Abstract
With the mortality rate of invasive aspergillosis caused by Aspergillus fumigatus reaching almost 100% among some groups of patients, and with the rapidly increasing resistance of A. fumigatus to available antifungal drugs, new antifungal agents have never been more desirable than now. Numerous bioactive compounds were isolated and characterized from marine resources. However, only a few exhibited a potent activity against A. fumigatus when compared to the multitude that did against some other pathogens. Here, we review the marine bioactive compounds that display a bioactivity against A. fumigatus. The challenges hampering the discovery of antifungal agents from this rich habitat are also critically analyzed. Further, we propose strategies that could speed up an efficient discovery and broaden the dimensions of screening in order to obtain promising in vivo antifungal agents with new modes of action.
Keywords: marine resources, Aspergillus fumigatus, bioactive compounds, screening model, fungi
1. Introduction
Aspergillus fumigatus is a saprophytic mold commonly found in the environment, with spores that are very light and easily disseminated [1]. It is also a potentially dangerous opportunistic pathogen that is reported as being the number one causative mold for mycoses, especially in immunocompromised patients [2]. Invasive aspergillosis (IA) caused by A. fumigatus is a severe systemic infection with high mortality and morbidity rates. IA has an annual global incidence of over 200,000, and the mortality rate could reach almost 100% among certain categories of patients if not properly treated [2,3,4].
With the already existing challenge of having only a limited repertoire of antifungal drugs for the treatment of aspergilloses, the continuous rise of drug resistance in A. fumigatus strains [5,6,7] further aggravates this challenge. Reports of A. fumigatus developing a resistance, in both clinical [8,9] and environmental [10,11] isolates, to antifungal drugs such as amphotericin B [12], azole-class drugs [13,14] and echinocandins [5,15], as well as developing a multidrug resistance [16], are increasing annually.
In the UK for example, Public Health England reported over a five-fold increase in A. fumigatus isolates that showed resistance to itraconazole (Minimum Inhibitory Concentration (MIC) ≥ 2.0 μg/mL) from 2012 (1.4%) to 2016 (8.5%), whereas 4.7% and 6.9% increased resistance to voriconazole (MIC ≥ 2.0 μg/mL) and posaconazole (MIC ≥ 0.25 μg/mL), respectively [17]. A study from Brazil by Reichert–Lima [18] showed one of the highest resistances (27%) of A. fumigatus to amphotericin B (MIC ≥ 2.0 μg/mL).
Due to its peculiar environment and rich biodiversity, the marine resource is a treasure box for isolating novel bioactive compounds. Over the years, antibacterial [19,20,21], antifungal [22,23], antiviral [24,25,26], anthelminths [27,28], antiprotozoan [29,30,31], antitumor [32,33], anticancer [34,35,36], anti-inflammatory [37,38], antioxidant [39,40], antiaging [41,42] and antidiabetics [43,44,45] compounds have been isolated from different marine organisms.
Among the reported antifungals isolated from the marine environment, only a few have been found to be effective against the recalcitrant fungus, A. fumigatus. Considering the threat of this important pathogen, in this review we have compiled and discussed bioactive compounds, isolated from the marine environment over the last two decades, which displayed an activity against A. fumigatus. We have also discussed the challenges restricting the discovery and isolation of effective agents against A. fumigatus from the marine environment. We have further proffered enhanced methods and multiple strategies that may improve the future discovery of marine antifungal agents.
2. A. fumigatus and Aspergillosis
Yu et al. [46] defined aspergillosis as a clinical infection caused by the genus Aspergillus, which can lead to allergic, superficial, saprophytic or invasive diseases. Cases of aspergillosis have constantly been on the increase since it was first reported over a century ago by Bennett [47]. Although aspergillosis is popularly associated with immunocompromised patients, over the years it has rapidly evolved in epidemiology, with increasingly new groups of patients at risk, even among the supposedly non-immunocompromised individuals [1,48].
Cases of varying kinds of aspergillosis have been reported among non-immunocompromised patients for years now, such as craniocerebral aspergillosis of sino-nasal origin [49,50], chronic invasive sinus aspergillosis [51], orbital aspergillosis [52,53,54], fatal invasive aspergillosis [55], intracranial aspergillosis [56], central nervous system aspergillosis [57], pulmonary aspergillosis [58,59], and adrenal and hepatic aspergillosis [60].
A. fumigatus has been reported as the most frequent etiological mold for invasive aspergillosis over the past two decades [61,62]. Unfortunately, the rapid evolution of drug resistant strains of this fungus complicates aspergillosis cases and limits clinical treatment by available antifungal drugs [6,8,63]. This has driven global searches for promising and potent new agents against A. fumigatus. Notwithstanding the fact that no new drug class has been discovered, some promising effective compounds have, however, been isolated and characterized from the marine environment.
3. Bioactive Compounds from Marine Organisms against A. fumigatus
A number of effective bioactive compounds against A. fumigatus have been isolated from marine organisms over the last two decades. Some of these compounds have been characterized and demonstrated as giving an appreciable potency against A. fumigatus. Other compounds, on the other hand, have been reported to have a weaker activity. These active compounds were isolated from different marine organisms, including bacteria [64,65], most of which were actinomycetes [66,67,68], fungi [69,70], algae and seaweed [71,72,73,74], sponges [75,76,77,78], and sea cucumbers [79,80,81].
3.1. A. fumigatus Effective Compounds from Marine Bacteria
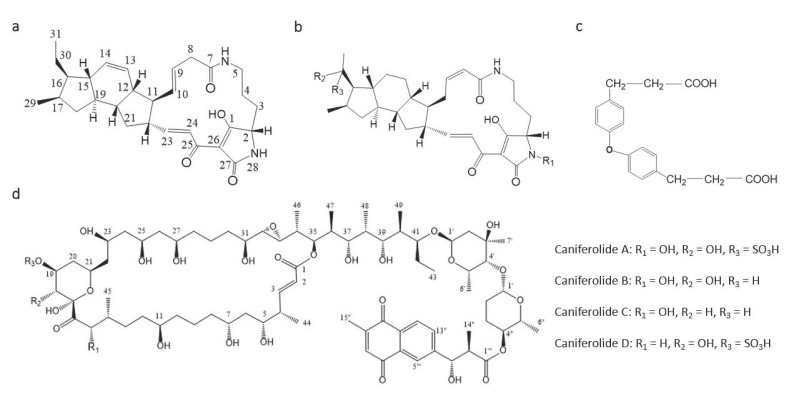
Compounds with antifungal activity against A. fumigatus have been isolated from marine bacteria such as Bacillus [65,82], and more frequently from the unicellular filamentous bacteria actinomycetes [83,84,85]. Certain compounds, such as the caniferolides [85], have been characterized and shown to have a good activity against A. fumigatus (Table 1; Figure 1). Some other compounds, such as 30-oxo-28-N-methylikarugamycin, isolated from Streptomyces zhaozhouensis, only showed a very weak activity against A. fumigatus, with MICs of ˃64 μg/mL [83]. On the other hand, extracts from some streptomycetes [86,87], and other bacteria like Micrococcus sp., Flavobacterium sp. and Streptomyces sp. [64], have also been demonstrated as possessing very effective activities against A. fumigatus (Table 2). More interestingly, some of these extracts also showed promising activities against A. fumigatus strains with multidrug resistance (MDR) (Table 2).
Table 1.
Compounds from marine Streptomyces and other bacteria with antifungal activity against A. fumigatus.
| Compound | Description/Source of Compound | Compound Class | Activity (MIC μg/mL) | Reference |
|---|---|---|---|---|
| Isoikarugamycin | Ethyl acetate extract from Streptomyces zhaozhouensis CA-185989 broth | polycyclic tetramic acid macrolactams | 4–8 | [83] |
| 28-N-methylikaguramycin | Ethyl acetate extract from Streptomyces zhaozhouensis CA-185989 broth | polycyclic tetramic acid macrolactams | 4–8 | [83] |
| Ikarugamycin | Ethyl acetate extract from Streptomyces zhaozhouensis CA-185989 broth | polycyclic tetramic acid macrolactams | 4–8 | [83] |
| Caniferolides A&B | Ethyl acetate extract from Streptomyces caniferus CA-271066. | polyol macrolides | 2–4 | [85] |
| Caniferolides C&D | Ethyl acetate extract from Streptomyces caniferus CA-271066. | polyol macrolides | 4–8 | [85] |
| 4,4′-oxybis(3-phenylpropionic acid) | Ethyl acetate concentrates of methanolic extract from Bacillus licheniformis | oxyneolignan | 7–10 mm * | [65] |
* Zone of inhibition given by 50 µg/disc.
Figure 1.
Chemical structures of compounds from marine bacteria with antifungal activity against A. fumigatus. (a) Isoikarugamycin, (b) 28-N-methylikaguramycin, (c) 4,4′-oxybis [3-phenylpropionic acid] and (d) Caniferolide A–D.
Table 2.
Antifungal activity of extracts from marine actinomycetes and other bacteria against A. fumigatus or MDR A. fumigatus.
| Isolate | Description | Activity (MIC μg/mL) | Reference |
|---|---|---|---|
| Streptomyces sp. VITSDK36 | Ethyl acetate extract | 1.32 | [87] |
| Streptomyces sp. VITSDK37 | Ethyl acetate extract | 0.74 | [87] |
| Streptomyces sp. VITSDK38 | Ethyl acetate extract | 1.64 | [87] |
| Streptomyces sp. VITSDK39 | Ethyl acetate extract | 1.78 | [87] |
| Streptomyces sp. VITSDK41 | Ethyl acetate extract | 2.68 | [87] |
| Streptomyces sp. VITSDK42 | Ethyl acetate extract | 1.58 | [87] |
| Actinopolyspora sp. VITSDK43 | Ethyl acetate extract | 1.58 | [87] |
| Actinopolyspora sp. VITSDK44 | Ethyl acetate extract | 2.54 | [87] |
| Micromonospora sp. VITSDK46 | Ethyl acetate extract | 1.40 | [87] |
| Sachharopolyspora sp. VITSDK47 | Ethyl acetate extract | 0.90 | [87] |
| Streptomyces VITSVK5 spp. | Ethyl acetate extract | 0.5 | [86] |
| Micrococcus sp. RRL-3 | Methanol extract | 0.5 | [64] |
| Flavobacterium sp. RRL-10 | Methanol extract | 5.5 | [64] |
| Pseudomonas sp. RRL-12 | Methanol extract | 0.9 | [64] |
| Streptomyces sp. RRL-13 | Methanol extract | 0.7 | [64] |
| Flavobacterium sp. RRL-20 | Methanol extract | 4.3 | [64] |
| Flavobacterium sp. RRL-32 | Methanol extract | 0.9 | [64] |
| Micrococcus sp. RRL-37 | Methanol extract | 0.8 | [64] |
| Bacillus sp. RRL-38 | Methanol extract | 2.0 | [64] |
| Flavobacterium sp. RRL-41 | Methanol extract | 1.3 | [64] |
| Flavobacterium sp. RRL-54 | Methanol extract | 0.5 | [64] |
| Alcaligenes sp. RRL-56 | Methanol extract | 0.3 | [64] |
| Bacillus sp. RRL-57 | Methanol extract | 2.5 | [64] |
| Streptomyces VITSVK5 spp. | Ethyl acetate extract against MDR9 | 4 * | [86] |
| Streptomyces VITSVK5 spp. | Ethyl acetate extract against MDR10 | 0.25 * | [86] |
| Streptomyces VITSVK5 spp. | Ethyl acetate extract against MDR11 | 2 * | [86] |
* Against multidrug resistant A. fumigatus strain.
3.2. A. fumigatus Effective Compounds from Marine Sponges
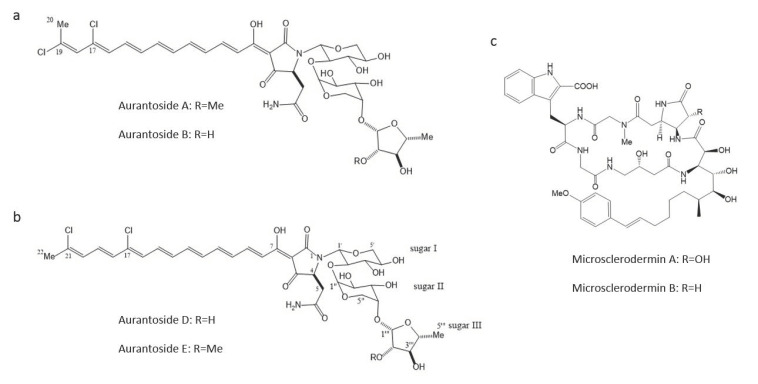
Marine sponges, such as Theonella swinhoei, Siliquariaspongia japonica and Microscleroderma herdmani, are other categories of interesting marine organisms demonstrated to produce effective antifungal compounds against A. fumigatus. Compounds such as Aurantosides E, A, B and Microsclerodermin B are the best antifungal bioactive compounds against A. fumigatus, with MICs of 0.04, 0.16, 0.16 and 0.6 μg/mL, respectively [77,88] (Table 3; Figure 2). Moreover, other Microsclerodermins (A, J and K) and Swinhoeiamide A also exhibit a good antifungal activity against A. fumigatus, with MICs ranging between 1 to 10 μg/mL [77,89] (Table 3).
Table 3.
Effective compounds against A. fumigatus from marine sponges.
| Compound | Description/Source of Compound | Location of Isolation | Compound Class | Activity (MIC μg/mL) | Reference |
|---|---|---|---|---|---|
| Swinhoeiamide A | Methanol extract from Theonella swinhoei | Karkar Island, Papua New Guinea | calyculinamide-related congener | 1.0 | [89] |
| 1,2-dioxane ring peroxide acid | Ethanol extract from Plakortis halichondrioides | Long Bay, Negril, Jamaica | peroxide | 5.6 1 | [90] |
| Aurantoside A & B | Ethanol extract from Siliquariaspongia japonica | Hachijo-jima Island, Tokyo, Japan | polyene tetramic acids | 0.16 | [88] |
| Aurantoside D | Ethanol extract from Siliquariaspongia japonica | Hachijo-jima Island, Tokyo, Japan | polyene tetramic acids | 11.0 2 | [88] |
| Aurantoside E | Ethanol extract from Siliquariaspongia japonica | Hachijo-jima Island, Tokyo, Japan | polyene tetramic acids | 0.04 | [88] |
| 3,5-dibromo-2-(3,5-dibromo-2-methoxyphenoxy) phenol | Ethyl acetate-methanol (EtOAc-MeOH) extract of Dysidea herbace | Coast of Zanzibar | phenol | 7.8 | [91] |
| Microsclerodermin J | Methananol extract of Microscleroderma herdmani | Mauritius | hexapeptides | 10.0 | [77] |
| Microsclerodermin K | Methananol extract of Microscleroderma herdmani | Mauritius | hexapeptides | 10.0 | [77] |
| Microsclerodermin A | Methananol extract of Microscleroderma herdmani | Mauritius | hexapeptides | 1.3 | [77] |
| Microsclerodermin B | Methananol extract of Microscleroderma herdmani | Mauritius | hexapeptides | 0.6 | [77] |
| Araguspongin C | Methanol extract of Haliclona exigua | Tamil Nadu coast of India | heteropentacyclic compound | 50 | [75] |
| Spongistatin 1 | Extract from Hyrtios erecta | Eastern Indian Ocean | macrocyclic lactone polyether | 12.5 3 | [92,93] |
1 IC90; 2 inhibition zone (mm); 3 minimum fungicidal concentration (MFC).
Figure 2.
Chemical structures of compounds from marine sponges with antifungal activities against A. fumigatus. (a) Aurantosides A & B, (b) Aurantosides D & E, and (c) Microsclerodermin A & B.
3.3. A. fumigatus Effective Compounds from Marine Algae
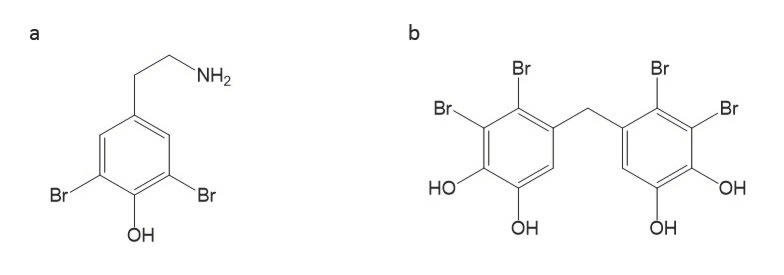
Both compounds and extracts from several marine algae have been demonstrated to exhibit antifungal activities against A. fumigatus. The bromophenol compound 2,20,3,30-tetrabromo-4,40,5,50-tetrahydroxydiphenylmethane is one of the most potent compounds from marine algae, with a potent activity against A. fumigatus (with an MIC of 0.78 μg/mL) [94] (Table 4; Figure 3). Furthermore, ethanol extracts from Laurencia majuscula and Padina pavonica displayed an effective activity against A. fumigatus at low MICs of 1.95 and 0.95 μg/mL, respectively [71] (Table 4).
Table 4.
Effective antifungal compounds/extracts against A. fumigatus from marine algae.
| Compound/Isolate | Description/Source of Compound | Activity (MIC μg/mL) | Reference |
|---|---|---|---|
| 2,20,3,30-tetrabromo-4,40,5,50-tetrahydroxydiphenylmethane | Compounds from Odonthalia corymbifera | 0.78 | [94] |
| 3-bromo-4-(2,3-dibromo-4,5-dihydroxybenzyl)-5-methoxymethylpyrocatechol | Compounds from Odonthalia corymbifera | 25 | [94] |
| (E)-2-{(E) tridec-2-en-2-yl} heptadec-2-enal | Chloroform/methanol extract of Laurencia papillosa | 200 | [95] |
| Extract | Ethanol extract from Laurencia catarinensis | 3.90 | [71] |
| Extract | Ethanol extract from Laurencia majuscula | 1.95 | [71] |
| Extract | Ethanol extract from Padina pavonica | 0.98 | [71] |
Figure 3.
Bromophenol compounds from marine algae with antifungal activities against A. fumigatus. (a) 2,20,3,30-tetrabromo-4,40,5,50 tetrahydroxydiphenylmethane and (b) 3-bromo-4-(2,3-dibromo-4,5-dihydroxybenzyl)-5 methoxymethylpyrocatechol.
3.4. A. fumigatus Effective Compounds from Sea Cucumbers
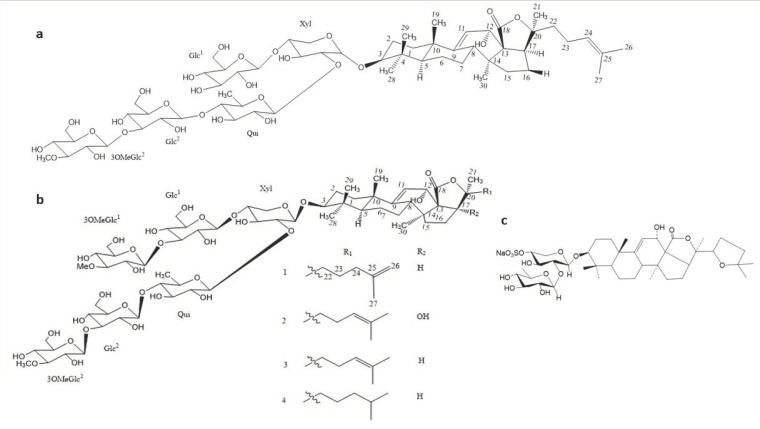
Several triterpene glycosides (Figure 4) with good antifungal activities against A. fumigatus have been isolated and characterized from sea cucumbers such as Holothuria scabra, Actinopyga lecanora, several species of Bohadschia, etc. [80,81,96,97]. Among the best bioactive compounds isolated from these marine echinoderms, potent activities with an MIC80 ranging from 1.0 to 4.0 μg/mL have been recorded in in vitro screenings (Table 5). Triterpene glycosides therefore present very good prospects for future antifungal drug development.
Figure 4.
Chemical structure of compounds from sea cucumbers with effective antifungal activities against A. fumigatus. (a) Impatienside B, (b) Marmoratoside A (1); 17α-hydroxy impatienside (2); Impatienside A (3); & Bivittoside D (4), and (c) Holothurin B.
Table 5.
Characterized compounds against A. fumigatus from sea cucumbers.
| Compounds | Sea Cucumber | Compound Class | Activity (MIC μg/mL) | Reference |
|---|---|---|---|---|
| Scabraside A | Holothuria scabra | Triterpene glycosides | 2.0 * | [97] |
| Echinoside A | Holothuria scabra | Triterpene glycosides | 1.0 * | [97] |
| Holothurin A1 | Holothuria scabra | Triterpene glycosides | 8.0 * | [97] |
| Holothurin B | Actinopyga lecanora | Triterpene glycosides | 3.12 | [82] |
| 3-O-b-D-xylopyranosyl-16b-acetoxyholost-7-ene | Actinopyga lecanora | Triterpene glycosides | 50.0 | [96] |
| Holothurin A | Actinopyga lecanora | Triterpene glycosides | 50.0 | [96] |
| Bivittoside-D | Bohadschia vitiensis Semper | Lanostane triterpenoid | 1.56 | [99] |
| Marmoratoside A | Bohadschia marmorata Jaeger. | Triterpene glycosides | 2.81 * | [80] |
| Marmoratoside B | Bohadschia marmorata Jaeger. | Triterpene glycosides | 44.44 * | [80] |
| 17α-hydroxy impatienside A | Bohadschia marmorata Jaeger. | Triterpene glycosides | 2.78 * | [80] |
| Impatienside A | Bohadschia marmorata Jaeger. | Triterpene glycosides | 2.81 * | [80] |
| Bivittoside D | Bohadschia marmorata Jaeger. | Triterpene glycosides | 2.80 * | [80] |
| 25-acetoxy bivittoside D | Bohadschia marmorata Jaeger. | Triterpene glycosides | 43.13 * | [80] |
| Arguside F | Holothuria (Microthele) axiloga | Holostan-type triterpene glycosides | 16.0 * | [81] |
| Impatienside B | Holothuria (Microthele) axiloga | Holostan-type triterpene glycosides | 4.0 * | [81] |
| Pervicoside D | Holothuria (Microthele) axiloga | Holostan-type triterpene glycosides | 64.0 * | [81] |
* MIC80.
Besides characterized compounds, both crude and partially purified extracts from different sea cucumbers have also been demonstrated to exhibit varying levels of antifungal activities against A. fumigatus. Ismail et al. [79] reported that crude and semipurified extracts from both aqueous body fluid extracts and methanolic wall extracts from Holothuria polii displayed varying activities against A. fumigatus in a concentration-dependent manner. Methanolic body-wall crude extracts showed better activities than those of aqueous body fluid. However, there was no difference in activities among the extracts after purification. Similarly, Adibpour et al. [98] also recorded an antifungal activity against A. fumigatus from Holothuria leucospilota body-wall and coelomic fluid extracts but none from its cuvierian organs’ extracts.
3.5. A. fumigatus Effective Compounds from Marine Fungi
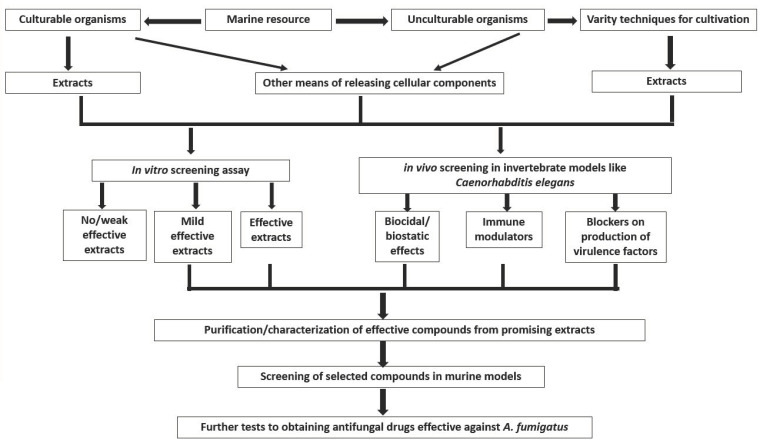
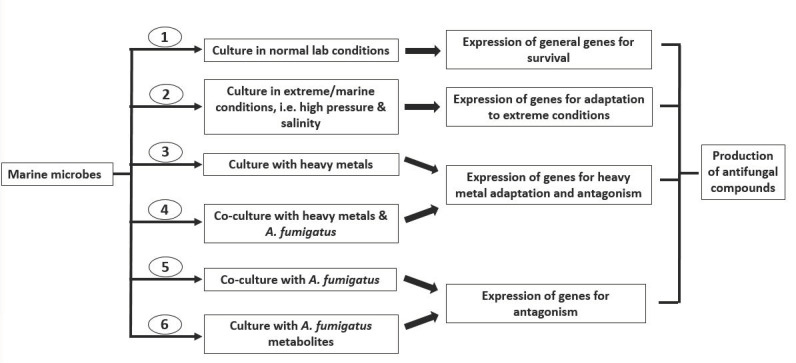
A few studies have reported bioactive compounds from marine fungi against pathogenic molds. With reference to A. fumigatus, to the best of our knowledge, only the marine fungus Phoma sp. produced a characterized bioactive compound, YM-202204, with an IC80 of 12.5 μg/mL against A. fumigatus [69]. We propose that most fungi do indeed produce necessary antagonism metabolites against their fellow fungi if “they feel threatened” or if there is competition. That is why we have proposed several enhanced pathways (e.g., the coculture approach) for isolating and screening bioactive compounds (Figure 5 and Figure 6).
Figure 5.
Flow chart of possible approaches to enhance the chances of discovering potent antifungal agents against A. fumigatus.
Figure 6.
Suggested culture approaches to increase the discovery of antifungal bioactive compounds.
4. Challenges and Future Prospects
One of the biggest challenges facing the discovery of effective antifungal agents against A. fumigatus and other pathogenic microorganisms from a rich marine environment is the limitation on culturable organisms. It has been proven that the vast majority of microorganisms in nature cannot be isolated through the usual cultural techniques and are therefore labelled as unculturable microorganisms [100,101], where marine microbes predominate. Therefore, obtaining these viable but unculturable marine microbes by using several methods, including molecular approaches [102] and simpler techniques such as a long incubation with low nutrition [101], will certainly open a new vista of opportunities for discovering new antimicrobial agents. This may consequently lead to obtaining and evaluating novel metabolites from those unisolated and uncharacterized microorganisms, increasing the possibility of discovering potent antimicrobials, including antifungal agents against A. fumigatus (Figure 5).
Furthermore, the techniques and methods currently adopted in the isolation and screening of these bioactive compounds require upgrading in order to increase the chances of identifying new broad spectrum antimicrobial agents. For example, mainly focusing on extracellular components from these marine microorganisms seriously limits the global progress in this search. Considering the fact that marine organisms are unique, with special cellular features and components to survive in extreme and peculiar environments, it will be desirable to isolate and test intracellular and membrane-bound polysaccharides, peptides and lipids [103,104].
Moreover, conducting an initial screening of these compounds with predominantly in vitro assays might just be another limitation in this search. It has previously been demonstrated that certain compounds with weak in vitro antimicrobial activities could still be quite effective in in vivo applications [105,106,107,108]. Such compounds could have alternative mechanisms of “conquering the pathogen(s) of interest” beyond biocidal/biostatic activities. Other mechanisms than just the usual growth inhibition of pathogens, such as the modulation of the host immune system or blocking the production of virulence factor(s), are enviable attributes of new compounds that can only be discovered with in vivo assays [107,108,109,110]. Therefore, in vivo screening assays are highly recommended to discover bioactive compounds with broader modes of action.
Using invertebrate models such as Caenorhabditis elegans for a high-throughput in vivo screening has numerous advantages, including the absence of an ethical license requirement, reduced cost, labor and resources, as well as the possibility to simultaneously determine the cytotoxity of bioactive compounds [105,110]. We have recently established a C. elegans-based A. fumigatus infection model [111], making the high-throughput evaluation of the efficacy of bioactive compounds possible. Furthermore, we also discovered that the in vivo efficacy of some antifungal agents was different from what was observed in in vitro screenings, as demonstrated by the different killing modes of amphotericin B and the azole drugs. These therefore provide confidence in high-throughput in vivo assays as the primary screening approach.
Based on the general numbers of bioactive compounds from marine microbes, there are multiple strategies for inducing the production of some essential bioactive metabolites that possess antifungal properties, such as coculturing marine microbes with stimulators (other microorganisms, pathogens/their metabolites) or exposing them to stress conditions. Heavy metal stress could induce the expression of genes that lead to the production of desired metabolites, some of which have also been discovered to possess antimicrobial properties [112,113,114,115,116]. The use of heavy metals in the culture media of certain marine organisms is necessary for inducing the production of some essential metabolites, which may have antifungal properties. Evaluating such antifungal potency on A. fumigatus would really help to broaden the future search and discovery of antimicrobial compounds.
We have therefore suggested cultural approaches in Figure 6 in order to broaden and hasten the search for antifungal agents against A. fumigatus. Six culture approaches, ranging from monocultures to cocultures under different conditions, are suggested to stimulate genes involved in the production of uncommon metabolites, thereby increasing the possibilities of finding novel bioactive compounds that may otherwise not be identified through conventional approaches.
5. Conclusions
The search for antifungal agents effective against A. fumigatus from the marine habitat has not been all that productive (compared to other similar leading opportunistic pathogens like Candida albicans) for years now. A change of strategy is therefore indispensable and urgent if we must win the raging menacing war against this mold pathogen. Adoption of alternative cultural methods and evaluation of compounds for other treatment mechanisms would help to broaden the horizon of this search and may as well just be the needed breakthrough that we are waiting for.
Author Contributions
C.S.A. and B.C.E. wrote the initial manuscript. J.C.O.; A.N.M., A.C.I., B.W., C.J. and W.F. revised the manuscript. W.F. supervised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by National Natural Science Foundation of China (31960032, 32071279), Guangxi Natural Science Foundation (2018GXNSFAA138012, 2020GXNSFDA238008) and Research Start-up Funding of Guangxi Academy of Sciences (2017YJJ025) to W.F., Bagui Scholar Program Fund (2016A24) of Guangxi Zhuang Autonomous Region to C.J.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Latgé J.P., Chamilos G. Aspergillus fumigatus and aspergillosis in 2019. Clin. Microbiol. Rev. 2019;33:e00140-18. doi: 10.1128/CMR.00140-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geißel B., Loiko V., Klugherz I., Zhu Z., Wagener N., Kurzai O., van den Hondel C.A.M.J.J., Wagener J. Azole-induced cell wall carbohydrate patches kill Aspergillus fumigatus. Nat. Commun. 2018;9 doi: 10.1038/s41467-018-05497-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Darling B.A., Milder E.A. Invasive aspergillosis. Pediatr. Rev. 2018;39:476–478. doi: 10.1542/pir.2017-0129. [DOI] [PubMed] [Google Scholar]
- 4.Linder K.A., McDonald P.J., Kauffman C.A., Revankar S.G., Chandrasekar P.H., Miceli M.H. Invasive aspergillosis in patients following umbilical cord blood transplant. Bone Marrow Transpl. 2019;54:308–311. doi: 10.1038/s41409-018-0230-5. [DOI] [PubMed] [Google Scholar]
- 5.Jiménez-Ortigosa C., Aimanianda C., Muszkieta L., Mouyna I., Alsteens D., Pire S., Beau R., Krappmann S., Beauvais A., Dufrêne Y.F., et al. Chitin synthases with a myosin motor-like domain control the resistance of Aspergillus fumigatus to echinocandins. Antimicrob. Agents Chemother. 2012;56:6121–6131. doi: 10.1128/AAC.00752-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Abdolrasouli A., Scourfield A., Rhodes J., Shah A., Elborn J.S., Fisher M.C., Schelenz S., Armstrong-James D. High prevalence of triazole resistance in clinical Aspergillus fumigatus isolates in a spe-cialist cardio-thoracic centre. Int. J. Antimicrob. Agents. 2018;52:637–642. doi: 10.1016/j.ijantimicag.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 7.Wirmann L., Ross B., Reimann O., Steinmann J., Rath P.-M. Airborne Aspergillus fumigatus spore concentration during demolition of a building on a hospital area and patient risk determination for invasive aspergillosis including azole resistance. J. Hosp. Infect. 2018;100:e91–e97. doi: 10.1016/j.jhin.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 8.Prigitano A., Esposto M.C., Biffi A., De Lorenzis G., Favuzzi V., Koncan R., Lo Cascio G., Barao Ocampo M., Colombo C., Pizzamiglio G., et al. Triazole resistance in Aspergillus fumigatus isolates from patients with cystic fibrosis in Italy. J. Cyst. Fibros. 2017;16:64–569. doi: 10.1016/j.jcf.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Hagiwara D., Arai T., Takahashi H., Kusuya Y., Watanabe A., Kamei K. Non-cyp51A azole-resistant Aspergillus fumigatus isolates with mutation in HMG-CoA reductase. Emerg. Infect. Dis. 2018;24:1889–1897. doi: 10.3201/eid2410.180730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J., Snelders E., Zwaan B.J., Schoustra S.E., Meis J.F., van Dijk K., Hagen F., van der Beek M.T., Kampinga G.A., Zoll J., et al. A novel environmental azole resistance mutation in Aspergillus fumigatus and a possible role of sexual reproduction in its emergence. mBio. 2017;8:e00791-17. doi: 10.1128/mBio.00791-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Prigitano A., Esposto M.C., Romanò L., Auxilia F., Tortorano A.M. Azole-resistant Aspergillus fumigatus in the Italian environment. J. Glob. Antimicrob. Resist. 2019;16:220–224. doi: 10.1016/j.jgar.2018.10.017. [DOI] [PubMed] [Google Scholar]
- 12.Ashu E.E., Korfanty G.A., Samarasinghe H., Pum N., You M., Yamamura D., Xu J. Widespread amphotericin B-resistant strains of Aspergillus fumigatus in Hamilton, Canada. Infect. Drug Resist. 2018;11:1549–1555. doi: 10.2147/IDR.S170952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hagiwara D., Miura D., Shimizu K., Paul S., Ohba A., Gonoi T., Watanabe A., Kamei K., Shintani T., Moye-Rowley W.S., et al. A novel Zn2 Cys6 transcription factor AtrR plays a key role in an azole resistance mechanism of Aspergillusfumigatus by co-regulating cyp51A and cdr1B expressions. PLoS Pathog. 2017;13:e1006096. doi: 10.1371/journal.ppat.1006096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharma C., Nelson-Sathi S., Singh A., Pillai M.R., Chowdhary A. Genomic perspective of triazole resistance in clinical and environmental Aspergillus fumigatus isolates without cyp51A mutations. Fungal Genet. Biol. 2019;132 doi: 10.1016/j.fgb.2019.103265. [DOI] [PubMed] [Google Scholar]
- 15.Lamoth F., Juvvadi P.R., Soderblom E.J., Moseley M.A., Asfaw Y.G., Steinbach W.J. Identification of a key lysine residue in heat shock protein 90 required for azole and echinocandin resistance in Aspergillus fumigatus. Antimicrob. Agents Chemother. 2014;58:1889–1896. doi: 10.1128/AAC.02286-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Beer K.D., Farnon E.C., Jain S., Jamerson C., Lineberger S., Miller J., Berkow E.L., Lockhart S.R., Chiller T., Jackson B.R. Multidrug-resistant Aspergillus fumigatus carrying mutations linked to environmental fungicide exposure—Three states, 2010–2017. MMWR-Morb. Mortal. Wkly. Rep. 2018;67:1064–1067. doi: 10.15585/mmwr.mm6738a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Public Health England . English Surveillance Programme for Antimicrobial Utilization and Resistance (ESPAUR) Public Health England; London, UK: 2017. pp. 1–143. [Google Scholar]
- 18.Reichert-Lima F., Lyra L., Pontes L., Moretti M.L., Pham C.D., Lockhart S.R., Schreiber A.Z. Surveillance for azoles resistance in Aspergillus spp. highlights a high number of amphotericin B-resistant isolates. Mycoses. 2018;61:360–365. doi: 10.1111/myc.12759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mondol M.A., Shin H.J. Antibacterial and antiyeast compounds from marine-derived bacteria. Mar. Drugs. 2014;12:2913–2921. doi: 10.3390/md12052913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matobole R., van Zyl L., Parker-Nance S., Davies-Coleman M., Trindade M. Antibacterial activities of bacteria isolated from the marine sponges Isodictya compressa and Higginsia bidentifera collected from Algoa Bay, South Africa. Mar. Drugs. 2017;15:47. doi: 10.3390/md15020047. [DOI] [Google Scholar]
- 21.Kamarudheen N., Rao K.V.B. Fatty acyl compounds from marine Streptomyces griseoincarnatus strain HK12 against two major bio-film forming nosocomial pathogens; an in vitro and in silico approach. Microb. Pathog. 2019;127:121–130. doi: 10.1016/j.micpath.2018.11.050. [DOI] [PubMed] [Google Scholar]
- 22.Mickymaray S., Alturaiki W. Antifungal efficacy of marine macroalgae against fungal isolates from bronchial asthmatic cases. Molecules. 2018;23:3032. doi: 10.3390/molecules23113032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meng L., Sun C., Zhang C., Song S., Sun X., Ju J., Deng Y. Efficacy of compounds isolated from Streptomyces olivaceus against the morphogenesis and virulence of Candida albicans. Mar. Drugs. 2019;17:442. doi: 10.3390/md17080442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sivakumar K.C., Sajeevan T.P., Bright Singh I.S. Marine derived compounds as binders of the White spot syndrome virus VP28 envelope protein: In silico insights from molecular dynamics and binding free energy calculations. Comput. Biol. Chem. 2016;64:359–367. doi: 10.1016/j.compbiolchem.2016.08.006. [DOI] [PubMed] [Google Scholar]
- 25.Jia K., Yuan Y., Liu W., Liu L., Qin Q., Yi M. Identification of inhibitory compounds against Singapore grouper iridovirus infection by cell viability-based screening assay and droplet digital PCR. Mar. Biotechnol. 2017;20:35–44. doi: 10.1007/s10126-017-9785-1. [DOI] [PubMed] [Google Scholar]
- 26.Guo P., Li G., Liu Y., Lu A., Wang Z., Wang Q. Naamines and naamidines as novel agents against a plant virus and phytopathogenic fungi. Mar. Drugs. 2018;16:311. doi: 10.3390/md16090311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Melek F.R., Tadros M.M., Yousif F., Selim M.A., Hassan M.H. Screening of marine extracts for schistosomicidal activity in vitro. Isolation of the triterpene glycosides echinosides A and B with potential activity from the sea cucumbers Actinopyga echinites and Holothuria polii. Pharm. Biol. 2012;50:490–496. doi: 10.3109/13880209.2011.615842. [DOI] [PubMed] [Google Scholar]
- 28.Tasdemir D., MacIntosh A.J.J., Stergiou P., Kaiser M., Mansour N.R., Bickle Q., Huffman M.A. Antiprotozoal and antihelminthic properties of plants ingested by wild Japanese macaques (Macaca fuscata yakui) in Yakushima Island. J. Ethnopharmacol. 2019;247 doi: 10.1016/j.jep.2019.112270. [DOI] [PubMed] [Google Scholar]
- 29.Orhan I., Sener B., Kaiser M., Brun R., Tasdemir D. Inhibitory activity of marine sponge-derived natural products against parasitic protozoa. Mar. Drugs. 2010;8:47–58. doi: 10.3390/md8010047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Khanavi M., Toulabi P.B., Abai M.R., Sadati N., Hadjiakhoond F., Hadjiakhoondi A., Vatandoost H. Larvicidal activity of marine algae, Sargassum swartzii and Chondria dasyphylla, against malaria vector Anopheles stephensi. J. Vector Dis. 2011;48:241–244. [PubMed] [Google Scholar]
- 31.Martins L.F., Mesquita J.T., Pinto E.G., Costa-Silva T.A., Borborema S.E.T., Galisteo Junior A.J., Galisteo Junior A.J., Neves B.J., Andrade C.H., Shuhaib Z.A., et al. Analogues of marine guanidine alkaloids are in vitro effective against Trypanosoma cruzi and selectively eliminate Leishmania (L.) infantum intracellular amastigotes. J. Nat. Prod. 2016;79:2202–2210. doi: 10.1021/acs.jnatprod.6b00256. [DOI] [PubMed] [Google Scholar]
- 32.Peters T.L., Tillotson J., Yeomans A.M., Wilmore S.C., Lemm E., Jiménez-Romero C., Amador L.A., Li L., Amin A.D., Pongtornpipat P., et al. Target-based screening against eIF4A1 reveals the marine natural Product elatol as a novel inhibitor of translation initiation with in vivo antitumor activity. Clin. Cancer Res. 2018;24:4256–4270. doi: 10.1158/1078-0432.CCR-17-3645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howarth A., Simms C., Kerai N., Allen O., Mihajluk K., Madureira P.A., Sokratous G., Cragg S., Lee S.Y., Morley A.D., et al. DIVERSet JAG compounds inhibit topoisomerase II and are effective against adult and pediatric high-grade gliomas. Transla. Oncol. 2019;12:1375–1385. doi: 10.1016/j.tranon.2019.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rath B., Hochmair M., Plangger A., Hamilton G. Anticancer activity of fascaplysin against lung cancer cell and small cell lung cancer circulating tumor cell lines. Mar. Drugs. 2018;16:383. doi: 10.3390/md16100383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cowan J., Shadab M., Nadkarni D.H., Kc K., Velu S.E., Yusuf N.A. Novel marine natural product derived pyrroloiminoquinone with potent activity against skin cancer cells. Mar. Drugs. 2019;17:443. doi: 10.3390/md17080443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang W., Park C., Oh E., Sung Y., Lee J., Park K.H., Kang H. Benzophenone compounds, from a marine-derived strain of the fungus Pestalotiopsis neglecta, inhibit proliferation of pancreatic cancer cells by targeting the MEK/ERK pathway. J. Nat. Prod. 2019;82:3357–3365. doi: 10.1021/acs.jnatprod.9b00646. [DOI] [PubMed] [Google Scholar]
- 37.Oh S., Son M., Lee H.S., Kim H.S., Jeon Y.J., Byun K. Protective effect of pyrogallol-phloroglucinol-6, 6-bieckol from Ecklonia cava on monocyte-associated vascular dysfunction. Mar. Drugs. 2018;16:441. doi: 10.3390/md16110441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chakraborty K., Antony T. First report of spiro-compounds from marine macroalga Gracilaria salicornia: Prospective natural anti-inflammatory agents attenuate 5-lipoxygenase and cyclooxygenase-2. Nat. Prod. Res. 2019:1–12. doi: 10.1080/14786419.2019.1608545. [DOI] [PubMed] [Google Scholar]
- 39.Fernando I.P.S., Kim M., Son K.-T., Jeong Y., Jeon Y.-J. Antioxidant activity of marine algal polyphenolic compounds: A mechanistic approach. J. Med. Food. 2016;19:615–628. doi: 10.1089/jmf.2016.3706. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen V.B., Nguyen T.H., Doan C.T., Tran T.N., Nguyen A.D., Kuo Y.-H., Wang S.-L. Production and bioactivity-guided isolation of antioxidants with α-glucosidase inhibitory and anti-NO properties from marine chitinous materials. Molecules. 2018;23:1124. doi: 10.3390/molecules23051124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sun Y., Lin Y., Cao X., Xiang L., Qi J. Sterols from Mytilidae show anti-aging and neuroprotective effects via anti-oxidative activity. Int. J. Mol. Sci. 2014;15:21660–21673. doi: 10.3390/ijms151221660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Prastya M.E., Astuti R.I., Batubara I., Takagi H., Wahyudi A.T. Chemical screening identifies an extract from marine Pseudomonas sp.-PTR-08 as an anti-aging agent that promotes fission yeast longevity by modulating the Pap1-ctt1+ pathway and the cell cycle. Mol. Biol. Rep. 2019;47:33–43. doi: 10.1007/s11033-019-05102-0. [DOI] [PubMed] [Google Scholar]
- 43.Lauritano C., Ianora A. Marine organisms with anti-diabetes properties. Mar. Drugs. 2016;14:220. doi: 10.3390/md14120220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.de Alencar Alves M.F., de Almeida Barreto F.K., de Vasconcelos M.A., do Nascimento Netro L.G., Carneiro R.F., Silva L.T.D., Nagano C.S., Sampaio A.H., Teixeira E.H. Antihyperglycemic and antioxidant activities of a lectin from the marine red algae, Bryothamnion seaforthii, in rats with streptozotocin-induced diabetes. Int. J. Biol. Macromol. 2010;158:773–780. doi: 10.1016/j.ijbiomac.2020.04.238. [DOI] [PubMed] [Google Scholar]
- 45.Nasab B.S., Homaei A., Pletschke B.I., Salinas-Solazar C., Castillo-Zacarias C., Parra-Saldívar R. Marine resources effective in controlling and treating diabetes and its associated complications. Process. Biochem. 2020;92:313–342. doi: 10.1016/j.procbio.2020.01.024. [DOI] [Google Scholar]
- 46.Yu J., Proctor R.H., Brown D.W., Abe K., Gomi K., Machida M., Hasegawa F., Nierman W.C., Bhatnagar D., Cleveland T.E. Genomics of economically significant Aspergillus and Fusarium species. In: Arora D.K., Khachatourians G.G., editors. Applied Mycology and Biotechnology. Elsevier; Amsterdam, The Netherlands: 2004. pp. 249–283. [DOI] [Google Scholar]
- 47.Bennett J.H. On the parasitic vegetable structures found growing in living animals. Trana. R. Soc. Edinb. 1844;15:277–294. doi: 10.1017/S0080456800029963. [DOI] [Google Scholar]
- 48.Stevens D.A., Melikian G.L. Aspergillosis in the ‘nonimmunocompromised’ host. Immunol. Investig. 2011;40:751–766. doi: 10.3109/08820139.2011.614307. [DOI] [PubMed] [Google Scholar]
- 49.Siddiqui A.A., Shah A.A., Bashir S.H. Craniocerebral aspergillosis of sinonasal origin in immunocompetent patients: Clinical spectrum and outcome in 25 cases. Neurosurgery. 2004;55:602–613. doi: 10.1227/01.NEU.0000134597.94269.48. [DOI] [PubMed] [Google Scholar]
- 50.Siddiqui A.A., Bashir S.H., Ali Shah A., Sajjad Z., Ahmed N., Jooma R., Enam S.A. Diagnostic MR imaging features of craniocerebral aspergillosis of sino-nasal origin in immunocompetent patients. Acta Neurochir. 2006;148:155–166. doi: 10.1007/s00701-005-0659-3. [DOI] [PubMed] [Google Scholar]
- 51.Webb B.J., Vikram H.R. Chronic invasive sinus aspergillosis in immunocompetent hosts: A geographic comparison. Mycopathologia. 2010;170:403–410. doi: 10.1007/s11046-010-9338-x. [DOI] [PubMed] [Google Scholar]
- 52.Mody K.H., Ali M.J., Vemuganti G.K., Nalamada S., Naik M.N., Honavar S.G. Orbital aspergillosis in immunocompetent patients. Br. J. Ophthalmol. 2014;98:1379–1384. doi: 10.1136/bjophthalmol-2013-303763. [DOI] [PubMed] [Google Scholar]
- 53.Aggarwal E., Mulay K., Menon V., Sundar G., Honavar S.G., Sharma M. Isolated orbital aspergillosis in immunocompetent patients: A multicenter study. Am. J. Ophthalmol. 2016;165:125–132. doi: 10.1016/j.ajo.2016.03.007. [DOI] [PubMed] [Google Scholar]
- 54.Baeesa S.S., Bakhaidar M., Ahamed N.A.B., Madani T.A. Invasive orbital apex aspergillosis with mycotic aneurysm formation and subarachnoid hemorrhage in immunocompetent patients. World Neurosurg. 2017;102:42–48. doi: 10.1016/j.wneu.2017.02.096. [DOI] [PubMed] [Google Scholar]
- 55.Blaize M., Mayaux J., Nabet C., Lampros A., Marcelin A.G., Thellier M., Piarroux R., Demoule A., Fekkar A. Fatal Invasive aspergillosis and coronavirus disease in an immunocompetent patient. Emerg. Infect. Dis. 2020;26:1636–1637. doi: 10.3201/eid2607.201603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bora S., Kumar A., Mishra S., Satyarthee G.D., Singh P.K., Sawarkar D., Verma S., Borkar S., Sharma R., Chandra S.P., et al. Intracranial aspergillosis amongst immunocompetent patients: An experience with combined surgical and medical management of 18 patients. Clin. Neurol. Neurosurg. 2019;186:105511. doi: 10.1016/j.clineuro.2019.105511. [DOI] [PubMed] [Google Scholar]
- 57.Ma Y., Li W., Ao R., Lan X., Li Y., Zhang J., Yu S. Central nervous system aspergillosis in immunocompetent patients: Case series and literature review. Medicine. 2020;99:e22911. doi: 10.1097/MD.0000000000022911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Yoon S.H., Park C.M., Goo J.M., Lee H.J. Pulmonary aspergillosis in immunocompetent patients without air-meniscus sign and underlying lung disease: CT findings and histopathologic features. Acta Radiol. 2011;52:756–761. doi: 10.1258/ar.2011.100481. [DOI] [PubMed] [Google Scholar]
- 59.Chen L., Han X., Li Y., Zhang C., Xing X. Invasive pulmonary aspergillosis in immunocompetent patients hospitalised with influenza A-related pneumonia: A multicenter retrospective study. BMC Pulm Med. 2020;20:239. doi: 10.1186/s12890-020-01257-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen L., Liu Y., Wang W., Liu K. Adrenal and hepatic aspergillosis in an immunocompetent patient. Infect. Dis. 2015;47:428–432. doi: 10.3109/00365548.2014.995697. [DOI] [PubMed] [Google Scholar]
- 61.Snelders E., Huisin’t Veld R.A.G., Rijs A.J.M.M., Kema G.H.J., Melchers W.J.G., Verweij P.E. Possible environmental origin of resistance of Aspergillus fumigatus to medical triazoles. Appl. Environ. Microb. 2009;75:4053–4057. doi: 10.1128/AEM.00231-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fang W., Latgé J.P. Microbe profile: Aspergillus fumigatus: A saprotrophic and opportunistic fungal pathogen. Microbiology. 2018;164:1009–1011. doi: 10.1099/mic.0.000651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Vaezi A., Fakhim H., Javidnia J., Khodavaisy S., Abtahian Z., Vojoodi M., Nourbakhsh F., Badali H. Pesticide behavior in paddy fields and development of azole-resistant Aspergillus fumigatus: Should we be concerned? J. Mycol. Med. 2018;28:59–64. doi: 10.1016/j.mycmed.2017.12.007. [DOI] [PubMed] [Google Scholar]
- 64.Mohapatra B.R., Bapuji M., Sree A. Antifungal efficacy of bacteria isolated from marine sedentary organisms. Folia Microbiol. 2002;47:51–55. doi: 10.1007/BF02818565. [DOI] [PubMed] [Google Scholar]
- 65.Devi P., Wahidullah S., Rodrigues C., Souza L.D. The sponge-associated bacterium Bacillus licheniformis SAB1: A source of antimicrobial compounds. Mar. Drugs. 2010;8:1203–1212. doi: 10.3390/md8041203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Chen H., Cai K., Yao R. A new macrolactam derivative from the marine actinomycete HF-11225. J. Antibiot. 2018;71:477–479. doi: 10.1038/s41429-017-0021-z. [DOI] [PubMed] [Google Scholar]
- 67.Santos J.D., Vitorino I., De la Cruz M., Díaz C., Cautain B., Annang F., Pérez-Moreno G., Gonzalez Martinez I., Tormo J.R., Martín J.M., et al. Bioactivities and extract dereplication of Actinomycetales isolated from marine sponges. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.00727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Subramani R., Sipkema D. Marine rare actinomycetes: A promising source of structurally diverse and unique novel natural products. Mar. Drugs. 2019;17:249. doi: 10.3390/md17050249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Nagai K., Kamigiri K., Matsumoto H., Kawano Y., Yamaoka M., Shimoi H., Watanabe M., Suzuki K. YM-202204, a new antifungal antibiotic produced by marine fungus Phoma sp. J. Antibiot. 2002;55:1036–1041. doi: 10.7164/antibiotics.55.1036. [DOI] [PubMed] [Google Scholar]
- 70.Orwa P., Mugambi G., Wekesa V., Mwirichia R. Isolation of haloalkaliphilic fungi from Lake Magadi in Kenya. Heliyon. 2020;6:e02823. doi: 10.1016/j.heliyon.2019.e02823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Al-Enazi N.M., Awaad A.S., Zain M.E., Alqasoumi S.I. Antimicrobial, antioxidant and anticancer activities of Laurencia catarinensis, Laurencia majuscula and Padina pavonica extracts. Saudi Pharm. J. 2018;26:44–52. doi: 10.1016/j.jsps.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Al-Enazi N.M., Awaad A.S., Alqasoumi S.I., Alwethairi M.F. Biological activities of the red algae Galaxaura rugosa and Liagora hawaiiana butters. Saudi Pharm. J. 2018;26:25–32. doi: 10.1016/j.jsps.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Silva P., Fernandes C., Barros L., Ferreira I.C.F.R., Pereira L., Gonçalves T. The antifungal activity of extracts of Osmundea pinnatifida, an edible seaweed, indicates its usage as a safe environmental fungicide or as a food additive preventing post-harvest fungal food contamination. Food Funct. 2018;9:6187–6195. doi: 10.1039/C8FO01797B. [DOI] [PubMed] [Google Scholar]
- 74.Martínez K.A., Lauritano C., Druka D., Romano G., Grohmann T., Jaspars M., Martín J., Díaz C., Cautain B., de la Cruz M., et al. Amphidinol 22, a new cytotoxic and antifungal amphidinol from the dinoflagellate Amphidinium carterae. Mar. Drugs. 2019;17:385. doi: 10.3390/md17070385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lakshmi V., Mishra S.K., Srivastava S., Chaturvedi A., Srivastava M.N., Shukla P.K. Antifungal activity of marine sponge Haliclona exigua (Krikpatrick) J. Mycol. Méd. 2010;20:31–35. doi: 10.1016/j.mycmed.2009.12.001. [DOI] [Google Scholar]
- 76.Xu T., Feng Q., Jacob M.R., Avula B., Mask M.M., Baerson S.R., Tripathi S.K., Mohammed R., Hamann M.T., Khan I.A., et al. The marine sponge-derived polyketide endoperoxide plakortide F acid mediates its antifungal activity by interfering with calcium homeostasis. Antimicrob. Agents Chemother. 2011;55:1611–1621. doi: 10.1128/AAC.01022-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhang X., Jacob M.R., Rao R.R., Wang Y.-H., Agarwal A.K., Newman D.J. Antifungal cyclic peptides from the marine sponge Microscleroderma herdmani. Res. Rep. Med. Chem. 2010;22:7–14. doi: 10.2147/RRMC.S30895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Nazemi M., Alidoust Salimi M., Alidoust Salimi P., Motallebi A., Tamadoni Jahromi S., Ahmadzadeh O. Antifungal and antibacterial activity of Haliclona sp. from the Persian Gulf, Iran. J. Mycol. Méd. 2014;24:220–224. doi: 10.1016/j.mycmed.2014.03.005. [DOI] [PubMed] [Google Scholar]
- 79.Ismail H., Lemriss S., Aoun B.Z., Mhadhebi L., Dellai A., Kacem Y., Boiron P., Bouraoui A. A activity of aqueous and methanolic extracts from the Mediterranean sea cucumber, Holothuria polii. J. Mycol. Méd. 2008;18:23–26. doi: 10.1016/j.mycmed.2008.01.002. [DOI] [Google Scholar]
- 80.Yuan W.-H., Yi Y.-H., Tang H.-F., Liu B.-S., Wang Z.-L., Sun G.-Q., Zhang W., Li L., Sun P. Antifungal triterpene glycosides from the sea cucumber Bohadschia marmorata. Planta Med. 2009;75:168–173. doi: 10.1055/s-0028-1088348. [DOI] [PubMed] [Google Scholar]
- 81.Yuan W.-H., Yi Y.-H., Tan R.-X., Wang Z.-L., Sun G.-Q., Xue M., Zhang H.-W., Tang H.-F. Antifungal triterpene glycosides from the sea cucumber Holothuria (Microthele) axiloga. Planta Med. 2009;75:647–653. doi: 10.1055/s-0029-1185381. [DOI] [PubMed] [Google Scholar]
- 82.Barsby T., Kelly M.T., Andersen R.J. Tupuseleiamides and basiliskamides, new acyldipeptides and antifungal polyketides produced in culture by a Bacillus laterosporus isolate obtained from a tropical marine habitat. J. Nat. Prod. 2002;65:1447–1451. doi: 10.1021/np0201321. [DOI] [PubMed] [Google Scholar]
- 83.Lacret R., Oves-Costales D., Gómez C., Díaz C., de la Cruz M., Pérez-Victoria I., Vicente F., Genilloud O., Reyes F. New ikarugamycin derivatives with antifungal and antibacterial properties from Streptomyces zhaozhouensis. Mar. Drugs. 2015;13:128–140. doi: 10.3390/md13010128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lacret R., Pérez-Victoria I., Oves-Costales D., de la Cruz M., Domingo E., Martín J., Díaz C., Vicente F., Genilloud O., Reyes F. MDN-0170, a new napyradiomycin from Streptomyces sp. strain CA-271078. Mar. Drugs. 2016;14:188. doi: 10.3390/md14100188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pérez-Victoria I., Oves-Costales D., Lacret R., Martín J., Sánchez-Hidalgo M., Diaz C., Cautain B., Vicente F., Genilloud O., Reyes F. Structure elucidation and biosynthetic gene cluster analysis of caniferolides A-D, new bioactive glycosylated 36-membered polyol macrolides from the marine-derived Streptomyces caniferus CA-271066. Org. Biomol. Chem. 2019;17 doi: 10.1039/C8OB03115K. [DOI] [PubMed] [Google Scholar]
- 86.Kumar S., Kannabiran K. Antifungal activity of Streptomyces VITSVK5 spp. against drug resistant Aspergillus clinical isolates from pulmonary tuberculosis patients. J. Mycol. Méd. 2010;20:101–107. doi: 10.1016/j.mycmed.2010.04.005. [DOI] [Google Scholar]
- 87.Suthindhiran K., Kannabiran K. Diversity and exploration of bioactive marine actinomycetes in the Bay of Bengal of the Puducherry coast of India. Indian J. Microbiol. 2010;50:76–82. doi: 10.1007/s12088-010-0048-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Sata N.U., Matsunaga S., Fusetani N., van Soest R.W.M. Aurantosides D, E, and F: New antifungal tetramic acid glycosides from the marine sponge Siliquariaspongia japonica. J. Nat. Prod. 1999;62:969–971. doi: 10.1021/np9900021. [DOI] [PubMed] [Google Scholar]
- 89.Edrada R.A., Ebel R., Supriyono A., Wray V., Schupp P., Steube K., van Soest R., Proksch P. Swinhoeiamide A, a new highly active calyculin derivative from the marine sponge Theonella swinhoei. J. Nat. Prod. 2002;65:1168–1172. doi: 10.1021/np020049d. [DOI] [PubMed] [Google Scholar]
- 90.Chen Y., McCarthy P.J., Harmody D.K., Schimoler-O’Rourke R., Chilson K., Selitrennikoff C., Pomponi S.A., Wright A.E. New bioactive peroxides from marine sponges of the family Plakiniidae. J. Nat. Prod. 2002;65:1509–1512. doi: 10.1021/np0200414. [DOI] [PubMed] [Google Scholar]
- 91.Sionov E., Roth D., Sandovsky-Losica H., Kashman Y., Rudi A., Chill L., Berdicevsky I., Segal E. Antifungal effect and possible mode of activity of a compound from the marine sponge Dysidea herbacea. J. Infect. 2005;50:453–460. doi: 10.1016/j.jinf.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 92.Pettit G.R., Chicacz Z.A., Gao F., Herald C.L., Boyd M.R., Schmidt J.M., Hooper J.N.A. Antineoplastic agents. 257. Isolation and structure of spongistatin 1. J. Org. Chem. 1993;58:1302–1304. doi: 10.1021/jo00058a004. [DOI] [Google Scholar]
- 93.Pettit R.K., McAllister S.C., Pettit G.R., Herald C.L., Johnson J.M., Cichacz Z.A. A broad-spectrum antifungal from the marine sponge Hyrtios erecta. Int. J. Antimicrob. Agents. 1998;9:147–152. doi: 10.1016/S0924-8579(97)00044-7. [DOI] [PubMed] [Google Scholar]
- 94.Oh K.-B., Lee J.H., Chung S.-C., Shin J., Shin H.J., Kim H.-K., Lee H.-S. Antimicrobial activities of the bromophenols from the red alga Odonthalia corymbifera and some synthetic derivatives. Bioorg. Med. Chem. Lett. 2008;18:104–108. doi: 10.1016/j.bmcl.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Alarif W.M., Al-Lihaibi S.S., Abdel-Lateff A., Ayya S.-E.N. New antifungal cholestane and aldehyde derivatives from the red alga Laurencia papillosa. Nat. Prod. Commun. 2011;6:1821–1824. doi: 10.1177/1934578X1100601208. [DOI] [PubMed] [Google Scholar]
- 96.Kumar R., Chaturvedi A.K., Shukla P.K., Lakshmi V. Antifungal activity in triterpene glycosides from the sea cucumber Actinopyga lecanora. Bioorg. Med. Chem. Lett. 2007;17:4387–4391. doi: 10.1016/j.bmcl.2006.12.052. [DOI] [PubMed] [Google Scholar]
- 97.Han H., Yi Y.-H., Li L., Liu B.-S., La M.-P., Zhang H.-W. Antifungal active triterpene glycosides from sea cucumber Holothuria scabra. Acta Pharm. Sin. 2009;44:620–624. [PubMed] [Google Scholar]
- 98.Adibpour N., Nasr F., Nematpour F., Shakouri A., Ameri A. Antibacterial and antifungal activity of Holothuria leucospilota isolated from Persian Gulf and Oman Sea. Jundishapur. J. Microb. 2014;7:e8708. doi: 10.5812/jjm.8708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lakshmi V., Srivastava S., Mishra S.K., Shukla P.K. Antifungal activity of bivittoside-D from Bohadschia vitiensis (Semper) Nat. Prod. Res. 2012;26:913–918. doi: 10.1080/14786419.2010.534096. [DOI] [PubMed] [Google Scholar]
- 100.Austin B. The value of cultures to modern microbiology. Antonie Van Leeuwenhoek. 2017;110:1247–1256. doi: 10.1007/s10482-017-0840-8. [DOI] [PubMed] [Google Scholar]
- 101.Pulschen A.A., Bendia A.G., Fricker A.D., Pellizari V.H., Galante D., Rodrigues F. Isolation of uncultured bacteria from Antarctica using long incubation periods and low nutritional media. Front. Microbiol. 2017;8 doi: 10.3389/fmicb.2017.01346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Bodor A., Bounedjoum N., Vincze G.E., Erdeiné Kis Á., Laczi K., Bende G., Szilágyi Á., Kovács T., Perei K., Rákhely G. Challenges of unculturable bacteria: Environmental perspectives. Rev. Environ. Sci. Biotechnol. 2020;19:1–22. doi: 10.1007/s11157-020-09522-4. [DOI] [Google Scholar]
- 103.Kovalchuk V., Voronkina A., Binnewerg B., Schubert M., Muzychka L., Wysokowski M., Tsurkan M.V., Bechmann N., Petrenko I., Fursov A., et al. Naturally drug-loaded chitin: Isolation and applications. Mar. Drugs. 2019;17:574. doi: 10.3390/md17100574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rahman M.A. Collagen of extracellular matrix from marine invertebrates and its medical applications. Mar. Drug. 2019;17:118. doi: 10.3390/md17020118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Breger J., Fuchs B.B., Aperis G., Moy T.I., Asbel F.M., Mylonakis E. Antifungal chemical compounds identified using a C. elegans pathogenicity assay. PLoS Pathog. 2007;3:e18. doi: 10.1371/journal.ppat.0030018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Moy T.I., Conery A.L., Larkins-Ford J., Wu G., Mazitschek R., Casadei G., Lewis K., Carpenter A.E., Ausubel F.M. High-throughput screen for novel antimicrobials using a whole animal infection model. ACS Chem. Biol. 2009;4:527–533. doi: 10.1021/cb900084v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Graham C.E., Cruz M.R., Garsin D.A., Lorenz M.C. Enterococcus faecalis bacteriocin EntV inhibits hyphal morphogenesis, biofilm formation, and virulence of Candida albicans. Proc. Natl. Acad. Sci. USA. 2017;25:4507–4512. doi: 10.1073/pnas.1620432114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Manoharan R.K., Lee J.H., Kim Y.G., Kim S.I., Lee J. Inhibitory effects of the essential oils α-longipinene and linalool on biofilm formation and hyphal growth of Candida albicans. Biofouling. 2017;33:143–155. doi: 10.1080/08927014.2017.1280731. [DOI] [PubMed] [Google Scholar]
- 109.Okoli I., Coleman J.J., Tempakakis E., An W.F., Holson E., Wagner F., Conery A.L., Larkins-Ford J., Wu G., Stern A., et al. Identification of antifungal compounds active against Candida albicans using an improved high-throughput Caenorhabditis elegans assay. PLoS ONE. 2009;4:e7025. doi: 10.1371/journal.pone.0007025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Peterson N.D., Pukkila-Worley R. Caenorhabditis elegans in high-throughput screens for anti-infective compounds. Curr. Opin. Immunol. 2018;54:59–65. doi: 10.1016/j.coi.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ahamefule C.S., Qin Q., Odiba A.S., Li S., Moneke A.N., Ogbonna J.C., Jin C., Wang B., Fang W. Caenorhabditis elegans-based Aspergillus fumigatus infection model for evaluating pathogenicity and drug efficacy. Front. Cell. Infect. Microbiol. 2020;10 doi: 10.3389/fcimb.2020.00320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Haferburg G., Groth I., Möllmann U., Kothe E., Sattler I. Arousing sleeping genes: Shifts in secondary metabolism of metal tolerant actinobacteria under conditions of heavy metal stress. Biometals. 2009;22:225–234. doi: 10.1007/s10534-008-9157-4. [DOI] [PubMed] [Google Scholar]
- 113.Morgenstern A., Paetz C., Behrend A., Spiteller D. Divalent transition-metal-ion stress induces prodigiosin biosynthesis in Streptomyces coelicolor M145: Formation of coeligiosins. Chemistry. 2015;21:6027–6032. doi: 10.1002/chem.201405733. [DOI] [PubMed] [Google Scholar]
- 114.Shi Y., Pan C., Auckloo B.N., Chen X., Chen C.-T.A., Wang K., Wu X., Ye Y., Wu B. Stress-driven discovery of a cryptic antibiotic produced by Streptomyces sp. WU20 from Kueishantao hydrothermal vent with an integrated metabolomics strategy. Appl. Microbiol. Biotechnol. 2016;101:1395–1408. doi: 10.1007/s00253-016-7823-y. [DOI] [PubMed] [Google Scholar]
- 115.Hassan S.S., Shah S.A.A., Pan C., Fu L., Cao X., Shi Y., Wu X., Wang K., Wu B. Production of an antibiotic enterocin from a marine actinobacteria strain H1003 by metal-stress technique with enhanced enrichment using response surface methodology. Pak. J. Pharm. Sci. 2017;30:313–324. [PubMed] [Google Scholar]
- 116.Zhu C., Liu X., Chi H., Chen C., Chen Z., Fu G., Gong H., Huang Y. System for the heterologous expression of NS1 protein of H9N2 avian influenza virus in the ciliate Tetrahymena thermophila. J. Vet. Med. Sci. 2018;80:1610–1618. doi: 10.1292/jvms.18-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]