SEAP 182 | SEOM | Spain | 2017 |
| 152 | Provincial consensus recommendations for adjuvant systemic therapy for breast cancer 183 | CCM 2017 183 | CCM | Canada | 2017 |
| 153 | Postoperative radiotherapy for breast cancer: UK consensus statements 184 | RCR postoperative RT 184 | RCR | UK | 2016 |
| 154 | Consensus Guideline on Accelerated Partial Breast Irradiation 185 | ASBS RT 185 | ASBS | USA | 2018 |
| 155 | Consensus Guideline on the Use of Transcutaneous and Percutaneous Ablation for the Treatment of Benign and Malignant Tumors of the Breast 186 | ASBS ablation 186 | ASBS | USA | 2018 |
| 156 | Consensus Guideline on the Management of the Axilla in Patients With Invasive/In‐Situ Breast Cancer 187 | ASBS axilla 187 | ASBS | USA | 2019 |
| 157 | Consensus Guideline on Breast Cancer Lumpectomy Margins 188 | ASBS margins 188 | ASBS | USA | 2017 |
| 158 | Consensus Guideline on Concordance Assessment of Image‐Guided Breast Biopsies and Management of Borderline or High‐Risk Lesions 189 | ASBS borderline lesions 188 | ASBS | USA | 2016 |
| 159 | Contralateral Prophylactic Mastectomy (CPM) Consensus Statement from the American Society of Breast Surgeons: Data on CPM Outcomes and Risks 190 | ASBS CPM 190 | ASBS | USA | 2016 |
| 160 | Consensus Guideline on Venous Thromboembolism (VTE) Prophylaxis for Patients Undergoing Breast Operations 191 | ASBS VTE prophylaxis BC 191 | ASBS | USA | 2011 |
| 161 | The American Brachytherapy Society consensus statement on intraoperative radiation therapy 192 | AB intraoperative RT 192 | AB | USA | 2017 |
| 162 | The American Brachytherapy Society consensus report for accelerated partial breast irradiation using interstitial multicatheter brachytherapy 193 | AB partial RT BC 193 | AB | USA | 2017 |
| 163 | Society of Surgical Oncology Breast Disease Working Group Statement on Prophylactic (Risk‐Reducing) Mastectomy 194 | SSO prophylactic mastectomy 194 | SSO | USA | 2016 |
| 164 | SSO‐ASTRO Consensus Guideline on Margins for Breast‐Conserving Surgery with Whole‐Breast Irradiation in Ductal Carcinoma In Situ 195 | SSO margins 195 | SSO | USA | 2016 |
| 165 | SSO‐ASTRO Consensus Guideline on Margins for Breast‐Conserving Surgery with Whole Breast Irradiation in Stage I and II Invasive Breast Cancer 196 | SSO–ASTRO invasive BC 196 | SSO ‐ ASTRO | USA | 2014 |
| 166 | Margins for Breast‐Conserving Surgery With Whole‐Breast Irradiation in Stage I and II Invasive Breast Cancer: American Society of Clinical Oncology Endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology Consensus Guideline 197 | ASCO margin BC CSs 197 | ASCO | USA | 2014 |
| 167 | International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment 198 | International expert panel BC 198 | IEP | International | 2010 |
Table 2.
Characteristics of the CPGs and CSs regarding SDM
| Characteristics |
CPGs or CSs without SDM (n = 101) |
CPGs or CSs with SDM (n = 66) |
P value |
|---|---|---|---|
| Published after 2015 | 42 (42.0 %) | 46 (69.7 %) | .0003 |
| CPG | 83 (82.1 %) | 54 (81.8 %) | .95 |
| European guidelines | 45 (44.5 %) | 25 (37.0 %) | .21 |
| North American guidelines | 43 (42.5 %) | 28 (42.4 %) | .98 |
| South American guidelines | 2 (1.9 %) | 5 (7.5 %) | .1 |
| Asia guidelines | 9 (8.9 %) | 3 (4.5 %) | .15 |
| Oceania guidelines | 3 (2.9 %) | 3 (4.5 %) | .3 |
| Published in a journal | 44 (43.5 %) | 17 (25.7 %) | .009 |
Table 3.
Update frequency of each CPGs/CSs where SDM appears
| Entity | First year of publication | 2010 | 2011 | 2012 | 2013 | 2014 | 2015 | 2016 | 2017 | 2018 | 2019 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CPGs | |||||||||||||
| 3 | Japanese RT BC CPG 34 | JBCS | 2015 | * | |||||||||
| 9 | ESMO BC 2019 40 | ESMO | 2010 | * | * | * | |||||||
| 11 | EUSOMA 2012 42 | EUSOMA | 2012 | * | |||||||||
| 12 | AGO early BC 43 | AGO | 2012 | * | * | * | * | * | |||||
| 14 | AGO DCIS 45 | AGO | 2002 | * | * | * | * | * | * | * | * | * | |
| 16 | AGO oncoplastic 47 | AGO | 2012 | * | * | * | * | * | * | * | |||
| 17 | AGO adjuvant endocrine 48 | AGO | 2012 | * | * | * | * | * | * | * | * | ||
| 27 | AGO CT MBC 58 | AGO | 2012 | * | * | * | * | * | * | * | * | ||
| 41 | IKNL BC 72 | IKNL | 2008 | * | |||||||||
| 42 | Fisterra BC 73 | Fisterra | 2011 | * | * | * | |||||||
| 47 | NICE abemaciclib 78 | NICE | 2019 | * | |||||||||
| 48 | NICE ribociclib 79 | NICE | 2019 | * | |||||||||
| 49 | NICE early and advanced BC 80 | NICE | 2018 | * | |||||||||
| 50 | NICE BC 81 | NICE | 2011 | * | |||||||||
| 51 | NICE familial BC 82 | NICE | 2013 | * | |||||||||
| 52 | NICE lipomodelling 83 | NICE | 2012 | * | |||||||||
| 53 | NICE gene expression 84 | NICE | 2013 | * | |||||||||
| 54 | NICE pertuzumab BC 85 | NICE | 2016 | * | |||||||||
| 56 | AHS reconstruction BC 87 | AHS | 2013 | * | * | ||||||||
| 57 | AHS early BC 88 | AHS | 2014 | * | * | * | * | ||||||
| 63 | CCO surgical management BC 94 | CCO | 1996 | * | * | ||||||||
| 70 | CCO sQT for node‐negative BC 101 | CCO | 1998 | * | |||||||||
| 71 | CCO ovarian ablation early stage 102 | CCO | 2010 | * | |||||||||
| 73 | CCO trastuzumab MBC 104 | CCO | 1999 | * | |||||||||
| 76 | CCO LABC 107 | CCO | 2014 | * | |||||||||
| 79 | CCO bone‐modifying agents BC 110 | CCO | 2016 | * | |||||||||
| 86 | ABS/BAPRAS oncoplastic 117 | ABS, BAPRAS | 2012 | * | |||||||||
| 88 | SCT quality indicators 119 | SCT | 2016 | * | |||||||||
| 98 | ASCO postmastectomy RT 129 | ASCO | 2001 | * | * | ||||||||
| 100 | ASCO treatment for early BC 131 | ASCO | 2016 | * | * | ||||||||
| 104 | ASCO EGFR2 advanced BC 135 | ASCO | 2014 | * | |||||||||
| 105 | ASCO bone‐modifying agent MBC 136 | ASCO | 2000 | * | * | ||||||||
| 108 | ASCO ovarian suppression BC 139 | ASCO | 2016 | * | |||||||||
| 109 | ASCO factors in early BC 140 | ASCO | 2019 | ||||||||||
| 110 | ASCO use bone‐modifying agent BC 141 | ASCO | 2017 | * | |||||||||
| 116 | ASCO endocrine BC 147 | ASCO | 2016 | * | |||||||||
| 117 | NCCN invasive BC basic 148 | NCCN | 2015 | * | * | * | |||||||
| 118 | NCCN invasive BC core 149 | NCCN | 2015 | * | * | * | |||||||
| 119 | NCCN invasive BC enhanced 150 | NCCN | 2015 | * | * | * | |||||||
| 120 | NCCN evidence block BC 151 | NCCN | 2015 | * | * | * | * | ||||||
| 121 | NCCN BC 152 | NCCN | 2015 | * | * | * | |||||||
| 122 | MHM BC 153 | MHM | 2002 | * | |||||||||
| 123 | Australia BC 154 | AG | 2016 | * | |||||||||
| 124 | CA axilla 155 | CA | 2011 | * | |||||||||
| 129 | GPC Colombia 160 | INC | 2013 | * | * | ||||||||
| 130 | IHCAI GPC Costa Rica 161 | IHCAI | 2011 | * | |||||||||
| 131 | GPC Perú 162 | IETSI | 2017 | * | |||||||||
| 132 | GPC Venezuela 163 | SAV | 2015 | * | |||||||||
| 133 | New Zealand BC 164 | MHNZ | 2009 | * | |||||||||
| 134 | Würzburg BC 165 | UHW | 2018 | * | |||||||||
| 136 | AEC BC 167 | AEC | 2007 | * | |||||||||
| 137 | NCA BC 168 | NCA | 2019 | * | |||||||||
| 138 | BCMA management and follow‐up 169 | BCMA | 2013 | * | |||||||||
| 139 | WMCA BC 170 | WMCA BC | 2016 | ||||||||||
| CSs | |||||||||||||
| 140 | CS Costa Rica 171 | CMCCR | 2016 | ||||||||||
| 141 | GPC México 172 | SSM | 1994 | * | * | * | * | * | |||||
| 145 | Indian ICMR CS 176 | ICMR | 2016 | * | |||||||||
| 146 | ABC4 177 | ESMO | 2012 | * | * | * | * | ||||||
| 147 | St. Gallen 2019 178 | St. Gallen | 2015 | * | * | * | |||||||
| 152 | CCM 2017 183 | CCM | 2017 | * | |||||||||
| 154 | ASBS RT 185 | ASBS | 2018 | * | |||||||||
| 156 | ASBS axilla 187 | ASBS | 2019 | * | |||||||||
| 158 | ASBS borderline lesions 189 | ASBS | 2016 | * | |||||||||
| 159 | ASBS CPM 190 | ASBS | 2016 | * | |||||||||
| 163 | SSO prophylactic mastectomy 194 | SSO | 2007 | * | |||||||||
| 164 | SSO margins 195 | SSO | 2014 | * |
3.4. SDM in CPGs and CSs concerning BC
The analysis of the compliance of the items valued is presented in Figure 2 and Appendix 4. SDM appeared in any section of 66 CPGs and CSs (12/28 (43%) CSs vs 54/139 (39%) CPGs, P = .69). SDM appeared in glossary or indexes in only two documents, and only in one, its basis was explained. In general, CSs had higher overall quality than CPGs (CSs' mean 2.833 vs CPGs' mean 1.12 items, P < .001) (Appendix ).
Figure 2.
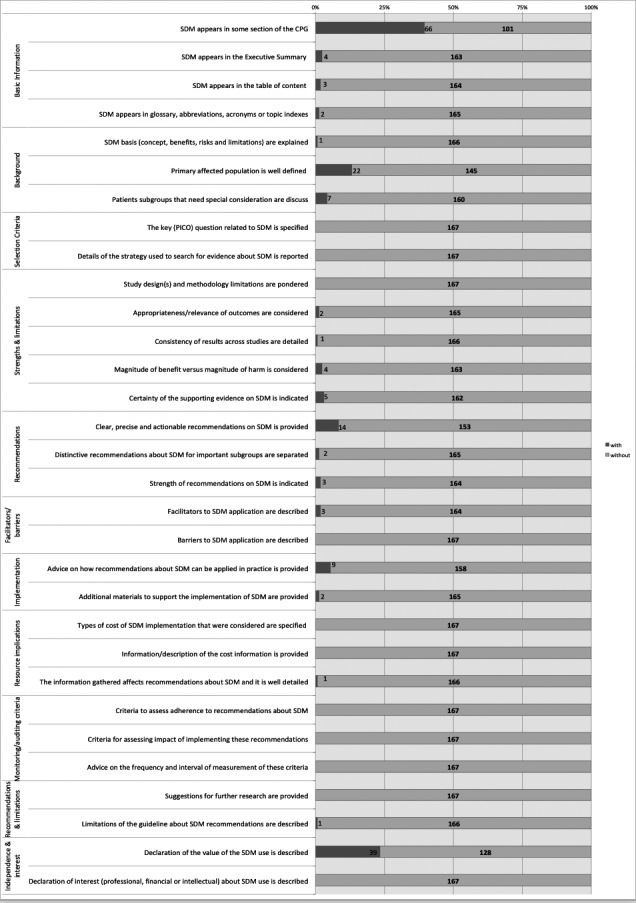
The analysis of the compliance of the data extraction items
Overall, 39 (23%) stated the value of SDM as an option in the decision‐making process, 14 (8%) provided clear and precise SDM recommendations, 4 (3%) considered benefits versus harms of using SDM, and 4 (2%) identified evidence supporting the use of SDM. Only 9 (5%) of these CPGs and CSs gave advice for the SDM application in practice. The strength of recommendations on SDM was indicated in three (2%). Support for the implementation of SDM was well‐detailed in two documents (1%). The information gathered about SDM affected recommendations and was detailed in one (<1%). Limitations of the CPG or CS about SDM recommendations were described in just one of them (<1%).
Only 4 (2%) of these guides emphasized their interest in SDM appearing in the executive summary. Only in three (2%) of the CPGs and CSs, the table of content talked about SDM. Primary affected population with BC was well‐defined in 22 (13%) articles, and patients’ subgroups with special consideration were discussed in 7 (4%) documents. Appropriateness and relevance of outcomes were considered in only 2 (1%) CPGs. Only one document detailed the consistency of results across studies. Recommendations about SDM for subgroups were separated in only two articles (1%). Facilitators and barriers to SDM application were described in only two articles too (1%).
Ten items (32%) measured in the data extraction instrument were not included in any CPGs and CSs (n = 10/31). The PICO question related to SDM was not specified, search strategy was not reported, the study design and limitations were not pondered, barriers were not described, the cost of SDM implementation was not specified, adherence to recommendations and the impact were not assessed, description of the cost information and suggestions for further research were not provided and finally, professional, financial or intellectual interest about SDM was not described (Figure 2 and Appendix ). Finally, there were 101 (61%) CPGs or CSs did not talk about SDM.
All three reviewers categorized that the 'Alberta Health Services' 88 , 'Australian Government' 155 , 'Ministry of Health from New Zealand' 165 and Costa Rica 'IHCAI' 162 CPGs and 'CMCCR' 172 CS had the highest overall quality in analysing the decision‐making process in BC treatment (Appendix ). In the United States of America, we highlighted two of the 'American Society of Clinical Oncology (ASCO)' 140 , 141 , 142 , 143 , 144 , 145 , 146 , 147 , 148 guidelines and the last version of NCCN 153 , but with a lower mark if you compare with the ones we named before. In Europe, we found the 'European Society for Medical Oncology (ESMO)' 41 , the 'Asociación Española de Cirujanos (AEC)' 80 and the 'ABS‐BAPRAS' 118 CPGs with a score of 6 as the best paradigm of a guide that talks about SDM.
4. DISCUSSION
4.1. Main findings
We developed a standardized quality assessment tool for assessing the coverage of SDM in recommendation documents. Our review and analysis showed that SDM description, clarification and recommendations CPGs and CSs concerning BC treatment were poor, leaving a large scope for improvement in this area. SDM more frequently reported in CPGs and CSs in recent years but surprising SDM was less often covered in medical journals (Figure 3).
Figure 3.
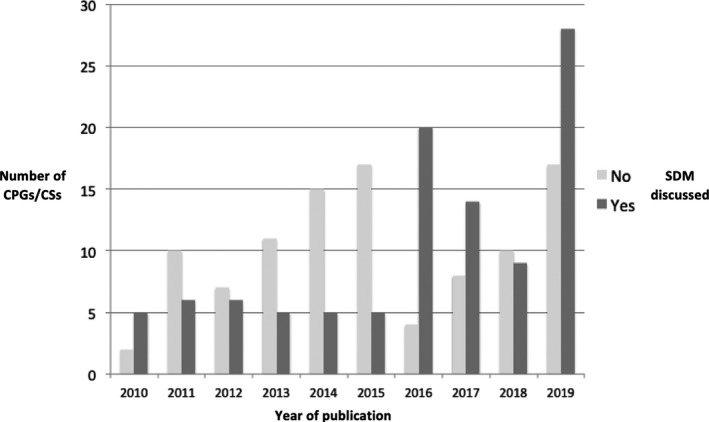
Comparison between the year of publication of the guide according to whether or not SDM appearance
4.2. Strengths and weaknesses
The validity of findings depends on the strength and limitations of methods, which should be understood first before assessing their implications 202 . A key strength of this study was a global perspective with a big number of CPGs and CSs included, without language restrictions or data sources limitations. We developed and deployed a prospective protocol with a specific SDM quality assessment tool incorporating the AGREE II instrument 201 , RIGHT statement 200 and other related papers 9 , 21 , 24 , 25 , 27 . Unfortunately, as there were no other similar studies, we could not compare our results with other findings. There have been evaluations of risk of bias in other papers, but our focus was on examining the reporting of guidance about SDM. One perceived limitation of this study could be related to the subjective nature of the data extraction; however, as we used duplicate data extraction with arbitration, we minimized this methodological issue. Quality assessment tool performance may be a further issue, and we addressed this by following a standard methodology for tool development. Not all quality items can have the same relevance and weight, and future research should focus on scoring them creating a threshold for rating quality. Because the items mainly came from two wide‐used indexes 200 , 201 , demonstrably our tool should be considered to have face validity. Therefore, we are confident that our finding of poverty of SDM information in practice recommendations is trustworthy and merits further consideration.
Inter‐examiner reliability should be calculated in systematic reviews as the data extracted should be the same by different reviewers 203 . Intra‐examiner reliability is a pre‐condition for inter‐observer reliability, and so was not calculated or reported 31 . In our paper, the inter‐examiner reliability score was found to be excellent (ICC = 0.97).
4.3. Implications
To our knowledge, information and recommendations about SDM in BC CPGs and CSs have not been systematically analysed previously. Neither did we find a tool to evaluate SDM reporting quality. This is surprising because SDM is a legal obligation 11 , 12 , 13 and a key component for high‐quality patient‐centred cancer care 6 , 7 , 8 , 9 , 10 .
Breast cancer is the paradigm of the situation where a two‐way exchange not only of information but also of treatment preferences is needed to find the best option for a particular patient, as different strategies may show a priori similar advantages and disadvantages but possible outcomes are deeply related to the patient’s values and personal situation 10 , 203 .
Formal recommendations should promote SDM application in clinical routine practice, but this has proved difficult and slow 18 , 19 , 20 , 21 , 23 , 24 . It would require changing attitudes, acquiring new skills, developing specific tools and ensuring an environment where communication and sharing perspectives are valued 10 , 21 , 25 , 26 , 27 . Effective implementation strategies could be underpinned by SDM detailed in CPGs and CSs as these documents should be expected to provide this specific content 11 , 12 , 13 . Our work has identified a gap that offers an important contribution in directing further research and debate, including assessment of risk of bias in guidelines. It highlights the need for more objective‐specific tools for SDM assessment, evaluation of their psychometric properties and promotion in CPGs and CSs for diverse malignancies. Future studies should be required in that direction.
5. CONCLUSIONS
This systematic review found that BC treatment CPGs and CSs insufficiently addressed SDM. Implementation of this practice is important for high‐quality patient‐centred cancer care, but lack of knowledge is a known barrier. SDM descriptions and recommendations in CPGs and CSs concerning BC treatment need improvement. SDM was more frequently reported in CPGs and CSs in recent years, but surprisingly it was less often covered in medical journals, a feature that needs attention. In the future, SDM should be suitably explained and encouraged and specific tools should be applied to assess its dealing and promotion in specific cancer treatment CPGs and CSs. Medical journals should play a strong role in promoting SDM in CPGs and CSs they publish in the future.
CONFLICTS OF INTEREST
The study was conducted in Granada, Spain. There are no conflicts of interest.
AUTHOR CONTRIBUTIONS
Each author certifies that he/she has made a direct and substantial contribution to the conception and design of the study, development of the search strategy, the establishment of the inclusion and exclusion criteria, data extraction, analysis and interpretation. MMC was involved in the design of the study, literature search, data collection and analysis, quality appraisal and writing. IMMN was involved in the literature search and data collection. MMD was involved in the design of this study, analysis of data and writing. LM was involved in writing. KSK was involved in the design of this study, conducted the quality appraisal, in the writing, and provided critical revision of the paper. ABC was involved in the design of this study and provided critical revision of the paper. All authors read and provided the final approval of the version to be published.
Supporting information
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Acknowledgements
We gratefully thank the 'Programa Beatriz Galindo. Modalidad Senior. Ministerio de Ciencia, Innovación y Universidades' for making possible to link the distinguish researcher Khalid S. Khan to the University of Granada.
Maes‐Carballo M, Muñoz‐Núñez I, Martín‐Díaz M, Mignini L, Bueno‐Cavanillas A, Khan KS. Shared decision making in breast cancer treatment guidelines: Development of a quality assessment tool and a systematic review. Health Expect. 2020;23:1045–1064. 10.1111/hex.13112
DATA AVAILABILITY STATEMENT
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
REFERENCES
- 1. Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68(6):394‐424. [DOI] [PubMed] [Google Scholar]
- 2. World Health Organization . Global Health Observatory. Geneva: World Health Organization ; 2019. who.int/gho/database/en/. Accessed December 3, 2019. [Google Scholar]
- 3. Acebal Blanco M, Alba Conejo E, Alvarez Benito M, et al. Cáncer de mama: proceso asistencial integrado , 3rdª edn. Sevilla: Consejería de Salud; 2011. [Google Scholar]
- 4. Chan CWH, Law BMH, So WKW, et al. Novel strategies on personalized medicine for breast cancer treatment: an update. Int J Mol Sci. 2017;18(11):2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA. 2019;321(3):288‐300. [DOI] [PubMed] [Google Scholar]
- 6. Soukup T, Lamb BW, Arora S, et al. Successful strategies in implementing a multidisciplinary team working in the care of patients with cancer: an overview and synthesis of the available literature. J Multidiscip Healthc. 2018;11:49‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Waljee JF, Hawley S, Alderman AK, et al. Patient satisfaction with treatment of breast cancer: does surgeon specialization matter? J Clin Oncol. 2007;25(24):3694‐3698. [DOI] [PubMed] [Google Scholar]
- 8. Unidades asistenciales del área del cáncer . Estándares y recomendaciones de calidad y seguridad. Madrid, Spain: Ministerio de Sanidad, Servicios Sociales e Igualdad; 2013. [Google Scholar]
- 9. Levit LBE, Nass S, Ganz P. Delivering High‐Quality Cancer Care: Charting a New Course for a System in Crisis. The National Academies Press; 2013. [PubMed] [Google Scholar]
- 10. Elwyn G, Frosch D, Thomson R, et al. Shared decision making: a model for clinical practice. J Gen Intern Med. 2012;27(10):1361‐1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Estado BOd . Ley 41/2002 básica reguladora de la autonomía del paciente y de derechos y obligaciones en materia de información y documentación clínica. BOE. 2002. [Google Scholar]
- 12. Senate and House of Representatives . Patient Protection and Affordable Care Act. HR 3590. Washington, D.C.: Senate and House of Representatives; 2010. [Google Scholar]
- 13. Department of Health . Equity and Excellence: Liberating the NHS. London, UK: Department of Health; 2010. [Google Scholar]
- 14. Schoenfeld EM, Mader S, Houghton C, et al. The effect of shared decisionmaking on patients' likelihood of filing a complaint or lawsuit: a simulation study. Ann Emerg Med. 2019;74(1):126‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. International Shared Decision Making Society . 2018.
- 16. AECC . Estudio de investigación “Necesidades no clínicas de los pacientes con cáncer y sus acompañantes en España: una visión multidisciplinar”. La mitad de los pacientes oncológicos no participa en la toma de decisiones sobre su tratamiento. 2018.
- 17. The Patients Association. UK; 2018. [Google Scholar]
- 18. Legare F, Thompson‐Leduc P. Twelve myths about shared decision making. Patient Education and Counseling. 2014;96(3):281‐286. [DOI] [PubMed] [Google Scholar]
- 19. Savelberg W, Boersma LJ, Smidt M, et al. Does lack of deeper understanding of shared decision making explains the suboptimal performance on crucial parts of it? An example from breast cancer care. Eur J Oncol Nurs. 2019;38:92‐97. [DOI] [PubMed] [Google Scholar]
- 20. Stacey D, Hill S, McCaffery K, et al. Shared decision making interventions: theoretical and empirical evidence with implications for health literacy. Stud Health Technol Inform. 2017;240:263‐283. [PubMed] [Google Scholar]
- 21. Gillick MR. Re‐engineering shared decision‐making. J Med Ethics. 2015;41(9):785‐788. [DOI] [PubMed] [Google Scholar]
- 22. Volk RJ, Llewellyn‐Thomas H, Stacey D, et al. Ten years of the international patient decision aid standards collaboration: evolution of the core dimensions for assessing the quality of patient decision aids. BMC Med Inform Decis Mak. 2013;13(Suppl 2):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Staveley I, Sullivan P. We need more guidance on shared decision making. Br J Gen Pract. 2015;65(641):663‐664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Légaré FAR, Stacey D, Turcotte S, KryworuchkoIan J, Lyddiatt GA, Politi MC, Thomson R, Elwyn G, Donner‐Banzhoff N. Interventions for increasing the use of shared decision making by healthcare professionals. Cochrane Database of Syst Rev. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Elwyn G, Frosch DL, Kobrin S. Implementing shared decision‐making: consider all the consequences. Implement Sci. 2016;11:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Agoritsas T, Heen AF, Brandt L, et al. Decision aids that really promote shared decision making: the pace quickens. BMJ. 2015;350:g7624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Elwyn G, Lloyd A, Joseph‐Williams N, et al. Option Grids: shared decision making made easier. Patient Educ Couns. 2013;90(2):207‐212. [DOI] [PubMed] [Google Scholar]
- 28. Holmes‐Rovner M, Valade D, Orlowski C, et al. Implementing shared decision‐making in routine practice: barriers and opportunities. Health Expect. 2000;3(3):182‐191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta‐analyses: the PRISMA Statement. Open Med. 2009;3(3):e123‐e130. [PMC free article] [PubMed] [Google Scholar]
- 30. Liberati A, Altman DG, Tetzlaff J, et al. The PRISMA statement for reporting systematic reviews and meta‐analyses of studies that evaluate health care interventions: explanation and elaboration. Ann Intern Med. 2009;151(4):W65‐W94. [DOI] [PubMed] [Google Scholar]
- 31. Koo TK, Li MY. A guideline of selecting and reporting intraclass correlation coefficients for reliability research. J Chiropr Med. 2016;15(2):155‐163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Higgins JTJ. Cochrane Handbook for Systematic Reviews of Interventions (version 6); 2019.
- 33. Zhang BN, Cao XC, Chen JY, et al. Guidelines on the diagnosis and treatment of breast cancer (2011 edition). Gland Surg 2012;1(1):39‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. National Health Commission Of The People's Republic Of C . Chinese guidelines for diagnosis and treatment of breast cancer 2018 (English version). Chin J Cancer Res. 2019;31(2):259‐277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Yamauchi C, Sekiguchi K, Nishioka A, et al. The Japanese Breast Cancer Society Clinical Practice Guideline for radiation treatment of breast cancer, 2015 edition. Breast Cancer 2016;23(3):378‐390. [DOI] [PubMed] [Google Scholar]
- 36. Mukai H, Aihara T, Yamamoto Y, et al. The Japanese Breast Cancer Society Clinical Practice Guideline for systemic treatment of breast cancer. Breast Cancer. 2015;22(1):5‐15. [DOI] [PubMed] [Google Scholar]
- 37. Mukai H, Noguchi S, Akiyama F, et al. 2013 Clinical practice guidelines (The Japanese Breast Cancer Society): history, policy and mission. Breast Cancer. 2015;22(1):1‐4. [DOI] [PubMed] [Google Scholar]
- 38. Singapore Cancer Network Breast Cancer W . Singapore Cancer Network (SCAN) Guidelines for Adjuvant Trastuzumab Use in Early Stage HER2 Positive Breast Cancer. Ann Acad Med Singapore 2015;44(10):360‐7. [PubMed] [Google Scholar]
- 39. Singapore Cancer Network Breast Cancer W . Singapore Cancer Network (SCAN) Guidelines for Bisphosphonate Use in the Adjuvant Breast Cancer Setting. Ann Acad Med Singapore 2015;44(10):368‐378. [PubMed] [Google Scholar]
- 40. (KCE) Bhkc . Breast Cancer in Women: Diagnosis, Treatment and Follow‐up. 2015.
- 41. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow‐up. Ann Oncol. 2019;30(10):1674. [DOI] [PubMed] [Google Scholar]
- 42. Lin NU, Thomssen C, Cardoso F, et al. International guidelines for management of metastatic breast cancer (MBC) from the European School of Oncology (ESO)‐MBC Task Force: Surveillance, staging, and evaluation of patients with early‐stage and metastatic breast cancer. Breast. 2013;22(3):203‐210. [DOI] [PubMed] [Google Scholar]
- 43. Cardoso F, Loibl S, Pagani O, et al. The European Society of Breast Cancer Specialists recommendations for the management of young women with breast cancer. Eur J Cancer. 2012;48(18):3355‐3377. [DOI] [PubMed] [Google Scholar]
- 44. (AGO) AGO . Diagnosis and treatment of patients with primary and metastatic. Breast Cancer. 2019. [Google Scholar]
- 45. (AGO) AGO . Lesions of Uncertain Malignant Potential (B3) (ADH, LIN, FEA, Papilloma, Radial Scar). 2019.
- 46. (AGO) AGO . Ductal Carcinoma in Situ. (DCIS). 2019.
- 47. (AGO) AGO . Breast Cancer Surgery Oncological Aspects. 2019.
- 48. (AGO) AGO . Oncoplastic and Reconstructive Surgery. 2019.
- 49. (AGO) AGO . Adjuvant Endocrine Therapy in Pre‐ and Postmenopausal Patients. 2019.
- 50. (AGO) AGO .Adjuvant Cytotoxic and Targeted Therapy. 2019.
- 51. (AGO) AGO . Neoadjuvant (Primary) Systemic Therapy. 2019.
- 52. (AGO) AGO . Adjuvant Radiotherapy. 2019.
- 53. (AGO) AGO . Therapy Side Effects. 2019.
- 54. (AGO) AGO . Supportive Care. 2019.
- 55. (AGO) AGO . Breast Cancer: Specific Situations. 2019.
- 56. (AGO) AGO . Breast Cancer Follow‐Up. 2019.
- 57. (AGO) AGO . Loco‐Regional Recurrence. 2019.
- 58. (AGO) AGO . Endocrine and “Targeted” Therapy in Metastatic Breast Cancer. 2019.
- 59. (AGO) AGO . Chemotherapy With or Without Targeted Drugs* in Metastatic Breast Cancer. 2019.
- 60. (AGO) AGO . Osteooncology and Bone. Health. 2019.
- 61. (AGO) AGO . Specific Sites of Metastases. 2019.
- 62. (AGO) AGO .CNS Metastases in Breast Cancer. 2019.
- 63. (AGO) AGO . Complementary Therapy Survivorship. 2019.
- 64. (AGO) AGO . AGO Recommendations for the Diagnosis and Treatment of Patients with Advanced and Metastatic Breast Cancer: Update 2019. 2019.
- 65. (AGO) AGO . Recommendations for the Diagnosis and Treatment of Patients with Early Breast Cancer: Update 2019. 2019. [DOI] [PMC free article] [PubMed]
- 66. Harms W, Budach W, Dunst J, et al. DEGRO practical guidelines for radiotherapy of breast cancer VI: therapy of locoregional breast cancer recurrences. Strahlenther Onkol. 2016;192(4):199‐208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Sedlmayer F, Sautter‐Bihl ML, Budach W, et al. DEGRO practical guidelines: radiotherapy of breast cancer I: radiotherapy following breast conserving therapy for invasive breast cancer. Strahlenther Onkol. 2013;189(10):825‐833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Wenz F, Sperk E, Budach W, et al. DEGRO practical guidelines for radiotherapy of breast cancer IV: radiotherapy following mastectomy for invasive breast cancer. Strahlenther Onkol. 2014;190(8):705‐714. [DOI] [PubMed] [Google Scholar]
- 69. Sautter‐Bihl ML, Sedlmayer F, Budach W, et al. DEGRO practical guidelines: radiotherapy of breast cancer III–radiotherapy of the lymphatic pathways. Strahlenther Onkol. 2014;190(4):342‐351. [DOI] [PubMed] [Google Scholar]
- 70. (NCCP) NCCP . Diagnosis, staging and treatment of patients with breast cancer. National Clinical Guideline No. 7. 2015.
- 71. Richtlijnendatabase . Breast Cancer. 2018.
- 72. Mureau MAM. Breast Reconstruction Guideline Working G. Dutch breast reconstruction guideline. J Plast Reconstr Aesthet Surg. 2018;71(3):290‐304. [DOI] [PubMed] [Google Scholar]
- 73. Nederland IK. Breast Cancer. 2012.
- 74. Fisterra . Cáncer de mama/Breast Cancer. 2019.
- 75. Ayala de la Pena F, Andres R, Garcia‐Saenz JA, et al. SEOM clinical guidelines in early stage breast cancer (2018). Clin Transl Oncol 2019;21(1):18‐30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chacon Lopez‐Muniz JI, de la Cruz Merino L, Gavila Gregori J, et al. SEOM clinical guidelines in advanced and recurrent breast cancer (2018). Clin Transl Oncol. 2019;21(1):31‐45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Gavila J, Lopez‐Tarruella S, Saura C, et al. SEOM clinical guidelines in metastatic breast cancer 2015. Clin Transl Oncol. 2015;17(12):946‐955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Gonzalez‐Santiago S, Ramon YCT, Aguirre E, et al. SEOM clinical guidelines in hereditary breast and ovarian cancer (2019). Clin Transl Oncol. 2020;22(2):193‐200. [DOI] [PubMed] [Google Scholar]
- 79. NICE . Abemaciclib with fulvestrant for treating hormone receptor‐positive, HER2‐negative advanced breast cancer after endocrine the therapy. 2019.
- 80. NICE . Ribociclib with fulvestrant for treating hormone receptor‐positive, HER2‐negative, advanced breast cancer. 2019.
- 81. NICE . Early and locally advanced breast cancer: diagnosis and management. 2018.
- 82. NICE . Breast cancer. 2011.
- 83. NICE . Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer. 2013. [PubMed]
- 84. NICE . Breast reconstruction using lipomodelling after breast cancer treatment. 2012.
- 85. NICE . Gene expression profiling and expanded immunohistochemistry tests for guiding adjuvant chemotherapy decisions in early breast cancer management: MammaPrint, Oncotype DDX, X, IHC4 and Mammostrat. 2013.
- 86. NICE . Pertuzumab for the neoadjuvant treatment of HER2‐positive breast cancer. 2016.
- 87. NICE . Intraoperative tests (RD‐100i OSNA system and Metasin test) for detecting sentinel lymph node metastases in breast cancer. 2013. [DOI] [PMC free article] [PubMed]
- 88. Services AH . Breast reconstruction following prophylactic or therapeutic mastectomy for breast cancer. 2017.
- 89. Services AH . Adjuvant systemic therapy for early stage (lymph node negative and lymph node positive) breast cancer. 2018.
- 90. Services AH . Optimal use of taxanes in metastatic breast cancer (MBC) . 2013.
- 91. Services AH . Adjuvant radiation therapy for invasive breast cancer. 2015.
- 92. Services AH . Adjuvant radiation therapy for ductal carcinoma in situ. 2015.
- 93. Services AH . Neo‐adjuvant (pre‐operative) therapy for breast cancer ‐ general considerations. 2014.
- 94. Health COMo . The Role of Trastuzumab in Adjuvant and Neoadjuvant Therapy in Women with HER2/neu‐overexpressing Breast Cancer. 2011.
- 95. Health COMo . Surgical management of early‐stage invasive breast cancer. 2015.
- 96. Health COMo . Breast irradiation in women with early stage invasive breast cancer following breast conserving surgery. 2016.
- 97. Health COMo . The role of the taxanes in the management of metastatic breast cancer. 2011.
- 98. Health COMo . Vinorelbine in stage iv breast cancer. 2012.
- 99. Health COMo . The role of aromatase inhibitors in the treatment of postmenopausal women with metastatic breast cancer. 2012.
- 100. Health COMo . Epirubicin, as a single agent or in combination, for metastatic breast cancer. 2011.
- 101. Health COMo . Adjuvant taxane therapy for women with early‐stage, invasive breast cancer. 2011.
- 102. Health COMo . Adjuvant systemic therapy for node‐negative breast cancer. 2011.
- 103. Health COMo . Adjuvant ovarian ablation in the treatment of premenopausal women with early stage invasive breast cancer. 2010.
- 104. Health COMo . The role of gemcitabine in the management of metastatic breast cancer. 2011.
- 105. Health COMo . The role of trastuzumab (herceptin) in the treatment of women with Her2/neu‐overexpressing metastatic breast cancer. 2010.
- 106. Health COMo . Capecitabine in stage IV breast cancer. 2011.
- 107. Health COMo . The role of her2/neu in systemic and radiation therapy for women with breast cancer. 2012.
- 108. Health COMo . Locoregional therapy of locally advanced breast cancer (LABC) . 2014.
- 109. Health COMo . The role of taxanes in neoadjuvant chemotherapy for women with non‐metastatic breast cancer. 2011.
- 110. Health COMo . Optimal systemic therapy for early female breast cancer. 2014.
- 111. Health COMo . Use of adjuvant bisphosphonates and other bone‐modifying agents in breast cancer. 2016. [DOI] [PubMed]
- 112. Health COMo . The Role of Aromatase Inhibitors in Adjuvant Therapy for Postmenopausal Women with Hormone Receptor‐positive Breast Cancer. 2012.
- 113. (ABS) AoBs . Margin width in breast conservation Surgery. 2015.
- 114. (ABS) AoBs . Antibiotic prophylaxis In breast surgery. 2015.
- 115. (ABS) AoBs . Management of the malignant axilla in early breast cancer. 2015.
- 116. (ABS) AoBs . Breast operation note Documentation. 2015.
- 117. (ABS) AoBs . Update on optimal duration of adjuvant antihormonal therapy. 2015.
- 118. Association of Breast surgery (ABS) BAoP, Reconstructive and Aesthetic Surgeons (BAPRAS) . Oncoplastic breast reconstruction. 2012.
- 119. Association of Breast surgery (ABS) BAoP, Reconstructive and Aesthetic Surgeons (BAPRAS) . Acellular dermal matrix (ADM) assisted breast reconstruction procedures. 2012. [DOI] [PubMed]
- 120. Taskforce SC . Breast Cancer Clinical Quality Performance Indicators. 2016.
- 121. (SING) SIGN . Treatment of primary breast cancer. 2013.
- 122. Associations JGfBS . Lipomodelling Guidelines for Breast Surgery. 2012.
- 123. (ASBS) ASoBS . Performance and Practice Guidelines for the Use of Neoadjuvant Systemic Therapy in the Management of Breast Cancer. 2017.
- 124. (ASBS) ASoBS . Performance and Practice Guidelines for Mastectomy. 2014.
- 125. (ASBS) ASoBS . Performance and Practice Guidelines for Breast‐Conserving Surgery/Partial Mastectomy. 2014.
- 126. (ASBS) ASoBS . Performance and Practice Guidelines for Axillary Lymph Node Dissection in Breast Cancer Patients. 2014.
- 127. (ASBS) ASoBS . Performance and Practice Guidelines for Sentinel Lymph Node Biopsy in Breast Cancer Patients. 2014.
- 128. Lee BT, Agarwal JP, Ascherman JA, et al. Evidence‐Based clinical practice guideline: autologous breast reconstruction with DIEP or Pedicled TRAM Abdominal Flaps. Plast Reconstr Surg. 2017;140(5):651e‐664e. [DOI] [PubMed] [Google Scholar]
- 129. Visvanathan K, Fabian CJ, Bantug E, et al. Use of endocrine therapy for breast cancer risk reduction: ASCO Clinical Practice Guideline Update. J Clin Oncol. 2019;37(33):3152‐3165. [DOI] [PubMed] [Google Scholar]
- 130. Recht A, Comen EA, Fine RE, et al. Postmastectomy Radiotherapy: An American Society of Clinical Oncology, American Society for Radiation Oncology, and Society of Surgical Oncology Focused Guideline Update. Pract Radiat Oncol. 2017;6(6):e219‐e234. [DOI] [PubMed] [Google Scholar]
- 131. Smith TJ. Breast cancer surveillance guidelines. J Oncol Pract. 2013;9(1):65‐67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132. Denduluri N, Somerfield MR, Giordano SH. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Clinical Practice Guideline Focused Update Summary. J Oncol Pract. 2018;14(8):508‐510. [DOI] [PubMed] [Google Scholar]
- 133. Giordano SH, Temin S, Davidson NE. Systemic therapy for patients with advanced human epidermal growth factor receptor 2‐positive breast cancer: ASCO Clinical Practice Guideline Update Summary. J Oncol Pract. 2018;14(8):501‐504. [DOI] [PubMed] [Google Scholar]
- 134. Ramakrishna N, Temin S, Lin NU. Recommendations on disease management for patients with advanced human epidermal growth factor receptor 2‐positive breast cancer and brain metastases: ASCO Clinical Practice Guideline Update Summary. J Oncol Pract. 2018;14(8):505‐507. [DOI] [PubMed] [Google Scholar]
- 135. Lyman GH, Bohlke K, Cohen L. Integrative Therapies During and After Breast Cancer Treatment: ASCO Endorsement of the SIO Clinical Practice Guideline Summary. J Oncol Pract. 2018;14(8):495‐499. [DOI] [PubMed] [Google Scholar]
- 136. Partridge AH, Rumble RB, Carey LA, et al. Chemotherapy and targeted therapy for women with human epidermal growth factor receptor 2‐negative (or unknown) advanced breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2014;32(29):3307‐3329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137. Van Poznak C, Somerfield MR, Barlow WE, et al. Role of bone‐modifying agents in metastatic breast cancer: an American society of clinical oncology‐cancer care Ontario focused guideline update. J Clin Oncol. 2017;35(35):3978‐3976. [DOI] [PubMed] [Google Scholar]
- 138. Wolff AC, Hammond ME, Hicks DG, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997‐4013. [DOI] [PubMed] [Google Scholar]
- 139. Khatcheressian JL, Hurley P, Bantug E, et al. Breast cancer follow‐up and management after primary treatment: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(7):961‐965. [DOI] [PubMed] [Google Scholar]
- 140. Burstein HJ, Lacchetti C, Griggs JJ. Adjuvant endocrine therapy for women with hormone receptor‐positive breast cancer: ASCO Clinical Practice Guideline Focused Update. J Oncol Pract. 2019;15(2):106‐107. [DOI] [PubMed] [Google Scholar]
- 141. Henry NL, Somerfield MR, Abramson VG, et al. Role of patient and disease factors in adjuvant systemic therapy decision making for early‐stage, operable breast cancer: American society of clinical oncology endorsement of cancer care Ontario guideline recommendations. J Clin Oncol. 2016;34(19):2303‐2311. [DOI] [PubMed] [Google Scholar]
- 142. (ASCO) ASoCO . Use of Adjuvant Bisphosphonates and Other Bone‐Modifying Agents in Breast Cancer: A Cancer Care Ontario and American Society of Clinical Oncology Clinical Practice Guideline. 2017. [DOI] [PubMed]
- 143. Krop I, Ismaila N, Stearns V. Use of biomarkers to guide decisions on adjuvant systemic therapy for women with early‐stage invasive breast cancer: American Society of Clinical Oncology Clinical Practice Focused Update Guideline Summary. J Oncol Pract. 2017;13(11):763‐766. [DOI] [PubMed] [Google Scholar]
- 144. Van Poznak C, Harris LN, Somerfield MR. Use of biomarkers to guide decisions on systemic therapy for women with metastatic breast cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Oncol Pract. 2017;11(6):514‐516. [DOI] [PubMed] [Google Scholar]
- 145. Griggs JJ, Somerfield MR, Anderson H, et al. American Society of Clinical Oncology endorsement of the cancer care Ontario practice guideline on adjuvant ovarian ablation in the treatment of premenopausal women with early‐stage invasive breast cancer. J Clin Oncol. 2011;29(29):3939‐3942. [DOI] [PubMed] [Google Scholar]
- 146. Hammond ME, Hayes DF, Wolff AC, et al. American society of clinical oncology/college of american pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J Oncol Pract. 2010;6(4):195‐197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147. Visvanathan K, Hurley P, Bantug E, et al. Use of pharmacologic interventions for breast cancer risk reduction: American Society of Clinical Oncology clinical practice guideline. J Clin Oncol. 2013;31(23):2942‐2962. [DOI] [PubMed] [Google Scholar]
- 148. Rugo HS, Rumble RB, Macrae E, et al. Endocrine therapy for hormone receptor‐positive metastatic breast cancer: American Society of Clinical Oncology Guideline. J Clin Oncol. 2016;34(25):3069–3103. [DOI] [PubMed] [Google Scholar]
- 149. NCCN . Invasive Breast Cancer. Basic resources. Version 1.2019. 2019.
- 150. NCCN . Invasive Breast Cancer. Core resources. Version 1.2019. 2019.
- 151. NCCN . NCCN. Invasive Breast Cancer. Enhanced resources. Version 1.2019. 2019.
- 152. NCCN . NCCN Evidence Blocks. Version 1.2019. 2019.
- 153. NCCN . Breast Cancer. Version 3.2018. 2019.
- 154. Malaysia MoH . Management of Breast Cancer (2nd Edition). 2010.
- 155. Government A . Influencing best practice in breast cancer. 2016.
- 156. Australia C . Recommendations for staging and managing the axilla. 2011.
- 157. Australia C . Recommendations for use of hypofractionated radiotherapy for early operable breast cancer. 2011.
- 158. Australia C . Recommendations for use of Biphosphonates. 2011.
- 159. Australia C . Recommendations for the management of early breast cancer in women with an identified BRCA1 or BRCA2 gene mutation or at high risk of a gene mutation. 2014.
- 160. Chile MdSd . Guía de Práctica Clínica AUGE Cáncer de Mama. 2015.
- 161. Colombia INd . Guía de práctica clínica (GPC) para la detección temprana, tratamiento integral, seguimiento y rehabilitación del cáncer de mama. 2017.
- 162. Institution IHCA . Guía de Práctica Clínica del Tratamiento para el Cáncer de Mama. 2011.
- 163. IETSI . Guía de Práctica Clínica para el Tratamiento del Cáncer de Mama. 2017.
- 164. Venezuela SAd . Guía para el Cáncer de Mama en Venezuela. 2015.
- 165. Zealand MoHfN . Management of Early Breast Cancer. 2014.
- 166. Wockel A, Albert US, Janni W, et al. The screening, diagnosis, treatment, and follow‐up of breast cancer. Dtsch Arztebl Int. 2018;115(18):316‐323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167. Gil‐Gil MJ, Martinez‐Garcia M, Sierra A, et al. Breast cancer brain metastases: a review of the literature and a current multidisciplinary management guideline. Clin Transl Oncol. 2014;16(5):436‐446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168. Cirugía AEd . Cirugía de la Mama. 2017.
- 169. Alliance BEAGNC . NCA Breast Cancer Clinical Guidelines. 2019.
- 170. (BCMA) BCMA . Breast Cancer: Management and Follow‐Up. 2013.
- 171. (WMCA) WMEAGfBC . Clinical Guidelines for the Management of Breast Cancer. 2016.
- 172. Rica CdMyCdC . Consenso costarricense sobre prevención, diagnóstico y tratamiento del cáncer mamario. 2016.
- 173. México SdSd . Consenso Mexicano sobre diagnóstico y tratamiento del cáncer mamario. 2019.
- 174. Xu B, Hu X, Jiang Z, et al. National consensus in China on diagnosis and treatment of patients with advanced breast cancer. Ann Transl Med. 2015;3(17):242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175. Parikh PM, Gupta S, Dawood S, et al. ICON 2013: practical consensus recommendations for hormone receptor‐positive Her2‐negative advanced or metastatic breast cancer. Indian J Cancer. 2014;51(1):73‐79. [DOI] [PubMed] [Google Scholar]
- 176. Somashekhar SP, Agarwal G, Deo SVS et al Indian solutions for indian problems‐association of breast surgeons of India (ABSI) practical consensus statement, recommendations, and guidelines for the treatment of breast cancer in India. Indian J Surg. 2017;79(4):275‐285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177. (ICMR) ICoMR . Consensus document for management of breast cancer. 2016.
- 178. Cardoso F, Senkus E, Costa A, et al. 4th ESO‐ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4)dagger. Ann Oncol. 2018;29(8):1634‐1657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179. Gnant M, Harbeck N, Thomssen C. St. Gallen/Vienna 2017: A Brief summary of the consensus discussion about escalation and de‐escalation of primary breast cancer treatment. Breast Care (Basel). 2017;12(2):102‐107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180. Offersen BV, Boersma LJ, Kirkove C, et al. ESTRO consensus guideline on target volume delineation for elective radiation therapy of early stage breast cancer, version 1.1. Radiother Oncol. 2016;118(1):205‐208. [DOI] [PubMed] [Google Scholar]
- 181. Paluch‐Shimon S, Pagani O, Partridge AH, et al. ESO‐ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast. 2017;35:203‐217. [DOI] [PubMed] [Google Scholar]
- 182. Juozaityte E, Aleknavicius E, Janciauskiene R, et al. Guidelines for diagnostics and treatment of aromatase inhibitor‐induced bone loss in women with breast cancer: a consensus of Lithuanian medical oncologists, radiation oncologists, endocrinologists, and family medicine physicians. Medicina (Kaunas). 2014;50(4):197‐203. [DOI] [PubMed] [Google Scholar]
- 183. Colomer R, Aranda‐Lopez I, Albanell J, et al. Biomarkers in breast cancer: A consensus statement by the Spanish Society of Medical Oncology and the Spanish Society of Pathology. Clin Transl Oncol. 2018;20(7):815‐826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184. Manitoba C. Provincial consensus recommendations for adjuvant systemic therapy for breast cancer. Cancer Guidelines Database. 2017. [Google Scholar]
- 185. Radiologist TRCo . Postoperative radiotherapy for breast cancer: UK consensus statements. RCR Consensus. 2016. [DOI] [PubMed] [Google Scholar]
- 186. (ASBS) ASoBS . Consensus Guideline on Accelerated Partial Breast Irradiation. 2018.
- 187. (ASBS) ASoBS . Consensus Guideline on the Use of Transcutaneous and Percutaneous Ablation for the Treatment of Benign and Malignant Tumors of the Breast. 2018.
- 188. (ASBS) ASoBS . Consensus Guideline on the Management of the Axilla in Patients With Invasive/In‐Situ Breast Cancer. 2019.
- 189. (ASBS) ASoBS . Consensus Guideline on Breast Cancer Lumpectomy Margins. 2017.
- 190. (ASBS) ASoBS . Consensus Guideline on Concordance Assessment of Image‐Guided Breast Biopsies and Management of Borderline or High‐Risk Lesions. 2016.
- 191. Boughey JC, Attai DJ, Chen SL, et al. Contralateral Prophylactic Mastectomy (CPM) Consensus Statement from the American Society of Breast Surgeons: Data on CPM Outcomes and Risks. Ann Surg Oncol. 2016;23(10):3100‐3105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192. (ASBS) ASoBS . Consensus Guideline on Venous Thromboembolism (VTE) Prophylaxis for Patients Undergoing Breast Operations. ASBS Consensus. 2011.
- 193. Tom MC, Joshi N, Vicini F, et al. The American Brachytherapy Society consensus statement on intraoperative radiation therapy. Brachytherapy. 2019;18(3):242‐257. [DOI] [PubMed] [Google Scholar]
- 194. Hepel JT, Arthur D, Shaitelman S, et al. American Brachytherapy Society consensus report for accelerated partial breast irradiation using interstitial multicatheter brachytherapy. Brachytherapy. 2017;16(5):919‐928. [DOI] [PubMed] [Google Scholar]
- 195. Hunt KK, Euhus DM, Boughey JC, et al. Society of Surgical Oncology Breast Disease Working Group Statement on Prophylactic (Risk‐Reducing) Mastectomy. Ann Surg Oncol. 2017;24(2):375‐397. [DOI] [PubMed] [Google Scholar]
- 196. Morrow M, Van Zee KJ, Solin LJ, et al. Society of Surgical Oncology‐American Society for Radiation Oncology‐American Society of Clinical Oncology Consensus Guideline on Margins for Breast‐Conserving Surgery With Whole‐Breast Irradiation in Ductal Carcinoma in Situ. Pract Radiat Oncol. 2016;6(5):287‐295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197. Moran MS, Schnitt SJ, Giuliano AE, et al. Society of Surgical Oncology‐American Society for Radiation Oncology consensus guideline on margins for breast‐conserving surgery with whole‐breast irradiation in stages I and II invasive breast cancer. Int J Radiat Oncol Biol Phys. 2014;88(3):553‐564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198. Buchholz TA, Somerfield MR, Griggs JJ, et al. Margins for breast‐conserving surgery with whole‐breast irradiation in stage I and II invasive breast cancer: American Society of Clinical Oncology endorsement of the Society of Surgical Oncology/American Society for Radiation Oncology consensus guideline. J Clin Oncol. 2014;32(14):1502‐1506. [DOI] [PubMed] [Google Scholar]
- 199. Dawood S, Merajver SD, Viens P, et al. International expert panel on inflammatory breast cancer: consensus statement for standardized diagnosis and treatment. Ann Oncol. 2011;22(3):515‐523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200. Chen Y, Yang K, Marusic A, et al. A Reporting Tool for Practice Guidelines in Health Care: The RIGHT Statement. Ann Intern Med. 2017;166(2):128‐132. [DOI] [PubMed] [Google Scholar]
- 201. Brouwers MC, Kerkvliet K, Spithoff K, et al. The AGREE Reporting Checklist: a tool to improve reporting of clinical practice guidelines. BMJ. 2016;352:i1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202. M HG . How to write a paper (3er edition). BMJ Book. 2013.
- 203. Wieringa TH, Kunneman M, Rodriguez‐Gutierrez R, et al. A systematic review of decision aids that facilitate elements of shared decision‐making in chronic illnesses: a review protocol. Syst Rev. 2017;6(1):155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1
Appendix S2
Appendix S3
Appendix S4
Appendix S5
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.


