Abstract
Simple Summary
Colorectal cancer is the third most common cause of cancer-related deaths. The Wnt signaling pathway is activated by genetic mutations in most patients with colorectal cancer. A number of different types of Wnt pathway mutation have been described: some increase the sensitivity of tumor cells to Wnt ligands produced by stromal cells (ligand-dependent), while others drive downstream activation of the pathway (ligand-independent). Ligand-dependent tumors are of particular interest as there are a number of emerging treatment options, such as porcupine inhibitors, that can specifically target these tumors. In this review, we discuss what is known about these different types of Wnt activating mutations. We propose that ligand-dependent tumors should be viewed as a separate subset of colorectal cancer with its own biomarkers, prognosis and targeted therapies.
Abstract
Wnt signaling is ubiquitously activated in colorectal tumors and driver mutations are identified in genes such as APC, CTNNB1, RNF43 and R-spondin (RSPO2/3). Adenomatous polyposis coli (APC) and CTNNB1 mutations lead to downstream constitutive activation (ligand-independent), while RNF43 and RSPO mutations require exogenous Wnt ligand to activate signaling (ligand-dependent). Here, we present evidence that these mutations are not equivalent and that ligand-dependent and ligand-independent tumors differ in terms of underlying Wnt biology, molecular pathogenesis, morphology and prognosis. These non-overlapping characteristics can be harnessed to develop biomarkers and targeted treatments for ligand-dependent tumors, including porcupine inhibitors, anti-RSPO3 antibodies and asparaginase. There is emerging evidence that these therapies may synergize with immunotherapy in ligand-dependent tumors. In summary, we propose that ligand-dependent tumors are an underappreciated separate disease entity in colorectal cancer.
Keywords: Wnt, signaling, colorectal, cancer, porcupine, R-spondin, serrated, immunotherapy
1. Introduction
Metastatic colorectal cancer (CRC) is a lethal malignancy with a five-year survival of less than 15% [1]. Patients with metastatic CRC are treated with combination cytotoxic chemotherapy alongside monoclonal antibodies targeting angiogenesis or epidermal growth factor receptor (EGFR) [2]. There is a need to develop new therapeutic strategies for metastatic cancer, especially in light of evidence showing rapid increases in CRC incidence affecting younger patients [3]. Molecular profiling of CRC has shown considerable disease heterogeneity, suggesting that patients might benefit from precision medicine, in which treatments are personalized for their tumor profile [4]. For example, immunotherapy targeting PD-1/PD-L1 signaling is only active in hypermutated tumors, while anti-EGFR antibodies are only effective in tumors without downstream mutations [5,6,7].
Colorectal cancer is characterized by near-ubiquitous activation of the Wnt signaling pathway [8]. The Wnt pathway is an evolutionarily conserved mechanism for intercellular communication, with essential roles in embryogenesis and adult tissue development [9]. In the colonic crypt, Wnt signaling is necessary to maintain the adult intestinal stem cell niche and epithelial homeostasis [10]. Colorectal tumors are dependent upon aberrant Wnt signaling to maintain stemness and a de-differentiated phenotype and genetic Wnt inhibition leads to rapid tumor regression [11]. Additionally, Wnt signaling can protect cells from immune surveillance, thus restricting anti-tumoral immunity [12,13]. Altogether, this suggests that the Wnt pathway could be a viable therapeutic target for patients with CRC.
Colorectal tumors are thought to evolve through the sequential acquisition of mutations driving progression from a normal founder cell to adenoma and then carcinoma [14]. Adenomas can be histologically classified as either conventional, such as tubular or tubulovillous (TVA), or serrated, such as sessile serrated lesions (SSL) or traditional serrated adenomas (TSA) [15]. Serrated adenomas are characterized histologically by a saw-tooth morphology. The cell-of-origin for TVA is likely the crypt-based columnar stem cell [16] while the cell-of-origin for serrated lesions is unknown, but may derive from ectopic crypt foci in the rare traditional serrated adenoma subtype [17].
Here, we present evidence for a new model of CRC in which Wnt pathway activation can take one of two distinct trajectories, ligand-dependent (LD) and ligand-independent (LI), with implications spanning tumor biology, screening, diagnosis and treatment. We will first outline the Wnt signaling pathway in the normal colon and types of recurrent Wnt mutations in CRC. We will then discuss morphological and molecular biomarkers that can be used to identify LD tumors in the clinic. Finally, we will argue that this model has the potential to transform the landscape of precision medicine in CRC.
2. Wnt Signaling Pathway
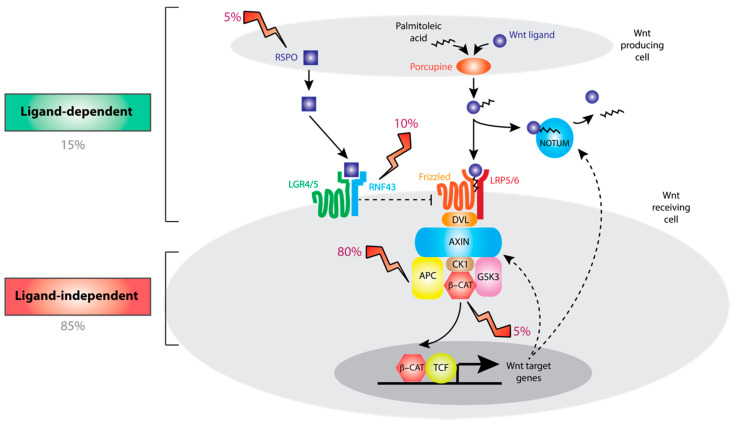
The Wnt signaling pathway in normal colon crypts is summarized in Figure 1. Briefly, canonical Wnt ligands are secreted by the cells in the stem cell niche following O-acylation by porcupine [18,19]. Wnt ligands bind to Frizzled (FZD) and lipoprotein receptor-related protein (LRP) receptor complexes on the plasma membrane of neighboring cells [20]. Both Wnt ligand secretion and binding to FZD depend upon acylation of Wnt ligands [21]. The downstream effector of Wnt signaling is the transcriptional co-activator β-catenin (CTNNB1). In the absence of Wnt ligands, CTNNB1 is degraded by the action of a destruction complex containing adenomatous polyposis coli (APC), axin-like protein (AXIN1/2), glycogen synthase kinase (GSK3) and casein kinase (CSNK1A) [22]. Wnt ligand binding inhibits the destruction complex, thus stabilizing CTNNB1 and activating expression of Wnt target genes. An additional level of regulation comes from E3 ubiquitin ligases ring finger protein 43 (RNF43) and zinc and ring finger 3 (ZNRF3), which constitutively degrade FZD to repress Wnt signaling [23]. R-spondin (RSPO) ligands bind to leucine-rich repeat-containing G protein-coupled (LGR) receptors, inhibiting RNF43/ZNRF3 and substantially amplifying Wnt signaling [24]. There are four homologous human RSPO ligands (RSPO1-4) and, while all four can bind to LGR-family receptors, the EC50 for activation of Wnt signaling varies 100-fold, with RSPO2 and RSPO3 demonstrating the highest potency (0.02–0.05 nM) [25]. R-spondins are produced by stromal cells adjacent to the stem cell niche [26]. Consistent with this, R-spondin signaling is necessary to maintain the stem cell niche, both in vivo and as part of organoid culture systems [24,27,28]. Wnt target genes, such as notum palmitoleoyl-protein carboxylesterase (NOTUM) and AXIN2 are negative regulators of Wnt signaling, functioning as negative feedback loops to fine-tune and limit downstream signaling [29]. AXIN2 is an inducible component of the destruction complex, while NOTUM works in the extracellular space to deacetylate and inactivate Wnt ligands [30].
Figure 1.
Overview of Wnt signaling pathway. Wnt ligands secreted from stromal cells are activated by porcupine-mediated post-translational modification and bind to Frizzled (FZD) receptors on Wnt receiving cells. This functions to inhibit a destruction complex containing axin-like protein (AXIN)1/2 and adenomatous polyposis coli (APC) thus disinhibiting β-catenin (CTNNB1), the master transcriptional regulator of Wnt singaling. Frizzled receptors are degraded due to the action of ring finger protein 43 (RNF43), which is in turn inhibited by binding of R-spondin (RSPO) ligands to leucine-rich repeat-containing G protein-coupled (LGR) family receptors, thus augmenting Wnt signaling tone. Wnt pathway activation is regulated at multiple levels by negative feedback loops, including those mediated by AXIN2 and notum palmitoleoyl-protein carboxylesterase (NOTUM). Recurrent mutations in CTNNB1 and APC result in ligand-independent pathway activation while mutations in RSPO and RNF43 depend upon binding of Wnt ligands to Frizzled receptors. GSK: glycogen synthase kinase; LRP: lipoprotein receptor-related protein.
3. Ligand-Dependent and Ligand-Independent Alterations in Colorectal Cancer
Large-scale sequencing studies in CRC have established the presence of pervasive Wnt pathway mutations. Recurrent mutations include loss-of-function mutations in APC and RNF43, and gain-of-function mutations in CTNNB1 and RSPO2/3 (Figure 1, Table 1) [8,31,32]. Consistent with driver mutation status, in vivo modeling indicates that these mutations can be sufficient for colorectal tumorigenesis [33,34,35,36]. For tumor suppressors APC and RNF43, we only consider protein-truncating mutations or deletions as potential driver alterations [37]. ZNRF3 is a homolog of RNF43 but truncating mutations are rare in colorectal tumors, potentially reflecting its comparably low mRNA expression in normal colon and colorectal tumors [8,38]. This is in contrast to the situation in murine intestine, in which Znrf3 and Rnf43 gene expression is comparable, and loss-of-function alterations in both Znrf3 and Rnf43 are necessary to activate Wnt signaling [36,39].
Table 1.
Driver Wnt alterations in colorectal cancer. Prevalence refers to the frequency of each mutation in the subset of colorectal tumors with a detectable driver Wnt alteration, as derived from [42]. Loss-of-function alterations in APC and RNF43 are frequently accompanied by loss of heterozygosity (LOH) affecting the second allele [13].
| Mutation Type | Gene | Type of Alteration | Prevalence in CRC |
|---|---|---|---|
| Ligand-dependent | RNF43 | Loss-of-function | 10% |
| - Nonsense | |||
| - Frameshift | |||
| R-spondin (RSPO2, RSPO3) | Gain-of-function | 8% | |
| - Stromal overexpression | |||
| - Epithelial gene fusions | |||
| Ligand-independent | APC | Loss-of-function | 81% |
| - Nonsense | |||
| - Frameshift | |||
| CTNNB1 | Gain-of-function | 2% | |
| - Missense (affecting codons 31–35, 37, 40, 41, 45, 383 and 387) |
While APC and CTNNB1 alterations drive downstream, constitutive activation of the Wnt pathway that is independent of Wnt ligand binding (ligand-independent, LI), RSPO and RNF43 alterations disrupt the synergistic RSPO axis and by doing so, amplify endogenous and otherwise intact, Wnt ligand signaling (ligand-dependent, LD). APC mutations are characteristically nonsense or frameshift alterations affecting the “mutation cluster region”, often with a second “hit” from loss of heterozygosity [40]. CTNNB1 mutations are gain-of-function missense mutations affecting specific amino acid residues that are phosphorylation sites for components of the destruction complex [41].
RSPO mutations induce R-spondin ligand overexpression either from epithelial cells (autocrine), as RSPO fusion genes [42]. R-spondin gain-of-function is only observed for RSPO2 and RSPO3, consistent with their enhanced potency to induce Wnt signaling in vitro [25]. RSPO3 fusion genes commonly result in the replacement of RSPO3 exon one and promoter with that of a gene with higher basal expression, resulting in a functional epithelial-expressed protein [32]. A wide range of fusion partners have been identified including PTPRK, EIF3E, NRIP1 and PIEZO1 [43,44], all of which are associated with relatively high constitutive gene expression [38]. RSPO fusions cannot be reliably identified even from whole-genome sequencing due to large inconsistency in genomic alterations, while the transcript breakpoints are more stereotypical [32]. Alternatively, in a rare subset of colorectal tumors, we identified R-spondin overexpression in the absence of RSPO fusions or any other detectable Wnt driver alteration [42]. In situ hybridization demonstrated high stromal RSPO3 expression in these tumors, implicating a role for paracrine R-spondin signaling driven by stromal overexpression [42]. RSPO3 overexpression in the absence of gene fusions has been previously detected in lung cancer, where it was associated with RSPO3 hypomethylation [45]. The concept that RSPO overexpression can derive from either epithelial or stromal sources is consistent with previous evidence that RSPO3 expression is significantly and positively correlated with stromal expression signatures [46]. RNF43 mutations are mostly recurrent frameshift mutations at amino acid positions 117 and 659 that result in a truncated gene product [31]. These recurrent mutations occur at tandem repeats called microsatellites whose stability is dependent upon proficient mismatch repair (MMR) [47]. As a result, these mutations tend to occur in tumors with MMR deficiency, detected as microsatellite instability (MSI), which is often caused by promoter hypermethylation of MLH1 [8,48].
Recently, there has been some controversy about whether the RNF43 G659Vfs*41 mutation demonstrably leads to impaired protein function. In vitro transfection experiments have indicated that this mutant RNF43 protein retains the ability to bind R-spondin and repress Frizzled [49]. However, this alteration is associated with significantly reduced RNF43 expression, potentially consistent with nonsense-mediated decay [49], and CRISPR-Cas9 editing of the endogenous RNF43 locus to mimic the G659Vfs*41 mutation, was sufficient to increase cell surface Frizzled expression [50]. Furthermore, the G659Vfs*41 mutation occurs substantially more often than would be expected by chance in microsatellite-unstable tumors, indicating strong positive selection [31,51].
It is important to note that driver Wnt alterations affecting APC, CTNNB1, RNF43 and RSPO in pre-cancerous polyps and tumors show marked mutual exclusivity [42]. There are two logical implications from this: firstly, these alterations are redundantly able to activate Wnt signaling. Secondly, there may be selection against the accumulation of driver alterations in more than one gene. This is consistent with the “just right” theory of Wnt signaling: that there is an optimal level of Wnt activation to drive tumorigenesis. It has been observed that there is a non-random distribution of second “hit” mutations in APC that is consistent with selection for APC genotypes that retain some CTNNB1 repression [52]. Additionally, ectopic expression of R-spondin in APC-mutant mice results in reduced proliferation and increased apoptosis [53], consistent with evidence that Wnt can directly promote apoptosis [54].
4. Mutation Selection in Lesion Subtypes
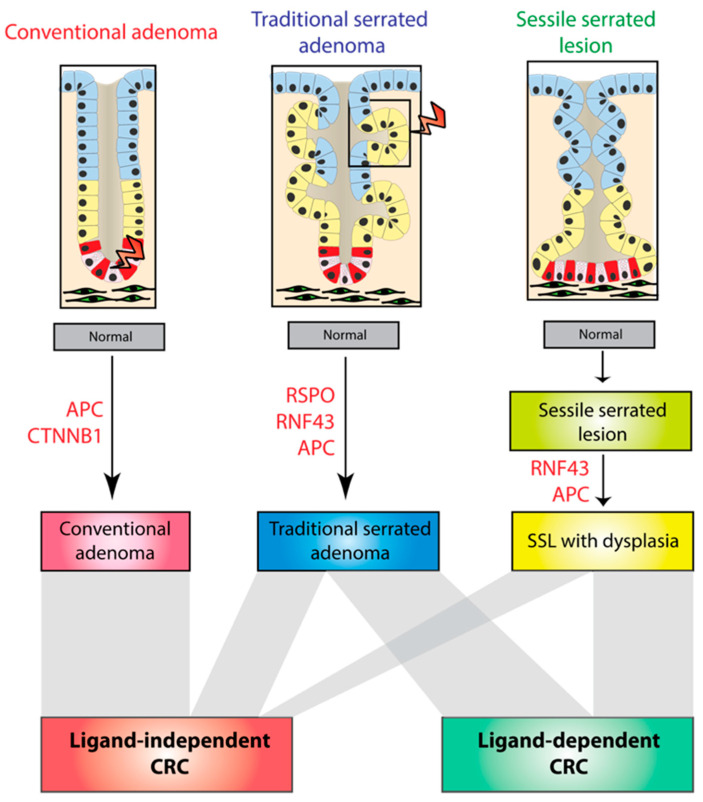
Molecular profiling in pre-cancerous polyps has shown that ligand-dependent alterations are predominantly seen in the serrated pathway (Figure 2) [44,55]. A total of 55% of TSAs have ligand-dependent alterations, namely truncating RNF43 mutations or RSPO fusions (mostly PTPRK-RSPO3) [44]. In sessile serrated lesions (SSL), mutations in the Wnt signaling pathway are not thought to be initiating lesions as Wnt disruption is observed predominantly in dysplastic rather than non-dysplastic lesions. A total of 50% of SSLs had ligand-dependent RNF43 mutations [55], whereas APC mutations are much rarer in serrated lesions, being detected in 13% and 9% of TSAs and dysplastic SSLs, respectively [44,55]. In contrast, conventional adenomas have a high frequency (>85%) of ligand-independent alterations [56]. APC mutation is sufficient to initiate adenoma pathogenesis [57] and no ligand-dependent alterations have been reported in conventional adenomas [44].
Figure 2.
Molecular pathogenesis of different colorectal precursor subtypes. Colorectal cancer develops from three types of pre-cancerous polyps: conventional and serrated adenomas—divided into traditional serrated adenomas (TSAs) and sessile serrated lesions (SSLs). Conventional adenomas are driven by ligand-independent mutations that likely arise in the crypt base columnar (CBC) stem cells. TSAs arise from APC, RSPO or RNF43 mutations, possibly in ectopic crypts. SSL pathogenesis is characterized by the late acquisition of APC or RNF43 mutations, concurrent with the onset of the detectable dysplasia. Ligand-dependent CRC arises from TSAs and SSLs while ligand-independent CRC arises from all three types of polyp (bottom panel).
Altogether, this raises the possibility that different intestinal lesions follow distinct molecular carcinogenesis pathways. These different evolutionary trajectories appear to result in the selection of either ligand-dependent or independent mutations. This may also partly explain the mutual exclusivity of Wnt driver mutations discussed above. Why polyp subtypes acquire apparent obligatory Wnt disruption through these different mechanisms is unknown, but may be influenced by the variable cell-of-origin in different lesion subtypes (Figure 2). Indeed, APC mutations induce tumorigenesis in vivo if introduced into the LGR5+ intestinal stem cell but not transit-amplifying cells [16], while RSPO fusions significantly co-occur with loss-of-function mutations in the Bone morphogenic protein (BMP) signaling pathway that are known to induce ectopic crypt formation [58,59]. These data would also suggest that RSPO-mutant colorectal tumors are wholly derived from TSAs.
5. Negative Regulation of Wnt Signaling
In some ways, it is surprising that despite multiple levels of negative feedback, ligand-dependent mutations, which act upstream in an otherwise normal pathway, can induce activation of Wnt signaling at all. Ligand-dependent pathway activation would be expected to induce physiological expression of Wnt negative regulators such as AXIN2 or NOTUM, which would function to proportionately constrain activation of the pathway. In contrast, ligand-independent alterations result in downstream, constitutive activation that is uncoupled from the action of negative regulators. We have recently shown that tumors with ligand-dependent alterations are associated with significant repression of Wnt negative regulators, especially AXIN2 [42]. This repression may be at least partly explained by AXIN2 methylation [60]. This raises the possibility that ligand-dependent Wnt activation requires two ”hits”—firstly a driver mutation affecting RNF43 or RSPO, and secondly, epigenetic downregulation of Wnt negative regulators. Indeed, serrated adenomas which are enriched for ligand dependent mutations, have lower AXIN2 expression and increased AXIN2 methylation compared to conventional tubulovillous adenomas [61,62], as do MSI-high cancers that progress via this pathway [45,53]. AXIN2 expression is also decreased in an in vivo model of ligand-dependent tumors, generated by orthotopic engraftment of CRISPR-edited organoids [63]. Furthermore, ectopic expression of AXIN2, leading to re-activation of Wnt negative feedback in an RNF43-mutant cell line (HCT116) resulted in rapid cell death [60,61]. In fact, AXIN2 is not the only Wnt negative regulator known to be silenced by promoter hypermethylation in colorectal cancer: hypermethylation has been detected in negative regulators including WIF1, SFRP1/2/4, DKK1–3 and NOTUM [42,62,64]. These genes are predominantly hypermethylated in ligand-dependent or microsatellite-unstable tumors. This suggests that repression of negative regulators is a more global phenomenon in ligand-dependent CRC, with loss of negative feedback mechanisms at multiple levels of the Wnt signaling pathway.
6. Application of AXIN2 as a Biomarker for Ligand-Dependent Wnt Biology
Our finding that ligand-dependent tumors exhibit suppressed expression of negative regulators of Wnt can be harnessed to utilize AXIN2 as a single-gene biomarker to distinguish between ligand-dependent and ligand-independent tumors at the point of diagnosis. This is particularly important as otherwise ligand-dependent tumors would need to be identified from expensive and time-consuming analysis of paired DNA (for APC, CTNNB1 and RNF43) and RNA sequencing (RSPO fusions). Paired DNA and RNA sequencing is simply not practical for routine diagnostic assessment in the clinic, both in terms of cost and the relatively high failure rate of sequencing (>10%) from diagnostic clinical samples [65].
We recently demonstrated that AXIN2 mRNA expression could be used as a discriminatory biomarker with an area under the curve (AUC) greater than 0.93 in three independent cohorts, indicating excellent diagnostic performance. This analysis incorporated both RNA sequencing and microarray profiling to assay gene expression in resection and biopsy specimens [42]. The diagnostic performance corresponded to sensitivity and specificity >90%. We also demonstrated similar results with high-throughput AXIN2 profiling by quantitative real-time polymerase chain reaction (qRT-PCR). These findings were recently supported by the use of an organoid biobank derived from patients with colorectal cancer, in which organoids with RSPO fusions or RNF43 mutations exhibited lower AXIN2 expression than APC-mutant organoids [58]. Our analysis of paired qRT-PCR and immunohistochemistry for AXIN2 showed that there was only weak correlation between AXIN2 mRNA and scored AXIN2 protein expression, suggesting that AXIN2 may undergo significant translational regulation, as has been described previously [66]. This would suggest that profiling of AXIN2 mRNA expression would be the preferred approach to translate this biomarker into the clinic.
It is worth noting that AXIN2 gene expression is widely used as a read-out of global Wnt pathway activation [67] and our findings suggest that this should be interpreted with caution, as AXIN2 expression can be confounded by the type of acquired Wnt disrupting pathway mutation. This confounding has important implications for the interpretation of analyses that have demonstrated inverse correlations between AXIN2 (used as a read-out of Wnt activation) and immune infiltration [13]. Tumors with low AXIN2 expression are enriched with RNF43-mutant MSI-high tumors that have enhanced anti-tumoral immune responses, thought to result from an increased neoantigen load [68]. As a result, the inverse relationship between AXIN2 and immune infiltration may be partly explained by increased mutational load in RNF43-mutant ligand-dependent tumors, rather than reduced Wnt activation.
In summary, the distinction between ligand-dependent and ligand-independent tumors is clinically-actionable because tumors can be robustly discriminated using a low-cost single-gene molecular biomarker.
7. Non-Overlapping Clinicopathological Features of Ligand-Dependent Tumors
Consistent with altered Wnt pathway biology and an altered trajectory through the serrated pathway, ligand-dependent tumors have non-overlapping morphological and clinical characteristics with ligand-independent tumors, reflecting an underappreciated separate disease entity in colorectal cancer. Using manual and automated digital pathological approaches, we have demonstrated that ligand-dependent tumors are enriched with mucin [13,42]. Mucin is a high molecular-weight glycoprotein that is secreted by goblet cells and forms a key component of the mucous layer that provides physical protection in the gastrointestinal tract [69]. Mucinous differentiation has long been recognized in a subset of colorectal tumors (around 10%) and is diagnosed in tumors where mucin comprises >50% of the tumor volume [70]. Indeed, mucinous differentiation is associated with microsatellite instability, implicating a link with RNF43-mutant tumors. We have demonstrated that computational-scored mucin area alone could discriminate between ligand-dependent and ligand-independent tumors with an AUC > 0.75. Based on our findings, we propose that mucinous differentiation may well either be induced by ligand-dependent Wnt signaling or reflect the association with the serrated pathway. Consistent with the former hypothesis, the induction of ligand-dependent alterations in organoids is sufficient to generate orthotopic colon tumors with mucinous differentiation [63]. Furthermore, RNF43 mutations in biliary malignancies are associated with mucin hypersecretion [71]. Altogether, this suggests that mucin content, which is routinely scored by histopathologists, can be used as a phenotypic biomarker for ligand-dependent tumors with good diagnostic performance.
In our comparison of ligand-dependent and ligand-independent tumors in a pooled cohort of over 600 tumors with available outcome data, we did not identify any significant differences in prognosis [42]. However, this is likely to mask, considerably, the prognostic heterogeneity between the subsets of ligand-dependent tumors. One way to examine this is to compare specific subsets with their consensus molecular subtype (CMS) classifications, as this study was well-powered to identify prognostic associations incorporating over 2000 patients [72]. Ligand-dependent tumors appear to lie on a continuum between RNF43-mutant tumors which mostly classify as CMS1 (associated with good prognosis) and tumors with stromal RSPO overexpression which mostly classify as CMS4 (associated with poor prognosis). Consistent with this, we observed a high frequency of tumor budding and enriched desmoplastic stroma in tumors with stromal RSPO overexpression, both of which are associated with poor prognosis [73,74]. Of note, mucinous differentiation is associated with marginally reduced overall survival [75]. These data suggest that the prognostic implications of ligand-dependent Wnt biology are likely to be highly heterogenous.
8. Selective Vulnerabilities in Ligand-Dependent Tumors
Downstream ligand-independent Wnt signaling has proved difficult to target in solid tumors, reflecting challenges in designing small-molecule inhibitors to inhibit constitutive pathway activation through transcription factors such as beta-catenin [76]. In contrast, from a conceptual and experimental standpoint, ligand-dependent Wnt activation is inherently “druggable” through deprivation of extracellular ligand (Wnt or R-spondin) or attenuation of negative regulator suppression with demethylating agents (Figure 3) [27,60,77]. Furthermore, emerging evidence would indicate that these selective vulnerabilities in ligand-dependent tumors could synergize with immunotherapy targeting PD-1/PD-L1 signaling in tumors [78]. This makes ligand-dependent tumors a fascinating subset of colorectal cancer, with the real possibility of new transformative treatments.
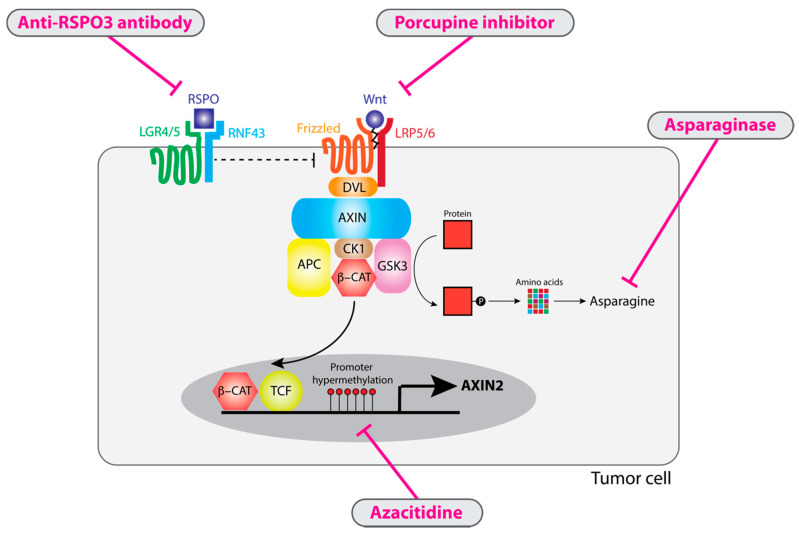
Figure 3.
Target therapies for ligand-dependent tumors. All ligand-dependent tumors require Wnt ligands for pathway activation and so are sensitive to porcupine inhibitors that impair Wnt ligand activation. RSPO3 overexpression can be antagonized by anti-RSPO3 antibodies. Ligand-dependent GSK3 inhibition results in reduced proteasomal degradation to generate amino acids such as asparagine, making tumor cells sensitive to asparagine depletion with asparaginase treatment. Ligand-dependent tumors are characterized by AXIN2 repression which can be antagonized with licensed demethylating agents such as azacitidine.
By definition, ligand-dependent Wnt alterations can only induce downstream Wnt pathway activation in the presence of Wnt ligand. As a result, depletion and inactivation of Wnt ligand by inhibition of porcupine is a viable therapeutic approach for ligand-dependent tumors. In vitro models of ligand-dependent tumors, including organoids with RNF43 mutations [79] and cell lines with RSPO fusions [46], are exquisitely sensitive to porcupine inhibitors. This has also been demonstrated in various in vivo settings, including xenografts with RSPO fusions [77] and autochthonous Rnf43/Znrf3-null intestinal tumors [80]. Porcupine inhibition is associated with marked repression of Wnt pathway activity, reduced tumor size and substantial remodeling the transcriptomic landscape that includes increased intestinal differentiation [77,80]. Porcupine inhibitors have entered early-phase clinical trials (NCT01351103, NCT03447470, NCT03507998). Preliminary evidence from a phase 1 trial identified a partial response in one patient with a detectable RNF43 mutation [81] while porcupine inhibition was associated with reduced AXIN2 expression, suggesting on-target effects [82].
However, in vitro modeling of porcupine inhibition in ligand-dependent CRC cell lines has identified selection for resistance mutations, such as loss-of-function alterations to AXIN1, leading to loss of function of the destruction complex and downstream constitutive pathway activation [46]. It is worth noting that AXIN2 repression seen in ligand-dependent tumors does not result in downstream pathway activation because of redundancy with AXIN1. AXIN1 is a constitutive component of the destruction complex and not a Wnt pathway target. This would suggest that AXIN1 inactivation alone would not be sufficient to drive Wnt pathway activation unless AXIN2 was concurrently repressed—we would hypothesize that this situation could only arise in ligand-dependent tumors. This might explain the relatively low frequency (<0.05%) of truncating AXIN1 mutations seen in CRC [8].
In tumors with epithelial RSPO fusions, the autocrine signaling loop can be blocked by an anti-RSPO3 antibody. For example, treatment with anti-RSPO3 antibody has been shown to result in inhibition of xenograft tumor growth with tumor regression in some cases [27,83,84,85]. As with porcupine inhibitors, this was associated with evidence of increased intestinal differentiation on morphological and transcriptomic analysis [27,83]. This differentiated phenotype was associated with reduced expression of stem cell markers and key Wnt targets such as LGR5 and ASCL2. A phase 1 trial of an anti-RSPO3 antibody in patients with metastatic colorectal cancer was associated with partial responses in some patients, although this was not clearly associated with baseline RSPO3 expression [86]. In addition, while it has not been formally tested, it is entirely plausible that anti-RSPO3 therapy would also be effective for tumors with stromal RSPO overexpression.
The Wnt pathway plays a critical role in bone homeostasis [87] and unsurprisingly inhibition of ligand-dependent Wnt signaling via porcupine inhibitors or anti-RSPO3 antibodies results in on-target bone toxicity, including reduced bone strength and pathological fractures [86,88,89]. Consistent with this, porcupine-null mice have widespread bone defects, while germline loss-of-function Wnt ligand mutations in humans are associated with high fracture risk [90,91,92]. Preliminary evidence has shown that bone toxicity could be reduced with co-administration of denosumab, which inhibits bone degradation [89]. Altogether, concerns about resistance and on-target toxicity would likely limit the use of direct Wnt inhibitions (porcupine, anti-RSPO3) to short durations of time, likely in conjunction with other treatments.
In light of evidence that ligand-dependent tumors may depend upon repression of negative regulators, possibly via promoter hypermethylation [42], demethylating agents could be a viable therapeutic strategy in ligand-dependent tumors. Demethylation treatment with azacitidine in HCT116, a colorectal cancer cell line with an RNF43 mutation and comparatively low AXIN2 expression, resulted in increased AXIN2 expression and increased cell death [60,93]. Azacitidine is an approved treatment for myelodysplastic syndrome with a well-established toxicity profile suggesting that this would be a feasible treatment for ligand-dependent CRC [94].
Unexpectedly, recent work in acute myeloid leukemia found that asparaginase treatment was synthetically lethal with inhibition of GSK3 [95]. Asparaginase functions to deaminate and so degrade the nonessential amino acid asparagine, which is required for leukemic cell growth [96]. GSK3 mediates ubiquitination of a wide range of proteins, such as APC, and resulting proteasomal degradation provides a source of asparagine in the cell. Asparaginase treatment has a relatively favorable toxicity profile and is licensed for acute myeloid leukemia [97]. In contrast to ligand-independent alterations, which act downstream and by-pass GSK3, ligand-dependent mutations directly lead to inhibition of GSK3 (Figure 1) through activation of the canonical Wnt pathway, thus explaining a unique selective vulnerability for asparaginase treatment in ligand-dependent tumors. Specifically, asparaginase treatments were highly toxic for organoids with RSPO fusions but had no activity against organoids with APC or CTNNB1 mutations [98]. Treatment of mice with subcutaneous implantation of RSPO-mutant organoids was associated with marked tumor regression and prolonged progression-free survival, with no evidence of early therapy resistance [98]. No benefit was seen for implanted APC-mutant organoids. Altogether, these data would suggest that asparaginase could be a viable and well-tolerated treatment for patients with ligand-dependent CRC.
In summary, ligand-dependent Wnt biology is associated with a range of therapeutic vulnerabilities that could be exploited as effective anti-cancer therapy.
9. Combination Therapy for Ligand-Dependent Tumors
Considering that direct inhibition of the Wnt pathway is unlikely to be feasible for extended periods of time, it is important to consider how treatments for ligand-dependent tumors might synergize with existing anti-cancer therapy. Wnt pathway activation is often detected as a marker of resistance to cytotoxic chemotherapy [99]. Resistance to paclitaxel, which is a type of cytotoxic chemotherapy that inhibits microtubule detachment from centrosomes, is associated with Wnt pathway activation, detected as increased CTNNB1 protein expression. Considering that Wnt functions as a regulator of centrosome separation [100], it is feasible that Wnt activation could directly promote survival of tumor cells. Consistent with this, anti-Wnt treatments such as anti-RSPO3 antibodies synergize with paclitaxel in patient-derived xenografts with RSPO3 fusions [84].
More generally, inhibition of Wnt signaling in ligand-dependent tumors is consistently shown to skew cells from a stem-like phenotype to a more differentiated phenotype [27,101,102]. Resistance to cancer radiotherapy and chemotherapy is often driven by acquisition of stem-like phenotypes, with enrichment of tumor cells that are able to repopulate a tumor on transplantation, often termed cancer stem cells [103,104]. This suggests that short courses of Wnt pathway inhibitors could be synergistic with a wide range of existing and innovative drug regimens, especially if Wnt inhibitors are early in the treatment schedule.
The Wnt signaling pathway appears to play a role in protecting cells from immune surveillance. As a result, there is considerable interest in the combination of immunotherapy that targets PD-L1/PD-1 signaling and direct inhibition of the Wnt signaling pathway. Signaling through the PD-1 receptor is thought to promote an exhausted phenotype in cytotoxic T cells that impairs effective anti-tumoral immunity [105]. While immunotherapy has demonstrated activity in diverse tumor types, it has proved ineffective in unselected patients with CRC [5]. There are multiple lines of evidence that the Wnt signaling pathway can directly promote an immune suppressive environment [106]. Early data from trials of porcupine inhibitors have shown evidence for increased expression of activated immune signatures [82]. Furthermore, porcupine inhibition was synergistic with anti-CTLA4 immunotherapy in a murine melanoma model [107]. Altogether, this raises the question of whether anti-Wnt therapies would act synergistically with immunotherapy in colorectal tumors and this hypothesis is under active investigation in several early-phase clinical trials (NCT01351103, NCT02521844, NCT02675946).
Furthermore, as discussed above, the microsatellite-unstable subset of colorectal tumors ligand-dependent tumors is enriched with tumors, which have enhanced responses to immunotherapy [6]. Unexpectedly, a recent analysis incorporating a large cohort of patients with colorectal cancer who were treated with immunotherapy, demonstrated that RNF43-mutant tumors responded significantly better to immunotherapy than would have been expected from their mutational burden [108]. This is an exciting finding that raises the possibility the ligand-dependent Wnt biology might be independently associated with responses to immunotherapy and warrants further investigation in additional cohorts.
10. Outlook—Landscape of Precision Medicine in CRC
Approximately 15% of colorectal tumors have ligand-dependent alterations in the Wnt signaling pathway, affecting RNF43 or RSPO2/3. This unique Wnt biology is associated with a range of specific therapeutic vulnerabilities, especially to depletion of Wnt ligand by porcupine inhibitors. There is a strong theoretical basis for the combination of immunotherapy with a time-limited course of porcupine inhibition. Inhibitors of ligand-dependent Wnt signaling are known to be ineffectual in tumors with ligand-independent alterations such as APC mutations [79]. As a result, due to the low frequency of ligand-dependent alterations, clinical trials of these selective treatments will fail in unselected patients. Precision medicine depends upon the ability to stratify patients into clinically meaningful subsets, followed by targeting with biologically appropriate therapies. It is contingent on the existence of biomarkers specific for each subset that can be feasibly adopted into routine clinic practice. We propose that AXIN2 is one such biomarker and could be measured at low cost from routine clinical specimens. It can be measured by high-throughput qRT-PCR and does not require costly and time-consuming DNA and RNA sequencing. On the basis of AXIN2 expression, it would be possible to identify patients with ligand-dependent Wnt biology who could then be targeted with effective personalized therapies. In summary, we propose that the concept of ligand-dependent tumors as an individual disease entity has the potential to revolutionize precision medicine and improve the outcomes for patients with colorectal cancer.
Author Contributions
S.O.K. and S.J.L. co-wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by Wellcome Trust Senior Clinical Research Fellowship (206314/Z/17/Z).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Siegel R.L., Miller K.D., Sauer A.G., Fedewa S.A., Butterly L.F., Anderson J.C., Cercek A., Smith R.A., Jemal A. Colorectal cancer statistics, 2020. CA Cancer J. Clin. 2020;70:145–164. doi: 10.3322/caac.21601. [DOI] [PubMed] [Google Scholar]
- 2.Cutsem E.V., Cervantes A., Nordlinger B., Arnold D., Group E.G.W. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2014;25:iii1–iii9. doi: 10.1093/annonc/mdu260. [DOI] [PubMed] [Google Scholar]
- 3.Araghi M., Soerjomataram I., Bardot A., Ferlay J., Cabasag C.J., Morrison D.S., De P., Tervonen H., Walsh P.M., Bucher O., et al. Changes in colorectal cancer incidence in seven high-income countries: A population-based study. Lancet Gastroenterol. Hepatol. 2019;4:511–518. doi: 10.1016/S2468-1253(19)30147-5. [DOI] [PubMed] [Google Scholar]
- 4.Dienstmann R., Vermeulen L., Guinney J., Kopetz S., Tejpar S., Tabernero J. Consensus molecular subtypes and the evolution of precision medicine in colorectal cancer. Nat. Rev. Cancer. 2017;17:79–92. doi: 10.1038/nrc.2016.126. [DOI] [PubMed] [Google Scholar]
- 5.Brahmer J.R., Tykodi S.S., Chow L.Q.M., Hwu W.-J., Topalian S.L., Hwu P., Drake C.G., Camacho L.H., Kauh J., Odunsi K., et al. Safety and Activity of Anti–PD-L1 Antibody in Patients with Advanced Cancer. N. Engl. J. Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Overman M.J., McDermott R., Leach J.L., Lonardi S., Lenz H.-J., Morse M.A., Desai J., Hill A., Axelson M., Moss R.A., et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): An open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18:1182–1191. doi: 10.1016/S1470-2045(17)30422-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Xie Y.-H., Chen Y.-X., Fang J.-Y. Comprehensive review of targeted therapy for colorectal cancer. Signal Transduct. Target. Ther. 2020;5:22. doi: 10.1038/s41392-020-0116-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Muzny D.M., Bainbridge M.N., Chang K., Dinh H.H., Drummond J.A., Fowler G., Kovar C.L., Lewis L.R., Morgan M.B., Newsham I.F., et al. Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiese K.E., Nusse R., Amerongen R. Van Wnt signalling: Conquering complexity. Development. 2018;145:dev165902. doi: 10.1242/dev.165902. [DOI] [PubMed] [Google Scholar]
- 10.Fevr T., Robine S., Louvard D., Huelsken J. Wnt/β-Catenin Is Essential for Intestinal Homeostasis and Maintenance of Intestinal Stem Cells. Mol. Cell Biol. 2007;27:7551–7559. doi: 10.1128/MCB.01034-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dow L.E., O’Rourke K.P., Simon J., Tschaharganeh D.F., van Es J.H., Clevers H., Lowe S.W. Apc Restoration Promotes Cellular Differentiation and Reestablishes Crypt Homeostasis in Colorectal Cancer. Cell. 2015;161:1539–1552. doi: 10.1016/j.cell.2015.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spranger S., Bao R., Gajewski T.F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature. 2015;523:231–235. doi: 10.1038/nature14404. [DOI] [PubMed] [Google Scholar]
- 13.Grasso C.S., Giannakis M., Wells D.K., Hamada T., Mu X.J., Quist M., Nowak J.A., Nishihara R., Qian Z.R., Inamura K., et al. Genetic mechanisms of immune evasion in colorectal cancer. Cancer Discov. 2018;8:730–749. doi: 10.1158/2159-8290.CD-17-1327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fearon E.R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990;61:759–767. doi: 10.1016/0092-8674(90)90186-I. [DOI] [PubMed] [Google Scholar]
- 15.Bettington M., Walker N., Clouston A., Brown I., Leggett B., Whitehall V. The serrated pathway to colorectal carcinoma: Current concepts and challenges. Histopathology. 2013;62:367–386. doi: 10.1111/his.12055. [DOI] [PubMed] [Google Scholar]
- 16.Barker N., Ridgway R.A., Van Es J.H., Van De Wetering M., Begthel H., Born M.V.D., Danenberg E., Clarke A.R., Sansom O.J., Clevers H. Crypt stem cells as the cells-of-origin of intestinal cancer. Nat. Cell Biol. 2009;457:608–611. doi: 10.1038/nature07602. [DOI] [PubMed] [Google Scholar]
- 17.Davis H., Irshad S., Bansal M., Rafferty H., Boitsova T., Bardella C., Jaeger E., Lewis A., Freeman-Mills L., Giner F.C., et al. Aberrant epithelial GREM1 expression initiates colonic tumorigenesis from cells outside the stem cell niche. Nat. Med. 2015;21:62–70. doi: 10.1038/nm.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Takada R., Satomi Y., Kurata T., Ueno N., Norioka S., Kondoh H., Takao T., Takada S. Monounsaturated Fatty Acid Modification of Wnt Protein: Its Role in Wnt Secretion. Dev. Cell. 2006;11:791–801. doi: 10.1016/j.devcel.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Shoshkes-Carmel M., Wang Y.J., Wangensteen K.J., Tóth B., Kondo A., Massasa E.E., Itzkovitz S., Kaestner K.H. Subepithelial telocytes are an important source of Wnts that supports intestinal crypts. Nature. 2018;557:242–246. doi: 10.1038/s41586-018-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Niehrs C. The complex world of WNT receptor signalling. Nat. Rev. Mol. Cell Biol. 2012;13:767–779. doi: 10.1038/nrm3470. [DOI] [PubMed] [Google Scholar]
- 21.Janda C.Y., Waghray D., Levin A.M., Thomas C., Garcia K.C. Structural Basis of Wnt Recognition by Frizzled. Science. 2012;337:59–64. doi: 10.1126/science.1222879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stamos J.L., Weis W.I. The β-Catenin Destruction Complex. Cold Spring Harb. Perspect. Biol. 2013;5:a007898. doi: 10.1101/cshperspect.a007898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.De Lau W., Peng W.C., Gros P., Clevers H. The R-spondin/Lgr5/Rnf43 module: Regulator of Wnt signal strength. Genes Dev. 2014;28:305–316. doi: 10.1101/gad.235473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yan K.S., Janda C.Y., Chang J., Zheng G.X.Y., Larkin K.A., Luca V.C., Chia L.A., Mah A.T., Han A., Terry J.M., et al. Non-equivalence of Wnt and R-spondin ligands during Lgr5+ intestinal stem-cell self-renewal. Nature. 2017;545:238–242. doi: 10.1038/nature22313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Park S., Cui J., Yu W., Wu L., Carmon K.S., Liu Q.J. Differential activities and mechanisms of the four R-spondins in potentiating Wnt/β-catenin signaling. J. Biol. Chem. 2018;293:9759–9769. doi: 10.1074/jbc.RA118.002743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Greicius G., Kabiri Z., Sigmundsson K., Liang C., Bunte R., Singh M.K., Virshup D.M. PDGFRα+ pericryptal stromal cells are the critical source of Wnts and RSPO3 for murine intestinal stem cells in vivo. Proc. Natl. Acad. Sci. USA. 2018;115:201713510. doi: 10.1073/pnas.1713510115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Storm E.E., Durinck S., Melo F.D.S.E., Tremayne J., Kljavin N.M., Tan C., Ye X., Chiu C., Pham T., Hongo J.-A., et al. Targeting PTPRK-RSPO3 colon tumours promotes differentiation and loss of stem-cell function. Nat. Cell Biol. 2016;529:97–100. doi: 10.1038/nature16466. [DOI] [PubMed] [Google Scholar]
- 28.Sato T., Stange D.E., Ferrante M., Vries R.G., Van Es J.H., Brink S.V.D., Van Houdt W.J., Pronk A., Van Gorp J., Siersema P.D., et al. Long-term Expansion of Epithelial Organoids From Human Colon, Adenoma, Adenocarcinoma, and Barrett’s Epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 29.Filipovich A., Gehrke I., Poll-Wolbeck S.J., Kreuzer K. Physiological inhibitors of Wnt signaling. Eur. J. Haematol. 2011;86:453–465. doi: 10.1111/j.1600-0609.2011.01592.x. [DOI] [PubMed] [Google Scholar]
- 30.Kakugawa S., Langton P.F., Zebisch M., Howell S.A., Chang T.-H., Liu Y., Feizi T., Bineva G., O’Reilly N., Snijders A.P., et al. Notum deacylates Wnt proteins to suppress signalling activity. Nature. 2015;519:187–192. doi: 10.1038/nature14259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Giannakis M., Hodis E., Mu X.J., Yamauchi M., Rosenbluh J., Cibulskis K., Saksena G., Lawrence M.S., Qian Z.R., Nishihara R., et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 2014;46:1264–1266. doi: 10.1038/ng.3127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Seshagiri S., Stawiski E.W., Durinck S., Modrusan Z., Storm E.E., Conboy C.B., Chaudhuri S., Guan Y., Janakiraman V., Jaiswal B.S., et al. Recurrent R-spondin fusions in colon cancer. Nature. 2012;488:660–664. doi: 10.1038/nature11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han T., Schatoff E.M., Murphy C., Zafra M.P., Wilkinson J.E., Elemento O., Dow L.E. R-Spondin chromosome rearrangements drive Wnt-dependent tumour initiation and maintenance in the intestine. Nat. Commun. 2017;8:15945. doi: 10.1038/ncomms15945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harada N., Tamai Y., Ishikawa T., Sauer B., Takaku K., Oshima M., Taketo M.M. Intestinal polyposis in mice with a dominant stable mutation of the β-catenin gene. EMBO J. 1999;18:5931–5942. doi: 10.1093/emboj/18.21.5931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pollard P., Deheragoda M., Segditsas S., Lewis A., Rowan A., Howarth K., Willis L., Nye E., McCart A., Mandir N., et al. The Apc1322T Mouse Develops Severe Polyposis Associated with Submaximal Nuclear β-Catenin Expression. Gastroenterology. 2009;136:2204–2213.e13. doi: 10.1053/j.gastro.2009.02.058. [DOI] [PubMed] [Google Scholar]
- 36.Koo B.-K., Spit M., Jordens I., Low T.Y., Stange D.E., Van De Wetering M., Van Es J.H., Mohammed S., Heck A.J.R., Maurice M.M., et al. Tumour suppressor RNF43 is a stem-cell E3 ligase that induces endocytosis of Wnt receptors. Nat. Cell Biol. 2012;488:665–669. doi: 10.1038/nature11308. [DOI] [PubMed] [Google Scholar]
- 37.Vogelstein B., Papadopoulos N., Velculescu V.E., Zhou S., Diaz L.A., Kinzler K.W. Cancer Genome Landscapes. Science. 2013;339:1546–1558. doi: 10.1126/science.1235122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melé M., Ferreira P.G., Reverter F., DeLuca D.S., Monlong J., Sammeth M., Young T.R., Goldmann J.M., Pervouchine D.D., Sullivan T.J., et al. The human transcriptome across tissues and individuals. Science. 2015;348:660–665. doi: 10.1126/science.aaa0355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Carninci P., Kasukawa T., Katayama S., Gough J., Frith M.C., Maeda N., Oyama R., Ravasi T., Lenhard B., Wells C., et al. The Transcriptional Landscape of the Mammalian Genome. Science. 2005;309:1559–1563. doi: 10.1126/science.1112014. [DOI] [PubMed] [Google Scholar]
- 40.Mori Y., Nagse H., Ando H., Horii A., Ichii S., Nakatsuru S., Aoki T., Miki Y., Mori T., Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: Mutation cluster region in the APC gene. Hum. Mol. Genet. 1992;1:229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- 41.Gao C., Wang Y., Broaddus R., Sun L., Xue F., Zhang W. Exon 3 mutations of CTNNB1 drive tumorigenesis: A review. Oncotarget. 2017;9:5492–5508. doi: 10.18632/oncotarget.23695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kleeman S.O., Koelzer V.H., Jones H.J., Vazquez E.G., Davis H., East J.E., Arnold R., Koppens M.A., Blake A., Domingo E., et al. Exploiting differential Wnt target gene expression to generate a molecular biomarker for colorectal cancer stratification. Gut. 2020;69:1092–1103. doi: 10.1136/gutjnl-2019-319126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hashimoto T., Ogawa R., Yoshida H., Taniguchi H., Kojima M., Saito Y., Sekine S. EIF3E–RSPO2 and PIEZO1–RSPO2 fusions in colorectal traditional serrated adenoma. Histopathology. 2019;75:266–273. doi: 10.1111/his.13867. [DOI] [PubMed] [Google Scholar]
- 44.Sekine S., Yamashita S., Tanabe T., Hashimoto T., Yoshida H., Taniguchi H., Kojima M., Shinmura K., Saito Y., Hiraoka N., et al. Frequent PTPRK–RSPO3 fusions and RNF43 mutations in colorectal traditional serrated adenoma. J. Pathol. 2016;239:133–138. doi: 10.1002/path.4709. [DOI] [PubMed] [Google Scholar]
- 45.Gong X., Yi J., Carmon K.S., Crumbley C.A., Xiong W., Thomas A., Fan X., Guo S., An Z., Chang J.T., et al. Aberrant RSPO3-LGR4 signaling in Keap1-deficient lung adenocarcinomas promotes tumor aggressiveness. Oncogene. 2015;34:4692–4701. doi: 10.1038/onc.2014.417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Picco G., Petti C., Centonze A., Torchiaro E., Crisafulli G., Novara L., Acquaviva A., Bardelli A., Medico E. Loss of AXIN1 drives acquired resistance to WNT pathway blockade in colorectal cancer cells carrying RSPO3 fusions. EMBO Mol. Med. 2017;9:293–303. doi: 10.15252/emmm.201606773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ellegren H. Microsatellites: Simple sequences with complex evolution. Nat. Rev. Genet. 2004;5:435–445. doi: 10.1038/nrg1348. [DOI] [PubMed] [Google Scholar]
- 48.Maruvka Y.E., Mouw K.W., Karlic R., Parasuraman P., Kamburov A., Polak P., Haradhvala N.J., Hess J.M., Rheinbay E., Brody Y., et al. Analysis of somatic microsatellite indels identifies driver events in human tumors. Nat. Biotechnol. 2017;35:951–959. doi: 10.1038/nbt.3966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tu J., Park S., Yu W., Zhang S., Wu L., Carmon K., Liu Q.J. The most common RNF43 mutant G659Vfs*41 is fully functional in inhibiting Wnt signaling and unlikely to play a role in tumorigenesis. Sci. Rep. 2019;9:18557. doi: 10.1038/s41598-019-54931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Yu J., Yuso P.A.B.M., Woutersen D.T., Goh P., Harmston N., Smits R., Epstein D., Virshup D.M., Madan B. The functional landscape of patient-derived RNF43 mutations predicts sensitivity to Wnt inhibiton. Cancer Res. 2020 doi: 10.1158/0008-5472.CAN-20-0957. [DOI] [PubMed] [Google Scholar]
- 51.Hause R.J., Pritchard C.C., Shendure J., Salipante S.J. Classification and characterization of microsatellite instability across 18 cancer types. Nat. Med. 2016;22:1342–1350. doi: 10.1038/nm.4191. [DOI] [PubMed] [Google Scholar]
- 52.Albuquerque C., Breukel C., van der Luijt R., Fidalgo P., Lage P., Slors F.J.M., Leitão C.N., Fodde R., Smits R. The ‘just-right’ signaling model: APC somatic mutations are selected based on a specific level of activation of the β-catenin signaling cascade. Hum. Mol. Genet. 2002;11:1549–1560. doi: 10.1093/hmg/11.13.1549. [DOI] [PubMed] [Google Scholar]
- 53.Lähde M., Heino S., Högström J., Kaijalainen S., Anisimov A., Flanagan D., Kallio P., Leppänen V.-M., Ristimäki A., Ritvos O., et al. Expression of R-spondin1 in Apc Min/+ Mice Reduces Growth of Intestinal Adenomas by Altering Wnt and TGFB Signaling. Gastroenterology. 2020 doi: 10.1053/j.gastro.2020.09.011. [DOI] [PubMed] [Google Scholar]
- 54.Biechele T.L., Kulikauskas R.M., Toroni R.A., Lucero O.M., Swift R.D., James R.G., Robin N.C., Dawson D.W., Moon R.T., Chien A.J. Wnt/{beta}-Catenin Signaling and AXIN1 Regulate Apoptosis Triggered by Inhibition of the Mutant Kinase BRAFV600E in Human Melanoma. Sci. Signal. 2012;5:ra3. doi: 10.1126/scisignal.2002274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hashimoto T., Yamashita S., Yoshida H., Taniguchi H., Ushijima T., Yamada T., Saito Y., Ochiai A., Sekine S., Hiraoka N. WNT Pathway Gene Mutations Are Associated With the Presence of Dysplasia in Colorectal Sessile Serrated Adenoma/Polyps. Am. J. Surg. Pathol. 2017;41:1188–1197. doi: 10.1097/pas.0000000000000877. [DOI] [PubMed] [Google Scholar]
- 56.Borowsky J., Dumenil T., Bettington M., Pearson S.-A., Bond C., Fennell L., Liu C., McKeone D., Rosty C., Brown I., et al. The role of APC in WNT pathway activation in serrated neoplasia. Mod. Pathol. 2018;31:495–504. doi: 10.1038/modpathol.2017.150. [DOI] [PubMed] [Google Scholar]
- 57.Lamlum H., Papadopoulou A., Ilyas M., Rowan A., Gillet C., Hanby A., Talbot I., Bodmer W., Tomlinson I. APC mutations are sufficient for the growth of early colorectal adenomas. Proc. Natl. Acad. Sci. USA. 2000;97:2225–2228. doi: 10.1073/pnas.040564697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yan H.H.N., Siu H.C., Ho S.L., Yue S.S.K., Gao Y., Tsui W.Y., Chan D., Chan A.S., Wong J.W.H., Man A.H.Y., et al. Organoid cultures of early-onset colorectal cancers reveal distinct and rare genetic profiles. Gut. 2020 doi: 10.1136/gutjnl-2019-320019. [DOI] [PubMed] [Google Scholar]
- 59.Perekatt A.O., Shah P.P., Cheung S., Jariwala N., Wu A., Gandhi V., Kumar N., Feng Q., Patel N., Chen L., et al. SMAD4 suppresses WNT-driven de-differentiation and oncogenesis in the differentiated gut epithelium. Cancer Res. 2018;78 doi: 10.1158/0008-5472.CAN-18-0043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Koinuma K., Yamashita Y., Liu W., Hatanaka H., Kurashina K., Wada T., Takada S., Kaneda R., Choi Y.L., Fujiwara S.-I., et al. Epigenetic silencing of AXIN2 in colorectal carcinoma with microsatellite instability. Oncogene. 2006;25:139–146. doi: 10.1038/sj.onc.1209009. [DOI] [PubMed] [Google Scholar]
- 61.Muto Y., Maeda T., Suzuki K., Kato T., Watanabe F., Kamiyama H., Saito M., Koizumi K., Miyaki Y., Konishi F., et al. DNA methylation alterations of AXIN2 in serrated adenomas and colon carcinomas with microsatellite instability. BMC Cancer. 2014;14:466. doi: 10.1186/1471-2407-14-466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Murakami T., Mitomi H., Saito T., Takahashi M., Sakamoto N., Fukui N., Yao T., Watanabe S. Distinct WNT/β-catenin signaling activation in the serrated neoplasia pathway and the adenoma-carcinoma sequence of the colorectum. Mod. Pathol. 2015;28:146–158. doi: 10.1038/modpathol.2014.41. [DOI] [PubMed] [Google Scholar]
- 63.Lannagan T.R.M., Lee Y.K., Wang T., Roper J., Bettington M.L., Fennell L., Vrbanac L., Jonavicius L., Somashekar R., Gieniec K., et al. Genetic editing of colonic organoids provides a molecularly distinct and orthotopic preclinical model of serrated carcinogenesis. Gut. 2019;68:684. doi: 10.1136/gutjnl-2017-315920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Belshaw N.J., Elliott G.O., Foxall R.J., Dainty J.R., Pal N., Coupe A., Garg D., Bradburn D.M., Mathers J.C., Johnson I.T. Profiling CpG island field methylation in both morphologically normal and neoplastic human colonic mucosa. Br. J. Cancer. 2008;99:136–142. doi: 10.1038/sj.bjc.6604432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zehir A., Benayed R., Shah R.H., Syed A., Middha S., Kim H.R., Srinivasan P., Gao J., Chakravarty D., Devlin S.M., et al. Mutational landscape of metastatic cancer revealed from prospective clinical sequencing of 10,000 patients. Nat. Med. 2017;23:703–713. doi: 10.1038/nm.4333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hughes T.A., Brady H.J.M. Regulation of axin2 expression at the levels of transcription, translation and protein stability in lung and colon cancer. Cancer Lett. 2006;233:338–347. doi: 10.1016/j.canlet.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 67.Jho E., Zhang T., Domon C., Joo C.-K., Freund J.-N., Costantini F. Wnt/β-Catenin/Tcf Signaling Induces the Transcription of Axin2, a Negative Regulator of the Signaling Pathway. Mol. Cell Biol. 2002;22:1172–1183. doi: 10.1128/MCB.22.4.1172-1183.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Giannakis M., Mu X.J., Shukla S.A., Qian Z.R., Cohen O., Nishihara R., Bahl S., Cao Y., Amin-Mansour A., Yamauchi M., et al. Genomic Correlates of Immune-Cell Infiltrates in Colorectal Carcinoma. Cell Rep. 2016;15:857–865. doi: 10.1016/j.celrep.2016.03.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim Y.S., Ho S.B. Intestinal Goblet Cells and Mucins in Health and Disease: Recent Insights and Progress. Curr. Gastroenterol. Rep. 2010;12:319–330. doi: 10.1007/s11894-010-0131-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Luo C., Cen S., Ding G., Wu W. Mucinous colorectal adenocarcinoma: Clinical pathology and treatment options. Cancer Commun. 2019;39:1–13. doi: 10.1186/s40880-019-0361-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Tsai J., Liau J., Yuan C., Cheng M., Yuan R., Jeng Y. RNF43 mutation frequently occurs with GNAS mutation and mucin hypersecretion in intraductal papillary neoplasms of the bile duct. Histopathology. 2017;70:756–765. doi: 10.1111/his.13125. [DOI] [PubMed] [Google Scholar]
- 72.Guinney J., Dienstmann R., Wang X., De Reyniès A., Schlicker A., Soneson C., Marisa L., Roepman P., Nyamundanda G., Angelino P., et al. The consensus molecular subtypes of colorectal cancer. Nat. Med. 2015;21:1350–1356. doi: 10.1038/nm.3967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koelzer V.H., Zlobec I., Lugli A. Tumor budding in colorectal cancer—ready for diagnostic practice? Hum. Pathol. 2016;47:4–19. doi: 10.1016/j.humpath.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 74.Sis B., Sarioglu S., Sokmen S., Sakar M., Kupelioglu A., Fuzun M. Desmoplasia measured by computer assisted image analysis: An independent prognostic marker in colorectal carcinoma. J. Clin. Pathol. 2005;58:32. doi: 10.1136/jcp.2004.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Verhulst J., Ferdinande L., Demetter P., Ceelen W. Mucinous subtype as prognostic factor in colorectal cancer: A systematic review and meta-analysis. J. Clin. Pathol. 2012;65:381. doi: 10.1136/jclinpath-2011-200340. [DOI] [PubMed] [Google Scholar]
- 76.Cui C., Zhou X., Zhang W., Qu Y., Ke X. Is β-Catenin a Druggable Target for Cancer Therapy? Trends Biochem. Sci. 2018;43:623–634. doi: 10.1016/j.tibs.2018.06.003. [DOI] [PubMed] [Google Scholar]
- 77.Madan B., Ke Z., Harmston N., Ho S.Y., Frois A.O., Alam J., Jeyaraj D.A., Pendharkar V., Ghosh K., Virshup I.H., et al. Wnt addiction of genetically defined cancers reversed by PORCN inhibition. Oncogene. 2016;35:2197–2207. doi: 10.1038/onc.2015.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang B., Tian T., Kalland K.-H., Ke X., Qu Y. Targeting Wnt/β-Catenin Signaling for Cancer Immunotherapy. Trends Pharmacol. Sci. 2018;39:648–658. doi: 10.1016/j.tips.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 79.Van de Wetering M., Francies H.E., Francis J.M., Bounova G., Iorio F., Pronk A., van Houdt W., van Gorp J., Taylor-Weiner A., Kester L., et al. Prospective Derivation of a Living Organoid Biobank of Colorectal Cancer Patients. Cell. 2015;161:933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Koo B.-K., Van Es J.H., Born M.V.D., Clevers H. Porcupine inhibitor suppresses paracrine Wnt-driven growth of Rnf43;Znrf3-mutant neoplasia. Proc. Natl. Acad. Sci. USA. 2015;112:7548–7550. doi: 10.1073/pnas.1508113112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Janku F., Connolly R., Lorusso P., De Jonge M., Vaishampayan U., Rodon J., Argilés G., Myers A., Schmitz S.-F.H., Ji Y., et al. Abstract C45: Phase I study of WNT974, a first-in-class Porcupine inhibitor, in advanced solid tumors. Clin. Trials. 2015;14:C45. doi: 10.1158/1535-7163.targ-15-c45. [DOI] [Google Scholar]
- 82.Rodon J., Argilés G., Connolly R.M., Vaishampayan U., De Jonge M., Garralda E., Giannakis M., Smith D.C., Dobson J.R., McLaughlin M., et al. Abstract CT175: Biomarker analyses from a phase I study of WNT974, a first-in-class Porcupine inhibitor, in patients (pts) with advanced solid tumors. Clin. Trials. 2018;78:CT175. doi: 10.1158/1538-7445.am2018-ct175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Chartier C., Raval J., Axelrod F., Bond C., Cain J., Dee-Hoskins C., Ma S., Fischer M.M., Shah J., Wei J., et al. Therapeutic Targeting of Tumor-Derived R-Spondin Attenuates -Catenin Signaling and Tumorigenesis in Multiple Cancer Types. Cancer Res. 2015;76:713–723. doi: 10.1158/0008-5472.CAN-15-0561. [DOI] [PubMed] [Google Scholar]
- 84.Fischer M.M., Yeung V.P., Cattaruzza F., Hussien R., Yen W.-C., Murriel C., Evans J.W., O’Young G., Brunner A.L., Wang M., et al. RSPO3 antagonism inhibits growth and tumorigenicity in colorectal tumors harboring common Wnt pathway mutations. Sci. Rep. 2017;7:15270. doi: 10.1038/s41598-017-15704-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li C., Cao J., Zhang N., Tu M., Xu F., Wei S., Chen X., Xu Y. Identification of RSPO2 Fusion Mutations and Target Therapy Using a Porcupine Inhibitor. Sci. Rep. 2018;8:14244. doi: 10.1038/s41598-018-32652-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bendell J., Eckhardt G.S., Hochster H.S., Morris V.K., Strickler J., Kapoun A.M., Wang M., Xu L., McGuire K., Dupont J., et al. 68 Initial results from a phase 1a/b study of OMP-131R10, a first-in-class anti-RSPO3 antibody, in advanced solid tumors and previously treated metastatic colorectal cancer (CRC) Eur. J. Cancer. 2016;69:S29–S30. doi: 10.1016/S0959-8049(16)32668-5. [DOI] [Google Scholar]
- 87.Monroe D.G., McGee-Lawrence M.E., Oursler M.J., Westendorf J.J. Update on Wnt signaling in bone cell biology and bone disease. Gene. 2012;492:1–18. doi: 10.1016/j.gene.2011.10.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Funck-Brentano T., Nilsson K.H., Brommage R., Henning P., Lerner U.H., Koskela A., Tuukkanen J., Cohen-Solal M., Movérare-Skrtic S., Ohlsson C. Porcupine inhibitors impair trabecular and cortical bone mass and strength in mice. J. Endocrinol. 2018;238:13–23. doi: 10.1530/JOE-18-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Tan D., Ng M., Subbiah V., Messersmith W., Teneggi V., Diermayr V., Ethirajulu K., Yeo P., Gan B.H., Lee L.H., et al. 71O Phase I extension study of ETC-159 an oral PORCN inhibitor administered with bone protective treatment, in patients with advanced solid tumours. Ann. Oncol. 2018;29:ix23–ix24. doi: 10.1093/annonc/mdy430.002. [DOI] [Google Scholar]
- 90.Barrott J.J., Cash G.M., Smith A.P., Barrow J.R., Murtaugh L.C. Deletion of mouse Porcn blocks Wnt ligand secretion and reveals an ectodermal etiology of human focal dermal hypoplasia/Goltz syndrome. Proc. Natl. Acad. Sci. USA. 2011;108:12752–12757. doi: 10.1073/pnas.1006437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Fahiminiya S., Majewski J., Mort J., Moffatt P., Glorieux F.H., Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J. Med. Genet. 2013;50:345. doi: 10.1136/jmedgenet-2013-101567. [DOI] [PubMed] [Google Scholar]
- 92.Zheng H.-F., Tobias J.H., Duncan E.L., Evans D.M., Eriksson J., Paternoster L., Yerges-Armstrong L.M., Lehtimäki T., Bergström U., Kähönen M., et al. WNT16 Influences Bone Mineral Density, Cortical Bone Thickness, Bone Strength, and Osteoporotic Fracture Risk. PLoS Genet. 2012;8:e1002745. doi: 10.1371/journal.pgen.1002745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tsai H.-C., Li H., Van Neste L., Cai Y., Robert C., Rassool F.V., Shin J.J., Harbom K.M., Beaty R., Pappou E., et al. Transient Low Doses of DNA-Demethylating Agents Exert Durable Antitumor Effects on Hematological and Epithelial Tumor Cells. Cancer Cell. 2012;21:430–446. doi: 10.1016/j.ccr.2011.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Howell P.M., Liu Z., Khong H.T. Demethylating Agents in the Treatment of Cancer. Pharm. 2010;3:2022–2044. doi: 10.3390/ph3072022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hinze L., Pfirrmann M., Karim S., Degar J., McGuckin C., Vinjamur D., Sacher J., Stevenson K.E., Neuberg D.S., Orellana E., et al. Synthetic Lethality of Wnt Pathway Activation and Asparaginase in Drug-Resistant Acute Leukemias. Cancer Cell. 2019;35:664–676.e7. doi: 10.1016/j.ccell.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rizzari C., Conter V., Starý J., Colombini A., Moericke A., Schrappe M. Optimizing asparaginase therapy for acute lymphoblastic leukemia. Curr. Opin. Oncol. 2013;25:S1–S9. doi: 10.1097/CCO.0b013e32835d7d85. [DOI] [PubMed] [Google Scholar]
- 97.Pieters R., Hunger S.P., Boos J., Rizzari C., Silverman L., Baruchel A., Goekbuget N., Schrappe M., Pui C. L-asparaginase treatment in acute lymphoblastic leukemia. Cancer. 2011;117:238–249. doi: 10.1002/cncr.25489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Hinze L., Labrosse R., Degar J., Han T., Schatoff E.M., Schreek S., Karim S., McGuckin C., Sacher J.R., Wagner F., et al. Exploiting the Therapeutic Interaction of WNT Pathway Activation and Asparaginase for Colorectal Cancer Therapy. Cancer Discov. 2020;10:1690–1705. doi: 10.1158/2159-8290.CD-19-1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mohammed M.K., Shao C., Wang J., Wei Q., Wang X., Collier Z., Tang S., Liu H., Zhang F., Huang J., et al. Wnt/β-catenin signaling plays an ever-expanding role in stem cell self-renewal, tumorigenesis and cancer chemoresistance. Genes Dis. 2016;3:11–40. doi: 10.1016/j.gendis.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Mbom B.C., Siemers K.A., Ostrowski M.A., Nelson W.J., Barth A.I.M. Nek2 phosphorylates and stabilizes β-catenin at mitotic centrosomes downstream of Plk1. Mol. Biol. Cell. 2014;25:977–991. doi: 10.1091/mbc.e13-06-0349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kabiri Z., Greicius G., Zaribafzadeh H., Hemmerich A., Counter C.M., Virshup D.M. Wnt signaling suppresses MAPK-driven proliferation of intestinal stem cells. J. Clin. Investig. 2018;128:3806–3812. doi: 10.1172/JCI99325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Clarke M.F., Dick J.E., Dirks P.B., Eaves C.J., Jamieson C.H.M., Jones D.L., Visvader J., Weissman I.L., Wahl G.M. Cancer Stem Cells—Perspectives on Current Status and Future Directions: AACR Workshop on Cancer Stem Cells. Cancer Res. 2006;66:9339–9344. doi: 10.1158/0008-5472.CAN-06-3126. [DOI] [PubMed] [Google Scholar]
- 103.Todaro M., Alea M.P., Stefano A.B.D., Cammareri P., Vermeulen L., Iovino F., Tripodo C., Russo A., Gulotta G., Medema J.P., et al. Colon Cancer Stem Cells Dictate Tumor Growth and Resist Cell Death by Production of Interleukin-4. Cell Stem Cell. 2007;1:389–402. doi: 10.1016/j.stem.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 104.Bao S., Wu Q., McLendon R.E., Hao Y., Shi Q., Hjelmeland A.B., Dewhirst M.W., Bigner D.D., Rich J.N. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature. 2006;444:756–760. doi: 10.1038/nature05236. [DOI] [PubMed] [Google Scholar]
- 105.Keir M.E., Butte M.J., Freeman G.J., Sharpe A.H. PD-1 and Its Ligands in Tolerance and Immunity. Annu. Rev. Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galluzzi L., Spranger S., Fuchs E., López-Soto A. WNT Signaling in Cancer Immunosurveillance. Trends Cell Biol. 2018;29:44–65. doi: 10.1016/j.tcb.2018.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Holtzhausen A., Zhao F., Evans K.S., Tsutsui M., Orabona C., Tyler D.S., Hanks B.A. Melanoma-Derived Wnt5a Promotes Local Dendritic-Cell Expression of IDO and Immunotolerance: Opportunities for Pharmacologic Enhancement of Immunotherapy. Cancer Immunol. Res. 2015;3:1082–1095. doi: 10.1158/2326-6066.CIR-14-0167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Jun T., Qing T., Dong G., Signaevski M., Hopkins J.F., Frampton G.M., Albacker L.A., Cordon-Cardo C., Samstein R., Pusztai L., et al. Cancer-specific associations of driver genes with immunotherapy outcome. Biorxiv. 2020 doi: 10.1101/2020.06.16.155895. [DOI] [Google Scholar]