Abstract
OBJECTIVES
Recurrent laryngeal nerve (RLN) injury during thoracic surgery may result in life-threatening postoperative complications including recurrent aspiration and pneumonia. Anatomical details of the intrathoracic course are scarce. However, only an in-depth understanding of the anatomy will help reduce nerve injury. The aim of this study was to assess the anatomic variations of the intrathoracic left RLN.
METHODS
Left-sided vagal nerves and RLN were dissected in 100 consecutive Caucasian cadavers during routine autopsy. Anatomical details were documented. Available demographic data were assessed for possible correlations.
RESULTS
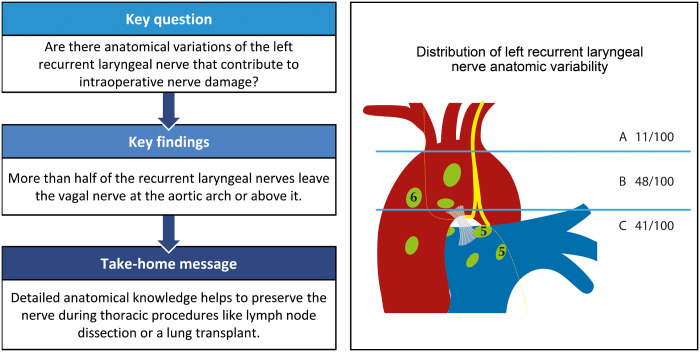
All nerves were identified during dissection. Variant courses were classified in 3 different groups according to the level at which the RLN separated from the vagal nerve: above the aortic arch, level with the aortic arch and below the aortic arch. We found 11% of RLN separating above the aortic arch and crossing the aortic arch at a considerable distance to the vagal nerve. In 48% of the RLN, the nerve split off when it was level with the aortic arch, and 41% of the RLN leave the vagal nerve in a perpendicular direction below the aortic arch. All nerves crossed the ligamentum arteriosum on the posterior side. No gender-specific differences were observed.
CONCLUSIONS
Mediastinal lymph node dissection in left-sided lung cancer patients puts the RLN at risk. With more detailed anatomical knowledge about its course, it is possible to avoid risking the nerve. Visualization will help protect the nerve.
Keywords: Recurrent laryngeal nerve, Anatomy, Variation, Lymph node dissection
Left recurrent laryngeal nerve (L-RLN) palsy is a severe complication following various thoracic surgical procedures.
INTRODUCTION
Left recurrent laryngeal nerve (L-RLN) palsy is a severe complication following various thoracic surgical procedures. Major lung resections and bilateral lung transplantation rely on patent vocal cord closure to avoid postoperative complications. Rates of RLN injury vary in the literature and are reported to be as high as 40% in high-risk procedures including left-sided lobectomy and pneumonectomy [1]. Vocal cord paralysis due to nerve injury was associated with a significantly higher rate of postoperative morbidity and a trend towards higher mortality [2].
Despite cautious dissection of lymph nodes in the anterior mediastinal area (American Thoracic Society lymph node station 6) and aorto-pulmonary window (lymph node station 5) during lung cancer surgery, L-RLN palsies occur at various rates [3]. We assume that anatomical variations in the course of L-RLN might contribute to the risk of injury.
Surprisingly, a detailed anatomical description of the L-RLN course in the area of the aortic arch is missing in the literature. Precise information on L-RLN variations and their frequency might improve the ability to safely dissect lymph nodes during lung cancer surgery and might prevent L-RLN injury during lung transplantation. The aim of this study was to evaluate and document anatomical variations of the course of L-RLN in unselected fresh cadavers.
MATERIALS AND METHODS
In cooperation with the Institute of Legal Medicine, we evaluated 100 consecutive fresh cadavers between October 2017 and January 2018. All cadavers were subject to post-mortem examination at the Institute of Legal Medicine. During routine autopsy, the course of L-RLN was dissected and documented.
Description of routine autopsy and dissection of the left recurrent laryngeal nerve
All nerve dissections were performed during routine autopsy according to forensic standards. After documenting external peculiarities, a skin incision is performed from the neck to the lower abdomen and the sternum is removed at the bone-cartilage border of the ribs. Then, after opening the pericardium, the inferior vena cava is transected close to the diaphragm and the distal oesophagus clamped to avoid spillage of gastric content. Subsequently, the neck and chest organ block is removed, including tongue, larynx, thyroid gland, trachea, oesophagus, thoracic aorta and the heart and the lungs. Before continuing with inspection of the neck and chest organ block, the L-RLN was dissected. All vagal nerves and the L-RLN were dissected by opening the mediastinal pleura covering the nerves. Both nerves, left vagal and L-RLN, were identified in every cadaver. The level at which the L-RLN separated from the vagal nerve was documented in a schematic drawing. Once the course was documented, the routine autopsy was completed by inspecting the neck and chest organ block and the intra-abdominal organs and the brain. Dissection of the L-RLN did not alter the results of the routine autopsy.
Cadaver demographics were recorded to analyse any possible correlations. SPSS 24.0 was used for statistical analysis. A P-level of <0.05 was deemed significant.
Approval of the study was waived by the local ethics committee.
RESULTS
The study group consisted of 72 male and 28 female Caucasian cadavers. The median age was 63 (range 13–97) years.
All anatomical courses of L-RLNs were classified in 3 groups: the first group consisted of L-RLNs that separated from the vagal nerve above the aortic arch and crossed the aortic arch at a distance to the vagal nerve (Fig. 1). In the second group, the L-RLN split off from the vagal nerve when it was level with the aortic arch (Fig. 2). All L-RLNs that split off below the aortic arch were allocated to the third group (Fig. 3).
Figure 1:
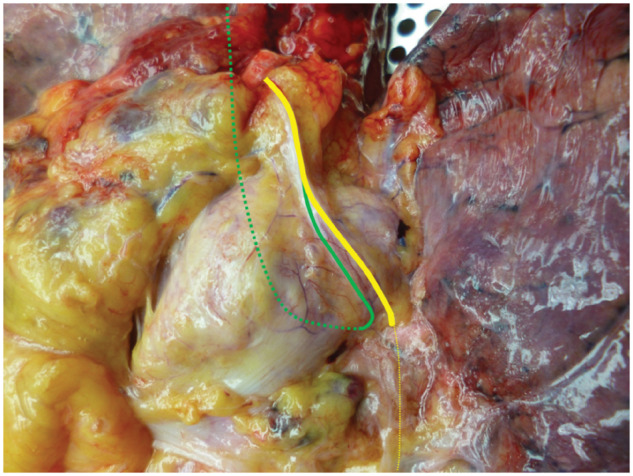
Left recurrent laryngeal nerve separating from the vagal nerve above the aortic arch. Yellow line: left vagal nerve and green line: left recurrent laryngeal nerve.
Figure 2:
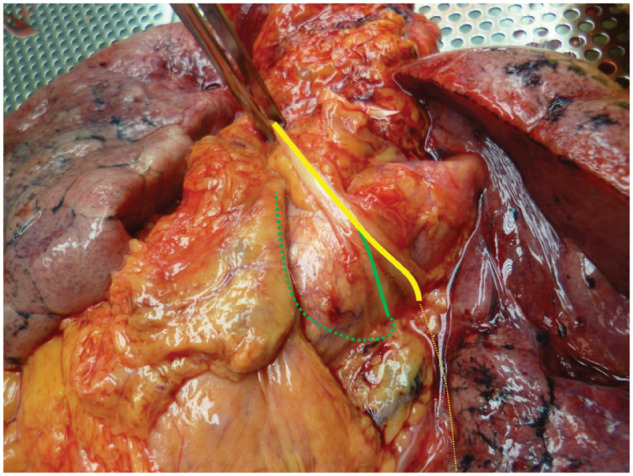
Left recurrent laryngeal nerve separating from the vagal nerve at the level of the aortic arch.
Figure 3:
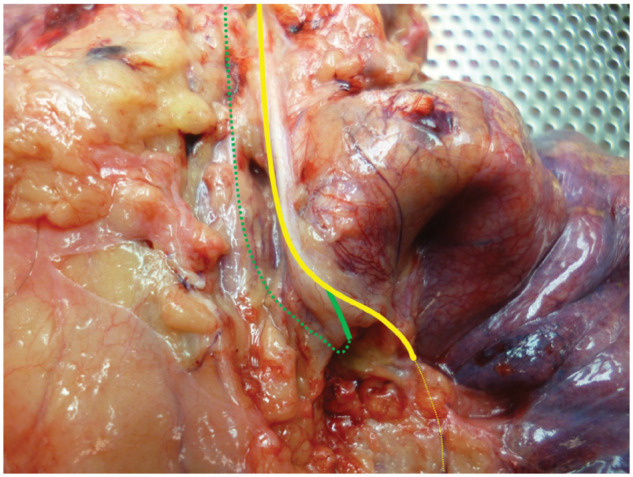
Left recurrent laryngeal nerve separating from the vagal nerve below the aortic arch.
Close to half of the L-RLNs left the vagal nerve at the level of the aortic arch (48%). Of the L-RLNs 41% split off below the aortic arch in an almost perpendicular direction. Only 11% of the L-RLNs separated above the aortic arch and crossed the aortic arch at a noticeable distance to the vagal nerve (Fig. 4).
Figure 4:
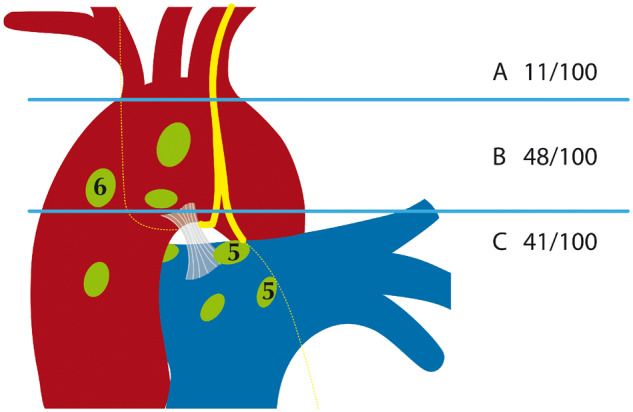
Distribution of left recurrent laryngeal nerve anatomic variability.
To assess possible correlations between the L-RLN course and demographic data, we performed a χ2 test. No association was observed between gender and L-RLN course (P = 0.386).
DISCUSSION
Injury of the RLN during thoracic surgery has a considerable impact on the early postoperative course and on long-term outcome. Nerve injury usually occurs during lymph node dissection, which should be performed in the triangle between the phrenic nerve, the vagal nerve and the aortic arch in left-sided lung cancer patients [3, 4]. In addition to procedure-related pain and functional limitations of lung parenchyma resection per se, vocal cord paralysis leads to compromised coughing, reduced mucus clearance and pneumonia [2]. Moreover, impaired vocal cord closure when swallowing may lead to (silent) aspiration and pneumonia. L-RLN injury may also affect lung transplant patients, especially cystic fibrosis patients, where the view of the hilar structures is impaired by the presence of numerous enlarged lymph nodes around the hilum. Reduced mucus clearance and risk of aspiration and aspiration pneumonia in an immuno-compromised patient may have catastrophic outcomes.
RLN injury may be caused by different mechanisms [5]. In minimally invasive thoracic surgery, 3 of these seem possible: first, nerve transection caused by sharp dissection; second, traction injury during blunt dissection; and third, thermic injury during dissection with electrocautery or other energy devices.
Thermic injury and traction injury seem to be most relevant as sharp dissection in this delicate area is usually avoided. Also, these types of injury mechanisms do not necessarily need direct contact with the nerve. Thermal spread may lead to nerve injury if energy devices are used at a distance of 2 mm or less to the nerve [6]. In a recent study by Fourdrain et al. [2], the group analysed the usefulness of a routine assessment of laryngeal lesions following lung cancer surgery. They reported a significant increase in postoperative pneumonia, a need for postoperative bronchoscopy, reintubation and a trend towards increased 90-day mortality in patients with vocal cord paralysis. Not all studies report an equally high rate of nerve injury. In the study by Fourdrain et al., the frequency of L-RLN palsy was 10% in left-sided lung cancer procedures. Seeliger et al. [7] report an incidence of 8.9% vocal cord paralysis after lung transplantation. In contrast to the study by Fourdrain et al., the Seeliger group did not find any difference in the length of hospital stay, post-transplant complications including lower respiratory tract infections within 24 months, or onset of chronic lung allograft dysfunction. Schneider et al. [1] found a 41% rate of recurrent nerve paralysis in patients with high-risk procedures including left-sided anatomic lung resections, lymph node dissection in the aorto-pulmonary window and tracheal resection. Lymph node dissection in the left paratracheal region might account for the highest risk of L-RLN injury. In contrast to this rather high rate, some authors report an almost negligible low rate of L-RLN injury despite extensive lymph node dissection in the critical areas [8].
One way to reduce the risk of nerve injury would be to avoid dissection in the area of the L-RLN if preoperative mediastinal staging with positron emission tomography–computed tomography is negative. However, nodal upstaging in clinically nodal negative patients is in the range of 10–20% [9]. Moreover, the number of dissected lymph node stations is associated with significantly more nodal upstaging [10]. Clearly, if we follow principles of oncological surgery, waiving lymph node dissection in the aorto-pulmonary window for left-sided tumours is not appropriate. The need to dissect mediastinal lymph nodes on the left side close to the L-RLN was highlighted in a recent study by Zhao et al. [11], where patients with lymph node dissection in station 4L had better disease-free and overall survival, especially for tumours >3 cm.
Once we agree on the necessity of lymph node dissection, we need to identify ways to protect the nerve. Avoiding the use of monopolar cautery or other energy devices will reduce thermic injury to the nerve. Moreover, improved anatomical knowledge of the L-RLN will help prevent injury during blunt or sharp dissection.
Our own lung cancer database revealed an L-RLN palsy rate of about 10% for left-sided procedures, depending on the lobe involved (data not shown). While left-sided paratracheal lymph nodes (station 4L) are not routinely approached during nodal dissection, lymph nodes in the aorto-pulmonary window and para-aortal nodes are dissected in every left-sided lung cancer surgery. As the technique for lymph node dissection is standardized in our unit, we hypothesized that anatomic variations in the course of the L-RLN might contribute to the risk of nerve injury.
In an effort to reduce the nerve injury rate, we attempted to find literature or figures describing the intrathoracic course of the L-RLN. Anatomic variations of the L-RLN are well known in the neck. However, documentation of the intrathoracic course in the literature is only vague.
In our study of 100 fresh cadavers, we were able to identify the nerve in every case. We grouped the variations in 3 different L-RLN courses determined according to the level at which the L-RLN separates from the vagal nerve: above the aortic arch, at the aortic arch or below the aortic arch. This grouping has a clinical impact on the route of dissection in lymph node stations 5 and 6 as follows: in almost 60% of cases, the L-RLN separates from the vagal nerve in the area of lymph node dissection or above and may cross the aortic arch at a considerable distance to the vagal nerve. Consequently, using the triangle between phrenic nerve, vagal nerve and aortic arch as landmarks for lymph node dissection would put the nerve at risk and might result in a high L-RLN palsy rate. However, there is a safe area of dissection that is defined by the ligamentum arteriosum: no L-RLN was found anterior to the ligament (Fig. 5). If dissection has to be carried out beyond that line, we would recommend that dissection be commenced from caudal, dividing the pleura covering the vagal nerve, up to the point where the L-RLN splits off (Figs 6 and 7). Once the L-RLN is identified, it is possible to preserve it, even though lymph node dissection is extended to station 4L. It goes without saying that an in-depth understanding of L-RLN anatomy will help reduce the injury rate. Using the phrenic and the vagal nerve as landmarks for lymph node dissection in left-sided lung cancer patients puts the L-RLN at risk in almost 60% of patients. Our results help clarify the intrathoracic course of the L-RLN at the level of the aortic arch, an area where lymph nodes are dissected during lung cancer surgery for appropriate staging and treatment.
Figure 5:
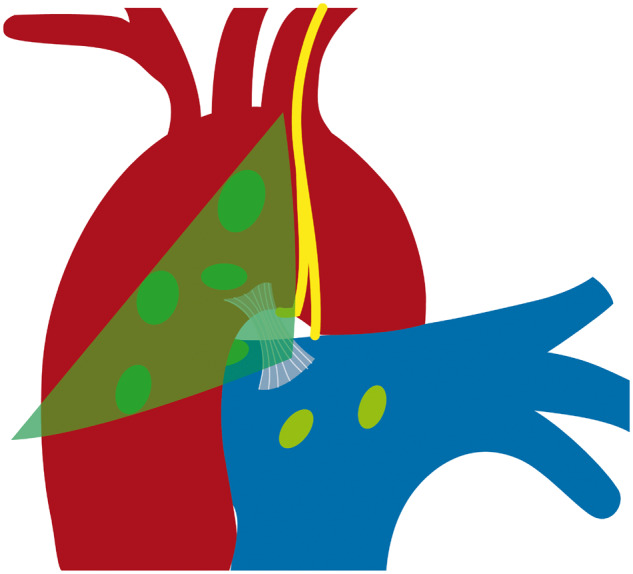
Safe triangle for lymph node dissection anterior to the ligamentum arteriosum.
Figure 6:
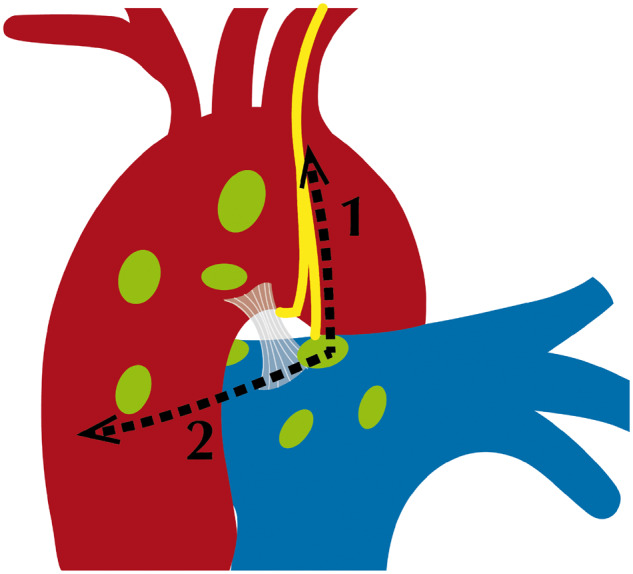
Proposed direction of dissection for lymph nodes close to the left recurrent laryngeal nerve. (1) Open the mediastinal pleura dorsal to the vagal nerve and in a cranial direction and then (2) dissect the pleura anterior to preserve the nerve.
Figure 7:
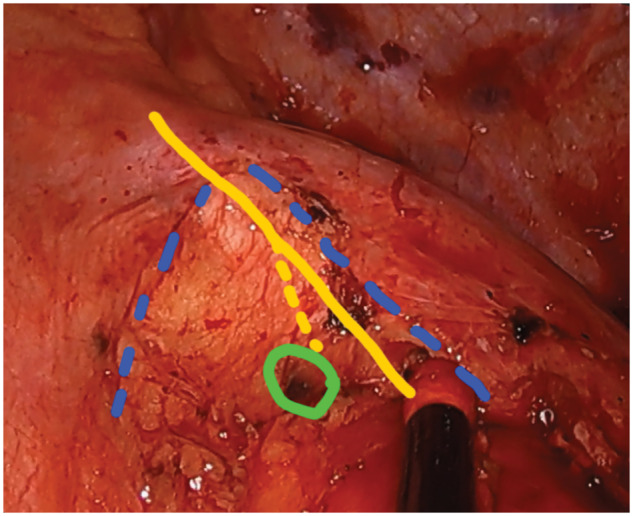
Intraoperative view during video-assisted left upper lobectomy: blue line: mediastinal pleura, yellow line: vagal nerve, yellow line dotted: left recurrent laryngeal nerve, and green line: lymph node.
ACKNOWLEDGEMENTS
The authors wish to thank Richard Scheithauer, head of the Institute of Legal Medicine Innsbruck, for his support in conducting this study. They also wish to thank Mary Heaney Margreiter for proofreading the manuscript.
Conflict of interest: none declared.
Author contributions
Caecilia Ng: Conceptualization; Data curation; Investigation; Visualization; Writing—original draft. Claudia Woess: Data curation; Investigation; Visualization; Writing—original draft. Herbert Maier: Conceptualization; Investigation; Writing—review & editing. Verena-Maria Schmidt: Data curation; Investigation; Writing—review & editing. Paolo Lucciarini: Formal analysis. Dietmar Öfner: Writing—review & editing. Walter Rabl: Data curation; Investigation; Writing—review & editing. Florian Augustin: Conceptualization; Investigation; Methodology; Project administration; Visualization; Writing—original draft; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Pascal Alexandre Thomas, Rui Haddad, Alexander Wahba and the other, anonymous reviewer(s) for their contribution to the peer review process of this article.
ABBREVIATIONS
- L-RLN
Left recurrent laryngeal nerve
- RLN
Recurrent laryngeal nerve
REFERENCES
- 1. Schneider B, Schickinger-Fischer B, Zumtobel M, Mancusi G, Bigenzahn W, Klepetko W et al. Concept for diagnosis and therapy of unilateral recurrent laryngeal nerve paralysis following thoracic surgery. Thorac Cardiovasc Surg 2003;51:327–31. [DOI] [PubMed] [Google Scholar]
- 2. Fourdrain A, De Dominicis F, Iquille J, Lafitte S, Merlusca G, Witte Pfister A et al. Usefulness of a routine endoscopic assessment of laryngeal lesions after lung cancer surgery. Respirology 2018;23:107–10. [DOI] [PubMed] [Google Scholar]
- 3. Rusch VW, Asamura H, Watanabe H, Giroux DJ, Rami-Porta R, Goldstraw P; Members of IASLC Staging Committee. The IASLC lung cancer staging project: a proposal for a new international lymph node map in the forthcoming seventh edition of the TNM classification for lung cancer. J Thorac Oncol 2009;4:568–77. [DOI] [PubMed] [Google Scholar]
- 4. Asamura H, Chansky K, Crowley J, Goldstraw P, Rusch VW, Vansteenkiste JF et al. ; International Association for the Study of Lung Cancer Staging and Prognostic Factors Committee, Advisory Board Members, and Participating Institutions. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: proposals for the revision of the N descriptors in the forthcoming 8th edition of the TNM classification for lung cancer. J Thorac Oncol 2015;10:1675–84. [DOI] [PubMed] [Google Scholar]
- 5. Chiang FY, Lu IC, Kuo WR, Lee KW, Chang NC, Wu CW. The mechanism of recurrent laryngeal nerve injury during thyroid surgery—the application of intraoperative neuromonitoring. Surgery 2008;143:743–9. [DOI] [PubMed] [Google Scholar]
- 6. Applewhite MK, White MG, James BC, Abdulrasool L, Kaplan EL, Angelos P et al. Ultrasonic, bipolar, and integrated energy devices: comparing heat spread in collateral tissues. J Surg Res 2017;207:249–54. [DOI] [PubMed] [Google Scholar]
- 7. Seeliger B, Drick N, Avsar M, Tudorache I, Welte T, Gottlieb J et al. Risk factors and outcomes of vocal cord paralysis after lung transplantation—a retrospective cohort study. Transpl Int 2019;32:626–34. [DOI] [PubMed] [Google Scholar]
- 8. Nagashima T. Thoracoscopic left mediastinal lymph node dissection. Ann Transl Med 2016;4:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Decaluwé H, Petersen RH, Brunelli A, Pompili C, Seguin-Givelet A, Gust L et al. ; MITIG-ESTS. Multicentric evaluation of the impact of central tumour location when comparing rates of N1 upstaging in patients undergoing video-assisted and open surgery for clinical stage I non-small-cell lung cancer. Eur J Cardiothorac Surg 2018;53:359–65. [DOI] [PubMed] [Google Scholar]
- 10. Zhou H, Tapias LF, Gaissert HA, Muniappan A, Wright CD, Wain JC et al. Lymph node assessment and impact on survival in video-assisted thoracoscopic lobectomy or segmentectomy. Ann Thorac Surg 2015;100:910–16. [DOI] [PubMed] [Google Scholar]
- 11. Zhao K, Wei S, Mei J, Guo C, Hai Y, Chen N et al. Survival benefit of left lower paratracheal (4L) lymph node dissection for patients with left-sided non-small cell lung cancer: once neglected but of great importance. Ann Surg Oncol 2019;26:2044–52. [DOI] [PubMed] [Google Scholar]