Abstract
The fibrinolytic system consists of a balance between rates of plasminogen activation and fibrin degradation, both of which are finely regulated by spatio-temporal mechanisms. Three distinct inhibitors of the fibrinolytic system that differently regulate these two steps are plasminogen activator inhibitor type-1 (PAI-1), α2-antiplasmin, and thrombin activatable fibrinolysis inhibitor (TAFI). In this review, we focus on the mechanisms by which PAI-1 governs total fibrinolytic activity to provide its essential role in many hemostatic disorders, including fibrinolytic shutdown after trauma. PAI-1 is a member of the serine protease inhibitor (SERPIN) superfamily and inhibits the protease activities of plasminogen activators (PAs) by forming complexes with PAs, thereby regulating fibrinolysis. The major PA in the vasculature is tissue-type PA (tPA) which is secreted from vascular endothelial cells (VECs) as an active enzyme and is retained on the surface of VECs. PAI-1, existing in molar excess to tPA in plasma, regulates the amount of free active tPA in plasma and on the surface of VECs by forming a tPA-PAI-1 complex. Thus, high plasma levels of PAI-1 are directly related to attenuated fibrinolysis and increased risk for thrombosis. Since plasma PAI-1 levels are highly elevated under a variety of pathological conditions, including infection and inflammation, the fibrinolytic potential in plasma and on VECs is readily suppressed to induce fibrinolytic shutdown. A congenital deficiency of PAI-1 in humans, in turn, leads to life-threatening bleeding. These considerations support the contention that PAI-1 is the primary regulator of the initial step of fibrinolysis and governs total fibrinolytic activity.
Keywords: Plasminogen activator inhibitor type 1 (PAI-1), tissue-type plasminogen activator (tPA), fibrinolysis, fibrinolytic potential, trauma, fibrinolysis shutdown
1. INTRODUCTION
The fibrinolytic system is sequentially composed of plasminogen activation and fibrin degradation [1, 2]. Three distinct inhibitors of the fibrinolytic system that differently regulate these two steps are plasminogen activator inhibitor type-1 (PAI-1), α2-antiplasmin (α2AP) and thrombin activatable fibrinolysis inhibitor (TAFI) [3]. PAI-1 limits the amount of free tissue-type plasminogen activator (tPA) both in plasma and on vascular endothelial cells (VECs), and regulates plasminogen activation potential to dissolve fibrin [4]. α2AP inhibits plasmin activity in plasma and also attenuates plasmin-catalyzed fibrin degradation after it is cross-linked to fibrin by the transglutaminase, activated coagulation factor XIII [5]. Activated TAFI, a carboxypeptidase, catalyzes the removal of C-terminal lysines of fibrin, which are essential for both effective plasminogen activation and effective fibrinolysis [6, 7]. These regulators function in a spatio-temporal manner, and enable the fibrinolytic system to quickly dissolve pathological thrombi, while at the same time protecting physiological haemostatic thrombi from premature lysis [3]. Disruption of the spatio-temporal regulation of the fibrinolytic system naturally leads to the development of either lethal bleeding or thrombotic disorders. A comprehension of the general scheme of fibrinolysis is necessary to evaluate the specific function of a particular component in a holistic manner. An accurate assessment of the contribution of each regulator to the global process of fibrinolysis, however, is problematic, since these regulators function differently in different environments. The lack of an apparent haemostatic phenotype in gene-deficient animals of these regulators [4, 8] also makes it difficult to extensively assess the functions of each regulatory component.
In the present review article, we focus on PAI-1 and discuss how this protein regulates the expression of fibrinolytic activity and how modification of this PAI-1 dependent regulation is causally related to either thrombotic or bleeding disorders. Although attention has focused on the pleiotropic functions of PAI-1 for drug development, we stress its principal function in the vasculature as a PA inhibitor.
2. PAI-1 GOVERNS THE “FIBRINOLYTIC POTENTIAL” IN PLASMA
Plasminogen activation in plasma takes place only in the presence of fibrin, and occurs immediately when a thrombus is formed. This is known as a coagulation-associated enhancement of fibrinolysis, in which the assembly of plasminogen and tPA on the fibrin surface occurs [1]. A template mechanism, as well as a conformational change of Gluplasminogen, the mature native plasminogen having glutamic acid in its N-terminus, to an easily activatable form after binding to fibrin through its lysine binding site (LBS) in kringle 5 is at the basis of these considerations [2, 3, 9, 10] (Fig. 1). Thus, fibrin formation is required to assess the full competence of fibrinolysis in plasma. However, this makes it difficult to identify the extent of the contribution of each regulator to the whole process of fibrinolysis in which all the regulators are involved in a complex manner. We here define the potential to express fibrinolytic activity when fibrin is formed as “fibrinolytic potential” and discuss how this is regulated.
Fig. (1).
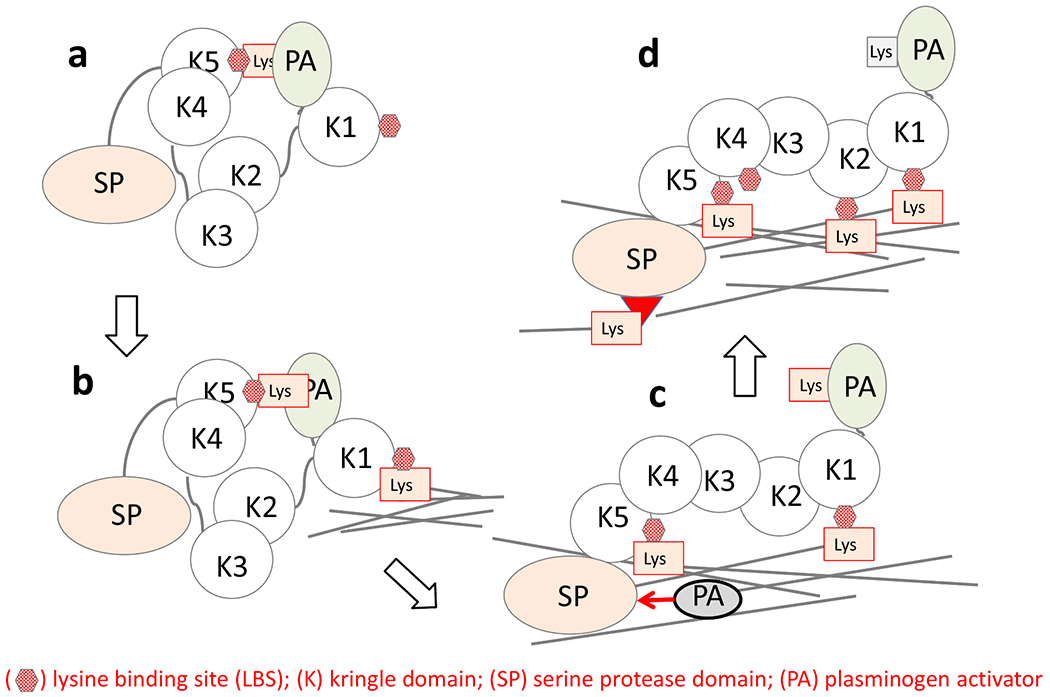
Glu-plasminogen changes its conformation from a tight to loose after binding to the fibrin surface.
Glu-plasminogen adopts a tight conformation (a) in the fluid phase in Cl−, but changes its conformation to a more relaxed conformation (c) after binding to fibrin through its lysine binding site in kringle 5. In the relaxed form, PAs easily cleave the 561-562 peptide bond in Glu-plasminogen to generate plasmin. Modified from [9].
The fibrinolytic potential of individual plasma samples varies widely. Several different assay methods have been developed to assess global fibrinolytic activity. Spontaneous plasma clot lysis occurs over days, and is impractical as a laboratory test [11]. The tPA-supplemented plasma clot lysis time is frequently used, but it is also not suitable since large amounts of tPA in excess of PAI-1 are required to obtain measurable clot lysis, which largely modifies the innate fibrinolytic potential. Thus, an euglobulin clot lysis time (ECLT) has been developed to more rapidly assess fibrinolytic potential. The euglobulin fraction of plasma, an isoelectric precipitate at acidic pH (pH 5.2-5.9), contains fibrinogen, plasminogen, tPA, and PAI-1 but does not contain major plasmin inhibitors of α2AP and α2-macroglobulin [12]. Thus, its lysis time is reasonably short and suitable for use as a laboratory test.
The ECLT varies to a large extent among plasma samples obtained from different individuals and shows circadian variations [13]. We observed a statistically significant inverse correlation between ECLT and tPA activity, and significant positive correlations between ECLT and both total and free PAI-1 levels in plasma [14–16]. Notably, the calculated amounts of free tPA, based upon the assumption that tPA and PAI-1 form a high molecular weight complex in plasma, showed significant positive correlations with tPA activity and a negative correlation with ECLT (Fig. 2) [15]. These data clearly suggest that the plasma concentration of PAI-1 in excess of tPA would directly govern free active tPA levels in the plasma, as well as the fibrinolytic potential in plasma, which is responsible for triggering plasmin generation when fibrin is formed. Such regulation of the initial step of fibrinolysis is different from those of other steps of the coagulation and fibrinolysis cascades. Whereas most of the other steps in hemostasis are regulated by more complex cascade-type mechanisms, in which the efficacy of the enzyme generation, as well as the balance between the amounts of the generated enzymes and the corresponding SERPINs are involved, the plasminogen activation step is basically reflected by the balance of plasma concentrations of tPA and PAI-1. This is mainly due to a unique characteristic of tPA of having endogenous activity to interact with both plasminogen and PAI-1 even in its single-chain form [17, 18].
Fig. (2).
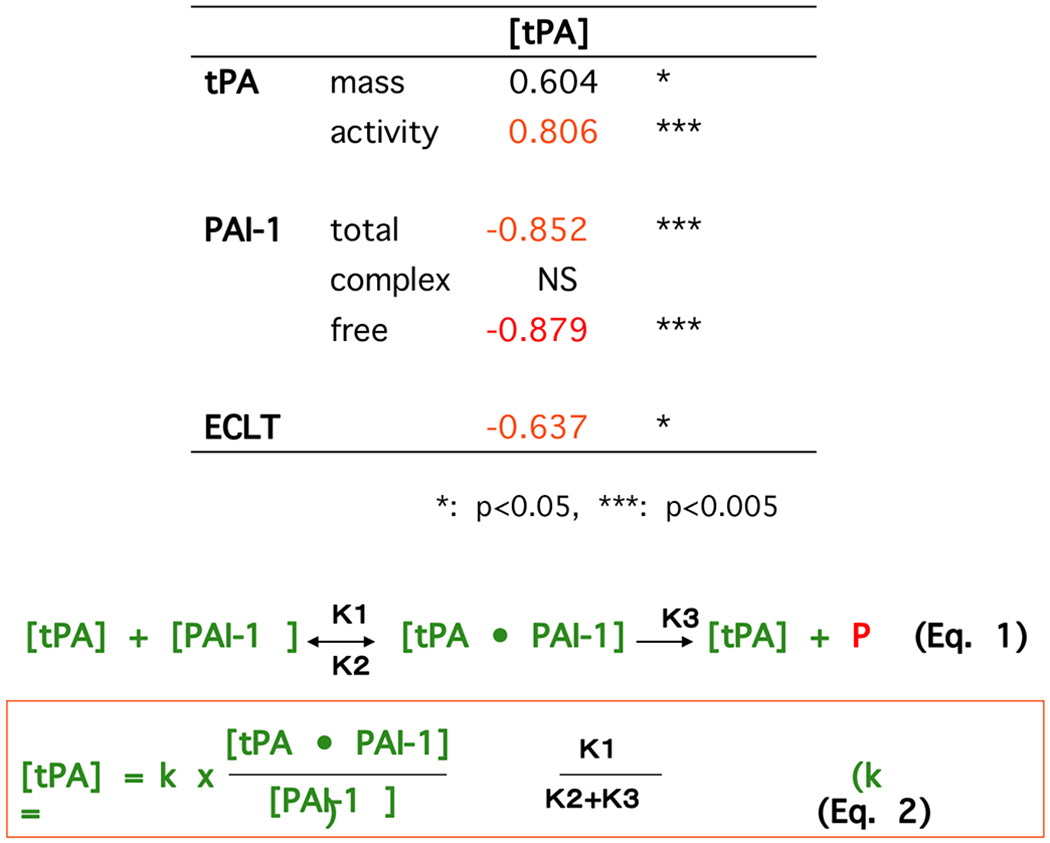
Balance between PAI-1 and tPA governs the amounts of free tPA, PA activity and fibrinolytic potential in plasma.
Concentrations of tPA (mass) and PAI-1 (total, complex and free form) as well as tPA activity in plasma, and ECLTs were measured using plasma obtained from 25 male normal volunteers. The amounts of free tPA ([tPA]) were calculated by equation (2) based upon an assumption that tPA and PAI-1 interacts according to equation (1) even in plasma milieu. Calculated free tPA level showed a significant strong correlation (Spearman’s rank correlation coefficient) with tPA activity in plasma, and a negative correlation with ECLT. Modified from [15].
3. REGULATION OF THE FIBRINOLYTIC POTENTIAL ON THE SURFACE OF VECs
tPA possesses another unique characteristic. This protein is secreted from VECs by both regulated and constitutive secretory mechanisms [19]. Employing tPA attached to green fluorescence protein (GFP), we analyzed the secretory dynamics of tPA-GFP by total internal reflection-fluorescence (TIR-F) microscopy. After activation and opening of tPA-containing granules, the released tPA-GFP appeared to be retained on the surface of VECs for longer than several seconds [20]. This is unique to tPA since other peptide substances including insulin are secreted within milliseconds after activation of the corresponding granules. This unique characteristic of tPA contributes to the maintenance of a high fibrinolytic potential on VEC surfaces [21], and the retained tPA on the VEC contributes to rapid fibrin dissolution. Modification of affinities of tPA to the surfaces of both VECs and fibrin naturally alter the efficacy of the dissolution of the fibrin clot on the surface of VECs [22].
The fibrinolytic potential on VEC surfaces is also governed by PAI-1. PAI-1 facilitates the detachment of tPA from VEC surfaces after forming the tPA-PAI-1 complex [20]. When the PAI-1 concentration is elevated in plasma, the amounts of tPA and its activity on VECs are suppressed, and the levels of the tPA-PAI-1 complex increase in plasma. This is similar to the amounts of free tPA and its activity in plasma both of which are principally determined by the balance of tPA and PAI-1 [14, 15]. All of this is governed by the unique characteristic of tPA which possess endogenous activity as a single chain form [17, 23].
4. SUPPRESSED FIBRINOLYTIC POTENTIAL BY PAI-1 IS RELATED TO THROMBOGENESIS
An increase in PAI-1 in plasma increases the risk of thrombosis by lowering the free tPA concentration and fibrinolytic potential in plasma [24]. High plasma levels of tPA antigen have been also reported as a risk factor for stroke and mitral infarction [25, 26]. An elevated level of plasma tPA antigen, in the form of PA-PAI-1 complex, indicates suppression of fibrinolytic potential on the surface of VECs. This results from the dissociation of retained tPA as a result of the formation of the tPA-PAI-1 complex [20]. The inner surface of the vascular wall is the site for thrombus formation, and thus rapid lysis after thrombus formation through highly maintained fibrinolytic potential is essential to maintain vascular patency. The dissociation of free active tPA from VEC surfaces by PAI-1 naturally increases the risk of thrombosis.
PAI-1 contained in platelets also plays an important role under physiological and pathological conditions [27, 28]. Although the regulatory mechanism of its secretion has not yet been clarified, PAI-1 originating in platelets contributes to thrombogenesis under shear stress [29–31], which is related to arterial thrombogenicity.
Fluctuations in fibrinolytic activity due to gender difference, aging, circadian regulation, exercise loading, lipid metabolism disorders, and infection/inflammation, have been shown to be caused by changes in plasma PAI-1 levels due to its modified gene expression [24, 32]. Several cytokines are known to enhance PAI-1 gene expression and to increase plasma PAI-1 concentrations as much as several hundredfold, which is a major mechanism for thrombogenesis and/or multiple organ failure in infection/inflammation [33].
5. INCREASED PAI-1 CAUSES FIBRINOLYSIS SHUTDOWN AFTER TRAUMA
Trauma-induced coagulopathy (TIC) is a well-known phenomenon in which uncontrollable bleeding occurs immediately after severe brain injury. This has been postulated to be associated with inflammation, dysfunctional vascular endothelial cells, shock, and organ failure [34]. Effective reduction of blood loss after tranexamic acid infusion, and/or blood component transfusion are typically seen [35]. TIC patients also show an increase in coagulation markers, including soluble fibrin monomer and the thrombin-antithrombin complex, as well as fibrinolysis markers, including the plasmin-α2AP complex in plasma [34]. In animal models, brain-derived microparticles bearing tissue factor on their surface has been shown to exist in circulation, which was then rapidly transferred to lung tissue [36]. These data suggest that the pathogenesis of the uncontrollable bleeding in TIC is considered to be consumptive coagulopathy associated with enhanced fibrinolysis, which is typically seen in disseminated intravascular coagulation (DIC). The loss of platelet function observed in TIC [37], however, seems an exception since this has not been reported in DIC. It is possible that TIC could be considered as a form of DIC which develops a wide range of phenotypes depending on the pathogenesis of the underlying disease [38]. A fibrinolysis-dominant phenotype of DIC, typically seen in abdominal aortic aneurysms having large thrombi [39], and in vascular intimal carcinogenesis, having high expression of annexin A2 [40], seems similar to TIC. Possible impairment of platelet function in these DIC patients should be analyzed in order to assess whether a highly elevated fibrinolytic activity is involved in the shedding of platelets’ receptors.
Recently, a stage of suppressed fibrinolysis termed “fibrinolytic shutdown” has received attention. This is typically seen in TIC following a hyperfibrinolytic stage, and contributes to the development of organ failure through microthrombi accumulation [41, 42]. A sudden increase in PAI-1 in plasma, as an acute phase reactant corresponding to systemic inflammation, is considered responsible for this phenomenon [43].
For the uncontrollable bleeding in TIC, treatment by tranexamic acid (TXA) is recommended and successful reduction in the amounts of bleeding has been reported [35]. Tranexamic acid suppresses plasminogen activation on the fibrin surface by inhibition of plasminogen binding to C-terminal Lys residues on the fibrin surface [2]. Success in retarding bleeding by TXA further confirms that the uncontrollable bleeding in TIC is caused by elevated fibrinolytic activity. Based on the success in TIC, TXA is now used in the treatment of other uncontrollable bleeding states, including postpartum hemorrhage [44]. However, considering that a stage of “fibrinolytic shutdown” would follow a “hyperfibrinolysis” stage, a question naturally arises as to whether tranexamic acid treatment is necessary and/or safe in TIC [42, 45]. TXA treatment should be considered in underlying diseases only regarding patients whose fibrinolytic activity is highly enhanced. For the safe use of TXA, rapid and accurate laboratory tests are required to assess fibrinolytic activity. Further, understanding the pathogenesis of the sequential modification of fibrinolytic activity after severe insults is prerequisite.
6. CONGENITAL PAI-1 DEFICIENCIES IN HUMAN DEVELOP LIFE-THREATENING BLEEDING
A genetically confirmed PAI-1 deficiency was first reported in an Amish family, the proband of which showed repeated severe bleeding episodes [46]. Two distinct Japanese cases also showed repeated severe bleeding after tooth extractions, surgery, and obstetric complications [4, 47, 48]. Delayed bleeding is the phenotype frequently seen in fibrinolytic disorders [4, 49]. The observations that plasma levels of fibrinogen and plasminogen are maintained in these patients when they are not exposed to insults [47, 48] suggest that fibrinolytic activity is expressed at low levels if fibrin is not formed, even in the absence of PAI-1. In turn, when fibrin is formed, fibrinolytic activity is highly expressed resulting from premature lysis of haemostatic thrombi and delayed bleeding. Pregnancy is such an occasion in which uncontrollable bleeding can occur. In order to maintain pregnancy, highly intensive interventions may be required although the amounts of bleeding are still extraordinary [4, 47, 48].
While PAI-1-deficient animals did not show a bleeding phenotype, these phenotypes observed in human homozygote cases of PAI-1 deficiency clearly demonstrate its principal role in fibrinolysis. Further, these results revealed the limitations of some animal models in the analyses of human pathology [4].
7. PLEIOTROPIC FUNCTION OF PAI-1 AND PROSPECTS OF PAI-1 INHIBITORS
In addition to its primary function to regulate PAs’ activity, PAI-1 has a wide range of pleiotropic activities [50]. Modifications of cellular functions including mobility and binding capacity to matrix proteins are well known, which are related to tumor metastasis, migration of inflammatory cells, angiogenesis, hematopoiesis, etc. These may also explain impaired wound healing in the PAI-1 deficient patients [4, 47, 48]. Underlying mechanisms are explained by the specific function of PAI-1 to alter cellular binding capacity to vitronectin, either through integrins or urokinase-type PA (uPA) / uPA receptor (uPAR) [50]. Modification of cellular binding to matrix proteins naturally alters the associated signal transductions and cellular function as well [51, 52]. Inhibition of the activity of uPAR-bound uPA by PAI-1 also modulates cellular functions [51]. Another intriguing function of PAI-1 is related to aging which was evidenced by a longer leukocyte telomere length in heterozygotes of a congenital PAI-1 deficient family [53]. Retardation of the early senescence found in Klotho-deficient mice by either a simultaneous PAI-1 gene knock-out or a treatment by PAI-1 inhibitor [54] also suggests that PAI-1 plays a role in senescence.
To reduce the risk for thrombosis caused by increased levels of plasma PAI-1, many pharmaceutical companies have developed PAI-1 inhibitors [55, 56], but none of these have been employed in the clinic as anti-thrombotic drugs at this time. To facilitate mobilization of hematopoietic stem cells, however, a phase-1 clinical trial for one of the small molecular weight PAI-1 inhibitors, TM5614, has been completed [57]. Interest in the clinical use of PAI-1 inhibitors is expanding for targeting a wide range of pleiotropic functions of PAI-1.
CONCLUSION
In this review, we considered how PAI-1 directly governs fibrinolytic potential in plasma, as well as on VECs, and why increased PAI-1 levels in the plasma directly induce thrombogenesis. The implication of an elevated PAI-1 level over that of tPA is totally different from the modification of other SERPIN concentrations over those of their target proteases. We believe that such knowledge is mandatory to understand the underlying mechanisms of hemostasis, as well as to establish novel treatments for fibrinolytic shutdown in which appropriate use of PAI-1 inhibitors under development would be one of the choices.
ACKNOWLEDGEMENTS
Declared none.
FUNDING
The study is funded by Japan Society for the Promotion of Science (JSPS) KAKENHI (16K08492, 19K08577), Foundation for the National Institutes of Health (HL013423), Japanese Ministry of Health, Labour and Welfare (H27NN018B1), Smoking Research foundation.
Footnotes
CONSENT FOR PUBLICATION
Not applicable.
CONFLICT OF INTEREST
The authors declare no conflict of interest, financial or otherwise.
REFERENCES
- [1].Collen D The plasminogen (fibrinolytic) system. Thromb Haemost 1999; 82(2): 259–70. [PubMed] [Google Scholar]
- [2].Castellino FJ, Ploplis VA. Structure and function of the plasminogen/plasmin system. Thromb Haemost 2005; 93(4): 647–54. [ 10.1160/TH04-12-0842] [DOI] [PubMed] [Google Scholar]
- [3].Urano T, Castellino FJ, Suzuki Y. Regulation of plasminogen activation on cell surfaces and fibrin. J Thromb Haemost 2018. [ 10.1111/jth.14157] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Iwaki T, Urano T, Umemura K. PAI-1, progress in understanding the clinical problem and its aetiology. Br J Haematol 2012; 157(3): 291–8. [ 10.1111/j.1365-2141.2012.09074.x] [DOI] [PubMed] [Google Scholar]
- [5].Rijken DC, Uitte de Willige S. Inhibition of Fibrinolysis by Coagulation Factor XIII. BioMed Res Int 2017; 20171209676 [ 10.1155/2017/1209676] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Colucci M, Semeraro N. Thrombin activatable fibrinolysis inhibitor: At the nexus of fibrinolysis and inflammation. Thromb Res 2012; 129(3): 314–9. [ 10.1016/j.thromres.2011.10.031] [DOI] [PubMed] [Google Scholar]
- [7].Plug T, Meijers JC. Structure-function relationships in thrombina-ctivatable fibrinolysis inhibitor. J Thromb Haemost 2016; 14(4): 633–44. [ 10.1111/jth.13261] [DOI] [PubMed] [Google Scholar]
- [8].Morser J, Gabazza EC, Myles T, Leung LL. What has been learnt from the thrombin-activatable fibrinolysis inhibitor-deficient mouse? J Thromb Haemost 2010; 8(5): 868–76. [ 10.1111/j.1538-7836.2010.03787.x] [DOI] [PubMed] [Google Scholar]
- [9].Law RH, Caradoc-Davies T, Cowieson N, et al. The X-ray crystal structure of full-length human plasminogen. Cell Rep 2012; 1(3): 185–90. [ 10.1016/j.celrep.2012.02.012] [DOI] [PubMed] [Google Scholar]
- [10].Castellino FJ. Plsminogen.Molecular Basis on Thrombosis and Haemostasis. Marcel Dekker; 1995; pp. 495–515. [Google Scholar]
- [11].Tomczyk M, Suzuki Y, Sano H, Brzoska T, Tanaka H, Urano T. Bidirectional functions of thrombin on fibrinolysis: Evidence of thrombin-dependent enhancement of fibrinolysis provided by spontaneous plasma clot lysis. Thromb Res 2016; 143: 28–33. [ 10.1016/j.thromres.2016.04.018] [DOI] [PubMed] [Google Scholar]
- [12].Urano T, Nishikawa T, Nagai N, Takada Y, Takada A. Amounts of tPA and PAI-1 in the euglobulin fraction obtained at different pH: Their relation to the euglobulin clot lysis time. Thromb Res 1997; 88(1): 75–80. [ 10.1016/S0049-3848(97)00193-X] [DOI] [PubMed] [Google Scholar]
- [13].Urano T, Sumiyoshi K, Nakamura M, Mori T, Takada Y, Takada A. Fluctuation of tPA and PAI-1 antigen levels in plasma: Difference of their fluctuation patterns between male and female. Thromb Res 1990; 60(2): 133–9. [ 10.1016/0049-3848(90)90292-K] [DOI] [PubMed] [Google Scholar]
- [14].Urano T, Sakakibara K, Rydzewski A, Urano S, Takada Y, Takada A. Relationships between euglobulin clot lysis time and the plasma levels of tissue plasminogen activator and plasminogen activator inhibitor 1. Thromb Haemost 1990; 63(1): 82–6. [ 10.1055/s-0038-1645691] [DOI] [PubMed] [Google Scholar]
- [15].Urano T, Sumiyoshi K, Pietraszek MH, Takada Y, Takada A. PAI-1 plays an important role in the expression of t-PA activity in the euglobulin clot lysis by controlling the concentration of free t-PA. Thromb Haemost 1991; 66(4): 474–8. [ 10.1055/s-0038-1646441] [DOI] [PubMed] [Google Scholar]
- [16].Urano T, Suzuki Y, Arakida M, Kanamori M, Takada A. The expression of exercise-induced tPA activity in blood is regulated by the basal level of PAI-1. Thromb Haemost 2001; 85(4): 751–2. [ 10.1055/s-0037-1615669] [DOI] [PubMed] [Google Scholar]
- [17].Madison EL, Kobe A, Gething MJ, Sambrook JF, Goldsmith EJ. Converting tissue plasminogen activator to a zymogen: A regulatory triad of Asp-His-Ser. Science 1993; 262(5132): 419–21. [ 10.1126/science.8211162] [DOI] [PubMed] [Google Scholar]
- [18].Urano T, deSerrano VS, Urano S, Castellino FJ. Stimulation by fibrinogen of the amidolytic activity of single-chain tissue plasminogen activator. Arch Biochem Biophys 1989; 270(1): 356–62. [ 10.1016/0003-9861(89)90038-6] [DOI] [PubMed] [Google Scholar]
- [19].Emeis JJ, van den Eijnden-Schrauwen Y, van den Hoogen CM, de Priester W, Westmuckett A, Lupu F. An endothelial storage gran ule for tissue-type plasminogen activator. J Cell Biol 1997; 139(1): 245–56. [ 10.1083/jcb.139.1.245] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Suzuki Y, Mogami H, Ihara H, Urano T. Unique secretory dynamics of tissue plasminogen activator and its modulation by plasminogen activator inhibitor-1 in vascular endothelial cells. Blood 2009; 113(2): 470–8. [ 10.1182/blood-2008-03-144279] [DOI] [PubMed] [Google Scholar]
- [21].Suzuki Y, Yasui H, Brzoska T, Mogami H, Urano T. Surface-retained tPA is essential for effective fibrinolysis on vascular endothelial cells. Blood 2011; 118(11): 3182–5. [ 10.1182/blood-2011-05-353912] [DOI] [PubMed] [Google Scholar]
- [22].Suzuki Y, Sano H, Tomczyk M, Brzoska T, Urano T. Activities of wild-type and variant tissue-type plasminogen activators retained on vascular endothelial cells. FEBS Open Bio 2016; 6(5): 469–76. [ 10.1002/2211-5463.12057] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lamba D, Bauer M, Huber R, et al. The 2.3 A crystal structure of the catalytic domain of recombinant two-chain human tissue-type plasminogen activator. J Mol Biol 1996; 258(1): 117–35. [ 10.1006/jmbi.1996.0238] [DOI] [PubMed] [Google Scholar]
- [24].Juhan-Vague I, Alessi MC, Mavri A, Morange PE. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost 2003; 1(7): 1575–9. [ 10.1046/j.1538-7836.2003.00279.x] [DOI] [PubMed] [Google Scholar]
- [25].Jansson JH, Olofsson BO, Nilsson TK. Predictive value of tissue plasminogen activator mass concentration on long-term mortality in patients with coronary artery disease. A 7-year follow-up. Circulation 1993; 88(5 Pt 1): 2030–4. [ 10.1161/01.CIR.88.5.2030] [DOI] [PubMed] [Google Scholar]
- [26].Thompson SG, Kienast J, Pyke SD, Haverkate F, van de Loo JC. Hemostatic factors and the risk of myocardial infarction or sudden death in patients with angina pectoris. N Engl J Med 1995; 332(10): 635–41. [ 10.1056/NEJM199503093321003] [DOI] [PubMed] [Google Scholar]
- [27].Fay WP, Eitzman DT, Shapiro AD, Madison EL, Ginsburg D. Platelets inhibit fibrinolysis in vitro by both plasminogen activator inhibitor-1-dependent and -independent mechanisms. Blood 1994; 83(2): 351–6. [PubMed] [Google Scholar]
- [28].Brogren H, Karlsson L, Andersson M, Wang L, Erlinge D, Jern S. Platelets synthesize large amounts of active plasminogen activator inhibitor 1. Blood 2004; 104(13): 3943–8. [ 10.1182/blood-2004-04-1439] [DOI] [PubMed] [Google Scholar]
- [29].Stringer HAR, Vanswieten P, Heijnen HFG, Sixma JJ, Pannekoek H. On the function of pai-1 in platelet-rich thrombi produced in the chandler loop. Thromb Haemost 1993; 69(6): 582. [Google Scholar]
- [30].Stringer HA, van Swieten P, Heijnen HF, Sixma JJ, Pannekoek H. Plasminogen activator inhibitor-1 released from activated platelets plays a key role in thrombolysis resistance. Studies with thrombi generated in the Chandler loop. Arterioscler Thromb 1994; 14(9): 1452–8. [ 10.1161/01.ATV.14.9.1452] [DOI] [PubMed] [Google Scholar]
- [31].Hosokawa K, Ohnishi-Wada T, Sameshima-Kaneko H, et al. Plasminogen activator inhibitor type 1 in platelets induces thrombogenicity by increasing thrombolysis resistance under shear stress in an in-vitro flow chamber model. Thromb Res 2016; 146: 69–75. [ 10.1016/j.thromres.2016.09.002] [DOI] [PubMed] [Google Scholar]
- [32].Urano T, Kojima Y, Takahashi M, et al. Impaired fibrinolysis in hypertension and obesity due to high plasminogen activator inhibitor-1 level in plasma. Jpn J Physiol 1993; 43(2): 221–8. [ 10.2170/jjphysiol.43.221] [DOI] [PubMed] [Google Scholar]
- [33].Schmitt FCF, Manolov V, Morgenstern J, et al. Acute fibrinolysis shutdown occurs early in septic shock and is associated with increased morbidity and mortality: Results of an observational pilot study. Ann Intensive Care 2019; 9(1): 19 [ 10.1186/s13613-019-0499-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kornblith LZ, Moore HB, Cohen MJ. Trauma-induced coagulopathy: The past, present, and future. J Thromb Haemost 2019; 17(6): 852–62. [ 10.1111/jth.14450] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].collaborators C-, Roberts I, Shakur H, Afolabi A, Brohi K, Coats T. The importance of early treatment with tranexamic acid in bleeding trauma patients: An exploratory analysis of the CRASH-2 randomised controlled trial. Lancet 377(9771): 1096–10101.2011; [DOI] [PubMed] [Google Scholar]
- [36].Yasui H, Donahue DL, Walsh M, Castellino FJ, Ploplis VA. Early coagulation events induce acute lung injury in a rat model of blunt traumatic brain injury. Am J Physiol Lung Cell Mol Physiol 2016; 311(1): L74–86. [ 10.1152/ajplung.00429.2015] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Castellino FJ, Liang Z, Davis PK, et al. Abnormal whole blood thrombi in humans with inherited platelet receptor defects. PLoS One 2012; 7(12): e52878 [ 10.1371/journal.pone.0052878] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Gando S, Levi M, Toh CH. Disseminated intravascular coagulation. Nat Rev Dis Primers 2016; 2: 16037 [ 10.1038/nrdp.2016.37] [DOI] [PubMed] [Google Scholar]
- [39].Jeleńska MM. Coagulation parameters as predictors of DIC in patients with intact aortic aneurysm. Hamostaseologie 2004; 24(3): 162–6. [ 10.1055/s-0037-1619626] [DOI] [PubMed] [Google Scholar]
- [40].Madoiwa S, Someya T, Hironaka M, et al. Annexin 2 and hemorrhagic disorder in vascular intimal carcinomatosis. Thromb Res 2007; 119(2): 229–40. [ 10.1016/j.thromres.2006.01.017] [DOI] [PubMed] [Google Scholar]
- [41].Madoiwa S Recent advances in disseminated intravascular coagulation: endothelial cells and fibrinolysis in sepsis-induced DIC. J Intensive Care 2015; 3: 8 [ 10.1186/s40560-015-0075-6] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Moore HB, Moore EE, Huebner BR, et al. Fibrinolysis shutdown is associated with a fivefold increase in mortality in trauma patients lacking hypersensitivity to tissue plasminogen activator. J Trauma Acute Care Surg 2017; 83(6): 1014–22. [ 10.1097/TA.0000000000001718] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gando S, Nakanishi Y, Tedo I. Cytokines and plasminogen activator inhibitor-1 in posttrauma disseminated intravascular coagulation: relationship to multiple organ dysfunction syndrome. Crit Care Med 1995; 23(11): 1835–42. [ 10.1097/00003246-199511000-00009] [DOI] [PubMed] [Google Scholar]
- [44].Sentilhes L, Winer N, Azria E, et al. Tranexamic Acid for the Prevention of Blood Loss after Vaginal Delivery. N Engl J Med 2018; 379(8): 731–42. [ 10.1056/NEJMoa1800942] [DOI] [PubMed] [Google Scholar]
- [45].Meizoso JP, Dudaryk R, Mulder MB, et al. Increased risk of fibrinolysis shutdown among severely injured trauma patients receiving tranexamic acid. J Trauma Acute Care Surg 2018; 84(3): 426–32. [ 10.1097/TA.0000000000001792] [DOI] [PubMed] [Google Scholar]
- [46].Fay WP, Shapiro AD, Shih JL, Schleef RR, Ginsburg D. Brief report: Complete deficiency of plasminogen-activator inhibitor type 1 due to a frame-shift mutation. N Engl J Med 1992; 327(24): 1729–33. [ 10.1056/NEJM199212103272406] [DOI] [PubMed] [Google Scholar]
- [47].Iwaki T, Nagahashi K, Takano K, et al. Mutation in a highly conserved glycine residue in strand 5B of plasminogen activator inhibitor 1 causes polymerisation. Thromb Haemost 2017; 117(5): 860–9. [ 10.1160/TH16-07-0572] [DOI] [PubMed] [Google Scholar]
- [48].Iwaki T, Tanaka A, Miyawaki Y, et al. Life-threatening hemorrhage and prolonged wound healing are remarkable phenotypes manifested by complete plasminogen activator inhibitor-1 deficiency in humans. J Thromb Haemost 2011; 9(6): 1200–6. [ 10.1111/j.1538-7836.2011.04288.x] [DOI] [PubMed] [Google Scholar]
- [49].Saes JL, Schols SEM, van Heerde WL, Nijziel MR. Hemorrhagic disorders of fibrinolysis: A clinical review. J Thromb Haemost 2018. [ 10.1111/jth.14160] [DOI] [PubMed] [Google Scholar]
- [50].Lijnen HR. Pleiotropic functions of plasminogen activator inhibitor-1. J Thromb Haemost 2005; 3(1): 35–45. [ 10.1111/j.1538-7836.2004.00827.x] [DOI] [PubMed] [Google Scholar]
- [51].Andreasen PA, Kjøller L, Christensen L, Duffy MJ. The urokinase-type plasminogen activator system in cancer metastasis: A review. Int J Cancer 1997; 72(1): 1–22. [] [DOI] [PubMed] [Google Scholar]
- [52].Gramling MW, Church FC. Plasminogen activator inhibitor-1 is an aggregate response factor with pleiotropic effects on cell signaling in vascular disease and the tumor microenvironment. Thromb Res 2010; 125(5): 377–81. [ 10.1016/j.thromres.2009.11.034] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [53].Khan SS, Shah SJ, Klyachko E, Baldridge AS, Eren M, Place AT, et al. A null mutation in SERPINE1 protects against biological aging in humans. Sci Adv 3(11)2017; [ 10.1126/sciadv.aao1617] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Eren M, Boe AE, Murphy SB, et al. PAI-1-regulated extracellular proteolysis governs senescence and survival in Klotho mice. Proc Natl Acad Sci USA 2014; 111(19): 7090–5. [ 10.1073/pnas.1321942111] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Wyseure T, Declerck PJ. Novel or expanding current targets in fibrinolysis. Drug Discov Today 2014; 19(9): 1476–82. [ 10.1016/j.drudis.2014.05.025] [DOI] [PubMed] [Google Scholar]
- [56].Simone TM, Higgins SP, Higgins CE, Lennartz MR, Higgins PJ. Chemical antagonists of plasminogen activator inhibitor-1: Mechanisms of action and therapeutic potential in vascular disease. J Mol Genet Med 2014; 8(3): 125 [ 10.4172/1747-0862.1000125] [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Ibrahim AA, Yahata T, Onizuka M, et al. Inhibition of plasminogen activator inhibitor type-1 activity enhances rapid and sustainable hematopoietic regeneration. Stem Cells 2014; 32(4): 946–58. [ 10.1002/stem.1577] [DOI] [PubMed] [Google Scholar]


