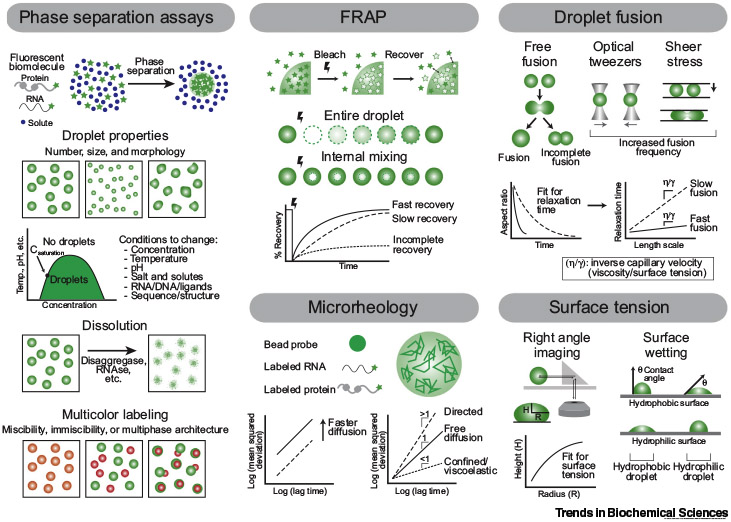
Many methods have been developed to examine the bulk properties and underlying molecular interactions of biomolecular condensates that form through liquid–liquid phase separation (LLPS). Phase separation assays using bright-field imaging or fluorescence microscopy track droplets over time and can assess the effect of various conditions on LLPS, measure droplet formation and dissolution kinetics, and test the miscibility of differentially labeled molecules. Fluorescence recovery after photobleaching (FRAP) and microrheology by particle tracking probe the diffusion of molecules within droplets, while droplet fusion assays, right angle imaging, and surface wetting determine droplet surface tension.
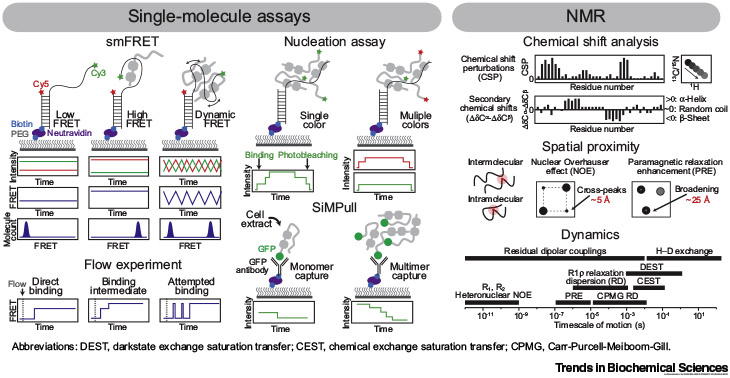
Single-molecule fluorescence resonance energy transfer (smFRET) monitors distance changes over time between FRET pair dyes conjugated to an immobilized molecule. Nucleation and single-molecule pull-down (SiMPull) assays determine the stoichiometry of complexes by counting photobleaching steps of labeled molecules at in vitro and cellular levels, respectively. NMR reports on the chemical environment and secondary structure via chemical shifts, proximal interactions via nuclear Overhauser effects (NOEs) and paramagnetic relaxation enhancement (PREs), and dynamics using multiple experiments.
ADVANTAGES:
Ensemble experiments: Direct measurement of the liquid-like properties of droplets over time. In vitro systems are readily perturbed to systematically test the effect of many factors. Multicolor labeling schemes allow for testing multiple species simultaneously. Most assays can be recapitulated in cells.
smFRET: Optimal for observing conformational changes and other dynamic processes in a time-resolved manner without population averaging. Unique tool to measure stoichiometry and nucleation process. Full-length proteins and complexes can be applied. Can readily change solution conditions and experimental schemes as desired.
NMR: Probe atomic-level structure and dynamics in solution. Multiple NMR methods probe distinct features, including dynamic processes occurring at various timescales.
CHALLENGES:
Ensemble experiments: No molecular-level information. Fluorophores may disrupt LLPS. In vitro reconstitution of cellular condensates is challenging due to their complexity and the difficulty in purifying phase separating proteins.
smFRET: Technical challenges with high-fluorescence background. Does not provide atomic-level detail. Experimental design often requires knowledge of the system a priori.
NMR: Samples must be highly pure and concentrated. Limitations due to spectral overlap and signal broadening caused by low sequence complexity, high molecular size, or increased viscosity. Data processing and interpretation can be difficult.
Literature
- 1.Mitrea DM et al. (2018) Methods for physical characterization of phase separated bodies and membrane-less organelles. J. Mol. Biol 430, 4773–4805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang J et al. (2018) A molecular grammar governing the driving forces for phase separation of prion-like RNA binding proteins. Cell 174, 688–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feric M et al. (2016) Coexisting liquid phases underlie nucleolar subcompartments. Cell 165, 1686–1697 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Banani S et al. (2016) Compositional control of phase-separated cellular bodies. Cell 166, 651–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niaki AG et al. (2020) Loss of dynamic RNA interaction and aberrant phase separation induced by two distinct types of ALS/FTD-linked FUS mutations. Mol. Cell 77, 82–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elbaum-Garfinkle S et al. (2015) The disordered P granule protein LAF-1 drives phase separation into droplets with tunable viscosity and dynamics. Proc. Natl. Acad. Sci. U. S. A 112, 7189–7194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jain A et al. (2012) Single-molecule pull-down for studying protein interactions. Nat. Protoc 7, 445–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy AC and Fawzi NL (2020) The (un)structural biology of biomolecular liquid–liquid phase separation using NMR spectroscopy. J. Biol. Chem 295, 2375–2384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martin E et al. (2020) Valence and patterning of aromatic residues determine the phase behavior of disordered prion-like domains. Science 367, 694–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brady JP et al. (2017) Structural and hydrodynamic properties of an intrinsically disordered region of a germ cell-specific protein on phase separation. Proc. Natl. Acad. Sci. U. S. A 114, E8194–E8203 [DOI] [PMC free article] [PubMed] [Google Scholar]


