Abstract
Flaviviruses are vector-borne RNA viruses that can emerge unexpectedly in human populations and cause a spectrum of potentially severe diseases including hepatitis, vascular shock syndrome, encephalitis, acute flaccid paralysis, and congenital abnormalities and fetal death. This epidemiological pattern has occurred numerous times during the last seventy years, including epidemics of Dengue virus and West Nile virus, and the most recent explosive epidemic of Zika virus in the Americas. Flaviviruses now are globally distributed and infect up to 400 million people annually. Of significant concern, outbreaks other less well-characterized flaviviruses have been reported in humans and animals in different regions of the world. The potential for these viruses to sustain epidemic transmission among humans is poorly understood. In this Review, we discuss the basic biology of flaviviruses, their infectious cycles, the diseases they cause and underlying host immune responses to infection. We describe flaviviruses that represent an established ongoing threat to global health and those that have recently emerged in new populations to cause significant disease. We also provide examples of lesser known flaviviruses that circulate in restricted areas of the world but have the potential to emerge more broadly in human populations. Finally, we discuss how an understanding of the epidemiology, biology, structure, and immunity of flaviviruses can inform the rapid development of countermeasures to treat or prevent human infections as they emerge.
Flaviviruses are single-stranded RNA viruses vectored principally by arthropods that cause severe illnesses in humans. The extensive global spread and epidemic transmission of flaviviruses during the last seven decades has been remarkable. The mosquito-borne dengue viruses (DENV) infect an estimated 400 million humans each year; more than a quarter of the world’s population lives in areas where DENV is now endemic 1. By comparison, only sporadic DENV epidemics were documented before the second World War 2. The introductions of West Nile (WNV) and Zika (ZIKV) viruses into the Western Hemisphere was followed by rapid geographical spread, large numbers of human infections, and considerable morbidity 3,4. Ongoing yellow fever virus (YFV) transmission and its encroachment on urban environments, despite the existence of an effective vaccine, pose a serious public health challenge 5–7. Other flaviviruses present ongoing health risks or are beginning to emerge in different parts of the world, including Japanese encephalitis virus (JEV), tick-borne encephalitis virus (TBEV), and Usutu virus (USUV).
The epidemic potential of flaviviruses reflects many factors related to the unique characteristics of their insect vectors, the consequences of poorly planned urbanization that creates ideal arthropod breeding habitats, the geographical expansion of vectors, changing environmental conditions, and extensive global travel 8,9. Beyond arthropods and humans, flaviviruses are also known to infect a wide array of animal species and can be important veterinary pathogens that threaten economically important domesticated animals 10–14. These vertebrate animal hosts may constitute important stable reservoirs and contribute to defining conditions that support the introduction of new viral species and transmission among humans 15. The continued threat of flavivirus emergence and re-emergence highlights a need for a detailed fundamental understanding of the biology of these viruses, the immune responses that can contain them, and the possible countermeasures that can blunt their impact on public health should new outbreaks occur.
FLAVIVIRUS STRUCTURE AND REPLICATION
Flaviviruses are small (~50 nm) spherical virus particles that incorporate a single genomic RNA of positive-sense polarity encoding three structural and seven non-structural proteins (Figure 1a). Our knowledge of the biology of flaviviruses has advanced considerably with the availability of high-resolution structures of viral structural proteins and of virions at different stages of the replication cycle or in complex with antibodies or host factors 16. Crystal structures of the enzymatic non-structural proteins also have been solved, accelerating advances in an understanding of virus replication and pathogenesis 17–19 and enabling structure-guided drug discovery, as reviewed elsewhere 20.
Figure 1. Organization and structure of flaviviruses.
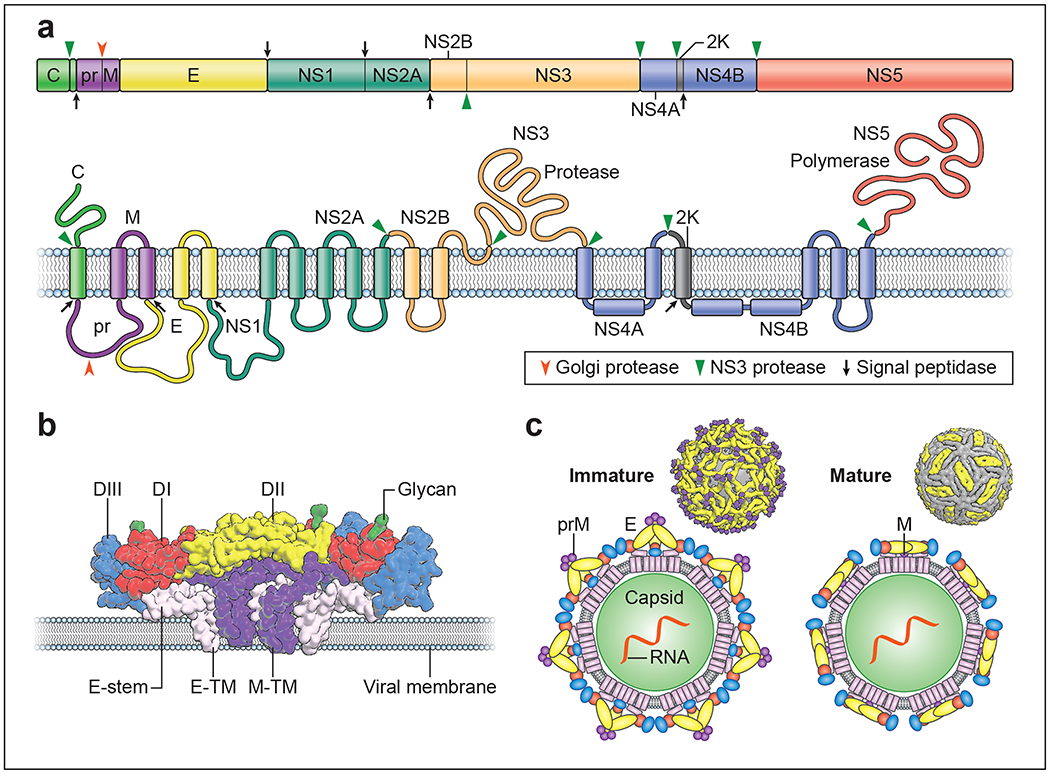
a. Flaviviruses encode a single open reading frame that is translated at the endoplasmic reticulum into a polyprotein, which subsequently is cleaved by viral and host cell proteases. This processing results in ten functional proteins including the three structural proteins C, prM, and E and seven non-structural proteins. NS4A exists in two forms that differ with respect to cleavage of the 2K domain at its carboxy-terminus. b. Flavivirus E proteins are elongated three domain structures tethered to the viral membrane by a stem and two antiparallel transmembrane domains. E protein domains are indicated in red, yellow, and blue (domain I-III, respectively). The M protein, also attached to the viral membrane by two transmembrane domains, is shown in purple. c. The distinct arrangement of E proteins on immature (left) and mature (right) forms of the virion are depicted.
Virion structure and morphogenesis.
Flaviviruses are assembled using three viral structural proteins (C, prM and E), a host lipid envelope, and the viral genomic RNA. The structure of the envelope (E) protein, which mediates virus entry steps of the replication cycle, was solved first for TBEV 21, and thereafter for multiple flaviviruses including DENV, WNV, and ZIKV (reviewed in 22). The E protein is a three-domain structure (referred to as domains E-DI, E-DII, and E-DIII) tethered to the viral membrane by a helical stem and two antiparallel transmembrane domains (Figure 1b). Most flavivirus E proteins are modified post-translationally by the addition of one or two asparagine-linked carbohydrates. The folding of the E protein in the endoplasmic reticulum (ER) is facilitated by interactions with the structural premembrane (prM) protein shortly after synthesis 23. prM is incorporated into the viral envelope during virion morphogenesis as heterotrimeric prM-E spikes with icosahedral symmetry (Figure 1c) 24 and prevents conformational changes in the E protein that would allow adventitious fusion of virions with host membranes during egress. Cleavage of prM to M during transit of immature virions through the trans-Golgi network by a host furin-like serine protease is required for the formation of infectious mature forms of the virion 25. On mature virions, E proteins are arranged as antiparallel dimers via extensive contacts between adjacent E-DIIs 26–29. Ninety E dimers are incorporated into each mature virion and arranged in a herringbone pattern with T = 3 icosahedral-like symmetry (Figure 1c). The viral capsid (C) protein is a small helical protein with surfaces that bind either viral nucleic acids or host lipids and directs the incorporation of the viral genome into the virion 30. Establishing the physical connection between membrane-anchored structural proteins and the C protein or RNA has been elusive. The application of asymmetric reconstruction techniques to the cryo-electron microscopy (cryo-EM) analysis of ZIKV provides evidence that capsid interacts transiently with the other structural proteins during particle biogenesis 31. C protein incorporation into the virion is regulated further by the coordinated cleavage of the polyprotein by the viral NS2B/3 serine protease 32.
Flavivirus entry.
Flaviviruses bind to an array of mammalian cell types through interactions of asparagine-linked sugars on structural proteins with multiple C-type lectins including DC-SIGN 33,34, the binding of charged surfaces of the E protein to glycosaminoglycans on cell surfaces 35, and interactions between the viral lipid envelope and proteins of the TIM and TAM family of phosphatidylserine receptors 36 (Figure 2). The role of specific host proteins in the attachment and entry of viruses into cells varies. Host proteins classically defined as receptors are essential for the entry of viruses because they catalyze critical conformational events. For example, the CD4 molecule on T lymphocytes enables conformational transitions in the HIV-1 gp120 protein required for viral membrane fusion 14. While host factors have been identified that increase the efficiency of flavivirus binding and infection of cells, they are not required to trigger the structural transitions that propel viral membrane fusion; instead, these are defined as attachment factors. Flaviviruses bound to synthetic lipid membranes devoid of host proteins are capable of stimulating E protein-mediated fusion once exposed to an acidic environment 37,38. Identifying virus-host receptor interactions important for pathogenesis in humans and other vertebrate animals has been challenging, and even less is known about entry pathways in invertebrate host cells. Relationships between host attachment factor expression and viral tropism in vivo have not been established. Some flavivirus attachment factors (e.g., TAM and integrin receptors) capable of binding virions also transduce signals into target cells, which has the potential to augment infection and further complicates the role and definition of host attachment molecules 39–42.
Figure 2. The flavivirus replication cycle.
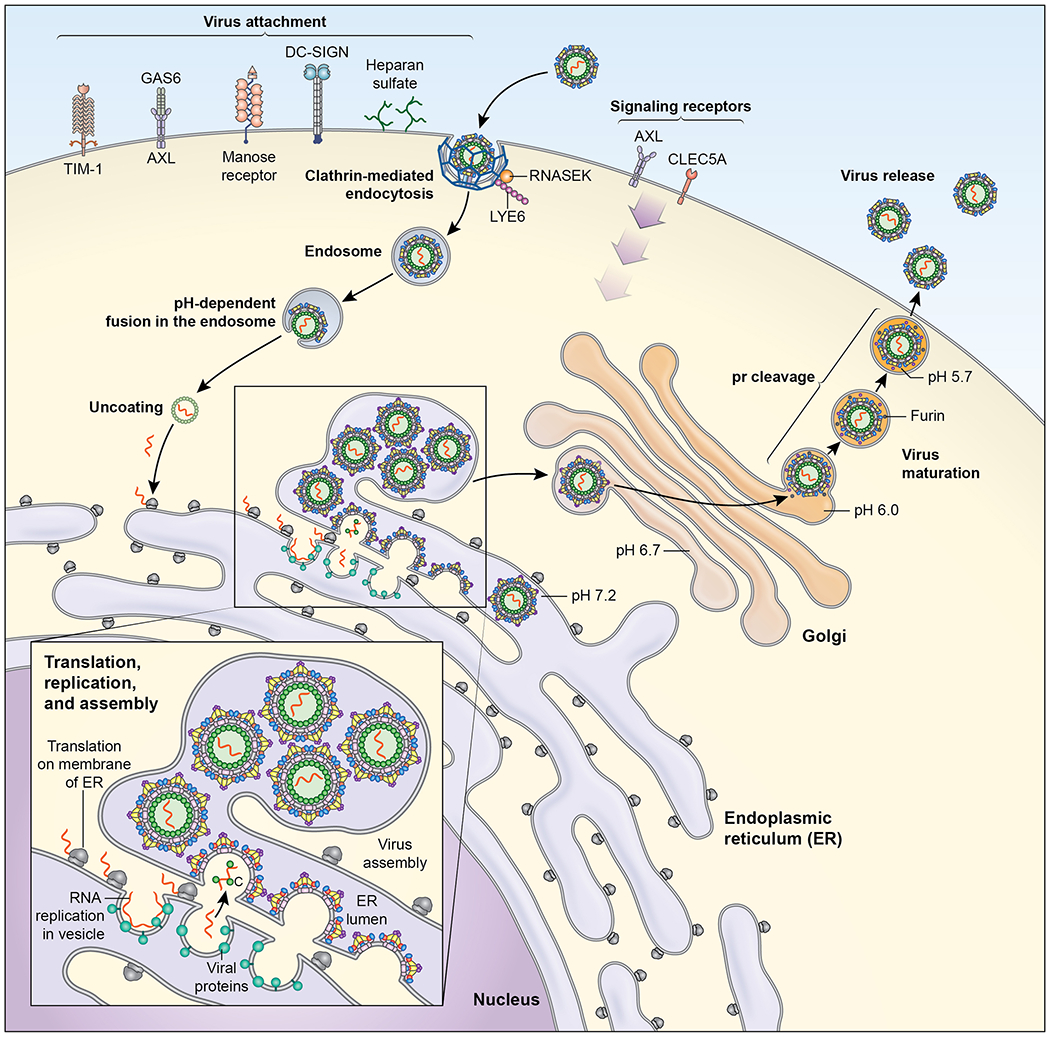
Flaviviruses infect mammalian cells via interactions with multiple types of host attachment factors, including molecules that bind to the viral membrane or virion-associated N-linked carbohydrates. Interactions with cell surface host factors, such as CLEC5a, may also initiate signaling pathways that modulate the host immune response. Virions are internalized by clathrin-dependent mechanisms that usurp host factors involved in the uptake of large macromolecules, including RNASEK. Viral fusion with host membranes occurs in the endosome in a low pH-dependent manner. Viral RNA replication occurs on membranes of the host reorganized through the actions of the non-structural proteins. These virus-induced membrane structures spatially coordinate viral genomic RNA replication and virion morphogenesis, and shield replication products from host innate immune sensors. Virus particles assemble at and bud into the endoplasmic reticulum and traffic out of the cell. Virion maturation, defined by the cleavage of prM by a furin-like protease, occurs during egress.
Once attached to cells, flaviviruses are taken up by clathrin-dependent endocytic vesicles. While this same host machinery is involved in the internalization of multiple types of cellular cargo, recent studies identified host molecules required by flaviviruses to exploit the endocytic pathway for infectious entry including RNASEK, lymphocyte antigen locus 6 (LYE6), and microtubules 43–45. Flavivirus membrane fusion occurs in the low pH compartments of the endosome and is catalyzed by conformational changes in the E protein that involve the formation of E protein trimers, penetration of the highly conserved E-DII fusion loop (E-DII-FL) into the adjacent host membranes, and the folding of the E protein helical stem against the exterior surface of the newly formed E protein trimer 46. A structural and kinetic understanding of flavivirus membrane fusion has informed the design of antiviral molecules that disrupt the entry process 47 (Figure 2).
Flavivirus replication.
The flavivirus genomic RNA encodes a single open reading frame flanked by highly structured untranslated regions (UTR) that coordinate viral translation, replication, and regulation of the innate immune response 48. The penetration of the viral genome into the cytoplasm allows for the cap-dependent translation of the viral polyprotein in association with membranes of the ER49. Viral translation products are believed to stimulate a shift in the use of the incoming viral genome from a substrate for translation to a template for genomic RNA replication. Flavivirus replication occurs on complex virus-induced membrane structures incorporating host and viral factors 50. The ultrastructure of these flavivirus replication complexes (RCs) was solved using cryo-EM tomography, revealing invaginations of the ER that form spherical compartments in which viral components required for RNA replication can be located, including NS1, NS2A, NS3, NS4A, and NS5 50,51 (Figure 3). Although the contents of these vesicle packets are protected from surveillance by cytoplasmic innate immune sensors, narrow connections exist to allow movement of viral RNA replication products to sites of translation and virion morphogenesis. Changes in host cell metabolism are important for the generation of RCs including an increase in cholesterol, fatty acid, and sphingomyelin synthesis; regulation of autophagy also has been suggested to contribute to virus-induced changes in lipid metabolism 52,53. Host factors such as the reticulon protein 3.1A and DNAJC14 also are critical for RC formation 54,55. As many of the enzymes involved in these metabolic changes are targets for therapeutics, a more detailed understanding of the host pathways and networks required to support flavivirus replication may identify new classes of antiviral agents 56.
Figure 3. Disease syndromes of flavivirus infection.
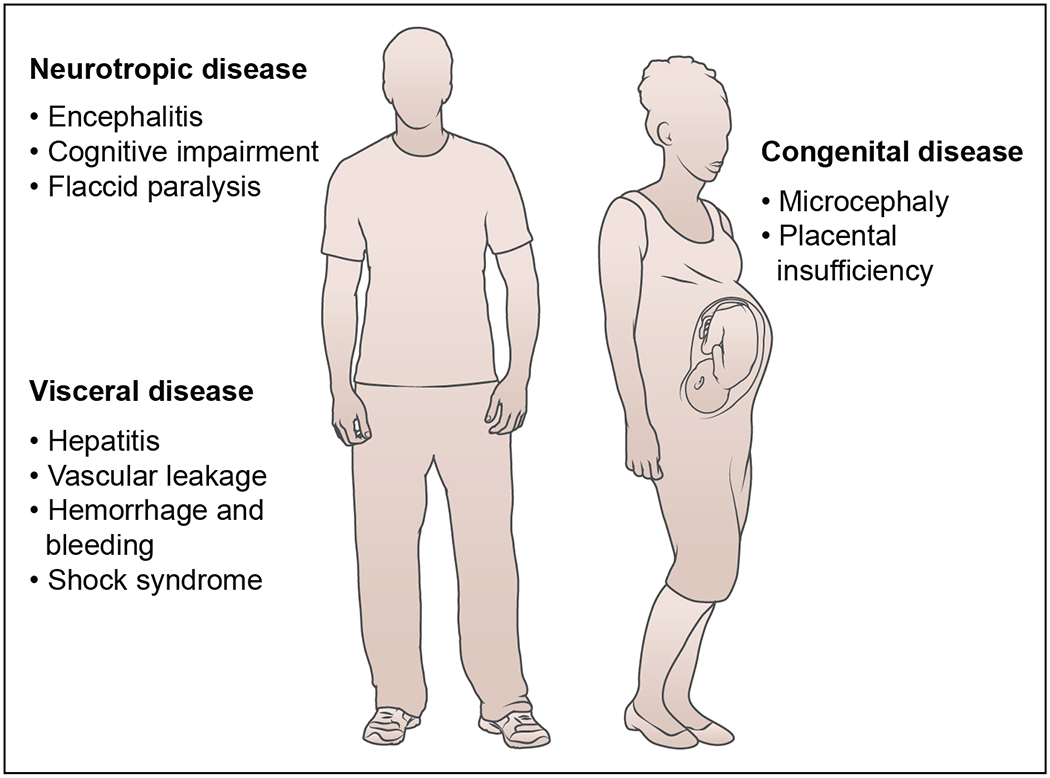
Flaviviruses cause different febrile syndromes depending on the virus and the affected patient. Several flaviviruses are neurotropic (e.g., West Nile, Japanese encephalitis, TBEV, Usutu, Zika, and Ilheus), can spread to the brain and spinal cord, and cause severe neurological syndromes including meningitis, encephalitis, and acute flaccid paralysis. These can result in death or long-term disability in survivors. Other flaviviruses (e.g., Yellow fever, Dengue, and Zika) cause visceral disease resulting in liver failure, hemorrhagic syndromes, and vascular compromise, also resulting in death. Uniquely, ZIKV can infect the tissues of the male and female reproductive tracts leading to sexual transmission. ZIKV infection during pregnancy can cause injury to the placenta as well as transmit to the developing fetus, resulting in placental insufficiency, microcephaly, congenital malformations, and fetal demise.
FLAVIVIRUS-INDUCED DISEASE
The clinical presentation of acute flavivirus infection in humans ranges from mild illness (asymptomatic infection or self-limiting febrile episodes) to severe and life-threatening disease (hemorrhagic fever, shock syndrome, encephalitis, paralysis, congenital defects, hepatitis, and hepatic failure). Individual flavivirus infections fall into two broad categories, visceral and neurotropic, although some have features of both (e.g., ZIKV) (Figure 3). Variability in disease presentation among individual flaviviruses likely reflects the unique cellular and tissue tropism of each virus, differences in a capacity to evade or antagonize host immunity, and the interplay between the direct pathogenic effects of virus infection and injury caused by the requisite host response. Approximately 50 to 80% of flavivirus infections are asymptomatic and cause no or minimal illness 57–59. Most symptomatic flavivirus infections result in self-limiting flu-like febrile illnesses with headache, myalgia, arthralgia, and a rash without long-term consequences. The factors that determine the penetrance of more severe disease phenotypes for different flaviviruses are not fully characterized, but likely reflect polymorphisms in key host genes (e.g., CCR5 for WNV 60, DC-SIGN for DENV 61), age 62, immune status and co-morbidities, and prior flavivirus immunity (e.g., DENV 63), in addition to differential pathogenicity of particular virus strains and perhaps other acquired factors including the microbiome 64.
Visceral disease.
DENV, YFV, and ZIKV are the principal flaviviruses that cause visceral disease in humans. DENV infection of myeloid cells in blood and tissues is believed to induce an immunopathogenesis cascade that results in vascular leakage, thrombocytopenia, abnormal bleeding, hemoconcentration and hypotension 65,66. The flavivirus NS1 protein may contribute to hypotension by virtue of its ability to bind endothelial cells, disrupt the integrity of underlying glycocalyx, and alter vascular permeability 67,68. YFV replicates to high levels in liver cells, and this results in severe hepatitis, renal failure, hemorrhage, shock, and death 69,70. ZIKV infects progenitor cells, epithelium, and myeloid cells, and in peripheral tissues causes injury to the male and female reproductive tracts and the eye 71. ZIKV persists in human semen for months 72, and may cause oligospermia, lower levels of sex hormones, and possibly, compromised fertility 73. The high viral load in seminal fluid also can lead to sexual transmission of ZIKV 74.
Neurotropic disease.
WNV, Japanese encephalitis virus (JEV), TBEV, Powassan virus (POWV), and ZIKV are neurotropic viruses that can cause encephalitis, cognitive impairment, seizure disorders, and paralysis 75. The neurological and functional disability associated with these neurotropic flavivirus infections represents a considerable source of morbidity in patients long after their recovery from acute illness. These viruses infect and cause injury to neurons (or neuroprogenitor cells in the case of ZIKV) through direct (viral-induced) and indirect (immune-mediated) mechanisms 75,76. Microscopic examination of the brain reveals neuronal cell death, activation of microglia and infiltrating macrophages, and accumulation of CD4+ and CD8+ T cells. Depending on the flavivirus, these lesions can occur in the brainstem, cerebral cortex, the hippocampus, thalamus, cerebellum, or spinal cord 77.
Congenital disease.
ZIKV also is teratogenic, in part, because it infects and causes injury to the developing placenta 78. The tropism of ZIKV for the placenta 71 may not be unique among flaviviruses, as inoculation of human placental explants or pregnant mice with the WNV or POWV also resulted in infection and injury to the placenta 79.
IMMUNE RESPONSE TO FLAVIVIRUS INFECTION
In this section, we highlight recent advances relating to cell-intrinsic host defense activation and innate and adaptive immune response-dependent restriction of flavivirus infections. We discuss here and below how these findings affect the development of candidate therapeutics.
Innate immunity.
The mammalian host detects and responds to flavivirus infection by recognizing viral RNA through several pathogen recognition receptors (PRRs), including the cell surface and endosomal RNA sensors Toll-like receptors 3 and 7 (TLR3 and TLR7), the cytoplasmic RNA sensors retinoic acid-inducible gene I (RIG-I), and melanoma-differentiation-associated gene 5 (MDA5) 80,81. Binding of single and/or double-stranded viral RNA results in downstream activation of adaptor molecules, such as mitochondrial antiviral signaling protein (MAVS), MyD88, TIR domain-containing adaptor inducing IFN-β (TRIF), nuclear translocation of interferon (IFN) regulatory transcription factors 3 and 7 (IRF3 and IRF7), and NF-κB, which induce expression of type I and III interferons (IFNs). The cytoplasmic adaptor molecule stimulator of IFN genes (STING) also participates in immune responses generated against flaviviruses in the context of RIG-I recognition by acting as a scaffold for the recruitment of signaling components required for IRF3 activation and IFN induction 82–84.
Type I interferons (IFN-α/β) promote an antiviral state by inducing IFN-stimulated genes (ISGs) with direct and indirect antiviral functions (reviewed by 85,86). Pretreatment of cells with type I IFNs inhibits flavivirus replication in vitro, but treatment after infection is less effective. Although flaviviruses can antagonize IFN-induced responses after infection by preventing induction of IFNs and disrupting their signaling pathways 87, (IFN) still restricts replication and spread in vivo. Mice lacking the type I IFN receptor (Ifnar1−/−) show expanded tropism and greater morbidity and mortality than wild-type mice after infection with multiple different flaviviruses 88,89. Type III IFN-λ is an antiviral cytokine that binds a unique receptor and primarily functions at barrier surfaces 90. In cell culture, IFN-λ has direct antiviral effects against flaviviruses through induction of ISGs 91,92. IFN-λ also has inhibitory activity against ZIKV in the context of infection of the maternal-derived decidua and fetal-derived placenta during pregnancy in mice and humans 93–95.
Some of the recently identified ISGs that display antiviral activity against flaviviruses 85 in vitro include: C6orf150, DDX24, HPSE, MAFK, NAMPT, PAK3, PHF15, SAMD9L, SC4MOL, C19orf66, CH25H, IFI44L, IFIT1, IFIT2, IFI6, IFITM2, IFITM3, ISG20, and RSAD2 (viperin). ISGs with demonstrated antiviral activity against flaviviruses in vivo include: PKR, RNase L, viperin, IFIT1, IFIT2, IFITM3, IFI27L2a, and CH25H 96–99. The inhibitory mechanisms of some well-described ISGs have been reviewed 98,100, with some targeting flavivirus entry/fusion (IFITM3, CH25H), translation (IFIT1/2, PKR, C19orf66) or replication (RNAse L, viperin). However, the mechanisms by which many other ISGs restrict flavivirus infections remain to be determined. Further delineation of how specific ISGs restrict flaviviruses could create opportunities for pharmacological targeting and enhanced resistance to infection.
B cell immunity.
The importance of antiviral antibodies against flaviviruses is well-established 101. Passive transfer of virus-reactive monoclonal or polyclonal antibodies confers significant protection in animal models 102,103. Anti-flavivirus antibodies also may exert protective effects via effector functions mediated by the Fc portion of the antibody molecule, including complement fixation, antibody-mediated cellular cytotoxicity, and antibody-mediated opsonization, all of which can facilitate viral clearance 104,105. Protective antibodies against flaviviruses predominantly recognize epitopes on the E protein of the virion, but also can bind to regions of the cell surface and secreted forms of non-structural protein NS1 106,107.
Neutralizing anti-flavivirus antibodies can inhibit infection at multiple steps in the virus lifecycle, including a blockade of virus attachment to host cells 108, presumably by disrupting interactions with attachment factors or receptors. Flavivirus-reactive antibodies also may block infection after the attachment step. Many potently neutralizing and protective antibodies inhibit the pH-dependent structural changes required for endosomal fusion and nucleocapsid release 109. In contrast, some flavivirus-reactive antibodies increase the efficiency of infection under certain conditions. Such antibody-dependent enhancement (ADE) of infection occurs when non-neutralizing amounts of antibody bind virions and promote more efficient infection of cells expressing activating Fc–γ receptors via enhancement of virion attachment and internalization 110. While readily demonstrated in vitro with multiple flaviviruses using cell lines or primary Fc–γ–expressing cells, a role for ADE in vivo has only been demonstrated convincingly for DENV 111,112 (see below).
T cell immunity.
Studies have established important roles for both CD4+ and CD8+ T cells in flavivirus pathogenesis and immunity (reviewed in 107,113,114). The protective roles of CD4+ T cells may differ during primary and memory responses. In mice, CD4+ T cells control primary WNV, YFV, ZIKV, and JEV infection and disease 115. In comparison, CD4+ T cells were not required for controlling primary DENV infection, yet instead contributed to viral clearance after immunization and challenge 116. CD4+ T cells can also protect against flavivirus infection by providing help for antibody responses, sustaining CD8+ T cell responses that enable viral clearance, producing antiviral cytokines, and lysing some infected cell targets. In humans, impaired JEV-specific CD4+ T cell function was seen preferentially in patients with encephalitis and neurological sequelae 117. As DENV-specific CD4+ T cells show cytolytic activity ex vivo and are associated with a protective class II MHC allele, they are believed to control DENV infection in humans 118.
Memory CD4+ T cells can have protective or pathological consequences depending on the context. For DENV, immunization schemes that elicit antigen-specific CD4+ T cells prior to infection of mice resulted in diminished viral burden after challenge with homologous DENV 116. Memory T cell responses elicited by prior infection with DENV recognize ZIKV-derived peptides and influence the magnitude and quality of the ZIKV T cell response 119. Although cross-reactive CD4+ T cells against conserved peptides can be detected across flaviviruses, their effect on viral infection and disease remains uncertain. In some settings, the memory response may also have pathological consequences. For example, CD4+ T cells primed against one serotype of DENV can result in over-exuberant production of inflammatory cytokines and an increased risk for severe disease in the context of infection with a second, heterologous DENV serotype 120.
CD8+ T cells, by virtue of their ability to lyse infected target cells and produce pro-inflammatory cytokines, can also have protective or pathologic effects against flaviviruses depending on the context. In mice, CD8+ T cells can be an essential component of protection against and resolution of primary infection by several different flaviviruses (e.g., WNV, ZIKV, and (DENV) 121–123. Flavivirus-specific cytotoxic CD8+ T cells proliferate, release proinflammatory cytokines including IFN-γ and TNF, and lyse cells through the delivery of perforin and granzymes, or via Fas-Fas ligand or TRAIL interactions 113. Consequently, mice deficient in these molecules had increased viral burden 124,125. Heterologous, memory T cell responses also can have protective functions, as cross-reactive DENV-immune CD8+ T cells restrict ZIKV infection and disease, including in pregnancy 126,127. Reciprocally, ZIKV-immune CD8+ T cells can protect against DENV infection in mice 128.
In certain circumstances, flavivirus-specific CD8+ T cells can cause immunopathology. The antiviral activity of CD8+ T cells within the brain markedly limited ZIKV infection of neurons, but also triggered ZIKV-associated paralysis in mice 129. CD8+ T cells induce immunopathology in the brain after infection with TBEV 130. For DENV, a pathogenic role of CD8+ T cells has been described during secondary infection. Serotype cross-reactive CD8+ T cells are preferentially activated during secondary infection in humans 131. These cross-reactive CD8+ T cells exhibit altered cytokine production and reduced cytolytic activity 132,133. Aberrant cytokine production by CD8+ T cells could contribute to severe DENV disease by promoting endothelial cell dysfunction or damage and plasma leakage 134. Notwithstanding these data, other human studies suggest that CD8+ T cell responses, in the context of secondary DENV infection, may have beneficial consequences 114,135.
Given this background on the how flaviviruses replicate, are recognized by the host immune system, and the clinical diseases they cause, in the next sections, we will describe the flaviviruses that are considered established threats, those that have recently emerged as global health threats, and finally those which may emerge to cause future (epidemics)[MSD15].
ESTABLISHED THREATS
Dengue virus.
After mosquito inoculation, the four serotypes of DENV can cause human clinical disease ranging from self-limited dengue fever to a life-threatening syndrome, termed “Severe Dengue”. DENV now causes an estimated 390 million total infections, 100 million clinically apparent cases, and 500,000 presentations of Severe Dengue per year worldwide, with at least 2.5 billion people at risk 1 (Table 1). Over the past seventy years, the number of people infected has risen steadily such that today DENV is the most prevalent arthropod-borne viral disease in the world. Severe Dengue routinely occurs in multiple parts of the world with more than 100 countries affected including those in the Americas, Asia, Africa, and Australia; in essence, wherever the primary mosquito vector Aedes aegypti is present (Figure 4). In the continental United States, although some regions (Gulf Coast and Southeast) periodically experience dengue outbreaks 136,137, sustained transmission has not occurred, possibly due to indoor lifestyles and rapid mosquito control efforts (e.g., spraying and larvicide strategies) implemented once DENV cases are detected.
Table 1.
Flaviviruses: Transmission Routes and Disease
| Virus | Antigenic Group | Primary Geographic Distribution | Zoonotic Reservoir | Transmission Vector and Route | Human Disease | # of Human Infections |
|---|---|---|---|---|---|---|
| Dengue | Dengue | South America Central America North America Asia Australia Africa |
Non-human primates (sylvatic cycle) |
Aedes aegypti Aedes albopictus |
Dengue Fever Severe Dengue (vascular leakage, shock) |
390 million infections per year (~30-50% are symptomatic) |
| Zika | Spondweni | Central America South America Africa Asia North America |
Non-human primates (sylvatic cycle) |
Aedes aegypti Aedes albopictus Sexual route Vertical (mother to fetus) |
Febrile syndrome Guillian-Barré syndrome Congenital anomaly Microcephaly |
Thousands to millions depending on the year (since 2013) |
| West Nile | Japanese encephalitis | North America Middle East Africa Europe Australia |
Birds |
Culex pipiens Culex tarsalis |
Febrile syndrome Meningitis Encephalitis Acute Flaccid Paralysis |
<10,000 cases per year |
| Japanese encephalitis | Japanese encephalitis | Asia Australia |
Birds, pigs |
Culex tritaeniorhynchus Culex annulirostris |
Febrile syndrome Meningitis Encephalitis |
70,000 cases per year |
| Yellow fever | Yellow fever | Africa South America |
Non-human primates (sylvatic cycle) | Aedes aegypti | Febrile syndrome Liver failure Hemorrhagic syndrome |
130,000 severe cases per year (>50% case fatality rate) |
| Powassan | Tick-borne flavivirus | North America Eastern Europe |
Rodents Lagomorphs Deer |
Ixodes cookei Ixodes scapularis |
Febrile syndrome Meningitis Encephalitis |
Hundreds |
| Usutu | Japanese encephalitis | Africa Europe |
Birds | Culex pipiens | Febrile syndrome Meningitis Encephalitis Acute Flaccid Paralysis |
Hundreds to Thousands |
| Ilheus | Japanese encephalitis | South America Central America |
Birds Non-human primates (?) Horses |
Culex pipiens Ochlerotatus serratus Sabethes, Haemagogus |
Febrile syndrome Encephalitis |
Unknown |
| Rocio | Japanese encephalitis | South America (Brazil only) |
Birds (?) |
Culex pipiens Culex tarsalis Psorophora ferox |
Febrile syndrome Encephalitis |
Unknown |
| Wesselsbron | Yellow fever | Africa | Cattle Sheep Rats |
Aedes spp (A. caballus and A. circumluteolus) |
Febrile syndrome | Unknown |
| Spondweni | Spondweni | Africa North America (?) |
Non-human primates (sylvatic cycle) | Aedes, Culex, Eretmapodites, and Mansonia species | Febrile syndrome Vascular leakage (shock) Neurological impairment |
Unknown |
This Table describes the primary geographic distribution, zoonotic reservoir, insect vector, clinical syndrome and estimated number of infections for a given flavivirus.
Figure 4. Global distribution of flaviviruses.
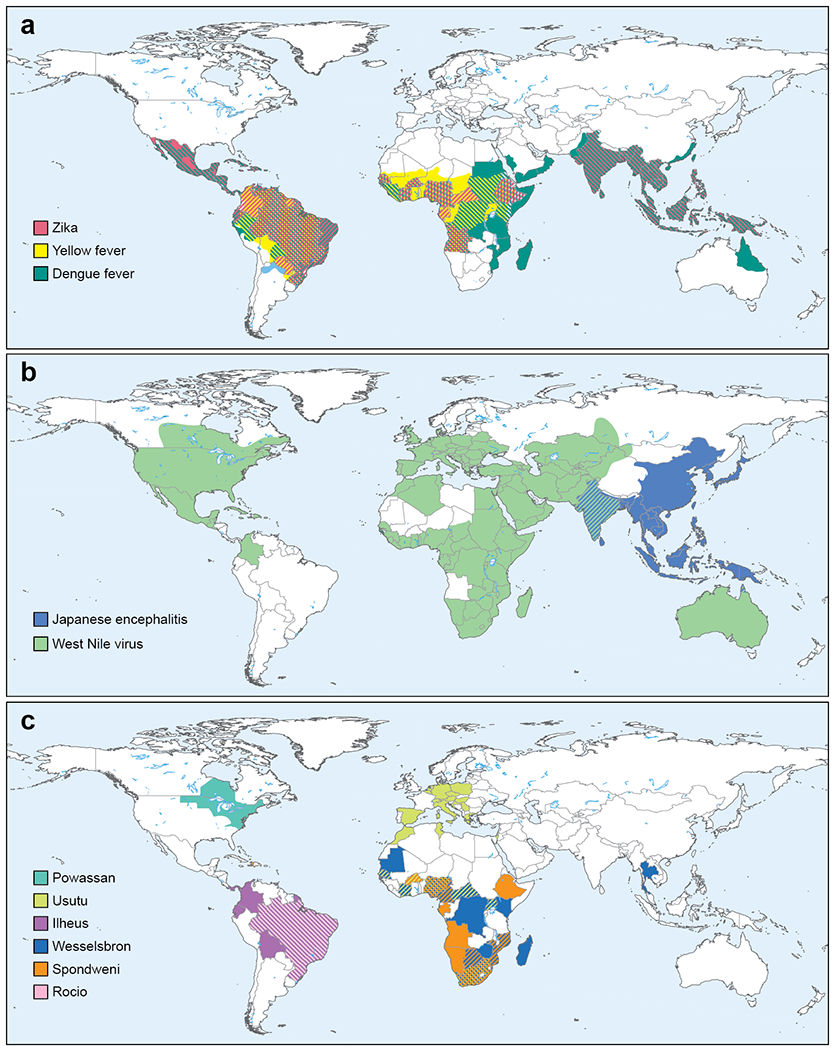
a. The global distribution of Aedes-transmitted flaviviruses ZIKV, YFV, and DENV are shown. b. The global distribution JEV and WNV is shown c. The approximate geographic locations of flaviviruses with the potential for emergence in human populations.
The incidence of Severe Dengue varies between primary and secondary infections. A secondary DENV infection results when a person previously infected with one serotype is exposed to a different serotype, and is the single most important risk factor for severe dengue disease 138,139. Severe Dengue is characterized by rapid onset of capillary leakage accompanied by thrombocytopenia and mild to moderate liver damage 140. Although hemorrhagic manifestations occur (e.g., epistaxis, gastrointestinal tract bleeding, and menorrhagia), fluid loss into tissue spaces and the resulting hypotension carries the greatest risk of mortality 141. Whereas Severe Dengue occurs principally after secondary infection in children and adults 142, in infants less than age one born to dengue-immune mothers, a primary DENV infection can cause substantial morbidity and mortality 143. Maternal anti-dengue antibody titers and age of the infant correlated with disease. Severe Dengue often occurs in infants (peak at 7 months) when maternal serum antibodies wane and enhance rather than neutralize infection of monocytes via ADE 112. Severe Dengue is more prevalent in infants 144 and has a higher mortality rate compared to other age groups 145.
West Nile virus.
WNV, which was first isolated in 1937 146, cycles in nature between Culex mosquitoes and birds but also infects and causes disease in humans, horses, and other mammals (Table 1). Although its enzootic cycle is between mosquitoes and birds, with mammals serving as “dead-end” hosts because of low-level and transient viremia, non-viremic transmission of WNV between co-feeding mosquitoes suggests that some mammals could act as additional reservoirs 147. Historically, WNV caused sporadic outbreaks of a febrile illness in regions of Africa, the Middle East, Asia, and Australia that were not associated with severe human disease. However, in the 1990s, the epidemiology of infection changed. Cases in Eastern Europe were associated with neurological disease 148. In 1999, WNV entered North America and caused seven human fatalities in the New York area as well a large number of avian and equine deaths. In the United States, some avian species are particularly vulnerable with a large number of deaths in crows, jays, and hawks recorded during the epidemic. Over the past two decades, WNV has spread to and circulated in all continental United States as well in Canada, Mexico, the Caribbean, and South America (Figure 4). Because of the increased range, the number of human cases has continued to rise: in the United States between 1999 and 2019, 51,747 cases were confirmed. Forty-eight percent of these cases caused acute flaccid paralysis, meningitis, and/or encephalitis, and were associated with 2,381 deaths (https://www.cdc.gov/westnile/statsmaps/index.html). Based on blood supply screening, 2 to 4 million total infections likely occurred in the United States between 1999 and 2010 149. Moreover, WNV continues to emerge in parts of Eastern Europe 150 with severe neurological disease and fatalities caused by a different genetic lineage, termed lineage 2 WNV151 (In)[MSD16] 2018, an unusually high number of infections in horses and people were reported in southern parts of Europe 152. Although sequence determinants responsible for greater virulence in birds have been identified (e.g., a T249P amino acid substitution in NS3 153), the basis for enhanced pathogenicity of contemporary American and European isolates in humans remains an unanswered question.
Japanese encephalitis virus.
JEV causes severe neurological disease, primarily in Asia, where it accounts for ~35,000 to 50,000 cases and 10,000 to 15,000 deaths annually 154. JEV epidemics originally were described in Japan in the 19th century, and the virus was first recovered in 1935 from an infected human in Tokyo. While the majority of human infections are asymptomatic, many symptomatic cases result in meningitis, encephalitis, and/or flaccid paralysis and are fatal or cause devastating long-term neurological sequelae 155 (Table 1). In one study of children with JEV encephalitis 156, only 44% of patients had full recovery, with 8% dying during the acute phase and 31% having persistent neurological, developmental, and psychiatric disease. The enzootic cycle of JEV is between waterbirds and Culex mosquitoes, with pigs also serving as an amplifying host. Humans are considered incidental dead-end hosts, and generally do not produce viremia sufficient to infect mosquitoes. Despite the introduction of inactivated and live-attenuated vaccines 157 (see below), JEV remains an important global cause of viral encephalitis. JEV is classified into a single serotype with five genotypes, and infection and disease occurs across a large range of Asian countries with outbreaks occurring in Japan, China, Taiwan, Korea, the Philippines, India, and the eastern region of Russia (Figure 4). Epidemic activity in India, Nepal, and other parts of Southeast Asia appears to be escalating, and JEV more recently has been described in Pakistan, Papua New Guinea, and Australia, suggesting that its geographic range may be expanding 158. Indeed, autochthonous transmission of JEV was detected for the first time in Africa in a febrile patient from Angola 159. Of concern, the more divergent genotype V strains (amino acid divergence from 8.4% to 10.0% compared to genotypes I-IV) have been detected in Malaysia 160, Korea 161, and China 162 and may be covered poorly by existing genotype III-based vaccines. Currently, approximately 50 percent of the world’s population is living in regions that are endemic to JEV 163. There also is concern that JEV could spread to the Americas, much like WNV did, since North American field-collected Culex mosquitoes are susceptible to JEV infection 164, and several avian species in North America are susceptible to JEV and can potentially serve as amplification hosts.
EMERGING and RE-EMERGING THREATS
Yellow fever virus.
YFV is the prototype and namesake of the flavivirus genus owing to the jaundice that characterizes severe infections. While most infections are asymptomatic, YFV causes an acute febrile illness that may result in hepatitis, renal failure, hemorrhage, and shock 70,165 (Table 1). Infection is fatal in 20 to 60% of severe symptomatic cases 166. Trade between Africa, where YFV is thought to have originated, and the New World or Europe drove devastating outbreaks in coastal cities during the 18th and 19th century that shaped the development and economies of the Americas 167. These outbreaks ultimately were blunted by the deployment of a vaccine and measures to control mosquito populations.
Despite the existence of an effective vaccine (see below), YFV remains endemic in many parts of the world (Figure 4) 168,169. YFV has an equatorial distribution across the African continent bounded in the north by the Sahara Desert and Angola in the south. Periodic outbreaks of varying intensity occur, most frequently in West and East Africa 70. It is estimated that 90% of YFV cases occur in Africa 170. However, the burden disease in Africa has proven difficult to measure due to the heterogeneity of clinical presentation of YFV. Modeling suggests ~130,000 severe cases of YFV occur each year, resulting in ~78,000 deaths 169, mostly in West Africa. Only 12% of human YFV infections in Africa are estimated to cause severe illness 165. Prior to the late 1990’s, the distribution of YFV in South America occurred predominantly in the river basins of the Orinoco, Amazon, and Araguaia rivers. Multiple outbreaks in humans and non-human primates (NHPs) have occurred since outside this endemic region in Brazil, Columbia, Argentina, Ecuador, and Peru 171. This expanding activity is characterized by human infections proximal to major urban centers and large numbers of unvaccinated individuals.
The epidemiology of YFV is determined by the distribution of its mosquito vector. In South America, YFV is maintained in an enzootic cycle between canopy mosquitoes of the Haemogogus and Sabethes genera and a variety of NHP species, whereas transmission among African primates is vectored by Aedes species mosquitoes 168. These sylvatic cycles provide a reservoir for YFV and an opportunity for transmission when human activity encroaches on forest ecosystems. The presence of this reservoir virtually eliminates the possibility of YFV eradication through vaccination. Urban cycles of YFV transmission involving transmission cycles of Aedes aegypti and humans have not contributed significantly to YFV outbreaks in South America 172. A study of the 2016-2017 YFV outbreak in Minas Gerais, Brazil identified a temporal correlation between human infections and virus detection in NHPs, and established that YFV-infected individuals lived an average of 1.4 km from a YFV+ NHP sampled by this study (as compared to 39 km for non-exposed human controls) 5. The distribution of YFV cases in this outbreak also supported a model by which human infections originated from a sylvatic rather than urban cycle of enzootic transmission. While many factors contribute to the potential for YFV emergence in urban areas, the widespread distribution of Aedes aegypti populations capable of YFV transmission creates a significant risk for public health.
Zika virus.
Prior to 2007, ZIKV was an obscure virus that caused a mild febrile illness in a small number of humans in Africa and parts of Asia. In late 2013 or early 2014, ZIKV was introduced into Brazil and other regions of the Americas 173 with millions of infections occurring (Figure 4). As part of this epidemic, some of the unique clinical features of ZIKV infection (e.g., congenital malformations) were identified 174,175 (Table 1). A key question is how did ZIKV change to cause an epidemic of fetal microcephaly and other congenital anomalies.
Ecological factors have been proposed to explain the increase number of ZIKV infections in humans as a function of greater transmission by Aedes species mosquitoes. Potential factors that could have enhanced Aedes mosquito populations and transmission include land use change (e.g., deforestation), climate change, population growth, and human movement into urban areas 176. Beyond this, changes in the ZIKV sequence during the pre- to post-epidemic transition may explain the expanded vector transmission. An alanine to valine (A188V) substitution in NS1 of epidemic ZIKV strains facilitated greater infectivity in Aedes aegypti laboratory mosquitoes and thus is postulated to enhance epidemic transmission 177.
Genetic changes in ZIKV also may have affected its ability to replicate and cause injury to key neuroprogenitor cells in the brain. Initial phylogenetic analysis revealed 11 amino acid changes on between ancestral strains and French Polynesian and American ZIKV isolates, and these differences were dispersed in prM, NS1, NS3, and NS5 proteins 178. Subsequent experiments showed that a serine to asparagine substitution (S139N of the polyprotein) in prM resulted in increased ZIKV infectivity in neuroprogenitor cells and more severe microcephaly in neonatal mice 179. The S139N substitution arose just prior to the 2013 outbreak in French Polynesia and has been maintained in virtually all American strains. The basis for how the S139N mutation in prM mediates increased pathogenicity is uncertain, although it is speculated to affect the maturation state and/or physical structure of the ZIKV particle 180.
Sequence changes in the 3′-UTR also may contribute to pathogenic effects in neural cells. One group identified a putative Musashi protein binding element in the SL2 stem-loop of the 3′-UTR, with changes immediately upstream of this site in epidemic strains 181. As Musashi proteins regulate progenitor cell growth and differentiation through posttranscriptional control of gene expression, they speculated that the binding elements in the 3′-UTR of ZIKV would affect the fate of neuronal progenitor cells in infected cells and pathogenesis. A second group showed that Musashi-1 interacts with the ZIKV RNA and facilitates viral replication 182. ZIKV infection disrupted the binding of Musashi-1 to its endogenous targets, which altered expression of factors implicated in neural stem cell function and differentiation. Thus, Musashi protein interactions with RNA elements from epidemic strains of ZIKV may contribute to the vulnerability of the fetal brain to infection and development.
The same amino acid change in NS1 (A188V) in epidemic strains that is speculated to affect vector transmission also may affect replication in human cells. A188V variants of NS1 show enhanced binding to human TBK1, an enzyme that regulates the activity and nuclear translocation of IRF3. NS1 binding to TBK1 resulted in reduced levels of TBK1 phosphorylation and diminished IFN-β expression in human cells and mice 183. Thus, this recent sequence change in NS1 can promote evasion of the innate immune response, enhance viremia, and possibly enhance ZIKV transmissibility from hosts to vectors, all of which facilitates epidemic transmission.
The immune status of the host also may influence ZIKV pathogenesis. While cross-reactive anti-DENV antibodies can readily enhance ZIKV infection in cell culture 184,185, the significance of this finding to the epidemiology of ZIKV disease severity and transmission remains uncertain 186. Indeed, passive transfer of cross-reactive, neutralizing E-dimer epitope antibodies raised against DENV prevented ZIKV pathogenesis in mice and NHPs 187,188. However, in some settings, pre-existing anti-flavivirus antibodies have augmented ZIKV infection and disease: passive transfer of immune plasma raised against DENV or WNV enhanced ZIKV pathogenesis in Stat2−/− mice 189,190. Yet in another study in Ifnar1−/− (A129) or Ifnar1−/− Ifngr−/− (AG129) mice, whereas inactivated ZIKV vaccination enhanced dengue disease severity, ADE was not observed after ZIKV infection in animals that were passively immunized or pre-infected with DENV 180. Apart from the contrasting results, a major caveat to the passive transfer of antibody model is that these mice lack immune, cross-reactive CD8+ T cells, which can limit the pathological effects of ADE in the context of DENV immunity and subsequent ZIKV infection including during pregnancy 127,191.
In NHPs, the effects of pre-existing flavivirus immunity on ZIKV and DENV pathogenesis also are uncertain. In one study, no substantive differences in ZIKV infection viral titers, neutralizing antibody levels, or immune cell kinetics were observed after inoculation of naïve and flavivirus-immune rhesus macaques 192. Other groups also have found no evidence of enhancement of ZIKV pathogenesis in DENV-immune macaques 193,194. However, in a study in rhesus macaques, prior exposure to ZIKV resulted in enhanced DENV peak viremia 195, and this was associated with delayed induction of memory cross-neutralizing antibody responses 196. This observation may have implications for ZIKV vaccine development in areas endemic for DENV infections. More epidemiological studies in humans are necessary to establish whether clinically relevant ADE of ZIKV pathogenesis occurs. An analysis of Brazilian cohorts has not shown evidence of ADE, greater disease severity, or effects on birth outcomes in DENV-experienced patients with acute ZIKV infection 197,198.
THE NEXT EMERGING FLAVIVIRUSES?
The ZIKV epidemic showed that flaviviruses of relative obscurity can emerge as significant public health threats within a compressed time frame. Are there other esoteric flaviviruses that will appear soon and cause epidemics in vulnerable hosts? While it is difficult to predict the rise of a particular pathogen in the human population, six less well known flaviviruses could emerge to cause significant human disease in the near future (Figure 4; Table 1).
Spondweni virus.
Spondweni virus (SPOV) is the flavivirus most closely related to ZIKV. In the 1950s, SPOV was isolated from patients in Nigeria and South Africa 199,200, and subsequently circulated in sub-Saharan Africa. Although most symptomatic SPOV infections result in mild illness, a subset reportedly progress to more serious disease, including vascular leakage and shock or neurological involvement 201. The enzootic cycle of SPOV likely is between mosquitoes and NHPs 202. Historically, SPOV infection was not observed in Aedes aegypti, Aedes albopictus, and Culex quinquefasciatus mosquitoes, and instead was isolated from other mosquitoes in the genera Aedes, Culex, Eretmapodites, and Mansonia. Based on this vector biology, the potential for urban epidemic cycles of SPOV was considered low. However, the epidemiology may be changing, as SPOV reportedly was detected in field-caught Culex quinquefasciatus mosquitoes in Haiti in 2016 203. This finding suggests that SPOV may adapt to mosquito species that preferentially feed on humans. Given its relationship to ZIKV (~75% amino acid identity), there is concern that SPOV also might have the capacity to infect cells of the reproductive tract and be sexually transmitted, as was reported in mice 204.
Usutu virus.
Usutu virus (USUV) is a mosquito-transmitted flavivirus that belongs to the JEV antigenic complex. USUV is classified into eight lineages with two major African and European groups 205. USUV shares the same mosquito vectors (e.g., Culex pipiens) with WNV and similar bird populations as amplifying hosts, and the two viruses can co-circulate 206. Initially isolated in 1959 in South Africa, USUV appeared in Europe in 1996 in Italy (based on retrospective analysis of archived tissues) and 2001 in central Europe where it was associated with deaths in selected avian populations 207. In 2015-2016, widespread USUV activity was reported in Germany, France, Austria, Belgium, and the Netherlands, with mortality observed in blackbirds and grey owls 208. USUV infection occurs in humans, and seroprevalence studies suggest that it may be higher than WNV in areas of co-circulation 209. Neuroinvasive disease in humans caused by USUV appears less common than WNV, although reports of meningoencephalitis, meningitis, and paralysis exist 210. As WNV and USUV are related (~76% amino acid identity), serological distinction may be challenging and thus, it is possible that USUV infection and disease are underestimated.
Ilheus virus.
Ilheus virus (ILHV) is a mosquito-transmitted flavivirus that is closely related to viruses of the JEV serocomplex. It was first described in Brazil in 1944 and now circulates in South America where it sporadically causes a febrile syndrome in humans that can progress to encephalitis. ILHV infection in humans has been reported in Trinidad, Panama, Colombia, French Guyana, Brazil, Ecuador, and Bolivia 211. ILHV cycles in nature between birds and mosquitoes and has been isolated from mosquitoes. sentinel monkeys, man212 and birds. Moreover, high seroprevalence rates of ILHV have been detected in horses in parts of Brazil 213. As this virus can propagate in some mosquitoes that feed on humans (e.g., Aedes and Culex species) 214, there is potential for more extensive zoonotic emergence in the human population.
Rocio virus.
Rocio virus (ROCV) is a flavivirus in the JEV serocomplex and is closely related to ILHV. It was first isolated in 1975 from the brain of an affected individual during an epidemic of encephalitis in São Paulo, Brazil 215. Its spread to more than 20 municipalities resulted in approximately 1,000 diagnosed cases 216. During the epidemic, there was a case-fatality rate of 13% with approximately 20% of survivors developing long-term neurological sequelae. Laboratory studies suggest that ROCV is mosquito-transmitted, as Culex tarsalis and Culex pipiens were efficient experimental vectors 217, and that birds may act as amplifying hosts218. Although no cases of ROCV infection and encephalitis have been reported after the initial outbreak, serological surveys suggest ROCV transmission among humans and animals in different regions of Brazil is still actively occurring 219,220.
Wesselsbron virus.
Wesselsbron virus (WSLV) is a mosquito-transmitted zoonotic agent that causes disease in sheep and other ruminants in Africa with spillover into human populations. WSLV infection initially was reported on a sheep farm in South Africa in 1955 and caused substantial mortality in newborn lambs and abortion in pregnant ewes 212. In humans, WSLV infection can cause a sudden onset influenza-like illness characterized by fever, rigors, headache, myalgia, and arthralgia. Historical studies have suggested that WSLV circulation is widespread, at least in southern Africa 212, and more recent analysis has demonstrated infection of rats, which could serve as a reservoir 221. WSLV likely is present in many areas of Africa as viral isolations from mosquitoes have been reported in South Africa, Botswana, Zimbabwe, Uganda, Mozambique, Cameroon, Central African Republic, Mauritania, Senegal, Nigeria, Democratic Republic of Congo and Madagascar 212. There is concern that WSLV could emerge beyond its traditional borders, spread more extensively, and cause infection and disease in naïve human populations. Indeed, WSLV was isolated in Thailand from mosquitoes in 1966, although there is no recent evidence of circulation or transmission in Asia.
Tick-borne flaviviruses.
Transmission of tick-borne flaviviruses has been increasing worldwide. This group includes TBEV, which can cause fatal neurological syndrome principally in regions of northern China and Japan, Russia, and Central and Eastern Europe. TBEV causes several thousands of human cases per year, with recent increases attributed to changes in climate, population dynamics, range of permissive ticks, and shifts in land usage 222,223. Other antigenically related tick-borne flaviviruses can cause severe human disease. This group includes Omsk hemorrhagic fever virus (OHFV), Powassan virus (POWV), Kyasanur Forest disease virus (KFDV), Alkhurma hemorrhagic fever virus (AHFV), and Karshi virus (KSIV), with some causing encephalitis (KSIV and POWV) and others resulting in hemorrhagic fever (OHFV, KFDV, and AHFV).
POWV is the only known tick-borne flavivirus that circulates in North America. POWV was first isolated from a child who died of encephalitis in Powassan, Ontario in 1958. Human cases of POWV occur in the United States, Canada, and also Russia 224. Two genetic lineages of POWV circulate in North America, lineage I and lineage II (also called deer-tick virus (DTV)) that share at least 96% amino acid identity in their E proteins. POWV lineage I strains are predominantly maintained in Ixodes cookei ticks, whereas lineage II strains are found in Ixodes scapularis deer ticks 225.
The natural cycle of POWV includes some small mammals (rodents and lagomorphs), deer, and ticks 226, with peak transmission occurring during spring and summer. In humans, POWV infections can cause severe neuroinvasive disease including meningitis and encephalitis with an estimated 10 to 30% case-fatality rate, and many survivors suffering long-term disabling sequelae. While POWV-induced disease can occur in all age groups, epidemiological studies suggest a greater risk in the elderly (> 60 years of age) 223, which is similar to other encephalitic flaviviruses including WNV 227. POWV is emerging, as increasing numbers of cases have been diagnosed over the past decade 228 and up to 3-5% of Ixodes scapularis ticks isolated in certain parts of the United States now test positive for POWV 229,230. Moreover, seroprevalence rates of POWV infection in other mammals (e.g., white-tailed deer) are rising and may be associated with the expanded range of Ixodes scapularis in the United States 231. Thus, an abundance of evidence suggests that POWV is an emerging flavivirus threat, which has triggered the development of countermeasures to minimize severe disease 232.
COMBATING FLAVIVIRUS EMERGENCE
Given the ongoing, and likely future threats of flavivirus infections, the continued development and deployment of countermeasures that limit epidemic spread and disease in humans is urgent. This section focuses on the past successes and future challenges of flavivirus vaccines and the issues related to the development of direct-acting antiviral agents.
Flavivirus vaccines.
Licensed vaccines exist for five flaviviruses (YFV, DENV, JEV, KFDV, and TBEV), and several others have been evaluated in preclinical and clinical studies. The live-attenuated YFV vaccine is among the most successful of all vaccines to prevent viral infections. Developed by Max Theiler in 1939 by iterative passage of the pathogenic Asibi strain in mouse and chicken embryos, more than 500 million doses of YFV-17D vaccine have been administered worldwide 233. An extensively passaged vaccine for JEV (SA14-14-2) also is efficacious, and used extensively in Asia and India 234. Molecular clone technology enabled the development of rationally-attenuated vaccines for DENV 235–239 and JEV 240 via the construction of chimeric viruses or those encoding deletions in the 3′-UTR of the genome. Additional modes of attenuation (e.g., mutations in E, NS1, or NS5 genes) have been evaluated as flavivirus vaccine candidates in preclinical models 241,242. Chemically-inactivated viruses of cell culture-derived viruses currently are used as vaccines for JEV 243, TBEV 244, and KFDV 245. While they are protective, they require frequent iterative boosting to maintain protective immunity.
The severe clinical outcomes following DENV infections have made the development of a vaccine a global health imperative. However, vaccine design and development has been hampered by the theoretical risk that incomplete vaccine immunity against all four serotypes might paradoxically enhance pathogenesis in the setting of subsequent natural infection. As a result, the goal is to develop a vaccine that simultaneously elicits a balanced tetravalent neutralizing response against all four DENV serotypes. The live-attenuated, tetravalent Dengvaxia® (from Sanofi Pasteur) was the first anti-DENV vaccine licensed in 2016, although it was restricted to individuals greater than 9 years of age 246. The FDA later approved Dengvaxia® in 2019 but only for use in individuals 9 through 16 years of age with laboratory-confirmed prior dengue infection and living in endemic areas. These relatively narrow indications are based in part on the finding that in the clinical trials, vaccinated children aged 2-5 were at greater risk of hospitalization as compared to controls 247. Serological studies later demonstrated that individuals that were DENV seropositive at the time of vaccine administration experienced benefit from Dengvaxia 248, whereas DENV-naïve individuals were at increased risk for disease over this interval 249. Further follow-up is required to evaluate the public health impact of the use of this vaccine candidate on children since its licensure. As two other live-attenuated tetravalent DENV vaccines (TV003 from NIAID and TAK-003 from Takeda) are in advanced stages of clinical trial 250,251, the question remains as to whether they will provide superior protection to naïve individuals without the risk of sensitizing them to symptomatic or severe disease from subsequent natural DENV infection.
Despite the success of vaccines for some flaviviruses, challenges exist for the development of vaccine candidates to blunt epidemics caused by emerging flaviviruses. First, the extensive cross-reactivity of flavivirus-immune sera complicates the development and use of diagnostics to track and manage outbreaks. While neutralization assays provide some capacity to resolve antibody responses to homologous and heterologous viruses in convalescent sera, these approaches have limitations in sera from acutely infected individuals 252,253. Since viremia is typically transient, molecular assays to detect flavivirus infection are sensitive only for relatively small intervals after exposure, the timing of which is often unknown. While the discovery that RNA persists in the urine and semen of ZIKV-infected individuals extended the utility of these approaches during the 2015 epidemic 72, serological assays remain an important tool for the management of the epidemics and evaluation of vaccine candidates 254,255. Second, the presence of cross-reactive antibodies may shape the immune response to vaccination and influence the outcome of disease following infection, as detailed above and reviewed elsewhere (ref 110). Third, while promising new platforms have been applied to create flavivirus vaccines, including synthetic nucleic expression systems, small differences in antigen design unpredictably modulate the potency of the immune response to vaccination, highlighting the need for additional study of the biology, structure, and heterogeneity of vaccine antigens 256. Fourth, even large epidemics of flavivirus infection and disease can be transient relative to the interval required to the development and evaluation of vaccine candidates. Despite the unprecedented speed of generating vaccine candidates for early clinical evaluation, a requirement for advanced clinical trials in larger numbers of individuals to reveal efficacy and provide insights into correlates of protection may be jeopardized by the smaller number of new infections characteristic of a waning epidemic 257. Finally, limited availability or insufficient deployment may limit the utility of vaccines once developed. Notably, vaccine shortages have exacerbated ongoing YFV activity in South America and Africa, prompting vaccine sparing studies 258. Moreover, considerable numbers of JEV and TBEV infectious continue to occur in Asia and Europe despite the availability of safe and effective vaccine programs. Even when available, effective vaccines have not always had the impact on global health as intended.
Anti-flavivirus drugs.
The development of antiviral therapeutics will enable new approaches for the management of flavivirus outbreaks due to their potential for use as treatment and prophylaxis. Flaviviruses encode multiple potential targets for small molecule drugs. Extensive drug-discovery efforts have focused on the NS5 and NS3 proteins encoding enzymatic activity required for viral genome replication and polyprotein processing. Nucleoside 259 and allosteric inhibitors 260 of NS5 encoded RNA-dependent RNA polymerase activity have been described (reviewed in 261). Compounds with broad activity against multiple classes of viruses, including flaviviruses, have also been characterized, including the adenosine analog BCX4430 262,263 and the nucleotide analog prodrug Sofosbuvir 264. The methyltransferase domain that comprises the amino-terminus of NS5 responsible for the N-7 and 2′-O methylation of the viral RNA cap also is a potential target for small molecules 265,266. Inhibition of viral protease activity has yielded important classes of drugs for multiple viruses, including HCV, and has been aggressively pursued for other flaviviruses. While inhibitor design was guided by numerous structures of the NS3 protease in complex with NS2B, this complex have proven to be a challenging target due to the relatively flat structure of the substrate pocket, that ligands binding this motif are charged, and the conformational flexibility of the protease target 267,268. Both small molecule and peptide protease inhibitors have been characterized; some of these function via an allosteric mechanism. Of interest, multiple repurposed compounds have been shown to inhibit flavivirus proteases, including several FDA-approved drugs capable of inhibiting ZIKV replication in cell culture and mice 269,270. Flavivirus helicase inhibitors also have been characterized in preclinical studies 271.
Structural proteins of the virion also may be targeted by antiviral compounds. Crystallographic studies of the E protein of DENV2 identified a lipid molecule in a hydrophobic pocket formed at the junction between E-II and E-DI 272. Compounds that target this pocket have been identified and are thought to block infection by interfering with the viral membrane fusion process 273,274. Peptides derived from sequences present in the stem anchor domains of E also have antiviral activity 275,276. The internal capsid protein has also been targeted for drug discovery efforts. High-throughput screening identified the small molecule ST-148 as capable of inhibiting cell death in a DENV propagation assay 277. The proposed mechanism of this molecule is the stabilization of the capsid protein, which results in altered assembly and disassembly during virus entry 278. A second chemically related compound has been described that also binds DENV capsid and inhibits infection 279.
Targeting the vector.
Progress has been made on reducing flavivirus transmission by limiting infection of the mosquito host 280. For example, the infection of Aedes aegypti mosquitoes with selected strains of endosymbiotic Wolbachia resulted in bacterial invasion of mosquito populations and interference with DENV and ZIKV replication 281,282. The wMel strain of Wolbachia-infected Aedes aegypti, when directly fed on viremic dengue patients, have lower DENV transmission potential than their wild-type counterparts 283. Mechanistic studies suggest that infection with Wolbachia reduces flavivirus replication, is associated with rapid viral RNA degradation in the cytoplasm, and is mediated by the mosquito XRN1 enzyme 284. The establishment of Aedes aegypti strains with Wolbachia infection in an endemic setting could abolish or reduce flavivirus transmission 285. Wolbachia-infected Aedes aegypti mosquitoes have been released in Australia where outbreaks of Dengue fever occur, and have been stable over several years 286. The AWED trial (Applying Wolbachia to Eliminate Dengue) is underway to assess the efficacy of Wolbachia-infected mosquito deployments to reduce DENV incidence in Indonesia 287.
Other groups have created genetically engineered Aedes aegypti mosquitoes resistant to DENV infection through the induction of an antiviral RNA interference response 288. More recently, a polycistronic cluster of engineered synthetic small RNAs targeting ZIKV was expressed in the midgut of mosquitoes, a site of early virus infection. Engineered Aedes aegypti mosquitoes harboring the anti-ZIKV transgene had markedly reduced viral infection, dissemination, and transmission rates of ZIKV in the laboratory 289.
CONCLUSIONS
The recent outbreaks of less well-known flaviviruses highlight the transmission potential and dynamic state of emergence. While it is challenging to predict which flavivirus will transition next from relative obscurity to worldwide notoriety, their changing epidemiology raises concern for large-scale emergence and disease. Sustained research efforts on flaviviruses and likely other arboviruses (e.g., alphaviruses, bunyaviruses, and some orthomyxoviruses) are needed. Such a concerted program can prepare us to respond rapidly with countermeasures to new viral epidemics that cause known and unanticipated clinical syndromes.
A requirement to respond rapidly to an explosive ZIKV outbreak in the Americas identified aspects of flavivirus biology that may be particularly important for future preparedness efforts. While expensive to establish and maintain, surveillance programs to identify the changes in pathogen distribution that provide early signals to public health officials are critical. The emergence of WNV in North America in 1999 resulted in a considerable increase in arbovirus surveillance capacity to manage this outbreak, but this was not sustained 290. The development of sensitive and specific flavivirus diagnostics is a challenge due to serological cross-reactivity and the relatively limited persistence of viral RNA in those infected. These technical obstacles hamper the management of an outbreak response, including the evaluation of vaccines. Enhanced and sustained investment in these areas are critical for an effective response to future flavivirus threats. Antibody discovery efforts for emerging flaviviruses will be a powerful component of preparedness efforts because they inform the development of diagnostics, allow for characterization of vaccine antigens, and identify protective features of the immune response. Moreover, in vivo expression of potent flavivirus-reactive neutralizing antibodies using recently developed synthetic gene expressing platforms, such as modified messenger RNA, provides a rapid pathway for the development of therapeutics 291. While these gene-expression platforms also enable the rapid development of vaccine candidates, an understanding of structure-immunogen relationships and the correlates of protection may be insufficient to ensure rapid success for understudied flaviviruses in an outbreak setting. A continued emphasis on obtaining a fundamental understanding of the structure(s) of flavivirus vaccine antigens, the genetic and functional components of the antibody response to infection and vaccination, and viral pathogenesis in animal models strengthens our capacity to respond quickly to the next flavivirus threat. Because flaviviruses share an overall similar structure, antigen designs that lack features recognized by cross-reactive antibodies and are compatible with increasingly powerful antigen expression or display platforms may be particularly important first-generation vaccine candidates for use in an increasingly flavivirus-experienced world.
Acknowledgements.
This work is dedicated to the memory of Dr. Michael Rossmann (1930-2019), who pioneered our structural understanding of flaviviruses and their interactions with the host. This work was supported by the intramural program of the National Institute of Allergy and Infectious Diseases, NIH and NIH grants (R01 AI073755, R01 AI127828, and R01 HD091218 to M.S.D). We thank Ethan Tyler (NIH OD) for preparation of the figures. We also thank Nikos Vasilakis and Scott Weaver for their editorial comments.
Declaration of interests. M.S.D. is a consultant for Inbios, on the Scientific Advisory Board of Moderna, and receives funding from Emergent BioSolutions.
REFERENCES
- 1.Bhatt S et al. The global distribution and burden of dengue. Nature 496, 504–507, doi: 10.1038/nature12060 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gubler DJ Dengue/dengue haemorrhagic fever: history and current status. Novartis Found Symp 277, 3–16; discussion 16-22, 71-13, 251-253 (2006). [DOI] [PubMed] [Google Scholar]
- 3.Pierson TC & Diamond MS The emergence of Zika virus and its new clinical syndromes. Nature 560, 573–581, doi: 10.1038/s41586-018-0446-y (2018). [DOI] [PubMed] [Google Scholar]
- 4.Roehrig JT West nile virus in the United States - a historical perspective. Viruses 5, 3088–3108, doi: 10.3390/v5123088 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Faria NR et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 361, 894–899, doi: 10.1126/science.aat7115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ingelbeen B et al. Urban yellow fever outbreak-Democratic Republic of the Congo, 2016: Towards more rapid case detection. PLoS Negl Trop Dis 12, e0007029, doi: 10.1371/journal.pntd.0007029 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ling Y et al. Yellow Fever in a Worker Returning to China from Angola, March 2016. Emerg Infect Dis 22, 1317–1318, doi: 10.3201/eid2207.160469 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Young PR Arboviruses: A Family on the Move. Adv Exp Med Biol 1062, 1–10, doi: 10.1007/978-981-10-8727-1_1 (2018). [DOI] [PubMed] [Google Scholar]
- 9.Tabachnick WJ Climate Change and the Arboviruses: Lessons from the Evolution of the Dengue and Yellow Fever Viruses. Annu Rev Virol 3, 125–145, doi: 10.1146/annurev-virology-110615-035630 (2016). [DOI] [PubMed] [Google Scholar]
- 10.Mansfield KL, Hernandez-Triana LM, Banyard AC, Fooks AR & Johnson N Japanese encephalitis virus infection, diagnosis and control in domestic animals. Vet Microbiol 201, 85–92, doi: 10.1016/j.vetmic.2017.01.014 (2017). [DOI] [PubMed] [Google Scholar]
- 11.Jeffries CL et al. Louping ill virus: an endemic tick-borne disease of Great Britain. J Gen Virol 95, 1005–1014, doi: 10.1099/vir.0.062356-0 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McLean RG, Ubico SR, Bourne D & Komar N West Nile virus in livestock and wildlife. Curr Top Microbiol Immunol 267, 271–308, doi: 10.1007/978-3-642-59403-8_14 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Venter M Assessing the zoonotic potential of arboviruses of African origin. Curr Opin Virol 28, 74–84, doi: 10.1016/j.coviro.2017.11.004 (2018). [DOI] [PubMed] [Google Scholar]
- 14.Zhang W, Chen S, Mahalingam S, Wang M & Cheng A An updated review of avian-origin Tembusu virus: a newly emerging avian Flavivirus. J Gen Virol 98, 2413–2420, doi: 10.1099/jgv.0.000908 (2017). [DOI] [PubMed] [Google Scholar]
- 15.Pandit PS et al. Predicting wildlife reservoirs and global vulnerability to zoonotic Flaviviruses. Nat Commun 9, 5425, doi: 10.1038/s41467-018-07896-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sirohi D & Kuhn RJ Zika Virus Structure, Maturation, and Receptors. J Infect Dis 216, S935–S944, doi: 10.1093/infdis/jix515 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Akey DL et al. Flavivirus NS1 structures reveal surfaces for associations with membranes and the immune system. Science 343, 881–885, doi: 10.1126/science.1247749 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murthy HM, Clum S & Padmanabhan R Dengue virus NS3 serine protease. Crystal structure and insights into interaction of the active site with substrates by molecular modeling and structural analysis of mutational effects. J Biol Chem 274, 5573–5580 (1999). [DOI] [PubMed] [Google Scholar]
- 19.Wu J, Bera AK, Kuhn RJ & Smith JL Structure of the Flavivirus helicase: implications for catalytic activity, protein interactions, and proteolytic processing. J Virol 79, 10268–10277, doi: 10.1128/JVI.79.16.10268-10277.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi Y & Gao GF Structural Biology of the Zika Virus. Trends Biochem Sci 42, 443–456, doi: 10.1016/j.tibs.2017.02.009 (2017). [DOI] [PubMed] [Google Scholar]
- 21.Rey FA, Heinz FX, Mandl C, Kunz C & Harrison SC The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Nature 375, 291–298, doi: 10.1038/375291a0 (1995). [DOI] [PubMed] [Google Scholar]
- 22.Rey FA, Stiasny K & Heinz FX Flavivirus structural heterogeneity: implications for cell entry. Curr Opin Virol 24, 132–139, doi: 10.1016/j.coviro.2017.06.009 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lorenz IC, Allison SL, Heinz FX & Helenius A Folding and dimerization of tick-borne encephalitis virus envelope proteins prM and E in the endoplasmic reticulum. J Virol 76, 5480–5491, doi: 10.1128/jvi.76.11.5480-5491.2002 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasad VM et al. Structure of the immature Zika virus at 9 A resolution. Nat Struct Mol Biol 24, 184–186, doi: 10.1038/nsmb.3352 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elshuber S, Allison SL, Heinz FX & Mandl CW Cleavage of protein prM is necessary for infection of BHK-21 cells by tick-borne encephalitis virus. J Gen Virol 84, 183–191 (2003). [DOI] [PubMed] [Google Scholar]
- 26.Kostyuchenko VA et al. Structure of the thermally stable Zika virus. Nature 533, 425–428, doi: 10.1038/nature17994 (2016). [DOI] [PubMed] [Google Scholar]
- 27.Sirohi D et al. The 3.8 A resolution cryo-EM structure of Zika virus. Science 352, 467–470, doi: 10.1126/science.aaf5316 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mukhopadhyay S, Kim BS, Chipman PR, Rossmann MG & Kuhn RJ Structure of West Nile virus. Science 302, 248, doi: 10.1126/science.1089316 (2003). [DOI] [PubMed] [Google Scholar]
- 29.Kuhn RJ et al. Structure of dengue virus: implications for flavivirus organization, maturation, and fusion. Cell 108, 717–725, doi: 10.1016/s0092-8674(02)00660-8 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Byk LA & Gamarnik AV Properties and Functions of the Dengue Virus Capsid Protein. Annu Rev Virol 3, 263–281, doi: 10.1146/annurev-virology-110615-042334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Therkelsen MD et al. Flaviviruses have imperfect icosahedral symmetry. Proc Natl Acad Sci U S A 115, 11608–11612, doi: 10.1073/pnas.1809304115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Amberg SM & Rice CM Mutagenesis of the NS2B-NS3-mediated cleavage site in the flavivirus capsid protein demonstrates a requirement for coordinated processing. J Virol 73, 8083–8094 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tassaneetrithep B et al. DC-SIGN (CD209) mediates dengue virus infection of human dendritic cells. J Exp Med 197, 823–829, doi: 10.1084/jem.20021840 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Navarro-Sanchez E et al. Dendritic-cell-specific ICAM3-grabbing non-integrin is essential for the productive infection of human dendritic cells by mosquito-cell-derived dengue viruses. EMBO Rep 4, 723–728, doi: 10.1038/sj.embor.embor866 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chen Y et al. Dengue virus infectivity depends on envelope protein binding to target cell heparan sulfate. Nat Med 3, 866–871 (1997). [DOI] [PubMed] [Google Scholar]
- 36.Meertens L et al. The TIM and TAM families of phosphatidylserine receptors mediate dengue virus entry. Cell Host Microbe 12, 544–557, doi: 10.1016/j.chom.2012.08.009 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Corver J et al. Membrane fusion activity of tick-borne encephalitis virus and recombinant subviral particles in a liposomal model system. Virology 269, 37–46 (2000). [DOI] [PubMed] [Google Scholar]
- 38.Gollins SW & Porterfield JS pH-dependent fusion between the flavivirus West Nile and liposomal model membranes. J Gen Virol 67 (Pt 1), 157–166 (1986). [DOI] [PubMed] [Google Scholar]
- 39.Miner JJ et al. The TAM receptor Mertk protects against neuroinvasive viral infection by maintaining blood-brain barrier integrity. Nat Med, doi: 10.1038/nm.3974 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen J et al. AXL promotes Zika virus infection in astrocytes by antagonizing type I interferon signalling. Nat Microbiol 3, 302–309, doi: 10.1038/s41564-017-0092-4 (2018). [DOI] [PubMed] [Google Scholar]
- 41.Wang S et al. Integrin αvβ5 Internalizes Zika Virus during Neural Stem Cells Infection and Provides a Promising Target for Antiviral Therapy. Cell Reports, doi: 10.1016/j.celrep.2019.11.020 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu Z et al. Zika Virus Targets Glioblastoma Stem Cells through a SOX2-Integrin αvβ5 Axis. Cell Stem Cell, doi: 10.1016/j.stem.2019.11.016 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hackett BA & Cherry S Flavivirus internalization is regulated by a size-dependent endocytic pathway. Proc Natl Acad Sci U S A 115, 4246–4251, doi: 10.1073/pnas.1720032115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hackett BA et al. RNASEK is required for internalization of diverse acid-dependent viruses. Proc Natl Acad Sci U S A 112, 7797–7802, doi: 10.1073/pnas.1424098112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Perreira JM et al. RNASEK Is a V-ATPase-Associated Factor Required for Endocytosis and the Replication of Rhinovirus, Influenza A Virus, and Dengue Virus. Cell Rep 12, 850–863, doi: 10.1016/j.celrep.2015.06.076 (2015). [DOI] [PubMed] [Google Scholar]
- 46.Chao LH, Klein DE, Schmidt AG, Pena JM & Harrison SC Sequential conformational rearrangements in flavivirus membrane fusion. Elife 3, e04389, doi: 10.7554/eLife.04389 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chao LH et al. How small-molecule inhibitors of dengue-virus infection interfere with viral membrane fusion. Elife 7, doi: 10.7554/eLife.36461 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gebhard LG, Filomatori CV & Gamarnik AV Functional RNA elements in the dengue virus genome. Viruses 3, 1739–1756, doi: 10.3390/v3091739 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Barrows NJ et al. Biochemistry and Molecular Biology of Flaviviruses. Chem Rev 118, 4448–4482, doi: 10.1021/acs.chemrev.7b00719 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aktepe TE & Mackenzie JM Shaping the flavivirus replication complex: It is curvaceous! Cell Microbiol 20, e12884, doi: 10.1111/cmi.12884 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Welsch S et al. Composition and three-dimensional architecture of the dengue virus replication and assembly sites. Cell Host Microbe 5, 365–375, doi: 10.1016/j.chom.2009.03.007 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jordan TX & Randall G Flavivirus modulation of cellular metabolism. Curr Opin Virol 19, 7–10, doi: 10.1016/j.coviro.2016.05.007 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Heaton NS & Randall G Dengue virus and autophagy. Viruses 3, 1332–1341, doi: 10.3390/v3081332 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Aktepe TE, Liebscher S, Prier JE, Simmons CP & Mackenzie JM The Host Protein Reticulon 3.1A Is Utilized by Flaviviruses to Facilitate Membrane Remodelling. Cell Rep 21, 1639–1654, doi: 10.1016/j.celrep.2017.10.055 (2017). [DOI] [PubMed] [Google Scholar]
- 55.Yi Z, Yuan Z, Rice CM & MacDonald MR Flavivirus replication complex assembly revealed by DNAJC14 functional mapping. J Virol 86, 11815–11832, doi: 10.1128/JVI.01022-12 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acosta EG & Bartenschlager R The quest for host targets to combat dengue virus infections. Curr Opin Virol 20, 47–54, doi: 10.1016/j.coviro.2016.09.003 (2016). [DOI] [PubMed] [Google Scholar]
- 57.Burger-Calderon R et al. Zika virus infection in Nicaraguan households. PLoS Negl Trop Dis 12, e0006518, doi: 10.1371/journal.pntd.0006518 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Endy TP et al. Epidemiology of inapparent and symptomatic acute dengue virus infection: a prospective study of primary school children in Kamphaeng Phet, Thailand. Am J Epidemiol 156, 40–51 (2002). [DOI] [PubMed] [Google Scholar]
- 59.Mostashari F et al. Epidemic West Nile encephalitis, New York, 1999: results of a household-based seroepidemiological survey. Lancet 358, 261–264, doi: 10.1016/S0140-6736(01)05480-0 (2001). [DOI] [PubMed] [Google Scholar]
- 60.Lim JK et al. Genetic deficiency of chemokine receptor CCR5 is a strong risk factor for symptomatic West Nile virus infection: a meta-analysis of 4 cohorts in the US epidemic. J Infect Dis 197, 262–265, doi: 10.1086/524691 (2008). [DOI] [PubMed] [Google Scholar]
- 61.Sakuntabhai A et al. A variant in the CD209 promoter is associated with severity of dengue disease. Nat Genet 37, 507–513, doi: 10.1038/ng1550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murray K et al. Risk factors for encephalitis and death from West Nile virus infection. Epidemiol Infect 134, 1325–1332, doi: 10.1017/S0950268806006339 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iwamoto M et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med 348, 2196–2203, doi: 10.1056/NEJMoa022987 (2003). [DOI] [PubMed] [Google Scholar]
- 64.Thackray LB et al. Oral Antibiotic Treatment of Mice Exacerbates the Disease Severity of Multiple Flavivirus Infections. Cell reports 22, 3440–3453.e3446, doi: 10.1016/j.celrep.2018.03.001 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ngo NT et al. Acute management of dengue shock syndrome: a randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clin Infect Dis 32, 204–213, doi: 10.1086/318479 (2001). [DOI] [PubMed] [Google Scholar]
- 66.Rothman AL Immunity to dengue virus: a tale of original antigenic sin and tropical cytokine storms. Nat Rev Immunol 11, 532–543, doi: 10.1038/nri3014 (2011). [DOI] [PubMed] [Google Scholar]
- 67.Beatty PR et al. Dengue virus NS1 triggers endothelial permeability and vascular leak that is prevented by NS1 vaccination. Sci Transl Med 7, 304ra141, doi: 10.1126/scitranslmed.aaa3787 (2015). [DOI] [PubMed] [Google Scholar]
- 68.Puerta-Guardo H, Glasner DR & Harris E Dengue Virus NS1 Disrupts the Endothelial Glycocalyx, Leading to Hyperpermeability. PLoS Pathog 12, e1005738, doi: 10.1371/journal.ppat.1005738 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vieira WT, Gayotto LC, de Lima CP & de Brito T Histopathology of the human liver in yellow fever with special emphasis on the diagnostic role of the Councilman body. Histopathology 7, 195–208 (1983). [DOI] [PubMed] [Google Scholar]
- 70.Monath TP & Vasconcelos PF Yellow fever. J Clin Virol 64, 160–173, doi: 10.1016/j.jcv.2014.08.030 (2015). [DOI] [PubMed] [Google Scholar]
- 71.Miner JJ & Diamond MS Zika Virus Pathogenesis and Tissue Tropism. Cell Host Microbe 21, 134–142, doi: 10.1016/j.chom.2017.01.004 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mansuy JM et al. Zika virus in semen and spermatozoa. Lancet Infect Dis 16, 1106–1107, doi: 10.1016/S1473-3099(16)30336-X (2016). [DOI] [PubMed] [Google Scholar]
- 73.Joguet G et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: a prospective observational study. Lancet Infect Dis 17, 1200–1208, doi: 10.1016/S1473-3099(17)30444-9 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Counotte MJ et al. Sexual transmission of Zika virus and other flaviviruses: A living systematic review. PLoS Med 15, e1002611, doi: 10.1371/journal.pmed.1002611 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Maximova OA & Pletnev AG Flaviviruses and the Central Nervous System: Revisiting Neuropathological Concepts. Annu Rev Virol 5, 255–272, doi: 10.1146/annurev-virology-092917-043439 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Cain MD, Salimi H, Diamond MS & Klein RS Mechanisms of Pathogen Invasion into the Central Nervous System. Neuron 103, 771–783, doi: 10.1016/j.neuron.2019.07.015 (2019). [DOI] [PubMed] [Google Scholar]
- 77.Ludlow M et al. Neurotropic virus infections as the cause of immediate and delayed neuropathology. Acta Neuropathol 131, 159–184, doi: 10.1007/s00401-015-1511-3 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Coyne CB & Lazear HM Zika virus - reigniting the TORCH. Nat Rev Microbiol 14, 707–715, doi: 10.1038/nrmicro.2016.125 (2016). [DOI] [PubMed] [Google Scholar]
- 79.Platt DJ et al. Zika virus-related neurotropic flaviviruses infect human placental explants and cause fetal demise in mice. Sci Transl Med 10, doi: 10.1126/scitranslmed.aao7090 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suthar MS, Diamond MS & Gale M Jr. West Nile virus infection and immunity. Nat Rev Microbiol 11, 115–128, doi: 10.1038/nrmicro2950 (2013). [DOI] [PubMed] [Google Scholar]
- 81.Ngono AE & Shresta S Immune Response to Dengue and Zika. Annu Rev Immunol 36, 279–308, doi: 10.1146/annurev-immunol-042617-053142 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Ishikawa H & Barber GN STING is an endoplasmic reticulum adaptor that facilitates innate immune signalling. Nature 455, 674–678, doi: 10.1038/nature07317 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Maringer K & Fernandez-Sesma A Message in a bottle: lessons learned from antagonism of STING signalling during RNA virus infection. Cytokine Growth Factor Rev 25, 669–679, doi: 10.1016/j.cytogfr.2014.08.004 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.McGuckin Wuertz K et al. STING is required for host defense against neuropathological West Nile virus infection. PLoS Pathog 15, e1007899, doi: 10.1371/journal.ppat.1007899 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schoggins JW Recent advances in antiviral interferon-stimulated gene biology. F1000Res 7, 309, doi: 10.12688/f1000research.12450.1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schoggins JW Interferon-Stimulated Genes: What Do They All Do? Annu Rev Virol 6, 567–584, doi: 10.1146/annurev-virology-092818-015756 (2019). [DOI] [PubMed] [Google Scholar]
- 87.Miorin L, Maestre AM, Fernandez-Sesma A & Garcia-Sastre A Antagonism of type I interferon by flaviviruses. Biochemical and biophysical research communications 492, 587–596, doi: 10.1016/j.bbrc.2017.05.146 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Samuel MA & Diamond MS Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol 79, 13350–13361, doi: 10.1128/JVI.79.21.13350-13361.2005 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lazear HM et al. A Mouse Model of Zika Virus Pathogenesis. Cell Host Microbe 19, 720–730, doi: 10.1016/j.chom.2016.03.010 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lazear HM, Nice TJ & Diamond MS Interferon-lambda: Immune Functions at Barrier Surfaces and Beyond. Immunity 43, 15–28, doi: 10.1016/j.immuni.2015.07.001 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ma D et al. Antiviral effect of interferon lambda against West Nile virus. Antiviral Res 83, 53–60, doi: 10.1016/j.antiviral.2009.03.006 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palma-Ocampo HK et al. Interferon lambda inhibits dengue virus replication in epithelial cells. Virol J 12, 150, doi: 10.1186/s12985-015-0383-4 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bayer A et al. Type III Interferons Produced by Human Placental Trophoblasts Confer Protection against Zika Virus Infection. Cell Host Microbe 19, 705–712, doi: 10.1016/j.chom.2016.03.008 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chen J et al. Outcomes of Congenital Zika Disease Depend on Timing of Infection and Maternal-Fetal Interferon Action. Cell Rep 21, 1588–1599, doi: 10.1016/j.celrep.2017.10.059 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jagger BW et al. Gestational Stage and IFN-lambda Signaling Regulate ZIKV Infection In Utero. Cell Host Microbe 22, 366–376 e363, doi: 10.1016/j.chom.2017.08.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Gorman MJ, Poddar S, Farzan M & Diamond MS The Interferon-Stimulated Gene Ifitm3 Restricts West Nile Virus Infection and Pathogenesis. J Virol 90, 8212–8225, doi: 10.1128/JVI.00581-16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lucas TM, Richner JM & Diamond MS The Interferon-Stimulated Gene Ifi2712a Restricts West Nile Virus Infection and Pathogenesis in a Cell-Type- and Region-Specific Manner. J Virol 90, 2600–2615, doi: 10.1128/JVI.02463-15 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Schoggins JW Interferon-stimulated genes: roles in viral pathogenesis. Curr Opin Virol 6, 40–46, doi: 10.1016/j.coviro.2014.03.006 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li C et al. 25-Hydroxycholesterol Protects Host against Zika Virus Infection and Its Associated Microcephaly in a Mouse Model. Immunity 46, 446–456, doi: 10.1016/j.immuni.2017.02.012 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Diamond MS & Farzan M The broad-spectrum antiviral functions of IFIT and IFITM proteins. Nat Rev Immunol 13, 46–57, doi: 10.1038/nri3344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Slon Campos JL, Mongkolsapaya J & Screaton GR The immune response against flaviviruses. Nature immunology 19, 1189–1198, doi: 10.1038/s41590-018-0210-3 (2018). [DOI] [PubMed] [Google Scholar]
- 102.Fernandez E et al. Mouse and Human Monoclonal Antibodies Protect against Infection by Multiple Genotypes of Japanese Encephalitis Virus. MBio 9, doi: 10.1128/mBio.00008-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Williams KL et al. Therapeutic efficacy of antibodies lacking Fcgamma receptor binding against lethal dengue virus infection is due to neutralizing potency and blocking of enhancing antibodies [corrected]. PLoS Pathog 9, e1003157, doi: 10.1371/journal.ppat.1003157 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Vogt MR et al. Poorly neutralizing cross-reactive antibodies against the fusion loop of West Nile virus envelope protein protect in vivo via Fcgamma receptor and complement-dependent effector mechanisms. J Virol 85, 11567–11580, doi: 10.1128/jvi.05859-11 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bournazos S, DiLillo DJ & Ravetch JV The role of Fc-FcgammaR interactions in IgG-mediated microbial neutralization. J Exp Med 212, 1361–1369, doi: 10.1084/jem.20151267 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Muller DA & Young PR The flavivirus NS1 protein: molecular and structural biology, immunology, role in pathogenesis and application as a diagnostic biomarker. Antiviral Res 98, 192–208, doi: 10.1016/j.antiviral.2013.03.008 (2013). [DOI] [PubMed] [Google Scholar]
- 107.Reyes-Sandoval A & Ludert JE The Dual Role of the Antibody Response Against the Flavivirus Non-structural Protein 1 (NS1) in Protection and Immuno-Pathogenesis. Front Immunol 10, 1651, doi: 10.3389/fimmu.2019.01651 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Crill WD & Roehrig JT Monoclonal antibodies that bind to domain III of dengue virus E glycoprotein are the most efficient blockers of virus adsorption to Vero cells. J Virol 75, 7769–7773, doi: 10.1128/JVI.75.16.7769-7773.2001 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pierson TC, Fremont DH, Kuhn RJ & Diamond MS Structural insights into the mechanisms of antibody-mediated neutralization of flavivirus infection: implications for vaccine development. Cell Host Microbe 4, 229–238, doi: 10.1016/j.chom.2008.08.004 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rey FA, Stiasny K, Vaney MC, Dellarole M & Heinz FX The bright and the dark side of human antibody responses to flaviviruses: lessons for vaccine design. EMBO Rep 19, 206–224, doi: 10.15252/embr.201745302 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Katzelnick LC et al. Antibody-dependent enhancement of severe dengue disease in humans. Science 358, 929–932, doi: 10.1126/science.aan6836 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Kliks SC, Nisalak A, Brandt WE, Wahl L & Burke DS Antibody-dependent enhancement of dengue virus growth in human monocytes as a risk factor for dengue hemorrhagic fever. Am J Trop Med Hyg 40, 444–451 (1989). [DOI] [PubMed] [Google Scholar]
- 113.Netland J & Bevan MJ CD8 and CD4 T cells in west nile virus immunity and pathogenesis. Viruses 5, 2573–2584, doi: 10.3390/v5102573 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Weiskopf D & Sette A T-cell immunity to infection with dengue virus in humans. Front Immunol 5, 93, doi: 10.3389/fimmu.2014.00093 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Aberle JH, Koblischke M & Stiasny K CD4 T cell responses to flaviviruses. J Clin Virol 108, 126–131, doi: 10.1016/j.jcv.2018.09.020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Yauch LE et al. CD4+ T cells are not required for the induction of dengue virus-specific CD8+ T cell or antibody responses but contribute to protection after vaccination. J Immunol 185, 5405–5416, doi: 10.4049/jimmunol.1001709 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kumar P et al. Impaired T helper 1 function of nonstructural protein 3-specific T cells in Japanese patients with encephalitis with neurological sequelae. J Infect Dis 189, 880–891, doi: 10.1086/381768 (2004). [DOI] [PubMed] [Google Scholar]
- 118.Weiskopf D et al. Dengue virus infection elicits highly polarized CX3CR1+ cytotoxic CD4+ T cells associated with protective immunity. Proc Natl Acad Sci U S A 112, E4256–4263, doi: 10.1073/pnas.1505956112 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Grifoni A et al. Prior Dengue Virus Exposure Shapes T Cell Immunity to Zika Virus in Humans. J Virol 91, doi: 10.1128/JVI.01469-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Beaumier CM & Rothman AL Cross-reactive memory CD4+ T cells alter the CD8+ T-cell response to heterologous secondary dengue virus infections in mice in a sequence-specific manner. Viral Immunol 22, 215–219, doi: 10.1089/vim.2008.0089 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Elong Ngono A et al. Mapping and Role of the CD8(+) T Cell Response During Primary Zika Virus Infection in Mice. Cell Host Microbe 21, 35–46, doi: 10.1016/j.chom.2016.12.010 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Brien JD, Uhrlaub JL & Nikolich-Zugich J Protective capacity and epitope specificity of CD8(+) T cells responding to lethal West Nile virus infection. Eur J Immunol 37, 1855–1863, doi: 10.1002/eji.200737196 (2007). [DOI] [PubMed] [Google Scholar]
- 123.Yauch LE et al. A protective role for dengue virus-specific CD8+ T cells. J Immunol 182, 4865–4873, doi: 10.4049/jimmunol.0801974 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Shrestha B & Diamond MS Fas ligand interactions contribute to CD8+ T-cell-mediated control of West Nile virus infection in the central nervous system. J Virol 81, 11749–11757, doi: 10.1128/JVI.01136-07 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Shrestha B, Samuel MA & Diamond MS CD8+ T cells require perforin to clear West Nile virus from infected neurons. J Virol 80, 119–129, doi: 10.1128/JVI.80.1.119-129.2006 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wen J et al. Identification of Zika virus epitopes reveals immunodominant and protective roles for dengue virus cross-reactive CD8(+) T cells. Nat Microbiol 2, 17036, doi: 10.1038/nmicrobiol.2017.36 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Regla-Nava JA et al. Cross-reactive Dengue virus-specific CD8(+) T cells protect against Zika virus during pregnancy. Nat Commun 9, 3042, doi: 10.1038/s41467-018-05458-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Huang H et al. CD8(+) T Cell Immune Response in Immunocompetent Mice during Zika Virus Infection. J Virol 91, doi: 10.1128/JVI.00900-17 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jurado KA et al. Antiviral CD8 T cells induce Zika-virus-associated paralysis in mice. Nat Microbiol 3, 141–147, doi: 10.1038/s41564-017-0060-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Ruzek D et al. CD8+ T-cells mediate immunopathology in tick-borne encephalitis. Virology 384, 1–6, doi: 10.1016/j.virol.2008.11.023 (2009). [DOI] [PubMed] [Google Scholar]
- 131.Mongkolsapaya J et al. Original antigenic sin and apoptosis in the pathogenesis of dengue hemorrhagic fever. Nat Med 9, 921–927, doi: 10.1038/nm887 (2003). [DOI] [PubMed] [Google Scholar]
- 132.Mongkolsapaya J et al. T cell responses in dengue hemorrhagic fever: are cross-reactive T cells suboptimal? J Immunol 176, 3821–3829, doi: 10.4049/jimmunol.176.6.3821 (2006). [DOI] [PubMed] [Google Scholar]
- 133.Bashyam HS, Green S & Rothman AL Dengue virus-reactive CD8+ T cells display quantitative and qualitative differences in their response to variant epitopes of heterologous viral serotypes. J Immunol 176, 2817–2824, doi: 10.4049/jimmunol.176.5.2817 (2006). [DOI] [PubMed] [Google Scholar]
- 134.Mathew A & Rothman AL Understanding the contribution of cellular immunity to dengue disease pathogenesis. Immunol Rev 225, 300–313, doi: 10.1111/j.1600-065X.2008.00678.x (2008). [DOI] [PubMed] [Google Scholar]
- 135.Weiskopf D et al. Comprehensive analysis of dengue virus-specific responses supports an HLA-linked protective role for CD8+ T cells. Proc Natl Acad Sci U S A 110, E2046–2053, doi: 10.1073/pnas.1305227110 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Anez G, Heisey DA, Espina LM, Stramer SL & Rios M Phylogenetic analysis of dengue virus types 1 and 4 circulating in Puerto Rico and Key West, Florida, during 2010 epidemics. Am J Trop Med Hyg 87, 548–553, doi: 10.4269/ajtmh.2012.12-0091 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Dengue hemorrhagic fever--U.S.-Mexico border, 2005. MMWR. Morbidity and mortality weekly report 56, 785–789 (2007). [PubMed] [Google Scholar]
- 138.Halstead SB, Nimmannitya S & Cohen SN Observations related to pathogenesis of dengue hemorrhagic fever. IV. Relation of disease severity to antibody response and virus recovered. Yale J Biol Med 42, 311–328 (1970). [PMC free article] [PubMed] [Google Scholar]
- 139.Burke DS, Nisalak A, Johnson DE & Scott RM A prospective study of dengue infections in Bangkok. Am J Trop Med Hyg 38, 172–180 (1988). [DOI] [PubMed] [Google Scholar]
- 140.Halstead SB Dengue. Lancet 370, 1644–1652, doi:S0140-6736(07)61687-0 [pii] 10.1016/S0140-6736(07)61687-0 (2007). [DOI] [PubMed] [Google Scholar]
- 141.Ngo NT et al. Acute management of dengue shock syndrome: a randomized double-blind comparison of 4 intravenous fluid regimens in the first hour. Clinical infectious diseases : an official publication of the Infectious Diseases Society of America 32, 204–213, doi: 10.1086/318479 (2001). [DOI] [PubMed] [Google Scholar]
- 142.Graham RR et al. A prospective seroepidemiologic study on dengue in children four to nine years of age in Yogyakarta, Indonesia I. studies in 1995-1996. Am J Trop Med Hyg 61, 412–419 (1999). [DOI] [PubMed] [Google Scholar]
- 143.Simmons CP et al. Maternal antibody and viral factors in the pathogenesis of dengue virus in infants. J Infect Dis 196, 416–424 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hammond SN et al. Differences in dengue severity in infants, children, and adults in a 3-year hospital-based study in Nicaragua. Am J Trop Med Hyg 73, 1063–1070, doi:73/6/1063 [pii] (2005). [PubMed] [Google Scholar]
- 145.Kalayanarooj S & Nimmannitya S Clinical presentations of dengue hemorrhagic fever in infants compared to children. J Med Assoc Thai 86 Suppl 3, S673–680 (2003). [PubMed] [Google Scholar]
- 146.Smithburn KC, Hughes TP, Burke AW & Paul JH A neurotropic virus isolated from the blood of a native of Uganda. Am J Trop Med Hyg 20, 471–492 (1940). [Google Scholar]
- 147.Higgs S, Schneider BS, Vanlandingham DL, Klingler KA & Gould EA Nonviremic transmission of West Nile virus. Proc Natl Acad Sci U S A 102, 8871–8874 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hubalek Z & Halouzka J West Nile fever - a reemerging mosquito-borne viral disease in Europe. Emerg Inf Dis 5, 643–650 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Petersen LR et al. Estimated cumulative incidence of West Nile virus infection in US adults, 1999–2010. Epidemiology and infection 141, 591–595, doi: 10.1017/s0950268812001070 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Erdelyi K et al. Clinical and pathologic features of lineage 2 West Nile virus infections in birds of prey in Hungary. Vector Borne Zoonotic Dis 7, 181–188, doi: 10.1089/vbz.2006.0586 (2007). [DOI] [PubMed] [Google Scholar]
- 151.Veo C et al. Evolutionary Dynamics of the Lineage 2 West Nile Virus That Caused the Largest European Epidemic: Italy 2011–2018. Viruses 11, doi: 10.3390/v11090814 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Phipps P, Johnson N, McElhinney LM & Roberts H West Nile virus season in Europe. The Veterinary record 183, 224, doi: 10.1136/vr.k3497 (2018). [DOI] [PubMed] [Google Scholar]
- 153.Brault AC et al. A single positively selected West Nile viral mutation confers increased virogenesis in American crows. Nature genetics 39, 1162–1166, doi: 10.1038/ng2097 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Tsai TF New initiatives for the control of Japanese encephalitis by vaccination: minutes of a WHO/CVI meeting, Bangkok, Thailand, 13–15 October 1998. Vaccine 18 Suppl 2, 1–25, doi:S0264410X00000372 [pii] (2000). [DOI] [PubMed] [Google Scholar]
- 155.Solomon T Flavivirus encephalitis. N Engl J Med 351, 370–378, doi: 10.1056/NEJMra030476 351/4/370[pii] (2004). [DOI] [PubMed] [Google Scholar]
- 156.Ooi MH et al. The epidemiology, clinical features, and long-term prognosis of Japanese encephalitis in central sarawak, malaysia, 1997–2005. Clin Infect Dis 47, 458–468, doi: 10.1086/590008 (2008). [DOI] [PubMed] [Google Scholar]
- 157.Halstead SB & Thomas SJ New Japanese encephalitis vaccines: alternatives to production in mouse brain. Expert Rev Vaccines 10, 355–364, doi: 10.1586/erv.11.7 (2011). [DOI] [PubMed] [Google Scholar]
- 158.Hanna JN et al. Japanese encephalitis in north Queensland, Australia, 1998. Med J Aust 170, 533–536 (1999). [DOI] [PubMed] [Google Scholar]
- 159.Simon-Loriere E et al. Autochthonous Japanese Encephalitis with Yellow Fever Coinfection in Africa. N Engl J Med 376, 1483–1485, doi: 10.1056/NEJMc1701600 (2017). [DOI] [PubMed] [Google Scholar]
- 160.Mohammed MA et al. Molecular phylogenetic and evolutionary analyses of Muar strain of Japanese encephalitis virus reveal it is the missing fifth genotype. Infect Genet Evol 11, 855–862, doi:S1567-1348(11)00023-2 [pii] 10.1016/j.meegid.2011.01.020 (2011). [DOI] [PubMed] [Google Scholar]
- 161.Kim H et al. Detection of Japanese encephalitis virus genotype V in Culex orientalis and Culex pipiens (Diptera: Culicidae) in Korea. PLoS One 10, e0116547, doi: 10.1371/journal.pone.0116547 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Li MH et al. Genotype v Japanese encephalitis virus is emerging. PLoS neglected tropical diseases 5, e1231, doi: 10.1371/journal.pntd.0001231 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Connor B & Bunn WB The changing epidemiology of Japanese encephalitis and New data: the implications for New recommendations for Japanese encephalitis vaccine. Tropical diseases, travel medicine and vaccines 3, 14, doi: 10.1186/s40794-017-0057-x (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Huang YJ et al. Susceptibility of a North American Culex quinquefasciatus to Japanese Encephalitis Virus. Vector Borne Zoonotic Dis 15, 709–711, doi: 10.1089/vbz.2015.1821 (2015). [DOI] [PubMed] [Google Scholar]
- 165.Johansson MA, Vasconcelos PF & Staples JE The whole iceberg: estimating the incidence of yellow fever virus infection from the number of severe cases. Trans R Soc Trop Med Hyg 108, 482–487, doi: 10.1093/trstmh/tru092 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Tuboi SH, Costa ZG, da Costa Vasconcelos PF & Hatch D Clinical and epidemiological characteristics of yellow fever in Brazil: analysis of reported cases 1998–2002. Trans R Soc Trop Med Hyg 101, 169–175, doi: 10.1016/j.trstmh.2006.04.001 (2007). [DOI] [PubMed] [Google Scholar]
- 167.Bryant JE, Holmes EC & Barrett AD Out of Africa: a molecular perspective on the introduction of yellow fever virus into the Americas. PLoS Pathog 3, e75, doi: 10.1371/journal.ppat.0030075 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Barrett AD & Higgs S Yellow fever: a disease that has yet to be conquered. Annu Rev Entomol 52, 209–229, doi: 10.1146/annurev.ento.52.110405.091454 (2007). [DOI] [PubMed] [Google Scholar]
- 169.Garske T et al. Yellow Fever in Africa: estimating the burden of disease and impact of mass vaccination from outbreak and serological data. PLoS Med 11, e1001638, doi: 10.1371/journal.pmed.1001638 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hamlet A et al. The seasonal influence of climate and environment on yellow fever transmission across Africa. PLoS Negl Trop Dis 12, e0006284, doi: 10.1371/journal.pntd.0006284 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hamer DH et al. Fatal Yellow Fever in Travelers to Brazil, 2018. MMWR Morb Mortal Wkly Rep 67, 340–341, doi: 10.15585/mmwr.mm6711e1 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Rezende IM et al. Persistence of Yellow fever virus outside the Amazon Basin, causing epidemics in Southeast Brazil, from 2016 to 2018. PLoS Negl Trop Dis 12, e0006538, doi: 10.1371/journal.pntd.0006538 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Metsky HC et al. Zika virus evolution and spread in the Americas. Nature 546, 411–415, doi: 10.1038/nature22402 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Musso D et al. Zika virus in French Polynesia 2013–14: anatomy of a completed outbreak. Lancet Infect Dis 18, e172–e182, doi: 10.1016/S1473-3099(17)30446-2 (2018). [DOI] [PubMed] [Google Scholar]
- 175.Mlakar J et al. Zika Virus Associated with Microcephaly. N Engl J Med 374, 951–958, doi: 10.1056/NEJMoa1600651 (2016). [DOI] [PubMed] [Google Scholar]
- 176.Ali S et al. Environmental and Social Change Drive the Explosive Emergence of Zika Virus in the Americas. PLoS Negl Trop Dis 11, e0005135, doi: 10.1371/journal.pntd.0005135 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Liu Y et al. Evolutionary enhancement of Zika virus infectivity in Aedes aegypti mosquitoes. Nature 545, 482–486, doi: 10.1038/nature22365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Faria NR et al. Zika virus in the Americas: Early epidemiological and genetic findings. Science 352, 345–349, doi: 10.1126/science.aaf5036 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Yuan L et al. A single mutation in the prM protein of Zika virus contributes to fetal microcephaly. Science 358, 933–936, doi: 10.1126/science.aam7120 (2017). [DOI] [PubMed] [Google Scholar]
- 180.Watanabe S, Tan NWW, Chan KWK & Vasudevan SG Dengue Virus and Zika Virus Serological Cross-reactivity and Their Impact on Pathogenesis in Mice. J Infect Dis 219, 223–233, doi: 10.1093/infdis/jiy482 (2019). [DOI] [PubMed] [Google Scholar]
- 181.Klase ZA et al. Zika Fetal Neuropathogenesis: Etiology of a Viral Syndrome. PLoS Negl Trop Dis 10, e0004877, doi: 10.1371/journal.pntd.0004877 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Chavali PL et al. Neurodevelopmental protein Musashi-1 interacts with the Zika genome and promotes viral replication. Science 357, 83–88, doi: 10.1126/science.aam9243 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Xia H et al. An evolutionary NS1 mutation enhances Zika virus evasion of host interferon induction. Nat Commun 9, 414, doi: 10.1038/s41467-017-02816-2 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Dejnirattisai W et al. Dengue virus sero-cross-reactivity drives antibody-dependent enhancement of infection with zika virus. Nat Immunol 17, 1102–1108, doi: 10.1038/ni.3515 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Li M et al. Dengue immune sera enhance Zika virus infection in human peripheral blood monocytes through Fc gamma receptors. PLoS One 13, e0200478, doi: 10.1371/journal.pone.0200478 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Halstead SB Biologic Evidence Required for Zika Disease Enhancement by Dengue Antibodies. Emerg Infect Dis 23, 569–573, doi: 10.3201/eid2304.161879 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Fernandez E et al. Human antibodies to the dengue virus E-dimer epitope have therapeutic activity against Zika virus infection. Nat Immunol 18, 1261–1269, doi: 10.1038/ni.3849 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Abbink P et al. Therapeutic and protective efficacy of a dengue antibody against Zika infection in rhesus monkeys. Nat Med 24, 721–723, doi: 10.1038/s41591-018-0056-0 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Bardina SV et al. Enhancement of Zika virus pathogenesis by preexisting antiflavivirus immunity. Science 356, 175–180, doi: 10.1126/science.aal4365 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Duehr J et al. Tick-Borne Encephalitis Virus Vaccine-Induced Human Antibodies Mediate Negligible Enhancement of Zika Virus Infection InVitro and in a Mouse Model. mSphere 3, doi: 10.1128/mSphereDirect.00011-18 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wen J et al. Dengue virus-reactive CD8(+) T cells mediate cross-protection against subsequent Zika virus challenge. Nat Commun 8, 1459, doi: 10.1038/s41467-017-01669-z (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.McCracken MK et al. Impact of prior flavivirus immunity on Zika virus infection in rhesus macaques. PLoS Pathog 13, e1006487, doi: 10.1371/journal.ppat.1006487 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pantoja P et al. Zika virus pathogenesis in rhesus macaques is unaffected by pre-existing immunity to dengue virus. Nat Commun 8, 15674, doi: 10.1038/ncomms15674 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Breitbach ME et al. Primary infection with dengue or Zika virus does not affect the severity of heterologous secondary infection in macaques. PLoS Pathog 15, e1007766, doi: 10.1371/journal.ppat.1007766 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.George J et al. Prior Exposure to Zika Virus Significantly Enhances Peak Dengue-2 Viremia in Rhesus Macaques. Sci Rep 7, 10498, doi: 10.1038/s41598-017-10901-1 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Valiant WG et al. Zika convalescent macaques display delayed induction of anamnestic cross-neutralizing antibody responses after dengue infection. Emerg Microbes Infect 7, 130, doi: 10.1038/s41426-018-0132-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Terzian ACB et al. Viral Load and Cytokine Response Profile Does Not Support Antibody-Dependent Enhancement in Dengue-Primed Zika Virus-Infected Patients. Clin Infect Dis 65, 1260–1265, doi: 10.1093/cid/cix558 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Halai UA et al. Maternal Zika Virus Disease Severity, Virus Load, Prior Dengue Antibodies, and Their Relationship to Birth Outcomes. Clin Infect Dis 65, 877–883, doi: 10.1093/cid/cix472 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Draper CC Infection with the Chuku Strain of Spondweni Virus. West Afr Med J 14, 16–19 (1965). [PubMed] [Google Scholar]
- 200.Kokernot RH, Smithburn KC, Muspratt J & Hodgson B Studies on arthropod-borne viruses of Tongaland. VIII. Spondweni virus, an agent previously unknown, isolated from Taeniorhynchus (Mansonioides) uniformis. S Afr J Med Sci 22, 103–112 (1957). [PubMed] [Google Scholar]
- 201.Haddow AD & Woodall JP Distinguishing between Zika and Spondweni viruses. Bull World Health Organ 94, 711–711A, doi: 10.2471/BLT.16.181503 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Haddow AD et al. Genetic Characterization of Spondweni and Zika Viruses and Susceptibility of Geographically Distinct Strains of Aedes aegypti, Aedes albopictus and Culex quinquefasciatus (Diptera: Culicidae) to Spondweni Virus. PLoS Negl Trop Dis 10, e0005083, doi: 10.1371/journal.pntd.0005083 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.White SK, Lednicky JA, Okech BA, Morris JG Jr. & Dunford JC Spondweni Virus in Field-Caught Culex quinquefasciatus Mosquitoes, Haiti, 2016. Emerg Infect Dis 24, 1765–1767, doi: 10.3201/eid2409.171957 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.McDonald EM, Duggal NK & Brault AC Pathogenesis and sexual transmission of Spondweni and Zika viruses. PLoS Negl Trop Dis 11, e0005990, doi: 10.1371/journal.pntd.0005990 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Engel D et al. Reconstruction of the Evolutionary History and Dispersal of Usutu Virus, a Neglected Emerging Arbovirus in Europe and Africa. MBio 7, e01938–01915, doi: 10.1128/mBio.01938-15 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Barzon L Ongoing and emerging arbovirus threats in Europe. J Clin Virol 107, 38–47, doi: 10.1016/j.jcv.2018.08.007 (2018). [DOI] [PubMed] [Google Scholar]
- 207.Weissenbock H et al. Emergence of Usutu virus, an African mosquito-borne flavivirus of the Japanese encephalitis virus group, central Europe. Emerg Infect Dis 8, 652–656, doi: 10.3201/eid0807.020094 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Cadar D et al. Widespread activity of multiple lineages of Usutu virus, western Europe, 2016. Euro Surveill 22, doi: 10.2807/1560-7917.ES.2017.22.4.30452 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Pierro A et al. Detection of specific antibodies against West Nile and Usutu viruses in healthy blood donors in northern Italy, 2010–2011. Clin Microbiol Infect 19, E451–453, doi: 10.1111/1469-0691.12241 (2013). [DOI] [PubMed] [Google Scholar]
- 210.Gaibani P & Rossini G An overview of Usutu virus. Microbes Infect 19, 382–387, doi: 10.1016/j.micinf.2017.05.003 (2017). [DOI] [PubMed] [Google Scholar]
- 211.Pauvolid-Correa A et al. Ilheus virus isolation in the Pantanal, west-central Brazil. PLoS Negl Trop Dis 7, e2318, doi: 10.1371/journal.pntd.0002318 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Weyer J et al. Human cases of Wesselsbron disease, South Africa 2010–2011. Vector Borne Zoonotic Dis 13, 330–336, doi: 10.1089/vbz.2012.1181 (2013). [DOI] [PubMed] [Google Scholar]
- 213.Pauvolid-Correa A et al. Neutralising antibodies for West Nile virus in horses from Brazilian Pantanal. Mem Inst Oswaldo Cruz 106, 467–474, doi: 10.1590/s0074-02762011000400014 (2011). [DOI] [PubMed] [Google Scholar]
- 214.Vieira C et al. Detection of Ilheus virus in mosquitoes from southeast Amazon, Brazil. Trans R Soc Trop Med Hyg 113, 424–427, doi: 10.1093/trstmh/trz031 (2019). [DOI] [PubMed] [Google Scholar]
- 215.de Souza Lopes O, Coimbra TL, de Abreu Sacchetta L & Calisher CH Emergence of a new arbovirus disease in Brazil. I. Isolation and characterization of the etiologic agent, Rocio virus. American journal of epidemiology 107, 444–449, doi: 10.1093/oxfordjournals.aje.a112563 (1978). [DOI] [PubMed] [Google Scholar]
- 216.Medeiros DB, Nunes MR, Vasconcelos PF, Chang GJ & Kuno G Complete genome characterization of Rocio virus (Flavivirus: Flaviviridae), a Brazilian flavivirus isolated from a fatal case of encephalitis during an epidemic in Sao Paulo state. The Journal of general virology 88, 2237–2246, doi: 10.1099/vir.0.82883-0 (2007). [DOI] [PubMed] [Google Scholar]
- 217.Mitchell CJ, Monath TP & Cropp CB Experimental transmission of Rocio virus by mosquitoes. Am J Trop Med Hyg 30, 465–472, doi: 10.4269/ajtmh.1981.30.465 (1981). [DOI] [PubMed] [Google Scholar]
- 218.Monath TP, Kemp GE, Cropp CB & Bowen GS Experimental infection of house sparrows (Passer domesticus) with Rocio virus. Am J Trop Med Hyg 27, 1251–1254, doi: 10.4269/ajtmh.1978.27.1251 (1978). [DOI] [PubMed] [Google Scholar]
- 219.Pauvolid-Correa A et al. Serological evidence of widespread circulation of West Nile virus and other flaviviruses in equines of the Pantanal, Brazil. PLoS neglected tropical diseases 8, e2706, doi: 10.1371/journal.pntd.0002706 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Straatmann A et al. [Serological evidence of the circulation of the Rocio arbovirus (Flaviviridae) in Bahia]. Revista da Sociedade Brasileira de Medicina Tropical 30, 511–515, doi: 10.1590/s0037-86821997000600012 (1997). [DOI] [PubMed] [Google Scholar]
- 221.Diagne MM et al. Emergence of Wesselsbron virus among black rat and humans in Eastern Senegal in 2013. One Health 3, 23–28, doi: 10.1016/j.onehlt.2017.02.001 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Gritsun TS, Nuttall PA & Gould EA Tick-borne flaviviruses. Adv Virus Res 61, 317–371 (2003). [DOI] [PubMed] [Google Scholar]
- 223.Kemenesi G & Banyai K Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin Microbiol Rev 32, doi: 10.1128/CMR.00106-17 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Hermance ME & Thangamani S Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America. Vector Borne Zoonotic Dis 17, 453–462, doi: 10.1089/vbz.2017.2110 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ebel GD, Spielman A & Telford SR 3rd. Phylogeny of North American Powassan virus. J Gen Virol 82, 1657–1665, doi: 10.1099/0022-1317-82-7-1657 (2001). [DOI] [PubMed] [Google Scholar]
- 226.Dupuis AP 2nd et al. Isolation of deer tick virus (Powassan virus, lineage II) from Ixodes scapularis and detection of antibody in vertebrate hosts sampled in the Hudson Valley, New York State. Parasit Vectors 6, 185, doi: 10.1186/1756-3305-6-185 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Montgomery RR & Murray KO Risk factors for West Nile virus infection and disease in populations and individuals. Expert Rev Anti Infect Ther 13, 317–325, doi: 10.1586/14787210.2015.1007043 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Krow-Lucal ER, Lindsey NP, Fischer M & Hills SL Powassan Virus Disease in the United States, 2006–2016. Vector Borne Zoonotic Dis 18, 286–290, doi: 10.1089/vbz.2017.2239 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Aliota MT et al. The prevalence of zoonotic tick-borne pathogens in Ixodes scapularis collected in the Hudson Valley, New York State. Vector Borne Zoonotic Dis 14, 245–250, doi: 10.1089/vbz.2013.1475 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Knox KK et al. Powassan/Deer Tick Virus and Borrelia Burgdorferi Infection in Wisconsin Tick Populations. Vector Borne Zoonotic Dis 17, 463–466, doi: 10.1089/vbz.2016.2082 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Eisen RJ & Eisen L The Blacklegged Tick, Ixodes scapularis: An Increasing Public Health Concern. Trends Parasitol 34, 295–309, doi: 10.1016/j.pt.2017.12.006 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.VanBlargan LA et al. An mRNA Vaccine Protects Mice against Multiple Tick-Transmitted Flavivirus Infections. Cell Rep 25, 3382–3392 e3383, doi: 10.1016/j.celrep.2018.11.082 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Gardner CL & Ryman KD Yellow fever: a reemerging threat. Clin Lab Med 30, 237–260, doi:S0272-2712(10)00002-8 [pii] 10.1016/j.cll.2010.01.001 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Halstead SB & Jacobson J in Vaccines (eds Plotkin SA, Orenstein WA, & Offit PA) 311–352 (Saunders Elsevier, 2008). [Google Scholar]
- 235.Huang CY et al. Chimeric dengue type 2 (vaccine strain PDK-53)/dengue type 1 virus as a potential candidate dengue type 1 virus vaccine. Journal of virology 74, 3020–3028 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Huang CY et al. Dengue 2 PDK-53 virus as a chimeric carrier for tetravalent dengue vaccine development. Journal of virology 77, 11436–11447 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Guy B et al. Preclinical and clinical development of YFV 17D-based chimeric vaccines against dengue, West Nile and Japanese encephalitis viruses. Vaccine 28, 632–649, doi:S0264-410X(09)01445-5 [pii] 10.1016/j.vaccine.2009.09.098 (2010). [DOI] [PubMed] [Google Scholar]
- 238.Guirakhoo F et al. Construction, safety, and immunogenicity in nonhuman primates of a chimeric yellow fever-dengue virus tetravalent vaccine. J Virol 75, 7290–7304 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Whitehead SS Development of TV003/TV005, a single dose, highly immunogenic live attenuated dengue vaccine; what makes this vaccine different from the Sanofi-Pasteur CYD vaccine? Expert Rev Vaccines 15, 509–517, doi: 10.1586/14760584.2016.1115727 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Appaiahgari MB & Vrati S IMOJEV((R)): a Yellow fever virus-based novel Japanese encephalitis vaccine. Expert Rev Vaccines 9, 1371–1384, doi: 10.1586/erv.10.139 (2010). [DOI] [PubMed] [Google Scholar]
- 241.Zust R et al. Rational design of a live attenuated dengue vaccine: 2′-o-methyltransferase mutants are highly attenuated and immunogenic in mice and macaques. PLoS Pathog 9, e1003521, doi: 10.1371/journal.ppat.1003521 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Richner JM et al. Vaccine Mediated Protection Against Zika Virus-Induced Congenital Disease. Cell 170, 273–283.e212, doi: 10.1016/j.cell.2017.06.040 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Fischer M, Lindsey N, Staples JE & Hills S Japanese encephalitis vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 59, 1–27, doi:rr5901a1 [pii] (2010). [PubMed] [Google Scholar]
- 244.Rendi-Wagner P Advances in vaccination against tick-borne encephalitis. Expert Rev Vaccines 7, 589–596, doi: 10.1586/14760584.7.5.589 (2008). [DOI] [PubMed] [Google Scholar]
- 245.Kasabi GS et al. Coverage and effectiveness of Kyasanur forest disease (KFD) vaccine in Karnataka, South India, 2005–10. PLoS neglected tropical diseases 7, e2025, doi: 10.1371/journal.pntd.0002025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Hadinegoro SR et al. Efficacy and Long-Term Safety of a Dengue Vaccine in Regions of Endemic Disease. N Engl J Med 373, 1195–1206, doi: 10.1056/NEJMoa1506223 (2015). [DOI] [PubMed] [Google Scholar]
- 247.Addendum to report of the Global Advisory Committee on Vaccine Safety (GACVS), 10–11 June 2015(1). Safety of CYD-TDV dengue vaccine. Wkly Epidemiol Rec 90, 421–423 (2015). [PubMed] [Google Scholar]
- 248.Sridhar S et al. Effect of Dengue Serostatus on Dengue Vaccine Safety and Efficacy. N Engl J Med 379, 327–340, doi: 10.1056/NEJMoa1800820 (2018). [DOI] [PubMed] [Google Scholar]
- 249.Halstead SB Dengvaxia sensitizes seronegatives to vaccine enhanced disease regardless of age. Vaccine 35, 6355–6358, doi: 10.1016/j.vaccine.2017.09.089 (2017). [DOI] [PubMed] [Google Scholar]
- 250.Biswal S et al. Efficacy of a Tetravalent Dengue Vaccine in Healthy Children and Adolescents. N Engl J Med 381, 2009–2019, doi: 10.1056/NEJMoa1903869 (2019). [DOI] [PubMed] [Google Scholar]
- 251.Whitehead SS et al. In a randomized trial, the live attenuated tetravalent dengue vaccine TV003 is well-tolerated and highly immunogenic in subjects with flavivirus exposure prior to vaccination. PLoS neglected tropical diseases 11, e0005584, doi: 10.1371/journal.pntd.0005584 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Collins MH et al. Lack of Durable Cross-Neutralizing Antibodies Against Zika Virus from Dengue Virus Infection. Emerg Infect Dis 23, 773–781, doi: 10.3201/eid2305.161630 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Montoya M et al. Longitudinal Analysis of Antibody Cross-neutralization Following Zika Virus and Dengue Virus Infection in Asia and the Americas. J Infect Dis 218, 536–545, doi: 10.1093/infdis/jiy164 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Balmaseda A et al. Antibody-based assay discriminates Zika virus infection from other flaviviruses. Proc Natl Acad Sci U S A 114, 8384–8389, doi: 10.1073/pnas.1704984114 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Lindsey NP et al. Ability To Serologically Confirm Recent Zika Virus Infection in Areas with Varying Past Incidence of Dengue Virus Infection in the United States and U.S. Territories in 2016. J Clin Microbiol 56, doi: 10.1128/JCM.01115-17 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Richner JM et al. Modified mRNA Vaccines Protect against Zika Virus Infection. Cell 168, 1114–1125 e1110, doi: 10.1016/j.cell.2017.02.017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Diamond MS, Ledgerwood JE & Pierson TC Zika Virus Vaccine Development: Progress in the Face of New Challenges. Annu Rev Med 70, 121–135, doi: 10.1146/annurev-med-040717-051127 (2019). [DOI] [PubMed] [Google Scholar]
- 258.Casey RM et al. Immunogenicity of Fractional-Dose Vaccine during a Yellow Fever Outbreak - Final Report. N Engl J Med 381, 444–454, doi: 10.1056/NEJMoa1710430 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Eyer L, Nencka R, de Clercq E, Seley-Radtke K & Ruzek D Nucleoside analogs as a rich source of antiviral agents active against arthropod-borne flaviviruses. Antivir Chem Chemother 26, 2040206618761299, doi: 10.1177/2040206618761299 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Niyomrattanakit P et al. Inhibition of dengue virus polymerase by blocking of the RNA tunnel. J Virol 84, 5678–5686, doi: 10.1128/JVI.02451-09 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Chen YL, Yokokawa F & Shi PY The search for nucleoside/nucleotide analog inhibitors of dengue virus. Antiviral Res 122, 12–19, doi: 10.1016/j.antiviral.2015.07.010 (2015). [DOI] [PubMed] [Google Scholar]
- 262.Warren TK et al. Protection against filovirus diseases by a novel broad-spectrum nucleoside analogue BCX4430. Nature 508, 402–405, doi: 10.1038/nature13027 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Julander JG et al. Efficacy of the broad-spectrum antiviral compound BCX4430 against Zika virus in cell culture and in a mouse model. Antiviral Res 137, 14–22, doi: 10.1016/j.antiviral.2016.11.003 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Bullard-Feibelman KM et al. The FDA-approved drug sofosbuvir inhibits Zika virus infection. Antiviral Res 137, 134–140, doi: 10.1016/j.antiviral.2016.11.023 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Dong H, Zhang B & Shi PY Flavivirus methyltransferase: a novel antiviral target. Antiviral Res 80, 1–10, doi: 10.1016/j.antiviral.2008.05.003 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Lim SP et al. Small molecule inhibitors that selectively block dengue virus methyltransferase. J Biol Chem 286, 6233–6240, doi: 10.1074/jbc.M110.179184 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Majerova T, Novotny P, Krysova E & Konvalinka J Exploiting the unique features of Zika and Dengue proteases for inhibitor design. Biochimie 166, 132–141, doi: 10.1016/j.biochi.2019.05.004 (2019). [DOI] [PubMed] [Google Scholar]
- 268.Nitsche C Strategies Towards Protease Inhibitors for Emerging Flaviviruses. Advances in experimental medicine and biology 1062, 175–186, doi: 10.1007/978-981-10-8727-1_13 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Li Z et al. Existing drugs as broad-spectrum and potent inhibitors for Zika virus by targeting NS2B-NS3 interaction. Cell Res 27, 1046–1064, doi: 10.1038/cr.2017.88 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Yuan S et al. Structure-based discovery of clinically approved drugs as Zika virus NS2B-NS3 protease inhibitors that potently inhibit Zika virus infection in vitro and in vivo. Antiviral Res 145, 33–43, doi: 10.1016/j.antiviral.2017.07.007 (2017). [DOI] [PubMed] [Google Scholar]
- 271.Luo D, Vasudevan SG & Lescar J The flavivirus NS2B-NS3 protease-helicase as a target for antiviral drug development. Antiviral Res 118, 148–158, doi: 10.1016/j.antiviral.2015.03.014 (2015). [DOI] [PubMed] [Google Scholar]
- 272.Modis Y, Ogata S, Clements D & Harrison SC A ligand-binding pocket in the dengue virus envelope glycoprotein. Proc Natl Acad Sci U S A 100, 6986–6991 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Poh MK et al. A small molecule fusion inhibitor of dengue virus. Antiviral Res 84, 260–266, doi: 10.1016/j.antiviral.2009.09.011 (2009). [DOI] [PubMed] [Google Scholar]
- 274.Schmidt AG, Lee K, Yang PL & Harrison SC Small-molecule inhibitors of dengue-virus entry. PLoS Pathog 8, e1002627, doi: 10.1371/journal.ppat.1002627 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Schmidt AG, Yang PL & Harrison SC Peptide inhibitors of flavivirus entry derived from the E protein stem. Journal of virology 84, 12549–12554, doi:JVI.01440-10 [pii] 10.1128/JVI.01440-10 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Schmidt AG, Yang PL & Harrison SC Peptide inhibitors of dengue-virus entry target a late-stage fusion intermediate. PLoS Pathog 6, e1000851, doi: 10.1371/journal.ppat.1000851 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Byrd CM et al. A novel inhibitor of dengue virus replication that targets the capsid protein. Antimicrob Agents Chemother 57, 15–25, doi: 10.1128/AAC.01429-12 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Scaturro P et al. Characterization of the mode of action of a potent dengue virus capsid inhibitor. J Virol 88, 11540–11555, doi: 10.1128/JVI.01745-14 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Smith JL et al. Characterization and structure-activity relationship analysis of a class of antiviral compounds that directly bind dengue virus capsid protein and are incorporated into virions. Antiviral Res 155, 12–19, doi: 10.1016/j.antiviral.2018.04.019 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Shaw WR & Catteruccia F Vector biology meets disease control: using basic research to fight vector-borne diseases. Nat Microbiol 4, 20–34, doi: 10.1038/s41564-018-0214-7 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Moreira LA et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, Chikungunya, and Plasmodium. Cell 139, 1268–1278, doi: 10.1016/j.cell.2009.11.042 (2009). [DOI] [PubMed] [Google Scholar]
- 282.Dutra HL et al. Wolbachia Blocks Currently Circulating Zika Virus Isolates in Brazilian Aedes aegypti Mosquitoes. Cell Host Microbe 19, 771–774, doi: 10.1016/j.chom.2016.04.021 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Carrington LB et al. Field- and clinically derived estimates of Wolbachia-mediated blocking of dengue virus transmission potential in Aedes aegypti mosquitoes. Proc Natl Acad Sci U S A 115, 361–366, doi: 10.1073/pnas.1715788115 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Thomas S, Verma J, Woolfit M & O’Neill SL Wolbachia-mediated virus blocking in mosquito cells is dependent on XRN1-mediated viral RNA degradation and influenced by viral replication rate. PLoS Pathog 14, e1006879, doi: 10.1371/journal.ppat.1006879 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Ferguson NM et al. Modeling the impact on virus transmission of Wolbachia-mediated blocking of dengue virus infection of Aedes aegypti. Sci Transl Med 7, 279ra237, doi: 10.1126/scitranslmed.3010370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Hoffmann AA et al. Stability of the wMel Wolbachia Infection following invasion into Aedes aegypti populations. PLoS Negl Trop Dis 8, e3115, doi: 10.1371/journal.pntd.0003115 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Anders KL et al. The AWED trial (Applying Wolbachia to Eliminate Dengue) to assess the efficacy of Wolbachia-infected mosquito deployments to reduce dengue incidence in Yogyakarta, Indonesia: study protocol for a cluster randomised controlled trial. Trials 19, 302, doi: 10.1186/s13063-018-2670-z (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Franz AW et al. Fitness impact and stability of a transgene conferring resistance to dengue-2 virus following introgression into a genetically diverse Aedes aegypti strain. PLoS Negl Trop Dis 8, e2833, doi: 10.1371/journal.pntd.0002833 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Buchman A et al. Engineered resistance to Zika virus in transgenic Aedes aegypti expressing a polycistronic cluster of synthetic small RNAs. Proc Natl Acad Sci U S A 116, 3656–3661, doi: 10.1073/pnas.1810771116 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Hadler JL et al. Assessment of Arbovirus Surveillance 13 Years after Introduction of West Nile Virus, United States. Emerg Infect Dis 21, 1159–1166, doi: 10.3201/eid2107.140858 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Kose N et al. A lipid-encapsulated mRNA encoding a potently neutralizing human monoclonal antibody protects against chikungunya infection. Sci Immunol 4, doi: 10.1126/sciimmunol.aaw6647 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


