Abstract
Mitochondria are the powerhouses of the cell, whilst their malfunction is related to several human pathologies, including neurodegenerative diseases, cardiovascular diseases, and various types of cancer. In mitochondrial metabolism, cytochrome c is a small soluble heme protein that acts as an essential redox carrier in the respiratory electron transport chain. However, cytochrome c is likewise an essential protein in the cytoplasm acting as an activator of programmed cell death. Such a dual role of cytochrome c in cell life and death is indeed fine-regulated by a wide variety of protein post-translational modifications. In this work, we show how these modifications can alter cytochrome c structure and functionality, thus emerging as a control mechanism of cell metabolism but also as a key element in development and prevention of pathologies.
Keywords: cytochrome c, mitochondrial diseases, post-translational modifications
1. Introduction
Mitochondria are the powerhouses of the cell. Their dysfunction favors the development of neurodegenerative diseases, cardiovascular pathologies, and several types of cancer [1]. A key protein in mitochondrial metabolism and control of redox signaling is cytochrome c (Cc) [2]. Cc is a small soluble protein (ca. 12 kDa, 104 amino acids) with four α-helices and a heme group, which is covalently bound by two cysteine residues (Cys14 and Cys17). The heme group is wrapped in a hydrophobic crevice, being only slightly exposed to the solvent. This conformation allows Cc to efficiently exchange electrons with its redox partners. Several residues of Cc undergo post-translational modifications, which provide a fine-tuned regulation at the functional level [3]. In the absence of stimuli, Cc is located in the mitochondrial intermembrane space, participating in redox metabolism [4]. However, several stimuli—such as apoptotic or DNA damage signals—promote Cc release from mitochondria. The first extramitochondrial target reported for Cc was Apoptosis protease-activating factor-1 (Apaf-1) [5]. The interaction of Cc with Apaf-1 in the cytosol is an early event of the mitochondrial apoptotic pathway [3]. Actually, the mitochondrial and extramitochondrial network of Cc encompasses a variety of proteins that are located in different organelles and cytosol [3,6,7]. These interactions can modulate the cell fate decision between cell life and death [7], which in turn relate to health and disease. The objective of this review is to carry out an in-depth analysis of how Cc modifications affect its functions and their impact on the development of certain diseases and pathologies.
2. The Pleiotropic Role of Cytochrome c in Cell Homeostasis and Diseases
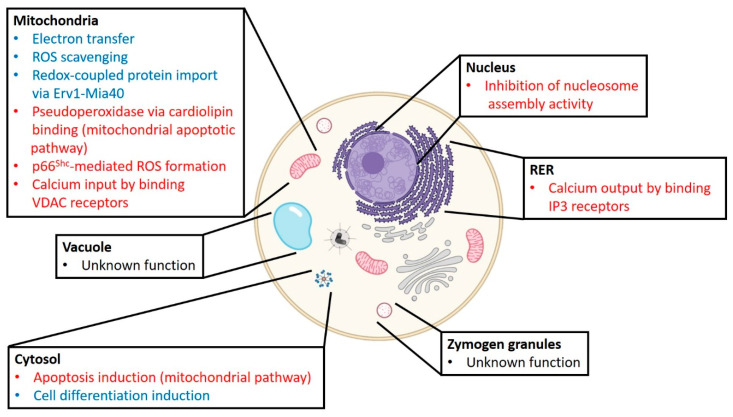
Cc is a moonlighting protein whose localization and functions depend on cellular conditions (Figure 1) [3,6,7]. Under homeostasis, it is a key component of cellular energy metabolism, acting as an electron carrier between the cytochrome bc1 complex (complex III, CIII) and cytochrome c oxidase (complex III, CIV) in the mitochondrial electron transport chain (ETC) [4]. In this context, two binding sites have been described in cytochrome c1 (Cc1; from CIII) and CIV for Cc: a proximal site, optimal for electron transfer, and a distal site, which is not productive in terms of electronic transfer. It has been postulated that the interaction at the distal site could have physiological relevance in the dynamics and organization of electronic flow. It also seems to increase the local concentration of Cc available near the proximal site, allowing a rapid turnover of Cc molecules [8,9]. Recently, the molecular basis of electron transfer from Cc1 in CIII to Cc has been described [10,11]. According to the data, the coupling of redox potential shifts the conformational cycle of the Rieske subunit and the binding of Cc to Cc1 causes electrons to flow in a single direction [11]. Cc has other redox functions, acting as a reactive oxygen species (ROS) scavenger and participating in the import of redox-coupled cysteine-rich proteins via Erv1-Mia40 [12,13,14]. The heme protein is also localized in vacuoles and zymogen granules during cell homeostasis, although its role there is unknown [15].
Figure 1.
Cell localization and functions of cytochrome c. Cc is located in the mitochondrial intermembrane space, vacuole and zymogen granules under homeostasis. However, during DNA damage and apoptosis stimuli, mitochondrial Cc travels into the nucleus, the rough endoplasmic reticulum (RER) and the cytoplasm, respectively. The most relevant functions performed by Cc in each location are explained in the boxes. Color key: blue, physiological functions; red, functions performed under stress; and black, unknown functions. Created with BioRender.com (https://biorender.com/).
Under nitro-oxidative stress conditions, Cc contributes to ROS production via the p66 redox cycle and acts as an inducer of programmed cell death (PCD) (Figure 1) [16]. Alterations in PCD signaling are relevant in many diseases [17,18]. Recent data indicates that the apoptotic network involving Cc is complex, and several target proteins are functionally equivalent in humans, plants, and Drosophila melanogaster [19,20,21,22,23,24,25]. At the onset of apoptosis, a Cc population—tightly bound to the inner mitochondrial membrane—catalyzes peroxidation of phospholipids, particularly cardiolipin (CL) (Figure 1). The nature of the Cc/CL interaction is subject of intense research [26]. Some authors proposed that CL-adducted Cc undergoes a profound tertiary conformational rearrangement that opens an entry channel for H2O2 molecules, enhancing the peroxidase activity of Cc [5,27]. Extensive data in the literature hints that the interaction with the CL affects the dynamics and conformation of a set of loops that determine the heme environment and iron coordination of Cc. In particular, the bond between the Sδ atom of Met80 and iron can be disrupted, leading to high spin penta-coordinated species resembling that described for myoglobin [28]. It has been proposed that Cc/CL conjugates are sufficient for the formation of mitochondrial pores, which allow the release of the hemeprotein into the cytosol during apoptosis and redistribution of CL from the inner to the outer mitochondrial membrane [29,30]. In the early stages of apoptosis, Cc interacts with the inositol 1,4,5-trisphosphate (IP3) receptor in the rough endoplasmic reticulum (RER) and with the voltage-dependent anion channel (VDAC) in the outer mitochondrial membrane. These interactions, in turn, promote calcium output and input, respectively (Figure 1) [31,32,33]. This traffic of calcium ions is restricted in space since both the IP3 receptors and the VDAC are located in mitochondria-associated endoplasmic reticulum membranes (MAMs) [34,35]. MAMs are microdomains involving both RER and mitochondrial proteins. These interconnection zones have fundamental roles in the transmission of calcium ions but are closely related to cellular lipid and energy metabolism [36,37]. The interaction of Cc with the IP3 receptor prevents its calcium-induced inhibition, triggering the release of calcium [38]. Calcium binds to VDAC dimers, resulting in oligomerization [39]. This event increases the conductance of the channel and allows the release of Cc through it [40,41,42]. It is worth mentioning that the release of Cc into the cytosol may not always lead to cell death, and the heme protein can sometimes act as a cell differentiation inductor (Figure 1) [20,43]. Cc also reaches the cell nucleus upon DNA damage where it sequesters histone chaperones and inhibits chromatin remodeling (Figure 1) [23,24]. The pleiotropy of the effects of extramitochondrial Cc can be modulated by the cytosolic diflavin reductase NDOR1, which has NADPH-dependent Cc reductase activity [44].
3. Post-Translational Modifications of Cytochrome c: Regulation, Functionality, and Structural Changes
Post-translational modifications (PTMs) in proteins are regulatory mechanisms which control an ample set of cell metabolic processes and provide a tool to increase the functional diversity of proteins [45,46,47]. Indeed, PTMs play an integral role in regulating Cc functions [48,49]. The heme protein can undergo different PTMs (see below) that affect its physicochemical properties, interactions with physiological partners and, consequently, its functions. However, these effects depend on which residues are post-translationally modified.
3.1. Phosphorylation
The functionality of Cc is controlled in vivo by phosphorylation of the following residues: Thr28, Ser47, Tyr48, Thr58, and Tyr97 [50,51,52,53,54,55]. Since Thr58 is replaced by isoleucine in humans, this position will not be a subject of this review. The low yield of phosphorylated Cc during purification procedures makes functional and structural analysis challenging. Furthermore, the specific kinases responsible for phosphorylating the protein remain unknown—although some authors suggest that it could be the AMP kinase [53]. Therefore, it is common to mimic targeted phosphorylation with site-directed mutations that encode canonical or non-canonical (AMBER-type stop codons) substitutions. The evolved tRNA synthetase technique allows the site-specific incorporation of a non-canonical amino acid into the protein sequence [56]. The method is based on the use of an orthogonal aminoacyl-tRNA synthetases/tRNA pair. The aminoacyl-tRNA synthetase identifies the non-canonical amino acid and charges it in the tRNA. This is a special tRNA that recognizes AMBER-type stop codons, allowing the introduction of the residue into the desired position. The evolved tRNA synthetase technique can be used for the incorporation of non-canonical amino acids into proteins being produced in both prokaryotic and eukaryotic cells [57,58,59].
The use of non-canonical amino acids aims at a better emulating the physical properties of the target residues—e.g., the volume and/or charge—as compared to canonical substitutions (Table S1) [60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77]. A good example is the non-natural residue p-carboxymethyl-L-phenylalanine (pCMF), which has been widely used to mimic tyrosine phosphorylation [77]. Non-canonical amino acids are indeed resistant to hydrolysis, making them ideal candidates for use in pharmacological therapy (see below).
The structural and functional effects of phosphorylations at positions 28 and 47 have been studied using samples of Cc both, phosphorylated in vivo and via the use of phosphomimetic species. The isolated Cc in vivo phosphorylated at positions 28 and 47 donates electrons to CIV less efficiently than the wild-type species [53,55]. Phosphomimetic species at Thr28 show enhanced peroxidase activity at a low Cc/CL ratio [52,77]. This function correlates with the ability of Cc to be translocated from the mitochondria into the cytoplasm, where it triggers apoptosis. In addition, mutations at Ser47 impairs Cc–mediated caspase cascade activation, which is an essential step proceeding programmed cell death (Table 1) [55,78].
Table 1.
Post-translational modifications of cytochrome c reported in the literature.
| Modification | Sites | Effects | References |
|---|---|---|---|
| Phosphorylation | Thr28, Tyr46, Ser47, Tyr48, Thr58 *, Tyr74, Tyr97 | Increase of Cc peroxidase activity. Decrease of electron transfer efficiency (Thr28, Ser47 and Tyr48). Increase of electron transfer efficiency under supercomplex formation (Tyr97). Modification of redox potential (Tyr48). Inhibition of caspase activation. |
[53,55,77,78,79,80,81,82] |
| Nitration | Tyr46, Tyr48, Tyr67, Tyr74, Tyr97 (only nitration of Tyr74 and Tyr67 are detected in vivo) | Proteolytic degradation (Y46 and Y48). Increase of peroxidase activity (Y46, Y48 and Y74). Inhibition of caspase activation. |
[83,84,85,86,87] |
| Nitrosylation | Heme and Met80 | Inhibition of Cc/CL complex peroxidase activity. Changes in protein conformation and heme coordination |
[88,89] |
| Acetylation | Lysines (only acetylation of Lys8 and Lys53 are detected in vivo) | Decrease of electron transfer efficiency in the respiratory chain. Changes in the protein configuration. Inhibition of caspase activation |
[90,91,92,93,94,95,96] |
| Glycosylation | Lysines 1 | Down-regulation of proteolytic degradation. Enhancement of thermodynamic stability. Inhibition of caspase activation. |
[97,98] |
| Glycation | Arg91, Lys72, Lys87, Arg92 | Monomer aggregation. Reduction of conformational stability. Decrease of electron transfer efficiency in the respiratory chain. Decrease of ability to bind membrane. Enhance peroxidase activity. |
[99,100,101,102] |
| Deamidation | Gln42, Asn31, Asn52 and Asn70 | Conformational changes. Modification of redox potential. |
[103,104] |
| Sulfoxidation | Met80 | Loss of autoxidizable function. Decrease of electron transfer efficiency in the respiratory chain. Enhance peroxidase activity. Increase of apoptosis induction. |
[105,106,107,108,109,110] |
| Homocysteinylation | Lys8 or Lys13, Lys86 or Lys87, Lys99, and Lys100 | Protein denaturation. Increase of resistance to proteolysis. Protein aggregation. Enhancement of peroxidase activity. |
[111,112,113,114,115] |
| Carbonylation | Lys53, Lys55, Lys60 †, Lys72/Lys73 |
Enhancement of peroxidase activity. Impairment of CL binding. Protein aggregation. |
[116,117,118] |
* Thr58 is replaced by isoleucine in human Cc, so its effects are not objective of this study. † Lys60 is replaced by glycine in human Cc, so its effects are not objective of this study. 1 Unidentified specific modification residues.
In relation to tyrosine phosphorylation, the presence of negative charges at position 48 affects the redox properties of Cc and lowers the pKa value of the alkaline transition towards physiological pH values [77,79,80]. In turn, in vivo phosphorylated Cc and its phosphomimetics at position 48 diminish oxygen consumption [51,79,81]. In fact, during supercomplex formation the Y48pCMF mutant shows decreased electron transfer to CIV. This finding was attributed to the lower binding affinity of the aforementioned mutant for the distal sites of Cc1 (in CIII) and CIV. These changes in binding equilibria alter the preferential diffusion pathway that channels Cc molecules through the CIII/CIV supercomplex [81]. On the other hand, the phosphomimetic species display a higher ROS scavenger activity and a more efficient peroxidase activity when binding to CL [79,80,81]. Phosphorylation also alters the apoptotic function of Cc by inhibiting its ability to activate the caspase cascade [79,80,81]. The mutant Y97pCMF Cc likewise displays decreased electron transfer to CIV in the context of supercomplex formation, despite no substantial changes in Cc peroxidase activity. However, Y97pCMF Cc acts as an inefficient caspase activator [82].
3.2. Nitration and Nitrosylation
The effects of tyrosine nitration and nitrosylation in Cc have been studied extensively due to their roles in cell metabolism under stress [85,86,87,119,120,121,122]. Nitric oxide (NO) is a signaling molecule with pleiotropic effects. A significant number of these effects are exerted through PTMs mediated by nitric oxide-derived reactive nitrogen species (RNS) under physiological and stress conditions [123]. Nitration and nitrosylation consist of non-enzymatic covalent modifications that introduce a nitro group (-NO2) at the phenolic ring of tyrosine or a nitrosyl group (-NO) at the thiol group of cysteine residues, respectively. Cc has no free cysteines—Cys14 and Cys17 are covalently linked to the heme group—but the Met80 axial ligand and/or heme Fe atom are susceptible to nitrosylation [124]. -NO2 and -NO primarily originate from RNS, such as peroxynitrite [119,125]. However, recent studies have shown that these modifications can be produced by diffusion of the NO radical into the mitochondria [126].
Nitration of Cc tyrosine residues 46 and 48 has not been observed in vivo. In fact, Díaz-Moreno and co-workers showed that the nitration of these residues enhances proteolytic degradation of Cc by cell extracts [83]. In vitro assays demonstrate that nitration of any tyrosine from Cc increases its peroxidase activity and impairs membrane potential formation [84]. The impact on the redox properties of Cc increases with the number of nitrated tyrosines [126,127]. This explains why electron transfer increases when Cc is doubly nitrated [128,129] (Table 1). Notably, nitration at Tyr46, Tyr48, and Tyr74 decreases the pKa value of Cc alkaline transition [83,86].
On the other hand, nitrosylation of Cc induces changes in protein conformation and heme configuration/coordination [89]. Such effects on redox Cc properties inhibit its peroxidase activity and enhance its ability to activate the caspase cascade. For this reason, it has been proposed that Cc nitrosylation is a proapoptotic modification [88,89,124].
3.3. Acetylation
Cc acetylation has been studied for decades after Minakami et al. first explored the properties of acetylated Cc [91]. Acetylation of lysine residues is an enzymatic process carried out by lysine acetyltransferases [130]. However, it has been observed that in some cases the acetylation process takes place via a non-enzymatic mechanism in the mitochondrial matrix, where the transfer of an acetyl group is promoted by high concentrations of acetyl-CoA and an alkaline pH [92].
In general, the acetylation of lysine residues results in a decrease of Cc-mediated electron transfer in the respiratory chain [90], as the rate of Cc reduction/oxidation is greatly reduced [91]. The effect on oxidation-reduction efficiency is explained by the loss of the positively charged lysine residues 72, 73 and 79 which would induce changes in the heme environment (Table 1) [93]. Conservation of the environment is crucial in preserving Cc redox stability [91]. Likewise, changes in the pI value also influence the interaction of Cc with CIII and CIV. Lower pI values prevent these interactions, which in turn impact on the electron transport chain [94]. This downregulation of mitochondrial electron flow causes the “Warburg effect” (cell respiration inhibition) [96]. The effect of net charge decrease is less pronounced on the native structure when the acetylated groups are located on the protein surface [92]. However, it was found that acetylation of more than six lysine residues resulted in a decrease in the overall positive charge of the protein, which plays a key role in the interaction between Cc and CIV, leading to a complete loss of the ability of Cc to transfer electrons to CIV [92]. Moreover, Korshunov et al. showed that in vitro acetylation of horse heart Cc prevents ROS scavenger activity [94]. Only acetylation of Lys8 and Lys53 in mammalian Cc has been described in vivo [96,131]. Although Cc acetylation has been studied for decades, scope of this PTM and its cellular impact remains unknown, leaving the door open for further investigations [48].
3.4. Glycosylation and Glycations
Recent studies have elucidated the main features of chemical Cc glycosylation, which comprises addition of a carbohydrate moiety to a protein molecule. Méndez et al. have studied the effect of equine heart Cc glycosylation via an activated lactose, finding improved thermodynamic and colloidal stability of the glycosylated Cc [97] which was resistant to protein denaturation or unfolding [132]. Glycosylation of Cc also protects the protein surface due to the shielding effect of the glycan, which decreases the proteolytic degradation process carried out by proteases. It has also been demonstrated that that glycosylation of lysine residues leads to some minor perturbations in protein tertiary structure, whose integrity is crucial for apoptosis induction [98].
Whereas Cc glycosylation is an enzyme-directed mechanism, glycation is a non-enzymatic chemical process [133]. The in vitro glycation of Arg91 in horse heart Cc induces conformational changes in the protein structure due to an increase in the α-helical content which alters the secondary structure and, therefore, protein folding [99]. Cc glycation leads to an aggregation process through monomer addition, driven by the exposition of new hydrophobic segments to the solvent [100].
Other Cc glycation target residues include Lys72 and Lys87/88, which are located in a cationic patch involved in the association of Cc with CL-containing membranes [101]. The glycation of these residues lowers the positive charge of the protein, hindering the interaction between Cc and the CL membrane. The effect of Cc glycation on the electron transfer chain highlights the importance of lysine residues on the ability of heme protein to interact with its partners [12,134,135,136].
In fact, a recent study showed that glycation of bovine heart Cc by glyoxal caused conformational alteration in protein structure [102]. This alteration resulted in perturbation of tertiary structural interactions induced by the formation of a penta-coordinated structure that may also promote the reduction of heme, altering the redox state of Cc. Godoy et al. have shown that it also occurs in a Cc M80A mutant, in which the methionine ligand is replaced with an alanine residue, disrupting the Met80-Fe interaction [137]. Moreover, Cc glycation activates peroxidase activity, which is a crucial step in the release of Cc from mitochondria [102].
3.5. Deamidations
Mammalian Cc deamidation was discovered by Flatmark in 1964 as a result of Cc fractions with different electrophoresis mobility [138]. Cc exposed to different pH and temperature values leads to the deamidation at glutamine and/or asparagine residues in consecutive steps [103,139]. These residues are involved in the maintenance of Cc native structure. In particular, residues Asn31 and Gln42, Asn52 and Asn70 have a structural and/or functional role [104], thus these modifications affect the biological activity of Cc (Table 1).
Cc deamidation is a nonenzymatic process [140]. However, as mammalian tissues are rich in hydrolyze-amide enzymes, Flatmark’s work suggested that the in vivo deamidation of Cc takes place by an enzymatic mechanism [141].
3.6. Sulfoxidation
Sulfoxidation is a PTM involving oxidation of a sulfur group on a methionine residue. Generally, this is Met80 in Cc [108]. The key role of Met80 is to coordinate the heme center together with His18 and covalently-bound Cys14 and Cys17, maintaining a packed and hydrophobic environment for the heme iron that ensures a suitable redox potential for Cc biological functions [109]. Oxidation of Met80 to methionine sulfoxide opens the heme coordination pocket [137], altering the protein configuration. Freeing the heme coordination could facilitate the interaction between the iron atom and lysine residues, changing the pKa value of the alkaline transition. This change in protein configuration enables the iron atom to interact with ligands such as NO, CO, O2, and H2O2 [30,125]. This interaction likely disrupts the electron transport chain and restrains energy transduction in mitochondria [110].
Sulfoxidation of Met80 also enhances the peroxidase activity of Cc [107], causing the simultaneous reduction of H2O2 and oxidization of associated CL. This diminishes electron transport as it increases heme center accessibility [105]. In fact, Rouco et al. have recently measured the binding affinity between sulfoxidized Cc and CL, finding an increase of ca. 4 times in affinity in comparison to WT Cc [107]. This finding would explain the enhancement of peroxidase activity shown by the sulfoxidized species. Moreover, Yin et al. have shown that Met80 sulfoxidation followed by lysine carbonylation results in an even greater Cc peroxidase activity when compared to the native protein (see below) [117]. Finally, the Gly41Ser mutation renders Cc more susceptible to Met80 oxidation [142].
3.7. Homocysteinylation
According to the literature, homocysteinylation of Cc results from high homocysteine levels in the cell, known as hyperhomocysteinemia [111]. Homocysteine residues bind to the amino group of lysines via amide bonds. In vitro, this PTM is reproduced using homocysteine-tiolactone which reacts spontaneously with free amino groups. The homocysteinylation of lysine amino groups results in a change in the protein surface charge due to the incorporation of homocysteine, which is a less basic amino group. This modification leads to slight changes in the secondary structure which affect the fraction of α-helices [112]. These changes expose some thiol groups, thereby leading to spontaneous formation of protein multimers by intermolecular disulfide bonds [115]. Moreover, the homocysteinylation causes a change in the redox state as a consequence of a homocysteine thiol group incorporation, making the reduced species more thermodynamically stable than the oxidized one and, consequently, homocysteinylated Cc is more resistant to proteolysis by pronase, trypsin, and chymotrypsin than the non-modified protein [143].
Since lysines are involved in the interaction of Cc with CIV and Cc1 (from CIII), the homocysteinylation of lysine residues could also affect to the electron transport chain [11,113,134,135,136].
As with sulfoxidation, Sharma et al. found that homocysteinylation by homocysteine tiolactone also confers conformational changes in Cc which disrupt the heme-Met80 interaction and, consequently, activates Cc peroxidase activity [114,144], as previously described for the M80A Cc mutant [137].
3.8. Carbonylation
The accumulation of carbonylated proteins has been implicated in cell aging and certain neurodegenerative diseases [145,146]. Carbonylation is an oxidative modification that affects the amino group of lysine residues and is often used as a measure of oxidative damage. Several authors have verified that the carbonylation of Cc is a consequence of oxidation at other residues, such as Met65/Met80 and Tyr67 or Tyr74, which act as co-activators of lysine carbonylation [12,13,14,147]. Lysine residues are important in the configuration of Cc. Carbonylation of the Lys72/Lys73 pair impairs its ability to act as heme ligand, thereby generating a free distal site—as it takes place when the Met ligand is already oxidized—that facilitates the entry of peroxide molecules, increasing the peroxidase activity of the hemeprotein [116]. Moreover, the decrease in the positive net charge of Cc impairs its ability to bind CL. Both hallmarks—enhanced peroxidase activity and impaired CL-containing adduct assembly—contribute to the release of Cc from mitochondria [118]. Unfortunately, specific consequences of Cc carbonylation on the electron transfer reaction have not been reported in the literature yet.
4. Conservation and Evolution of Cytochrome c Residues
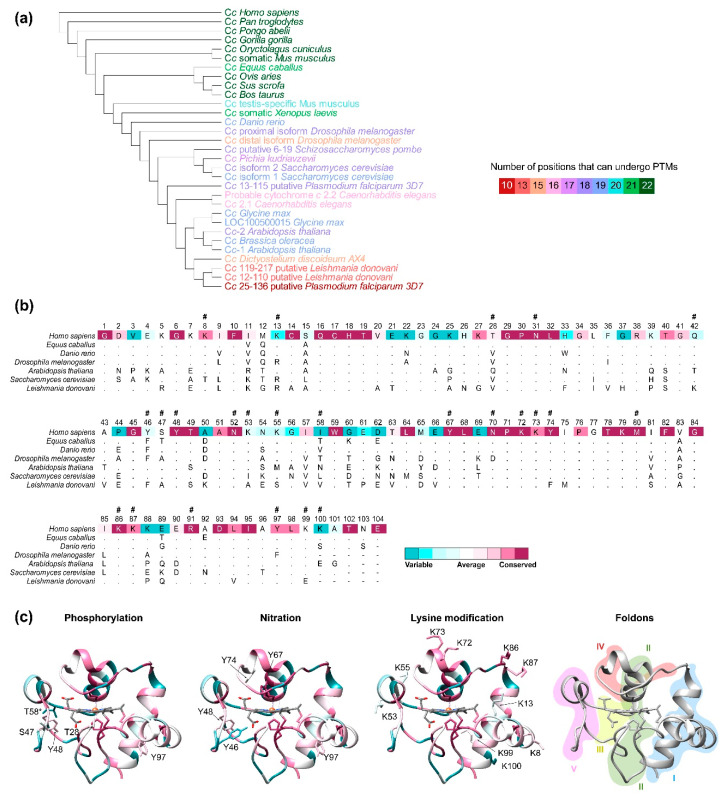
Cc is a highly conserved protein throughout evolution in terms of functionality. Zaidi and co-workers analyzed the conservation of Cc-related proteins over 285 unique sequences [148]. Their analysis showed that majority of Cc-related proteins have a length of 104 amino acids, but only 14% of their residues are conserved along the phylogenetic tree. Given that PTMs finely regulate the functions of Cc, we aim to analyze whether these conserved residues are targeted for modifications. To achieve this, 31 different respiratory Cc sequences spanning organisms from five life kingdoms, including model organisms, were analyzed (Figure 2a). Model organisms’ sequences were selected from the landmark BLAST database and sequences of the relevant organisms were manually selected from UniProt database. The analysis revealed that 34% of the residues are highly conserved among the analyzed sequences (Figure 2b). Notably, the number of positions susceptible of PTMs was greater in mammals and closely related species (Figure 2a). Cc shows a folding model governed by five regions—named foldons—that differ in their stability (Figure 2c) [149,150]. The most stable region is foldon I following by foldon II, the neck or foldon III, the main Ω-loop (foldon IV) and the less stable region, the nested Ω-loop (foldon V). If we focus on the residues that undergo most of the human Cc PTMs—phosphorylation and nitration of tyrosines, and modifications of lysine residues (Table 1)—we find a correlation with their position and foldon stability (Figure 2c). As previously mentioned, lysine residues play a fundamental role in the interactions of Cc with its physiological targets, constituting positive patches. Actually, conserved lysine residues undergoing PTMs cluster at the most stable foldon of the protein. The resulting positive surface patch participates in a variety of protein-protein interactions [2,8,9,23,24]. On the other hand, phosphorylated and nitrated residues are mainly in foldons IV and V. The dynamic/flexibility that shows these foldons are substantially affected by PTMs [77,81], modulating the access to heme crevice, which is essential for redox function of Cc. Other highly conserved residues take part of the heme environment (Cys14, Cys17, Lys72, Lys73, Lys79, and Met80). However, only residues providing the sixth ligand—Met 80 during physiological condition and Lys72, Lys73, or Lys 79 under alkaline transition phenomenon—undergo modifications. This highlights the relevance of the cofactor environment as regards the function of proteins implicated in the redox metabolism [151,152,153,154,155,156,157]. In summary, modulation of Cc functionality by PTMs is a key factor influencing the evolution of the protein.
Figure 2.
Cytochrome c sequence evolutionary conservation and post-translational modifications. (a) Evolutionary analysis by Maximum Likelihood method. The evolutionary history was inferred by using the Maximum Likelihood method and General Reversible Mitochondrial + Freq. model [158]. The bootstrap consensus tree inferred from 500 replicates [159] represents the evolutionary history of the taxa analyzed [160]. Branches corresponding to partitions reproduced in less than 50% bootstrap replicates are collapsed. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (500 replicates) are shown next to the branches [160]. Initial tree(s) for the heuristic search were obtained automatically by applying Neighbor-Join and BioNJ algorithms to a matrix of pairwise distances estimated using the JTT model, and then selecting the topology with superior log likelihood value. This analysis involved 31 amino acid sequences. There was a total of 122 positions in the final dataset. Evolutionary analyses were conducted in MEGA X [161]. Labels are colored as a function of the number of amino acid positions that can undergo post-translational modifications (PTMs). (b) Sequence alignment and amino acid evolutionary conservation of Cc sequences. Residues are colored according to ConSurf conservation score [162]. Amino acids that can be modified are indicated with (#). (c) Ribbon representation of human Cc structure (PDB ID: 2N9I [163]). Left, residues are colored according to conservation score. Side chain of amino acids that undergo PTMs are labelled. Right, the different foldon units are colored as follows: foldon I in blue, foldon II in green, foldon III in yellow, foldon IV in red, and foldon V in purple.
5. Clinical Relevance of Post-Translationally Modified Cytochrome c
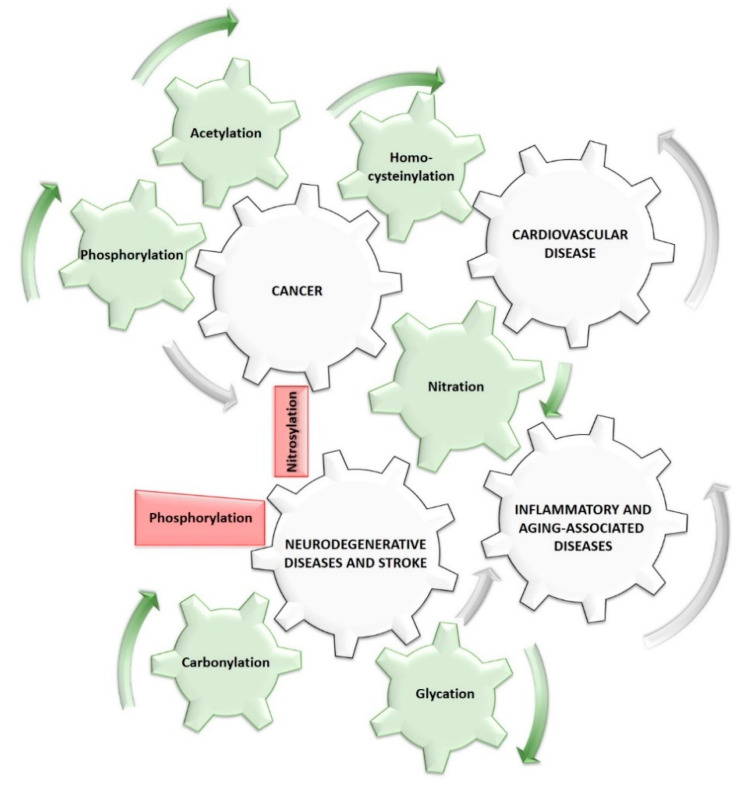
Proper working of Cc is essential for cell homeostasis and detoxification. Consequently, its malfunction is related to several mitochondrial diseases and aging [159,164,165]. The cause–effect relationships between post-translationally modified Cc and several pathologies have been widely studied. Figure 3 graphically summarizes all the described modifications of Cc that are present in human pathologies. Carbonylation, along with glycation, promote protein aggregation―this includes Cc, which is one of the principal features in neurodegenerative diseases [99,146]. On the contrary, Cc phosphorylated in positions Ser47 and Tyr97, or nitrosylated has been revealed as a neuroprotective agent against these neuronal disorders [49,55,81,123]. A plausible explanation is that these modifications inhibit PCD and enhance electron transfer under oxygen deprivation [81]. These findings, along with the fact that non-canonical amino acid-based phosphorylated Cc mutants are resistant to phosphatases, lead us to propose them as neuroprotectors with promising therapeutic applications. Cc homocysteinilation occurs in cardiovascular diseases [113] and, together with phosphorylation, in tumors [166]. Cancer cells are characterized by a high growth rate, which derives from enhanced mitochondrial metabolism, as well as an evasion of PCD (Table 1) [131,167]. Bazylianska and co-workers identified the acetylation of Lys53 in Cc in prostate cancer. In fact, they showed that acetylation strongly inhibits the role of Cc in apoptosis, allowing the survival of cancer cells [95]. Interestingly, tyrosine nitration and phosphorylation are mutually exclusive modification events. Thus, the Tyr-nitrated Cc species could function as antagonists to the Tyr-phosphorylated species, acting as an anticancer agent [123]. Moreover, proteins nitrated by peroxynitrite ion (including Cc) trigger inflammatory processes [168]. Even though Cc sulfoxidation has been identified in patients with thrombocytopenia, there is no evidence that this PTM directly causes the disease [169].
Figure 3.
Cause–effect of post-translational modifications of cytochrome c in human pathologies. Carbonylation, glycation and nitration of specific residues of Cc promote the development of several neurodegenerative diseases and strokes. However, phosphorylated Cc at positions 47 and 97, or nitrosylated in its tyrosine residues has been revealed to be a neuroprotective agent against this type of pathology. Cancer cells show many modifications of heme protein, such as phosphorylations, acetylations, homocysteinilations, and nitrations. Nitrosylation and phosphorylation are mutually exclusive modifications, so the presence of nitrosylated Cc species could be act as an anticancer agent. Notably, homocysteinilation is also related to cardiovascular diseases. Finally, it has been described that peroxynitrite ion and nitrated proteins (including Cc) trigger inflammatory processes. The green wheels represent Cc modifications that favor the development of diseases (see the direction of rotation of the gear indicated by the green arrows), white wheels represent the pathologies—gray arrows represent the direction of rotation that produces the development of the disease—and the red wedges indicate PTMs that impede them.
Acknowledgments
Figure 1 were created using BioRender icons [https://biorender.com/). Evolutionary analyses were performed with the ConSurf Server [https://consurf.tau.ac.il/).
Abbreviations
| AcK Apaf-1 |
N-ε-acetyl-L-Lys Apoptosis protease-activating factor-1 |
| CL | Cardiolipin |
| CIII | Complex III |
| CIV | Complex IV |
| Cytochrome c | Cc |
| Cytochrome c1 | Cc1 |
| CcO | Cytochrome c oxidase |
| ECT | Electron transport chain |
| IP3 | Inositol 1,4,5-trisphosphate |
| MAMs | Mitochondria-associated endoplasmic reticulum membranes |
| PCD | Programmed cell death |
|
pCMF pI |
p-carboxymethyl-L-phenylalanine Isoelectric point |
| PTMs | Post-translational modifications |
| RER | Rugose endoplasmic reticulum |
| RNS | Reactive nitrogen species |
| ROS | Reactive oxygen species |
| VDAC | Voltage-dependent anion channel |
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/1422-0067/21/22/8483/s1.
Author Contributions
Conceptualization, A.G.-C., I.M., G.P.-M., A.D.-Q., M.A.D.l.R. and I.D.-M.; resources, A.G.-C., I.M. and G.P.-M.; writing—original draft preparation, A.G.-C., I.M. and G.P.-M.; writing—review and editing, M.A.D.l.R. and I.D.-M.; supervision, M.A.D.l.R. and I.D.-M.; funding acquisition, M.A.D.l.R. and I.D.-M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Innovation [PGC2018-096049-B-I00), European Regional Development Fund [FEDER), Andalusian Government [BIO-198, US-1254317, US-1257019 and P18-FR-3487), VI PPIT-US and TA Instruments. G.P.-M. were awarded a PhD fellowship from the Spanish Ministry of Education, Culture and Sport [FPU17/04604).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Pieczenik S.R., Neustadt J. Mitochondrial dysfunction and molecular pathways of disease. Exp. Mol. Pathol. 2007;83:84–92. doi: 10.1016/j.yexmp.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 2.Díaz-Moreno I., García-Heredia J.M., Díaz-Quintana A., De La Rosa M.Á. Cytochrome c signalosome in mitochondria. Eur. Biophys. J. 2011;40:1301–1315. doi: 10.1007/s00249-011-0774-4. [DOI] [PubMed] [Google Scholar]
- 3.Hüttemann M., Pecina P., Rainbolt M., Sanderson T.H., Kagan V.E., Samavati L., Doan J.W., Lee I. The multiple functions of cytochrome c and their regulation in life and death decisions of the mammalian cell: From respiration to apoptosis. Mitochondrion. 2011;11:369–381. doi: 10.1016/j.mito.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou C., Zhang J., Ying W. Mitochondrial Electron Transport Chain Inhibition Suppresses LPS-Induced Inflammatory Responses via TREM1/STAT3 Pathway in BV2 Microglia. Int. J. Mol. Med. 2019;44:3–15. doi: 10.1101/2019.12.25.888529. ROS generation and uncoupling (Review) [DOI] [Google Scholar]
- 5.Liu X., Kim C.N., Yang J., Jemmerson R., Wang X. Induction of Apoptotic Program in Cell-Free Extracts: Requirement for dATP and Cytochrome c. Cell. 1996;86:147–157. doi: 10.1016/S0092-8674(00)80085-9. [DOI] [PubMed] [Google Scholar]
- 6.D’Herde K., De Prest B., Mussche S., Schotte P., Beyaert R., Van Coster R., Roels F. Ultrastructural localization of cytochrome c in apoptosis demonstrates mitochondrial heterogeneity. Cell Death Differ. 2000;7:331–337. doi: 10.1038/sj.cdd.4400655. [DOI] [PubMed] [Google Scholar]
- 7.González-Arzola K., Velázquez-Cruz A., Guerra-Castellano A., Casado-Combreras M.A., Pérez-Mejías G., Quintana A.D., Díaz-Moreno I., De La Rosa M.A. New moonlighting functions of mitochondrial cytochrome c in the cytoplasm and nucleus. FEBS Lett. 2019;593:3101–3119. doi: 10.1002/1873-3468.13655. [DOI] [PubMed] [Google Scholar]
- 8.Moreno-Beltrán B., Díaz-Quintana A., González-Arzola K., Velazquez-Campoy A., De La Rosa M.Á., Díaz-Moreno I. Cytochrome c1 exhibits two binding sites for cytochrome c in plants. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2014;1837:1717–1729. doi: 10.1016/j.bbabio.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 9.Moreno-Beltrán B., Díaz-Moreno I., González-Arzola K., Castellano A.G., Velazquez-Campoy A., De La Rosa M.Á., Díaz-Quintana A. Respiratory complexes III and IV can each bind two molecules of cytochromecat low ionic strength. FEBS Lett. 2015;589:476–483. doi: 10.1016/j.febslet.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Lagunas A., Castellano A.G., Nin-Hill A., Díaz-Moreno I., De La Rosa M.A., Samitier J., Rovira C., Gorostiza P. Long distance electron transfer through the aqueous solution between redox partner proteins. Nat. Commun. 2018;9:5157. doi: 10.1038/s41467-018-07499-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pérez-Mejías G., Olloqui-Sariego J.L., Guerra-Castellano A., Díaz-Quintana A., Calvente J.J., Andreu R., De La Rosa M.A., Díaz-Moreno I. Physical contact between cytochrome c1 and cytochrome c increases the driving force for electron transfer. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2020:148277. doi: 10.1016/j.bbabio.2020.148277. [DOI] [PubMed] [Google Scholar]
- 12.Pasdois P., Parker J.E., Griffiths E.J., Halestrap A.P. The role of oxidized cytochrome c in regulating mitochondrial reactive oxygen species production and its perturbation in ischaemia. Biochem. J. 2011;436:493–505. doi: 10.1042/BJ20101957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen S., Balabanidou V., Sideris D.P., Lisowsky T., Tokatlidis K. Erv1 Mediates the Mia40-dependent Protein Import Pathway and Provides a Functional Link to the Respiratory Chain by Shuttling Electrons to Cytochrome c. J. Mol. Biol. 2005;353:937–944. doi: 10.1016/j.jmb.2005.08.049. [DOI] [PubMed] [Google Scholar]
- 14.Dabir D.V., Leverich E.P., Kim S.-K., Tsai F.D., Hirasawa M., Knaff D.B., Koehler C.M. A role for cytochrome c and cytochrome c peroxidase in electron shuttling from Erv1. EMBO J. 2007;26:4801–4811. doi: 10.1038/sj.emboj.7601909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Soltys B.J., Andrews D.W., Jemmerson R., Gupta R.S. Cytochrome-c localizes in secretory granules in pancreas and anterior pituitary. Cell Biol. Int. 2001;25:331–338. doi: 10.1006/cbir.2000.0651. [DOI] [PubMed] [Google Scholar]
- 16.Ow Y.-L.P., Green D.R., Hao Z., Mak T.W. Cytochrome c: Functions beyond respiration. Nat. Rev. Mol. Cell Biol. 2008;9:532–542. doi: 10.1038/nrm2434. [DOI] [PubMed] [Google Scholar]
- 17.Gilchrist D.G. PROGRAMMED CELL DEATH IN PLANT DISEASE: The Purpose and Promise of Cellular Suicide. Annu. Rev. Phytopathol. 1998;36:393–414. doi: 10.1146/annurev.phyto.36.1.393. [DOI] [PubMed] [Google Scholar]
- 18.Fuchs Y., Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dorstyn L., Read S., Cakouros D., Huh J.R., Hay B.A., Kumar S. The role of cytochrome c in caspase activation in Drosophila melanogaster cells. J. Cell Biol. 2002;156:1089–1098. doi: 10.1083/jcb.200111107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrido C., Galluzzi L., Brunet M., E Puig P., Didelot C., Kroemer G. Mechanisms of cytochrome c release from mitochondria. Cell Death Differ. 2006;13:1423–1433. doi: 10.1038/sj.cdd.4401950. [DOI] [PubMed] [Google Scholar]
- 21.Martínez-Fábregas J., Díaz-Moreno I., González-Arzola K., Janocha S., Navarro J.A., Hervás M., Bernhardt R., Díaz-Quintana A., De La Rosa M.Á. NewArabidopsis thalianaCytochromecPartners: A Look Into the Elusive Role of Cytochromecin Programmed Cell Death in Plants. Mol. Cell. Proteom. 2013;12:3666–3676. doi: 10.1074/mcp.M113.030692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martínez-Fábregas J., Díaz-Moreno I., González-Arzola K., Díaz-Quintana A., A De La Rosa M. A common signalosome for programmed cell death in humans and plants. Cell Death Dis. 2014;5:e1314. doi: 10.1038/cddis.2014.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González-Arzola K., Díaz-Moreno I., Cano-González A., Díaz-Quintana A., Velázquez-Campoy A., Moreno-Beltrán B., López-Rivas A., De la Rosa M.A. Structural basis for inhibition of the histone chaperone activity of SET/TAF-Iβ by cytochrome c. Proc. Natl. Acad. Sci. USA. 2015;112:9908–9913. doi: 10.1073/pnas.1508040112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González-Arzola K., Díaz-Quintana A., Rivero-Rodríguez F., Velázquez-Campoy A., De La Rosa M.A., Díaz-Moreno I. Histone chaperone activity of Arabidopsis thaliana NRP1 is blocked by cytochrome c. Nucleic Acids Res. 2017;45:2150–2165. doi: 10.1093/nar/gkw1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Elena-Real C.A., Díaz-Quintana A., González-Arzola K., Velázquez-Campoy A., Orzáez M., López-Rivas A., Gil-Caballero S., De la Rosa M.Á., Díaz-Moreno I. Cytochrome c speeds up caspase cascade activation by blocking 14-3-3ε-dependent Apaf-1 inhibition. Cell Death Dis. 2018;9:65. doi: 10.1038/s41419-018-0408-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Díaz-Quintana A., Pérez-Mejías G., Guerra-Castellano A., De La Rosa M.A., Díaz-Moreno I. Wheel and Deal in the Mitochondrial Inner Membranes: The Tale of Cytochrome c and Cardiolipin. Oxidative Med. Cell. Longev. 2020;2020:1–20. doi: 10.1155/2020/6813405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Everse J., Liu C.-J.J., Coates P.W. Physical and catalytic properties of a peroxidase derived from cytochrome c. Biochim. et Biophys. Acta (BBA)-Mol. Basis Dis. 2011;1812:1138–1145. doi: 10.1016/j.bbadis.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 28.Ascenzi P., Coletta M., Wilson M.T., Fiorucci L., Marino M., Polticelli F., Sinibaldi F., Santucci R. Cardiolipin-cytochromeccomplex: Switching cytochromecfrom an electron-transfer shuttle to a myoglobin- and a peroxidase-like heme-protein. IUBMB Life. 2015;67:98–109. doi: 10.1002/iub.1350. [DOI] [PubMed] [Google Scholar]
- 29.Jemmerson R., Liu J., Hausauer D., Lam K.-P., Mondino A.A., Nelson R.D. A Conformational Change in Cytochromecof Apoptotic and Necrotic Cells Is Detected by Monoclonal Antibody Binding and Mimicked by Association of the Native Antigen with Synthetic Phospholipid Vesicles†. Biochemistry. 1999;38:3599–3609. doi: 10.1021/bi9809268. [DOI] [PubMed] [Google Scholar]
- 30.Kagan V.E., Borisenko G.G., Tyurina Y.Y., Tyurin V.A., Jiang J., Potapovich A.I., Kini V., Amoscato A.A., Fujii Y. Oxidative lipidomics of apoptosis: Redox catalytic interactions of cytochrome c with cardiolipin and phosphatidylserine. Free. Radic. Biol. Med. 2004;37:1963–1985. doi: 10.1016/j.freeradbiomed.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Shoshan-Barmatz V., Zalk R., Gincel D., Vardi N. Subcellular localization of VDAC in mitochondria and ER in the cerebellum. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2004;1657:105–114. doi: 10.1016/j.bbabio.2004.02.009. [DOI] [PubMed] [Google Scholar]
- 32.Shah S.Z.A., Zhao D., Khan S.H., Yang L. Regulatory Mechanisms of Endoplasmic Reticulum Resident IP3 Receptors. J. Mol. Neurosci. 2015;56:938–948. doi: 10.1007/s12031-015-0551-4. [DOI] [PubMed] [Google Scholar]
- 33.Camara A.K.S., Zhou Y., Wen P.-C., Tajkhorshid E., Kwok W.-M. Mitochondrial VDAC1: A Key Gatekeeper as Potential Therapeutic Target. Front. Physiol. 2017;8:460. doi: 10.3389/fphys.2017.00460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Patergnani S., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Giorgi C., Retta S.F., Missiroli S., Poletti F., et al. Calcium signaling around Mitochondria Associated Membranes (MAMs) Cell Commun. Signal. 2011;9:19. doi: 10.1186/1478-811X-9-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhu Z., Ma Q., Wang Q., Sun X., Zhang Z., Ji L., Huang Q. The relationship between mitochondria Ca2+ intake mediated by mitochondria-associated endoplasmic reticulum membranes and tumor genesis. J. Cell Signal. 2019;4:199. [Google Scholar]
- 36.Vance J.E. Phospholipid synthesis in a membrane fraction associated with mitochondria. J. Biol. Chem. 1990;265:7248–7256. [PubMed] [Google Scholar]
- 37.Theurey P., Rieusset J. Mitochondria-Associated Membranes Response to Nutrient Availability and Role in Metabolic Diseases. Trends Endocrinol. Metab. 2017;28:32–45. doi: 10.1016/j.tem.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Boehning D., Patterson R.L., Sedaghat L., Glebova N.O., Kurosaki T., Snyder S.H. Cytochrome c binds to inositol (1,4,5) trisphosphate receptors, amplifying calcium-dependent apoptosis. Nat. Cell Biol. 2003;5:1051–1061. doi: 10.1038/ncb1063. [DOI] [PubMed] [Google Scholar]
- 39.Zalk R., Israelson A., Garty E.S., Azoulay-Zohar H., Shoshan-Barmatz V. Oligomeric states of the voltage-dependent anion channel and cytochrome c release from mitochondria. Biochem. J. 2005;386:73–83. doi: 10.1042/BJ20041356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Szabadkai G., Bianchi K., Várnai P., De Stefani D., Wieckowski M.R., Cavagna D., Nagy A.I., Balla T., Rizzuto R. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J. Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shoshan-Barmatz V., Keinan N., Abu-Hamad S., Tyomkin D., Aram L. Apoptosis is regulated by the VDAC1 N-terminal region and by VDAC oligomerization: Release of cytochrome c, AIF and Smac/Diablo. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2010;1797:1281–1291. doi: 10.1016/j.bbabio.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 42.Shoshan-Barmatz V., Maldonado E.N., Krelin Y. VDAC1 at the crossroads of cell metabolism, apoptosis and cell stress. Cell Stress. 2017;1:11–36. doi: 10.15698/cst2017.10.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ameisen J.C. On the origin, evolution, and nature of programmed cell death: A timeline of four billion years. Cell Death Differ. 2002;9:367–393. doi: 10.1038/sj.cdd.4400950. [DOI] [PubMed] [Google Scholar]
- 44.Paine M.J.I., Garner A.P., Powell D., Sibbald J., Sales M., Pratt N., Smith T., Tew D.G., Wolf C.R. Cloning and characterization of a novel human dual flavin reductase. J. Biol. Chem. 2000;275:1471–1478. doi: 10.1074/jbc.275.2.1471. [DOI] [PubMed] [Google Scholar]
- 45.Walsh C. Posttranslational Modification of Proteins: Expanding Nature’s Inventory. Roberts and Company Publishers; Greenwood Village, CO, USA: 2006. p. 490. [Google Scholar]
- 46.Karve T.M., Cheema A.K. Small Changes Huge Impact: The Role of Protein Posttranslational Modifications in Cellular Homeostasis and Disease. J. Amino Acids. 2011;2011:1–13. doi: 10.4061/2011/207691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Müller M.M. Post-Translational Modifications of Protein Backbones: Unique Functions, Mechanisms, and Challenges. Biochemistry. 2018;57:177–185. doi: 10.1021/acs.biochem.7b00861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kalpage H.A., Bazylianska V., Recanati M.A., Fite A., Liu J., Wan J., Mantena N., Malek M.H., Podgorski I., Heath E.I., et al. Tissue-specific regulation of cytochrome c by post-translational modifications: Respiration, the mitochondrial membrane potential, ROS, and apoptosis. FASEB J. 2018;33:1540–1553. doi: 10.1096/fj.201801417R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kalpage H.A., Wan J., Morse P.T., Zurek M.P., Turner A.A., Khobeir A., Yazdi N., Hakim L., Liu J., Vaishnav A., et al. Cytochrome c phosphorylation: Control of mitochondrial electron transport chain flux and apoptosis. Int. J. Biochem. Cell Biol. 2020;121:105704. doi: 10.1016/j.biocel.2020.105704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee I., Salomon A.R., Yu K., Doan J.W., I Grossman L., Hüttemann M. New Prospects for an Old Enzyme: Mammalian CytochromecIs Tyrosine-Phosphorylated in Vivo. Biochemistry. 2006;45:9121–9128. doi: 10.1021/bi060585v. [DOI] [PubMed] [Google Scholar]
- 51.Yu H., Lee I., Salomon A.R., Yu K., Hüttemann M. Mammalian liver cytochrome c is tyrosine-48 phosphorylated in vivo, inhibiting mitochondrial respiration. Biochim. Biophys. Acta Bioenerg. 2008;1777:1066–1071. doi: 10.1016/j.bbabio.2008.04.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanderson T.H., Mahapatra G., Pecina P., Ji Q., Yu K., Sinkler C., Varughese A., Kumar R., Bukowski M.J., Tousignant R.N., et al. Cytochrome c Is Tyrosine 97 Phosphorylated by Neuroprotective Insulin Treatment. PLOS ONE. 2013;8:e78627. doi: 10.1371/journal.pone.0078627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mahapatra G., Varughese A., Ji Q., Lee I., Liu J., Vaishnav A., Sinkler C., Kapralov A.A., Moraes C.T., Sanderson T.H., et al. Phosphorylation of cytochrome c threonine 28 regulates electron transport chain activity in kidney: Implications for amp kinase. J. Biol. Chem. 2017;292:64–79. doi: 10.1074/jbc.M116.744664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wan J., Kalpage H.A., Vaishnav A., Liu J., Lee I., Mahapatra G., Turner A.A., Zurek M.P., Ji Q., Moraes C.T., et al. Regulation of Respiration and Apoptosis by Cytochrome c Threonine 58 Phosphorylation. Sci. Rep. 2019;9:15–16. doi: 10.1038/s41598-019-52101-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kalpage H.A., Vaishnav A., Liu J., Varughese A., Wan J., Turner A.A., Ji Q., Zurek M.P., Kapralov A.A., Kagan V.E., et al. Serine-47 phosphorylation of cytochrome c in the mammalian brain regulates cytochrome c oxidase and caspase-3 activity. FASEB J. 2019;33:13503–13514. doi: 10.1096/fj.201901120R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liu D.R., Magliery T.J., Pastrnak M., Schultz P.G. Engineering a tRNA and aminoacyl-tRNA synthetase for the site-specific incorporation of unnatural amino acids into proteins in vivo. Proc. Natl. Acad. Sci. USA. 1997;94:10092–10097. doi: 10.1073/pnas.94.19.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ewals K., Ovaa H. Unnatural amino acid incorporation in E. coli: Current and future applications in the design of therapeutic proteins. Front. Chem. 2014;2:15. doi: 10.3389/fchem.2014.00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Leisle L., I Valiyaveetil F., Mehl R.A., Ahern C.A. Incorporation of Non-Canonical Amino Acids. Adv. Exp. Med. Biol. 2015;869:119–151. doi: 10.1007/978-1-4939-2845-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Meineke B., Heimgärtner J., Eirich J., Landreh M., Elsässer S.J. Site-Specific Incorporation of Two ncAAs for Two-Color Bioorthogonal Labeling and Crosslinking of Proteins on Live Mammalian Cells. Cell Rep. 2020;31:107811. doi: 10.1016/j.celrep.2020.107811. [DOI] [PubMed] [Google Scholar]
- 60.Kawahata N., Yang M.G., Luke G.P., Shakespeare W.C., Sundaramoorthi R., Wang Y., Johnson D., Merry T., Violette S., Guan W., et al. A novel phosphotyrosine mimetic 4′-carboxymethyloxy-3′-phosphonophenylalanine (cpp): Exploitation in the design of nonpeptide inhibitors of pp60Src SH2 domain. Bioorganic Med. Chem. Lett. 2001;11:2319–2323. doi: 10.1016/S0960-894X(01)00446-2. [DOI] [PubMed] [Google Scholar]
- 61.A Erlanson D., McDowell R.S., He M.M., Randal M., Simmons R.L., Kung J., Waight A., Hansen S.K. Discovery of a New Phosphotyrosine Mimetic for PTP1B Using Breakaway Tethering. J. Am. Chem. Soc. 2003;125:5602–5603. doi: 10.1021/ja034440c. [DOI] [PubMed] [Google Scholar]
- 62.Rothman D.M., Petersson E.J., Vázquez M.E., Brandt G.S., Dougherty D.A., Imperiali B. Caged Phosphoproteins. J. Am. Chem. Soc. 2005;127:846–847. doi: 10.1021/ja043875c. [DOI] [PubMed] [Google Scholar]
- 63.Liu C.C., Schultz P.G. Recombinant expression of selectively sulfated proteins in Escherichia coli. Nat. Biotechnol. 2006;24:1436–1440. doi: 10.1038/nbt1254. [DOI] [PubMed] [Google Scholar]
- 64.Xie J., Supekova L., Schultz P.G. A Genetically Encoded Metabolically Stable Analogue of Phosphotyrosine in Escherichia coli. ACS Chem. Biol. 2007;2:474–478. doi: 10.1021/cb700083w. [DOI] [PubMed] [Google Scholar]
- 65.Serwa R., Wilkening I., Del Signore G., Mühlberg M., Claussnitzer I., Weise C., Gerrits M., Hackenberger C.P.R. Chemoselective Staudinger-Phosphite Reaction of Azides for the Phosphorylation of Proteins. Angew. Chem. Int. Ed. 2009;48:8234–8239. doi: 10.1002/anie.200902118. [DOI] [PubMed] [Google Scholar]
- 66.Fan C., Ip K., Söll D. Expanding the genetic code of Escherichia coli with phosphotyrosine. FEBS Lett. 2016;590:3040–3047. doi: 10.1002/1873-3468.12333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hoppmann C., Wong A., Yang B., Li S., Hunter T., Shokat K.M., Wang L. Site-specific incorporation of phosphotyrosine using an expanded genetic code. Nat. Chem. Biol. 2017;13:842–844. doi: 10.1038/nchembio.2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Luo X., Fu G., Wang R.E., Zhu X., Zambaldo C., Liu R., Liu T., Lyu X., Du J., Xuan W., et al. Genetically encoding phosphotyrosine and its nonhydrolyzable analog in bacteria. Nat. Chem. Biol. 2017;13:845–849. doi: 10.1038/nchembio.2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chin J.W., Santoro S.W., Martin A.B., King D.S., Wang L., Schultz P.G. Addition of p-azido-L-phenylaianine to the genetic code of Escherichia coli. J. Am. Chem. Soc. 2002;124:9026–9027. doi: 10.1021/ja027007w. [DOI] [PubMed] [Google Scholar]
- 70.Zhang M.S., Brunner S.F., Huguenin-Dezot N., Liang A.D., Schmied W.H., Rogerson D.T., Chin J.W. Biosynthesis and genetic encoding of phosphothreonine through parallel selection and deep sequencing. Nat. Methods. 2017;14:729–736. doi: 10.1038/nmeth.4302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rogerson D.T., Sachdeva A., Wang K., Haq T., Kazlauskaite A., Hancock S.M., Huguenin-Dezot N., Muqit M.M.K., Fry A.M., Bayliss R., et al. Efficient genetic encoding of phosphoserine and its nonhydrolyzable analog. Nat. Chem. Biol. 2015;11:496–503. doi: 10.1038/nchembio.1823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sokolovsky M., Riordan J.F., Vallee B.L. Conversion of 3-nitrotyrosine to 3-aminotyrosine in peptides and proteins. Biochem. Biophys. Res. Commun. 1967;27:20–25. doi: 10.1016/S0006-291X(67)80033-0. [DOI] [PubMed] [Google Scholar]
- 73.Neumann H., Peak-Chew S.Y., Chin J.W. Genetically encoding N(epsilon)-acetyllysine in recombinant proteins. Nat. Chem. Biol. 2008;4:232–234. doi: 10.1038/nchembio.73. [DOI] [PubMed] [Google Scholar]
- 74.Hancock S.M., Uprety R., Deiters A., Chin J.W. Expanding the Genetic Code of Yeast for Incorporation of Diverse Unnatural Amino Acids via a Pyrrolysyl-tRNA Synthetase/tRNA Pair. J. Am. Chem. Soc. 2010;132:14819–14824. doi: 10.1021/ja104609m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang Y., Wan W., Russell W.K., Pai P.-J., Wang Z., Russell D.H., Liu W.R. Genetic incorporation of an aliphatic keto-containing amino acid into proteins for their site-specific modifications. Bioorganic Med. Chem. Lett. 2010;20:878–880. doi: 10.1016/j.bmcl.2009.12.077. [DOI] [PubMed] [Google Scholar]
- 76.Pérez-Mejías G., Velázquez-Cruz A., Guerra-Castellano A., Baños-Jaime B., Díaz-Quintana A., González-Arzola K., De La Rosa M.Á., Díaz-Moreno I. Exploring protein phosphorylation by combining computational approaches and biochemical methods. Comput. Struct. Biotechnol. J. 2020;18:1852–1863. doi: 10.1016/j.csbj.2020.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Castellano A.G., Díaz-Quintana A., Moreno-Beltrán B., López-Prados J., Nieto P.M., Meister W., Staffa J., Teixeira M., Hildebrandt P., De La Rosa M.Á., et al. Mimicking Tyrosine Phosphorylation in Human Cytochrome cby the Evolved tRNA Synthetase Technique. Chem.-A Eur. J. 2015;21:15004–15012. doi: 10.1002/chem.201502019. [DOI] [PubMed] [Google Scholar]
- 78.Castellano A.G., Díaz-Moreno I., Velazquez-Campoy A., De La Rosa M.Á., Díaz-Quintana A. Structural and functional characterization of phosphomimetic mutants of cytochrome c at threonine 28 and serine 47. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2016;1857:387–395. doi: 10.1016/j.bbabio.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 79.Pecina P., Borisenko G.G., Belikova N.A., Tyurina Y.Y., Pecinova A., Lee I., Samhan-Arias A.K., Przyklenk K., Kagan V.E., Hüttemann M. Phosphomimetic substitution of cytochrome c tyrosine 48 decreases respiration and binding to cardiolipin and abolishes ability to trigger downstream caspase activation. Biochemistry. 2010;49:6705–6714. doi: 10.1021/bi100486s. [DOI] [PubMed] [Google Scholar]
- 80.García-Heredia J.M., Díaz-Quintana A., Salzano M., Orzáez M., Pérez-Payá E., Teixeira M., De La Rosa M.A., Díaz-Moreno I. Tyrosine phosphorylation turns alkaline transition into a biologically relevant process and makes human cytochrome c behave as an anti-apoptotic switch. JBIC J. Biol. Inorg. Chem. 2011;16:1155–1168. doi: 10.1007/s00775-011-0804-9. [DOI] [PubMed] [Google Scholar]
- 81.Moreno-Beltrán B., Guerra-Castellano A., Díaz-Quintana A., Del Conte R., García-Mauriño S.M., Díaz-Moreno S., González-Arzola K., Santos-Ocaña C., Velázquez-Campoy A., De La Rosa M.A., et al. Structural basis of mitochondrial dysfunction in response to cytochrome c phosphorylation at tyrosine 48. Proc. Natl. Acad. Sci. USA. 2017;114:E3041–E3050. doi: 10.1073/pnas.1618008114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guerra-Castellano A., Díaz-Quintana A., Pérez-Mejías G., Elena-Real C.A., González-Arzola K., García-Mauriño S.M., De La Rosa M.A., Díaz-Moreno I. Oxidative stress is tightly regulated by cytochrome c phosphorylation and respirasome factors in mitochondria. Proc. Natl. Acad. Sci. USA. 2018;115:7955–7960. doi: 10.1073/pnas.1806833115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Díaz-Moreno I., García-Heredia J.M., Díaz-Quintana A., Teixeira M., De La Rosa M.Á. Nitration of tyrosines 46 and 48 induces the specific degradation of cytochrome c upon change of the heme iron state to high-spin. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2011;1807:1616–1623. doi: 10.1016/j.bbabio.2011.09.012. [DOI] [PubMed] [Google Scholar]
- 84.Nakagawa H., Ohshima Y., Takusagawa M., Ikota N., Takahashi Y., Shimizu S., Ozawa T. Functional Modification of Cytochrome c by Peroxynitrite in an Electron Transfer Reaction. Chem. Pharm. Bull. 2001;49:1547–1554. doi: 10.1248/cpb.49.1547. [DOI] [PubMed] [Google Scholar]
- 85.Díaz-Moreno I., Nieto P.M., Del Conte R., Gairí M., García-Heredia J.M., De La Rosa M.Á., Díaz-Quintana A. A Non-damaging Method to Analyze the Configuration and Dynamics of Nitrotyrosines in Proteins. Chem.-A Eur. J. 2012;18:3872–3878. doi: 10.1002/chem.201103413. [DOI] [PubMed] [Google Scholar]
- 86.García-Heredia J.M., Díaz-Moreno I., Nieto P.M., Orzáez M., Kocanis S., Teixeira M., Pérez-Payá E., Díaz-Quintana A., De La Rosa M.Á. Nitration of tyrosine 74 prevents human cytochrome c to play a key role in apoptosis signaling by blocking caspase-9 activation. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2010;1797:981–993. doi: 10.1016/j.bbabio.2010.03.009. [DOI] [PubMed] [Google Scholar]
- 87.García-Heredia J.M., Díaz-Moreno I., Díaz-Quintana A., Orzáez M., Navarro J.A., Hervás M., De La Rosa M.Á. Specific nitration of tyrosines 46 and 48 makes cytochromecassemble a non-functional apoptosome. FEBS Lett. 2011;586:154–158. doi: 10.1016/j.febslet.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 88.Vlasova I.I., Tyurin V.A., Kapralov A.A., Kurnikov I.V., Osipov A.N., Potapovich M.V., Stoyanovsky D.A., Kagan V.E. Nitric oxide inhibits peroxidase activity of cytochrome c.cardiolipin complex and blocks cardiolipin oxidation. J. Biol. Chem. 2006;281:14554–14562. doi: 10.1074/jbc.M509507200. [DOI] [PubMed] [Google Scholar]
- 89.Kruglik S.G., Yoo B.-K., Lambry J.C., Martin J.-L., Negrerie M. Structural changes and picosecond to second dynamics of cytochrome c in interaction with nitric oxide in ferrous and ferric redox states. Phys. Chem. Chem. Phys. 2017;19:21317–21334. doi: 10.1039/C7CP02634J. [DOI] [PubMed] [Google Scholar]
- 90.Azzi A., Montecucco C., Richter C. The use of acetylated ferricytochrome C for the detection of superoxide radicals produced in biological membranes. Biochem. Biophys. Res. Commun. 1975;65:597–603. doi: 10.1016/S0006-291X(75)80188-4. [DOI] [PubMed] [Google Scholar]
- 91.Minakami S., Titani K., Ishikura H. The structure of cytochrome c. II. Properties of acetylated cytochrome c. J. Biochem. 1958;45:341–348. doi: 10.1093/oxfordjournals.jbchem.a126874. [DOI] [Google Scholar]
- 92.Wada K., Okunuki K. Studies on Chemically Modified Cytochrome cI. The Acetylated Cytochrome c. J. Biochem. 1968;64:667–687. doi: 10.1093/oxfordjournals.jbchem.a128945. [DOI] [PubMed] [Google Scholar]
- 93.Hagihara Y., Tan Y., Goto Y. Comparison of the Conformational Stability of the Molten Globule and Native States of Horse Cytochrome c. J. Mol. Biol. 1994;237:336–348. doi: 10.1006/jmbi.1994.1234. [DOI] [PubMed] [Google Scholar]
- 94.Takemori S., Wada K., Sekuzu I., Okunuki K., Takemori K.W.S. Reaction of Cytochrome a with Chemically Modified Cytochrome c and Basic Proteins. Nat. Cell Biol. 1962;195:456–457. doi: 10.1038/195456a0. [DOI] [PubMed] [Google Scholar]
- 95.Korshunov S.S., Krasnikov B.F., O Pereverzev M., Skulachev V.P. The antioxidant functions of cytochrome c. FEBS Lett. 1999;462:192–198. doi: 10.1016/S0014-5793(99)01525-2. [DOI] [PubMed] [Google Scholar]
- 96.Bazylianska V. The Effect of Acetylation of Cytochrome c on Its Functions in Prostate Cancer. Wayne State University; Detroit, MI, USA: 2017. [Google Scholar]
- 97.Méndez J., Cruz M.M., Delgado Y., Figueroa C.M., Orellano E.A., Morales M., Monteagudo A., Griebenow K. Delivery of Chemically Glycosylated Cytochrome c Immobilized in Mesoporous Silica Nanoparticles Induces Apoptosis in HeLa Cancer Cells. Mol. Pharm. 2014;11:102–111. doi: 10.1021/mp400400j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Delgado Y., Morales-Cruz M., Hernández-Román J., Martínez Y., Griebenow K. Chemical glycosylation of cytochrome c improves physical and chemical protein stability. BMC Biochem. 2014;15:16. doi: 10.1186/1471-2091-15-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mercado-Uribe H., Andrade-Medina M., Espinoza-Rodríguez J.H., Carrillo-Tripp M., Scheckhuber C.Q. Analyzing structural alterations of mitochondrial intermembrane space superoxide scavengers cytochrome-c and SOD1 after methylglyoxal treatment. PLoS ONE. 2020;15:e0232408. doi: 10.1371/journal.pone.0232408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.A Oliveira L.M., A Gomes R., Yang D., Dennison S.R., Família C., Lages A., Coelho A., Murphy R.M., Phoenix D.A., Quintas A. Insights into the molecular mechanism of protein native-like aggregation upon glycation. Biochim. et Biophys. Acta (BBA)-Proteins Proteom. 2013;1834:1010–1022. doi: 10.1016/j.bbapap.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 101.Hildick-Smith G.J., Downey M.C., Gretebeck L.M., Gersten R.A., Sandwick R.K. Ribose 5-Phosphate Glycation Reduces CytochromecRespiratory Activity and Membrane Affinity. Biochemistry. 2011;50:11047–11057. doi: 10.1021/bi2012977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sharma G.S., Warepam M., Bhattacharya R., Singh L.R. Covalent Modification by Glyoxals Converts Cytochrome c Into its Apoptotically Competent State. Sci. Rep. 2019;9:4781. doi: 10.1038/s41598-019-41282-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Flatmark T. On the heterogeneity of beef heart cytochrome c III.A kinetic study of the non-enzymic deamidation of the main sub-fractions. Acta Chem Scand. 1966;20:1487–1496. doi: 10.3891/acta.chem.scand.20-1487. [DOI] [PubMed] [Google Scholar]
- 104.Flatmark T. Multiple molecular forms of bovine heart cytochrome c. A comparative study of their physiochemical properties and their reactions in biological systems. J. Biol. Chem. 1967;242:2454–2459. [PubMed] [Google Scholar]
- 105.Aluri H.S., Simpson D.C., Allegood J.C., Hu Y., Szczepanek K., Gronert S., Chen Q., Lesnefsky E.J. Electron flow into cytochrome c coupled with reactive oxygen species from the electron transport chain converts cytochrome c to a cardiolipin peroxidase: Role during ischemia–reperfusion. Biochim. et Biophys. Acta (BBA)-Gen. Subj. 2014;1840:3199–3207. doi: 10.1016/j.bbagen.2014.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Capdevila D.A., Marmisollé W.A., Tomasina F., Demicheli V., Portela M., Radi R., Murgida D.H. Specific methionine oxidation of cytochrome c in complexes with zwitterionic lipids by hydrogen peroxide: Potential implications for apoptosis. Chem. Sci. 2015;6:705–713. doi: 10.1039/C4SC02181A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.A Capdevila D., Rouco S.O., Tomasina F., Tortora V., Demicheli V., Radi R., Murgida D.H. Active Site Structure and Peroxidase Activity of Oxidatively Modified Cytochrome c Species in Complexes with Cardiolipin. Biochemistry. 2015;54:7491–7504. doi: 10.1021/acs.biochem.5b00922. [DOI] [PubMed] [Google Scholar]
- 108.Wang Z., Ando Y., Nugraheni A.D., Ren C., Nagao S., Hirota S. Self-oxidation of cytochrome c at methionine80 with molecular oxygen induced by cleavage of the Met–heme iron bond. Mol. BioSyst. 2014;10:3130–3137. doi: 10.1039/C4MB00285G. [DOI] [PubMed] [Google Scholar]
- 109.Ivanetich K.M., Bradshaw J.J., Kaminsky L.S. Methionine sulfoxide cytochrome c. Biochemistry. 1976;15:1144–1153. doi: 10.1021/bi00650a029. [DOI] [PubMed] [Google Scholar]
- 110.Chen Y.-R., Deterding L.J., Sturgeon B.E., Tomer K.B., Mason R.P. Protein Oxidation of Cytochrome c by Reactive Halogen Species Enhances Its Peroxidase Activity. J. Biol. Chem. 2002;277:29781–29791. doi: 10.1074/jbc.M200709200. [DOI] [PubMed] [Google Scholar]
- 111.Gates A.T., Moore J.L., Sylvain M.R., Jones C.M., Lowry M., El-Zahab B., Robinson J.W., Strongin R.M., Warner I.M. Mechanistic Investigation ofN-Homocysteinylation-Mediated Protein−Gold Nanoconjugate Assembly. Langmuir. 2009;25:9346–9351. doi: 10.1021/la900798q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Perła-Kaján J., Marczak Ł., Kaján L., Skowronek P., Twardowski T., Jakubowski H. Modification by Homocysteine Thiolactone Affects Redox Status of Cytochromec. Biochemistry. 2007;46:6225–6231. doi: 10.1021/bi602463m. [DOI] [PubMed] [Google Scholar]
- 113.Dopner S., Hildebrandt P., Rosell F.I., Mauk A.G., Von Walter M., Buse G., Soulimane T. The structural and functional role of lysine residues in the binding domain of cytochrome c in the electron transfer to cytochrome c oxidase. JBIC J. Biol. Inorg. Chem. 1999;261:379–391. doi: 10.1046/j.1432-1327.1999.00249.x. [DOI] [PubMed] [Google Scholar]
- 114.Sharma G.S., Singh L.R. Conformational status of cytochrome c upon N-homocysteinylation: Implications to cytochrome c release. Arch. Biochem. Biophys. 2017;614:23–27. doi: 10.1016/j.abb.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 115.Jakubowski H. Protein homocysteinylation: Possible mechanism underlying pathological consequences of elevated homocysteine levels. FASEB J. 1999;13:2277–2283. doi: 10.1096/fasebj.13.15.2277. [DOI] [PubMed] [Google Scholar]
- 116.Yin V., Shaw G.S., Konermann L. Cytochrome c as a Peroxidase: Activation of the Precatalytic Native State by H2O2-Induced Covalent Modifications. J. Am. Chem. Soc. 2017;139:15701–15709. doi: 10.1021/jacs.7b07106. [DOI] [PubMed] [Google Scholar]
- 117.Yin V., Mian S.H., Konermann L. Lysine carbonylation is a previously unrecognized contributor to peroxidase activation of cytochrome c by chloramine-T. Chem. Sci. 2019;10:2349–2359. doi: 10.1039/C8SC03624A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Barayeu U., Lange M., Méndez L., Arnhold J., Shadyro O.I., Fedorova M., Flemmig J. Cytochrome c autocatalyzed carbonylation in the presence of hydrogen peroxide and cardiolipins. J. Biol. Chem. 2018;294:1816–1830. doi: 10.1074/jbc.RA118.004110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Cassina A.M., Hodara R., Souza J.M., Thomson L., Castro L., Ischiropoulos H., Freeman B.A., Radi R. Cytochrome c nitration by peroxynitrite. J. Biol. Chem. 2000;275:21409–21415. doi: 10.1074/jbc.M909978199. [DOI] [PubMed] [Google Scholar]
- 120.Abriata L.A., Cassina A., Tórtora V., Marín M., Souza J.M., Castro L., Vila A.J., Radi R. Nitration of Solvent-exposed Tyrosine 74 on CytochromecTriggers Heme Iron-Methionine 80 Bond Disruption. J. Biol. Chem. 2008;284:17–26. doi: 10.1074/jbc.M807203200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Su J., Groves J.T. Mechanisms of Peroxynitrite Interactions with Heme Proteins. Inorg. Chem. 2010;49:6317–6329. doi: 10.1021/ic902157z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Díaz-Moreno I., García-Heredia J.M., González-Arzola K., Díaz-Quintana A., De La Rosa M.Á. Recent Methodological Advances in the Analysis of Protein Tyrosine Nitration. ChemPhysChem. 2013;14:3095–3102. doi: 10.1002/cphc.201300210. [DOI] [PubMed] [Google Scholar]
- 123.Corpas F.J., Del Río L.A., Barroso J.B. Post-translational modifications mediated by reactive nitrogen species. Plant Signal. Behav. 2008;3:301–303. doi: 10.4161/psb.3.5.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schonhoff C.M., Gaston B., Mannick J.B. Nitrosylation of Cytochromecduring Apoptosis. J. Biol. Chem. 2003;278:18265–18270. doi: 10.1074/jbc.M212459200. [DOI] [PubMed] [Google Scholar]
- 125.Radi R. Nitric oxide, oxidants, and protein tyrosine nitration. Proc. Natl. Acad. Sci. USA. 2004;101:4003–4008. doi: 10.1073/pnas.0307446101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pacher P., Beckman J.S., Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007;87:315–424. doi: 10.1152/physrev.00029.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Rodríguez-Roldán V., García-Heredia J.M., Navarro J.A., De La Rosa M.A., Hervás M. Effect of Nitration on the Physicochemical and Kinetic Features of Wild-Type and Monotyrosine Mutants of Human Respiratory Cytochromec†. Biochemistry. 2008;47:12371–12379. doi: 10.1021/bi801329s. [DOI] [PubMed] [Google Scholar]
- 128.Ly H.K., Utesch T., Díaz-Moreno I., García-Heredia J.M., De La Rosa M.Á., Hildebrandt P. Perturbation of the Redox Site Structure of Cytochrome c Variants upon Tyrosine Nitration. J. Phys. Chem. B. 2012;116:5694–5702. doi: 10.1021/jp302301m. [DOI] [PubMed] [Google Scholar]
- 129.Souza J.M., Castro L., Cassina A., Batthyány C., Radi R. Nitrocytochrome c: Synthesis, Purification, and Functional Studies. Methods in Enzymology. 2008;441:197–215. doi: 10.1016/s0076-6879(08)01211-1. [DOI] [PubMed] [Google Scholar]
- 130.Hosp F., Lassowskat I., Santoro V., De Vleesschauwer D., Fliegner D., Redestig H., Mann M., Christian S., Hannah M.A., Finkemeier I. Lysine acetylation in mitochondria: From inventory to function. Mitochondrion. 2017;33:58–71. doi: 10.1016/j.mito.2016.07.012. [DOI] [PubMed] [Google Scholar]
- 131.Kim S.C., Sprung R., Chen Y., Xu Y., Ball H., Pei J., Cheng T., Kho Y., Xiao H., Xiao L., et al. Substrate and Functional Diversity of Lysine Acetylation Revealed by a Proteomics Survey. Mol. Cell. 2006;23:607–618. doi: 10.1016/j.molcel.2006.06.026. [DOI] [PubMed] [Google Scholar]
- 132.Romero-Garcia S., Lopez-Gonzalez J.S., B´ez-Viveros J.L., Aguilar-Cazares D., Prado-Garcia H. Tumor cell metabolism. Cancer Biol. Ther. 2011;12:939–948. doi: 10.4161/cbt.12.11.18140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tak I.-U.-R., Ali F., Dar J.S., Magray A.R., Ganai B.A., Chishti M. Protein Modificomics. Elsevier BV; Amsterdam, The Netherlands: 2019. Posttranslational Modifications of Proteins and Their Role in Biological Processes and Associated Diseases; pp. 1–35. [Google Scholar]
- 134.König B., Osheroff N., Wilms J., Muijsers A., Dekker H.L., Margoliash E. Mapping of the interaction domain for purified cytochrome c 1 on cytochrome c. FEBS Lett. 1980;111:395–398. doi: 10.1016/0014-5793(80)80835-0. [DOI] [PubMed] [Google Scholar]
- 135.Smith H.T., Ahmed A.J., Millett F. Electrostatic interaction of cytochrome c with cytochrome c1 and cytochrome oxidase. J. Biol. Chem. 1981;256:4984–4990. [PubMed] [Google Scholar]
- 136.Kokhan O., Wraight C.A., Tajkhorshid E. The Binding Interface of Cytochrome c and Cytochrome c1 in the bc1 Complex: Rationalizing the Role of Key Residues. Biophys. J. 2010;99:2647–2656. doi: 10.1016/j.bpj.2010.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Godoy L.C., Muñoz-Pinedo C., Castro L., Cardaci S., Schonhoff C.M., King M., Tórtora V., Marín M., Miao Q., Jiang J.F., et al. Disruption of the M80-Fe ligation stimulates the translocation of cytochromecto the cytoplasm and nucleus in nonapoptotic cells. Proc. Natl. Acad. Sci. USA. 2009;106:2653–2658. doi: 10.1073/pnas.0809279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flatmark T. On the heterogeneity of beef heart cytochrome C. I. Separation and isolation of subfractions by dise electrophoresis and column chromatography. Acta Chem. Scand. 1964;18:1656–1666. doi: 10.3891/acta.chem.scand.18-1656. [DOI] [Google Scholar]
- 139.Robinson A.B., McKerrow J.H., Legaz M. Sequence dependent deamidation rates for model peptides of cytochrome C. Int. J. Pept. Protein Res. 1974;6:31–35. doi: 10.1111/j.1399-3011.1974.tb02355.x. [DOI] [PubMed] [Google Scholar]
- 140.Flatmark T., Sletten K. Life span of rat kidney c. In: Okunuti K., Kamen M.D., Sekuzu I., editors. Structure and Function of Cytochromes. University Press; Tokyo, Japan: 1968. pp. 413–421. [Google Scholar]
- 141.Flatmark T., Sletten K. Multiple forms of cytochrome c in the rat. Precursor-product relationship between the main component Cy I and the minor components Cy II and Cy 3 in vivo. J. Biol. Chem. 1968;243:1623–1629. [PubMed] [Google Scholar]
- 142.Parakra R.D., Kleffmann T., Jameson G.N.L., Ledgerwood E.C. The proportion of Met80-sulfoxide dictates peroxidase activity of human cytochrome c. Dalton Trans. 2018;47:9128–9135. doi: 10.1039/C8DT02185F. [DOI] [PubMed] [Google Scholar]
- 143.Wang L., Kallenbach N.R. Proteolysis as a measure of the free energy difference between cytochrome c and its derivatives. Protein Sci. 1998;7:2460–2464. doi: 10.1002/pro.5560071124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Birk A.V., Chao W.M., Liu S., Soong Y., Szeto H.H. Disruption of cytochrome c heme coordination is responsible for mitochondrial injury during ischemia. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2015;1847:1075–1084. doi: 10.1016/j.bbabio.2015.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dalle-Donne I., Aldini G., Carini M., Colombo R., Rossi R., Milzani A. Protein carbonylation, cellular dysfunction, and disease progression. J. Cell. Mol. Med. 2006;10:389–406. doi: 10.1111/j.1582-4934.2006.tb00407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kim N.H., Jeong M.S., Choi S.Y., Kang J.H. Oxidative modification of cytochrome c by hydrogen peroxide. Mol. Cells. 2006;22:220–227. [PubMed] [Google Scholar]
- 147.Tomášková N., Varinská L., Sedlák E. Rate of oxidative modification of cytochrome c by hydrogen peroxide is modulated by Hofmeister anions. Gen. Physiol. Biophys. 2010;29:255–265. doi: 10.4149/gpb_2010_03_255. [DOI] [PubMed] [Google Scholar]
- 148.Zaidi S., Hassan I., Islam A., Ahmad F. The role of key residues in structure, function, and stability of cytochrome-c. Cell. Mol. Life Sci. 2013;71:229–255. doi: 10.1007/s00018-013-1341-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Maity H., Maity M., Englander S.W. How Cytochrome c Folds, and Why: Submolecular Foldon Units and their Stepwise Sequential Stabilization. J. Mol. Biol. 2004;343:223–233. doi: 10.1016/j.jmb.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 150.Hu W., Kan Z.-Y., Mayne L., Englander S.W. Cytochrome c folds through foldon-dependent native-like intermediates in an ordered pathway. Proc. Natl. Acad. Sci. USA. 2016;113:3809–3814. doi: 10.1073/pnas.1522674113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Navarro J.A., Hervás M., Genzor C., Cheddar G., Fillat M., De La Rosa M.Á., Gomezmoreno C., Cheng H., Xia B., Chae Y., et al. Site-Specific Mutagenesis Demonstrates That the Structural Requirements for Efficient Electron Transfer in Anabaena Ferredoxin and Flavodoxin Are Highly Dependent on the Reaction Partner: Kinetic Studies with Photosystem I, Ferredoxin:NADP+ Reductase, and Cytochrome c. Arch. Biochem. Biophys. 1995;321:229–238. doi: 10.1006/abbi.1995.1390. [DOI] [PubMed] [Google Scholar]
- 152.Frazão C., Enguita F.J., Coelho R., Sheldrick G.M., Navarro J.A., Hervás M., De La Rosa M.Á., Carrondo M.A. Crystal structure of low-potential cytochrome c549 from Synechocystis sp. PCC 6803 at 1.21 A resolution. JBIC J. Biol. Inorg. Chem. 2001;6:324–332. doi: 10.1007/s007750100208. [DOI] [PubMed] [Google Scholar]
- 153.Navarro J.A., Hervás M., De La Cerda B., De La Rosa M.Á. Purification and Physicochemical Properties of the Low Potential Cytochrome C549 from the Cyanobacterium Synechocystis Sp PCC 6803. Arch. Biochem. Biophys. 1995;318:46–52. doi: 10.1006/abbi.1995.1202. [DOI] [PubMed] [Google Scholar]
- 154.Medina M., Hervás M., Navarro J.A., De La Rosa M.Á., Gómez-Moreno C., Tollin G., Medina M. A laser flash absorption spectroscopy study of Anabaena sp. PCC 7119 flavodoxin photoreduction by photosystem I particles from spinach. FEBS Lett. 1992;313:239–242. doi: 10.1016/0014-5793(92)81200-6. [DOI] [PubMed] [Google Scholar]
- 155.Sun J., Xu W., Hervás M., Navarro J.A., Rosa M.A., Chitnis P.R. Oxidizing side of the cyanobacterial photosystem I. Evidence for interaction between the electron donor proteins and a luminal surface helix of the PsaB subunit. J. Biol. Chem. 1999;274:19048–19054. doi: 10.1074/jbc.274.27.19048. [DOI] [PubMed] [Google Scholar]
- 156.Goñi G., Herguedas B., Hervás M., Peregrina J.R., De La Rosa M.A., Gómez-Moreno C., Navarro J.A., Hermoso J.A., Martínez-Júlvez M., Medina M. Flavodoxin: A compromise between efficiency and versatility in the electron transfer from Photosystem I to Ferredoxin-NADP+ reductase. Biochim. et Biophys. Acta (BBA)-Bioenerg. 2009;1787:144–154. doi: 10.1016/j.bbabio.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 157.De La Cerda B., Navarro J.A., Herv́s >M., De La Rosa M.Á. Changes in the Reaction Mechanism of Electron Transfer from Plastocyanin to Photosystem I in the CyanobacteriumSynechocystissp. PCC 6803 As Induced by Site-Directed Mutagenesis of the Copper Protein. Biochemistry. 1997;36:10125–10130. doi: 10.1021/bi9708601. [DOI] [PubMed] [Google Scholar]
- 158.Adachi J., Hasegawa M. Model of amino acid substitution in proteins encoded by mitochondrial DNA. J. Mol. Evol. 1996;42:459–468. doi: 10.1007/BF02498640. [DOI] [PubMed] [Google Scholar]
- 159.Gao L., Laude K., Cai H. Mitochondrial Pathophysiology, Reactive Oxygen Species, and Cardiovascular Diseases. Vet. Clin. N. Am. Small Anim. Pract. 2008;38:137–155. doi: 10.1016/j.cvsm.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.1111/j.1558-5646.1985.tb00420.x. [DOI] [PubMed] [Google Scholar]
- 161.Kumar S., Stecher G., Li M., Knyaz C., Tamura K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018;35:1547–1549. doi: 10.1093/molbev/msy096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Berezin C., Glaser F., Rosenberg J., Paz I., Pupko T., Fariselli P., Casadio R., Ben-Tal N. ConSeq: The identification of functionally and structurally important residues in protein sequences. Bioinformatics. 2004;20:1322–1324. doi: 10.1093/bioinformatics/bth070. [DOI] [PubMed] [Google Scholar]
- 163.Imai M., Saio T., Kumeta H., Uchida T., Inagaki F., Ishimori K. Investigation of the redox-dependent modulation of structure and dynamics in human cytochrome c. Biochem. Biophys. Res. Commun. 2016;469:978–984. doi: 10.1016/j.bbrc.2015.12.079. [DOI] [PubMed] [Google Scholar]
- 164.Storz P. Mitochondrial ROS – radical detoxification, mediated by protein kinase D. Trends Cell Biol. 2007;17:13–18. doi: 10.1016/j.tcb.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 165.Liesa M., Shirihai O. Mitochondrial Dynamics in the Regulation of Nutrient Utilization and Energy Expenditure. Cell Metab. 2013;17:491–506. doi: 10.1016/j.cmet.2013.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Hüttemann M., Lee I., Grossman L.I., Doan J.W., Sanderson T.H. Phosphorylation of mammalian cytochrome c and cytochrome c oxidase in the regulation of cell destiny: Respiration, apoptosis, and human disease. Single Mol. Single Cell Seq. 2012;748:237–264. doi: 10.1007/978-1-4614-3573-0_10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Pfeffer C.M., Singh A.T.K. Apoptosis: A Target for Anticancer Therapy. Int. J. Mol. Sci. 2018;19:448. doi: 10.3390/ijms19020448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.MacMillan-Crow L.A. Mitochondrial tyrosine nitration precedes chronic allograft nephropathy. Free. Radic. Biol. Med. 2001;31:1603–1608. doi: 10.1016/S0891-5849(01)00750-X. [DOI] [PubMed] [Google Scholar]
- 169.Morison I.M., Bordé E.M.C., Cheesman E.J., Cheong P.L., Holyoake A.J., Fichelson S., Weeks R.J., Lo A., Davies S.M.K., Wilbanks S.M., et al. A mutation of human cytochrome c enhances the intrinsic apoptotic pathway but causes only thrombocytopenia. Nat. Genet. 2008;40:387–389. doi: 10.1038/ng.103. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.