Abstract
A pyrimidine moiety exhibiting a wide range of pharmacological activities has been employed in the design of privileged structures in medicinal chemistry. To prepare libraries of novel heterocyclic compounds with potential biological activities, a series of novel 2-(pyridin-2-yl) pyrimidine derivatives were designed, synthesized and their biological activities were evaluated against immortalized rat hepatic stellate cells (HSC-T6). Fourteen compounds were found to present better anti-fibrotic activities than Pirfenidone and Bipy55′DC. Among them, compounds ethyl 6-(5-(p-tolylcarbamoyl)pyrimidin-2-yl)nicotinate (12m) and ethyl 6-(5-((3,4-difluorophenyl)carbamoyl)pyrimidin-2-yl)nicotinate (12q) show the best activities with IC50 values of 45.69 μM and 45.81 μM, respectively. Furthermore, the study of anti-fibrosis activity was evaluated by Picro-Sirius red staining, hydroxyproline assay and ELISA detection of Collagen type I alpha 1 (COL1A1) protein expression. Our study showed that compounds 12m and 12q effectively inhibited the expression of collagen, and the content of hydroxyproline in cell culture medium in vitro, indicating that compounds 12m and 12q might be developed the novel anti-fibrotic drugs.
Keywords: anti-fibrosis, collagen prolyl 4-hydroxylases, pyrimidine, COL1A1, synthesis
1. Introduction
Construction of novel heterocyclic compound libraries with potential biological activities is an important component of medicinal chemistry and chemical biology [1]. The pyrimidine moiety has been considered as a privileged structure in medicinal chemistry [2] and the compounds containing pyrimidine as the core are reported to exhibit diverse types of biological and pharmaceutical activities. For example, pyrimidine derivatives are known as antimicrobial [3,4,5], antiviral [6,7], antitumor [8,9,10] and antifibrotic compounds [11].
The discovery of anti-fibrotic drugs has attracted great attention from organic and medicinal chemists. As shown in Figure 1a, vascular endothelial growth factor receptor 2 (VEGFR-2) and Platelet derived growth factor-β (PDGF-β) inhibitor sorafinib reduced collagen deposition by nearly 63% in a liver fibrosis model [12]; N2,N4-bis(2-methoxyethyl)pyridine-2,4-dicarboxamide (HOE-077), which is a prodrug of pyridine-2,4-dicarboxylic acid (24PDC), can inhibit collagen synthesis in rat and dog models by inactivating hepatic stellate cells that are mainly responsible for collagen synthesis in liver fibrosis [13,14]; Ethyl 3,4-dihydroxybenzoate and S4682 were effective in inhibiting collagen synthesis in keloid fibroblasts or the CCl4 model of chronic hepatic injury, by inhibition of collagen prolyl-4-hydroxylase (CP4H) [14,15]; 2-(benzo[d][1,3]dioxol-5-yl)thiazole (CW209292) displays anti-fibrotic activity in rats with dimethylnitrosamine (DMN)-induced hepatic fibrosis by blocking the mRNA expression of transforming growth factor-β1 (TGF-β1) in hepatic stellate cells [16]; nicotinic acid prevented fibrosis by its antioxidant properties and reducing the expression of TGF-β in thioacetamide (TAA)-induced hepatic fibrogenesis [17]. All of those small molecules contain the similarly structure fragment.
Figure 1.
(a) Examples of small molecules with similarly active structure fragments; (b) design strategy.
CP4Hs have been considered an important target for treating fibrotic disease and a few small molecular inhibitors have been reported in the literature [18,19]. However, most of these compounds usually have poor activities in cultured cells [20]. It is our ongoing interest to discover novel compounds with potent anti-fibrotic activities [21,22,23]. In this report, the novel compounds containing pyrimidine scaffold as the core were designed by combining pyrimidine ring with the similarly structure fragment through a molecular hybridization strategy, as shown in Figure 1b. Herein, we report the preparation of pyrimidine–pyridine compounds, CP4H activity in cultured cells and their anti-fibrotic activities against immortalized rat hepatic stellate cells (HSC-T6).
2. Results
2.1. Chemistry
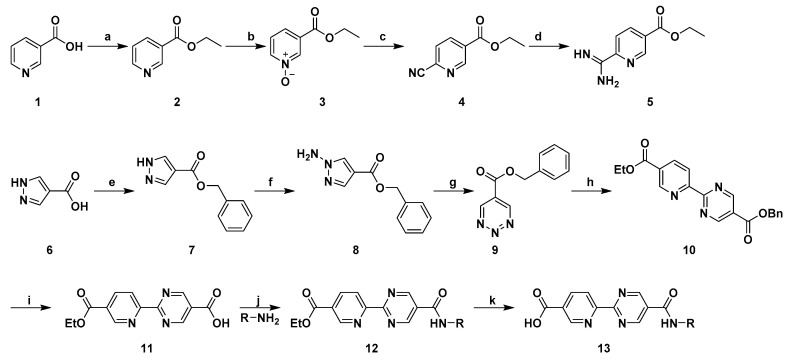
In this work, the key intermediate 5 was obtained by a convenient four-step procedure in moderate yields and the synthetic route was depicted in Scheme 1: First, esterification of nicotinic acid was undertaken to yield 2; then, compound 2 was oxidated with 3-chloroperoxybenzoic acid (mCPBA) to afford pyridine N-Oxides 3; next, a nucleophilic substitution of the ortho-position of pyridine N-Oxides 3 with trimethylsilyl cyanide (TMSCN) was conducted to generate 4 [24]; finally, compound 4 was reacted with Na and NH4Cl in EtOH solution to get compound 5 [25].
Scheme 1.
Synthetic route of compound 12 and 13. Reagent and conditions: (a) H2SO4, EtOH, reflux, 8 h; (b) 3-chloroperbenzoic acid, Dichloromethane (DCM), rt, 24 h; (c) TMSCN, Triethylamine (TEA), Acetonitrile (ACN), reflux, 10 h; (d) Na, EtOH, NH4Cl, reflux, 28 h; (e) BnOH, EDCI, HOBT, DCM, rt, 24 h; (f) amino 4-nitrobenzoate, NMP, rt, 8 h; (g) NaIO4, DCM, H2O, 2 h; (h) compound 5, K2CO3, Dioxane, rt, 8 h; (i) Pd/C, H2, MeOH, rt, 24 h; (j) EDCI, HOBT, DMF, rt, 30 h; (k) NaOH, EtOH, rt, 4 h.
The target compounds (12a–12t and 13a–13t) were prepared according to Scheme 1, starting from the commercially available 4-pyrazolecarboxylic acid. Briefly, condensation of 4-pyrazolecarboxylic acid 6 with benzyl alcohol in the presence of 1-ethyl-3(3-dimethylpropylamine) carbodiimide (EDCI) and 1-hydroxybenzotriazole (HOBT) afforded compound 7 [26]. Intermediate 8 was prepared by using amino 4-nitrobenzoate in 1-methyl-2-pyrrolidinone (NMP). This step was followed by oxidation of 8 with NaIO4 at a low temperature [27]. Subsequently, a Diels–Alder reaction between the key intermediate 5 and Benzyl 1,2,3-triazine-5-carboxylate (9) led to the formation of the correspondent compound 10 [28], which in turn was converted to the carboxylic acid intermediate 11 by reaction with hydrogen at room temperature. Finally, EDCI and HOBT mediated an amide coupling reaction between the activated intermediate carboxylic acid 11 and various substituted amines formed the desired compounds 12a–12t in moderate to good yields and compounds 12a–12t were also converted into the desired product compounds 13a–13t by hydrolysis reactions.
2.2. Biology
2.2.1. MTT Assay on HSC-T6 Cell Proliferation
To evaluate the anti-proliferation activities of target compounds, HSC-T6 cells (Rat Hepatic Stellate Cell) were employed since they have been recognized as an effective and convenient method for anti-proliferation screening model in vitro [29,30]. In our experiment, HSC-T6 cells were seeded to the 96-well plate and were cultured with serum-free medium. This process resulted in HSC-T6 cells transforming into a static period and synchronizing, then the culture medium was refreshed with 2% fetal bovine serum to stimulate the activation of HSC-T6 cells. The inhibitory activities of all synthesized compounds were evaluated by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay with Bipy55′DC, 24PDC and clinical drug Pirfenidone (PFD) as control (Bipy55′DC and 24PDC are typical CP4H inhibitors; Pirfenidone (PFD) can treat idiopathic pulmonary fibrosis with good tolerance and little side effects [31]). It showed that the cell growth increased in speed after cells were cultured with 2% fetal bovine serum in the control group. Moreover, the floating cell is rare in the 100 μM group when co-culturing with the 12m and 12q compounds for about 48 h, indicating that the death cell is rare, but we can observe that the hepatic stellate cells become slim. The half-maximal inhibitory concentration (IC50) values of compounds are shown in Table 1.
Table 1.
The IC50 values of the compounds 12a–12t and 13a–13t by MTT assay.

| |||||||
|---|---|---|---|---|---|---|---|
| Compd | R | R1 | IC50 a/(μmol·L−1) | Compd | R | R1 | IC50 a/(μmol·L−1) |
| 12a | Et | p-F-C6H4CH2- | >1000 | 13a | H | p-F-C6H4CH2- | >1000 |
| 12b | Et | p-Cl-C6H4CH2- | >1000 | 13b | H | p-Cl-C6H4CH2- | >1000 |
| 12c | Et | p-Br-C6H4CH2- | >1000 | 13c | H | p-Br-C6H4CH2- | 174.553 ± 1.872 |
| 12d | Et | p-CH3-C6H4CH2- | 71.465 ± 1.854 | 13d | H | p-CH3-C6H4CH2- | >1000 |
| 12e | Et | p-CH3O-C6H4CH2- | >1000 | 13e | H | p-CH3O-C6H4CH2- | >1000 |
| 12f | Et | m-Br-C6H4CH2- | >1000 | 13f | H | m-Br-C6H4CH2- | >1000 |
| 12g | Et | m-CH3-C6H4CH2- | >1000 | 13g | H | m-CH3-C6H4CH2- | 266.06 ± 2.22 |
| 12h | Et | o-F-C6H4CH2- | >1000 | 13h | H | o-F-C6H4CH2- | >1000 |
| 12i | Et | m,p-2F-C6H3CH2- | >1000 | 13i | H | m,p-2F-C6H3CH2- | >1000 |
| 12j | Et | m-F-C6H4CH2- | >1000 | 13j | H | m-F-C6H4CH2- | >1000 |
| 12k | Et | p-Cl-C6H4- | 47.415 ± 1.351 | 13k | H | p-Cl-C6H4- | >1000 |
| 12l | Et | p-Br-C6H4- | 51.532 ± 1.062 | 13l | H | p-Br-C6H4- | 156.539 ± 2.981 |
| 12m | Et | p-CH3-C6H4- | 45.693 ± 1.660 | 13m | H | p-CH3-C6H4- | >1000 |
| 12n | Et | m-Br-C6H4- | 200.946 ± 2.303 | 13n | H | m-Br-C6H4- | >1000 |
| 12o | Et | p-CH3O-C6H4- | 67.379 ± 1.240 | 13o | H | p-CH3O-C6H4- | >1000 |
| 12p | Et | m-CH3-C6H4- | 259.261 ± 2.414 | 13p | H | m-CH3-C6H4- | 220.356 ± 2.691 |
| 12q | Et | m,p-2F-C6H3- | 45.819 ± 1.747 | 13q | H | m,p-2F-C6H3- | >1000 |
| 12r | Et | m-F-C6H4- | 63.728 ± 1.804 | 13r | H | m-F-C6H4- | >1000 |
| 12s | Et | o-F-C6H4- | 92.777 ± 1.967 | 13s | H | o-F-C6H4- | >1000 |
| 12t | Et | Et | >1000 | 13t | H | Et | >1000 |
| 12u | Et | p-F-C6H4- | 369.324 ± 2.351 | 13u | H | p-F-C6H4- | >1000 |
| 24PDC b | >1000 | Bipy55′DC b | >1000 | ||||
| PFD b | 3025 ± 0.481 | ||||||
a Inhibitory activity was assayed by exposure to substances for 48 h and expressed as concentration required to inhibit HSC-T6 by 50% (IC50). b Positive control.
From the screening results in Table 1, some of the target compounds displayed better anti-fibrosis activity than Pirfenidone (PFD), Bipy55′DC and 24PDC on HSC-T6 cells. Regarding the activity of the tested compounds, compounds (12k, 12l, 12m, 12n, 12o, 12p and 12q) with ethyl groups on the substituent group R exhibited better activity effectiveness than compounds (13k, 13l, 13m, 13n, 13o, 13p and 13q) with hydrogen atoms on the substituent group R. In addition, compounds (12k, 12l, 12m, 12n, 12o, 12q and 12r) with phenyl rings on the substituent group R1 presented more highly inhibitory activities than those (12b, 12c, 12d, 12f, 12e, 12i and 12j) with benzyl groups on the substituent group R1. Similarly, compounds (12l, 12m) with the 4-substituent group R1 showed better activity compared to compounds (12n, 12p) with the 3-substituent group R1. According to a series of chemical structural modifications, we got two highly active compounds: 12m and 12q. To summarize what has been mentioned above, the results encouraged us to further investigate the anti-fibrotic activity of the two compounds 12m and 12q.
2.2.2. 12m and 12q Screened for Prolyl-4-hydroxylase Content In Vitro
Initial screening for prolyl-4-hydroxylase inhibitory activity was performed by determining the hydroxyproline content in HSC-T6 cells [20] and collagen was also determined by estimating the content of hydroxyproline [32]. As shown in Figure 2, the content of hydroxyproline was significantly reduced in the presence of 12m and 12q at a concentration of 50 μM or 100 μM. The result of the hydroxyproline assay displayed that 12m and 12q are potent inhibitors of collagen prolyl-4-hydroxylase. The result also suggested that 12m and 12q do have a potential effect on suppressing the production of collagen in vitro.
Figure 2.
The effect of PFD, 12q and 12m on the expression of hydroxyproline in HSC-T6 cell line.
2.2.3. 12m and 12q Suppressed the Total Collagen Accumulation In Vitro
Extracellular collagen deposition was established by Picro-Sirius red (PSR) staining to test inhibitory activity of 12m and 12q against total collagen accumulation [33,34]. As depicted in Figure 3, treatment with 12m and 12q reduced the cell density and inhibited the total collagen accumulation in a dose-dependent manner. In addition, it is worth noting that the total collagen accumulation was significantly decreased in the presence of 12m and 12q at a concentration of 100 μM.
Figure 3.
Picro-Sirius red (PSR) staining for total collagen accumulation in HSC-T6 cells.
2.2.4. 12m and 12q Suppressed the Protein Expression of COL1A1 In Vitro
Collagen type I alpha 1 (COL1A1), which has been generally recognized as a fibrotic marker, is overexpressed in the common fibrotic diseases. To further study the anti-fibrotic activity of compounds 12m and 12q, their abilities of inhibiting protein expression of COL1A1 in vitro were investigated through the ELISA method. As displayed in Figure 4, it was observed that 12m could reduce the expression of COL1A1 in HSC-T6 cells, which means that the extracellular matrix molecules’ deposition was also reduced.
Figure 4.
The effect of PFD, 12q and 12m on the expression of collagen α1 in HSC-T6 cell line.
3. Experimental Section
3.1. General Information
All commercial reagents were used as received without additional purification. The melting point was uncorrected. Mass spectra were obtained using the electro spray ionization (ESI) method. The 1H and 13C Nuclear Magnetic Resonance (NMR) data were obtained on a 300 MHz NMR spectrometer (Bruker, Rheinstetten, Germany) with tetramethylsilane (TMS) as the internal standard, using Chloroform-d (CDCl3), DMSO-d6 as solvents. All the NMR spectra can be found in Supplementary Materials (Figures S1–S101). Multiplicities are indicated as follows: s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet; dd, doubled doublet; coupling constants is in Hertz (Hz); chemicalshift (δ) is in parts per million (ppm).
3.2. Synthesis of Compounds
Ethyl nicotinate (2): ethanol (50 mL) and nicotinic acid 1 (2.0 g, 16.24 mmol) was added in a 100 mL round bottomed flask. To this, a catalytic amount of concentrated H2SO4 was added at room temperature and was heated under reflux with continuous stirring for 8 h at 85 °C. After 8 h, the reaction was monitored by TLC using an ethyl acetate and hexane (1:4) system. Stirring was continued until TLC indicated the completion of reaction. Then, the reaction mixture was allowed to reach room temperature. Ethanol was distilled off under reduced pressure and the residue was dissolved in water and then extracted twice with ethyl acetate. The combined organic solutions were washed with saturated NaHCO3 solution, and finally washed with water and dried over anhydrous Na2SO4. The solvent was removed under vacuum to get ethyl nicotinate (2.4 g, 98%): 1H NMR (300 MHz, DMSO-d6) δ 9.07 (dd, J = 2.2, 0.8 Hz, 1H), 8.80 (dd, J = 4.8, 1.7 Hz, 1H), 8.33–8.21 (m, 1H), 7.55 (ddd, J = 8.0, 4.8, 0.9 Hz, 1H), 4.34 (q, J = 7.1 Hz, 2H), 1.32 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.64, 153.56, 149.90, 136.73, 125.73, 123.84, 61.11, 14.00.
3-(ethoxycarbonyl)pyridine 1-oxide (3): A solution of ethyl nicotinate 2 (2.0 g, 14.6 mmol) in DCM (150 mL) was added m-CPBA (6.5 g, 29.2 mmol) at 0 °C. After stirring at 0 °C for 1 h and then at room temperature overnight, the mixture was poured into ice water. Then, 2N NaOH was added to adjust the pH to 8–9 and the resultant mixture was extracted with DCM (3 × 200 mL). The organic layer was dried over Na2SO4, filtered and concentrated. The crude product was purified by flash column chromatography on silica gel (2% v/v MeOH in DCM) to afford the title compound (2.19 g, 98%) as a white solid; melting point: 101–102 °C; 1H NMR (300 MHz, CDCl3) δ 8.82–8.75 (m, 1H), 8.34 (ddd, J = 6.5, 1.8, 1.1 Hz, 1H), 7.91–7.82 (m, 1H), 7.37 (dd, J = 7.8, 6.6 Hz, 1H), 4.43 (q, J = 7.1 Hz, 2H), 1.41 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 162.70, 142.45, 138.78, 129.60, 126.83, 125.19, 61.84, 13.89.
Ethyl 6-cyanonicotinate (4): To a solution of 3-(ethoxycarbonyl)pyridine N-oxide (2.19 g, 14.3 mmol) in acetonitrile (ACN) (30 mL) was added trimethylsilyl cyanide (7.2 mL, 57.2 mmol) and triethylamine (TEA) (3.74 mL, 28.6 mmol). The solution was heated at reflux for 10 h. The reaction was then concentrated and diluted with DCM and water before adding 1N HCl. The mixture was extracted with DCM and the organic solutions were combined and washed with brine, dried over Na2SO4, filtered and concentrated in vacuo. The crude product was purified by flash column chromatography on silica gel (2% v/v MeOH in DCM) to afford the title compound (1.26 g, 50%) as a white solid: melting point: 56–57 °C; 1H NMR (300 MHz, DMSO-d6) δ 9.19 (dd, J = 2.1, 0.9 Hz, 1H), 8.51 (dd, J = 8.1, 2.1 Hz, 1H), 8.21 (dd, J = 8.1, 0.9 Hz, 1H), 4.39 (q, J = 7.1 Hz, 2H), 1.35 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 163.56, 151.72, 137.95, 136.83, 128.82, 127.94, 116.50, 62.21, 14.10.
Ethyl 6-carbamimidoylnicotinate hydrochloride (5): A solution of sodium metal (8 mg, 0.35 mmol) in EtOH (20 mL) was added to a solution of ethyl 6-cyanonicotinate (1.26 g, 7.15 mmol) in EtOH (20 mL) and the mixture was stirred for 24 h at room temperature. Ammonium chloride (574 mg, 10.7 mmol) was added and the mixture was stirred at 50 °C for 24 h. The resulting suspension was filtered and the solvent removed under reduced pressure. The solid obtained was washed with ethyl ether (3 × 20 mL) to give compound 5 (982 mg, 60%) as a yellow solid: 1H NMR (300 MHz, DMSO-d6) δ 9.53 (s, 3H), 9.22 (dd, J = 2.0, 0.7 Hz, 1H), 8.62 (dd, J = 8.3, 2.1 Hz, 1H), 8.47 (d, J = 8.3 Hz, 1H), 4.40 (q, J = 7.1 Hz, 2H), 1.36 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 163.63, 161.60, 149.61, 147.36, 138.84, 129.31, 123.57, 61.77, 13.97. MS(ESI): m/z 194.20 [M + H]+.
Benzyl 1H-pyrazole-4-carboxylate (7): EDCI (2.6 g, 13.38 mmol) and HOBT (1.8 g, 13.38 mmol) were added at 0 °C. to a solution of 1H-pyrazole-4-carboxylic acid (1 g, 8.92 mmol) and benzyl alcohol (1.39 mL, 13.38 mmol) in dichloromethane followed by stirring at room temperature for 24 h. The reaction mixture was extracted three times with EtOAc (20 mL). The combined organic layers were washed with brine, dried (Na2SO4), filtered and concentrated in vacuo. The crude product was purified by flash column chromatography on silica gel (33% v/v ethyl acetate in Petroleum ether (60–90 °C) to afford the title compound (514 mg, 23%) as a white solid: melting point: 130–131 °C; 1H NMR (300 MHz, CDCl3) δ 8.09 (s, 2H), 7.46–7.33 (m, 5H), 5.31 (s, 2H). 13C NMR (75 MHz, DMSO-d6) δ 162.51, 136.51, 128.43, 127.90, 127.77, 113.21, 64.94.
Benzyl 1-amino-1H-pyrazole-4-carboxylate (8): A solution of potassium tert-butoxide (KOBu-t) (264 mg, 2.35 mmol) in NMP (20 mL) was added to a stirring solution of 7 (300 mg, 2.14 mmol) in NMP (30 mL). The resulting light pink solution was stirred at room temperature for 20 min. After 20 min, a solution of O-(4-nitrobenzoyl)hydroxylamine(449 mg, 2.46 mmol) in NMP (10 mL) was added to the reaction mixture. The reaction mixture was stirred at room temperature for 8 h. After 8 h, the reaction mixture was treated with saturated aqueous NaCl (7%, 60 mL) and ethyl acetate (60 mL). The organic layer was separated and the aqueous layer was extracted with ethyl acetate (60 mL). The combined organic layers were washed with aqueous sodium bicarbonate (5%, 45 mL), H2O (45 mL), dried (Na2SO4), and concentrated on a rotary evaporator. The crude product was purified by flash chromatography on silica gel (20% v/v ethyl acetate in Petroleum ether (60–90°C) to afford the title compound (160 mg, 50%) as a yellow oil: 1H NMR (300 MHz, DMSO-d6) δ 8.11 (d, J = 0.7 Hz, 1H), 7.81 (d, J = 0.9 Hz, 1H), 7.49–7.30 (m, 5H), 6.75 (s, 2H), 5.28 (s, 2H). 13C NMR (75 MHz, DMSO-d6) δ 162.01, 137.58, 136.45, 131.74, 128.44, 127.91, 127.79, 127.72, 111.76, 64.96. MS(ESI): m/z 218.32 [M + H]+.
Benzyl 1,2,3-triazine-5-carboxylate (9): Compound 8 (400 mg, 1.84 mmol) was taken up in CH2Cl2 (30 mL) and cooled to 0 °C. NaIO4 (787 mg, 3.68 mmol) in H2O (15 mL), which was cooled to 0 °C and added to the stirring solution of 8 at 0 °C. The reaction mixture was warmed to room temperature and stirred for 2 h. After 2 h, the organic layer was separated and the aqueous layer was extracted with CH2Cl2 (20 mL × 3). The combined organic layers were washed with saturated aqueous NaCl (30 mL), dried (Na2SO4) and concentrated on a rotary evaporator. The crude product was purified by flash chromatography on silica gel (20% v/v ethyl acetate in Petroleum ether (60–90 °C) to afford the title compound (272 mg, 81%) as a white solid: melting point: 47–48 °C; 1H NMR (300 MHz, CDCl3) δ 9.48 (s, 2H), 7.48–7.39 (m, 5H), 5.47 (s, 2H). 13C NMR (75 MHz, CDCl3) δ 162.12, 147.86, 133.99, 129.14, 128.85, 128.77, 119.21, 77.41, 76.98, 76.56, 68.75. MS(ESI): m/z 216.30 [M + H]+.
Benzyl 2-(5-(ethoxycarbonyl)pyridin-2-yl)pyrimidine-5-carboxylate (10): benzyl 1,2,3-triazine-5-carboxylate (80 mg, 0.5 mmol) was added to a stirring solution of ethyl 6-carbamimidoylnicotinate hydrochloride (100 mg, 0.5 mmol) and K2CO3 (139 mg, 1 mmol) in CH3CN (20 mL) followed by stirring at room temperature for 8 h. The reaction mixture was extracted three times with EtOAc (20 mL). The combined organic layers were washed with brine, dried (Na2SO4), filtered and concentrated in vacuo. The crude product was purified by flash column chromatography on silica gel (2% v/v MeOH in DCM) to afford the title compound (127 mg, 70%) as a white solid: 1H NMR (300 MHz, CDCl3) δ 9.47 (d, J = 0.6 Hz, 2H), 9.45–9.40 (m, 1H), 8.68 (dd, J = 8.2, 0.7 Hz, 1H), 8.49 (dd, J = 8.3, 2.1 Hz, 1H), 7.51–7.37 (m, 5H), 5.46 (s, 2H), 4.46 (q, J = 7.1 Hz, 2H), 1.44 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, CDCl3) δ 165.12, 164.72, 163.28, 158.84, 156.68, 151.18, 138.11, 134.93, 128.67, 128.65, 128.39, 127.63, 123.90, 123.15, 67.54, 61.60, 14.15. MS(ESI): m/z 364.29 [M + H]+.
2-(5-(ethoxycarbonyl)pyridin-2-yl)pyrimidine-5-carboxylic acid (11): a solution of benzyl 2-(5-(ethoxycarbonyl)pyridin-2-yl)pyrimidine-5-carboxylate (5.7 g, 15.69 mmol) in MeOH (150 mL) was added to palladium on carbon (200 mg, 0.18 mmol) and the reaction mixture was subjected to an atmosphere of hydrogen for 24 h. The catalyst was removed via filtration over Celite and the solvent removed in vacuo. The crude product was purified by flash column chromatography on silica gel (2% v/v MeOH in DCM) to afford the title compound (2.7 g, Yield 73%) as a yellow solid: melting point: 231–232 °C; 1H NMR (300 MHz, DMSO-d6) δ 13.91 (s, 1H), 9.40 (s, 2H), 9.27 (d, J = 1.4 Hz, 1H), 8.61 (d, J = 8.3 Hz, 1H), 8.51 (dd, J = 8.2, 2.2 Hz, 1H), 4.41 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). MS(ESI): m/z 275.25 [M + H]+.
Ethyl 6-(5-((4-fluorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12a): A solution of 2-(5-(ethoxycarbonyl)pyridin-2-yl)pyrimidine-5-carboxylic acid (200 mg, 0.731 mmol) in DMF (2 mL) and DCM (6 mL) at 0 °C was treated with HOBT (114 mg, 0.841 mmol), and EDCI (162 mg, 0.841 mmol). The mixture was stirred at 0 °C for 1 h. Then, 4-Fluorobenzylamine (167 μL, 1.463 mmol) and triethylamine (150 μL, 0.407 mmol) were added and the mixture was warmed to room temperature for 30 h. The reaction mixture was extracted three times with EtOAc (15 mL). The combined organic layers were washed with brine, dried (MgSO4), filtered and concentrated in vacuo. The crude product was purified by flash column chromatography on silica gel (1.5% v/v MeOH in DCM) to afford the title compound (163 mg, 58%) as a yellow solid, melting point: 225–226 °C, 1H NMR (300 MHz, DMSO-d6) δ 9.50 (t, J = 5.8 Hz, 1H), 9.39 (s, 2H), 9.26 (dd, J = 2.2, 0.9 Hz, 1H), 8.59 (dd, J = 8.3, 0.9 Hz, 1H), 8.50 (dd, J = 8.3, 2.2 Hz, 1H), 7.50–7.32 (m, 2H), 7.25–7.11 (m, 2H), 4.54 (d, J = 5.9 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.33, 163.32, 162.84, 162.74, 156.95, 156.80, 150.13, 137.98, 134.95, 129.45, 129.35, 126.69, 126.54, 123.79, 115.17, 114.89, 61.35, 42.00, 14.01. MS(ESI): m/z 381.38 [M + H]+.
6-(5-((4-fluorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13a): ethyl ethyl 6-(5-((4-fluorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (100 mg, 0.26 mmol) was taken up in methanol (12 mL) to which NaOH 1 mol·L−1 (5 mL) was added and the solution was stirred at room temperature for about 5 h. The solvent was evaporated and the residue dissolved in a minimum amount of water. The solution was extracted with dichloromethane (2 × 15 mL) and the aqueous layer acidified to pH~3 with concentrated HCl. The product precipitated as an almost white solid. After filtration and washing with water, the solid was dried (67 mg, 72%): melting point: 294–295 °C; 1H NMR (300 MHz, DMSO-d6) δ 13.61 (s, 1H), 9.56 (t, J = 5.8 Hz, 1H), 9.41 (s, 2H), 9.24 (s, 1H), 8.56 (d, J = 8.2 Hz, 1H), 8.46 (dd, J = 8.2, 2.0 Hz,1H), 7.42–7.30 (m, 4H), 7.27 (dt, J = 9.5, 4.1 Hz, 1H), 4.55 (d, J = 5.8 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.93, 163.48, 162.79, 156.89, 156.73, 150.48, 138.86, 138.21, 128.36, 127.42, 126.96, 126.62, 126.57, 123.80, 42.71. MS(ESI): m/z 353.41 [M + H]+.
Ethyl 6-(5-((4-chlorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12b): Yellow solid, melting point: 224–225 °C, Yield: 63%, 1H NMR (300 MHz, DMSO-d6) δ 9.52 (t, J = 5.9 Hz, 1H), 9.39 (s, 2H), 9.26 (dd, J = 2.2, 0.8 Hz, 1H), 8.59 (dd, J = 8.3, 0.8 Hz, 1H), 8.49 (dd, J = 8.3, 2.2 Hz, 1H), 7.41 (s, 4H), 4.54 (d, J = 5.9 Hz, 2H), 4.40 (q, J = 7.1 Hz,2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.32, 163.33, 162.79, 156.92, 156.82, 150.14, 138.00, 137.84, 131.49, 129.25, 128.25, 126.69, 126.48, 123.81, 61.36, 42.03, 14.02. MS(ESI): m/z 397.33 [M + H]+.
6-(5-((4-chlorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13b): White solid, melting point: 306–307 °C, Yield: 78%, 1H NMR (300 MHz, DMSO-d6) δ 13.65 (s, 1H), 9.57 (t, J = 5.9 Hz, 1H), 9.41 (s, 2H), 9.25 (s, 1H), 8.58 (d, J = 8.3 Hz,1H), 8.48 (dd, J = 8.2, 2.1 Hz, 1H), 7.42 (s, 4H), 4.54 (d, J = 5.9 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.90, 163.47, 162.83, 156.91, 156.69, 150.48, 138.20, 137.95, 131.50, 129.31, 128.27, 127.68, 126.49, 123.78, 42.05. MS(ESI): m/z 369.32 [M + H]+.
Ethyl 6-(5-((4-bromobenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12c): Yellow solid, melting point: 227–228 °C, Yield: 69%, 1H NMR (300 MHz, DMSO-d6) δ 9.52 (t, J = 5.9 Hz, 1H), 9.39 (s, 2H), 9.26 (dd, J = 2.2, 0.8 Hz, 1H), 8.61–8.57 (m, 1H), 8.50 (dd, J = 8.2, 2.2 Hz, 1H), 7.57–7.52 (m, 2H), 7.35 (d, J = 8.5 Hz, 2H), 4.52 (d, J = 5.9 Hz, 2H), 4.40 (q, J = 7.0 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.32, 163.35, 162.79, 156.93, 156.80, 150.13, 138.27, 137.96, 131.16, 129.62, 126.69, 126.48, 123.79, 119.96, 61.35, 42.11, 14.02. MS(ESI): m/z 441.29 [M + H]+.
6-(5-((4-bromobenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13c): White solid, melting point: 314–315 °C, Yield: 81%, 1H NMR (300 MHz, DMSO-d6) δ 13.64 (s, 1H), 9.52 (t, J = 5.9 Hz, 1H), 9.39 (s, 2H), 9.25 (s, 1H), 8.57 (d, J = 8.4 Hz, 1H), 8.47 (dd, J = 8.2, 2.1 Hz, 1H), 7.60–7.49 (m, 2H), 7.35 (d, J = 8.4 Hz, 2H), 4.52 (d, J = 5.9 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.92, 163.53, 162.88, 156.86, 156.74, 150.47, 138.34, 138.18, 131.21, 129.67, 127.64, 126.50, 123.80, 120.00, 42.14. MS(ESI): m/z 413.33 [M + H]+.
Ethyl 6-(5-((4-methylbenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12d): Yellow solid, melting point: 210–211 °C, Yield: 70%, 1H NMR (300 MHz, DMSO-d6) δ 9.45 (t, J = 5.9 Hz, 1H), 9.39 (s, 2H), 9.26 (dd, J = 2.2, 0.9 Hz, 1H), 8.59 (dd, J = 8.3, 0.9 Hz, 1H), 8.50 (dd, J = 8.3, 2.2 Hz, 1H), 7.27 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 7.8 Hz, 2H), 4.51 (d, J = 5.8 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 2.28 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.37, 163.36, 162.67, 157.04, 156.78, 150.14, 137.98, 136.02, 135.74, 128.85, 127.39, 126.73, 126.71, 123.80, 61.35, 42.48, 20.60, 14.03. MS(ESI): m/z 377.39 [M + H]+.
6-(5-((4-methylbenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13d): White solid, melting point: 295–296 °C, Yield: 76%, 1H NMR (300 MHz, DMSO-d6) δ 13.64 (s, 1H), 9.46 (t, J = 5.9 Hz, 1H), 9.39 (s, 2H), 9.24 (d, J = 1.4 Hz, 1H), 8.57 (d, J = 8.3 Hz, 1H), 8.47 (dd, J = 8.2, 2.1 Hz, 1H), 7.26 (d, J = 8.0 Hz, 2H), 7.16 (d, J = 7.8 Hz, 2H), 4.50 (d, J = 5.8 Hz, 2H), 2.28 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 165.92, 163.46, 162.70, 156.84, 156.75, 150.47, 138.18, 136.03, 135.80, 128.88, 127.62, 127.43, 126.64, 123.77, 42.48, 20.65. MS(ESI): m/z 349.42 [M + H]+.
Ethyl 6-(5-((4-methoxybenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12e): Yellow solid, melting point: 218¨C219 °C, Yield: 76%, 1H NMR (300 MHz, DMSO-d6) δ 9.43 (t, J = 6.0 Hz, 1H), 9.38 (s,2H), 9.26 (dd, J = 2.2, 0.9 Hz, 1H), 8.59 (dd, J = 8.3, 0.9 Hz, 1H), 8.50 (dd, J = 8.3, 2.2 Hz, 1H), 7.31 (d, J = 8.8 Hz, 2H), 6.97–6.86 (m, 2H), 4.48 (d, J = 5.8 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 3.74 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.37, 163.34, 162.61, 158.35, 157.04, 156.78, 150.14, 137.99, 130.71, 128.79, 128.68, 126.73, 123.81, 113.76, 61.35, 55.05, 42.21, 14.03. MS(ESI): m/z 393.43 [M + H]+.
6-(5-((4-methoxybenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13e): Grey solid, melting point: 296–297 °C, Yield: 65%, 1H NMR (300 MHz, DMSO-d6) δ 13.63 (s, 1H), 9.44 (t, J = 5.8 Hz, 1H), 9.38 (s, 2H), 9.24 (s, 1H), 8.57 (d, J = 8.3 Hz, 1H), 8.47 (dd, J = 8.2, 2.1 Hz, 1H), 7.42–7.21 (m, 2H), 7.01–6.85 (m, 2H), 4.48 (d, J = 5.7 Hz, 2H), 3.73 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 165.89, 163.49, 162.66, 158.38, 156.82, 150.44, 138.16, 130.79, 128.84, 127.63, 126.71, 123.77, 113.78, 55.08, 42.24. MS(ESI): m/z 365.05 [M + H]+.
Ethyl 6-(5-((3-bromobenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12f): Yellow solid, melting point: 209–210 °C, Yield: 70%, 1H NMR (300 MHz, DMSO-d6) δ 9.52 (t, J = 6.0 Hz, 1H), 9.40 (s, 2H), 9.26 (dd, J = 2.2, 0.9 Hz, 1H), 8.59 (dd, J = 8.3, 0.9 Hz, 1H), 8.50 (dd, J = 8.3, 2.2 Hz, 1H), 7.59 (s, 1H), 7.51–7.44 (m, 1H), 7.40 (d, J = 7.9 Hz, 1H), 7.32 (t, J = 7.7 Hz, 1H), 4.56 (d, J = 5.8 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.36, 163.38, 162.86, 156.97, 156.89, 150.19, 141.68, 138.04, 130.56, 130.17, 129.85, 126.73, 126.52, 126.46, 123.85, 121.67, 61.40, 42.19, 14.07. MS(ESI): m/z 441.26 [M + H]+.
6-(5-((3-bromobenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13f): White solid, melting point: 270–271 °C, Yield: 71%, 1H NMR (300 MHz, DMSO-d6) δ 13.64 (s, 1H), 9.56 (t, J = 5.9 Hz, 1H), 9.41 (s, 2H), 9.25 (s, 1H), 8.57 (d, J = 8.2 Hz, 1H), 8.47 (dd, J = 8.2, 2.1 Hz, 1H), 7.59 (t, J = 1.6 Hz, 1H), 7.50–7.46 (m, 1H), 7.40 (dd, J = 6.3, 1.5 Hz, 1H), 7.32 (t, J = 7.7 Hz, 1H), 4.55 (d, J = 5.8 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.89, 163.54, 162.89, 156.91, 156.73, 150.46, 141.75, 138.17, 130.52, 130.19, 129.82, 127.70, 126.54, 126.47, 123.78, 121.63, 42.20. MS(ESI): m/z 413.32 [M + H]+.
Ethyl 6-(5-((3-methylbenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12g): White solid, melting point: 180–181 °C, Yield: 70%, 1H NMR (300 MHz, DMSO-d6) δ 9.46 (t, J = 5.9 Hz, 1H), 9.40 (s, 2H), 9.26 (dd, J = 2.2, 0.9 Hz, 1H), 8.59 (dd, J = 8.3, 0.9 Hz,1H), 8.49 (dd, J = 8.3, 2.2 Hz, 1H), 7.28–7.14 (m, 3H), 7.08 (d, J = 7.4 Hz, 1H), 4.52 (d, J = 5.8 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 2.30 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.34, 163.30, 162.66, 156.96, 156.84, 150.15, 138.69, 137.98, 137.44, 128.25, 128.05, 127.59, 126.68, 126.60, 124.54, 123.80, 61.38, 42.71, 20.97, 14.04. MS(ESI): m/z 377.37 [M + H]+.
6-(5-((3-methylbenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13g): White solid, melting point: 276–277 °C, Yield: 81%, 1H NMR (300 MHz, DMSO-d6) δ 13.64 (s, 1H), 9.48 (t, J = 5.9 Hz, 1H), 9.40 (s, 2H), 9.25 (s, 1H), 8.57 (d, J = 8.3 Hz, 1H), 8.47 (dd, J = 8.2, 2.1 Hz, 1H), 7.24 (t, J = 7.4 Hz, 1H), 7.22–7.12 (m, 2H), 7.08 (d, J = 7.3 Hz, 1H), 4.52 (d, J = 5.8 Hz, 2H), 2.30 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 165.93, 163.45, 162.73, 156.89, 156.75, 150.48, 138.75, 138.21, 137.45, 128.28, 128.06, 127.60, 126.62, 124.56, 123.80, 42.71, 21.01. MS(ESI): m/z 349.42 [M + H]+.
Ethyl 6-(5-((2-fluorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12h): Yellow solid, melting point: 235–236 °C, Yield: 70%, 1H NMR (300 MHz, DMSO-d6) δ 9.49 (t, J = 5.8 Hz, 1H), 9.40 (s, 2H), 9.26 (dd, J = 2.2, 0.9 Hz, 1H), 8.59 (dd, J = 8.3, 0.9 Hz,1H), 8.50 (dd, J = 8.3, 2.2 Hz, 1H), 7.47 (dd, J = 8.6, 6.7 Hz, 1H), 7.40–7.29 (m, 1H), 7.27–7.16 (m, 1H), 4.59 (d, J = 5.7 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.40, 163.44, 162.92, 161.75, 158.50, 157.04, 156.87, 150.18, 138.02, 129.89, 129.84, 129.18, 129.08, 126.79, 126.55, 125.45, 125.25, 124.39, 123.85, 115.27, 114.99, 61.39, 36.68, 14.05. MS(ESI): m/z 381.38 [M + H]+.
6-(5-((2-fluorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13h): White solid, melting point: 280–281 °C, Yield: 77%, 1H NMR (300 MHz, DMSO-d6) δ 13.64 (s, 1H), 9.51 (t, J = 5.7 Hz, 1H), 9.40 (s, 2H), 9.24 (s, 1H), 8.57 (d, J = 8.2 Hz, 1H), 8.47 (dd, J = 8.2, 2.1 Hz, 1H), 7.47 (td, J = 7.5, 1.6 Hz, 1H), 7.40–7.31 (m, 1H), 7.26–7.16 (m, 2H), 4.59 (d, J = 5.6 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.90, 163.54, 162.96, 161.75, 158.50, 156.89, 156.77, 150.47, 138.17, 129.89, 129.83, 129.16, 129.06, 127.66, 126.51, 125.49, 125.30, 124.40, 124.36, 123.79, 115.26, 114.98, 36.65, 36.59. MS(ESI): m/z 353.41 [M + H]+.
Ethyl 6-(5-((2,3-difluorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12i): White solid, melting point: 181–182 °C, Yield: 66%, 1H NMR (300 MHz, DMSO-d6) δ 9.52 (t, J = 6.0 Hz, 1H), 9.40 (s, 2H), 9.26 (dd, J = 2.2, 0.9 Hz, 1H), 8.59 (dd, J = 8.3, 0.9 Hz, 1H), 8.50 (dd, J = 8.3, 2.2 Hz, 1H), 7.51–7.34 (m, 2H), 7.24 (s, 1H), 4.54 (d, J = 5.7 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.37, 163.38, 162.93, 156.97, 156.89, 150.98, 150.82, 150.18, 150.00, 146.93, 146.77, 145.96, 144.97, 138.03, 136.72, 136.68, 136.65, 136.61, 126.73, 126.49, 124.16, 124.12, 124.07, 124.03, 123.84, 117.42, 117.20, 116.53, 116.30, 61.39, 41.78, 14.05. MS(ESI): m/z 399.37 [M + H]+.
6-(5-((2,3-difluorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13i): White solid, melting point: 283–284 °C, Yield: 54%, 1H NMR (300 MHz, DMSO-d6) δ 13.64 (s, 1H), 9.54 (t, J = 5.9 Hz, 1H), 9.40 (s, 2H), 9.25 (s, 1H), 8.57 (d, J = 8.1 Hz, 1H), 8.47 (dd, J = 8.2, 2.1 Hz, 1H), 7.52–7.32 (m, 2H), 7.31–7.15 (m, 1H), 4.54 (d, J = 5.8 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.92, 163.52, 162.96, 156.90, 156.74, 150.98, 150.81, 150.47, 150.16, 150.00, 147.72, 147.55, 146.92, 146.75, 138.19, 136.70, 127.64, 126.46, 124.16, 124.12, 124.08, 124.03, 123.79, 117.41, 117.19, 116.54, 116.31, 41.78. MS(ESI): m/z 371.48 [M + H]+.
Ethyl 6-(5-((3-fluorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinate (12j): Yellow solid, melting point: 209–210 °C, Yield: 67%, 1H NMR (300 MHz, DMSO-d6) δ 9.52 (t, J = 6.0 Hz, 1H), 9.41 (s, 2H), 9.26 (dd, J = 2.2, 0.9 Hz, 1H), 8.60 (dd, J = 8.3, 0.8 Hz, 1H), 8.50 (dd, J = 8.3, 2.2 Hz, 1H), 7.46–7.35 (m, 1H), 7.27–7.17 (m, 2H), 7.11 (t, J = 9.1 Hz, 1H), 4.57 (d, J = 5.8 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.37, 163.38, 162.91, 156.99, 156.90, 150.19, 141.78, 138.05, 130.36, 130.25, 126.73, 126.53, 123.85, 123.37, 123.34, 114.22, 113.94, 113.87, 113.59, 61.40, 42.23, 14.06. MS(ESI): m/z 381.35 [M + H]+.
6-(5-((3-fluorobenzyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13j): White solid, melting point: 280–281 °C, Yield: 59%, 1H NMR (300 MHz, DMSO-d6) δ 13.64 (s, 1H), 9.56 (t, J = 5.9 Hz, 1H), 9.41 (s, 2H), 9.25 (s, 1H), 8.58 (d, J = 8.1 Hz, 1H), 8.47 (dd, J = 8.2, 2.1 Hz, 1H), 7.46–7.35 (m, 1H), 7.26–7.17 (m, 2H), 7.11 (ddd, J = 10.7, 5.3, 1.4 Hz, 1H), 4.57 (d, J = 5.9 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.93, 163.86, 162.94, 160.63, 156.95, 156.73, 150.49, 141.94, 141.85, 138.23, 130.35, 130.24, 126.52, 123.82, 123.40, 123.37, 114.25, 113.97, 113.86, 113.58, 42.24. MS(ESI): m/z 353.40 [M + H]+.
Ethyl 6-(5-((4-chlorophenyl)carbamoyl)pyrimidin-2-yl)nicotinate (12k): Yellow solid, melting point: 249–250 °C, Yield: 50%, 1H NMR (300 MHz, DMSO-d6) δ 10.80 (s, 1H), 9.46 (s, 2H), 9.28 (dd, J = 2.2, 0.8 Hz, 1H), 8.62 (dd, J = 8.3, 0.8 Hz, 1H), 8.51 (dd, J = 8.3, 2.2 Hz, 1H), 7.82 (d, J = 8.9 Hz, 2H), 7.54–7.39 (m, 2H), 4.41 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.43, 163.54, 162.07, 157.20, 157.03, 150.26, 138.10, 137.49, 128.74, 127.99, 127.43, 126.89, 123.96, 121.97, 61.43, 14.09. MS(ESI): m/z 383.33 [M + H]+.
6-(5-((4-chlorophenyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13k): Grey solid, melting point: 319–320 °C, Yield: 71%, 1H NMR (300 MHz, DMSO-d6) δ 13.50 (s, 1H), 10.87 (s, 1H), 9.46 (s, 2H), 9.25 (s, 1H), 8.57 (d, J = 7.9 Hz, 1H), 8.47 (dd, J = 8.2, 1.7 Hz, 1H), 7.83 (d, J = 8.9 Hz, 2H), 7.44 (d, J = 8.8 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.93, 163.62, 162.03, 157.17, 156.63, 150.50, 138.14, 137.51, 128.66, 127.93, 127.56, 127.28, 123.83, 121.96. MS(ESI): m/z 355.39 [M + H]+.
Ethyl 6-(5-((4-bromophenyl)carbamoyl)pyrimidin-2-yl)nicotinate (12l): Yellow solid, melting point: 269–270 °C, Yield: 58%, 1H NMR (300 MHz, DMSO-d6) δ 10.81 (s, 1H), 9.46 (s, 2H), 9.28 (dd, J = 2.2, 0.8 Hz, 1H), 8.62 (dd, J = 8.3, 0.8 Hz, 1H), 8.52 (dd, J = 8.3, 2.2 Hz, 1H), 7.85–7.72 (m, 2H), 7.69–7.53 (m, 2H), 4.41 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.41, 163.54, 162.07, 157.19, 157.01, 150.25, 138.09, 137.90, 131.65, 127.43, 126.87, 123.94, 122.30, 116.06, 61.42, 14.08. MS(ESI): m/z 427.34 [M + H]+.
6-(5-((4-bromophenyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13l): Yellow solid, melting point: 320–321 °C, Yield: 86%, 1H NMR (300 MHz, DMSO-d6) δ 10.93 (s, 1H), 9.48 (s, 2H), 9.25 (s, 1H), 8.59 (d, J = 8.1 Hz, 1H), 8.48 (dd, J = 8.2, 2.0 Hz, 1H), 7.80 (d, J = 8.9 Hz, 2H), 7.57 (d, J = 8.8 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.82, 163.49, 162.02, 157.21, 156.54, 150.37, 138.34, 137.95, 131.56, 127.74, 127.30, 123.89, 122.33, 116.02. MS(ESI): m/z 339.36 [M + H]+.
Ethyl 6-(5-(p-tolylcarbamoyl)pyrimidin-2-yl)nicotinate (12m): Yellow solid, melting point: 272–273 °C, Yield: 71%, 1H NMR (300 MHz, DMSO-d6) δ 10.61 (s, 1H), 9.45 (s, 2H), 9.27 (dd, J = 2.2, 0.8 Hz, 1H), 8.61 (dd, J = 8.3, 0.8 Hz, 1H), 8.51 (dd, J = 8.3, 2.2 Hz, 1H), 7.67 (d, J = 8.4 Hz, 2H), 7.21 (d, J = 8.3 Hz, 2H), 4.40 (q, J = 7.1 Hz, 2H), 2.30 (s, 3H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.35, 163.29, 161.57, 157.06, 156.96, 150.20, 138.02, 135.94, 133.31, 129.13, 127.51, 126.73, 123.84, 120.36, 61.38, 20.49, 14.04. MS(ESI): m/z 363.37 [M + H]+.
6-(5-(p-tolylcarbamoyl)pyrimidin-2-yl)nicotinic acid (13m): Yellow solid, melting point: 330–331 °C, Yield: 76%, 1H NMR (300 MHz, DMSO-d6) δ 10.69 (s, 1H), 9.46 (s, 2H), 9.25 (s, 1H), 8.59 (d, J = 8.3 Hz, 1H), 8.48 (d, J = 7.6 Hz, 1H), 7.68 (d, J = 7.6 Hz, 2H), 7.19 (d, J = 7.4 Hz, 2H), 2.29 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 165.86, 163.40, 161.65, 157.10, 156.68, 150.42, 138.27, 136.00, 133.33, 129.11, 127.69, 127.54, 123.82, 120.46, 20.50. MS(ESI): m/z 335.37 [M + H]+.
Ethyl 6-(5-((3-bromophenyl)carbamoyl)pyrimidin-2-yl)nicotinate (12n): White solid, melting point: 216–217 °C, Yield: 50%, 1H NMR (300 MHz, DMSO-d6) δ 10.82 (s,1H), 9.46 (s, 2H), 9.28 (d, J = 1.4 Hz, 1H), 8.62 (d, J = 8.2 Hz, 1H), 8.52 (dd, J = 8.3, 2.2 Hz, 1H), 8.11 (d, J = 1.7 Hz,1H), 7.81–7.70 (m, 1H), 7.47–7.29 (m, 2H), 4.41 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.44, 163.62, 162.24, 157.29, 157.24, 150.28, 140.11, 138.12, 130.84, 127.01, 126.95, 123.99, 122.75, 121.49, 119.91, 119.17, 61.44, 14.10. MS(ESI): m/z 427.33 [M + H]+.
6-(5-((3-bromophenyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13n): White solid, melting point: 310–311 °C, Yield: 54%, 1H NMR (300 MHz, DMSO-d6) δ 13.64 (s, 1H), 10.98 (s, 1H), 9.49 (s, 2H), 9.26 (s, 1H), 8.58 (s, 1H), 8.49 (s, 1H), 8.13 (s, 1H), 7.80 (s, 1H), 7.35 (s, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.90, 163.64, 162.19, 157.28, 156.62, 150.51, 140.16, 138.23, 130.74, 127.84, 127.15, 126.84, 123.88, 122.70, 121.44, 119.13. MS(ESI): m/z 399.41 [M + H]+.
Ethyl 6-(5-((4-methoxyphenyl)carbamoyl)pyrimidin-2-yl)nicotinate (12o): Yellow solid, melting point: 269–270 °C, Yield: 70%, 1H NMR (300 MHz, DMSO-d6) δ 10.57 (s, 1H), 9.45 (s, 2H), 9.27 (dd, J = 2.2, 0.8 Hz, 1H), 8.62 (dd, J = 8.3, 0.8 Hz, 1H), 8.51 (dd, J = 8.3, 2.2 Hz, 1H), 7.75–7.64 (m, 2H), 7.02–6.93 (m, 2H), 4.41 (q, J = 7.1 Hz, 2H), 3.76 (s, 3H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.41, 163.36, 161.38, 157.06, 157.02, 156.00, 150.22, 138.04, 131.51, 127.58, 126.80, 123.86, 122.04, 113.92, 61.39, 55.21, 14.07. MS(ESI): m/z 379.35 [M + H]+.
6-(5-((4-methoxyphenyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13o): Grey solid, melting point: 285–286 °C, Yield: 65%, 1H NMR (300 MHz, DMSO-d6) δ 10.72 (s, 1H), 9.49 (s, 2H), 9.27 (s, 1H), 8.61 (d, J = 7.6 Hz, 1H), 8.49 (dd, J = 8.2, 1.8 Hz, 1H), 7.74 (d, J = 9.0 Hz, 2H), 7.04–6.92 (m, 2H), 3.77 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 165.86, 163.34, 161.38, 157.08, 156.70, 155.97, 150.43, 138.24, 131.59, 127.74, 127.52, 123.81, 122.07, 113.87, 55.21. MS(ESI): m/z 351.35 [M + H]+.
Ethyl 6-(5-(m-tolylcarbamoyl)pyrimidin-2-yl)nicotinate (12p): Yellow solid, melting point: 204–205 °C, Yield: 60%, 1H NMR (300 MHz, DMSO-d6) δ 10.60 (s, 1H), 9.45 (s, 2H), 9.27 (d, J = 1.4 Hz, 1H), 8.61 (d, J = 8.2 Hz, 1H), 8.51 (dd, J = 8.2, 2.1 Hz, 1H), 7.59 (d, J = 9.1 Hz, 2H), 7.28 (t, J = 7.6 Hz, 1H), 6.99 (d, J = 7.6 Hz, 1H), 4.41 (q, J = 7.1 Hz, 2H), 2.33 (s, 3H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.37, 163.35, 161.76, 157.10, 156.97, 150.21, 138.37, 138.05, 137.96, 128.59, 127.54, 126.77, 124.99, 123.87, 120.89, 117.56, 61.39, 21.15, 14.05. MS(ESI): m/z 363.35 [M + H]+.
6-(5-(m-tolylcarbamoyl)pyrimidin-2-yl)nicotinic acid (13p): White solid, melting point: 311–312 °C, Yield: 60%, 1H NMR (300 MHz, DMSO-d6) δ 13.67 (s, 1H), 10.61 (s, 1H), 9.46 (s, 2H), 9.28 (s, 1H), 8.60 (d, J = 7.9 Hz, 1H), 8.49 (dd, J = 8.2, 1.9 Hz, 1H), 7.61 (d, J = 9.2 Hz, 2H), 7.29 (t, J = 7.8 Hz, 1H), 6.99 (d, J = 7.6 Hz, 1H), 2.34 (s, 3H). 13C NMR (75 MHz, DMSO-d6) δ 165.94, 163.52, 161.82, 157.11, 156.73, 150.51, 138.41, 138.20, 137.97, 128.61, 127.65, 127.51, 124.99, 123.84, 120.89, 117.56, 21.17. MS(ESI): m/z 355.42 [M + H]+.
Ethyl 6-(5-((3,4-difluorophenyl)carbamoyl)pyrimidin-2-yl)nicotinate (12q): Yellow solid, melting point: 256–257 °C, Yield: 61%, 1H NMR (300 MHz, DMSO-d6) δ 10.88 (s, 1H), 9.45 (s, 2H), 9.27 (dd, J = 2.2, 0.9 Hz, 1H), 8.61 (dd, J = 8.3, 0.9 Hz, 1H), 8.50 (dd, J = 8.3, 2.2 Hz, 1H), 7.94 (ddd, J = 9.9, 7.3, 2.0 Hz, 1H), 7.59–7.41 (m, 2H), 4.40 (q, J = 7.1 Hz, 2H), 1.37 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.38, 163.57, 162.11, 157.20, 156.92, 150.25, 138.10, 135.46, 127.18, 126.85, 123.96, 117.69, 117.45, 116.86, 109.55, 109.27, 61.42, 14.07. MS(ESI): m/z 385.35 [M + H]+.
6-(5-((3,4-difluorophenyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13q): White solid, melting point: 304–305 °C, Yield: 70%, 1H NMR (300 MHz, DMSO-d6) δ 13.63 (s, 1H), 10.90 (s, 1H), 9.46 (s, 2H), 9.26 (s, 1H), 8.57 (s, 1H), 8.48 (dd, J = 8.2, 1.8 Hz, 1H), 7.95 (ddd, J = 13.1, 7.5, 2.4 Hz, 1H), 7.63–7.36 (m, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.90, 163.82, 162.07, 157.17, 156.61, 150.49, 147.54, 147.31, 147.14, 144.32, 144.16, 138.18, 135.58, 135.54, 135.46, 135.42, 127.63, 127.08, 123.86, 117.62, 117.39, 116.81, 116.77, 116.73, 116.69, 109.52, 109.23. MS(ESI): m/z 357.42 [M + H]+.
Ethyl 6-(5-((3-fluorophenyl)carbamoyl)pyrimidin-2-yl)nicotinate (12r): White solid, melting point: 239–240 °C, Yield: 65%, 1H NMR (300 MHz, DMSO-d6) δ 10.87 (s, 1H), 9.46 (s, 2H), 9.32–9.24 (m, 1H), 8.62 (d, J = 8.3 Hz, 1H), 8.52 (dd, J = 8.3, 2.2 Hz, 1H), 7.76 (d, J = 11.4 Hz, 1H), 7.56 (d, J = 9.1 Hz, 1H), 7.45 (dd, J = 15.0, 8.1 Hz, 1H), 7.02 (dd, J = 11.3, 5.6 Hz, 1H), 4.41 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.32, 163.60, 163.45, 162.11, 160.40, 157.16, 156.86, 150.20, 140.26, 140.11, 138.01, 130.47, 130.35, 127.21, 126.77, 123.88, 116.03, 110.89, 110.61, 107.22, 106.87, 61.38, 14.03. MS(ESI): m/z 367.33 [M + H]+.
6-(5-((3-fluorophenyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13r): Light green solid, melting point: 296–297 °C, Yield: 76%, 1H NMR (300 MHz, DMSO-d6) δ 13.64 (s, 1H), 10.92 (s, 1H), 9.48 (s, 2H), 9.27 (s, 1H), 8.60 (d, J = 7.7 Hz, 1H), 8.49 (dd, J = 8.2, 1.8 Hz, 1H), 7.78 (dt, J = 11.6, 2.2 Hz, 1H), 7.65–7.56 (m, 1H), 7.44 (td, J = 8.2, 6.9 Hz, 1H), 7.00 (td, J = 8.3, 1.8 Hz, 1H). 13C NMR (75 MHz, DMSO-d6) δ 165.88, 163.63, 162.22, 160.43, 157.19, 156.69, 150.48, 140.32, 140.17, 138.18, 130.46, 130.33, 127.70, 127.26, 123.86, 116.13, 116.09, 110.90, 110.62, 107.32, 106.97. MS(ESI): m/z 339.45 [M + H]+.
Ethyl 6-(5-((2-fluorophenyl)carbamoyl)pyrimidin-2-yl)nicotinate (12s): Yellow solid, melting point: 189–190 °C, Yield: 62%, 1H NMR (300 MHz, DMSO-d6) δ 10.65 (s, 1H), 9.47 (s, 2H), 9.28 (dd, J = 2.2, 0.9 Hz, 1H), 8.63 (dd, J = 8.3, 0.9 Hz, 1H), 8.52 (dd, J = 8.3, 2.2 Hz, 1H), 7.73 (dt, J = 7.9, 4.1 Hz, 1H), 7.47–7.17 (m, 3H), 4.41 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.37, 163.58, 162.14, 157.27, 156.94, 153.69, 150.22, 138.07, 127.33, 127.23, 126.81, 126.74, 126.56, 125.07, 124.90, 124.48, 124.43, 123.94, 116.06, 115.80, 61.41, 14.06. MS(ESI): m/z 367.35 [M + H]+.
6-(5-((2-fluorophenyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13s): White solid, melting point: 285–286 °C, Yield: 42%, 1H NMR (300 MHz, DMSO-d6) δ 13.68 (s, 1H), 10.81 (s, 1H), 9.52 (s, 2H), 9.28 (s, 1H), 8.61 (d, J = 8.2 Hz, 1H), 8.50 (dd, J = 8.2, 2.1 Hz, 1H), 7.73 (td, J = 7.9, 1.6 Hz, 1H), 7.43–7.20 (m, 3H). 13C NMR (75 MHz, DMSO-d6) δ 165.89, 163.64, 162.12, 157.35, 157.10, 156.60, 153.82, 150.52, 138.26, 127.78, 127.36, 127.26, 126.72, 125.08, 124.92, 124.45, 124.40, 123.88, 116.06, 115.80. MS(ESI): m/z 339.26 [M + H]+.
Ethyl 2-(5-(ethoxycarbonyl)pyridin-2-yl)pyrimidine-5-carboxylate (12t): Yellow solid, melting point: 214–215 °C, Yield: 75%, 1H NMR (300 MHz, DMSO-d6) δ 9.34 (s, 2H), 9.26 (dd, J = 2.2, 0.9 Hz, 1H), 8.95 (t, J = 5.4 Hz, 1H), 8.58 (dd, J = 8.3, 0.9 Hz, 1H), 8.49 (dd, J = 8.3, 2.2 Hz, 1H), 4.40 (q, J = 7.1 Hz, 2H), 3.46–3.25 (m, 2H), 1.37 (t, J = 7.1 Hz, 3H), 1.18 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.38, 163.23, 162.40, 157.05, 156.66, 150.15, 137.96, 126.87, 126.71, 123.77, 61.37, 34.19, 14.51, 14.05. MS(ESI): m/z 301.32 [M + H]+.
6-(5-(ethoxycarbonyl)pyrimidin-2-yl)nicotinic acid (13t): White solid, melting point: 284–285 °C, Yield: 66%, 1H NMR (300 MHz, DMSO-d6) δ 13.61 (s, 1H), 9.35 (s, 2H), 9.24 (s, 1H), 8.99 (t, J = 5.4 Hz, 1H), 8.56 (d, J = 8.2 Hz, 1H), 8.47 (dd, J = 8.2, 2.1 Hz, 1H), 3.48–3.19 (m, 2H), 1.18 (t, J = 7.2 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 165.89, 163.28, 162.39, 156.70, 156.57, 150.43, 138.19, 127.56, 126.79, 123.73, 34.17, 14.53. MS(ESI): m/z 273.28 [M + H]+.
Ethyl 6-(5-((4-fluorophenyl)carbamoyl)pyrimidin-2-yl)nicotinate (12u): White solid, melting point: 198–199 °C, Yield: 72%, 1H NMR (300 MHz, DMSO-d6) δ 10.74 (s,1H), 9.46 (s, 2H), 9.28 (dd, J = 2.1, 0.8 Hz, 1H), 8.62 (dd, J = 8.3, 0.8 Hz, 1H), 8.51 (dd, J = 8.3, 2.2 Hz, 1H), 7.81 (dd, J = 5.7, 3.5 Hz, 2H), 7.33–7.19 (m, 2H), 4.41 (q, J = 7.1 Hz, 2H), 1.38 (t, J = 7.1 Hz, 3H). 13C NMR (75 MHz, DMSO-d6) δ 164.39, 163.49, 161.82, 160.23, 157.12, 157.03, 150.23, 138.05, 134.85, 127.44, 126.84, 123.90, 122.38, 122.27, 115.55, 115.25, 61.40, 14.07. MS(ESI): m/z 367.35 [M + H]+.
6-(5-((4-fluorophenyl)carbamoyl)pyrimidin-2-yl)nicotinic acid (13u): Grey solid, melting point: 290–291 °C, Yield: 65%, 1H NMR (300 MHz, DMSO-d6) δ 13.62 (s, 1H), 10.81 (s, 1H), 9.46 (s, 2H), 9.25 (s, 1H), 8.58 (d, J = 7.8 Hz, 1H), 8.47 (d, J = 7.5 Hz, 1H), 7.82 (dd, J = 8.7, 5.1 Hz, 2H), 7.23 (t, J = 8.8 Hz, 2H). 13C NMR (75 MHz, DMSO-d6) δ 165.91, 163.47, 161.80, 160.17, 157.16, 156.98, 150.49, 138.21, 134.86, 127.66, 127.32, 123.83, 122.33, 122.23, 115.51, 115.22. MS(ESI): m/z 339.26 [M + H]+.
3.3. MTT Assay
HSC-T6 cells were obtained from National Science and Technology Infrastructure. To determine the half-maximal inhibitory concentration (IC50) of the compounds we synthesized, HSC-T6 cells were seeded into 96-well with a density of 2000 cells/well in DMEM culture medium with 10% fetal bovine serum in a humidified atmosphere containing 5% CO2. After attachment (about 24 h), the cells were starved in a serum-free medium for about 12 h to induce HSC-T6 transformation into a static period as well as synchronization, and then compounds to be tested were added to corresponding wells with different concentrations in culture medium with 2% fetal bovine serum. After 48 h of incubation, 20 μL of 3-[4,5-dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide (MTT) solution (5 mg/mL) was added to each well and the plates were incubated for an additional 4 h. Subsequently, the medium was aspirated carefully, and 150 μL of DMSO was added to dissolve the crystal. The optical density was measured at 490 nm using a RT-2100C Microplate Reader (Rayto, Shenzhen, China). The results were used to calculate IC50 values. Each experiment was repeated three times.
3.4. Hydroxyproline Assay
Cells were plated on 96-wells with a density of 7000 cells/well. After attachment (24 h), each compound, at different concentrations, was added to cells. After 48 h of incubation, cell culture supernatants were taken out for the next step. The hydroxyproline contents in cell culture supernatants were measured with a conventional hydroxyproline assay kit (A030-2-1, Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Subsequently, the hydroxyproline analysis was performed using Chloramine-T spectrophotometric absorbance.
3.5. Picro-Sirius Red (PSR) Staining
The HSC-T6 cells cultured in 6-well plates were carefully washed twice with phosphate buffered saline (PBS), xylene, ethanol and incubated in the PSR staining solution (SIRIUS RED.0.1% in saturated picric acid 26357-02, Electron Microscopy Organizer Sciences, Lot no.:190628-01) at room temperature for 1 h. The staining solution was then removed and the cells were washed three times with ethanol. Then, the HSC-T6 cells were incubated in the hematoxylin staining solution at room temperature for 10 min. The hematoxylin staining solution was removed and the cells were washed three times with ethanol and kept in xylene for 5 min. The stained cells were dried and a picture was taken with a microscope.
3.6. ELISA Detection for COL1A1
HSC-T6 cells were plated on 96-wells with a density of 7000 cells/well. After attachment (24 h), each compound, at different concentrations, was added to the remaining cell lines mentioned above. After 48 h of incubation, cell culture supernates were centrifuged for 20 min at 1000× g. A quantity of 50 μL of standard or cell culture supernates was added to the appropriate wells and 100 μL of Enzymeconjugate was added to standard wells and sample wells, except for the blank well. After 1 h of incubation at 37 °C, the Microtiter Plate was washed 4 times. Then, Substrate A 50 μL and Substrate B 50 μL were added to each well (Rat ColIELISA KIT, RUIXIN Biology, Guangzhou, China, lot:07/2020). After 15 min of incubation at 37 °C, 50 μL Stop Solution was added to each well and the optical density was measured at 490 nm using RT-2100C Microplate Reader.
4. Conclusions
In conclusion, we have successfully prepared a representative library of 2-(pyridin-2-yl)pyrimidine derivatives by starting from the readily available nicotinic acid and 1H-pyrazole-4-carboxylic acid. In addition, those compounds were evaluated for their anti-fibrotic activities in vitro. Among them, fourteen compounds exhibited inhibitory activities stronger than Pirfenidone, 24PDC and Bipy55′DC, with 12m and 12q displaying activities with IC50 values of 45.69 μM and 45.81 μM against HSC-T6, respectively. Furthermore, the results of hydroxyproline assay displayed that 12m and 12q might be inhibitors of collagen prolyl-4-hydroxylase. In addition, hydroxyproline assay and Picro-Sirius red (PSR) staining of compounds 12m and 12q exhibited potent anti-fibrosis abilities in alleviating the total collagen accumulation in HSC-T6 cells in a dose-dependent manner. Further, ELISA results for compounds 12m and 12q showed better activity in alleviating COL1A1 in HSC-T6 cells. The in-depth mechanisms of action of the compounds 12m and 12q remain to be further investigated.
Supplementary Materials
The following are available online. Figures are 1H NMR and 13C NMR of the synthesized compounds.
Author Contributions
Manuscript writing, Y.-F.G. and Z.-q.Z.; biological work, J.L., Y.Z., F.-l.Y. and S.-t.L.; chemical work, Y.-F.G.; design and supervision of the study, Y.-F.G. and X.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Fund of China (NO. 81872741) and the Innovation Project for College Students (NO. 202010183098).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of the compounds 12q and 12m are available from the authors on request.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Xiang J., Wen D., Xie H., Dang Q., Bai X. Synthesis of Novel 8,9-Dihydro-5H-pyrimido[4,5-e][1,4]diazepin-7(6H)-ones. J. Comb. Chem. 2010;12:503–509. doi: 10.1021/cc100039w. [DOI] [PubMed] [Google Scholar]
- 2.Dolle R.E., Bourdonnec B.L., Goodman A.J., Morales G.A., Thomas C.J., Zhang W. Comprehensive Survey of Chemical Libraries for Drug Discovery and Chemical Biology: 2007. J. Comb. Chem. 2008;10:753–802. doi: 10.1021/cc800119z. [DOI] [PubMed] [Google Scholar]
- 3.Seenaiah D., Reddy P.R., Reddy G.M., Padmaja A., Padmavathi V., Siva krishna N. Synthesis, antimicrobial and cytotoxic activities of pyrimidinyl benzoxazole, benzothiazole and benzimidazole. Eur. J. Med. Chem. 2014;77:1–7. doi: 10.1016/j.ejmech.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 4.Raj T., Singh N., Ishar M.P.S. Unusual transformation of substituted-3-formylchromones to pyrimidine analogues: Synthesis and antimicrobial activities of 5-(o-hydroxyaroyl)pyrimidines. Bioorg. Med. Chem. Lett. 2013;23:6093–6096. doi: 10.1016/j.bmcl.2013.09.024. [DOI] [PubMed] [Google Scholar]
- 5.Al-Turkistani A.A., Al-Deeb O.A., El-Brollosy N.R., El-Emam A.A. Synthesis and Antimicrobial Activity of Some Novel 5-Alkyl-6-Substituted Uracils and Related Derivatives. Molecules. 2011;16:4764–4774. doi: 10.3390/molecules16064764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Romeo R., Iannazzo D., Veltri L., Gabriele B., Macchi B., Frezza C., Marino-Merlo F., Giofre S.V. Pyrimidine 2,4-diones in the design of new HIV RT inhibitors. Molecules. 2019;24:1718. doi: 10.3390/molecules24091718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wagner E., Becan L., Nowakowska E. Synthesis and pharmacological assessment of derivatives of isoxazolo[4,5-d]pyrimidine. Biorg. Med. Chem. 2004;12:265–272. doi: 10.1016/j.bmc.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 8.Chen P.-J., Yang A., Gu Y.-F., Zhang X.-S., Shao K.-P., Xue D.-Q., He P., Jiang T.-F., Zhang Q.-R., Liu H.-M. Synthesis, in vitro antimicrobial and cytotoxic activities of novel pyrimidine–benzimidazol combinations. Bioorg. Med. Chem. Lett. 2014;24:2741–2743. doi: 10.1016/j.bmcl.2014.04.037. [DOI] [PubMed] [Google Scholar]
- 9.Cortes-Percino A., Vega-Baez J.L., Romero-Lopez A., Puerta A., Merino-Montiel P., Meza-Reyes S., Padron J.M., Montiel-Smith S. Synthesis and evaluation of pyrimidine steroids as antiproliferative agents. Molecules. 2019;24:3676. doi: 10.3390/molecules24203676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sirakanyan S.N., Spinelli D., Geronikaki A., Hakobyan E.K., Sahakyan H., Arabyan E., Zakaryan H., Nersesyan L.E., Aharonyan A.S., Danielyan I.S., et al. Synthesis, Antitumor Activity, and Docking Analysis of New Pyrido[3′,2′:4,5]furo(thieno)[3,2-d]pyrimidin-8-amines. Molecules. 2019;24:3952. doi: 10.3390/molecules24213952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin J.-R., Xiong F., Zhang X.-Y., Liu W.-J. Effect of minoxidil on transforming growth factor β1-induced rat lung fibroblasts proliferation. Guangdong Yixue. 2015;36:2933–2936. [Google Scholar]
- 12.Ma R., Chen J., Liang Y., Lin S., Zhu L., Liang X., Cai X. Sorafenib: A potential therapeutic drug for hepatic fibrosis and its outcomes. Biomed. Pharmacother. 2017;88:459–468. doi: 10.1016/j.biopha.2017.01.107. [DOI] [PubMed] [Google Scholar]
- 13.Vasta J.D., Raines R.T. Collagen Prolyl 4-Hydroxylase as a Therapeutic Target. J. Med. Chem. 2018;61:10403–10411. doi: 10.1021/acs.jmedchem.8b00822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rose N.R., McDonough M.A., King O.N.F., Kawamura A., Schofield C.J. Inhibition of 2-oxoglutarate dependent oxygenases. Chem. Soc. Rev. 2011;40:4364–4397. doi: 10.1039/c0cs00203h. [DOI] [PubMed] [Google Scholar]
- 15.Bickel M., Baringhaus K.-H., Gerl M., Gunzler V., Kanta J., Schmidts L., Stapf M., Tscank G., Weidmann K., Werner U. Selective inhibition of hepatic collagen accumulation in experimental liver fibrosis in rats by a new prolyl 4-hydroxylase inhibitor. Hepatology. 1998;28:404–411. doi: 10.1002/hep.510280217. [DOI] [PubMed] [Google Scholar]
- 16.Oh S.-W., Kim D.-H., Ha J.-R., Kim D.-Y. Anti-fibrotic Effects of a Methylenedioxybenzene Compound, CW209292 on Dimethylnitrosamine-Induced Hepatic Fibrosis in Rats. Biol. Pharm. Bull. 2009;32:1364–1370. doi: 10.1248/bpb.32.1364. [DOI] [PubMed] [Google Scholar]
- 17.Weiskirchen R. Hepatoprotective and Anti-fibrotic Agents: It’s Time to Take the Next Step. Front. Pharmacol. 2016;6:303. doi: 10.3389/fphar.2015.00303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vasta J.D., Andersen K.A., Deck K.M., Nizzi C.P., Eisenstein R.S., Raines R.T. Selective Inhibition of Collagen Prolyl 4-Hydroxylase in Human Cells. ACS Chem. Biol. 2016;11:193–199. doi: 10.1021/acschembio.5b00749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasta J.D., Raines R.T. Selective inhibition of prolyl 4-hydroxylases by bipyridinedicarboxylates. Bioorg. Med. Chem. 2015;23:3081–3090. doi: 10.1016/j.bmc.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Seo J.S., Joo Y.-H., Yi J.B., Lee E.J., Lee N., Cho Y.-B., Kwak W.J., Hwang J.Y., Jeon Y.S., Jeon H.S., et al. Novel inhibitors of prolyl 4-hydroxylase; solid-phase synthesis of 2,2-dimethyl-3,4-dialkoxy-substituted 6-aminobenzopyran derivatives. Bull. Korean Chem. Soc. 2006;27:909–917. [Google Scholar]
- 21.Xu G., Bai X., Dang Q. Aromatic Heterocycles as Productive Dienophiles in the Inverse Electron-Demand Diels-Alder Reactions of 1,3,5-Triazines. Acc. Chem. Res. 2020;53:773–781. doi: 10.1021/acs.accounts.9b00604. [DOI] [PubMed] [Google Scholar]
- 22.Yang K., Dang Q., Cai P.-J., Gao Y., Yu Z.-X., Bai X. Reaction of Aldehydes/Ketones with Electron-Deficient 1,3,5-Triazines Leading to Functionalized Pyrimidines as Diels-Alder/Retro-Diels-Alder Reaction Products: Reaction Development and Mechanistic Studies. J. Org. Chem. 2017;82:2336–2344. doi: 10.1021/acs.joc.6b02570. [DOI] [PubMed] [Google Scholar]
- 23.Xiang J., Leung C., Zhang Z., Hu C., Geng C., Liu L., Yi L., Li Z., Berenson J., Bai X. Synthesis and Evaluation of 2-Alkylthio-4-(N-substituted sulfonamide)pyrimidine Hydroxamic Acids as Antimyeloma Agents. Chem. Biol. Drug Des. 2016;87:472–477. doi: 10.1111/cbdd.12678. [DOI] [PubMed] [Google Scholar]
- 24.Skerlj R., Bridger G., Zhou Y., Bourque E., McEachern E., Danthi S., Langille J., Harwig C., Veale D., Carpenter B., et al. Mitigating hERG Inhibition: Design of Orally Bioavailable CCR5 Antagonists as Potent Inhibitors of R5 HIV-1 Replication. ACS Med. Chem. Lett. 2012;3:216–221. doi: 10.1021/ml2002604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Q., Shi D., Fang F. A convenient synthetic route for amidine salts. Huaxue Shiji. 1993;15:376. [Google Scholar]
- 26.Shinozuka T., Namiki H., Ohkawa N., Hoshino M., Matsushita K., Yamanoi S., Ogiyama T., Asoh Y., Tsuruoka H. Preparation of Five-Membered-Heterocycle Derivatives Useful as Hypoglycemic Agents. WO2016204135A1. 2016 Dec 22;
- 27.Anderson E.D., Duerfeldt A.S., Zhu K., Glinkerman C.M., Boger D.L. Cycloadditions of noncomplementary substituted 1,2,3-triazines. Org. Lett. 2014;16:5084–5087. doi: 10.1021/ol502436n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Anderson E.D., Boger D.L. Inverse electron demand Diels-Alder reactions of 1,2,3-triazines: Pronounced substituent effects on reactivity and cycloaddition scope. J. Am. Chem. Soc. 2011;133:12285–12292. doi: 10.1021/ja204856a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y., Peng Z., Ji W., Li X., Lin X., Qian L., Li X., Chai X., Wu Q., Gao Q., et al. A Novel Matrine Derivative WM130 Inhibits Activation of Hepatic Stellate Cells and Attenuates Dimethylnitrosamine-Induced Liver Fibrosis in Rats. BioMed Res. Int. 2015;2015:203978. doi: 10.1155/2015/203978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Y., Chai L., Lu G., Wang L., Lv J. In-Vitro Screening Method of Active Fraction of Kadsura Coccinea for Reversing Hepatic Fibrosis, and Its Chemical Component Analysis. CN109142606A. 2019 Jan 4;
- 31.Yang X., Yuan Y.-S., Zhong H. Application of pirfenidone anti-fibrosis in ophthalmology. Guoji Yanke Zazhi. 2013;13:1569–1571. [Google Scholar]
- 32.Kim H.-K., Yang T.-H., Cho H.-Y. Antifibrotic effects of green tea on in vitro and in vivo models of liver fibrosis. World J. Gastroenterol. 2009;15:5200–5205. doi: 10.3748/wjg.15.5200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Deng D., Pei H., Lan T., Zhu J., Tang M., Xue L., Yang Z., Zheng S., Ye H., Chen L. Synthesis and discovery of new compounds bearing coumarin scaffold for the treatment of pulmonary fibrosis. Eur. J. Med. Chem. 2020;185:111790. doi: 10.1016/j.ejmech.2019.111790. [DOI] [PubMed] [Google Scholar]
- 34.Zhu J., He L., Ma L., Wei Z., He J., Yang Z., Pu Y., Cao D., Wu Y., Xiang M., et al. Synthesis and biological evaluation of 4-oxoquinoline-3-carboxamides derivatives as potent anti-fibrosis agents. Bioorg. Med. Chem. Lett. 2014;24:5666–5670. doi: 10.1016/j.bmcl.2014.10.071. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.