Abstract
Intrauterine adhesion (IUA), which mainly occurs after intrauterine surgery or an inflammatory process, is an important but often neglected condition in women of reproductive age. The presentation of IUA varies greatly, ranging from symptom-free to severe, with amenorrhea or infertility. With much advanced development of intrauterine instruments, more intrauterine diseases can be successfully cured by hysteroscopic surgery. Among these, submucosal myoma is one of the best examples. Submucosal myomas are often related to abnormal bleeding, anemia, and possible infertility or miscarriage. However, submucosal myoma after hysteroscopic myomectomy may be complicated by IUA in various grades of severity, and its incidence and prevalence might be nearly one-quarter to one-third of patients, suggesting an urgent need for efforts to decrease the risk of developing IUA after hysteroscopic myomectomy. Many strategies have been reported to be useful for this purpose, and intrauterine application of anti-adhesive gels, such as polyethylene oxide–sodium carboxymethylcellulose (PEO-NaCMC) or auto-crosslinked hyaluronic acid (ACHA), has become increasingly popular in routine clinical practice. This meta-analysis is aimed at investigating the effect of ACHA on the primary prevention of IUA formation after hysteroscopic myomectomy. A pooled analysis of three studies (hysteroscopic surgeries for fibroids, polyps, and septum) including 242 women showed that using PEO-NaCMC or ACHA gel decreased the IUA rate with an odds ratio (OR) of 0.364 (95% confidence interval (CI) 0.189–0.703, p = 0.03). Pooled analysis of two studies that limited the use of ACHA in 119 women showed that the application of ACHA gel for the primary prevention of IUA in patients after hysteroscopic myomectomy led to a statistically significant reduction of the development of IUA postoperatively (OR 0.285, 95% CI 0.116–0.701, p = 0.006). All of this suggests that the use of ACHA gel in patients after hysteroscopic myomectomy could significantly reduce de novo IUA, although more evidence is needed.
Keywords: anti-adhesive gel, hyaluronic acid, hysteroscopic myomectomy, intrauterine adhesion, prevention, reduction
1. Introduction
Intrauterine adhesion (IUA) is a potentially chronic complication developed by the pathophysiology of trauma to the vascular basal layer of the endometrium, mainly as a result of hysteroscopic surgery, uterine curettage, termination of pregnancy, cesarean section, or genital tuberculosis or other severe inflammation processes [1,2,3,4,5,6,7,8,9]. IUA presents a challenge to the endometrial model of scar-free wound healing. In fact, the healing process of the endometrium is similar to the classical wound healing process, including three separate, continuous, and overlapping steps: hemostasis/inflammatory, proliferative, and remodeling phases [10,11,12,13,14,15]. In order to achieve scar-free regeneration and maintain endometrial integrity, at least three key components of endometrial biology should exist: (1) limited inflammation to prevent excessive tissue destruction, (2) cyclic activation of stem cells for regeneration, and (3) scar-free repair following menstrual shedding. Several postulated mechanisms for the loss of scar-free regeneration and repair have been proposed. They include hypoxic injury, unbalanced inflammatory process, decreased angiogenesis, disturbance of immune and molecular mechanisms, unregulated epithelial–mesenchymal transition, aberrant myofibroblast differentiation, bizarre stem cell regeneration, and interrupted normal endometrial cell proliferation [16,17]. IUA is a severe form of disruption of normal endometrial regeneration.
The basic histological finding of IUA is endometrial fibrosis. Avascular fibrous tissues and spindle-shaped myofibroblasts take the place of the originally normal stroma structure of the uterus [2,8]. Additionally, the normal endometrial glands are replaced by inactive cubo-columnar endometrial epithelium, which cannot be distinguished between stratum functionalis and stratum basalis [2,8]. Furthermore, this inactive single layer of cubo-columnar epithelium is almost completely nonresponsive to hormonal stimulation. Finally, fibrotic synechiae form across the entire uterine cavity, resulting in the most severe form of IUA, sometimes called Asherman syndrome [8,18,19,20,21,22,23]. According to Foix’s classification, three types of IUA have been proposed: (1) The most common type is in the form of avascular fibrous strands joining the uterine wall. In this type of IUA, thin-walled telangiectatic vessels can sometimes be found in the avascular fibrous strand. In addition, calcification and/or ossification can be found in the stroma area accompanied by spare and inactive or cystically dilated gland. (2) The second common type is muscular adhesion composed of collagen bundles, fibrous strips, or muscle with the same characteristics as normal myometrium, of which there is more than 50–80% of fibrous tissue in biopsy specimens. (3) The third type is sclerotic, atrophic endometrium [2,8,20]. Han and Du summarized the pathological changes of IUA, including endometrial fibrosis, endometrial scarring, loss or thinning of endometrium with different degrees of damage to the basal layer, atrophic gland, lack of vascular stromal tissue and hypoxia, and pale microenvironment in the adhesion area [22].
Women with IUA may present with various kinds of symptoms, and some are persistent. These symptoms include abnormal uterine bleeding, amenorrhea, dysmenorrhea, infertility, abnormal placentation, and recurrent miscarriage [1,2,4,7,8,9,24,25,26,27,28,29]. As there is continuous progression in hysteroscopic surgeries and they are widely performed for the treatment of various kinds of intrauterine lesions, there is increased concern about IUA-associated morbidities and the subsequent significant impairment of reproductive performance in women of reproductive age [3,5,7,30,31,32,33,34,35,36,37,38]. Among these surgeries, hysteroscopic myomectomy is one of the best examples, since it is considered as the best choice of therapy in the management of women with submucosal myomas [39,40,41,42,43,44,45,46]. However, the significantly increased risk of IUA after hysteroscopic myomectomy compared to other intrauterine surgeries, such as polypectomy, is well known [1,2,7,8,26,27,28,29,30,31,47,48,49,50,51].
Because of the wide variation of symptoms in women with the complication of IUA, late diagnosis is common. Some patients with IUA may have troublesome or even life-threatening clinical situations. These symptoms can be minimal but unpleasant, such as abnormal vaginal bleeding and/or intermittent vaginal spotting. Sometimes, symptoms can be severe, resulting in amenorrhea, and can be associated with pregnancy-related catastrophic diseases such as severe postpartum hemorrhage (PPH) and abnormal placentation, such as placenta accrete, increta, or percreta [52,53]. These IUA patients can be treated by hysteroscopic adhesiolysis after resolution of IUA and immediate restoration of the normal uterine cavity contour. However, hysteroscopic adhesiolysis is a relatively complicated surgery, associated with not only a high risk of surgery-related morbidity but also short-term therapeutic outcomes [1,2,7,8,26,27,37,54,55,56,57,58,59,60,61,62,63,64,65]. It is reported that in up to 62.5% of patients, IUA will recur after hysteroscopic adhesiolysis [54,55,56,57,58,59,60,61,62,63,64,65]. Taken together, this suggests the urgent need to focus on the primary prevention of IUA after intrauterine surgeries [3,5,7,30,31,32,33,34,35,36,37,38]. Several techniques have been proposed to prevent de novo IUA, which is postoperative adhesion without initial evidence of IUA at the same sites [66]. Physical barriers such as balloon catheters and intrauterine devices (IUD) have been used to decrease IUA after hysteroscopic surgery [24,26,30,31,35,38,46,58,59,60,61,64]. However, foreign body-related discomfort, inconvenience, increased infection rates, and possible uterine perforation are concerns [67]. In contrast, semi-solid agents can overcome the disadvantages of physical barriers. These materials include polyethylene oxide–sodium carboxymethylcellulose (PEO-NaCMC) gels and auto-crosslinked hyaluronic acid (ACHA) or hyaluronic acid (HA) gels, which have been proposed or investigated over the past few years [3,6,24,30,31,32,33,34,36,37,57,58,62,63,64,65,66,67]. ACHA and HA, in theory, show their effect on preventing the development of IUA based on their high affinity to the traumatic site of the postoperative endometrium [64]. However, a limited number of randomized controlled trials evaluating their efficacy in the primary prevention of IUA after hysteroscopic myomectomy have been conducted. The current meta-analysis was aimed at exploring the efficacy of ACHA and HA gels in hysteroscopic myomectomy for the primary prevention of de novo IUA.
2. Materials and Methods
2.1. Search Strategy and Study Selection
The meta-analysis was conducted based on the recommendation of Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) and was registered in PROSPERO (ID: CRD42020176878) on 28/04/2020. We searched the PubMed, Embase, and ClinicalTrials.gov databases for relevant randomized controlled trials (RCTs) published online from their inception to May 2020. The search was performed without restrictions regarding language and country. Combined search terms included “hyaluronic acid”, “adhesion”, “intrauterine adhesion”, and “hysteroscopic surgery”. RCTs were eligible according to the following inclusion criteria: women undergoing hysteroscopic surgery for benign gynecologic disease, adhesion barrier of HA gel applied primarily at the end of surgery, and second-look hysteroscopy performed to identify the incidence and severity of IUA. Endpoints were reported as relative risk (RR) or odds ratio (OR) with corresponding 95% confidence interval (CI). Studies were excluded according to the following conditions: (1) patients with IUA before receiving surgery; (2) case reports, observational studies, or conference abstracts without adequate information for data synthesis; and (3) animal testing. Two reviewers (M.C. and P.-H.W.) independently evaluated all relevant articles retrieved from the databases according to the inclusion and exclusion criteria. Disagreements were resolved by discussion with the third author (W.-L.L.).
2.2. Procedures
Two investigators (M.C. and P.-H.W.) independently extracted data from each article, including authors’ names, publication year, study period, sample size, indication for surgery, type of hysteroscopic surgery, and incidence of primary IUA after surgery. Risk of bias was assessed by using the Cochrane Collaboration’s risk of bias tool covering allocation concealment, sequence generation, blinding, detection bias, attrition bias, and reporting bias.
2.3. Statistical Analysis and Data Synthesis
Heterogeneity between studies was evaluated using Cochran’s Q test and measured by I2 statistics. Low, moderate, and high heterogeneity were defined as I2 values of 25%, 50%, and 75%, respectively. A two-sided p-value of ≤0.05 was regarded as statistically significant. Comprehensive Meta-analysis Version 3.0 (Biostat Inc., Englewood, NJ, USA) was used for data synthesis [68]. A random effect model was used to calculate effect size in meta-analysis due to potential clinical heterogeneity from different surgical indications and investigated populations. Odds ratios (ORs) were calculated for dichotomous outcomes, with 95% CI measuring the effect of applying HA gels in hysteroscopic surgery versus no administration of anti-adhesion products according to the Cochrane Handbook for Systematic Reviews of Interventions Version 6, 2019.
3. Results
3.1. Strategy to Include Studies in the Current Meta-Analysis
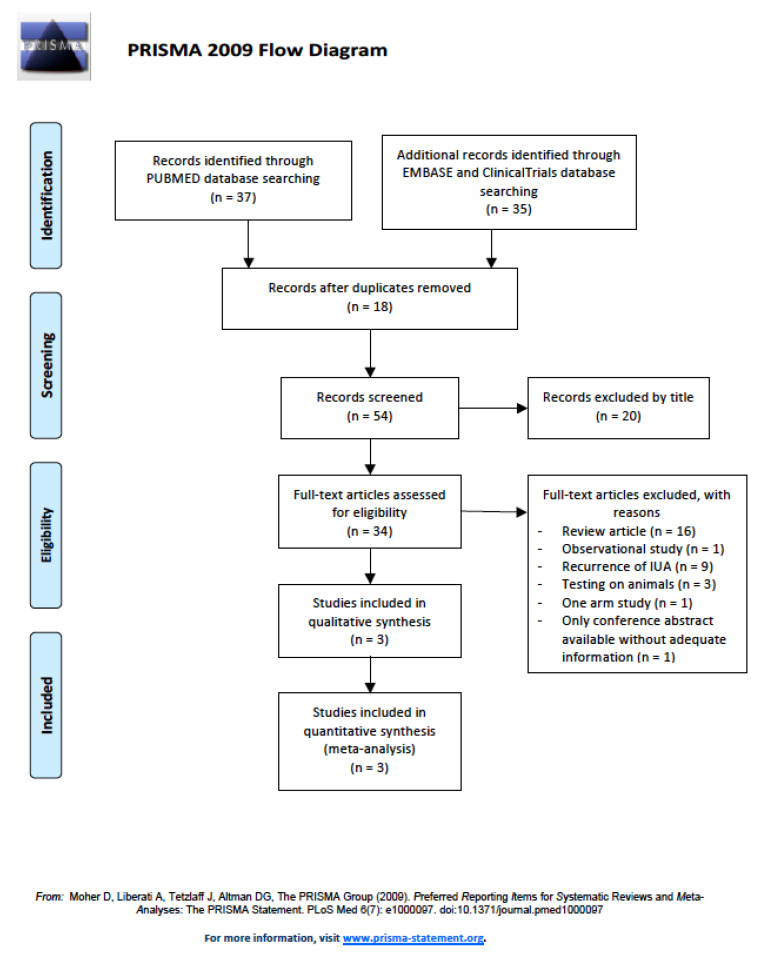
After removing duplications and articles with unrelated topics, a total of 34 studies were reviewed in detail for eligibility; 31 studies were excluded, including 16 articles in review form, 1 observational study, 9 studies with evaluations of secondary intrauterine adhesion, 3 animal studies, 1 study with only one arm, and 1 conference abstract. Figure 1 shows the flowchart for identifying studies that met the criteria for the current meta-analysis. In the end, three randomized controlled studies [67,69,70] were included for meta-analysis.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) 2009 flowchart of studies in the current report.
3.2. Characteristics of Included Studies
The indications for hysteroscopic surgery were not consistent among the three studies [67,69,70]. Table 1 shows the basic characteristics of the included studies.
Table 1.
Characteristics of included randomized controlled trials in the systematic review.
| Study [Ref.] |
n | Age (y) | Indication | Exclusion Criteria | Tools | Intervention | Follow-up Evaluation | DR |
|---|---|---|---|---|---|---|---|---|
| De Iaco, 2003 [69] | 40 | 18–65 | Fibroid Polyp Septum |
Not stated | MR | HA/NaCMC, 10.5 ± 5.5 mL | 9 weeks | Not stated |
| Guida, 2004 [67] | 132 | <50 | Fibroid (n = 49) Polyp (n = 67) Septum (n = 16) |
Postmenopause Pregnancy Prolapse Current illness Age > 50 y BW > 100 kg Other intrauterine lesions |
BR | ACHA, 10 mL | 3 months | 4.3% |
| Huang, 2020 [70] | 70 | 20–65 | Fibroid (n = 70) |
|
BR | 3 or 4% ACHA, 10 mL | 12 weeks | 1.4% |
Fibroid, submucosal myoma; BW, body weight; MR, monopolar resectoscope; BR, bipolar resectoscope; HA, hyaluronic acid; NaCMC, sodium carboxymethylcellulose; ACHA, auto-crosslinked hyaluronic acid; DR, dropout rate.
3.3. Quality of Included Studies
Table 2 shows an assessment of risk of bias, which was composed of five domains according to RoB 2, a revised Cochrane risk of bias tool for RCTs [71]. One study may have been at risk of randomization bias since it failed to report on allocation concealment [69]. Two studies failed to keep the investigation blind, which may have led to a higher risk of deviation from the intended intervention [67,70]. One study did not report the dropout rate [69], and the other two had dropout rates of 1.4% and 4.3% [67,70]. All dropout cases resulted from failing to attend follow-up hysteroscopy and outcomes were not evaluated based on intention to treat. However, dropout rates of the two studies were low, which may offset the risk of bias on missing data.
Table 2.
Risk of bias assessment of included studies.
| Study [Ref.] | Bias Due to Randomization Process | Bias Due to Deviation from Intended Intervention | Bias Due to Missing Data | Bias Due to Outcome Measurement | Bias Due to Selection of Reported Results | Overall Risk of Bias |
|---|---|---|---|---|---|---|
| De Iaco, 2003 [69] | No information | No information | No information | No information | Some concerns | High |
| Guida, 2004 [67] | Low | Some concerns | Some concerns | Low | Low | Some concerns |
| Huang, 2020 [70] | Low | Low | Low | Low | Low | Low |
3.4. Effectiveness of Primary Prevention of Developing Intrauterine Adhesion in Patients Undergoing Hysteroscopic Surgery, Including Fibroid, Polyp, and Septum
On the evaluation of primary IUA rates, two of the three studies demonstrated a significant reduction (Table 3). The time of follow-up after operation ranged from 9 to 12 weeks. Guida et al. included cases of hysteroscopic surgery for myomectomy, polypectomy, and intrauterine septum resection, revealing 10.4% of IUA in the treatment group compared with 26.2% of IUA in the control group [67]. Huang et al. limited the patients with submucosal myoma treated by hysteroscopic myomectomy, and the results showed that 12.8% of patients had postoperative IUA in the treatment group compared with 39.1% in the control group [70]. On the other hand, De Iaco et al. did not show a significant difference in IUA rates between intervention and control groups, and their study also included different indications for hysteroscopic surgery, including myomectomy, polypectomy, and intrauterine septum resection [69].
Table 3.
Summarized primary postoperative intrauterine adhesion rates of included studies.
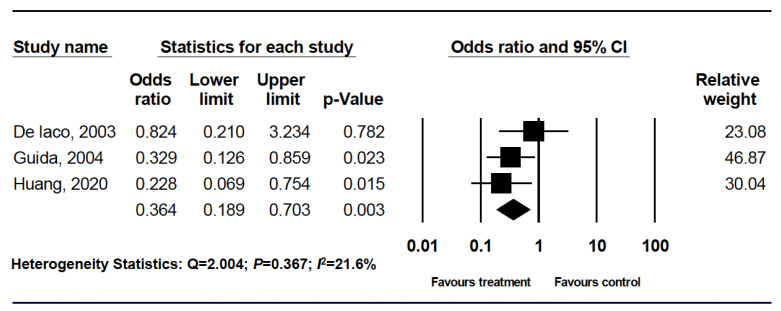
All three of the analyzed studies included information on the IUA rate. For analysis, all three categories were included and pooled into the meta-analysis [67,69,70]. For these 242 patients, there was a significantly reduced risk of developing IUA in the ACHA and HA/NaCMC groups based on a random effect model (Figure 2).
Figure 2.
Forest plot comparing hyaluronic acid with control for intrauterine adhesion prevention.
3.5. Significant Reduction of Intrauterine Adhesion Rates in Patients Undergoing Hysteroscopic Myomectomy
While we focused on evaluating the effectiveness of applying ACHA in the primary prevention of IUA in patients after hysteroscopic myomectomy, two of the studies were included and pooled into the meta-analysis (Table 4) [67,71]. In Guida’s study, 49 patients were included in the analysis, contributing to an incidence of IUA of 16% in the ACHA treatment group and one-third in the group without ACHA [67]. Since all patients in Huang’s study were undergoing hysteroscopic myomectomy, upon further examination of their report, we found that two concentrations of ACHA (3% and 4%) were applied in the intervention group [70]. There was no statistically significant difference in the development of IUA between 3% and 4% ACHA application, although the trend showed a higher effect of 4% ACHA not only on the reduction of IUA incidence (17.4% vs. 8.3%, p = 0.352), but also on decreased severity (all had a mild degree of IUA in the 4% ACHA group and one-quarter had a moderate degree of IUA in the 3% ACHA group) [70]. However, compared with no use of ACHA in patients after hysteroscopic myomectomy, application of ACHA successfully decreased the incidence of IUA with both concentrations of ACHA gel (12.8% vs. 39.1%, p = 0.012) [70].
Table 4.
Summarized primary postoperative intrauterine adhesion rates of included studies (hysteroscopic myomectomy).
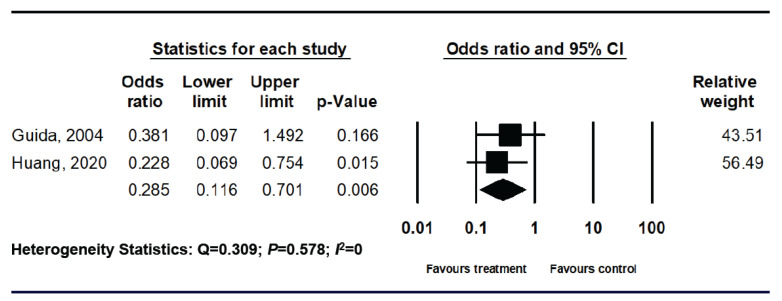
For these 119 patients, there was a significantly reduced risk of developing IUA in the ACHA application groups based on the random effect model (Figure 3).
Figure 3.
Forest plot comparing hyaluronic acid with control for intrauterine adhesion prevention in subgroup of hysteroscopic myomectomy.
4. Discussion
The incidence of IUA after hysteroscopic surgery varies greatly depending on surgical indications and time of postoperative evaluation [1,2,3,4,5,6,7,8,9,24,26,30,47]. Taskin et al. reported IUA following hysteroscopic removal of a single myoma in 31.3% of cases, hysteroscopic resection for multiple myomas in 45.5% of cases, and hysteroscopic resection of intrauterine septum in 6.7% of cases [72]. On the other hand, a study by Yang et al. showed an incidence of IUA of 88% in patients who had undergone hysteroscopic septum resection and 40% in patients after hysteroscopic myomectomy, suggesting a significant proportion of IUA development after hysteroscopic surgery for more complicated diseases, such as uterine septation or myoma [35,36]. Although we found that a number of randomized controlled trials were performed to evaluate the application of ACHA gels as a barrier for the prevention of postoperative IUA, most of the studies did not exclude patients with IUA, and some studies also allowed adhesiolysis as one of the indications for hysteroscopic surgery, which could potentially show a relatively higher incidence of IUA resulting from intrauterine surgeries and underestimate the efficacy of ACHA gels on the primary prevention of IUA after hysteroscopic myomectomy. This meta-analysis focused on studies that enrolled patients who did not have IUA, with the expectation of conducting a more precise evaluation of the effect of ACHA gels on the primary prevention of IUA after hysteroscopic myomectomy.
Under similar clinical circumstances, including time of follow-up and surgical instruments used, Guida et al. [67] and Huang et al. [71] presented relatively consistent results on the efficacy of ACHA gels in the primary prevention of de novo IUA. Both studies revealed a significant reduction of the rate of IUA with the use of ACHA gels. This may be the first meta-analysis focusing on an evaluation of the incidence rate of de novo IUA with ACHA gels in patients who have undergone hysteroscopic myomectomy, demonstrating the low heterogeneity of eligible studies and a more conclusive effect with the use of a single anti-adhesion agent. However, the small sample size and limited number of available studies meeting our inclusion criteria were the major limitations of this meta-analysis, indicating an urgent need to include more randomized controlled trials to clarify the effect of ACHA as a tool for the primary prevention of IUA in patients following hysteroscopic myomectomy.
5. Conclusions
Applying ACHA gels in patients after hysteroscopic myomectomy could significantly reduce de novo IUA, although more evidence is needed.
Acknowledgments
This research was supported by grants from the Taipei Veterans General Hospital (V109C-108; V109E-005-5; and V109A-022) and the Ministry of Science and Technology, Executive Yuan (MOST: 106-2314-B-075-061-MY3; MOST 109-2314-B-075B-014-MY2 and MOST 109-2314-B-075-056), Taipei, Taiwan.
Abbreviations
The following abbreviations are used in this manuscript:
| ACHA | auto-crosslinked hyaluronic acid |
| CI | confidence interval |
| D&C | dilation and curettage |
| IUA | intrauterine adhesion or intrauterine adhesions |
| NaCMC | sodium carboxymethylcellulose |
| OR | odds ratio |
| PEO | polyethylene oxide |
| PRISMA | Preferred Reporting Items for Systematic reviews and Meta-analyses |
| RCTs | randomized controlled trials |
| RR | relative risk |
Author Contributions
Conceptualization, M.C.,, W.-H.C., and P.-H.W.; formal analysis, H.-Y.H., W.-H.C., and P.-H.W.; investigation, M.C., S.-T.Y., K.-H.T., C.-P.C., W.-L.L., and P.-H.W.; resources, H.-Y.H., K.-H.T., W.-L.L., and P.-H.W.; data curation, M.C., W.-H.C., S.-T.Y., W.-L.L, and P.-H.W.; methodology, M.C., H.-Y.H., W.-L.L., and P.-H.W.; writing—original draft, M.C., W.-L.L., and P.-H.W.; writing—review and editing, M.C., W.-L.L., and P.-H.W.; supervision, W.-L.L., and P.-H.W.; project administration, W.-H.C., K.-H.T., and P.-H.W. All authors have read and agreed to the published version of the manuscript.
Funding
The authors appreciate the financial support from the Female Cancer Foundation, Taipei, Taiwan, and SciVision Biotech Inc., Kaohsiung, Taiwan. The sources of funding provided no conflict of interest that would affect this study’s impartiality.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Yu D., Wong Y.M., Cheong Y., Xia E., Li T.C. Asherman syndrome-one century later. Fertil. Steril. 2008;89:759–779. doi: 10.1016/j.fertnstert.2008.02.096. [DOI] [PubMed] [Google Scholar]
- 2.Deans R., Abbott J. Review of intrauterine adhesions. J. Minim. Invasive Gynecol. 2010;17:555–569. doi: 10.1016/j.jmig.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 3.Fuchs N., Smorgick N., Ben Ami I., Vaknin Z., Tovbin Y., Halperin R., Pansky M. Intercoat (Oxiplex/AP gel) for preventing intrauterine adhesions after operative hysteroscopy for suspected retained products of conception: Double-blind, prospective, randomized pilot study. J. Minim. Invasive Gynecol. 2014;21:126–130. doi: 10.1016/j.jmig.2013.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Hooker A.B., Lemmers M., Thurkow A.L., Heymans M.W., Opmeer B.C., Brolmann H.A.M., Mol B.W., Huirne J.A.F. Systematic review and meta-analysis of intrauterine adhesions after miscarriage: Prevalence, risk factors and long-term reproductive outcome. Hum. Reprod. Update. 2014;20:262–278. doi: 10.1093/humupd/dmt045. [DOI] [PubMed] [Google Scholar]
- 5.Barel O., Krakov A., Pansky M., Vaknin Z., Halperin R., Smorgick N. Intrauterine adhesions after hysteroscopic treatment for retained products of conception: What are the risk factors? Fertil. Steril. 2015;103:775–779. doi: 10.1016/j.fertnstert.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 6.Hooker A.B., de Leeuw R., van de Ven P.M., Bakkum E.A., Thurkow A.L., Vogel N.E.A., van Vliet H.A.A.M., Bongers M.Y., Emanuel M.H., Verdonkschot A.E.M., et al. Prevalence of intrauterine adhesions after the application of hyaluronic acid gel after dilatation and curettage in women with at least one previous curettage: Short-term outcomes of a multicenter, prospective randomized controlled trial. Fertil. Steril. 2017;107:1223–1231. doi: 10.1016/j.fertnstert.2017.02.113. [DOI] [PubMed] [Google Scholar]
- 7.Salazar C.A., Isaacson K., Morris S. A comprehensive review of Asherman’s syndrome: Causes, symptoms and treatment options. Curr. Opin. Obstet. Gynecol. 2017;29:249–256. doi: 10.1097/GCO.0000000000000378. [DOI] [PubMed] [Google Scholar]
- 8.Dreisler E., Kjer J.J. Asherman’s syndrome: Current perspectives on diagnosis and management. Int. J. Womens Health. 2019;11:191–198. doi: 10.2147/IJWH.S165474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dawood A., Al-Talib A., Tulandi T. Predisposing factors and treatment outcome of different stages of intrauterine adhesions. J. Obstet. Gynaecol. Can. 2010;32:767–770. doi: 10.1016/S1701-2163(16)34618-7. [DOI] [PubMed] [Google Scholar]
- 10.Jiang D., Rinkevich Y. Scars or regeneration?—Dermal fibroblasts as drivers of diverse skin wound responses. Int. J. Mol. Sci. 2020;21:617. doi: 10.3390/ijms21020617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Ayadi A., Jay J.W., Prasai A. Current approaches targeting the wound healing phases to attenuate fibrosis and scarring. Int. J. Mol. Sci. 2020;21:1105. doi: 10.3390/ijms21031105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Akita S. Wound repair and regeneration: Mechanisms, signaling. Int. J. Mol. Sci. 2019;20:6328. doi: 10.3390/ijms20246328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogawa R. Recent advances in scar biology. Int. J. Mol. Sci. 2018;19:1749. doi: 10.3390/ijms19061749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang P.H., Huang B.S., Horng H.C., Yeh C.C., Chen Y.J. Wound healing. J. Chin. Med. Assoc. 2018;81:94–101. doi: 10.1016/j.jcma.2017.11.002. [DOI] [PubMed] [Google Scholar]
- 15.Horng H.C., Chang W.H., Yeh C.C., Huang B.S., Chang C.P., Chen Y.J., Tsui K.H., Wang P.H. Estrogen effects on wound healing. Int. J. Mol. Sci. 2017;18:2325. doi: 10.3390/ijms18112325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Owusu-Akyaw A., Krishnamoorthy K., Goldsmith L.T., Morelli S.S. The role of mesenchymal-epithelial transition in endometrial function. Hum. Reprod. Update. 2019;25:114–133. doi: 10.1093/humupd/dmy035. [DOI] [PubMed] [Google Scholar]
- 17.Wei C., Pan Y., Zhang Y., Dai Y., Jiang L., Shi L., Yang W., Xu S., Zhang Y., Xu W., et al. Overactivated sonic hedgehog signaling aggravates intrauterine adhesion via inhibiting autophagy in endometrial stromal cells. Cell. Death. Dis. 2020;11:755. doi: 10.1038/s41419-020-02956-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F., Hu S., Wang S., Cheng K. Cell and biomaterial-based approaches to uterus regeneration. Regen. Biomater. 2019;6:141–148. doi: 10.1093/rb/rbz021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhou Q., Wu X., Hu J., Yuan R. Abnormal expression of fibrosis markers, estrogen receptor α and stromal derived factor-1/chemokine (C-X-C motif) receptor-4 axis in intrauterine adhesions. Int. J. Mol. Med. 2018;42:81–90. doi: 10.3892/ijmm.2018.3586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Foix A., Bruno R.O., Davison T., Lema B. The pathology of postcurettage adhesions. Am. J. Obstet. Gynecol. 1966;96:1027–1033. doi: 10.1016/0002-9378(66)90452-2. [DOI] [PubMed] [Google Scholar]
- 21.Yaffe H., Ron M., Polishuk W. Amenorrhoea, hypomenorrhoea and uterine fibrosis. Am. J. Obstet. Gynecol. 1978;130:599–601. doi: 10.1016/0002-9378(78)90093-5. [DOI] [PubMed] [Google Scholar]
- 22.Han Q., Du Y. Advances in the application of biomimetic endometrium interfaces for uterine bioengineering in female infertility. Front. Bioeng. Biotechnol. 2020;8:153. doi: 10.3389/fbioe.2020.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan Y., Xu D. The Effect of adjuvant treatment to prevent and treat intrauterine adhesions: A network meta-analysis of randomized controlled trials. J. Minim. Invasive Gynecol. 2018;25:589–599. doi: 10.1016/j.jmig.2017.09.006. [DOI] [PubMed] [Google Scholar]
- 24.Chen Y.Y., Tsai C.C., Kung F.T., Lan K.C., Ou Y.C. Association between hysteroscopic findings of previous cesarean delivery scar defects and abnormal uterine bleeding. Taiwan J. Obstet. Gynecol. 2019;58:541–544. doi: 10.1016/j.tjog.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 25.Doroftei B., Dabuleanu A.M., Ilie O.D., Maftei R., Anton E., Simionescu G., Matei T., Armeanu T. Mini-review of the new therapeutic possibilities in Asherman syndrome—Where are we after one hundred and twenty-six years? Diagnostics. 2020;10:706. doi: 10.3390/diagnostics10090706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valle R.F., Sciarra J.J. Intrauterine adhesions: Hysteroscopic diagnosis, classification, treatment, and reproductive outcome. Am. J. Obstet. Gynecol. 1988;158:1459–1470. doi: 10.1016/0002-9378(88)90382-1. [DOI] [PubMed] [Google Scholar]
- 27.Menzies D. Postoperative adhesions: Their treatment and relevance in clinical practice. Ann. R. Coll. Surg. Engl. 1993;75:147–153. [PMC free article] [PubMed] [Google Scholar]
- 28.Schenker J.G. Etiology of and therapeutic approach to synechia uteri. Eur. J. Obstet. Gynecol. Reprod. Biol. 1996;65:109–113. doi: 10.1016/0028-2243(95)02315-J. [DOI] [PubMed] [Google Scholar]
- 29.Healy M.W., Schexnayder B., Connell M.T., Terry N., DeCherney A.H., Csokmay J.M., Yauger B.J., Hill M.J. Intrauterine adhesion prevention after hysteroscopy: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. 2016;215:267–275. doi: 10.1016/j.ajog.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 30.Bosteels J., Weyers S., D’Hooghe T.M., Torrance H., Broekmans F.J., Chua S.J., Mol B.W.J. Anti-adhesion therapy following operative hysteroscopy for treatment of female subfertility. Cochrane. Database Syst. Rev. 2017;11:CD011110. doi: 10.1002/14651858.CD011110.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Di Spiezio Sardo A., Spinelli M., Bramante S., Scognamiglio M., Greco E., Guida M., Cela V., Nappi C. Efficacy of a polyethylene oxide-sodium carboxymethylcellulose gel in prevention of intrauterine adhesions after hysteroscopic surgery. J. Minim. Invasive Gynecol. 2011;18:462–469. doi: 10.1016/j.jmig.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 32.Bosteels J., Weyers S., Mol B.W., D’Hooghe T. Anti-adhesion barrier gels following operative hysteroscopy for treating female infertility: A systematic review and meta-analysis. Gynecol. Surg. 2014;11:113–127. doi: 10.1007/s10397-014-0832-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zheng F., Xin X., He F., Liu J., Cui Y. Meta-analysis on the use of hyaluronic acid gel to prevent intrauterine adhesion after intrauterine operations. Exp. Ther. Med. 2020;19:2672–2678. doi: 10.3892/etm.2020.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Matsubara S. A novel uterine stent for preventing intrauterine adhesion: Not only gynecologic but also obstetric significance. Ann. Transl. Med. 2020;8:614. doi: 10.21037/atm.2020.03.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee D.Y., Lee S.R., Kim S.K., Joo J.K., Lee W.S., Shin J.H., Cho S., Park J.C., Kim S.H. A new thermo-responsive hyaluronic acid sol-gel to prevent intrauterine adhesions after hysteroscopic surgery: A randomized, non-inferiority trial. Yonsei Med. J. 2020;61:868–874. doi: 10.3349/ymj.2020.61.10.868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ducarme G., Davitian C., Zarrouk S., Uzan M., Poncelet C. Interest of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: A case-control study. J. Gynecol. Obstet. Biol. Reprod. 2006;35:691–695. doi: 10.1016/S0368-2315(06)76465-1. [DOI] [PubMed] [Google Scholar]
- 37.Huang H., Xu B., Cheng C., Xu D. A novel intrauterine stent for prevention of intrauterine adhesions. Ann. Transl. Med. 2020;8:61. doi: 10.21037/atm.2019.12.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mettler L., Schollmeyer T., Tinelli A., Malvasi A., Alkatout I. Complications of uterine fibroids and their management, surgical management of fibroids, laparoscopy and hysteroscopy versus hysterectomy, haemorrhage, adhesions, and complications. Obstet. Gynecol. Int. 2012;2012:791248. doi: 10.1155/2012/791248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Capmas P., Levaillant J.M., Fernandez H. Surgical techniques and outcome in the management of submucous fibroids. Curr. Opin. Obst. Gyn. 2013;25:332–338. doi: 10.1097/GCO.0b013e3283630e10. [DOI] [PubMed] [Google Scholar]
- 40.Mazzon I., Favilli A., Cocco P., Grasso M., Horvath S., Bini V., Di Renzo G.C., Gerli S. Does cold loop hysteroscopic myomectomy reduce intrauterine adhesions? A retrospective study. Fertil. Steril. 2014;101:294–298. doi: 10.1016/j.fertnstert.2013.09.032. [DOI] [PubMed] [Google Scholar]
- 41.Litta P., Leggieri C., Conte L., Dalla Toffola A., Multinu F., Angioni S. Monopolar versus bipolar device: Safety, feasibility, limits and perioperative complications in performing hysteroscopic myomectomy. Clin. Exp. Obstet. Gynecol. 2014;41:335–338. [PubMed] [Google Scholar]
- 42.Haber K., Hawkins E., Levie M., Chudnoff S. Hysteroscopic morcellation: Review of the manufacturer and user facility device experience (MAUDE) database. J. Minim. Invasive Gynecol. 2015;22:110–114. doi: 10.1016/j.jmig.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 43.Ciebiera M., Łoziński T., Wojtyła C., Rawski W., Jakiel G. Complications in modern hysteroscopic myomectomy. Ginekol. Pol. 2018;89:398–404. doi: 10.5603/GP.a2018.0068. [DOI] [PubMed] [Google Scholar]
- 44.Friedman J.A., Wong J.M.K., Chaudhari A., Tsai S., Milad M.P. Hysteroscopic myomectomy: A comparison of techniques and review of current evidence in the management of abnormal uterine bleeding. Curr. Opin. Obstet. Gynecol. 2018;30:243–251. doi: 10.1097/GCO.0000000000000475. [DOI] [PubMed] [Google Scholar]
- 45.Chiu C.S., Hwu Y.M., Lee R.K., Lin M.H. Intrauterine adhesion prevention with Malecot catheter after hysteroscopic myomectomy: A novel approach. Taiwan J. Obstet. Gynecol. 2020;59:56–60. doi: 10.1016/j.tjog.2019.11.008. [DOI] [PubMed] [Google Scholar]
- 46.March C.M. Management of Asherman’s syndrome. Rerpod. BioMed. Online. 2011;23:63–76. doi: 10.1016/j.rbmo.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 47.Santamaria X., Isaacson K., Simón C. Asherman’s Syndrome: It may not be all our fault. Hum. Reprod. 2018;33:1374–1380. doi: 10.1093/humrep/dey232. [DOI] [PubMed] [Google Scholar]
- 48.Yang J.H., Chen M.J., Chen C.D., Chen S.U., Ho H.N., Yang Y.S. Optimal waiting period for subsequent fertility treatment after various hysteroscopic surgeries. Fertil. Steril. 2013;99:2092–2096. doi: 10.1016/j.fertnstert.2013.01.137. [DOI] [PubMed] [Google Scholar]
- 49.Yang J.H., Chen M.J., Wu M.Y., Chao K.H., Ho H.N., Yang Y.S. Office hysteroscopic early lysis of intrauterine adhesion after transcervical resection of multiple apposing submucous myomas. Fertil. Steril. 2008;89:1254–1259. doi: 10.1016/j.fertnstert.2007.05.027. [DOI] [PubMed] [Google Scholar]
- 50.Liao W.L., Ying T.H., Shen H.P., Wu P.J. Combined treatment for big submucosal myoma with High Intensity Focused Ultrasound and hysteroscopic resection. Taiwan J. Obstet. Gynecol. 2019;58:888–890. doi: 10.1016/j.tjog.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 51.Zhang L.P., Wang M., Shang X., Zhang Q., Yang B.J., Xu Y., Li J.H., Feng L.M. The incidence if placeta related diseases after the hysteroscopic adhesiolysis in patients with intrauterine adhesions. Taiwan J. Obstet. Gynecol. 2020;59:575–579. doi: 10.1016/j.tjog.2020.05.018. [DOI] [PubMed] [Google Scholar]
- 52.Dogan O., Pulatoglu C., Yassa M. A new facilitating technique for postpartum hysterectomy at full dilatation: Cervical clamp. J. Chin. Med. Assoc. 2018;81:366–369. doi: 10.1016/j.jcma.2017.05.010. [DOI] [PubMed] [Google Scholar]
- 53.Yamamoto N., Takeuchi R., Izuchi D., Yuge N., Miyazaki M., Yasunaga M., Egashira K., Ueoka Y., Inoue Y. Hysteroscopic adhesiolysis for patients with Asherman’s syndrome: Menstrual and fertility outcomes. Reprod. Med. Biol. 2013;12:159–166. doi: 10.1007/s12522-013-0149-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Di Guardo F., Della Corte L., Vilos G.A., Carugno J., Török P., Giampaolino P., Manchanda R., Vitale S.G. Evaluation and treatment of infertile women with Asherman syndrome: An updated review focusing on the role of hysteroscopy. Reprod. Biomed. Online. 2020;41:55–61. doi: 10.1016/j.rbmo.2020.03.021. [DOI] [PubMed] [Google Scholar]
- 55.Donnez J., Nisolle M. Hysteroscopic lysis of intrauterine adhesions (Asherman syndrome) In: Donnez J., editor. Atlas of Laser Operative Laparoscopy and Hysteroscopy. Parthenon; New York, NY, USA: 1994. pp. 305–322. [Google Scholar]
- 56.Zhou Q., Shi X., Saravelos S., Huang X., Zhao Y., Huang R., Xia E., Li T.C. Auto-cross-linked hyaluronic acid gel for prevention of intrauterine adhesions after hysteroscopic adhesiolysis: A randomized controlled trial. J. Minim. Invasive Gynecol. 2020 doi: 10.1016/j.jmig.2020.06.030. [DOI] [PubMed] [Google Scholar]
- 57.Lin X., Wei M., Li T.C., Huang Q., Huang D., Zhou F., Zhang S. A comparison of intrauterine balloon, intrauterine contraceptive device and hyaluronic acid gel in the prevention of adhesion reformation following hysteroscopic surgery for asherman syndrome: A cohort study. Eur. J. Obstet. Gynecol. Reprod. Biol. 2013;170:512–516. doi: 10.1016/j.ejogrb.2013.07.018. [DOI] [PubMed] [Google Scholar]
- 58.Salma U., Xue M., Md Sayed A.S., Xu D. Efficacy of intrauterine device in the treatment of intrauterine adhesions. Biomed. Res. Int. 2014;2014:589296. doi: 10.1155/2014/589296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Azumaguchi A., Henmi H., Saito T. Efficacy of silicone sheet as a personalized barrier for preventing adhesion reformation after hysteroscopic adhesiolysis of intrauterine adhesions. Reprod. Med. Biol. 2019;18:378–383. doi: 10.1002/rmb2.12294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhu R., Duan H., Gan L., Wang S. Comparison of intrauterine suitable balloon and Foley balloon in the prevention of adhesion after hysteroscopic adhesiolysis. Biomed. Res. Int. 2018;2018:9494101. doi: 10.1155/2018/9494101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thubert T., Dussaux C., Demoulin G., Rivain A.L., Trichot C., Deffieux X. Influence of auto-cross-linked hyaluronic acid gel on pregnancy rate and hysteroscopic outcomes following surgical removal of intra-uterine adhesions. Eur. J. Obstet. Gynecol. Reprod. Biol. 2015;193:65–69. doi: 10.1016/j.ejogrb.2015.06.025. [DOI] [PubMed] [Google Scholar]
- 62.Passos I.M.P.E., Britto R.L. Diagnosis and treatment of müllerian malformations. Taiwan. J. Obstet. Gynecol. 2020;59:183–188. doi: 10.1016/j.tjog.2020.01.003. [DOI] [PubMed] [Google Scholar]
- 63.Fei Z., Bin Z., Xin X., Fei H., Yuechong C. Meta-analysis on the use of hyaluronic acid gel to prevent recurrence of intrauterine adhesion after hysteroscopic adhesiolysis. Taiwan. J. Obstet. Gynecol. 2019;58:731–736. doi: 10.1016/j.tjog.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 64.Krajcovicova R., Hudeck R., Ventruba P., Surgentova K. The role of hyaluronan in Asherman’s syndrome therapy. J. Gynecol. Surg. 2015;31:250–254. doi: 10.1089/gyn.2014.0129. [DOI] [Google Scholar]
- 65.Diamond M.P., Daniell J.F., Feste J., Surrey M.W., McLaughlin D.S., Friedman S., Vaughn W.K., Martin D.C. Adhesion reformation and de novo adhesion formation after reproductive pelvic surgery. Fertil. Steril. 1987;47:864–866. doi: 10.1016/S0015-0282(16)59181-X. [DOI] [PubMed] [Google Scholar]
- 66.Guida M., Acunzo G., Di Spiezio Sardo A., Bifulco G., Piccoli R., Pellicano M., Cerrota G., Cirillo D., Nappi C. Effectiveness of auto-crosslinked hyaluronic acid gel in the prevention of intrauterine adhesions after hysteroscopic surgery: A prospective, randomized, controlled study. Hum. Reprod. 2004;19:1461–1464. doi: 10.1093/humrep/deh238. [DOI] [PubMed] [Google Scholar]
- 67.Mensitieri M., Ambrosio L., Nicolais L., Bellini D., O’Regan M. Viscoelastic properties modulation of a novel autocrosslinked hyaluronic acid polymer. J. Mater. Sci. Mater. Med. 1996;7:695–698. doi: 10.1007/BF00123409. [DOI] [Google Scholar]
- 68.Lee H.H., Huang B.S., Cheng M., Yeh C.C., Lin I.C., Horng H.C., Huang H.Y., Lee W.L., Wang P.H. Intracervical Foley catheter plus intravaginal misoprostol vs intravaginal misoprostol alone for cervical ripening: A meta-analysis. Int. J. Environ. Res. Health. 2020;17:1825. doi: 10.3390/ijerph17061825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.De Iaco P.A., Muzzupapa G., Bigon E., Pressato D., Dona M., Pavesio A., Bovicelli L. Efficacy of a hyaluronan derivative gel in postsurgical adhesion prevention in the presence of inadequate hemostasis. Surgery. 2001;130:60–64. doi: 10.1067/msy.2001.115102. [DOI] [PubMed] [Google Scholar]
- 70.Huang C.Y., Chang W.H., Cheng M., Huang H.Y., Horng H.C., Chen Y.J., Lee W.L., Wang P.H. Crosslinked hyaluronic acid gels for the prevention of intrauterine adhesions after a hysteroscopic myomectomy in women with submucosal myomas: A prospective, randomized, controlled trial. Life. 2020;10:67. doi: 10.3390/life10050067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sterne J.A.C., Savović J., Page M.J., Elbers R.G., Blencowe N.S., Boutron I., Cates C.J., Cheng H.Y., Corbett M.S., Eldridge S.M., et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 72.Taskin O., Sadik S., Onoglu A., Gokdeniz R., Erturan E., Burak F., Wheeler J.M. Role of endometrial suppression on the frequency of intrauterine adhesions after resectoscopic surgery. J. Am. Assoc. Gynecol. Laparosc. 2000;7:351–354. doi: 10.1016/S1074-3804(05)60478-1. [DOI] [PubMed] [Google Scholar]