Abstract
Histone deacetylases (HDACs) play important roles in transcriptional regulation in eukaryotic cells. Class I deacetylase HDAC1/2 often associates with repressor complexes, such as Sin3 (Switch Independent 3), NuRD (Nucleosome remodeling and deacetylase) and CoREST (Corepressor of RE1 silencing transcription factor) complexes. It has been shown that HDAC1 interacts with and modulates all essential transcription factors for erythropoiesis. During erythropoiesis, histone deacetylase activity is dramatically reduced. Consistently, inhibition of HDAC activity promotes erythroid differentiation. The reduction of HDAC activity not only results in the activation of transcription activators such as GATA-1 (GATA-binding factor 1), TAL1 (TAL BHLH Transcription Factor 1) and KLF1 (Krüpple-like factor 1), but also represses transcription repressors such as PU.1 (Putative oncogene Spi-1). The reduction of histone deacetylase activity is mainly through HDAC1 acetylation that attenuates HDAC1 activity and trans-repress HDAC2 activity through dimerization with HDAC1. Therefore, the acetylation of HDAC1 can convert the corepressor complex to an activator complex for gene activation. HDAC1 also can deacetylate non-histone proteins that play a role on erythropoiesis, therefore adds another layer of gene regulation through HDAC1. Clinically, it has been shown HDACi can reactivate fetal globin in adult erythroid cells. This review will cover the up to date research on the role of HDAC1 in modulating key transcription factors for erythropoiesis and its clinical relevance.
Keywords: erythropoiesis, histone deacetylase, HDAC1, GATA-1, PU.1, hemoglobin, reactivation of fetal globin, HDAC inhibitor
1. Introduction
Histone deacetylases (HDACs) catalyze the removal of acetyl groups from the ε-amino groups of lysine residues. The reversible acetylation of histones and non-histone proteins by histone acetyltransferases (HATs) and HDACs play critical roles in transcriptional regulation in eukaryotic cells. Histone acetylation is commonly associated with transcriptional activation of genes, and is thought to be responsible for the formation of a local “open chromatin” structure required for the binding of multiple transcription factors (Reviewed in [1]). In contrast, the removal of acetyl groups by histone deacetylases frequently accompanies the suppression of gene activity (Reviewed in [2]). However, non-histone protein lysine acetylation plays a diverse role in the regulation of all aspects of cellular processes that may result in transcription activation or repression [3,4,5,6].
Mammalian HDACs are classified into four classes (I, II, III, and IV) based on the sequence homology of the yeast histone deacetylases Rpd3 (reduced potassium dependency), Hda1 (histone deacetylase 1), and Sir2 (silent information regulator 2), respectively. Class I HDACs include HDAC1, 2, 3 and 8, class II HDACs contain HDACs 4, 5, 6, 7, 9 and 10. Class III enzymes, however, require the coenzyme NAD+ (Nicotinamide adenine dinucleotide) as a cofactor. HDAC11 belongs to the class IV family. Class I HDACs are ubiquitously expressed nuclear proteins. Although these enzymes share a high level of sequence homology and common substrates, and HDAC2 can partially compensate for HDAC1 loss, each of the enzymes has a unique role in cell function, as deletion of each member of the class I deacetylase leads to lethality (reviewed in [7]). HDAC1 knockout mice die before embryonic day 10.5 with severe growth defects [8,9]. The total deacetylase enzymatic activity is greatly reduced in HDAC1 null ES cells, indicating that HDAC1 is a major deacetylase in ES cells [10]. Conditional HDAC1/2 double knockout mice show significant hematopoietic defect, mainly in the erythroid/megakaryocyte compartment [11]. Hematopoietic deficiency has also been reported in clinical trials with various HDACi (HDAC inhibitors), including anemia, thrombocytopenia, neutropenia, and lymphopenia (reviewed in [12]), indicating that HDACi affects hematopoiesis in multiple lineages. Therefore, it is extremely important to understand how HDAC affects normal hematopoiesis.
2. HDAC1: Modifications and Associated Corepressor Complexes
2.1. Histone Deacetylase 1 (HDAC1) and Its Associated Complexes
HDAC1 is found in at least three evolutionally conserved, distinct protein complexes: the Sin3, the CoREST and the Mi-2/NuRD complexes (Reviewed in [2,13]). HDAC1 and HDAC2 are highly related enzymes with 82% overall sequence identity and often coexist in these complexes [14,15,16]. All complexes are recruited to target genes through interactions with DNA binding transcription factors. Sin3, originally identified as the transcription corepressor of Mad-Max (Max dimerization protein, Myc-associated factor X), requires HDAC1 and HDAC2 deacetylase activities for its full repression [17,18,19,20]. Sin3 complexes also contain RbAp46/48 (retinoblastoma associated protein-46/48) [18], SAP18 (Sin3A Associated Protein 18) and SAP30 (Sin3A Associated Protein 30) [21]. The Sin3 complex may also contain ING1 (Inhibitor of Growth Family Member 1), RBP1 (Retinoblastoma-binding protein 1), RBP-like protein SAP180 (Sin3A Associated Protein 180), SDS3 (Suppressor of defective silencing 3) and BRMS1 (Breast cancer metastasis suppressor 1) [22,23,24,25,26]. Sin3 has been identified in most eukaryotes; it contains the conserved basic structure of multiple PAH (Paired amphipathic helix) domains for protein-protein interaction. Sin3 does not bind to DNA and has no known enzymatic activity of its own. It may function, however, through its ability to interact with other proteins [27]. Mammals have two isoforms, Sin3A (SIN3 Transcription Regulator Family Member A) and Sin3B (SIN3 Transcription Regulator Family Member B), which provide more diverse protein complexes for gene regulation.
The CoREST complex is a multi-subunit complex containing the lysine demethylase LSD1 (Lysine-specific demethylase 1), corepressor CtBP (C-terminal binding protein), BHC80 (BRAF35-HDAC complex protein BHC80), CoREST, HDAC1 and HDAC2 [28,29,30,31,32]. LSD1 can demethylate mono- or di-methyl histone H3 lysine 4 or lysine 9 for gene repression or activation [33,34,35]. Thus, this complex is capable of deacetylating as well as demethylating nucleosomes. CoREST is required to tether HDAC1 to nucleosomes for nucleosomal deacetylation [35]. Interestingly, LSD1 can further enhance deacetylation on nucleosomes, suggesting that LSD1 demethylase activity is required for optimal deacetylase activity. In reverse, deacetylation of nucleosomes is required for LSD1 enzymatic activity [29], thus unveiling the functional cross-talk between the demethylase and deacetylase.
The NuRD complex includes the ATPase/helicase Mi-2, HDAC1/2, MTA (Metastasis-associated) proteins, MBD2 (methyl CpG-binding domain-2), and RbAp46/48 [36,37]. Mi-2 belongs to the CHD (chromo-helicase DNA-binding) protein family [38]. There are two isoforms of Mi-2, the Mi-2α(CHD3) and Mi-2β(CHD4), both of which are integral components of the NuRD complex. Further studies have shown that the NuRD complex is itself part of a larger protein complex of 10 subunits, the so-called MeCP1 (methyl CpG-binding protein 1) complex [39,40,41,42]. NuRD/MeCP1 is recruited to target genes through interactions with DNA binding transcription factors. Interestingly, a recent report suggests that LSD1 is also associated with the NuRD complex [43,44,45]. NuRD/MeCP1 is a repressor complex in most case. For example, the NuRD/MeCP1 complex is associated with gene repression during development [46]. However, interaction of GATA-3 with NuRD in differentiating Th2 (Type 2 helper T) cells was shown to activate transcription of the IL-4 (Interleukin 4) gene after displacing MBD2 from the chromatin [47]. NuRD is also required for GATA-1 mediated gene activation [48,49]. These observations suggest that Mi-2 containing NuRD or MeCP1 complexes can either repress or activate gene transcription.
In addition to these three complexes, HDAC1 also associates with other sub-complexes during development [13], including a variety of proteins, such as transcription factors, coactivators, chromatin remodeling proteins, etc., thus adding to the complexity of HDAC1 functions [13,50].
2.2. Regulation of HDAC1 Activity through Posttranslational Modification
HDAC1 activity is modulated through various post translational modifications including phosphorylation, acetylation, ubiquitination, sumoylation, and nitrosylation [51]. HDAC1 is phosphorylated at serine 421 and 423 by casein kinase II and dephosphorylated by mitogen-activated protein kinase phosphatase-3 (MKP-3) [52,53]. Mutagenesis analysis suggested that phosphorylation on these sites are important for histone deacetylase activity and for HDAC1 to interact with corepressor complexes [53]. However, treatment with phosphatases did not significantly affect HDAC1 histone deacetylase activity in vivo [52,53,54,55]. Therefore, it is suggested that phosphorylation may be required for initial folding and may be dispensable for subsequent functional events [56]. HDAC1 phosphorylation increases during the G1 phase and was significantly reduced during the late S/G2 phase [57]. It is unclear whether the increase of phosphorylation is linked to the increase of HDAC1 protein levels during the cell cycle [56]. There are also other minor sites that may be phosphorylated [55,58]. It remains to be elucidated whether phosphorylation on these sites affect HDAC1 function.
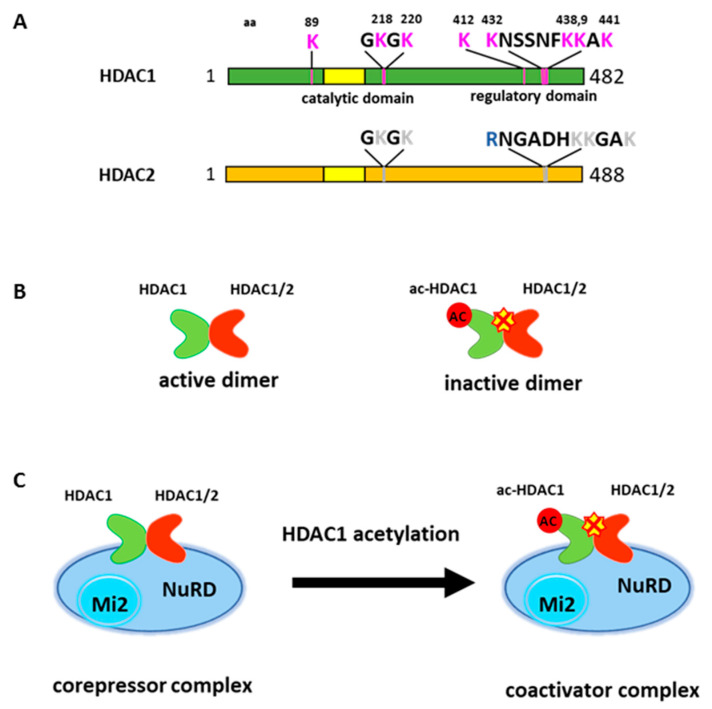
HDAC1 can also undergo acetylation modification. Qiu et al. (2006) initially reported HDAC1 acetylation at six lysine residues [6]. Two acetylated lysines (K218, 220) are located near the catalytic core domain and other four lysines (K432, 438, 439 and 441) are located at the C-terminal region of the protein (Figure 1A). The C-terminal domain does not have any catalytic activity. However, the deletion of this region greatly reduces deacetylase activity, therefore this region is considered a regulatory domain [18]. Acetylation on HDAC1 abolishes deacetylase activity in vitro and in vivo [6,59]. Lysine 432 acetylation seems to be crucial for subsequent acetylation on other lysines and appears to be important in regulating histone deacetylase activity [16,60]. The mutation on lysine 432 to glutamine, which mimics acetylated lysine, profoundly reduced histone deacetylase activity [6,16]. Subsequent studies found additional sites for acetylation, but the function of these acetylation sites remain elusive [60].
Figure 1.
HDAC1 acetylation abolishes deacetylase activity and convers its associated corepressor complex to coactivator complex. (A) Schematic representation of HDAC1 acetylation sites. HDAC2 is not acetylated. Note lysine 432 in HDAC1 corresponds to an arginine in HDAC2. (B) HDAC1 and 2 exist as a homo or heterodimer. Acetylation on HDAC1 represses its deacetylase activity and results in inactive deacetylase dimer. (C) HDAC1 acetylation converse NuRD complex to a coactivator complex.
Unlike constitutive phosphorylation, acetylation on HDAC1 is an inducible event. Glucocorticoid receptor (GR) associated HDAC1 becomes acetylated by p300 when GR mediated transcription activation is down regulated on the GR responsive promoter [6]. Cellular stress, such as heat shock or DNA damage, can also induce HDAC1 acetylation to facilitate cellular response [60,61]. HDAC1 acetylation is also increased during erythroid differentiation to promote this process [49].
HDAC1 and HDAC2 often coexist in multi-component protein complexes and are highly related enzymes [14,15,16]. Interestingly, the major acetylation modifications of HDAC1 occur in the C-terminal domain, which reveals a lesser degree of homology to HDAC2. Further, the activity of HDAC2 is trans-repressed when forming heterodimer with acetylated HDAC1 [16] (Figure 1B). Thus, HDAC1 acetylation results in the inhibition of overall deacetylase activity within HDAC1 containing complexes (Figure 1C).
3. HDAC1 Is a Master Regulator for Erythropoiesis
Blood cells consist of cells from multiple lineages, which are specialized for oxygen delivery, homeostasis, and defense from infection. Diverse lineages of blood cells originate from hematopoietic stem cells (HSC), which reside within the bone marrow. HSC give rise to multipotent progenitors (MPPs) that continue to differentiate into common myeloid progenitors (CMPs) and common lymphocyte progenitors (CLPs), which further differentiate toward the specific cell lineages. CMPs differentiate to generate megakaryocytes, red blood cells, neutrophils and macrophages, while CLPs differentiate into B cells and T cells [62,63].
Hematopoietic deficiencies such as anemia, thrombocytopenia, neutropenia, and lymphopenia have been reported in clinical trials with various HDACi [12], indicating the importance of HDACs in hematopoietic development. The role of HDAC1 in development was first studied in knockout mice. Deletion of Hdac1 in mice results in embryonic lethality before E10.5 due to severe proliferation defects and retardation in development [9]. The Hdac1/2 double knockout in hematopoietic compartment was established in mice harboring the interferon-inducible MxCre (Mx dynamin-like GTPase/Cyclic recombinase) transgene and conditional knockout alleles for Hdac1 and/or Hdac2. The Hdac1/2 double knockout mice die approximately nine days after pI/pC (Polyinosinic/polycytidylic acid) injections, displaying significant hematopoietic defects that reduced bone-marrow cell numbers and affected differentiation of all major hematopoietic lineages. The reduction of bone marrow cell numbers was associated with an increase in apoptotic bone marrow cells. The most significant defect was in erythroid/megakaryocyte compartment, including reduction of mature differentiated cells and increased apoptosis [11,64]. Individual knockout of Hdac1 and Hdac2 show compensatory and overlapping functions in hematopoiesis. However, Hdac1 knockout mice expressing mono-allelic Hdac2 showed severe erythropoiesis defect, with mice dying 12 days after pI/pC induction. The defect included reduction of proerythroblast and mature erythroblast in bone marrow and compensatory extramedullary hematopoiesis. Hdac2 knockout mice expressing mono-allelic Hdac1 developed normally, indicating the essential role of HDAC1 in erythropoiesis [64].
Experiments conducted in hematopoietic progenitor cells show that HDAC1 deacetylase activity plays diverse roles in erythropoiesis, including promoting cell survival and proliferation [65,66,67]. Importantly, HDAC1 associated deacetylase activity is significantly decreased during the process of erythropoiesis [68]. Consistently, HDAC1 acetylation levels increased after the induction of erythroid differentiation in multiple cell models, including in human CD36+ cells, mouse G1E erythroblast cells, and mouse erythroleukemia cells [49]. The acetylation of HDAC1 correlates to the reduction of overall histone deacetylase activity in the cell after the induction of differentiation.
Erythropoiesis is regulated through lineage specific transcription factors for the proliferation and differentiation of multipotent progenitors into the erythroid lineage. Among them, GATA-1, GATA-2 (GATA-binding factor 2), FOG-1 (Friend of GATA Protein 1), TAL1/SCL/LMO2/Ldb1/E2A (TAL BHLH Transcription Factor 1/Stem cell leukemia/ LIM Domain Only 2/ LIM-domain-binding protein 1/ Early 2A protein), KLF1, Gfi-1b (Growth factor independent protein 1b), BCL11A (B-cell lymphoma/leukemia 11A), NF-E2 (Nuclear factor, erythroid-derived 2), and PU.1 are hematopoietic-specific transcription factors required for erythropoiesis [69]. Importantly, HDAC1 interacts with all factors and regulates their recruitment and transcription activity. Further, acetylated HDAC1 incorporates into their associated complex and regulates transcription activity, thus confirming HDAC1 as a master regulator for erythropoiesis.
4. GATA-1 Interacts with HDAC1 Containing NuRD Complex for Activation and Repression
Hematopoietic lineage specific transcription factor GATA-1 is the founding member of the GATA factor family. GATA-1 is primarily expressed in erythroid lineage. It is also expressed in megakaryocytes, eosinophils, mast cells, and sertoli cells of the testis (reviewed in [70,71]). GATA-1 is essential for normal hematopoiesis, especially for erythropoiesis and megakaryopoiesis [72]. Mutations of the GATA-1 gene cause characteristic hematologic diseases [71,73]. GATA-1 knockout mice die between embryonic days 10.5 and 11.5 due to severe anemia. Embryonic stem cells or erythroid cells of mice deficient in GATA-1 fail to develop into mature erythroid cells [74,75,76]. GATA binding sites are present in the regulatory regions of virtually all erythroid-specific genes, suggesting that GATA-1 represents an erythroid-specific master regulator [71,72,77,78]. GATA-1 has been reported to regulate genes involved in many pathways, such as anti-apoptotic regulation, hemoglobin synthesis, cell signaling as well as the cell cycle [79].
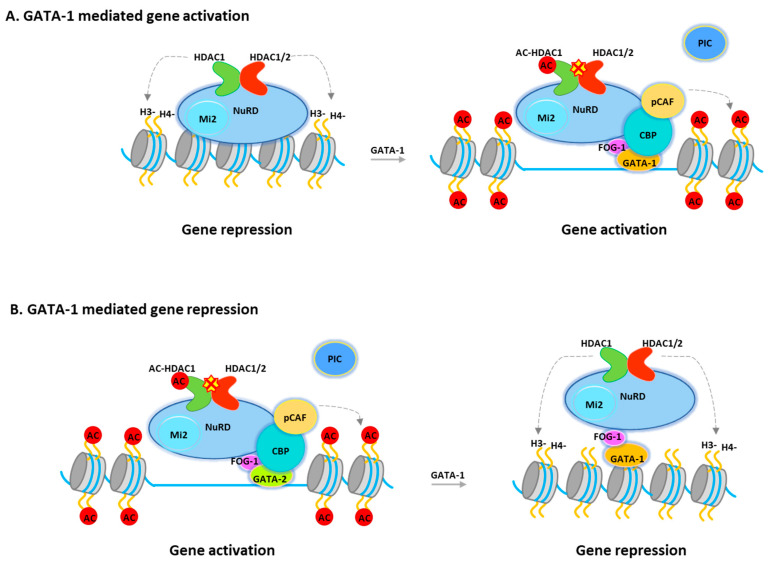
The transcriptional activity of GATA-1 is regulated through interactions with a variety of transcription factors and cofactors. GATA-1 interacts with TAL1, KLF1, PU.1, and SP1 (Sp1 transcription factor) [80]. GATA-1 has also been reported to interact with chromatin remodeling/modification proteins, including the CBP/p300 histone acetyltransferases, and the SWI/SNF (SWItch/Sucrose Non-Fermentable) chromatin remodeling complexes [77,81]. Recent studies showed that GATA-1 is associated with NuRD or MeCP1 corepressor complexes through its interaction with FOG-1 [82,83,84] in either uninduced or induced terminally differentiated murine erytholeukemia (MEL) cells. It was suggested that these complexes play important roles in GATA-1 mediated repression of target genes such as GATA-2, c-Myc (Cellular myelocytomatosis oncogene), and c-kit (Mast/stem cell growth factor receptor Kit), which are all required for the proliferation of hematopoietic progenitors. However, the NuRD/MeCP1 complex is also recruited to GATA-1 sites, which mediate gene activation in induced cells, such as the β-globin gene, where GATA-1 binding also requires FOG-1 [48,82,83]. Hence, the question will be how the corepressor complex regulates GATA-1 mediated gene activation. In fact, GATA-1-associated HDAC1 is increasingly acetylated after differentiation [49]. Consistently, overexpression of an HDAC1 mutant, which mimics acetylated HDAC1, promotes GATA-1-mediated transcription and erythroid differentiation. Furthermore, during erythroid differentiation, acetylated HDAC1 recruitment is increased at GATA-1-activated genes while decreased at GATA-1-repressed genes. (Figure 2) Interestingly, deacetylase activity is not required for Mi-2 remodeling activity, suggesting that remodeling activity may be required for both gene activation and repression. Therefore, NuRD can function as a coactivator or a repressor and that acetylated HDAC1 converts the NuRD complex from a repressor to an activator during GATA-1-directed erythroid differentiation (Figure 2). Notably, the NuRD complex become MBD2 free during erythropoiesis. Therefore, the complex that acts as a transcriptional coactivator may have different configurations to facilitate gene activation [85].
Figure 2.
HDAC1 in NuRD complex mediates GATA-1 function. (A) A model for GATA-1 mediated gene activation. GATA-1 is recruited to the target sites and subsequently recruits NuRD complex and p300/CBP coactivators. These coactivators acetylate histones, GATA-1 and HDAC1, resulting in gene activation. (B) A model for GATA-1 mediated gene repression. GATA-1 displaces GATA-2 and recruits the NuRD complex with full deacetylase activity for repression. PIC, Pre Initiation Complex. PCAF, p300/CBP-associated factor.
GATA-1 can be acetylated at both N- and C-terminal zinc-fingers at conserved lysine residues. Acetylation of GATA-1 may or may not affect DNA binding depending on the GATA-1 binding sites [86,87]. Lysine to alanine mutations of acetylated sites affect chromatin binding, transcription activation and erythroid differentiation [88,89,90]. Acetylation can also target GATA-1 to ubiquitin dependent degradation [91]. It remains to be investigated whether acetylated GATA-1 can be dynamically deacetylated and whether NuRD or HDAC1 can deacetylate GATA-1. It has been reported that HDAC5 can interact with GATA-1 and repress GATA-1 mediated transcription [92], but there is no evidence that HDAC5 can deacetylate GATA-1.
5. HDAC1 in Sin3 Complex Modulates TAL1 Function in Hematopoiesis
TAL1 is a member of the basic helix-loop-helix (bHLH) family of transcription factors and is required for the development of all hematopoietic cell lineages [93]. TAL1 forms heterodimer with the products of the ubiquitously expressed bHLH genes, E2A or HEB, and recognizes a hexanucleotide sequence CANNTG termed “E box” to act as regulators of transcription. Knockout of Tal1 in mice leads to embryonic lethality at E8.5 due to complete loss of yolk sac hematopoietic cells [94,95]. Consistently, Tal1 null embryonic stem cells are unable to generate both primitive and definitive erythroid cells in vitro and do not contribute to hematopoiesis in vivo in chimeric mice [93,96]. The data suggest a key role of TAL1 in hematopoiesis and subsequently, erythropoiesis. Interestingly, TAL1 associates with many transcription regulators, including GATA-1, LMO2, Ldb1, E2A, Bcl11a, NF-E2, KLF11 (Krüpple-like factor 11), BACH1 (BTB and CNC homolog 1), Myb (Myeloblastosis oncogene), and Myc [97]. All of these proteins have been implicated in the regulation of erythropoiesis. The TAL1/LMO2/GATA-1 containing protein complex specifically recognizes composite binding motifs consisting of a GATA site and an E-Box motif. This composite element is found in the regulatory regions of many erythroid-specific target genes including p4.2, KLF1, GATA-1, the locus control region (LCR) and the β-major globin promoter [98,99,100,101].
During hematopoiesis, TAL1 can function as a repressor or an activator of transcription depending on the DNA sequence and/or cellular contexts [102]. TAL1 recruits HDAC1/2-associated Sin3A corepressor complexes which mediate its transcription repressive activity in certain stages of erythroid differentiation [103]. The TAL1 associated deacetylase activity that appears to be important for proliferation of erythroid progenitors/precursors are markedly decreased during differentiation [103]. Inhibition of HDAC activities, such as treatment with TSA (Trichostatin A), a non-specific histone deacetylase inhibitor, synergizes with TAL1 to promote erythroid differentiation [103], suggesting that HDAC activity blocks erythroid progenitor cell differentiation via modulating TAL1 transcriptional activity. Consistent with the above findings, two groups recently undertook biochemical purification of TAL1-associated complexes from murine erythroleukemia (MEL) cells and showed that TAL1 is associated with the co-repressor ETO-2 (Eight twenty one protein, MTG8-related protein 2) [104,105]. Similar to its interaction with mSin3A, the interaction between TAL1 and ETO-2 decreased during erythroid differentiation of primary fetal liver cells [104]. Interestingly, ETO-2 also associates with HDACs and NCoR (Nuclear receptor corepressor) to repress target gene transcription [106,107,108].
6. HDAC1 Is Required for PU.1 Promoter Activation
PU.1 belongs to ETS (Erythroblast Transformation Specific) family of transcription factor that plays an essential role in hematopoietic stem cell self-renewal and in directing multipotent hematopoietic stem cells towards lineage commitment [79,109,110,111]. The expression of PU.1 fluctuates in various hematopoietic differentiation pathways. PU.1 protein level is high in human HSC cells [112,113,114,115] and remains high in common myeloid progenitors (CMPs), common lymphoid progenitors (CLPs), but changes to low as myeloid/erythroid precursor cells commit to the erythroid lineage [116,117,118,119]. Inappropriate expression of PU.1 in specific hematopoietic cells can result in leukemic transformation, such as T-cell lymphomas, acute myeloid leukemia and erythroleukemias [120,121,122]. Therefore, maintaining proper levels of PU.1 is critical for hematopoiesis.
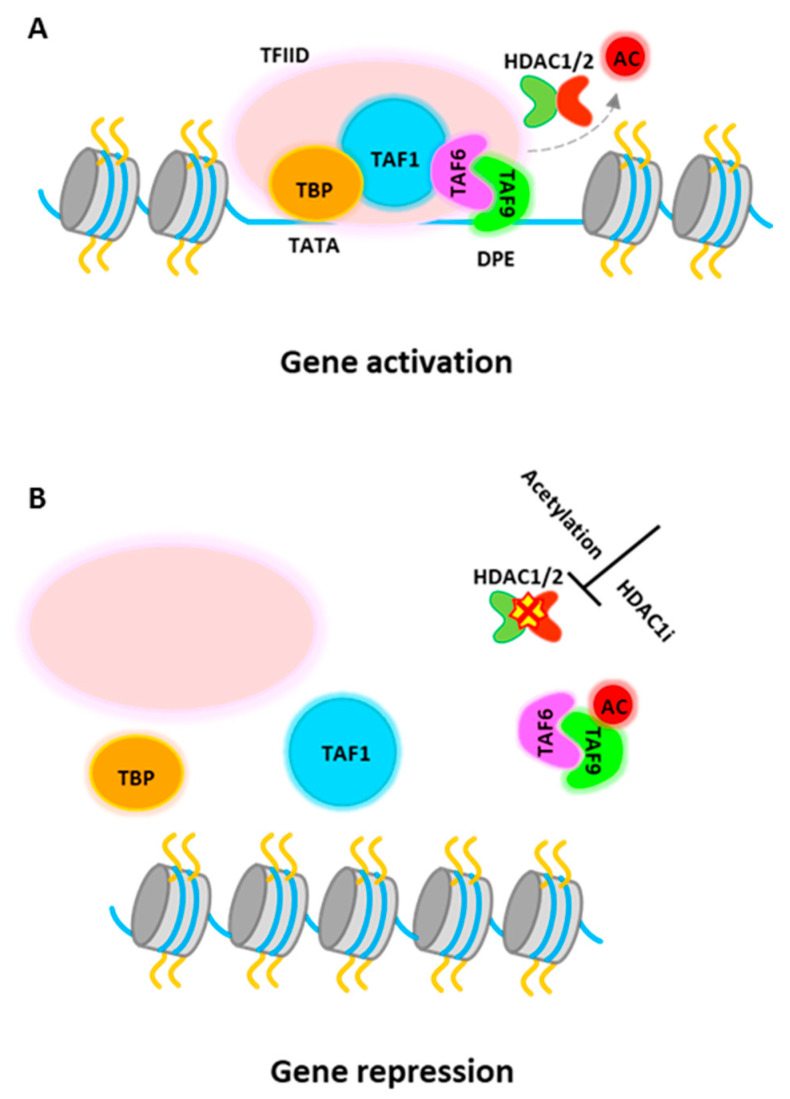
PU.1 gene expression can be regulated epigenetically [5,123,124]. It is reported that inhibition of HDAC activity by HDACi treatment represses PU.1 gene expression [124], although it is generally considered that HDACs associate with corepressor complexes and repress gene transcription. Recent study further demonstrates that PU.1 directly requires histone deacetylase activity for gene activation [5]. HDAC1 is recruited to active PU.1 promoter in progenitor cells to promote PU.1 gene transcription. Detailed study shows that TATA-box binding protein associated factor 9 (TAF9), a member of the TFIID complex, is a target for HDAC1-mediated deacetylation and this process is required for PU.1 gene activation. The transcription factor IID (TFIID) is the first complex to be recruited to the core promoter element for preinitiation complex (PIC) assembly and that also serves to mediate signals between various activators and the basal transcription machinery [125,126,127]. TAF9 binds to a consensus DNA sequence, the downstream promoter element (DPE), which is found in both TATA-containing and TATA-less promoters, although more commonly in TATA-less promoters [128,129]. The study from Jian et al. shows that HDAC1 associates with TAF9 to maintain it in an unacetylated stage. The non-acetylated TAF9 is able to associate with the PU.1 promoter and promotes gene activation (Figure 3A). Upon induction of erythropoiesis, acetylated HDAC1 becomes accumulated at the PU.1 promoter and no longer be able to deacetylate TAF9. Acetylated TAF9 not only fails to bind to the promoter, but also results in the disassembly of the entire TFIID complex from the promoter, leading to transcriptional repression [5] (Figure 3B). Thus, this study demonstrate a key role of HDAC1 in PU.1 gene transcription and more importantly, the study shows that acetylation of HDAC1 during erythropoiesis has multiple roles, turning on transcription activators such as GATA-1 or other activators, and turning off counter regulators, such as PU.1.
Figure 3.
HDAC1 dependent dynamic transcription activation and repression at PU.1 promoter. (A) TAF9 can be acetylated by an acetyltransferase and subsequently deacetylated by HDAC1. Non-acetylated TAF9 binds to DPE site and associates with TFIID complex to promote PU.1 gene activation. (B) HDAC1 is acetylated or inactivated by HDACi, causing an inability to deacetylate TAF9. Acetylated TAF9 is displaced from DNA. Acetylated TAF9 also causes the disassembly of the TFIID complex from the promoter, resulting in gene repression.
In addition, HDAC1 can directly modulate PU.1 activity. It was found that the C-terminal domain of PU.1 formed a complex with mSin3A and HDAC1 in vivo, which mediates PU.1 induced transcriptional repression [130]. Further study shows that PU.1 directly associates with MeCP2 (Methyl CpG-binding protein 2) corepressor, which facilitates PU.1-mSin3A-HDAC complex formation. The complex disassociates from the region during the course of erythroid differentiation of MEL cells [131]. PU.1 itself can be acetylated and its repressor activity was reduced when the putative acetylation motifs in the Ets domain were mutated. The acetyl-mimicking mutant also insufficiently interacts with GATA-1 and mSin3A [132]. Therefore, HDAC1 plays important roles on the repressive function of PU.1 for erythroid differentiation blockage.
7. KLF1 Regulates Erythroid Differentiation by Interacting with HDAC1 Containing Complexes
KLF1 (EKLF) is another transcription factor essential for erythropoiesis. KLF1 expression is restricted to hematopoietic compartment and expressed in both primitive and definitive erythroid populations [133]. Gene ablation studies showed that KLF1 null mice suffered profound β-thalassemia resulting in embryonic lethality [134,135].
KLF1 regulates erythropoiesis mainly through activation of gene transcription [133,136,137]. Genome wide analysis of KLF1 chromatin occupancy revealed that it binds to the proximity sites to GATA-1, suggesting co-occupation of these factors at the same promoter/enhance region [137].
Although KLF1 has been primarily characterized as a transcriptional activator, it can also exert transcriptional repression. p300/CBP (E1A binding protein p300/CREB-binding protein) can acetylate KLF1 at lysine 288. Acetylation leads to increased association of KLF1 with the chromatin remodeling SWI/SNF-related complex, resulting in an open chromatin domain and transcription of the adult β-globin promoter [138,139,140]. However, acetylation also promotes interaction with Sin3A and HDAC1 corepressors [141]. These studies suggest that dynamic coactivator-corepressor interaction through acetylation status of KLF1 modulates downstream transcriptional effects [133,139,140,142]. On the other hand, sumoylation of K74 facilitates KLF1 interaction with the Mi-2β of the NuRD repressor complex, which is important for suppression of megakaryocyte differentiation and direct MEP to erythroid lineage [143].
Interestingly, KLF1 also physically interact with TAF9, a component of TFIID complex. This interaction is required for a subset of KLF1 activated genes, including β-globin promoter, and is necessary for its activation. Since the interaction is not required at all KLF1 activation target promoters, it is conceivable that the requirement of TAF9 by KLF1 may depend on specific promoter sequences DPE, which is present at the β-globin promoter [144,145]. Further analysis is necessary to determine whether the DPE site is required for co-recruitment of TAF9 and KLF1. This is particularly interesting since TAF9 is modulated by HDAC1 in a DPE dependent manner [5].
8. Gfi-1b Repressed Erythroid and Megakaryocytic Differentiation through Association with CoREST Corepressor Complex
The family of Gfi-1 zinc finger oncoproteins consists of Gfi-1 and Gfi-1b. Studies from gene targeting and mutational screening in humans have revealed an essential role for Gfi-1 and Gfi-1b in hematopoiesis [146]. In mice, Gfi-1b is required for the development of two related blood lineages, erythroid and megakaryocytic. Disruption of Gfi-1b is embryonic lethal due to delayed maturation of primitive erythrocytes and failure to produce definitive enucleated erythrocytes [146,147]. Induced disruption of Gfi-1b at the adult stage was also lethal within 3 weeks after induction, with severely reduced hemoglobin levels and platelet counts. Genome-wide gene expression analyses revealed that Gfi-1b directly regulates a wide spectrum of megakaryocytic and erythroid genes, predominantly through gene repression [148]. Transcriptional repression by Gfi-1b requires the conserved SNAG (Snail/Gfi-1) domain. Gfi-1b associates with CoREST corepressor complex, which contains the corepressor CoREST, the histone demethylase LSD1, and HDAC1 and 2. Gfi-1b recruits these cofactors to the majority of target gene promoters in vivo. Inhibition of factors in CoREST complex perturbs differentiation of erythroid, megakaryocytic, and granulocytic cells as well as primary erythroid progenitors [149].
Gfi-1b p32 is an alternative spliced form that lacks the first two zinc finger domains of the protein. Gfi-1b p32 isoform binds to Gfi-1b target gene promoters and associates with the LSD1-CoREST repressor complex more efficiently than the major Gfi-1b p37 isoform. Selective knock down of Gfi-1b p32 compromises erythroid differentiation, further demonstrates that association of CoREST complex is essential for the repressive effect of Gfi-1b [150].
9. BCL11A Interacts with Multiple HDAC1/2 Containing Corepressor Complex for Fetal Globin Gene Suppression
BCL11A is a zinc finger transcription repressor. Genome-wide association studies (GWAS) have linked BCL11A to fetal globin level, which directly associates with the clinical severity of sickle cell disease and β-thalassemia [151,152]. Genetic study further shows that BCL11A is a repressor for fetal globin gene expression. Knockout of Bcl11a in mice prevents silencing of endogenous mouse fetal globin and transgenic human γ-globin [153]. Inducible deletion of Bcl11a in adult mice results in derepression of fetal globin, indicating that BCL11A actively repress fetal globin expression in the adult stage and that repression is reversible. Importantly, knock down of BCL11A in transgenic sickle cell disease mice stimulated therapeutic levels of fetal globin, suggesting the importance of BCL11A in hemoglobinopathy treatments [154].
BCL11A binds to the globin locus control region (LCR) and multiple other regions at the β-globin gene locus to regulate globin gene expression [155,156,157]. In adult erythroid cells, BCL11A interacts and co-operates with SOX6 (SRY-box transcription factor 6) in silencing γ-globin transcripts [157]. Liu et al. (2018) showed that BCL11A can directly bind to the γ-globin gene promoter to mediate γ-globin repression during hemoglobin switching [158]. BCL11A is found within multiprotein complexes consisting of erythroid transcription factors, transcriptional corepressors, and chromatin-modifying enzymes [156]. Affinity purification result shows that BCL11A associates with multiple corepressor complexes. These include members of the NuRD, LSD1/CoREST, NCoR/SMRT (Silencing mediator for retinoid and thyroid hormone receptors), SIN3, and SWI/SNF complexes [156]. Interestingly, these complexes bind to distinct sites at the globin locus and they all require HDAC1 and HDAC2 for repression. Knock down of HDAC1/2 significantly elevates fetal globin expression without significantly altering β-globin expression.
10. EpoR/JAK2/Stat5 Signaling Requires HDAC1
Erythropoietin (EPO) is the primary hormone that regulates erythroid cell maturation. EPO binds to its cognate receptor EpoR and subsequently activates the Janus Kinase 2 (JAK2), which activates signaling pathways, including mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3 kinase), signal transducer and activator of transcription 5 (Stat5) [159,160]. EPO-stimulated STAT5 activation is essential and sufficient for erythropoiesis [161]. A genome wide mapping on EPO activated STAT5 recruitment on chromatin shows that STAT5 binds to various genes necessary for erythroid differentiation and maturation. In addition, STAT5 binds within the proximity of binding sites for other transcription factors, such as TAL1, GATA1, and KLF1, to direct erythroid differentiation [162]. How HDAC1 regulates STAT5 during erythroid differentiation is not fully studied. However, in murine T and B lymphocytes, treatment with HDACi represses transactivation of all cytokine induced STAT5 target genes. The deacetylase activity appear to be required to control proper assembly of the basal transcription machinery on STAT5 target genes and is independent of histone acetylation [163]. A detailed study on the role of HDAC1 was reported on STAT5 target gene Id1 (Inhibitor of DNA Binding 1). The study shows that both STAT5 and C/EBPβ (CCAAT-enhancer-binding protein beta) binding to Id1 promoter are required for Id1 transcription activation. HDAC1 physically interacts with STAT5 on Id1 promoter and subsequently deacetylate C/EBPβ. Deacetylation enable C/EBPβ to retain on chromatin and activate Id1 promoter activity [164]. It remains for future investigation whether deacetylation of C/EBPβ is a general mechanism for STAT5 mediated gene activation.
11. Use of Histone Deacetylase Inhibitor for Hemoglobinopathies
Sickle cell disease and β-thalassemia represent the most common hemoglobinopathies. The expression of normally silenced fetal globin in adult erythroid cells can ameliorate the pathophysiological consequences. Therefore, mechanisms of fetal globin reactivation have been intensively studied in the past few decades.
In addition to factors such as BCL11A that mediate silencing of the γ-globin gene in adult stage erythroid cells, epigenetic factors, such as chromatin remodelers and histone modification enzymes have been intensively studied for their role in fetal globin gene expression [165,166]. The knock down of Mi-2β, the chromatin remodeler within NuRD corepressor complex, relieves γ-globin gene silencing in β-YAC (Yeast artificial chromosome) transgenic murine erythroid cells and in CD34(+) progenitor-derived human primary adult erythroid cells [167]. Depletion of MBD2, a component of the NuRD complex, increased γ to β mRNA ratios in adult erythroid cells [168]. γ-globin expression is also regulated by HDACs through chromatin modification, and several transcription factors that regulate HbF are associated with these HDAC proteins [166]. Indeed, HDAC1 and HDAC2 have been identified as potential new molecular targets for mediating fetal hemoglobin induction by chemical library screening [169]. The targeted inactivation of HDAC1 and HDAC2 is effective to enhance fetal globin expression in human adult erythroid cells without altering cell cycle [170]. Consistently, treatment with HDAC1/2 specific inhibitor Romidepsin [171] induces fetal globin expression. The combination of BCL11A inhibitor and Romidepsin further increased γ-globin gene expression [172]. MS-275 (Entinostat), a HDAC1/3 inhibitor, also increases γ-globin gene expression [173]. HDAC inhibitors can activate γ-globin expression via a p38 mitogen-activating protein kinase (MAPK)-dependent mechanism [174]. A recent report shows that selective HDAC inhibitors have been developed for the activation of γ-globin. The small molecule ACY-957 screened from a chemical library can selectively inhibit HDAC1 and HDAC2 and induces γ-globin transcripts and protein through activation of GATA-2 in primary erythroid progenitor cells [175].
Two clinical trials have been conducted with two pan-HDAC inhibitors for sickle cell disease therapy. One trial used Vorinostat and another trial used Panobinostat as a single drug for therapy (information received from clinicaltrials.gov). The Vorinostat treatment shows effective elevation of γ-globin level and increased γ to β globin ratios. However, the trial was terminated due to blood related adverse events [12,176], which further suggests that HDACi affects hematopoietic differentiation. More study is needed to evaluate the efficacy of the treatment and adverse effect of HDACi in the treatment.
12. Closing Remark and Future Prospective
HDACs have been known to associate with many hematopoietic lineage specific transcription factors and mediate transcription regulation through these factors. GATA-1 and PU.1 are master regulators which functionally antagonize each other’s activity. While PU.1 directs myeloid differentiation, GATA-1 promotes erythroid differentiation (reviewed in [79]). HDAC1 plays an important role in counter regulating PU.1 and GATA-1 [5,49]. Deacetylase activity of HDAC1 represses GATA-1 activity but activates PU.1. During erythropoiesis, increased level of HDAC1 acetylation reduces deacetylase activity, which results in GATA-1 activation and PU.1 repression. Therefore, HDAC1 is an essential regulator of the master regulators for erythropoiesis.
The aberrant recruitment of HDAC1 is linked to various types of leukemia [177,178,179]. HDAC inhibitors (HDACi) have shown clinical and preclinical promise when used alone or in conjunction with other therapeutic drugs [180,181,182]. In addition, the inhibition of histone deacetylases results in the reactivation of fetal globin in adult erythroid cells [174,183,184,185,186,187]. Clinical trials have tested the effectiveness of HDACi for reactivation of fetal globin in patients suffering thalassemia and sickle cell diseases. However, blood related side effects led to termination of that study. Therefore, it is extremely important to understand how HDAC impacts normal hematopoiesis before HDACi is administered to patients.
Acknowledgments
The authors thank Rachael Mills for editing the manuscript.
Abbreviations
| BACH1 | BTB and CNC homolog 1 |
| BCL11A | B-cell lymphoma/leukemia 11A |
| BHC80 | BRAF35-HDAC complex protein BHC80 |
| bHLH | Basic helix-loop-helix |
| BRMS1 | Breast cancer metastasis suppressor 1 |
| C/EBP | CCAAT-enhancer-binding protein |
| CHD3/4 | Chromodomain Helicase DNA binding protein 3/4 |
| c-kit | Mast/stem cell growth factor receptor Kit |
| CLPs | Common lymphocyte progenitors |
| CMPs | Common myeloid progenitors |
| c-Myc | Cellular myelocytomatosis oncogene |
| CoREST | Corepressor of RE1 silencing transcription factor |
| CtBP | C-terminal binding protein |
| DPE | Downstream promoter element |
| E2A | Early 2A protein |
| EPO | Erythropoietin |
| ETO-2 | Eight twenty one protein, MTG8-related protein 2 |
| Ets | Erythroblast Transformation Specific |
| FOG-1 | Friend of GATA Protein 1 |
| GATA-1/2 | GATA-binding factor 1/2 |
| Gfi-1 | Growth factor independent protein 1 |
| GR | Glucocorticoid receptor |
| GWAS | Genome-wide association studies |
| HAT | Histone acetyltransferases |
| Hda1 | Histone deacetylase HDA1 |
| HDAC | Histone deacetylase |
| HDACi | HDAC inhibitors |
| HEB | Helix-loop-helix, E-box-binding protein |
| HSC | Hematopoietic stem cells |
| Id1 | Inhibitor of DNA Binding 1 |
| IL-4 | Interleukin 4 |
| ING1 | Inhibitor of Growth Family Member 1 |
| JAK2 | Janus Kinase 2 |
| K | Lysine |
| KLF1 | Krüpple-like factor 1 |
| KLF11 | Krüpple-like factor 11 |
| LCR | Locus control region |
| Ldb1 | LIM-domain-binding protein 1 |
| LMO2 | LIM Domain Only 2 |
| LSD1 | Lysine-specific demethylase 1 |
| Mad | Max dimerization protein |
| MAPK | Mitogen-activated protein kinase |
| Max | Myc-associated factor X |
| MBD2 | Methyl CpG-binding domain 2 |
| MeCP1/2 | Methyl CpG-binding protein 1/2 |
| MEL | Murine erytholeukemia |
| MEP | Megakaryocyte/Erythroid progenitor |
| Mi-2 | ATP-dependent helicase Mi-2 |
| MKP-3 | Mitogen-activated protein kinase phosphatase-3 |
| MPPs | Multipotent progenitors |
| MS-275 | Entinostat, HDAC1 and 3 inhibitor |
| MTA | Metastasis-associated protein |
| MxCre | Mx dynamin-like GTPase/Cyclic recombinase |
| Myb | Myeloblastosis oncogene |
| NAD | Nicotinamide adenine dinucleotide |
| NCoR | Nuclear receptor corepressor |
| NF-E2 | Nuclear factor, erythroid-derived 2 |
| NuRD | Nucleosome remodeling and deacetylase |
| p300/CBP | E1A binding protein p300/CREB-binding protein |
| PAH | Paired amphipathic helix |
| pI/pC | Polyinosinic/polycytidylic acid |
| PI3 kinase | Phosphatidylinositol 3-kinase |
| PIC | Pre Initiation Complex |
| PU.1 | Putative oncogene Spi-1 |
| RbAp46/48 | Retinoblastoma-associated proteins 46/48 |
| RBP1 | Retinoblastoma-binding protein 1 |
| Rpd3 | Reduced potassium dependency 3 |
| SAP18 | Sin3A Associated Protein 18 |
| SAP180 | Sin3A Associated Protein 180 |
| SAP30 | Sin3A Associated Protein 30 |
| SCL | Stem cell leukemia |
| SDS3 | Suppressor of defective silencing 3 |
| Sin3 | Switch Independent 3 |
| Sin3A | SIN3 Transcription Regulator Family Member A |
| Sin3B | SIN3 Transcription Regulator Family Member B |
| Sir2 | Silent information regulator 2 |
| SMRT | Silencing mediator for retinoid and thyroid hormone receptors |
| SNAG | Snail/Gfi-1 |
| SOX6 | SRY-box transcription factor 6 |
| SP1 | Sp1 transcription factor |
| STAT5 | Signal transducer and activator of transcription 5 |
| SWI/SNF | SWItch/Sucrose Non-Fermentable |
| TAF9 | TATA-Box Binding Protein Associated Factor 9 |
| TAL1 | TAL BHLH Transcription Factor 1 |
| TFIID | Transcription factor IID |
| Th2 | Type 2 helper T cells |
| TSA | Trichostatin A |
| YAC | Yeast artificial chromosome |
Author Contributions
M.Y.K., B.Y., S.H. and Y.Q. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the grants from the National Institute of Health (R01HL144712 to Y.Q.; R01DK110108, R01CA204044, R01HL141950 to S.H.) and the Four Diamonds Fund (S.H.).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sterner D.E., Berger S.L. Acetylation of histones and transcription-related factors. Microbiol. Mol. Biol. Rev. 2000;64:435–459. doi: 10.1128/MMBR.64.2.435-459.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Seto E., Yoshida M. Erasers of histone acetylation: The histone deacetylase enzymes. Cold Spring Harb. Perspect. Biol. 2014;6:a018713. doi: 10.1101/cshperspect.a018713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang X.J., Seto E. HATs and HDACs: From structure, function and regulation to novel strategies for therapy and prevention. Oncogene. 2007;26:5310–5318. doi: 10.1038/sj.onc.1210599. [DOI] [PubMed] [Google Scholar]
- 4.Smith C.L. A shifting paradigm: Histone deacetylases and transcriptional activation. Bioessays. 2008;30:15–24. doi: 10.1002/bies.20687. [DOI] [PubMed] [Google Scholar]
- 5.Jian W., Yan B., Huang S., Qiu Y. Histone deacetylase 1 activates PU.1 gene transcription through regulating TAF9 deacetylation and transcription factor IID assembly. FASEB J. 2017;31:4104–4116. doi: 10.1096/fj.201700022R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qiu Y., Zhao Y., Becker M., John S., Parekh B.S., Huang S., Hendarwanto A., Martinez E.D., Chen Y., Lu H., et al. HDAC1 acetylation is linked to progressive modulation of steroid receptor-induced gene transcription. Mol. Cell. 2006;22:669–679. doi: 10.1016/j.molcel.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Haberland M., Montgomery R.L., Olson E.N. The many roles of histone deacetylases in development and physiology: Implications for disease and therapy. Nat. Rev. Genet. 2009;10:32–42. doi: 10.1038/nrg2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Montgomery R.L., Davis C.A., Potthoff M.J., Haberland M., Fielitz J., Qi X., Hill J.A., Richardson J.A., Olson E.N. Histone deacetylases 1 and 2 redundantly regulate cardiac morphogenesis, growth, and contractility. Genes Dev. 2007;21:1790–1802. doi: 10.1101/gad.1563807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lagger G., O’Carroll D., Rembold M., Khier H., Tischler J., Weitzer G., Schuettengruber B., Hauser C., Brunmeir R., Jenuwein T., et al. Essential function of histone deacetylase 1 in proliferation control and CDK inhibitor repression. EMBO J. 2002;21:2672–2681. doi: 10.1093/emboj/21.11.2672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zupkovitz G., Tischler J., Posch M., Sadzak I., Ramsauer K., Egger G., Grausenburger R., Schweifer N., Chiocca S., Decker T., et al. Negative and positive regulation of gene expression by mouse histone deacetylase 1. Mol. Cell. Biol. 2006;26:7913–7928. doi: 10.1128/MCB.01220-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilting R.H., Yanover E., Heideman M.R., Jacobs H., Horner J., van der Torre J., DePinho R.A., Dannenberg J.H. Overlapping functions of Hdac1 and Hdac2 in cell cycle regulation and haematopoiesis. EMBO J. 2010;29:2586–2597. doi: 10.1038/emboj.2010.136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wagner J.M., Hackanson B., Lubbert M., Jung M. Histone deacetylase (HDAC) inhibitors in recent clinical trials for cancer therapy. Clin. Epigenetics. 2010;1:117–136. doi: 10.1007/s13148-010-0012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moser M.A., Hagelkruys A., Seiser C. Transcription and beyond: The role of mammalian class I lysine deacetylases. Chromosoma. 2014;123:67–78. doi: 10.1007/s00412-013-0441-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeng Y., Tang C.M., Yao Y.L., Yang W.M., Seto E. Cloning and characterization of the mouse histone deacetylase-2 gene. J. Biol. Chem. 1998;273:28921–28930. doi: 10.1074/jbc.273.44.28921. [DOI] [PubMed] [Google Scholar]
- 15.Taplick J., Kurtev V., Kroboth K., Posch M., Lechner T., Seiser C. Homo-oligomerisation and nuclear localisation of mouse histone deacetylase 1. J. Mol. Biol. 2001;308:27–38. doi: 10.1006/jmbi.2001.4569. [DOI] [PubMed] [Google Scholar]
- 16.Luo Y., Jian W., Stavreva D., Fu X., Hager G., Bungert J., Huang S., Qiu Y. Trans-regulation of histone deacetylase activities through acetylation. J. Biol. Chem. 2009;284:34901–34910. doi: 10.1074/jbc.M109.038356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ayer D.E., Lawrence Q.A., Eisenman R.N. Mad-Max transcriptional repression is mediated by ternary complex formation with mammalian homologs of yeast repressor Sin3. Cell. 1995;80:767–776. doi: 10.1016/0092-8674(95)90355-0. [DOI] [PubMed] [Google Scholar]
- 18.Hassig C.A., Fleischer T.C., Billin A.N., Schreiber S.L., Ayer D.E. Histone deacetylase activity is required for full transcriptional repression by mSin3A. Cell. 1997;89:341–347. doi: 10.1016/S0092-8674(00)80214-7. [DOI] [PubMed] [Google Scholar]
- 19.Ayer D.E. Histone deacetylases: Transcriptional repression with SINers and NuRDs. Trends Cell Biol. 1999;9:193–198. doi: 10.1016/S0962-8924(99)01536-6. [DOI] [PubMed] [Google Scholar]
- 20.Laherty C.D., Yang W.M., Sun J.M., Davie J.R., Seto E., Eisenman R.N. Histone deacetylases associated with the mSin3 corepressor mediate mad transcriptional repression. Cell. 1997;89:349–356. doi: 10.1016/S0092-8674(00)80215-9. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Y., Iratni R., Erdjument-Bromage H., Tempst P., Reinberg D. Histone deacetylases and SAP18, a novel polypeptide, are components of a human Sin3 complex. Cell. 1997;89:357–364. doi: 10.1016/S0092-8674(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 22.Doyon Y., Cayrou C., Ullah M., Landry A.J., Cote V., Selleck W., Lane W.S., Tan S., Yang X.J., Cote J. ING tumor suppressor proteins are critical regulators of chromatin acetylation required for genome expression and perpetuation. Mol. Cell. 2006;21:51–64. doi: 10.1016/j.molcel.2005.12.007. [DOI] [PubMed] [Google Scholar]
- 23.Fleischer T.C., Yun U.J., Ayer D.E. Identification and characterization of three new components of the mSin3A corepressor complex. Mol. Cell. Biol. 2003;23:3456–3467. doi: 10.1128/MCB.23.10.3456-3467.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kuzmichev A., Zhang Y., Erdjument-Bromage H., Tempst P., Reinberg D. Role of the Sin3-histone deacetylase complex in growth regulation by the candidate tumor suppressor p33(ING1) Mol. Cell. Biol. 2002;22:835–848. doi: 10.1128/MCB.22.3.835-848.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nikolaev A.Y., Papanikolaou N.A., Li M., Qin J., Gu W. Identification of a novel BRMS1-homologue protein p40 as a component of the mSin3A/p33(ING1b)/HDAC1 deacetylase complex. Biochem. Biophys. Res. Commun. 2004;323:1216–1222. doi: 10.1016/j.bbrc.2004.08.227. [DOI] [PubMed] [Google Scholar]
- 26.Skowyra D., Zeremski M., Neznanov N., Li M., Choi Y., Uesugi M., Hauser C.A., Gu W., Gudkov A.V., Qin J. Differential association of products of alternative transcripts of the candidate tumor suppressor ING1 with the mSin3/HDAC1 transcriptional corepressor complex. J. Biol. Chem. 2001;276:8734–8739. doi: 10.1074/jbc.M007664200. [DOI] [PubMed] [Google Scholar]
- 27.Li Y., Seto E. HDACs and HDAC Inhibitors in Cancer Development and Therapy. Cold Spring Harb. Perspect. Med. 2016;6 doi: 10.1101/cshperspect.a026831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Humphrey G.W., Wang Y., Russanova V.R., Hirai T., Qin J., Nakatani Y., Howard B.H. Stable histone deacetylase complexes distinguished by the presence of SANT domain proteins CoREST/kiaa0071 and Mta-L1. J. Biol. Chem. 2001;276:6817–6824. doi: 10.1074/jbc.M007372200. [DOI] [PubMed] [Google Scholar]
- 29.Lee M.G., Wynder C., Bochar D.A., Hakimi M.A., Cooch N., Shiekhattar R. Functional interplay between histone demethylase and deacetylase enzymes. Mol. Cell. Biol. 2006;26:6395–6402. doi: 10.1128/MCB.00723-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee M.G., Wynder C., Cooch N., Shiekhattar R. An essential role for CoREST in nucleosomal histone 3 lysine 4 demethylation. Nature. 2005;437:432–435. doi: 10.1038/nature04021. [DOI] [PubMed] [Google Scholar]
- 31.Shi Y., Sawada J., Sui G., Affar E.B., Whetstine J.R., Lan F., Ogawa H., Luke M.P., Nakatani Y., Shi Y. Coordinated histone modifications mediated by a CtBP co-repressor complex. Nature. 2003;422:735–738. doi: 10.1038/nature01550. [DOI] [PubMed] [Google Scholar]
- 32.You A., Tong J.K., Grozinger C.M., Schreiber S.L. CoREST is an integral component of the CoREST- human histone deacetylase complex. Proc. Natl. Acad. Sci. USA. 2001;98:1454–1458. doi: 10.1073/pnas.98.4.1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi Y., Lan F., Matson C., Mulligan P., Whetstine J.R., Cole P.A., Casero R.A., Shi Y. Histone demethylation mediated by the nuclear amine oxidase homolog LSD1. Cell. 2004;119:941–953. doi: 10.1016/j.cell.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 34.Metzger E., Wissmann M., Yin N., Muller J.M., Schneider R., Peters A.H., Gunther T., Buettner R., Schule R. LSD1 demethylates repressive histone marks to promote androgen-receptor-dependent transcription. Nature. 2005;437:436–439. doi: 10.1038/nature04020. [DOI] [PubMed] [Google Scholar]
- 35.Garcia-Bassets I., Kwon Y.S., Telese F., Prefontaine G.G., Hutt K.R., Cheng C.S., Ju B.G., Ohgi K.A., Wang J., Escoubet-Lozach L., et al. Histone methylation-dependent mechanisms impose ligand dependency for gene activation by nuclear receptors. Cell. 2007;128:505–518. doi: 10.1016/j.cell.2006.12.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Allen H.F., Wade P.A., Kutateladze T.G. The NuRD architecture. Cell. Mol. Life Sci. 2013;70:3513–3524. doi: 10.1007/s00018-012-1256-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Basta J., Rauchman M. The nucleosome remodeling and deacetylase complex in development and disease. Transl. Res. 2015;165:36–47. doi: 10.1016/j.trsl.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kunert N., Brehm A. Novel Mi-2 related ATP-dependent chromatin remodelers. Epigenetics. 2009;4:209–211. doi: 10.4161/epi.8933. [DOI] [PubMed] [Google Scholar]
- 39.Fatemi M., Wade P.A. MBD family proteins: Reading the epigenetic code. J. Cell Sci. 2006;119:3033–3037. doi: 10.1242/jcs.03099. [DOI] [PubMed] [Google Scholar]
- 40.Feng Q., Cao R., Xia L., Erdjument-Bromage H., Tempst P., Zhang Y. Identification and functional characterization of the p66/p68 components of the MeCP1 complex. Mol. Cell. Biol. 2002;22:536–546. doi: 10.1128/MCB.22.2.536-546.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng Q., Zhang Y. The MeCP1 complex represses transcription through preferential binding, remodeling, and deacetylating methylated nucleosomes. Genes Dev. 2001;15:827–832. doi: 10.1101/gad.876201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ng H.H., Zhang Y., Hendrich B., Johnson C.A., Turner B.M., Erdjument-Bromage H., Tempst P., Reinberg D., Bird A. MBD2 is a transcriptional repressor belonging to the MeCP1 histone deacetylase complex. Nat. Genet. 1999;23:58–61. doi: 10.1038/12659. [DOI] [PubMed] [Google Scholar]
- 43.Magliulo D., Bernardi R., Messina S. Lysine-Specific Demethylase 1A as a Promising Target in Acute Myeloid Leukemia. Front. Oncol. 2018;8:255. doi: 10.3389/fonc.2018.00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Snow J.W., Orkin S.H. Translational isoforms of FOG1 regulate GATA1-interacting complexes. J. Biol. Chem. 2009;284:29310–29319. doi: 10.1074/jbc.M109.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang Y., Zhang H., Chen Y., Sun Y., Yang F., Yu W., Liang J., Sun L., Yang X., Shi L., et al. LSD1 is a subunit of the NuRD complex and targets the metastasis programs in breast cancer. Cell. 2009;138:660–672. doi: 10.1016/j.cell.2009.05.050. [DOI] [PubMed] [Google Scholar]
- 46.Ahringer J. NuRD and SIN3 histone deacetylase complexes in development. Trends Genet. 2000;16:351–356. doi: 10.1016/S0168-9525(00)02066-7. [DOI] [PubMed] [Google Scholar]
- 47.Hutchins A.S., Mullen A.C., Lee H.W., Sykes K.J., High F.A., Hendrich B.D., Bird A.P., Reiner S.L. Gene silencing quantitatively controls the function of a developmental trans-activator. Mol. Cell. 2002;10:81–91. doi: 10.1016/S1097-2765(02)00564-6. [DOI] [PubMed] [Google Scholar]
- 48.Miccio A., Wang Y., Hong W., Gregory G.D., Wang H., Yu X., Choi J.K., Shelat S., Tong W., Poncz M., et al. NuRD mediates activating and repressive functions of GATA-1 and FOG-1 during blood development. EMBO J. 2010;29:442–456. doi: 10.1038/emboj.2009.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang T., Jian W., Luo Y., Fu X., Noguchi C., Bungert J., Huang S., Qiu Y. Acetylation of histone deacetylase 1 regulates NuRD corepressor complex activity. J. Biol. Chem. 2012;287:40279–40291. doi: 10.1074/jbc.M112.349704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Winter M., Moser M.A., Meunier D., Fischer C., Machat G., Mattes K., Lichtenberger B.M., Brunmeir R., Weissmann S., Murko C., et al. Divergent roles of HDAC1 and HDAC2 in the regulation of epidermal development and tumorigenesis. EMBO J. 2013;32:3176–3191. doi: 10.1038/emboj.2013.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Segre C.V., Chiocca S. Regulating the regulators: The post-translational code of class I HDAC1 and HDAC2. J. Biomed. Biotechnol. 2011;2011:690848. doi: 10.1155/2011/690848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Schultz B.E., Misialek S., Wu J., Tang J., Conn M.T., Tahilramani R., Wong L. Kinetics and comparative reactivity of human class I and class IIb histone deacetylases. Biochemistry. 2004;43:11083–11091. doi: 10.1021/bi0494471. [DOI] [PubMed] [Google Scholar]
- 53.Pflum M.K., Tong J.K., Lane W.S., Schreiber S.L. Histone deacetylase 1 phosphorylation promotes enzymatic activity and complex formation. J. Biol. Chem. 2001;276:47733–47741. doi: 10.1074/jbc.M105590200. [DOI] [PubMed] [Google Scholar]
- 54.Galasinski S.C., Resing K.A., Goodrich J.A., Ahn N.G. Phosphatase inhibition leads to histone deacetylases 1 and 2 phosphorylation and disruption of corepressor interactions. J. Biol. Chem. 2002;277:19618–19626. doi: 10.1074/jbc.M201174200. [DOI] [PubMed] [Google Scholar]
- 55.Cai R., Kwon P., Yan-Neale Y., Sambuccetti L., Fischer D., Cohen D. Mammalian histone deacetylase 1 protein is posttranslationally modified by phosphorylation. Biochem. Biophys. Res. Commun. 2001;283:445–453. doi: 10.1006/bbrc.2001.4786. [DOI] [PubMed] [Google Scholar]
- 56.Karwowska-Desaulniers P., Ketko A., Kamath N., Pflum M.K. Histone deacetylase 1 phosphorylation at S421 and S423 is constitutive in vivo, but dispensable in vitro. Biochem. Biophys. Res. Commun. 2007;361:349–355. doi: 10.1016/j.bbrc.2007.06.167. [DOI] [PubMed] [Google Scholar]
- 57.Gnad F., Ren S., Cox J., Olsen J.V., Macek B., Oroshi M., Mann M. PHOSIDA (phosphorylation site database): Management, structural and evolutionary investigation, and prediction of phosphosites. Genome Biol. 2007;8:R250. doi: 10.1186/gb-2007-8-11-r250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Olsen J.V., Blagoev B., Gnad F., Macek B., Kumar C., Mortensen P., Mann M. Global, in vivo, and site-specific phosphorylation dynamics in signaling networks. Cell. 2006;127:635–648. doi: 10.1016/j.cell.2006.09.026. [DOI] [PubMed] [Google Scholar]
- 59.Qiu Y., Stavreva D.A., Luo Y., Indrawan A., Chang M., Hager G.L. Dynamic Interaction of HDAC1 with a Glucocorticoid Receptor-regulated Gene Is Modulated by the Activity State of the Promoter. J. Biol. Chem. 2011;286:7641–7647. doi: 10.1074/jbc.M110.185488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Dobbin M.M., Madabhushi R., Pan L., Chen Y., Kim D., Gao J., Ahanonu B., Pao P.C., Qiu Y., Zhao Y., et al. SIRT1 collaborates with ATM and HDAC1 to maintain genomic stability in neurons. Nat. Neurosci. 2013;16:1008–1015. doi: 10.1038/nn.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yang H., Yan B., Liao D., Huang S., Qiu Y. Acetylation of HDAC1 and degradation of SIRT1 form a positive feedback loop to regulate p53 acetylation during heat-shock stress. Cell Death Dis. 2015;6:e1747. doi: 10.1038/cddis.2015.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hattangadi S.M., Wong P., Zhang L., Flygare J., Lodish H.F. From stem cell to red cell: Regulation of erythropoiesis at multiple levels by multiple proteins, RNAs, and chromatin modifications. Blood. 2011;118:6258–6268. doi: 10.1182/blood-2011-07-356006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Palis J., Segel G.B. Developmental biology of erythropoiesis. Blood Rev. 1998;12:106–114. doi: 10.1016/S0268-960X(98)90022-4. [DOI] [PubMed] [Google Scholar]
- 64.Heideman M.R., Lancini C., Proost N., Yanover E., Jacobs H., Dannenberg J.H. Sin3a-associated Hdac1 and Hdac2 are essential for hematopoietic stem cell homeostasis and contribute differentially to hematopoiesis. Haematologica. 2014;99:1292–1303. doi: 10.3324/haematol.2013.092643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wada T., Kikuchi J., Nishimura N., Shimizu R., Kitamura T., Furukawa Y. Expression levels of histone deacetylases determine the cell fate of hematopoietic progenitors. J. Biol. Chem. 2009;284:30673–30683. doi: 10.1074/jbc.M109.042242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fujieda A., Katayama N., Ohishi K., Yamamura K., Shibasaki T., Sugimoto Y., Miyata E., Nishi K., Masuya M., Ueda H., et al. A putative role for histone deacetylase in the differentiation of human erythroid cells. Int. J. Oncol. 2005;27:743–748. [PubMed] [Google Scholar]
- 67.Yamamura K., Ohishi K., Katayama N., Yu Z., Kato K., Masuya M., Fujieda A., Sugimoto Y., Miyata E., Shibasaki T., et al. Pleiotropic role of histone deacetylases in the regulation of human adult erythropoiesis. Br. J. Haematol. 2006;135:242–253. doi: 10.1111/j.1365-2141.2006.06275.x. [DOI] [PubMed] [Google Scholar]
- 68.Huang S., Brandt S.J. mSin3A regulates murine erythroleukemia cell differentiation through association with the TAL1 (or SCL) transcription factor. Mol. Cell. Biol. 2000;20:2248–2259. doi: 10.1128/MCB.20.6.2248-2259.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cantor A.B., Orkin S.H. Transcriptional regulation of erythropoiesis: An affair involving multiple partners. Oncogene. 2002;21:3368–3376. doi: 10.1038/sj.onc.1205326. [DOI] [PubMed] [Google Scholar]
- 70.Migliaccio A.R., Rana R.A., Vannucchi A.M., Manzoli F.A. Role of GATA-1 in normal and neoplastic hemopoiesis. Ann. N. Y. Acad. Sci. 2005;1044:142–158. doi: 10.1196/annals.1349.019. [DOI] [PubMed] [Google Scholar]
- 71.Crispino J.D. GATA1 in normal and malignant hematopoiesis. Semin Cell Dev. Biol. 2005;16:137–147. doi: 10.1016/j.semcdb.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 72.Ferreira R., Ohneda K., Yamamoto M., Philipsen S. GATA1 function, a paradigm for transcription factors in hematopoiesis. Mol. Cell. Biol. 2005;25:1215–1227. doi: 10.1128/MCB.25.4.1215-1227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Harigae H. GATA transcription factors and hematological diseases. Tohoku J. Exp. Med. 2006;210:1–9. doi: 10.1620/tjem.210.1. [DOI] [PubMed] [Google Scholar]
- 74.Fujiwara Y., Browne C.P., Cunniff K., Goff S.C., Orkin S.H. Arrested development of embryonic red cell precursors in mouse embryos lacking transcription factor GATA-1. Proc. Natl. Acad. Sci. USA. 1996;93:12355–12358. doi: 10.1073/pnas.93.22.12355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Pevny L., Lin C.S., D’Agati V., Simon M.C., Orkin S.H., Costantini F. Development of hematopoietic cells lacking transcription factor GATA-1. Development. 1995;121:163–172. doi: 10.1242/dev.121.1.163. [DOI] [PubMed] [Google Scholar]
- 76.Pevny L., Simon M.C., Robertson E., Klein W.H., Tsai S.F., D’Agati V., Orkin S.H., Costantini F. Erythroid differentiation in chimaeric mice blocked by a targeted mutation in the gene for transcription factor GATA-1. Nature. 1991;349:257–260. doi: 10.1038/349257a0. [DOI] [PubMed] [Google Scholar]
- 77.Blobel G.A., Nakajima T., Eckner R., Montminy M., Orkin S.H. CREB-binding protein cooperates with transcription factor GATA-1 and is required for erythroid differentiation. Proc. Natl. Acad. Sci. USA. 1998;95:2061–2066. doi: 10.1073/pnas.95.5.2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bresnick E.H., Lee H.Y., Fujiwara T., Johnson K.D., Keles S. GATA switches as developmental drivers. J. Biol. Chem. 2010;285:31087–31093. doi: 10.1074/jbc.R110.159079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Wolff L., Humeniuk R. Concise review: Erythroid versus myeloid lineage commitment: Regulating the master regulators. Stem Cells. 2013;31:1237–1244. doi: 10.1002/stem.1379. [DOI] [PubMed] [Google Scholar]
- 80.Cantor A.B., Katz S.G., Orkin S.H. Distinct domains of the GATA-1 cofactor FOG-1 differentially influence erythroid versus megakaryocytic maturation. Mol. Cell. Biol. 2002;22:4268–4279. doi: 10.1128/MCB.22.12.4268-4279.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kadam S., Emerson B.M. Mechanisms of chromatin assembly and transcription. Curr. Opin. Cell Biol. 2002;14:262–268. doi: 10.1016/S0955-0674(02)00330-7. [DOI] [PubMed] [Google Scholar]
- 82.Rodriguez P., Bonte E., Krijgsveld J., Kolodziej K.E., Guyot B., Heck A.J., Vyas P., de Boer E., Grosveld F., Strouboulis J. GATA-1 forms distinct activating and repressive complexes in erythroid cells. EMBO J. 2005;24:2354–2366. doi: 10.1038/sj.emboj.7600702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hong W., Nakazawa M., Chen Y.Y., Kori R., Vakoc C.R., Rakowski C., Blobel G.A. FOG-1 recruits the NuRD repressor complex to mediate transcriptional repression by GATA-1. EMBO J. 2005;24:2367–2378. doi: 10.1038/sj.emboj.7600703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Snow J.W., Kim J., Currie C.R., Xu J., Orkin S.H. Sumoylation regulates interaction of FOG1 with C-terminal-binding protein (CTBP) J. Biol. Chem. 2010;285:28064–28075. doi: 10.1074/jbc.M109.096909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kim M.Y., Kim J.S., Son S.H., Lim C.S., Eum H.Y., Ha D.H., Park M.A., Baek E.J., Ryu B.Y., Kang H.C., et al. Mbd2-CP2c loop drives adult-type globin gene expression and definitive erythropoiesis. Nucleic Acids Res. 2018;46:4933–4949. doi: 10.1093/nar/gky193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Boyes J., Byfield P., Nakatani Y., Ogryzko V. Regulation of activity of the transcription factor GATA-1 by acetylation. Nature. 1998;396:594–598. doi: 10.1038/25166. [DOI] [PubMed] [Google Scholar]
- 87.Hung H.L., Lau J., Kim A.Y., Weiss M.J., Blobel G.A. CREB-Binding protein acetylates hematopoietic transcription factor GATA-1 at functionally important sites. Mol. Cell. Biol. 1999;19:3496–3505. doi: 10.1128/MCB.19.5.3496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Lamonica J.M., Deng W., Kadauke S., Campbell A.E., Gamsjaeger R., Wang H., Cheng Y., Billin A.N., Hardison R.C., Mackay J.P., et al. Bromodomain protein Brd3 associates with acetylated GATA1 to promote its chromatin occupancy at erythroid target genes. Proc. Natl. Acad. Sci. USA. 2011;108:E159–E168. doi: 10.1073/pnas.1102140108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamonica J.M., Vakoc C.R., Blobel G.A. Acetylation of GATA-1 is required for chromatin occupancy. Blood. 2006;108:3736–3738. doi: 10.1182/blood-2006-07-032847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zheng W.W., Dong X.M., Yin R.H., Xu F.F., Ning H.M., Zhang M.J., Xu C.W., Yang Y., Ding Y.L., Wang Z.D., et al. EDAG positively regulates erythroid differentiation and modifies GATA1 acetylation through recruiting p300. Stem Cells. 2014;32:2278–2289. doi: 10.1002/stem.1723. [DOI] [PubMed] [Google Scholar]
- 91.Hernandez-Hernandez A., Ray P., Litos G., Ciro M., Ottolenghi S., Beug H., Boyes J. Acetylation and MAPK phosphorylation cooperate to regulate the degradation of active GATA-1. EMBO J. 2006;25:3264–3274. doi: 10.1038/sj.emboj.7601228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Watamoto K., Towatari M., Ozawa Y., Miyata Y., Okamoto M., Abe A., Naoe T., Saito H. Altered interaction of HDAC5 with GATA-1 during MEL cell differentiation. Oncogene. 2003;22:9176–9184. doi: 10.1038/sj.onc.1206902. [DOI] [PubMed] [Google Scholar]
- 93.Porcher C., Swat W., Rockwell K., Fujiwara Y., Alt F.W., Orkin S.H. The T cell leukemia oncoprotein SCL/tal-1 is essential for development of all hematopoietic lineages. Cell. 1996;86:47–57. doi: 10.1016/S0092-8674(00)80076-8. [DOI] [PubMed] [Google Scholar]
- 94.Shivdasani R.A., Mayer E.L., Orkin S.H. Absence of blood formation in mice lacking the T-cell leukaemia oncoprotein tal-1/SCL. Nature. 1995;373:432–434. doi: 10.1038/373432a0. [DOI] [PubMed] [Google Scholar]
- 95.Robb L., Lyons I., Li R., Hartley L., Köntgen F., Harvey R.P., Metcalf D., Begley C.G. Absence of yolk sac hematopoiesis from mice with a targeted disruption of the scl gene. Proc. Natl. Acad. Sci. USA. 1995;92:7075–7079. doi: 10.1073/pnas.92.15.7075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Robb L., Elwood N.J., Elefanty A.G., Köntgen F., Li R., Barnett L.D., Begley C.G. The scl gene product is required for the generation of all hematopoietic lineages in the adult mouse. EMBO J. 1996;15:4123–4129. doi: 10.1002/j.1460-2075.1996.tb00787.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Anantharaman A., Lin I.J., Barrow J., Liang S.Y., Masannat J., Strouboulis J., Huang S., Bungert J. Role of helix-loop-helix proteins during differentiation of erythroid cells. Mol. Cell. Biol. 2011;31:1332–1343. doi: 10.1128/MCB.01186-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Anderson K.P., Crable S.C., Lingrel J.B. The GATA-E box-GATA motif in the EKLF promoter is required for in vivo expression. Blood. 2000;95:1652–1655. doi: 10.1182/blood.V95.5.1652.005k23_1652_1655. [DOI] [PubMed] [Google Scholar]
- 99.Xu Z., Huang S., Chang L.S., Agulnick A.D., Brandt S.J. Identification of a TAL1 target gene reveals a positive role for the LIM domain-binding protein Ldb1 in erythroid gene expression and differentiation. Mol. Cell. Biol. 2003;23:7585–7599. doi: 10.1128/MCB.23.21.7585-7599.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vyas P., McDevitt M.A., Cantor A.B., Katz S.G., Fujiwara Y., Orkin S.H. Different sequence requirements for expression in erythroid and megakaryocytic cells within a regulatory element upstream of the GATA-1 gene. Development. 1999;126:2799–2811. doi: 10.1242/dev.126.12.2799. [DOI] [PubMed] [Google Scholar]
- 101.Song S.H., Hou C., Dean A. A positive role for NLI/Ldb1 in long-range beta-globin locus control region function. Mol. Cell. 2007;28:810–822. doi: 10.1016/j.molcel.2007.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hu X., Ybarra R., Qiu Y., Bungert J., Huang S. Transcriptional regulation by TAL1: A link between epigenetic modifications and erythropoiesis. Epigenetics. 2009;4:357–361. doi: 10.4161/epi.4.6.9711. [DOI] [PubMed] [Google Scholar]
- 103.Huang S.Z., Ren Z.R., Chen M.J., Xu H.P., Zeng Y.T., Rodgers G.P., Zeng F.Y., Schechter A.N. Treatment of beta-thalassemia with hydroxyurea (HU)—Effects of HU on globin gene expression. Sci. China B. 1994;37:1350–1359. [PubMed] [Google Scholar]
- 104.Schuh A.H., Tipping A.J., Clark A.J., Hamlett I., Guyot B., Iborra F.J., Rodriguez P., Strouboulis J., Enver T., Vyas P., et al. ETO-2 associates with SCL in erythroid cells and megakaryocytes and provides repressor functions in erythropoiesis. Mol. Cell. Biol. 2005;25:10235–10250. doi: 10.1128/MCB.25.23.10235-10250.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Goardon N., Lambert J.A., Rodriguez P., Nissaire P., Herblot S., Thibault P., Dumenil D., Strouboulis J., Romeo P.H., Hoang T. ETO2 coordinates cellular proliferation and differentiation during erythropoiesis. EMBO J. 2006;25:357–366. doi: 10.1038/sj.emboj.7600934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Amann J.M., Nip J., Strom D.K., Lutterbach B., Harada H., Lenny N., Downing J.R., Meyers S., Hiebert S.W. ETO, a target of t(8;21) in acute leukemia, makes distinct contacts with multiple histone deacetylases and binds mSin3A through its oligomerization domain. Mol. Cell. Biol. 2001;21:6470–6483. doi: 10.1128/MCB.21.19.6470-6483.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gelmetti V., Zhang J., Fanelli M., Minucci S., Pelicci P.G., Lazar M.A. Aberrant recruitment of the nuclear receptor corepressor-histone deacetylase complex by the acute myeloid leukemia fusion partner ETO. Mol. Cell. Biol. 1998;18:7185–7191. doi: 10.1128/MCB.18.12.7185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang J., Hoshino T., Redner R.L., Kajigaya S., Liu J.M. ETO, fusion partner in t(8;21) acute myeloid leukemia, represses transcription by interaction with the human N-CoR/mSin3/HDAC1 complex. Proc. Natl. Acad. Sci. USA. 1998;95:10860–10865. doi: 10.1073/pnas.95.18.10860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Staber P.B., Zhang P., Ye M., Welner R.S., Nombela-Arrieta C., Bach C., Kerenyi M., Bartholdy B.A., Zhang H., Alberich-Jorda M., et al. Sustained PU.1 levels balance cell-cycle regulators to prevent exhaustion of adult hematopoietic stem cells. Mol. Cell. 2013;49:934–946. doi: 10.1016/j.molcel.2013.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yamada T., Abe M., Higashi T., Yamamoto H., Kihara-Negishi F., Sakurai T., Shirai T., Oikawa T. Lineage switch induced by overexpression of Ets family transcription factor PU.1 in murine erythroleukemia cells. Blood. 2001;97:2300–2307. doi: 10.1182/blood.V97.8.2300. [DOI] [PubMed] [Google Scholar]
- 111.Mak K.S., Funnell A.P.W., Pearson R.C.M., Crossley M. PU.1 and Haematopoietic Cell Fate: Dosage Matters. Int. J. Cell Biol. 2011;2011 doi: 10.1155/2011/808524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Arinobu Y., Mizuno S., Chong Y., Shigematsu H., Iino T., Iwasaki H., Graf T., Mayfield R., Chan S., Kastner P., et al. Reciprocal activation of GATA-1 and PU.1 marks initial specification of hematopoietic stem cells into myeloerythroid and myelolymphoid lineages. Cell Stem Cell. 2007;1:416–427. doi: 10.1016/j.stem.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 113.Iwasaki H., Somoza C., Shigematsu H., Duprez E.A., Iwasaki-Arai J., Mizuno S., Arinobu Y., Geary K., Zhang P., Dayaram T., et al. Distinctive and indispensable roles of PU.1 in maintenance of hematopoietic stem cells and their differentiation. Blood. 2005;106:1590–1600. doi: 10.1182/blood-2005-03-0860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Richardson C.L., Delehanty L.L., Bullock G.C., Rival C.M., Tung K.S., Kimpel D.L., Gardenghi S., Rivella S., Goldfarb A.N. Isocitrate ameliorates anemia by suppressing the erythroid iron restriction response. J. Clin. Investig. 2013;123:3614–3623. doi: 10.1172/JCI68487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang Q., Zhang M., Wang X., Yuan W., Chen D., Royer-Pokora B., Zhu T. A novel transcript of the LMO2 gene, LMO2-c, is regulated by GATA-1 and PU.1 and encodes an antagonist of LMO2. Leukemia. 2007;21:1015–1025. doi: 10.1038/sj.leu.2404644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Rekhtman N., Radparvar F., Evans T., Skoultchi A.I. Direct interaction of hematopoietic transcription factors PU.1 and GATA-1: Functional antagonism in erythroid cells. Genes Dev. 1999;13:1398–1411. doi: 10.1101/gad.13.11.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhang P., Behre G., Pan J., Iwama A., Wara-Aswapati N., Radomska H.S., Auron P.E., Tenen D.G., Sun Z. Negative cross-talk between hematopoietic regulators: GATA proteins repress PU.1. Proc. Natl. Acad. Sci. USA. 1999;96:8705–8710. doi: 10.1073/pnas.96.15.8705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Back J., Allman D., Chan S., Kastner P. Visualizing PU.1 activity during hematopoiesis. Exp. Hematol. 2005;33:395–402. doi: 10.1016/j.exphem.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 119.Nutt S.L., Metcalf D., D’Amico A., Polli M., Wu L. Dynamic regulation of PU.1 expression in multipotent hematopoietic progenitors. J. Exp. Med. 2005;201:221–231. doi: 10.1084/jem.20041535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Rosenbauer F., Owens B.M., Yu L., Tumang J.R., Steidl U., Kutok J.L., Clayton L.K., Wagner K., Scheller M., Iwasaki H., et al. Lymphoid cell growth and transformation are suppressed by a key regulatory element of the gene encoding PU.1. Nat. Genet. 2006;38:27–37. doi: 10.1038/ng1679. [DOI] [PubMed] [Google Scholar]
- 121.Moreau-Gachelin F., Wendling F., Molina T., Denis N., Titeux M., Grimber G., Briand P., Vainchenker W., Tavitian A. Spi-1/PU.1 transgenic mice develop multistep erythroleukemias. Mol. Cell. Biol. 1996;16:2453–2463. doi: 10.1128/MCB.16.5.2453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Will B., Vogler T.O., Narayanagari S., Bartholdy B., Todorova T.I., da Silva Ferreira M., Chen J., Yu Y., Mayer J., Barreyro L., et al. Minimal PU.1 reduction induces a preleukemic state and promotes development of acute myeloid leukemia. Nat. Med. 2015;21:1172–1181. doi: 10.1038/nm.3936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Amaravadi L., Klemsz M.J. DNA methylation and chromatin structure regulate PU.1 expression. DNA Cell Biol. 1999;18:875–884. doi: 10.1089/104454999314737. [DOI] [PubMed] [Google Scholar]
- 124.Laribee R.N., Klemsz M.J. Loss of PU.1 expression following inhibition of histone deacetylases. J. Immunol. 2001;167:5160–5166. doi: 10.4049/jimmunol.167.9.5160. [DOI] [PubMed] [Google Scholar]
- 125.Bieniossek C., Papai G., Schaffitzel C., Garzoni F., Chaillet M., Scheer E., Papadopoulos P., Tora L., Schultz P., Berger I. The architecture of human general transcription factor TFIID core complex. Nature. 2013;493:699–702. doi: 10.1038/nature11791. [DOI] [PubMed] [Google Scholar]
- 126.Cler E., Papai G., Schultz P., Davidson I. Recent advances in understanding the structure and function of general transcription factor TFIID. Cell Mol. Life Sci. 2009;66:2123–2134. doi: 10.1007/s00018-009-0009-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Juven-Gershon T., Kadonaga J.T. Regulation of gene expression via the core promoter and the basal transcriptional machinery. Dev. Biol. 2010;339:225–229. doi: 10.1016/j.ydbio.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Burke T.W., Kadonaga J.T. Drosophila TFIID binds to a conserved downstream basal promoter element that is present in many TATA-box-deficient promoters. Genes Dev. 1996;10:711–724. doi: 10.1101/gad.10.6.711. [DOI] [PubMed] [Google Scholar]
- 129.Burke T.W., Kadonaga J.T. The downstream core promoter element, DPE, is conserved from Drosophila to humans and is recognized by TAFII60 of Drosophila. Genes Dev. 1997;11:3020–3031. doi: 10.1101/gad.11.22.3020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kihara-Negishi F., Yamamoto H., Suzuki M., Yamada T., Sakurai T., Tamura T., Oikawa T. In vivo complex formation of PU.1 with HDAC1 associated with PU.1-mediated transcriptional repression. Oncogene. 2001;20:6039–6047. doi: 10.1038/sj.onc.1204756. [DOI] [PubMed] [Google Scholar]
- 131.Suzuki M., Yamada T., Kihara-Negishi F., Sakurai T., Oikawa T. Direct association between PU.1 and MeCP2 that recruits mSin3A-HDAC complex for PU.1-mediated transcriptional repression. Oncogene. 2003;22:8688–8698. doi: 10.1038/sj.onc.1207182. [DOI] [PubMed] [Google Scholar]
- 132.Kihara-Negishi F., Suzuki M., Yamada T., Sakurai T., Oikawa T. Impaired repressor activity and biological functions of PU.1 in MEL cells induced by mutations in the acetylation motifs within the ETS domain. Biochem. Biophys. Res. Commun. 2005;335:477–484. doi: 10.1016/j.bbrc.2005.07.098. [DOI] [PubMed] [Google Scholar]
- 133.Siatecka M., Bieker J.J. The multifunctional role of EKLF/KLF1 during erythropoiesis. Blood. 2011;118:2044–2054. doi: 10.1182/blood-2011-03-331371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Perkins A.C., Sharpe A.H., Orkin S.H. Lethal beta-thalassaemia in mice lacking the erythroid CACCC-transcription factor EKLF. Nature. 1995;375:318–322. doi: 10.1038/375318a0. [DOI] [PubMed] [Google Scholar]
- 135.Nuez B., Michalovich D., Bygrave A., Ploemacher R., Grosveld F. Defective haematopoiesis in fetal liver resulting from inactivation of the EKLF gene. Nature. 1995;375:316–318. doi: 10.1038/375316a0. [DOI] [PubMed] [Google Scholar]
- 136.Hodge D., Coghill E., Keys J., Maguire T., Hartmann B., McDowall A., Weiss M., Grimmond S., Perkins A. A global role for EKLF in definitive and primitive erythropoiesis. Blood. 2006;107:3359–3370. doi: 10.1182/blood-2005-07-2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tallack M.R., Whitington T., Yuen W.S., Wainwright E.N., Keys J.R., Gardiner B.B., Nourbakhsh E., Cloonan N., Grimmond S.M., Bailey T.L., et al. A global role for KLF1 in erythropoiesis revealed by ChIP-seq in primary erythroid cells. Genome Res. 2010;20:1052–1063. doi: 10.1101/gr.106575.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Zhang W., Kadam S., Emerson B.M., Bieker J.J. Site-specific acetylation by p300 or CREB binding protein regulates erythroid Krüppel-like factor transcriptional activity via its interaction with the SWI-SNF complex. Mol. Cell. Biol. 2001;21:2413–2422. doi: 10.1128/MCB.21.7.2413-2422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sengupta T., Chen K., Milot E., Bieker J.J. Acetylation of EKLF is essential for epigenetic modification and transcriptional activation of the beta-globin locus. Mol. Cell. Biol. 2008;28:6160–6170. doi: 10.1128/MCB.00919-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Mas C., Lussier-Price M., Soni S., Morse T., Arseneault G., Di Lello P., Lafrance-Vanasse J., Bieker J.J., Omichinski J.G. Structural and functional characterization of an atypical activation domain in erythroid Kruppel-like factor (EKLF) Proc. Natl. Acad. Sci. USA. 2011;108:10484–10489. doi: 10.1073/pnas.1017029108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Chen X., Bieker J.J. Stage-specific repression by the EKLF transcriptional activator. Mol. Cell. Biol. 2004;24:10416–10424. doi: 10.1128/MCB.24.23.10416-10424.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Yien Y.Y., Bieker J.J. EKLF/KLF1, a tissue-restricted integrator of transcriptional control, chromatin remodeling, and lineage determination. Mol. Cell. Biol. 2013;33:4–13. doi: 10.1128/MCB.01058-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Siatecka M., Xue L., Bieker J.J. Sumoylation of EKLF promotes transcriptional repression and is involved in inhibition of megakaryopoiesis. Mol. Cell. Biol. 2007;27:8547–8560. doi: 10.1128/MCB.00589-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sengupta T., Cohet N., Morle F., Bieker J.J. Distinct modes of gene regulation by a cell-specific transcriptional activator. Proc. Natl. Acad. Sci. USA. 2009;106:4213–4218. doi: 10.1073/pnas.0808347106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ohler U., Wassarman D.A. Promoting developmental transcription. Development. 2010;137:15–26. doi: 10.1242/dev.035493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Duan Z., Horwitz M. Gfi-1 oncoproteins in hematopoiesis. Hematology. 2003;8:339–344. doi: 10.1080/10245330310001612116. [DOI] [PubMed] [Google Scholar]
- 147.Saleque S., Cameron S., Orkin S.H. The zinc-finger proto-oncogene Gfi-1b is essential for development of the erythroid and megakaryocytic lineages. Genes Dev. 2002;16:301–306. doi: 10.1101/gad.959102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Foudi A., Kramer D.J., Qin J., Ye D., Behlich A.S., Mordecai S., Preffer F.I., Amzallag A., Ramaswamy S., Hochedlinger K., et al. Distinct, strict requirements for Gfi-1b in adult bone marrow red cell and platelet generation. J. Exp. Med. 2014;211:909–927. doi: 10.1084/jem.20131065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Saleque S., Kim J., Rooke H.M., Orkin S.H. Epigenetic regulation of hematopoietic differentiation by Gfi-1 and Gfi-1b is mediated by the cofactors CoREST and LSD1. Mol. Cell. 2007;27:562–572. doi: 10.1016/j.molcel.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 150.Laurent B., Randrianarison-Huetz V., Frisan E., Andrieu-Soler C., Soler E., Fontenay M., Dusanter-Fourt I., Duménil D. A short Gfi-1B isoform controls erythroid differentiation by recruiting the LSD1-CoREST complex through the dimethylation of its SNAG domain. J. Cell Sci. 2012;125:993–1002. doi: 10.1242/jcs.095877. [DOI] [PubMed] [Google Scholar]
- 151.Uda M., Galanello R., Sanna S., Lettre G., Sankaran V.G., Chen W., Usala G., Busonero F., Maschio A., Albai G., et al. Genome-wide association study shows BCL11A associated with persistent fetal hemoglobin and amelioration of the phenotype of beta-thalassemia. Proc. Natl. Acad. Sci. USA. 2008;105:1620–1625. doi: 10.1073/pnas.0711566105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Vinjamur D.S., Bauer D.E., Orkin S.H. Recent progress in understanding and manipulating haemoglobin switching for the haemoglobinopathies. Br. J. Haematol. 2018;180:630–643. doi: 10.1111/bjh.15038. [DOI] [PubMed] [Google Scholar]
- 153.Sankaran V.G., Xu J., Ragoczy T., Ippolito G.C., Walkley C.R., Maika S.D., Fujiwara Y., Ito M., Groudine M., Bender M.A., et al. Developmental and species-divergent globin switching are driven by BCL11A. Nature. 2009;460:1093–1097. doi: 10.1038/nature08243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Xu J., Peng C., Sankaran V.G., Shao Z., Esrick E.B., Chong B.G., Ippolito G.C., Fujiwara Y., Ebert B.L., Tucker P.W., et al. Correction of sickle cell disease in adult mice by interference with fetal hemoglobin silencing. Science. 2011;334:993–996. doi: 10.1126/science.1211053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bauer D.E., Kamran S.C., Lessard S., Xu J., Fujiwara Y., Lin C., Shao Z., Canver M.C., Smith E.C., Pinello L., et al. An erythroid enhancer of BCL11A subject to genetic variation determines fetal hemoglobin level. Science. 2013;342:253–257. doi: 10.1126/science.1242088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Xu J., Bauer D.E., Kerenyi M.A., Vo T.D., Hou S., Hsu Y.J., Yao H., Trowbridge J.J., Mandel G., Orkin S.H. Corepressor-dependent silencing of fetal hemoglobin expression by BCL11A. Proc. Natl. Acad. Sci. USA. 2013;110:6518–6523. doi: 10.1073/pnas.1303976110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Xu J., Sankaran V.G., Ni M., Menne T.F., Puram R.V., Kim W., Orkin S.H. Transcriptional silencing of γ-globin by BCL11A involves long-range interactions and cooperation with SOX6. Genes Dev. 2010;24:783–798. doi: 10.1101/gad.1897310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liu N., Hargreaves V.V., Zhu Q., Kurland J.V., Hong J., Kim W., Sher F., Macias-Trevino C., Rogers J.M., Kurita R., et al. Direct Promoter Repression by BCL11A Controls the Fetal to Adult Hemoglobin Switch. Cell. 2018;173:430–442.e417. doi: 10.1016/j.cell.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Koulnis M., Porpiglia E., Hidalgo D., Socolovsky M. Erythropoiesis: From molecular pathways to system properties. Adv. Exp. Med. Biol. 2014;844:37–58. doi: 10.1007/978-1-4939-2095-2_3. [DOI] [PubMed] [Google Scholar]
- 160.Perreault A.A., Venters B.J. Integrative view on how erythropoietin signaling controls transcription patterns in erythroid cells. Curr. Opin. Hematol. 2018;25:189–195. doi: 10.1097/MOH.0000000000000415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Grebien F., Kerenyi M.A., Kovacic B., Kolbe T., Becker V., Dolznig H., Pfeffer K., Klingmüller U., Müller M., Beug H., et al. Stat5 activation enables erythropoiesis in the absence of EpoR and Jak2. Blood. 2008;111:4511–4522. doi: 10.1182/blood-2007-07-102848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gillinder K.R., Tuckey H., Bell C.C., Magor G.W., Huang S., Ilsley M.D., Perkins A.C. Direct targets of pSTAT5 signalling in erythropoiesis. PLoS ONE. 2017;12:e0180922. doi: 10.1371/journal.pone.0180922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Kosan C., Ginter T., Heinzel T., Krämer O.H. STAT5 acetylation: Mechanisms and consequences for immunological control and leukemogenesis. JAK-STAT. 2013;2:e26102. doi: 10.4161/jkst.26102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Xu M., Nie L., Kim S.H., Sun X.H. STAT5-induced Id-1 transcription involves recruitment of HDAC1 and deacetylation of C/EBPbeta. EMBO J. 2003;22:893–904. doi: 10.1093/emboj/cdg094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Bauer D.E., Orkin S.H. Hemoglobin switching’s surprise: The versatile transcription factor BCL11A is a master repressor of fetal hemoglobin. Curr. Opin. Genet. Dev. 2015;33:62–70. doi: 10.1016/j.gde.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Ginder G.D. Epigenetic regulation of fetal globin gene expression in adult erythroid cells. Transl. Res. 2015;165:115–125. doi: 10.1016/j.trsl.2014.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Amaya M., Desai M., Gnanapragasam M.N., Wang S.Z., Zu Zhu S., Williams D.C., Jr., Ginder G.D. Mi2β-mediated silencing of the fetal γ-globin gene in adult erythroid cells. Blood. 2013;121:3493–3501. doi: 10.1182/blood-2012-11-466227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yu X., Azzo A., Bilinovich S.M., Li X., Dozmorov M., Kurita R., Nakamura Y., Williams D.C., Jr., Ginder G.D. Disruption of the MBD2-NuRD complex but not MBD3-NuRD induces high level HbF expression in human adult erythroid cells. Haematologica. 2019;104:2361–2371. doi: 10.3324/haematol.2018.210963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Bradner J.E., Mak R., Tanguturi S.K., Mazitschek R., Haggarty S.J., Ross K., Chang C.Y., Bosco J., West N., Morse E., et al. Chemical genetic strategy identifies histone deacetylase 1 (HDAC1) and HDAC2 as therapeutic targets in sickle cell disease. Proc. Natl. Acad. Sci. USA. 2010;107:12617–12622. doi: 10.1073/pnas.1006774107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Esrick E.B., McConkey M., Lin K., Frisbee A., Ebert B.L. Inactivation of HDAC1 or HDAC2 induces gamma globin expression without altering cell cycle or proliferation. Am. J. Hematol. 2015;90:624–628. doi: 10.1002/ajh.24019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Furumai R., Matsuyama A., Kobashi N., Lee K.H., Nishiyama M., Nakajima H., Tanaka A., Komatsu Y., Nishino N., Yoshida M., et al. FK228 (depsipeptide) as a natural prodrug that inhibits class I histone deacetylases. Cancer Res. 2002;62:4916–4921. [PubMed] [Google Scholar]
- 172.Habibi H., Atashi A., Abroun S., Noruzinia M. Synergistic Effect of Simvastatin and Romidepsin on Gamma-globin Gene Induction. Cell J. 2019;20:576–583. doi: 10.22074/cellj.2019.5589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Dai Y., Sangerman J., Luo H.Y., Fucharoen S., Chui D.H., Faller D.V., Perrine S.P. Therapeutic fetal-globin inducers reduce transcriptional repression in hemoglobinopathy erythroid progenitors through distinct mechanisms. Blood Cells Mol. Dis. 2016;56:62–69. doi: 10.1016/j.bcmd.2015.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Sangerman J., Lee M.S., Yao X., Oteng E., Hsiao C.H., Li W., Zein S., Ofori-Acquah S.F., Pace B.S. Mechanism for fetal hemoglobin induction by histone deacetylase inhibitors involves gamma-globin activation by CREB1 and ATF-2. Blood. 2006;108:3590–3599. doi: 10.1182/blood-2006-01-023713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Shearstone J.R., Golonzhka O., Chonkar A., Tamang D., van Duzer J.H., Jones S.S., Jarpe M.B. Chemical Inhibition of Histone Deacetylases 1 and 2 Induces Fetal Hemoglobin through Activation of GATA2. PLoS ONE. 2016;11:e0153767. doi: 10.1371/journal.pone.0153767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Subramanian S., Bates S.E., Wright J.J., Espinoza-Delgado I., Piekarz R.L. Clinical Toxicities of Histone Deacetylase Inhibitors. Pharmaceuticals. 2010;3:2751–2767. doi: 10.3390/ph3092751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Zhao J., Xie C., Edwards H., Wang G., Taub J.W., Ge Y. Histone deacetylases 1 and 2 cooperate in regulating BRCA1, CHK1, and RAD51 expression in acute myeloid leukemia cells. Oncotarget. 2017;8:6319–6329. doi: 10.18632/oncotarget.14062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Schafer C., Goder A., Beyer M., Kiweler N., Mahendrarajah N., Rauch A., Nikolova T., Stojanovic N., Wieczorek M., Reich T.R., et al. Class I histone deacetylases regulate p53/NF-kappaB crosstalk in cancer cells. Cell Signal. 2017;29:218–225. doi: 10.1016/j.cellsig.2016.11.002. [DOI] [PubMed] [Google Scholar]
- 179.Moreno D.A., Scrideli C.A., Cortez M.A., de Paula Queiroz R., Valera E.T., da Silva Silveira V., Yunes J.A., Brandalise S.R., Tone L.G. Differential expression of HDAC3, HDAC7 and HDAC9 is associated with prognosis and survival in childhood acute lymphoblastic leukaemia. Br. J. Haematol. 2010;150:665–673. doi: 10.1111/j.1365-2141.2010.08301.x. [DOI] [PubMed] [Google Scholar]
- 180.Yan B., Chen Q., Shimada K., Tang M., Li H., Gurumurthy A., Khoury J.D., Xu B., Huang S., Qiu Y. Histone deacetylase inhibitor targets CD123/CD47-positive cells and reverse chemoresistance phenotype in acute myeloid leukemia. Leukemia. 2019;33:931–944. doi: 10.1038/s41375-018-0279-6. [DOI] [PubMed] [Google Scholar]
- 181.San Jose-Eneriz E., Gimenez-Camino N., Agirre X., Prosper F. HDAC Inhibitors in Acute Myeloid Leukemia. Cancers. 2019;11:1794. doi: 10.3390/cancers11111794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Ungerstedt J.S. Epigenetic Modifiers in Myeloid Malignancies: The Role of Histone Deacetylase Inhibitors. Int. J. Mol. Sci. 2018;19:3091. doi: 10.3390/ijms19103091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Pace B.S., Chen Y.R., Thompson A., Goodman S.R. Butyrate-inducible elements in the human gamma-globin promoter. Exp. Hematol. 2000;28:283–293. doi: 10.1016/S0301-472X(99)00153-8. [DOI] [PubMed] [Google Scholar]
- 184.McCaffrey P.G., Newsome D.A., Fibach E., Yoshida M., Su M.S. Induction of gamma-globin by histone deacetylase inhibitors. Blood. 1997;90:2075–2083. doi: 10.1182/blood.V90.5.2075. [DOI] [PubMed] [Google Scholar]
- 185.Fathallah H., Weinberg R.S., Galperin Y., Sutton M., Atweh G.F. Role of epigenetic modifications in normal globin gene regulation and butyrate-mediated induction of fetal hemoglobin. Blood. 2007;110:3391–3397. doi: 10.1182/blood-2007-02-076091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Migliaccio A.R., Rotili D., Nebbioso A., Atweh G., Mai A. Histone deacetylase inhibitors and hemoglobin F induction in beta-thalassemia. Int. J. Biochem. Cell Biol. 2008;40:2341–2347. doi: 10.1016/j.biocel.2008.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Okam M.M., Ebert B.L. Novel approaches to the treatment of sickle cell disease: The potential of histone deacetylase inhibitors. Expert Rev. Hematol. 2012;5:303–311. doi: 10.1586/ehm.12.20. [DOI] [PubMed] [Google Scholar]