Abstract
Virtual memory T (TVM) cells are a recently described population of conventional CD8+ T cells that, in spite of their antigen inexperience, express markers of T cell activation. TVM cells exhibit rapid responsiveness to both antigen-specific and innate stimuli in youth but acquire intrinsic antigen-specific response defects in the elderly. In this article, we review how the identification of TVM cells necessitates a re-evaluation of accepted paradigms for conventional memory T (TMEM) cells, the potential for heterogeneity within the TVM population, and the defining characteristics of TVM cells. Further, we highlight recent literature documenting the development of TVM cells as a distinct CD8+ T cell lineage as well their biological significance in the context of disease.
Keywords: virtual memory T cells, Eomes, IL-15, CD8+ T cells
1. Introduction
Traditionally, CD8+ T cells have been considered to exist along a single spectrum; resting naïve CD8+ T (TN) cells, upon recognition of cognate antigen and subsequent activation, differentiate into effector T cells, which contract upon antigen clearance, leaving a conventional memory T (TMEM) cell population. The TMEM cell population is comprised largely of effector memory T cells (TEM), found predominantly in tissues and primed for rapid effector function, and central memory T (TCM) cells, found mainly in lymph nodes and responsible for self-renewal and supplying the pipeline of effector T cells [1]. TMEM cells are quiescent but poised for activation, and present at a relatively high antigen-specific frequency. These features of CD8+ T cell memory underpin their ability to respond rapidly after reencounter with the same antigen and are a hallmark of adaptive immunity. Recently, a novel population(s) of CD8+ T cells has been identified, referred to variously as virtual memory T (TVM) cells, memory phenotype (MP) T cells, antigen-inexperienced memory T (TAIM) cells, and innate memory T (TIM) cells, that exhibit many characteristics of TMEM cells—including cell surface phenotype and rapid responsiveness to both antigen-specific and innate stimuli—despite having not previously encountered specific antigen. Herein, we generally refer to this population of antigen-naïve memory phenotype CD8+ T cells as TVM cells. The interest in TVM cells stems from their ability to exert robust and rapid effector functions never previously attributed to antigen-inexperienced T cells, their responsiveness to both antigen-specific and innate stimuli, their superior survival capacity and their intrinsic dysfunction in elderly mice and humans [2,3,4,5,6]. The following review highlights how TVM cells have blurred the traditional boundaries between TN cells and TMEM cells and have driven the need for a re-evaluation of conventionally accepted TMEM characteristics. In addition, we discuss recent advances in our understanding of TVM cells, including their development as a distinct cell lineage and their biological relevance in protection from infection and cancers [7,8,9,10,11].
2. Conflation of Mouse TVM Cells with Conventional TMEM Cells
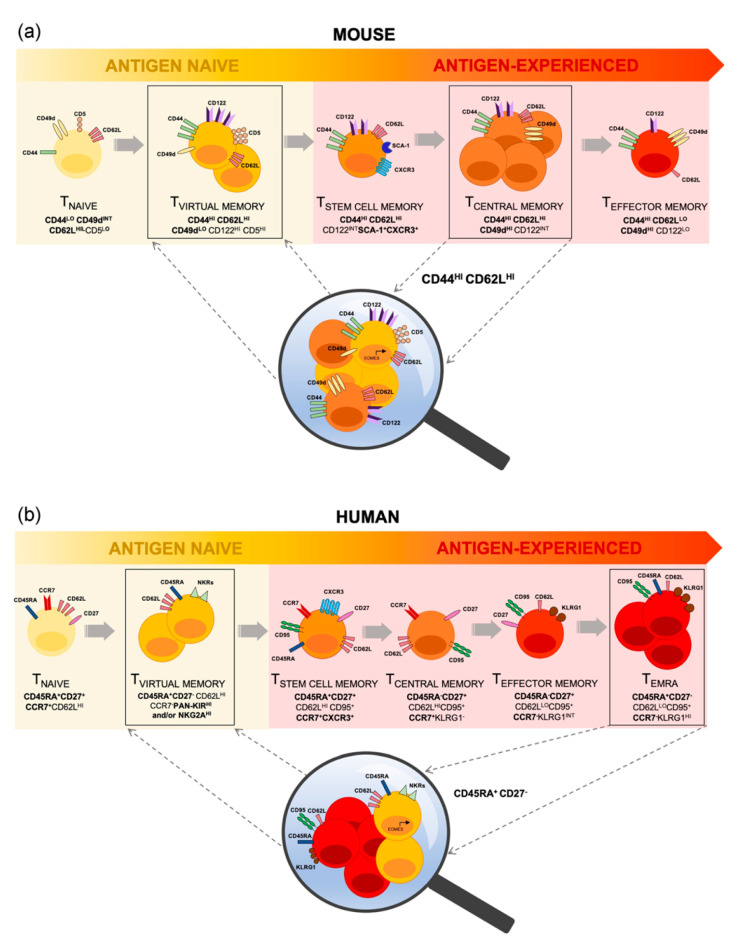
In mice, the phenotype of conventional TMEM cell populations is well established [12,13], with all antigen-experienced memory T cells expressing the definitive activation marker, CD44, and differential expression of the lymph node homing receptor, CD62L, allowing distinction between TCM and TEM subsets [14]. However, the discovery of TVM cells has revealed a substantial overlap in the cell surface phenotype of conventional TCM cells and TVM cells in mice, with both expressing high levels of CD44 and CD62L [6,15,16]. Consequently, TVM cells have historically fallen into the conventional phenotypic definition of antigen-experienced CD8+ TCM population (Figure 1a). This can be easily overcome by the inclusion of CD49d, an integrin involved in cell trafficking, which is stably upregulated on TMEM cells but, even with advanced age or certain infection models, remains low on TVM cells [3,6]. TVM cells can also be identified by high level expression of IL-2Rβ/IL-15Rβ (CD122) compared to lower expression on TMEM cells, reflecting TVM cell sensitivity to IL-15 [17]. It has recently been demonstrated that conventionally defined TCM cells are, in both young and aged mice, comprised predominantly (~80%) of TVM cells [3]. Even in mice recently infected with LCMV, which induces a robust CD8+ T cell memory population [3], over 60% of CD8+ CD44hi CD62Lhi cells (i.e. conventionally defined TCM cells) are TVM cells [3,18]. Findings regarding the TCM population may therefore be influenced by the inclusion of TVM cells.
Figure 1.
The Differentiation Continuum and Defining Phenotype of Steady-State CD8+ T cells. CD8+ TVM cells are a semi-differentiated yet antigenically naïve T cell population, indicating the potential for antigen-independent T cell differentiation and representing a link between antigen naïve (yellow shaded) and antigen-experienced (red shaded) memory T cells. TVM cells have historically been phenotypically included within (a) the TCM cell population in mice, and (b) the TEMRA cell population in humans. Lineage-defining markers (in bold), in conjunction with other additional markers, demarcate TVM cells and reinforce their phenotypic and functional uniqueness.
Inaccurate attribution of characteristics as a consequence of the conflation of TVM and TCM cells is exemplified by our understanding of the reliance of CD8+ TMEM cells on IL-15 for survival [3,6]. The current paradigm indicates that IL-15 is critical for TMEM cell survival. However, recent studies have shown that in young and aged mice lacking IL-15 there is a complete loss of TVM cells (CD44hi CD49dlo) whilst TMEM (CD44hi CD49dhi) cells are relatively unaffected [3,19,20]. Similarly, in mice lacking CD122, the generation and maintenance of TMEM cells remains relatively intact whilst TVM cells fail to develop [17]. These findings call into question other widely accepted characteristics of TCM cells. Of particular interest is the dependence of TVM and TCM cells on tonic peptide + Major Histocompatibility Complex I molecule (MHCI)-TCR signalling for survival in the periphery. It has long been appreciated that circulating naïve CD8+ T cells require low affinity self-pMHCI:TCR interactions in order to provide tonic signals for survival [21], contrasting memory cells whose survival is independent of MHCI but dependent on homeostatic cytokines such as IL-7, IL-15 [22]. However, the requirements for survival of TVM cells is contentious. Early adoptive transfer experiments demonstrated the survival of LCMV-specific memory phenotype (MP) cells in β2m−/− hosts [23]. However, transferred MP cells in this experiment were defined only by high expression of CD44. Expanding on this finding, later adoptive transfer experiments demonstrated that MP cells expressing high levels of CD122 (characteristic of TVM cells) were maintained in the periphery of MHC-Ia−/− mice, whilst MP cells expressing low levels of CD122 failed to survive [24]. These CD122lo MP cells expressed a cell surface phenotype reminiscent of recently activated effector CD8+ T cells, including low expression of CD62L. Similarly, inspection of the endogenous population of peripheral CD8+ T cells in MHC-I−/− hosts revealed the majority of these cells exhibited CD44hi CD122hi phenotype reminiscent of TVM cells [25]. Thus, the extent to which conventional TCM cells and TVM cells rely on MHC-I for peripheral survival is yet to be definitively determined.
3. TVM Cells Are Contained within the Human TEMRA Cell Population
While largely characterized in mice, a putative TVM population has also been identified in humans, which displays both functional and phenotypic similarities to mouse TVM cells [20,26]. These human TVM cells display a differentiated phenotype typically associated with effector memory T cells re-expressing CD45RA (TEMRA) (CD45RA+CD27−), express NK cell receptors (NKRs) such as KIRs and NKG2A, show high expression of Nur77 (indicative of high self-pMHCI affinity) [20], and show high expression of the transcription factor Eomes, with rapid production of IFNγ upon innate-like stimulation [26]. Furthermore, human TVM cells accumulate with age and acquire defects in TCR-mediated proliferation [2,20]. In addition to these parallels with mouse TVM cells, human TVM cells have been detected in human cord blood and thus their development appears to be independent of antigen exposure [26]. The limited study of human TVM cells can be attributed to the lack of definitive surface markers that distinguish them from TEMRA cells (CD8+CD45RA+CCR7−). Currently, identification of human TVM cells is based on the additional expression of NK cell markers, pan-KIR2D and KIR3DL, and/or NKG2A, which separates TVM cells from the entire CD45RA+ subset of CD8+ T cells. Non-TVM CD45RA+ cells are further subdivided into TN cells (CD27+CD45RA+CD95−), TSCM cells (CD27+CD45RA+CD95+) [27] and TEMRA cells. Owing to the overlap in surface marker expression between TEMRA and TVM cells, and the lack of routine inclusion of defining NKRs, TVM cells are typically included within the TEMRA population [28] (Figure 1b).
Despite their apparent similarities, there are key differences that distinguish TVM cells from TEMRA cells. Firstly, TVM cells are considered to be antigen-inexperienced [5,29] whilst TEMRA cells are antigen-experienced memory cells, as evidenced by the observation that they can comprise up to 39% of the CD8+ T cells within a given epitope-specific population [30]. Secondly, TVM cells have a higher proliferative capacity than TEMRA cells which are non-proliferative in both young and aged individuals [2]. Thirdly, although a direct comparison between human TVM and TEMRA cell metabolism has not been performed to date, our recent work in mouse models have shown that TVM cells not only have the highest oxygen consumption rate (OCR) of all CD8+ subsets in steady state but that it is further increased with infection and ageing [3]. In addition, our study indicates that there is no difference in basal mitochondrial characteristics, such as mitochondrial mass and number of mitochondria per cell, between TVM cells compared to other CD8+ subsets [3]. In contrast, TEMRA cells have a lower basal OCR and extracellular acidification rate (ECAR), following overnight CD3 stimulation [31], as well as lower basal mitochondrial mass and fewer mitochondria per cell compared to conventional memory subsets [32].
The inclusion of NKRs to separate putative TVM cells from TEMRA cells in humans marks the beginning of the quest to better investigate this distinct cell population. It is clear that this putative TVM population parallels many of the functional characteristics observed in murine TVM cells, emphasising the need to identify unique and definitive markers for future studies. Indeed, a recent single cell transcriptional analysis of human memory T cells has identified novel subsets of stem-like CD8+ memory T cells [27], highlighting the heterogeneity that has confounded a complete understanding of memory phenotype T cells in humans.
4. Heterogeneity within the TVM Cell Compartment
Broadly, two populations of antigen-inexperienced MP cells have been described—TVM cells and TIM cells. Whilst TVM cells and TIM cells were originally distinguished from one another by the thymic expression of CD49d, their dependence on IL-15 vs IL-4, and their emergence in the periphery versus the thymus, the two populations are indistinguishable once in the periphery [6,17,33,34]. It is likely that these cells represent the same population, but their original identification in different mouse strains has resulted in the attribution of distinct characteristics. TIM cells are highly abundant in BALB/c mice due to the ability of unconventional PLZF+ NKT cells in this strain to produce large amounts of IL-4, facilitating TIM differentiation [33,34,35]. In contrast, TIM cells are not readily detectable in C57Bl/6 mice, however, genetic alterations in these mice, such as knockout of tyrosine kinases ITK and RLK, increases the number of IL-4 producing PLZF+ NKT cells and, in turn, increases the number of detectable TIM cells within the thymus and periphery [34,36,37,38,39]. Moreover, while not readily detectable in a WT C57Bl/6 mouse thymus, recent evidence indicates TVM cell differentiation is programmed during thymic development (see below) [40,41,42].
Although peripheral TVM cells in mice are readily identified using cell surface markers such as CD49d, CD44 and CD122, whether or not this population represents a homogenous population of cells or an amalgamation of disparate cell subsets is unclear. Heterogeneity within the TVM population has been suggested by a recent study which used tamoxifen-induced time stamping to analyse TVM cells generated in the neonatal period (day 1) or later in life (day 28) [43]. TVM cells generated early in life (day 1) exhibit a transcriptional profile more akin to a short-lived effector cell (SLEC), as indicated by expression of tbx21, Ifng and gzma genes, compared to those generated later in life (day 28). This heterogeneity translated to differences in functionality, with day 1 TVM cells responding more rapidly to antigen and inflammatory cues linked with increases in effector molecules such as granzyme B and IFNγ. This exaggerated effector response also translated to a greater propensity to adopt a terminally differentiated (KLRG1hi CD62Llo) phenotype 41 days post-infection.
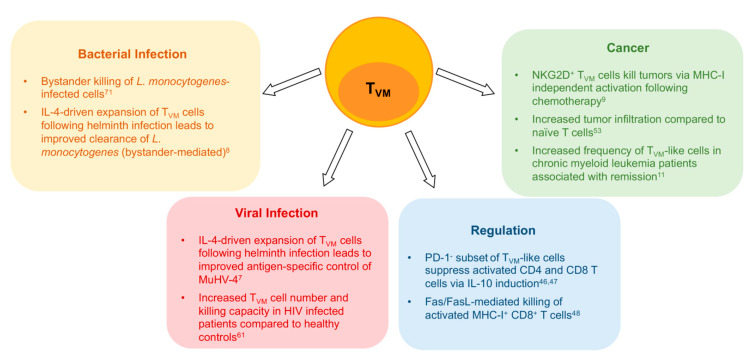
Functional heterogeneity is further observed in a subset of TVM cells that selectively express NK cell markers [20]. NKR expression is a distinguishing feature of mouse TVM cells [6,20,44], and a defining characteristic of human TVM cells. Whilst NKR expression on memory CD8+ T cells has conventionally been associated with senescence [45], in TVM cells this subset appears to show heightened functionality, as evidenced by an increased ability to kill MHC-I deficient tumour cells following chemotherapy treatment in both humans and mice [9] (Figure 2).
Figure 2.
Virtual memory CD8+ T cells (TVM) participate in various immune responses to pathogens and tumors and may be involved in immune regulation. Boxes indicate the role and possible mechanism of action of TVM cells in different disease or immune contexts.
Adding to evidence of functional heterogeneity within the TVM population, a number of studies have indicated a regulatory role of a subset of CD8+CD44hiCD122hi cells. Early reports have suggested MP CD8+ T cells function similar to that of CD4 Treg cells via IL-10-induced suppression of effector function in activated CD4 and CD8+ cells [46] (Figure 2). Later studies revealed that only the PD-1 negative MP subset displayed regulatory functions [47]. In addition, Akane and colleagues characterised these CD8+CD44hiCD122hi Treg cells and determined they could be further defined from other CD8+ MP cells via a lack of CD49d expression, suggesting they were in fact TVM cells [48]. Taken together, these data suggest both phenotypic and functional heterogeneity within the TVM population.
5. Heightened TCR Reactivity and Cytokine Sensitivity Are Key TVM Cell Characteristics
TCR reactivity appears to be a key determinant in driving TVM differentiation, phenotype and effector function. Firstly, TVM cells have been shown to express heightened levels of CD5 in mice [49], and Nur77 in humans [20], which are surrogate markers for TCR signal strength [50,51] and thus are indicative of heightened TCR self-reactivity [52]. In addition, TCR repertoire analyses shows a TCR bias in CD8+ MP cells [49,53,54], further suggesting the TCR dependence of TVM differentiation. It is likely that this high self-peptide:MHCI reactivity during TVM cell development drives the heightened TVM cell cytokine sensitivity in the periphery, which can, at least in part, be attributed to Eomes expression. Eomes is a Tbox transcription factor which, in CD8+ T cells, shows increased expression following activation [55]. In a study by Miller and colleagues, it was shown that Eomes expression could be upregulated during thymic maturation of CD44hiCD122+ cells, which was attributed to heightened TCR reactivity to self-ligands [53]. Eomes expression has also been shown to bind to the il2rb promoter leading to activation and a subsequent increase in CD122 expression [56]. Thus, the heightened self-peptide MHC reactivity of TVM cells appears to upregulate Eomes expression, which in turn leads to increased CD122 expression, driving TVM cell dependence on, and sensitivity to, IL-15. This is also supported by Gett and colleagues who showed that strong TCR engagement, and subsequent signalling, enhanced survival and responsiveness to IL-15, and other cytokines, through increased expression of cytokine receptors [57].
Eomes expression in TVM cells has also been shown to be augmented by type I IFN signaling [58]. Indeed, IFNβ signalling resulted in an Eomes-dependent increase of both peripheral TVM cells and thymic TIM cells, and TVM cells were significantly diminished in IFNAR−/− mice [58]. Given the observation that tonic type I IFN signalling is received by SP thymocytes as a normal part of T cell development [59], it seems plausible that type I IFN signalling is essential both in the thymus for TVM lineage differentiation at this SP stage [53], as well as in the periphery for the peripheral maintenance of Eomes expression.
Although a characteristic of memory cell subsets in general, TVM cells are particularly sensitive to a range of homeostatic cytokines, such as IL-12, IL-18, IL-4, and IL-7 [17,20,60,61,62]. As mentioned, their particularly high sensitivity to IL-15 is likely to be due to increased expression of CD122, which increases further with age [3], and leads to a downstream increase in STAT5 phosphorylation following stimulation with IL-15, compared to TMEM cells [2,5,60,63,64,65]. Although the selective impact of cytokines on TVM cells may correspond in part to changes in cytokine receptor expression, age-related changes in TVM cell frequency and function may also be explained by an increase in the levels of these cytokines with aging. For example, there is evidence for elevated IL-15, IL-6, IL-18 and TNF cytokine levels with advanced age, as part of the ‘inflammaging’ process [66,67,68,69]. Outside of IL-15, TVM cells from both mice and humans can be directly activated by other cytokines. Previous in vitro studies of TVM cells have shown that IFNγ production in these cells can be driven by IL-12 and IL-18 stimulation and result in an antigen-independent acquisition of cytotoxic capacity [6,20,60].
The role of cytokines in mediating the expansion and effector function of TVM cells is further reinforced in vivo in the context of infection. Baez and colleagues demonstrated that mice infected with Trypanosoma cruzi showed enhanced expansion of CD44hi CD8+ T cells, owing to increased levels of thymic IL-15 and IL-4, which ultimately promoted antigen-independent proliferation and subsequent protection from parasitemia by this population [70] (Figure 2). Furthermore, the ability of CD44hiNKG2D+ CD8+ T cells to directly kill Listeria monocytogenes-infected target cells occurred independently of strong TCR signalling, but was instead NKG2D-dependent and promoted by direct cytokine exposure (IL-12, IL-15 and IL-18) [71] (Figure 2). This innate-like response was required for effective bacterial clearance during the acute stages of infection [71]. In the context of helminth infections, studies have shown that the robust IL-4 production following infection of B6 or BALB/c mice drives antigen-independent TVM cell expansion, which in turn offered significant protection following subsequent viral or bacterial infections, via either innate or antigen-specific mechanisms [7,8,70].
Given the heightened cytokine sensitivity of TVM cells, it will be of interest to determine whether changes in the cytokine milieu associated with infections over a life course, or that occur as a natural part of the aging process (‘inflammaging’), are responsible for changes in TVM cell number and function with age. In this way, it seems possible that the same cytokine responsiveness that may impart the rapid responses and semi-differentiated phenotype in TVM cells from young mice and humans, might also be responsible for the acquisition of a senescent phenotype in advanced age.
6. NKR Expression as Markers of Functionality and Senescence on TVM Cells
It has been shown in both mice and humans that a subset of memory phenotype CD8+ T cells express NK cell markers, with their frequencies increasing as a result of the ageing process [72,73,74] as well as during infection [75,76]. TVM cells have been reported to express NKRs [20] and appear to demarcate a subset of functionally distinct cells within this population. Following TCR-mediated stimulation, human CD8+ T cells expressing NKRs, particular inhibitory NKRs such as NKG2A, KIR2DL and KIR3DL, show reduced effector cytokine production, compared to NKR− CD8+ T cells, [77]. Despite this, KIR/NKG2A+ CD8+ T cells, in contrast to their KIR/NKG2A− counterparts, were able to elicit strong TCR-independent cytotoxic responses via CD16 ligation and enhanced degranulation upon recognition of MHC class I-deficient target cells [26]. Thus, at a functional level, NKR-expressing CD8+ T cells appear to display reduced TCR− mediated responses coincident with heightened innate responsiveness.
T cell expression of NKRs is broadly used as an indicator of cellular senescence. As reviewed by Michel and colleagues, the expression of NKRs on CD8+ T cells has been linked with classical properties of senescence, including increased expression of cell cycle arrest molecules, such as p53, as well as the markers KLRG1 and CD57, which have been shown to be expressed on terminally differentiated T cells which show poor TCR-dependent signalling activity [78]. Certainly, senescent cells, including senescent TVM cells in aged mice, are characterised both by an upregulation of NKRs and enhanced longevity associated with high Bcl-2 expression [2]. Studies of both mouse and human T cells have shown that expression of inhibitory NKRs results in a decrease in activation-induced cell death in vitro [72,79,80,81]. Moreover, transgenic mice expressing the human KIR2DL3 and HLA-Cw3 ligand showed that ligation of inhibitory KIRs by MHC class I directly resulted in an in vivo accumulation of TVM-like cells [72], directly demonstrating a linkage between NKR expression on T cells and enhanced survival, a key characteristic of senescent cells.
NKG2D is another important NKR that can be upregulated on both conventional TMEM cells and TVM cells after activation, and is typically associated with a senescent phenotype when expressed on CD8+ T cells [82]. However, despite being a marker of senescence and TCR-mediated dysfunction, the activity of NKG2D on these cells actually facilitates enhanced innate responsiveness. TCR-independent cytotoxicity has been observed following NKG2D engagement on memory phenotype CD8+ T cells, through measurement of increased granzyme and perforin production [26,83,84]. In addition, NKG2D has been shown to be expressed on senescent human CD8+ T cells and, through its interaction with the ITAM-containing DAP12 protein and Sestrin-2 (SESN2), was able to result in direct activation, cytokine production and cytotoxicity following ligation with its ligand, MICA, or through stimulation with anti-NKG2D antibody [45]. The SESN2-mediated augmentation of NKG2D expression and signalling, along with other NKRs, was concomitant with a downregulation of TCR-mediated signalling. Accordingly, inhibition of SESN2 resulted in a restoration of TCR-mediated signalling and a reduction in NKG2D-mediated, antigen-independent effector function. In mice, this reciprocal TCR-mediated and innate functionality of T cells is exemplified in a study by Anfossi and colleagues who showed that expression of inhibitory Ly49 molecules diminished TCR-mediated T cell activation without loss of sensitivity to IL-15-mediated activation [85]. These data provide a mechanistic explanation for the aforementioned observations of reduced TCR-mediated signalling in T cells expressing NKRs and indicate a reciprocal relationship between innate and adaptive functionality in CD8+ MP T cells.
This reciprocal functionality is relevant to the remodelling of function that is observed in both highly differentiated CD8+ TEMRA cells in humans (that contain the TVM population) and in TVM cells from aged mice. Namely, the heightened expression of NKRs and sensitivity to cytokines such as IL-15 in advanced age, likely indicates a transition from classical TCR mediated functionality in young individuals, to a primary role in innate effector function, which has been proposed to be beneficial for tumour surveillance and the elimination of senescent cells in tissues [45]. These data highlight the importance of understanding the changing dynamic of T cell stimuli and effector functions over time, and the consideration that the observed ‘senescence’ of CD8+ T cells may simply indicate an advantageous functional adaptation in advanced age.
7. Transcriptional Regulation of TVM Development and Function
The formation of long-lived CD8+ T cell memory after primary responses relies heavily on the binding of early transcription factors (TFs), such as RUNX3, TCF-1 and FOXO1, at pro-memory genes [86,87,88] facilitating chromatin remodelling. These early changes allow memory CD8+ T cells to adopt a ‘poised’ chromatin conformation, allowing rapid recall responses via binding of TFs, such as T-bet, at effector gene loci [87]. Similarly, particular TFs have also been implicated in the development or differentiation of TVM cells. As mentioned earlier, the T-box TF Eomes is highly expressed by TVM cells in mice and humans and is critical for TVM formation [6,17]. Importantly these studies demonstrated that early Eomes upregulation in developing CD8+ T cells in the thymus prior their adoption of a memory-like (CD44hiCD122+) phenotype is critical for their development [53]. Other groups have also shown that overexpression of Eomes alone was sufficient to drive antigen inexperienced cells towards a memory phenotype [89]. These studies not only solidify the importance of Eomes in TVM cell development, but also suggest that these cells represent a distinct lineage of CD8+ T development (rather than an activation or differentiation stage) that is ratified in the periphery through IL-15 exposure [53]. This substantially changes the paradigm for TVM cell development, which previously considered these cells to arise largely as a consequence of homeostatic proliferation.
Eomes expression likely drives TVM differentiation via its promotion of CD122 expression by binding directly to the il2rb promoter [53,56,90]. In addition, it has been shown that Eomes is important for the programming of active enhancers in antigen-naïve MP cells, including TVM cells [89], via binding to RUNX3-bound enhancers, allowing for the chromatin remodelling protein BRG1 to bind and facilitate loop formation. RUNX3 is a critical transcription factor responsible for memory cell reprogramming following TCR stimulation via repression of gene transcription associated with terminal differentiation [88].
TVM development also appears to be controlled by negative transcriptional regulators, such as Bcl11b. Bcl11b is first expressed at the DN2 stage of thymocyte development and its maintenance is required to suppress innate gene expression, including some NKRs and Runx3 [41]. Despite its critical role in regulating positive and negative selection within the thymus [91,92], mice lacking a functional form of Bcll1b accumulated thymocytes and peripheral CD8+ T cells with a memory phenotype (CD44hiCD122hi) that remain functionally active [40,41,42]. These MP cells upregulated transcripts of NKRs as well as increased Eomes expression contributing to their high expression of CD122 [40]. The increase of these cells was found to be due to a cell intrinsic action of Bcl11b and not secondary to elevated thymic IL-4 availability as observed in other genetically modified mouse models [29,34,36,37,38,39,44]. Thus, TVM cell differentiation appears to be tightly controlled through the coordinated action of both positive and negative transcriptional regulation acting in the thymus during T cell development.
8. Epigenetic Regulation of TVM Cells and Age-Related T Cell Dysfunction
The epigenome has been shown to be a critical determinant of CD8+ T cell development as well as the ability of CD8+ T cells to mount a robust antigen-specific effector response to infections such as Influenza A virus [93,94,95,96,97]. However, little is currently known about the role of epigenetics in the development of TVM CD8+ T cells, particularly the development and changing function of TVM cells with age [3,6,18,44]. In the previously mentioned study by Smith and colleagues, it was demonstrated that TVM cell developmental timing determined responsiveness to effector cues and also impacted TVM cell epigenetic identity [43]. The heightened semi-differentiated phenotype seen in day 1 compared to day 28 TVM cells was associated with an increase in chromatin accessibility at gene loci associated with effector function (e.g., gzma, Ifng, tbx21) facilitating rapid transcription. These epigenetic changes resulted in increases in granzyme B and IFNγ production as well as increases in early response to bacterial challenge [43]. Thus, day 1 TVM cells displayed an epigenome pre-programmed towards a SLEC response rather than an MPEC response [98]. These data indicate that TVM functionality, and dysfunction, is likely controlled via underlying epigenetic mechanisms reflected by chromatin accessibility.
Of particular interest is whether the key functional changes associated with TVM cells in aged mice (>18 months), namely the development of cellular senescence and the retention of cytokine responsiveness, are also reflected by epigenetic changes. Further, it is unclear whether the epigenome of aged TVM cells reflects that which is typically observed in conventional memory T cells that have undergone proliferative senescence [99,100]. Broadly, ageing cells tend to exhibit many different epigenetic changes, including the loss of histone proteins, imbalances in post translational modifications, site-specific gains in heterochromatin and aberrant changes in chromatin accessibility [101]. All of these changes result in chromatin instability, transcriptional noise and impaired DNA damage repair all of which contribute to the accumulation of senescent cells as well as the pathology observed with age [102,103]. In an effort to understand the role of epigenetics in the regulation of T-cell specific age-related changes, studies have used ATAC-seq to examined the changes in CD8+ T cell chromatin accessibility [104,105]. These experiments demonstrated concentrated changes in chromatin accessibility within aged CD8+ T cells, specifically those possessing a memory phenotype. Moskowitz et al demonstrated that CD8+ TN and TCM cells adopted a chromatin organisation more akin to differentiated CD8+ T cells via their increased accessibility of the bZIP family of transcription proteins, such as BATF, which is critical for effector differentiation [106].
Another significant observation made by these studies was a broad preferential loss in accessibility at promoters rather than enhancers4, with affected promoters enriched for NRF1 binding [105] as well as the observed closing at the il7r gene promoter [104]. Sites of chromatin closing were also found to be enriched for genes associated with TCR signalling, emphasising the profound effect ageing has on normal T cell function. These studies have provided powerful insight into the molecular mechanisms that underpin human CD8+ T cell ageing, but detailed studies on the epigenetics of dysfunctional CD8+ T cell subsets, such as TVM cells, are lacking.
Although clearly important in the differentiation of TN cells to effector T cells and then in the establishment of antigen-specific memory, the role of histone post-translational modifications (PTMs) on TVM cell development and function is poorly studied. However, one key study has revealed an important role for the H3K79 methyltransferase, DOT1L, in TVM cell development [107]. In this study, it was demonstrated that, in the absence of DOT1L from T cells, almost all CD8+ T cells exhibited a TVM cell phenotype. Due to the almost complete absence of circulating TN cells in these mice, the authors suggested a role for H3K79me in sustaining the naive state in CD8+ T cells, and that deletion of DOT1L may drive cells down a semi-differentiated state independently of antigen exposure [107]. These TVM-like cells also appeared during thymic development, suggesting that, similar to Bcl11b, Dot1L is required to suppress development of T cells down a TVM lineage. Further, detailed studies investigating histone PTMs, and epigenetic changes more generally, in the development and maintenance of TVM cells is essential for our understanding of the biology of this population and, by extension and comparison, other CD8+ T cell populations.
9. Concluding Remarks
As new information emerges it is becoming clearer that memory phenotype TVM cells have a unique function within the immune system, that may evolve quite dramatically over a life course. The identification of this distinct CD8+ T cell lineage necessitates a re-evaluation of many of the current paradigms associated with conventional antigen-experienced memory CD8+ T cells. Further study should ascertain the heterogeneity of phenotype and function within the TVM population, which should in turn allow for a more precise definition of this population, especially in the human context. Finally, the recent delineation of TVM cells as a distinct developmental pathway, and the identification of several transcriptional and epigenetic controllers of this fate, has highlighted the active mechanisms in place to maintain the naïve CD8+ T cell state and prevent excessive T cell activation/innate functionality. Thus, further investigation of TVM populations, while inherently important for our understanding of TVM cell biology, should also serve to advance our understanding of molecular controls that are essential for maintaining adaptive functionality and naïve state of conventional CD8+ T cells.
Author Contributions
All authors contributed to the conceptualization and writing of this review. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by funding from the Australian Research Council and the Australian National Health and Medical Research Council.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sallusto F., Lenig D., Förster R., Lipp M., Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nat. Cell Biol. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 2.Quinn K.M., Fox A., Harland K.L., Russ B.E., Li J., Nguyen T.H.O., Loh L., Olshanksy M., Naeem H., Tsyganov K., et al. Age-Related Decline in Primary CD8+ T Cell Responses Is Associated with the Development of Senescence in Virtual Memory CD8+ T Cells. Cell Rep. 2018;23:3512–3524. doi: 10.1016/j.celrep.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 3.Quinn K.M., Hussain T., Kraus F., Formosa L.E., Lam W.K., Dagley M.J., Saunders E.C., Assmus L.M., Wynne-Jones E., Loh L., et al. Metabolic characteristics of CD8+ T cell subsets in young and aged individuals are not predictive of functionality. Nat. Commun. 2020;11:1–13. doi: 10.1038/s41467-020-16633-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Decman V., Laidlaw B.J., Doering T.A., Leng J., Ertl H.C.J., Goldstein D.R., Wherry E.J. Defective CD8 T Cell Responses in Aged Mice Are Due to Quantitative and Qualitative Changes in Virus-Specific Precursors. J. Immunol. 2012;188:1933–1941. doi: 10.4049/jimmunol.1101098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Haluszczak C., Akue A.D., Hamilton S.E., Johnson L.D., Pujanauski L., Teodorovic L., Jameson S.C., Kedl R.M. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory phenotype cells bearing markers of homeostatic expansion. J. Exp. Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee J.-Y., Hamilton S.E., Akue A.D., Hogquist K.A., Jameson S.C. Virtual memory CD8 T cells display unique functional properties. Proc. Natl. Acad. Sci. USA. 2013;110:13498–13503. doi: 10.1073/pnas.1307572110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rolot M., Dougall A.M., Chetty A., Javaux J., Chen T., Xiao X., Machiels B., Selkirk M.E., Maizels R.M., Hokke C., et al. Helminth-induced IL-4 expands bystander memory CD8+ T cells for early control of viral infection. Nat. Commun. 2018;9:1–16. doi: 10.1038/s41467-018-06978-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin J.S., Mohrs K., Szaba F.M., Kummer L.W., Leadbetter E.A., Mohrs M. Virtual memory CD8 T cells expanded by helminth infection confer broad protection against bacterial infection. Mucosal Immunol. 2018;12:258–264. doi: 10.1038/s41385-018-0100-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang X., Waschke B.C., Woolaver R.A., Chen S.M.Y., Chen Z., Wang J.H. MHC class I-independent activation of virtual memory CD8 T cells induced by chemotherapeutic agent-treated cancer cells. Cell. Mol. Immunol. 2020:1–12. doi: 10.1038/s41423-020-0463-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rufer N., Brümmendorf T.H., Kolvraa S., Bischoff C., Christensen K., Wadsworth L., Schulzer M., Lansdorp P.M. Telomere Fluorescence Measurements in Granulocytes and T Lymphocyte Subsets Point to a High Turnover of Hematopoietic Stem Cells and Memory T Cells in Early Childhood. J. Exp. Med. 1999;190:157–168. doi: 10.1084/jem.190.2.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cayssials E., Jacomet F., Piccirilli N., Lefèvre L., Roy L., Guilhot F., Chomel J., Leleu X., Gombert J., Herbelin A., et al. Sustained treatment-free remission in chronic myeloid leukaemia is associated with an increased frequency of innate CD8(+) T-cells. Br. J. Haematol. 2019;186:54–59. doi: 10.1111/bjh.15858. [DOI] [PubMed] [Google Scholar]
- 12.Kaech S.M., Ahmed R. Memory CD8+ T cell differentiation: Initial antigen encounter triggers a developmental program in naïve cells. Nat. Immunol. 2001;2:415–422. doi: 10.1038/87720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wherry E.J., Ahmed R. Memory CD8 T-Cell Differentiation during Viral Infection. J. Virol. 2004;78:5535–5545. doi: 10.1128/JVI.78.11.5535-5545.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Harty J.T., Badovinac V.P. Shaping and reshaping CD8+ T-cell memory. Nat. Rev. Immunol. 2008;8:107–119. doi: 10.1038/nri2251. [DOI] [PubMed] [Google Scholar]
- 15.Kieper W.C., Jameson S.C. Homeostatic expansion and phenotypic conversion of naïve T cells in response to self peptide/MHC ligands. Proc. Natl. Acad. Sci. USA. 1999;96:13306–13311. doi: 10.1073/pnas.96.23.13306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldrath A.W., Bogatzki L.Y., Bevan M.J. Naive T Cells Transiently Acquire a Memory-like Phenotype during Homeostasis-Driven Proliferation. J. Exp. Med. 2000;192:557–564. doi: 10.1084/jem.192.4.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sosinowski T., White J.T., Cross E.W., Haluszczak C., Marrack P., Gapin L., Kedl R.M. CD8α+Dendritic CellTransPresentation of IL-15 to Naive CD8+T Cells Produces Antigen-Inexperienced T Cells in the Periphery with Memory Phenotype and Function. J. Immunol. 2013;190:1936–1947. doi: 10.4049/jimmunol.1203149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chiu B.-C., Martin B.E., Stolberg V.R., Chensue S.W. Cutting Edge: Central Memory CD8 T Cells in Aged Mice Are Virtual Memory Cells. J. Immunol. 2013;191:5793–5796. doi: 10.4049/jimmunol.1302509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Judge A.D., Zhang X., Fujii H., Surh C.D., Sprent J. Interleukin 15 Controls both Proliferation and Survival of a Subset of Memory-Phenotype CD8+ T Cells. J. Exp. Med. 2002;196:935–946. doi: 10.1084/jem.20020772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.White J.T., Cross E.W., Burchill M.A., Danhorn T., McCarter M.D., Rosen H.R., O’Connor B., Kedl R.M. Virtual memory T cells develop and mediate bystander protective immunity in an IL-15-dependent manner. Nat. Commun. 2016;7:11291. doi: 10.1038/ncomms11291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jameson S.C. Maintaining the norm: T-cell homeostasis. Nat. Rev. Immunol. 2002;2:547–556. doi: 10.1038/nri853. [DOI] [PubMed] [Google Scholar]
- 22.Surh C.D., Sprent J. Homeostasis of Naive and Memory T Cells. Immunity. 2008;29:848–862. doi: 10.1016/j.immuni.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 23.Murali-Krishna K., Lau L.L., Sambhara S., Lemonnier F., Altman J., Ahmed R. Persistence of Memory CD8 T Cells in MHC Class I-Deficient Mice. Science. 1999;286:1377–1381. doi: 10.1126/science.286.5443.1377. [DOI] [PubMed] [Google Scholar]
- 24.Boyman O., Cho J.-H., Tan J.T., Surh C.D., Sprent J. A major histocompatibility complex class I–dependent subset of memory phenotype CD8+ cells. J. Exp. Med. 2006;203:1817–1825. doi: 10.1084/jem.20052495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurepa Z., Su J., Forman J. Memory phenotype of CD8+ T cells in MHC class Ia-deficient mice. J. Immunol. 2003;170:5414–5420. doi: 10.4049/jimmunol.170.11.5414. [DOI] [PubMed] [Google Scholar]
- 26.Jacomet F., Cayssials E., Basbous S., Levescot A., Piccirilli N., Desmier D., Robin A., Barra A., Giraud C., Guilhot F., et al. Evidence for eomesodermin-expressing innate-like CD8+KIR/NKG2A+T cells in human adults and cord blood samples. Eur. J. Immunol. 2015;45:1926–1933. doi: 10.1002/eji.201545539. [DOI] [PubMed] [Google Scholar]
- 27.Galletti G., De Simone G., Mazza E.M.C., Puccio S., Mezzanotte C., Bi T.M., Davydov A.N., Metsger M., Scamardella E., Alvisi G., et al. Two subsets of stem-like CD8+ memory T cell progenitors with distinct fate commitments in humans. Nat. Immunol. 2020:1–11. doi: 10.1038/s41590-020-0791-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Geginat J., Lanzavecchia A., Sallusto F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood. 2003;101:4260–4266. doi: 10.1182/blood-2002-11-3577. [DOI] [PubMed] [Google Scholar]
- 29.White J.T., Cross E.W., Kedl R.M. Antigen-inexperienced memory CD8+ T cells: Where they come from and why we need them. Nat. Rev. Immunol. 2017;17:391–400. doi: 10.1038/nri.2017.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Appay V., Dunbar P.R., Callan M., Klenerman P., Gillespie G.M.A., Papagno L., Ogg G.S., King A., Lechner F., Spina C.A., et al. Memory CD8+ T cells vary in differentiation phenotype in different persistent virus infections. Nat. Med. 2002;8:379–385. doi: 10.1038/nm0402-379. [DOI] [PubMed] [Google Scholar]
- 31.Henson S.M., Lanna A., Riddell N.E., Franzese O., Macaulay R., Griffiths S.J., Puleston D.J., Watson A.S., Simon A.K., Tooze S.A., et al. p38 signaling inhibits mTORC1-independent autophagy in senescent human CD8+ T cells. J. Clin. Investig. 2014;124:4004–4016. doi: 10.1172/JCI75051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zinser M., Highton A.J., Kurioka A., Kronsteiner B., Hagel J., Leng T., Marchi E., Phetsouphanh C., Willberg C.B., Dunachie S.J., et al. Human MAIT cells show metabolic quiescence with rapid glucose-dependent upregulation of granzyme B upon stimulation. Immunol. Cell Biol. 2018;96:666–674. doi: 10.1111/imcb.12020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tripathi P., Morris S.C., Perkins C., Sholl A., Finkelman F.D., Hildeman D.A. IL-4 and IL-15 promotion of virtual memory CD8+T cells is determined by genetic background. Eur. J. Immunol. 2016;46:2333–2339. doi: 10.1002/eji.201646404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lee Y.J., Holzapfel K.L., Zhu J., Jameson S.C., Hogquist K.A. Steady-state production of IL-4 modulates immunity in mouse strains and is determined by lineage diversity of iNKT cells. Nat. Immunol. 2013;14:1146–1154. doi: 10.1038/ni.2731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lai D., Zhu J., Wang T., Hu-Li J., Terabe M., Berzofsky J.A., Clayberger C., Krensky A.M. KLF13 sustains thymic memory-like CD8+ T cells in BALB/c mice by regulating IL-4–generating invariant natural killer T cells. J. Exp. Med. 2011;208:1093–1103. doi: 10.1084/jem.20101527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jameson S.C., Lee Y.J., Hogquist K.A. Innate Memory T cells. Adv. Immunol. 2015;126:173–213. doi: 10.1016/bs.ai.2014.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atherly L.O., Lucas J.A., Felices M., Yin C.C., Reiner S.L., Berg L.J. The Tec Family Tyrosine Kinases Itk and Rlk Regulate the Development of Conventional CD8+ T Cells. Immunology. 2006;25:79–91. doi: 10.1016/j.immuni.2006.05.012. [DOI] [PubMed] [Google Scholar]
- 38.Broussard C., Fleischecker C., Horai R., Chetana M., Venegas A.M., Sharp L.L., Hedrick S.M., Fowlkes B., Schwartzberg P.L. Altered Development of CD8+ T Cell Lineages in Mice Deficient for the Tec Kinases Itk and Rlk. Immunity. 2006;25:93–104. doi: 10.1016/j.immuni.2006.05.011. [DOI] [PubMed] [Google Scholar]
- 39.Weinreich M.A., Odumade O.A., Jameson S.C., Hogquist K.A. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat. Immunol. 2010;11:709–716. doi: 10.1038/ni.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hirose S., Touma M., Go R., Katsuragi Y., Sakuraba Y., Gondo Y., Abe M., Sakimura K., Mishima Y., Kominami R. Bcl11b prevents the intrathymic development of innate CD8 T cells in a cell intrinsic manner. Int. Immunol. 2014;27:205–215. doi: 10.1093/intimm/dxu104. [DOI] [PubMed] [Google Scholar]
- 41.Kastner P., Chan S., Vogel W.K., Zhang L.-J., Topark-Ngarm A., Golonzhka O., Jost B., Le Gras S., Gross M.K., Leid M. Bcl11b represses a mature T-cell gene expression program in immature CD4+CD8+ thymocytes. Eur. J. Immunol. 2010;40:2143–2154. doi: 10.1002/eji.200940258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hosokawa H., Romero-Wolf M., Yui M.A., Ungerback J., Quiloan M.L.G., Matsumoto M., Nakayama K.I., Tanaka T., Rothenberg E.V. Bcl11b sets pro-T cell fate by site-specific cofactor recruitment and by repressing Id2 and Zbtb16. Nat. Immunol. 2018;19:1427–1440. doi: 10.1038/s41590-018-0238-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Smith N.L., Patel R.K., Reynaldi A., Grenier J.K., Wang J., Watson N.B., Nzingha K., Mon K.J.Y., Peng S.A., Grimson A., et al. Developmental Origin Governs CD8+ T Cell Fate Decisions during Infection. Cell. 2018;174:117–130.e14. doi: 10.1016/j.cell.2018.05.029. [DOI] [PubMed] [Google Scholar]
- 44.Hussain T., Quinn K.M. Similar but different: Virtual memory CD8 T cells as a memory-like cell population. Immunol. Cell Biol. 2019;97:675–684. doi: 10.1111/imcb.12277. [DOI] [PubMed] [Google Scholar]
- 45.Pereira B.I., De Maeyer R.P.H., Covre L.P., Nehar-Belaid D., Lanna A., Ward S., Marches R., Chambers E.S., Gomes D.C.O., Riddell N.E., et al. Sestrins induce natural killer function in senescent-like CD8+ T cells. Nat. Immunol. 2020;21:684–694. doi: 10.1038/s41590-020-0643-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith T.R., Kumar V. Revival of CD8+ Treg–mediated suppression. Trends Immunol. 2008;29:337–342. doi: 10.1016/j.it.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 47.Dai Z., Wan N., Zhang S., Moore Y., Wan F. Cutting Edge: Programmed Death-1 Defines CD8+CD122+ T Cells as Regulatory versus Memory T Cells. J. Immunol. 2010;185:803–807. doi: 10.4049/jimmunol.1000661. [DOI] [PubMed] [Google Scholar]
- 48.Akane K., Kojima S., Mak T.W., Shiku H., Suzuki H. CD8+CD122+CD49dlow regulatory T cells maintain T-cell homeostasis by killing activated T cells via Fas/FasL-mediated cytotoxicity. Proc. Natl. Acad. Sci. USA. 2016;113:2460–2465. doi: 10.1073/pnas.1525098113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Quinn K.M., Zaloumis S.G., Cukalac T., Kan W.-T., Sng X.Y.X., Mirams M., Watson K.A., McCaw J.M., Doherty P.C., Thomas P.G., et al. Heightened self-reactivity associated with selective survival, but not expansion, of naïve virus-specific CD8+ T cells in aged mice. Proc. Natl. Acad. Sci. USA. 2016;113:1333–1338. doi: 10.1073/pnas.1525167113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moran A.E., Holzapfel K.L., Xing Y., Cunningham N.R., Maltzman J.S., Punt J., Hogquist K.A. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 2011;208:1279–1289. doi: 10.1084/jem.20110308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ashouri J.F., Weiss A. Endogenous Nur77 Is a Specific Indicator of Antigen Receptor Signaling in Human T and B Cells. J. Immunol. 2017;198:657–668. doi: 10.4049/jimmunol.1601301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Azzam H.S., Grinberg A., Lui K., Shen H., Shores E.W., Love P.E. CD5 Expression Is Developmentally Regulated By T Cell Receptor (TCR) Signals and TCR Avidity. J. Exp. Med. 1998;188:2301–2311. doi: 10.1084/jem.188.12.2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miller C.H., Klawon D.E.J., Zeng S., Lee V., Socci N.D., Savage P.A. Eomes identifies thymic precursors of self-specific memory-phenotype CD8+ T cells. Nat. Immunol. 2020;21:567–577. doi: 10.1038/s41590-020-0653-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rudd B.D., Venturi V., Li G., Samadder P., Ertelt J.M., Way S.S., Davenport M.P., Nikolich-Žugich J. Nonrandom attrition of the naive CD8+ T-cell pool with aging governed by T-cell receptor:pMHC interactions. Proc. Natl. Acad. Sci. USA. 2011;108:13694–13699. doi: 10.1073/pnas.1107594108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pearce E.L., Mullen A.C., Martins G.A., Krawczyk C.M., Hutchins A.S., Zediak V.P., Banica M., DiCioccio C.B., Gross D.A., Mao C.-A., et al. Control of Effector CD8+ T Cell Function by the Transcription Factor Eomesodermin. Science. 2003;302:1041–1043. doi: 10.1126/science.1090148. [DOI] [PubMed] [Google Scholar]
- 56.Intlekofer A.M., Takemoto N., Wherry E.J., A Longworth S., Northrup J.T., Palanivel V.R., Mullen A.C., Gasink C.R., Kaech S.M., Miller J.D., et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 2005;6:1236–1244. doi: 10.1038/ni1268. [DOI] [PubMed] [Google Scholar]
- 57.Gett A.V., Sallusto F., Lanzavecchia A., Geginat J. T cell fitness determined by signal strength. Nat. Immunol. 2003;4:355–360. doi: 10.1038/ni908. [DOI] [PubMed] [Google Scholar]
- 58.Martinet V., Tonon S., Torres D., Azouz A., Nguyen M., Köhler A., Flamand V., Mao C.-A., Klein W.H., Leo O., et al. Type I interferons regulate eomesodermin expression and the development of unconventional memory CD8+ T cells. Nat. Commun. 2015;6:7089. doi: 10.1038/ncomms8089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Xing Y., Wang X., Jameson S.C., A Hogquist Y.X.X.W.S.C.J.K. Late stages of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling. Nat. Immunol. 2016;17:565–573. doi: 10.1038/ni.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Akue A.D., Lee J.-Y., Jameson S.C. Derivation and Maintenance of Virtual Memory CD8 T Cells. J. Immunol. 2012;188:2516–2523. doi: 10.4049/jimmunol.1102213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin J.-H., Huang H.-H., Zhou M.-J., Li J., Hu W., Huang L., Xu Z., Tu B., Yang G., Shi M., et al. Virtual memory CD8+ T cells restrain the viral reservoir in HIV-1-infected patients with antiretroviral therapy through derepressing KIR-mediated inhibition. Cell. Mol. Immunol. 2020:1–9. doi: 10.1038/s41423-020-0408-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tan J.T., Ernst B., Kieper W.C., Leroy E., Sprent J., Surh C.D. Interleukin (IL)-15 and IL-7 Jointly Regulate Homeostatic Proliferation of Memory Phenotype CD8+ Cells but Are Not Required for Memory Phenotype CD4+ Cells. J. Exp. Med. 2002;195:1523–1532. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Berg R.E., Crossley E., Murray S., Forman J. Memory CD8+ T Cells Provide Innate Immune Protection against Listeria monocytogenes in the Absence of Cognate Antigen. J. Exp. Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Liu K., Catalfamo M., Li Y., Henkart P.A., Weng N.-P. IL-15 mimics T cell receptor crosslinking in the induction of cellular proliferation, gene expression, and cytotoxicity in CD8+ memory T cells. Proc. Natl. Acad. Sci. USA. 2002;99:6192–6197. doi: 10.1073/pnas.092675799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang X., Sun S., Hwang I., Tough D.F., Sprent J. Potent and Selective Stimulation of Memory-Phenotype CD8+ T Cells In Vivo by IL-15. Immunity. 1998;8:591–599. doi: 10.1016/S1074-7613(00)80564-6. [DOI] [PubMed] [Google Scholar]
- 66.Pangrazzi L., Naismith E., Meryk A., Keller M., Jenewein B., Trieb K., Grubeck-Loebenstein B. Increased IL-15 Production and Accumulation of Highly Differentiated CD8+ Effector/Memory T Cells in the Bone Marrow of Persons with Cytomegalovirus. Front. Immunol. 2017;8:715. doi: 10.3389/fimmu.2017.00715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ershler W.B., Keller E.T. Age-Associated Increased Interleukin-6 Gene Expression, Late-Life Diseases, and Frailty. Annu. Rev. Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- 68.Ferrucci L., Corsi A., Lauretani F., Bandinelli S., Bartali B., Taub D.D., Guralnik J.M., Longo D.L. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bruunsgaard H., Skinhoj P., Pedersen A.N., Schroll M., Pedersen B.K. Ageing, tumour necrosis factor-alpha (TNF-alpha) and atherosclerosis. Clin. Exp. Immunol. 2000;121:255–260. doi: 10.1046/j.1365-2249.2000.01281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baez N.S., Cerbán F., Savid-Frontera C., Hodge D.L., Tosello J., Acosta-Rodriguez E., Almada L., Gruppi A., Viano M.E., Young H.A., et al. Thymic expression of IL-4 and IL-15 after systemic inflammatory or infectious Th1 disease processes induce the acquisition of "innate" characteristics during CD8+ T cell development. PLOS Pathog. 2019;15:e1007456. doi: 10.1371/journal.ppat.1007456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Chu T., Tyznik A.J., Roepke S., Berkley A.M., Woodward-Davis A., Pattacini L., Bevan M.J., Zehn D., Prlic M. Bystander-Activated Memory CD8 T Cells Control Early Pathogen Load in an Innate-like, NKG2D-Dependent Manner. Cell Rep. 2013;3:701–708. doi: 10.1016/j.celrep.2013.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ugolini S., Arpin C., Anfossi N., Walzer T., Cambiaggi A., Förster R., Lipp M., Toes R.E.M., Melief C.J., Marvel J., et al. Involvement of inhibitory NKRs in the survival of a subset of memory-phenotype CD8+ T cells. Nat. Immunol. 2001;2:430–435. doi: 10.1038/87740. [DOI] [PubMed] [Google Scholar]
- 73.Anfossi N., Pascal V., Vivier E., Ugolini S. Biology of T memory type 1 cells. Immunol. Rev. 2001;181:269–278. doi: 10.1034/j.1600-065X.2001.1810123.x. [DOI] [PubMed] [Google Scholar]
- 74.Coles M.C., McMahon C.W., Takizawa H., Raulet D.H. Memory CD8 T lymphocytes express inhibitory MHC-specific Ly49 receptors. Eur. J. Immunol. 2000;30:236–244. doi: 10.1002/1521-4141(200001)30:1<236::AID-IMMU236>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 75.Kambayashi T., Assarsson E., Chambers B.J., Ljunggren H.-G. Expression of the DX5 antigen on CD8+ T cells is associated with activation and subsequent cell death or memory during influenza virus infection. Eur. J. Immunol. 2001;31:1523–1530. doi: 10.1002/1521-4141(200105)31:5<1523::AID-IMMU1523>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 76.Bonorino P., Leroy V., Dufeu-Duchesne T., Tongiani-Dashan S., Sturm N., Pernollet M., Vivier E., Zarski J.-P., Marche P.N., Jouvin-Marche E. Features and distribution of CD8 T cells with human leukocyte antigen class I-specific receptor expression in chronic hepatitis C. Hepatology. 2007;46:1375–1386. doi: 10.1002/hep.21850. [DOI] [PubMed] [Google Scholar]
- 77.Björkström N.K., Béziat V., Cichocki F., Liu L.L., Levine J., Larsson S., Koup R.A., Anderson S.K., Ljunggren H.-G., Malmberg K.-J. CD8 T cells express randomly selected KIRs with distinct specificities compared with NK cells. Blood. 2012;120:3455–3465. doi: 10.1182/blood-2012-03-416867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Michel J.J., Griffin P., Vallejo A.N. Functionally Diverse NK-Like T Cells Are Effectors and Predictors of Successful Aging. Front. Immunol. 2016;7:530. doi: 10.3389/fimmu.2016.00530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Young N.T., Uhrberg M., Phillips J.H., Lanier L.L., Parham P. Differential Expression of Leukocyte Receptor Complex-Encoded Ig-Like Receptors Correlates with the Transition from Effector to Memory CTL. J. Immunol. 2001;166:3933–3941. doi: 10.4049/jimmunol.166.6.3933. [DOI] [PubMed] [Google Scholar]
- 80.Roger J., Chalifour A., Lemieux S., Duplay P. Cutting edge: Ly49A inhibits TCR/CD3-induced apoptosis and IL-2 secretion. J. Immunol. 2001;167:6–10. doi: 10.4049/jimmunol.167.1.6. [DOI] [PubMed] [Google Scholar]
- 81.Alter G., Rihn S., Streeck H., Teigen N., Piechocka-Trocha A., Moss K., Cohen K., Meier A., Pereyra F., Walker B., et al. Ligand-Independent Exhaustion of Killer Immunoglobulin-Like Receptor-Positive CD8+ T Cells in Human Immunodeficiency Virus Type 1 Infection. J. Virol. 2008;82:9668–9677. doi: 10.1128/JVI.00341-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Prajapati K., Perez C., Rojas L.B.P., Burke B., Guevara-Patino J.A. Functions of NKG2D in CD8+ T cells: An opportunity for immunotherapy. Cell. Mol. Immunol. 2018;15:470–479. doi: 10.1038/cmi.2017.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wong H.C., Jeng E.K., Rhode P.R. The IL-15-based superagonist ALT-803 promotes the antigen-independent conversion of memory CD8+T cells into innate-like effector cells with antitumor activity. OncoImmunology. 2013;2:e26442. doi: 10.4161/onci.26442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tietze J.K., Wilkins D.E.C., Sckisel G.D., Bouchlaka M.N., Alderson K.L., Weiss J.M., Ames E., Bruhn K.W., Craft N., Wiltrout R.H., et al. Delineation of antigen-specific and antigen-nonspecific CD8+ memory T-cell responses after cytokine-based cancer immunotherapy. Blood. 2012;119:3073–3083. doi: 10.1182/blood-2011-07-369736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Anfossi N., Robbins S.H., Ugolini S., Georgel P., Hoebe K., Bouneaud C., Ronet C., Kaser A., DiCioccio C.B., Tomasello E., et al. Expansion and Function of CD8+T Cells Expressing Ly49 Inhibitory Receptors Specific for MHC Class I Molecules. J. Immunol. 2004;173:3773–3782. doi: 10.4049/jimmunol.173.6.3773. [DOI] [PubMed] [Google Scholar]
- 86.He B., Xing S., Chen C., Gao P., Teng L., Shan Q., Gullicksrud J.A., Martin M.D., Yu S., Harty J.T., et al. CD8+ T Cells Utilize Highly Dynamic Enhancer Repertoires and Regulatory Circuitry in Response to Infections. Immunity. 2016;45:1341–1354. doi: 10.1016/j.immuni.2016.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Chang J.T., Wherry E.J., Goldrath B.Y.J.J.M.C.T.A.W. Molecular regulation of effector and memory T cell differentiation. Nat. Immunol. 2014;15:1104–1115. doi: 10.1038/ni.3031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang D., Diao H., Getzler A.J., Rogal W., Frederick M.A., Milner J., Yu B., Crotty S., Goldrath A.W., Pipkin M.E. The Transcription Factor Runx3 Establishes Chromatin Accessibility of cis-Regulatory Landscapes that Drive Memory Cytotoxic T Lymphocyte Formation. Immunity. 2018;48:659–674.e6. doi: 10.1016/j.immuni.2018.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Istaces N., Splittgerber M., Silva V.L., Nguyen M., Thomas S., Le A., Achouri Y., Calonne E., Defrance M., Fuks F., et al. EOMES interacts with RUNX3 and BRG1 to promote innate memory cell formation through epigenetic reprogramming. Nat. Commun. 2019;10:1–17. doi: 10.1038/s41467-019-11233-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Gordon S.M., Carty S.A., Kim J.S., Zou T., Smith-Garvin J., Alonzo E.S., Haimm E., Sant’Angelo D.B., Koretzky G.A., Reiner S.L., et al. Requirements for eomesodermin and promyelocytic leukemia zinc finger in the development of innate-like CD8+ T cells. J. Immunol. 2011;186:4573–4578. doi: 10.4049/jimmunol.1100037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Albu D.I., Feng D., Bhattacharya D., Jenkins N.A., Copeland N.G., Liu P., Avram D. BCL11B is required for positive selection and survival of double-positive thymocytes. J. Exp. Med. 2007;204:3003–3015. doi: 10.1084/jem.20070863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Avram D., Califano D. The Multifaceted Roles of Bcl11b in Thymic and Peripheral T Cells: Impact on Immune Diseases. J. Immunol. 2014;193:2059–2065. doi: 10.4049/jimmunol.1400930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Barrero M.J., Boué S., Belmonte J.C.I. Epigenetic Mechanisms that Regulate Cell Identity. Cell Stem Cell. 2010;7:565–570. doi: 10.1016/j.stem.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 94.Allis C.D., Jenuwein T. The molecular hallmarks of epigenetic control. Nat. Rev. Genet. 2016;17:487–500. doi: 10.1038/nrg.2016.59. [DOI] [PubMed] [Google Scholar]
- 95.Russ B.E., Olshanksy M., Smallwood H.S., Li J., Denton A.E., Prier J.E., Stock A.T., Croom H.A., Cullen J.G., Nguyen M.L.T., et al. Distinct Epigenetic Signatures Delineate Transcriptional Programs during Virus-Specific CD8+ T Cell Differentiation. Immunity. 2014;41:853–865. doi: 10.1016/j.immuni.2014.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Araki Y., Wang Z., Zang C., Wood W.H., E Schones D., Cui K., Roh T.-Y., Lhotsky B., Wersto R.P., Peng W., et al. Genome-wide Analysis of Histone Methylation Reveals Chromatin State-Based Regulation of Gene Transcription and Function of Memory CD8+ T Cells. Immunity. 2009;30:912–925. doi: 10.1016/j.immuni.2009.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Henning A.N., Roychoudhuri R., Restifo N.P. Epigenetic control of CD8+ T cell differentiation. Nat. Rev. Immunol. 2018;18:340–356. doi: 10.1038/nri.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Joshi N.S., Cui W., Chandele A., Lee H.K., Urso D.R., Hagman J., Gapin L., Kaech S.M. Inflammation Directs Memory Precursor and Short-Lived Effector CD8+ T Cell Fates via the Graded Expression of T-bet Transcription Factor. Immunity. 2007;27:281–295. doi: 10.1016/j.immuni.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Chou J.P., Effros R.B. T CELL REPLICATIVE SENESCENCE IN HUMAN AGING. Curr. Pharm. Des. 2013;19:1680–1698. doi: 10.2174/138161213805219711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hodes R.J., Hathcock K.S., Weng N.-P. Telomeres in T and B cells. Nat. Rev. Immunol. 2002;2:699–706. doi: 10.1038/nri890. [DOI] [PubMed] [Google Scholar]
- 101.Sen P., Shah P.P., Nativio R., Berger S.L. Epigenetic Mechanisms of Longevity and Aging. Cell. 2016;166:822–839. doi: 10.1016/j.cell.2016.07.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.López-Otín C., Blasco M.A., Partridge L., Serrano M., Kroemer G. The Hallmarks of Aging. Cell. 2013;153:1194–1217. doi: 10.1016/j.cell.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rando T.A., Chang H.Y. Aging, Rejuvenation, and Epigenetic Reprogramming: Resetting the Aging Clock. Cell. 2012;148:46–57. doi: 10.1016/j.cell.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ucar D., Márquez E.J., Chung C.-H., Marches R., Rossi R.J., Uyar A., Wu T.-C., George J., Stitzel M.L., Palucka A.K., et al. The chromatin accessibility signature of human immune aging stems from CD8+ T cells. J. Exp. Med. 2017;214:3123–3144. doi: 10.1084/jem.20170416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Moskowitz D.M., Zhang D.W., Hu B., Le Saux S., Yanes R.E., Ye Z., Buenrostro J.D., Weyand C.M., Greenleaf W.J., Goronzy J.J. Epigenomics of human CD8 T cell differentiation and aging. Sci. Immunol. 2017;2:eaag0192. doi: 10.1126/sciimmunol.aag0192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kurachi M., Barnitz R.A., Yosef N., Odorizzi P.M., Dilorio M.A., Lemieux M.E., Yates K., Godec J., Klatt M.G., Regev A., et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat. Immunol. 2014;15:373–383. doi: 10.1038/ni.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kwesi-Maliepaard E.M., Aslam M.A., Alemdehy M.F., Brand T.V.D., McLean C., Vlaming H., Van Welsem T., Korthout T., Lancini C., Hendriks S., et al. The histone methyltransferase DOT1L prevents antigen-independent differentiation and safeguards epigenetic identity of CD8+T cells. Proc. Natl. Acad. Sci. USA. 2020;117:20706–20716. doi: 10.1073/pnas.1920372117. [DOI] [PMC free article] [PubMed] [Google Scholar]