Ischaemic heart disease remains the leading global cause of mortality and is rising in prevalence with population growth, ageing effects and shifting epidemiological trends [1, 2].
Keywords: Omentum, Cardiac regeneration, Omental flap, Omentopexy, In vivo models, Vascularization
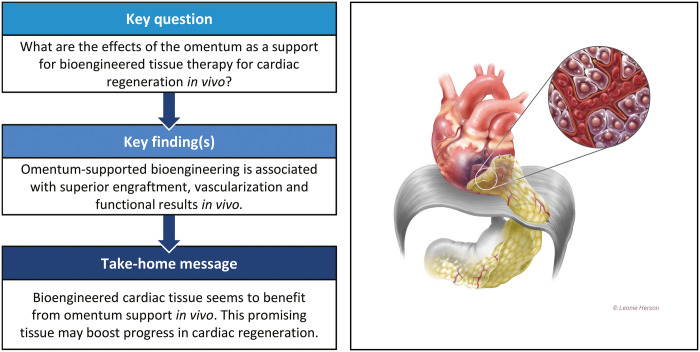
Abstract
OBJECTIVES
Preclinical in vivo studies using omental tissue as a biomaterial for myocardial regeneration are promising and have not previously been collated. We aimed to evaluate the effects of the omentum as a support for bioengineered tissue therapy for cardiac regeneration in vivo.
METHODS
A systematic scoping review was performed. Only English-language studies that used bioengineered cardio-regenerative tissue, omentum and ischaemic cardiomyopathy in vivo models were included.
RESULTS
We initially screened 1926 studies of which 17 were included in the final qualitative analysis. Among these, 11 were methodologically comparable and 6 were non-comparable. The use of the omentum improved the engraftment of bioengineered tissue by improving cell retention and reducing infarct size. Vascularization was also improved by the induction of angiogenesis in the transplanted tissue. Omentum-supported bioengineered grafts were associated with enhanced host reverse remodelling and improved haemodynamic measurements.
CONCLUSIONS
The omentum is a promising support for myocardial regenerative bioengineering in vivo. Future studies would benefit from more homogenous methodologies and reporting of outcomes to allow for direct comparison.
INTRODUCTION
Ischaemic heart disease remains the leading global cause of mortality and is rising in prevalence with population growth, ageing effects and shifting epidemiological trends [1, 2]. For end-stage heart failure patients, transplantation and mechanical circulatory assistance devices are 2 of the limited options to restore a better quality of life [3]. Donor shortage and the limited regenerative potential of myocardium have led to the recent development of numerous cell-based therapies for cardiac tissue engineering [2, 4–10].
The omentum has been used as a support for cardiac bioengineering to overcome some of the challenges in myocardial regeneration, such as poor vascularization and engraftment of bioengineered tissue [2, 11–14]. It has regenerative properties that have been exploited in surgical techniques, such as omental transposition, where the omentum is extended or wrapped around another tissue to promote healing, including the heart in cardio-omentopexy [15]. It is thought that these regenerative capabilities are linked to the presence of angiogenic factors, including vascular endothelial growth factor, basic fibroblast growth factor and an abundance of progenitor cells [16]. Its abundance of collagens, glycosaminoglycans and adhesive proteins is hypothesized to support the morphological, physiological and biochemical properties of bioengineered cardiac tissues to be more akin to native myocardium [17, 18].
Rapid preclinical progress with omental-cardiac support has not previously been collated; therefore, we conducted a systematic scoping review [19]. The primary aim was to determine what is currently known about the effectiveness of the omentum as a biomaterial in regenerative strategies for in vivo models of myocardial infarction (MI). The outcomes of interest that will be explored include: (i) engraftment of bioengineered cardiac tissues, (ii) tissue vascularization, (iii) reduction in pathological cardiac remodelling and (iv) functional cardiac and haemodynamic improvement. Gaps in the literature will be identified, and future research directions indicated.
MATERIALS AND METHODS
Eligibility criteria for initial database search
Any English-language study in a peer-reviewed journal reporting on the use of the omentum in bioengineered cardiac tissue was considered in the original database search. Only original scientific articles were included. Conference abstracts, letters, case reports, editorials without a full text and reviews were excluded.
Search strategy and screening process
The databases Embase, Medline, PubMed, Scopus and Web of Science were searched by 1 reviewer (H.W.) from inception until 6 August 2019. The search terms used were: (omentum OR oment*) AND (cardiac OR heart).
Identified studies were imported into bibliographic management software, Endnote X9 (Clarivate Analytics, Philadelphia, PA, USA), and duplicated studies were deleted. One reviewer (H.W.) screened the title and abstract of each citation. For each eligible citation, the full text was obtained and independently screened by 2 reviewers (H.W. and C.D.R.) for the assessment of full-text inclusion. Reference lists of included articles were also searched for additional studies not captured by the original search. Disagreements were resolved by discussion. The criteria for full-text inclusion were as follows:
The use of the greater omentum as a biomaterial, flap or in omentopexy;
An ischaemic cardiomyopathy model (animal and/or human tissue);
The implantation of biomaterials, including non-cardiac cell types, onto the infarcted heart; and
Implantation efficacy expressed in terms of morphological, biochemical or physiological integration with host tissue.
Data extraction
Extraction tables were used to standardize the collection of data from the included studies (Tables 1–6). One reviewer (H.W.) extracted the data initially, and the second reviewer verified the data (C.D.R.).
Table 1:
Studies which used a pedicled omental flap as support for bioengineered tissue to regenerate the myocardium
| First author | Year | In vivo model | Coronary artery for MI | Intervention interval after MI | N per groupa | Bioengineered cardiac tissue | Mode of tissue delivery |
|---|---|---|---|---|---|---|---|
| Kainuma et al. [20] | 2015 | Pig | LCA | 2 weeks | 11 | Skeletal myoblast cell sheet | Transplantation onto MI/peri-infarct area |
| Kanamori et al. [21] | 2006 | Minipig | OM1 + 2 Distal D1 | 1 h | 5 | Autologous bone marrow-derived mononuclear cells | Injection into MI/peri-infarct area |
| Kawamura et al. [22] | 2017 | Pig | LAD | 1 month | 7 | Human iPSC cardiomyocyte cell sheets | Transplantation onto MI area |
| Lilyanna et al. [23] | 2013 | Rat | LAD | 2 weeks | 11 | Fibrin graft containing cord-lining mesenchymal stem cells | Transplantation onto MI area. Attached using fibrin glue |
| Shudo et al. [24] | 2011 | Minipig | LAD | 4 weeks | 6 | Cell sheets consisting of skeletal myoblast cells | Transplantation onto MI/peri-infarct area |
| Suzuki et al. [25] | 2009 | Rat | LAD | At initial procedure | 10 | Myocardial cell sheets | Transplantation onto MI area |
| Takaba et al. [26] | 2006 | Rabbit | Cx | 4 weeks | 8 | Gelatine hydrogel sheet with bFGF applied | Transplantation onto MI area |
| Ueyama et al. [27] | 2004 | Rabbit | Cx | At initial procedure | 10 | Gelatine hydrogel sheet with bFGF applied | Transplantation onto MI area |
| Yajima et al. [28] | 2018 | Pig | LAD | 4 weeks | 6 | Gelatine compressed sponge immersed in ONO-13301ST (slow-releasing synthetic prostacyclin agonist) | Transplantation onto MI area |
| Zhang et al. [29] | 2011 | Rat | LCA | 3 weeks | 17 | Autologous tissue patch from left atrial appendage | Transplantation onto MI area |
| Zhou et al. [30] | 2010 | Rat | LCA | 8 weeks | 16 | Cell patch of polylactic acid-co-glycolic acid polymer seeded with mesenchymal stem cells | Transplantation onto MI area |
Defined as the treatment group in which both the bioengineered cardiac tissue and greater omentum were applied.
bFGF: basic fibroblast growth factor; Cx: circumflex coronary artery; D1: first diagonal artery; iPSC: induced pluripotent stem cell; LAD: left anterior descending coronary artery; LCA: left coronary artery; MI: myocardial infarction; OM1 + 2: obtuse marginal coronary artery 1 and 2.
RESULTS
Study selection and characteristics of studies
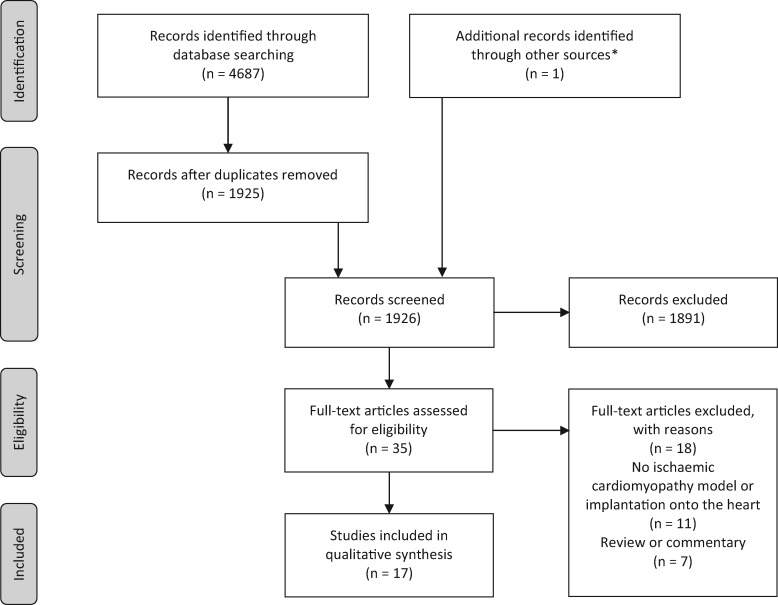
The process of study selection into the review is represented in Fig. 1, a PRISMA flowchart [37]. A total of 17 studies met the inclusion criteria. The 11 comparable studies using a pedicled omental flap technique underwent comparable data extraction (Tables 1–4). Those using non-comparable methodologies [31–33] or control groups [34–36] were separated out and are displayed in Tables 5 and 6, respectively.
Figure 1:
PRISMA flowchart of pathway for papers in the review. *Ueyama et al. [27] identified through reference list of an article accepted for full-text assessment.
Table 5:
Studies that did not use an omental pedicled flap method
| First author | Year | MI model in vivo | Coronary artery for MI | Intervention interval after MI | Subjects (n)/group | Bioengineered cardiac tissue | Method utilizing omentum | Mode of tissue delivery |
|---|---|---|---|---|---|---|---|---|
| Bourahla et al. [31] | 2010 | Sheep |
|
3 weeks | 10 | Omental cells or skeletal myoblast cells | Isolation and expansion of autologous omental mesothelial cells | Injection into MI area |
| De Siena et al. [32] | 2010 | Pig | LAD | 45 min | 13 | Human fat omentum-derived stromal cells | Isolation and expansion of human fat omentum-derived stromal cells | Injection into proximal MI border zone |
| Dvir et al. [33] | 2009 | Rat | LAD | 1 week | 11 | Alginate-based cardiac patch containing neonatal cardiac cells and pro-survival and angiogenic factors (stromal cell-derived factor-1, IGF-1, VEGF) | Cardiac patch was vascularized on the omentum | Transplantation onto MI area |
D2: second diagonal coronary artery; IGF-1: insulin-like growth factor 1; LAD: left anterior descending coronary artery; MI: myocardial infarction; VEGF: vascular endothelial growth factor.
Table 6:
Studies that did not use a control group allowing for the comparison of bioengineered tissue with or without omentum support
| Author | Year | MI model in vivo | Coronary artery for MI | Intervention interval after MI | Subjects (n)/group | Bioengineered cardiac tissue | Method utilizing omentum | Mode of tissue delivery |
|---|---|---|---|---|---|---|---|---|
| Kainuma et al. [34] | 2018 | Minipig | LAD (distal) | 4 weeks | 2 | Skeletal myoblast cell sheet | Pedicled omentum flap | Transplantation onto MI area using transphrenic peritoneoscopy-assisted omentopexy |
| Shao et al. [35] | 2008 | Rat | LAD | 30 min | 11 | Hepatic tissue resected from the left lobe of the liver | Pedicled omentum flap | Transplantation onto MI area |
| Taheri et al. [36] | 2008 | Rabbit | LAD | At initial procedure | 6 | Autologous graft using uterine segment | ‘Reinforcement’ of myometrial patches | Transplantation onto MI area |
LAD: left anterior descending coronary artery; MI: myocardial infarction.
Of the 17 selected studies, 6 used a rat MI model [23, 25, 29, 30, 33, 35], 7 used a porcine MI model [20–22, 24, 28, 32, 34], 3 used a rabbit MI model [26, 27, 36] and 1 used a sheep MI model [31].
Bioengineering cardiac tissue involved a variety of approaches, including the use of skeletal myoblast cells [20, 24, 25, 31, 34], cells derived from the omentum itself [31, 32], scaffolds for factor delivery [26–28, 33], atrial tissue [29], hepatic tissue [35], uterine tissue [36] and stem cells [21–23, 30].
Fourteen studies transplanted the bioengineered tissue onto the MI and/or peri-infarct area whereas the remaining 3 [21, 31, 32] reported the injection of cells into the same areas.
Effects of omentum support on bioengineered tissue engraftment
Measures of engraftment were reported in 9 methodologically comparable studies (those using a pedicled omental flap to support bioengineered tissue) using various metrics at various time points (Table 2). They were tested between the time period of 7 days to 3 months across these studies, with most reporting effects in 4 weeks or less after treatment.
Table 2:
Measures of engraftment outcomes of bioengineered cardiac tissue with omentum
| First author | Cell retention |
Fibre organization and contacts formed |
Infarct size, scar and wall changes |
|||
|---|---|---|---|---|---|---|
| Omentum- supported bioengineered tissue | Comparison group: bioengineered tissue no omentum support | Omentum- supported bioengineered tissue | Comparison group: bioengineered tissue no omentum support | Omentum- supported bioengineered tissue | Comparison group: bioengineered tissue no omentum support | |
| Kainuma et al. [20] | Engrafted area remaining with time | Collagen content | ||||
|
|
8% | 13% | |||
| Day 28 = 0.15 mm2 | Day 28 = 0.05 mm2 | LV wall thickness | ||||
| 912 µm | 688 µm | |||||
| Myocyte size | ||||||
| 16 µm | 20 µm | |||||
| Key findings | ∼3–4× increased area of grafted cells remained in situ with omentum supporta | Scar collagen attenuation, less thick LV wall, reduced hypertrophy with omentum support | ||||
| Kawamura et al. [22] | Cell % survival rate | Myosin heavy chain/myosin light chain-2 positive (striated filaments) | ||||
|
|
Present | Not reported | |||
| Key findings | Improved grafted cell survival with omentum supporta | Well-organized sarcomere structure in cells with omentum support (not compared to control) | ||||
| Lilyanna et al. [23] | Bioluminescence photon emission flux of labelled live donor cells (photons/s) | Scar size (LV cross sectional area % containing fibrosis) | ||||
|
|
34.7% | 35.7% | |||
| Key findings | Donor cell attrition rate in vivo over time comparable with or without omentum support | Minimal difference in scar with or without omentum support | ||||
| Shudo et al. [24] | Infarct area | |||||
| ∼6% | ∼11% | |||||
| Key findings | Infarct size (infarcted LV/total LV estimated by computer-based planimetry of Masson trichrome-staining) reduced with omentum supporta | |||||
| Suzuki et al. [25] | Cardiomyocyte survival | |||||
| 46% | 31% | |||||
| Cell sheet thickness | ||||||
| 120 μm | 70 μm | |||||
| Key findings | Improved graft survival with omentum support | |||||
| Takaba et al. [26] | Dynamic % wall thickening of infarct region | |||||
| 49% | 41% | |||||
| Key findings | % fractional wall thickening (assessed by cine MRI for quantitative wall motion) increased with omentum support | |||||
| Ueyama et al. [27] | Infarct size | |||||
| 10% | 16% | |||||
| LV circumference | ||||||
| 48 mm | 56 mm | |||||
| Scar circumference | ||||||
| 16 mm | 24 mm | |||||
| Infarct area wall thickness | ||||||
| 2.5 mm (ns) | 2.0 mm (ns) | |||||
| Key findings | Reduced infarct size, dilatation and scar. No significant difference in wall thickness | |||||
| Zhang et al. [29] | Atrial tissue patch graft presence after 4 weeks | Scar thickness | ||||
| In situ | Not seen | ∼0.4 mm (ns) | ∼0.35 mm (ns) | |||
| Infarct size | ||||||
| ∼38% (ns) | ∼39% (ns) | |||||
| Key findings | Troponin-stained graft survived with omentum support but did not without omentum support | No significant difference in scar thickness or infarct size with or without omentum supporta | ||||
| Zhou et al. [30] | Quantification PCR of grafted cellsb | Connective protein Cx-43 expressionc | Collagen (scar) density | |||
|
|
0.23 units | 0.19 units | 16% | 26% | |
| Key findings | Cell survival rate in vivo over time improved with omentum support | Higher levels of Cx-43 suggested enhanced structural coupling of transplanted cells to host myocardium. Sham group (baseline) level = 0.31; MI with no treatment group level = 0.11 | Reduced % fibrillar collagen in the infarction zone (semiquantitatively measured by picrosirius red staining under polarized light microscopy) | |||
Numerical data extrapolated from graphical figure.
Units expressed as ratio of optical density under UV light compared to reference sample at the same time.
Cx-43 protein expression determined by western blot. Units expressed as ratio to the level of β-actin which was run on all blots.
Cx-43: connexin-43; LV: left ventricle; MI: myocardial infarction; MRI: magnetic resonance imaging; ns: result not statistically significant; PCR: polymerase chain reaction; UV: ultra-violet.
Transplanted cell retention
In 6 methodologically comparable studies, cell survival was evaluated following transplantation (Table 2) [22, 23, 25, 29, 30, 34]. Only one study [23] found that the omentum had no effects in promoting cell survival. All remaining studies reported greater cell survival and/or decreased apoptosis for omentum-supported treatment compared to bioengineered tissue applied without supportive omentopexy (Table 2).
Cell markers
From all of the 17 selected studies, the most common report of a structural integration marker was the presence of connexin-43, a gap junction protein, critical for propagation of the depolarization impulse between transplanted cells and host myocardium [30, 32, 33, 36]. In 2 of these studies, a higher expression of connexin-43 was observed in omentum-supported groups compared to treatment without omentum [30, 32]. Only one paper reported on the presence of troponin-T and actinin staining to corroborate microscopic observations of distinctive bundled cardiac muscle structures in transplanted tissue [33]. However, this was not compared to their frequency in control groups.
Structural integration
Two of 17 studies described fibre organization of the bioengineered tissue [22, 33]. Omentum-supported neonatal cardiac cells in an alginate scaffold and cardiomyocyte cell sheets transplanted onto ischaemic myocardium both exhibited desirable attributes, such as striation and elongation [22, 33]. Kawamura et al. [22] reported that the omentum contributed to the further maturation of induced pluripotent stem cell-derived cardiomyocytes, characterized by larger cells with well-aligned and organized sarcomere structures with positive staining for myosin heavy chain and myosin light chain-2 in the transplanted area at 2 months after omentum-supported treatment.
Infarct size
In the 4 methodologically comparable studies examining changes in infarct size, 2 reported a decrease after omentum-supported treatment compared to the control group not using the omentum [24, 27] and 2 reported no difference [23, 29]. Omentum support was shown to increase myocardial wall thickness in 2 methodologically comparable studies [20, 26] and one that did not use a pedicled omental flap [33], although 2 studies showed no significant difference with omental flap support [27, 29]. All studies that examined percentage collagen in the myocardium demonstrated collagen attenuation, leading to decreased cardiac fibrosis, in omentum-supported treatment [20, 30, 35].
Overall results showed that omentum support had a favourable effect on the engraftment of cells for bioengineering strategies to regenerate the heart after MI.
Effects of omentum support on vascularization
Blood vessel formation
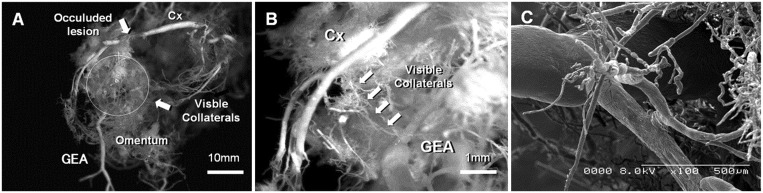
Direct blood vessel communication between the bioengineered tissue and omentum was observed in 4 methodologically comparable studies as contributing to a network of vessels that would anastomose with the host myocardium (Table 3 and Fig. 2) [20, 21, 26, 27]. Whilst most comparable studies demonstrated that support with a pedicled omental flap led to greater vessel density in the transplantation area, there were variable reports of whether arteriolar or capillary density was increased (Table 3).
Table 3:
Measures of vascularization outcomes of bioengineered cardiac tissue with omentum
| First author | Blood vessel character |
Blood vessel dynamics |
Up-regulated vascular markers in omentum-supported tissue | ||
|---|---|---|---|---|---|
| Omentum-supported bioengineered tissue | Comparison group: bioengineered tissue no omentum support or omentopexy alone | Omentum-supported bioengineered tissue | Comparison group: bioengineered tissue no omentum support or omentopexy alone | ||
| Kainuma et al. [20] | Total CD31+ endothelial cells (mature and immature vessels) | 1st branching order vessel diameter |
|
||
| ∼425 cells/mm2 | ∼275 cells/mm2 | ∼225 µm | ∼170 µm | ||
| Functionally mature vessels (CD31+/Lecithin+) | 2nd–4th branch vessel diameter | ||||
| ∼375 cells/mm2 | ∼225 cells/mm2 | No difference | No difference | ||
| Structurally mature vessels (CD31+/SMA+) | Resistance vessels (3rd–4th order) | ||||
| ∼120 cells/mm2 | ∼30 cells/mm2 | ∼2–3× more vessels | ∼2–3× fewer vessels | ||
| % Maturation (structurally mature vessels/total) | Acetylcholine challenge (resistance vessel diameter dilation) | ||||
| ∼31% | ∼12% |
|
|
||
| Gastroepiploic-coronary anastomoses | Dobutamine challenge (resistance vessel diameter constriction) | ||||
| Present | (Absent)b |
|
|
||
| Gastroepiploic-coronary anastomotic tight junctions | Global CFR change (ratio pre:post-treatment) | ||||
| Present | (Absent)b | 1.3 | 0.9 | ||
| Gastroepiploic-coronary anastomotic ink leakage | MBF (resting or stressed) | ||||
| Minimal | (Widespread)b | No difference | No difference | ||
| Key findings |
|
|
Up-regulation of multiple vascular molecular markers suggesting increased vascular cellularity with omentum support | ||
| Kanamori et al. [21] | Arteriole (>50 µm) density | Regional MBF (infarct or non-infarct wall, resting or stressed) | |||
| 27/mm2 | 18/mm2 | No difference | No difference | ||
| Capillaries (<50 µm) density | Regional MBF ratio infarct: non-infarct wall (resting or stressed) | ||||
| 109/mm2 (ns) | 88/mm2 (ns) | No difference | No difference | ||
| Gastroepiploic-coronary anastomoses via omentum-supported tissue | |||||
| Present | No comparison data | ||||
| Key findings |
|
No difference in regional MBF (assessed by spectrophotometry of coloured microsphere cardiac injection with femoral arterial blood reference sampling) with omentum support compared to bioengineered tissue without omentum support | |||
| Kawamura et al. [22] | Capillary density |
|
|||
| 111 units/mm2 | 51 units/mm2 | ||||
| Key findings | Increased capillary density at the transplanted area (assessed by semiquantitative immunohistochemistry for vWF) with omentum support | Up-regulation of markers suggesting increased endothelial cells and angiogenesis with omentum support | |||
| Lilyanna et al. [23] | Functional blood vessels as % of LV scar area | ||||
| 18% | 8% | ||||
| Structural blood vessels | |||||
| 6/hpf (400×) | 3/hpf (400×) | ||||
| Key findings | Increased vascularity with functional staining (infused DiI+ vesselsc) and structural staining (Masson’s trichrome) with omentum support | ||||
| Shudo et al. [24] | Capillary density |
|
|||
| 170/mm2 | 125/mm2 | ||||
| Key findings | Increased capillaries (anti-vWF antibody immunolabelled capillaries) with omentum support | Up-regulation of markers suggesting increased endothelial cells | |||
| Suzuki et al. [25] | Small vessels |
|
|||
| 70/hpf | 20/hpf | ||||
| Key findings | Increased small vessels observed (anti-vWF antibody immunolabelled vessels) with omentum supporta | Up-regulation of markers suggesting increased endothelial cells | |||
| Takaba et al. [26] | Arteriole (>50 µm) density | Regional MBF | |||
| 31 vessels/mm2 | 26 vessels/mm2 | 2.8 ml/min/g | 2.3 ml/min/g | ||
| Gastroepiploic-coronary anastomoses via omentum-supported tissue | Regional MBF drop on clamping gastroepiploic artery pedicle | ||||
| Present | No comparison data | 2.8–1.9 ml/min/g | No comparison data | ||
| Key findings |
|
|
|||
| Ueyama et al. [27] | Arteriole (20–100 μm) density | Subjects with LV collateral vessels on angiography via gastroepiploic artery pedicle | |||
| 23/mm2 | 14/mm2 | 7/7 | (2/7)b | ||
| Collateral vessel description | |||||
| Rich | (Poor)b | ||||
| Patent collateral vessel proportion (angiographic score) | |||||
| 0.8 | (0.1)b | ||||
| Key findings | Increased arterioles (anti-SMA antibody immunolabelled arterioles) with omentum support | Dye injection into gastroepiploic pedicle at immediate post-mortem angiography showed favourable collateral vessel patency for omentum-supported bioengineered tissue compared to omentopexy alone | Up-regulation of markers suggesting increased endothelial cells | ||
| Yajima et al. [28] | Arteriole (CD31+/SMA+) density | Global MBF | CD31 (endothelial cells) SMA (smooth muscle cells) VEGF (endothelial cells) (ns) bFGF (fibroblasts/angiogenesis) (ns) | ||
| 31/mm2 | 20/mm2 | ∼1.3 (ns) | ∼1.0 (ns) | ||
| Capillary (CD31+) density | Territorial and regional MBF | ||||
| ∼98/mm2 (ns) | ∼90/mm2 (ns) | No difference | No difference | ||
| Vessels >100 µm diameter | CFR proportional change on occlusion of Cx artery with gastroepiploic pedicle not occluded | ||||
| ∼1.5/mm2 (ns) | ∼1.2/mm2 (ns) | ∼1.0 | (∼0.7)b | ||
| Key findings | Increased arteriole (CD31+ and SMA+ vessels) density and no difference for capillaries (CD31+ vessels) or >100 µm diameter vessels in peri-infarct area with omentum supporta |
|
Up-regulation of markers suggesting increased endothelial cells | ||
| Zhang et al. [29] | Capillary (VEGF+) density | VEGF (endothelial cells) (ns) | |||
| ∼48/0.2 mm2 (ns) | ∼28/0.2 mm2 (ns) | ||||
| Key findings | No difference in capillary (VEGF+ vessels) density with omentum support versus bioengineered tissue alonea | No difference in up-regulation of VEGF | |||
| Zhou et al. [30] | Microvessel (vWF+) density | VEGF (endothelial cells) | |||
| 226/mm2 | 109/mm2 | ||||
| Key findings | Increased vessel (anti-vWF antibody immunolabelled microvessels) density with omentum support | Up-regulation of VEGF suggesting increased endothelial cells | |||
Numerical data extrapolated from graphical figure.
Comparison to bioengineered tissue without omentum support is not applicable for this assay as no connection to gastroepiploic circulation is possible in this group. Therefore control group result is for omentopexy alone (no bioengineered tissue).
DiI is DiIC18 (1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate) fluorescent dye.
Ang-1: angiopoietin 1; bFGF: basic fibroblast growth factor; CFR: coronary flow reserve; Cx: circumflex coronary artery; LV: left ventricle; MBF: myocardial blood flow; ns: result not statistically significant; PDGF-β: platelet-derived growth factor-β; PECAM: platelet endothelial cell adhesion molecule; SMA: smooth muscle actin; VEGF: vascular endothelial growth factor; vWF: von Willebrand factor.
Figure 2:
Collateral blood vessel formation between the Cx and the GEA in omentum-supported bioengineered tissue applied to the heart in a rabbit model of Cx infarction. (A) The whole specimen (scale bar = 10 mm). (B) Collateral formation between occluded Cx and GEA (scale bar = 1 mm). (C) Scanning electron micrograph of collaterals between occluded Cx and GEA. Reproduced with permission from [36]. Cx: circumflex coronary artery; GEA: gastroepiploic artery.
Of all 17 selected studies, 7 reported that arteriolar density was improved [21, 23, 25–28, 35], whilst 5 reported that capillary density had improved [22, 25, 30, 31, 35] and 2 did not specify vessel diameter [20, 33]. No negative relationship between blood vessel density and use of omentum support was reported in any study.
Angiogenic markers
Of all 17 selected studies, many corroborated the observation of increased vascularization with the up-regulated expression of genes related to angiogenesis [20, 22, 24, 25, 28–30, 33, 35]. The most commonly reported up-regulated gene in omentum-supported tissue was vascular endothelial growth factor [20, 22, 24, 25, 30, 35]. There were also reports of increased basic fibroblast growth factor [22, 35] and smooth muscle actin [28, 33].
Blood flow
Taken together, these results suggested that omentum support conveyed a proangiogenic effect. However, despite the potential for this to lead to increased myocardial blood flow or coronary flow reserve, only 2 studies in total reported that treatment supported by the omentum was superior to that of other treatment groups for blood flow [20, 26]. Two studies reported that omentum support made no significant difference to observed blood flow [21, 28].
Effects of omentum-supported bioengineered tissue on cardiac remodelling and function
Remodelling
Eight studies reported that bioengineered tissue supported with a pedicled omental flap decreased cardiac remodelling (Table 4). Seven studies reported a decrease in left ventricular end-diastolic diameter in the range of 2–25%, and 5 studies reported a decrease in left ventricular end-systolic diameter in the range of 10–27% (Table 4). For reverse remodelling, the study that reported the most beneficial effect did not involve a pedicled omental flap, but rather pre-vascularization of a cardiac patch on the omentum, supplemented with angiogenic factors, before transplanting the patch without omentopexy onto the heart [33]. Nevertheless, combining bioengineered tissue with an omental flap favoured reverse remodelling, especially at 4 weeks or later after intervention (Table 4).
Table 4:
Cardiac functional outcomes of bioengineered tissue with omentum support compared to bioengineered tissue without omentum supporta
| First author | LVEDD % decrease | LVESD % decrease | LVEF % increase | FS % increase | FAC % increase | Measurement interval after treatment |
|---|---|---|---|---|---|---|
| Kainuma et al. [20] | 10% (ns)b | 13% (ns)b | 12% (ns)b | 2 weeks | ||
| 16%b | 16%b | 24%b | 4 weeks | |||
| Kawamura et al. [22] | 5% (ns) | 1 month | ||||
| 8% (ns) | 2 months | |||||
| 25% | 26% | 16% | 3 months | |||
| Lilyanna et al. [23] | 15% (ns) | 15% (ns) | 6% (ns) | 4 weeks | ||
| Shudo et al. [24] | 24% (ns)b | 36%b | 26%b | 4 weeks | ||
| 25% (ns)b | 27%b | 22%b | 8 weeks | |||
| Suzuki et al. [25] | 0% (ns)b | 3%b | 1 week | |||
| 10% (ns)b | 8%b | 4 weeks | ||||
| 12%b | 18%b | 8 weeks | ||||
| Takaba et al. [26] | −3% (ns)b | 82% | 5% (ns)b | 4 weeks | ||
| 2% | 36% | 8 weeks | ||||
| Ueyama et al. [27] | 26%b | 26%b | 2 weeks | |||
| 21% | 41% | 4 weeks | ||||
| Yajima et al. [28] | 5% (ns) | 14% (ns) | 34% (ns) | 4 weeks | ||
| Zhang et al. [29] | 8% | 10% | 10% | 6.3% | 4 weeks | |
| Zhou et al. [30] | 13% | 12% | 13% | 11% | 4 weeks |
Data expressed as % decrease or % increase (whichever is the desirable outcome) between the absolute values for the omentum-supported and non-omentum-supported groups.
Numerical data extrapolated from graphical figure.
FAC: fractional area change; FS: fractional shortening; LVEDD: left ventricular end-diastolic diameter; LVEF: left ventricular ejection fraction; LVESD: left ventricular end-systolic diameter; ns: result not stastically significant.
Function
The most common measure of functional improvement reported was the left ventricular ejection fraction (LVEF). Omentum-supported bioengineered tissue improved the LVEF by up to 82% as a relative increase on absolute values compared to controls receiving bioengineered tissue alone (Table 4). Conversely, omentopexy alone without a bioengineered tissue was not enough to significantly improve LVEF [25, 29]. Results for fractional shortening and fractional area change were reported with less frequency than LVEF with only 3 studies reporting a significant increase in fractional shortening [26, 29, 30] and 1 study reporting an increase in fractional area change [27] with omentum support (Table 4).
DISCUSSION
This is the first review that systematically evaluates the effects of omentum support for bioengineering of cardiac tissues in MI models in vivo. Although all the included studies demonstrated that the omentum conferred a benefit in at least one of the outcomes assessed (engraftment, vascularization, remodelling, function), only a few studies reported on all outcomes. Furthermore, a few did not contain optimal control groups. This makes it difficult to draw conclusions of how effective the omentum is compared to controls or other bioengineering strategies. Our results highlight the variability of methodologies and results between studies (such as the treatment modality combined with the omentum, the model of MI and the outcome measures). This limits the extent to which the benefit of the omentum can be compared across studies.
The synergistic proangiogenic potential of omentum-supported bioengineered tissue was instrumental in most studies to promoting greater vascularization than bioengineered treatment or omentopexy alone. The development of a microvasculature between the coronary and gastroepiploic circulation was reported (Fig. 2) [20, 21, 26, 28]. The up-regulation of several angiogenic genes and proteins (e.g. vascular endothelial growth factor and smooth muscle actin) suggested that angiogenesis and vessel maturation are supported by the omentum (Table 3). However, most studies demonstrated that enhanced vascularization of the bioengineered tissue did not ultimately correlate with increased myocardial blood flow [20, 21, 28, 34]. Therefore, additional studies are needed to make progress from these results before they can be translated into clinical trials.
As shown in Table 4, bioengineered tissues with omentum support reported positive effects on cardiac function at 4 weeks in 6 studies. Suzuki et al. [25] reported an improvement at 1 week, and Kawamura et al. [22] reported an improvement at 3 months. All studies reporting a significant positive effect on function (Table 4) also reported enhanced vascularization (Table 3). Five studies reported both improved engraftment and cardiac function (Tables 2 and 4). Altogether, this suggests that both vascularization and engraftment are required for a cardiac functional improvement. Furthermore, 2 studies [25, 29] showed that the omentum by itself did not significantly improve cardiac function. Despite promising functional results, future studies would benefit from observations of long-term outcomes as some measurements, such as LVEF, have limited prognostic power in predicting clinical benefit across long time horizons.
Limitations
Limitations of this review include those inherent to the scoping review methodology, namely that other relevant studies may not have been included. Aside from those not in English, there remain innovative in vitro studies utilizing the omentum for bioengineered cardiac tissue that fell outside the scope of this review because they were not tested in vivo. Most studies captured by our scoping review used a pedicled omental flap, which is feasible in human surgery. This is perhaps why it featured so prominently and may lend itself to a smooth translation from the laboratory into clinical practice. However, only 17 publications out of 1926 were admissible for the lack of translation of in vitro work into in vivo experiments, which highlights a gap between scientists and clinicians. This should be addressed in all future studies to facilitate translating preclinical in vivo studies to human trials.
The tendency towards positive results from the studies found in this review may also present a publication bias. No studies in this review reported a detrimental effect and only a few reported no overall difference as a result of omentum support. This was despite the cardiac and diaphragmatic impairment that an omentopexy might cause in animal models. The results may also present attrition bias whereby animals that died as the result of the initial grafting procedure were not analysed. Furthermore, preclinical studies that pioneer new techniques are susceptible to scientific design weaknesses such as operator skill variability, tweaking of methods during experiments, non-randomization of animal subjects, small sample sizes and non-blinding of researchers [38]. Future in vivo experiments should explicitly address all of these points, adhering to an established experimental planning guideline, uploading protocols to un-editable repositories before work begins and including more systematic reporting on cardiac and respiratory functional outcomes beyond the LVEF.
The omentum has also been used in non-cardiac tissues for the promotion of regeneration and superior bioengineering techniques. In particular, the pedicled omental flap has been used in vivo for spinal wound repair [39] and synthetic patch reconstruction of the anterior abdominal wall [40]. Hepatocytes on biodegradable scaffolds [41] and tracheal [42] tissue have also been shown to grow successfully on the omentum. The common mechanism behind the regenerative potential of the omentum is likely due to its numerous paracrine factors and immunological mediators promoting the optimal stem cell niche [43]. A deeper understanding of the mechanisms regulating non-cardiac tissue regeneration may lead to future innovative approaches in cardiac bioengineering.
CONCLUSION
The omentum is a promising tissue for cardiac bioengineering. It has demonstrated its ability to enhance transplanted cell engraftment, vascularization and host cardiac function. The mechanisms that confer functional cardiac benefit are not fully understood and require further experimental consideration. Future studies that examine these mechanisms and outcomes would benefit from a more homogenous approach to methodology that promotes a more detailed understanding of mechanistic processes and outcomes, which is important for clinical translation.
ACKNOWLEDGEMENTS
The authors thank Yulia Ulyannikova, Academic Liaison Librarian, University of Sydney for her guidance on the design of the literature search. They also thank Leonie Herson for her work in the design and generation of the central image.
Funding
C.D.R. was supported by a Sir John Loewenthal Scholarship 2019 (University of Sydney), the Le Gros Legacy Fund New Zealand [PhD012019] and a Heart Research Australia Scholarship [PhD2019-02]. C.G. was supported by a University of Sydney Kick-Start Grant, University of Sydney Chancellor's Doctoral Incentive Programme Grant, a Sydney Medical School Foundation Cardiothoracic Surgery Research Grant, UTS Seed Funding and the Catholic Archdiocese of Sydney Grant for Adult Stem Cell Research (2019).
Conflict of interest: none declared.
Author contributions
Hogan Wang: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Resources; Software; Validation; Visualization; Writing—original draft; Writing—review & editing. Christopher D. Roche: Conceptualization; Data curation; Formal analysis; Funding acquisition; Investigation; Methodology; Project administration; Supervision; Validation; Writing—review & editing. Carmine Gentile: Conceptualization; Data curation; Funding acquisition; Methodology; Project administration; Supervision; Writing—review & editing.
Reviewer information
European Journal of Cardio-Thoracic Surgery thanks Claudia Heilmann, Luiz Felipe P. Moreira and Peter Zilla for their contribution to the peer review process of this article.
ABBREVIATIONS
- LVEF
Left ventricular ejection fraction
- MI
Myocardial infarction
REFERENCES
- 1. Naghavi M, Abajobir AA, Abbafati C, Abbas KM, Abd-Allah F, Abera SF et al. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 2017;390:1151–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Roche CD Brereton RJL Ashton AW Jackson C Gentile C. Current challenges in three-dimensional bioprinting heart tissues for cardiac surgery. Eur J Cardiothorac Surg 2020;doi: 10.1093/ejcts/ezaa093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Boilson BA, Raichlin E, Park SJ, Kushwaha SS. Device therapy and cardiac transplantation for end-stage heart failure. Curr Probl Cardiol 2010;35:8–64. [DOI] [PubMed] [Google Scholar]
- 4. Reis LA, Chiu LLY, Feric N, Fu L, Radisic M. Biomaterials in myocardial tissue engineering. J Tissue Eng Regen Med 2016;10:11–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Duan B. State-of-the-art review of 3D bioprinting for cardiovascular tissue engineering. Ann Biomed Eng 2017;45:195–209. [DOI] [PubMed] [Google Scholar]
- 6. Sui R, Liao X, Zhou X, Tan Q. The current status of engineering myocardial tissue. Stem Cell Rev Rep 2011;7:172–80. [DOI] [PubMed] [Google Scholar]
- 7. Chachques JC Lila N Soler-Botija C Martinez-Ramos C Valles A Autret G et al. Elastomeric cardiopatch scaffold for myocardial repair and ventricular support. Eur J Cardiothorac Surg 2020;57:545–55. [DOI] [PubMed] [Google Scholar]
- 8. Chachques JC Trainini JC Lago N Cortes-Morichetti M Schussler O Carpentier A. Myocardial assistance by grafting a new bioartificial upgraded myocardium (MAGNUM trial): clinical feasibility study. Ann Thorac Surg 2008;85:901–8. [DOI] [PubMed] [Google Scholar]
- 9. Menasché P Vanneaux V Hagège A Bel A Cholley B Parouchev A et al. Transplantation of human embryonic stem cell–derived cardiovascular progenitors for severe ischemic left ventricular dysfunction. J Am Coll Cardiol 2018;71:429–38. [DOI] [PubMed] [Google Scholar]
- 10. Menasché P Vanneaux V Hagège A Bel A Cholley B Cacciapuoti I et al. Human embryonic stem cell-derived cardiac progenitors for severe heart failure treatment: first clinical case report. Eur Heart J 2015;36:2011–7. [DOI] [PubMed] [Google Scholar]
- 11. Tee R, Lokmic Z, Morrison WA, Dilley RJ. Strategies in cardiac tissue engineering. ANZ J Surg 2010;80:683–93. [DOI] [PubMed] [Google Scholar]
- 12. Noor N, Shapira A, Edri R, Gal I, Wertheim L, Dvir T. 3D printing of personalized thick and perfusable cardiac patches and hearts. Adv Sci 2019;6:1900344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chachques JC, Pradas MM, Bayes-Genis A, Semino C. Creating the bioartificial myocardium for cardiac repair: challenges and clinical targets. Expert Rev Cardiovasc Ther 2013;11:1701–11. [DOI] [PubMed] [Google Scholar]
- 14. Vunjak-Novakovic G, Tandon N, Godier A, Maidhof R, Marsano A, Martens TP et al. Challenges in cardiac tissue engineering. Tissue Eng Part B Rev 2010;16:169–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. O'Shaughnessy L. Surgical treatment of cardiac ischaemia. Lancet 1937;229:185–94. [Google Scholar]
- 16. Litbarg NO, Gudehithlu KP, Sethupathi P, Arruda JA, Dunea G, Singh AK. Activated omentum becomes rich in factors that promote healing and tissue regeneration. Cell Tissue Res 2007;328:487–97. [DOI] [PubMed] [Google Scholar]
- 17. Shevach M, Soffer-Tsur N, Fleischer S, Shapira A, Dvir T. Fabrication of omentum-based matrix for engineering vascularized cardiac tissues. Biofabrication 2014;6:024101. [DOI] [PubMed] [Google Scholar]
- 18. Soffer-Tsur N, Shevach M, Shapira A, Peer D, Dvir T. Optimizing the biofabrication process of omentum-based scaffolds for engineering autologous tissues. Biofabrication 2014;6:035023. [DOI] [PubMed] [Google Scholar]
- 19. Peters MDJ, Godfrey CM, Khalil H, McInerney P, Parker D, Soares CB. Guidance for conducting systematic scoping reviews. Int J Evid Based Healthc 2015;13:141–6. [DOI] [PubMed] [Google Scholar]
- 20. Kainuma S, Miyagawa S, Fukushima S, Pearson J, Chen YC, Saito A et al. Cell-sheet therapy with omentopexy promotes arteriogenesis and improves coronary circulation physiology in failing heart. Mol Ther 2015;23:374–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kanamori T, Watanabe G, Yasuda T, Nagamine H, Kamiya H, Koshida Y. Hybrid surgical angiogenesis: omentopexy can enhance myocardial angiogenesis induced by cell therapy. Ann Thorac Surg 2006;81:160–7. [DOI] [PubMed] [Google Scholar]
- 22. Kawamura M, Miyagawa S, Fukushima S, Saito A, Miki K, Funakoshi S et al. Enhanced therapeutic effects of human iPS cell derived-cardiomyocyte by combined cell-sheets with omental flap technique in porcine ischemic cardiomyopathy model. Sci Rep 2017;7:8824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Lilyanna S, Martinez EC, Vu TD, Ling LH, Gan SU, Tan AL et al. Cord lining-mesenchymal stem cells graft supplemented with an omental flap induces myocardial revascularization and ameliorates cardiac dysfunction in a rat model of chronic ischemic heart failure. Tissue Eng Part A 2013;19:1303–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Shudo Y, Miyagawa S, Fukushima S, Saito A, Shimizu T, Okano T et al. Novel regenerative therapy using cell-sheet covered with omentum flap delivers a huge number of cells in a porcine myocardial infarction model. J Thorac Cardiovasc Surg 2011;142:1188–96. [DOI] [PubMed] [Google Scholar]
- 25. Suzuki R, Hattori F, Itabashi Y, Yoshioka M, Yuasa S, Manabe-Kawaguchi H et al. Omentopexy enhances graft function in myocardial cell sheet transplantation. Biochem Biophys Res Commun 2009;387:353–9. [DOI] [PubMed] [Google Scholar]
- 26. Takaba K, Jiang C, Nemoto S, Saji Y, Ikeda T, Urayama S et al. A combination of omental flap and growth factor therapy induces arteriogenesis and increases myocardial perfusion in chronic myocardial ischemia: evolving concept of biologic coronary artery bypass grafting. J Thorac Cardiovasc Surg 2006;132:891–9. [DOI] [PubMed] [Google Scholar]
- 27. Ueyama K, Bing G, Tabata Y, Ozeki M, Doi K, Nishimura K et al. Development of biologic coronary artery bypass grafting in a rabbit model: revival of a classic concept with modern biotechnology. J Thorac Cardiovasc Surg 2004;127:1608–15. [DOI] [PubMed] [Google Scholar]
- 28. Yajima S, Miyagawa S, Fukushima S, Sakai Y, Isohashi K, Watabe T et al. A prostacyclin agonist and an omental flap increased myocardial blood flow in a porcine chronic ischemia model. J Thorac Cardiovasc Surg 2018;156:229–41.e14. [DOI] [PubMed] [Google Scholar]
- 29. Zhang C, Hou J, Zheng S, Zheng Z, Hu S. Vascularized atrial tissue patch cardiomyoplasty with omentopexy improves cardiac performance after myocardial infarction. Ann Thorac Surg 2011;92:1435–42. [DOI] [PubMed] [Google Scholar]
- 30. Zhou Q, Zhou JY, Zheng Z, Zhang H, Hu SS. A novel vascularized patch enhances cell survival and modifies ventricular remodeling in a rat myocardial infarction model. J Thorac Cardiovasc Surg 2010;140:1388–96.e1–3. [DOI] [PubMed] [Google Scholar]
- 31. Bourahla B, Shafy A, Meilhac O, Elmadbouh I, Michel JB, Chachques JC. Mesothelial cells vs. skeletal myoblasts for myocardial infarction. Asian Cardiovasc Thorac Ann 2010;18:153–60. [DOI] [PubMed] [Google Scholar]
- 32. De Siena R, Balducci L, Blasi A, Montanaro MG, Saldarelli M, Saponaro V et al. Omentum-derived stromal cells improve myocardial regeneration in pig post-infarcted heart through a potent paracrine mechanism. Exp Cell Res 2010;316:1804–15. [DOI] [PubMed] [Google Scholar]
- 33. Dvir T, Kedem A, Ruvinov E, Levy O, Freeman I, Landa N et al. Prevascularization of cardiac patch on the omentum improves its therapeutic outcome. Proc Natl Acad Sci USA 2009;106:14990–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kainuma S, Nakajima K, Miyagawa S, Fukushima S, Saito A, Harada A et al. Novel regenerative therapy combined with transphrenic peritoneoscopy-assisted omentopexy. Interact CardioVasc Thorac Surg 2018;26:993–1001. [DOI] [PubMed] [Google Scholar]
- 35. Shao ZQ, Kawasuji M, Takaji K, Katayama Y, Matsukawa M. Therapeutic angiogenesis with autologous hepatic tissue implantation and omental wrapping. Circ J 2008;72:1894–9. [DOI] [PubMed] [Google Scholar]
- 36. Taheri SA, Yeh J, Batt RE, Fang Y, Ashraf H, Heffner R et al. Uterine myometrium as a cell patch as an alternative graft for transplantation to infarcted cardiac myocardium: a preliminary study. Int J Artif Organs 2008;31:62–7. [DOI] [PubMed] [Google Scholar]
- 37. Moher D, Liberati A, Tetzlaff J, Altman DG; The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Sade RM, Rylski B, Swain JA, Entwistle JWC, Ceppa DP, Blitzer D et al. ; Members of the Cardiothoracic Ethics Forum who contributed to this work. Transatlantic editorial: institutional investigations of ethically flawed reports in cardiothoracic surgery journals. Eur J Cardiothorac Surg 2020;57:617–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Sambri A, Gasbarrini A, Cialdella S, De Iaco P, Boriani S. Pedicled omental flaps in the treatment of complex spinal wounds after en bloc resection of spine tumors. J Plast Reconstr Aesthet Surg 2017;70:1267–71. [DOI] [PubMed] [Google Scholar]
- 40. Uchibori T, Takanari K, Hashizume R, Amoroso NJ, Kamei Y, Wagner WR. Use of a pedicled omental flap to reduce inflammation and vascularize an abdominal wall patch. J Surg Res 2017;212:77–85. [DOI] [PubMed] [Google Scholar]
- 41. Lee H, Cusick RA, Utsunomiya H, Ma PX, Langer R, Vacanti JP. Effect of implantation site on hepatocytes heterotopically transplanted on biodegradable polymer scaffolds. Tissue Eng 2003;9:1227–32. [DOI] [PubMed] [Google Scholar]
- 42. Li J, Xu P, Chen H, Yang Z, Zhang Q. Improvement of tracheal autograft survival with transplantation into the greater omentum. Ann Thorac Surg 1995;60:1592–6. [DOI] [PubMed] [Google Scholar]
- 43. Behfar A, Crespo-Diaz R, Terzic A, Gersh BJ. Cell therapy for cardiac repair—lessons from clinical trials. Nat Rev Cardiol 2014;11:232–46. [DOI] [PubMed] [Google Scholar]