Abstract
The emergence of phosphatidylinositol 3-kinase (PI3Kα) in cancer development has accentuated its significance as a potential target for anticancer drug design. Twenty one derivatives of N-phenyl-4-hydroxy-6-methyl-2-quinolone-3-carboxamide were synthesized and characterized using NMR (1H and 13C) and HRMS. The derivatives displayed inhibitory activity against human epithelial colorectal adenocarcinoma (Caco-2) and human colon cancer (HCT-116) cell lines: compounds 8 (IC50 Caco-2 = 98 µM, IC50 HCT-116 = 337 µM) and 16 (IC50 Caco-2 = 13 µM, IC50 HCT-116 = 240.2 µM). Results showed that compound 16 significantly affected the gene encoding AKT, BAD, and PI3K. The induced-fit docking (IFD) studies against PI3Kα demonstrated that the scaffold accommodates the kinase domains and forms H-bonds with significant binding residues.
Keywords: anticancer, colon cancer, PI3Kα, AKT, docking, quinolone-3-carboxamide
1. Introduction
Phosphatidylinositol 3-kinases (PI3Ks) phosphorylate the hydroxyl moiety on position 3 (3-OH) of inositol rings, producing phosphatidylinositol 3,4,5 triphosphates (PIP3). The generated triphosphates induce downstream messengers and signaling receptors such as protein kinase B (AKT), and consequently promote cell proliferation and division [1,2].
The phosphatase and tensin homolog (PTEN) dephosphorylate the 3-OH group of PIP3 and adversely control PI3Ks [2,3]. PI3Ks are classified into three classes (I, II, and III) based on their substrate binding and primary structures. Class IA PI3Ks compromises PI3Kα, β, and δ isozymes expressed by their discrete genes PIK3CA (PI3Kα), PIK3CB (PI3Kβ), and PIK3CD (PI3Kδ) [4]. Abnormal activation of the PI3Kα/AKT signaling cascade has been detected in diverse human cancers [5]. The gene PIK3CA is exaggerated, over encrypted, and mutated in many human cancers [5]. Mutations in the helical (E545K and E542K) and kinase (H1047R) domains of PI3Kα are identified in brain, breast, endometrial, and GIT cancers [6,7,8,9]. These oncogenic alterations distort PI3Kα conformation and have therefore lead to the design of selective PI3Kα inhibitors [8]. The prevalence of PI3Kα and PTEN mutations in various human cancers identify PI3Kα inhibition as a promising goal for anticancer drug design [10,11].
Employing pharmacophore modeling and database searching against the national cancer institute (NCI) database [12], we previously disclosed N-benzyl-4-hydroxy-2-quinolone-3-carboxamide (1) as a lead PI3Kα inhibitor against the wild-type (WT) (IC50 = 1.1 μM) and mutant (MUT) H1047R PI3Kα (IC50 = 0.73 μM) (Figure 1; Figure 2) [13]. Furthermore, we determined the structural changes between the WT and MUT PI3Kα that could be targeted to identify selective inhibitors [14].
Figure 1.
Chemical structures of 1, 2 and 3.
Figure 2.
PI3Kα inhibitor pharmacophore model with (1) and (2) depicted in green and orange color, respectively. Picture made by MOE [15].
Furthermore, we identified the key binding residues of MUT H1047R PI3Kα inhibitors through binding free energy calculations recruiting the molecular mechanics/generalized born surface area (MM/GBSA) protocol for PI3Kα molecular dynamic (MD) extracted assemblies [16]. Additionally, excessive lead optimization strategies released N-phenyl-4-hydroxy-2-quinolone-3-carboxamide derivatives as selective MUT H1047R PI3Kα inhibitors exemplified by 2 [17]. Lately, we released a series of N-substituted 4-hydroxy-2-quinolone-3-carboxamides that suppressed human colon carcinoma (HCT116) cell line proliferation and enhanced apoptosis through inducing caspase-3 activity and destructing cellular DNA illustrated by 3 [18]. Our ceaseless research has uncovered diverse scaffolds [19,20,21,22,23,24] targeting PI3Kα.
In this context, we designed and synthesized a series of N-phenyl-4-hydroxy-6-methyl-2-quinolone-3-carboxamides to address the effect of incorporating a methyl moiety on the biological activity and to better understand the structural activity relation (SAR) of this series. This work delineates the design and synthesis of novel N-phenyl-4-hydroxy-6-methyl-2-quinolone-3-carboxamides employing structure-based drug design and molecular modeling tactics. Biological evaluation of the prepared compounds along with pan PI3K inhibitors (LY294002) has been investigated in vitro against human epithelial colorectal adenocarcinoma (Caco-2) and human colon carcinoma (HCT-116) cell lines.
2. Results and Discussion
2.1. Chemistry
Target compounds (7–27) were designed to probe the influence of introducing different functionalities at the carboxamide side chain on the bioactivity of the 4-hydroxy-6-methylquinolinone scaffold. The reaction of 3 and 4 in basic medium under reflux generated the target scaffold 5 with 70% yield, as outlined in Scheme 1. Monitoring the reaction progress was applied by employing thin layer chromatography (TLC); the disappearance of the ethyl anthranilate spot indicated that the reaction was completed. Prospective compounds 7–27 have been synthesized by reacting 5 with an excess of the corresponding amine (ArNH2) using DMF under reflux, as delineated in Table 1 and Scheme 1. The absence of the limiting reactant spot (5) on the TLC plate indicated that the reaction was completed. Confirmation of the identity of the chemical structures was carried out using NMR and HRMS. The detailed data in the experimental section accord with the target structures.
Scheme 1.
Conditions: (a): (i) NaOC2H5, DMSO, 130–140 °C, 72 h. (ii) 0.3M HCl (b): (i) THF, DMF, 150 °C, 48 h.
Table 1.
Chemical structures of synthesized N-substituted-4-hydroxy-6-methyl-2-quinolone-3- carboxamides (7–27).

| Compound | Ar | Compound | Ar |
|---|---|---|---|
| 7 |
|
18 |

|
| 8 |

|
19 |

|
| 9 |

|
20 |

|
| 10 |

|
21 |

|
| 11 |

|
22 |

|
| 12 |

|
23 |
|
| 13 |

|
24 |

|
| 14 |

|
25 |
|
| 15 |

|
26 |

|
| 16 |

|
27 |

|
| 17 |

|
||
2.2. Biological Evaluation of the Synthesized Compounds
In order to investigate the inhibitory activity of the verified compounds (5, 7–27) against PI3Kα, we probed their antiproliferative activity in human epithelial colorectal adenocarcinoma (Caco-2) and human colon cancer (HCT-116) cell lines as shown in Table 2. The epithelial colorectal adenocarcinoma (Caco-2) expresses the wild-type (WT) PI3Kα [25,26,27]. The malignant (HCT-116) carcinoma encodes both wild-type (WT) and mutant (MUT) (H1047R) PI3Kα [28]. Therefore, the difference in activity against Caco-2 and HCT-116 accounts for MUT (H1047R) PI3Kα.
Table 2.
The inhibitory activity (IC50 µM) of 5, 7–27. SD does not exceed 5%.
| Compound’s Number | IC50 (µM) ± SD | Compound’s Number | IC50 (µM) ± SD | ||
|---|---|---|---|---|---|
| Caco-2 | HCT-116 | Caco-2 | HCT-116 | ||
| LY294002 | 7.4 | 6.5 | 17 | 2321 | 615 |
| 5 | 338.5 | 346.8 | 18 | 486.6 | 422.5 |
| 7 | 264 | 212.2 | 19 | 402.3 | 304.2 |
| 8 | 98 | 337 | 20 | 1559 | 789.4 |
| 9 | 356 | 383.7 | 21 | 664.5 | 16.04 |
| 10 | 275 | 390.4 | 22 | 458.2 | 184.6 |
| 11 | 204 | 92.61 | 23 | 261.4 | 46.38 |
| 12 | 260 | 207.4 | 24 | 298 | 241.1 |
| 13 | 687 | 178.6 | 25 | 302 | 140.0 |
| 14 | 354 | 441.2 | 26 | 282.5 | 405.7 |
| 15 | 212 | 282.2 | 27 | 289.2 | 462.7 |
| 16 | 13 | 240.2 | |||
All examined compounds were examined against skin fibroblast cells at concentration ranges between the IC50 and double IC50 values for each compound. At each concentration point, cell viability was compared to the negative control of untreated cells. We found less than 10% growth reduction for all examined compounds at any concentration point.
The inhibitory activity against Caco-2 for compounds 14, 15, and 16 reveals that attaching the p-F moiety on the phenyl carboxamide side chain (13) induces activity that suggests that a hydrophobic and/or H-bond interaction mediate(s) ligand/PI3Kα complex formation. The activity of 14, 15, and 16 against HCT-116 indicates distinct inhibitory activity and highlights the activity of 16 in Caco-2; 16 exerted potent inhibitory activity in Caco-2. In contrast, the activity of 17, 18, and 19 in Caco-2 and HCT-116 shows that a steric factor might hinder their proper orientation in the binding site.
The biological data of 11, 12, and 13 in Caco-2 suggests that an H-bond interaction presides ligand/PI3Kα complex formation. Furthermore, comparing the activity of p-F (16) with that of p-OH (11) clarifies that a steric factor affects the accommodation of –OH in the binding domain. Additionally, contrasting the inhibitory activity of p-OCH3 (12) and of p-CH3 (13) in Caco-2 implies that an H-bond interaction might drive ligand/PI3Kα binding and/or an oxygen atom might push –CH3 deeply into the binding cleft. Furthermore, the inhibitory activity of 11, 12, and 13 in HCT-116 declares that an H-bond interaction mediates ligand/PI3Kα interaction; 11 provides an H-bond donor and acceptor, whereas 12 offers an H-bond acceptor. Interestingly, the activity of p-OCH3 (12) and p-CH3 (13) affirms that an H-bond donor, exemplified by (11), dominates ligand/PI3Kα complex formation and suggests that a small hydrophobic cleft encloses the –CH3 motif.
Comparing the activity of 7 and 8 in Caco-2 infers that a nitrogen atom in 8 might mediate the H-bond interaction with PI3Kα backbones and/or the solubility and polarity factor of pyridine might influence the compound’s distribution in the cell line. Comparing the activity of 9 and 10 in Caco-2 reveals that elongation of the carboxamide side chain by the –NH motif (10) improves the activity and thus infers that the H-bond might drive ligand/PI3Kα interactions. Such a result shows the low activity of 9 and underlines the significance of the –NH motif.
The biological data of 7, 8, 9, and 10 in HCT-116 show that introducing the phenyl moiety on the carboxamide side chain (7) provokes the activity interrogating the tightness of the binding cleft, and thus, 7 accommodates the binding cleft. Contrasting the activity of 7 and 8 suggests that a hydrophobic lining encloses the phenyl motif and understates the significance of the H-bond in HCT-116. Furthermore, the activity of 9 and 10 confirms the narrowness of the binding cleft and provides a further clue to the proper orientation of 7 in the kinase domain. Furthermore, the antiproliferative activity of 7, 8, 9, and 10 in Caco-2 and HCT-116 displays comparable activity for 7 and 9 and distinct activity for 8 and 10 in both cell lines.
Tailoring the derivatives with –COOH (20, 21, and 22) confirms that the H-bond mediates ligand/PI3Kα complex formation in Caco-2 on the p-position (22). Such a finding emphasizes the importance of the H-bond on the ligand/PI3Kα interaction and further explains the activity of p-F (16) and p-OH (11) in Caco-2. Additionally, the result confirms that the steric factor impedes their proper location in the binding site. Distinctly, p-F (16) and p-OH (11) exerted higher activity than that of p-COOH (22) in Caco-2.
The activity of 20, 21, and 22 in HCT-116 demonstrates that ionic and/or H-bond(s) guide(s) ligand/PI3Kα complex formation on the m-position (21). Furthermore, the activity of m-F (15), m-COOH (16), and m-CF3 (18) in HCT-116 accentuates the significance of the H-bond donor and/or ionic bond on the ligand/PI3Kα interaction. Contrasting the activity of 20 and 23 in Caco-2 and HCT-116 infers that the ester moiety improves cell membrane permeability and consequently enhances the activity.
The inhibitory activity of 24 and 25 in Caco-2 declares that tailoring the benzoic acid with –Cl (24) and –CH3 (25) improves the activity, suggesting that –Cl and –CH3 might orientate the derivatives properly in the binding site and/or provide an extra binding interaction. Contrasting the activity of 20, 24, and 25 in HCT-116 shows that attaching –Cl (24) and –CH3 (25) induces the activity, suggesting that –Cl and –CH3 might place the ligands suitably in the binding cleft and/or furnish an extra binding interaction.
The activity of p-SH (26) in Caco-2 provides an extra clue for the H-bond interaction on the p-site. Comparing the activity of p-OH (11) and p-SH (26) confirms that the H-bond drives the ligand/PI3Kα interaction. Moreover, contrasting the activity of p-SCH3 (27) to that of p-OCH3 (12) in Caco-2 suggests that S and O push –CH3 deeply into the binding site and thus in turn improves the activity. The difference in activity between 12 and 27 might be due to the H bond supplied by the O atom. It is worth noting that the series exhibited distinct antiproliferative activity in both cell lines, suggesting differences in the binding sites of WT PI3Kα and MUT (H1047R) PI3Kα, which accords with our previous data on PI3Kα [13,14,16,17,18,19,23,24].
Comparing the biological data of p-OH (11) and p-SH (26) in HCT-116 highlights the significance of the H-bond interaction on p-site. Furthermore, the difference in activity between p-OCH3 (12) and p-SCH3 (27) in HCT-116 shows the significance of the H bond assigned by the O atom and/or the steric interference of the S atom, which impedes the proper conformation of 27 in the binding cleft. Finally, the activity of 5 against Caco-2 and HCT-116 cell lines highlights the significance of the tailored carboxamide motif.
The explored cell lines encode both WT and MUT PI3Kαs; therefore, isolating either gene is highly recommended to confirm the inhibitory activity against purified PI3Kα. Future validations should be performed through knocking down any gene to interpret the mechanism of inhibition against each purified enzyme.
The effect of compound 16 on the PI3K/AKT signaling pathway was investigated using real time PCR. According to our results, the relative gene expression of PI3K, AKT, and BAD was significantly affected by treatment with compound 16 (1 µM) in a manner consistent with the positive control treatment (1 uM) (Figure 3). A significant decrease in PI3K and AKT gene expression was evident upon treatment with either compound 16 or the positive control LY-294002. On the other hand, the expression of the pro-apoptotic gene BAD was significantly increased in treated cells (for both compound 16 and the positive control) when compared to the negative control where there was no treatment applied.
Figure 3.
Relative gene expression of PI3K, AKT, and BAD in HCT116 cells after treatment with Compound 16 and the positive control LY-294002. Bars represent mean values ± SEM (lines) of three replicates. Asterisks represent significant change relative to the negative control (* p < 0.05).
2.3. Computational Studies
2.3.1. Molecular Docking
In order to test whether the anticancer activity of the synthesized compounds (5, 7–27) in Caco-2 and HCT-116 cell lines (both strongly upregulate PI3Kα) can be partly attributed to PI3Kα modulation, we performed molecular docking studies in PI3Kα crystal structures. For that, we employed the coordinates of WT (PDB ID: 2RD0) [4] and MUT (H1047R) (PDB ID: 3HHM) [29] PI3Kα to determine the structural basis of the PI3Kα/ligand interaction.
In order to explore the binding interaction of PI3Kα and the verified compounds (5, 7–27) in the kinase domain of PI3Kα, we carried out induced-fit docking (IFD) studies [30,31,32] against the kinase domains of 2RD0 and 3HHM. IFD data illustrated that 5, 7–27 reside in PI3Kαs kinase domains and the docked pose of 14 superposes the crystal structure of X6K in the kinase domain of PI3Kα (PDB ID: 4L23) [33] (Figure 4).
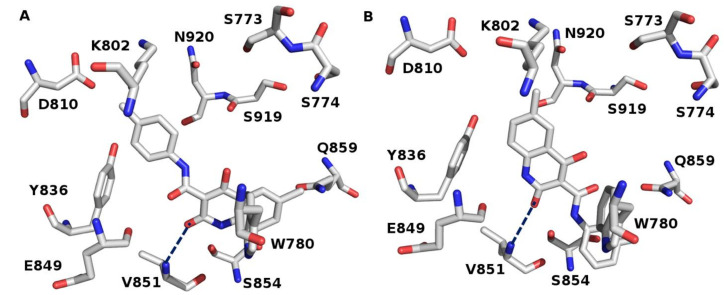
Figure 4.
The kinase cleft of (A) 2RD0 bears the IF docked poses of some of the synthesized molecules and (B) overlaying of the IF docked conformation of 14 (represented in pink color) and the co-crystallized ligand (X6K) (yellow color). Some of key binding residues are disclosed and H atoms are unseen for clarification. Picture visualized by PYMOL [32].
The IFD approach probes the conformational changes in proteins in the following manner: ligands are docked to a protein’s binding site recruiting Glide docking and the top ligand geometries are minimized along with protein binding site using the Prime module. Next, a redocking procedure is employed against the relaxed protein. Therefore, protein plasticity is considered during the docking tactic. The backbones of the synthesized molecules form H-bonds with S773, S774, A775, K776, W780, K802, D810, Y836, E849, V851, N853, S854, Q859, D915, H917, S919, N920, and D933 (Table 3, Figure 5). Furthermore, other computational [14,16,17,19,20,34] and experimental studies [4] have assigned the contribution of these key binding amino acids in the PI3Kα/ligand binding interaction. Interestingly, 5, 7–27 displayed comparable affinity toward both WT (2RD0) and MUT (H1047R) (3HHM) PI3Kα. Moreover, the prevalence of S774 in the ligand/PI3Kα interaction suggests that the series might be selective PI3Kα inhibitors [14].
Table 3.
The IFD scores (Kcal/mol) and H-bond interactions between 2RD0 and 3HHM.
| 2RD0 | 3HHM | |||
|---|---|---|---|---|
| Compound | Docking Score | Binding Residues | Docking Score | Binding Residues |
| 5 | −7.58 | K802, D933 | −7.77 | V851 |
| 7 | −8.04 | V851 | −8.91 | S774, D933, N920 |
| 8 | −7.84 | S774, D933 | −8.73 | S774, D933, S919 |
| 9 | −8.98 | E849, V851 | −8.71 | S774, D933 |
| 10 | −9.39 | W780, E849, V851, S854 | −8.72 | S774, D933, S919, N920 |
| 11 | −9.40 | V851 | −9.68 | S774, D933, S919, F1059 |
| 12 | −8.69 | V851, Q859 | −9.32 | Y836, V851 |
| 13 | −8.59 | V851 | −7.92 | S774, D933 |
| 14 | −8.37 | Y836, D933 | −8.09 | S774, S919, D933 |
| 15 | −8.79 | V851 | −8.99 | S774, S919, D933 |
| 16 | −9.20 | W780, V851 | −8.41 | Y836 |
| 17 | −8.44 | S854, Q859 | −8.39 | N920, D933 |
| 18 | −8.65 | W780, V851 | −8.33 | S774, D933, N920 |
| 19 | −8.42 | K802, D933 | −8.95 | S773, D933 |
| 20 | −8.89 | S774, K802, D933 | −9.98 | S774, A775, K776, D933, N920 |
| 21 | −9.15 | S774, K776, D805, D933 | −9.08 | S774, D915, H917, N920, D933 |
| 22 | −10.32 | K802, D810, V851 | −9.58 | S774, E849, D933 |
| 23 | −8.11 | S774, K802, D933 | −8.47 | S774, N920 |
| 24 | −8.39 | W780, S854, Q859 | −9.46 | S774, H917, N920 |
| 25 | −9.83 | V851, S854, D933 | −10.04 | S774, K776, N920, D933 |
| 26 | −8.59 | D933 | −8.52 | S773, N920 |
| 27 | −8.81 | V851 | −10.0 | D810, Y836, D933 |
Figure 5.
Binding geometries of (A) 13 and (B) 15 in the kinase site of 2RD0. H-bonds are depicted by blue dotted lines. H atoms are masked for clarification. Picture visualized by PYMOL [32].
In order to get further details about the tailored functionalities of 7–27, we screened them against an adopted pharmacophore model of active PI3Kα inhibitors [13]. The backbones of 7–27 endorse the fingerprint of active PI3Kα inhibitors (Figure 6), represented as (F1), pointing to one aromatic ring; (F2), one aromatic or H-bond acceptor; (F3), one aromatic or hydrophobic or H-bond acceptor; and (F4) or (F5), one H-bond acceptor. Such a result rationalizes the affinity of the series against PI3Kα. Furthermore, the accommodation of 7–27 in the kinase cleft anticipates potential PI3Kα inhibitory activity.
Figure 6.
PI3Kα active inhibitors pharmacophore model with 20 (yellow color), 24 (light pink color), and 25 (green color). Picture made by MOE [15].
2.3.2. Descriptor Analysis
Molecular descriptors comprising two main categories of [35], drug-like indices and molecular properties, were calculated for molecules 5 and 7–27. All descriptor values were then analyzed using principal component analysis. Our analysis revealed a diversity of lead-like, drug-like, and molecular properties of the synthesized compounds (Figure 7A). All calculated descriptors for all analyzed molecules are reported in Supplementary Materilas Table S1.
Figure 7.
Molecular descriptor analysis. (A) A graphical 2D representation of the principal component analysis of molecular descriptors. PC1 explaining 44.20% of the variation in descriptor values is on the X-axis, and PC2 explaining 28.83% of the variation in descriptor values is on the y-axis. The descriptors used for the analysis belong to two major categories of molecular descriptors: (1) drug-like indices; and (2) molecular properties. Structures shown in red boxes, which correspond to the red dots on the figure, serve as structural examples. (B) Radar plot of drug-like molecular descriptors. Both drug-like scores (DLS) and lead-like scores (LLS) were calculated for molecules 5 and 7–22. A molecule is drug-like or lead-like if its DLS or LLS is 0.50.
Our analysis further revealed that our synthesized molecules differ in their lead-like and drug-like properties as shown in Figure 7B. All synthesized molecules passed the lead-like scoring filter LLS-02 [36], but only molecules 5, 7 and 8 passed the lead-like scoring filter DDL-01 [37]. Our molecules also showed diverse drug-like properties based on three drug-like scoring filters: DLS-04 [38], DLS-05 [39], and Dragon Consensus Score for drug-likeness [40]. For example, molecule 5 was the only molecule that passed the DLS-05 drug-like filter, while molecules 5, 7, 9–13, 23, 25–27 passed the DLS-04 drug-like filter. All values for all calculated drug-like scores are found in Supplementary Materilas Table S1. Drugs that have higher overall drug-like scores have better chances to succeed in further experimental testing including animal studies.
3. Materials and Methods
3.1. Chemistry
All chemicals, reagents, and solvents were of analytical grade and used directly without further purification. Chemicals were purchased from the corresponding companies: SD Fine-Chem Limited (SDFCL) (Mumbai, India), Acros Organics (Fair Lawn, NJ, USA), Sigma-Aldrich (St. Louis, MO, USA), Fluka (Buchs, Switzerland), Sharlau (Barcelona, Spain), Tedia (Fairfield, OH, USA), and Gainland Chemical Company (GCC) (Sandycroft, Flintshire, UK). Rota vapor (1) model R-215 (Buchi, Switzerland) connected to vacuum pump model v-700 and water bath B-491 were used to evaporate ordinary solvents and vacuum controller v-855. Melting points (MP) were measured using Gallenkamp melting point apparatus (Loughborough, UK). The hot plate and magnetic stirrer were purchased from vision scientific CO, LTD, Westland, MI, USA. Thin layer chromatography (TLC) was performed on 20 × 20 cm, with layer thickness 0.2 mm, aluminum cards pre-coated with fluorescent silica gel GF254 DC (Fluka analytical, Munich, Germany) and visualized by UV light indicator (at 254 and/or 360 nm).
Nuclear magnetic resonance (NMR) 1H- and 13C-NMR spectra were measured on a Bruker (Mundelein, IL, USA), Avance DPX- 500 MHz spectrophotometer (The University of Jordan) and Bruker NanoBay 400 MHz spectrophotometer (the Hashemite University). Chemical shifts are given in δ (ppm) using TMS as an internal reference; the samples are dissolved in DMSO-d6. High-resolution mass spectra (HRMS) were recorded using a Bruker APEX-IV (7 Tesla) instrument. External calibration was executed using an arginine cluster at a mass range of m/z 175–871, and the samples were dissolved in methanol and drops of formic acid.
3.2. Synthesis of Target Compounds
3.2.1. Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5)
Sodium ethoxide (6.27 g, 92.16 mmol) was added to a solution of ethyl 2-amino-5-methylbenzoate (3) (5.506 g, 30.72, mmol) and diethylmalonate (4) (46.86 mL, 307.2 mmol) in DMSO (30 mL). The mixture was refluxed at 130–140 °C for 72 h. Completion of the reaction was achieved by the absence of the compound (3) spot on TLC. After cooling, the reaction mixture was acidified with 0.3 M HCl and the precipitate was collected by suction filtration then dried under reduced pressure.
A beige powder (3.35 g) with % yield: 70.2, Rƒ = 0.51 (CHCl3: MeOH-9.8: 0.2); m.p: 215–216 °C; 1H-NMR (500 Hz, DMSO-d6): δ = 13.34 (s, 1H, OH), 11.38 (s, 1H, NH), 7.65 (s, 1H, Ar-H), 7.36 (d, J = 8.1 Hz, 1H, Ar-H), 7.16 (d, J = 8.1 Hz, 1H, Ar-H), 4.29 (q, J = 7.0 Hz, 2H, OCH2), 2.27 (s, 3H, CH3), 1.25 (t, J = 7.0 Hz, 3H, CH2CH3) ppm; 13C-NMR (125 Hz, DMSO-d6): δ = 170.9 (1C), 169.2 (1C), 159.6 (1C), 138.4 (1C), 135.4 (1C), 131.2 (1C), 123.8 (1C), 115.7 (1C), 113.6 (1C), 100.3 (1C), 61.8 (1C), 20.9 (1C), 14.5 (1C) ppm.
3.2.2. N-Phenyl-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (7)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and aniline (6a) (1.13 g, 12.13 mmol) were dissolved in 25 mL THF. A few drops of DMF were added, then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by suction filtration, washed with absolute ethanol, and dried under reduced pressure.
A beige powder (0.67 g) with %yield: 56, Rƒ = 0.68 (CHCl3: MeOH-9.7: 0.3); m.p: 302–304 °C; 1H-NMR (500 Hz, DMSO-d6 + NaOD): δ = 7.72 (s, 1H, Ar-H), 7.64 (d, J = 8.0 Hz, 2H, Ar-H), 7.17 (t, J = 8.0 Hz, 2H, Ar-H), 7.05 (d, J = 8 Hz, 1H, Ar-H), 6.94 (d, J = 8.0 Hz, 1H, Ar-H), 6.87 (t, J = 7.0 Hz, 1H, Ar-H), 2.31 (s, 3H, CH3) ppm;13C-NMR (125 Hz, DMSO-d6+NaOD): 177.4 (1C), 168.8 (1C), 168.4 (1C), 141.2 (1C), 131.8 (1C), 129.0 (3C), 127.5 (1C), 125.5 (1C), 122.2 (1C), 121.9 (1C), 119.9 (2C), 117.6 (1C), 99.8 (1C), 21.2 (1C) ppm. High-resolution mass spectrum, m/z: calculated 295.10827 for C17H15N2O3 [M + H]+; found 295.10804.
3.2.3. N-(Pyridin-3-yl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (8)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 3-aminopyridine (6b) (1.13 g, 12.03 mmol) were dissolved in 25 mL THF. A few drops of DMF were added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A beige powder (0.96 g) with %yield: 80, Rƒ = 0.6 (CHCl3: MeOH-9.5: 0.5); m.p: 296–298 °C; 1H-NMR (500 Hz, DMSO-d6 +NaOD): δ = 8.81 (s, 1H, Ar-H), 8.21 (d, J = 6.7 Hz, 1H, Ar-H), 8.07 (d, J = 6.7 Hz, 1H, Ar-H), 7.69 (s, 1H, Ar-H), 7.23 (t, J = 7 Hz, 1H, Ar-H), 6.97 (d, J = 7.2 Hz, 1H, Ar-H), 6.93 (d, J = 7.2 Hz, 1H, Ar-H), 2.26 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6+NaOD): δ = 177.7 (1C), 173.1 (1C), 170.8 (1C), 148.2 (1C), 141.9 (1C), 141.4 (1C), 138.6 (1C), 130.8 (1C), 126.5 (1C), 125.0 (1C), 124.4 (1C), 124.0 (1C), 122.9 (1C), 100.6 (1C), 21.1 (1C) ppm. High-resolution mass spectrum, m/z: calculated 296.10352 for C16H14N3O3 [M + H]+; found 296.10341.
3.2.4. N-Benzyl-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (9)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (0.95 g, 3.84 mmol) and benzylamine (6c) (1.23 g, 11.52 mmol) were dissolved in 25 mL THF. A few drops of DMF were added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by suction filtration, washed with absolute ethanol, and dried under reduced pressure.
A white powder (0.87 g) with %yield: 74, Rƒ = 0.75 (CHCl3: MeOH-9.7: 0.3); m.p: 233–235 °C; 1H-NMR (500 Hz, DMSO-d6): δ = 17.01 (s, 1H, OH), 11.78 (s, 1H, NH), 10.71 (s, 1H, NH), 7.75 (s,1H, Ar-H), 7.37 (m, 7H, Ar-H), 4.6 (s, 2H, CH2), 2.38 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6): δ = 172.5 (1C), 171.2 (1C), 162.9 (1C), 138.7 (1C), 137.4 (1C), 135.6 (1C), 132.0 (1C), 129.0 (2C), 128.0 (2C), 127.7 (1C), 123.6 (1C), 116.3 (1C), 114.6 (1C), 96.6 (1C), 42.6 (1C), 20.9 (1C) ppm. High-resolution mass spectrum, m/z: calculated 309.12392 for C18H17N2O3 [M + H]+; found 309.12345.
3.2.5. N-(Anilino)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (10)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (0.95 g, 3.82 mmol) and phenyl hydrazine (6d) (1.23 g, 11.38 mmol) were dissolved in 25 mL THF. A few drops of DMF were added and the mixture was refluxed at 150–160 °C for 72 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A beige powder (0.67 g) with %yield: 57, Rƒ = 0.475 (CHCl3: MeOH-9.7: 0.3); m.p: 245–247 °C; 1H-NMR (500 Hz, DMSO-d6): δ = 16.3 (s, 1H, OH), 11.93 (s, 1H, CONH), 11.58 (s, 1H, CONH), 8.17 (s, 1H, HNNH), 7.74 (s, 1H, Ar-H), 7.51 (d, J = 4.6 Hz, 1H, Ar-H), 7.29 (d, J = 4.6 Hz, 1H, Ar-H), 7.20 (m, 2H, Ar-H), 6.80 (m, 3H, Ar-H), 2.37 (s, 3H, CH3); 13C-NMR (125 Hz, DMSO-d6): δ = 172.3 (1C), 171.6 (1C), 162.7 (1C), 148.7 (1C), 137.4 (1C), 135.9 (1C), 132.2 (1C), 129.4 (2C), 123.6 (1C), 119.7 (1C), 116.3 (1C), 114.3 (1C), 112.8 (2C), 96.6 (1C), 20.9 (1C) ppm. High-resolution mass spectrum, m/z: calculated 310.11917 for C17H16N3O3 [M + H]+; found 310.11895.
3.2.6. N-(4-hydroxyphenyl) 4-hydroxy-6-methyl-2-quinolone-3-carboxamide (11)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate carboxylate (5) (1 g, 4.044 mmol) and 4-aminophenol (6e) (1.32 g, 12.12 mmol) were dissolved in 25 mL THF. A few drops of DMF were added then the mixture was heated at 150 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
An off-white powder (0.78 g) with %yield: 62, Rƒ = 0.6 (CHCl3:MeOH-9.5: 0.5); m.p: 298–300 °C; 1H-NMR (500 Hz, DMSO-d6): δ = 16.64 (s, 1H, OH), 12.41 (s,1H, NH), 11.87 (s,1H, NH), 9.43 (s, 1H, Ar-OH), 7.73 (s, 1H, Ar-H), 7.48 (d, J = 8.0 Hz, 1H, Ar-H), 7.43 (d, J = 8.4 Hz, 2H, Ar-H), 7.29 (d, J = 8.0 Hz, 1H, Ar-H), 6.8 (d, J = 8.4 Hz, 2H, Ar-H), 2.36 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6): δ = 172.4 (1C), 168.8 (1C), 163.1 (1C), 155.1 (1C), 137.1 (1C), 135.7 (1C), 132.2 (1C), 128.8 (1C), 123.5 (1C), 122.9 (2C), 116.3 (1C), 116.0 (2C), 114.6 (1C), 96.8 (1C), 20.9 (1C) ppm. High-resolution mass spectrum, m/z: calculated 311.10318 for C17H15N2O4 [M + H]+; found 311.10345.
3.2.7. N-(4-methoxyphenyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (12)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (0.95 g, 3.82 mmol) and p-anisidine (6f) (0.95 g, 7.64 mmol) were dissolved in 25 mL THF. A few drops of DMF were added then the mixture was refluxed at 150–176 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by suction filtration, washed with absolute ethanol, and dried under reduced pressure.
An off-white powder (1.09 g) with %yield: 88, Rƒ = 0.65 (CHCl3: MeOH-9.7: 0.3); m.p: 275–277 °C; 1H-NMR (500 Hz, DMSO-d6): δ = 16.58 (s,1H, OH), 12.52 (s, 1H, NH), 11.95 (s, 1H, NH), 7.81 (s, 1H, Ar-H), 7.57 (d, J= 8.0 Hz, 3H, Ar-H), 7.34 (d, J = 8.0 Hz, 1H, Ar-H), 6.99 (d, J = 8.0 Hz, 2H, Ar-H), 3.78 (s, 3H, OCH3), 2.40 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6): δ = 177.1 (1C), 173.1 (1C), 170.2 (1C), 154.2 (1C), 147.6 (1C), 135.1 (1C), 130.7 (1C), 125.0 (1C), 124.6 (1C), 122.9 (1C), 122.3 (1C), 121.4 (2C), 114.1 (2C), 100.9 (1C), 55.5 (1C), 21.4 (1C) ppm. High-resolution mass spectrum, m/z: calculated 325.11883 for C18H17N2O4 [M + H]+; found 325.11825.
3.2.8. N-(p-tolyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (13)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and p-toluidine (1.2 g, 11.20 mmol) (6g) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A light yellow powder (0.73 g) with %yield: 71, Rƒ = 0.65 (CHCl3: MeOH-9.8: 0.2); m.p: 298–300 °C; 1H-NMR (500 Hz, DMSO-d6 +NaOD): δ = 7.66 (s, 1H, Ar-H), 7.54 (d, J = 7.7 Hz, 2H, Ar-H), 6.96-6.88 (m, 4H, Ar-H), 2.22 (s, 3H, CH3), 2.17 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6+NaOD): δ = 177.1 (1C), 172.5 (1C), 170.1 (1C), 146.9 (1C), 139.0 (1C), 130.9 (1C), 130.2 (1C), 129.4 (2C), 125.1 (1C), 125.0 (1C), 122.7 (1C), 121.8 (1C), 120.1 (2C), 100.93 (1C), 21.3 (1C), 20.9 (1C) ppm. High-resolution mass spectrum, m/z: calculated 309.12392 for C18H17N2O3 [M + H]+; found 309.12289.
3.2.9. N-(2-fluorophenyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (14)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 2-fluoroaniline (6h) (1.20 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A beige powder (0.51 g) with %yield: 41, Rƒ = 0.66 (CHCl3: MeOH-9.8: 0.2); m.p: 296–298 °C; 1H-NMR (400 Hz, DMSO-d6 +NaOD): δ = 8.75-8.65 (m, 1H, Ar-H), 7.72 (s, 1H, Ar-H), 7.16–7.02 (m, 1H, Ar-H), 7.0–6.94 (m, 2H, Ar-H), 6.93-6.90 (m, 2H, Ar-H), 2.27 (s, 3H, CH3) ppm; 13C-NMR (100 Hz, DMSO-d6+NaOD): δ = 177.7 (1C), 174.8 (1C), 170.9 (1C), 169.8 (1C), 153.7(1C), 151.3 (1C), 144.9 (1C), 138.1 (1C), 130.1 (1C), 129.9 (1C), 124.6 (1C), 123.3 (1C), 122.1 (1C), 121.2 (1C), 114.8 (1C), 100.2 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 313.09885 for C17H14FN2O3 [M + H]+; found 313.09824.
3.2.10. N-(3-fluorophenyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (15)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 3-fluoroaniline (6i) (1.20 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A beige powder (0.58 g) with %yield: 46, Rƒ = 0.70 (CHCl3: MeOH-9.8: 0.2); m.p: 294–296 °C; 1H-NMR (500 Hz, DMSO-d6 +NaOD): δ = 7.89–7.87 (m, 1H, Ar-H), 7.63 (s, 1H, Ar-H), 7.21–7.11 (m, 2H, Ar-H), 6.92 (d, J = 8.7 Hz, 1H, Ar-H), 6.88 (d, J = 8 Hz, 1H, Ar-H), 6.65-6.55 (m, 1H, Ar-H), 2.20 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6+NaOD): δ = 177.5 (1C), 172.8 (1C), 170.4 (1C), 163.9 (1C), 161.9 (1C), 147.9 (1C), 143.7 (1C), 143.6 (1C), 130.2 (1C), 130.1 (1C), 124.9 (1C), 122.7 (1C), 115.5 (1C), 107.5 (1C), 106.6 (1C), 100.8 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 313.09885 for C17H14FN2O3 [M + H]+; found 313.09879.
3.2.11. N-(4-fluorophenyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (16)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 2-fluoroaniline (6j) (1.20 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A beige powder (0.71 g) with %yield: 56, Rƒ = 0.61 (CHCl3: MeOH-9.8: 0.2); m.p: 303–305 °C; 1H-NMR (400 Hz, DMSO-d6 +NaOD): δ = 7.71–7.69 (m, 3H, Ar-H), 7.02–6.96 (m, 3H, Ar-H), 6.91 (s, 1H, Ar-H), 2.27 (s, 3H, CH3) ppm; 13C-NMR (100 Hz, DMSO-d6+NaOD): δ = 177.3 (1C), 172.4 (1C), 170.0 (1C), 158.3 (1C), 155.9 (1C), 147.2 (1C), 143.9 (1C), 138.3 (1C), 130.7 (1C), 125.0 (1C), 124.5 (1C), 122.8 (2C), 115.3 (1C), 115.09 (1C), 100.5 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 313.09885 for C17H14FN2O3 [M + H]+; found 313.09758.
3.2.12. N-(2-(trifluoromethyl)phenyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (17)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 2-trifluromethyl aniline (6k) (1.8 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A white powder (0.89 g) with %yield: 67, Rƒ = 0.64 (CHCl3: MeOH-9.8: 0.2); m.p: 285–287 °C; 1H-NMR (400 Hz, DMSO-d6 +NaOD): δ = 8.43 (d, J = 8.4 Hz, 1H, Ar-H), 7.68 (s, 1H, Ar-H), 7.57 (d, J = 8.0 Hz, 1H, Ar-H), 7.43 (t, J = 7.6 Hz, 1H, Ar-H), 7.07 (t, J = 8.0 Hz, 1H, Ar-H), 6.99-6.97 (m, 1H, Ar-H), 6.91-6.89 (m, 1H, Ar-H), 2.27 (s, 3H, CH3) ppm; 13C-NMR (100 Hz, DMSO-d6+NaOD): δ = 178.0 (1C), 172.5 (1C), 170.2 (1C), 139.7 (1C), 132.4 (1C), 130.8 (1C), 128.9 (1C), 125.2 (1C), 124.3 (1C), 123.4 (1C), 122.7 (1C), 121.7 (1C), 121.4 (1C), 120.7 (1C), 119.2 (1C), 118.9 (1C), 100.0 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 363.09565 for C18H14F3N2O3 [M + H]+; found 363.09516.
3.2.13. N-(3-(trifluoromethyl)phenyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (18)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 3-trifluromethyl aniline (6l) (1.8 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A white powder (0.95 g) with %yield: 75, Rƒ = 0.60 (CHCl3: MeOH-9.8: 0.2); m.p: 302–304 °C; 1H-NMR (400 Hz, DMSO-d6 +NaOD): δ = 8.25 (s, 1H, Ar-H), 7.76 (d, J = 7.6 Hz, 1H, Ar-H), 7.70 (s, 1H, Ar-H), 7.43 (t, J =8.0 Hz, 1H, Ar-H), 7.23–7.15 (m, 1H, Ar-H), 6.99–6.89 (m, 2H, Ar-H), 2.26 (s, 3H, CH3) ppm; 13C-NMR (100 Hz, DMSO-d6+NaOD): δ = 177.6 (1C), 170.4 (1C), 170.0 (1C), 129.9 (1C), 125.1 (1C), 125.0 (3C), 123.5 (1C), 123.1 (1C), 122.9 (3C), 122.7 (1C), 120.9 (2C), 100.5 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 363.09565 for C18H14F3N2O3 [M + H]+; found 363.09524.
3.2.14. N-(4-(trifluoromethyl)phenyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (19)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 4-trifluromethyl aniline (6m) (1.8 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A white powder (1.08 g) with %yield: 85, Rƒ = 0.59 (CHCl3: MeOH-9.8: 0.2); m.p: 317–319 °C; 1H-NMR (400 Hz, DMSO-d6 +NaOD): δ = 7.88 (d, J = 8.8 Hz, 2H, Ar-H), 7.53 (s, 1H, Ar-H), 7.51 (d, J = 8.4 Hz, 2H, Ar-H), 7.09–6.79 (m, 2H, Ar-H), 2.29 (s, 3H, CH3) ppm; 13C-NMR (100 Hz, DMSO-d6+NaOD): δ = 177.6 (1C), 170.4 (1C), 170.0 (1C), 129.9 (1C), 125.1 (1C), 125.0 (3C), 123.5 (1C), 123.1 (1C), 122.9 (3C), 122.7 (1C), 120.9 (2C), 100.5 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 363.09565 for C18H14F3N2O3 [M + H]+; found 363.09587.
3.2.15. N-(2-benzoic acid)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (20)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 2-amino benzoic acid (6n) (1.50 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A white powder (0.93 g) with %yield: 80, Rƒ = 0.53 (CHCl3: MeOH-9.0: 1.0); m.p: 290–292 °C; 1H-NMR (400 Hz, DMSO-d6 +NaOD): δ = 8.60 (d, J = 8.4 Hz, 1H, Ar-H), 7.79–7.69 (m, 2H, Ar-H), 7.11 (t, J = 7.6 Hz, 1H, Ar-H), 6.95–6.89 (m, 2H, Ar-H), 6.82–6.79 (m, 1H, Ar-H), 2.23 (s, 3H, CH3) ppm; 13C-NMR (100 Hz, DMSO-d6+NaOD): δ = 176.8 (2C), 172.9 (1C), 170.2 (1C), 146.9 (1C), 140.1 (1C), 130.7 (1C), 130.4 (1C), 130.1 (1C), 128.4 (1C), 125.4 (1C), 125.0 (1C), 124.6 (1C), 124.5 (1C), 123.7 (1C), 123.5 (1C), 102.1 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 339.09810 for C18H15N2O5 [M + H]+; found 339.09898.
3.2.16. N-(3-benzoic acid)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (21)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 3-amino benzoic acid (6o) (1.50 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A beige powder (1.03 g) with %yield: 88, Rƒ = 0.34 (CHCl3: MeOH-9.5: 0.5); m.p: 303–305 °C; 1H-NMR (400 Hz, DMSO-d6 +NaOD): δ = 8.17 (s, 1H, Ar-H), 7.74 (d, J = 7.6 Hz, 1H, Ar-H), 7.69 (s, 1H, Ar-H), 7.45 (d, J = 7.6 Hz, 1H, Ar-H), 7.14 (t, J = 7.6 Hz, 1H, Ar-H), 6.98–6.89 (m, 2H, Ar-H), 2.27 (s, 3H, CH3) ppm; 13C-NMR (100 Hz, DMSO-d6+NaOD): δ = 177.2 (1C), 173.1 (1C), 171.5 (1C), 170.3 (1C), 147.9 (1C), 140.9 (1C), 140.2 (1C), 127.6 (1C), 125.0 (1C), 124.0 (1C), 122.9 (1C), 122.5 (1C), 122.3 (1C), 121.4 (1C), 120.8 (1C), 100.9 (1C), 21.4 (1C) ppm. High-resolution mass spectrum, m/z: calculated 339.09810 for C18H15N2O5 [M + H]+; found 339.09854.
3.2.17. N-(4-benzoic acid)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (22)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 4-amino benzoic acid (6p) (1.50 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A yellow powder (0.99 g) with %yield: 83, Rƒ = 0.2 (CHCl3: MeOH-9.5: 0.5); m.p: 330–332 °C; 1H-NMR (500 Hz, DMSO-d6 +NaOD): δ = 7.79 (s, 1H, Ar-H), 7.64–7.54 (m, 2H, Ar-H), 6.93 (d, J = 8 Hz, 2H, Ar-H), 6.87 (d, J = 8 Hz, 2H, Ar-H), 2.23 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6+NaOD): δ = 177.3 (1C), 172.9 (1C), 171.7 (1C), 170.3 (1C), 147.8 (1C), 142.9 (1C), 132.3 (1C), 130.8 (1C), 130.3 (2C), 124.9 (1C), 124.5 (1C), 122.8 (1C), 122.2 (1C), 118.4 (2C), 100.9 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 339.09810 for C18H15N2O5 [M + H]+; found 339.09817.
3.2.18. N-(2-methyl benzoate)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (23)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and methyl 2-amino benzoate (6q) (1.69 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A white powder (1.2 g) with %yield: 93, Rƒ = 0.65 (CHCl3: MeOH-9.8:0.2); m.p: 237–239 °C; 1H-NMR (400 Hz, DMSO-d6 +NaOD): δ = 8.58 (d, J = 8.0 Hz, 1H, Ar-H), 7.78 (s, 1H, Ar-H), 7.66 (d, J = 8.0 Hz, 1H, Ar-H), 7.10 (t, J = 7.2 Hz, 1H, Ar-H), 6.96-6.89 (m, 2H, Ar-H), 6.83 (t, J = 7.6 Hz, 1H, Ar-H), 3.7 (s, 3H, -COOCH3), 2.23 (s, 3H, CH3) ppm; 13C-NMR (100 Hz, DMSO-d6+NaOD): δ = 172.9 (1C), 172.8 (1C), 171.9 (1C), 170.4 (1C), 146.4 (1C), 140.9 (1C), 130.7 (1C), 130.4 (2C), 128.4 (2C), 125.3 (1C), 124.2 (1C), 123.6 (1C), 122.2 (1C), 121.9 (1C), 102.1 (1C), 56.4 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 353.11375 for C19H17N2O5 [M + H]+; found 353.11375.
3.2.19. N-(4-chlorobenzoic acid)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (24)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 2-amino-4- chloro- benzoic acid (6r) (1.90 g, 11.20 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A white powder (0.15 g) with %yield: 10, Rƒ = 0.21 (CHCl3: MeOH-8.0: 2.0); m.p: 286–289 °C; 1H-NMR (400 Hz, DMSO-d6 +NaOD): δ = 8.83 (s, 1H, Ar-H), 7.79-7.72 (m, 2H, Ar-H), 6.93–6.85 (m, 3H, Ar-H), 2.24 (s, 3H, CH3) ppm; 13C-NMR (100 Hz, DMSO-d6+NaOD): δ = 176.9 (2C), 171.1 (1C), 169.3 (1C), 141.2 (2C), 133.1 (2C), 132.1 (1C), 131.1 (1C), 127.8 (1C), 125.8 (1C), 123.1 (1C), 120.7 (2C), 119.7 (1C), 101.3 (1C), 21.2 (1C) ppm. High-resolution mass spectrum, m/z: calculated 373.05912 for C18H14ClN2O5 [M + H]+; found 373.05899.
3.2.20. N-(5-methylbenzoic acid)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (25)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 2-amino-5-methyl benzoic acid (6s) (1.83 g, 12.13 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A yellow powder (1.2 g) with %yield: 84, Rƒ = 0.6 (CHCl3: MeOH-1: 9); m.p: 298–300 °C; 1H-NMR (500 Hz, DMSO-d6): δ = 18.8 (s, 1H, OH), 13.1 (s, 1H, COOH), 11.76 (s, 1H, NH), 10.67 (s, 1H, NH), 8.08 (s, 1H, Ar-H), 7.07-7.8 (m, 4H, Ar-H), 7.07 (s, 1H, Ar-H), 2.40 (s, 3H, CH3), 2.35 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6): δ = 176.5 (1C), 173.3 (1C), 172.1 (1C), 170.1 (1C), 146.6 (1C), 137.1 (1C), 130.6 (1C), 130.2 (1C), 130.1 (1C), 129.2 (1C), 129.0 (1C), 125.2 (1C), 124.9 (1C), 123.4 (1C), 122.3 (1C), 121.4 (1C), 102.3 (1C), 21.3 (1C), 20.9 (1C) ppm. High-resolution mass spectrum, m/z: calculated 353.11375 for C19H17N2O5 [M + H]+; found 353.11301.
3.2.21. N-(4-mercaptophenyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (26)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 4-amino- benzenethiol (6t) (1.57 g, 12.52 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A yellow powder (1.0 g) with %yield: 84, Rƒ = 0.7 (CHCl3: MeOH-9.5: 0.5); m.p: 299–303 °C; 1H-NMR (500 Hz, DMSO-d6+NaOD): δ = 7.66 (s, 1H, Ar-H), 7.09 (d, J = 8.3 Hz, 1H, Ar-H), 6.98 (d, J = 8.2 Hz, 4H, Ar-H), 6.89 (d, J = 8.2 Hz, 1H, Ar-H), 2.31 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6+NaOD): δ = 177.3 (1C), 176.7 (1C), 171.1 (1C), 168.9 (1C), 147.5 (1C), 144.6 (1C), 133.0 (1C), 132.6 (1C), 131.0 (1C), 125.7 (1C), 125.1 (1C), 122.7 (1C), 120.2 (1C), 119.7 (1C), 119.2 (1C), 100.8 (1C), 21.3 (1C) ppm. High-resolution mass spectrum, m/z: calculated 327.08034 for C17H15N2O3S [M + H]+; found 327.08078.
3.2.22. N-(4-(methylthio) phenyl)-4-hydroxy-6-methyl-2-quinolone-3-carboxamide (27)
Ethyl 4-hydroxy-6-methyl-2-quinolone-3-carboxylate (5) (1 g, 4.044 mmol) and 4-(methylthio) benzenamine (6u) (1.68 g, 12.13 mmol) were dissolved in 25 mL THF. A few drops of DMF was added then the mixture was refluxed at 150–160 °C for 48 h. Completion of the reaction was indicated by absence of 5 on TLC and appearance of precipitate at RT. The solid was isolated by filtration, washed with absolute ethanol, and dried under reduced pressure.
A green powder (0.641 g) with %yield: 56, Rƒ = 0.7 (CHCl3: MeOH-9.5: 0.5); m.p: 297–299 °C; 1H-NMR (500 Hz, DMSO-d6+NaOD): δ = 14.1 (s, 1H, Ar-OH), 7.73 (s, 1H, Ar-H), 7.62 (d, J = 8.6 Hz, 2H, Ar-H), 7.09 (d, J = 8.5 Hz, 2H, Ar-H), 6.96 (d, J = 8.2 Hz, 2H, Ar-H), 2.31 (s, 3H, CH3), 2.12 (s, 3H, CH3) ppm; 13C-NMR (125 Hz, DMSO-d6+NaOD): δ = 177.5 (1C), 168.5 (1C), 167.1 (1C), 139.2 (1C), 139.0 (1C), 132.0 (1C), 131.9 (1C), 129.5 (1C), 128.2 (2C), 125.7 (1C), 122.1 (1C), 120.6 (1C), 120.5 (1C), 116.20 (1C), 99.3 (1C), 21.2 (1C), 16.6 (1C) ppm. High-resolution mass spectrum, m/z: calculated 341.09599 for C18H17N2O3S [M + H]+; found 341.09519.
3.3. Biology
3.3.1. Culture Conditions
Two human colon cancer cell lines; Caco-2 and HCT-116 were maintained in DMEM culture medium (Dulbecco’s modified essential medium, Gibco) (Waltham, MA, USA), supplemented with 5% (v/v) fetal calf serum (JS Bioscience, Redland, Queensland, Australia), and 1% (v/v) antibiotic (2 mM L-glutamine, 100 U/mL Penicillin and 0.1 mg/mL Streptomycin; Gibco). Cells were cultured at 37 °C in a humidified 5% CO2 incubator. Enzymatic detachment of the confluent cell layers was carried out using Trypsin/ EDTA (Gibco, Waltham, MA, USA), and trypan blue vital staining (0.4% (w/v); Sigma, USA) was used to assess cell viability with cell number determined using a light microscope.
3.3.2. MTT Assay
All cells were plated at density of 8 × 103 cells per well in 96-well plates and incubated to allow attachment for 24 h. The in vitro evaluation of the antiproliferative activities of the examined series was accomplished using the 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) colorimetric assay, as previously described [41]. In brief, compounds were diluted in culture media to yield the required concentration and applied to test wells for 48 h at 37 °C in a 5% CO2 incubator. Three triplicates of each concentration for all tested compounds were evaluated in three independent assays (n = 9). DMEM samples were employed as negative controls, and doxorubicin as a positive control. At the end of the exposure period, 20 µL of 0.5 mg/mL of MTT was added to each well and incubated for 4 h, afterword its reduction to formazan by metabolically active cells was calculated via measuring the absorbance at 570 nm. Cell viability was calculated based on the measured absorbance relative to the absorbance of the cells exposed to the negative control, which represented 100% cell viability.
3.3.3. Statistical Analysis
Data analysis was performed using GraphPad Prism software version 7 (San Diego, CA, USA). The differences between treatment groups were determined by t-test or one-way analysis of variance (ANOVA) followed by Tukey post hoc T-test as appropriate. Data were expressed as mean ± SD and p < 0.05 was considered a statistically significant difference. A non-linear regression analysis was used to calculate IC50 values.
3.3.4. Quantitative Real-Time PCR
RNA Extraction
Total RNA was extracted from the cultured cells using Quick-RNA MiniPrep according to the manufacturer’s instructions as follows: harvested cells were re-suspended in RNA lysis buffer and centrifuged, the supernatant then was transferred into a Spin-Away Filter in a collection tube and centrifuged, and then 95% ethanol was added and mixed well. The mixture was then transferred to a Zymo-Spin IIICG Column in a collection tube and centrifuged, followed by adding RNA Prep Buffer to the column, followed by two washing steps by adding RNA wash buffer to the column and then centrifuged. Finally, the RNA was eluted by adding DNase/RNase-free water to the column and it was centrifuged. Samples were quantified using a spectrophotometer via absorbance at 260/280 nm.
Complementary DNA (cDNA) Synthesis
Complementary cDNA synthesis was performed using ProtoScript First Strand cDNA Synthesis Kit following the manufacturer’s instructions. In brief: 1000 ng from total RNA was added to a total of 20 µL reaction volume that included 60 µM random primer mix, reaction mix, 10× enzyme mix, and nuclease-free water. The tubes were then incubated at 25 °C for 5 min followed by incubation at 42 °C for 1 h. The cDNA was then then stored at −20 °C until further analysis.
Real-Time PCR
The sequences of the primers that were used in real-time PCR assay are shown in Table 4. The reaction mixtures consisted of 200 ng cDNA template, 10 µM of each primer, 10 µL of 2× SYBER Premix Ex Taq II (Takara BIO INC, Shiga, Japan), and the total reaction volume was 20 µL. The reaction was carried out using a BIO RAD iQ5 Multicolor real-time PCR Detection System thermal cycler under the following reaction conditions: 1 cycle of 2 min at 95.0 °C, followed by 45 cycles of 10 s at 95.0 °C, 25 s at 57.0 °C, 25 s at 60.0 °C, and a final cycle of 30 s at 55.0 °C. To confirm that only one PCR product was amplified, dissociation curve analysis of amplification products was performed at the end of the last amplification cycle.
Table 4.
Sequences of primers that were used to detect the expression of β-actin, BAD, PI3K, and AKT.
| Target | Forward Primers (5′→3′) | Reverse Primers (5′→3′) |
|---|---|---|
| β-actin | ACGGGGTCACCCACACTGTGC | CTAGAAGCATTTGCGGTGGACGATG |
| BAD | CCTCAGGCCTATGCAAAAAG | AAACCCAAAACTTCCGATGG |
| PI3K | ACCCAGCAACAGAAAAATGG | GCGCTGTGAATTTAGCCTTC |
| AKT | AACCTGTGCTCCATGACCTC | CCCTTCTACAACCAGGACCA |
3.4. Computational Methods
3.4.1. Preparation of PI3Kα Structure
The X-ray structures of WT PI3Kα (PDB ID: 2RD0) [4] and (PDB ID: 4L23) [33] as well as MUT (H1047R) PI3Kα (PDB ID: 3HHM) [29] were retrieved from the RCSB Protein Data Bank. The coordinates of X6K in 4L23 [33] were transferred to 2RD0 and assigned as a ligand to derive the grid file. The homology modeled structures of 2RD0 and 3HHM were adopted for this study [14]. Energy minimization was employed to reduce steric clash.
Further treatment of the minimized structures was performed using Protein Preparation module in Schrödinger enterprise [32] to optimize H-bond interactions between backbones.
3.4.2. Preparation of Ligand Structures
The synthesized compounds (ligands) were built using wortmannin’s coordinates in 3HHM. The ligands were modeled using MAESTRO [32] Build wizard and energetically minimized by the MacroModel module using OPLS2005 force field.
3.4.3. Induced-Fit Docking (IFD)
The co-crystallized ligand (wortmannin) was assigned as a centroid in the kinase binding site of 2RD0 [4] and 3HMM [29]. The Vander Waals scaling factors for receptor and ligand were adjusted to 0.5 to furnish flexibility for the best docked ligand conformation. Other parameters were set as default. The ligand pose with the highest XP Glide score was identified.
3.4.4. Molecular Descriptors
All molecular structures, sketched in ChemDraw [42] and saved in SDF file format, were standardized according to the methods described by Hajjo et al. [43]. Next, two groups of molecular descriptors, comprising ‘Drug-like Indices’ and ‘Molecular Properties’ calculated using alvaDesc software from Kode Cheminformatics [40], were generated for compounds 5, 7–27.
3.4.5. Principal Component Analysis (PCA)
A principal component analysis was performed on structures 5 and 7–27 using drug-like indices and molecular properties. All calculations and PCA analysis were performed using alvaDesc software from Kode Cheminformatics [40].
4. Conclusions
Phosphatidylinositol 3-kinase (PI3Kα) has been underlined as a potential target for anticancer drug design. We identified a series of N-phenyl-4-hydroxy-6-methyl-2-quinolone-3-carboxamide as possible PI3Ká inhibitors. Biological evaluation showed that the series exhibited high inhibitory activity against Caco-2 and HCT-116 cell lines. Results revealed that compound 16 has a substantial effect on AKT, BAD, and PI3K gene expression. Docking studies against PI3Kαs displayed that the scaffold orientates in PI3Kαs kinase domains and form H-bonds with key binding residues. We are looking to optimize the core structure of this series to induce its anticancer activity and selectivity against a panel of kinases.
Acknowledgments
We thank the University of Jordan and Hashemite University, the Chemistry Departments for spectroscopic facilities. We are grateful to the College of Pharmacy, the University of Jordan for use of cell culture laboratory and equipment.
Supplementary Materials
The following are available online, Table S1: Drug-like and alvaDesc molecular properties.
Author Contributions
D.A.S. is responsible for the study design, hypothesis, monitoring synthesis, modeling studies, and writing the manuscript. S.E.H., conducted the synthesis reaction and purification of verified molecules. R.A.K. and K.A.S. analyzed NMR charts and HRMS data and edited the chemistry part. S.K.B. and K.M.A. performed cancer biology experiments and wrote biological results and discussion. R.H. performed molecular descriptor analysis and wrote the corresponding results and discussion. A.M.A.-Z. assisted collaborators in synthesis, purification, and spectroscopy analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This work was financially supported by the Scientific Research Support Fund of the Ministry of Higher Education & Scientific Research and the Deanship of Scientific Research and Graduate Studies at Al-Zaytoonah University of Jordan (Grant number: MPH/1/8/2017).
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability: Samples of the compounds are available from the authors.
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Vanhaesebroeck B., Waterfield M.D. Signaling by distinct classes of phosphoinositide 3-kinases. Exp. Cell Res. 1999;253:239–254. doi: 10.1006/excr.1999.4701. [DOI] [PubMed] [Google Scholar]
- 2.Vanhaesebroeck B., Guillermet-Guibert J., Graupera M., Bilanges B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010;11:329–341. doi: 10.1038/nrm2882. [DOI] [PubMed] [Google Scholar]
- 3.Cantley L.C. The phosphoinositide 3-kinase pathway. Science. 2002;296:1655–1657. doi: 10.1126/science.296.5573.1655. [DOI] [PubMed] [Google Scholar]
- 4.Huang C.-H., Mandelker D., Schmidt-Kittler O., Samuels Y., Velculescu V.E., Kinzler K.W., Vogelstein B., Gabelli S.B., Amzel L.M. The structure of a human p110 alpha/p85 alpha complex elucidates the effects of oncogenic PI3K alpha mutations. Science. 2007;318:1744–1748. doi: 10.1126/science.1150799. [DOI] [PubMed] [Google Scholar]
- 5.Vivanco I., Sawyers C.L. The phosphatidylinositol 3-kinase-AKT pathway in human cancer. Nat. Rev. Cancer. 2002;2:489–501. doi: 10.1038/nrc839. [DOI] [PubMed] [Google Scholar]
- 6.Samuels Y., Wang Z.H., Bardelli A., Silliman N., Ptak J., Szabo S., Yan H., Gazdar A., Powell D.M., Riggins G.J., et al. High frequency of mutations of the PIK3CA gene in human cancers. Science. 2004;304:554. doi: 10.1126/science.1096502. [DOI] [PubMed] [Google Scholar]
- 7.Samuels Y., Diaz L.A., Schmidt-Kittler O., Cummins J.M., DeLong L., Cheong I., Rago C., Huso D.L., Lengauer C., Kinzler K.W., et al. Mutant PIK3CA promotes cell growth and invasion of human cancer cells. Cancer Cell. 2005;7:561–573. doi: 10.1016/j.ccr.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 8.Zhao L., Vogt P.K. Helical domain and kinase domain mutations in p110 alpha of phosphatidylinositol 3-kinase induce gain of function by different mechanisms. Proc. Natl. Acad. Sci. USA. 2008;105:2652–2657. doi: 10.1073/pnas.0712169105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu P., Cheng H., Roberts T.M., Zhao J.J. Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 2009;8:627–644. doi: 10.1038/nrd2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cully M., You H., Levine A.J., Mak T.W. Beyond PTEN mutations: The PI3K pathway as an integrator of multiple inputs during tumorigenesis. Nat. Rev. Cancer. 2006;6:184–192. doi: 10.1038/nrc1819. [DOI] [PubMed] [Google Scholar]
- 11.Carracedo A., Pandolfi P.P. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene. 2008;27:5527–5541. doi: 10.1038/onc.2008.247. [DOI] [PubMed] [Google Scholar]
- 12.Release 3; National Cancer Institute. [(accessed on 15 June 2012)]; Available online: http://cactus.nci.nih.gov/download/nci.
- 13.Sabbah D.A., Simms N.A., Brattain M.G., Vennerstrom J.L., Zhong H. Biological evaluation and docking studies of recently identified inhibitors of phosphoinositide-3-kinases. Bioorg. Med. Chem. Lett. 2012;22:876–880. doi: 10.1016/j.bmcl.2011.12.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sabbah D.A., Vennerstrom J.L., Zhong H. Docking studies on isoform-specific inhibition of phosphoinositide-3-kinases. J. Chem. Inf. Model. 2010;50:1887–1898. doi: 10.1021/ci1002679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.MOE . Version 2016. The Molecular Operating, Environment Chemical Computing Group, Inc.; Montreal, QC, Canada: [Google Scholar]
- 16.Sabbah D.A., Vennerstrom J.L., Zhong H.A. Binding selectivity studies of phosphoinositide 3-kinases using free energy calculations. J. Chem. Inf. Model. 2012;52:3213–3224. doi: 10.1021/ci3003057. [DOI] [PubMed] [Google Scholar]
- 17.Sabbah D.A., Simms N.A., Wang W., Dong Y., Ezell E.L., Brattain M.G., Vennerstrom J.L., Zhong H.A. N-Phenyl-4-hydroxy-2-quinolone-3-carboxamides as selective inhibitors of mutant H1047R phosphoinositide-3-kinase (PI3Kα) Bioorg. Med. Chem. 2012;20:7175–7183. doi: 10.1016/j.bmc.2012.09.059. [DOI] [PubMed] [Google Scholar]
- 18.Sabbah D.A., Hishmah B., Sweidan K., Bardaweel S., AlDamen M., Zhong H.A., Abu-Khalaf R., Hasan-Ibrahim A., Al-Qirim T., Abu-Sheikha G. Structure-based design: Synthesis, X-ray crystallography, and biological evaluation of N-substituted-4-hydroxy-2-quinolone-3-carboxamides as potential cytotoxic agents. Anticancer Agents Med. Chem. 2018;18:263–276. doi: 10.2174/1871520617666170911171152. [DOI] [PubMed] [Google Scholar]
- 19.Sabbah D.A., Saada M., Khalaf R.A., Bardaweel S., Sweidan K., Al-Qirim T., Al-Zughier A., Halim H.A., Sheikha G.A. Molecular modeling based approach, synthesis, and cytotoxic activity of novel benzoin derivatives targeting phosphoinostide 3-kinase (PI3Kα) Bioorg. Med. Chem. Lett. 2015;25:3120–3124. doi: 10.1016/j.bmcl.2015.06.011. [DOI] [PubMed] [Google Scholar]
- 20.Sweidan K., Sabbah D.A., Bardaweel S., Dush K.A., Sheikha G.A., Mubarak M.S. Computer-aided design, synthesis, and biological evaluation of new indole-2-carboxamide derivatives as PI3Kα/EGFR inhibitors. Bioorg. Med. Chem. Lett. 2016;26:2685–2690. doi: 10.1016/j.bmcl.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 21.Sweidan K., Sabbah D.A., Bardaweel S., Abu-Sheikha G., Al-Qirim T., Salih H., El-Abadelah M.M., Mubarak M.S., Voelter W. Facile synthesis, characterization, and cytotoxicity study of new 3-(indol-2-yl)bicyclotetrazatridecahexaens. Can. J. Chem. 2017;95:858–862. doi: 10.1139/cjc-2017-0120. [DOI] [Google Scholar]
- 22.Sweidan K., Zalloum H., Sabbah D.A., Idris G., Abudosh K., Mubarak M.S. Synthesis, characterization, and anticancer evaluation of some new N1-(anthraquinon-2-yl) amidrazone derivatives. Can. J. Chem. 2018;96:1123–1128. doi: 10.1139/cjc-2018-0145. [DOI] [Google Scholar]
- 23.Sabbah D.A., Al-Tarawneh F., Talib W.H., Sweidan K., Bardaweel S.K., Al-Shalabi E., Zhong H.A., Abu Sheikha G., Abu Khalaf R., Mubarak M.S. Benzoin schiff bases: Design, synthesis, and biological evaluation as potential antitumor agents. Med. Chem. 2018;14:695–708. doi: 10.2174/1573406414666180412160142. [DOI] [PubMed] [Google Scholar]
- 24.Sabbah D.A., Ibrahim A.H., Talib W.H., Alqaisi K.M., Sweidan K., Bardaweel S.K., Sheikha G.A., Zhong H.A., Al-Shalabi E., Khalaf R.A. Ligand-based drug design: Synthesis and biological evaluation of substituted benzoin derivatives as potential antitumor agents. Med. Chem. 2019;15:417–429. doi: 10.2174/1573406414666180912111846. [DOI] [PubMed] [Google Scholar]
- 25.Wang Q., Wang X., Hernandez A., Kim S., Evers B.M. Inhibition of the phosphatidylinositol 3-kinase pathway contributes to HT29 and Caco-2 intestinal cell differentiation. Gastroenterology. 2001;120:1381–1392. doi: 10.1053/gast.2001.24044. [DOI] [PubMed] [Google Scholar]
- 26.Sambuy Y., de Angelis I., Ranaldi G., Scarino M., Stammati A., Zucco F. The Caco-2 cell line as a model of the intestinal barrier: Influence of cell and culture-related factors on Caco-2 cell functional characteristics. Cell Biol. Toxicol. 2005;21:1–26. doi: 10.1007/s10565-005-0085-6. [DOI] [PubMed] [Google Scholar]
- 27.Sheng H., Shao J., Townsend C.M., Evers B.M. Phosphatidylinositol 3-kinase mediates proliferative signals in intestinal epithelial cells. Gut. 2003;52:1472–1478. doi: 10.1136/gut.52.10.1472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brattain M.G., Levine A.E., Chakrabarty S., Yeoman L.C., Willson J.K.V., Long B. Heterogeneity of human colon carcinoma. Cancer Metastasis Rev. 1984;3:177–191. doi: 10.1007/BF00048384. [DOI] [PubMed] [Google Scholar]
- 29.Mandelker D., Gabelli S.B., Schmidt-Kittler O., Zhu J., Cheong I., Huang C.-H., Kinzler K.W., Vogelstein B., Amzel L.M. A frequent kinase domain mutation that changes the interaction between PI3K alpha and the membrane. Proc. Natl. Acad. Sci. USA. 2009;106:16996–17001. doi: 10.1073/pnas.0908444106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Friesner R.A., Banks J.L., Murphy R.B., Halgren T.A., Klicic J.J., Mainz D.T., Repasky M.P., Knoll E.H., Shelley M., Perry J.K., et al. Glide: A new approach for rapid, accurate docking and scoring. 1. Method and assessment of docking accuracy. J. Med. Chem. 2004;47:1739–1749. doi: 10.1021/jm0306430. [DOI] [PubMed] [Google Scholar]
- 31.Friesner R.A., Murphy R.B., Repasky M.P., Frye L.L., Greenwood J.R., Halgren T.A., Sanschagrin P.C., Mainz D.T. Extra precision glide: Docking and scoring incorporating a model of hydrophobic enclosure for protein-ligand complexes. J. Med. Chem. 2006;49:6177–6196. doi: 10.1021/jm051256o. [DOI] [PubMed] [Google Scholar]
- 32.Schrödinger . Protein Preparation Wizard, Maestro, Macromodel, QPLD-dock, and Pymol. Schrödinger, LLC; Portland, OR, USA: 2016. [Google Scholar]
- 33.Zhao Y., Zhang X., Chen Y., Lu S., Peng Y., Wang X., Guo C., Zhou A., Zhang J., Luo Y., et al. Crystal structures of PI3Kalpha complexed with PI103 and its derivatives: New directions for inhibitors design. ACS Med. Chem. Lett. 2013;5:138–142. doi: 10.1021/ml400378e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sweidan K., Sabbah D.A., Engelmann J., Halim H.A., Sheikha G.A. Computational Docking Studies of Novel Heterocyclic Carboxamides as Potential PI3Kα Inhibitors. Lett. Drug Des. Discov. 2015;12:856–863. doi: 10.2174/1570180812666150529205248. [DOI] [Google Scholar]
- 35.alvaDesc. [(accessed on 30 September 2020)]; Available online: https://chm.kode-solutions.net/products_alvadesc.php.
- 36.Monge A., Arrault A., Marot C., Morin-Allory L. Managing, profiling and analyzing a library of 2.6 million compounds gathered from 32 chemical providers. Mol. Divers. 2006;10:389–403. doi: 10.1007/s11030-006-9033-5. [DOI] [PubMed] [Google Scholar]
- 37.Congreve M., Carr R., Murray C., Jhoti H. A ‘rule of three’ for fragment-based lead discovery? Drug Discov. Today. 2003;8:876–877. doi: 10.1016/S1359-6446(03)02831-9. [DOI] [PubMed] [Google Scholar]
- 38.Chen G., Zheng S., Luo X., Shen J., Zhu W., Liu H., Gui C., Zhang J., Zheng M., Puah C.M. Focused combinatorial library design based on structural diversity, druglikeness and binding affinity score. J. Comb. Chem. 2005;7:398–406. doi: 10.1021/cc049866h. [DOI] [PubMed] [Google Scholar]
- 39.Zheng S., Luo X., Chen G., Zhu W., Shen J., Chen K., Jiang H. A new rapid and effective chemistry space filter in recognizing a druglike database. J. Chem. Inf. Model. 2005;45:856–862. doi: 10.1021/ci050031j. [DOI] [PubMed] [Google Scholar]
- 40.Kode. [(accessed on 10 September 2020)]; Available online: https://chm.kode-solutions.net/products_dragon.php.
- 41.Bardaweel S.K., Abu-Dahab R., Almomani N.F. An in vitro based investigation into the cytotoxic effects of D-amino acids. Acta Pharm. 2013;63:467–478. doi: 10.2478/acph-2013-0032. [DOI] [PubMed] [Google Scholar]
- 42.Mendelsohn L.D. ChemDraw 8 ultra, windows and macintosh versions. J. Chem. Inf. Model. 2004;44:2225–2226. doi: 10.1021/ci040123t. [DOI] [Google Scholar]
- 43.Hajjo R., Setola V., Roth B.L., Tropsha A. Chemocentric informatics approach to drug discovery: Identification and experimental validation of selective estrogen receptor modulators as ligands of 5-hydroxytryptamine-6 receptors and as potential cognition enhancers. J. Med. Chem. 2012;55:5704–5719. doi: 10.1021/jm2011657. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.