Abstract
In this study, the relationship between nematicidal activity and chemical composition of ten essential oils (EOs) from different plant species was investigated both in in vitro assays on juveniles (J2) and eggs of the root-knot nematode Meloidogyne incognita and in experiments on tomato in soil infested by M. incognita. Nematode J2 were exposed for 4, 8 or 24 h to 0.78–100 μg mL−1 concentrations of each EO, whereas 24, 48 or 96 h exposures to 250, 500 and 1000 μg mL−1 solutions were tested on M. incognita egg masses. Treatments with 50, 100 or 200 μg kg soil rates of each EO were applied in the experiment on potted tomato. The highest nematicidal potential resulted for the C. verum EO, as highly toxic to both M. incognita J2 and eggs and strongly suppressive on nematode multiplication on tomato roots. The infestation of M. incognita on tomato roots was also strongly reduced by the EOs from E. citriodora and S. aromaticum, both highly toxic to M. incognita J2 but less active on nematode eggs. Adversely, R. graveolens EO strongly inhibited the egg hatch but was limitedly toxic to the infective J2. Chemical composition of the EOs was determined by GC-FID and GC-MS. The ten EOs showed a very different chemical composition in terms of major phytochemicals, with one or two dominant components totally amounting up to 85%. The structure–activity relationship based on the main phytochemicals identified in the assayed EOs and their nematicidal effects on M. incognita was also discussed. Results from this study confirmed that the selection of suitable EO raw materials can lead to the formulation on new effective nematicidal products.
Keywords: essential oils, bioactive components, nematicidal activity, Meloidogyneincognita, sustainable management
1. Introduction
The management of root-knot nematodes (Meloidogyne species) represents a serious issue in many agricultural systems due to the heavy yield losses caused to a large number of herbaceous and fruit crops [1]. Criteria of environmental sustainability imposed by EU politics and public opinion have been reducing the use of synthetic nematicides in favour of more sustainable nematode management strategies [2], such as the use of plant-derived nematicidal compounds [3]. A large number of plant products have been tested for their nematicidal properties throughout the past decades, though only few, such as derivatives of Brassicaceae plants or neem (Azadirachta indica Juss) have been converted into effective industrial nematicidal formulates [4,5,6].
In recent years, researchers’ attention focused on a further promising source of botanical nematicides, such as the essential oils (EOs) produced by a large number of aromatic and medicinal plants, as extensively proved for a biocidal activity on phytoparasitic nematodes also including root-knot species [7,8].
EOs are composite mixtures of different classes of chemical compounds, mainly monoterpenes and sesquiterpenes, with a variable activity on phytoparasitic nematodes and targeting different nematode metabolic sites [8,9,10]. Investigation of structure–activity relationships regulating EOs’ effects against these phytoparasites is a key issue for their application as nematicidal products, as their nematotoxic effectiveness strictly depends on their quanti-qualitative composition and synergistic or antagonistic interactions among present components [11]. Laquale et al. [7] related the strong suppressiveness to the root-knot nematode Meloidogyne incognita Kofoid et White (Chitw.) of two EOs from Monarda didyma L. and M. fistulosa L. to their content of carvacrol and thymol, as repeatedly confirmed for a high nematicidal activity on Meloidogyne species [10,12,13,14,15]. Adversely, the low activity on M. incognita of an EO from Citrus sinensis (L.) Osbeck was attributed to the poor activity of the EO dominant component, limonene [15].
This study focused on the EOs from ten plant species of different botanical and geographical origin, i.e., Cinnamomum camphora (L.) J. Presl. (camphorwood), C. verum J. Presl. (cinnamom), Eucalyptus citriodora Hook (lemon eucalyptus), E. globulus Labill. (blue gum), Mentha piperita L. (peppermint), Pelargonium asperum Ehrh. ex Willd. (bourbon geranium), Ruta graveolens L. (common rue), Schinus molle L. (false pepper) and Syzygium aromaticum (L.) Merr. et Perry (clove). Most of these EOs were already documented for a nematicidal activity, though generally based on limited in vitro bioassays and without any specific correlation to their compositional profile. An in vitro activity on infective root-knot nematode juveniles (J2) and/or eggs was already documented for the EOs of C. verum [16,17], E. citriodora [12,18], M. piperita [10,18], P. asperum [10,18,19], R. graveolens [20,21] and S. aromaticum [22]. Moreover, soil treatments with some of these ten EOs were also documented for variable effects on M. incognita infestation on tomato [23,24].
The aim of this study was a comparative investigation of the nematicidal potential of these ten EOs against M. incognita through both in vitro bioassays on M. incognita infective J2 and eggs and an experiment in soil on potted tomato as well as to elucidate the relationship between nematicidal activity and chemical structure of each EO.
2. Results
2.1. Chemical Composition of the EOs
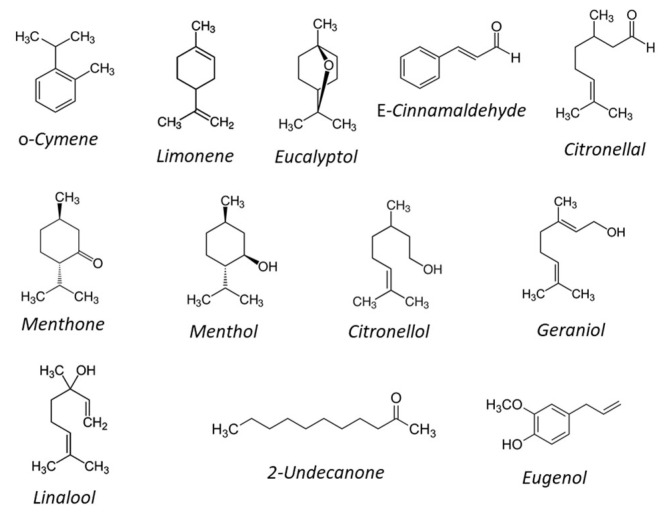
Each of the ten EOs presented a distinctive chemical profile of major components (Table 1; Figure 1).
Table 1.
Chemical composition of the ten essential oils (EOs) as determined by GC-FID and GC-MS analyses.
| Compounds | AI tab a | AI b | Amount% ± SD |
|---|---|---|---|
| C. camphora | |||
| α-Pinene | 932 | 932 | 1.0 ± 0.01 |
| o-Cymene | 1022 | 1023 | 16.8 ± 0.10 |
| Limonene | 1024 | 1025 | 59.3 ± 2.20 |
| Eucalyptol | 1026 | 1026 | 21.8 ± 2.10 |
| Terpinen-4-olo | 1174 | 1175 | 0.5 ± 0.01 |
| α-Terpineol | 1186 | 1186 | 0.36 ± 0.01 |
| C. verum | |||
| Z-Cinnamaldehyde | 1217 | 1217 | 1.8 ± 0.06 |
| E-Cinnamaldehyde | 1267 | 1265 | 84.8 ± 0.29 |
| Eugenol | 1356 | 1356 | 13.4 ± 0.34 |
| C. aurantium | |||
| α-Pinene | 932 | 932 | 0.6 ± 0.006 |
| Sabinene | 969 | 969 | 0.3 ± 0.001 |
| β-Pinene | 974 | 974 | 0.2 ± 0.002 |
| β-Myrcene | 988 | 988 | 1.6 ± 0.002 |
| Limonene | 1024 | 1025 | 94.9 ± 0.02 |
| Linalool | 1095 | 1095 | 1.0 ± 0.01 |
| Linalyl acetate | 1254 | 1255 | 1.5 ± 0.006 |
| E. citriodora | |||
| α-Pinene | 932 | 932 | 1.4 ± 0.01 |
| β-Pinene | 974 | 974 | 1.0 ± 0.03 |
| Eucalyptol | 1026 | 1026 | 1.0 ± 0.20 |
| Isopulegol | 1145 | 1148 | 7.0 ± 0.15 |
| Citronellal | 1148 | 1150 | 83.9 ± 1.50 |
| Citronellol | 1223 | 1223 | 4.7 ± 1.71 |
| Citronellyl formate | 1271 | 1270 | 1.0 ± 0.02 |
| β-Caryophyllene | 1417 | 1417 | 1.0 ± 0.02 |
| E. globulus | |||
| α-Pinene | 932 | 932 | 2.5 ± 0.02 |
| β-Pinene | 974 | 974 | 0.5 ± 0.02 |
| β-Myrcene | 988 | 988 | 0.6 ± 0.02 |
| α-Terpinene | 1014 | 1014 | 0.2 ± 0.02 |
| p.Cymene | 1020 | 1021 | 3.3 ± 0.02 |
| Eucalyptol | 1026 | 1026 | 91.7 ± 0.02 |
| γ-Terpinene | 1054 | 1055 | 0.7 ± 0.02 |
| Terpinen-4-ol-acetate | 1299 | 1298 | 0.2 ± 0.02 |
| α-Terpinyl-acetate | 1346 | 1345 | 0.3 ± 0.02 |
| M. piperita | |||
| α-Pinene | 932 | 932 | 0.4 ± 0.01 |
| β-Pinene | 974 | 974 | 0.6 ± 0.01 |
| Limonene | 1024 | 1026 | 4.5 ± 0.01 |
| Linalool | 1095 | 1095 | 0.4 ± 0.01 |
| iso-Pulegol | 1145 | 1143 | 1.2 ± 0.03 |
| Menthone | 1148 | 1148 | 20.5 ± 0.08 |
| iso-Menthone | 1158 | 1156 | 11.3 ± 0.07 |
| Menthol | 1167 | 1167 | 54.8 ± 0.20 |
| iso-Menthol | 1179 | 1178 | 0.5 ± 0.01 |
| Menthol neo-iso | 1184 | 1182 | 0.2 ± 0.02 |
| α-Terpineol | 1186 | 1186 | 0.3 ± 0.04 |
| Pulegone | 1233 | 1232 | 0.7 ± 0.01 |
| Carvone | 1239 | 1239 | 0.6 ± 0.01 |
| Menthyl-acetate | 1294 | 1292 | 4.0 ± 0.03 |
| P. asperum | |||
| α-Pinene | 932 | 932 | 1.0 ± 0.01 |
| Eucaliptol | 1026 | 1026 | 1.8 ± 0.05 |
| Linalool | 1095 | 1095 | 12.8 ± 0.27 |
| cis-Rose oxide | 1106 | 1108 | 1.3 ± 0.03 |
| trans-Rose oxide | 1122 | 1121 | 0.5 ± 0.02 |
| Menthone | 1148 | 1148 | 1.4 ± 0.02 |
| iso-Menthone | 1158 | 1156 | 5.5 ± 0.24 |
| Citronellol | 1223 | 1223 | 35.0 ± 0.37 |
| Geraniol | 1249 | 1249 | 22.1 ± 0.30 |
| Cytronellyl formate | 1271 | 1273 | 7.0 ± 0.16 |
| Geranyl formate | 1298 | 1296 | 3.8 ± 0.20 |
| α-Copaene | 1374 | 1373 | 0.5 ± 0.10 |
| Geranyl acetate | 1379 | 1379 | 0.5 ± 0.02 |
| α-Bourbonene | 1384 | 1386 | 1.0 ± 0.03 |
| β-Caryophyllene | 1417 | 1417 | 2.0 ± 0.01 |
| trans-Bergamotene | 1432 | 1433 | 1.2 ± 0.04 |
| trans-Murola-3,5-diene | 1451 | 1450 | 1.0 ± 0.04 |
| Citronellyl tiglate | 1656 | 1655 | 1.6 ± 0.05 |
| R. graveolens | |||
| Eucalyptol | 1026 | 1026 | 0.4 ± 0.01 |
| 2-Nonanone | 1087 | 1088 | 0.5 ± 0.02 |
| Camphor | 1141 | 1141 | 0.4 ± 0.01 |
| 2-Decanone | 1190 | 1190 | 0.5 ± 0.04 |
| 2-Undecanone | 1293 | 1294 | 83.2 ± 0.25 |
| Carvacrol | 1298 | 1299 | 15.0 ± 0.31 |
| S. molle | |||
| α-Pinene | 932 | 932 | 14.8 ± 0.16 |
| Sabinene | 969 | 969 | 3.4 ± 0.05 |
| β-Pinene | 974 | 974 | 7.0 ± 0.10 |
| β-Myrcene | 988 | 988 | 1.0 ± 0.05 |
| δ-3-Carene | 1008 | 1008 | 5.5 ± 0.07 |
| p.Cymene | 1021 | 1021 | 6.7 ± 0.09 |
| Limonene | 1024 | 1024 | 7.6 ± 0.09 |
| Eucalyptol | 1026 | 1026 | 0.3 ± 0.04 |
| Linalool | 1095 | 1095 | 10.0 ± 0.17 |
| Terpinene-4-ol | 1174 | 1174 | 1.2 ± 0.03 |
| α-Terpineol | 1186 | 1186 | 1.0 ± 0.05 |
| Linalyl acetate | 1254 | 1255 | 7.3 ± 0.16 |
| Carvacrol | 1299 | 1299 | 3.7 ± 0.14 |
| δ-Elemene | 1335 | 1333 | 0.5 ± 0.01 |
| Eugenol | 1356 | 1356 | 12.0 ± 0.13 |
| β-Caryophyllene | 1417 | 1417 | 5.4 ± 0.07 |
| γ-Curcumene | 1481 | 1480 | 0.8 ± 0.003 |
| Myristicin | 1517 | 1518 | 1.7 ± 0.06 |
| Eugenil acetate | 1521 | 1522 | 1.3 ± 0.03 |
| Cedryl acetate | 1767 | 1766 | 8.8 ± 0.39 |
| S. aromaticum | |||
| Eugenol | 1356 | 1356 | 89.6 ± 0.40 |
| β-Caryophyllene | 1417 | 1417 | 8.0 ± 0.40 |
| α-Humulene | 1452 | 1453 | 2.4 ± 0.01 |
a Arithmetic indexes (AI)from library files (see 4.3)]; b AI calculated by GC relative to a homologous series of n-hydrocarbons (C6-C32) eluted on a DB-5 column.
Figure 1.
Chemical structure of main constituents of the ten EOs.
Thus, both EOs from the two Cinnamomum species, C. camphora and C. verum, contained only few constituents but showed an extremely different compositional pattern. The C. camphora EO was characterized by terpenes, with limonene (59.3%), eucalyptol (21.8%) and o-cymene (16.8%) as the most abundant components, while the EO from C. verum contained phenolics such as E-cinnamaldehyde (84.8%) and eugenol (13.4%). A different chemical composition was also shown by the EOs from the two Eucalyptus species, E. citriodora and E. globulus. Citronellal (83.9%) was the main constituent of E. citriodora EO, whereas eucalyptol (91.7%) was almost the only component of the EO from E. globulus. In both these two EOs, very small amounts of other monoterpenoids were also detected. Limonene was the dominant component of C. aurantium EO, accounting for 94.9% of the total EO, though small amounts of β-myrcene (1.6%), linalool (1.0%) and linalyl acetate (1.5%) were also detected. The sample of M. piperita EO contained mainly menthane-type monoterpenoids, with menthol (54.8%), menthone (20.5%), iso-menthone (11.3%) and menthyl acetate (4.0%) making up the bulk of constituents. The EO from P. asperum was a mixture of several monoterpenoids, with citronellol (35.0%), geraniol (22.1%) and linalool (12.8%) as major components. The dominant component of the R. graveolens EO was the aliphatic ketone 2-undecanone (83.2%), with a very poor content of terpenoids but carvacrol (15.0%). The EO of S. molle was a mixture of several mono- and sesquiterpenes, with α-pinene (14.7%), eugenol (12.0%) and linalool (10.0%) as main monoterpene constituents and cedryl acetate (8.8%) and β-caryophyllene (5.4%) as the two main sesquiterpenes. Finally, only eugenol (89.6%) and the two sesquiterpenes β-caryophyllene (8.0%) and α-humulene (2.4%) were detected in the EO from S. aromaticum.
2.2. Juvenile Mortality Assay
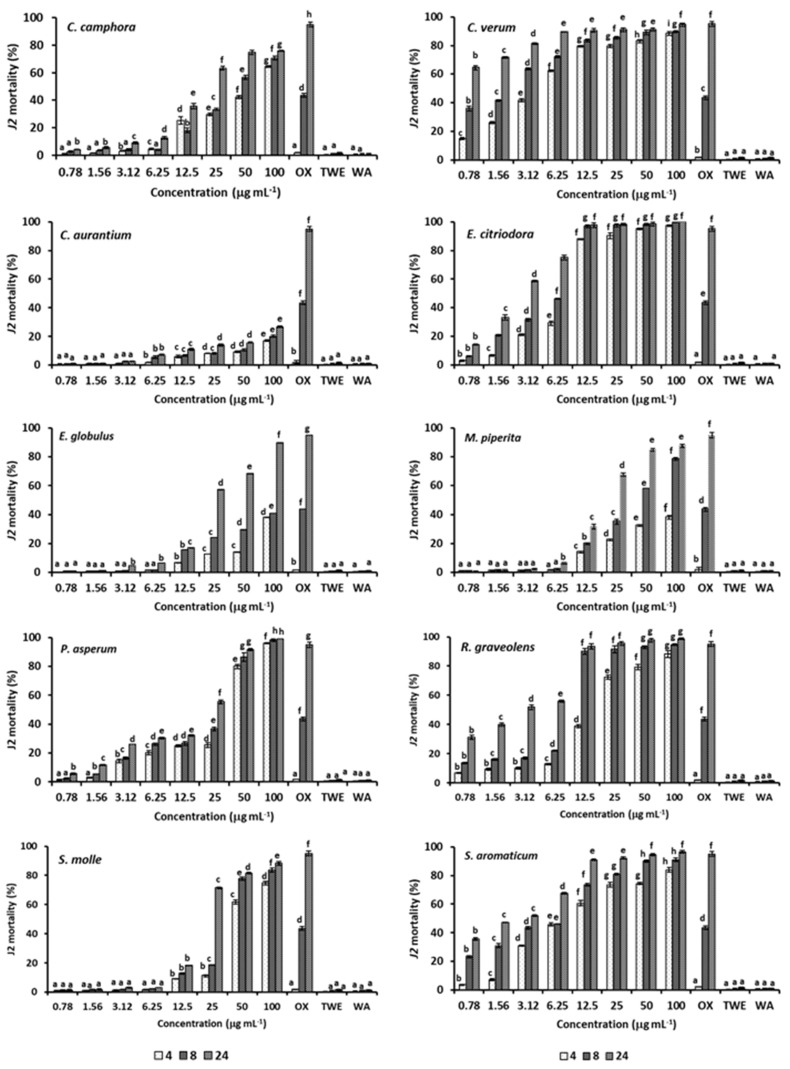
The EOs from the two Cinnamomum species showed a different activity on M. incognita J2, as a 64% mortality rate was reached after 24 exposure to 0.78 and 25 μg mL−1 solutions of C. verum and C. camphora EOs, respectively (Figure 2).
Figure 2.
Mortality (%) of M. incognita J2 after 4, 8 or 24 h exposure to a 0.78–100 μg mL−1 range of concentrations of the ten tested EOs. Data are means of four replicates. At each exposure time, bars marked with the same letter are not significantly different (p ≤ 0.05) according to the least significant difference test.
In addition, both 4 and 8 h treatments with the C. verum EO were also significantly more active than the nematicide Oxamyl at almost all the tested concentrations. Toxicity to M. incognita J2 largely differed also between the two Eucalyptus EOs, as the E. citriodora EO caused an almost complete mortality even after 8 h exposure to a 12.5 μg mL−1 concentration while the EO from E. globulus peaked a 90% J2 mortality only at the maximum concentration x time combination. The EO from P. asperum was strongly toxic at the 50 and 100 μg mL−1 solutions but limitedly active at lower concentrations. At a less instance, a similar performance was provided also by the EOs of M. piperita and S. molle, as both causing more than 80% mortality only at the two highest concentrations. Mortality of J2 ranged 31–56% after a 24 h immersion in 0.78–6.25 μg mL−1 solutions of the R. graveolens EO, but raised above 90% since 8 h exposure to a 12 μg mL−1 concentration. The EO of S. aromaticum resulted in highly toxic to M. incognita J2, as 24 h exposure caused 28.7–31.0% mortality rates even at 0.78 and 1.56 μg mL−1 EO concentrations, respectively, as well as an almost complete mortality (95–97%) at the two highest concentrations. Adversely, the C. aurantium EO was poorly active on nematode J2, as causing only 26.7% mortality after 24 h exposure to the 100 μg mL−1 solution. At all the exposure times, the lowest LC50 values were calculated for the C. verum EO, as confirming the highest activity of this EO within the ten EO samples in comparison (Table 2). Low LC50 values were also estimated for the 24 h treatment with the EOs from S. aromaticum, E. citriodora and R. graveolens EOs, 1.8, 1.6 and 1.4 μg mL−1, respectively. LC50 values of the C. aurantium EO always resulted largely above the range of tested concentrations, as confirming the poor activity on nematode J2.
Table 2.
LD50 values of the ten tested EOs at 4, 8 and 24 h exposures of M. incognita J2.
| EOs | 4 h | 8 h | 24 h | ||||||
|---|---|---|---|---|---|---|---|---|---|
| LD50 | 95% Fiducial CI | LD50 | 95% Fiducial CI | LD50 | 95% Fiducial CI | ||||
| Lower | Upper | Lower | Upper | Lower | Upper | ||||
| C. camphora | 63.3 | 34.1 | 117.6 | 53.9 | 28.7 | 101.1 | 22.9 | 13.1 | 40.0 |
| C. verum | 4.9 | 2.6 | 9.5 | 1.6 | 0.7 | 3.9 | 0.1 | 0.02 | 0.4 |
| C. aurantium | 1704.0 | 416.7 | 6967.4 | 1996.9 | 459.4 | 8681.7 | 447.3 | 154.1 | 1298.4 |
| E. citriodora | 7.6 | 5.0 | 11.6 | 3.9 | 2.7 | 5.8 | 2.4 | 1.6 | 3.6 |
| E. globulus | 264.8 | 112.5 | 623.7 | 470.6 | 141.5 | 1564.7 | 26.7 | 16.8 | 42.6 |
| M. piperita | 167.7 | 76.2 | 369.4 | 44.4 | 26.2 | 75.2 | 20.7 | 13.4 | 32.0 |
| P. asperum | 20.6 | 12.8 | 33.1 | 31.7 | 17.2 | 58.3 | 13.0 | 7.6 | 22.1 |
| R. graveolens | 13.9 | 8.3 | 23.4 | 6.4 | 3.9 | 10.3 | 2.3 | 1.4 | 4.0 |
| S. molle | 70.3 | 39.9 | 123.9 | 43.8 | 26.3 | 72.8 | 22.6 | 14.5 | 35.3 |
| S. aromaticum | 11.8 | 6.9 | 20.3 | 4.4 | 2.9 | 8.4 | 1.8 | 0.9 | 3.6 |
2.3. Egg Hatchability Bioassay
Hatchability of M. incognita eggs was strongly affected by the EOs from R. graveolens and C. verum, as reduced to only 1.2 and 7.0%, respectively, after 96 h egg mass exposure to a 500 μg mL−1 solution, and also resulted always significantly lower than Oxamyl (Table 3). Compared to C. verum, R. graveolens EO solutions were largely more toxic to nematode eggs at the lowest concentration, but significantly less active at 500 and 1000 μg mL−1. The EOs from E. citriodora, P. asperum and S. molle reduced egg hatchability to 54–56% and 40–43% only after a 96 h egg mass exposure to the 500 and 1000 μg mL−1 solutions, respectively. Analogously, the E. globulus EO resulted in a discrete reduction of nematode egg hatchability only at the maximum concentration x time combination. Limited activity on M. incognita eggs was recorded also for the S. aromaticum EO, as 70.5 and 54.2% cumulative hatch values were found even after an egg mass exposure to a 1000 μg mL−1 solution for 24 and 48 h, respectively. Finally, egg hatchability was poorly affected by M. piperita and C. aurantium EOs, as not significantly different or only slightly lower compared to the non-treated control.
Table 3.
Percentage hatchability (means of four replicates ± SE) of M. incognita eggs after 24, 48 or 96 h exposure of egg masses to 125–1000 μg mL−1 solutions of the ten tested EOs.
| Concentrations (μg mL−1) |
Exposure Time (h) | ||
|---|---|---|---|
| 24 | 48 | 96 | |
| C. camphora | |||
| 250 | 93.0 ± 1.7 | 83.7 ± 0.7 | 81.7 ± 0.6 |
| 500 | 87.7 ± 0.8 | 82.7 ± 0.9 | 77.5 ± 1.2 |
| 1000 | 85.2 ± 1.4 | 81.0 ± 0.9 | 73.5 ± 1.6 |
| C. verum | |||
| 250 | 69.5 ± 2.1 | 52.5 ± 1.6 | 42.0 ± 2.2 |
| 500 | 11.0 ± 0.8 | 2.7 ± 0.5 | 1.2 ± 0.2 |
| 1000 | 1.7 ± 0.5 | 1.2 ± 0.2 | 1.0 ± 0.4 |
| C. aurantium | |||
| 250 | 88.5 ± 0.8 | 85.0 ± 1.3 | 80.2 ± 1.2 |
| 500 | 85.0 ± 1.6 | 84.0 ± 0.4 | 79.5 ± 1.9 |
| 1000 | 83.5 ± 1.2 | 79.2 ± 2.6 | 67.5 ± 2.2 |
| E. citriodora | |||
| 250 | 83.7 ± 1.8 | 68.5 ± 1.7 | 63.2 ± 1.6 |
| 500 | 75.7 ± 0.6 | 64.0 ± 1.3 | 55.7 ± 2.2 |
| 1000 | 67.7 ± 1.1 | 60.5 ± 1.0 | 43.2 ± 1.2 |
| E. globulus | |||
| 250 | 86.5 ± 0.6 | 83.5 ± 1.0 | 81.5 ± 2.2 |
| 500 | 84.7 ± 0.7 | 81.2 ± 1.4 | 77.2 ± 1.4 |
| 1000 | 84.2 ± 1.4 | 76.5 ± 1.2 | 66.7 ± 1.5 |
| M. piperita | |||
| 250 | 93.5 ± 0.6 | 90.2 ± 0.9 | 88.5 ± 0.3 |
| 500 | 92.5 ± 0.9 | 89.0 ± 1.5 | 84.0 ± 1.2 |
| 1000 | 90.0 ± 1.9 | 85.2 ± 0.6 | 79.0 ± 1.1 |
| P. asperum | |||
| 250 | 82.2 ± 1.3 | 76.2 ± 0.7 | 65.5 ± 2.7 |
| 500 | 71.2 ± 1.1 | 68.5 ± 0.9 | 54.5 ± 0.9 |
| 1000 | 56.2 ± 5.9 | 52.0 ± 2.2 | 39.7 ± 1.1 |
| R. graveolens | |||
| 250 | 26.7 ± 1.7 | 20.0 ± 0.4 | 13.5 ± 0.3 |
| 500 | 22.7 ± 0.6 | 16.0 ± 0.7 | 7.0 ± 0.4 |
| 1000 | 18.5 ± 1.4 | 5.0 ± 0.7 | 3.2 ± 0.6 |
| S. molle | |||
| 250 | 79.5 ± 1.3 | 74.0 ± 0.4 | 62.2 ± 0.8 |
| 500 | 70..7 ± 1.3 | 65.5 ± 1.0 | 53.7 ± 1.6 |
| 1000 | 65.7 ± 1.4 | 59.7 ± 0.8 | 43.2 ± 1.3 |
| S. aromaticum | |||
| 250 | 82.5 ± 0.9 | 76.0 ± 1.2 | 68.0 ± 0.9 |
| 500 | 77.5 ± 0.9 | 70.0 ± 1.8 | 68.0 ± 1.6 |
| 1000 | 70.5 ± 1.2 | 62.5 ± 1.4 | 54.2 ± 1.1 |
| Oxamyl | 86.2 ± 0.8 | 77.5 ± 0.6 | 66.2 ± 1.5 |
| Tween 20 | 94.2 ± 0.6 | 92.7 ± 0.8 | 92.2 ± 0.8 |
| Non treated | 93.2 ± 1.3 | 93.2 ± 1.3 | 93.2 ± 1.3 |
| LSD 0.05 | 4.5 | 3.3 | 3.9 |
2.4. Experiment in Soil
All the soil treatments with the ten EOs significantly suppressed M. incognita multiplication and gall formation on tomato roots in comparison with the non-treated control, though always resulting significantly less suppressive than the nematicide Oxamyl (Table 4). Soil treatments with the EOs of S. aromaticum, C. verum and E. citriodora resulted in the lowest number of nematode eggs and J2 on tomato roots from, whereas P. asperum and R. graveolens EOs were mainly active on gall formation. The EOs from C. aurantium and M. piperita confirmed their poor activity also in soil, as resulting in the lowest suppressive effect on the root-knot nematode infestation.
Table 4.
Effects (means of five replicates ± SE) of soil treatments with different rates of the ten tested EOs on the infestation of the root-knot nematode M. incognita and the growth of tomato (cv. Roma).
| Dose (μg kg−1 Soil) |
Nematode Infestation Parameters | Plant Fresh Weight | ||
|---|---|---|---|---|
| J2 and Eggs g−1 Roots | Gall Index (0–5) |
Aerial Parts | Roots | |
| C. camphora | ||||
| 50 | 23376 ± 379 | 4.2 ± 0.2 | 37.2 ± 0.2 | 9.4 ± 0.2 |
| 100 | 20579 ± 264 | 4.0 ± 0.0 | 40.2 ± 0.4 | 10.6 ± 0.2 |
| 200 | 12053 ± 179 | 2.8 ± 0.2 | 42.2 ± 0.4 | 11.0 ± 0.3 |
| C. verum | ||||
| 50 | 12297 ± 259 | 2.4 ± 0.2 | 61.0 ± 0.5 | 10.4 ± 0.2 |
| 100 | 11945 ± 125 | 2.2 ± 0.2 | 70.6 ± 1.2 | 11.8 ± 0.2 |
| 200 | 9244 ± 198 | 2.0 ± 0.0 | 77.8 ± 2.3 | 14.2 ± 0.8 |
| C. aurantium | ||||
| 50 | 21492 ± 244 | 5.0 ± 0.0 | 34.4 ± 0.2 | 8.8 ± 0.2 |
| 100 | 18685 ± 212 | 4.6 ± 0.2 | 37.4 ± 0.2 | 9.2 ± 0.2 |
| 200 | 16246 ± 244 | 3.8 ± 0.2 | 38.2 ± 0.5 | 9.4 ± 0.2 |
| E. citriodora | ||||
| 50 | 15302 ± 168 | 4.0 ± 0.0 | 60.6 ± 1.4 | 13.0 ± 0.8 |
| 100 | 11222 ± 157 | 3.0 ± 0.0 | 72.8 ± 1.6 | 14.8 ± 0.7 |
| 200 | 9551 ± 209 | 2.2 ± 0.2 | 86.4 ± 3.1 | 18.4 ± 0.5 |
| E. globulus | ||||
| 50 | 22321 ± 212 | 4.0 ± 0.0 | 40.2 ± 0.5 | 9.6 ± 0.2 |
| 100 | 16963 ± 219 | 3.2 ± 0.2 | 41.6 ± 0.5 | 10.6 ± 0.2 |
| 200 | 14938 ± 266 | 3.0 ± 0.0 | 42.2 ± 0.6 | 12.4 ± 0.2 |
| M. piperita | ||||
| 50 | 20830 ± 484 | 3.8 ± 0.2 | 39.4 ± 0.4 | 9.8 ± 0.5 |
| 100 | 18137 ± 433 | 3.2 ± 0.2 | 40.4 ± 0.4 | 11.4 ± 0.2 |
| 200 | 14183 ± 335 | 2.4 ± 0.2 | 43.4 ± 0.2 | 12.6 ± 0.4 |
| P. asperum | ||||
| 50 | 20197 ± 268 | 3.8 ± 0.2 | 60.2 ± 1.8 | 11.4 ± 0.2 |
| 100 | 15182 ± 307 | 2.6 ± 0.2 | 54.6 ± 0.9 | 10.8 ± 0.9 |
| 200 | 11738 ± 273 | 2.0 ± 0.0 | 47.4 ± 0.9 | 8.0 ± 0.3 |
| R. graveolens | ||||
| 50 | 17871 ± 367 | 4.2 ± 0.4 | 53.6 ± 2.2 | 10.4 ± 0.7 |
| 100 | 15700 ± 458 | 2.8 ± 0.2 | 59.0 ± 2.9 | 11.6 ± 0.2 |
| 200 | 10126 ± 255 | 1.8 ± 0.2 | 67.4 ± 1.2 | 13.8 ± 0.2 |
| S. molle | ||||
| 50 | 20368 ± 213 | 4.2 ± 0.4 | 43.8 ± 0.7 | 10.0 ± 0.3 |
| 100 | 16840 ± 203 | 3.0 ± 0.0 | 46.8 ± 0.8 | 11.0 ± 0.3 |
| 200 | 12419 ± 273 | 2.2 ± 0.2 | 49.8 ± 0.4 | 12.6 ± 0.2 |
| S. aromaticum | ||||
| 50 | 12567 ± 131 | 3.0 ± 0.3 | 49.6 ± 2.2 | 9.6 ± 0.2 |
| 100 | 10633 ± 75 | 2.8 ± 0.4 | 61.4 ± 0.7 | 11.6 ± 0.6 |
| 200 | 9160 ± 94 | 2.2 ± 0.2 | 67.4 ± 1.7 | 13.4 ± 1.0 |
| Oxamyl | 7832 ± 275 | 2.0 ± 0.0 | 61.8 ± 0.9 | 11.6 ± 0.2 |
| Tween20 | 28550 ± 1269 | 4.8 ± 0.2 | 40.8 ± 0.4 | 9.2 ± 0.2 |
| Non treated | 28318 ± 432 | 4.8 ± 0.2 | 41.0 ± 0.3 | 8.4 ± 0.4 |
| Non infested | - | - | 43.6 ± 0.2 | 10.4 ± 0.4 |
| LSD (p = 0.05) | 979 | 0.57 | 3.49 | 1.26 |
Most treatments with the ten EOs also caused a significant increase of tomato aerial and root biomass (Table 4). Growth effect of the EOs of C. verum and E. citriodora was significantly larger than that of the other EOs and, at high application rates, also of Oxamyl. Adversely, the lowest tomato growth performance was always recorded in soil treated with the C. aurantium and M. piperita EOs.
2.5. Analysis of Aggregated Data
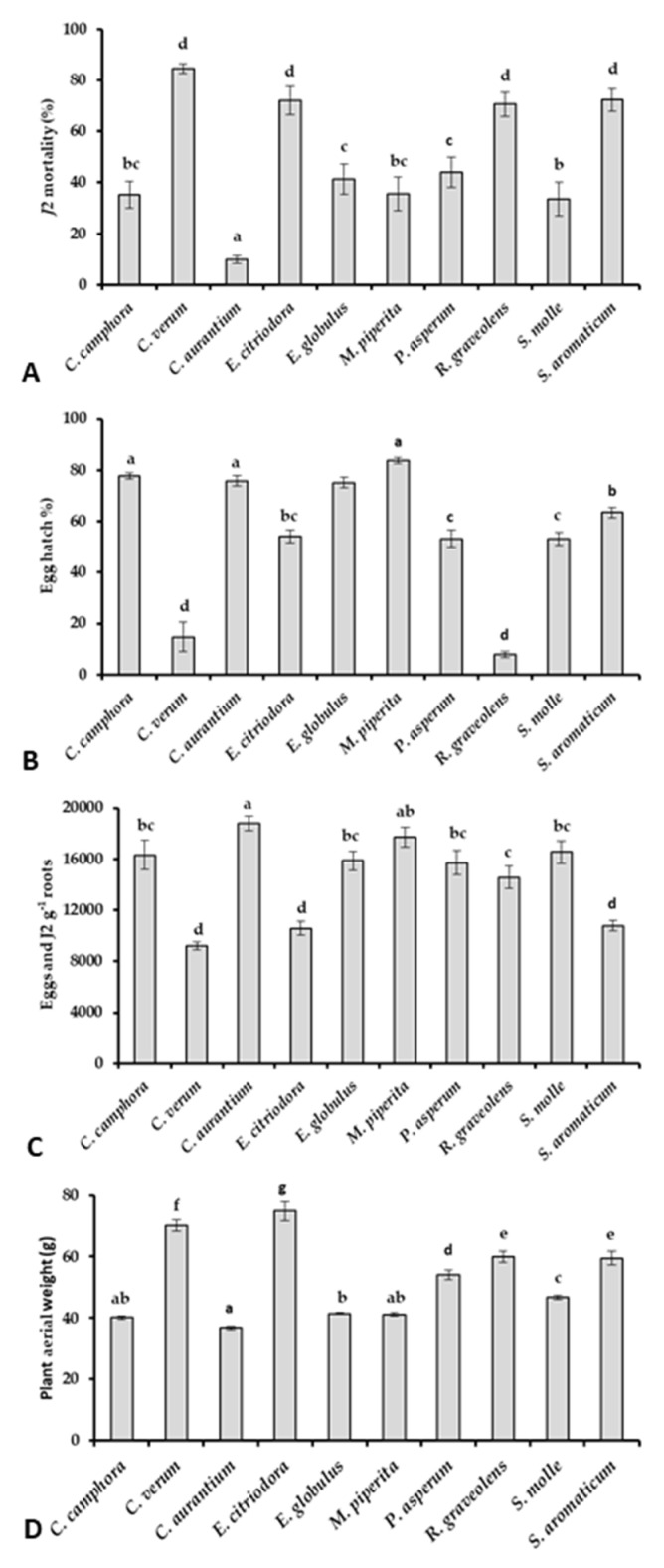
A summary analysis of aggregated data showed that the highest larvicidal activity was provided by the EOs from C. verum, E. citriodora, R. graveolens and S. aromaticum, whereas the ovicidal effect of C. verum and R. graveolens EOs was largely higher than that of the other EOs (Figure 3).
Figure 3.
EOs’ aggregated effects on J2 mortality (A), egg hatchability (B), M. incognita multiplication on roots (C) and tomato growth (D). Bars marked with the same letters are not significantly different according to Least Significant Difference Test (p ≤ 0.05).
In the experiment in soil, the highest aggregated suppressiveness to M. incognita was shown by the EOs of C. verum, E. citriodora and S. aromaticum followed by the R. graveolens EO. In addition, the EOs from E. citrodora and C. verum and, at a lesser instance, those from R. graveolens and S. aromaticum showed also the strongest effect on plant growth.
The lowest in vitro and in vivo nematicidal performance, as well as the lowest plant growth effect, were summarily found for the EOs of C. aurantium and M. piperita.
3. Discussion
Data displayed in this study evidenced strong differences among the nematicidal activity of the tested EOs and also when derived from plant species of the same genus. Within the two Cinnamomum EOs, the C. verum EO was largely more active than that from C. camphora both on in vitro J2 and eggs as well as more suppressive on M. incognita infestation on tomato. These findings are in accordance with the literature data, as in previous in vitro comparative studies on a wide range of different EOs, C. verum EO always showed the most potent nematicidal activity both on the pinewood nematode Bursaphelenchus xylophilus (Steiner et Buhrer) Nickle [25,26] and root-knot nematodes M. incognita [16] and M. graminicola Golden and Birchfield [17]. In addition, soil treatments with C. verum EO resulted in a strong suppression of M. incognita infestation on tomato also in a previous preliminary study of our group [23], and were found for a significant reduction of root gall formation on rice seedlings in soil infested by M. graminicola [17]. Interestingly, aqueous extracts of C. verum were also documented for causing a complete J2 mortality and a strong egg hatch inhibition on the root-knot nematode M. exigua Goeldi [22]. Adversely to that of C. verum EO, the nematicidal activity of the EO from C. camphora is poorly documented, as limited to the report of a moderate suppressiveness on M. incognita on tomato [23]. However, soil treatments with C. camphora ethanolic extracts in greenhouse were found to significantly suppress the infestation of M. javanica (Treub) Chitwood on greenhouse tomato, and to reduce the incidence and severity of wilt disease complex due to root-knot nematode association with the fungus Fusarium oxysporum f.sp lycopersici Snyder et Hansen [27].
The EO from C. verum, as prevalently constituted by E-cinnamaldehyde, had a composition consistent with that previously described [28], while the composition of the EO from C. camphora resulted quite unusal for the high content of limonene along with eucalyptol, which instead characterizes some chemotypes of this species [29]. Plant EOs have been reported to have a large number of biological activities which are determined by their specific chemical composition. Thus, the highest nematicidal efficacy of C. verum EO can be likely attributed to the presence of E-cinnamaldehyde as its major component. The toxicity of this compound and/or EOs containing E-cinnamaldehyde to different pests, including nematodes, has been demonstrated in several studies and related to its aldehydic structure (Figure 1). In specific investigations [26,30], E-cinnamaldehyde resulted in fact more active as nematicide or acaricide than other cinnamic acids characterized by different functional groups. Analogously, the lower nematicidal performance of the C. camphora EO was expected on the base of its chemical composition. We have in fact previously shown [15] that limonene, the main phytochemical of the EO from C. camphora, has a very poor nematicidal activity, independently of the nematode species. In addition, a limited nematicidal activity on M. incognita was also shown for an EO from C. sinensis also containing limonene as dominant component [15]. Limonene is an aliphatic hydrocarbon and the lack of any functional group in its structure might possibly reduce its reactivity.
The strong nematicidal activity of the E. citriodora EO is also in agreement with literature data. An E. citriodora EO was reported for the highest toxicity to M. incognita J2 in an in vitro bioassay on eight different EOs [18], and EOs and extracts from different Eucalyptus species, also including E. citriodora, were found to reduce root-knot nematode J2 mobility and viability and egg hatchability [12]. Moreover, soil fumigation with an E. citriodora EO resulted in a strong reduction of M. incognita multiplication and egg formation on tomato roots [24]. In the same study, the fumigant activity of E. globulus EO on M. incognita was consistently lower than that of an EO from E. citriodora, as confirming the different nematicidal effectiveness of the two Eucalyptus EOs observed in this study.
In agreement with literature findings [24], the bulk of the tested E. citriodora EO was made up by aliphatic terpenes such as citronellal, the major compound (83.9%), citronellol and citronellyl formate, while E. globulus was prevalently (92%) constituted by the byciclic ether eucalyptol. As for the two terpenes described above, the higher nematicidal activity of E. citriodora EO can be ascribed to the functional groups present in citronellal (aldehydic function) and its derivatives compared to eucalyptol (Figure 1). In specific studies aimed to evaluate the toxicity of a series of terpenes against bacterial and microbial systems [31,32], it was found that the presence of an oxygen-related function (as in citronellal and citronellol) and a double bond (as in citronellal) contributes to enhance the reactivity of the molecule towards biological processes involving the transfer of electrons by increasing its electronegativity.
In the present study the EO of P. asperum was found more active on M. incognita J2 than in previous in vitro bioassays, which reported for this EO a significant toxicity to M. incognita J2 only at high concentrations and/or exposure times [18,19]. Moreover, a weak in vitro sensitivity to P. asperum EO was also described for the infective J2 of M. javanica [10]. Adversely, the satisfactory suppressiveness to M. incognita on tomato of soil treatments with the P. asperum EO seems to be in good agreement with the effects previously shown in literature [23].
The P. asperum EO used in this study was a complex mixture of terpenes prevalently made up by three major components, linalool (12.8%), citronellol (35.0%) and geraniol (22.1%), as agreeing with previous literature data [24,33]. These compounds are all characterized by a hydroxyl function considered to be crucial for reactivity towards biological systems (Figure 1). However, in spite of the structural features of its components, the P. asperum EO showed a lower nematicidal activity compared to C. verum, E. citriodora, R. graveolens or S. aromaticum EOs, all characterized by only one major component accounting for 83–89%. This result may suggest that constituents of P. asperum EO do not act synergistically.
The EO of R. graveolens was particularly toxic to M. incognita eggs, as almost completely inhibiting egg hatch, though strongly active on nematode J2 only at 24 h exposure. Previous in vitro studies also indicated a strong activity of R. graveolens EO on the root-knot nematode M. chitwoodi Golden, O’Bannon, Santo and Finley [21] and B. xylophilus [34], whereas da Silva et al. [20] reported high mortality rates of M. incognita J2 only at high (500–1000 μg mL−1) R. graveolens EO concentrations. In the present study, the R. graveolens EO was less suppressive to M. incognita on tomato than the EO of E. citriodora, in agreement with the effects of soil biofumigation with the same two EOs reported by Laquale et al. [24].
As previously documented [20,24], the EO of R. graveolens is very poor in terpenoids except carvacrol, while the dominant component is a dialkyl ketone, 2-undecanone. This compound can structurally form a keto-enol tautomer and then can be considered as an oxygenated highly reactive hydrocarbon responsible for toxicity to nematodes (Figure 1). In addition, the EO of R. graveolens is also characterized by a discrete amount of carvacrol, a monoterpenoid phenol with many biological properties [10,12,35,36,37], that can likely act in a synergistic way with 2-undecanone. Moreover, this phytochemical is an isomer of thymol, which was also shown highly toxic to M. incognita [15].
The high toxicity to J2 and, at a lesser instance, eggs of M. incognita of the S. aromaticum EO also agrees with literature studies, as EOs and extracts from this plant species were previously documented for a high J2 mortality and a strong egg hatch inhibition on M. exigua [22], as well as for a nematicidal activity on different stages of B. xylophilus [25]. In our experiment in soil, the S. aromaticum EO resulted one of the most effective at reducing M. incognita infestation on tomato roots, in accordance with the high suppressive performance previously reported for soil drench treatments with S. aromaticum EO water emulsions [23]. In contrast, Meyer et al. [38] did not find any consistent reduction of M. incognita infestation on different greenhouse vegetable crops by not phytotoxic concentrations of a S. aromaticum EO formulation resulted highly active in preliminary lab assays.
As described by previous studies [39], the EO from S. aromaticum was almost completely made up by eugenol (89.6%), which should be then considered as the main responsible of its nematotoxic activity. Eugenol is a 2-alkyl(oxy)phenol which shares some chemical features with the above discussed phenolic monoterpenes carvacrol and thymol, also reported as very active against bacteria [32] and insects [40]. As demonstrated in a specific study aimed to evaluate structure–activity relationships of eugenol and its congeners against some insects, eugenol allylic double bond and phenolic proton are essential for the molecule bioactivity [41]. A neurotoxic mode of action has been highlighted specifically for some compounds such as eugenol. Docking experiments have in fact shown that this phytochemical may act as an acetylcholinesterase inhibitor and can block octopamine receptors, thus supporting a nematicidal action based on nervous system targets [42,43]. However, mechanisms of action of EOs and their components against nematodes are still debated and further hypotheses, such as an effect on membranes permeability, can be found in literature studies [8,10].
Effects of the S. molle EO on M. incognita J2 and eggs and on nematode infestation on tomato were significant only at high solution concentrations and soil application dosages, respectively. The EO from S. molle was previously documented for antifungal and antibacterial properties [44] as well as for insecticidal and acaricidal activities [45,46]. The only literature report of a S. molle EO nematicidal activity described a higher suppressiveness to M. incognita on tomato compared to that emerged from this study [23], whereas there are no previous data on an in vitro toxicity to nematode J2 and eggs. The tested EO of S. molle was a mixture of several monoterpenes and sesquiterpenes without any dominant compound, as also documented by literature data [47]. Thus, its effect on nematodes may reasonably be ascribed to a synergistic activity of all its components. Indeed, the S. molle EO was among the less active in this study and, according to above considerations, this result could be attributed to the high presence of terpenes without reactive functional groups. The EO of S. molle also contains low amounts of carvacrol and eugenol, 3.7 and 12.0%, respectively, but their expected bioactivity did not seem to emerge and to be determinant against M. incognita.
The EOs from M. piperita and C. aurantium showed the lowest activity on M. incognita both in the in vitro assays and in the experiment in soil. The low activity of our M. piperita EO confirms the literature data, which generally documented a limited in vitro toxicity of this EO to root-knot nematode J2 and eggs [10,18]. Similarly, a poor nematicidal effect of soil treatments with M. piperita EO solutions was reported also in a study of Walker and Melin [48], as describing not significant effects of M. piperita EO in soil infested by M. arenaria on tomato. Our sample of M. piperita EO contained menthane-type monoterpenoids, with menthol and menthone as the two dominant compounds (54.8 and 20.5%, respectively), as also documented in previous studies [24,49]. Therefore, the low nematicidal effect of M. piperita EO in our studies can be considered quite unexpected, as the structural features of the above two terpenes could adversely suggest a high nematicidal effectiveness.
This study is the first investigation of the nematicidal properties of C. aurantium EO, whereas it was widely proved for a biocidal and/or repellent activity on crop insect pest insects, such as Bemisia tabaci, or stored product parasites as Callosobruchus maculatus [50,51]. Our C. aurantium EO confirmed the typical chemical composition documented by previous literature reports [51], characterized by the presence of limonene as the almost exclusive component (94.9%). Therefore, according to the considerations above reported for the nematicidal properties of the C. camphora EO and limonene, the poor nematicidal activity of C. aurantium EO is largely expected.
4. Materials and Methods
4.1. Essential Oils
The analyzed samples consisted in ten commercially available (Erboris Orientis Dacor, Milan, Italy) distilled pure EOs from Cinnamomum camphora (L.) J. Presl. (camphorwood), C. verum J. Presl. (cinnamon), Eucalyptus citriodora Hook (lemon eucalyptus), E. globulus Labill. (blue gum), Mentha piperita L. (peppermint), Pelargonium asperum Ehrh. ex Willd. (bourbon geranium), Ruta graveolens L. (common rue), Schinus molle L.(false pepper) and Syzygium aromaticum (L.) Merr. & L.M. Perry (clove).
4.2. Chemical Analysis of the EOs
A Trace gas chromatograph (GC)-FID Ultra Thermo Finnigan was used for the compositional analysis of the ten EOs. A sample (1µL) of each EO solubilized in hexane was injected in the cold on-column mode in a Agilent DB-5 (J&W Scientific, Milan, Italy) fused silica capillary column (30 m × 0.25 mm; 0.25 µm film thickness). Analytical conditions were as follows: Detector temperature 300 °C; column temperature was programmed from 60 °C (5 min isothermal) to 280 °C (30 min isothermal) at 4 °C/min. Hydrogen was the carrier gas (35 kP; 2.0 mL/min); data were processed using the Chrom-Card 32-bit computing software.
Gas chromatography-Mass spectrometry (GC-MS) analyses were performed with a Hewlett Packard 6890–5973 (Milan, Italy) mass spectrometer interfaced with a HP Chemstation (Milan, Italy). Chromatographic conditions were set as follows: Column oven program from 60 °C (5 min isothermal) to 240 °C (15 min isothermal) at 3 °C/min; injector, 280 °C. Helium was the carrier gas (flow rate, 1 mL/min). A HP-5 MS capillary column (30 m × 0.25 mm; 0.25 µm film thickness) was used. MS operating parameters were: Ion source, 70 eV; ion source temperature, 200 °C; electron current, 34.6 µA; vacuum 10−5 torr. Mass spectra were acquired over a 40–800 amu range at 1 scan/s. The ion source was operating in the electron impact mode. Samples (1µl) were injected using the splitless sampling technique.
4.3. Identification and Quantitation of the EOs Components
Chemical composition of the analysed EOs was achieved by comparison of GC retention times of their constituents with authentic reference compounds in combinations with arithmetic indexes (AI) and by means of reference mass spectra from standard compounds and/or from library files [15,52,53].
AI index values were calculated using an n-alkane series (C6–C32) under the same GC conditions as that for the samples. The relative amount of individual components of the oil were expressed as percent peak area relative to total peak area from the GC-FID analysis of the whole extracts without the use of correction factors. A linear proportion between the areas was used, assuming an equal response factor for all detected compounds.
4.4. Juvenile Mortality Bioassays
The Italian population of M. incognita used for both in vitro and in vivo experiments was collected from infested tomato roots in a field at Castellaneta (Taranto province, Apulia region) and then multiplicated on tomato (cv. Roma) plants at a constant 25 ± 2 °C temperature in a glasshouse. Mature nematode egg masses picked from the tomato roots were directly used in the hatching assay or incubated in distilled water at 25 °C in a growth chamber. The J2 emerged during the incubation period were recovered and maintained in a refrigerator (5 °C) until their use in the toxicity bioassay.
4.5. Nematode Mortality Bioassays
A 0.5 mL volume of a distilled water suspension of M. incognita J2 (about 200 specimens mL−1 water) was poured in 1.5 mL Eppendorf tubes and then added with the same volume of 1.56, 3.12, 6.25, 12.5, 25, 50, 100 and 200 μg mL−1 solutions of the ten EOS in a 0.3% Tween 20 water solution, as to reach 0.78, 1.56, 3.12, 6.25, 12.5, 25, 50 and 100 μg mL−1 treatment EO concentrations. Nematode suspensions were treated with each test solution for 4, 8 or 24 h, with four replicates of each concentration x exposure time combination. Distilled water, the 0.3% Tween 20 solution and a 2 mL L−1 water solution of a commercial formulation (Vydate® 10 L) of the nematicide Oxamyl (10% a.i.) were also included as controls. The J2 from each tube were microscopically checked for their motility at the end of each exposure time and then transferred into distilled water for 72 h, after which persistence of immobility was stated as mortality. The Abbott’s formula m = 100 × (1 − nt/nc), in which m = percent mortality; nt = number of J2 still viable after the treatment; nc = number of viable J2 in water control was applied to calculate J2 mortality rates [54].
4.6. Egg Hatchability Bioassays
Fifty M. incognita egg masses, ranging about 400 eggs per mass, were immersed in 0.5 mL of distilled water in 1.5 mL Eppendorf tubes and then added with the same volume of 500, 1000 and 2000 μg mL−1 0.3% Tween 20 water solutions of each of the ten EOs, as to reach 250, 500 and 1000 μg mL−1 final concentrations. Distilled water and the 0.3% Tween 20 water solution were included as controls. All concentration x exposure time combinations and the two controls were replicated four times. After each treatment with EOs, the egg masses were repeatedly washed in distilled water and then transferred into 2 cm diameter sieves (215 μm aperture) placed in a 3.5 cm diameter Petri dish. The egg masses were then submerged by 3 mL of distilled water and placed into Petri dishes in a growth chamber at 25 °C. Number of emerged J2 was microscopically counted and distilled water was renewed at weekly intervals throughout 5 weeks, after which the egg masses were shaked for 3 min in a 1% sodium hypochlorite solution [55] and released unhatched eggs were counted under a microscope. Egg hatchability was expressed as percentage of hatched eggs compared to the total (hatched + unhatched) egg content of egg masses.
4.7. Experiment in Soil
Roots from the infested tomato plants previously reared in a glasshouse were minutely cut and thoroughly mixed, then five 10 g samples were picked on which M. incognita egg and J2 density was assessed by their extraction (55) followed by microscopical count. Steam sterilised soil (64.4% sand, 18.7% silt, 16.9% clay, 0.8% organic matter and 7.5 pH) was artificially infested with the root inoculum to reach up to a 20 eggs and J2 mL−1 soil initial nematode population density and then poured into 1 L clay pots. Potted soil was then treated with 50, 100 and 200 µg kg−1 soil rates of each of the ten EOs carried by 400 mL volume of a 0.3% water solution of Tween 20. Infested soil, either non-treated or treated with a 2 mL kg−1 soil rate of the same commercial formulation of the nematicide Oxamyl used in the in vitro bioassay on nematode J2 and sterilised soil were also included as controls. There were five replicates of each treatment, arranged in a randomised block design on the benches of a glasshouse maintained at 25 ± 2 °C constant temperature.
Three weeks after the treatments with EOs, the soil of each pot was transplanted with a 1-month-old tomato (cv. Roma) seedling. The tomato plants were uprooted two-months after the transplant, recording fresh weight of aerial parts and roots of each plant. Root gall formation caused by M. incognita infestation was evaluated on each tomato root according to a 0–5 scale (0 = no galls, 1 = 1–2 galls, 2 = 3–10 galls, 3 = 11–30 galls, 4 = 31–100 galls and 5 > 100 galls) [56]. Nematode multiplication was evaluated by extracting using the sodium hypochlorite method [55] and then microscopically counting eggs and J2 from the roots of each replicate.
4.8. Data Analysis
The three experiments were repeated twice and, in the absence of significant experiment x treatment interactions, data from the two experimental runs were pooled [54]. Error variances were homogenized by arcsin-transforming the pooled data before their statistical analysis by the PlotIT 3.2 (Scientific Programming Enterprises, Haslett, MI, USA) software. One or two-way analysis of variance were performed and means were compared by Fisher’s Least Significant Difference Test at p ≤ 0.05. The LC50 values of each EO on M. incognita J2 were calculated by probit analysis [54] of mortality data from the in vitro bioassay.
5. Conclusions
EOs are generally acknowledged for their strong nematicidal activity, but this study indicated that nematicidal potential can consistently vary among different EOs according to their chemical composition, and that not all EOs can be suitable to the formulation of new nematicidal products. Therefore, the choice of an EO candidate for this specific use should be always preceded by a chemical analysis of its constituents [57], also taking into account the quanti-qualitative variability of their composition related to agronomical and climatic factors as well as to the distilled plant parts.
A further indication is that the selection of EO raw materials for new potential nematicides should also consider the target nematode stage, as an EO could be strongly toxic to infective J2 but less or poorly active on eggs.
Moreover, the final choice of nematicidal EO materials should also result from the evaluation of technical and economic aspects, such as the suitability for slow-release micro- or nanoencapsulated formulations easily distributable in field and a prompt availability at reasonable costs.
However, as clearly evidenced by Isman et al. [57], plant EOs have a large number of positive aspects that strongly recommend their industrial use for the production of new nematicides in spite of the complexity of their selection process. EOs and their components can be strongly active on phytonematodes but poorly toxic to mammals and, as highly volatile, environmental safe. In addition, the coexistence of multiple mechanisms of activity and metabolic target sites [30,36,42,43] can avoid the insurgence of resistant populations frequently occurring for synthetic nematicides. Finally, most of EOs can be abundantly purchased at low prices due to their large worldwide production and trade, as ensuring a regular supply to nematicide industries.
Finally, the recent registration of new nematicides based on mixtures of synthetically derived terpenes such as thymol and geraniol indicates that EOs can also represent a model for new synthetic formulations of highly active components as an alternative to direct use as industrial raw materials.
Acknowledgments
The authors acknowledge the technical assistance of Fabio Catalano (IPSP-CNR).
Author Contributions
Conceptualization, T.D., P.A. and V.C.; methodology, S.L. and M.P.A.; software, S.L.; validation, curation, formal analysis, T.D., P.A., V.C.; investigation, T.D., S.L., M.P.A.; data curation, T.D., S.L., M.P.A., P.A.; writing—original draft preparation, T.D., P.A.; writing—review and editing, T.D., P.A., V.C.; visualization, T.D., P.A. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Nicol J.M., Turner S.J., Coyne D.L., Nijs L., Hockland S., Maafi Z.T. Current Nematode Threats to World Agriculture. In: Jones J., Gheysen G., Fenoll C., editors. Genomics and Molecular Genetics of Plant-Nematode Interactions. Springer; Dordrecht, The Netherlands: 2011. pp. 21–43. [Google Scholar]
- 2.Tilman D., Cassman K.G., Matson P.A., Naylor R., Polasky S. Agricultural sustainability and intensive production practices. Nature. 2002;418:671–677. doi: 10.1038/nature01014. [DOI] [PubMed] [Google Scholar]
- 3.Ntalli N.G., Caboni P. Botanical nematicides: A review. J. Agric. Food Chem. 2012;60:9929–9940. doi: 10.1021/jf303107j. [DOI] [PubMed] [Google Scholar]
- 4.D’Addabbo T., Laquale S., Perniola M., Candido V. Biostimulants for plant growth promotion and sustainable management of phytoparasitic nematodes in vegetable crops. Agronomy. 2019;9:616. doi: 10.3390/agronomy9100616. [DOI] [Google Scholar]
- 5.Benelli G., Pavela R., Maggi F., Petrelli R., Nicoletti M. Commentary: Making green pesticides greener? The potential of plant products for nanosynthesis and pest control. J. Clust. Sci. 2017;28:3–10. doi: 10.1007/s10876-016-1131-7. [DOI] [Google Scholar]
- 6.Avato P., D’Addabbo T., Leonetti P., Argentieri M.P. Nematicidal potential of Brassicaceae. Phytochem. Rev. 2013;12:791–802. doi: 10.1007/s11101-013-9303-7. [DOI] [Google Scholar]
- 7.Laquale S., Avato P., Argentieri M.P., Bellardi M.G., D’Addabbo T. Nematotoxic activity of essential oils from Monarda species. J. Pest Sci. 2018;91:1115–1125. doi: 10.1007/s10340-018-0957-1. [DOI] [Google Scholar]
- 8.Andrés M.F., Gonzáles-Coloma A., Sanz J., Burillo J., Sainz P. Nematicidal activity of essential oils: A review. Phytochem. Rev. 2013;11:371–390. doi: 10.1007/s11101-012-9263-3. [DOI] [Google Scholar]
- 9.Echeverrigaray S., Zacaria J., Beltrão R. Nematicidal activity of monoterpenoids against the root-knot nematode Meloidogyne incognita. Nematology. 2010;100:199–203. doi: 10.1094/PHYTO-100-2-0199. [DOI] [PubMed] [Google Scholar]
- 10.Oka Y., Nacar S., Putievsky E., Ravid U., Yaniv Z., Spiegel Y. Nematicidal activity of essential oils and their components against the root-knot nematode. Phytopathology. 2000;90:710–715. doi: 10.1094/PHYTO.2000.90.7.710. [DOI] [PubMed] [Google Scholar]
- 11.Ntalli N., Ferrari F., Giannakou I., Menkissoglu-Spiroudi U. Synergistic and antagonistic interactions of terpenes against Meloidogyne incognita and the nematicidal activity of essential oils from seven plants indigenous to Greece. Pest Manag. Sci. 2011;67:341–351. doi: 10.1002/ps.2070. [DOI] [PubMed] [Google Scholar]
- 12.Ibrahim S.K., Traboulsi A.F., El-Haj S. Effect of essential oilsand plant extracts on hatching, migration and mortality of Meloidogyne incognita. Phytopathol. Medit. 2006;45:238–246. [Google Scholar]
- 13.Ntalli N., Ferrari F., Giannakou I., Menkissoglu-Spiroudi U. Phytochemistry and nematicidal activity of the essential oils from 8 Greek Lamiaceae aromatic plants and 13 terpene components. J. Agric. Food Chem. 2010;58:7856–7863. doi: 10.1021/jf100797m. [DOI] [PubMed] [Google Scholar]
- 14.Santana O., Andrès M.F., Sanz J., Errahmani N., Lamiri A., Gonzalez-Coloma A. Valorization of essential oils from Moroccan aromatic plants. Nat. Prod. Commun. 2014;9:1109–1114. doi: 10.1177/1934578X1400900812. [DOI] [PubMed] [Google Scholar]
- 15.Avato P., Laquale S., Argentieri M.P., Lamiri A., Radicci V., D’Addabbo T. Nematicidal activity of essential oils from aromatic plants of Morocco. J. Pest Sci. 2017;90:711–722. doi: 10.1007/s10340-016-0805-0. [DOI] [Google Scholar]
- 16.Eloh K., Kpegba K., Sasanelli N., Koumaglo H.K., Caboni P. Nematicidal activity of some essential plant oils from tropical West Africa. Int. J. Pest Manag. 2020;66:131–141. doi: 10.1080/09670874.2019.1576950. [DOI] [Google Scholar]
- 17.Amarasinghe L.D., Wijesinghe W.K.A.G.A., Jayawardhane B.K. Efficacy of essential oils from bark and leaf of Cinnamomum zeylanicum on root knot nematode, Meloidogyne graminicola in rice seedlings and young rice plants. J. Sci. Univ. Kelaniya. 2011;6:45–54. doi: 10.4038/josuk.v6i0.4220. [DOI] [Google Scholar]
- 18.Pandey R.C., Dwivedi B.K. Comparative study of different plant extracts for their nematicidal potential. Curr. Nematol. 2000;11:39–43. [Google Scholar]
- 19.Leela N.K., Khan R.M., Reddy P.P., Nidiry E.S.J. Nematicidal activity of essential oil of Pelargonium graveolens against the root-knot nematode Meloidogyne incognita. Nematol. Medit. 1992;20:57–58. [Google Scholar]
- 20.Da Silva F.G.E., Mendes F.R.D.S., Assunção J.C.D.C., Pinheiro Santiago G.M., Xavier Bezerra M.A., Barbosa F.G., Mafezoli J., Rodrigues Rocha R. Seasonal variation, larvicidal and nematicidal activities of the leaf essential oil of Ruta graveolens L. J. Essent. Oil Res. 2014;26:204–209. doi: 10.1080/10412905.2014.882276. [DOI] [Google Scholar]
- 21.Faria J.M.S., Sena I., Ribeiro B., Rodrigues A.M., Maleita C.M.N., Abrantes I., Bennett R., Mota M., da Silva Figueiredo A.C. First report on Meloidogyne chitwoodi hatching inhibition activity of essential oils and essential oils fractions. J. Pest Sci. 2016;89:207–217. doi: 10.1007/s10340-015-0664-0. [DOI] [Google Scholar]
- 22.Salgado S.M.L., Campos V.P. Eclosão e mortalidade de Meloidogyne exigua em extratos e em produtos naturais. Fitopatol. Bras. 2003;28:166–170. doi: 10.1590/S0100-41582003000200008. [DOI] [Google Scholar]
- 23.Laquale S., Sasanelli N., D’Addabbo T. Book of Abstracts of the I National Symposium of the Italian Society of Research on Essential Oils (S.I.R.O.E.) Natural 1; Milan, Italy: 2013. Biocidal activity of essential oils from Eucalyptus species on the root-knot nematode Meloidogyne incognita; p. 55. [Google Scholar]
- 24.Laquale S., Candido V., Avato P., Argentieri M.P., D’Addabbo T. Essential oils as soil biofumigants for the control of the root-knot nematode Meloidogyne incognita on tomato. Ann. Appl. Biol. 2015;167:217–224. doi: 10.1111/aab.12221. [DOI] [Google Scholar]
- 25.Park I.K., Kim K.H., Choi K.S., Kim C.S., Choi I.H., Park J.Y., Shin S.C. Nematicidal activity of plant essential oils and components from garlic (Allium sativum) and cinnamon (Cinnamomum verum) oils against the pine wood nematode (Bursaphelenchus xylophilus) Nematology. 2005;7:767–774. doi: 10.1163/156854105775142946. [DOI] [Google Scholar]
- 26.Kong J.O., Lee S.M., Moon Y.S., Lee S.G., Ahn Y.J. Nematicidal activity of cassia and cinnamon oil compounds and related compounds toward Bursaphelenchus xylophilus (Nematoda: Parasitaphelenchidae) J. Nematol. 2007;39:31–36. [PMC free article] [PubMed] [Google Scholar]
- 27.El-Shennawy M.Z., Abo-Kora M.S. Management of wilt disease complex caused by Meloidogyne javanica and Fusarium oxysporum f. sp. lycopersici on tomato using some plant extracts. J. Plant Prot. Pathol. 2016;7:797–802. [Google Scholar]
- 28.Li Y.Q., Kong D.X., Wu H. Analysis and evaluation of essential oil components of cinnamon barks using GC–MS and FTIR spectroscopy. Ind. Crop Prod. 2013;41:269–278. doi: 10.1016/j.indcrop.2012.04.056. [DOI] [Google Scholar]
- 29.Xu Y., Qin J., Wang P., Li Q., Yu S., Zhang Y., Wang Y. Chemical composition and larvicidal activities of essential oil of Cinnamomum camphora (L.) leaf against Anopheles stephensi. Rev. Soc. Brasil. Medic. Trop. 2020;53:e20190211. doi: 10.1590/0037-8682-0211-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Z., Kim H.K., Tao W., Wang M., Ahn Y.J. Contact and fumigant toxicity of cinnamaldehyde and cinnamic acid and related compounds to Dermatophagoides farinae and Dermatophagoides pteronyssinus (Acari: Pyroglyphidae) J. Med. Entomol. 2011;48:366–371. doi: 10.1603/ME10127. [DOI] [PubMed] [Google Scholar]
- 31.Perestrelo R., Silva C., Fernandes M.X., Câmara J.S. Prediction of terpenoid toxicity based on a quantitative structure-activity relationship model. Foods. 2019;8:628. doi: 10.3390/foods8120628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mahizan N.A., Yang S.K., Moo C.L., Song A.L., Chong C.M., Chong C.W., Abushelaibi A., Lim S.H.E., Lai K.S. Terpene Derivatives as a Potential Agent against Antimicrobial Resistance (AMR) Pathogens. Molecules. 2019;24:2631. doi: 10.3390/molecules24142631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hassane S.O.S., Ghanmi M., Satrani B., Mansouri N., Mohamed H., El Hajaji H., Chaouch A. Composition chimique et activités antibactériennes, antifongiques et antioxydante de l’huile essentielle de Pelargonium asperum Ehrh. ex Wilde des Comores. Acta Bot. Gallica. 2011;158:225–237. doi: 10.1080/12538078.2011.10516269. [DOI] [Google Scholar]
- 34.Faria J.M.S., Barbosa P., Bennett R.N., Mota M., Figueiredo A.C. Bioactivity against Bursaphelenchus xylophilus: Nematotoxics from essential oils, essential oils fractions and decoction waters. Phytochemistry. 2013;94:220–228. doi: 10.1016/j.phytochem.2013.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Zotti M., Colaianna M., Morgese M.G., Tucci P., Schiavone S., Avato P., Trabace L. Carvacrol: From ancient flavoring to neuromodulatory agent. Molecules. 2013;18:6161–6172. doi: 10.3390/molecules18066161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Trabace L.Z.M., Morgese M.G., Tucci P., Colaianna M., Schiavone S., Avato P., Cuomo V. Estrous cycle affects the neurochemical and neurobehavioral profile of carvacrol-treated female rats. Toxicol. Appl. Pharmacol. 2011;255:169–175. doi: 10.1016/j.taap.2011.06.011. [DOI] [PubMed] [Google Scholar]
- 37.Can Baser K.H. Biological and pharmacological activities of carvacrol and carvacrol bearing essential oils. Curr. Pharm. Design. 2008;14:3106–3119. doi: 10.2174/138161208786404227. [DOI] [PubMed] [Google Scholar]
- 38.Meyer S.L., Lakshman D.K., Zasada I.A., Vinyard B.T., Chitwood D.J. Phytotoxicity of clove oil to vegetable crop seedlings and nematotoxicity to root-knot nematodes. HortTechnology. 2008;18:631–638. doi: 10.21273/HORTTECH.18.4.631. [DOI] [Google Scholar]
- 39.Srivastava A.K., Srivastava S.K., Syamsundar K.V. Bud and leaf essential oil composition of Syzygium aromaticum from India and Madagascar. Flav. Fragr. J. 2005;20:51–53. doi: 10.1002/ffj.1364. [DOI] [Google Scholar]
- 40.Huang Y., Ho S.H., Lee H.C., Yap Y.L. Insecticidal properties of eugenol, isoeugenol and methyleugenol and their effects on nutrition of Sitophilus zeamais Motsch. (Coleoptera: Curculionidae) and Tribolium castaneum (Herbst)(Coleoptera: Tenebrionidae) J. Stored Prod. Res. 2002;38:403–412. doi: 10.1016/S0022-474X(01)00042-X. [DOI] [Google Scholar]
- 41.Barbosa J.D., Silva V.B., Alves P.B., Gumina G., Santos R.L., Sousa D.P., Cavalcanti S.C. Structure–activity relationships of eugenol derivatives against Aedes aegypti (Diptera: Culicidae) larvae. Pest Manag. Sci. 2012;68:1478–1483. doi: 10.1002/ps.3331. [DOI] [PubMed] [Google Scholar]
- 42.Lee S.E., Lee B.H., Choi W., Park B.S., Kim J.G., Campbell B.C. Fumigant toxicity of volatile natural products from Korean spices and medicinal plants towards the riceweevil, Sitophilus oryzae (L) Pest Manag. Sci. 2001;57:548–553. doi: 10.1002/ps.322. [DOI] [PubMed] [Google Scholar]
- 43.Kostyukovsky M., Rafaeli A., Gileadi C., Demchenko N., Shaaya E. Activation of octopaminergic receptors by essential oil constituents isolated from aromatic plants: Possible mode of action against insect pests. Pest Manag. Sci. 2002;58:1101–1106. doi: 10.1002/ps.548. [DOI] [PubMed] [Google Scholar]
- 44.do Prado A., Garces H., Bagagli E., Rall V., Furlanetto A., Fernandes Junior A., Furtado F. Schinus molle essential oil as a potential source of bioactive compounds: Antifungal and antibacterial properties. J. Appl. Microbiol. 2019;126:516–522. doi: 10.1111/jam.14157. [DOI] [PubMed] [Google Scholar]
- 45.López A., Castro S., Andina M.J., Ures X., Munguía B., Llabot J.M., Elder H., Dellacassa E., Palma S., Domínguez L. Insecticidal activity of microencapsulated Schinus molle essential oil. Ind. Crop Prod. 2014;53:209–216. doi: 10.1016/j.indcrop.2013.12.038. [DOI] [Google Scholar]
- 46.Rey-Valeirón C., Pérez K., Guzmán L., López-Vargas J., Valarezo E. Acaricidal effect of Schinus molle (Anacardiaceae) essential oil on unengorged larvae and engorged adult females of Rhipicephalus sanguineus (Acari: Ixodidae) Exp. Appl. Acarol. 2018;76:399–411. doi: 10.1007/s10493-018-0303-6. [DOI] [PubMed] [Google Scholar]
- 47.Bernhard R.A., Shibamoto T., Yamaguchi K., White E. The volatile constituents of Schinus molle L. J. Agric. Food Chem. 1983;31:463–466. doi: 10.1021/jf00116a075. [DOI] [Google Scholar]
- 48.Walker J.T., Melin J.B. Mentha x piperita, Mentha spicata and effects of their essential oils on Meloidogyne in soil. J. Nematol. 1996;28:629–635. [PMC free article] [PubMed] [Google Scholar]
- 49.Shahi A.K., Chandra S., Dutt P., Kaul B.L., Tava A., Avato P. Essential oil composition of Mentha x piperita L. from different environments of north India. Flavour Fragr. J. 1999;14:5–8. doi: 10.1002/(SICI)1099-1026(199901/02)14:1<5::AID-FFJ768>3.0.CO;2-3. [DOI] [Google Scholar]
- 50.Saeidi M., Moharramipour S., Sefidkon F., Aghajanzadeh S. Insecticidal and repellent activities of Citrus reticulata, Citrus limon and Citrus aurantium essential oils on Callosobruchus maculatus. Int. Prot. Stored Prod. IOBC/WPRS Bull. 2011;69:289–293. [Google Scholar]
- 51.Zarrad K., Hamouda A.B., Chaieb I., Laarif A., Jemâa J.M.B. Chemical composition, fumigant and anti-acetylcholinesterase activity of the Tunisian Citrus aurantium L. essential oils. Ind. Crop Prod. 2015;76:121–127. doi: 10.1016/j.indcrop.2015.06.039. [DOI] [Google Scholar]
- 52.Avato P., Morone-Fortunato I., Ruta C., D’Elia R. Glandular hairs and essential oils in micropropagated plants of Salvia officinalis L. Plant Sci. 2005;169:29–36. doi: 10.1016/j.plantsci.2005.02.004. [DOI] [Google Scholar]
- 53.Adams R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry. 4th ed. Allured Publishing Corporation; Carol Stream, IL, USA: 2007. p. 469. [Google Scholar]
- 54.Finney D.J. Statistical Method in Biological Assay. 3rd ed. Charles Griffin & Company Ltd.; High Wycombe, UK: 1978. p. 508. [Google Scholar]
- 55.Hussey R.S., Barker K.R. A comparison of methods of collecting inocula of Meloidogyne spp. including a new technique. Plant Dis. Rep. 1973;57:1025–1028. [Google Scholar]
- 56.Taylor A.L., Sasser J.N. Biology, Identification and Control of Root-Knot Nematodes (Meloidogyne spp.) North Carolina State University Graphics; Raleigh, NC, USA: 1978. p. 111. [Google Scholar]
- 57.Isman M.B., Miresmailli S., Machial C. Commercial opportunities for pesticides based on plant essential oils in agriculture, industry and consumer products. Phytochem. Rev. 2011;10:197–204. doi: 10.1007/s11101-010-9170-4. [DOI] [Google Scholar]