Abstract
Beech trees of the genus Fagus (Fagaceae) are monoecious and distributed in the Northern Hemisphere. They represent an important component of mixed broad-leaved evergreen–deciduous forests and are an economically important source of timber. Despite their ecological and economical importance, however, little is known regarding the overall plastome evolution among Fagus species in East Asia. In particular, the taxonomic position and status of F. multinervis, a beech species endemic to Ulleung Island of Korea, remains unclear even today. Therefore, in this study, we characterized four newly completed plastomes of East Asian Fagus species (one accession each of F. crenata and F. multinervis and two accessions of F. japonica). Moreover, we performed phylogenomic analyses comparing these four plastomes with F. sylvatica (European beech) plastome. The four plastomes were highly conserved, and their size ranged from 158,163 to 158,348 base pair (bp). The overall GC content was 37.1%, and the sequence similarity ranged from 99.8% to 99.99%. Codon usage patterns were similar among species, and 7 of 77 common protein-coding genes were under positive selection. Furthermore, we identified five highly variable hotspot regions of the Fagus plastomes (ccsA/ndhD, ndhD/psaC, ndhF/rpl32, trnS-GCU/trnG-UCC, and ycf1). Phylogenetic analysis revealed the monophyly of Fagus as well as early divergence of the subgenus Fagus and monophyletic Engleriana. Finally, phylogenetic results supported the taxonomic distinction of F. multinervis from its close relatives F. engleriana and F. japonica. However, the sister species and geographic origin of F. multinervis on Ulleung Island could not be determined.
Keywords: plastome, eastern asia beeches, Fagus multinervis, Ulleung Island, Fagaceae
1. Introduction
Genus Fagus L. (Fagaceae), commonly distributed in the Northern Hemisphere, is an ecologically and economically important tree lineage of broad-leaved deciduous-evergreen forests of North America and East Asia as well as the most abundant broad-leaved tree genus in Europe and West Asia [1,2,3,4]. With the center of diversity in East Asia, Fagus comprises 10 monoecious broad-leaved deciduous tree species within two subgenera, namely Engleriana (three species) and Fagus (seven species) [1,5]. While subgenus Engleriana is restricted to East Asia (China, Korea, and Japan), Fagus is distributed almost throughout the Northern Hemisphere. Fagus japonica Maxim. and F. okamotoi Shen [5] of subgenus Engleriana are endemic to Japan, while F. engleriana Seemen is distributed in China; the taxonomic entity of F. okamotoi is uncertain in Japan and it is not currently recognized as a distinct species. Of the seven species of subgenus Fagus, F. sylvatica L. is widely distributed in Europe and southwestern Asia and F. grandifolia Ehrh. in eastern North America and Mexico [2]. Meanwhile, some species are geographically restricted to specific regions, such as F. crenata Blume to Japan, F. longipetiolata Seemen and F. lucida Rehder and Wilson to China, and F. chienii Cheng to western China. F. hayatae Palibin is distributed in a disjunct manner in Mainland China and Taiwan. Moreover, F. okamotoi has been recorded from few localities in Japan, while F. chienii is recorded from a single locality in western China [2]. In addition, former distinct species but current synonyms of F. longipetiolata show restricted geographical distribution; for instance, F. brevipetiolata Hu is recorded from few localities in China and F. bijiensis C.F. Wei & Y.Y. Chang and F. tientaiensis T.N. Liou are recorded from a single locality in western China [1,2,5].
Based on numerous fossil records (see references in Denk [1] and Renner et al. [6]), Fagus has been speculated to have already existed in the early Cenozoic in the northern Pacific Basin, extending to the Axel Heiberg Island and western Greenland and further spreading westward to Central Asia and Europe during the Oligocene. Based on 53 fossil records and nuclear sequences of nine Fagus species, Renner et al. [6] constructed a fossilized birth-death model to estimate the divergence time of major lineages within this genus. The model revealed that the crown group originated in the early Eocene, nearly 53 million years ago (Ma); F. grandifolia (America) diverged 44 Ma; F. sylvatica (western Eurasia) diverged from F. crenata (Japan) nearly 23 Ma; and F. sylvatica (Central Europe) diverged from F. orientalis (eastern Mediterranean; now treated as a conspecific of F. sylvatica) nearly 9 Ma. In addition to molecular dating, several phylogenetic analyses have been performed to infer species relationships within the genus. For instance, the non-monophyly of subgenus Engleriana and overall diverse species relationships within the genus have been demonstrated [7,8]. Meanwhile, a morphological study has revealed the monophyly of subgenus Engleriana, which is either deeply nested within or reciprocally monophyletic with subgenus Fagus [1]. Another study based on nuclear internal transcribed spacer (ITS) region sequences and morphological data [2] showed the early divergence of Eurasian species and paraphyly of subgenus Fagus. Given this tree topology, subgenus Engleriana has been inferred to be nested within subgenus Fagus. Morphological data have also suggested the early divergence of F. hayatae and F. longipeiolata from Taiwan and China and identified two intercontinental disjunct distributed taxa, namely F. crenata (Japan) and F. sylvatica (Europe) as well as F. grandifolia (eastern North America) and F. engleriana/F. japonica/F. okamotoi (East Asia). Based on nuclear ITS region and LEAFY sequence data, Renner et al. [6] demonstrated the early divergence of monophyletic subgenus Engleriana (F. engleriana and F. japonica) and its sister relationship to monophyletic subgenus Fagus. Furthermore, Oh et al. [9] reconstructed the phylogeny of Fagus based on both nuclear (LEAFY) and chloroplast (cpDNA) sequence data but yielded largely unsupported and conflicting topologies. Specifically, the tree based on cpDNA sequences suggested unresolved relationships among the lineages of F. grandifolia, F. sylvatica, F. crenata/F. japonica, and F. engleriana (including F. multinervis)/F. longipetiolata/F. japonica. Meanwhile, the tree based on LEAFY sequences identified clades containing F. engleriana (including F. multinervis) and F. japonica as well as F. lucida/F. longipetiolata. Therefore, a large-scale phylogenomic study is warranted to resolve phylogenetic relationships of species within the genus as well as to infer hybridization and introgression.
Similar to several other taxonomically controversial taxa (e.g., F. orientalis, F. brevipetiolata, F. bijiensis, and F. tientaiensis, among others), the phylogenetic position and species delimitation of F. multinervis have remained unclear for several decades. In the Korean Peninsula, a single Fagus species, F. multinervis, occurs on Ulleung Island but its taxonomic status and relationship to other Fagus species remains controversial. Ulleung Island (with the estimated age of ca. 1.8 Ma) is an oceanic and a volcanic island located at about 130 km from the eastern coast of the Korean Peninsula. As a dominant tree in mixed evergreen-deciduous forests at elevations ranging from 400 to 940 m, F. multinervis represents an ecologically, economically and culturally important component of the island [9]. Considering leaf, anther, and pollen characteristics, F. multinervis was considered an ecological variant of F. japonica, which is endemic to central and southern Japan [10]. Meanwhile, based on cupule characteristics, F. multinervis has been more frequently and recently treated as a synonym of F. engleriana, implying disjunct distribution in western and eastern China as well as Ulleung Island in Korea [1,2,5,11]. However, F. multinervis has also been proposed as a taxonomically distinct species endemic to Ulleung Island [12,13,14,15]. Specifically, Oh et al. [9] demonstrated the monophyly of F. multinervis and its unresolved relationships with F. engleriana and F. japonica. They supported the recognition of F. multinervis as a distinct species endemic to Ulleung Island and its potential hybrid origin based on the topological incongruence. Nonetheless, despite several aforementioned previous reports, the sister relationships and geographical origin of F. multinervis require additional evidence.
Recently, whole plastome phylogenomic analyses have gained popularity due to unique characteristics of plastomes and technical advantages [16]. Owing to high structural conservation and slow evolutionary rate, plastid phylogenomics has significantly advanced our understanding of unresolved high- or deep-level relationships among angiosperms [17,18,19,20,21]. In addition, plastome sequencing has revealed considerable variation within and between plant species [16]. Therefore, such information is particularly fundamental to provide a greater resolution and stronger support at lower taxonomic levels [22,23,24,25,26,27,28,29]. Recently, several studies have reported the whole plastome sequences of Fagus and related genera, such as Quercus and Castanea, in Fagaceae [30,31,32,33,34]. Previous phylogenetic studies of Fagus were based primarily on nuclear ITS region and LEAFY intron and rarely on plastid coding or noncoding regions, which are either highly variable or highly conserved, providing unsupported congeneric relationships [2,9]. Therefore, recent characterization studies have been particularly helpful to understand plastome organization and structure in congeners of Fagus as well as to determine their phylogenetic relationships. To clarify the phylogenetic position and taxonomic status of F. multinervis endemic to Ulleung Island, two accessions of this species were sequenced, which revealed intraspecific variation [31,33]. However, these studies, which did not include F. japonica endemic to Japan, suggested that F. engleriana is sister to two accessions of F. multinervis and that F. crenata, another species endemic to Japan, is sister to the clade containing F. sylvatica/F. engleriana/F. multinervis. To determine the precise phylogenetic position of F. multinervis based on plastome data, F. japonica must be included in the analysis. Furthermore, structural variation and molecular evolution of plastomes within Fagus have been addressed only to a limited extent based on two species (F. crenata and F. engleriana) [32,34], which cannot clarify the whole scenario of plastome evolution within this genus.
To this end, in the present study, we characterized the first two plastome sequences of F. japonica and the second accession of F. crenata, both of which are endemic to Japan. We also sequenced an additional accession of F. multinervis from the northern part of Ulleung Island and compared it with two previously reported accessions from the eastern and southeastern parts of the island. Furthermore, we performed comparative analyses across nine plastomes in two subgenera, namely Fagus (F. crenata and F. sylvatica) and Engleriana (F. engleriana, F. japonica, and F. multinervis). We aimed to (1) characterize the plastome structure and evolution within Fagus, (2) gain insight into the phylogenetic position of F. multinervis on Ulleung Island, and (3) identify useful chloroplast markers, including mutation hotspots, to construct strongly supported and highly resolved phylogenetic trees of Fagus species. Given the limited samples and geographic coverage used in this study, we cautiously interpret our results for the first two objectives, requiring further evidence from a large-scale plastid phylogenomic study and independent nuclear loci data.
2. Materials and Methods
2.1. Plant Sampling, DNA Isolation, and Plastome Sequencing and Annotation
We sampled one accession of F. crenata and two accessions of F. japonica from Sasari, Nantan, Kyoto Prefecture, Japan (35°16′39.4″ N 135°43′50.2″ E) and one accession of F. multinervis from Buk-myeon, Ulleung Island, Korea (37°30′57 N 130°52′10 E). A previously sequenced accession of F. crenata (NC041252) was collected from Daisengen Peak on the northern island Hokkaido, Japan (41.616° N, 140.1333° E) [32]. The accession sequenced in this study represents a sample from southern Japan. The F. multinervis accession sequenced in this study was collected from the northern part of Ulleung Island (~400 m elevation), while the two previously sequenced accessions [31,33] were collected from the eastern (37°30′ N, 130°54′ E, 216 m elevation) and southeastern (37°29′19.0″ N, 130°53′15.9″ E, 471 m elevation) parts of the island. Voucher specimens are deposited in the Ha Eun Herbarium of Sungkyunkwan University, Korea.
Fresh leaves were collected and dried with silica gel before DNA extraction. Total DNA was extracted using the DNeasy Plant Mini Kit (Qiagen, Carlsbad, CA, USA) and sequenced with Illumina HiSeq 4000 (Illumina, Inc., San Diego, CA, USA), yielding 150 bp paired-end read length, at Macrogen Co. (Seoul, Korea). The resulting paired-end reads were assembled de novo using Velvet v1.2.10 with multiple k-mers [35]. tRNAs were confirmed using tRNAscan-SE [36]. Sequences were annotated using Geneious R10 [37] and deposited in GenBank [F. crenata (MT762292), F. japonica7-1 (MT762294), F. japonica10-1 (MT762295), and F. multinervis (MT762296)]. Annotated sequence files in the GenBank format were used to draw a circular map with OGDRAW v1.2 [38].
2.2. Comparative Plastome Analysis
Using the Shuffle-LAGAN mode [39] of mVISTA [40], six complete plastomes of Fagus species were compared: one plastome each of F. crenata, F. engleriana, F. multinervis, and F. sylvatica and two plastomes of F. japonica. Sequences of the six Fagus plastomes were aligned using the back-translation approach with MAFFT ver.7 [41] and manually edited with Geneious R10 [37]. Using DnaSP 6.10 [42], sliding window analysis with a step size of 200 bp and window length of 800 bp was performed to determine nucleotide diversity (Pi) of the plastomes. Codon usage frequency was calculated using MEGA 7 [43] based on the relative synonymous codon usage (RSCU) value [44], which is a simple measure of non-uniform usage of synonymous codons in a coding sequence. The DNA code used by bacteria, archaea, prokaryotic viruses, and chloroplast proteins was used [45]. Protein-coding genes were run using the PREP suite [46] with 35 reference genes and a cut off value of 0.8 to predict possible RNA editing sites in five Fagus plastomes that were newly reported in the present study. Analyses based on complete plastomes and concatenated sequences of 77 common protein-coding genes of the studied Fagus species were performed using MAFFT ver.7 [41] in Geneious R10 (Kearse et al., 2012). A maximum likelihood (ML) phylogenetic tree was constructed using IQ-TREE 1.4.2 [47]. To evaluate natural selection pressure on the protein-coding genes of the five Fagus plastomes, a site-specific model was developed using EasyCodeML [48] with the CODEML algorithm [49]. Seven codon substitution models (M0, M1a, M2A, M3, M7, M8, and M8a) were constructed and compared to detect positively selected sites based on likelihood ratio test (LRT).
2.3. Phylogenetic Analysis
For phylogenetic analysis, complete plastome sequences of 17 representative species of Fagaceae were aligned with MAFFT ver.7 [41] in Geneious R10 [37]: two Castanea species, including C. henryi (NC033881) and C. pumila (KM360048); five Quercus species, including Q. aquifolioides (NC026913), Q. spinosa (NC026907), Q. rubra (NC020152), Q. tarokoensis (NC036370), and Q. variabillis (NC031356); four Fagus species, including F. crenata (NC041252), F. engleriana (NC036929), F. multinervis (MK518070), and F. sylvatica (NC041437); one Betula species, namely B. nana (NC033978). ML analysis based on the best-fit model of “K3Pu+F+R3” was conducted with IQ-TREE 1.4.2 [47]. B. nana was used as the outgroup, and non-parametric bootstrap analysis was performed with 1000 replicates.
3. Results
3.1. Genome Size and Characteristics
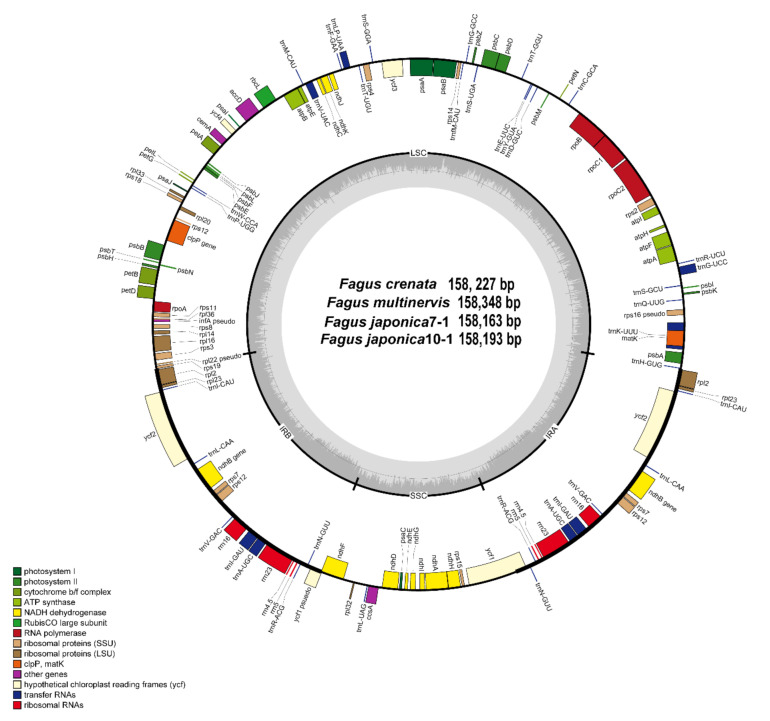
The plastomes of four accessions of East Asian Fagus species were newly characterized, including the plastome of F. japonica for the first time. The size of complete plastome sequences ranged from 158,348 (F. multinervis) to 158,163 bp (F. japonica7-1) (Table 1). The plastomes were highly conserved, with no structural variations or content rearrangements (Figure 1). The four plastomes of East Asian Fagus species contained 131 genes, including 82 protein-coding, 8 rRNA, and 37 tRNA genes. Consistent with previous reported values for Fagus plastomes (35.5% for F. crenata [32]; 37.0% for F. engleriana [34]; and 37.1% for F. multinervis [31,33]), the overall GC content was 37.1% (Table 1). Moreover, 17 genes were duplicated in the inverted repeat (IR) regions, including 7 tRNA, 4 rRNA, and 6 protein-coding genes. A total of 15 genes (ndhA, ndhB, petB, petD, rpl2, rpl16, rpoC1, rps16, rps12, trnA-UGC, trnG-UCC, trnI-GAU, trnK-UUU, trnL-UAA, and trnV-UAC) contained 1 intron, while 2 genes (clpP and ycf3) contained 2 introns.
Table 1.
Summary of characteristics of four Fagus plastomes in eastern Asia.
| Taxa | F. crenata | F. japonica7-1 | F. japonica10-1 | F. multinervis |
|---|---|---|---|---|
| Total cpDNA size (bp) | 158,227 | 158,163 | 158,193 | 158,348 |
| GC content (%) | 37.1% | 37.1% | 37.1% | 37.1% |
| LSC size (bp)/GC content (%) | 87,552/35.1% | 87,590/35.1% | 87,620/35.1% | 87,659/35.0% |
| IR size (bp)/GC content (%) | 25,873/42.7% | 25,894/42.7% | 25,893/42.7% | 25,893/42.7% |
| SSC size (bp)/GC content (%) | 18,929/31.1% | 18,785/31.2% | 18,787/31.2% | 18,903/31.1% |
| Number of genes | 131 | 131 | 131 | 131 |
| Number of protein-coding genes | 82 | 82 | 82 | 82 |
| Number of tRNA genes | 37 | 37 | 37 | 37 |
| Number of rRNA genes | 8 | 8 | 8 | 8 |
| Number of duplicated genes | 17 | 17 | 17 | 17 |
| Accession Number | MT762292 | MT762294 | MT762295 | MT762296 |
LSC: Large single-copy region, IR: Inverted repeat, SSC: Small single-copy region.
Figure 1.
The four Fagus plastomes in Fagaceae. The genes located outside of the circle are transcribed clockwise, while those located inside are transcribed counterclockwise. The gray bar area in the inner circle denotes the guanine-cytosine (GC) content of the genome, whereas the lighter gray area indicates the adenosine-thymine (AT) content of the genome. Large single copy, small single copy, and inverted repeat are indicated with LSC, SSC, and IR, respectively.
Partial ycf1 (1113–1131 bp) was located in the IRb/SSC junction region, and complete ycf1 (5670–5688 bp) was located in the IR region at the SSC/IRa junction. Similar to those of F. engleriana [34], three protein-coding genes, namely infA, rps16, and rpl22, of the four East Asian Fagus plastomes were pseudogenes.
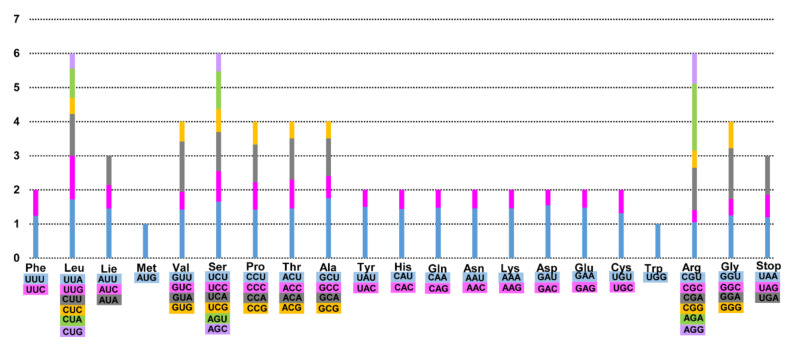
Codon usage frequency of the five complete East Asia Fagus plastomes was calculated using sequences of protein-coding and tRNA genes (Figure 2). Average codon usage frequency ranged from 25,748 (F. crenata) to 26,053 (F. multinervis), although the distribution of codon types was consistent. Codon usage in the four East Asian Fagus plastomes was biased toward high RSCU values of U and A at the third codon position.
Figure 2.
Codon distribution and Relative Synonymous Codon Usage (RSCU) in five Fagus plastomes. The RSCU values are represented on the y-axis, while the codon families for each amino acid are denoted on the x-axis.
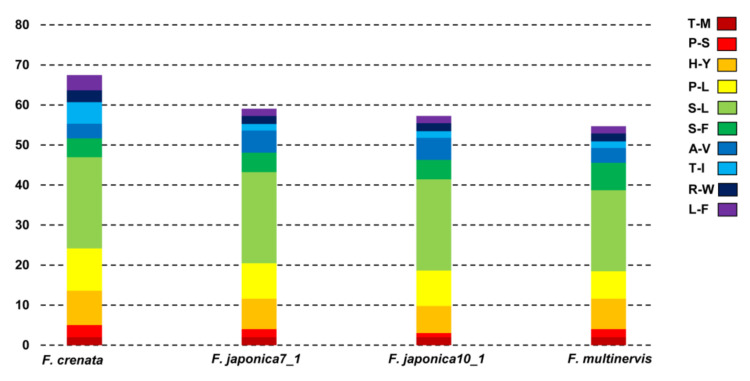
Prediction of RNA editing sites in the five East Asian Fagus species indicated 75 sites with the same cut-off value in 23 of the 35 protein-coding genes (Table S1). These included photosynthesis-related genes (atpA, atpF, atpI, ndA, ndhB, ndhD, ndhF, ndhG, petB, psaI, psbE, and psbF), self-replication-related genes (rpoA, rpoB, rpoC2, rps2, rps14, and rps16), and others (accD, clpP, ccsA, and matK). The ndhF in F. multinervis showed a significantly higher frequency of RNA editing sites (8 sites) than in other species. Moreover, rpoC2 in F. crenata showed the highest frequency of RNA editing sites (13 in F. crenata vs. 3 in others). In F. japonica and F. multinervis, ndhB was predicted to contain the highest number of potential editing sites (10 sites), followed by ndhD (seven sites). All editing sites showed base transition from C to T, and the most frequent conversion was serine to leucine (Figure 3). Consequently, amino acids with hydrophobic chains (isoleucine, leucine, methionine, phenylalanine, tryptophan, and valine) were formed in 82.3% of the 23 RNA editing sites.
Figure 3.
Amino acid changes in potential RNA editing sites of the chloroplast genomes of five Fagus. Color bricks indicate RNA editing effect. T-M: Threonine to Methionine, P-S: Proline to Serine, H-Y: Histidine to Tyrosine, P-L: Proline to Leucine, S-L: Serine to Leucine, S-F: Serine to Phenylalanine, A-V: Alanine to Valine, T-I: Threonine to lsoleucine, R-W: Arginine to Tryptophan, L-F: Leucine to Phenylalanine.
To assess intraspecific variations in three East Asian Fagus species, we compared four newly completed Fagus plastomes with previously reported ones (F. crenata, NC041252; F. multinervis, MK518070 and MN894556). The plastome of F. crenata (158,227 bp) from southern Japan known to have chlorotype “BF” [50] was compared with a previously reported plastome from northern Japan (NC041252, 158,372 bp) known to have chlorotype “A.” The sequences of two intraspecific plastomes shared 99.8% identity, with 59 indels and 45 substitutions. Moreover, 11 protein-coding genes (atpB, atpF, matK, ndhD, petA, rpl16, rpoB, rpoC2, rps16, and ycf1) were polymorphic, with 8 indels and 9 substitutions. Of these, rpoB and petA harbored a single nonsynonymous substitution. In addition, 51 indels and 34 substitutions were detected in intergenic regions in the two F. creanata plastomes. Specifically, the intergenic region trnE-UUU/trnT-GGU/psbD harbored 33 indels and 26 substitutions.
Two newly sequenced accessions of F. japonica in the present study shared 99.98% sequence identity, with three indels and one substitution. The total lengths of the two plastomes of F. japonica 7-1 and F. japonica10-1 were 158,163 and 158,193 bp, respectively. We also compared three plastomes of F. multinervis from Ulleung Island, which shared 99.99% sequence identity. The newly assembled plastome of F. multinervis in this study was identical to the accession MK518070 (158,348 bp) sampled from the eastern part of the island. Compared to the other accession, MN894556, sampled from the southeastern part of the island, a frameshift with one base-pair insertion (T) was detected in atpB, resulting in a shorter gene with early termination. In addition, as previously reported, one synonymous substitution in psbM and one nonsynonymous substitution in ccsA were detected.
3.2. Comparative Analysis of Genome Structure
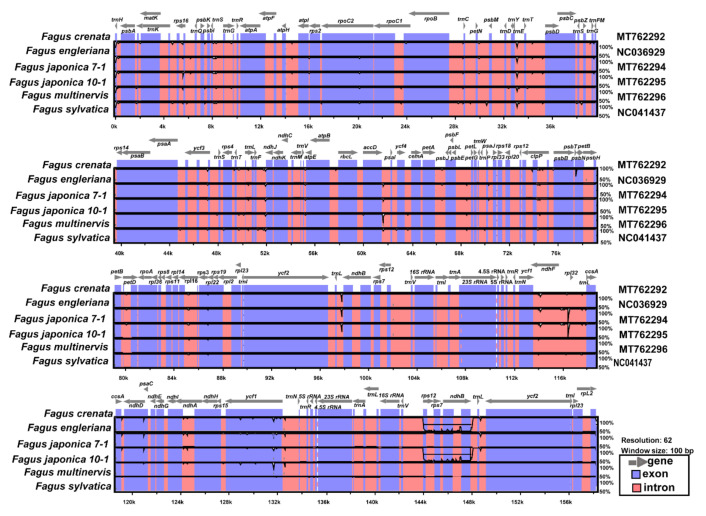
Five complete plastome sequences of East Asian Fagus species and one European Fagus (F. sylvatica) were plotted using mVISTA [40], with the annotated F. crenata plastome as a reference (Figure 4). As expected, the large single-copy (LSC) region was the most divergent, and the two IR regions were highly conserved. Moreover, the non-coding regions were more divergent and variable than the coding regions. Overall, all Fagus plastomes showed high sequence similarity (i.e., 99.6% sequence identity; 157,284 bp identical sites) to the F. crenata plastome.
Figure 4.
Visualization of alignment of the six Fagus plastome sequences of Fagaceae species. Vertical scale indicates the percent identity from 50% to 100%. Coding and non-coding regions are in blue and pink, respectively. Gray arrows above the alignment indicate the position and direction of each gene.
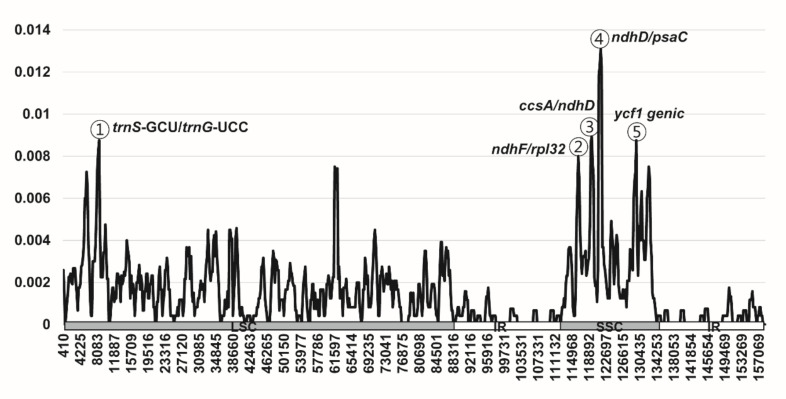
Sliding window analysis using DnaSP [42] revealed highly variable regions in Fagus plastomes (Figure 5). When plastomes of five East Asian and one European Fagus accessions were compared, average Pi over the whole plastome was 0.0016. The ndhD/psaC intergenic region was the most variable region, with a Pi value of 0.01308. Moreover, highly variable regions included three other intergenic regions, namely trnS-GCU/trnG-UCC (Pi = 0.00875), ccsA/ndhD (Pi = 0.00892), and ndhF/rpl32 (Pi = 0.008), and one ycf1 genic region (Pi = 0.00875). Overall, five highly variable regions with a Pi value greater than 0.008 were identified in six Fagus plastomes.
Figure 5.
Sliding window analysis of the six whole-chloroplast genomes of Fagus species. X-axis: position of the window midpoint, Y-axis: nucleotide diversity within each window.
The site-specific model developed using EasyCodeML [48] with the CodeML algorithm [49] identified positively selected genes among six Fagus plastomes (Table 2). Seven conserved genes across Fagus plastomes were predicted to be under positive selection, with significant LRT p values. Moreover, in the six comparison groups, 70 of the 77 genes had an average Ka/Ks ratio below 1, suggesting that these genes were subjected to strong purifying selection in the Fagus plastomes. The remaining seven genes had an average Ka/Ks ratio greater than 1, suggesting that these genes were positively selected in the six Fagus plastomes. These genes include two photosynthesis-related genes encoding NADH-dehydrogenase subunits (ndhD and ndhJ), two genes encoding DNA-dependent RNA polymerase (rpoB and rpoC2), one self-replication-related gene encoding the ribosomal small subunit (rps16), and two unknown genes (ycf1 and ycf2). Based on the M8 model, ycf1 had the highest number of positive sites (9 sites), followed by rpoC2 (2 sites), while the other five genes had only number of positive sites (9 sites), followed by rpoC2 (2 sites), while the other five genes had only one positive site.
Table 2.
Log-Likelihood values of the site-specific models, with detected sites having dN/dS values > 1.
| Gene Name | Models | np | ln L | Likelihood Ratio Test p-Value | Positively Selected Sites |
|---|---|---|---|---|---|
| ndhD | M8 | 15 | −2087.368596 | 0.000000130 | 385 T 0.951 * |
| M7 | 13 | −2103.224804 | |||
| ndhJ | M8 | 15 | −670.195417 | 0.000000009 | 151 F 0.995 ** |
| M7 | 13 | −688.698395 | |||
| rpoB | M8 | 15 | −4347.768637 | 0.000000009 | 248 Q 0.959 * |
| M7 | 13 | −4372.590740 | |||
| rpoC2 | M8 | 15 | −5635.415430 | 0.000000000 | 748 Y 0.984 *,1338 I 0.983 * |
| M7 | 13 | −5656.937844 | |||
| rps16 | M8 | 15 | −242.458940 | 0.000000720 | 1 M 0.995 ** |
| M7 | 13 | −256.602491 | |||
| ycf1 | M8 | 15 | −7160.045295 | 0.000000000 | 372 K 1.000 **, 373 T 1.000 **, 842 F 0.991 **, 905 P 0.991 **, 1066 R 0.992 **, 1142 F 0.991 **, 1284 N 1.000 **, 1286 D 0.991 **, 1359 F 0.999 ** |
| M7 | 13 | −7218.177588 | |||
| ycf2 | M8 | 15 | −9220.322295 | 0.000000000 | 388 T 0.982 * |
| M7 | 13 | −9248.972947 |
* p < 0.05; ** p < 0.01. np represents the degree of freedom.
3.3. Phylogenetic Analysis
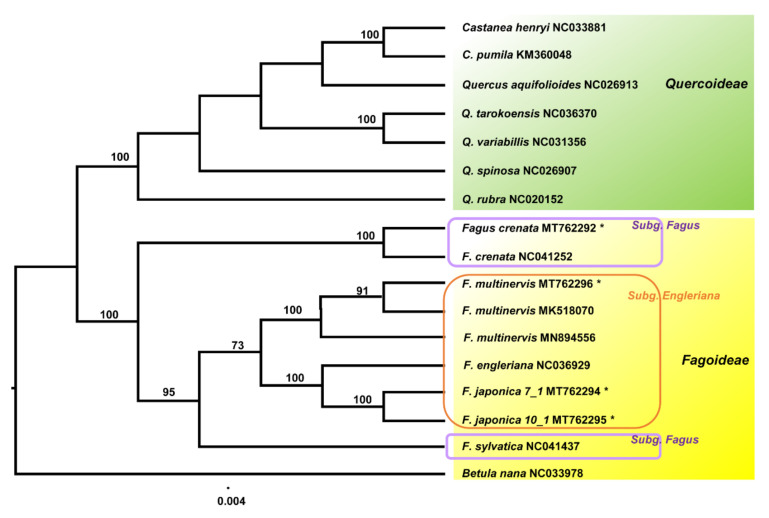
ML of the best-fit model of “K3Pu+F+R3” suggested well-resolved phylogenetic relationships within Fagaceae (Figure 6). Phylogenetic analysis of 16 representative plastomes of Fagaceae supported the monophyly of Fagus (100% bootstrap support (BS)) as well as the sister relationship between Fagus and the clade containing Castanea and Quercus (weak support, <60% BS). Within the subfamily Quercoideae, the ML tree suggested that monophyletic Castanea is deeply nested within Quercus, making it paraphyletic. Within Fagus, the plastome phylogenomic tree suggested that Japanese F. crenata diverged first, followed by European F. sylvatica. Based on three accessions, F. multinervis endemic to Ulleung Island is monophyletic (100% BS), whereas F. engleriana and the newly sequenced F. japonica are sister to each other (100% BS). Finally, F. multinervis is sister to the clade containing F. engleriana and F. japonica (moderate support, 73% BS), although its sister species remain undetermined.
Figure 6.
Maximum likelihood tree inferred from 17 representative taxa of Fagaceae. Bootstrap values based on 1000 replicates are shown on each node. Asterisk (*) represents the newly assembled plastomes of Fagus (Fagaceae) in this study.
4. Discussion
4.1. Intraspecific Variation and Plastome Evolution in Fagus
In the present study, we characterized two complete plastomes of the Japanese endemic F. japonica, which represents subgenus Engleriana, for the first time. These two plastomes showed very high similarity (i.e., 99.98% identity), with few indels and substitutions. Interestingly, sequencing of the second accession of F. crenata (subgenus Fagus), another species endemic to Japan, albeit with a much broader geographical distribution, revealed numerous indels and substitutions. These results indicate that F. crenata, which appears to have diverged much earlier than F. japonica, may show much greater intraspecific plastome variation. Since the two accessions of F. japonica were sampled from geographically close areas, this species may show much smaller variation than F. crenata. Therefore, this result warrants further confirmation through more extensive geographical sampling. Whole plastomes of three accessions of F. multinervis, which is endemic to Ulleung Island, from geographically different areas of the island showed very little variation. One frameshift insertion in atpB, resulting in early termination, one synonymous substitution in psbM, and one nonsynonymous substitution in ccsA were detected within F. multinervis plastomes. Similarly, very little variation in plastomes (i.e., just one haplotype) has been reported based on the trnH/psbA intergenic region, perhaps due to repeated colonization from a narrow source with a geographically structured chloroplast haplotype and a single long-distance seed dispersal event for the progenitor of F. multinervis [51]. Since very little variation was found in plastomes of F. multinervis, it is necessary to survey five highly variable regions within Fagus identified in current study (i.e., ndhD/psaC, ccsA/ndhD, trnS/trnG, ycf1, and ndhF/rpl32) to uncover the species’ phylogeographic structure on Ulleung Island.
Similar to the plastomes of several other congeners of Fagaceae [34,52], the East Asian species of Fagus tested in the present study had highly conserved plastomes, with no structural variations and content rearrangements. In addition, codon usage patterns of the five whole plastomes of East Asian Fagus species, including one plastome each of F. crenata, F. engleriana, and F. multinervis and two plastomes of F. japonica, were comparable. In these plastomes, codon usage was biased toward high RSCU values of U and A at the third codon position. Similar patterns have been observed in other angiosperm [53] and algal [54] lineages. Prediction of RNA editing sites in the five East Asian Fagus plastomes obtained consistent results with previous reports [55,56,57]. The ndhB (10 sites) had the highest number of potential editing sites, followed by ndhD (7 sites), in F. japonica and F. multinervis, whereas rpoC2 had an exceptionally high number of editing sites (13 sites) in the single-stemmed F. crenata. The most frequent conversion was serine to leucine, and most RNA editing sites (82.3%) increased protein hydrophobicity.
Plastomes of all Fagus congeners in East Asia and Europe showed pseudogenization of three protein-coding genes, namely infA, rpl22, and rps16. While infA is intact in Quercus, the sister lineage of Fagus, it is lost in Castanea. Similarly, infA is lost in all studied Fagus species, indicating independent loss of this gene within Fagaceae [34]. Within Fagaceae, rps16 became a pseudogene in Fagus but not in its sister lineages Castanea and Quercus. The rpl22 became a pseudogene in all studied Castanea, Quercus, and Fagus species within Fagaceae, representing family synapomporphy. Based on comparative plastome analyses among members of the related order Malpighiales, Menezes et al. [58] reported the presence or absence of three protein-coding genes (infA, rpl32, and rps16) and two pseudogenes (ycf1 and rps19). infA was present or absent in the plastome within the families of the order Malpighiales. The rps16 and rpl32 were independently lost in Violaceae and Salicaceae within this order. Given the evidence that some genes (infA, rpl32, and rps16) absent from the plastome in certain taxa are transferred to the nuclear genome in angiosperms [59,60,61], whether the three genes absent from the plastome of Fagus species were transferred to another genome or were completely lost should be investigated [62,63,64].
Most genes of the plastome evolve under purifying selection due to functional limitations during the course of evolution [65,66,67,68]. In the five Fagus plastomes studied, nearly 7.8% of protein-coding genes, including two photosynthesis-related genes encoding NADH-dehydrogenase subunits (ndhD and ndhJ), two genes encoding DNA-dependent RNA polymerase (rpoB and rpoC2), one self-replication-related gene encoding the ribosomal small subunit (rps16), and two unknown genes (ycf1 and ycf2), were under positive selection pressure. Similar sets of genes (ndhA, ndhK, petA, and ycf1) were found to be under positive selection in the sister lineage Quercus [52]. Positive selection of genes encoding NADH-dehydrogenase and ribosomal complex has also been reported in other angiosperms, such as Iodes (Icacinaceae) and Citrus (Rutaceae) [69,70]. Moreover, positive selection of ycf1 and ycf2 has been suggested in Iodes (Icacinaceae [69]), Panax (Araliaceae [67]), and Sileneae (Caryophyllaceae [65]). Therefore, genes that protect plants from excess light and high temperature, such as NADH-dehydrogenase and ribosomal complex genes, may be positively selected in East Asian Fagus species [71,72]. Positive selection is considered to be indicative of adaptation to environmental changes, ecological niches, or coevolutionary processes [73,74]. Therefore, we speculate that the plastomes of Fagus species have contributed to their divergence and adaptation to temperate mixed deciduous forests in East Asia, although this topic warrants further research.
Identification and application of a highly variable or hotspot region in the plastome can achieve better resolution among closely related species or recently radiated groups. Recently, several hotspot regions, including genic and non-coding regions, across whole plastomes were reported based on the comparison between F. crenata (subgenus Fagus) and F. engleriana (subgenus Engleriana) [32]. The study found substantially lower pairwise nucleotide differences (p-distances) between the two species of Fagus than between those of its sister genus Quercus (0.0018 vs. 0.0042) [32]. In addition, Worth et al. [32] identified six highly variable regions (in decreasing order of variability, ndhD/psaC, ndhI/ndhH, trnV, rpl32, trnG/psbfM, and psbK/psbI) between two Fagus species. Sampling of additional East Asian Fagus species and their comparison with European species in the present study revealed five highly variable regions (in decreasing order of variability, ndhD/psaC, ccsA/ndhD, trnS/trnG, ycf1, and ndhF/rpl32). Thus, the ndhD/psaC intergenic region is the only and the most variable region in genus Fagus based on both the present and previous studies. As closely related species and conspecific accessions were included to identify hotspots, previously identified regions (ndhI/ndhH, trnV, rpl32, trnG/psbfM, and psbK/psbI) [32] showed significantly lower Pi values (<0.00492 for ndhI/ndhH) than the four regions (ccsA/ndhD, trnS/trnG, ycf1, and ndhF/rpl32; Pi > 0.008) identified in the present study. Therefore, the five highly variable regions identified in this study can be effective chloroplast DNA markers for population genetic and phylogeographic studies of Fagus species. When these hotspots were compared to those in the closest sister lineage Quercus, some regions (e.g., ycf1 and ccsA/ndhD) were consistently recognized as hotspots between the two genera, while others (e.g., rpl22, rps16, trnR/atpA, and trnM/atpE, among others) were identified in Quercus alone, indicating that these regions may serve as effective taxon-specific phylogenetic and DNA barcoding markers.
4.2. Phylogenetic Position and Relationship of Fagus multinervis on Ulleung Island
Since there are no Fagus populations in the Korean Peninsula, the origin and evolution of F. multinervis endemic to Ulleung Island with respect to its close relatives F. engleriana and F. japonica have long been controversial. F. engleriana is distributed in western China (Sichuan, Guizhou, and western Hubei; above 1200 m elevation) and eastern China (southern Anhui and northwestern Zhejiang; between 900 and 1700 m elevations) [5,9]. Based on similar cupule characteristics between F. engleriana and F. multinervis (i.e., the base of cupules is covered with leaf-like bracts), Shen [5] treated F. multinervis as a conspecific of F. engleriana in China, indicating disjunct distribution in western China, Central China, and Ulleung Island, Korea. Meanwhile, based on the similarity of leaves, anthers, and pollen, Lee [10] considered F. multinervis an ecological variant of F. japonica, which is distributed in central and southern Japan. Some authors have also treated F. multinervis as a synonym of F. engleriana [5,11], while others have treated it as a distinct species based on floristics, allozymes, and comparative descriptions [12,13,15]. Based on whole plastomes of Fagus species, we hoped to determine the closest relative of F. multinervis on Ulleung Island. Unfortunately, however, even with whole plastome data, we could not determine the closest species of F. multinervis. The plastome phylogenomic tree suggested that genus Fagus is monophyletic (100% BS support) and that monophyletic subgenus Engleriana is deeply nested within subgenus Fagus (Figure 6). Moreover, F. crenata, which is endemic to Japan, diverged first, followed by the European species F. sylvatica. Within subgenus Engleriana, F. multinervis is a distinct and monophyletic clade and is sister to the clade containing two closely related species, F. japonica and F. engleriana. Based on combined plastid sequence data, Oh et al. [9] demonstrated that F. japonica accessions cultivated in the Arnold Arboretum (USA) formed a clade with F. crenata cultivated in the Tsukuba Botanical Garden (Japan) and Arnold Arboretum. Conversely, F. japonica cultivated in the Arnold Arboretum was sister to the clade containing F. engleriana and F. multinervis on Ulleung Island and was, in turn, sister to the clade containing F. engleriana, F. lucida, F. longipetiolata, and F. japonica. These relationships imply plastid capture via hybridization or introgression, both of which are known frequent processes in Fagaceae [75,76]. The nuclear LEAFY phylogeny, however, is highly unresolved in terms of the phylogenetic position of F. multinervis; the strongly supported clade (100% BS and posterior probability (PP) 1.0) includes monophyletic F. multinervis (72% BS and PP 1.0), monophyletic F. engleriana (65% BS and PP 1.0), and non-monophyletic F. japonica. Oh et al. [9] further argued that given the patterns of phylogenetic incongruence between plastid and nuclear phylogenies and certain shared morphological traits, F. multinervis could be a hybrid between F. engleriana in China and F. japonica in Japan. The divergence of two close relatives F. engleriana and F. japonica was estimated to be 9.3 to 10.1 Ma, but the fossils of Fagus in the Korean Peninsula date back to the early Miocene (16.8 Ma) [6,77]. Given the very young age of Ulleung Island (ca. 1.8 Ma), it is difficult to determine the precise timing of the origin of F. multinervis on this island. If F. multinervis is indeed a hybrid of F. engleriana and F. japonica, this hybridization event might have occurred between 10 and 2 Ma on the Korean mainland or the Japanese archipelago, and the ancestor of F. multinervis became extinct from these regions later or simultaneously dispersed to Ulleung Island as early as 1.8 Ma. Alternatively, a different position of F. multinervis on plastid and nuclear trees [9] may be obtained due to lack of sufficient resolution and/or incomplete lineage sorting of nuclear genes, given their greater coalescence time than that of plastid genes. Although previous studies do not support the direct divergence of F. multinervis from F. engleriana in China or F. japonica in Japan, this possibility cannot be completely ruled out. If these hypotheses are supported, the shared morphological traits between F. multinervis and F. engleriana or F. japonica could be a result of shared ancestral traits (symplesiomorphy) or convergent evolution. Lack of determination of sister species and the precise geographical origin of F. multinervis was further confirmed by the present study. Nonetheless, the present results, consistent with previous reports [78], strongly support the recognition of F. multinervis as a distinct species endemic to Ulleung Island. As previously suggested [9], extensive sampling of F. engleriana populations from China and F. japonica populations from Japan as well as phylogenomic and molecular dating analyses are warranted to gain insight into the origin and evolution of F. multinervis on Ulleung Island.
Acknowledgments
We thank Woong Lee and Myong-Suk Cho for their assistance during field work on Ulleung Island, Korea and Mount Miya, Japan.
Supplementary Materials
The following are available online at https://www.mdpi.com/2073-4425/11/11/1338/s1, Table S1. The predicted RNA editing sites in the complete plastome of five Fagus species in eastern Asia.
Author Contributions
J.Y. and S.-C.K. conceived and designed the experiment. J.Y., J.-S.Y., K.T. and S.-C.K. collected plant materials. J.Y. performed experiments and analyzed data. J.Y. wrote the first draft of the manuscript and K.T., J.-H.P. and S.-C.K. revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), which is funded by the Ministry of Education (2016R1A6A1A0511910 and 2019R1A2C1011221), to J.-H.P. and J.Y.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Denk T. Phylogeny of Fagus L. (Fagaceae) based on morphological data. Plant. Syst. Evol. 2003;240:55–81. doi: 10.1007/s00606-003-0018-x. [DOI] [Google Scholar]
- 2.Denk T., Grimm G.W., Hemleben V. Patterns of molecular and morphological differentiation in Fagus (Fagaceae): Phylogenetic implications. Amer. J. Bot. 2005;92:1006–1016. doi: 10.3732/ajb.92.6.1006. [DOI] [PubMed] [Google Scholar]
- 3.Zhou G., Li X. Species composition and structure of Chinese beech forests. Nat. Hist. Res. 1994;3:21–26. [Google Scholar]
- 4.Peters R. Beech Forests. Kluwer Academic Publishers; Dordrecht, The Netherlands: 1997. [Google Scholar]
- 5.Shen C.-F. Ph.D. Thesis. The City University of New York; New York, NY, USA: 1992. A monograph of the genus Fagus Tourn. ex L. (Fagaceae) [Google Scholar]
- 6.Renner S.S., Grimm G.W., Kapli P., Denk T. Species relationships and divergence times in beeches: New insights from the inclusion of 53 young and old fossils in a birth–death clock model. Phil. Trans. R. Soc. B. 2016;371:20150135. doi: 10.1098/rstb.2015.0135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stanford A.M. Ph.D. Thesis. University of North Carolina; Chapel Hill, NC, USA: 1998. The Biogeography and Phylogeny of Castanea, Fagus, and Juglans Based on matK and ITS Sequence Data. [Google Scholar]
- 8.Manos P.S., Stanford A.M. The historical biogeography of Fagaceae: Tracking the Tertiary history of temperate and subtropical forests of the Northern Hemisphere. Int. J. Plant. Sci. 2001;162(Suppl. S6):S77–S93. doi: 10.1086/323280. [DOI] [Google Scholar]
- 9.Oh S.-H., Youn J.-W., Kim Y.-I., Kim Y.-D. Phylogeny and evolution of endemic species on Ulleungdo Island, Korea: The case of Fagus multinervis (Fagaceae) Syst. Bot. 2016;41:617–625. doi: 10.1600/036364416X692271. [DOI] [Google Scholar]
- 10.Lee Y.N. Taxonomic studies on the Fagus multinervis, Fagus japonica and Fagus crenata. Bull. Korean Inst. Cult. Res. 1967;10:373–377. [Google Scholar]
- 11.Chang C.-S., Fagus L. In: The Genera of Vascular Plants of Korea. Park C.W., editor. Academy Publishing Co.; Seoul, Korea: 2007. pp. 268–269. [Google Scholar]
- 12.Sun B.-Y., Shin H., Hyun J.-O., Kim Y.-D., Oh S.-H. Vascular Plants of Dokdo and Ulleungdo Islands in Korea. National Institute of Biological Resources; Incheon, Korea: 2014. [Google Scholar]
- 13.Chung H.G., Chung J.M., Chung M.G. Allozyme variation in six flowering plant species characterizing Ullung Island, Korea. J. Jpn Bot. 1998;73:241–247. [Google Scholar]
- 14.Ohkawa T., Kitamura K., Takasu H., Kawano S. Genetic variation in Fagus multinervis Nakai (Fagaceae), a beech species endemic to Ullung Island, South Korea. Plant. Spec. Biol. 2006;21:135–145. doi: 10.1111/j.1442-1984.2006.00159.x. [DOI] [Google Scholar]
- 15.Hukusima T., Matsui T., Nishio T., Pignatti S., Yang L., Lu S.Y., Kim M.-H., Yoshikawa M., Honma H., Wang Y. Phytosociology of the beech (Fagus) forest in East Asia. In: Pedrotii F., editor. Syntaxonomy of the East Asiatic Fagus Forests. Springer; Berlin/Heidelberg, Germany: 2013. pp. 9–47. [Google Scholar]
- 16.Daniell H., Lin C.-S., Yu M., Chang W.-J. Chloroplast genomes: Diversity, evolution, and applications in genetic engineering. Genome Biol. 2016;17:134. doi: 10.1186/s13059-016-1004-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jansen R.K., Cai Z., Raubeson L.A., Daniell H., DePamphilis C.W., Leebens-Mack J., Muller K.F., Guisinger-Bellian M., Haberle R.C., Hansen A.K., et al. Analysis of 81 genes from 64 plastid genomes resolves relationships in angiosperms and identifies genome-scale evolutionary patterns. Proc. Natl. Acad. Sci. USA. 2007;104:19369–19374. doi: 10.1073/pnas.0709121104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore M.J., Bell C.D., Soltis P.S., Soltis D.E. Using plastid genome scale data to resolve enigmatic relationships among basal angiosperms. Proc. Natl. Acad. Sci. USA. 2007;104:19363–19368. doi: 10.1073/pnas.0708072104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barrett C.F., Davis J.I., Leebens-Mack J., Conran J.G., Stevenson D.W. Plastid genomes and deep relationships among the commelinid monocot angiosperms. Cladistics. 2013;29:65–87. doi: 10.1111/j.1096-0031.2012.00418.x. [DOI] [PubMed] [Google Scholar]
- 20.Barrett C.F., Baker W.J., Comer J.R., Conran J.G., Lahmeyer S.C., Leebens-Mack J.H., Li J., Lim G.S., Mayfield-Jones D.R., Perez L., et al. Plastid genomes reveal support for deep phylogenetic relationships and extensive rate variation among palms and other commelinid monocots. New Phytol. 2016;209:855–870. doi: 10.1111/nph.13617. [DOI] [PubMed] [Google Scholar]
- 21.Ma P.F., Zhang Y.X., Zeng C.X., Guo Z.H., Li D.Z. Chloroplast phylogenomic analysis resolve deep-level relationships of an intractable bamboo tribe Arundinarieae (Poaceae) Syst. Biol. 2014;63:933–950. doi: 10.1093/sysbio/syu054. [DOI] [PubMed] [Google Scholar]
- 22.Parks M., Cronn R., Liston A. Increasing phylogenetic resolution at low taxonomic levels using massively parallel sequencing of chloroplast genomes. BMC Biol. 2009;7:84. doi: 10.1186/1741-7007-7-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nikiforova S.V., Cavalieri D., Velasco R., Goremykin V. Phylogenetic analysis of 47 chloroplast genomes clarifies the contribution of wild species to the domesticated apple maternal line. Mol. Biol. Evol. 2013;30:1751–1760. doi: 10.1093/molbev/mst092. [DOI] [PubMed] [Google Scholar]
- 24.Carbonell-Caballero J., Alonso R., Ibañez V., Terol J., Talon M., Dopazo J. A phylogenetic analysis of 34 chloroplast genomes elucidates the relationships between wild and domestic species within the genus Citrus. Mol. Biol. Evol. 2015;32:2015–2035. doi: 10.1093/molbev/msv082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Viljeon E., Odeny D.A., Coetzee M.P.A., Berger D.K., Rees D.J.G. Application of chloroplast phylogenomics to resolve species relationships within the plant genus Amaranthus. J. Mol. Evol. 2018;86:216–239. doi: 10.1007/s00239-018-9837-9. [DOI] [PubMed] [Google Scholar]
- 26.Cho M.-S., Kim J.H., Kim C.-S., Mejias J.A., Kim S.-C. Sow thistle chloroplast genomes: Insights into the plastome evolution and relationship of two weedy species, Sonchus asper and Sonchus oleraceus (Asteraceae) Genes. 2019;10:881. doi: 10.3390/genes10110881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jeon J.-H., Kim S.-C. Comparative analysis of the complete chloroplast genome sequences of three closely related east-Asian wild roses (Rosa sect. Synstylae; Rosaceae) Genes. 2019;10:23. doi: 10.3390/genes10010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu L.-S., Herrando-Moraira S., Susanna A., Galbany-Casals M., Chen Y.-S. Phylogeny, origin and dispersal of Saussurea (Asteraceae) based on chloroplast genome data. Mol. Phylogenet. Evol. 2019;141:106613. doi: 10.1016/j.ympev.2019.106613. [DOI] [PubMed] [Google Scholar]
- 29.Kyalo C.M., Lim Z.-Z., Mkala E.M., Malombe I., Hu G.-W., Wang Q.-F. The first glimpse of Streptocarpus ionanthus (Gesneriaceae) phylogenomics: Analysis of five subspecies’ chloroplast genomes. Plants. 2020;9:456. doi: 10.3390/plants9040456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mader M., Liesebach H., Liesebach M., Kersten B. The complete chloroplast genome sequence of Fagus sylvatica L. (Fagaceae) Mitochondrial DNA B. 2019;4:1818–1819. doi: 10.1080/23802359.2019.1612712. [DOI] [Google Scholar]
- 31.Park J.-S., Jin D.-P., Park J.-W., Choi B.-H. Complete chloroplast genome of Fagus multinervis, a beech species endemic to Ulleung Island in South Korea. Mitochondrial DNA B. 2019;4:1698–1699. doi: 10.1080/23802359.2019.1607581. [DOI] [Google Scholar]
- 32.Worth J.R.P., Liu L., Wei F.-J., Tomaru N. The complete chloroplast genome of Fagus crenata (subgenus Fagus) and comparison with F. engleriana (subgenus Engleriana) PeerJ. 2019;7:e7026. doi: 10.7717/peerj.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park J.S., Oh S.-H. A second complete chloroplast genome sequence of Fagus multinervis Nakai (Fagaceae): Intraspecific variations on chloroplast genome. Mitochondrial DNA B. 2020;5:1868–1869. doi: 10.1080/23802359.2020.1752837. [DOI] [Google Scholar]
- 34.Yang Y., Zhu J., Feng L., Zhou T., Bai G., Yang J., Zhao G. Plastid Genome Comparative and Phylogenetic Analyses of the Key Genera in Fagaceae: Highlighting the Effect of Codon Composition Bias in Phylogenetic Inference. Front. Plant. Sci. 2018;9:82. doi: 10.3389/fpls.2018.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zerbino D.R., Birney E. Velvet: Algorithms for de novo short read assembly using de Bruijn graphs. Genome Res. 2008;18:821–829. doi: 10.1101/gr.074492.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lowe T.M., Eddy S.R. tRNAscan-SE: A program for improved detection of transfer RNA genes in genomic sequence. Nucleic Acids Res. 1997;25:955–964. doi: 10.1093/nar/25.5.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kearse M., Moir R., Wilson A., Stones-Havas S., Cheung M., Sturrock S., Buxton S., Cooper A., Markowitz S., Duran C., et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lohse M., Drechsel O., Bock R. Organellar genome DRAW (OGDRAW): A tool for the easy generation of high-quality custom graphical maps of plastid and mitochondrial genomes. Curr. Genet. 2009;25:1451–1452. doi: 10.1007/s00294-007-0161-y. [DOI] [PubMed] [Google Scholar]
- 39.Brundo M., Malde S., Poliakov A., Do C.B., Couronne O., Dubchak I., Batzoglou S. Global Alignment: Finding rearrangements during alignment. Bioinformatics. 2003;19(Suppl. S1):i54–i62. doi: 10.1093/bioinformatics/btg1005. [DOI] [PubMed] [Google Scholar]
- 40.Frazer K.A., Pachter L., Poliakov A., Rubin E.M., Dubchak I. VISTA: Computational tools for comparative genomics. Nucleic Acids Res. 2004;32:W273–W279. doi: 10.1093/nar/gkh458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katoh K., Standley D.M. MAFFT multiple sequence alignment software v7: Improvements in performance and usability. Mol. Biol. Evol. 2013;30:772–780. doi: 10.1093/molbev/mst010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rozas J., Ferrer-Mata A., Sánchez-DelBarrio J.C., Guirao-Rico S., Librado P., Ramos-Onsins S.E., Sánchez-Gracia A. DnaSP v6: DNA sequence polymorphism analysis of large datasets. Mol. Biol. Evol. 2017;34:3299–3302. doi: 10.1093/molbev/msx248. [DOI] [PubMed] [Google Scholar]
- 43.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sharp P.M., Li W.H. The codon adaptation index–a measure of directional synonymous codon usage bias, and its potential applications. Nucleic Acids Res. 1987;15:1281–1295. doi: 10.1093/nar/15.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kozak M. Comparison of initiation of protein synthesis in procaryotes, eucaryotes, and organelles. Microbiol. Rev. 1983;47:1–45. doi: 10.1128/MMBR.47.1.1-45.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mower J.P. The PREP suite: Predictive RNA editors for plant mitochondrial genes, chloroplast genes and user-defined alignments. Nucleic Acids Res. 2009;37:W253–W259. doi: 10.1093/nar/gkp337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nguyen L.-T., Schmidt H.A., von Haeseler A., Minh B.Q. IQ-TREE: A fast and effective stochastic Algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gao F., Chen C., Arab D.A., Du Z., He Y., Ho S.Y.W. EasyCodeML: A visual tool for analysis of selection using CodeML. Ecol. Evolut. 2019;9:3891–3898. doi: 10.1002/ece3.5015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yang Z. PAML: A program package for phylogenetic analysis by maximum likelihood. Bioinformatics. 1997;13:555–556. doi: 10.1093/bioinformatics/13.5.555. [DOI] [PubMed] [Google Scholar]
- 50.Fujii N., Tomaru N., Okuyama K., Koike T., Mikami T., Ueda K. Chloroplast DNA phylogeography of Fagus crenata (Fagaceae) in Japan. Plant. Syst. Evol. 2002;232:21–33. doi: 10.1007/s006060200024. [DOI] [Google Scholar]
- 51.Oh S.-H. Sea, wind, or bird: Origin of Fagus multinervis (Fagaceae) inferred from chloroplast DNA sequences. Korean J. Plant Taxon. 2015;45:213–220. doi: 10.11110/kjpt.2015.45.3.213. [DOI] [Google Scholar]
- 52.Yang Y., Zhou T., Duan D., Yang J., Feng L., Zhao G. Comparative analysis of the complete chloroplast genomes of five Quercus species. Front. Plant Sci. 2016;7:959. doi: 10.3389/fpls.2016.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ravi V., Khurana J.P., Tyagi A.K., Khurana P. An update chloroplast genomes. Plant Syst. Evol. 2008;271:101–122. doi: 10.1007/s00606-007-0608-0. [DOI] [Google Scholar]
- 54.Morton B.R. Selection on the codon bias of chloroplast and cyanelle genes in different plant and algal lineages. J. Mol. Evol. 1998;46:449–459. doi: 10.1007/PL00006325. [DOI] [PubMed] [Google Scholar]
- 55.Rabah S.O., Lee C., Hajrah N.H., Makki R.M., Alharby H.F., Alhebshi A.M., Sabir J.S.M., Jansen R.K., Ruhlman T.A. Plastome sequencing of ten nonmodel crop species uncovers a large insertion of mitochondrial DNA in cashew. Plant Genome. 2017;10:1–14. doi: 10.3835/plantgenome2017.03.0020. [DOI] [PubMed] [Google Scholar]
- 56.Pinard D., Myburg A.A., Mizrachi E. The plastid and mitochondrial genomes of Eucalyptus grandis. BMC Genom. 2019;20:1–14. doi: 10.1186/s12864-019-5444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kim S.-H., Yang J.Y., Park J.S., Yamada T., Maki M., Kim S.-C. Comparison of whole plastome sequences between thermogenic skunk cabbage Symplocarpus renifolius and nonthermogenic S. nipponicus (Orontioideae; Araceae) in East Asia. Int. J. Mol. Sci. 2019;20:4678. doi: 10.3390/ijms20194678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Menezes A.P.A., Resende-Moreira L.C., Buzatti R.S.O., Nazareno A.G., Carlsen M., Lobo F.P., Kalapothakis E., Lovato M.B. Chloroplast genomes of Byrsonima species (Malpighiaceae): Comparative analysis and screening of high divergence sequences. Sci. Rep. 2018;8:2210. doi: 10.1038/s41598-018-20189-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Millen R.S., Olmstead R.G., Adams K.L., Palmer J.D., Lao N.T., Heggie L., Kavanagh T.A., Hibberd J.M., Gray J.C., Morden C.M., et al. Many parallel losses of infA from chloroplast DNA during angiosperm evolution with multiple independent transfers to the nucleus. Plant Cell. 2001;13:645–658. doi: 10.1105/tpc.13.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ueda M., Nishikawa T., Fujimoto M., Takanashi H., Arimura S.-I., Tsutsumi N., Kadowaki K.-I. Substitution of the gene for chloroplast rps16 was assisted by generation of a dual targeting signal. Mol. Biol. Evol. 2008;25:1566–1575. doi: 10.1093/molbev/msn102. [DOI] [PubMed] [Google Scholar]
- 61.Park S.J., Jansen R.K., Park S.J. Complete plastome sequence of Thalictrum coreanum (Ranunculadeae) and transfer of the rpl32 gene to the nucleus in the ancestor of the subfamily Thalictroideae. BMC Plant Biol. 2015;15:40. doi: 10.1186/s12870-015-0432-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Stegemann S., Hartmann S., Ruf S., Bock R. High-frequency gene transfer from the chloroplast genome to the nucleus. Proc. Natl. Acad. Sci. USA. 2003;100:882–8833. doi: 10.1073/pnas.1430924100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Timmis J.N., Ayliffe M.A., Huang C.Y., Martin W. Endosymbiotic gene transfer: Organelle genomes forge eukaryotic chromosomes. Nature Rev. Genet. 2004;5:123–135. doi: 10.1038/nrg1271. [DOI] [PubMed] [Google Scholar]
- 64.Noutsos C., Richly E., Leister D. Generation and evolutionary fate of insertions of organelle DNA in the nuclear genomes of flowering plants. Genome Res. 2005;15:616–628. doi: 10.1101/gr.3788705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sloan D.B., Triant D.A., Forrester N.J., Bergner L.M., Wu M., Taylor D.R. A recurring syndrome of accelerated plastid genome evolution in the angiosperm tribe Sileneae (Caryophyllaceae) Mol. Phylogenet. Evol. 2014;72:82–89. doi: 10.1016/j.ympev.2013.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Cheng H., Li J., Zhang H., Cai B., Gao Z., Qiao Y., Mi L. The complete chloroplast genome sequence of strawberry (Fragaria×ananassa Duch.) and comparison with related species of Rosaceae. PeerJ. 2017;5:e3919. doi: 10.7717/peerj.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jiang P., Shi F.X., Li M.R., Liu B., Wen J., Xiao H.X., Li L.F. Positive selection driving cytoplasmic genome evolution of the medicinally important ginseng plant genus Panax. Front. Plant Sci. 2018;9:359. doi: 10.3389/fpls.2018.00359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li P., Lou G., Cai X., Zhang B., Cheng Y., Wang H. Comparison of the complete plastomes and the phylogenetic analysis of Paulownia species. Sci. Rep. 2020;10:2225. doi: 10.1038/s41598-020-59204-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang L.Q., Zhang H., Jiang M., Chen H., Huang L., Liu C. Complete plastome sequence of Iodes cirrhosa Turcz., the first in the Icacinaceae, comparative genomic analyses and possible split of Idoes species in response to climate changes. PeerJ. 2019;7:e6663. doi: 10.7717/peerj.6663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Caspermeyer J. Most comprehensive study to date reveals evolutionary history of Citrus. Mol. Biol. Evol. 2015;32:2217–2218. doi: 10.1093/molbev/msv101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fan X., Zhang J., Li W., Peng L. The ndhV subunit is required to stabilize the chloroplast NADH dehydrogenase-like complex in Arabidopsis. Plant J. 2015;82:221–231. doi: 10.1111/tpj.12807. [DOI] [PubMed] [Google Scholar]
- 72.Horvath E.M., Peter S.O., Joët T., Rumeau D., Cournac L., Horváth G.V., Kavanagh T.A., Schafer C., Peltier G., Medgyesy P. Targeted inactivation of the plastid ndhB gene in tobacco results in an enhanced sensitivity of photosynthesis to moderate stomatal closure. Plant Physiol. 2000;123:1337–1350. doi: 10.1104/pp.123.4.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Burri R., Salamin N., Studer R.A., Roulin A., Fumagalli L. Adaptive divergence of ancient gene duplicates in the avian MHC class II β. Mol. Biol. Evol. 2010;27:2360–2374. doi: 10.1093/molbev/msq120. [DOI] [PubMed] [Google Scholar]
- 74.Piot A., Hackel J., Christin P.A., Besnard G. One-third of the plastid genes evolved under positive selection in PACMAD grasses. Planta. 2018;247:255–266. doi: 10.1007/s00425-017-2781-x. [DOI] [PubMed] [Google Scholar]
- 75.Okaura T., Harada K. Phylogeographical structure revealed by chloroplast DNA variation in Japanese Beech (Fagus crenata Blume) Heredity. 2002;88:322–329. doi: 10.1038/sj.hdy.6800048. [DOI] [PubMed] [Google Scholar]
- 76.Cannon C.H., Manos P.S. Phylogeography of the Southeast Asian stone oaks (Lithocarpus) J. Biogeogr. 2003;30:211–226. doi: 10.1046/j.1365-2699.2003.00829.x. [DOI] [Google Scholar]
- 77.Paik I.-S., Kim H.-J., Kim K., Jeong E.-K., Kang H.-C., Lee H.-I., Uemura K. Leaf beds in the Early Miocene lacustrine deposits of the Geumgwangdong Formation, Korea: Occurrence, plant-insect interaction records, taphonomy and paleoenviromental implications. Rev. Palaeobot. Palynol. 2012;170:1–14. doi: 10.1016/j.revpalbo.2011.10.011. [DOI] [Google Scholar]
- 78.Nakai T. Notulae ad plantas Japoniae et Koreae XVII. Bot. Mag. 1918;32:103–110. doi: 10.15281/jplantres1887.32.377_103. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.