Abstract
Background. Atrial fibrillation (AF) increases the risk for stroke but also for non-stroke major adverse cardiovascular events (MACE). The 2MACE score was recently proposed to predict these events. Since the interest of microRNAs (miRNAs) in cardiovascular diseases is increasing, we aimed to investigate whether miRNA levels may improve the predictive performance of the 2MACE score. Methods. We included consecutive AF patients stable on vitamin K antagonist therapy. Blood samples were drawn at baseline and plasma expression of miRNAs was assessed. During a median of 7.6 (interquartile range (IQR) 5.4–8.0) years, the occurrence of any MACE (nonfatal myocardial infarction/cardiac revascularization and cardiovascular death) was recorded. Results. We conducted a miRNA expression analysis in plasma from 19 patients with and without cardiovascular events. The miRNAs selected (miR-22-3p, miR-107, and miR-146a-5p) were later measured in 166 patients (47% male, median age 77 (IQR 70–81) years) and all were associated with a higher risk of MACE. The addition of miR-107 and miR-146a-5p to the 2MACE score significantly increased the predictive performance (c-indexes: 0.759 vs. 0.694, p = 0.004), and the model with three miRNAs also improved the predictive performance compared to the original score (c-indexes: 0.762 vs. 0.694, p = 0.012). 2MACE models with the addition of miRNAs presented higher net benefit and potential clinical usefulness. Conclusions. Higher miR-22-3p andmiR-107 and lower miR-146a-5p levels were associated with a higher risk of MACE. The addition of these miRNAs to the 2MACE score significantly increased the predictive performance for MACE, which may aid to some extent in the decision-making process about risk stratification in AF.
Keywords: miRNAs, atrial fibrillation, risk stratification, adverse cardiovascular event, 2MACE
1. Introduction
Atrial fibrillation (AF) is the most frequent cardiac arrhythmia and it implies a high morbidity and mortality [1,2]. There is evidence supporting the presence of a prothrombotic or hypercoagulable state in AF, which is associated with an increased risk of stroke and thromboembolism [3]. However, a non-negligible proportion of patients suffer from adverse cardiovascular events regardless of stroke, including myocardial infarction (MI) [4]. For this reason, the 2MACE score was proposed to predict major adverse cardiovascular events (MACE; MI, cardiac revascularization, and cardiovascular death) in AF patients [5], and external validations have shown promising results [6,7].
On the other hand, the study of biomarkers as prognostic or risk stratification markers has gained attention. In this context, microRNAs (miRNAs) are post-transcriptional regulators that have been widely recognized as active effectors of the cardiovascular system [8]. The recognition of miRNAs as plasma biomarkers of cardiovascular disease is being consolidated because their collect is minimally invasive, only a blood sample is required, and they are remarkably stable in the bloodstream, and sensitive to acute or chronic environmental alterations [9,10]. In addition to their great accessibility, circulating miRNAs are relatively easy to measure [11]. While a few works have reported profiles of circulating miRNAs as predictors for cardiovascular events in patients presenting MI [12], coronary artery disease [13], or acute coronary syndrome [14], their role as prognostic markers in AF is still unknown.
In the present study, we aimed to determine a profile of circulating miRNAs with the ability to predict MACE in AF, and to assess whether plasma miRNA levels can improve the predictive performance of the 2MACE score in a “real-world” cohort of AF patients under vitamin K antagonist (VKA) therapy.
2. Methods
We included a prospective cohort of permanent/paroxysmal AF patients from our outpatient anticoagulation clinic in a tertiary hospital (Murcia, southeast of Spain). All patients were on VKA therapy with stable international normalized ratios (INR 2.0–3.0) during at least the previous 6 months. This 6-month period of good anticoagulation control with VKA before inclusion was required to have a baseline homogeneity that would allow us to avoid the potential bias of poor anticoagulation control on clinical outcomes. Patients with prosthetic heart valves, rheumatic AF, or patients with acute coronary syndrome, stroke (ischemic or embolic), unstable chest pain, hemodynamic instability, hospital admission, or surgical intervention in the preceding 6 months were excluded.
The recruitment period was from May 2007 to December 2007. At inclusion, a complete medical history was recorded, and stroke risk (CHA2DS2-VASc) and bleeding risk (HAS-BLED) were estimated. The time in the therapeutic range at 6 months after entry was calculated using the Rosendaal method.
All subjects who met eligibility criteria were enrolled after providing written informed consent. The study was approved by the ethical committee of our institution (code: AVAL03/11) and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its subsequent amendments.
2.1. Assessment of the 2MACE Score
The baseline 2MACE score was retrospectively calculated in all patients as described by Pastori et al. [5]. This score was described as a simple risk score to identify AF patients with a high residual risk of cardiovascular events and to improve cardiovascular risk stratification. The 2MACE score includes 2 points for metabolic syndrome and age ≥75, and 1 point for MI/revascularization, congestive heart failure (ejection fraction ≤40%) and thromboembolism (stroke/transient ischemic attack). The original publication stated that the 2MACE score was proposed to predict MACE (i.e., any of the following: MI, cardiac revascularization, and cardiovascular death) in AF patients.
2.2. Blood Samples Collection and miRNome Analysis
Blood samples were atraumatically drawn at study entry and without stasis into syringes preloaded with trisodium citrate (0.011 mol/L), i.e., all patients were stable under VKA therapy for at least 6 months when the blood sample was collected. Samples were centrifuged at 2200× g and 4 °C for 10 min, and the supernatants were stored in aliquots at −80 °C until further use. miRNAs from plasma were purified using the Nucleo Spin miRNA Plasma Kit (Macherey–Nagel) in accordance with the manufacturer’s protocol. Plasma miRNAs were screened using the plasma/serum focus microRNA PCR Panel V4 (Exiqon) composed of 179 miRNAs as has been previously described [15]. Data were normalized by cycle threshold (Ct) mean value for miR-103a and miR-191-5p, which showed a high correlation (Pearson correlation) with both the global geometrical mean value and the top 20 expressed miRNA geometric mean values, resulting in an r = 0.8607 (p-value < 0.0001) and r = 0.8902 (p-value < 0.0001), respectively. Plasma expression of miRNAs was calculated using the 2−ΔCt method.
2.3. Follow-Up and Endpoints
The primary endpoint was the occurrence of any MACE (the composite of nonfatal MI or cardiac revascularization and cardiovascular death (death caused by sudden death, progressive congestive heart failure, fatal MI, or procedure-related death), during the follow-up period. We excluded from MACE all embolic events; i.e., stroke, transient ischemic attack, and peripheral or systemic embolism.
The median follow-up was 7.6 (interquartile range (IQR) 5.4–8.0) years. Follow-up was performed through routine visits to the anticoagulation clinic, the hospital electronic medical records, or, when unavailable, by telephone interview. No specific visits were performed regarding the study. Of note, no patient was lost to follow-up. The investigators identified, confirmed, and recorded all adverse events and outcomes.
2.4. Statistical Analysis
Continuous variables were presented as mean±SD or median (interquartile range (IQR)), according to the Kolmogorov–Smirnov test. Categorical variables were presented as absolute frequencies and percentages. The Pearson χ2 test was used to compare frequencies and comparisons of miRNA levels were analyzed using the Mann–Whitney U test or the Student t-test, as appropriate.
Cox regression analyses were performed to investigate the association of each miRNA with the risk of MACE.
Receiver operating characteristic curves were used to evaluate the predictive ability (expressed as c-indexes) of the original 2MACE score and the miRNA-modified ones. The cut-off point of each miRNA with the best combination of specificity and sensitivity was assessed by the Youden index. Comparisons of receiver operating characteristic curves were performed as described by DeLong et al. [16].
Discrimination and reclassification performances were evaluated by calculating the integrated discrimination improvement (IDI) and the net reclassification improvement (NRI), according to the methods of Pencina et al. [17].
The clinical usefulness and the net benefit of the original score in comparison with the miRNA-modified scores was estimated by using the decision curve analysis (DCA), as was proposed by Vickers et al. [18]. The DCA shows the clinical usefulness of each new model based on a continuum of potential thresholds for adverse events (x axis) and the net benefit of using the model to stratify patients at risk (y axis) relative to assuming that no patient will have an adverse event. Here, those models that are the farthest away from the slanted dashed line (i.e., assumes all MACEs) and the horizontal line (i.e., assumes no MACE) at a particular threshold probability demonstrate the higher net clinical benefit.
A p-value < 0.05 was accepted as statistically significant. Statistical analyses were performed using SPSS 21.0 (SPSS Inc., Chicago, IL, USA), MedCalc v. 16.4.3 (MedCalc Software bvba, Ostend, Belgium), STATA v. 12.0 (Stata Corp, College Station, TX, USA), and survIDINRI package for R v. 3.3.1 for Windows.
3. Results
3.1. Pilot Study
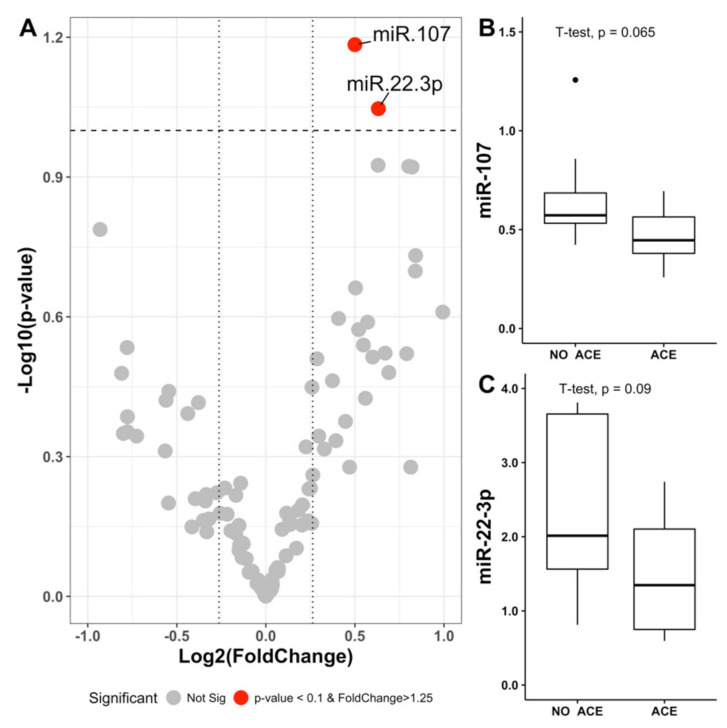
To test if miRNA levels were associated with the development of cardiovascular events, we firstly conducted a miRNA expression pattern analysis in plasma from 9 patients with and 10 patients without cardiovascular events. Since ischemic stroke is the classic main efficacy outcome in AF, we initially selected this endpoint to homogenize the samples. As shown in Supplementary Table S1, cases with stroke and controls without stroke were matched and no clinical differences between these two groups were observed. Of the 178 miRNAs on the panel, we selected those that were detected in all the samples (n = 110) for further analysis. Assuming the criteria of: (i) Fold change in log2 greater than 1.25 and (ii) a statistical significance level of 0.1, due to the limited number of samples; differences of plasma miRNA levels between cases and controls were found only for miR-22-3p and miR-107 (Figure 1). In addition to the two miRNAs selected from this pilot study, we also included miR-146a-5p in the validation study due to its role in the development of cardiovascular events in AF patients [19,20].
Figure 1.
Levels and selection of miRNAs in the pilot study. (A) Volcano plot representing the fold change values in logarithm of base 2 (X-axis) and the p-values in logarithm of base 10 (Y-axis). (B,C) Box plots representing the miRNA levels selected for meeting the criteria of p-value less than 0.1 and fold change greater than 1.25. ACE = adverse cardiovascular events.
3.2. Validation Study
The three miRNAs selected were measured in 166 patients (47% male, median age 77 (IQR 70–81) years). The median CHA2DS2-VASc and HAS-BLED were 4 (IQR 3–5) and 2 (IQR 2–3), respectively, whereas the median 2MACE score was 2.5 (IQR 1–4). A summary of baseline clinical characteristics is shown in Table 1.
Table 1.
Validation study cohort baseline clinical characteristics.
| Overall N = 166 |
Patients Without MACE N = 117 |
Patients with MACE N = 49 |
p | |
|---|---|---|---|---|
| Demographic | ||||
| Male sex, n (%) | 78 (47.0) | 54 (46.2) | 24 (49.0) | 0.739 |
| Age (years), median (IQR) | 77 (70–81) | 74 (68–79) | 80 (77–84) | <0.001 |
| Comorbidities, n (%) | ||||
| Hypertension | 140 (84.3) | 96 (82.1) | 44 (89.8) | 0.210 |
| Diabetes mellitus | 41 (24.7) | 25 (21.4) | 16 (32.7) | 0.124 |
| Heart failure | 68 (41.0) | 37 (31.6) | 31 (63.3) | 0.001 |
| History of stroke/TIA/thromboembolism | 32 (19.3) | 17 (14.5) | 15 (30.6) | 0.016 |
| Renal impairment | 13 (7.8) | 6 (5.1) | 7 (14.3) | 0.045 |
| Coronary artery disease | 36 (21.7) | 22 (18.8) | 14 (28.6) | 0.163 |
| Hypercholesterolemia | 54 (32.5) | 41 (35.0) | 13 (26.5) | 0.286 |
| Current smoking habit | 26 (15.7) | 12 (10.3) | 14 (28.6) | <0.01 |
| Current alcohol consumption | 3 (1.8) | 3 (2.6) | 0 (0.0) | 0.622 |
| History of previous bleeding | 12 (7.2) | 5 (4.3) | 7 (14.3) | 0.052 |
| Concomitant treatment, n (%) | ||||
| Amiodarone | 13 (7.8) | 10 (8.5) | 3 (6.1) | 0.596 |
| Digoxin | 28 (16.9) | 17 (14.5) | 11 (22.4) | 0.214 |
| Calcium antagonist | 41 (24.7) | 24 (20.5) | 17 (34.7) | 0.053 |
| Beta-blockers | 53 (31.9) | 39 (33.3) | 14 (28.6) | 0.548 |
| Statins | 35 (21.1) | 27 (23.1) | 8 (16.3) | 0.331 |
| Diuretics | 81 (48.8) | 52 (44.4) | 29 (59.2) | 0.083 |
| Antiplatelet therapy | 25 (15.1) | 16 (13.7) | 9 (18.4) | 0.441 |
| ACE inhibitors/ARBs | 80 (48.2) | 51 (43.6) | 29 (59.2) | 0.067 |
| TTR at 6 months of entry, n (%) | 80 (60–100) | 80 (60–100) | 80 (60–83) | 0.250 |
| CHA2DS2-VASc score, median (IQR) | 4 (3–5) | 4 (3–5) | 5 (4–6) | <0.001 |
| HAS-BLED score, median (IQR) | 2 (2–3) | 2 (2–3) | 3 (2–3) | <0.001 |
ACE inhibitors = angiotensin-converting-enzyme inhibitors; ARBs = angiotensin II receptor blockers; IQR = interquartile range; TIA = transient ischemic attack; TTR = time in therapeutic range.
We first aimed to find an association between the levels of these three miRNAs and MACE in the cohort of 166 patients. During a median of 7.6 (IQR 5.4–8.0) years, 49 (29.5%; annual rate 3.88%/year) patients suffered a MACE. As shown in Table 2, all miRNAs were associated with the risk of MACE. Hence, miR-22-3p presented a hazard ratio (HR) of 1.07 (95% CI 1.02–1.14), miR-107 presented an HR of 3.66 (95% CI 1.19–11.24), and miR-146a-5p showed an inverse association with MACE, with an HR of 0.86 (95% CI 0.74–0.99).
Table 2.
Crude risk of major adverse cardiovascular events (MACE) according to miRNAs.
| HR | 95% CI | p-Value | |
|---|---|---|---|
| miR-22-3p | 1.07 | 1.02–1.14 | 0.013 |
| miR-107 | 3.66 | 1.19–11.24 | 0.023 |
| miR-146a-5p | 0.86 | 0.74–0.99 | 0.042 |
HR = hazard ratio; CI = confidence interval.
When the predictive ability for MACE was tested, receiver operating characteristic (ROC)curve analyses demonstrated that miR-22-3p and miR-107 presented poor c-indexes (0.523; 95% CI 0.431–0.632 and 0.555; 95% CI 0.476–0.632, respectively) whereas miR-146a-5p exhibited a moderate c-index (0.656; 95% CI 0.578–0.728). In order to test if any of these miRNAs could enhance the predictive ability of the 2MACE score, we identified cut-off points with the best combination of sensitivity and specificity. The best cut-offs were 0.191, 0.137, and 1.979 for miR-22-3p, miR-107, and miR-146a-5p, respectively. Therefore, patients with miR-22-3p or miR-107 levels over the cut-off points and patients with miR-146a-5p levels under the cut-off point were categorized as being at “high risk” of MACE.
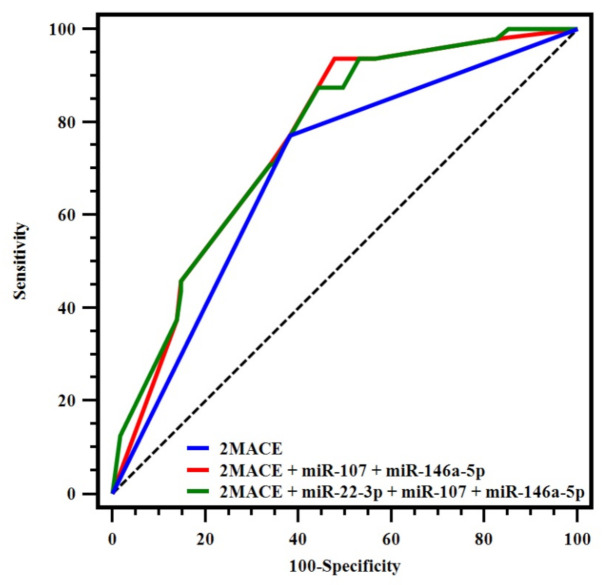
Combinations of miRNAs were then included in the 2MACE score, according to the previously established miRNA risk category. Thus, the addition of miR-107 and miR-146a-5p to the 2MACE significantly increased the predictive performance (c-indexes: 0.759 vs. 0.694, p = 0.004). Although miR-22-3p alone showed a poor c-index, we also tested a model with this miRNA in addition to miR-107 and miR-146a-5p into the 2MACE score. Similarly, this model also improved the predictive performance of the original 2MACE (0.762 vs. 0.694, p = 0.012). In both models of miRNAs, the sensitivity was also enhanced, as is shown by the results of IDI (Table 3, Figure 2).
Table 3.
C-indexes, c-indexes comparison, integrated discrimination improvement (IDI), and net reclassification improvement (NRI) after the addition of miRNAs to the 2MACE score.
| C-index | 95% CI | Z Score * | p * | IDI | 95% CI | p | NRI | 95% CI | p | |
|---|---|---|---|---|---|---|---|---|---|---|
| 2MACE | 0.694 | 0.617–0.764 | ||||||||
| + miR-107 + miR-146a-5p | 0.759 | 0.686–0.822 | 2.876 | 0.004 | 0.053 | 0.011/0.096 | 0.014 | 0.345 | −0.327/0.518 | 0.736 |
| + miR-107 + miR-146a-5p+ miR-22-3p | 0.762 | 0.689–0.825 | 2.518 | 0.012 | 0.056 | 0.012/0.101 | 0.015 | 0.047 | −0.274/0.519 | 0.627 |
* for C-index comparison. CI = confidence interval; IDI = integrated discrimination improvement; NRI = net reclassification improvement.
Figure 2.
Receiver operating characteristic-curves for the 2MACE score and the 2MACE models with the addition of miRNAs.
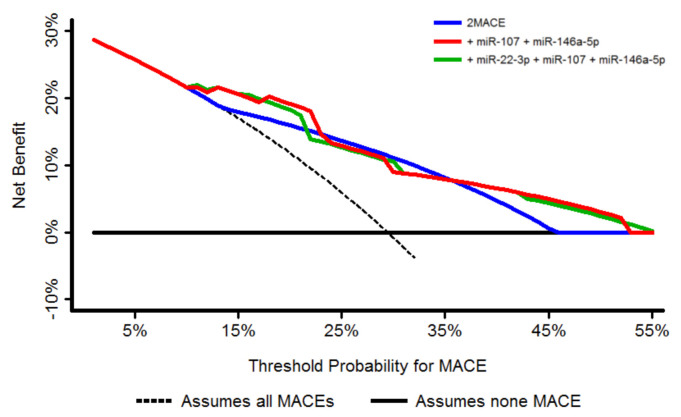
Finally, a DCA was plotted, showing that both 2MACE models with the addition of miRNAs presented higher net benefit and clinical usefulness compared to the original 2MACE for a large threshold of probabilities (from 10 to 25% and 35 to 55%) (Figure 3).
Figure 3.
Decision curve analysis for the 2MACE score and the 2MACE models with the addition of miRNAs. MACE = major adverse cardiovascular event.
4. Discussion
In this study including steadily anticoagulated AF patients taking VKAs, we observed that plasma levels of miR-22-3p, miR-107, and miR-146a-5p were associated with a higher risk of MACE. Importantly, the predictive ability of the 2MACE score for this event was enhanced with the addition of miRNAs.
During the last years, the interest of miRNAs in AF is increasing [21,22,23]. Despite most of the evidence suggesting a relationship of miRNAs with incident AF or recurrent AF after ablation [24,25,26,27], the therapeutic potential of miRNAs in AF has also emerged [28,29].
For example, miR-22-3p showed to play a role in several cardiovascular diseases [30]. Thus, a recent study has demonstrated that miR-22-3p plasma levels tend to be higher in patients with AF and a high CHA2DS2-VASc score. This suggests that this miRNA is more expressed in patients with high thromboembolic risk, which could provide some information about the pathophysiological conditions of AF patients [31]. Previously, elevated miR-22-3p serum levels have been shown in patients with heart failure, suggesting that high miR-22-3p circulating levels in serum may reflect intracellular levels in certain tissues and thus may explain the potential functional effect of the miRNA [32]. This is of particular importance since different in vivo models have shown that miR-22-3p overexpression promotes heart hypertrophy while downregulation protects mice from the pathology [33,34]. It is thus tempting to speculate that the higher levels of miR-22-3p in patients with MACE may induce a functional effect in tissues, still to be determined, and therefore may not act only as a mere biomarker of comorbidities.
In another recent study, miR-107 was investigated in AF patients undergoing catheter ablation. Although this miRNA was downregulated in sera from patients with AF recurrence compared to patients without AF recurrence, differences in expression were not significant [35]. However, the role of miR-107 in cardiovascular diseases is not well documented.
There is, however, more prognosis information regarding miR-146a-5p. A preliminary analysis of patients from this cohort at ~3 years of follow-up already showed an association of miR-146a-5p with adverse cardiovascular events (any of the following: Stroke/transient ischemic attack, systemic embolism, MI, and acute heart failure). In that study, lower levels of miR-146a-5p due to the presence of a functional single-nucleotide polymorphism rs2431697 were related to poor prognosis [20]. These data have been later confirmed at ~8 years, where miR-146a-5p was suggested as a regulator of neutrophil extracellular trap formation, and thus associated with cardiovascular risk [19]. Then, it could be hypothesized that dysregulation of miRNAs involved in inflammation may be associated with a higher risk of adverse events in patients with AF [36,37].
Nevertheless, the clinical usefulness of biomarkers when they are evaluated alone may be only limited. In fact, a common criticism of biomarkers is their high variability and lack of specificity in different clinical settings [38,39,40]. However, an advantage of miRNAs compared to other biomarkers is their plasma stability, which makes them less likely to be altered due to acute conditions [9,41], even though results from miRNAs levels should be interpreted with caution and contextualized according to clinical risk factors. In the present analysis, we considered this approach and included several miRNAs into the 2MACE score, which is a clinical score encompassing traditional risk factors for MI. In a similar way, a previous study also suggested that the use of a scoring system that would incorporate both circulating biomarkers and clinical factors might be more useful [42].
Limitations
There are some limitations that we aim to acknowledge. First, this study was performed in a single center, and it was composed of a Caucasian-based population. Second, all patients were hemodynamically stable and had good anticoagulation control during the previous 6 months after entry, in an attempt to homogenize the sample and avoid the potential impact of acute conditions or poor anticoagulation control in the development of adverse events. Therefore, our results might not be extrapolated to different clinically contexts. In addition, our study is limited only to AF patients treated with VKAs, since all patients were under this therapy at inclusion. Although VKAs are still the most commonly prescribed oral anticoagulants in Spain, the use of direct-acting oral anticoagulants (DOACs) is increasing worldwide. Thus, our results require further validation in AF patients under VKAs and DOACs in order to broaden their clinical usefulness. Finally, we only measured miRNAs at inclusion, and no other determinations have been performed throughout the follow-up. Comorbidities change over time and how miRNAs could evolve during the follow-up period has not been determined. For the above reason, further studies are warranted to confirm our results.
5. Conclusions
In this study including AF patients taking VKAs, we found that higher miR-22-3p and miR-107 and lower miR-146a-5p plasma levels were associated with higher risk of MACE at follow-up. The addition of these miRNAs to the 2MACE score significantly increased the predictive performance for MACE and enhanced its clinical usefulness. A combination of miRNAs and clinical risk factors could aid to some extent in the decision-making process about risk stratification in patients with AF.
Supplementary Materials
The following are available online at https://www.mdpi.com/2077-0383/9/11/3645/s1, Table S1: Pilot study cohort baseline clinical characteristics.
Author Contributions
Data curation, J.M.R.-C. and R.T.-M.; formal analysis, J.M.R.-C. and R.T.-M.; funding acquisition, R.G.-C. and C.M.; investigation, A.M.d.l.R.-G., S.Á., and M.P.F.-P.; methodology, R.C.-R., J.A.C.-M., L.Z.-M., and N.G.-B.; resources, L.R.-G.; supervision, R.G.-C. and C.M.; writing–original draft, J.M.R.-C. and R.T.-M.; writing–review and editing, V.R., V.V., F.M., R.G.-C., and C.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by research grants from Instituto de Salud Carlos III, Fondo Europeo de Desarrollo Regional ((P17/0051), (PFIS18/0045: A.M. de los Reyes-García) (CD18/00044: S. Águila) (CM19/00037: L. Reguilón-Gallego) and Fundación Séneca (19873/GERM/15 and 20644/JLI/18).
Conflicts of Interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Lip G., Freedman B., de Caterina R., Potpara T.S. Stroke prevention in atrial fibrillation: Past, present and future. Comparing the guidelines and practical decision-making. Thromb. Haemost. 2017;117:1230–1239. doi: 10.1160/TH16-11-0876. [DOI] [PubMed] [Google Scholar]
- 2.Hindricks G., Potpara T., Dagres N., Arbelo E., Bax J.J., Blomström-Lundqvist C., Boriani G., Castella M., Dan G.A., Dilaveris P.E., et al. 2020 ESC Guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association of Cardio-Thoracic Surgery (EACTS) Eur. Heart J. 2020 doi: 10.1093/eurheartj/ehaa612. [DOI] [PubMed] [Google Scholar]
- 3.Khan A.A., Lip G.Y.H. The prothrombotic state in atrial fibrillation: Pathophysiological and management implications. Cardiovasc. Res. 2019;115:31–45. doi: 10.1093/cvr/cvy272. [DOI] [PubMed] [Google Scholar]
- 4.Violi F., Soliman E.Z., Pignatelli P., Pastori D. Atrial fibrillation and myocardial infarction: A systematic review and appraisal of pathophysiologic mechanisms. J. Am. Heart Assoc. 2016;5:e003347. doi: 10.1161/JAHA.116.003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pastori D., Farcomeni A., Poli D., Antonucci E., Angelico F., Del Ben M., Cangemi R., Tanzilli G., Lip G.Y., Pignatelli P., et al. Cardiovascular risk stratification in patients with non-valvular atrial fibrillation: The 2MACE score. Intern. Emerg. Med. 2016;11:199–204. doi: 10.1007/s11739-015-1326-1. [DOI] [PubMed] [Google Scholar]
- 6.Polovina M., Đikić D., Vlajković A., Vilotijević M., Milinković I., Ašanin M., Ostojić M., Coats A.J.S., Seferović P.M. Adverse cardiovascular outcomes in atrial fibrillation: Validation of the new 2MACE risk score. Int. J. Cardiol. 2017;249:191–197. doi: 10.1016/j.ijcard.2017.09.154. [DOI] [PubMed] [Google Scholar]
- 7.Rivera-Caravaca J.M., Marín F., Esteve-Pastor M.A., Raña-Míguez P., Anguita M., Muñiz J., Cequier Á., Bertomeu-Martínez V., Valdés M., Vicente V., et al. Usefulness of the 2MACE score to predicts adverse cardiovascular events in patients with atrial fibrillation. Am. J. Cardiol. 2017;120:2176–2181. doi: 10.1016/j.amjcard.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 8.Halushka P.V., Goodwin A.J., Halushka M.K. Opportunities for microRNAs in the crowded field of cardiovascular biomarkers. Annu. Rev. Pathol. 2019;14:211–238. doi: 10.1146/annurev-pathmechdis-012418-012827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Viereck J., Thum T. Circulating Noncoding RNAs as Biomarkers of Cardiovascular Disease and Injury. Circ. Res. 2017;120:381–399. doi: 10.1161/CIRCRESAHA.116.308434. [DOI] [PubMed] [Google Scholar]
- 10.Vrijens K., Bollati V., Nawrot T.S. MicroRNAs as potential signatures of environmental exposure or effect: A systematic review. Environ. Health Perspect. 2015;123:399–411. doi: 10.1289/ehp.1408459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitchell P.S., Parkin R.K., Kroh E.M., Fritz B.R., Wyman S.K., Pogosova-Agadjanyan E.L., Peterson A., Noteboom J., O’Briant K.C., Allen A., et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc. Natl. Acad. Sci. USA. 2008;105:10513–10518. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jakob P., Kacprowski T., Briand-Schumacher S., Heg D., Klingenberg R., Stähli B.E., Jaguszewski M., Rodondi N., Nanchen D., Räber L., et al. Profiling and validation of circulating microRNAs for cardiovascular events in patients presenting with ST-segment elevation myocardial infarction. Eur. Heart J. 2017;38:511–515. doi: 10.1093/eurheartj/ehw563. [DOI] [PubMed] [Google Scholar]
- 13.Rizzacasa B., Morini E., Mango R., Vancheri C., Budassi S., Massaro G., Maletta S., Macrini M., D’Annibale S., Romeo F., et al. MiR-423 is differentially expressed in patients with stable and unstable coronary artery disease: A pilot study. PLoS ONE. 2019;14:e0216363. doi: 10.1371/journal.pone.0216363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barraclough J.Y., Joan M., Joglekar M.V., Hardikar A.A., Patel S. MicroRNAs as prognostic markers in acute coronary syndrome patients—A systematic review. Cells. 2019;8:1572. doi: 10.3390/cells8121572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manna I., Iaccino E., Dattilo V., Barone S., Vecchio E., Mimmi S., Filippelli E., Demonte G., Polidoro S., Granata A., et al. Exosome-associated miRNA profile as a prognostic tool for therapy response monitoring in multiple sclerosis patients. FASEB J. 2018;32:4241–4246. doi: 10.1096/fj.201701533R. [DOI] [PubMed] [Google Scholar]
- 16.DeLong E.R., DeLong D.M., Clarke-Pearson D.L. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics. 1988;44:837–845. doi: 10.2307/2531595. [DOI] [PubMed] [Google Scholar]
- 17.Pencina M.J., D’Agostino R.B., Sr., D’Agostino R.B., Jr., Vasan R.S. Evaluating the added predictive ability of a new marker: From area under the ROC curve to reclassification and beyond. Stat. Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 18.Vickers A.J., Elkin E.B. Decision curve analysis: A novel method for evaluating prediction models. Med. Decis. Mak. 2006;26:565–574. doi: 10.1177/0272989X06295361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arroyo A.B., de Los Reyes-García A.M., Rivera-Caravaca J.M., Valledor P., García-Barberá N., Roldán V., Vicente V., Martínez C., González-Conejero R. MiR-146a regulates neutrophil extracellular trap formation that predicts adverse cardiovascular events in patients with atrial fibrillation. Arterioscler. Thromb. Vasc. Biol. 2018;38:892–902. doi: 10.1161/ATVBAHA.117.310597. [DOI] [PubMed] [Google Scholar]
- 20.Roldán V., Arroyo A.B., Salloum-Asfar S., Manzano-Fernández S., García-Barberá N., Marín F., Vicente V., González-Conejero R., Martínez C. Prognostic role of MIR146A polymorphisms for cardiovascular events in atrial fibrillation. Thromb. Haemost. 2014;112:781–788. doi: 10.1160/TH14-01-0092. [DOI] [PubMed] [Google Scholar]
- 21.Komal S., Yin J.J., Wang S.H., Huang C.Z., Tao H.L., Dong J.Z., Han S.N., Zhang L.R. MicroRNAs: Emerging biomarkers for atrial fibrillation. J. Cardiol. 2019;74:475–482. doi: 10.1016/j.jjcc.2019.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Franco D., Aranega A., Dominguez J.N. Non-coding RNAs and atrial fibrillation. Adv. Exp. Med. Biol. 2020;1229:311–325. doi: 10.1007/978-981-15-1671-9_19. [DOI] [PubMed] [Google Scholar]
- 23.Jiang S., Guo C., Zhang W., Che W., Zhang J., Zhuang S., Wang Y., Zhang Y., Liu B. The integrative regulatory network of circRNA, microRNA, and mRNA in Atrial Fibrillation. Front. Genet. 2019;10:526. doi: 10.3389/fgene.2019.00526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Briasoulis A., Sharma S., Telila T., Mallikethi-Reddy S., Papageorgiou N., Oikonomou E., Tousoulis D. MicroRNAs in Atrial Fibrillation. Curr. Med. Chem. 2019;26:855–863. doi: 10.2174/0929867324666170920151024. [DOI] [PubMed] [Google Scholar]
- 25.da Silva A.M., de Araújo J.N., de Freitas R.C., Silbiger V.N. Circulating MicroRNAs as potential biomarkers of atrial fibrillation. BioMed. Res. Int. 2017;2017:7804763. doi: 10.1155/2017/7804763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kapodistrias N., Theocharopoulou G., Vlamos P. A hypothesis of circulating MicroRNAs’ implication in high incidence of atrial fibrillation and other electrocardiographic abnormalities in cancer patients. Adv. Exp. Med. Biol. 2020;1196:1–9. doi: 10.1007/978-3-030-32637-1_1. [DOI] [PubMed] [Google Scholar]
- 27.Zhang P.P., Sun J., Li W. Genome-wide profiling reveals atrial fibrillation-related circular RNAs in atrial appendages. Gene. 2020;728:144286. doi: 10.1016/j.gene.2019.144286. [DOI] [PubMed] [Google Scholar]
- 28.Xu X., Zhao Z., Li G. The therapeutic potential of MicroRNAs in Atrial Fibrillation. Mediat. Inflamm. 2020;2020:3053520. doi: 10.1155/2020/3053520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lozano-Velasco E., Garcia-Padilla C., Aránega A.E., Franco D. Genetics of Atrial Fibrilation: In search of novel therapeutic targets. Cardiovasc. Hematol. Disord. Drug Targets. 2019;19:183–194. doi: 10.2174/1871529X19666190206150349. [DOI] [PubMed] [Google Scholar]
- 30.Huang Z.P., Wang D.Z. miR-22 in cardiac remodeling and disease. Trends Cardiovasc. Med. 2014;24:267–272. doi: 10.1016/j.tcm.2014.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kiyosawa N., Watanabe K., Morishima Y., Yamashita T., Yagi N., Arita T., Otsuka T., Suzuki S. Exploratory analysis of circulating miRNA signatures in Atrial Fibrillation patients determining potential biomarkers to support decision-making in anticoagulation and catheter ablation. Int. J. Mol. Sci. 2020;21:2444. doi: 10.3390/ijms21072444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goren Y., Kushnir M., Zafrir B., Tabak S., Lewis B.S., Amir O. Serum levels of microRNAs in patients with heart failure. Eur. J. Heart Fail. 2012;14:147–154. doi: 10.1093/eurjhf/hfr155. [DOI] [PubMed] [Google Scholar]
- 33.Huang Z.P., Chen J., Seok H.Y., Zhang Z., Kataoka M., Hu X., Wang D.Z. MicroRNA-22 regulates cardiac hypertrophy and remodeling in response to stress. Circ. Res. 2013;112:1234–1243. doi: 10.1161/CIRCRESAHA.112.300682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xu X.D., Song X.W., Li Q., Wang G.K., Jing Q., Qin Y.W. Attenuation of microRNA-22 derepressed PTEN to effectively protect rat cardiomyocytes from hypertrophy. J. Cell. Physiol. 2012;227:1391–1398. doi: 10.1002/jcp.22852. [DOI] [PubMed] [Google Scholar]
- 35.Kiliszek M., Maciak K., Maciejak A., Krzyżanowski K., Wierzbowski R., Gora M., Burzynska B., Segiet A., Skrobowski A. Serum microRNA in patients undergoing atrial fibrillation ablation. Sci. Rep. 2020;10:4424. doi: 10.1038/s41598-020-61322-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li Y., Tan W., Ye F., Wen S., Hu R., Cai X., Wang K., Wang Z. Inflammation as a risk factor for stroke in atrial fibrillation: Data from a microarray data analysis. J. Int. Med Res. 2020;48 doi: 10.1177/0300060520921671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Y., Shen H., Wang P., Min J., Yu Y., Wang Q., Wang S., Xi W., Nguyen Q.M., Xiao J., et al. Identification and characterization of circular RNAs in atrial appendage of patients with atrial fibrillation. Exp. Cell Res. 2020;389:111821. doi: 10.1016/j.yexcr.2020.111821. [DOI] [PubMed] [Google Scholar]
- 38.Camelo-Castillo A., Rivera-Caravaca J.M., Marín F., Vicente V., Lip G.Y.H., Roldán V. Predicting adverse events beyond stroke and bleeding with the ABC-stroke and ABC-bleeding scores in patients with atrial fibrillation: The murcia AF project. Thromb. Haemost. 2020;120:1200–1207. doi: 10.1055/s-0040-1712914. [DOI] [PubMed] [Google Scholar]
- 39.Rivera-Caravaca J.M., Esteve-Pastor M.A. Heart failure and cardiac events: Is a consecutive measurement of biomarkers a simple and practical approach? Thromb. Haemost. 2019;119:1891–1893. doi: 10.1055/s-0039-3400274. [DOI] [PubMed] [Google Scholar]
- 40.Esteve-Pastor M.A., Roldán V., Rivera-Caravaca J.M., Ramírez-Macías I., Lip G.Y.H., Marín F. The use of biomarkers in clinical management guidelines: A critical appraisal. Thromb. Haemost. 2019;119:1901–1919. doi: 10.1055/s-0039-1696955. [DOI] [PubMed] [Google Scholar]
- 41.Wang Y., Liu B. Circular RNA in diseased heart. Cells. 2020;9:1240. doi: 10.3390/cells9051240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ioannou A., Papageorgiou N., Falconer D., Rehal O., Sewart E., Zacharia E., Toutouzas K., Vlachopoulos C., Siasos G., Tsioufis C., et al. Biomarkers associated with stroke risk in Atrial Fibrillation. Curr. Med. Chem. 2019;26:803–823. doi: 10.2174/0929867324666170718120651. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.