Abstract
Aging is a complicated biological process in which functional and structural alterations in a living organism take place over time. Reactive oxygen species is one of the main factors responsible for aging and is associated with several chronic pathologies. The relationship between aging and diet is quite interesting and has attained worldwide attention. Healthy food, in addition to dietary antioxidants, are required to delay the process of aging and improve the quality of life. Many healthy foods such as fruits are a good source of dietary nutrients and natural bioactive compounds which have antioxidant properties and are involved in preventing aging and other age-related disorders. Health benefits linked with healthy consumption of fruit have drawn increased interest. A significant number of studies have documented the advantages of fruit intake, as it suppresses free-radical development that further reduces the oxidative stress created in the body and protects against several types of diseases such as cancer, type 2 diabetes, inflammatory disorders, and other cardiovascular diseases that ultimately prevent aging. In addition, fruits have numerous other properties like anti-inflammatory, anti-cancerous, anti-diabetic, neuroprotective, and have health-promoting effects. Mechanisms of various bioactive compounds that aids in preventing various diseases and increases longevity are also described. This manuscript provides a summary of various bioactive components present in fruits along with their health-promoting and antiaging properties.
Keywords: bioactive compounds, anti-aging, edible fruits, life extension, antioxidants, free radicals, health benefits
1. Introduction
Aging is a slow process of physiological deterioration that each living organism experiences with time. In fact, aging is a primary risk factor connected with considerably raised incidences of several degenerative diseases in particular type 2 diabetes, cancer, Alzheimer’s disease, cardiovascular disease (CVD), and these chronic diseases account for deaths among peoples [1]. Aging at the biological level is distinguished by the accumulation of cell and molecular damage resulting in functional and structural changes in tissues and cells, such as impaired intercellular contact, senescence, loss of mitochondrial homeostasis, and reduced regenerative ability [2]. Approximately 150,000 people across the world die every day of aging and around two-thirds die of age-related diseases [3]. Among several agents that are believed to play a significant part in the age-linked decline of functions, are free radicals that include reactive nitrogen species (RNS) and reactive oxygen species (ROS) and play a vital role [4,5].
Free radicals and reactive species are natural byproducts that are generated in organisms through both physiological and environmental processes [6]. Free radicals are normally generated as a result of ATP (adenosine triphosphate) production in the mitochondria when cells use oxygen (an essential element for life) to produce energy. Thus, an imbalance between overproduction or accretion of free radicals in the body and the potential of a biological system to detoxify the reactive substances results in oxidative stress, which is the leading factor in the development of several degenerative and age-linked chronic disorders [7,8]. A plethora of evidence proposed that the oxidative stress produced in mitochondria as a byproduct of cellular respiration is the main reason for aging [9]. Delay or inhibition in the pathogenesis of such diseases by plants is also an attractive strategy to encourage healthy aging [3]. So, a proper nutritional diet is recognized in combating these diseases as it has a substantial influence on aging and health without any side effects [10]. Furthermore, the optimistic connection between aging and diet has escalated the consumer interest in gaining more knowledge of a functional diet, rich in antioxidants, such as vegetables, fruits, and their related products [11,12,13,14]. Antioxidants are the natural substances present in fruits and vegetables that protect the cell from free radical damage by neutralizing and scavenging them. Among these, fruits are of great importance and have attracted worldwide attention from researchers due to their nutritional value, delicious taste, vitamins, minerals, and fiber content. Several research findings have also reported that healthy fruit intake is related to a lower prevalence of chronic diseases [15,16,17,18,19].
Various fruits and their derivatives are well-known to hold an elevated level of naturally occurring polyphenolic compounds [18,20,21,22]. Polyphenols are the plant secondary metabolites with antioxidant properties that function as free radical inhibitors and play a vital part in reducing oxidative stress that ultimately prevents aging and their associated diseases [23,24]. Moreover, several bioactive compounds such as catechins, anthocyanins, and isoflavones have potent antioxidant activity against ROS. Frequently consumed fruits, particularly apples, grapes, berries, oranges, and cherries contain different polyphenolic compounds that have a beneficial impact on human health [25,26]. The presence of high bioactive content in these fruits aid in delaying the processes of aging and alleviates the risk of various age-linked chronic disorders like CVD and cancer. Polyphenols, however, contain a broad diversity of compounds and are categorized into various groups like stilbenes, tannins, flavonoids (flavanones, flavanols, flavones, flavonols, isoflavones, proanthocyanidins, anthocyanins), phenolic acids, and lignans [27,28,29]. It has been documented that an extensive variety of antioxidant activities and phytochemical levels occur inside and across fruit genera [22,30].
Pterostilbene, resveratrol, and quercetin are the naturally occurring phytochemicals or the polyphenolic antioxidants present in a variety of fruits such as cranberries, bilberries, and blueberries (Vaccinium sp.) [31]. Recent findings have shown that these have positive effects such as anti-aging and a tendency to prolong lifespan by controlling the signs of aging, inflammation, cell senescence, oxidative damage, and telomeric attrition [32,33]. The main purpose of this manuscript is to develop an outline about various nutraceuticals and bioactive compounds present in fruits, and their anti-aging, and other health-promoting properties that increase life expectancy in humans.
2. Free Radicals and Aging
Aging refers to the universal, progressive, and deleterious changes in organisms that occur with time and which intensifies the probability of several diseases and sometimes leads to death [34]. Interestingly, chronic diseases and aging both are highly linked with DNA mutations, low-grade inflammation, and increased metabolic and oxidative stress, including escalated levels of its damage [3]. The human body is in a continuous fight to stay itself away from aging. One of the well-studied and most prominent theories about aging is the free radical theory of aging [5].
Free radicals are unstable, highly reactive, and self-existent molecules, comprising one or more unpaired electron in a nuclear orbit. They can either accept an electron or donate an electron to other molecules and thus serve as reductants or oxidants [35]. Free radicals are generally present in the body as a natural byproduct of chemical processes like metabolism that can upsurge the chances of various diseases and quicken the process of aging. ROS such as superoxide radical (O2•), peroxyl radical (ROO•), alkoxyl radical (RO•), hydroxyl radical (•OH), and RNS such as nitric oxide (NO) and nitrogen dioxide (NO2) are among the most common free radicals originating from both exogenous and endogenous sources [6]. Exogenous sources of ROS/RNS include environmental pollutants, radiations, industrial chemicals, drugs, xenobiotics, and smoke [35,36,37]. The endogenous sources include phagocytosis, inflammatory responses, and cellular metabolic processes such as mitochondrial electron transport [38,39].
Overproduction of ROS in the human body damages diverse biomolecules via redox reactions and leads to cellular damage, mutation, cell death, and aging [40,41,42,43]. ROS are also implicated in several chronic illnesses and other age-related disorders. Generally, two groups of antioxidants, viz. enzymatic and non-enzymatic antioxidants, regulate free radical reactions. The human body uses enzymatic antioxidant defense mechanisms to maintain the balance between free radicals and antioxidants by eliminating surplus ROS. The antioxidant enzymes minimize the level of H2O2 as it is essential in the preclusion of lipid peroxidation and retaining the structure and function of cell membranes. Various enzymatic antioxidant enzymes that are involved in free radical scavenging activity are superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GSHPx) as shown in the following reactions.
SOD are found in mitochondria and cytosol of the cell, catalytically convert the superoxide radical (O2•−) into hydrogen peroxide (H2O2) and oxygen (O2) in the presence of metal ion cofactors like zinc (Zn) and copper (Cu) [44]. The CAT enzyme located in peroxisome uses iron as a cofactor and catalyzes the reduction or degradation of hydrogen peroxide (H2O2) to form water (H2O) and molecular oxygen (O2), thus completing the detoxification process started by SOD [45]. GSHPx is an intracellular enzyme present mainly in mitochondria and cytosol, that breaks hydrogen peroxide (H2O2) into two water molecules (H2O) and oxidizes GSH (glutathione). The activity of GSHPx generally depends on the selenium [46].
Similarly, RNS such as nitric oxide (NO•) is produced in the human body from amino acid L-arginine in the presence of enzyme nitric oxide synthase (NOS) as shown in the equation:
Generation of free radicals occurs with the absorption of oxygen, the activation of NADPH oxidase, and the production of superoxide anion radicals is shown in the equation:
The inducible nitrogen oxide synthase (iNOS) is involved in the synthesis of NO• and reacts with oxygen radicals (O2)•−. The NO• and O2•− react together (radical-radical coupling) to yield peroxynitrite (ONOO−), which is a powerful oxidant that can attack a large range of biological targets [47].
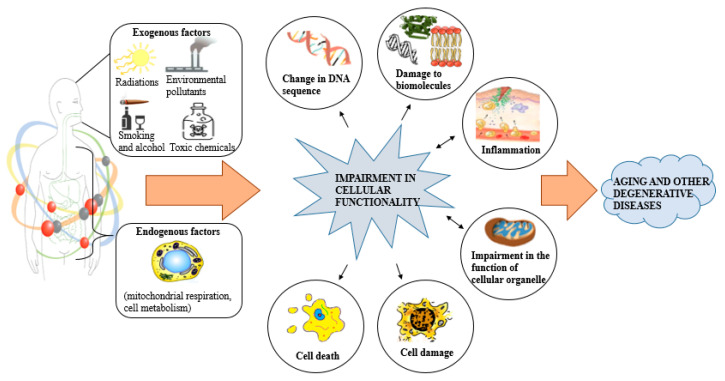
Insufficient antioxidant defense systems and a great amount of ROS/RNS cause the accretion of free radicals in the cells, which causes oxidative damage [48]. Cellular oxidative stress causes protein dysfunction, loss of structural integrity, deleterious damage to the cell membrane, DNA, mitochondrial DNA contributes considerably to chronic age-linked diseases mainly CVD, type 2 diabetes, cancer, hypertension, and atherosclerosis as shown in Figure 1 [39,49,50,51,52].
Figure 1.
Mechanism of free radical formation and its impact on the cell. Modified from Khanthapok et al. [53] with permission.
3. Nutraceuticals and Bioactive Compounds in Fruits: Source of Antioxidants
Plants synthesize various phenolic compounds that are present in different parts of the plant, but particularly in fruits, leaves, and seeds, where they are mainly used to protect against pathogens and UV radiations [54,55]. Many foods (plant-based) in a healthy diet like fruits and vegetables contain most of the naturally occurring polyphenols [24]. Fruits are not merely a source of non-nutritive compounds containing phenolics but a great source of a large variety of nutritional compounds containing minerals (iron, copper, zinc, manganese, and selenium), vitamins (C, A, E), and dietary fibers [15,17,56,57]. These minerals and vitamins serve as antioxidants that help in reducing several chronic and age-related diseases, mainly diabetes, cancer, coronary heart disease, CVD, and provide beneficial health benefits and encourage healthy aging [55]. Antioxidants also help in reducing inflammation [58]. As dietary compounds present in fruits activate the nuclear erythroid-2 like factor-2 (Nrf2), a key regulator of antioxidants that inhibits the activation of NF-κB (nuclear factor kappa B) pathway which is involved in developing inflammation. Nrf2 increases antioxidant defenses, which efficiently neutralizes ROS by regulating toll-like receptor 4-mediated NF-κB activation [59,60].
3.1. Nutraceuticals
Nutraceuticals are natural foods including vitamins, minerals, and have physiological benefits that protect various chronic pathologies. Nutraceuticals help in delaying the process of aging, improve health, and support the structure and function of the body or increase life expectancy.
3.1.1. Vitamins
Vitamin is a major micronutrient that the body requires for the proper functioning of its metabolism. Humans cannot naturally synthesize these nutrients in their bodies and try to meet their requirements through food sources that are rich in vitamins [11]. Various fruits such as oranges, berries, grapefruit, cherries, apples, etc. contain significant quantities of vitamins C, E, and A. These vitamins aid in boosting the immune system and decrease inflammation [22]. They also have powerful reducing properties which make them a better antioxidant and help in mitigating the effects of oxidative stress and contribute towards aging and associated diseases. Vitamin C (ascorbic acid), is water-soluble that acts as the first defense against free radicals and present in relatively high content in fruits like strawberries, oranges, and black currants (58.8, 53.2, and 41 mg per 100 g of fruit respectively) [61,62].
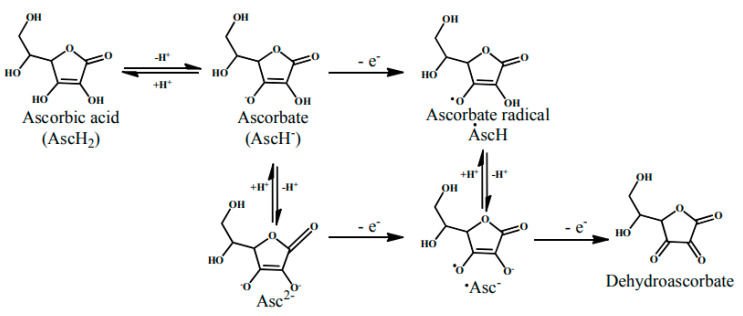
Vitamin C is a potent antioxidant and radical scavenger that avert free radicals from damaging DNA, tissues, cell membranes [63,64] and regenerates vitamin E, a lipid-soluble vitamin in lipoproteins and membranes. Vitamin C (ascorbic acid) changes to the ascorbate radical by giving an electron to the lipid radical to stop the lipid peroxidation chain reactions described in Figure 2.
Figure 2.
Mechanism of free radical scavenging activity of vitamin C [65].
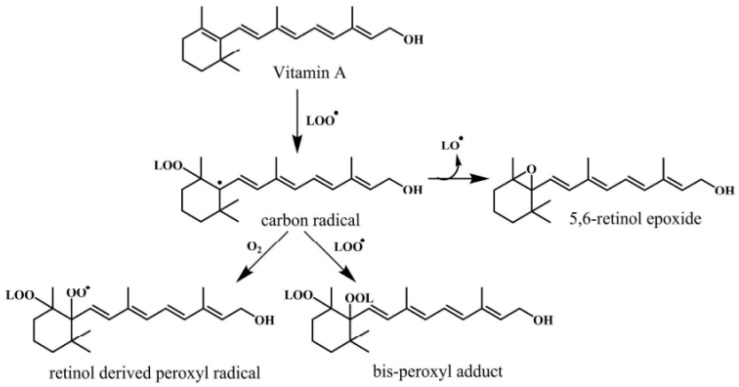
The pairs of ascorbate radicals then react and form a molecule of dehydroascorbate and ascorbate. The dehydroascorbate does not possess any antioxidant potential so it is transformed back by the addition of two electrons into the ascorbate [63,65]. During lipid peroxidation, vitamin E act as a chain breaker in several lipid particles such as low-density lipoprotein (LDL) and in cell membranes. It functions to intercept lipid peroxyl radicals and to terminate the lipid peroxidation chain reactions [65]. The combination of ascorbic acid with α-tocopherol (Vitamin E) is mainly efficient in preventing oxidation [66]. Vitamin A is also a lipid-soluble vitamin that acts as an antioxidant and helps in scavenging free radicals to prevent a variety of chronic pathologies are described in Figure 3. Monaghan and Schmitt [67] first identified the antioxidant potential of vitamin A and stated that this vitamin can protect lipids from rancidity. Vitamin A also has a major antioxidant impact in protecting human LDL against copper-stimulated oxidation [65,68].
Figure 3.
Mechanism of free radical scavenging activity of vitamin A [65].
3.1.2. Minerals
Minerals are those elements present on earth and in food that are required as essential nutrients for organisms to grow and perform various functions necessary for life. Fruits such as apples, berries, cherries, and grapes are abundant in both micro and macronutrients containing minerals. The key minerals present in these fruits are potassium, magnesium, calcium, phosphorus, iron, sodium, copper, zinc, selenium, and manganese. Berries accumulate a great deal of phosphorus, calcium, sodium, and iron minerals from the environment and hold supremacy over all other fruits [22]. Several microelements such as iron, selenium, zinc, copper, and manganese act as cofactors for various antioxidant enzymes and participate in redox metabolism which further helps in slowing the process of aging as they decrease ROS in cells, thus increasing the life expectancy of organisms [11,46]. Mineral nutrients are clinically acknowledged as necessary elements for consumer health since they provide strength to muscles and play a crucial part in the teeth and bones development. These major mineral elements are implicated in multiple essential biochemical and physiological processes that occur in humans. The mineral content of several fruits is shown in Table 1.
Table 1.
Nutritional composition of various antioxidant-rich fruits [62].
| Fruit | Macronutrients (in g) | Mineral Composition (mg/100 g) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| P | K | Mg | Ca | Fe | Zn | Na | Cu | Mn | Se | ||
Citrus sinensis

|
Carbohydrates = 11.75 Fats = 0.12 Proteins = 0.94 Dietary fibers = 2.4 |
14 | 181 | 10 | 40 | 0.1 | 0.07 | 0 | 0.045 | ND | 0.0005 |
Fragaria x. ananassa

|
Carbohydrates = 7.68 Fats = 0.3 Proteins = 0.67 Dietary fibers = 2 |
24 | 153 | 13 | 16 | 0.41 | 0.14 | 1 | 0.048 | ND | 0.0004 |
Malus pumila

|
Carbohydrates = 13.81 Fats = 0.17 Proteins = 0.26 Dietary fibers = 2.4 |
11 | 107 | 5 | 6 | 0.12 | 0.04 | 1 | 0.027 | ND | 0 |
Prunus avium

|
Carbohydrates = 16.01 Fats = 0.2 Proteins = 1.06 Dietary fibers = 2.1 |
21 | 222 | 11 | 13 | 0.36 | 0.07 | 0 | 0.06 | ND | 0 |
Ribes nigrum

|
Carbohydrates = 13.8 Fats = 0.2 Proteins = 1.4 Dietary fibers = 4.3 |
44 | 275 | 13 | 33 | 1 | 0.23 | 1 | 0.107 | ND | 0.0006 |
Rubus fruticosus

|
Carbohydrates = 9.61 Fats = 0.49 Proteins = 1.39 Dietary fibers = 5.3 |
22 | 162 | 20 | 29 | 0.62 | 0.53 | 1 | 0.165 | ND | 0.0004 |
Rubus idaeus

|
Carbohydrates = 11.94 Fats = 0.65 Proteins = 1.2 Dietary fibers = 6.5 |
29 | 151 | 22 | 25 | 0.69 | 0.42 | 1 | 0.09 | 0.67 | 0.0002 |
Vaccinium corymbosum

|
Carbohydrates = 14.49 Fats = 0.33 Proteins = 0.74 Dietary fibers = 2.4 |
12 | 77 | 6 | 6 | 0.28 | 0.16 | 1 | 0.057 | ND | 0.0001 |
Vaccinium macrocarpon

|
Carbohydrates = 11.97 Fats = 0.13 Proteins = 0.46 Dietary fibers = 3.6 |
11 | 80 | 6 | 8 | 0.23 | 0.09 | 2 | 0.056 | ND | 0.0001 |
Vitis vinifera

|
Carbohydrates = 18.1 Fats = 0.16 Proteins = 0.72 Dietary fibers = 0.9 |
20 | 191 | 7 | 10 | 0.36 | 0.07 | 2 | 0.127 | 0.071 | 0.0001 |
P = Phosphorus; K = Potassium; Mg = Magnesium; Ca = Calcium; Fe = Iron; Zn = Zinc; Na = Sodium; Cu = Copper; Mn = Manganese; Se = Selenium; ND = not defined.
3.2. Bioactive Compounds
Bioactive compounds are important complexes, found in foods and are efficient in regulating different metabolic activities and results in better health [69,70]. Furthermore, several fruits constitute a broad diversity and large content of bioactive compounds particularly tannins, stilbenes, flavonoids, and phenolic acids [14,56,71]. Polyphenols play a vital part in fruits and are used as antioxidants and colorants [72]. Intake of dietary antioxidants helps to uphold an adequate antioxidant status in the human body. A substantial amount of research on polyphenols is emphasized on their antioxidant properties after they are believed to have optimistic effects on age-related chronic pathologies. Various studies have also reported that polyphenols rich diet can prevent oxidative damage leading to aging [73]. Fruits like berries, cherries, apples, and grapes constitute approximately 200–300 mg polyphenols per 100 g of fresh weight [24,74]. The products derived from these fruits, constitute a great proportion of polyphenols. Several polyphenols including catechin, epicatechin, rutin, proanthocyanidin B2, phloretin glycosides, quercetin glycosides, and chlorogenic acid are mostly found in apples which have a strong antioxidant property [11]. Different polyphenols with antioxidant properties that are found in different fruits are shown in Table 2.
Table 2.
Dietary sources of polyphenols in fruits.
| Category | Main Class | Sub-Class | Chemical Compound | Chemical Structure | Fruit Resources | References |
|---|---|---|---|---|---|---|
| Phenolic compound | Phenolic acids | Hydroxybenzoic acids | Gallic acid |
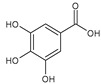
|
Strawberry, red grapes, raspberry, cranberry, blackberry | [75,76,77] |
| Vanillic acid |
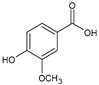
|
Strawberry | [78] | |||
| Syringic acid |
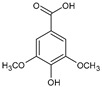
|
Strawberry | [78] | |||
| Hydroxycinnamic acids | Chlorogenic acid |
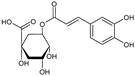
|
Apples, blackcurrant, blueberry, orange, cherry | [79,80,81,82] | ||
| Caffeic acid |
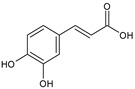
|
Strawberry, orange, cherry, blackberry | [83,84,85] | |||
| Ferulic acid |
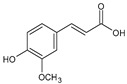
|
Orange | [84] | |||
| p-coumaric |
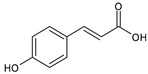
|
Strawberry, orange, blackcurrant, cranberry, cherry, blackberry | [81,82,84,86] | |||
| Flavonoids | Flavonols | Quercetin |
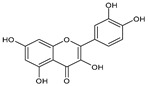
|
Apples, raspberry, blackcurrant, grapes, blueberry, blackberry | [80,82,87] | |
| Rutin |
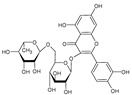
|
Apples, cherry, red grapes, oranges, blueberry, blackcurrant, raspberry | [82,83,87] | |||
| Kaempferol |
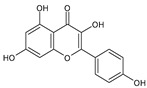
|
Strawberry, grapes, apples | [88] | |||
| Myricetin |
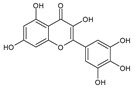
|
Grapes, berry, apple, strawberry, blueberry, cranberry | [82,89] | |||
| Flavanones | Naringenin |
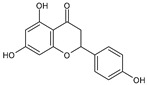
|
Orange | [84,90] | ||
| Hesperetin |
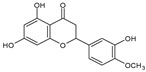
|
Orange | [84,90] | |||
| Flavan-3-ols | Catechin |
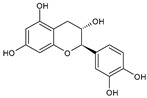
|
Apples, strawberry, red grapes, sweet cherry, blackcurrant | [80,83,87,91] | ||
| Epicatechin |
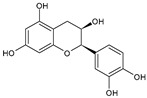
|
Red grapes, apples, cranberry, cherry | [79,80] | |||
| Flavones | Luteolin |
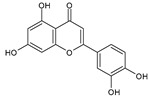
|
Orange, blackberry | [20,85] | ||
| Anthocyanins | Pelargonidin |
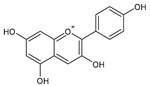
|
Strawberry, raspberry | [82] | ||
| Delphinidin |
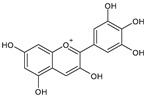
|
Blueberry, blackcurrant | [82] | |||
| Cyanidin |
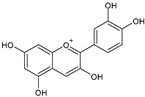
|
Raspberry, blueberry, cranberry | [82] | |||
| Malvidin |
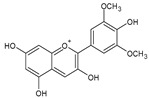
|
Red grapes, blueberry, cranberry | [82] | |||
| Peonidin |
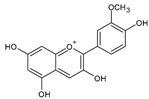
|
Blueberry, blackcurrant, cranberry | [82,92] | |||
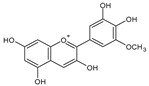
| Petunidin |
|
Blueberry, apple | [82,93] | |||
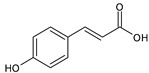
| Stilbenes | Resveratrol |
|
Red grapes, blueberry, cranberry | [31,94] | ||
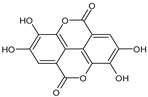
| Tannins | Ellagic acid |
|
Raspberry, strawberry, blueberry, blackberry | [82,89,95] |
These molecules can act as an antioxidant (in-vivo) in various ways: (i) by scavenging reactive species due to an elevated reactivity (measured as a rate constant) that allows it to scavenge oxidants before they can affect other biological targets such as nucleic acids and proteins; (ii) by inducing endogenous antioxidant responses through Nrf2-dependent gene expression to modulate the pathophysiological and physiological outcomes of oxidant exposure [96]; (iii) by inhibiting the production of ROS/RNS either by inhibiting the expression or activities of enzymes such as NADPH oxidases or xanthine oxidase, inhibiting inflammation or by decreasing mitochondrial electron leaking [97]. A study published in 2008, which investigated the effect of dietary supplementation with red grape juice (source of vitamin E and polyphenols) on neutrophil NADPH oxidase activity and cardiovascular risk factors in thirty-two patients on hemodialysis. The findings suggested that both red grape juice and vitamin E reduced ex-vivo neutrophil NADPH oxidase activity and plasma concentrations of oxidized LDL. Red grape juice also causes a reduction in cardiovascular risk factors [98]. Thus, findings indicate that natural antioxidants are the possible inhibitors of NADPH oxidase.
3.2.1. Phenolic Acids
Phenolic acid is abundantly present in fruits and is separated into two main classes: hydroxybenzoic acid and hydroxycinnamic acid. Most of the berries, particularly blackberries, raspberries, blueberries, cranberries, apples, oranges, and cherries are rich in both hydroxybenzoic and hydroxycinnamic acid [99,100]. The most prevalent hydroxybenzoic acids are vanillic, syringic, gallic, protocatechuic, and p-hydroxybenzoic acids, while the corresponding hydroxycinnamic acids are sinapic, p-coumaric, ferulic, and caffeic acids. These derivatives vary in methoxylations and hydroxylations patterns of their aromatic rings. Phenolic acids are mostly present in bound forms and serve as a potent antioxidant because of the reactivity of phenol moiety; a hydroxyl substituent on the aromatic ring [101]. A few derivatives of hydroxybenzoic acids are presently used as additives to decrease nutrient oxidation and to improve the nutritional value of fruits. A wide array of phenolic compounds have the potential to scavenge ROS including hydroxyl radicals and superoxide radicals, which decreases lipid peroxyl radicals and prevents lipid peroxidation. Phenolic acids act as a powerful anti-radical agent because of their redox properties, which makes them efficient hydrogen donors and metal chelators [101]. The phenolic content of several fruits is listed in Table 3.
Table 3.
Total phenolics, flavonoids, and anthocyanins content of several fruits.
| Fruits | TPC (mg/g Fresh Weight) | TFC (mg/g Fresh Weight) | TAC (mg/g Fresh Weight) | References |
|---|---|---|---|---|
| Citrus sinensis | 81.2 | - | - | [102] |
| Fragaria x. ananassa | 225 | - | 60–80 | [55] |
| Malus pumila | 296.3 | 48.6 | - | [102] |
| Prunus avium | 88–239 | - | 82–297 | [83] |
| Ribes nigrum | 560 | 46 | 44 | [55] |
| Rubus fructicosus | 248–486 | 276 | 82–326 | [55] |
| Rubus idaeus | 126 | 60 | 99 | [55] |
| Vaccinium corymbosum | 261–585 | 50 | 25–495 | [22] |
| 525 | - | 1562.2 | [80] | |
| Vaccinium macrocarpon | 315 | 157 | 67–140 | [22] |
| 120–315 | - | - | [55] | |
| Vitis vinifera | 538.6 | 214.5 | - | [55] |
| 500 | - | - | [103] |
TPC—Total phenolic content; TFC—Total flavonoid content; TAC—Total anthocyanin content.
Research on the structural activity of phenolic acids and their derivatives revealed that the derivatives of hydroxycinnamic acid had greater antioxidant ability in comparison to that of benzoic acid counterparts [104]. This potential was because of the existence of a propanoic side chain in cinnamic derivatives; the conjugated double bond in their side chains has an intense effect on the phenoxyl radical by resonance, thereby improving the antioxidant power. Gallic acid is also a powerful antioxidant, found abundantly in strawberries, raspberries, red grapes, grapefruit, cranberries, blackberries, also in juices made from these fruits [75]. Antioxidant activity of gallic acid is triple times more than that of vitamin E or C, representing that three hydroxyl groups of gallic acid can independently act as electron acceptors [77]. Derivatives of gallic acid, thus, also act as a strong antioxidant with a free hydroxyl group that is responsible for radical scavenging and apoptosis of cancer cells. The most intriguing benefits of gallic acid have been evaluated on the skin [105]. In the case of prostate cancer cells, gallic acid prevents multiplication and the death of cells. Phenolic acids have been reported to possess various useful therapeutic activities like anti-inflammatory, anti-viral, anti-bacterial, anti-allergic, anti-cancer, anti-mutagenic, and anti-melanogenic activities [77,106]. Ruifeng et al. [107] conducted an experiment to evaluate whether chlorogenic acid could ameliorate the inflammation response in lipopolysaccharide-induced mice mastitis. The findings revealed that chlorogenic acid significantly decreased the production of tumor necrosis factor-alpha (TNF-α), interleukins (IL-1β, IL-6) against lipopolysaccharide-induced mastitis. The western blot analysis indicated that chlorogenic acid could suppress the expression of toll-like receptor (TLR4), the phosphorylation of nuclear factor kappa B (NF-κB), and the inhibition of NF-κB (IκB) induced by lipopolysaccharide, and thus highlights the anti-inflammatory response.
3.2.2. Flavonoids
Flavonoids are diverse and the most studied group of polyphenols found abundantly in several fruits like blackberries, blueberries, raspberries, blackcurrants, strawberries, grapes, cranberries, apples, cherries, etc. Currently, more than 8000 flavonoids are recognized, some of which are responsible for the fascinating colors of the leaves, flowers, and fruits [108]. Phenolic compounds obtained from natural sources are considered much safer in terms of having no side effects than synthesized chemicals [109,110]. Synthetic antioxidants are involved in triggering diseases in humans beyond certain concentrations [111]. In fruits, flavonoids are found in glycosides or acyl glycosides form, while methylated, acylated, and sulfate molecules are rare and present in low concentrations. The flavonoid content in various fruits is listed in Table 3. The common basic structure of flavonoids comprises two aromatic rings that are joined to each other by three carbon atoms and forms a closed oxygenated heterocyclic pyran ring [112]. Flavonoids are classified into six subclasses based on differences in the type of heterocycle present in this group: flavanols (blueberries and apples), flavonols (grapes), flavanones (citrus fruits), flavones, isoflavones, and anthocyanins (grapes and berries) [113,114]. Flavonols, flavanols, and anthocyanins are ubiquitous and possess strong antioxidant properties, which mainly depends on the position and number of hydroxyl groups present within their structure. Flavonoids also exert a wide variety of biological actions namely antibacterial, antioxidant, anti-inflammatory, anti-hyperlipidemic, and hepatoprotective activities [115].
Flavonoids in citrus can suppress phosphodiesterase and kinases activation implicated in the initiation phase of inflammation. These enzymes can affect protein kinases and proinflammatory TNF-α expression. Some flavonoids can suppress the induction of endothelial cell adhesion molecules that are activated by cytokines. Inflammatory responses are also suppressed by impeding the monocytes, leukocytes, and neutrophils adhesion from injured regions, thus highlighting the anti-inflammatory impact [116,117]. Flavonoids also reduce the risk of coronary heart disease by averting low-density lipoproteins (LDLs) from oxidizing, reducing the ability of the platelets in the blood to clot, and by improving coronary vasodilatation [118]. Flavonoids can act in various developmental stages of malignant tumors by inactivating carcinogens, protecting DNA against oxidative damage, suppressing mutagenic genes, and enzymes expression accountable for triggering pro-carcinogenic substances, and triggering the systems accountable for xenobiotic detoxification [119]. Most of the studies have shown a structural-functional relationship, indicating that anti-proliferative, enzyme-inhibition, and antioxidant activities of flavonoids are reliant upon specific structural motifs [120,121,122].
Anthocyanins
Anthocyanins are the pigments that usually occur in nature, inhabiting a unique position in the group of polyphenols [123]. They are broadly distributed in a large number of fruits and vegetables and a great concentration of anthocyanins is observed in cranberries, blackcurrants, strawberries, bilberries, raspberries, blackberries, blueberries, and chokeberries [124,125]. They also constitute an immense group of colored water-soluble pigments that give the fruit purple, blue, and red colors [55]. However, anthocyanins are not solely responsible for the fruit color but are often used as a natural pigment in the food industry. Over 600 anthocyanins have been recognized in nature to date, but only six anthocyanins are widely found in fruits: malvidin, pelargonidin, peonidin, delphinidin, cyanidin, and petunidin [126,127]. The overall anthocyanin content in several fruits is mentioned in Table 3. Anthocyanins are also known as effective natural antioxidants [128]. The chemical structure of anthocyanin determines its efficacy as an antioxidant agent. The antioxidant activity of anthocyanin is allied with free hydroxyl numbers around the pyrone ring. Larger the hydroxyl number larger the antioxidant activity [129]. The anthocyanins have indicated characteristics that suppress free radical formation, reduces the threat of various age-linked diseases like cancers, CVD, improves aging and memory [130]. In the case of fruits, the anthocyanins are found mostly in the outer layer of the pericarp. Cyanidin-3-glucoside is the main anthocyanin that is present in maximum fruits. Anthocyanins include aglycones and their glycosides—anthocyanidins and anthocyanins, and also form different complexes [131].
Anthocyanins vary in terms of the hydroxyl groups number in a molecule, their methylation degree; place, form, and number of sugar molecules attached, number, and form of aromatic and aliphatic acids attached to sugars. In berries, anthocyanins are present in different glycosides forms i.e., mono-, di- or tri-, where residues of glycosides are commonly substituted at C3 or rarely, at C5 or C7 position [56]. The most predominant sugars are sophorose, sambubiose, rutinose, arabinose, rhamnose, galactose, and glucose [132]. The glycoside residues of anthocyanin are frequently acylated by ferulic, p-coumaric, caffeic acid, and by acetic or malonic acid, p-hydroxybenzoic acid [131,133].
Anthocyanins exert several biological properties like anti-tumor, anti-inflammatory, antioxidant, anti-diabetic, anticancer, and neuroprotective [134]. A researcher [135] experimented to assess the chemopreventive effect of anthocyanin-rich black currant skin extract with concentrations 100 and 500 mg/kg against diethyl nitrosamine-initiated hepatocarcinogenesis in rats for 18 weeks. The findings showed a decrease in iNOS expression, 3-nitrotyrosine, abnormal lipid peroxidation, and protein oxidation in a dose-dependent manner. Mechanistic studies have shown that black currant skin extract upregulated the gene expression of a number of carcinogens detoxifying and hepatic antioxidant enzymes, like uridine diphosphate-glucuronosyltransferase isozymes, glutathione S-transferase and NAD(P)H:quinone oxidoreductase in diethyl nitrosamine-initiated animals. This treatment significantly raised mRNA and protein expressions of nuclear factor E2-related factor 2 (Nrf2), providing evidence of a synchronized activation of the Nrf2-regulated antioxidant pathway, which results in the activation of multiple housekeeping genes. Moreover, the anti-inflammatory activity of anthocyanins can be attributed to their antioxidant property which results in the down-regulation of the redox-sensitive nuclear factor-κB signaling pathway [136].
Catechins
Catechin is a polyphenolic antioxidant present in several fruits such as blueberries, strawberries, gooseberries, cherries, black grapes, and apples. Catechin term refers to the flavonoid class and flavan-3-ols/flavanols sub-class. The most important dietary catechins are gallocatechin, epicatechin (EC), epicatechin 3-gallate (ECG), epigallocatechin 3-gallate (EGCG), and epigallocatechin (EGC). In strawberry, 2–50 mg/100 g total amount of catechins is present. Cherries contain 5–22 mg/100 g catechins whereas apples contain 10–43 mg/100 g catechins but both are abundant in EGCG [137]. Catechins are rich in external tissues of the fruit and are distinguished from the flavonoids containing ketones, in particular rutin and quercetin, which endorse the antioxidant defense system. Catechin present in cranberries is like the catechin present in green tea, that aids in protection against cancer [22]. The antioxidant action of catechin is effective against cancer, neurodegenerative and cardiovascular diseases [138]. A study conducted by researchers [139] reported that epigallocatechin gallate (EGCG), a major catechin strongly inhibits an enzyme called telomerase, which is necessary for unlocking the proliferative capacity of cancer cells by upholding the tips of their chromosomes. Thus, it may be another reason for the anticancer activity of catechins. The antioxidant property of catechin proved beneficial in reducing coronary heart diseases as it decreases cytotoxicity caused by amiodarone in fibroblast cells of the lungs [140]. Catechins also show anti-inflammatory activities in bowel disease in humans by effecting oxidative stress-related cell signaling pathways, such as transcription factor nuclear factor (erythroid-derived 2)-like 2 (Nrf2), mitogen-activated protein kinases (MAPKs), nuclear factor-kappa B (NF-κB), signal transducer and the activator of transcription 1/3 (STAT1/3) pathways [141]. Pan et al. [142] experimented to study the anti-inflammatory effects of catechins in an in vitro experiment using stimulated human nasal epithelial cells (HNEpCs) and in an ovalbumin-induced allergic rhinitis murine model. The results showed that catechin inhibited the expression of Thymic stromal lymphopoietin (a molecule that plays a main role in the development of allergy) in epithelial cells by influencing the NF-κB/TSLP pathway. Catechin effectively decreased the inflammation in allergic rhinitis. Catechins also reduce the degeneration of neurons by directly or indirectly decreasing oxidative stress, scavenging ROS, and improving antioxidant enzymes [140]. Thus, most of the benefits of catechins are achieved through the way of their antioxidant mechanism.
Quercetin
Quercetin is a natural polyphenolic compound, found abundantly in several fruits such as apples, grapes, blueberries, raspberries, cherries, and blackcurrant [82,143]. It is reported as one of the most powerful ROS scavengers in the flavonoid class and flavonol sub-class [144]. An experiment was proposed [145] to evaluate the free radical scavenging activity of flavonoids in H2O2 treated human myelogenous leukemia (K562) cells. The experimental findings revealed that quercetin and luteonin have shown the highest protective effects as compared to rutin, an apigenin towards H2O2 induced damage in leukemia cells. The antioxidant property of quercetin to scavenge free radicals is due to the presence of two antioxidant pharmacophores inside molecules that have the ideal structure for free radical scavengers [33,146]. Chondrogianni et al. [147] described in his studies that quercetin and its derivative quercetin caprylate (QU-CAP) can revitalize senescent human fibroblasts and extend their life expectancy by activating proteasome. It acts as an effective antioxidant that has significant pharmacological, biological, and medicinal properties. The antioxidant potential of quercetin is directly proportional to the number of free hydroxyl groups [148]. Moreover, it also exhibits potential anticancer and anti-inflammatory properties. Quercetin suppresses the production of inflammatory enzymes like lipoxygenase (LOX) and cyclooxygenase (COX) thus reducing the production of prostaglandins and leukotrienes, chemicals that promote inflammation [149]. The anti-inflammatory and antioxidant activities of quercetin and its derivatives contribute to the anti-aging effect since inflammation and chronic oxidative stress are considered to play a substantial part in activating the aging process [3].
3.2.3. Tannins
Tannins are the essential constituents that play a major part in delineating the sensory characteristics of fruits and their products. They include both ellagic acid or esters of gallic acid called hydrolyzable tannins, and condensed tannins called proanthocyanidins [150]. They are responsible for variations in the fruit color and tart taste [151]. Tannins stabilize anthocyanins, in fruits rich in it, by binding them to form copolymers. Hydrolyzable tannins are rarely found and have been present in blackberries, blueberries, strawberries, and raspberries [82,95].
Ellagic acid contains approximately 51% of the total phenolic content and occurs in free as well as in complex form as glucosides and ellagitannins esterified as glucose. The presence of ellagic acid and its derivatives makes the berries (cranberries, raspberries, blackberries, strawberries) and grapes consumption appropriate for possible health benefits. In addition, the ellagic acid has been described to possess potent antiviral, anti-inflammatory, anti-proliferative, antioxidant properties and also provide defense against cancer of the esophagus, lungs, and colon [152,153]. Ellagic acid has been identified in several fruits in which the total concentration of ellagic acid was computed by evaluating the concentration after acid hydrolysis of ellagic acid extracts [22]. In the case of raspberries, free ellagic acid contains a small portion of total ellagic acid, and the main source ellagitannins is discharged by acid hydrolysis [95].
3.2.4. Stilbenes
Stilbenes are low-molecular-weight compounds found in several fruits like berries and grapes. It comprises two phenyl moieties that are linked by a two-carbon methylene bridge. Generally, stilbenes are present in low concentrations in the human diet. One of the naturally occurring and most studied polyphenol stilbenes is resveratrol [154].
Resveratrol
Resveratrol (RES) is a phytoalexin that is formed by a broad diversity of plants as a response to fungal infections, injury, UV radiation, and stress. In 1940, the first resveratrol molecule was isolated from the Veratrum grandiflorum O. Loes roots (white hellebore) and later from berry-skins, grapevines, and Vitis spp. (grapes) leaves comprising their frequent and most examined source [31,155]. Research suggests that the resveratrol molecule is one of the main factors of the French Paradox that defines an epidemiological observation that connects fewer incidences of cardiovascular diseases and longer lifespan (despite a high-fat diet) in French people with the daily consumption of red wines [3,156,157]. As red wine comprises a substantial quantity of resveratrol, it has dragged great interest in the experimental community to explore its possible health benefits, whether the resveratrol might bestow these benefits.
Resveratrol is a polyphenolic antioxidant that belongs to the family stilbene and is synthesized by grapevine and various other plants as a response to pathogens attack from the precursor molecules of p-coumaroyl CoA and malonyl-coenzyme A (CoA), in the existence of enzyme stilbene synthase [158]. Resveratrol is found in various fruits like grapes and Vaccinium spp. (cranberry, bilberry, and blueberry). It has also been identified in plum fruit, increasing its natural wealth profile, particularly in dietary sources [31,159].
A plethora of evidence proposed that supplementation of dietary resveratrol exerts advantageous effects on aging and various other age-related chronic diseases in particular Alzheimer’s disease, cancer, and diabetes, etc. [160]. In addition, it was found to prolong lifespan by 70% in Saccharomyces cerevisiae, a typical model for aging studies. Over the past two decades, resveratrol has been the subject of extraordinary studies due to its anti-aging properties. It works both as a radical scavenger, as a chelating agent, and possesses anti-inflammatory activity [161].
4. An Insight into Pharmacological and Biological Benefits of Fruits
There is growing evidence from worldwide research that fruits are an imperative part of a balanced diet. Numerous phytochemicals from the fruits have antioxidant properties that safeguard against the detrimental effects of free radicals, which further lead to chronic pathologies linked with aging [162,163]. Fruits are also a good source of naturally occurring antioxidants which include vitamins, minerals, flavonoids, and phenolic acids. Presence of these antioxidants, aid in delaying or preventing oxidative damage of biomolecules by ROS which contain reactive free radicals including hydroxyl, peroxyl, alkoxyl, superoxide, and non-radicals such as hypochlorous, hydrogen peroxide, etc. The anthocyanins in fruits are also the most studied phenolic with a large variety of bioactivities including anti-aging, anticancer, anti-inflammatory, and antioxidant properties. Antioxidants scavenge these radicals by counteracting the formation of free radicals by attaching to metal ions or suppressing the initiation and chain-breaking propagation, by quenching superoxide and singlet oxygen, and by reducing hydrogen peroxide [164] and thus circumventing several age-linked diseases like type 2 diabetes, inflammation, cancer, and CVD. Various pharmacological and biological properties of several fruits are described in Table 4.
Table 4.
Pharmacological and biological properties of fruits.
| Fruits (Botanical Name/Common Name) | Phytoconstituents | Pharmaceutical Properties | References |
|---|---|---|---|
| Citrus sinensis/Orange |
|
Antibacterial/fungal, anti-tumoral, antioxidant, anti-microbial, anti-inflammatory, anti-allergic, neuroprotective, decreases cholesterol, promote cardiovascular diseases | [165,166] |
| Fragaria x. ananassa/Strawberry |
|
Anti-inflammatory, antioxidant, anti-diabetic, neuroprotective, anti-microbial, prevent development of cardiovascular diseases | [17,167,168] |
| Malus domestica/Apple |
|
Anticancer, anti-inflammatory, antioxidant, anti-radiation, boost digestive functions, anti-diabetic, prevent cardiovascular diseases, reduces cholesterol | [169,170,171] |
| Prunus avium/Cherry |
|
Anti-inflammatory, antioxidant, anti-diabetic, reduces cholesterol, promote cardiovascular diseases | [79,172] |
| Ribes nigrum/Blackcurrant |
|
Antioxidant, anti-inflammatory, anti-cancer, stimulates digestion, reduces blood cholesterol | [87,173] |
| Rubus fruticosus/Blackberry |
|
Antiviral/bacterial, antioxidant, antiseptic, anti-aging, anticancer, fights free radical damage, pain reliever | [22,174] |
| Rubus idaeus/Raspberry |
|
Prevents damage caused by free radical, enhances metabolic rate that burns fat, anticancer, antimicrobial, antioxidant | [22,87] |
| Vaccinium corymbosum/Blueberry |
|
Anti-diabetic, antioxidant, anti-inflammatory, anticancer, antimicrobial, reverses aging signs, decreases cholesterol, prevents Alzheimer’s disease | [21,82] |
| Vaccinium macrocarpon/Cranberry |
|
Antibacterial, diuretic, antiseptic, eradicate fat from the lymphatic system, promote cardiovascular health | [175,176] |
| Vitis vinifera/Red grapes |
|
Anticancer, antioxidant, anti-aging, anti-inflammatory, anti-microbial, hepatoprotective, anti-viral/bacterial, cardioprotective, neuroprotective, reduces blood pressure | [177,178] |
4.1. Anti-Aging Effects
Several vivid fruits and vegetables are extensively tested for their potential to neutralize free radicals that can destroy DNA and cell membranes via a mechanism called oxidative stress, which is responsible for most aging-related malfunctions and disorders [11]. While several fruits like blueberries, grapes, cranberries themselves are not a remedy for all of them, they contain a variety of substances such as vitamins, fibers, and antioxidants that have health benefits [14,22]. Antioxidants in fruits appear to have the most credible role in delaying or preventing aging, heart disease, cancer, and other age-related disorders. Clinical reports by nutritionists from laboratories across the world conclude that consuming raspberries is associated with health benefits [179]. Research conducted in 2018, investigated the effects of blueberry supplementation on lifespan and stress tolerance in Caenorhabditis elegans. The result showed that the mean lifespan of Caenorhabditis elegans was increased significantly on the dose-dependent basis by 22.2%, 36.5%, and 44.4%, respectively, when treated with blueberry extract at 50, 100, and 200 mg/mL for 4 days. In addition, blueberry treated Caenorhabditis elegans shows more resistance to stresses (heat, paraquat, and Ultraviolet-B radiation) than non-treated Caenorhabditis elegans [180]. Several experiments and trials with the various fruit extracts are displayed in Table 5.
Table 5.
Experimental studies (in-vitro, in-vivo) and human trials showing the pharmacological health benefits of different fruits and their derived products.
| Fruit Extracts/Bioactive Compound | Dose Per Day | Experimental Models/Subjects | Duration | Results | References |
|---|---|---|---|---|---|
| Orange | |||||
| In-Vitro | |||||
| Red Orange | 15, 30 μg/mL | Human keratinocytes (HaCaT) cell line | 7 h |
|
[181] |
| Orange peel extract | 400 mg/mL | Human esophageal cancer cell (YM1) | 24 h |
|
[182] |
| In-Vivo | |||||
| Orange peel extract | 0.25%, 0.5% | 10 ApcMin/+ mice (4 weeks old) per group | 9 weeks |
|
[183] |
| Orange extract | 100, 200, and 400 mg/mL | N2 wild-type Caenorhabditis elegans | 5 days |
|
[184] |
| Orange peel | 50 mg/kg | Xenografts 5 male mice (6–8 weeks old) | 14 days |
|
[182] |
| Human trials | |||||
| Red orange extract | 100 mg | 20 Caucasian subjects with skin erythema induced by UV irradiation (aged 26–47 years) | 15 days |
|
[185] |
| Red orange extract | 100 mg | 25 volunteers with tanning skin homogeneity (aged 45–70 years) | 5 weeks |
|
[185] |
| Red orange | 50 mg | 32 patients with Type 2 diabetes and 28 healthy volunteers | 2 months |
|
[186] |
| Strawberry | |||||
| In-Vitro | |||||
| Freeze dried strawberry | NS | Breast (MCF-7 and T47-D) and cervical (CaSki and SiHa) cancer cell lines | 48 h |
|
[187] |
| Strawberry rich extracts | 25–200 µg/mL | Human oral (KB, CAL-27), prostate (LNCaP), colon (HT-29, HCT-116) and breast (MCF-7) tumor cell lines | 24 to 72 h |
|
[188] |
| Crude extracts and purified compounds | 250 μg/mL (crude extract) and 100 μg/mL (pure compounds) | Human oral (CAL-27, KB), prostate (LNCaP, DU145) and colon (HT-29 and HCT-116) cancer cells | 48 h |
|
[189] |
| Kaempferol | 20, 40, 60 μmol/l | HT-29 colon cancer cells | 24 to 72 h |
|
[190] |
| In-Vivo | |||||
| Freeze-dried strawberry | 2.5%, 5.0% or 10.0% | AOM/DSS induced male Crj: CD-1 mice | 20 weeks |
|
[191] |
| Apples | |||||
| In-Vitro | |||||
| Kaempferol | 20, 40, 60 μmol/l | HT-29 colon cancer cells | 24 to 72 h |
|
[190] |
| Quercetin | 5, and 10 μg/mL | HT1080 human fibrosarcoma cells | 24 h |
|
[192] |
| In-Vivo | |||||
| Whole apple extract | 2.5, 5 and 10 mg/mL | Caenorhabditis elegans | 4 days |
|
[193] |
| Apple peel extract | NS | 8 male C57BL/6J mice (6 weeks old) | 10 weeks |
|
[194] |
| Cherry | |||||
| In-Vitro | |||||
| Cherry extracts | 0.025, 0.05, 0.25, and 0.5% dry wt. | Colon (HT-29) and breast (MCF-7) cancer cells | 24 h |
|
[195] |
| In-Vivo | |||||
| Cherry extract | 8 male C57BL/6J mice (6 weeks old) | 10 weeks |
|
[194] | |
| Blackcurrant | |||||
| In-Vitro | |||||
| Blackcurrant extracts | 0.025, 0.05, 0.25, and 0.5% dry wt. | Colon (HT-29) and breast (MCF-7) cancer cells | 24 h |
|
[195] |
| Blackcurrant press residue extracts | 20, 75, 100, or 125 μg GAE/mL | Caco-2, HT-29, and HCT-116 cells | 24 to 48 h |
|
[196] |
| Human trials | |||||
| FL and CAM30 prepared from blackcurrant extract | Both products contain 672 mg blackcurrant powder | 30 healthy volunteers (16 women, 14 men, Aged 20–60 years) | 2 weeks |
|
[197] |
| Blackberry | |||||
| In-Vitro | |||||
| Blackberry extract | 25–200 µg/mL | Human oral (KB, CAL-27), prostate (LNCaP), colon (HT-29, HCT-116) and breast (MCF-7) tumor cell lines | 24 to 72 h |
|
[188] |
| Anthocyanin-rich extracts from hull and crude blackberry | 13.6 to 49.2 µg of monomeric anthocyanins/mL | HT-29 colon tumor cells | 48 to 72 h |
|
[198] |
| In-Vivo | |||||
| Freeze-dried blackberry fruits | 50 g/kg | Fisher 344 male rats (3–4 years old) | 13 weeks |
|
[199] |
| Raspberry | |||||
| In-Vitro | |||||
| Red raspberry extracts | 5%, 7.5%, and 10% | AGS stomach, LoVo colon cancer cells, and MCF-7 breast cancer cell lines | 24 to 48 h |
|
[200] |
| Red raspberry | 10, 25, 50 mg/mL | Human hepatocellular carcinoma HepG2 and Huh7 cell lines | 24 to 96 h |
|
[201] |
| In-Vivo | |||||
| Fresh-dried raspberry | 29.0 g | Wild-type male C57BL/6J mice (6 weeks old) | 10 weeks |
|
[202] |
| Freeze-dried raspberry extracts | 100, 200, 300 mg/kg | C57BL/6J male mice (6 weeks old) | 8 weeks |
|
[203] |
| Blueberry | |||||
| In-Vitro | |||||
| Freeze-dried strawberry | NS | Breast (MCF-7 and T47-D) and cervical (CaSki and SiHa) cancer cell lines with different requirements for estrogen | 48 h |
|
[187] |
| Dried extracts and fractions | 50–10,000 µg/mL | HT-29 and Caco-2 colon cancer cell lines | 48 h |
|
[204] |
| Blueberry extracts | 25–200 µg/mL | Human oral (KB, CAL-27), prostate (LNCaP), colon (HT-29, HCT-116) and breast (MCF-7) tumor cell lines | 24 to 72 h |
|
[188] |
| Pterostilbene | 50 µM | Human colon carcinoma HT-29 cells | 4 h |
|
[205] |
| Blueberry | NS | Fischer 344 male rats | 10 weeks |
|
[206] |
| In-Vivo | |||||
| Blueberry extracts | 5 mg/mL | Drosophila melanogaster | 76 days |
|
[207] |
| Blueberry extracts | 50, 100, and 200 mg/mL | Caenorhabditis elegans | 7 days |
|
[180] |
| Freeze-dried blueberry extracts | 50 g/kg | Fisher 344 male rats (3–4 years old) | 13 weeks |
|
[199] |
| Pterostilbene | 40 p.p.m. (0.004%) | AOM induced Fisher 344 male rats | 45 weeks |
|
[205] |
| Blueberry juice | NS | 12 C57BL/6 male mice (4 weeks old) | 12 weeks |
|
[208] |
| Cranberry | |||||
| In-Vitro | |||||
| Crude cranberry extract | 1, 10, 50 and 100 µg/mL | HT-29 human colon adenocarcinoma cells | 6 h |
|
[209] |
| Quercetin | 1, 10, 50, and 100 µmol/L | HT-29 human colon adenocarcinoma cells | 6 h |
|
[209] |
| In-Vivo | |||||
| Flavonoid-rich fraction 6 (Fr6) and purified proanthocyanidin (PAC) | 100 mg/kg proanthocyanidin (PAC) and 250 mg/kg Fr6 | Xenografts Balb/c female mice (4–6 years old) | Every 2 days for 3 weeks |
|
[210] |
| Cranberry extracts and dried cranberry | 0.1% cranberry extract and 1.5% dry cranberry | Dextran sulfate sodium (DSS) induced murine colitis | 1 week |
|
[211] |
| Human trials | |||||
| Cranberry juice | 125, 250 and 500 mL/day | 30 abdominally obese men | 4 weeks |
|
[212] |
| Cranberry juice cocktail | 202 mL/day | Adults (≥19 years) | 2 days |
|
[213] |
| Cranberry juice | 480 mL/day | 30 women, 26 men | 8 weeks |
|
[214] |
| Cranberry beverage | 450 mL/day | 78 overweight or obese men and women | 8 weeks |
|
[215] |
| Red grapes | |||||
| Human trials | |||||
| Red grape cell powder | 200 mg, 400 mg/day |
Fifty adults (35 male, 15 female) with pre- and mild-hypertension (≥35 and <70 years) | 12 weeks |
|
[216] |
| Red grape juice | 100 mL/day | 15 healthy and 26 hemodialysis patients | 14 days |
|
[217] |
| Red grape juice | 100 mL/day | 32 hemodialysis patients | 14 days |
|
[98] |
| Red grape juice | 300 mL/day | 26 healthy and non-smokers males | 1 month |
|
[218] |
NS—not specified.
4.2. Antioxidant Effects
Antioxidants are the substances that retard or prevent oxidation of a substrate while present in low concentrations. They have been of great interest to biochemists and healthcare professionals as they aid in protecting the body from the damage caused by ROS and RNS [219]. Various phytochemicals, such as phenolics and flavonoids, are the most active dietary antioxidants that help in safeguarding cells against oxidative stress induced by free radicals [220]. Nutritional antioxidants work by various mechanisms in different compartments, they are primarily free radical scavengers: as they minimize peroxide concentrations and overhaul oxidized membranes; directly neutralize free radicals; quench iron to reduce the development of ROS, through lipid metabolism in which short-chain free fatty acids and cholesteryl esters counteract ROS [221]. Recently, more focus has been paid to the health benefits of fruit consumption. As antioxidants present in fruits help in protecting the human body from the detrimental effects of free radicals associated with aging. Orena et al. [59] performed an experiment to assess the antioxidant response element (ARE) activation capacity of vegetable and fruit extracts in human IMR-32 cells. The findings showed that the fruit components activate the antioxidant response element. Fresh fruits constitute various natural antioxidants like phenolic, resveratrol, anthocyanins, vitamins (C, E) which show antioxidant properties and prevent oxidation of low-density lipoprotein (LDL) [131].
4.3. Anti-Inflammatory Effects
Inflammation is a physiological process of the body’s response to various kinds of injuries, but the deregulation of the inflammatory systems can cause severe damage to the cells and tissues of the host and contribute to disease development. Chronic inflammation is associated with multiple diseases, which include Alzheimer’s disease, CVD, metabolic syndrome, cancer, type 2 diabetes, and atherosclerosis [222,223]. Significant studies indicate that the intake of a fruit-rich diet is related to a reduced risk of inflammation-associated diseases. In-vitro and in-vivo research have highlighted the potential beneficial effects of fruits on chronic inflammatory diseases [26,224]. Cai et al. [225] in his research stated that supplementation of cranberry decreases the colonic levels of pro-inflammatory cytokines in mice treated with dextran sodium sulfate. Phytochemicals in raspberry effectively modulate gene expression, enzyme activity, and cellular pathways. In addition, to reduce the production of oxidized-LDL through their antioxidant activity, raspberry phytochemicals have shown anti-inflammatory and anti-atherosclerotic properties that protect against CVD [22]. The systematic consumption of polyphenol-rich foods, strawberry, or antioxidant has been shown to prevent inflammatory and fibrinolytic factors in foods [167,226].
4.4. Anticancer Effects
Cancer is a major health issue and almost 10 million people around the world die every year from cancer. A significant amount of epidemiological evidence indicates a strong connection between the dietary intake of fruit and the lower prevalence of several forms of cancers [227]. Several in-vitro and in-vivo studies have shown that polyphenolic extracts (triterpenoids, proanthocyanidin oligomers, and flavonols) from cranberries and their derivatives suppress the growth and multiplication of lung, prostate, colon, breast, and other tumors [228]. Many research findings have also revealed that the intake of a diet rich in phytochemicals including fresh vegetables and fruit leads to a decrease in the incidence of various types of human cancers [99]. Berries have demonstrated genoprotective, antioxidative, and anti-carcinogenic properties in different types of cancer cells and animal models. It has been evaluated that raspberry phytochemicals have the potential to suppress the in-vitro growth rate and proliferation of cancer cells by persuading S-phase arrest via the PTEN/AKT pathway [201]. Various other berries such as strawberry, blueberry, and cranberry have been shown to suppress cancer development by regulating numerous pathways, such as Wnt/β-catenin, ERK/MAPK, JAK/STAT, NF-κB, and PI3K/AKT/mTOR [167,229,230,231], thus changing cellular processes implicated in growth, multiplication, and metastasis. Berries inhibit the initiation of tumors by influencing the metabolism of carcinogens, leading to decreased rates of carcinogen-induced DNA damage. They prevent promotion events by decreasing the tissue inflammatory parameters and the progression rate of pre-malignant cells, encouraging apoptosis, and preventing angiogenesis [232]. Fruits polyphenols confer anticancer benefits through number of mechanisms, including the activation of metabolizing enzymes, gene expression modulation, and their influence on cell multiplication, cell signal transduction pathways, and cell death. Multiple studies have also indicated that the fruits have anti-cancer property and some of which are described in Table 5.
4.5. Neuroprotective Effects
Neurodegenerative diseases are widely known as significant health problems and aging is the primary risk factor for these diseases. Various epidemiological data have shown strong correlations between the intake of bioactive compounds, antioxidants, and decreased rates of neurodegenerative pathologies, such as aging [167,233]. As antioxidants obtained from natural sources help in combating various types of stresses one of which is migraine, which is considered a neurodegenerative disorder and helps in preventing the production of oxidative stress by suppressing the activation, spread, and oxidative chain reactions [234]. Clinical trials have also shown that the consumption of Citrus sp. is effective in reducing the risk of migraine attacks [235]. In 2006, Galli et al. [206] proposed an experiment on thirty male rats to study the effect of blueberries for 10 weeks. The findings showed that the short-term stimulation/exposure of blueberries results in enhanced HSP70-mediated defense against a variety of neurodegenerative activity in the brain. Numerous observations (in-vitro and in-vivo) indicate that plant phenols (flavonoids and phenolic acids) have a potential effect on neurological dysfunctions, and high consumption of antioxidants appears to be actively involved in enhancing neurological functions [236]. A research study observed correlation between cognitive decline and prolonged berry consumption, suggesting that a higher intake of berries is connected with a slower rate of cognitive decline in aged people [237]. Several other experiments with the various fruits extracts are displayed in Table 5.
4.6. Anti-Diabetic Effects
Diabetes mellitus is a rising problem for human wellness, with presently 463 million patients suffering from diabetes worldwide. This condition is caused by persistent high blood glucose levels (hyperglycemia), which are accountable for the dysfunction and failure of several organs. Various epidemiological studies have shown a link between increased fruit consumption, especially berries, and decreased diabetic incidences [238,239]. Research studies indicated that intake of strawberries, or their purified fraction of flavonoid, suppressed the transport and uptake of glucose and balanced their level in blood. As increased intake of fruit is significantly associated with a lesser incidence of diabetes in the prospective longitudinal cohort study. The roles of strawberry-flavored drink and strawberry freeze-dried beverage consumption were investigated in subjects with type 2 diabetes, evaluating the blood pressure and lipid profiles [167,240]. Significant reductions in total cholesterol (TC) levels, blood pressure levels, and TC to high-density lipoproteins (HDL) cholesterol ratios were found and compared to the initial values. The findings indicated that certain threat factors for CVD in subjects with diabetes were improved by the intake of strawberries over a short period of time [226]. Erlund et al. [241] also described that supplementation of only moderate amounts of berries resulted in positive improvements in platelet function, HDL cholesterol, and blood pressure. The findings show that the intake of berry plays a significant part in controlling CVD. In 2006, Ruel et al. [212] experimented on thirty abdominally obese men to observe the effect of consuming cranberry juice cocktails over a span of four weeks with an increase in daily doses (125, 250, and 500 mL). The results showed a rise in plasma HDL-cholesterol concentration, indicating a positive outcome in the reduction of the risk factor for CVD. Similarly, a study published in 2016 by Snyder et al. [194] which investigated the effect of apple peel extract and cherry extract on male mice for 10 weeks. The findings showed a large decrease in the amount of C-reactive protein in blood glucose. Various other research showing the anti-diabetic effect of fruit consumption are shown in Table 5.
5. Conclusions
As there is an increase in the prevalence of oxidative stress-mediated aging and other associated chronic pathologies, there is a need to improve the body’s defense mechanism with the least side effects. This need has been recognized by using potent antioxidants (naturally occurring compounds). In this regard, polyphenolic compounds in the fruit have demonstrated beneficial activity as antioxidants. Many natural antioxidants are highly effective in controlling the oxidative damage generated by free radicals and serve as a free radical scavenger. Various fruits like apples, oranges, berries, grapes, and cherries are a rich source of natural antioxidants like anthocyanins, phenolic, vitamins, minerals, and other anti-aging phytochemicals such as resveratrol, quercetin that are actively involved in postponing the process of aging and have health encouraging properties. Research on these fruits is of great importance to both the scientific and the public community. However, mechanisms that are linked with anti-aging in humans still need a detailed understanding of newly discovered phytochemicals.
Author Contributions
Conceptualization, K.K. and E.N.; formal analysis, V.K. and S.P.; writing—original draft, R.D.; review writing, editing, and interpretation, A.T., R.V., and D.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the University of Hradec Kralove (Faculty of Science VT2019-2021) and Ministry of Health (No. NV19-09-00578).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ho Y.S., So K.F., Chang R.C.C. Anti-aging herbal medicine—How and why can they be used in aging-associated neurodegenerative diseases? Ageing Res. Rev. 2010;9:354–362. doi: 10.1016/j.arr.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Hou Y., Dan X., Babbar M., Wei Y., Hasselbalch S.G., Croteau D.L., Bohr V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019;15:565–581. doi: 10.1038/s41582-019-0244-7. [DOI] [PubMed] [Google Scholar]
- 3.Si H., Liu D. Dietary antiaging phytochemicals and mechanisms associated with prolonged survival. J. Nutr. Biochem. 2014;25:581–591. doi: 10.1016/j.jnutbio.2014.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Harman D. Free radical theory of aging: An update: Increasing the functional life span. Ann. N. Y. Acad. Sci. 2006;1067:10–21. doi: 10.1196/annals.1354.003. [DOI] [PubMed] [Google Scholar]
- 5.Peng C., Wang X., Chen J., Jiao R., Wang L., Li Y.M., Zuo Y., Liu Y., Lei L., Ma K.Y., et al. Biology of ageing and role of dietary antioxidants. BioMed Res. Int. 2014;2014:1–14. doi: 10.1155/2014/831841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bhattacharya S. Reactive Oxygen Species and Cellular Defense System. In: Rani V., Yadav U.C.S., editors. Free Radicals in Human Health and Disease. Springer; New Delhi, India: 2015. pp. 17–29. [Google Scholar]
- 7.Diaconeasa Z., Iuhas C.I., Ayvaz H., Rugină D., Stanilă A., Dulf F., Bunea A., Socaci S.A., Socaciu C., Pintea A. Phytochemical characterization of commercial processed blueberry, blackberry, blackcurrant, cranberry, and raspberry and their antioxidant activity. Antioxidants. 2019;8:540. doi: 10.3390/antiox8110540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Uttara B., Singh A.V., Zamboni P., Mahajan R.T. Oxidative stress and neurodegenerative diseases: A review of upstream and downstream antioxidant therapeutic options. Curr. Neuropharmacol. 2009;7:65–74. doi: 10.2174/157015909787602823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Martel J., Ojcius D.M., Ko Y.F., Chang C.J., Young J.D. Antiaging effects of bioactive molecules isolated from plants and fungi. Med. Res. Rev. 2019;39:1515–1552. doi: 10.1002/med.21559. [DOI] [PubMed] [Google Scholar]
- 10.Weindruch R., Walford R.L. Retardation of Aging and Disease by Dietary Restriction. CC Thomas; Springfield, IL, USA: 1988. pp. 339–397. [Google Scholar]
- 11.Gupta B., Kumar B., Sharma A., Sori D., Sharma R., Mehta S. Nutraceuticals for Antiaging. In: Gupta R.C., Srivastava A., Lall R., editors. Nutraceuticals in Veterinary Medicine. Springer; Cham, Switzerland: 2019. pp. 383–392. [Google Scholar]
- 12.Aversa R., Petrescu R.V., Apicella A., Petrescu F.I. One can slow down the aging through antioxidants. Am. J. Eng. Appl. Sci. 2016;9:1112–1126. doi: 10.3844/ajeassp.2016.1112.1126. [DOI] [Google Scholar]
- 13.Kaur C., Kapoor H.C. Antioxidants in fruits and vegetables–the millennium’s health. Int. J. Food Sci. Technol. 2001;36:703–725. doi: 10.1046/j.1365-2621.2001.00513.x. [DOI] [Google Scholar]
- 14.Paredes-López O., Cervantes-Ceja M.L., Vigna-Pérez M., Hernández-Pérez T. Berries: Improving human health and healthy aging, and promoting quality life—A review. Plant Foods Hum. Nutr. 2010;65:299–308. doi: 10.1007/s11130-010-0177-1. [DOI] [PubMed] [Google Scholar]
- 15.Gomes-Rochette N., Da Silveira Vasconcelos M., Nabavi S.M., Mota E.F., Nunes-Pinheiro D.C.S., Daglia M., de Melo D.F. Fruit as potent natural antioxidants and their biological effects. Curr. Pharm. Biotechnol. 2016;17:986–993. doi: 10.2174/1389201017666160425115401. [DOI] [PubMed] [Google Scholar]
- 16.Vauzour D., Vafeiadou K., Rendeiro C., Corona G., Spencer J.P. The inhibitory effects of berry-derived flavonoids against neurodegenerative processes. J. Berry Res. 2010;1:45–52. doi: 10.3233/BR-2010-005. [DOI] [Google Scholar]
- 17.Giampieri F., Tulipani S., Alvarez-Suarez J.M., Quiles J.L., Mezzetti B., Battino M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition. 2012;28:9–19. doi: 10.1016/j.nut.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Blumberg J.B., Camesano T.A., Cassidy A., Kris-Etherton P., Howell A., Manach C., Ostertag L.M., Sies H., Ray A.S., Vita J.A. Cranberries and their bioactive constituents in human health. Adv. Nutr. 2013;4:618–632. doi: 10.3945/an.113.004473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Acero N., Gradillas A., Beltran M., García A., Mingarro D.M. Comparison of phenolic compounds profile and antioxidant properties of different sweet cherry (Prunus avium L.) varieties. Food Chem. 2019;279:260–271. doi: 10.1016/j.foodchem.2018.12.008. [DOI] [PubMed] [Google Scholar]
- 20.Rinaldo D., Mbéguié-A-Mbéguié D., Fils-Lycaon B. Advances on polyphenols and their metabolism in sub-tropical and tropical fruits. Trends Food Sci. Technol. 2010;21:599–606. doi: 10.1016/j.tifs.2010.09.002. [DOI] [Google Scholar]
- 21.Williamson G. The role of polyphenols in modern nutrition. Nutr. Bull. 2017;42:226–235. doi: 10.1111/nbu.12278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nile S.H., Park S.W. Edible berries: Bioactive components and their effect on human health. Nutrition. 2014;30:134–144. doi: 10.1016/j.nut.2013.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Wang S.Y., Jiao H. Scavenging capacity of berry crops on superoxide radicals, hydrogen peroxide, hydroxyl radicals, and singlet oxygen. J. Agric. Food Chem. 2000;48:5677–5684. doi: 10.1021/jf000766i. [DOI] [PubMed] [Google Scholar]
- 24.Pandey K.B., Rizvi S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009;2:270–278. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lima G.P.P., Vianello F., Corrêa C.R., Campos R.A.D.S., Borguini M.G. Polyphenols in fruits and vegetables and its effect on human health. Food Nutr. Sci. 2014;5:1065–1082. doi: 10.4236/fns.2014.511117. [DOI] [Google Scholar]
- 26.Joseph S.V., Edirisinghe I., Burton-Freeman B.M. Fruit polyphenols: A review of anti-inflammatory effects in humans. Crit. Rev. Food Sci. Nutr. 2016;56:419–444. doi: 10.1080/10408398.2013.767221. [DOI] [PubMed] [Google Scholar]
- 27.Cutrim C.S., Cortez M.A.S. A review on polyphenols: Classification, beneficial effects and their application in dairy products. Int. J. Dairy Technol. 2018;71:564–578. doi: 10.1111/1471-0307.12515. [DOI] [Google Scholar]
- 28.Nurmi T., Mursu J., Heinonen M., Nurmi A., Hiltunen R., Voutilainen S. Metabolism of berry anthocyanins to phenolic acids in humans. J. Agric. Food Chem. 2009;57:2274–2281. doi: 10.1021/jf8035116. [DOI] [PubMed] [Google Scholar]
- 29.Belščak-Cvitanović A., Durgo K., Huđek A., Bačun-Družina V., Komes D. Overview of polyphenols and their properties. In: Galanakis C.M., editor. Polyphenols: Properties, Recovery, and Applications. Elsevier; Amsterdam, The Netherlands: 2018. pp. 3–44. [Google Scholar]
- 30.Moyer R.A., Hummer K.E., Finn C.E., Frei B., Wrolstad R.E. Anthocyanins, phenolics, and antioxidant capacity in diverse small fruits: Vaccinium, Rubus, and Ribes. J. Agric. Food Chem. 2002;50:519–525. doi: 10.1021/jf011062r. [DOI] [PubMed] [Google Scholar]
- 31.Kasiotis K.M., Pratsinis H., Kletsas D., Haroutounian S.A. Resveratrol and related stilbenes: Their anti-aging and anti-angiogenic properties. Food Chem. Toxicol. 2013;61:112–120. doi: 10.1016/j.fct.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 32.Li Y.R., Li S., Lin C.C. Effect of resveratrol and pterostilbene on aging and longevity. Biofactors. 2018;44:69–82. doi: 10.1002/biof.1400. [DOI] [PubMed] [Google Scholar]
- 33.Boots A.W., Haenen G.R., Bast A. Health effects of quercetin: From antioxidant to nutraceutical. Eur. J. Pharmacol. 2008;585:325–337. doi: 10.1016/j.ejphar.2008.03.008. [DOI] [PubMed] [Google Scholar]
- 34.Viña J., Borrás C., Miquel J. Theories of ageing. IUBMB Life. 2007;59:249–254. doi: 10.1080/15216540601178067. [DOI] [PubMed] [Google Scholar]
- 35.Lobo V., Patil A., Phatak A., Chandra N. Free radicals, antioxidants and functional foods: Impact on human health. Pharmacogn. Rev. 2010;4:118–126. doi: 10.4103/0973-7847.70902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lushchak V.I. Free radicals, reactive oxygen species, oxidative stress and its classification. Chem. Biol. Interact. 2014;224:164–175. doi: 10.1016/j.cbi.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 37.Bagchi K., Puri S. Free radicals and antioxidants in health and disease: A review. East. Mediterr. Health J. 1998;4:350–360. [Google Scholar]
- 38.Ebadi M. Antioxidants and free radicals in health and disease: An introduction to reactive oxygen species, oxidative injury, neuronal cell death and therapy in neurodegenerative diseases. Crit. Rev. Toxicol. 2001;38:13–71. [Google Scholar]
- 39.Scapagnini G., Caruso C., Spera G. Preventive medicine and healthy longevity: Basis for sustainable anti-aging strategies. In: Scuderi N., Toth B.A., editors. International Textbook of Aesthetic Surgery. Springer; Berlin/Heidelberg, Germany: 2016. pp. 1213–1227. [Google Scholar]
- 40.Young I.S., Woodside J.V. Antioxidants in health and disease. J. Clin. Pathol. 2001;54:176–186. doi: 10.1136/jcp.54.3.176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Vaiserman A.M., Lushchak V., Koliada A.K. Anti-aging pharmacology: Promises and pitfalls. Ageing Res. Rev. 2016;31:9–35. doi: 10.1016/j.arr.2016.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Ďuračková Z. Some current insights into oxidative stress. Physiol. Res. 2010;59:459–469. doi: 10.33549/physiolres.931844. [DOI] [PubMed] [Google Scholar]
- 43.Dhalaria R., Kumar D., Kumar H., Nepovimova E., Kuča K., Torequl Islam M., Verma R. Arbuscular mycorrhizal fungi as potential agents in ameliorating heavy metal stress in plants. Agronomy. 2020;10:815. doi: 10.3390/agronomy10060815. [DOI] [Google Scholar]
- 44.Marklund S.L. Extracellular superoxide dismutase and other superoxide dismutase isoenzymes in tissues from nine mammalian species. Biochem. J. 1984;222:649–655. doi: 10.1042/bj2220649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chelikani P., Fita I., Loewen P.C. Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 2004;61:192–208. doi: 10.1007/s00018-003-3206-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos--Sánchez N.F., Salas-Coronado R., Villanueva-Cañongo C., Hernández-Carlos B. Antioxidant compounds and their antioxidant mechanism. In: Shalaby E., editor. Antioxidants. IntechOpen; London, UK: 2019. pp. 1–28. [Google Scholar]
- 47.Alhasawi A., Legendre F., Jagadeesan S., Appanna V., Appanna V. Biochemical Strategies to Counter Nitrosative Stress: Nanofactories for Value-Added Products. In: Das S., Dash H.R., editors. Microbial Diversity in the Genomic Era. Elsevier; Amsterdam, The Netherlands: 2019. pp. 153–169. [Google Scholar]
- 48.Momtaz S., Abdollahi M. A comprehensive review of biochemical and molecular evidences from animal and human studies on the role of oxidative stress in aging: An epiphenomenon or the cause. [(accessed on 4 November 2020)];Asian J. Anim. Vet. Adv. 2012 :1–18. Available online: http://hdl.handle.net/2263/19369.
- 49.Jay D., Hitomi H., Griendling K.K. Oxidative stress and diabetic cardiovascular complications. Free Radic. Biol. Med. 2006;40:183–192. doi: 10.1016/j.freeradbiomed.2005.06.018. [DOI] [PubMed] [Google Scholar]
- 50.Valko M., Leibfritz D., Moncol J., Cronin M.T., Mazur M., Telser J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007;39:44–84. doi: 10.1016/j.biocel.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Reuter S., Gupta S.C., Chaturvedi M.M., Aggarwal B.B. Oxidative stress, inflammation, and cancer: How are they linked? Free Radic. Biol. Med. 2010;49:1603–1616. doi: 10.1016/j.freeradbiomed.2010.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhat A.H., Dar K.B., Anees S., Zargar M.A., Masood A., Sofi M.A., Ganie S.A. Oxidative stress, mitochondrial dysfunction and neurodegenerative diseases; a mechanistic insight. Biomed. Pharmacother. 2015;74:101–110. doi: 10.1016/j.biopha.2015.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Khanthapok P., Sukrong S. Anti-aging and health benefits from Thai food: Protective effects of bioactive compounds on the free radical theory of aging. J. Food Health Bioenviron. Sci. 2019;12:54–67. [Google Scholar]
- 54.Naczk M., Shahidi F. Extraction and analysis of phenolics in food. J. Chromatogr. A. 2004;1054:95–111. doi: 10.1016/S0021-9673(04)01409-8. [DOI] [PubMed] [Google Scholar]
- 55.Olas B. Berry phenolic antioxidants–implications for human health? Front. Pharmacol. 2018;9:1–14. doi: 10.3389/fphar.2018.00078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szajdek A., Borowska E.J. Bioactive compounds and health-promoting properties of berry fruits: A review. Plant Foods Hum. Nutr. 2008;63:147–156. doi: 10.1007/s11130-008-0097-5. [DOI] [PubMed] [Google Scholar]
- 57.Slavin J.L., Lloyd B. Health benefits of fruits and vegetables. Adv. Nutr. 2012;3:506–516. doi: 10.3945/an.112.002154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Skrovankova S., Sumczynski D., Mlcek J., Jurikova T., Sochor J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015;16:24673–24706. doi: 10.3390/ijms161024673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Orena S., Owen J., Jin F., Fabian M., Gillitt N.D., Zeisel S.H. Extracts of fruits and vegetables activate the antioxidant response element in IMR-32 cells. J. Nutr. 2015;145:2006–2011. doi: 10.3945/jn.115.216705. [DOI] [PubMed] [Google Scholar]
- 60.Ahmed S.M.U., Luo L., Namani A., Wang X.J., Tang X. Nrf2 signaling pathway: Pivotal roles in inflammation. Biochim. Biophys. Acta Mol. Basis Dis. 2017;1863:585–597. doi: 10.1016/j.bbadis.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 61.Pantelidis G.E., Vasilakakis M., Manganaris G.A., Diamantidis G.R. Antioxidant capacity, phenol, anthocyanin and ascorbic acid contents in raspberries, blackberries, red currants, gooseberries and Cornelian cherries. Food Chem. 2007;102:777–783. doi: 10.1016/j.foodchem.2006.06.021. [DOI] [Google Scholar]
- 62.U.S. Department of Agriculture, Agricultural Research Service Food Data Central. [(accessed on 24 July 2020)]; Available online: fdc.nal.usda.gov.
- 63.Arrigoni O., De Tullio M.C. Ascorbic acid: Much more than just an antioxidant. Biochem. Biophys. Acta Gen. Subj. 2002;1569:1–9. doi: 10.1016/S0304-4165(01)00235-5. [DOI] [PubMed] [Google Scholar]
- 64.Wu X., Cheng J., Wang X. Dietary antioxidants: Potential anticancer agents. Nutr. Cancer. 2017;69:521–533. doi: 10.1080/01635581.2017.1299872. [DOI] [PubMed] [Google Scholar]
- 65.Nimse S.B., Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006. doi: 10.1039/C4RA13315C. [DOI] [Google Scholar]
- 66.Niki E., Noguchi N., Tsuchihashi H., Gotoh N. Interaction among vitamin C, vitamin E, and beta-carotene. Am. J. Clin. Nutr. 1995;62:1322S–1326S. doi: 10.1093/ajcn/62.6.1322S. [DOI] [PubMed] [Google Scholar]
- 67.Monaghan B.R., Schmitt F.O. The effects of carotene and of vitamin A on the oxidation of linoleic acid. J. Biol. Chem. 1932;96:387–395. [Google Scholar]
- 68.Livrea M.A., Tesoriere L., Bongiorno A., Pintaudi A.M., Ciaccio M., Riccio A. Contribution of vitamin A to the oxidation resistance of human low density lipoproteins. Free Radic. Biol. Med. 1995;18:401–409. doi: 10.1016/0891-5849(94)00151-9. [DOI] [PubMed] [Google Scholar]
- 69.Biesalski H.K., Dragsted L.O., Elmadfa I., Grossklaus R., Müller M., Schrenk D., Weber P. Bioactive compounds: Definition and assessment of activity. Nutrition. 2009;25:1202–1205. doi: 10.1016/j.nut.2009.04.023. [DOI] [PubMed] [Google Scholar]
- 70.Ortega A.M.M., Campos M.R.S. Bioactive Compounds as Therapeutic Alternatives. In: Campos M.R.S., editor. Bioactive Compounds. Elsevier; Amsterdam, The Netherlands: 2019. pp. 247–264. [Google Scholar]
- 71.Liu R.H. Dietary bioactive compounds and their health implications. J. Food Sci. 2013;78:A18–A25. doi: 10.1111/1750-3841.12101. [DOI] [PubMed] [Google Scholar]
- 72.Fu L., Xu B.T., Xu X.R., Gan R.Y., Zhang Y., Xia E.Q., Li H.B. Antioxidant capacities and total phenolic contents of 62 fruits. Food Chem. 2011;129:345–350. doi: 10.1016/j.foodchem.2011.04.079. [DOI] [PubMed] [Google Scholar]
- 73.Haminiuk C.W., Maciel G.M., Plata-Oviedo M.S., Peralta R.M. Phenolic compounds in fruits—An overview. Int. J. Food Sci. Technol. 2012;47:2023–2044. doi: 10.1111/j.1365-2621.2012.03067.x. [DOI] [Google Scholar]
- 74.Tabart J., Franck T., Kevers C., Pincemail J., Serteyn D., Defraigne J.O., Dommes J. Antioxidant and anti-inflammatory activities of Ribes nigrum extracts. Food Chem. 2012;131:1116–1122. doi: 10.1016/j.foodchem.2011.09.076. [DOI] [Google Scholar]
- 75.Selma M.V., Espin J.C., Tomas-Barberan F.A. Interaction between phenolics and gut microbiota: Role in human health. J. Agric. Food Chem. 2009;57:6485–6501. doi: 10.1021/jf902107d. [DOI] [PubMed] [Google Scholar]
- 76.Rockenbach I.I., Rodrigues E., Gonzaga L.V., Caliari V., Genovese M.I., Gonçalves A.E.D.S.S., Fett R. Phenolic compounds content and antioxidant activity in pomace from selected red grapes (Vitis vinifera L. and Vitis labrusca L.) widely produced in Brazil. Food Chem. 2011;127:174–179. doi: 10.1016/j.foodchem.2010.12.137. [DOI] [Google Scholar]
- 77.Badhani B., Sharma N., Kakkar R. Gallic acid: A versatile antioxidant with promising therapeutic and industrial applications. RSC Adv. 2015;5:27540–27557. doi: 10.1039/C5RA01911G. [DOI] [Google Scholar]
- 78.Russell W.R., Scobbie L., Labat A., Duthie G.G. Selective bio-availability of phenolic acids from Scottish strawberries. Mol. Nutr. Food Res. 2009;53:S85–S91. doi: 10.1002/mnfr.200800302. [DOI] [PubMed] [Google Scholar]
- 79.Usenik V., Fabcic J., Stampar F. Sugars, organic acids, phenolic composition and antioxidant activity of sweet cherry (Prunus avium L.) Food Chem. 2008;107:185–192. doi: 10.1016/j.foodchem.2007.08.004. [DOI] [Google Scholar]
- 80.Boyer J., Liu R.H. Apple phytochemicals and their health benefits. Nutr. J. 2004;3:1–15. doi: 10.1186/1475-2891-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gavrilova V., Kajdzanoska M., Gjamovski V., Stefova M. Separation, Characterization and Quantification of Phenolic Compounds in Blueberries and Red and Black Currants by HPLC− DAD− ESI-MSn. J. Agric. Food Chem. 2011;59:4009–4018. doi: 10.1021/jf104565y. [DOI] [PubMed] [Google Scholar]
- 82.Borges G., Degeneve A., Mullen W., Crozier A. Identification of flavonoid and phenolic antioxidants in black currants, blueberries, raspberries, red currants, and cranberries. J. Agric. Food Chem. 2010;58:3901–3909. doi: 10.1021/jf902263n. [DOI] [PubMed] [Google Scholar]
- 83.Kelebek H., Selli S. Evaluation of chemical constituents and antioxidant activity of sweet cherry (Prunus avium L.) cultivars. Int. J. Food Sci. Technol. 2011;46:2530–2537. doi: 10.1111/j.1365-2621.2011.02777.x. [DOI] [Google Scholar]
- 84.Iglesias-Carres L., Mas-Capdevila A., Bravo F.I., Aragonès G., Muguerza B., Arola-Arnal A. Optimization of a polyphenol extraction method for sweet orange pulp (Citrus sinensis L.) to identify phenolic compounds consumed from sweet oranges. PLoS ONE. 2019;14:e0211267. doi: 10.1371/journal.pone.0211267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Jakobek L., Seruga M., Novak I., Medvidović-Kosanović M. Flavonols, phenolic acids and antioxidant activity of some red fruits. Deut. Lebensm Rundsch. 2007;103:369–378. [Google Scholar]
- 86.Chu N.T., Clydesdale F.M., Francis F.J. Isolation and identification of some fluorescent phenolic compounds in cranberries. J. Food Sci. 1973;38:1038–1042. doi: 10.1111/j.1365-2621.1973.tb02143.x. [DOI] [Google Scholar]
- 87.Okatan V. Antioxidant properties and phenolic profile of the most widely appreciated cultivated berry species: A comparative study. Folia Hortic. 2020;32:79–85. doi: 10.2478/fhort-2020-0008. [DOI] [Google Scholar]
- 88.Hernanz D., Recamales A.F., Meléndez-Martínez A.J., González-Miret M.L., Heredia F.J. Assessment of the differences in the phenolic composition of five strawberry cultivars (Fragaria × ananassa Duch.) grown in two different soilless systems. J. Agric. Food Chem. 2007;55:1846–1852. doi: 10.1021/jf063189s. [DOI] [PubMed] [Google Scholar]
- 89.Häkkinen S.H., Törrönen A.R. Content of flavonols and selected phenolic acids in strawberries and Vaccinium species: Influence of cultivar, cultivation site and technique. Food Res. Int. 2000;33:517–524. doi: 10.1016/S0963-9969(00)00086-7. [DOI] [Google Scholar]
- 90.Barreca D., Gattuso G., Bellocco E., Calderaro A., Trombetta D., Smeriglio A., Lagana G., Daglia M., Meneghini S., Nabavi S.M. Flavanones: Citrus phytochemical with health-promoting properties. BioFactors. 2017;43:495–506. doi: 10.1002/biof.1363. [DOI] [PubMed] [Google Scholar]
- 91.Mozetic B., Trebse P., Hribar J. Determination and quantitation of anthocyanins and hydroxycinnamic acids in different cultivars of sweet cherries (Prunus avium L.) from Nova Gorica region (Slovenia) Food Technol. Biotechnol. 2002;40:207–212. [Google Scholar]
- 92.Bakowska-Barczak A.M., Kolodziejczyk P.P. Black currant polyphenols: Their storage stability and microencapsulation. Ind. Crops Prod. 2011;34:1301–1309. doi: 10.1016/j.indcrop.2010.10.002. [DOI] [Google Scholar]
- 93.Kahle K., Kraus M., Scheppach W., Ackermann M., Ridder F., Richling E. Studies on apple and blueberry fruit constituents: Do the polyphenols reach the colon after ingestion? Mol. Nutr. Food Res. 2006;50:418–423. doi: 10.1002/mnfr.200500211. [DOI] [PubMed] [Google Scholar]
- 94.Huang X., Mazza G. Simultaneous analysis of serotonin, melatonin, piceid and resveratrol in fruits using liquid chromatography tandem mass spectrometry. J. Chromatogr. A. 2011;1218:3890–3899. doi: 10.1016/j.chroma.2011.04.049. [DOI] [PubMed] [Google Scholar]
- 95.Bobinaitė R., Viškelis P., Venskutonis P.R. Variation of total phenolics, anthocyanins, ellagic acid and radical scavenging capacity in various raspberry (Rubus spp.) cultivars. Food Chem. 2012;132:1495–1501. doi: 10.1016/j.foodchem.2011.11.137. [DOI] [PubMed] [Google Scholar]
- 96.Ma Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013;53:401–426. doi: 10.1146/annurev-pharmtox-011112-140320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lu J.M., Lin P.H., Yao Q., Chen C. Chemical and molecular mechanisms of antioxidants: Experimental approaches and model systems. J. Cell. Mol. Med. 2010;14:840–860. doi: 10.1111/j.1582-4934.2009.00897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Castilla P., Dávalos A., Teruel J.L., Cerrato F., Fernández-Lucas M., Merino J.L., Sanchez-Marti C.C., Ortuno J., Lasunción M.A. Comparative effects of dietary supplementation with red grape juice and vitamin E on production of superoxide by circulating neutrophil NADPH oxidase in hemodialysis patients. Am. J. Clin. Nutr. 2008;87:1053–1061. doi: 10.1093/ajcn/87.4.1053. [DOI] [PubMed] [Google Scholar]
- 99.Karasawa M.M.G., Mohan C. Fruits as prospective reserves of bioactive compounds: A review. Nat. Prod. Bioprospect. 2018;8:335–346. doi: 10.1007/s13659-018-0186-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Bastos C., Barros L., Dueñas M., Calhelha R.C., Queiroz M.J.R., Santos-Buelga C., Ferreira I.C. Chemical characterisation and bioactive properties of Prunus avium L.: The widely studied fruits and the unexplored stems. Food Chem. 2015;173:1045–1053. doi: 10.1016/j.foodchem.2014.10.145. [DOI] [PubMed] [Google Scholar]
- 101.Kumar N., Goel N. Phenolic acids: Natural versatile molecules with promising therapeutic applications. Biotechnol. Rep. 2019;24:1–10. doi: 10.1016/j.btre.2019.e00370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sun J., Chu Y.F., Wu X., Liu R.H. Antioxidant and antiproliferative activities of common fruits. J. Agric. Food Chem. 2002;50:7449–7454. doi: 10.1021/jf0207530. [DOI] [PubMed] [Google Scholar]
- 103.Singh J.P., Kaur A., Shevkani K., Singh N. Composition, bioactive compounds and antioxidant activity of common Indian fruits and vegetables. J. Food Sci. Technol. 2016;53:4056–4066. doi: 10.1007/s13197-016-2412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Natella F., Nardini M., Di Felice M., Scaccini C. Benzoic and cinnamic acid derivatives as antioxidants: Structure− activity relation. J. Agric. Food Chem. 1999;47:1453–1459. doi: 10.1021/jf980737w. [DOI] [PubMed] [Google Scholar]
- 105.Khan B.A., Mahmood T., Menaa F., Shahzad Y., Yousaf A.M., Hussain T., Ray S.D. New perspectives on the efficacy of gallic acid in cosmetics and nanocosmeceuticals. Curr. Pharm. Des. 2018;24:5181–5187. doi: 10.2174/1381612825666190118150614. [DOI] [PubMed] [Google Scholar]
- 106.Umar Lule S., Xia W. Food phenolics, pros and cons: A review. Food Rev. Int. 2005;21:367–388. doi: 10.1080/87559120500222862. [DOI] [Google Scholar]
- 107.Ruifeng G., Yunhe F., Zhengkai W., Yimeng L., Minjun Y., Xiaojing S., Zhengtao Y., Naisheng Z. Chlorogenic acid attenuates lipopolysaccharide-induced mice mastitis by suppressing TLR4-mediated NF-κB signaling pathway. Eur. J. Pharmacol. 2014;729:54–58. doi: 10.1016/j.ejphar.2014.01.015. [DOI] [PubMed] [Google Scholar]
- 108.Ozcan T., Akpinar-Bayizit A., Yilmaz-Ersan L., Delikanli B. Phenolics in human health. Int. J. Chem. Eng. Appl. 2014;5:393–396. doi: 10.7763/IJCEA.2014.V5.416. [DOI] [Google Scholar]
- 109.Kumar H., Bhardwaj K., Nepovimova E., Kamil K., Dhanjal D.S., Bhardwaj S., Bhatia S.K., Verma R., Kumar D. Antioxidant functionalized nanoparticles: A combat against oxidative stress. Nanomaterials. 2020;10:1334. doi: 10.3390/nano10071334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Gulcin I., Beydemir S. Phenolic compounds as antioxidants: Carbonic anhydrase isoenzymes inhibitors. Mini-Rev. Med. Chem. 2013;13:408–430. doi: 10.2174/138955713804999874. [DOI] [PubMed] [Google Scholar]
- 111.Thorat I.D., Jagtap D.D., Mohapatra D., Joshi D.C., Sutar R.F., Kapdi S.S. Antioxidants, their properties, uses in food products and their legal implications. Int. J. Food Stud. 2013;2:81–104. doi: 10.7455/ijfs/2.1.2013.a7. [DOI] [Google Scholar]
- 112.Balasundram N., Sundram K., Samman S. Phenolic compounds in plants and agri-industrial by-products: Antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. doi: 10.1016/j.foodchem.2005.07.042. [DOI] [Google Scholar]
- 113.Kumar S., Pandey A.K. Chemistry and biological activities of flavonoids: An overview. Sci. World J. 2013;2013:1–16. doi: 10.1155/2013/162750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kabera J.N., Semana E., Mussa A.R., He X. Plant secondary metabolites: Biosynthesis, classification, function and pharmacological properties. J. Pharm. Pharmacol. 2014;2:377–392. [Google Scholar]
- 115.Orhan D.D., Özçelik B., Özgen S., Ergun F. Antibacterial, antifungal, and antiviral activities of some flavonoids. Microbiol. Res. 2010;165:496–504. doi: 10.1016/j.micres.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 116.Manthey J.A., Guthrie N., Grohmann K. Biological properties of citrus flavonoids pertaining to cancer and inflammation. Curr. Med. Chem. 2001;8:135–153. doi: 10.2174/0929867013373723. [DOI] [PubMed] [Google Scholar]
- 117.Hwang S.L., Shih P.H., Yen G.C. Neuroprotective effects of citrus flavonoids. J. Agric. Food Chem. 2012;60:877–885. doi: 10.1021/jf204452y. [DOI] [PubMed] [Google Scholar]
- 118.Benavente-Garcia O., Castillo J. Update on uses and properties of citrus flavonoids: New findings in anticancer, cardiovascular, and anti-inflammatory activity. J. Agric. Food Chem. 2008;56:6185–6205. doi: 10.1021/jf8006568. [DOI] [PubMed] [Google Scholar]
- 119.Bravo L. Polyphenols: Chemistry, dietary sources, metabolism, and nutritional significance. Nutr. Rev. 1998;56:317–333. doi: 10.1111/j.1753-4887.1998.tb01670.x. [DOI] [PubMed] [Google Scholar]
- 120.Manthey J.A., Guthrie N. Antiproliferative activities of citrus flavonoids against six human cancer cell lines. J. Agric. Food Chem. 2002;50:5837–5843. doi: 10.1021/jf020121d. [DOI] [PubMed] [Google Scholar]
- 121.Casagrande F., Darbon J.M. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem. Pharmacol. 2001;61:1205–1215. doi: 10.1016/S0006-2952(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 122.Kawaii S., Tomono Y., Katase E., Ogawa K., Yano M. Antiproliferative activity of flavonoids on several cancer cell lines. Biosci. Biotechnol. Biochem. 1999;63:896–899. doi: 10.1271/bbb.63.896. [DOI] [PubMed] [Google Scholar]
- 123.Pervaiz T., Songtao J., Faghihi F., Haider M.S., Fang J. Naturally occurring anthocyanin, structure, functions and biosynthetic pathway in fruit plants. J. Plant Biochem. Physiol. 2017;5:1–9. doi: 10.4172/2329-9029.1000187. [DOI] [Google Scholar]
- 124.You Q., Wang B., Chen F., Huang Z., Wang X., Luo P.G. Comparison of anthocyanins and phenolics in organically and conventionally grown blueberries in selected cultivars. Food Chem. 2011;125:201–208. doi: 10.1016/j.foodchem.2010.08.063. [DOI] [Google Scholar]
- 125.Olivas-Aguirre F.J., Rodrigo-García J., Martínez-Ruiz N.D.R., Cárdenas-Robles A.I., Mendoza-Díaz S.O., Alvarez-Parrilla E., Gonzalez-Aguilar G.A., De la Rosa L.A., Ramos-Jimenez A., Wall-Medrano A. Cyanidin-3-O-glucoside: Physical-chemistry, foodomics and health effects. Molecules. 2016;21:1264. doi: 10.3390/molecules21091264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Smeriglio A., Barreca D., Bellocco E., Trombetta D. Chemistry, pharmacology and health benefits of anthocyanins. Phytother. Res. 2016;30:1265–1286. doi: 10.1002/ptr.5642. [DOI] [PubMed] [Google Scholar]
- 127.Kong J.M., Chia L.S., Goh N.K., Chia T.F., Brouillard R. Analysis and biological activities of anthocyanins. Phytochemistry. 2003;64:923–933. doi: 10.1016/S0031-9422(03)00438-2. [DOI] [PubMed] [Google Scholar]
- 128.Yazhen S., Wenju W., Panpan Z., Yuanyuan Y., Panpan D., Wusen Z., Yanling W. Anthocyanins: Novel Antioxidants in Diseases Prevention and Human Health. In: Badria F.A., Ananga A., editors. Flavonoids-A Coloring Model for Cheering up Life. IntechOpen; London, UK: 2019. pp. 1–16. [Google Scholar]
- 129.Miguel M.G. Anthocyanins: Antioxidant and/or anti-inflammatory activities. J. Appl. Pharm. Sci. 2011;1:7–15. [Google Scholar]
- 130.Srivastava A., Akoh C.C., Fischer J., Krewer G. Effect of anthocyanin fractions from selected cultivars of Georgia-grown blueberries on apoptosis and phase II enzymes. J. Agric. Food Chem. 2007;55:3180–3185. doi: 10.1021/jf062915o. [DOI] [PubMed] [Google Scholar]
- 131.Jimenez-Garcia S.N., Guevara-Gonzalez R.G., Miranda-Lopez R., Feregrino-Perez A.A., Torres-Pacheco I., Vazquez-Cruz M.A. Functional properties and quality characteristics of bioactive compounds in berries: Biochemistry, biotechnology, and genomics. Food Res. Int. 2013;54:1195–1207. doi: 10.1016/j.foodres.2012.11.004. [DOI] [Google Scholar]
- 132.Battino M., Beekwilder J., Denoyes-Rothan B., Laimer M., McDougall G.J., Mezzetti B. Bioactive compounds in berries relevant to human health. Nutr. Rev. 2009;67:S145–S150. doi: 10.1111/j.1753-4887.2009.00178.x. [DOI] [PubMed] [Google Scholar]
- 133.Viskelis P., Rubinskienė M., Jasutienė I., Šarkinas A., Daubaras R., Česonienė L. Anthocyanins, antioxidative, and antimicrobial properties of American cranberry (Vaccinium macrocarpon Ait.) and their press cakes. J. Food Sci. 2009;74:C157–C161. doi: 10.1111/j.1750-3841.2009.01066.x. [DOI] [PubMed] [Google Scholar]
- 134.Khoo H.E., Azlan A., Tang S.T., Lim S.M. Anthocyanidins and anthocyanins: Colored pigments as food, pharmaceutical ingredients, and the potential health benefits. Food Nutr. Res. 2017;61:1–21. doi: 10.1080/16546628.2017.1361779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Thoppil R.J., Bhatia D., Barnes K.F., Haznagy-Radnai E., Hohmann J., Darvesh A.S., Bishayee A. Black currant anthocyanins abrogate oxidative stress through Nrf2-mediated antioxidant mechanisms in a rat model of hepatocellular carcinoma. Curr. Cancer Drug Tar. 2012;12:1244–1257. doi: 10.2174/156800912803987968. [DOI] [PubMed] [Google Scholar]
- 136.Vendrame S., Klimis-Zacas D. Anti-inflammatory effect of anthocyanins via modulation of nuclear factor-κB and mitogen-activated protein kinase signaling cascades. Nutr. Rev. 2015;73:348–358. doi: 10.1093/nutrit/nuu066. [DOI] [PubMed] [Google Scholar]
- 137.Shukla A.S., Jha A.K., Kumari R., Rawat K., Syeda S., Shrivastava A. Role of Catechins in Chemosensitization. In: Bharti A.C., Aggarwal B.B., editors. Role of Nutraceuticals in Cancer Chemosensitization. Volume 2. Elsevier; Amsterdam, The Netherlands: 2018. pp. 169–198. [Google Scholar]
- 138.Zanwar A.A., Badole S.L., Shende P.S., Hegde M.V., Bodhankar S.L. Antioxidant role of catechin in health and disease. In: Watson R.R., Preedy V.R., Zibadi S., editors. Polyphenols in Human Health and Disease. Volume 1. Elsevier; Amsterdam, The Netherlands: 2014. pp. 267–271. [Google Scholar]
- 139.Naasani I., Seimiya H., Tsuruo T. Telomerase inhibition, telomere shortening, and senescence of cancer cells by tea catechins. Biochem. Biophys. Res. Commun. 1998;249:391–396. doi: 10.1006/bbrc.1998.9075. [DOI] [PubMed] [Google Scholar]
- 140.Cosarca S., Tanase C., Muntean D.L. Therapeutic aspects of catechins and its derivatives- an update. Acta Biol. Marisiensis. 2019;2:21–29. doi: 10.2478/abmj-2019-0003. [DOI] [Google Scholar]
- 141.Fan F.Y., Sang L.X., Jiang M. Catechins and Their Therapeutic Benefits to Inflammatory Bowel Disease. Molecules. 2017;22:484. doi: 10.3390/molecules22030484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pan Z., Zhou Y., Luo X., Ruan Y., Zhou L., Wang Q., Yan Y.J., Liu Q., Chen J. Against NF-κB/thymic stromal lymphopoietin signaling pathway, catechin alleviates the inflammation in allergic rhinitis. Int. Immunopharmacol. 2018;61:241–248. doi: 10.1016/j.intimp.2018.06.011. [DOI] [PubMed] [Google Scholar]
- 143.Williamson G., Manach C. Bioavailability and bioefficacy of polyphenols in humans. II. Review of 93 intervention studies. Am. J. Clin. Nutr. 2005;81:243S–255S. doi: 10.1093/ajcn/81.1.243S. [DOI] [PubMed] [Google Scholar]
- 144.Pocernich C.B., Lange M.L., Sultana R., Butterfield D.A. Nutritional approaches to modulate oxidative stress in Alzheimer’s disease. Curr. Alzheimer Res. 2011;8:452–469. doi: 10.2174/156720511796391908. [DOI] [PubMed] [Google Scholar]
- 145.Horvathova K., Novotny L., Tothova D., Vachalkova A. Determination of free radical scavenging activity of quercetin, rutin, luteolin and apigenin in H~ 2O~ 2-treated human ML cells K562. Neoplasma. 2004;51:395–399. [PubMed] [Google Scholar]
- 146.Heijnen C.G., Haenen G.R., Minou Oostveen R., Stalpers E.M., Bast A. Protection of flavonoids against lipid peroxidation: The structure activity relationship revisited. Free Radic. Res. 2002;36:575–581. doi: 10.1080/10715760290025951. [DOI] [PubMed] [Google Scholar]
- 147.Chondrogianni N., Kapeta S., Chinou I., Vassilatou K., Papassideri I., Gonos E.S. Anti-ageing and rejuvenating effects of quercetin. Exp. Gerontol. 2010;45:763–771. doi: 10.1016/j.exger.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 148.Lesjak M., Beara I., Simin N., Pintać D., Majkić T., Bekvalac K., Orcic D., Mimica-Dukić N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods. 2018;40:68–75. doi: 10.1016/j.jff.2017.10.047. [DOI] [Google Scholar]
- 149.David A.V.A., Arulmoli R., Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Bautista-Ortín A.B., Rodríguez-Rodríguez P., Gil-Muñoz R., Jiménez-Pascual E., Busse-Valverde N., Martínez-Cutillas A., Lopez-Roca J.M., Gómez-Plaza E. Influence of berry ripeness on concentration, qualitative composition and extractability of grape seed tannins. Aust. J. Grape Wine Res. 2012;18:123–130. doi: 10.1111/j.1755-0238.2012.00178.x. [DOI] [Google Scholar]
- 151.Puupponen-Pimiä R., Nohynek L., Alakomi H.L., Oksman-Caldentey K.M. Bioactive berry compounds—novel tools against human pathogens. Appl. Microbiol. Biotechnol. 2005;67:8–18. doi: 10.1007/s00253-004-1817-x. [DOI] [PubMed] [Google Scholar]
- 152.Bushman B.S., Phillips B., Isbell T., Ou B., Crane J.M., Knapp S.J. Chemical composition of cranberry (Rubus spp.) seeds and oils and their antioxidant potential. J. Agric. Food Chem. 2004;52:7982–7987. doi: 10.1021/jf049149a. [DOI] [PubMed] [Google Scholar]
- 153.Mullen W., McGinn J., Lean M.E., MacLean M.R., Gardner P., Duthie G.G., Yokota T., Crozier A. Ellagitannins, flavonoids, and other phenolics in red raspberries and their contribution to antioxidant capacity and vasorelaxation properties. J. Agric. Food Chem. 2002;50:5191–5196. doi: 10.1021/jf020140n. [DOI] [PubMed] [Google Scholar]
- 154.Han X., Shen T., Lou H. Dietary polyphenols and their biological significance. Int. J. Mol. Sci. 2007;8:950–988. doi: 10.3390/i8090950. [DOI] [Google Scholar]
- 155.De Ligt M., Timmers S., Schrauwen P. Resveratrol and obesity: Can resveratrol relieve metabolic disturbances? Biochim. Biophys. Acta Mol. Basis Dis. 2015;1852:1137–1144. doi: 10.1016/j.bbadis.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 156.Kopp P. Resveratrol, a phytoestrogen found in red wine. A possible explanation for the conundrum of the ‘French paradox’? Eur. J. Endocrinol. 1998;138:619–620. doi: 10.1530/eje.0.1380619. [DOI] [PubMed] [Google Scholar]
- 157.Catalgol B., Batirel S., Taga Y., Ozer N.K. Resveratrol: French paradox revisited. Front. Pharmacol. 2012;3:1–18. doi: 10.3389/fphar.2012.00141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Knutson M.D., Leeuwenburgh C. Resveratrol and novel potent activators of SIRT1: Effects on aging and age-related diseases. Nutr. Rev. 2008;66:591–596. doi: 10.1111/j.1753-4887.2008.00109.x. [DOI] [PubMed] [Google Scholar]
- 159.Sebastià N., Montoro A., Mañes J., Soriano J.M. A preliminary study of presence of resveratrol in skins and pulps of European and Japanese plum cultivars. J. Sci. Food Agric. 2012;92:3091–3094. doi: 10.1002/jsfa.5759. [DOI] [PubMed] [Google Scholar]
- 160.Di Franco R., Calvanese M., Murino P., Manzo R., Guida C., Di Gennaro D., Anania C., Ravo V. Skin toxicity from external beam radiation therapy in breast cancer patients: Protective effects of Resveratrol, Lycopene, Vitamin C and anthocianin (Ixor®) Radiat. Oncol. 2012;7:1–6. doi: 10.1186/1748-717X-7-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Risuleo G. Resveratrol: Multiple activities on the biological functionality of the cell. In: Gupta R.C., editor. Nutraceuticals. Elsevier; Amsterdam, The Netherlands: 2016. pp. 453–464. [Google Scholar]
- 162.Serra A.T., Duarte R.O., Bronze M.R., Duarte C.M. Identification of bioactive response in traditional cherries from Portugal. Food Chem. 2011;125:318–325. doi: 10.1016/j.foodchem.2010.07.088. [DOI] [Google Scholar]
- 163.Lee J., Koo N., Min D.B. Reactive oxygen species, aging, and antioxidative nutraceuticals. Compr. Rev. Food Sci. Food Saf. 2004;3:21–33. doi: 10.1111/j.1541-4337.2004.tb00058.x. [DOI] [PubMed] [Google Scholar]
- 164.Lim Y.Y., Lim T.T., Tee J.J. Antioxidant properties of several tropical fruits: A comparative study. Food Chem. 2007;103:1003–1008. doi: 10.1016/j.foodchem.2006.08.038. [DOI] [Google Scholar]
- 165.Favela-Hernández J.M.J., González-Santiago O., Ramírez-Cabrera M.A., Esquivel-Ferriño P.C., Camacho-Corona M.D.R. Chemistry and Pharmacology of Citrus sinensis. Molecules. 2016;21:247. doi: 10.3390/molecules21020247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Khan M.K., Dangles O. A comprehensive review on flavanones, the major citrus polyphenols. J. Food Compos. Anal. 2014;33:85–104. doi: 10.1016/j.jfca.2013.11.004. [DOI] [Google Scholar]
- 167.Afrin S., Gasparrini M., Forbes-Hernandez T.Y., Reboredo-Rodriguez P., Mezzetti B., Varela-Lόpez A., Giampieri F., Battino M. Promising health benefits of the strawberry: A focus on clinical studies. J. Agric. Food Chem. 2016;64:4435–4449. doi: 10.1021/acs.jafc.6b00857. [DOI] [PubMed] [Google Scholar]
- 168.Giampieri F., Alvarez-Suarez J.M., Battino M. Strawberry and human health: Effects beyond antioxidant activity. J. Agric. Food Chem. 2014;62:3867–3876. doi: 10.1021/jf405455n. [DOI] [PubMed] [Google Scholar]
- 169.Bai L., Guo S., Liu Q., Cui X., Zhang X., Zhang L., Yang X., Hou M., Ho C.T., Bai N. Characterization of nine polyphenols in fruits of Malus pumila Mill by high-performance liquid chromatography. J. Food Drug Anal. 2016;24:293–298. doi: 10.1016/j.jfda.2015.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rupasinghe H.V., Thilakarathna S., Nair S. Polyphenols: Chemistry, Dietary Sources and Health Benefits. Nova Science; New York, NY, USA: 2013. Polyphenols of apples and their potential health benefits; pp. 333–368. [Google Scholar]
- 171.Bondonno N.P., Bondonno C.P., Ward N.C., Hodgson J.M., Croft K.D. The cardiovascular health benefits of apples: Whole fruit vs. isolated compounds. Trends Food Sci. Technol. 2017;69:243–256. doi: 10.1016/j.tifs.2017.04.012. [DOI] [Google Scholar]
- 172.Kelley D.S., Adkins Y., Laugero K.D. A review of the health benefits of cherries. Nutrients. 2018;10:368. doi: 10.3390/nu10030368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Slimestad R., Solheim H. Anthocyanins from black currants (Ribes nigrum L.) J. Agric. Food Chem. 2002;50:3228–3231. doi: 10.1021/jf011581u. [DOI] [PubMed] [Google Scholar]
- 174.Jiao H., Wang S.Y. Correlation of antioxidant capacities to oxygen radical scavenging enzyme activities in blackberry. J. Agric. Food Chem. 2000;48:5672–5676. doi: 10.1021/jf000765q. [DOI] [PubMed] [Google Scholar]
- 175.Pappas E., Schaich K.M. Phytochemicals of cranberries and cranberry products: Characterization, potential health effects, and processing stability. Crit. Rev. Food Sci. Nutr. 2009;49:741–781. doi: 10.1080/10408390802145377. [DOI] [PubMed] [Google Scholar]
- 176.Bodet C., Grenier D., Chandad F., Ofek I., Steinberg D., Weiss E.I. Potential oral health benefits of cranberry. Crit. Rev. Food Sci. Nutr. 2008;48:672–680. doi: 10.1080/10408390701636211. [DOI] [PubMed] [Google Scholar]
- 177.Yang J., Xiao Y.Y. Grape phytochemicals and associated health benefits. Crit. Rev. Food Sci. Nutr. 2013;53:1202–1225. doi: 10.1080/10408398.2012.692408. [DOI] [PubMed] [Google Scholar]
- 178.Pezzuto J.M. Grapes and human health: A perspective. J. Agric. Food Chem. 2008;56:6777–6784. doi: 10.1021/jf800898p. [DOI] [PubMed] [Google Scholar]
- 179.Beekwilder J., Hall R.D., De Vos C.H. Identification and dietary relevance of antioxidants from raspberry. Biofactors. 2005;23:197–205. doi: 10.1002/biof.5520230404. [DOI] [PubMed] [Google Scholar]
- 180.Wang H., Liu J., Li T., Liu R.H. Blueberry extract promotes longevity and stress tolerance via DAF-16 in Caenorhabditis elegans. Food Funct. 2018;9:5273–5282. doi: 10.1039/C8FO01680A. [DOI] [PubMed] [Google Scholar]
- 181.Cimino F., Cristani M., Saija A., Bonina F.P., Virgili F. Protective effects of a red orange extract on UVB-induced damage in human keratinocytes. Biofactors. 2007;30:129–138. doi: 10.1002/biof.5520300206. [DOI] [PubMed] [Google Scholar]
- 182.Tajaldini M., Samadi F., Khosravi A., Ghasemnejad A., Asadi J. Protective and anticancer effects of orange peel extract and naringin in doxorubicin treated esophageal cancer stem cell xenograft tumor mouse model. Biomed. Pharmacother. 2020;121:1–8. doi: 10.1016/j.biopha.2019.109594. [DOI] [PubMed] [Google Scholar]
- 183.Fan K., Kurihara N., Abe S., Ho C.T., Ghai G., Yang K. Chemopreventive effects of orange peel extract (OPE) I. OPE inhibits intestinal tumor growth in ApcMin/+ mice. J. Med. Food. 2007;10:11–17. doi: 10.1089/jmf.2006.0214. [DOI] [PubMed] [Google Scholar]
- 184.Wang J., Deng N., Wang H., Li T., Chen L., Zheng B., Liu R.H. Effects of Orange Extracts on Longevity, Healthspan, and Stress Resistance in Caenorhabditis elegans. Molecules. 2020;25:351. doi: 10.3390/molecules25020351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Puglia C., Offerta A., Saija A., Trombetta D., Venera C. Protective effect of red orange extract supplementation against UV-induced skin damages: Photoaging and solar lentigines. J. Cosmet. Dermatol. 2014;13:151–157. doi: 10.1111/jocd.12083. [DOI] [PubMed] [Google Scholar]
- 186.Bonina F.P., Leotta C., Scalia G., Puglia C., Trombetta D., Tringali G., Roccazzello A.M., Rapisarda P., Saija A. Evaluation of oxidative stress in diabetic patients after supplementation with a standardised red orange extract. Diabetes Nutr. Metab. 2002;15:14–19. [PubMed] [Google Scholar]
- 187.Wedge D.E., Meepagala K.M., Magee J.B., Smith S.H., Huang G., Larcom L.L. Anticarcinogenic activity of strawberry, blueberry, and raspberry extracts to breast and cervical cancer cells. J. Med. Food. 2001;4:49–51. doi: 10.1089/10966200152053703. [DOI] [PubMed] [Google Scholar]
- 188.Seeram N.P., Adams L.S., Zhang Y., Lee R., Sand D., Scheuller H.S., Heber D. Blackberry, black raspberry, blueberry, cranberry, red raspberry, and strawberry extracts inhibit growth and stimulate apoptosis of human cancer cells in vitro. J. Agric. Food Chem. 2006;54:9329–9339. doi: 10.1021/jf061750g. [DOI] [PubMed] [Google Scholar]
- 189.Zhang Y., Seeram N.P., Lee R., Feng L., Heber D. Isolation and identification of strawberry phenolics with antioxidant and human cancer cell antiproliferative properties. J. Agric. Food Chem. 2008;56:670–675. doi: 10.1021/jf071989c. [DOI] [PubMed] [Google Scholar]
- 190.Cho H.J., Park J.H.Y. Kaempferol induces cell cycle arrest in HT-29 human colon cancer cells. J. Cancer Prev. 2013;18:257–263. doi: 10.15430/JCP.2013.18.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Shi N., Clinton S.K., Liu Z., Wang Y., Riedl K.M., Schwartz S.J., Zhang X., Pan Z., Chen T. Strawberry phytochemicals inhibit azoxymethane/dextran sodium sulfate-induced colorectal carcinogenesis in Crj: CD-1 mice. Nutrients. 2015;7:1696–1715. doi: 10.3390/nu7031696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Lee D.E., Chung M.Y., Lim T.G., Huh W.B., Lee H.J., Lee K.W. Quercetin suppresses intracellular ROS formation, MMP activation, and cell motility in human fibrosarcoma cells. J. Food Sci. 2013;78:H1464–H1469. doi: 10.1111/1750-3841.12223. [DOI] [PubMed] [Google Scholar]
- 193.Vayndorf E.M., Lee S.S., Liu R.H. Whole apple extracts increase lifespan, healthspan and resistance to stress in Caenorhabditis elegans. J. Funct. Foods. 2013;5:1235–1243. doi: 10.1016/j.jff.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Snyder S.M., Zhao B., Luo T., Kaiser C., Cavender G., Hamilton-Reeves J., Sullivan D.K., Shay N.F. Consumption of quercetin and quercetin-containing apple and cherry extracts affects blood glucose concentration, hepatic metabolism, and gene expression patterns in obese C57BL/6J high fat–fed mice. J. Nutr. 2016;146:1001–1007. doi: 10.3945/jn.115.228817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Olsson M.E., Gustavsson K.E., Andersson S., Nilsson A., Duan R.D. Inhibition of cancer cell proliferation in vitro by fruit and berry extracts and correlations with antioxidant levels. J. Agric. Food Chem. 2004;52:7264–7271. doi: 10.1021/jf030479p. [DOI] [PubMed] [Google Scholar]
- 196.Holtung L., Grimmer S., Aaby K. Effect of processing of black currant press-residue on polyphenol composition and cell proliferation. J. Agric. Food Chem. 2011;59:3632–3640. doi: 10.1021/jf104427r. [DOI] [PubMed] [Google Scholar]
- 197.Molan A.L., Liu Z., Plimmer G. Evaluation of the effect of blackcurrant products on gut microbiota and on markers of risk for colon cancer in humans. Phytother. Res. 2014;28:416–422. doi: 10.1002/ptr.5009. [DOI] [PubMed] [Google Scholar]
- 198.Dai J., Patel J.D., Mumper R.J. Characterization of blackberry extract and its antiproliferative and anti-inflammatory properties. J. Med. Food. 2007;10:258–265. doi: 10.1089/jmf.2006.238. [DOI] [PubMed] [Google Scholar]
- 199.Boateng J., Verghese M., Shackelford L., Walker L.T., Khatiwada J., Ogutu S., Williams D.S., Jones J., Guyton M., Asiamah D., et al. Selected fruits reduce azoxymethane (AOM)-induced aberrant crypt foci (ACF) in Fisher 344 male rats. Food Chem. Toxicol. 2007;45:725–732. doi: 10.1016/j.fct.2006.10.019. [DOI] [PubMed] [Google Scholar]
- 200.God J., Tate P.L., Larcom L.L. Red raspberries have antioxidant effects that play a minor role in the killing of stomach and colon cancer cells. Nutr. Res. 2010;30:777–782. doi: 10.1016/j.nutres.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 201.Zhang H., Liu J., Li G., Wei J., Chen H., Zhang C., Zhao J., Wang Y., Dang S., Li X., et al. Fresh red raspberry phytochemicals suppress the growth of hepatocellular carcinoma cells by PTEN/AKT pathway. Int. J. Biochem. Cell Biol. 2018;104:55–65. doi: 10.1016/j.biocel.2018.09.003. [DOI] [PubMed] [Google Scholar]
- 202.Bibi S., Du M., Zhu M.J. Dietary Red Raspberry Reduces Colorectal Inflammation and Carcinogenic Risk in Mice with Dextran Sulfate Sodium–Induced Colitis. J. Nutr. 2018;148:667–674. doi: 10.1093/jn/nxy007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Tu L., Sun H., Tang M., Zhao J., Zhang Z., Sun X., He S. Red raspberry extract (Rubus idaeus L shrub) intake ameliorates hyperlipidemia in HFD-induced mice through PPAR signaling pathway. Food Chem. Toxicol. 2019;133:110796. doi: 10.1016/j.fct.2019.110796. [DOI] [PubMed] [Google Scholar]
- 204.Yi W., Fischer J., Krewer G., Akoh C.C. Phenolic compounds from blueberries can inhibit colon cancer cell proliferation and induce apoptosis. J. Agric. Food Chem. 2005;53:7320–7329. doi: 10.1021/jf051333o. [DOI] [PubMed] [Google Scholar]
- 205.Paul S., DeCastro A.J., Lee H.J., Smolarek A.K., So J.Y., Simi B., Wang C.X., Zhou R., Rimando A.M., Suh N. Dietary intake of pterostilbene, a constituent of blueberries, inhibits the β-catenin/p65 downstream signaling pathway and colon carcinogenesis in rats. Carcinogenesis. 2010;31:1272–1278. doi: 10.1093/carcin/bgq004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Galli R.L., Bielinski D.F., Szprengiel A., Shukitt-Hale B., Joseph J.A. Blueberry supplemented diet reverses age-related decline in hippocampal HSP70 neuroprotection. Neurobiol. Aging. 2006;27:344–350. doi: 10.1016/j.neurobiolaging.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 207.Peng C., Zuo Y., Kwan K.M., Liang Y., Ma K.Y., Chan H.Y.E., Huang Y., Yu H., Chen Z.Y. Blueberry extract prolongs lifespan of Drosophila melanogaster. Exp. Gerontol. 2012;47:170–178. doi: 10.1016/j.exger.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 208.Wu T., Tang Q., Gao Z., Yu Z., Song H., Zheng X., Chen W. Blueberry and mulberry juice prevent obesity development in C57BL/6 mice. PLoS ONE. 2013;8:e77585. doi: 10.1371/journal.pone.0077585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Narayansingh R., Hurta R.A. Cranberry extract and quercetin modulate the expression of cyclooxygenase-2 (COX-2) and IκBα in human colon cancer cells. J. Sci. Food Agric. 2009;89:542–547. doi: 10.1002/jsfa.3471. [DOI] [Google Scholar]
- 210.Ferguson P.J., Kurowska E.M., Freeman D.J., Chambers A.F., Koropatnick J. In vivo inhibition of growth of human tumor lines by flavonoid fractions from cranberry extract. Nutr. Cancer. 2006;56:86–94. doi: 10.1207/s15327914nc5601_12. [DOI] [PubMed] [Google Scholar]
- 211.Xiao X., Kim J., Sun Q., Kim D., Park C.S., Lu T.S., Park Y. Preventive effects of cranberry products on experimental colitis induced by dextran sulphate sodium in mice. Food Chem. 2015;167:438–446. doi: 10.1016/j.foodchem.2014.07.006. [DOI] [PubMed] [Google Scholar]
- 212.Ruel G., Pomerleau S., Couture P., Lemieux S., Lamarche B., Couillard C. Favourable impact of low-calorie cranberry juice consumption on plasma HDL-cholesterol concentrations in men. Brit. J. Nutr. 2006;96:357–364. doi: 10.1079/BJN20061814. [DOI] [PubMed] [Google Scholar]
- 213.Duffey K.J., Sutherland L.A. Adult consumers of cranberry juice cocktail have lower C-reactive protein levels compared with nonconsumers. Nutr. Res. 2015;35:118–126. doi: 10.1016/j.nutres.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 214.Novotny J.A., Baer D.J., Khoo C., Gebauer S.K., Charron C.S. Cranberry juice consumption lowers markers of cardiometabolic risk, including blood pressure and circulating C-reactive protein, triglyceride, and glucose concentrations in adults. J. Nutr. 2015;145:1185–1193. doi: 10.3945/jn.114.203190. [DOI] [PubMed] [Google Scholar]
- 215.Chew B., Mathison B., Kimble L., McKay D., Kaspar K., Khoo C., Chen C.Y.O., Blumberg J. Chronic consumption of a low calorie, high polyphenol cranberry beverage attenuates inflammation and improves glucoregulation and HDL cholesterol in healthy overweight humans: A randomized controlled trial. Eur. J. Nutr. 2019;58:1223–1235. doi: 10.1007/s00394-018-1643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Vaisman N., Niv E. Daily consumption of red grape cell powder in a dietary dose improves cardiovascular parameters: A double blind, placebo-controlled, randomized study. Int. J Food Sci. Nutr. 2015;66:342–349. doi: 10.3109/09637486.2014.1000840. [DOI] [PubMed] [Google Scholar]
- 217.Castilla P., Echarri R., Dávalos A., Cerrato F., Ortega H., Teruel J.L., Lucas M.F., Gomes-Coronado D., Ortuno J., Lasunción M.A. Concentrated red grape juice exerts antioxidant, hypolipidemic, and antiinflammatory effects in both hemodialysis patients and healthy subjects. Am. J. Clin. Nutr. 2006;84:252–262. doi: 10.1093/ajcn/84.1.252. [DOI] [PubMed] [Google Scholar]
- 218.Khadem-Ansari M.H., Rasmi Y., Ramezani F. Effects of red grape juice consumption on high density lipoprotein-cholesterol, apolipoprotein AI, apolipoprotein B and homocysteine in healthy human volunteers. Open Biochem. J. 2010;4:96–99. doi: 10.2174/1874091X01004010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shahidi F. In: Natural Antioxidants: An Overview. Natural Antioxidants: Chemistry, Health Effects, and Applications. Shahidi F., editor. The American Oil Chemists Society; Champion, IL, USA: 1997. pp. 1–11. [Google Scholar]
- 220.Shahidi F., Naczk M., Griffiths W. Food phenolics: Sources, chemistry, effects, applications. Trends Food Sci. Technol. 1995;7:1–5. [Google Scholar]
- 221.Brewer M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011;10:221–247. doi: 10.1111/j.1541-4337.2011.00156.x. [DOI] [Google Scholar]
- 222.Mullenix P.S., Andersen C.A., Starnes B.W. Atherosclerosis as inflammation. Ann. Vasc. Surg. 2005;19:130–138. doi: 10.1007/s10016-004-0153-z. [DOI] [PubMed] [Google Scholar]
- 223.Rosenberg P.B. Clinical aspects of inflammation in Alzheimer’s disease. Int. Rev. Psychiatry. 2005;17:503–514. doi: 10.1080/02646830500382037. [DOI] [PubMed] [Google Scholar]
- 224.Giampieri F., Forbes-Hernandez T.Y., Gasparrini M., Alvarez-Suarez J.M., Afrin S., Bompadre S., Quiles J.L., Mezzetti B., Battino M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015;6:1386–1398. doi: 10.1039/C5FO00147A. [DOI] [PubMed] [Google Scholar]
- 225.Cai X., Han Y., Gu M., Song M., Wu X., Li Z., Li F., Goulette T., Xiao H. Dietary cranberry suppressed colonic inflammation and alleviated gut microbiota dysbiosis in dextran sodium sulfate-treated mice. Food Funct. 2019;10:6331–6341. doi: 10.1039/C9FO01537J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Forbes-Hernandez T.Y., Gasparrini M., Afrin S., Bompadre S., Mezzetti B., Quiles J.L., Giampieri F., Battino M. The healthy effects of strawberry polyphenols: Which strategy behind antioxidant capacity? Crit. Rev. Food Sci. Nutr. 2016;56:S46–S59. doi: 10.1080/10408398.2015.1051919. [DOI] [PubMed] [Google Scholar]
- 227.Van’t Veer P., Jansen M.C., Klerk M., Kok F.J. Fruits and vegetables in the prevention of cancer and cardiovascular disease. Public Health Nutr. 2000;3:103–107. doi: 10.1017/S1368980000000136. [DOI] [PubMed] [Google Scholar]
- 228.Neto C.C. Cranberry and its phytochemicals: A review of in vitro anticancer studies. J. Nutr. 2007;137:186S–193S. doi: 10.1093/jn/137.1.186S. [DOI] [PubMed] [Google Scholar]
- 229.Bishayee A., Haskell Y., Do C., Siveen K.S., Mohandas N., Sethi G., Stoner G.D. Potential benefits of edible berries in the management of aerodigestive and gastrointestinal tract cancers: Preclinical and clinical evidence. Crit. Rev. Food Sci. Nutr. 2016;56:1753–1775. doi: 10.1080/10408398.2014.982243. [DOI] [PubMed] [Google Scholar]
- 230.Chu S.C., Hsieh Y.S., Hsu L.S., Chen K.S., Chiang C.C., Chen P.N. Rubus idaeus L inhibits invasion potential of human A549 lung cancer cells by suppression epithelial-to-mesenchymal transition and Akt pathway in vitro and reduces tumor growth in vivo. Integr. Cancer Ther. 2014;13:259–273. doi: 10.1177/1534735413510559. [DOI] [PubMed] [Google Scholar]
- 231.Baby B., Antony P., Vijayan R. Antioxidant and anticancer properties of berries. Crit. Rev. Food Sci. Nutr. 2018;58:2491–2507. doi: 10.1080/10408398.2017.1329198. [DOI] [PubMed] [Google Scholar]
- 232.Stoner G.D., Wang L.S., Zikri N., Chen T., Hecht S.S., Huang C., Sardo. C., Lechner J.F. Seminars in Cancer Biology. Volume 17. Elsevier; Amsterdam, The Netherlands: 2007. Cancer prevention with freeze-dried berries and berry components; pp. 403–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Ames B.N., Shigenaga M.K., Hagen T.M. Oxidants, antioxidants, and the degenerative diseases of aging. Proc. Natl. Acad. Sci. USA. 1993;90:7915–7922. doi: 10.1073/pnas.90.17.7915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Goschorska M., Gutowska I., Baranowska-Bosiacka I., Barczak K., Chlubek D. The use of antioxidants in the treatment of migraine. Antioxidants. 2020;9:116. doi: 10.3390/antiox9020116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Jafarpour M., Yousefi G., Hamedi A., Shariat A., Salehi A., Heydari M. Effect of a traditional syrup from Citrus medica L. fruit juice on migraine headache: A randomized double blind placebo controlled clinical trial. J. Ethnopharmacol. 2016;179:170–176. doi: 10.1016/j.jep.2015.12.040. [DOI] [PubMed] [Google Scholar]
- 236.Martin A., Prior R., Shukitt-Hale B., Cao G., Joseph J.A. Effect of fruits, vegetables, or vitamin E–rich diet on vitamins E and C distribution in peripheral and brain tissues: Implications for brain function. J. Gerontol. A Biol. Sci. Med. Sci. 2000;55:B144–B151. doi: 10.1093/gerona/55.3.B144. [DOI] [PubMed] [Google Scholar]
- 237.Devore E.E., Kang J.H., Breteler M.M., Grodstein F. Dietary intakes of berries and flavonoids in relation to cognitive decline. Ann. Neurol. 2012;72:135–143. doi: 10.1002/ana.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Mursu J., Virtanen J.K., Tuomainen T.P., Nurmi T., Voutilainen S. Intake of fruit, berries, and vegetables and risk of type 2 diabetes in Finnish men: The Kuopio Ischaemic Heart Disease Risk Factor Study. Am. J. Clin. Nutr. 2014;99:328–333. doi: 10.3945/ajcn.113.069641. [DOI] [PubMed] [Google Scholar]
- 239.Muraki I., Imamura F., Manson J.E., Hu F.B., Willett W.C., van Dam R.M., Sun Q. Fruit consumption and risk of type 2 diabetes: Results from three prospective longitudinal cohort studies. Br. Med. J. 2013;347:12. doi: 10.1136/bmj.f5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Amani R., Moazen S., Shahbazian H., Ahmadi K., Jalali M.T. Flavonoid-rich beverage effects on lipid profile and blood pressure in diabetic patients. World J. Diabetes. 2014;5:962–968. doi: 10.4239/wjd.v5.i6.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Erlund I., Koli R., Alfthan G., Marniemi J., Puukka P., Mustonen P., Mattila P., Jula A. Favorable effects of berry consumption on platelet function, blood pressure, and HDL cholesterol. Am. J. Clin. Nutr. 2008;87:323–331. doi: 10.1093/ajcn/87.2.323. [DOI] [PubMed] [Google Scholar]