Abstract
Simple Summary
Splicing factor 1 (SF1) is a widely expressed alternative splicing factor that is able to process each piece of genetic information to generate different types of messenger RNAs (or alternate messages). The alternate messages can generate proteins with slightly different structure or function in the cell. Thus, alternative splicing is responsible for the large diversity of proteins that can finely tune cellular function to the cells’ physiological state. Using mouse models for our study, we found that mice expressing reduced SF1 levels develop fewer intestinal polyps. Lowered SF1 levels appear to limit the initiation of polyps. Thus, our studies point to a novel approach for reducing intestinal polyp burden.
Abstract
Background: Splicing factor 1 (SF1) is a conserved alternative splicing factor expressed in many different mammalian cell types. The genetically modified Sf1+/− (or Sf1β-geo/+) mice express reduced levels of SF1 protein in mouse tissues, including in cells of the intestines. Mutational inactivation of human adenomatous polyposis coli (APC) gene deregulates the Wnt signaling pathway and is a frequent genetic event in colon cancers. Mice with a point mutation in the Apc gene (ApcMin/+) also develop numerous intestinal polyps at a young age. Our aim was to determine the effect of reduced SF1 levels on polyp development due to the strong driver ApcMin/+ mutation. Methods: We utilized mice genetically deficient for expression of SF1 to assess how SF1 levels affect intestinal tumorigenesis. We crossed ApcMin/+ to Sf1+/− mice to generate a cohort of heterozygous mutant ApcMin/+;Sf1+/− mice and compared intestinal polyp development in these mice to that in a control cohort of sibling ApcMin/+ mice. We compared total polyp numbers, sizes of polyps and gender differences in polyp numbers between ApcMin/+;Sf1+/− and ApcMin/+ mice. Results: Our results showed that ApcMin/+ mice with lower SF1 expression developed 25–30% fewer intestinal polyps compared to their ApcMin/+ siblings with normal SF1 levels. Interestingly, this difference was most significant for females (ApcMin/+;Sf1+/− and ApcMin/+ females developed 39 and 55 median number of polyps, respectively). Furthermore, the difference in polyp numbers between ApcMin/+;Sf1+/− and ApcMin/+ mice was significant for smaller polyps with a size of 2 mm or less, whereas both groups developed similar numbers of larger polyps. Conclusions: Our results suggest that lower SF1 levels likely inhibit the rate of initiation of polyp development due to ApcMin/+ driver mutation in the mouse intestine. Thus, therapeutic lowering of SF1 levels in the intestine could attenuate intestinal polyp development.
Keywords: splicing factor 1 (SF1), APC (adenomatous polyposis coli), polyp
1. Introduction
Colorectal cancer (CRC) is the third most common cancer in the US, with the second highest in terms of mortality rates, and with higher incidence and mortality rates in African Americans [1,2]. Although there has been tremendous improvement in screening and detection for this cancer, much still remains to be elucidated regarding genetic susceptibility factors that predispose to this cancer.
Alternative splicing contributes to proteome diversity that is necessary for complex traits especially in mammalian systems [3]. Splicing factor 1 (SF1, also known as mammalian branch point-binding protein (mBBP), zinc finger gene in MEN1 locus (ZFM1), or zinc finger protein 162, ZNF162 or ZFP162) is a ubiquitously expressed and highly conserved splicing factor [4,5]. It is required for early spliceosome assembly and may function as a constitutive splicing factor in lower eukaryotes [6,7] but acts as an alternative splicing (AS) factor in mammalian cells [4,8,9]. SF1 participates in the assembly of the earliest spliceosome complex (E’ complex) during pre-mRNA splicing [10,11,12,13,14,15]. where it interacts with U2 snRNP auxiliary factor (U2AF65) to co-operatively bind to the branch point sequence and polypyrimidine tract within the intron of pre-mRNAs [6,16,17,18]. In mammalian cells, SF1 drives alternative splice site choices and can act as a negative or positive regulator of exon inclusion [8,9].
Crosslinking and immunoprecipitation (CLIP) of SF1-bound RNA in HeLa cells found that SF1 targets are mostly mRNAs of protein-coding genes that participate in a great number of cellular pathways, including metabolic pathways, base excision repair, DNA replication and RAS signaling pathways [8,9]. siRNA depletion of SF1 in HeLa cells resulted in changes in the ratio of inclusion or skipping of alternative exons. Moreover, depending on the transcript, the normal ratios of alternatively spliced isoforms were altered or stability of transcripts was affected. Thus, depletion of SF1 alters the expression levels of mRNA variants [8,9].
Splicing is progressively deregulated during normal aging [19,20,21]. Experimental overexpression of SF1 orthologue was found to be sufficient to restore “youthful” splicing patterns and extend lifespan in C. elegans [9]. SF1 was found to mediate lifespan extension in C. elegans by modulating the metabolic pathway TORC1 components. Interestingly, expression levels of specific splicing factors in some human populations are associated with longevity [21].
Alternative splicing is an important epigenetic mechanism in cellular transformation and cancer development [22,23,24,25,26,27]. Human cancer genome sequencing reveals recurrent, inactivating mutations in splicing factors and their gene targets in a number of malignancies. SF1 mutations, gene amplifications or deletions are found to occur with highest frequencies in human uterine cancers, melanomas, and stomach and colorectal cancers (cBioportal for Cancer Genomics).
In mice, SF1 is essential during early development. Mice with homozygous deletion of the Sf1 gene die during embryogenesis [28,29]. Heterozygous Sf1+/− mice are viable but have reduced SF1 expression in tissues. Our initial study combined Sf1+/− with mouse strains genetically predisposed to develop germ cell derived testicular tumors, where we found that congenital reduction of SF1 significantly decreased testicular tumors [29]. In contrast, it was reported that carcinogen treatment of Sf1+/− mice increased the number and size of intestinal polyps [28]. We therefore examined how SF1 deficiency affects intestinal adenoma development in mice in the context of germ line mutations in Apc.
Mutational inactivation of APC is one of the earliest and frequent genetic events in human colon cancers, and the majority of human sporadic colorectal cancers contain adenomatous polyposis coli (APC) mutations [30,31,32]. Germline mutations in APC deregulate the WNT signaling pathway to cause familial adenomatous polyposis (FAP). The (multiple intestinal neoplasia) Min mouse (ApcMin/+) carries a point mutation in the Apc gene and readily develops intestinal polyps [33]. Combining other genetic modifications with ApcMin/+ mice, such as Dnmt1N/+, loss of Trp53 or ER, has been shown to decrease or enhance polyp multiplicity or sizes [34,35,36]. Additionally, genetic modifiers of ApcMin/+ (Mom1 and others) that either enhance or diminish polyp multiplicity or characteristics have been mapped and, in some cases, candidate genes identified [37,38,39].
Our study aimed to determine the genetic consequences of lowered SF1 levels on ApcMin/+-driven intestinal polyp development. We therefore generated mice heterozygous mutant for both Sf1 (Sf1+/−) and ApcMin/+ and compared polyp numbers and sizes between ApcMin/+;Sf1+/− and ApcMin/+ mice. Our results show that congenitally reduced SF1 levels resulted in reduction of intestinal polyp development in ApcMin/+ mice. Thus, SF1 levels in intestinal cells influence cellular transformation and polyp development.
2. Materials and Methods
2.1. Mouse Strains
Animal studies were approved by Texas Southern University Institutional Animal Care and Use committee (IACUC) protocol number 9088. ApcMin/+ (002020: C57BL/6J-Apc<Min>/J) mice were purchased from JAX Labs (Bar Harbor, ME, USA). Sf1+/− mice (Sf1β-geo/+), generated in our lab, were maintained by crossing to 129/Sv [29].
2.2. Generation of ApcMin/+;Sf1+/− Mice
ApcMin/+ males were crossed to Sf1+/− female mice, and progeny were screened by PCR genotyping. ApcMin/+ mice were on a B6 strain background, and Sf1+/− was maintained on a 129 mouse strain genetic background. Thus, all progeny derived from the cross between ApcMin/+ male and Sf1+/− female mice were on a mixed B6/129 genetic background. v1F/v1R and gt1F/gt4R primers [29] were used to identify progeny carrying the Sf1 heterozygous mutant allele. Wild-type and Min mutant Apc alleles were detected by genotyping using a 3-primer PCR reaction, with primers APC1 (wild-type) (GCCATCCCTTCACGTTAG), common antisense APC2 (TTCCACTTTGGCATAAGGC) and (Min mutant) APC3 (TTCTGAGAAAGACAGAAGTTA) [40]. PCR-amplified DNA samples were loaded onto 1% agarose (GoldBio, St Louis, MO, USA) gel for visualization of products using a BIO-RAD ChemiDoc touch imaging system (version 1.2.0.12) (Bio-Rad, Hercules, CA, USA). Siblings positive for ApcMin/+ and ApcMin/+;Sf1+/− were allowed to age for at least 4 months, with most being between 4–6 months old before sacrifice to assess for intestinal polyps. Another sibling cohort of Sf1+/− and ++ mice, also derived from the same ApcMin/+ and Sf1+/− parents, was similarly aged prior to assessing polyp development.
2.3. Assessment of Intestinal Polyp Numbers and Size
For each mouse, the intestinal region stretching immediately distal to the stomach until the caecum (termed SI) was dissected out from the peritoneal cavity [41]. Additionally, the colonic region, immediately distal to the caecum stretching to the anus (termed LI), was isolated. The intestine and colon were repeatedly flushed with PBS. SI was divided into approximately 4 equal sections: SI-1, SI-2, SI-3 and SI-4. One side of the intestine/colon tube was snipped opened to visualize the inner surface. The sections SI-1 to SI-4 and L1 were examined under a Leica S9i Stereomicroscope integrated with a digital color camera with a CMOS sensor and an HDMI monitor (Leica Biosystems, Wetzlar, Germany). Starting from the top of SI-1 and systematically moving downwards, the inner surface of each intestinal and colon segment was scanned under the microscope to count polyps and measure the polyp sizes. The majority of polyps were between 1 mm to 5 mm size. Polyps less than 1 mm or greater than 5 mm were included in the 1mm and 5 mm category. Two individuals participated in identifying and counting the polyps. The count data collected from all the mice were collated according to size of polyps and gender for statistical analysis. The intestinal tissues were subsequently stored in formalin.
2.4. Immunoblotting
Protein lysates were prepared in radioimmunoprecipitation (RIPA) lysis buffer (sc-24948, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) containing protease inhibitor cocktail and sodium orthovanadate. The amount of protein in the samples was detected using BIO-RAD protein assay according to manufacturer’s instructions (Bio-Rad, Hercules, CA, USA). Proteins were separated by 12% SDS-PAGE gel and blotted onto Immobilon-P membranes (Millipore-Sigma, Burlington, MA, USA). After overnight blocking with 5% non-fat milk, the blots were incubated overnight with the primary antibodies at a concentration of 1/1000: Anti-SF1 (ab223256, Abcam, Cambridge, UK) or GAPDH (sc-365062, Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4 °C. The target proteins were detected with the relevant horseradish peroxidase-conjugated anti-human IgG antibody and ECL Western blotting detection reagents (Clarity Western ECL substrate, Bio-Rad, Hercules, CA, USA) and visualized using ChemiDoc touch imaging system, version 1.2.0.12) (Bio-Rad, Hercules, CA, USA) (Figure S1).
2.5. Immunohistochemistry
Immunohistochemistry was performed using anti-SF1 antibody (ab223256, Abcam, Cambridge, UK) at a concentration of 1/4000. Heat-mediated antigen retrieval with Tris/EDTA buffer pH 9.0 was performed before staining using a DAB Substrate Kit and peroxidase (HRP) with nickel, (3,3′-diaminobenzidine) (Vectorlabs, Burlingame, CA, USA).
2.6. Statistical Analysis
Data collected were sorted according to genotype, gender, number of polyps and size of polyps. Frequency distribution analysis found that data regarding polyp numbers and sizes were not normally distributed. Therefore, we used a nonparametric alternative statistical methodology to the t-test, the Mann–Whitney test. The Mann–Whitney test [42] does not make assumptions about the distribution of the data. Instead of testing the difference between means, the Mann–Whitney hypothesis tests for the difference between two medians. Both descriptive and inferential statistical methodologies were performed using the statistical software IBM SPSS (IBM, Armonk, NY, USA). Nonparametric statistical analyses was performed to determine if the total number of polyps differ between groups, for example, between ApcMin/+ (A) and ApcMin/+;Sf1+/− (AS) mice. Subsequently, we determined whether polyp sizes (1 mm to 5 mm) were statistically different between ApcMin/+ (A) and ApcMin/+;Sf1+/− (AS) mice and, further, if polyp numbers differed between males and females. Similar statistical analyses were used to determine whether polyp numbers differed between Sf1+/− and Sf1+/+ (wild-type) mice and whether polyp numbers differed between ApcMin/+ (A) or ApcMin/+;Sf1+/− (AS) mice and the control population (combination of Sf1+/− and Sf1+/+ mice).
3. Results
3.1. SF1 Expression in Nuclei of Intestinal Cells and Polyps
ApcMin/+;Sf1+/− and ApcMin/+ mice developed intestinal polyps by 4 months, and the majority of the polyps were of sizes between 1 mm to 5 mm. Polyps were easily visible on the inner intestinal surface, without staining, under a dissecting microscope (Figure 1a,b). The variation in size and number of polyps could be due to the age range, 4–6 months, of mice used for the study (Figure 2a,b). Intestines of mice in control cohorts, wild-type (Sf1+/+) and Sf1+/− mice, were similarly examined.
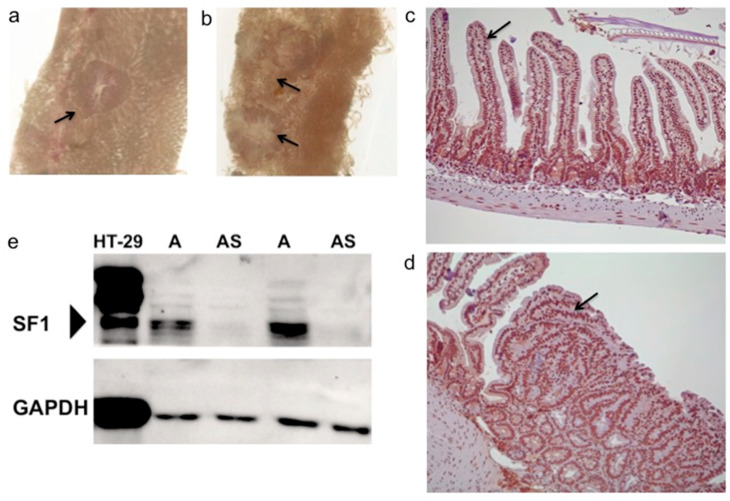
Figure 1.
SF1 expression in the intestine. (a,b) Representative polyps (arrow) in the small intestine of 4-month old ApcMin/+;Sf1+/− mice as viewed under the stereomicroscope. (c) Immunohistochemistry using anti-SF1 antibody of normal mouse intestine. Arrow indicates splicing factor 1 (SF1) expression in nuclei of intestinal villi and in (d) nuclei of adenoma cells of ApcMin/+ mice. (e) Immunoblotting using anti-SF1 antibody on cell lysate from HT-29 human colon cancer cells and lysates from ApcMin/+ (A) and ApcMin/+;Sf1+/− (AS) mouse intestines. (Bottom) GAPDH expression in the samples.
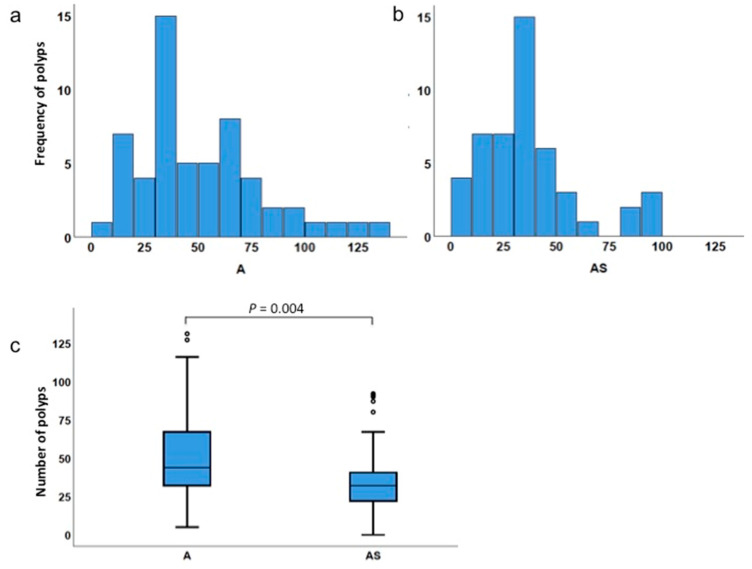
Figure 2.
Polyp incidence in ApcMin/+ and ApcMin/+;Sf1+/− intestines. (a,b) Frequency distribution of number of polyps in ApcMin/+ (A) and ApcMin/+;Sf1+/− (AS) mice. (c) Box plot comparing total number of polyps in ApcMin/+ (A) and ApcMin/+;Sf1+/− (AS) mice. The box plots are a graphical representation of the five-number-summary for the number of polyps: the minimum value (the bottom whisker), first quartile (lowest line of the box), median or second quartile (line inside the box), third quartile (top line of the box), and maximum value (top whisker). Actual values are listed in Table 4.
Immunohistochemical staining for SF1 in intestinal sections of wild-type B6 mice localized SF1 to the nuclei of epithelial cells of the intestinal villi and crypts (Figure 1c). SF1 was also detected in the nuclei of cells in the intestinal polyps from ApcMin/+ mice (Figure 1d). Further, immunoblotting was used to compare SF1 levels in ApcMin/+;Sf1+/− and ApcMin/+ mice. SF1 was significantly reduced in intestinal extracts of ApcMin/+;Sf1+/− compared to their ApcMin/+ siblings (Figure 1e). In comparison, very high levels of SF1 were detected in the HT-29 human colon cancer cell line (Figure 1e). Multiple isoforms of SF1, with higher molecular weights, appear to be present in the HT-29 cell line, as detected using the recombinant, monoclonal anti-SF1 antibody (abcam ab223256). Corresponding bands of higher molecular weight isoforms of SF1 were also detected in mouse intestinal lysates, although their expression levels appear to be much lower.
3.2. Haploinsufficiency of Sf1 Reduces Intestinal Polyp Formation
Sf1+/− was crossed to ApcMin/+ mice, and F1 generation progeny were genotyped to select the four cohorts, heterozygous mutant ApcMin/+;Sf1+/− mice, ApcMin/+ mice and controls of genotypes Sf1+/− and wild-type (Sf1+/+) siblings. Table 1 summarizes the number of ApcMin/+ (n = 57), ApcMin/+;Sf1+/− (n = 48) and control mice (total n = 39) examined. Mice were aged for 4–6 months before examination of intestinal polyps in the small and large intestines (SI-1, SI-2, SI-3, SI-4 and L1 sections, as described in the Methods). The inner surface of each intestinal segment was systematically scanned under the microscope to count the polyps and measure each polyp size. Overall, the greatest number of polyps was detected in the SI-3 segment. Most polyps were between 1 mm to 5 mm in size, with the 2 mm-sized polyps being the most numerous, as described below.
Table 1.
Number of mice examined. Mice were siblings derived from parents ApcMin/+ and Sf1+/−.
| Cohort Genotype | n | No. of Males | No. of Females |
|---|---|---|---|
| ApcMin/+ | 57 | 26 | 31 |
| ApcMin/+;Sf1+/− | 48 | 23 | 25 |
| Sf1+/− | 22 | 13 | 9 |
| WT (Sf1+/+) | 17 | 8 | 9 |
Histograms indicating the frequency distribution of the number of polyps in the 57 ApcMin/+ mice and 48 ApcMin/+;Sf1+/− mice are shown (Figure 2a,b). The lowest and highest numbers of polyps counted in ApcMin/+ mice were 5 and 131 respectively, and 0 and 92 respectively in ApcMin/+;Sf1+/− mice (Table 2). The larger range for ApcMin/+ indicates that the number of polyps in ApcMin/+ mice was more variable than for ApcMin/+;Sf1+/− mice.
Table 2.
Summary of polyp numbers and results from Mann–Whitney analysis.
| Type of Comparison | Genotype | Minimum No. of Polyps | Maximum No. of Polyps | Mean No. of Polyps | Median No. of Polyps | p Value |
|---|---|---|---|---|---|---|
| Total polyps | ApcMin/+ | 5 | 131 | 50.7 | 44.0 | 0.004 |
| ApcMin/+;Sf1+/− | 0 | 92 | 35.6 | 32.0 | ||
| 1 mm-sized polyps | ApcMin/+ | 0 | 71 | 20.0 | 18.0 | 0.029 |
| ApcMin/+;Sf1+/− | 0 | 57 | 12.5 | 7.5 | ||
| 2 mm-sized polyps | ApcMin/+ | 1 | 59 | 20.4 | 20.0 | 0.003 |
| ApcMin/+;Sf1+/− | 0 | 42 | 13.7 | 11.0 | ||
| 3 mm-sized polyps | ApcMin/+ | 0 | 25 | 5.9 | 4.0 | 0.487 |
| ApcMin/+;Sf1+/− | 0 | 28 | 5.4 | 3.5 | ||
| 4 mm-sized polyps | ApcMin/+ | 0 | 17 | 2.3 | 1.0 | 0.448 |
| ApcMin/+;Sf1+/− | 0 | 13 | 2.6 | 1.0 | ||
| 5 mm-sized polyps | ApcMin/+ | 0 | 34 | 2.1 | 0 | 0.729 |
| ApcMin/+;Sf1+/− | 0 | 12 | 1.4 | 0 | ||
| Number of polyps | ApcMin/+ female | 13 | 131 | 58.2 | 55.0 | 0.024 |
| ApcMin/+;Sf1+/−female | 5 | 92 | 41.7 | 39.0 | ||
| Number of polyps | ApcMin/+ male | 5 | 116 | 41.7 | 34.5 | 0.076 |
| ApcMin/+;Sf1+/−male | 0 | 90 | 29.0 | 28.0 | ||
| Number of polyps | ApcMin/+ male | 5 | 116 | 41.7 | 34.5 | 0.013 |
| ApcMin/+ female | 13 | 131 | 58.2 | 55.0 | ||
| Number of polyps | ApcMin/+;Sf1+/−male | 0 | 90 | 29.0 | 28.0 | 0.026 |
| ApcMin/+;Sf1+/−female | 5 | 92 | 41.7 | 39.0 | ||
| Number of polyps | WT (Sf1+/+) | 0 | 7 | 1.0 | 0 | 0.974 |
| Sf1+/− | 0 | 7 | 0.9 | 0 | ||
| Number of polyps | ApcMin/+ | 5 | 131 | 50.7 | 44.0 | 0 |
| 1 Control | 0 | 7 | 1.0 | 0 | ||
| Number of polyps | ApcMin/+;Sf1+/− | 0 | 92 | 35.6 | 32.0 | 0 |
| 1 Control | 0 | 7 | 1.0 | 0 |
1 Control includes both Sf1+/− and WT (Sf1+/+) mice.
The Mann–Whitney test was used to compare the differences between median polyp numbers in the two independent groups, namely, ApcMin/+ and ApcMin/+;Sf1+/− mice, because the variables (polyp numbers) were found to not be normally distributed. Mann–Whitney analysis, which determines if two distributions are equal or not, found that the total number of intestinal polyps in ApcMin/+;Sf1+/− (AS) mice was significantly lower compared to that in the ApcMin/+ (A) sibling cohort (p = 0.004) (Figure 2c and Table 2). The median numbers of polyps for the ApcMin/+ and ApcMin/+;Sf1+/− cohorts were 44 and 32, respectively. (The minimum, first quartile, median, third quartile and maximum values of each box plot are summarized in Table 4). Thus, we conclude that there is a statistically significant difference between the number of polyps in ApcMin/+ mice versus ApcMin/+;Sf1+/−.
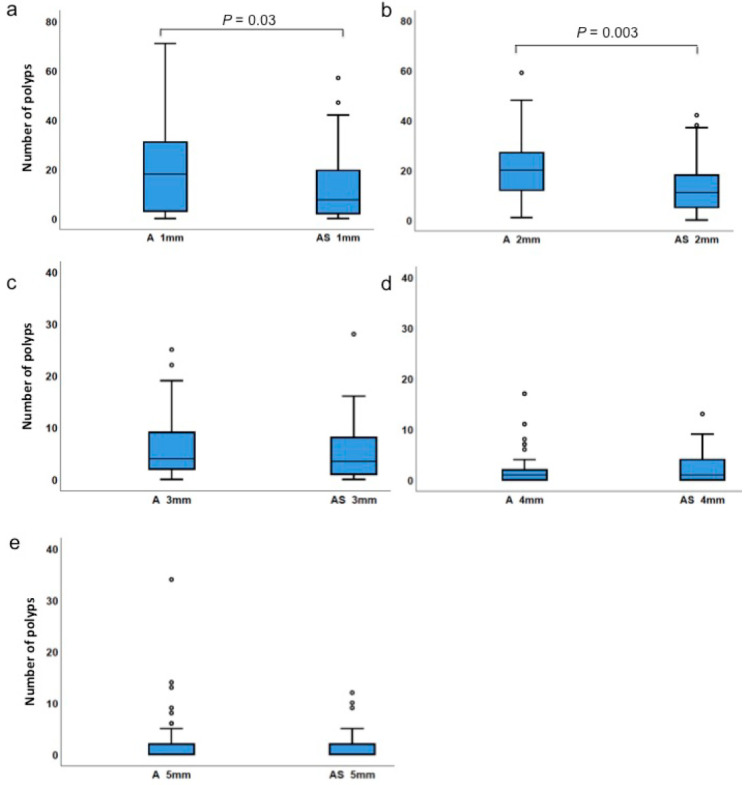
We then compared how polyp sizes (1 mm to 5 mm) differed between the two groups (Figure 3 and Table 2). When polyps of sizes 1 mm and 2 mm were compared, fewer polyps were detected in ApcMin/+;Sf1+/− than in ApcMin/+. Median values for the number of 1 mm-sized polyps were 18 and 7.5 (p = 0.029), and the medians for 2 mm-sized polyps were 20 and 11 (p = 0.003) in ApcMin/+ and ApcMin/+;Sf1+/− mice, respectively. However, the numbers of polyps with a size of 3–5 mm sizes were not significantly different in both cohorts (Figure 3 and Table 2). Thus, our data showed that congenital lowering of SF1 expression levels led to reduced development of the smaller sized polyps. Because the 1 mm- and 2 mm-sized polyps were more numerous, lowering the numbers of 1 mm- and 2 mm-sized polyps contributed to overall reduced incidence of polyps in ApcMin/+;Sf1+/− mice. Because the numbers of 3mm-sized polyps or larger were of similar counts in the two cohorts, this suggests that further growth and enlargement of polyps are independent of SF1 levels.
Figure 3.
Polyp size comparison. (a) Comparison of polyps in ApcMin/+ (A) and ApcMin/+;Sf1+/− (AS) mice of sizes of 1 mm, (b) 2 mm, (c) 3 mm, (d) 4 mm and (e) 5 mm. Quartile values are represented as described in the legend of Figure 2. Actual values are listed in Table 4.
3.3. Low Incidence of Polyps in Sf1+/− Mice
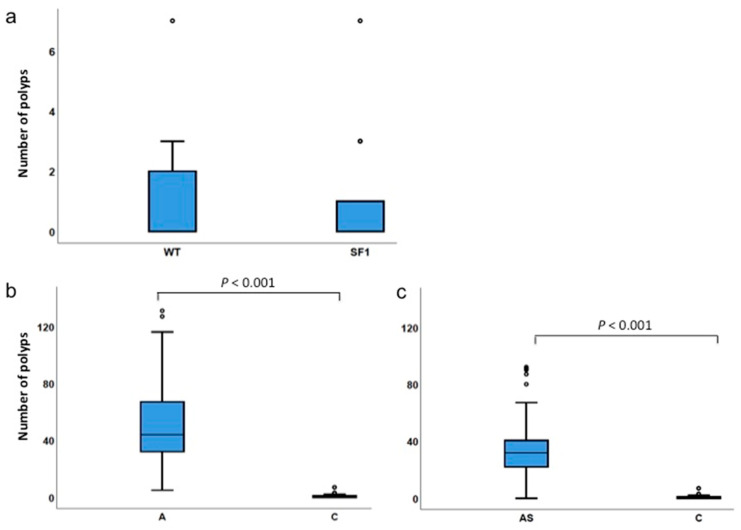
Intestinal polyps of control Sf1+/− and wild-type (Sf1+/+ or WT) mice were also examined. Statistical analysis did not detect significant differences in polyp numbers between wild-type and Sf1+/− mice (Figure 4a). Of the total 39 control mice (Sf1+/− and Sf1+/+) examined, most developed no polyps, but a few had up to seven polyps/mice (Table 3). Polyp numbers were significantly different between ApcMin/+ and control mice or ApcMin/+;Sf1+/− and control cohorts (Figure 4b,c, Table 2).
Figure 4.
Polyps in WT and Sf1+/− mice. (a) Comparison of polyp numbers in wild-type (WT) Sf1+/+ and Sf1+/− genotypes; (b) ApcMin/+ and control (Sf1+/+ and Sf1+/−) genotypes; and (c) ApcMin/+;Sf1+/− and control genotypes. Quartile values are represented as described in the legend of Figure 2. Actual values are listed in Table 4.
Table 3.
Frequency distribution of polyps in Sf1+/+ (WT) and Sf1+/− mice.
| Cohort Genotype | N | No. with 0 Polyps | No. with 1 to 7 Polyps |
|---|---|---|---|
| Sf1+/− | 22 | 14 | 8 |
| WT (Sf1+/+) | 17 | 11 | 6 |
3.4. Gender Differences Affect Polyp Incidences
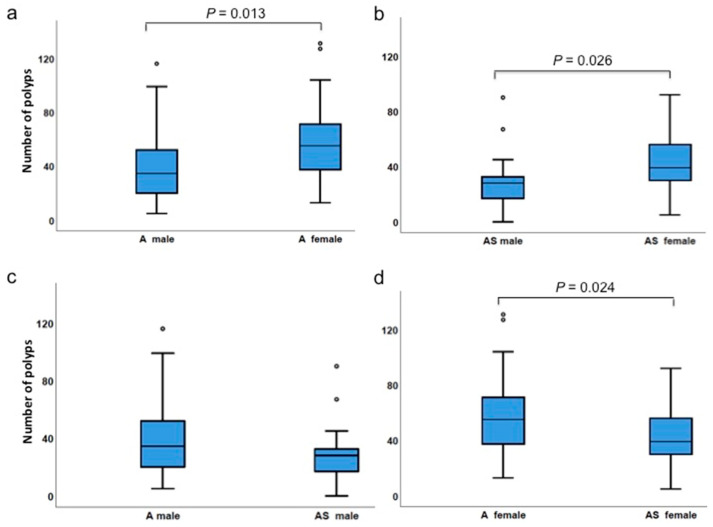
Next, we examined whether there were gender differences in polyp development. Interestingly, females of both ApcMin/+ and ApcMin/+;Sf1+/− cohorts had higher numbers of polyps compared to males of the same genotypes (p = 0.13 for ApcMin/+ and p = 0.026 for ApcMin/+;Sf1+/−) (Table 2, Figure 5a,b). Polyp numbers in ApcMin/+;Sf1+/− females were significantly lower than in ApcMin/+ females (p = 0.024; Figure 5d). Although polyp numbers in ApcMin/+;Sf1+/− males were lower than in ApcMin/+ males, the results were not statistically significantly different (medians of 34.5 and 28 in ApcMin/+ males and ApcMin/+;Sf1+/− males, respectively, p = 0.076; Figure 5c).
Figure 5.
Difference between genders. (a) Comparison of polyp numbers in ApcMin/+ (A) males and females; (b) ApcMin/+;Sf1+/− (AS) males and females; (c) ApcMin/+ and ApcMin/+;Sf1+/− males; (d) ApcMin/+ and ApcMin/+;Sf1+/− females. Quartile values are represented as described in the legend of Figure 2. Actual values are listed in Table 4.
4. Discussion
To date, two genetically modified Sf1 alleles have been characterized. Shitashige et al. created a gene trap inactivated Sf1+/− mouse line, where the trap was inserted in the Sf1 promoter [28], unlike our Sf1+/− mice, where the trap is within the first intron [29]. Notwithstanding this minor technical difference, homozygous Sf1−/− mice from the study of Shitashige et al. died due to embryonic lethality, and Sf1+/− mice expressed decreased SF1 in their tissues, similar to that observed in our strain. Shitashige et al. reported that treatment of Sf1+/− mice with the organotropic carcinogen, azoxymethane (AOM), resulted in a higher number and increased intestinal polyp volume compared to that in wild-type mice [28]. They compared AOM treatment of 4–6 Sf1+/− mice to 7–13 wild-type, +/+ mice.
To resolve the question of whether SF1 levels are important for genetic predisposition to intestinal tumorigenesis, we examined a larger cohort of 22 Sf1+/− mice and found that most Sf1+/− mice developed no polyps, whereas a few developed between one to seven polyps (Table 2 and Table 3). Thus, both Sf1+/− and WT (Sf1+/+) mice developed few polyps, there was no significant difference between the strains (Figure 4a), and we conclude that congenitally reduced SF1 levels does not predispose to intestinal tumorigenesis.
We then tested how lowered SF1 would affect colon tumorigenesis in genetically predisposed ApcMin/+ mice. Our studies found that the number of intestinal polyps in ApcMin/+;Sf1+/− mice was significantly lower compared to that in the ApcMin/+ sibling cohort, indicating that lowered SF1 can ameliorate intestinal polyp development. Thus, one possibility regarding the previously reported azoxymethane (AOM)-induced increase in polyp development in Sf1+/− mice [28] could be due to greater propensity of AOM-induced driver mutations to persist in intestinal epithelial cells of SF1-deficient mice. Another possibility is that alternatively spliced variants of drug-metabolizing enzymes in SF1-deficient mice may alter the response of intestinal tissues to azoxymethane, thus increasing polyp development in Sf1+/− mice.
Reduced SF1 expression in the intestines of ApcMin/+;Sf1+/− (Figure 1e) was consistent with previous studies where haploinsufficient expression of SF1 was detected in mouse testes from Sf1+/− mice [29]. However, our study detected SF1 in nuclei of villi and crypts of the intestine and in ApcMin/+ adenomas (Figure 1c,d). This was different from that reported by Shitashige et al. [28] where they found lower SF1 expression in the crypts and adenomas and correlated SF1 expression with differentiation status of intestinal cells. In contrast, our study found uniform expression of SF1 in nuclei of villus, crypts and adenomas. This difference in expression could be due to the antibody type and source used in the studies. The previous study [28] used polyclonal anti-SF1 antibody (Santa Cruz, CA, USA), which is not commercially available anymore. However, the monoclonal anti-SF1 used in our studies from abcam (abcam, Cambridge, UK) showed nuclear staining for SF1 plus the correct size for SF1 in immunoblotting experiments (Figure 1e). One explanation is that the different anti-SF1 antibodies may each detect specific SF1 isoforms, as immunoblotting (Figure 1e) of human and mouse cells indicates the presence of multiple SF1 isoforms. Thus, although our study confirms nuclear expression of SF1, we cannot draw definite conclusions regarding changes in SF1 with differentiation status of intestinal cells.
When polyps with sizes of 2 mm or less were compared, fewer polyps were detected in ApcMin/+;Sf1+/− compared to that in ApcMin/+ (p < 0.003). However, the numbers of larger sized polyps (>2 mm) were not significantly different in both cohorts. One explanation of this observation is that congenital lowering of SF1 expression in intestinal cells results in decreased initiation or generation of new polyps, resulting in the reduced incidence of small polyps observed in ApcMin/+;Sf1+/− mice.
The molecular mechanism of how lower SF1 levels reduce polyp development remains to be investigated. It is known that the mRNA targets of SF1 include genes in multiple pathways, including the RAS signaling pathways, base excision repair and DNA replication pathways [8,9]. Furthermore, it has been reported that SF1, together with TCF-4 and β-catenin, promotes gene transcription [43]. This could be one mechanism of generating SF1-associated cancer splice variants. Thus, depletion of SF1 in intestinal cells could result in changes in the expression ratios of alternatively spliced isoforms, stability of oncogenic transcripts or changes in transcriptional activity, which reduce production of cancer splice variants. We expect that SF1 reduction in ApcMin/+;Sf1+/− mice likely reduces production of multiple oncogenic variants in signaling pathway components, thus ameliorating the strong driver effect of ApcMin/+. Mechanistic studies remain to be performed to determine how SF1 directly or indirectly affects ApcMin/+ or pathway components to influence polyp development.
Interestingly, females of both ApcMin/+;Sf1+/− and ApcMin/+ cohorts had higher numbers of polyps compared to males. It is known that the genetic background of mouse strains [44,45] and male hormonal levels can influence polyp incidences [46]. The status of hormonal levels has not been examined in Sf1+/− male or female mice. Furthermore, we note that both the B6 and 129 strains have a Mom1S allele with an inactivated Pla2g2a gene [44]. Because our cohorts had mixed 129/B6 genetic background, our data indicate that SF1 functions independently of the Mom1 modifier to lower polyp numbers in ApcMin/+;Sf1+/−mice. The mixed 129/B6 genetic background may also be responsible for the wide range of polyp numbers (0 to 131) observed in our cohorts and as to why females of both ApcMin/+;Sf1+/− and ApcMin/+ cohorts developed higher numbers of intestinal polyps than males. However, lower SF1 levels appears to be more important for lowering polyp incidence in females than in males because polyp numbers in ApcMin/+;Sf1+/− females were significantly decreased than in ApcMin/+ females. In humans, males have higher colon tumor incidence than females [1]. Thus, it remains to be investigated how mouse genetic background together with changes in hormonal levels due to reduction in SF1 expression and function modulate intestinal tumorigenesis in the genders.
Polyp incidences in ApcMin/+;Sf1+/− mice was decreased by 25% to 30%. This level of reduction could be dependent on mouse genetic background, and changing the genetic background of Sf1+/− mice to that B6 strain background could further increase the level of suppression of polyp multiplicity in Sf1+/−;ApcMin/+ mice.
Our previous study found lower incidence of TGCT (testicular germ cell tumors) in DND1Ter mutant mice [29] that express reduced SF1. TGCTs initiate development during embryogenesis in males and develop into visible tumors in 1-month-old male DND1Ter mice. Similarly, intestinal polyp development is initiated in relatively young ApcMin/+ mice [41,45] as polyps are detected by 4 months. Thus, congenitally lowered SF1 reduces tumor initiation in both the DND1Ter and ApcMin/+ mouse model systems of cancers that occur in young mice. Whether lower SF1 expression is effective in decreasing incidence in other cancer model systems with longer initiation periods remains to be examined.
5. Conclusions
In conclusion, our studies provide important genetic evidence that SF1 levels can attenuate intestinal cancer initiation and development due to the strong driver ApcMin/+ mutation. Further studies using human tissues and cells will be important to understand the mechanistic and cellular role of SF1 in tumorigenesis and whether lowering SF1 could be used as a preventative therapeutic measure for patients predisposed to intestinal tumorigenesis.
Table 4.
Summary of quartile values of box plots. (a) Quartile values from Figure 2 and Figure 3. (b) Quartile values from Figure 4 and Figure 5.
| (a) | |||||||||||||
| Percentile | A | AS | A 1 | AS 1 | A 2 | AS 2 | A 3 | AS 3 | A 4 | AS 4 | A 5 | AS 5 | |
| Min | 5.0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 25 | 31.0 | 22.0 | 3.0 | 2.0 | 11.5 | 5.0 | 2.0 | 1.0 | 0 | 0 | 0 | 0 | |
| 50 | 44.0 | 32.0 | 18.0 | 7.5 | 20.0 | 11.0 | 4.0 | 3.5 | 1.0 | 1.0 | 0 | 0 | |
| 75 | 67.5 | 40.8 | 31.5 | 19.8 | 27.5 | 18.5 | 9.0 | 8.0 | 2.5 | 4.0 | 2.0 | 2.5 | |
| Max | 131.0 | 92.0 | 71.0 | 57.0 | 59.0 | 42.0 | 25.0 | 28.0 | 17.0 | 13.0 | 34.0 | 12.0 | |
| (b) | |||||||||||||
| Percentile | A F | AS F | A M | AS M | A M | A F | AS M | AS F | WT | Sf1+/- | A | AS | C |
| Min | 13.0 | 5 | 5 | 0 | 5 | 13.0 | 0 | 5 | 0 | 0 | 5.0 | 0 | 0 |
| 25 | 37.0 | 30.0 | 19.8 | 17.0 | 19.8 | 37.0 | 17.0 | 30.0 | 0 | 0 | 31.0 | 22.0 | 0 |
| 50 | 55.0 | 39.0 | 34.5 | 28.0 | 34.5 | 55.0 | 28.0 | 39.0 | 0 | 0 | 44.0 | 32.0 | 0 |
| 75 | 74.0 | 56.5 | 54.8 | 33.0 | 54.8 | 74.0 | 33.0 | 56.5 | 2.0 | 1.0 | 67.5 | 40.8 | 1.0 |
| Max | 131.0 | 92.0 | 116.0 | 90.0 | 116.0 | 131.0 | 90.0 | 92.0 | 7.0 | 7.0 | 131.0 | 92.0 | 7.0 |
A 1 = A 1 mm polyp, AS 1 = AS 1 mm polyp, etc. A F = A female, A M = A male, etc. WT = Sf1+/+; C = WT plus Sf1+/−.
Acknowledgments
We thank SM Benton, J Heaney and P Ogounu for technical assistance and MA Yakubu for the HT-29 cancer cell line.
Supplementary Materials
The following are available online at https://www.mdpi.com/2079-7737/9/11/398/s1, Figure S1: Western blot images.
Author Contributions
Conceptualization, A.M.; methodology, A.M. and A.S.; software, N.L.G.G.; validation, J.D.G., S.P. and L.N.; formal analysis, A.M., J.D.G. and N.L.G.G.; resources, A.M.; data curation, J.D.G., S.P. and L.N.; writing—original draft preparation, A.M.; writing—review and editing, A.M., J.D.G., S.P. and N.L.G.G.; funding acquisition, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by NCI R21CA186033, research infrastructure support from NIMHD U54MD007605 and CPRIT RP180748.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Centers for Disease Control and Prevention CDC 24/7: Saving Lives, Protecting People. Colorectal Cancer Statistics. [(accessed on 3 October 2020)]; Available online: https://www.cdc.gov/cancer/colorectal/statistics/index.htm.
- 2.Augustus G.J., Ellis N.A. Colorectal Cancer Disparity in African Americans. Am. J. Pathol. 2018;188:291–303. doi: 10.1016/j.ajpath.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen M., Manley J.L. Mechanisms of alternative splicing regulation: Insights from molecular and genomics approaches. Nat. Rev. Mol. Cell Biol. 2009;10:741–754. doi: 10.1038/nrm2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tanackovic G., Krämer A. Human Splicing Factor SF3a, but Not SF1, Is Essential for Pre-mRNA Splicing In Vivo. Mol. Biol. Cell. 2005;16:1366–1377. doi: 10.1091/mbc.e04-11-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arning S., Grüter P., Bilbe G., Krämer A. Mammalian splicing factor SF1 is encoded by variant cDNAs and binds to RNA. RNA. 1996;2:794–810. [PMC free article] [PubMed] [Google Scholar]
- 6.Rain J.-C., Rafi Z., Rhani Z., Legrain P., Krämer A. Conservation of functional domains involved in RNA binding and protein–protein interactions in human and Saccharomyces cerevisiae pre-mRNA splicing factor SF1. RNA. 1998;4:551–565. doi: 10.1017/S1355838298980335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abovich N., Rosbash M. Cross-Intron Bridging Interactions in the Yeast Commitment Complex Are Conserved in Mammals. Cell. 1997;89:403–412. doi: 10.1016/S0092-8674(00)80221-4. [DOI] [PubMed] [Google Scholar]
- 8.Corioni M., Antih N., Tanackovic G., Zavolan M., Krämer A. Analysis of in situ pre-mRNA targets of human splicing factor SF1 reveals a function in alternative splicing. Nucleic Acids Res. 2011;39:1868–1879. doi: 10.1093/nar/gkq1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Heintz C., Doktor T.K., Lanjuin A., Escoubas C.C., Zhang Y., Weir H.J., Dutta S., Silva-García C.G., Bruun G.H., Morantte I., et al. Splicing factor 1 modulates dietary restriction and TORC1 pathway longevity in C. elegans. Nat. Cell Biol. 2017;541:102–106. doi: 10.1038/nature20789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu Z., Luyten I., Bottomley M.J., Messias A.C., Houngninou-Molango S., Sprangers R., Zanier K., Krämer A., Sattler M. Structural Basis for Recognition of the Intron Branch Site RNA by Splicing Factor 1. Science. 2001;294:1098–1102. doi: 10.1126/science.1064719. [DOI] [PubMed] [Google Scholar]
- 11.Selenko P., Gregorovic G., Sprangers R., Stier G., Rhani Z., Kraemer A., Sattler M. Structural basis for the molecular recognition between human splicing factors U2AF65 and SF1/mBBP. Mol. Cell. 2003;11:965–976. doi: 10.1016/S1097-2765(03)00115-1. [DOI] [PubMed] [Google Scholar]
- 12.Jamison S.F., Crow A., A Garcia-Blanco M. The spliceosome assembly pathway in mammalian extracts. Mol. Cell. Biol. 1992;12:4279–4287. doi: 10.1128/MCB.12.10.4279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kent O.A., Ritchie D.B., Macmillan A.M. Characterization of a U2AF-Independent Commitment Complex (E′) in the Mammalian Spliceosome Assembly Pathway. Mol. Cell. Biol. 2005;25:233–240. doi: 10.1128/MCB.25.1.233-240.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Das R., Reed R. Resolution of the mammalian E complex and the ATP-dependent spliceosomal complexes on native agarose mini-gels. RNA. 1999;5:1504–1508. doi: 10.1017/S1355838299991501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Michaud S., Reed R. An ATP-independent complex commits pre-mRNA to the mammalian spliceosome assembly pathway. Genes Dev. 1991;5:2534–2546. doi: 10.1101/gad.5.12b.2534. [DOI] [PubMed] [Google Scholar]
- 16.Berglund J., Chua K., Abovich N., Reed R., Rosbash M. The Splicing Factor BBP Interacts Specifically with the Pre-mRNA Branchpoint Sequence UACUAAC. Cell. 1997;89:781–787. doi: 10.1016/S0092-8674(00)80261-5. [DOI] [PubMed] [Google Scholar]
- 17.Kramer A. Purification of splicing factor SF1, a heat-stable protein that functions in the assembly of a presplicing complex. Mol. Cell. Biol. 1992;12:4545–4552. doi: 10.1128/MCB.12.10.4545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berglund J.A., Abovich N., Rosbash M. A cooperative interaction between U2AF65 and mBBP/SF1 facilitates branchpoint region recognition. Genes Dev. 1998;12:858–867. doi: 10.1101/gad.12.6.858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang G.-S., Cooper T.A. Splicing in disease: Disruption of the splicing code and the decoding machinery. Nat. Rev. Genet. 2007;8:749–761. doi: 10.1038/nrg2164. [DOI] [PubMed] [Google Scholar]
- 20.Deschênes M., Chabot B. The emerging role of alternative splicing in senescence and aging. Aging Cell. 2017;16:918–933. doi: 10.1111/acel.12646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee B.P., Pilling L.C., Emond F., Flurkey K., Harrison D.E., Yuan R., Peters L.L., Kuchel G.A., Ferrucci L., Melzer D., et al. Changes in the expression of splicing factor transcripts and variations in alternative splicing are associated with lifespan in mice and humans. Aging Cell. 2016;15:903–913. doi: 10.1111/acel.12499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Srebrow A. The connection between splicing and cancer. J. Cell Sci. 2006;119:2635–2641. doi: 10.1242/jcs.03053. [DOI] [PubMed] [Google Scholar]
- 23.Skotheim R.I., Nees M. Alternative splicing in cancer: Noise, functional, or systematic? Int. J. Biochem. Cell Biol. 2007;39:1432–1449. doi: 10.1016/j.biocel.2007.02.016. [DOI] [PubMed] [Google Scholar]
- 24.Kalnina Z., Zayakin P., Silina K., Linē A. Alterations of pre-mRNA splicing in cancer. Genes Chromosom. Cancer. 2005;42:342–357. doi: 10.1002/gcc.20156. [DOI] [PubMed] [Google Scholar]
- 25.Venables J.P. Aberrant and Alternative Splicing in Cancer. Cancer Res. 2004;64:7647–7654. doi: 10.1158/0008-5472.CAN-04-1910. [DOI] [PubMed] [Google Scholar]
- 26.Kim E., Goren A., Ast G. Insights into the connection between cancer and alternative splicing. Trends Genet. 2008;24:7–10. doi: 10.1016/j.tig.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie W., Granjeaud S., Puthier D., Gautheret D. Entropy Measures Quantify Global Splicing Disorders in Cancer. PLoS Comput. Biol. 2008;4:e1000011. doi: 10.1371/journal.pcbi.1000011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shitashige M., Satow R., Honda K., Ono M., Hirohashi S., Yamada T. Increased susceptibility of Sf1+/- mice to azoxymethane-induced colon tumorigenesis. Cancer Sci. 2007;98:1862–1867. doi: 10.1111/j.1349-7006.2007.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhu R., Heaney J., Nadeau J.H., Ali S., Matin A. Deficiency of Splicing Factor 1 Suppresses the Occurrence of Testicular Germ Cell Tumors. Cancer Res. 2010;70:7264–7272. doi: 10.1158/0008-5472.CAN-10-0820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M., et al. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991;66:589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- 31.Kinzler K.W., Nilbert M.C., Su L.K., Vogelstein B., Bryan T.M., Levy D.B., Smith K.J., Preisinger A.C., Hedge P., McKechnie D., et al. Identification of FAP locus genes from chromosome 5q21. Science. 1991;253:661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- 32.Kinzler K.W., Vogelstein B. Lessons from Hereditary Colorectal Cancer. Cell. 1996;87:159–170. doi: 10.1016/S0092-8674(00)81333-1. [DOI] [PubMed] [Google Scholar]
- 33.Moser A.R., Pitot H.C., Dove W.F. A dominant mutation that predisposes to multiple intestinal neoplasia in the mouse. Science. 1990;247:322–324. doi: 10.1126/science.2296722. [DOI] [PubMed] [Google Scholar]
- 34.Halberg R., Katzung D., Hoff P., Moser A., Cole C., Lubet R., Donehower L., Jacoby R., Dove W. Tumorigenesis in the multiple intestinal neoplasia mouse: Redundancy of negative regulators and specificity of modifiers. Proc. Natl. Acad. Sci. USA. 2000;97:3461–3466. doi: 10.1073/pnas.97.7.3461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cho N., Javid S., Carothers A., Redston M., Bertagnolli M. Estrogen receptors alpha and beta are inhibitory modifiers of Apc dependent tumorigenesis in the proximal colon of Min/+ mice. Cancer Res. 2007;67:2366–2372. doi: 10.1158/0008-5472.CAN-06-3026. [DOI] [PubMed] [Google Scholar]
- 36.Cormier R., Dove W.F. Dnmt1 Reduces the Net Growth Rate and Multiplicity of Intestinal Adenomas in C57BL/6-Multiple Intestinal Neoplasia (Min)/+ Mice Independently of p53 but Demonstrates Strong Synergy with the Modifier of Min 1 Resistance Allele. Cancer Res. 2000;60:3965–3970. [PubMed] [Google Scholar]
- 37.McCart A.E., Vickaryous N.K., Silver A. Apc mice: Models, modifiers and mutants. Pathol. Res. Pract. 2008;204:479–490. doi: 10.1016/j.prp.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Dietrich W. Genetic identification of Mom-1, a major modifier locus affecting Min-induced intestinal neoplasia in the mouse. Cell. 1993;75:631–639. doi: 10.1016/0092-8674(93)90484-8. [DOI] [PubMed] [Google Scholar]
- 39.Nnadi S.C., Watson R., Innocent J., Gonye G.E., Buchberg A.M., Siracusa L.D. Identification of five novel modifier loci of ApcMin harbored in the BXH14 recombinant inbred strain. Carcinogenesis. 2012;33:1589–1597. doi: 10.1093/carcin/bgs185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ju J., Hong J., Zhou J.-N., Pan Z., Bose M., Liao J., Yang G.-Y., Liu Y.Y., Hou Z., Lin Y., et al. Inhibition of Intestinal Tumorigenesis in Apcmin/+ Mice by (−)-Epigallocatechin-3-Gallate, the Major Catechin in Green Tea. Cancer Res. 2005;65:10623–10631. doi: 10.1158/0008-5472.CAN-05-1949. [DOI] [PubMed] [Google Scholar]
- 41.Nalbantoglu I., Blanc V., Davidson N.O. Characterization of Colorectal Cancer Development in Apcmin/+ Mice. Antiviral RNAi. 2016;1422:309–327. doi: 10.1007/978-1-4939-3603-8_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gibbons J., Chakraborti S. Nonparametric Statistical Inference, Fourth Edition, Revised and Expanded. Marcel Dekker, Inc.; New York, NY, USA: 2003. [Google Scholar]
- 43.Shitashige M., Naishiro Y., Idogawa M., Honda K., Ono M., Hirohashi S., Yamada T. Involvement of Splicing Factor-1 in β-Catenin/T-cell factor-4-mediated gene transactivation and pre-mRNA splicing. Gastroenterology. 2007;132:1039–1054. doi: 10.1053/j.gastro.2007.01.007. [DOI] [PubMed] [Google Scholar]
- 44.Gould K.A., Dove W.F. Analysis of the Mom1 modifier of intestinal neoplasia in mice. Exp. Lung Res. 1998;24:437–453. doi: 10.3109/01902149809087379. [DOI] [PubMed] [Google Scholar]
- 45.Bilger A., Shoemaker A.R., Gould K.A., Dove W.F. Manipulation of the mouse germline in the study ofMin-induced neoplasia. Semin. Cancer Biol. 1996;7:249–260. doi: 10.1006/scbi.1996.0033. [DOI] [PubMed] [Google Scholar]
- 46.Amos-Landgraf J.M., Heijmans J., Wielenga M.C.B., Dunkin E., Krentz K.J., Clipson L., Ederveen A.G., Groothuis P.G., Mosselman S., Muncan V., et al. Sex disparity in colonic adenomagenesis involves promotion by male hormones, not protection by female hormones. Proc. Natl. Acad. Sci. USA. 2014;111:16514–16519. doi: 10.1073/pnas.1323064111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.