Abstract
Dementia is characterized by a long preclinical phase that may last years to decades before the onset of mild cognitive impairment. Slow gait speed and subjective memory complaint commonly co-occur during this preclinical phase, and each is a strong independent predictor of cognitive decline and dementia. Motoric cognitive risk (MCR) syndrome is a pre-dementia syndrome that combines these two early harbingers of dementia. The risk of cognitive decline or dementia is stronger for MCR than for either slow gait speed or subjective memory complaint alone. Slow gait speed and subjective memory complaint have several common risk factors: cardiovascular disease, diabetes mellitus, abnormal cortisol profiles, low vitamin D levels, brain atrophy with decreased hippocampal volume, and increased deposition of beta-amyloid in the brain. The underlying pathogenesis of MCR remains poorly understood. Metabolomics and proteomics have great potential to provide new insights into biological pathways involved in MCR during the long preclinical phase preceding dementia.
Keywords: Alzheimer’s disease, Cognitive decline, Dementia, Gait, Memory, Skeletal muscle
1. Introduction
An estimated 40–50 million people are currently living with dementia worldwide (GBD 2016 Dementia Collaborators, 2019,GBD 2016 Dementia Collaborators, 2019) of which 60–70 % is attributed to Alzheimer’s disease (AD) (World Health Organization, 2017). In the US alone, the number of people living with AD is estimated to grow to 13.8 million by 2050 (Alzheimer’s Association, 2017). Currently there is no effective therapy to prevent dementia. Total annual payments for health, long-term, and hospice care for people with Alzheimer’s disease in the US is projected to increase from $259 billion in 2017 to more than $1.1 trillion in 2050 (Alzheimer’s Association, 2017). Recent studies show AD is a multifactorial and heterogeneous disease with multiple contributors, including cerebrovascular disease (Schneider et al., 2007; Toledo et al., 2013; Attems and Jellinger, 2014; Sweeney et al., 2019; De Rueck et al., 2018). In spite of intensive research and new knowledge on the pathogenesis of dementia appears in the literature weekly if not daily, there are currently no effective therapies to prevent, slow, or stop dementia (Alzheimer’s Association, 2017).
Dementia is characterized by a long preclinical phase in which there are very subtle cognitive alterations that are detectable years before the clinical criteria for mild cognitive impairment (MCI) are met (Sperling et al., 2011). The preclinical phase of dementia may take several years to decades (Villemagne et al., 2013; Jack et al., 2013). Recent data show that older adults with subjective cognitive decline and normal cognitive testing during this preclinical phase are at an increased risk of developing MCI and dementia (Jessen et al., 2014a,b; Rabin et al., 2017). Subjective cognitive impairment may precede MCI by up to 15 years (Reisberg et al., 2008). The National Institute on Aging-Alzheimer’s Association preclinical AD working group has included subjective cognitive decline as a feature of this preclinical phase (Sperling et al., 2011).
Slow gait speed among cognitively normal older adults predicts cognitive decline and dementia (Cohen et al., 2016; Chhetri et al., 2017). Decline in gait speed may occur as early as 12 years before the onset of MCI (Buracchio et al., 2010), a transitional stage from usual aging to AD dementia. Cognition and gait share many common domains such as shared cortical regions in the brain, executive function, and risk factors such as cardiovascular disease and diabetes mellitus (Cohen et al., 2016; Chhetri et al., 2017; Montero-Odasso and Hachinski et al., 2014). These two harbingers of dementia have been integrated in a predementia syndrome known as motoric cognitive risk syndrome (MCR). MCR is characterized by the combination of slow gait speed and subjective cognitive complaint in older adults without dementia (Cohen et al., 2016; Chhetri et al., 2017). MCR may be an important research tool to understand the pathophysiology that occurs during the long preclinical phase that leads to dementia. The biological pathways that lead to MCR have not been well-characterized. The identification of specific pathophysiological processes, therapeutic targets, and pathways for intervention that occur during this long preclinical phase are a major priority to slow or prevent progression to dementia (Alzheimer’s Association, 2017; Sperling et al., 2011).
The purpose of this review is to gain insight into potential biological mechanisms of MCR during this preclinical phase and mechanisms by which MCR is connected with risk of dementia (Fig. 1).
Fig. 1.
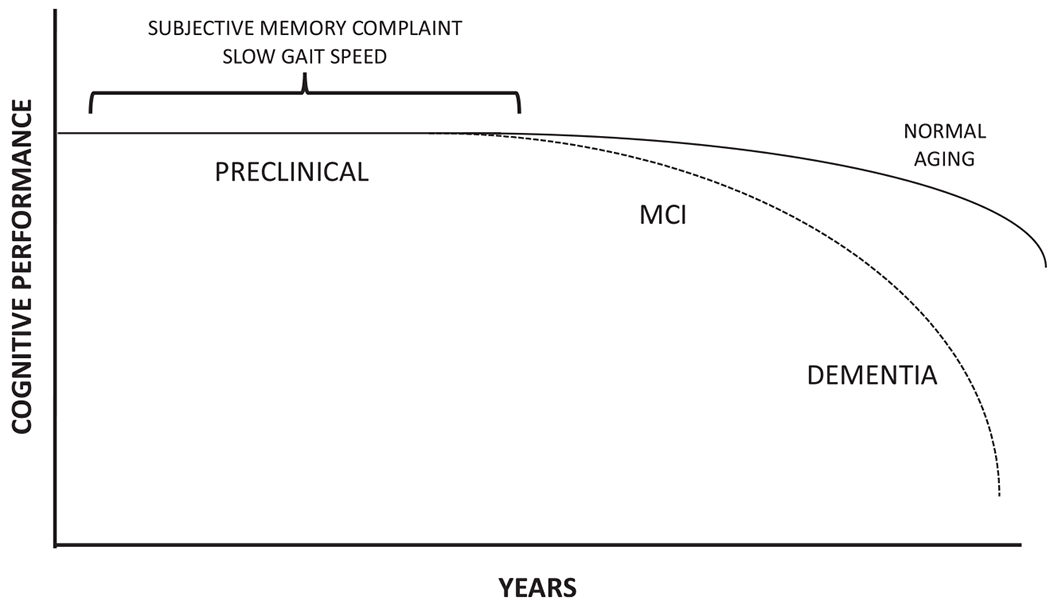
Hypothetical model of the trajectory of cognitive performance in relation to progression to Alzheimer’s disease (AD). Subjective memory complaint and slow gait speed can occur during the long preclinical phase and increase the risk of progression to MCI and AD. This is a hypothetical model and does not imply that all individuals with decline of cognitive performance will progress to dementia.
In light of the current literature, we identify specific gaps in knowledge that need further research. We have organized this review as a set of responses to specific questions: 1) What is the predictive value of slow gait speed and subjective memory complaint, respectively, for adverse aging outcomes including dementia? In this review, we focus on subjective memory complaint, since the memory domain has been most commonly addressed in general studies of subjective cognitive complaint (Rabin et al., 2015) and MCR (Chhetri et al., 2017). 2) What risk factors and biomarkers are shared between slow gait speed and subjective memory complaint? What is the predictive value of MCR for adverse aging outcomes including dementia? What do slow gait speed and subjective memory complaint have in common that might provide clues to the underlying biology of MCR? For this review, we identified relevant articles by searching PubMed under combinations of the terms “Alzheimer’s”, “biomarker”, “cognition”, “cognitive decline”, “dementia”, “epidemiology”, “gait”, “motoric cognitive risk”, “risk”, and “walking”.
2. Gait speed in older adults
Gait speed, or walking speed, has been termed the “sixth vital sign”, given its ease of use and strength in predicting adverse health outcomes (Fritz and Lusardi, 2009; Middleton et al., 2015). In most studies, normal gait speed is measured and timed over of a distance of 6 m (20 feet) with an allowance for acceleration and deceleration and with instructions to walk at a “normal” or “usual” pace and not as fast as possible. Reference data have been established for normal gait speed (Bohannon and Andrews, 2011). Normal gait speed in men is relatively unchanged at 1.43 m/s in ten-year age strata from 30 to 59 years and then declines thereafter (Bohannon and Andrews, 2011). In women, mean normal gait speed peaks at 1.39 m/s in the 40 – 49 year age strata and then declines with older age (Bohannon and Andrews, 2011).
3. Gait speed as a predictor of cognitive impairment and dementia
3.1. Cognitive decline
Slow gait speed predicted greater decline in cognition over time in many longitudinal cohort studies (Marquis et al., 2002; Inzitari et al., 2007; Alfaro-Acha et al., 2007; Soumaré et al., 2009a,b; Deshpande et al., 2009; Taniguchi et al., 2012; Mielke et al., 2013; Veronese et al., 2016; Tian et al., 2016; Hsu et al., 2017; Park et al., 2017). A systematic review showed that slow gait speed at baseline, defined using study-specific criteria, predicted the risk of cognitive impairment (HR 1.20, 95 % CI 1.10, 1.31) (Clouston et al., 2013). In meta-analyses involving 29,520 adults from 27 studies, slow gait speed was associated with poorer cognitive function in cross-sectional studies, and slow gait speed at baseline predicted great cognitive decline in longitudinal studies (Peel et al., 2019).
3.2. Dementia
Early studies showed that gait abnormalities or amount of walking predicted dementia (Verghese et al., 2002). These initial findings led to a large number of studies showing that slow gait speed itself predicted dementia. In 2002, Verghese and colleagues showed that gait abnormalities in community-dwelling adults, aged ≥75 years, predicted dementia (Verghese et al., 2002). Further studies showed that slow gait speed independently predicted dementia in study cohorts from Australia (Waite et al., 2005), the Netherlands (Ramakers et al., 2007), the US (Wang et al., 2006; Verghese et al., 2007; Gray et al., 2013; Camargo et al., 2016), France (Abellan van Kan et al., 2012; Dumurgier et al., 2017), and Sweden (Welmer et al., 2014). A meta-analysis involving 23,512 older adults from 13 studies showed that poor gait performance, which included both slow gait speed and clinical gait abnormalities, predicted dementia, with a pooled HR 1.53 (95 % CI 1.12, 1.43) for any dementia (Beauchet et al., 2016a,b). Another meta-analysis of 13,130 older adults from five studies showed that slow gait speed predicted dementia (HR 1.93, 95 % CI 1.41, 2.65) (Kueper et al., 2017).
4. Risk factors and biomarkers associated with slow gait speed
4.1. Demographic and lifestyle factors
Aging is accompanied by a progressive loss of muscle mass from middle-age at the rate of ~ 1 % per year (Wilkinson et al., 2018). In people aged 75 years, muscle mass is lost at a rate of 0.64-0.70 % per year in women and 0.80-0.98 % per year in men, and muscle strength is lost at a greater rate of 2.5–3.0 % in women and 3–4 % per year in men (Mitchell et al., 2012). The age-related loss of muscle mass and strength is characterized by muscle fiber atrophy and loss (Wilkinson et al., 2018), reduced satellite cell function (Snijders and Parise, 2017), alterations in the neuromuscular junction (Gonzalez-Freire et al., 2014), mitochondrial dysfunction (Habiballa et al., 2019), and increased oxidative stress and inflammation (Scicchitano et al., 2018).
Gait speed has a well-documented decline with older age, as noted above (Bohannon and Andrews, 2011). Slower gait speed has been described among blacks compared with whites (Blanco et al., 2012; Xie et al., 2017. Slow gait speed and greater decline in gait speed over four years were associated with lower total wealth among 7225 adults, aged ≥60 years, in the English Longitudinal Study of Aging Zaninotto et al., 2013). In France, adults aged 55–69 years in less skilled occupational categories had significantly slower gait speed compared with those in more skilled types of jobs (Plouvier et al., 2016). In the LIFEPATH project, higher socioeconomic status was associated with faster gait speed in an analysis of 109,107 adults, aged 45–90 years, from 37 different studies conducted worldwide (Stringhini et al., 2018).
Higher body mass index is associated with lower gait speed. In 6,229 adults in the Atherosclerosis Risk in Communities Study, among those who maintained normal, overweight, and obese status across the previous 25 years, mean gait speed measured in “late-life” (≥65 years) was 0.97, 0.89, and 0.81 m/s, respectively (Windham et al., 2017). Participants who had an increase in body mass index (BMI) over 25 years had slower late-life gait speed, suggesting that being overweight in older age was not protective of mobility function (Windham et al., 2017).
Socio-demographic correlates of slow gait speed included low social networks in 2565 adults, aged ≥60 years, in Singapore Shafie et al., 2017). Bus passes are offered for free to adults, aged ≥62 years, in the United Kingdom in order to reduce social exclusion (Webb et al., 2016. Among 4650 older adults who were eligible for free bus pass, those who were pass holders had significantly faster gait speed than non-holders Webb et al., 2016). The relationship of social networks and free bus passes with gait speed was attributed in part to greater physical activity among older adults with greater social interactions. Lower physical activity is associated with slow gait speed. Exercise and increased physical activity reduce the risk of slow gait speed (Pahor et al., 2014; Brach et al., 2017).
4.2. Diet
A high intake of fruit and vegetables, as indicated by higher plasma carotenoid concentrations, was associated with a slower decline of gait speed (Lauretani et al., 2008). A dietary pattern consistent with a Mediterranean diet was associated with slower decline of gait speed over time in community-dwelling adults in the US (Shahar et al., 2012) and lower risk of mobility disability among adults in rural Italy (Milaneschi et al., 2011). In the InCHIANTI Study, higher plasma n-3 polyunsaturated fatty acid (PUFA) concentrations were associated with faster gait speed (Abbatecola et al., 2009). Lower dietary protein intake was associated with slower gait speed in 554 community-dwelling women, aged 65–72 y in Finland (Isanejad et al., 2016). In a meta-analysis of 9,800 adults from seven studies, protein intake of < 0.8 g/kg/day was associated with slower gait speed compared to protein intake > 1.3g/kg/day (Coelho-Júnior et al., 2018). Low protein intake predicted mobility limitation in older community-dwelling adults (Houston et al., 2017). Vitamin K is a fat-soluble vitamin found primarily in green leafy vegetables. In 1089 adults, mean age 74 years, in the Health, Age and Body Composition Study, lower vitamin K status was associated with slow gait speed Shea et al., 2016).
4.3. Systemic inflammation
An age-related proinflammatory state is common in older adults and is characterized by increased circulating levels of cytokines and acute phase proteins (Ferrucci et al., 2005. Elevated systemic inflammation is associated with slow gait speed in cross-sectional studies and predicts decline of gait speed and incident mobility disability in longitudinal studies. High circulating C-reactive protein CRP was associated with slow gait speed among 1320 adults, aged 65–74 years, in the International Mobility Aging Study conducted in Canada, Colombia, and Brazil (Sousa et al., 2016). In 680 older adults in the MOBILIZE Boston Study, elevated soluble vascular cell adhesion molecule-1 (sVCAM-1) was associated with slow gait speed (< 0.6 m/s) in adults with a history of hypertension (Tchalla et al., 2015). sVCAM-1 is considered a marker of endothelial dysfunction (Tchalla et al., 2015. In 1680 adults, aged ≥60 years, in the National Health and Nutrition Examination Survey NHANES (1999–2002), circulating CRP was inversely associated with gait speed (Kuo et al., 2006). Another analysis from NHANES showed that a combination of high serum CRP and low serum 25(OH)D concentrations was associated with slow gait speed (Kositsawat et al., 2013. In the Health, Aging and Body Composition Study, eight serum inflammatory markers were associated with gait speed in 1269 adults, aged 70–79 years (Hsu et al., 2009).
Longitudinal studies show high circulating IL-6 predicted both decline of gait speed and increase in mobility disability. In 333 adults, aged ≥70 years, in the Einstein Aging Study, higher serum IL-6 was associated with slower gait speed. Participants in the highest IL-6 quartile had a 1.75 cm/s/y faster decline in gait speed compared with those in the lowest quartile (Verghese et al., 2011). Community-dwelling adults, aged ≥71 years, with elevated circulating IL-6 at baseline were at higher risk of developing mobility disability over four years of follow-up (Ferrucci et al., 1999). In 718 women, aged ≥65 years, in the Women’s Health and Aging Study I, participants with high serum IL-6 combined with low serum insulin-like growth factor I were at higher risk of developing mobility disability over three years of follow-up (Cappola et al., 2003). In 880 high-functioning adults, aged ≥65 years, in the MacArthur Studies of Successful Aging, slow gait speed at baseline was significantly associated with higher plasma IL-6 but not CRP. Neither plasma IL-6 or CRP predicted decline in gait speed assessed seven years later (Taaffe et al., 2000). The participants measured at follow-up were high-functioning older adults who were more physically active, which may partially explain the lack of association between systemic inflammation and gait speed decline (Taaffe et al., 2000).
Elevated serum CRP independently predicted decline of gait speed in 624 adults followed in the Einstein Aging Study (Verghese et al., 2012. In 2437 adults, aged 47–87 years, in the English Longitudinal Study of Aging, participants with a trajectory of serum CRP that was consistently low had faster gait speed than those with a consistently high or medium-to-high trajectories of serum CRP over ten years of follow-up (Lassale et al., 2019). Higher serum tumor necrosis factor receptor 1 concentrations predicted the inability to walk 400 m over six years of follow-up in 621 adults, aged ≥70 years, participating in the InCHIANTI Study (Vasunilashorn et al., 2013. In 2979 community-dwelling adults, aged 70–79 years, higher serum IL-6, TNF-α, and CRP, respectively, were predictive of incident mobility disability over 30 months of follow-up (Penninx et al., 2004).
4.4. Mitochondrial dysfunction
The post exercise recovery rate of phosphocreatine (PCr), kPCr, as measured by 31P-MRS, reflects the capacity of muscle mitochondria to synthesize ATP, or mitochondrial oxidative capacity. Low kPCr in the vastus lateralis was independently associated with slow gait speed in adults (Choi et al., 2016). The inefficiency in muscle bioenergetics partially accounted for the differences in gait speed (Zane et al., 2017). Low kPCr in the vastus lateralis was also associated with decreased plasma lysophosphatidylcholines (LPC) (Semba et al., 2019a,b,c). Lysophosphatidylcholines are involved in the biosynthesis and remodeling of cardiolipin and signaling of G-coupled protein receptors. Mitochondrial DNA (mtDNA) was sequenced in 1758 participants in the Lifestyle Interventions and Independence for Elders (LIFE) Study and 730 participants in the Health, Aging and Body Composition Study (Manini et al., 2018). The m.12705C > T, ND5 variant was significantly associated with gait speed. Significant pooled effects related to stopping during the long-distance walk test were noted across oxidative phosphorylation complexes I and III (Manini et al., 2018). Growth and differentiation factor 15 (GDF-15), a divergent member of the transforming growth factor-β (TGF-β) superfamily, has recently been implicated in muscle wasting, cachexia, and age-related mitochondrial dysfunction (Tsai et al., 2016; Fujita et al., 2016). Elevated plasma GDF-15 was associated with slow gait speed in healthy adults, aged 22–93 years, in the Baltimore Longitudinal Study of Aging (Semba et al., 2019a,b,c).
4.5. Endocrine abnormalities
Vitamin D has been implicated in skeletal muscle health because clinical vitamin D deficiency is characterized by muscle weakness and gait impairment, vitamin D receptors are found in skeletal muscle (Bischoff-Ferrari, 2012), and vitamin D supplementation improves skeletal muscle strength (Beaudart et al., 2014). Vitamin D deficiency was strongly associated with slow gait speed in a meta-analysis of circulating hydroxyvitamin D concentrations and gait in 22 studies (Annweiler et al., 2017. Low serum 25OHD concentrations predicted mobility disability in 2099 community-dwelling older adults Houston et al., 2013. In 1445 men participating in the Framingham Offspring Study, serum free testosterone was positively associated with usual gait speed and was associated with a lower risk of developing mobility disability Krasnoff et al., 2010).
Normal aging is associated with the general increase in daily serum cortisol levels without much alteration of the normal circadian rhythm pattern (Yiallouris et al., 2019). The activity of the hypothalamic-pituitary-adrenal-axis (HPA axis) activates cortisol secretion in response to stress. In humans there is a circadian rhythm in which cortisol levels are higher in the morning (cortisol awakening response, CAR) and then declines during the day. Chronically elevated cortisol levels can adversely affect the brain, skeletal muscle, bone, glucose metabolism, and immune function (Yiallouris et al., 2019). A meta-analysis of six studies of adults, aged 50–92 years, showed that a smaller diurnal drop of cortisol was associated with slow gait speed (Gardner et al., 2013). A reduced CAR was associated with slow gait speed in 86 adults, aged 56–72 years (Pulopulos et al., 2016).
4.6. Altered lipid metabolism
Targeted metabolics using liquid chromatography-tandem mass spectrometry (LC–MS/MS) showed that hexoses, sphingomyelin (SM) 16:1, SM 18:0, SM 18:1, phosphatidylcholine aa 32:3, lysophosphatidylcholine (LPC) 17:0, LPC 18:1, and LPC 18:2 were associated with slow gait speed in 504 adults, aged ≥50 years, in the Baltimore Longitudinal Study of Aging (Gonzalez-Freire et al., 2019). In longitudinal analyses, LPC 18:2 independently predicted the decline of gait speed over time (Gonzalez-Freire et al., 2019). LPC 18:2 can serve as ligand for specific G protein-coupled signaling receptors and as a precursor in the biosynthetic pathway of cardiolipin, an important dimeric phospholipid found almost exclusively in the inner mitochondrial membrane (Semba et al., 2019a,b,c). In 77 older men, mean age 79.2 years, elevated plasma acylcarnitines were associated with slow gait speed (Lum et al., 2011). Elevated plasma acylcarnitines are a marker of defective beta-oxidation of fatty acids and impaired mitochondrial oxidative capacity (Semba et al., 2019a,b,c). Low HDL cholesterol was associated with slow gait speed in women but not in men in a subsample of NHANES (1999–2002) (Okoro et al., 2006).
4.7. Chronic diseases
Slow gait speed in community-dwelling older adults was associated with subclinical atherosclerosis, such as increased coronary artery calcification and increased intima-media thickness (Elbaz et al., 2005; Hamer et al., 2010. Cardiovascular burden was associated with mobility disability in 2725 adults, aged ≥60 years, in the Swedish National Study of Aging and Care Welmer et al., 2013). Increased cardiovascular risk predicted slow gait speed (Elbaz et al., 2014) and mobility disability (Heiland et al., 2017. In 3604 adults, aged 65–85 years, in the Three-City Study in Dijon, France, those with hypertension at baseline had a greater decline in gait speed compared to normotensive adults over mean seven years of follow-up (Dumurgier et al., 2010). Increased metabolic syndrome indicators predicted gait speed decline in older women (Fredman et al., 2010). In older adults, slow gait speed was associated with chronic obstructive pulmonary disease (Eisner et al., 2008; Ilgin et al., 2011), knee osteoarthritis (Hayashi et al., 2016; White et al., 2017), chronic kidney disease (Zemp et al., 2019), and depression (Sanders et al., 2016; Hayashi et al., 2019). In a cross-sectional analysis of 32,683 female and 8,112 male participants of the National Walkers’ Health Study, compared with men and women who walked at a speed of < 1.2 m/s, those who walked > 2.1 m/s had 48 % and 52 % lower odds for antihypertensive, 68 % and 59 % lower odds for antidiabetic, and 53 % and 40 % lower odds for LDL cholesterol-lowering medications, respectively, when adjusted for age, smoking, and diet (Williams, 2008).
Diabetes mellitus was associated with gait abnormalities in the Baltimore Longitudinal Study Of Aging (Ko et al., 2011) and slow gait speed in the InCHIANTI Study (Volpato et al., 2012. In 2573 adults, aged ≥50 years, in NHANES (1999–2002), diabetes mellitus was associated with slower gait speed (Kalyani et al., 2013). Slow gait speed was associated with greater insulin resistance in 844 adults, aged ≥65 years, from a community-based study in Taiwan (Yang et al., 2017). In nondiabetic adults, aged 50–85 years, in NHANES (1999–2002), gait speed was associated with greater insulin resistance in men but not women (Kuo et al., 2009). Slow gait speed was associated with abnormal daily glucose profiles and 69 adults, mean age 75.0 years, with diabetes mellitus (Ogama et al., 2019).
4.8. Skeletal muscle alterations
Low knee extension strength was a strong predictor of gait speed decline in 934 adults, aged ≥65 years, in the InCHIANTI Study (Hicks et al., 2012. Low knee extension strength also predicted greater gait speed decline in 6766 adults, aged 67–93 years, in the Foundation of the National Institutes of Health Sarcopenia Project (Fragala et al., 2016. Higher intermuscular fat infiltration in the thigh predicted greater decline of gait speed in 2306 older adults in the Health, Aging and Body Composition Study Beavers et al., 2013).
4.9. Cognitive status
Brain cortex networks in the frontal and temporal lobes are involved in the regulation and control of gait (Montero-Odasso et al., 2014). Executive function, a higher order cognitive process that includes volition, planning, purposive action, and effective performance, is associated with gait speed (Martin et al., 2013). Longitudinal studies show that worsening of executive function are associated with slowing of gait speed (Callisaya et al., 2015; Best et al., 2015). In cross-sectional studies, meta-analyses show that slow gait speed was associated with poor cognition (Demnitz et al., 2016; Peel et al., 2019). Compared to gait speed in healthy controls, reductions in gait speed ranged from 0.11 m/s in cognitive impairment, 0.20 m/s in mild dementia, and 0.41 m/s in moderate dementia (Peel et al., 2019). Global and executive cognitive functions predicted decline in gait speed in among older adults in the Health, Aging and Body Composition Study (Atkinson et al., 2007).
4.10. Brain imaging
Slow gait speed was associated with β-amyloid (Aβ) deposition in the posterior and anterior putamen, occipital cortex, precuneus, and anterior cingulate, independent of age and APOE genotype, in a cross-sectional study of 128 non-demented older adults (del Campo et al., 2016). In 59 cognitively unimpaired adults, aged 56–89 years, in the Baltimore Longitudinal Study of Aging who underwent PET-PiB scans, higher mean cortical Aβ predicted greater decline of gait speed over mean follow-up of 4.7 years (Tian et al., 2017). Decline in lower extremity performance was associated with Aβ burden localized to early deposition regions of the brain important for motor planning, such as putamen, dorsolateral prefrontal cortex, lateral temporal lobe, and precuneus, but not later deposition regions such as primary motor cortex or hippocampus (Tian et al., 2017).
Cerebral small vessel disease is characterized by white matter hyperintensities (WMH) and lacunar infarcts. In 431 independently living, nondemented older adults, aged 50–85 years, WMH and lacunar infarcts were associated with slow gait speed (de Laat et al., 2010d). WMH were inversely associated with gait speed in 770 adults, mean age 57 years, in the population-based Shunyi study in China (Su et al., 2017), in 265 community-dwelling adults, mean age 83 years, in the Health, Aging and Body Composition Study (Rosario et al., 2016), in 321 adults, mean age 78 years, in the Cardiovascular Health Study (Rosano et al., 2006), in 803 adults, aged 40–75 years, in the Prospective Urban Rural Epidemiological Study (Smith et al., 2015), and in 429 adults, aged 50–85 years, with cerebral small vessel disease (de Laat et al., 2011d). In 633 nondisabled adults, aged 65–84 years, in the Leukoaraiosis and Disability (LADIS) Study, gait speed correlated with the severity of WMH (Baezner et al., 2008). In mild, moderate, and severe WMH groups, gait speed was 1.24, 1.18, and 1.09 m/s, respectively. Slow gait speed was defined as < 1.2 m/s. The ORs for slow gait speed in the moderate and severe WMH groups compared to the mild group, were 1.46 (95 % CI, 0.99–2.16) and 1.72 (95 % CI, 1.11–2.66), respectively, in multivariable analyses (Baezner et al., 2008).
Older adults with higher WMH had greater decline in gait speed (Silbert et al., 2008; Soumaré et al., 2009a,b). In a population-based study of 225 adults, aged 60–86 years, white matter atrophy and WMH were associated with decline in gait speed over mean follow-up of 30.6 months (Callisaya et al., 2013). Hippocampal atrophy was associated with decline in gait speed (Callisaya et al., 2013). In a longitudinal study of 275 adults with cerebral small vessel disease, age 50–85 years, gait decline over five years was associated with white matter atrophy and loss of white matter integrity (van der Holst et al., 2017v). Slow gait in older adults has also been associated with lower total brain volume (Aribisala et al., 2013), cerebellar gray matter volume (Nadkarni et al., 2014) and total gray matter volume and hippocampal volume (Ezzati et al., 2015). In 175 adults, mean age 73 years, a smaller right hippocampus was the only region of the brain that was related to both slow gait and cognitive impairment (Rosso et al., 2017).
Fluorodeoxyglucose positron emission tomography (FDG-PET) was used to study normalized regional cerebral metabolic rates of glucose (normalized-rCMRglc) in 182 community-dwelling women, mean age 69.4 years) who had no IADL and no mobility limitations (Sakurai et al., 2014). Both usual and maximal gait speed were assessed. Slower maximal gait speed was associated with lower normalized-rCMRglc in prefrontal, posterior cingulate, and parietal cortices (Sakurai et al., 2014). There was no association of normalized-rCMRglc with usual gait speed. In 149 well-functioning women, mean age 70.1 years, with no cognitive impairment or disability, both slower gait speed and lower executive function were associated with lower normalized-rCMRglc in posterior cingulate and primary sensorimotor cortices (Sakurai et al., 2017).
4.11. Other factors
The burden of the cellular senescence biomarker p16INK4a in vastus lateralis adipose tissue was negatively associated with gait speed in 11 older women, mean age 72.9 years (Justice et al., 2018). Elevated serum follistatin concentrations were associated with slow gait speed in 205 adults, aged ≥65 years (Liaw et al., 2016). In 665 adults, aged 65–96 years, high plasma heat shock protein 72, a stress-induced protein that plays a molecular chaperone function, was associated with slow gait speed (Ogawa et al., 2012. No genome-wide significant signals for gait speed were found in a genome-wide meta-analysis of 31,478 older adults from 17 cohorts in the CHARGE consortium that involved validation in 2588 older adults in four independent studies Ben-Avraham et al., 2017). The relationship of the apolipoprotein E genotype and gait speed decline was examined in 627 community-dwelling adults in the Einstein Aging Study (Verghese et al., 2013a,b). The apolipoprotein E ε4 allele was associated with increased risk of gait speed decline over median follow-up of three years in men but not women (Verghese et al., 2013a,b). Low serum levels of carnitine, a quaternary ammonium compound that is found in high concentrations in skeletal and cardiac muscle, were associated with slow gait speed in older adults (Nagai et al., 2017). Cadmium is an environmental toxicant that can accumulate in the body through cigarette smoking, diet (shellfish, offal, vegetables), and polluted air. High blood cadmium was associated with slow gait speed in adults, aged ≥50 years, in NHANES (1999–2000) (Kim et al., 2018).
5. Subjective memory complaint in older adults
Subjective memory complaint is common among older adults, with the prevalence generally ranging from about 20%–40%, depending upon the characteristics of the study population and the instrument used to measure subjective memory complaint (Jonker et al., 2000). In a meta-analysis, subjective memory complaint was found in 17.4 % of 15,553 older adults with normal cognition (Mitchell, 2008). Subjective memory complaint is considered one of the earliest warning signs of AD (Alzheimer’s Association, 2017).
6. Subjective memory complaint as a predictor of cognitive impairment and dementia
6.1. Cognitive decline
Subjective memory complaint predicted cognitive decline in 230 healthy, cognitively normal adults, aged > 50 years (Glodzik-Sobanska et al., 2007. In 213 cognitively normal adults, mean age 67.2 years, subjective memory complaint predicted cognitive decline during seven years of follow-up Resiberg et al., 2010. In 1107 cognitively normal community-dwelling women, aged > 65 years, subjective memory complaint predicted worse cognitive impairment over eighteen years of follow-up Kaup et al., 2015. In 1307 cognitively normal community-dwelling adults, aged > 70 years, in the GuidAge Study in France, subjective memory complaint was associated with a greater decline in cognition over five years of follow-up Dardenne et al., 2017). Subjective memory complaint predicted greater cognitive decline in 733 community-dwelling adults, aged 59–71 years, over four years of follow-up (Dufouil et al., 2005). The mean (SD) MMSE score in this study population was 27.6 (2.1) at baseline. The combination of WMH and subjective memory complaint was even a strong predictor of cognitive decline than subjective memory complaint alone (Dufouil et al., 2005). The annual conversion rate of subjective memory complaint to MCI was 6.6 % based upon a meta-analysis of 3642 older adults from 11 studies Mitchell et al., 2014). In patients with cardiovascular disease, subjective memory complaint projected decline of cognition (Haley et al., 2009).
6.2. Dementia
Many longitudinal studies have shown that older adults with subjective memory complaint are at higher risk of developing dementia (Tobiansky et al., 1995; Schofield et al., 1997; Geerlings et al., 1999; St John and Montgomery, 2002; Wang et al., 2004; Kim et al., 2006; van Oijen et al., 2007v; Verdelho et al., 2011; Waldorff et al., 2012; Jessen et al., 2014a,b; Mitchell et al., 2014; Abner et al., 2015; Ronnlund et al., 2015; Buckley et al., 2016; Tsutsumimoto et al., 2017; Peter et al., 2019). A meta-analysis of studies published up to 2014 involving 29,723 adults showed that the average annual conversion rate of people with subjective memory complaint to dementia was 2.33 % (95 % CI 1.93, 2.78 %) and a RR of dementia of 2.07 (95 % CI 1.76, 2.44) compared to those without subjective memory complaint (Mitchell et al., 2014). In 3,672 adults, mean age 71.7 years, among cognitively intact participants, subjective memory complaint was associated with higher risk of dementia (HR 4.95, 95 % CI 1.52, 16.11) (Tsutsumimoto et al., 2017). Subjective memory complaint predicted incident dementia or AD independent of objective memory performance in a prospective study of 1547 adults, aged 60–90 years, in Sweden (Rönnlund et al., 2015).
7. Risk factors and biomarkers associated with subjective memory complaint
7.1. Demographic and lifestyle factors
The proportion of adults with subjective memory complaint increased with age (Bassett and Folstein, 1993; Gagnon et al., 1994; Jonker et al., 1996; Blazer et al., 1997; Jonker et al., 2000; van Oijen et al., 2007v; Reisberg et al., 2010). Subjective memory complaint was associated with low education level (Bassett and Folstein, 1993; Gagnon et al., 1994; Schofield et al., 1997; Dufouil et al., 2005; van Oijen et al., 2007v; Miranda et al., 2008; Paradise et al., 2011; Kaup et al., 2015), and obesity (Paradise et al., 2011). In a randomized trial, a walking intervention was compared with a program of flexibility, toning, and balance in 175 community-dwelling adults, mean age 66.4 years, over a 12-month period (Zuniga et al., 2016). Subjective memory complaint did not change in either group from baseline through 6- and 12-month follow-up.
7.2. Diet
Although there is evidence, mostly from observational studies, that links diet with cognition (Power et al., 2019), the role of diet or nutritional factors with subject memory complaint has not been well characterized. Vitamin K, in addition to its well known role in blood coagulation, plays a role in sphingolipid synthesis and neuroprotection in the brain (Ferland, 2013) and may be involved in cognition (Alisi et al., 2019). Lower dietary intake of vitamin K associated with more severe subjective memory complaint in 160 adults, mean age 82.4 years, hospitalized or seen at a geriatric care unit in France (Soutif-Veillon et al., 2016).
7.3. Systemic inflammation
Systemic inflammation has been shown to predict cognitive decline in adults (Walker et al., 2019), but no studies, to our knowledge, have examined the relationship between subjective memory complaint and plasma biomarkers of inflammation.
7.4. Chronic diseases
Subjective memory complaint was associated with cardiovascular disease, stroke, and transient ischemic attack in 2546 adults, aged 60–64 years, in Australia (Jorm et al., 2004). Subjective memory complaint was associated with myocardial infarction (Kaup et al., 2015). In a cross-sectional study of 45,532 adults, aged 45–64 years, participating in the 45 and Up Study of healthy aging in New South Wales, Australia, subjective memory complaint was associated with two cardiovascular risk factors: smoking (OR 1.18, 95 % CI 1.03, 1.35) and hypercholesterolemia (OR 1.19, 95 % Ci 1.04, 1.36) and with diabetes (Paradise et al., 2011). In older African-Americans without depressive symptoms, those with subjective memory impairment had significantly higher cerebrovascular risk factors than those without impairment (Sperling et al., 2017). Community-based studies have shown that subjective memory complaint is associated with depressive symptoms (Bolla et al., 1991; Grut et al., 1993).
7.5. Endocrine abnormalities
Altered activity of the HPA axis has been associated with subjective memory complaint. In 46 healthy adults, mean age 61.8 years, those with subjective memory complaint had higher basal urinary cortisol concentrations and higher urinary cortisol concentrations after administration of dexamethasone compared with controls (Wolf et al., 2005). In 64 adults, mean age 78.6 years, without MCI or dementia, the averaged postpeak cortisol and cortisol awakening response were significantly associated with subjective memory complaint (Peavy et al., 2013). In 180 community-dwelling adults without dementia, mean age 71.1 years, those with subjective memory complaint about problems learning new information had lower serum vitamin D concentrations compared to those without subjective memory complaint (Annweiler et al., 2018).
7.6. Amino acid profiles
Serum amino acid profiles were characterized in 46 healthy controls, 24 adults with subjective memory complaint, 18 with MCI, 29 with probable AD, and (Corso et al., 2017). Across the four categories from healthy controls to AD, serum glutamic acid, asparatate, and phenylalanine progressively decreased and serum citrulline, argininosuccinic acid, and homocitrulline progressively increased (Corso et al., 2017). Serum hawkinsin, methionine, and succinylacetone were significantly lower in adults with subjective memory complaint compared with healthy controls (Corso et al., 2017).
7.7. Apolipoprotein E
Studies of the association between the APOE ε4 allele and subjective memory complaints have shown inconsistent results. Some studies reported a significant association of the APOE ε4 allele with subjective memory complaint (Small et al., 1999; Stewart et al., 2001; Laws et al., 2002; van der Flier et al., 2008v) while others reported no association (Kim et al., 2003; Harwood et al., 2004; Jorm et al., 2004; Dufouil et al., 2005; Lautenschlager et al., 2005; Bartley et al., 2012; Buckley et al., 2013).
7.8. Depressive symptoms
Subjective memory complaint has been associated with depression or depressive symptoms in cross-sectional studies that excluded subjects with dementia (Stewart et al., 2001; Bartley et al., 2012; Minett et al., 2005, 2008; Sperling et al., 2017).
7.9. Brain imaging
Neuroimaging studies have shown that older adults with subjective memory complaint have greater cortical atrophy in the medial temporal lobe, frontotemporal, and neocortical regions (Saykin et al., 2006), entorhinal cortex (Jessen et al., 2006; Schultz et al., 2015), fusiform, posterior cingulate, and inferior parietal cortices (Schultz et al., 2015), and medial temporal lobe and hippocampus (Striepens et al., 2010; Scheef et al., 2012; Buckley et al., 2016. In 1336 non-demented community-dwelling adults, aged ≥65 years, in the Three-City Study in France, subjective memory complaint at baseline predicted subsequent hippocampal volume change over four years of follow-up Stewart et al., 2011). In contrast, in a study of 305 community-dwelling adults, aged 60–64 years, who were free of MCI and dementia, hippocampal atrophy over four years was associated with subjective memory complaint at follow-up (Cherbuin et al., 2015). Neuroimaging studies have not shown a consistent relationship of subjective memory complaint with WMH (Minett et al., 2005; Miranda et al., 2008; Haley et al., 2009; Bartley et al., 2012). Neuroimaging studies show that subjective memory complaint is associated with increased amyloid burden in cognitively normal adults (Amariglio et al., 2012; Risacher et al., 2015; Snitz et al., 2015; Zwan et al., 2016). A higher amyloid burden has been described in adults with subjective memory complaint who have the APOE ε4 allele (Risacher et al., 2015; Zwan et al., 2016). In a study of healthy middle aged adults, participants with subjective memory complaint had reduced metabolic rates for glucose in the parietotemporal and parahippocampal gyrus compared with controls (Mosconi et al., 2008). In 109 adults with hypertension, mean age 56.1 years, subjective memory complaint was associated with cerebral microbleeds (Uiterwijk et al., 2014).
Sixty hypertensive adults, mean age 75 years, with subjective memory complaint and no objective cognitive impairment, were studied using FDG-PET and diffusion tensor magnetic resonance imaging (DTI) (Chetouani et al., 2017). Cross-sectional variation in the structure of the overall white matter was associated with metabolism of Alzheimer-like cortical areas (Chetouani et al., 2017). Brain glucose metabolism was studied using FDG-PET in 24 women, mean age 70.1 years, with subjective memory complaint (Jeong et al., 2017). The participants were evaluated at baseline and 24 months later. No women progressed to MCI or dementia. Regional cerebral metabolic rate of glucose significantly declined in the left superior temporal, right posterior cingulate, left parahippocampal, right lingual, and right angular gyri (Jeong et al., 2017). The reduction in the regional cerebral metabolic rate of glucose was associated with a decline in executive function (Jeong et al., 2017).
In a study of 18 adults with subjective memory complaint and 27 healthy controls, lower entorhinal cortical volumes were found in those with subjective memory complaint compared with controls (Ryu et al., 2017). In additional, changes in DTI were observed in the hippocampal body and entorhinal white matter compared with controls (Ryu et al., 2017). Cortical thickness was compared in predefined AD-related brain regions of the medial temporal lobe between 41 adults with subjective memory complaint and 69 healthy controls (Meiberth et al., 2015). Subjects with subjective memory complaint had significantly reduced cortical thickness in the left entorhinal cortex compared with controls (Meiberth et al., 2015). Subsequent memory effects were studied in 23 cognitively normal healthy older adults with subjective memory complaint and 41 healthy controls, aged 50–85 years. Those with subjective memory complaint showed lower subsequent memory effects in the occiptal lobe, superior parietal lobe, and posterior cingulate cortex compared with controls (Hayes et al., 2017). The intrinsic connectivity network was investigated in 44 adults with subjective memory complaint and 40 normal controls using resting-state functional magnetic resonance imaging (MRI) and pathological data (Li et al., 2018). Impaired local intrinsic connectivity networks were found in those with subjective memory complaint (Li et al., 2018).
7.10. Other factors
Subjective memory complaint was associated with lower hemoglobin, ferritin, and free thyroxine concentrations in 405 adults, aged ≥55 years, seen in outpatient clinics (Açikgöz et al., 2014). Macular thickness was previously reported as a biomarker for AD (Garcia-Martin et al., 2014), raising interest that this ocular measurement might have relevance in the pre-dementia stage. The macular thickness of 24 adults with subjective memory complaint, 33 adults with MCI, and 25 healthy controls was compared using optical coherence tomography (OCT) (Giménez Castejón et al., 2016). Macular thickness was significantly lower in the MCI and subjective memory complaint groups compared with controls (Giménez Castejón et al., 2016).
8. Motoric cognitive risk syndrome
Both slow gait speed and subjective memory complaint are early predictors of cognitive decline and dementia. The motoric cognitive risk syndrome (MCR) was proposed and validated in 2013 by Verghese and colleagues as a pre-dementia syndrome that identifies a subgroup of individuals that are at high risk for dementia (Verghese et al., 2013a,b). MCR was defined based upon four criteria: (1) subjective cognitive complaint from standardized questionnaires, (2) slow gait defined as one standard deviation or more below age- and sex-appropriate mean gait values, (3) ability to ambulate, and (4) absence of dementia (Ayers and Verghese, 2014). The presence of subjective cognitive complaint has not been harmonized across studies and was most often elicited by the standardized memory loss question from the 15-item Geriatric Depression Scale (GDS) (Yesavage et al., 1983) administered by trained research staff: “Do you feel you have more problems with memory than most?” with a “yes” responses defined as subjective memory complaint. The GDS was used to assess subjective memory complaint in 8 of 22 cohorts in the worldwide MCR prevalence study (Verghese et al., 2014a). In 6 of 22 cohorts, self-report cognitive questionnaire in the Study of Global Ageing and Adult Health (SAGE) used two self-reported memory questions: “How would you best describe your memory at present? Is it very good, good, moderate, bad or very bad?” and “Compared to 12 months ago, would you say your memory is now better, the same or worse than it was then?” (World Health Organization, 2006). The worldwide prevalence of MCR, based upon 26,802 adults without dementia and disability, aged ≥60 years, from 22 cohorts representing 17 countries was estimated at 9.7 % (95 % CI 8.2, 11.2 %) (Verghese et al., 2014a). The overall age- and sex-adjusted incidence rate of MCR, based upon four US cohorts involving 3,128 adults, aged ≥60 years, was 65.2/1000 person-years (95 % CI 53.3, 77.1) (Verghese et al., 2014b).
9. MCR as a predictor of cognitive impairment and dementia
9.1. Cognitive impairment
In a meta-analysis involving 4936 older community-dwelling adults from five studies, MCR predicted cognitive impairment HR 1.70, 95 % CI 1.46, 1.98) (Sekhon et al., 2019). The meta-analysis showed that MCR was a stronger predictor of cognitive impairment than either slow gait or subjective memory complaint alone (Sekhon et al., 2019. In a pooled analysis involving 4555 older community-dwelling adults from the Hispanic Established Populations for Epidemiologic Studies of the Elderly, InCHIANTI Study, Memory and Aging Project, and Religious Orders Study, MCR predicted cognitive impairment HR 2.00, 95 % CI 1.7, 2.4) (Verghese et al., 2014a,b).
9.2. Dementia
MCR predicted dementia in longitudinal studies conducted in Australia, Belgium, Canada, China, France, Ghana, India, Israel, Italy, Japan, Korea, Mexico, Russia, South Africa, Switzerland, United Kingdom, and the US (Kumai et al., 2016; Doi et al., 2017; Verghese et al., 2013a,b; Verghese et al., 2014a,b). In a pooled analysis of 26,802 older adults, MCR predicted dementia (HR 1.93, 95 % CI 1.59, 2.35) and AD (HR 2.21, 95 % CI 1.49, 3.28) (Verghese et al., 2014a). The results were robust even after removing those with possible cognitive impairment or any diagnostic overlap with other predementia syndromes (Verghese et al., 2014a). Notably, MCR predicted risk of dementia (HR 2.47, 95 % CI 1.93, 3.17) more than either slow gait alone (HR 1.77, 95 % CI 1.38, 2.27) or cognitive complaint alone (HR 1.27, 95 % CI 0.99, 1.63) (Verghese et al., 2014a). In a meta-analysis of 9,156 older adults from seven studies, MCR predicted dementia (OR 2.50, 95 % CI 1.75, 2.39) (Sekhon et al., 2019). In a study of 590 community dwelling adults, aged ≥75 years, in which the prevalence of MCI was 11.1 % at baseline, the incidence rate of dementia was 119.8/1000 person-years in the MCR group and 102.5/1000 persons-years in the non-MCR group (Kumai et al., 2016).
9.3. Other adverse aging-related outcomes
An analysis based upon five longitudinal studies, the Baltimore Longitudinal Study of Aging, LonGenity, InCHIANTI Study, Tasmanian Study of Cognition and Gait, and Einstein Aging Study involving 6,204 community-dwelling adults, aged ≥60 years, without dementia, showed that the pooled risk of MCR with falling was RR 1.44 (95 % CI 1.16, 1.79) (Callisaya et al., 2016). The risks of falls were lower for each separate component of MCR compared with MCR itself. Slow gait and cognitive complaint were associated with RR 1.30 (95 % CI 1.14, 1.47) and RR 1.25 (95 % 1.07, 1.46) for falling, respectively. In a study of 4,235 community-dwelling adults, mean age 72.0 years, MCR predicted the risk of disability, defined as a certified need for long-term care insurance (HR 1.69, 95 % CI 1.08, 2.02) (Doi et al., 2017).
In 11,867 adults without dementia, aged ≥65 years, from three established cohort studies, MCR predicted mortality (HR 1.69, 95 % CI 1.46,1.96) over median follow-up of 28 months (Ayers and Verghese, 2016). Notably, MCR significantly predicted mortality even after adjusting for both baseline gait speed and memory test scores (Ayers and Verghese, 2016. MCR predicted mortality among 3778 women, aged ≥75 years, followed for 19 years in the Epidémiologie de l’Ostéoporosis EPIDOS study in France HR 1.41, 95 % CI 1.25, 1.48) (Beauchet et al., 2019). In the same subjects, slow gait speed alone predicted mortality (HR 1.41, 95 % CI 1.27, 1.55), whereas subjective cognitive complaint was not a significant predictor of death (HR 1.05, 95 % CI 0.98, 1.14) (Beauchet et al., 2019). Interpretation of these results from the EPIDOS study should be made with caution, as the assessment of subjective cognitive complaint was based upon the short portable mental status questionnaire (Pfeiffer, 1975), which is an objective assessment, rather than that based upon subjective cognitive complaints.
10. Risk factors and biomarkers associated with MCR
10.1. Demographic and lifestyle factors
The prevalence and incidence of MCR increases with age (Verghese et al., 2014a,b; Doi et al., 2015). The prevalence of MCR was 8.9 % among adults aged 60–74 years and 10.6 % among adults aged ≥75 years (Verghese et al., 2014a). MCR prevalence and incidence do not appear to differ by sex (Verghese et al., 2013a,b; Verghese et al., 2014a,b). Blacks may be at a higher risk of MCR than whites (Verghese et al., 2013a,b). Lower education is associated with a greater risk of MCR (Verghese et al., 2014a; Doi et al., 2015; Maguire et al., 2018; Aguilar-Navarro et al., 2019; Beauchet et al., 2019). MCR was associated with low physical activity (Doi et al., 2015; Allali et al., 2016; Doi et al., 2017; Beauchet et al., 2019) and obesity (Doi et al., 2015; Allali et al., 2016; Doi et al., 2017; Maguire et al., 2018; Beauchet et al., 2018).
10.2. Genetic factors
The polygenic inheritance of MCR was examined in 4915 adults, aged ≥65 years, in the Health and Retirement Study Sathyan et al., 2019). Polygenic scores (PGS) were calculated as the weighted sum of single nucleotide polymorphisms with effects sizes derived from genome-wide association studies. The PGS for BMI and waist circumference were associated with MCR (Sathyan et al., 2019). MCR was associated with polymorphisms in IL-10 in an Ashkenazi Jewish population (Sathyan et al., 2017).
10.3. Chronic diseases
MCR was associated with hypertension (Beauchet et al., 2018), cardiovascular disease (Doi et al., 2015; Doi et al., 2017; Beauchet et al., 2019), osteoporosis (Doi et al., 2015), diabetes (Verghese et al., 2014a,b; Doi et al., 2015; Doi et al., 2017; Beauchet et al., 2018, 2019; Aguilar-Navarro et al., 2019). Meta-analyses showed that MCR was associated with hypertension, cardiovascular diseases, diabetes, and stroke (Beauchet et al., 2018). In a meta-analysis involving 50,025 older adults, MCR was associated with pooled risk factors for cardiovascular disease (Beauchet et al., 2018). MCR was associated with depression (Doi et al., 2015; Doi et al., 2017; Beauchet et al., 2019).
10.4. Brain imaging
Brain imaging was performed in 28 adults with MCR and 143 adults without MCR (Beauchet et al., 2016a,b). Participants with MCR did not differ in objective cognitive testing from controls without MCR. Adults with MCR had smaller volumes of total gray matter, total cortical gray matter, premotor cortex, prefrontal cortex, and dorsolateral segment of prefrontal cortext compared to those without MCR (Beauchet et al., 2016a,b). No association was found between WMH and MCR in a MRI study of 358 adults in France and India (Mergeche et al., 2016). In a study of 139 older adults in India, MCR was associated with frontal lacunar infarcts (Wang et al., 2016). Gray matter (GM) covariance networks were studied using voxel-based morphometry and multivariate covariance-based statistics in a pooled sample of 267 community-dwelling adults, mean age 75.6 years, from three different cohort studies (Blumen et al., 2018). A significant GM volume covariance network was associated with MCR that was primarily composed of supplementary motor, insular, and prefrontal cortex regions, suggesting that MCR is linked with GM atrophy in brain regions that are associated with control aspects rather than motor aspects of gait (Blumen et al., 2018). A systematic review showed that MCR was associated with lower gray matter volume mainly in the premotor cortex in the prefrontal cortex (Sekhon et al., 2019). There was no significant association of MCR with white matter abnormalities. These findings suggest that the brain pathology of MCR may involve mainly neurodegeneration (Sekhon et al., 2019).
11. What do slow gait speed and subjective memory complaint have in common that might provide clues to the underlying pathophysiology of MCR?
The underlying pathophysiology of MCR has not been well characterized. Studies aimed at gaining insight into altered biology in MCR have primarily involved neuroimaging. To date, there have been no studies, to our knowledge, on circulating proteins or metabolites that may be associated with MCR. Since a major component of MCR is subjective memory complaint, there is no animal model for MCR. The lack of an appropriate animal model greatly restricts mechanistic studies. There have been relatively more studies conducted to gain insight into the biological mechanisms that could explain slow gait speed compared with subjective memory complaint. Risk factors and biomarkers that have been associated with slow gait speed, subjective memory complaint, and MCR are summarized in Table 1. A conceptual model based upon known and likely risk factors for MCR is shown in Fig. 2.
Table 1.
Risk factors and biomarkers that have been associated with slow gait speed, subjective memory complaint, and motoric cognitive risk syndrome.
| Risk factor/biomarker | Slow gait speed | Subjective memory complaint | Motoric cognitive risk syndrome |
|---|---|---|---|
| Demographic, lifestyle | older age; low social network; low education level; higher risk in blacks; low physical activity; overweight; obesity |
older age; low education level; low physical activity |
older age; low education level; higher risk in blacks; low physical activity; obesity |
| Diet | low fruit/vegetable intake; non-Mediterranean diet; low protein intake low vitamin K status |
low vitamin K intake | ?? |
| Chronic disease | hypertension; cardiovascular disease; diabetes mellitus; chronic obstructive pulmonary disease; chronic kidney disease; depression |
cardiovascular disease; diabetes mellitus; depression |
hypertension; cardiovascular disease; diabetes mellitus; depression |
| Endocrine | low serum 25(OH)D; abnormal cortisol profile |
low serum 25(OH)D; abnormal cortisol profile |
?? |
| Systemic inflammation | elevated CRP; IL-6; VCAM-1; TNF-α | ?? | ?? |
| Plasma metabolites | low n-3 PUFA; low LPC; elevated acylcarnitines |
low serum hawkinsin, methionine, succinylacetone | ?? |
| Plasma proteins | elevated GDF-15 | ?? | ?? |
| Skeletal muscle | low knee strength; fat infiltration; impaired mitochondrial oxidative capacity |
?? | ?? |
| Brain | Aβ deposition; increased WMH; low total gray matter volume; low gray matter volume in hippocampus; decreased glucose metabolism in prefrontal, posterior cingulate, and parietal cortices |
Aβ deposition; increased WMH; cortical atrophy; hippocampal atrophy; cerebral microbleeds; decreased glucose metabolism in left superior temporal, right posterior cingulate, left parahippocampal, right lingual, and right angular gyri |
low total gray matter volume; smaller volume in premotor cortex, prefrontal cortex; frontal lacunar infarcts |
| Other | low serum carnitine; elevated blood cadmium; elevated serum follistatin; elevated plasma HSP 72 |
?? | ?? |
| APOE | no consistent relationship | no consistent relationship | ?? |
Abbreviations: APOE, apolipoprotein E; 25(OH)D, 25-hydroxyvitamin D; CRP, C-reactive protein; IL-6, interleukin-6; VCAM-1, vascular cell adhesion protein 1; TNF-α, tumor necrosis factor-α; PUFA, polyunsaturated fatty acid; LPC, lysophosphatidylcholine; GDF-15, growth and differentiation factor-15; HSP, heat shock protein.
Fig. 2.

Conceptual model of MCR showing known and probable risk factors. Broken arrows signify other modulating factors.
Slow gait speed and subjective memory complaint have several common risk factors: cardiovascular disease, diabetes mellitus, abnormal cortisol profiles, low vitamin D levels, low vitamin K status, brain atrophy with decreased hippocampal volume, and increased deposition of beta-amyloid in the brain. Brain regions and networks that are shared between gait and memory complaint can be affected by neurodegeneration and vascular factors (Montero-Odasso et al., 2014). However, gait speed is not explained solely by neural input from the brain, as there are pathological processes in skeletal muscle that can also contribute to a decline of gait speed with aging, such as loss of regenerative capacity, impaired proteostasis, accumulation of senescent cells, alterations in glucose metabolism, and impaired mitochondrial function (Aversa et al., 2019; Larsson et al., 2019). Low muscle strength in the lower extremities, especially in the quadriceps muscle, and impaired skeletal muscle mitochondrial oxidative capacity and the vastus lateralis, are strongly associated with slow gait speed. There is also evidence to suggest that the relationship between gait and the brain is bidirectional. Increased physical activity is known to protect against age-related decline in gait speed (Pahor et al., 2014; Brach et al., 2017) but also mitigates or even reverses age-related brain structural and functional deficits (Seidler et al., 2010; Bolandzadeh et al., 2015).
Increased circulating inflammatory biomarkers are associated with slow gait speed and may reflect greater cardiovascular disease burden (Ferrucci et al., 2005). Increased cardiovascular disease is associated with slow gait speed, subjective memory complaint, and MCR (Table 1). Increased plasma CRP and IL-6 have been associated with smaller total gray matter volume in non-demented older adults (Gu et al., 2017. In 1841 adults, aged 65–80 years, elevated circulating IL-6 was associated with decreased gray matter and hippocampal volumes (Satizabal et al., 2012). Decreased total gray matter volume and hippocampal atrophy have been associated with slow gait speed, subjective memory complaint, and MCR (Table 1).
Slow gait speed has been associated with low plasma LPC (Semba et al., 2019a,b,c) and elevated plasma acylcarnitines (Lum et al., 2011). Although plasma LPC and acylcarnitines have not been characterized in subjective memory complaint or MCR, it is notable that low plasma LPC have been associated with AD (Cui et al., 2014). In 525 healthy adults, ≥70 years, low plasma LPC 18:2 predicted progression to amnestic mild cognitive impairment or AD over 5 y of follow-up (Mapstone et al., 2014). Poor memory was associated with elevated plasma acylcarnitines in 100 adults, mean age 59.3 years, in the Fels Longitudinal Study (Peterson et al., 2019). Elevated plasma acylcarnitines were elevated in cognitively impaired adults with AD-related neurodegeneration (Toledo et al., 2017). Elevated plasma acylcarnitines reflect altered beta oxidation of fatty acids in mitochondria (Semba et al., 2019a,b,c).
Higher plasma n-3 polyunsaturated fatty acids (PUFA) were associated with faster gait speed (Abbatecola et al., 2009). A meta-analysis of intake of two n-3 PUFA, docosahexaenoic acid (DHA; 22:6) and eicosapentaenoic acid (EPA; 20:5), showed that DHA, alone or combined with EPA, improved memory function in older adults with mild memory complaints (Yurko-Mauro et al., 2015). Higher dietary intake of fruit and vegetables or adherence to the Mediterranean diet have been associated with faster gait speed (Lauretani et al., 2008; Shahar et al., 2012). Greater adherence to the Mediterranean diet has been associated with better cognition (Petersson and Philippou, 2016; Aridi et al., 2017).
Elevated plasma GDF-15 was associated with slow gait speed in healthy adults (Semba et al., 2019a,b,c). In 888 non-demented adults, aged 70–90 years, in the Sydney Memory and Aging Study, higher serum GDF-15 was associated with lower processing speed, memory, and executive function (Fuchs et al., 2013). Elevated serum GDF-15 was associated with a 20 % chance of decline from normal to MCI or dementia (Fuchs et al., 2013). In 80 adults with no cognitive impairment, 144 nondemented adults with cognitive impairment, and 100 adults with Alzheimer’s disease, neural imaging studies showed that WMH was associated with elevated plasma GDF-15 (OR 3.97, 95 % CI 1.79, 8.83) compared with those with no cognitive impairment (Chai et al., 2016).
12. Recommendations for future studies
Current proteomic and metabolomic platforms allow the detection and measurement of hundreds of circulating proteins and metabolites for discovery phase investigations of disease phenotypes (Semba et al., 2017; Tanaka et al., 2018; Feldreich et al., 2019; Semba et al., 2019a,b,c). These platforms could be applied to elucidate the roles of biological pathways in MCR that involve candidate biomarkers such as amino acids, proteins, peptides, biogenic amines, steroids, phenols, carbohydrates, bile acids, ceramides, fatty acids, diacylglycerols, triacylglycerols, lysophosphatidylcholines, sphingomyelins, cholesteryl esters, acylcarnitines, phosphatidylcholines, indoles, and hormones. Metabolomic investigations are showing promise in identifying specific lipids (Li et al., 2019) and amino acids (Tynkkynen et al., 2018) that predict cognitive decline. Other promising areas for biomarker studies in MCR include systemic inflammation, n-3 PUFA, GDF-15, and 25(OH) D concentrations. Cross-sectional studies of biomarkers can reveal potential associations but are limited in making causal inferences. Longitudinal study designs may provide stronger evidence for biomarkers as independent predictors of incident MCR.
13. Ethical and public health implications of MCR
MCR was originally proposed to have advantages in the clinical setting because of the relative simplicity of measuring gait speed and assessing subjective memory complaint (Verghese et al., 2013a,b; Ayers and Verghese, 2014). MCR could identify a subset of older adults who are at high risk for progressing to dementia (Verghese et al., 2014a). The advantage of MCR over MCI was that it did not require detailed neurocognitive assessment and evaluation by experts. MCR may also take on important significance as a screening tool and research tool to identify a high-risk population (Chhetri et al., 2017). However, it should be emphasized that a sizable portion of individuals with MCR do not progress to dementia. Although the natural history of MCR has not been well characterized, it is likely that many individuals with MCR remain stable or improve during follow-up. In the natural history of MCI, which has been better characterized than MCR, three quarters of patients remained cognitively stable or even improved over three years (Kaduszkiewicz et al., 2014). Slow gait speed and subjective memory complaint are heterogeneous risk factors that can have variable trajectories in older adults, with some individuals remaining stable or improving (Pahor et al., 2014; Best et al., 2018; Zarit et al., 1981). Thus, caution must be taken in the interpretation of risk, both in a clinical setting and for individuals who participate in research studies of MCR (Davis, 2015). MCR may be most useful as a research tool to could help uncover altered pathways, biomarkers, and therapeutic targets for intervention during the long preclinical phase of AD.
14. Conclusions
MCR is a relatively new pre-dementia syndrome that combines two early harbingers of dementia, slow gait speed and subjective memory complaint. Slowing of gait speed and the appearance of subjective memory complaint co-occur during the long preclinical phase of dementia. Slow gait speed and subjective memory complaint share several common risk factors and biomarkers such as cardiovascular disease, diabetes mellitus, abnormal cortisol profiles, low vitamin D levels, low vitamin K status, brain atrophy with decreased hippocampal volume, and increased deposition of beta-amyloid in the brain. Future studies may corroborate the association of these risk factors and biomarkers with MCR. The application of advanced platforms in proteomics and metabolomics holds great promise for the discovery of novel biomarkers in biological pathways that are associated with MCR and altered early in the development of dementia.
Acknowledgments
Support
The National Institutes of HealthR01 AG027012, R01 EY024596, R56 AG052973, R01 AG057723, and the Intramural Branch of the National Institute on Aging, Baltimore, Maryland.
References
- Abbatecola AM, Cherubini A, Guralnik JM, Andres Lacueva C, Ruggiero C, Maggio M, et al. 2009. Plasma polyunsaturated fatty acids and age-related physical performance decline. Rejuvenation Res. 12 (1), 25–32. 10.1089/rej.2008.0799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abellan van Kan G, Rolland Y, Gillette-Guyonnet S, Gardette V, Annweiler C, Beauchet O, et al. 2012. Gait speed, body composition, and dementia. The EPIDOS-Toulouse cohort. J. Gerontol. A Biol. Sci. Med. Sci 67 (4), 425–432. 10.1093/gerona/glr177. [DOI] [PubMed] [Google Scholar]
- Abner EL, Kryscio RJ, Caban-Holt AM, Schmitt FA, 2015. Baseline subjective memory complaints associate with increased risk of incident dementia: the PREADVISE trial. J. Prev. Alzheimers Dis 2 (1), 11–16. 10.14283/jpad.2015.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Açikgöz M, Özen Baru TB, Emre U, Taşçilar N, Atalay A, Köktürt F, 2014. Assessment of relation between subjective memory complaints and objective cognitive performance of elderly over 55 years old age. Noro Psikiyatr. Ars 51 (1), 57–62. 10.4274/npa.y6719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aguilar-Navarro SG, Mimenza-Alvarado AJ, Aguilar-Esquivel JE, Yeverino-Castro SG, Juárez-Cedillo T, Mejía-Arango S, 2019. Motoric cognitive risk syndrome: prevalence and risk of cognitive impairment in a population studied in the Mexican Health and Aging Study 2012–2015. J. Nutr. Health Aging 23 (3), 227–231. 10.1007/s12603-019-1160-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfaro-Acha A, Al Snih S, Raji MA, Markides KS, Ottenbacher KJ, 2007. Does 8-foot walk time predict cognitive decline in older Mexicans Americans? J. Am. Geriatr. Soc 55 (2), 245–251. 10.1111/j.1532-5415.2007.01039.x. [DOI] [PubMed] [Google Scholar]
- Alisi L, Cao R, De Angelis C, Cafolla A, Caramia F, Cartocci G, et al. 2019. The relationships between vitamin k and cognition: a review of current evidence. Front. Neurol 10, 239 10.3389/fneur.2019.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allali G, Ayers EI, Verghese J, 2016. Motoric cognitive risk syndrome subtypes and cognitive profiles. J. Gerontol. A Biol. Sci. Med. Sci 71 (3), 378–384. 10.1093/gerona/glv092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alzheimer’s Association, 2017. 2017 Alzheimer’s disease facts and figures. Alzheimer Dement. 13, 325–373. [Google Scholar]
- Amariglio RE, Becker JA, Carmasin J, Wadsworth LP, Lorius N, Sullivan C, et al. 2012. Subjective cognitive complaints and amyloid burden in cognitively normal older individuals. Neuropsychologia 50 (12), 2880–2886. 10.1016/j.neuropsychologia.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Annweiler C, Henni S, Walrand S, Montero-Odasso M, Duque G, Duval GT, 2017. Vitamin D and walking speed in older adults: systematic review and meta-analysis. Maturitas 106, 8–25. 10.1016/j.maturitas.2017.07.012. [DOI] [PubMed] [Google Scholar]
- Annweiler C, Doineau L, Gerigne L, Provendier A, Karras SN, Beauchet O, et al. 2018. Vitamin D and subjective memory complaint in community-dwelling older adults. Curr. Alzheimer Res 15 (7), 664–670. 10.2174/1567205015666180201153735. [DOI] [PubMed] [Google Scholar]
- Aribisala BS, Gow AJ, Bastin ME, del Carmen Valdés Hernández M, Murray C, Royle NA, et al. 2013. Associations between level and change in physical function and brain volumes. PLoS One 8 (11), e80386 10.1371/journal.pone.0080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aridi YS, Walker JL, Wright ORL, 2017. The association between the Mediterranean dietary pattern and cognitive health: a systematic review. Nutrients 9 (7). 10.3390/nu9070674 pii: E674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atkinson HH, Rosano C, Simonsick EM, Williamson JD, Davis C, Ambrosius WT, et al. 2007. Cognitive function, gait speed decline, and comorbidities: the Health, Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci 62 (8), 844–850. 10.1093/gerona/62.8.844. [DOI] [PubMed] [Google Scholar]
- Attems J, Jellinger KA, 2014. The overlap between vascular disease and Alzheimer’s disease–lessons from pathology. BMC Med. 12, 206 10.1186/s12916-014-0206-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aversa Z, Zhang X, Fielding RA, Lanza I, LeBrasseur NK, 2019 The clinical impact and biological mechanisms of skeletal muscle aging. Bone, 10.1016/j.bone.2019.05.021 2019. May 22 pii: S8756-3282(19)30196-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayers E, Verghese J, 2014. Diagnosing motoric cognitive risk syndrome to predict progression to dementia. Neurodegener. Dis. Manage 4 (5), 339–342. 10.2217/nmt.14.39. [DOI] [PubMed] [Google Scholar]
- Ayers E, Verghese J, 2016. Motoric cognitive risk syndrome and risk of mortality in older adults. Alzheimers Dement. 12 (5), 556–564. 10.1016/j.jalz.2015.08.167. [DOI] [PubMed] [Google Scholar]
- Baezner H, Blahak C, Poggesi A, Pantoni L, Inzitari D, Chabriat H, et al. 2008. Association of gait and balance disorders with age-related white matter changes: the LADIS study. Neurology 70 (12), 935–942. 10.1212/01.wnl.0000305959.46197.e6. [DOI] [PubMed] [Google Scholar]
- Bartley M, Bokde AL, Ewers M, Faluyi YO, Tobin WO, Snow A, et al. 2012. Subjective memory complaints in community dwelling healthy older people: the influence of brain and psychopathology. Int. J. Geriatr. Psychiatry 27 (8), 836–843. 10.1002/gps.2794. [DOI] [PubMed] [Google Scholar]
- Bassett SS, Folstein MF, 1993. Memory complaint, memory performance, and psychiatric diagnosis: a community study. J. Geriatr. Psychiatry Neurol 6 (2), 105–111. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Allali G, Annweiler C, Verghese J, 2016a. Association of motoric cognitive risk syndrome with brain volumes: results from the GAIT Study. J. Gerontol. A Biol. Sci. Med. Sci 71 (8), 1081–1088. 10.1093/gerona/glw012. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Annweiler C, Callisaya ML, De Cock AM, Helbostad JL, Kressig RW, et al. 2016b. Poor gait performance and prediction of dementia: results from a meta-analysis. J. Am. Med. Dir. Assoc 17 (6), 482–490. 10.1016/j.jamda.2015.12.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchet O, Sekhon H, Barden J, Liu-Ambrose T, Chester VL, Szturm T, et al. 2018. Association of motoric cognitive risk syndrome with cardiovascular disease and risk factors: results from an original study and meta-analysis. J. Alzheimers Dis 64 (3), 875–887. 10.3233/JAD-180203. [DOI] [PubMed] [Google Scholar]
- Beauchet O, Sekhon H, Launay CP, Chabot J, Rolland Y, Schott AM, et al. 2019. Motoric cognitive risk syndrome and mortality: results from the EPIDOS cohort. Eur. J. Neurol 26 (5), 794–e56. 10.1111/ene.13891. [DOI] [PubMed] [Google Scholar]
- Beaudart C, Buckinx F, Rabenda V, Gillain S, Cavalier E, Slomian J, et al. 2014. The effects of vitamin D on skeletal muscle strength, muscle mass, and muscle power: a systematic review and meta-analysis of randomized controlled trials. J. Clin. Endocrinol. Metab 99 (11), 4336–4345. 10.1210/jc.2014-1742. [DOI] [PubMed] [Google Scholar]
- Beavers KM, Beavers DP, Houston DK, Harris TB, Hue TF, Koster A, et al. 2013. Associations between body composition and gait-speed decline: results from the Health, Aging, and Body Composition study. Am. J. Clin. Nutr 97 (3), 552–560. 10.3945/ajcn.112.047860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Avraham D, Karasik D, Verghese J, Lunetta KL, Smith JA, Eicher JD, et al. 2017. The complex genetics of gait speed: genome-wide meta-analysis approach. Aging (Albany NY) 9 (1), 209–246. 10.18632/aging.101151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Davis JC, Liu-Ambrose T, 2015. Longitudinal analysis of physical performance, functional status, physical activity, and mood in relation to executive function in older adults who fall. J. Am. Geriatr. Soc 63 (6), 1112–1120. 10.1111/jgs.13444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Liu-Ambrose T, Metti AL, Rosso AL, Satterfield S, Studenski S, et al. 2018. Longitudinal associations between walking speed and amount of self-reported time spent walking over a 9-year period in older women and men. J. Gerontol. A Biol. Sci. Med. Sci 73 (9), 1265–1271. 10.1093/gerona/glx129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischoff-Ferrari HA, 2012. Relevance of vitamin D in muscle health. Rev. Endocr. Metab. Disord 13 (1), 71–77. 10.1007/s11154-011-9200-6. [DOI] [PubMed] [Google Scholar]
- Blanco I, Verghese J, Lipton RB, Putterman C, Derby CA, 2012. Racial differences in gait velocity in an urban elderly cohort. J. Am. Geriatr. Soc 60 (5), 922–926. 10.1111/j.1532-5415.2012.03927.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blazer DG, Hays JC, Fillenbaum GG, Gold DT, 1997. Memory complaint as a predictor of cognitive decline: a comparison of African American and White elders. J. Aging Health 9 (2), 171–184. [DOI] [PubMed] [Google Scholar]
- Blumen HM, Allali G, Beauchet O, Lipton RB, Verghese J, 2018. A gray matter volume covariance network associated with the motoric cognitive risk syndrome: a multi-cohort MRI Study. J. Gerontol. A Biol. Sci. Med. Sci 10.1093/gerona/gly158. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Bohannon RW, Williams Andrews A, 2011. Normal walking speed: a descriptive meta-analysis. Physiotherapy 97 (3), 182–189. 10.1016/j.physio.2010.12.004. [DOI] [PubMed] [Google Scholar]
- Bolandzadeh N, Tam R, Handy TC, Nagamatsu LS, Hsu CL, Davis JC, et al. 2015. Resistance training and white matter lesion progression in older women: exploratory analysis of a 12-month randomized controlled trial. J. Am. Geriatr. Soc 63 (10), 2052–2060. 10.1111/jgs.13644. [DOI] [PubMed] [Google Scholar]
- Bolla KI, Lindgren KN, Bonaccorsy C, Bleecker ML, 1991. Memory complaints in older adults. Fact or fiction? Arch. Neurol 48 (1), 61–64. [DOI] [PubMed] [Google Scholar]
- Brach JS, Perera S, Gilmore S, VanSwearingen JM, Brodine D, Nadkarni NK, et al. 2017. Effectiveness of a timing and coordination group exercise program to improve mobility in community-dwelling older adults: a randomized clinical trial. JAMA Intern. Med 177 (10), 1437–1444. 10.1001/jamainternmed.2017.3609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley R, Saling MM, Ames D, Rowe CC, Lautenschlager NT, Macaulay SL, et al. 2013. Factors affecting subjective memory complaints in the AIBL aging study: biomarkers, memory, affect, and age. Int. Psychogeriatr 25 (8), 1307–1315. 10.1017/S1041610213000665. [DOI] [PubMed] [Google Scholar]
- Buckley RF, Marufif P, Ames D, Bourgeat P, Martins RN, Masters CL, et al. 2016. Subjective memory decline predicts greater rates of clinical progression in preclinical Alzheimer’s disease. Alzheimers Dement. 12 (7), 796–804. 10.1016/j.jalz.2015.12.013. [DOI] [PubMed] [Google Scholar]
- Buracchio T, Dodge HH, Howieson D, Wasserman D, Kaye J, 2010. The trajectory of gait speed preceding mild cognitive impairment. Arch. Neurol 67 (8), 980–986. 10.1001/archneurol.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callisaya ML, Beare R, Phan TG, Blizzard L, Thrift AG, Chen J, et al. 2013. Brain structural change and gait decline: a longitudinal population-based study. J. Am. Geriatr. Soc 61 (7), 1074–1079. 10.1111/jgs.12331. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Blizzard CL, Wood AG, Thrift AG, Wardill T, Srikanth VK, 2015. Longitudinal relationships between cognitive decline and gait slowing: the Tasmanian Study of Cognition and Gait. J. Gerontol. A Biol. Sci. Med. Sci 70 (10), 1226–1232. 10.1093/gerona/glv066. [DOI] [PubMed] [Google Scholar]
- Callisaya ML, Ayers E, Barzilai N, Ferrucci L, Guralnik JM, Lipton RB, et al. 2016. Motoric cognitive risk syndrome and falls risk: a multi-center study. J. Alzheimers Dis 53 (3), 1043–1052. 10.3233/JAD-160230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camargo EC, Weinstein G, Beiser AS, Tan ZS, DeCarli C, Kelly-Hayes M, et al. 2016. Association of physical function with clinical and subclinical brain disease: the Framingham Offspring Study. J. Alzheimers Dis 53 (4), 1597–1608. 10.3233/JAD-160229. [DOI] [PubMed] [Google Scholar]
- Cappola AR, Xue QL, Ferrucci L, Guralnik JM, Volpato S, Fried LP, 2003. Insulin-like growth factor I and interleukin-6 contribute synergistically to disability and mortality in older women. J. Clin. Endocrinol. Metab 88 (5), 2019–2025. [DOI] [PubMed] [Google Scholar]
- Chai YL, Hilal S, Chong JP, Ng YX, Liew OW, Xu X, et al. 2016. Growth differentiation factor-15 and white matter hyperintensities in cognitive impairment and dementia. Medicine (Baltimore) 95 (33), e4566 10.1097/MD.0000000000004566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherbuin N, Sargent-Cox K, Easteal S, Sachdev P, Anstey KJ, 2015. Hippocampal atrophy is associated with subjective memory decline: the PATH through Life study. Am. J. Geriatr. Psychiatry 23 (5), 446–455. 10.1016/j.jagp.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Chetouani A, Chawki MB, Hossu G, Kearney-Schwartz A, Chauveau-Beuret F, Bracard S, et al. 2017. Cross-sectional variations of white and grey matter in older hypertensive patients with subjective memory complaints. Neuroimage Clin. 17, 804–810. 10.1016/j.nicl.2017.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chhetri JK, Chan P, Vellas B, Cesari M, 2017. Motoric cognitive risk syndrome: predictor of dementia and age-related negative outcomes. Front. Med. (Lausanne) 4, 166 10.3389/fmed.2017.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi S, Reiter DA, Shardell M, Simonsick EM, Studenski S, Spencer RG, et al. 2016. 31P magnetic resonance spectroscopy assessment of muscle bioenergetics as a predictor of gait speed in the Baltimore Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci 71 (12), 1638–1645. 10.1093/gerona/glw059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clouston SA, Brewster P, Kuh D, Richards M, Cooper R, Hardy R, 2013. The dynamic relationship between physical function and cognition in longitudinal aging cohorts. Epidemiol. Rev 35, 33–50. 10.1093/epirev/mxs004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coelho-Júnior HJ, Milano-Teixeira L, Rodrigues B, Bacurau R, Marzetti E, Uchida M, 2018. Relative protein intake and physical function in older adults: a systematic review and meta-analysis of observational studies. Nutrients 10 (9). 10.3390/nu10091330 pii: E1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen JA, Verghese J, Zwerling JL, 2016. Cognition and gait in older people. Maturitas 93, 73–77. 10.1016/j.maturitas.2016.05.005. [DOI] [PubMed] [Google Scholar]
- Corso G, Cristofano A, Sapere N, la Marca G, Angiolillo A, Vitale M, et al. 2017. Serum amino acid profiles in normal subjects and in patients with or at risk of Alzheimer dementia. Dement. Geriatr. Cogn. Dis. Extra 7 (1), 143–159. 10.1159/000466688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Liu X, Wang M, Liu L, Sun X, Ma L, et al. 2014. Lysophosphatidylcholine and amide as metabolites for detecting Alzheimer disease using ultrahigh-performance liquid chromatography-quadrupole time-of-flight mass spectrometry-based metabonomics. J. Neuropathol. Exp. Neurol 73 (10), 954–963. 10.1097/NEN.0000000000000116. [DOI] [PubMed] [Google Scholar]
- Dardenne S, Delrieu J, Sourdet S, Cantet C, Andrieu S, Mathiex-Fortunet H, et al. 2017. Memory complaints and cognitive decline: data from the GUIDAGE Study 1. J. Alzheimers Dis 60 (4), 1567–1578. 10.3233/JAD-170229. [DOI] [PubMed] [Google Scholar]
- Davis D, 2015. Ethical issues in interpretation of risk, from the perspective of a research subject. Narrat. Inq. Bioeth 5 (3), 203–206. 10.1353/nib.2015.0073. [DOI] [PubMed] [Google Scholar]
- de Laat KF, van Norden AG, Gons RA, van Oudheusden LJ, van Uden IW, Bloem BR, et al. 2010d. Gait in elderly with cerebral small vessel disease. Stroke 41 (8), 1652–1658. 10.1161/STROKEAHA.110.583229. [DOI] [PubMed] [Google Scholar]
- de Laat KF, Tuladhar AM, van Norden AG, Norris DG, Zwiers MP, de Leeuw FE, 2011d. Loss of white matter integrity is associated with gait disorders in cerebral small vessel disease. Brain 134 (Pt 1), 73–83. 10.1093/brain/awq343. [DOI] [PubMed] [Google Scholar]
- De Reuck J, Maurage CA, Deramecourt V, Pasquier F, Cordonnier C, Leys D, et al. 2018. Aging and cerebrovascular lesions in pure and in mixed neurodegenerative and vascular dementia brains: a neuropathological study. Folia Neuropathol. 56 (2), 81–87. 10.5114/fn.2018.76610. [DOI] [PubMed] [Google Scholar]
- Del Campo N, Payoux P, Djilali A, Delrieu J, Hoogendijk EO, Rolland Y, et al. 2016. Relationship of regional brain β-amyloid to gait speed. Neurology 86 (1), 36–43. 10.1212/WNL.0000000000002235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demnitz N, Esser P, Dawes H, Valkanova V, Johansen-Berg H, Ebmeier KP, et al. 2016. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture 50, 164–174. 10.1016/j.gaitpost.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande N, Metter EJ, Bandinelli S, Guralnik J, Ferrucci L, 2009. Gait speed under varied challenges and cognitive decline in older persons: a prospective study. Age Ageing 38 (5), 509–514. 10.1093/ageing/afp093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T, Verghese J, Shimada H, Makizako H, Tsutsumimoto K, Hotta R, et al. 2015. Motoric cognitive risk syndrome: prevalence and risk factors in Japanese seniors. J. Am. Med. Dir. Assoc 16 (12), 1103.e21–1103.e25. 10.1016/j.jamda.2015.09.003. [DOI] [PubMed] [Google Scholar]
- Doi T, Shimada H, Makizako H, Tsutsumimoto K, Verghese J, Suzuki T, 2017. Motoric cognitive risk syndrome: association with incident dementia and disability. J. Alzheimers Dis 59 (1), 77–84. 10.3233/JAD-170195. [DOI] [PubMed] [Google Scholar]
- Dufouil C, Fuhrer R, Alperovitch A, 2005. Subjective cognitive complaints and cognitive decline: consequence or predictor? The Epidemiology of Vascular Aging Study. J. Am. Geriatr. Soc 53 (4), 616–621. 10.1111/j.1532-5415.2005.53209.x. [DOI] [PubMed] [Google Scholar]
- Dumurgier J, Elbaz A, Dufouil C, Tavernier B, Tzourio C, 2010. Hypertension and lower walking speed in the elderly: the Three-City study. J. Hypertens 28 (7), 1506–1514. 10.1097/HJH.0b013e328338bbec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumurgier J, Artaud F, Touraine C, Rouaud O, Tavernier B, Dufouil C, et al. 2017. Gait speed and decline in gait speed as predictors of incident dementia. J. Gerontol. A Biol. Sci. Med. Sci 72 (5), 655–661. 10.1093/gerona/glw110. [DOI] [PubMed] [Google Scholar]
- Eisner MD, Blanc PD, Yelin EH, Sidney S, Katz PP, Ackerson L, et al. 2008. COPD as a systemic disease: impact on physical functional limitations. Am. J. Med 121 (9), 789–796. 10.1016/j.amjmed.2008.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elbaz A, Ripert M, Tavernier B, Février B, Zureik M, Gariépy J, et al. 2005. Common carotid artery intima-media thickness, carotid plaques, and walking speed. Stroke 36 (10), 2198–2202. [DOI] [PubMed] [Google Scholar]
- Elbaz A, Shipley MJ, Nabi H, Brunner EJ, Kivimaki M, Singh-Manoux A, 2014. Trajectories of the Framingham general cardiovascular risk profile in midlife and poor motor function later in life: the Whitehall II study. Int. J. Cardiol 172 (1), 96–102. 10.1016/j.ijcard.2013.12.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezzati A, Katz MJ, Lipton ML, Lipton RB, Verghese J, 2015. The association of brain structure with gait velocity in older adults: a quantitative volumetric analysis of brain MRI. Neuroradiology 57 (8), 851–861. 10.1007/s00234-015-1536-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldreich T, Nowak C, Fall T, Carlsson AC, Carrero JJ, Ripsweden J, et al. 2019. Circulating proteins as predictors of cardiovascular mortality in end-stage renal disease. J. Nephrol 32 (1), 111–119. 10.1007/s40620-018-0556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland G, 2013. Vitamin K and brain function. Semin. Thromb. Hemost 39 (8), 849–855. 10.1055/s-0033-1357481. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Harris TB, Guralnik JM, Tracy RP, Corti MC, Cohen HJ, et al. 1999. Serum IL-6 level and the development of disability in older persons. J. Am. Geriatr. Soc 47 (6), 639–646. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, et al. 2005. The origins of age-related proinflammatory state. Blood 105 (6), 2294–2299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragala MS, Alley DE, Shardell MD, Harris TB, McLean RR, Kiel DP, et al. 2016. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. J. Am. Geriatr. Soc 64 (1), 144–150. 10.1111/jgs.13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fredman L, Doros G, Cauley JA, Hillier TA, Hochberg MC, 2010. Caregiving, metabolic syndrome indicators, and 1-year decline in walking speed: results of Caregiver-SOF. J. Gerontol. A Biol. Sci. Med. Sci 65 (5), 565–572. 10.1093/gerona/glq025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritz S, Lusardi M, 2009. White paper: “walking speed: the sixth vital sign”. J. Geriatr. Phys. Ther 32 (2), 46–49. [PubMed] [Google Scholar]
- Fuchs T, Trollor JN, Crawford J, Brown DA, Baune BT, Samaras K, et al. 2013. Macrophage inhibitory cytokine-1 is associated with cognitive impairment and predicts cognitive decline - the Sydney Memory and Aging Study. Aging Cell 12 (5), 882–889. 10.1111/acel.12116. [DOI] [PubMed] [Google Scholar]
- Fujita Y, Taniguchi Y, Shinkai S, Tanaka M, Ito M, 2016. Secreted growth differentiation factor 15 as a potential biomarker for mitochondrial dysfunctions in aging and age-related disorders. Geriatr. Gerontol. Int 16 (Suppl 1), 17–29. 10.1111/ggi.12724. [DOI] [PubMed] [Google Scholar]
- Gagnon M, Dartigues JF, Mazaux JM, Dequae L, Letenneur L, Giroire JM, et al. 1994. Self-reported memory complaints and memory performance in elderly French community residents: results of the PAQUID Research Program. Neuroepidemiology 13 (4), 145–154. [DOI] [PubMed] [Google Scholar]
- Garcia-Martin ES, Rojas B, Ramirez AI, de Hoz R, Salazar JJ, Yubero R, et al. 2014. Macular thickness as a potential biomarker of mild Alzheimer’s disease. Ophthalmology 121 (5), 1149–1151. 10.1016/j.ophtha.2013.12.023.e3. [DOI] [PubMed] [Google Scholar]
- Gardner MP, Lightman S, Sayer AA, Cooper C, Cooper R, Deeg D, et al. 2013. Dysregulation of the hypothalamic pituitary adrenal (HPA) axis and physical performance at older ages: an individual participant meta-analysis. Psychoneuroendocrinology 38 (1), 40–49. 10.1016/j.psyneuen.2012.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GBD 2016 Dementia Collaborators, 2019. Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 18 (1), 88–106. 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geerlings MI, Jonker C, Bouter LM, Adèr HJ, Schmand B, 1999. Association between memory complaints and incident Alzheimer’s disease in elderly people with normal baseline cognition. Am. J. Psychiatry 156 (4), 531–537. [DOI] [PubMed] [Google Scholar]
- Giménez Castejón D, Dudekova M, Gómez Gallego M, Lajara Blesa J, 2016. Macular thickness in subjective memory complaints and mild cognitive impairment: a non-invasive biomarker. Neuroophthalmology 40 (1), 16–22. 10.3109/01658107.2015.1118516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glodzik-Sobanska L, Reisberg B, De Santi S, Babb JS, Pirraglia E, Rich KE, et al. 2007. Subjective memory complaints: presence, severity and future outcome in normal older subjects. Dement. Geriatr. Cogn. Disord 24 (3), 177–184. 10.1159/000105604. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Freire M, de Cabo R, Studenski SA, Ferrucci L, 2014. The neuromuscular junction: aging at the crossroad between nerves and muscle. Front. Aging Neurosci 6, 208 10.3389/fnagi.2014.00208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez-Freire M, Moaddel R, Sun K, Fabbri E, Zhang P, Khadeer M, et al. 2019. Targeted metabolomics shows low plasma lysophosphatidylcholine 18:2 predicts greater decline of gait speed in older adults: the Baltimore Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci 74 (1), 62–67. 10.1093/gerona/gly100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray SL, Anderson ML, Hubbard RA, LaCroix A, Crane PK, McCormick W, et al. 2013. Frailty and incident dementia. J. Gerontol. A Biol. Sci. Med. Sci 68 (9), 1083–1090. 10.1093/gerona/glt013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grut M, Jorm AF, Fratiglioni L, Forsell Y, Viitanen M, Winblad B, 1993. Memory complaints of elderly people in a population survey: variation according to dementia stage and depression. J. Am. Geriatr. Soc 41 (12), 1295–1300. [DOI] [PubMed] [Google Scholar]
- Gu Y, Vorburger R, Scarmeas N, Luchsinger JA, Manly JJ, Schupf N, et al. 2017. Circulating inflammatory biomarkers in relation to brain structural measurements in a non-demented elderly population. Brain Behav. Immun 65, 150–160. 10.1016/j.bbi.2017.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Habiballa L, Salmonowicz H, Passos JF, 2019. Mitochondria and cellular senescence: implications for musculoskeletal ageing. Free Radic. Biol. Med 2019 (132), 3–10. 10.1016/j.freeradbiomed.2018.10.417. [DOI] [PubMed] [Google Scholar]
- Haley AP, Hoth KF, Gunstad J, Paul RH, Jefferson AL, Tate DF, et al. 2009. Subjective cognitive complaints relate to white matter hyperintensities and future cognitive decline in patients with cardiovascular disease. Am. J. Geriatr. Psychiatry 17 (11), 976–985. 10.1097/JGP.0b013e3181b208ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamer M, Kivimaki M, Lahiri A, Yerramasu A, Deanfield JE, Marmot MG, et al. 2010. Walking speed and subclinical atherosclerosis in healthy older adults: the Whitehall II study. Heart 96 (5), 380–384. 10.1136/hrt.2009.183350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood DG, Barker WW, Ownby RL, Mullan M, Duara R, 2004. No association between subjective memory complaints and apolipoprotein E genotype in cognitively intact elderly. Int. J. Geriatr. Psychiatry 19 (12), 1131–1139. [DOI] [PubMed] [Google Scholar]
- Hayashi K, Kako M, Suzuki K, Hattori K, Fukuyasu S, Sato K, et al. 2016. Gait speeds associated with anxiety responses to pain in osteoarthritis patients. Pain Med. 17 (3), 606–613. 10.1111/pme.12897. [DOI] [PubMed] [Google Scholar]
- Hayashi T, Umegaki H, Makino T, Cheng XW, Shimada H, Kuzuya M, 2019. Association between sarcopenia and depressive mood in urban-dwelling older adults: a cross-sectional study. Geriatr. Gerontol. Int 10.1111/ggi.13650 [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Hayes JM, Tang L, Viviano RP, van Rooden S, Ofen N, Damoiseaux JS, 2017. Subjective memory complaints are associated with brain activation supporting successful memory encoding. Neurobiol. Aging 60, 71–80. 10.1016/j.neurobiolaging.2017.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heiland EG, Qiu C, Wang R, Santoni G, Liang Y, Fratiglioni L, et al. 2017. Cardiovascular risk burden and future risk of walking speed limitation in older adults. J. Am. Geriatr. Soc 65 (11), 2418–2424. 10.1016/j.jamda.2018.05.013. [DOI] [PubMed] [Google Scholar]
- Hicks GE, Shardell M, Alley DE, Miller RR, Bandinelli S, Guralnik J, et al. 2012. Absolute strength and loss of strength as predictors of mobility decline in older adults: the InCHIANTI study. J. Gerontol. A Biol. Sci. Med. Sci 67 (1), 66–73. 10.1093/gerona/glr055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DK, Neiberg RH, Tooze JA, Hausman DB, Johnson MA, Cauley JA, et al. 2013. Low 25-hydroxyvitamin D predicts the onset of mobility limitation and disability in community-dwelling older adults: the Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci 68 (2), 181–187. 10.1093/gerona/gls136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston DK, Tooze JA, Garcia K, Visser M, Rubin S, Harris TB, et al. 2017. Protein Intake and mobility limitation in community-dwelling older adults: the Health ABC Study. J. Am. Geriatr. Soc 65 (8), 1705–1711. 10.1111/jgs.14856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu FC, Kritchevsky SB, Liu Y, Kanaya A, Newman AB, Perry SE, et al. 2009. Association between inflammatory components and physical function in the Health, Aging, and Body Composition Study: a principal component analysis approach. J. Gerontol. A Biol. Sci. Med. Sci 64 (5), 581–589. 10.1093/gerona/glp005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu CL, Liang CK, Liao MC, Chou MY, Lin YT, 2017. Slow gait speed as a predictor of 1-year cognitive decline in a veterans’ retirement community in southern Taiwan. Geriatr. Gerontol. Int 17 (Suppl 1), 14–19. 10.1111/ggi.13034. [DOI] [PubMed] [Google Scholar]
- Ilgin D, Ozalevli S, Kilinc O, Sevinc C, Cimrin AH, Ucan ES, 2011. Gait speed as a functional capacity indicator in patients with chronic obstructive pulmonary disease. Ann. Thorac. Med 6 (3), 141–146. 10.4103/1817-1737.82448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inzitari M, Newman AB, Yafife K, Boudreau R, de Rekeneire N, Shorr R, 2007. Gait speed predicts decline in attention and psychomotor speed in older adults: the Health Aging and Body Composition Study. Neuroepidemiology 29 (3-4), 156–162. 10.1159/000111577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isanejad M, Mursu J, Sirola J, Kröger H, Rikkonen T, Tuppurainen M, et al. 2016. Dietary protein intake is associated with better physical function and muscle strength among elderly women. Br. J. Nutr 115 (7), 1281–1291. 10.1017/S000711451600012X. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Knopman DS, Jagust WJ, Petersen RC, Weiner MW, Aisen PS, 2013. Tracking pathophysiological processes in Alzheimer’s disease: an updated hypothetical model of dynamic biomarkers. Lancet Neurol. 12 (2), 207–216. 10.1016/S1474-4422(12)70291-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong HS, Park JS, Song IU, Chung YA, Rhie SJ, 2017. Changes in cognitive function and brain glucose metabolism in elderly women with subjective memory impairment: a 24-month prospective pilot study. Acta Neurol. Scand 135 (1), 108–114. 10.1111/ane.12569. [DOI] [PubMed] [Google Scholar]
- Jessen F, Feyen L, Freymann K, Tepest R, Maier W, Heun R, et al. 2006. Volume reduction of the entorhinal cortex in subjective memory impairment. Neurobiol. Aging 27 (12), 1751–1756. 10.1016/j.neurobiolaging.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Jessen F, Amariglio RE, van Boxtel M, Breteler M, Ceccaldi M, Chételat G, 2014a. A conceptual framework for research on subjective cognitive decline in preclinical Alzheimer’s disease. Alzheimers Dement. 10 (6), 844–852. 10.1016/j.jalz.2014.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen F, Wolfsgruber S, Wiese B, Bickel H, Mösch E, Kaduszkiewicz H, et al. 2014b. AD dementia risk in late MCI, in early MCI, and in subjective memory impairment. Alzheimers Dement. 10 (1), 76–83. 10.1016/j.jalz.2012.09.017. [DOI] [PubMed] [Google Scholar]
- Jonker C, Launer LJ, Hooijer C, Lindeboom J, 1996. Memory complaints and memory impairment in older individuals. J. Am. Geriatr. Soc 44 (1), 44–49. [DOI] [PubMed] [Google Scholar]
- Jonker C, Geerlings MI, Schmand B, 2000. Are memory complaints predictive for dementia? A review of clinical and population-based studies. Int. J. Geriatr. Psychiatry 15 (11), 983–991. [DOI] [PubMed] [Google Scholar]
- Jorm AF, Butterworth P, Anstey KJ, Christensen H, Easteal S, Mailer J, et al. 2004. Memory complaints in a community sample aged 60–64 years: associations with cognitive functioning, psychiatric symptoms, medical conditions, APOE genotype, hippocampus and amygdala volumes, and white-matter hyperintensities. Psychol. Med. (Paris) 34 (8), 1495–1506. [DOI] [PubMed] [Google Scholar]
- Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, et al. 2018. Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women. J. Gerontol. A Biol. Sci. Med. Sci 73 (7), 939–945. 10.1093/gerona/glx134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaduszkiewicz H, Eisele M, Wiese B, Prokein J, Luppa M, Luck T, et al. 2014. Prognosis of mild cognitive impairment in general practice: results of the German AgeCoDe study. Ann. Fam. Med 12 (2), 158–165. 10.1370/afm.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalyani RR, Tra Y, Yeh HC, Egan JM, Ferrucci L, Brancati FL, 2013. Quadriceps strength, quadriceps power, and gait speed in older U.S. Adults with diabetes mellitus: results from the National Health and Nutrition Examination Survey, 1999-2002. J. Am. Geriatr. Soc 61 (5), 769–775. 10.1111/jgs.12204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaup AR, Nettiksimmons J, LeBlanc ES, Yafife K, 2015. Memory complaints and risk of cognitive impairment after nearly 2 decades among older women. Neurology 85 (21), 1852–1858. 10.1212/WNL.0000000000002153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Shin IS, Choi SK, Yoon JS, 2003. Subjective memory impairment, cognitive function and depression–a community study in older Koreans. Dement. Geriatr. Cogn. Disord 15 (4), 218–225. [DOI] [PubMed] [Google Scholar]
- Kim JM, Stewart R, Kim SW, Yang SJ, Shin IS, Yoon JS, 2006. A prospective study of changes in subjective memory complaints and onset of dementia in South Korea. Am. J. Geriatr. Psychiatry 14 (11), 949–956. [DOI] [PubMed] [Google Scholar]
- Kim J, Garcia-Esquinas E, Navas-Acien A, Choi YH, 2018. Blood and urine cadmium concentrations and walking speed in middle-aged and older U.S. Adults. Environ Pollut 232, 97–104. 10.1016/j.envpol.2017.09.022. [DOI] [PubMed] [Google Scholar]
- Ko SU, Stenholm S, Chia CW, Simonsick EM, Ferrucci L, 2011. Gait pattern alterations in older adults associated with type 2 diabetes in the absence of peripheral neuropathy–results from the Baltimore Longitudinal Study of Aging. Gait Posture 34 (4), 548–552. 10.1016/j.gaitpost.2011.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kositsawat J, Barry LC, Kuchel GA, 2013. C-reactive protein, vitamin D deficiency, and slow gait speed. J. Am. Geriatr. Soc 61 (9), 1574–1579. 10.1111/jgs.12403. [DOI] [PubMed] [Google Scholar]
- Krasnoff JB, Basaria S, Pencina MJ, Jasuja GK, Vasan RS, Ulloor J, et al. 2010. Free testosterone levels are associated with mobility limitation and physical performance in community-dwelling men: the Framingham Offspring Study. J. Clin. Endocrinol. Metab 95 (6), 2790–2799. 10.1210/jc.2009-2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kueper JK, Speechley M, Lingum NR, Montero-Odasso M, 2017. Motor function and incident dementia: a systematic review and meta-analysis. Age Ageing 46 (5), 729–738. 10.1093/ageing/afx084. [DOI] [PubMed] [Google Scholar]
- Kumai K, Meguro K, Kasai M, Nakamura K, Nakatsuka M, 2016. Neuroepidemiologic and neurobehavioral characteristics of motoric cognitive risk syndrome in an old-old population: the Kurihara Project. Dement. Geriatr. Cogn. Dis. Extra 6 (2), 176–182. 10.1159/000445539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo HK, Bean JF, Yen CJ, Leveille SG, 2006. Linking C-reactive protein to late-life disability in the National Health and Nutrition Examination Survey (NHANES) 1999-2002. J. Gerontol. A Biol. Sci. Med. Sci 61 (4), 380–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo CK, Lin LY, Yu YH, Wu KH, Kuo HK, 2009. Inverse association between insulin resistance and gait speed in nondiabetic older men: results from the U.S. National Health and Nutrition Examination Survey (NHANES) 1999–2002. BMC Geriatr. 9, 49 10.1186/1471-2318-9-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson L, Degens H, Li M, Salviati L, Lee YI, Thompson W, et al. 2019. Sarcopenia: aging-related loss of muscle mass and function. Physiol. Rev 99 (1), 427–511. 10.1152/physrev.00061.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassale C, Batty GD, Steptoe A, Cadar D, Akbaraly TN, Kivimäki M, et al. 2019. Association of 10-year C-reactive protein trajectories with markers of healthy aging: findings from the English Longitudinal Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci 74 (2), 195–203. 10.1093/gerona/gly028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauretani F, Semba RD, Bandinelli S, Dayhoff-Brannigan M, Lauretani F, Corsi AM, et al. 2008. Carotenoids as protection against disability in older persons. Rejuvenation Res. 11 (3), 557–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lautenschlager NT, Flicker L, Vasikaran S, Leedman P, Almeida OP, 2005. Subjective memory complaints with and without objective memory impairment: relationship with risk factors for dementia. Am. J. Geriatr. Psychiatry 13 (8), 731–734. [DOI] [PubMed] [Google Scholar]
- Laws SM, Clarnette RM, Taddei K, Martins G, Paton A, Hallmayer J, et al. 2002. APOE-epsilon4 and APOE −491A polymorphisms in individuals with subjective memory loss. Mol. Psychiatry 7 (7), 768–775. [DOI] [PubMed] [Google Scholar]
- Li K, Luo X, Zeng Q, Jiaerken Y, Xu X, Huang P, et al. 2018. Aberrant functional connectivity network in subjective memory complaint individuals relates to pathological biomarkers. Transl. Neurodegener 7, 27 10.1186/s40035-018-0130-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Hagen C, Fett AR, Bui HH, Knopman D, Vemuri P, et al. 2019. Longitudinal association between phosphatidylcholines, neuroimaging measures of Alzheimer’s disease pathophysiology, and cognition in the Mayo Clinic Study of Aging. Neurobiol. Aging 79, 43–49. 10.1016/j.neurobiolaging.2019.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liaw FY, Kao TW, Fang WH, Han DS, Chi YC, Yang WS, 2016. Increased follistatin associated with decreased gait speed among old adults. Eur. J. Clin. Invest 46 (4), 321–327. 10.1111/eci.12595. [DOI] [PubMed] [Google Scholar]
- Lum H, Sloane R, Huffman KM, Kraus VB, Thompson DK, Kraus WE, et al. 2011. Plasma acylcarnitines are associated with physical performance in elderly men. J. Gerontol. A Biol. Sci. Med. Sci 66 (5), 548–553. 10.1093/gerona/glr006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire FJ, Killane I, Creagh AP, Donoghue O, Kenny RA, Reilly RB, 2018. Baseline association of motoric cognitive risk syndrome with sustained attention, memory, and global cognition. J. Am. Med. Dir. Assoc 19 (1), 53–58. 10.1016/j.jamda.2017.07.016. [DOI] [PubMed] [Google Scholar]
- Manini TM, Buford TW, Kairalla JA, McDermott MM, Vaz Fragoso CA, Fielding RA, et al. 2018. Meta-analysis identifies mitochondrial DNA sequence variants associated with walking speed. Geroscience 40 (5-6), 497–511. 10.1007/s11357-018-0043-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mapstone M, Cheema AK, Fiandaca MS, Zhong X, Mhyre TR, MacArthur LH, et al. 2014. Plasma phospholipids identify antecedent memory impairment in older adults. Nat. Med 20 (4), 415–418 10.1038/nm.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquis S, Moore MM, Howieson DB, Sexton G, Payami H, 2002. Independent predictors of cognitive decline in healthy elderly persons. Arch. Neurol 59 (4), 601–606. [DOI] [PubMed] [Google Scholar]
- Martin KL, Blizzard L, Wood AG, Srikanth V, Thomson R, Sanders LM, et al. 2013. Cognitive function, gait, and gait variability in older people: a population-based study. J. Gerontol. A Biol. Sci. Med. Sci 68 (6), 726–732. 10.1093/gerona/gls224. [DOI] [PubMed] [Google Scholar]
- Meiberth D, Scheef L, Wolfsgruber S, Boecker H, Block W, Träber F, et al. 2015. Cortical thinning in individuals with subjective memory impairment. J. Alzheimers Dis 45 (1), 139–146. 10.3233/JAD-142322. [DOI] [PubMed] [Google Scholar]
- Mergeche JL, Verghese J, Allali G, Wang C, Beauchet O, Kumar VGP, et al. 2016. White matter hyperintensities in older adults and motoric cognitive risk syndrome. J. Neuroimaging Psychiatry Neurol 1 (2), 73–78. 10.17756/jnpn.2016-009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton A, Fritz SL, Lusardi M, 2015. Walking speed: the functional vital sign. J. Aging Phys. Act 23 (2), 314–322. 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mielke MM, Roberts RO, Savica R, Cha R, Drubach DI, Christianson T, et al. 2013. Assessing the temporal relationship between cognition and gait: slow gait predicts cognitive decline in the Mayo Clinic Study of Aging. J. Gerontol. A Biol. Sci. Med. Sci 68 (8), 929–937. 10.1093/gerona/gls256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milaneschi Y, Bandinelli S, Corsi AM, Lauretani F, Paolisso G, Dominguez LJ, et al. 2011. Mediterranean diet and mobility decline in older persons. Exp. Gerontol 46 (4), 303–308. 10.1016/j.exger.2010.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minett TS, Dean JL, Firbank M, English P, O’Brien JT, 2005. Subjective memory complaints, white-matter lesions, depressive symptoms, and cognition in elderly patients. Am. J. Geriatr. Psychiatry 13 (8), 665–671. 10.1176/appi.ajgp.13.8.665. [DOI] [PubMed] [Google Scholar]
- Minett TS, Da Silva RV, Ortiz KZ, Bertolucci PH, 2008. Subjective memory complaints in an elderly sample: a cross-sectional study. Int. J. Geriatr. Psychiatry 23 (1), 49–54. 10.1002/gps.1836. [DOI] [PubMed] [Google Scholar]
- Miranda B, Madureira S, Verdelho A, Ferro J, Pantoni L, Salvadori E, et al. 2008. Self-perceived memory impairment and cognitive performance in an elderly independent population with age-related white matter changes. J. Neurol. Neurosurg. Psychiatry 79 (8), 869–873. 10.1136/jnnp.2007.131078. [DOI] [PubMed] [Google Scholar]
- Mitchell AJ, 2008. The clinical significance of subjective memory complaints in the diagnosis of mild cognitive impairment and dementia: a meta-analysis. Int. J. Geriatr. Psychiatry 23 (11), 1191–1202. 10.1002/gps.2053. [DOI] [PubMed] [Google Scholar]
- Mitchell WK, Williams J, Atherton P, Larvin M, Lund J, Narici M, 2012. Sarcopenia, dynapenia, and the impact of advancing age on human skeletal muscle size and strength; a quantitative review. Front. Physiol 3, 260 10.3389/fphys.2012.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell AJ, Beaumont H, Ferguson D, Yadegarfar M, Stubbs B, 2014. Risk of dementia and mild cognitive impairment in older people with subjective memory complaints: meta-analysis. Acta Psychiatr. Scand 130 (6), 439–451. 10.1111/acps.12336. [DOI] [PubMed] [Google Scholar]
- Montero-Odasso M, Hachinski V, 2014. Preludes to brain failure: executive dysfunction and gait disturbances. Neurol. Sci 35 (4), 601–604. 10.1007/si0072-013-1613-4. [DOI] [PubMed] [Google Scholar]
- Mosconi L, De Santi S, Brys M, Tsui WH, Pirraglia E, Glodzik-Sobanska L, et al. 2008. Hypometabolism and altered cerebrospinal fluid markers in normal apolipoprotein E E4 carriers with subjective memory complaints. Biol. Psychiatry 63 (6), 609–618. 10.1016/j.biopsych.2007.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadkarni NK, Nunley KA, Aizenstein H, Harris TB, Yafife K, Satterfield S, et al. 2014. Association between cerebellar gray matter volumes, gait speed, and information-processing ability in older adults enrolled in the Health ABC Study. J. Gerontol. A Biol. Sci. Med. Sci 69 (8), 996–1003. 10.1093/gerona/glt151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Koshiba H, Shibata S, Hirasawa A, Ebihara T, Kozaki K, 2017. Relationship of serum carnitine level with falls and gait disturbance in the elderly. J. Frailty Aging 6 (4), 178–182. 10.14283/jfa.2017.36. [DOI] [PubMed] [Google Scholar]
- Ogama N, Sakurai T, Kawashima S, Tanikawa T, Tokuda H, Satake S, et al. 2019. Association of glucose fluctuations with sarcopenia in older adults with type 2 diabetes mellitus. J. Clin. Med 8 (3). 10.3390/jcm8030319 pii: E319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa K, Kim HK, Shimizu T, Abe S, Shiga Y, Calderwood SK, 2012. Plasma heat shock protein 72 as a biomarker of sarcopenia in elderly people. Cell Stress Chaperones 17 (3), 349–359. 10.1007/s12192-011-0310-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okoro CA, Zhong Y, Ford ES, Balluz LS, Strine TW, Mokdad AH, 2006. Association between the metabolic syndrome and its components and gait speed among U.S. Adults aged 50 years and older: a cross-sectional analysis. BMC Public Health 6, 282 10.1186/1471-2458-6-282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahor M, Guralnik JM, Ambrosius WT, Blair S, Bonds DE, Church TS, et al. 2014. Effect of structured physical activity on prevention of major mobility disability in older adults: the LIFE study randomized clinical trial. JAMA 311 (23), 2387–2396. 10.1001/jama.2014.5616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradise MB, Glozier NS, Naismith SL, Davenport TA, Hickie IB, 2011. Subjective memory complaints, vascular risk factors and psychological distress in the middle-aged: a cross-sectional study. BMC Psychiatry 11, 108 10.1186/1471-244X-11-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park KY, Hwang HS, Kim YP, Park HK, 2017. Risk factors for cognitive decline associated with gait speed in community-dwelling elderly Koreans with MMSE scores of 30. Aging Clin. Exp. Res 29 (2), 183–189. 10.1007/s40520-016-0565-y. [DOI] [PubMed] [Google Scholar]
- Peavy GM, Santiago DP, Edland SD, 2013. Subjective memory complaints are associated with diurnal measures of salivary cortisol in cognitively intact older adults. Am. J. Geriatr. Psychiatry 21 (9), 925–928. 10.1016/j.jagp.2013.01.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peel NM, Alapatt LJ, Jones LV, Hubbard RE, 2019. The association between gait speed and cognitive status in community-dwelling older people: a systematic review and meta-analysis. J. Gerontol. A Biol. Sci. Med. Sci 74 (6), 943–948. 10.1093/gerona/gly140. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Kritchevsky SB, Newman AB, Nicklas BJ, Simonsick EM, Rubin S, et al. 2004. Inflammatory markers and incident mobility limitation in the elderly. J. Am. Geriatr. Soc 52 (7), 1105–1113. [DOI] [PubMed] [Google Scholar]
- Peterson MJ, Geoghegan S, Lawhorne LW, 2019. An exploratory analysis of potential new biomarkers of cognitive function. J. Gerontol. A Biol. Sci. Med. Sci 74 (3), 299–305. 10.1093/gerona/gly122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersson SD, Philippou E, 2016. Mediterranean Diet, cognitive function, and dementia: a systematic review of the evidence. Adv. Nutr 7 (5), 889–904. 10.3945/an.116.012138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer E, 1975. A short portable mental status questionnaire for the assessment of organic brain deficit in elderly patients. J. Am. Geriatr. Soc 23 (10), 433–441. [DOI] [PubMed] [Google Scholar]
- Plouvier S, Carton M, Cyr D, Sabia S, Leclerc A, Zins M, et al. 2016. Socioeconomic disparities in gait speed and associated characteristics in early old age. BMC Musculoskelet. Disord 17, 178 10.1186/s12891-016-1033-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Power R, Prado-Cabrero A, Mulcahy R, Howard A, Nolan JM, 2019. The role of nutrition for the aging population: implications for cognition and Alzheimer’s disease. Annu. Rev. Food Sci. Technol 10, 619–639. 10.1146/annurev-food-030216-030125. [DOI] [PubMed] [Google Scholar]
- Pulopulos MM, Puig-Perez S, Hidalgo V, Villada C, Salvador A, 2016. Cortisol awakening response and walking speed in older people. PLoS One 11 (5), e0152071 10.1371/journal.pone.0152071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Smart CM, Crane PK, Amariglio RE, Berman LM, Boada M, et al. 2015. Subjective cognitive decline in older adults: an overview of self-report measures used across 19 international research studies. J. Alzheimers Dis 48 (Suppl 1), S63–86. 10.3233/JAD-150154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabin LA, Smart CM, Amariglio RE, 2017. Subjective cognitive decline in preclinical Alzheimer’s disease. Annu. Rev. Clin. Psychol 13, 369–396. 10.1146/annurev-clinpsy-032816-045136. [DOI] [PubMed] [Google Scholar]
- Ramakers IH, Visser PJ, Aalten P, Boesten JH, Metsemakers JF, Jolles J, et al. 2007. Symptoms of preclinical dementia in general practice up to five years before dementia diagnosis. Dement. Geriatr. Cogn. Disord 24 (4), 300–306. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Prichep L, Mosconi L, John ER, Glodzik-Sobanska L, Boksay I, et al. 2008. The pre-mild cognitive impairment, subjective cognitive impairment stage of Alzheimer’s disease. Alzheimers Dement. 4 (1 Suppl. 1), S98–S108. 10.1016/j.jalz.2007.11.017. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Shulman MB, Torossian C, Leng L, Zhu W, 2010. Outcome over seven years of healthy adults with and without subjective cognitive impairment. Alzheimers Dement. 6 (1), 11–24. 10.1016/j.jalz.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Risacher SL, Kim S, Nho K, Foroud T, Shen L, Petersen RC, et al. 2015. APOE effect on Alzheimer’s disease biomarkers in older adults with significant memory concern. Alzheimers Dement. 11 (12), 1417–1429. 10.1016/j.jalz.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rönnlund M, Sundström A, Adolfsson R, Nilsson LG, 2015. Self-reported memory failures: associations with future dementia in a population-based study with long-term follow-up. J. Am. Geriatr. Soc 63 (9), 1766–1773. 10.1111/jgs.13611. [DOI] [PubMed] [Google Scholar]
- Rosano C, Brach J, Longstreth WT Jr, Newman AB, 2006. Quantitative measures of gait characteristics indicate prevalence of underlying subclinical structural brain abnormalities in high-functioning older adults. Neuroepidemiology 26 (1), 52–60. [DOI] [PubMed] [Google Scholar]
- Rosario BL, Rosso AL, Aizenstein HJ, Harris T, Newman AB, Satterfield S, et al. 2016. Cerebral white matter and slow gait: contribution of hyperintensities and normal-appearing parenchyma. J. Gerontol. A Biol. Sci. Med. Sci 71 (7), 968–973. 10.1093/gerona/glv224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosso AL, Verghese J, Metti AL, Boudreau RM, Aizenstein HJ, Kritchevsky S, et al. 2017. Slowing gait and risk for cognitive impairment: the hippocampus as a shared neural substrate. Neurology 89 (4), 336–342. 10.1212/WNL.0000000000004153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu SY, Lim EY, Na S, Shim YS, Cho JH, Yoon B, et al. 2017. Hippocampal and entorhinal structures in subjective memory impairment: a combined MRI volumetric and DTI study. Int. Psychogeriatr 29 (5), 785–792. 10.1017/S1041610216002349. [DOI] [PubMed] [Google Scholar]
- Sakurai R, Fujiwara Y, Yasunaga M, Takeuchi R, Murayama Y, Ohba H, et al. 2014. Regional cerebral glucose metabolism and gait speed in healthy community-dwelling older women. J. Gerontol. A Biol. Sci. Med. Sci 69 (12), 1519–1527. 10.1093/gerona/glu093. [DOI] [PubMed] [Google Scholar]
- Sakurai R, Ishii K, Yasunaga M, Takeuchi R, Murayama Y, Sakuma N, et al. 2017. The neural substrate of gait and executive function relationship in elderly women: a PET study. Geriatr. Gerontol. Int 17 (11), 1873–1880. 10.1111/ggi.12982. [DOI] [PubMed] [Google Scholar]
- Sanders JB, Bremmer MA, Comijs HC, Deeg DJ, Beekman AT, 2016. Gait speed and the natural course of depressive symptoms in late life; an independent association with chronicity? J. Am. Med. Dir. Assoc 17 (4), 331–335. 10.1016/j-jamda.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Sathyan S, Barzilai N, Atzmon G, Milman S, Ayers E, Verghese J, 2017. Association of anti-inflammatory cytokine IL10 polymorphisms with motoric cognitive risk syndrome in an Ashkenazi Jewish population. Neurobiol. Aging 58 (238). 10.1016/j.neurobiolaging.2017.06.006 e1–238.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sathyan S, Wang T, Ayers E, Verghese J, 2019. Genetic basis of motoric cognitive risk syndrome in the Health and Retirement Study. Neurology 92 (13), e1427–e1434. 10.1212/WNL.0000000000007141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satizabal CL, Zhu YC, Mazoyer B, Dufouil C, Tzourio C, 2012. Circulating IL-6 and CRP are associated with MRI findings in the elderly: the 3C-Dijon Study. Neurology. 78 (10), 720–727. 10.1212/WNL.0b013e318248e50f. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, et al. 2006. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology 67 (5), 834–842. 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheef L, Spottke A, Daerr M, Joe A, Striepens N, Kölsch H, et al. 2012. Glucose metabolism, gray matter structure, and memory decline in subjective memory impairment. Neurology 79 (13), 1332–1339. 10.1212/WNL.0b013e31826c1a8d. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA, 2007. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology 69 (24), 2197–2204. 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schofield PW, Marder K, Dooneief G, Jacobs DM, Sano M, Stern Y, 1997. Association of subjective memory complaints with subsequent cognitive decline in community-dwelling elderly individuals with baseline cognitive impairment. Am. J. Psychiatry 154 (5), 609–615. [DOI] [PubMed] [Google Scholar]
- Schultz SA, Oh JM, Koscik RL, Dowling NM, Gallagher CL, Carlsson CM, et al. 2015. Subjective memory complaints, cortical thinning, and cognitive dysfunction in middle-aged adults at risk for AD. Alzheimers Dement. (Amst.) 1 (1), 33–40. 10.1016/j.dadm.2014.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scicchitano BM, Pelosi L, Sica G, Musarò A, 2018. The physiopathologic role of oxidative stress in skeletal muscle. Mech. Ageing Dev 170, 37–44. 10.1016/j.mad.2017.08.009. [DOI] [PubMed] [Google Scholar]
- Seidler RD, Bernard JA, Burutolu TB, Fling BW, Gordon MT, Gwin JT, et al. 2010. Motor control and aging: links to age-related brain structural, functional, and biochemical effects. Neurosci. Biobehav. Rev 34 (5), 721–733. 10.1016/j.neubiorev.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon H, Allali G, Launay CP, Barden J, Szturm T, Liu-Ambrose T, et al. 2019. Motoric cognitive risk syndrome, incident cognitive impairment and morphological brain abnormalities: systematic review and meta-analysis. Maturitas 123, 45–54. 10.1016/j.maturitas.2019.02.006. [DOI] [PubMed] [Google Scholar]
- Semba RD, Trehan I, Li X, Salem N Jr., Moaddel R, Ordiz MI, et al. 2017. Low serum ω-3 and ω-6 polyunsaturated fatty acids and other metabolites are associated with poor linear growth in young children from rural Malawi. Am. J. Clin. Nutr 106 (6), 1490–1499. 10.3945/ajcn.117.164384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Gonzalez-Freire M, Tanaka T, Biancotto A, Zhang P, Shardell M, et al. 2019a. Elevated plasma growth and differentiation factor-15 is associated with slower gait speed and lower physical performance in healthy community-dwelling adults. J. Gerontol. A Biol. Sci. Med. Sci pii: glz071 10.1093/gerona/glz071 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Moaddel R, Zhang P, Ramsden CE, Ferrucci L, 2019b. Tetra-linoleoyl cardiolipin depletion plays a major role in the pathogenesis of sarcopenia. Med. Hypotheses 127, 142–149. 10.1016/j.mehy.2019.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semba RD, Zhang P, Adelnia F, Sun K, Gonzalez-Freire M, Salem N Jr, et al. 2019c. Low plasma lysophosphatidylcholines are associated with impaired mitochondrial oxidative capacity in adults in the Baltimore Longitudinal Study of Aging. Aging Cell 18 (2), e12915 10.1111/acel.12915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafie S, Shahwan S, Abdin E, Vaingankar J, Picco L, Sambasivam R, et al. 2017. The correlates of slow gait and its relation with social network among older adults in Singapore. Aging Ment. Health 21 (11), 1171–1176. 10.1080/13607863.2016.1202893. [DOI] [PubMed] [Google Scholar]
- Shahar DR, Houston DK, Hue TF, Lee JS, Sahyoun NR, Tylavsky FA, et al. 2012. Adherence to Mediterranean diet and decline in walking speed over 8 years in community-dwelling older adults. J. Am. Geriatr. Soc 60 (October (10)), 1881–1888. 10.1111/j.1532-5415.2012.04167.x.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shea MK, Loeser RF, Hsu FC, Booth SL, Nevitt M, Simonsick EM, et al. 2016. Vitamin K status and lower extremity function in older adults: the Health Aging and Body Composition Study. J. Gerontol. A Biol. Sci. Med. Sci 71 (10), 1348–1355. 10.1093/gerona/glv209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silbert LC, Nelson C, Howieson DB, Moore MM, Kaye JA, 2008. Impact of white matter hyperintensity volume progression on rate of cognitive and motor decline. Neurology 71 (2), 108–113. 10.1212/01.wnl.0000316799.86917.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small GW, Chen ST, Komo S, Ercoli L, Bookheimer S, Miller K, et al. 1999. Memory self-appraisal in middle-aged and older adults with the apolipoprotein E-4 allele. Am. J. Psychiatry 156 (7), 1035–1038. [DOI] [PubMed] [Google Scholar]
- Smith EE, O’Donnell M, Dagenais G, Lear SA, Wielgosz A, Sharma M, et al. 2015. Early cerebral small vessel disease and brain volume, cognition, and gait. Ann. Neurol 77 (2), 251–261. 10.1002/ana.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijders T, Parise G, 2017. Role of muscle stem cells in sarcopenia. Curr. Opin. Clin. Nutr. Metab. Care 20 (3), 186–190. 10.1097/MCO.0000000000000360. [DOI] [PubMed] [Google Scholar]
- Snitz BE, Weissfeld LA, Cohen AD, Lopez OL, Nebes RD, Aizenstein HJ, et al. 2015. Subjective cognitive complaints, personality and brain amyloid-beta in cognitively normal older adults. Am. J. Geriatr. Psychiatry 23 (9), 985–993. 10.1016/j.jagp.2015.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soumaré A, Elbaz A, Zhu Y, Maillard P, Crivello F, Tavernier B, et al. 2009a. White matter lesions volume and motor performances in the elderly. Ann. Neurol 65 (6), 706–715. 10.1002/ana.21674. [DOI] [PubMed] [Google Scholar]
- Soumaré A, Tavernier B, Alperovitch A, Tzourio C, Elbaz A, 2009b. A cross-sectional and longitudinal study of the relationship between walking speed and cognitive function in community-dwelling elderly people. J. Gerontol. A Biol. Sci. Med. Sci 64 (10), 1058–1065. 10.1093/gerona/glp077. [DOI] [PubMed] [Google Scholar]
- Sousa AC, Zunzunegui MV, Li A, Phillips SP, Guralnik JM, Guerra RO, 2016. Association between C-reactive protein and physical performance in older populations: results from the International Mobility in Aging Study (IMIAS). Age Ageing 45 (2), 274–280. 10.1093/ageing/afv202. [DOI] [PubMed] [Google Scholar]
- Soutif-Veillon A, Ferland G, Rolland Y, Presse N, Boucher K, Feart C, et al. 2016. Increased dietary vitamin K intake is associated with less severe subjective memory complaint among older adults. Maturitas 93, 131–136. 10.1016/j.maturitas.2016.02.004. [DOI] [PubMed] [Google Scholar]
- Sperling RA, Aisen PS, Beckett LA, Bennett DA, Craft S, Fagan AM, et al. 2011. Toward defining the preclinical stages of Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement. 7 (3), 280–292. 10.1016/j.jalz.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling SA, Tsang S, Williams IC, Park MH, Helenius IM, Manning CA, 2017. Subjective memory change, mood, and cerebrovascular risk factors in older African Americans. J. Geriatr. Psychiatry Neurol 30 (6), 324–330. 10.1177/0891988717732153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St John P, Montgomery P, 2002. Are cognitively intact seniors with subjective memory loss more likely to develop dementia? Int. J. Geriatr. Psychiatry 17 (9), 814–820. [DOI] [PubMed] [Google Scholar]
- Stewart R, Russ C, Richards M, Brayne C, Lovestone S, Mann A, 2001. Depression, APOE genotype and subjective memory impairment: a cross-sectional study in an African-Caribbean population. Psychol. Med. (Paris) 31 (3), 431–440. [PubMed] [Google Scholar]
- Stewart R, Godin O, Crivello F, Maillard P, Mazoyer B, Tzourio C, et al. 2011. Longitudinal neuroimaging correlates of subjective memory impairment: 4-year prospective community study. Br. J. Psychiatry 198 (3), 199–205. 10.1192/bjp.bp.110.078683. [DOI] [PubMed] [Google Scholar]
- Striepens N, Scheef L, Wind A, Popp J, Spottke A, Cooper-Mahkorn D, et al. 2010. Volume loss of the medial temporal lobe structures in subjective memory impairment. Dement. Geriatr. Cogn. Disord 29 (1), 75–81. 10.1159/000264630. [DOI] [PubMed] [Google Scholar]
- Stringhini S, Carmeli C, Jokela M, Avendano M, McCrory C, d’Errico A, et al. 2018. Socioeconomic status, non-communicable disease risk factors, and walking speed in older adults: multi-cohort population based study. BMJ 360, k1046 10.1136/bmj.kl046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su N, Zhai FF, Zhou LX, Ni J, Yao M, Li ML, et al. 2017. Cerebral small vessel disease burden is associated with motor performance of lower and upper extremities in community-dwelling populations. Front. Aging Neurosci 9, 313 10.3389/fnagi.2017.00313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney MD, Montagne A, Sagare AP, Nation DA, Schneider LS, Chui HC, et al. 2019. Vascular dysfunction-The disregarded partner of Alzheimer’s disease. Alzheimers Dement. 15 (1), 158–167. 10.1016/j.jalz.2018.07.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taaffe DR, Harris TB, Ferrucci L, Rowe J, Seeman TE, 2000. Cross-sectional and prospective relationships of interleukin-6 and C-reactive protein with physical performance in elderly persons: MacArthur Studies of Successful Aging. J. Gerontol. A Biol. Sci. Med. Sci 55 (12), M709–715. [DOI] [PubMed] [Google Scholar]
- Tanaka T, Biancotto A, Moaddel R, Moore AZ, Gonzalez-Freire M, Aon MA, et al. 2018. Plasma proteomic signature of age in healthy humans. Aging Cell 17 (5), e12799 10.1111/acel.12799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taniguchi Y, Yoshida H, Fujiwara Y, Motohashi Y, Shinkai S, 2012. A prospective study of gait performance and subsequent cognitive decline in a general population of older Japanese. J. Gerontol. A Biol. Sci. Med. Sci 67 (7), 796–803. 10.1093/gerona/glr243. [DOI] [PubMed] [Google Scholar]
- Tchalla AE, Wellenius GA, Travison TG, Gagnon M, Iloputaife I, Dantoine T, et al. 2015. Circulating vascular cell adhesion molecule-1 is associated with cerebral blood flow dysregulation, mobility impairment, and falls in older adults. Hypertension 66 (2), 340–346. 10.1093/gerona/glw099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, An Y, Resnick SM, Studenski S, 2016. The relative temporal sequence of decline in mobility and cognition among initially unimpaired older adults: results from the Baltimore Longitudinal Study of Aging. Age Ageing 46 (3), 445–451. 10.1093/ageing/afw185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Q, Resnick SM, Bilgel M, Wong DF, Ferrucci L, Studenski SA, 2017. β-amyloid burden predicts lower extremity performance decline in cognitively unimpaired older adults. J. Gerontol. A Biol. Sci. Med. Sci 72 (5), 716–723. 10.1093/gerona/glw183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiansky R, Blizard R, Livingston G, Mann A, 1995. The Gospel Oak Study stage IV: the clinical relevance of subjective memory impairment in older people. Psychol. Med. (Paris) 25 (4), 779–786. [DOI] [PubMed] [Google Scholar]
- Toledo JB, Arnold SE, Raible K, Brettschneider J, Xie SX, Grossman M, et al. 2013. Contribution of cerebrovascular disease in autopsy confirmed neurodegenerative disease cases in the National Alzheimer’s Coordinating Centre. Brain 136 (9), 2697–2706. 10.1093/brain/awt188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo JB, Arnold M, Kastenmuller G, Chang R, Baillie RA, Han X, et al. 2017. Metabolic network failures in Alzheimer’s disease: a biochemical road map. Alzheimers Dement. 13 (9), 965–984. 10.1016/j.jalz.2017.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai VW, Lin S, Brown DA, Salis A, Breit SN, 2016. Anorexia-cachexia and obesity treatment may be two sides of the same coin: role of the TGF-b superfamily cytokine MIC-1/GDF15. Int. J. Obes. (Lond) 40 (2), 193–197. 10.1038/ijo.2015.242. [DOI] [PubMed] [Google Scholar]
- Tsutsumimoto K, Makizako H, Doi T, Hotta R, Nakakubo S, Makino K, et al. 2017. Subjective memory complaints are associated with incident dementia in cognitively intact older people, but not in those with cognitive impairment: a 24-month prospective cohort study. Am. J. Geriatr. Psychiatry 25 (6), 607–616. 10.1016/j.jagp.2016.12.008. [DOI] [PubMed] [Google Scholar]
- Tynkkynen J, Chouraki V, van der Lee SJ, Hernesniemi J, Yang Q, Li S, et al. 2018. Association of branched-chain amino acids and other circulating metabolites with risk of incident dementia and Alzheimer’s disease: a prospective study in eight cohorts. Alzheimers Dement. 14 (6), 723–733. 10.1016/j.jalz.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uiterwijk R, Huijts M, Staals J, Duits A, Gronenschild E, Kroon AA, et al. 2014. Subjective cognitive failures in patients with hypertension are related to cognitive performance and cerebral microbleeds. Hypertension 64 (3), 653–657. 10.1161/HYPERTENSIONAHA.114.03621. [DOI] [PubMed] [Google Scholar]
- van der Flier WM, Pijnenburg YA, Schoonenboom SN, Dik MG, Blankenstein MA, Scheltens P, 2008v. Distribution of APOE genotypes in a memory clinic cohort. Dement. Geriatr. Cogn. Disord 25 (5), 433–438. 10.1159/000124750. [DOI] [PubMed] [Google Scholar]
- van der Holst HM, Tuladhar AM, Zerbi V, van Uden IWM, de Laat KF, van Leijsen EMC, et al. 2017v. White matter changes and gait decline in cerebral small vessel disease. Neuroimage Clin. 17, 731–738. 10.1016/j.nicl.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Oijen M, de Jong FJ, Hofman A, Koudstaal PJ, Breteler MM, 2007v. Subjective memory complaints, education, and risk of Alzheimer’s disease. Alzheimers Dement. 3 (2), 92–97. 10.1016/j.jalz.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Vasunilashorn S, Ferrucci L, Crimmins EM, Bandinelli S, Guralnik JM, Patel KV, 2013. Association of inflammation with loss of ability to walk 400 meters: longitudinal findings from the Invecchiare in Chianti Study. J. Am. Geriatr. Soc 61 (10), 1743–1749. 10.1111/jgs.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdelho A, Madureira S, Moleiro C, Santos CO, Ferro JM, Erkinjuntti T, et al. 2011. Self-perceived memory complaints predict progression to Alzheimer disease. The LADIS study. J. Alzheimers Dis 27 (3), 491–498. 10.3233/JAD-2011-110494. [DOI] [PubMed] [Google Scholar]
- Verghese J, Lipton RB, Hall CB, Kuslansky G, Katz MJ, et al. 2002. Abnormality of gait as a predictor of non-Alzheimer’s dementia. N. Engl. J. Med 347 (22), 1761–1768. [DOI] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R, Xue X, 2007. Quantitative gait dysfunction and risk of cognitive decline and dementia. J. Neurol. Neurosurg. Psychiatry 78 (9), 929–935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Oh-Park M, Derby CA, Lipton RB, Wang C, 2011. Inflammatory markers and gait speed decline in older adults. J. Gerontol. A Biol. Sci. Med. Sci 66 (10), 1083–1089. 10.1093/gerona/glr099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Lipton RB, Wang C, 2012. High-sensitivity C-reactive protein and mobility disability in older adults. Age Ageing 41 (4), 541–545. 10.1093/ageing/afs038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Holtzer R, Wang C, Katz MJ, Barzilai N, Lipton RB, 2013a. Role of APOE genotype in gait decline and disability in aging. J. Gerontol. A Biol. Sci. Med. Sci 68 (11), 1395–1401. 10.1093/gerona/glt115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Wang C, Lipton RB, Holtzer R, 2013b. Motoric cognitive risk syndrome and the risk of dementia. J. Gerontol. A Biol. Sci. Med. Sci 68 (4), 412–418. 10.1093/gerona/gls191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Annweiler C, Ayers E, Barzilai N, Beauchet O, Bennett DA, et al. 2014a. Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology 83 (8), 718–726. 10.1212/WNL.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese J, Ayers E, Barzilai N, Bennett DA, Buchman AS, Holtzer R, et al. 2014b. Motoric cognitive risk syndrome: multicenter incidence study. Neurology 83 (24), 2278–2284. 10.1212/WNL.0000000000001084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veronese N, Stubbs B, Trevisan C, Bolzetta F, De Rui M, Solmi M, 2016. What physical performance measures predict incident cognitive decline among intact older adults? A 4.4Year follow up study. Exp. Gerontol 81, 110–118. 10.1016/j.exger.2016.05.008. [DOI] [PubMed] [Google Scholar]
- Villemagne VL, Burnham S, Bourgeat P, Brown B, Ellis KA, Salvado O, et al. 2013. Amyloid β deposition, neurodegeneration, and cognitive decline in sporadic Alzheimer’s disease: a prospective cohort study. Lancet Neurol. 12 (4), 357–367. 10.1016/S1474-4422(13)70044-9. [DOI] [PubMed] [Google Scholar]
- Volpato S, Bianchi L, Lauretani F, Lauretani F, Bandinelli S, Guralnik JM, et al. 2012. Role of muscle mass and muscle quality in the association between diabetes and gait speed. Diabetes Care 35 (8), 1672–1679. 10.2337/dc11-2202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waite LM, Grayson DA, Piguet O, Creasey H, Bennett HP, Broe GA, 2005. Gait slowing as a predictor of incident dementia: 6-year longitudinal data from the Sydney Older Persons Study. J. Neurol. Sci 229-230, 89–93. [DOI] [PubMed] [Google Scholar]
- Waldorff FB, Siersma V, Vogel A, Waldemar G, 2012. Subjective memory complaints in general practice predicts future dementia: a 4-year follow-up study. Int. J. Geriatr. Psychiatry 27 (11), 1180–1188. 10.1002/gps.3765. [DOI] [PubMed] [Google Scholar]
- Walker KA, Gottesman RF, Wu A, Knopman DS, Gross AL, Mosley TH Jr., et al. 2019. Systemic inflammation during midlife and cognitive change over 20 years: the ARIC Study. Neurology 92 (11), e1256–e1267. 10.1001/jama.2019.10575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, van Belle G, Crane PK, Kukull WA, Bowen JD, McCormick WC, et al. 2004. Subjective memory deterioration and future dementia in people aged 65 and older. J. Am. Geriatr. Soc 52 (12), 2045–2051. [DOI] [PubMed] [Google Scholar]
- Wang L, Larson EB, Bowen JD, van Belle G, 2006. Performance-based physical 1115–1120 function and future dementia in older people. Arch. Intern. Med 166 (10), 1115–1120. [DOI] [PubMed] [Google Scholar]
- Wang N, Allah G, Kesavadas C, Noone ML, Pradeep VG, Blumen HM, et al. 2016. Cerebral Small vessel disease and motoric cognitive risk syndrome: results from the Kerala-Einstein Study. J. Alzheimers Dis 50 (3), 699–707. 10.3233/JAD-150523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb E, Laverty A, Mindell J, Millett C, 2016. Free bus travel and physical activity, gait speed, and adiposity in the English Longitudinal Study of Ageing. Am. J. Public Health 106 (1), 136–142. 10.2105/AJPH.2015.302907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welmer AK, Angleman S, Rydwik E, Fratiglioni L, Qiu C, 2013. Association of cardiovascular burden with mobility limitation among elderly people: a population-based study. PLoS One 8 (5), e65815 10.1371/journal.pone.0065815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welmer AK, Rizzuto D, Qiu C, Caracciolo B, Laukka EJ, 2014. Walking speed, processing speed, and dementia: a population-based longitudinal study. J. Gerontol. A Biol. Sci. Med. Sci 69 (12), 1503–1510. 10.1016/j.amjmed.2019.05.005. [DOI] [PubMed] [Google Scholar]
- White DK, Neogi T, Zhang Y, Niu J, Katz PP, 2017. Association of slow gait speed with trajectories of worsening depressive symptoms in knee osteoarthritis: an observational study. Arthritis Care Res. (Hoboken) 69 (2), 209–215. 10.1002/acr.22928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson DJ, Piasecki M, Atherton PJ, 2018. The age-related loss of skeletal muscle mass and function: measurement and physiology of muscle fibre atrophy and muscle fibre loss in humans. Ageing Res. Rev 47, 123–132. 10.1016/j.arr.2018.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PT, 2008. Reduced diabetic, hypertensive, and cholesterol medication use with walking. Med. Sci. Sports Exerc 40 (3), 433–443. 10.1249/MSS.0b013e31815f38f1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Windham BG, Griswold ME, Wang W, Kucharska-Newton A, Demerath EW, Gabriel KP, et al. 2017. The importance of mid-to-late-life body mass index trajectories on late-life gait speed. J. Gerontol. A Biol. Sci. Med. Sci 72 (8), 1130–1136. 10.1093/gerona/glw200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf OT, Dziobek I, McHugh P, Sweat V, de Leon MJ, Javier E, et al. 2005. Subjective memory complaints in aging are associated with elevated cortisol levels. Neurobiol. Aging 26 (10), 1357–1363. [DOI] [PubMed] [Google Scholar]
- World Health Organization, 2006. WHO SAGE Training Manual: The WHO Study on Global AGEing and Adult Health (SAGE), Evidence and Information for Policy, Multi-Country Studies. World Health Organization, Geneva. [Google Scholar]
- World Health Organization, 2017 Dementia Fact Sheet. 12 December 2017. https://www.who.int/en/news-room/fact-sheets/detail/dementia Accessed 04/03/19.
- Xie YJ, Liu EY, Anson ER, Agrawal Y, 2017. Age-related imbalance is associated with slower walking speed: an analysis from the National Health and Nutrition Examination Survey. J. Geriatr. Phys. Ther 40 (4), 183–189. 10.1519/JPT.0000000000000093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CW, Li CI, Li TC, Liu CS, Lin CH, Lin WY, et al. 2017. The joint association of insulin sensitivity and physical activity on the skeletal muscle mass and performance in community-dwelling older adults. Exp. Gerontol 95, 34–38. 10.1016/j.exger.2017.05.006. [DOI] [PubMed] [Google Scholar]
- Yesavage JA, Brink TL, Rose TL, Lum O, Huang V, Adey M, et al. 1983. Development and validation of a geriatric depression screening scale: a preliminary report. J. Psychiatr. Res 17 (1), 37–49. [DOI] [PubMed] [Google Scholar]
- Yiallouris A, Tsioutis C, Agapidaki E, Zafeiri M, Agouridis AP, Ntourakis D, et al. 2019. Adrenal aging and its implications on stress responsiveness in humans. Front. Endocrinol. (Lausanne) 10, 54 10.3389/fendo.2019.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurko-Mauro K, Alexander DD, Van Elswyk ME, 2015. Docosahexaenoic acid and adult memory: a systematic review and meta-analysis. PLoS One 10 (3), e0l20391 10.1371/journal.pone.0120391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zane AC, Reiter DA, Shardell M, Cameron D, Simonsick EM, Fishbein KW, et al. 2017. Muscle strength mediates the relationship between mitochondrial energetics and walking performance. Aging Cell 16 (3), 461–468. 10.1111/acel.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaninotto P, Sacker A, Head J, 2013. Relationship between wealth and age trajectories of walking speed among older adults: evidence from the English Longitudinal Study of Ageing. J. Gerontol. A Biol. Sci. Med. Sci 68 (12), 1525–1531. 10.1093/gerona/glt058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarit SH, Cole KD, Guider RL, 1981. Memory training strategies and subjective complaints of memory in the aged. Gerontologist 21 (2), 158–164. [DOI] [PubMed] [Google Scholar]
- Zemp DD, Giannini O, Quadri P, de Bruin ED, 2019. Gait characteristics of CKD patients: a systematic review. BMC Nephrol. 20 (1), 83 10.1186/s12882-019-1270-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuniga KE, Mackenzie MJ, Kramer A, McAuley E, 2016. Subjective memory impairment and well-being in community-dwelling older adults. Psychogeriatrics 16 (1), 20–26. 10.1111/psyg.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zwan MD, Villemagne VL, Dore V, Buckley R, Bourgeat P, Veljanoski R, et al. 2016. Subjective memory complaints in APOEε4 carriers are associated with high amyloid-β burden. J. Alzheimers Dis 49 (4), 1115–1122. 10.3233/JAD-150446. [DOI] [PubMed] [Google Scholar]


