Abstract
Simple Summary
The effects of two different concentrations of micro capsuled oregano essential oil (OEO) and purple garlic powder on biomarkers of oxidative status, stress, and inflammation, as well as on average daily gain (ADG) and feed conversion ratio (FCR), were evaluated in piglets during the postweaning period. The trial was carried out with 300 crossbred pigs of 21 days of age fed with different concentrations of OEO and purple garlic powder and ZnO. Saliva and serum samples were taken to evaluate a panel of biomarkers of oxidative status, stress, and inflammation. OEO and garlic powder at 0.4% did not produce significant changes in C-reactive protein (CRP) and cortisol and yielded higher levels of the antioxidant biomarker CUPRAC in serum than higher doses (p < 0.01); they also yielded a better ADG than the control and ZnO diets. OEO and garlic powder at higher concentrations than 0.4% showed higher concentrations of CRP (p < 0.05). Overall, doses of OEO and garlic powder at 0.4% did not lead to inflammation, stress, or negative changes in oxidative biomarkers in piglets during the postweaning period and gave better productive performance than the control and ZnO diets. High doses of OEO and garlic powder were ineffective.
Abstract
The effects of two different concentrations of micro capsuled oregano essential oil (OEO) and purple garlic powder on biomarkers of oxidative status, stress, and inflammation, as well as on average daily gain (ADG) and feed conversion ratio (FCR), were evaluated in piglets during the postweaning period. The trial was carried out with 300 crossbred pigs of 21 days of age fed with different concentrations of OEO and purple garlic powder and ZnO. Saliva and serum samples were taken to evaluate a panel of biomarkers of oxidative status, stress, and inflammation. OEO and garlic powder at 0.4% did not produce significant changes in C-reactive protein (CRP) and cortisol and yielded higher levels of the antioxidant biomarker CUPRAC in serum than higher doses (p < 0.01); they yielded a better ADG than the control and ZnO diets. OEO and garlic powder at higher concentrations than 0.4% showed higher concentrations of CRP (p < 0.05). Overall, doses of OEO and garlic powder at 0.4% did not lead to inflammation, stress, or negative changes in oxidative biomarkers in piglets during the postweaning period and gave better productive performance than the control and ZnO diets. High doses of OEO and garlic powder were ineffective and could negatively affect the animals. Therefore, our results highlight the importance of the dose used when OEO or garlic are supplemented to piglets.
Keywords: micro capsuled oregano essential oil, weaned piglets, purple garlic powder, biomarkers, pig production, oxidative status
1. Introduction
Weaning is a critical period in pig production that determines the productive performance of commercial farms. Practices such as mixing and changes in nutrition and environment can negatively affect the endocrine functions, growth, and welfare of weaned piglets [1,2]. Therefore, weaning is a stressful period that produces increases in salivary stress markers such as cortisol [3,4,5]. It also induces changes in oxidative stress markers and intestinal dysfunctions [6]. In addition, mucosal inflammation, digestive performance, and body weight gain are also affected by early weaning [7,8,9].
In order to reduce the impact of the abovementioned issues, therapeutic dietary addition of zinc as zinc oxide (ZnO) (2000 to 4000 mg/kg) has been widely used during the postweaning period, being effective in reducing diarrhoea and digestive dysfunctions [9,10,11,12,13]. ZnO improves postweaning performance and reduces the effects of postweaning colibacillosis by inhibiting cAMP-stimulated chloride secretion [14]. Bactericidal activity of ZnO has been observed in vitro, and, therefore, therapeutic levels of ZnO may reduce enterotoxigenic Escherichia coli (ETEC) colonization and bacterial population in the intestine [15]. Nevertheless, the use of ZnO at therapeutic levels has serious drawbacks such as environmental pollution due to the high proportion excreted in faeces [9,16,17] and its possible association with antimicrobial resistance [18,19]. These facts have led to the prohibition of its use at high doses from 2022 in the European Union (EU) [20].
Due to the problems surrounding the use of ZnO and its future restriction, research to find economically and environmentally friendly alternatives is being carried out in order to contribute to the sustainability and resilience of the pig production sector. Plant extracts are widely used as feed additives to enhance the productive performance of several animal species and represent a potential alternative to ZnO due to their properties [21]. Some of the plant extracts that could be used as alternatives are oregano (Origanum vulgare L.) and garlic (Allium sativum).
Oregano contains high concentrations of carvacrol and thymol (around 80%) [22] that have demonstrated, in vitro, significant antimicrobial, antifungal, and antioxidant activity [23,24,25]. Furthermore, its use appears to improve growth performance in weaned pigs [26,27], as well as in other species such as growing lambs and broilers [28,29,30]. In addition, Zhang et al. [31] showed that oregano could reduce serum cortisol levels and increase antioxidant enzyme activity after transportation stress in pigs. However, other reports have indicated that oregano administration can cause cell damage [32]. Oregano essential oil (OEO) can be micro capsuled to improve its stability, preserve the volatile compounds until consumed by animals, and ease its handling and administration. Several materials, such as sunflower oil, are used for this purpose [33].
On the other hand, garlic (Allium sativum) has the beneficial effects of antibacterial, antiviral, antifungal, antioxidant, and immunomodulatory activities [34,35,36,37,38]. The active compounds in garlic that seem to have beneficial effects are sulphide breakdown products such as alliin, diallylsulphides, and allicin [34]. Several studies have shown that dietary supplementation of garlic appears to improve the immune response and growth performance of growing pigs [39,40,41]. Horn et al. [42] observed that this plant could partially mitigate the stress effects of the postweaning period, producing a positive effect on growth performance, intestinal function, and antioxidant status. In addition, a study in chickens showed a reduction of heat stress due to dietary supplementation of garlic [43].
Although serum is usually used to measure biomarkers that can provide information about oxidative status in pigs [44,45], saliva can also be used in this species [46] with the advantage of being a sample that can be easily collected without producing stress to the pigs.
Research has been carried out on the effect of supplementation with OEO on the oxidative status in plasma and muscles of growing-finishing pigs, in the semen of boars, and in porcine small intestinal epithelial (IPEC-J2) cells in vitro. The anti-inflammatory effect of OEO has been associated with decreased cellular levels of reactive oxygen species (ROS), biomarkers of stress, such as cortisol, and biomarkers of inflammation, such as acute-phase proteins [47,48,49,50,51,52]. We hypothesized that garlic powder and OEO as feed additives at appropriate doses could improve the oxidative status, stress, and inflammation in weaned piglets. The purpose of this study is to evaluate and to compare the effects of dietary supplementation of two different concentrations of garlic powder and OEO with the supplementation of ZnO at therapeutic doses during the postweaning period of piglets in farm conditions, evaluating the oxidative status, stress, and inflammation of the animals. For this objective, a panel of biomarkers of oxidative stress in serum and saliva, a biomarker of stress in saliva (cortisol), and a biomarker of inflammation in serum (C-reactive protein (CRP)) were evaluated.
A panel of various analytes involving antioxidant and oxidant biomarkers were used, as this kind of panels has been recommended in order to have thorough information on the oxidative status [53]. Therefore this panel was comprised of thiol, catalase, uric acid, Trolox equivalent antioxidant capacity (TEAC), cupric reducing antioxidant capacity (CUPRAC), and ferric reducing ability of plasma (FRAP) as biomarkers of the antioxidant response and total oxidant status (TOS), advanced oxidation protein products (AOPP), and hydrogen peroxide (H2O2) as oxidant biomarkers. These biomarkers were measured in both serum and saliva in order to assess the possible use of saliva as an alternative or complement to serum in oxidative status evaluation. In saliva, the same biomarkers as serum were measured except for thiol and TOS because, to the author’s knowledge, there are currently no methods that can accurately measure these biomarkers in pig saliva [46].
2. Materials and Methods
2.1. Additives and Feed Composition
ZnO was administered as Zincotrax (Andrés Pintaluba S.A., Tarragona, Spain) containing 1000 mg of ZnO/g. The final dose in the ZnO group was 3100 mg of ZnO per kg of feed (equivalent to 2500 mg of Zn per kg of feed).
The essential oils of Origanum vulgare L. were acquired from Esencias Martínez Lozano S.A (Murcia, Spain). The extraction system was by steam distillation in an industrial boiler. The composition of the essential oil is detailed in Table 1, according to an analysis performed by gas chromatography-mass spectrometry (GC–MS) analysis. Oregano oil (1 µL diluted 5% in hexane) was subjected to analysis by GC–MS. An Agilent HP6890 gas chromatograph (GC), equipped with an HP-INNOWax (60 m × 0.25 mm i.d.) and 0.5 µm film thickness, was used. The stationary phase was supplied by Agilent Technologies (Palo Alto, CA, USA). Helium was used as the carrier gas (constant pressure 23 psi), and the split ratio was set to 100:1. The GC was linked to an Agilent model 5972 inert mass spectrometry detector (Agilent Technologies, Palo Alto, CA, USA). The initial oven temperature was set at 60 °C for 6 min, increased at 80 °C at a rate of 2 °C/min, then increased to 120 °C at 1 °C/min, and finally raised to 250 °C at a rate of 4 °C/min; the port and the transfer line to the mass selective detector were kept at 250 and 280 °C, respectively. The mass spectrometer was operated in electron impact ionization mode with ionizing energy of 70 eV, scanning from m/z 30 to 350 at 3.21 scan/s. The quadrupole temperature was 150 °C, and the electron multiplier voltage was maintained at 1300 V. The individual peaks were identified by retention times and by comparison of mass spectra using the NIST 75 K library (US National Bureau of Standards, 2002) and spectra obtained from the standard. Percentage compositions of samples were calculated according to the area of the chromatographic peaks using the total ion current.
Table 1.
Bioactive components of the oregano essential oil (OEO).
| Components | % |
|---|---|
| α-Pinene | 0.84 |
| α-Thuyene | 1.10 |
| Camphene | 0.07 |
| β-Pinene | 0.23 |
| β-Mircene | 1.56 |
| α-Phellandrene | 0.22 |
| α-Terpinene | 1.25 |
| Limonene | 0.31 |
| 1,8-Cineole+ β-Phellandrene | 0.30 |
| γ-Terpinene | 5.20 |
| 3-Octonone | 0.11 |
| p-Cymene | 5.99 |
| Terpinolene | 0.12 |
| 1-Octen-3-ol | 0.26 |
| (E)-Thuyanol | 0.10 |
| Linalool | 1.73 |
| (Z)-Thuyanol | 0.05 |
| 1-Octanol | 0.02 |
| Terpinene-4-ol | 0.79 |
| β-Caryophyllene | 2.59 |
| α-Humulene | 0.12 |
| α-Terpineol | 0.16 |
| Borneol | 0.22 |
| β-Bisabolene | 0.23 |
| Caryophellene oxyde | 0.14 |
| Thymol | 4.10 |
| Carvacrol Isomere | 0.05 |
| Carvacrol | 70.32 |
| Total | 98.19 |
The encapsulation was carried out by the company AT CAPSELOS SL. (Huesca, Spain). The 10% essential oil was encapsulated in mono and diglyceride coverage of edible fatty acids (as a low hydrophile–lipophile balance (HLB) emulsifier) and hydrogenated sunflower fat at a size of 800 microns.
Purple garlic powder (Allibia Fresh Flour, Adibio S.L., Teruel, Spain; see Table 2) containing 63% of purple garlic in the form of mashed and dried powder, with citric acid and silicic acid (E-551) as additives, was added to the basal diet in the Garlic 2% group.
Table 2.
Chemical composition and amino acid composition of the purple garlic powder.
| Analytical Components | Quantity |
|---|---|
| Protein (%) | 3.9 |
| Carbohydrates (%) | 15.4 |
| Sugars (%) | 1.4 |
| Crude Fibre (%) | 0.7 |
| Crude Fat (%) | 0.2 |
| Crude Ash (%) | 35.4 |
| Humidity (%) | 44.4 |
| Inuline (%) | 8.1 |
| Energy (kJ/kg) | 341 |
| Aminoacids (g/kg) | |
| Methionine | 0.5 |
| Lysine | 2.1 |
| Isoleucine | 0.9 |
| Leucine | 1.5 |
| Valine | 1.4 |
| Phenylalanine | 1.1 |
| Tryptophan | 0.4 |
| Threonine | 1.1 |
| Histidine | 0.6 |
| Arginine | 8.8 |
| Minerals (mg/kg) | |
| Calcium | 1100 |
| Phosphorus | 1060 |
| Potassium | 3500 |
| Sodium | 7247 |
| Copper | 1.6 |
| Cobalt | 0.12 |
| Iron | 20 |
| Manganese | 13.2 |
| Selenium | 0.2 |
| Zinc | 6.3 |
| Sulfur | 6000 |
| Vitamins | |
| Thiamine (mg/kg) | 2.6 |
| Niacin (mg/kg) | 17 |
| Ascorbic acid (mg/kg) | 116.5 |
| Cholecalciferol (µg) | 4 |
| Riboflavin (mg/kg) | 0.3 |
| Cyanocobalamin (µg) | 5 |
| Retinol (mg/kg) | 0.23 |
| Tocopherol (mg/kg) | 3 |
The composition of the control diet is shown in Table 3. It was a commercial basal diet formulated to meet or exceed the energy and other nutritional requirements for weaned piglets during the postweaning period [54]. The piglets received two diets: the first from 21 days of age until they reached 10 kg of weight (prestarter) and the second diet until the end of the trial at ten weeks of age (starter). Both diets were composed of the usual ingredients used in pig feed (Table 3). No antibiotic was included in the diets.
Table 3.
Composition of the control diet.
| Ingredients Prestarter (g/kg) | Prestarter | Starter |
|---|---|---|
| Maize | 299.8 | |
| Wheat | 200 | |
| Soybean | 183.5 | |
| Soybean meal | 135 | |
| Barley | 80 | |
| Whey | 75 | |
| Vitamins and oligoelements premix | 25 | |
| Lysine | 0.75 | |
| Threonine | 0.6 | |
| Tryptophan | 0.2 | |
| Methionine | 0.15 | |
| Ingredients Starter (g/kg) | ||
| Maize | 329.5 | |
| Barley | 250 | |
| Wheat | 170 | |
| Soybean | 129 | |
| Soybean meal | 61 | |
| Vitamins and oligoelements premix | 25 | |
| Soy husk | 10 | |
| Liquid lysine | 7.5 | |
| Monocalcic phosphate | 5.5 | |
| Lard | 5 | |
| Salt | 4 | |
| Calcium carbonate | 3.5 | |
| Vitamins | ||
| Vitamin A UI/kg | 6500 | 6500 |
| Vitamin D3 UI/kg | 1500 | 1500 |
| Vitamin E UI/kg | 92 | 92 |
| Coline chloride mg/kg | 1356 | 1356 |
| Oligoelements (mg/kg) | ||
| Fe | 120 | 120 |
| Mn | 30 | 30 |
| Zn | 110 | 110 |
| Se | 0.20 | 0.20 |
| I | 0.8 | 0.8 |
| Cu | 80 | 80 |
| Analytical Components (%) | ||
| Crude protein | 16.0 | 16.0 |
| Crude fibre | 2.7 | 4.0 |
| Crude fat | 3.8 | 3.0 |
| Crude ash | 6.3 | 4.3 |
| Calcium | 0.85 | 0.72 |
| Phosphorus | 0.67 | 0.50 |
| Sodium | 0.26 | 0.25 |
| Metionine | 0.49 | 0.44 |
| Lisine | 1.18 | 1.09 |
2.2. Animals, Housing, and Experimental Design
The Ethical Committee for Animal Experimentation (CEEA) of Murcia University approved all the experimental protocols used in this study (Authorization Code 471/2018). All animal handling protocols were carried out according to animal welfare legislation in force in the EU [55,56]. The trial was carried out from April to December 2019.
The animals were obtained from Dalland Hybrid España S.A. (Murcia, Spain) and kept on a commercial farm in field conditions. A total of 300 crossbred pigs (Pietrain × Large White × Landrace) were randomly allotted to one of 6 treatments and divided into 10 replicates, with five pigs per pen in each replication following weaning at 21 days of age. The initial body weight (BW) of the piglets was 5.65 ± 0.26 kg, and there were no significant differences in BW between groups at the start of the trial (p = 0.166, one-way ANOVA; Table 4). The same number of females and males were included in the study and sampling. The experimental unit for performance data analysis was the pen of 5 piglets.
Table 4.
Bodyweight at the beginning of the trial 1.
| Group | Mean (kg) | SEM (kg) |
|---|---|---|
| Control | 5.66 | 0.072 |
| ZnO | 5.59 | 0.070 |
| OEO 0.4% | 5.49 | 0.090 |
| OEO 1.2% | 5.87 | 0.061 |
| Garlic 0.4% | 5.68 | 0.082 |
| Garlic 2% | 5.67 | 0.174 |
1 Data are the means of 10 replicates of 5 pigs each per treatment.
The experimental unit for performance data analysis was the group of 50 piglets (25 females and 25 males in separated pens). The animals were under experimental conditions for 49 days. The prestarter phase lasted 23 days, and the second phase lasted 26 days. All groups had continuous ad libitum access to feed and water through nipple drinkers and feed troughs. At the end of the experiment, the animals were sent to a growing-finishing farm to continue their productive cycle under commercial conditions.
The dietary treatments were as follows: (1) control (basal diet; n = 50); (2) ZnO, containing 3100 mg/kg of ZnO added to the basal diet (n = 50); (3) micro capsuled OEO at a concentration of 0.4% in the basal diet (n = 50); (4) micro capsuled OEO at a concentration of 1.2% in the basal diet (n = 50); (5) garlic powder at a concentration of 0.4% in the basal diet (n = 50); (6) garlic powder at a concentration of 2% in the basal diet (n = 50).
2.3. Sample Collection and Productive Measures
At the end of the trial, blood and saliva samples were collected from 17 piglets of each group that were randomly selected, ensuring that the sample was evenly distributed between the replicates, so each replicate had at least one piglet sampled. Blood samples were collected from the jugular vein for analysis using a 5-mL vacuum test kit without anticoagulant (VACUTEST KIMA srl., Arzergrande, Padova, Italy) to obtain serum samples. Serum samples were separated within 2 h of collection by centrifugation for 5 min at 3500× g. Saliva samples were collected using Salivette® tubes (Sarstedt, Aktiengesellschaft & Co. D97 51588 Nümbrecht, Germany) containing a sponge instead of a cotton swab. The piglets were allowed to chew on the sponge, which was clipped to a flexible thin metal rod, for 1–2 min until thoroughly moist. Tubes were kept on ice until arrival at the laboratory and then centrifuged at 3500× g and 4 °C for 10 min to obtain saliva. Samples were stored at −80 °C until the day of analysis. No saliva samples with the presence of food or any other contamination were included in the study.
Body weight was measured at the beginning and the end of the trial. Feed intake was registered for every group during the whole trial. Average daily gain (ADG) was calculated by dividing the mean increase in weight in the group by the number of days that the trial lasted. To calculate the feed conversion ratio (FCR), the amount of feed consumed was divided by the weight gained during the studied period for each group. Average feed intake (AFI) was calculated for each group by dividing the amount of feed consumed per group between the number of animals. Final body weight (FBW) was calculated as the mean final animal weight for each group.
2.4. Measurements of Biomarkers
2.4.1. Oxidative Status
Serum thiol concentrations were measured according to the method described by Jocelyn [57] and modified by Costa et al. [58]. Uric acid was measured using a commercial kit (OSR6198, uric acid, Beckman Coulter Inc., Fullerton, CA, USA). Antioxidant activity of catalase in serum was assessed by an automatic method previously described by Slaughter and O’Brien [59].
Total antioxidant capacity of samples was assessed by three different methods: TEAC, CUPRAC, and FRAP. TEAC concentrations were evaluated according to the assay described by Arnao et al. [60], which is based on the inhibition of the radical ABTS by the sample. Serum CUPRAC was determined using a method based on the reduction of Cu2+ Cu1+ by the sample [61]. FRAP concentrations in serum and saliva (FRAS) were determined following the method of Benzie and Strain [62], which measures the ferric to ferrous ion reduction by the sample.
Serum TOS was measured by the method described by Erel [63]. AOPP in serum was determined according to a method described by Witko-Sarsat et al. [64]. H2O2 levels were analyzed using the assay described by Rhee et al. [65].
All assays in serum showed a between and within run imprecision that was lower than 15% and linear, with r > 0.9 after serial dilutions.
Uric acid, catalase, TEAC, CUPRAC, FRAS, AOPP, and H2O2 were also measured in saliva samples. Their measurements were based on previously validated methods for porcine saliva [46]. Assays were conducted on an Olympus AU400 automated chemistry analyzer (Olympus Diagnostica Europe GmbH, Ennis, Ireland).
2.4.2. Salivary Cortisol
Salivary cortisol was quantified using an automated chemiluminescent immunoassay (Immulite 1000 cortisol, Siemens Medical Solutions Diagnostics, Los Angeles, CA, USA) that was previously validated for porcine saliva [66].
2.4.3. Serum CRP
Serum CRP concentrations were determined with a commercial immunoturbidimetric assay (Beckman Coulter®, Inc., Fullerton, CA, USA) using an automated analyzer (Olympus AU600, Olympus Europe GmbH, Ennis, Ireland), as previously reported [67].
2.5. Statistical Analysis
Data analyses were performed using statistical procedures and software (GraphPad Software, San Diego, CA, USA). All data were evaluated for normality using the Shapiro–Wilk test. Regarding serum results, CRP, catalase, and TOS results in serum were not normally distributed; therefore, they were presented in median and interquartile range (IQR) values, and analysis of the results was made using a nonparametric Kruskal–Wallis test, followed by Dunn’s multiple comparisons test. Normally distributed data were presented as means ± standard deviation (SD) and compared using one-way ANOVA, followed by Tukey’s multiple comparisons test. Salivary results were not normally distributed and were compared using the Kruskal–Wallis test and Dunn’s multiple comparisons post-test.
Correlations between all the markers analyzed were determined using Spearman’s correlation analysis. A value of p < 0.05 was used to indicate significance in all analyses.
3. Results
3.1. Biomarkers of Oxidative Stress
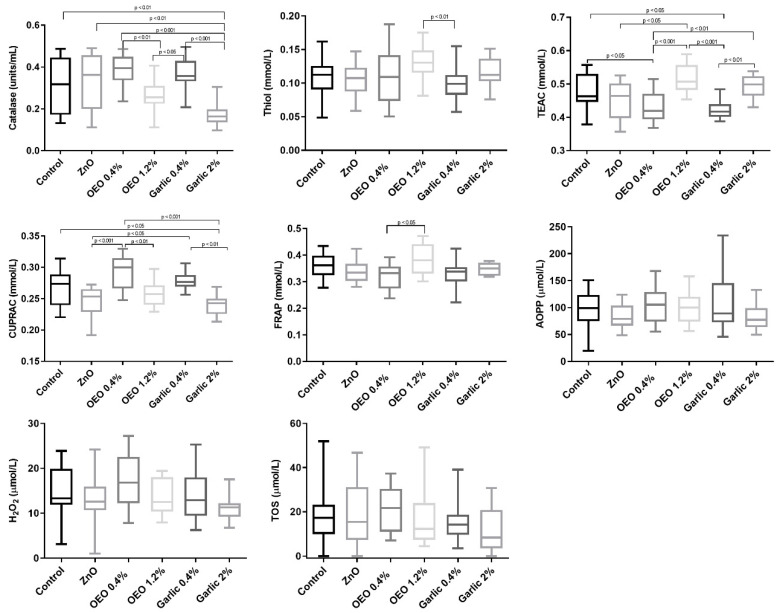
The effects of OEO and garlic supplementation on the serum oxidative stress biomarkers are presented in Figure 1.
Figure 1.
Results for serum catalase, thiol, Trolox equivalent antioxidant capacity (TEAC), cupric reducing antioxidant capacity (CUPRAC), ferric reducing ability of plasma (FRAP), advanced oxidation protein products (AOPP), hydrogen peroxide (H2O2), and total oxidant status (TOS) in piglets supplemented with ZnO, micro capsuled OEO at a concentration of 0.4% in the basal diet, micro capsuled OEO at a concentration of 1.2% in the basal diet, garlic powder at a concentration of 0.4% in the basal diet, and garlic powder at a concentration of 2% in the basal diet. The plots show median (line within box), 25th and 75th percentiles (box), and minimum and maximum values (whiskers).
When control and ZnO were compared with supplemented groups, control and ZnO groups showed higher serum catalase activity than garlic at 2% (p < 0.01). Serum TEAC was significantly higher (p < 0.05) in the control group than in OEO and garlic at 0.4% but significantly lower in the ZnO group when compared to OEO at 1.2% (p < 0.05). Serum CUPRAC was higher in the control group than in garlic at 2% (p < 0.05) and lower in ZnO when compared to OEO (p < 0.001) and garlic (p < 0.05) at 0.4% concentrations.
When the different concentrations of the supplemented groups were compared, serum catalase and CUPRAC were higher in OEO at 0.4% than in OEO at 1.2% (p < 0.01). In addition, they were higher in garlic at 0.4% than in garlic at 2% (p < 0.01). The group receiving OEO at 0.4% showed lower TEAC than OEO at 1.2%. TEAC was also lower in garlic at 0.4% than in garlic at 2% (p < 0.01).
Serum TOS, AOPP, and H2O2 were not different (p < 0.05) between the groups (Figure 1). Uric acid was below the detection limit in serum for all groups (data not shown).
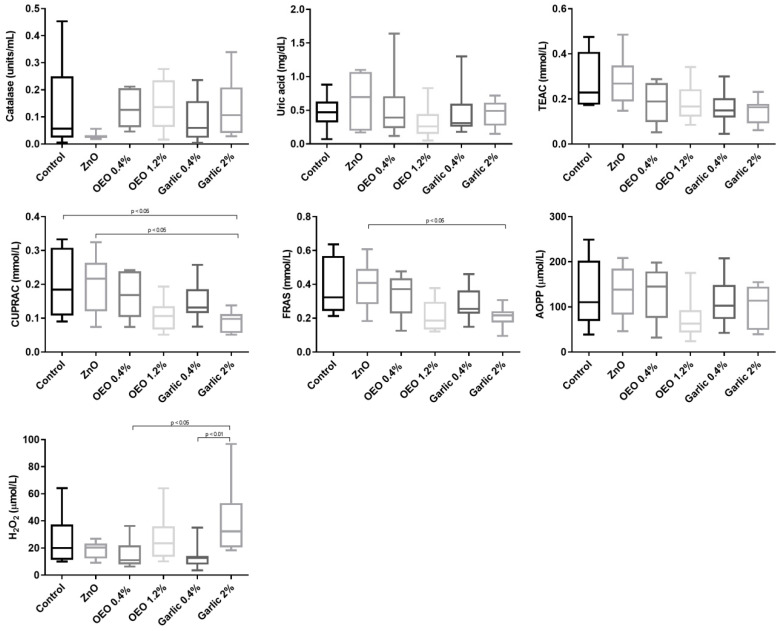
The effects of supplementation diets on salivary biomarkers of oxidative stress are shown in Figure 2. When control and ZnO were compared to supplemented groups, the control and ZnO groups presented higher (p < 0.05) salivary CUPRAC than the group supplemented with garlic at 2%. In addition, the ZnO group showed higher salivary FRAS when compared to the garlic at 2% group.
Figure 2.
Results for salivary catalase, uric acid, Trolox equivalent antioxidant capacity (TEAC), cupric reducing antioxidant capacity (CUPRAC), ferric reducing ability of saliva (FRAS), advanced oxidation protein products (AOPP), and hydrogen peroxide (H2O2) in piglets supplemented with ZnO, micro capsuled OEO at a concentration of 0.4% in the basal diet, micro capsuled OEO at a concentration of 1.2% in the basal diet, garlic powder at a concentration of 0.4% in the basal diet, and garlic powder at a concentration of 2% in the basal diet. The plots show median (line within box), 25th and 75th percentiles (box), and minimum and maximum values (whiskers).
When the different concentrations of the supplemented groups were compared, garlic at 2% presented higher salivary H2O2 than garlic at 0.4% (p < 0.01). Salivary TEAC, AOPP, uric acid, and catalase showed no differences (p < 0.05) between the groups (Figure 2).
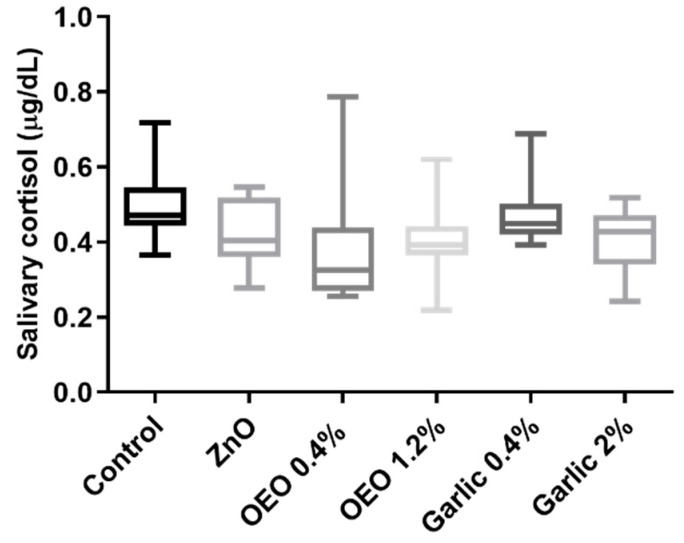
3.2. Salivary Cortisol
The cortisol results are presented in Figure 3. They were not different between the groups (p > 0.05).
Figure 3.
Results for salivary cortisol in piglets supplemented with ZnO, micro capsuled OEO at a concentration of 0.4% in the basal diet, micro capsuled OEO at a concentration of 1.2% in the basal diet, garlic powder at a concentration of 0.4% in the basal diet, and garlic powder at a concentration of 2% in the basal diet. The plots show median (line within box), 25th and 75th percentiles (box), and minimum and maximum values (whiskers).
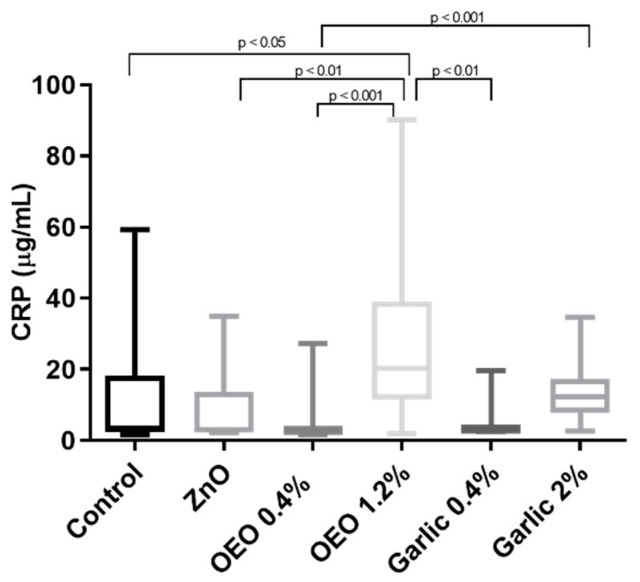
3.3. Serum CRP
Higher CRP concentrations were observed in OEO at 1.2% when compared with all groups (p < 0.05) except for garlic at 2% (Figure 4). In addition, CRP was significantly increased in garlic at 2% when compared with OEO at 0.4% (p < 0.001; see Figure 4).
Figure 4.
Results for serum C-reactive protein (CRP) in piglets supplemented with ZnO, micro capsuled OEO at a concentration of 0.4% in the basal diet, micro capsuled OEO at a concentration of 1.2% in the basal diet, garlic powder at a concentration of 0.4% in the basal diet, and garlic powder at a concentration of 2% in the basal diet. The plots show median (line within box), 25th and 75th percentiles (box), and minimum and maximum values (whiskers).
3.4. Correlations Between Serum and Saliva
CUPRAC and TEAC showed weak but significantly positive (r = 0.36, p < 0.01) and negative (r = −0.30, p < 0.01) correlations, respectively, between serum and saliva values.
3.5. Productive Parameters
The values of ADG (Table 5) in the OEO at low concentration group (mean ± SD: 0.3511 ± 0.02192 kg/day) were significantly higher than the values for the control (mean ± SD: 0.2650 ± 0.06253 kg/day; p = 0.001) and ZnO groups (mean ± SD: 0.2633 ± 0.03670 kg/day; p = 0.001).
Table 5.
Values of average daily gain (ADG) and feed conversion ratio (FCR) for the different groups 1,2.
| Group | ADG | FCR | Feed Intake | FBW | ||||
|---|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | Mean | SEM | Mean | SEM | |
| Control | 0.265 a | 0.025 | 2.120 | 0.187 | 26.763 | 1.839 | 18.645 a | 1.251 |
| ZnO | 0.263 a | 0.014 | 1.811 | 0.091 | 23.150 | 0.992 | 18.493 a | 0.734 |
| OEO 0.4% | 0.351 c | 0.007 | 1.456 | 0.089 | 24.974 | 1.373 | 22.701 b | 0.408 |
| OEO 1.2% | 0.307 abc | 0.007 | 1.787 | 0.064 | 26.903 | 0.877 | 20.937 ab | 0.368 |
| Garlic 0.4% | 0.342 bc | 0.011 | 1.876 | 0.217 | 31.338 | 3.442 | 22.523 b | 0.555 |
| Garlic 2% | 0.285 ab | 0.009 | 1.985 | 0.074 | 27.710 | 1.275 | 19.635 ab | 0.469 |
1 The different superscripts (a, b, c) indicate statistically significant differences between the groups (p ≤ 0.05). 2 Data are the means of 10 replicates of 5 pigs each per treatment.
Garlic at low concentration (mean ± SD: 0.3423 ± 0.03205 kg/day) led to a higher ADG values than in control (mean ± SD: 0.2650 ± 0.06253 kg/day; p = 0.001) and ZnO groups (mean ± SD: 0.2633 ± 0.3670 kg/day; p = 0.005) and did not show any significant difference with the OEO at low concentration group (p = 0.996) or with the groups with high concentrations of OEO and garlic (p = 0.615 and p = 0.126, respectively). Both groups with high concentrations of OEO and garlic did not show statistically significant differences with the rest of the groups.
Regarding FBW (Table 5), the OEO at low concentration (mean ± SD: 22.7013 ± 1.155 kg) and the garlic at low concentration groups (mean ± SD: 22.5238 ± 1.570 kg) showed higher values than the control (mean ± SD: 18.6450 ± 3.064 kg; p = 0.002 and p = 0.004, respectively) and ZnO groups (mean ± SD: 18.4933 ± 1.798 kg; p = 0.002 and p = 0.003 respectively). Both groups with high concentrations of OEO and garlic did not show statistically significant differences with the rest of the groups.
Regarding FCR and AFI, there were no significant differences between the groups (p = 0.069 and p = 0.149, respectively; Table 5).
4. Discussion
In this study, purple garlic powder and micro capsuled OEO were given at different concentrations as supplements to piglets during the weaning period, and their effects in biomarkers of oxidative status, stress, and inflammation, as well as in productive parameters, were evaluated.
The group supplemented with higher doses of OEO showed higher serum TEAC when compared to OEO and garlic at lower doses and ZnO groups. These increases could be related to the inflammation produced when higher concentrations of OEO were given, as evidenced by the increased CRP concentrations observed in this group. It could be hypothesized that the antioxidants represented by TEAC increase their concentrations to counteract adverse reactions related to inflammation [68]. The tendency of FRAP to also increase in the group receiving OEO at high doses, when compared to low doses of the same supplement, would support our hypothesis. In contrast, catalase, an endogenous antioxidant, was significantly diminished in the groups receiving higher doses of OEO and garlic in comparison with their respective lower doses. Catalase is one of the main parameters involved in the cellular defence against free radicals, and its decrease could be related to the inflammation caused by the higher concentrations of the supplements given. Previous research showed that weaned piglets challenged with lipopolysaccharide showed lower catalase activity [69], which could be in line with the finds of this study.
On the other hand, CUPRAC increased its concentration when low doses of OEO and garlic were given, when compared with the groups that received higher doses of the same products. This different behaviour of CUPRAC, compared to TEAC and FRAP, could be related to the different components that are in CUPRAC, such as thiol, β-carotene and α-tocopherol, that, for example, in the case of β-carotene and α-tocopherol, could have proven to be beneficial for health and reproduction [70,71,72]. Therefore, the increase in CUPRAC with OEO and garlic supplement at low doses could indicate an improvement in the antioxidant status of the animals.
Regarding salivary biomarkers, piglets supplemented with high doses of garlic exhibited decreased antioxidants biomarkers, such as CUPRAC, in comparison with ZnO. On the other hand, the same compound in high doses led to an increase in oxidant biomarkers such as H2O2 when compared with low concentrations of both OEO and garlic. This increase in oxidant compounds in saliva would be in line with the changes of biomarkers of oxidative stress in serum at higher doses of garlic powders, which could be due to the toxic effects described in farm animals after the consumption of garlic or onions at high doses, including oxidative damage to erythrocytes [73].
Based on the results of this trial, high concentrations of OEO or garlic power will lead to a worse situation in oxidative status compared to the use of lower concentrations of these supplements. This has been previously reported with some oregano compounds such as carvacrol and thymol since, at high doses, they induce DNA damage in human lymphocytes. In contrast, lower doses of thymol and carvacrol, which possess useful antioxidant properties, significantly reduced oxidative DNA damage in human lymphocytes [37].
The lack of significant changes in salivary cortisol between the different groups could indicate that the different supplements tested in this study do not produce a significant activation of the hypothalamic-pituitary-adrenal (HPA) axis in any group of piglets and, therefore, they do not induce a stress response. Although in this research, we did not evaluate the response of the different groups against a model of stress, serum cortisol concentrations after a model of transport were markedly lower in those animals receiving supplementation with OEO than in those fed with a control diet [31]. Considering this, an interesting line of future research would be the potential of using these supplements at low doses to reduce activation of the HPA axis in stressful situations.
In our study, higher serum CRP was observed when high doses of OEO were given. From a toxicological point of view, it has been reported that excessive oral doses of oregano can cause gastrointestinal irritation [74], which could explain the higher levels of serum CRP and inflammation observed in the group that received OEO at high concentrations. Conversely, the groups supplemented with low concentrations of OEO and garlic powder presented lower serum values of CRP, which is in line with the described anti-inflammatory effects of oregano and garlic in pigs [41,75].
Regarding productive parameters, OEO at low doses produced higher results of ADG in postweaning piglets, showing significantly higher values compared to the rest of the groups except for garlic at low concentration. Higher FBW values were also obtained for OEO and garlic at low doses, which were significantly higher than the control and ZnO FBW values. These results would be in line with the better results of the oxidative stress biomarkers, cortisol and CRP in the groups supplemented with low doses of OEO and garlic. Positive effects on ADG and average daily feed intake (ADFI) when low doses of fermented garlic were given to piglets have been described [76,77]. In addition, improved body weight due to supplementation of garlic or garlic-derived products in pigs has also been reported [39,41,78].
5. Conclusions
It could be concluded that doses of OEO and garlic powder at 0.4% did not lead to inflammation, stress, or negative changes in oxidative biomarkers in piglets during the postweaning period. In addition, they gave better productive performance than the control and ZnO diets. However, high doses of those supplements were shown to be ineffective and could negatively affect the animals. Therefore, our results highlight the importance of the dose used when OEO or garlic are supplemented to piglets.
Acknowledgments
The authors specially thank the project partners from DHESA (José Antonio Tornel, Antonio Martínez Abarca, and Rubén Brando), IMIDA (María José Jordán Bueso and José Antonio Sotomayor Sánchez), and UMU (María del Carmen Gallego Ruíz and Antonio Bernabé Salazar) for their valuable collaboration and support in this project.
Author Contributions
Conceptualization: M.J.C.P., J.J.C., and D.E.T.; results analysis: D.E.T., C.P.R., J.O.S., and J.R.-G.; funding and resources acquisition: M.J.C.P. and J.J.C.; methodology: C.M.C., M.J.C.P., J.J.C., D.E.T., C.P.R., and J.O.S.; supervision: M.J.C.P. and J.J.C.; writing, review, and editing: C.P.R., J.R.-G., D.E.T., J.J.C., M.J.C.P., and C.M.C. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the DALLAND CDTi IDI-20180830 grant “NATURPORKS” (2018–2021) from the Center for Industrial Technological Development (CDTI) of the Ministry of Science, Innovation, and Universities and cofinanced by FEDER funds through the Intelligent Growth Operational Program. D.E. (IJC2018-035105-I) has a postdoctoral grant “Juan de la Cierva” supported by the “Ministerio de Economía y Competitividad”, Spain. The funders had no role in study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Moberg G.P., Mench J.A. The Biology of Animal Stress: Basic Principles and Implications for Animal Welfare. CAB International; Wallingford, UK: 2000. [Google Scholar]
- 2.Srinongkote S., Smriga M., Nakagawa K., Toride Y. A Diet Fortified withl-lysine andl-arginine Reduces Plasma Cortisol and Blocks Anxiogenic Response to Transportation in Pigs. Nutr. Neurosci. 2003;6:283–289. doi: 10.1080/10284150310001614661. [DOI] [PubMed] [Google Scholar]
- 3.Gupta S., Earley B., Crowe M. Effect of 12-hour road transportation on physiological, immunological and haematological parameters in bulls housed at different space allowances. Veter J. 2007;173:605–616. doi: 10.1016/j.tvjl.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Sporer K.B., Weber P.S.D., Burton J.L., Earley B., Crowe M. Transportation of young beef bulls alters circulating physiological parameters that may be effective biomarkers of stress1. J. Anim. Sci. 2008;86:1325–1334. doi: 10.2527/jas.2007-0762. [DOI] [PubMed] [Google Scholar]
- 5.Escribano D., Ko H.-L., Chong Q., Llonch L., Manteca X., Llonch P. Salivary biomarkers to monitor stress due to aggression after weaning in piglets. Res. Veter Sci. 2019;123:178–183. doi: 10.1016/j.rvsc.2019.01.014. [DOI] [PubMed] [Google Scholar]
- 6.Zhu H., Jia Z., Misra H., Li Y.R. Oxidative stress and redox signaling mechanisms of alcoholic liver disease: Updated experimental and clinical evidence. J. Dig. Dis. 2012;13:133–142. doi: 10.1111/j.1751-2980.2011.00569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pié S., Lallès J.P., Blazy F., Laffitte J., Sève B., Oswald I.P. Weaning Is Associated with an Upregulation of Expression of Inflammatory Cytokines in the Intestine of Piglets. J. Nutr. 2004;134:641–647. doi: 10.1093/jn/134.3.641. [DOI] [PubMed] [Google Scholar]
- 8.Wijtten P.J.A., Van Der Meulen J., Verstegen M.W.A. Intestinal barrier function and absorption in pigs after weaning: A review. Br. J. Nutr. 2011;105:967–981. doi: 10.1017/S0007114510005660. [DOI] [PubMed] [Google Scholar]
- 9.Kim J., Hansen C., Mullan B., Pluske J. Nutrition and pathology of weaner pigs: Nutritional strategies to support barrier function in the gastrointestinal tract. Anim. Feed. Sci. Technol. 2012;173:3–16. doi: 10.1016/j.anifeedsci.2011.12.022. [DOI] [Google Scholar]
- 10.Hahn J.D., Baker D.H. Growth and plasma zinc responses of young pigs fed pharmacologic levels of zinc. J. Anim. Sci. 1993;71:3020–3024. doi: 10.2527/1993.71113020x. [DOI] [PubMed] [Google Scholar]
- 11.Patel A., Mamtani M., Dibley M.J., Badhoniya N., Kulkarni H. Therapeutic Value of Zinc Supplementation in Acute and Persistent Diarrhea: A Systematic Review. PLoS ONE. 2010;5:e10386. doi: 10.1371/journal.pone.0010386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sargeant H.R., Miller H.M., Shaw M.-A. Inflammatory response of porcine epithelial IPEC J2 cells to enterotoxigenic E. coli infection is modulated by zinc supplementation. Mol. Immunol. 2011;48:2113–2121. doi: 10.1016/j.molimm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 13.Hu C., Song J., Li Y., Luan Z., Zhu K. Diosmectite–zinc oxide composite improves intestinal barrier function, modulates expression of pro-inflammatory cytokines and tight junction protein in early weaned pigs. Br. J. Nutr. 2013;110:681–688. doi: 10.1017/S0007114512005508. [DOI] [PubMed] [Google Scholar]
- 14.Hoque K.M., Rajendran V.M., Binder H.J. Zinc inhibits cAMP-stimulated Cl secretion via basolateral K-channel blockade in rat ileum. Am. J. Physiol. Liver Physiol. 2005;288:G956–G963. doi: 10.1152/ajpgi.00441.2004. [DOI] [PubMed] [Google Scholar]
- 15.Söderberg T.A., Sunzel B., Holm S., Elmros T., Hallmans G., Sjöberg S. Antibacterial Effect of Zinc Oxide In Vitro. Scand. J. Plast. Reconstr. Surg. Hand Surg. 1990;24:193–197. doi: 10.3109/02844319009041278. [DOI] [PubMed] [Google Scholar]
- 16.Poulsen H.D., Larsen T. Zinc excretion and retention in growing pigs fed increasing levels of zinc oxide. Livest. Prod. Sci. 1995;43:235–242. doi: 10.1016/0301-6226(95)00039-N. [DOI] [Google Scholar]
- 17.Sargeant H.R., McDowall K.J., Miller H.M., Shaw M.-A. Dietary zinc oxide affects the expression of genes associated with inflammation: Transcriptome analysis in piglets challenged with ETEC K88. Veter Immunol. Immunopathol. 2010;137:120–129. doi: 10.1016/j.vetimm.2010.05.001. [DOI] [PubMed] [Google Scholar]
- 18.Cavaco L.M., Hasman H., Stegger M., Andersen P.S., Skov R., Fluit A.C., Ito T., Aarestrup F.M. Cloning and occurrence of czrC, a gene conferring cadmium and zinc resistance in methicillin-resistant Staphylococcus aureus CC398 isolates. Antimicrob. Agents Chemother. 2010;54:3605–3608. doi: 10.1128/AAC.00058-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cavaco L.M., Hasman H., Aarestrup F.M. Zinc resistance of Staphylococcus aureus of animal origin is strongly associated with methicillin resistance. Veter Microbiol. 2011;150:344–348. doi: 10.1016/j.vetmic.2011.02.014. [DOI] [PubMed] [Google Scholar]
- 20.Standing Committee on Veterinary Medicinal Products. 2017. Brussels, 26.6.2017 C (2017) 4529 Final. [(accessed on 14 April 2020)];2017 Available online: https://ec.europa.eu/health/documents/community-register/2017/20170626136754/dec_136754_en.pdf.
- 21.Allan P., Bilkei G. Oregano improves reproductive performance of sows. Theriogenology. 2005;63:716–721. doi: 10.1016/j.theriogenology.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 22.Pandey A., Rai M., Acharya D. Chemical Composition and Antimycotic Activity of the Essential Oils of Corn Mint (Mentha arvensis) and Lemon Grass (Cymbopogon flexuosus) against Human Pathogenic Fungi. Pharm. Biol. 2003;41:421–425. doi: 10.1076/phbi.41.6.421.17825. [DOI] [Google Scholar]
- 23.Daouk R.K., Dagher S.M., Sattout E.J. Antifungal Activity of the Essential Oil of Origanum syriacum L. J. Food Prot. 1995;58:1147–1149. doi: 10.4315/0362-028X-58.10.1147. [DOI] [PubMed] [Google Scholar]
- 24.Cervato G., Carabelli M., Gervasio S., Cittera A., Cazzola R., Cestaro B. Antioxbdant properties of oregano (origanum vulgare) leaf extracts. J. Food Biochem. 2000;24:453–465. doi: 10.1111/j.1745-4514.2000.tb00715.x. [DOI] [Google Scholar]
- 25.Dorman H.J.D., Deans S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 26.Marcin A., Lauková A., Mati R. Comparison of the effects of Enterococcus faecium and aromatic oils from sage and oregano on growth performance and diarrhoeal diseases of weaned pigs. Biologia. 2006;61 doi: 10.2478/s11756-006-0159-9. [DOI] [Google Scholar]
- 27.Neill C.R., Nelssen J.L., Tokach M.D., Goodband R.D., DeRouchey J.M., Dritz S.S. Effects of oregano oil on growth performance of nursery pigs. J. Swine Heal. Prod. 2006;14:312–316. [Google Scholar]
- 28.Bampidis V., Christodoulou V., Florou-Paneri P., Christaki E., Spais A., Chatzopoulou P.S. Effect of dietary dried oregano leaves supplementation on performance and carcass characteristics of growing lambs. Anim. Feed. Sci. Technol. 2005;121:285–295. doi: 10.1016/j.anifeedsci.2005.02.002. [DOI] [Google Scholar]
- 29.Hernández F., Madrid J., García V., Orengo J., Megías M.D. Influence of two plant extracts on broilers performance, digestibility, and digestive organ size. Poult. Sci. 2004;83:169–174. doi: 10.1093/ps/83.2.169. [DOI] [PubMed] [Google Scholar]
- 30.Hong J.-C., Steiner T., Aufy A., Lien T.-F. Effects of supplemental essential oil on growth performance, lipid metabolites and immunity, intestinal characteristics, microbiota and carcass traits in broilers. Livest. Sci. 2012;144:253–262. doi: 10.1016/j.livsci.2011.12.008. [DOI] [Google Scholar]
- 31.Zhang T., Zhou Y., Zou Y., Hu X., Zheng L., Wei H., Giannenas I., Jin L., Peng J., Jiang S. Effects of dietary oregano essential oil supplementation on the stress response, antioxidative capacity, and HSPs mRNA expression of transported pigs. Livest. Sci. 2015;180:143–149. doi: 10.1016/j.livsci.2015.05.037. [DOI] [Google Scholar]
- 32.Leyva-López N., Gutiérrez-Grijalva E.P., Vazquez-Olivo G., Heredia J.B. Essential Oils of Oregano: Biological Activity beyond Their Antimicrobial Properties. Molecules. 2017;22:989. doi: 10.3390/molecules22060989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bakry A.M., Abbas S., Ali B., Majeed H., Abouelwafa M.Y., Mousa A., Liang L. Microencapsulation of Oils: A Comprehensive Review of Benefits, Techniques, and Applications. Compr. Rev. Food Sci. Food Saf. 2015;15:143–182. doi: 10.1111/1541-4337.12179. [DOI] [PubMed] [Google Scholar]
- 34.Amagase H., Petesch B.L., Matsuura H., Kasuga S., Itakura Y. Intake of Garlic and Its Bioactive Components. J. Nutr. 2001;131:955S–962S. doi: 10.1093/jn/131.3.955S. [DOI] [PubMed] [Google Scholar]
- 35.Amagase H. Clarifying the Real Bioactive Constituents of Garlic. J. Nutr. 2006;136:716S–725S. doi: 10.1093/jn/136.3.716S. [DOI] [PubMed] [Google Scholar]
- 36.Sallam K.H., Ishioroshi I.M., Samejima K. Antioxid Antimicrob Eff garlic Chick sausage. Leb. Wiss U Technol. 2004;37:849–855. doi: 10.1016/j.lwt.2004.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aydın S., Başaran A.A., Başaran N., Aydin S. Modulating Effects of Thyme and Its Major Ingredients on Oxidative DNA Damage in Human Lymphocytes. J. Agric. Food Chem. 2005;53:1299–1305. doi: 10.1021/jf0402375. [DOI] [PubMed] [Google Scholar]
- 38.Li W.-R., Shi Q.-S., Dai H.-Q., Liang Q., Xie X.-B., Huang X.-M., Zhao G.-Z., Zhang L. Antifungal activity, kinetics and molecular mechanism of action of garlic oil against Candida albicans. Sci. Rep. 2016;6:22805. doi: 10.1038/srep22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tatara M.R., Sliwa E., Dudek K., Gawron A., Piersiak T., Dobrowolski P., Mosiewicz J., Siwicki A., Studzinski T. Aged garlic extract and allicin improve performance and gastrointestinal tract development of piglets reared in artificial sow. Ann. Agric. Environ. Med. 2008;15:63–69. [PubMed] [Google Scholar]
- 40.Huang R.H., Qiu X.S., Shi F.X., Hughes C.L., Lu Z.F., Zhu W.Y. Effects of dietary allicin on health and growth performance of weanling piglets and reduction in attractiveness of faeces to flies. Animals. 2011;5:304–311. doi: 10.1017/S1751731110001953. [DOI] [PubMed] [Google Scholar]
- 41.Wang J.P., Yoo J.S., Jang H.D., Lee J.H., Cho J.H., Kim I.H. Effect of dietary fermented garlic by Weissella koreensis powder on growth performance, blood characteristics, and immune response of growing pigs challenged with Escherichia coli lipopolysaccharide. J. Anim. Sci. 2011;89:2123–2131. doi: 10.2527/jas.2010-3186. [DOI] [PubMed] [Google Scholar]
- 42.Horn N., Miller G., Ajuwon K.M., Adeola O. Ability of garlic-derived diallyl disulfide and diallyl trisulfide supplemented by oral gavage to mitigate effects of an acute post weaning feed and water deprivation event in nursery pigs. J. Anim. Sci. 2017;95:3579. doi: 10.2527/jas.2017.1545. [DOI] [PubMed] [Google Scholar]
- 43.Prieto M., Campo J. Effect of heat and several additives related to stress levels on fluctuating asymmetry, heterophil:lymphocyte ratio, and tonic immobility duration in White Leghorn chicks. Poult. Sci. 2010;89:2071–2077. doi: 10.3382/ps.2010-00716. [DOI] [PubMed] [Google Scholar]
- 44.Buchet A., Belloc C., Leblanc-Maridor M., Merlot E. Effects of age and weaning conditions on blood indicators of oxidative status in pigs. PLoS ONE. 2017;12:e0178487. doi: 10.1371/journal.pone.0178487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Luo Z., Zhu W., Guo Q., Luo W., Zhang J., Xu W., Xu J. Weaning Induced Hepatic Oxidative Stress, Apoptosis, and Aminotransferases through MAPK Signaling Pathways in Piglets. Oxidative Med. Cell. Longev. 2016;2016:1–10. doi: 10.1155/2016/4768541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rubio C.P., Mainau E., Cerón J.J., Contreras-Aguilar M.D., Martínez-Subiela S., Navarro E., Tecles F., Manteca X., Escribano D. Biomarkers of oxidative stress in saliva in pigs: Analytical validation and changes in lactation. BMC Veter Res. 2019;15:1–7. doi: 10.1186/s12917-019-1875-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wei H., Chen G., Wang R.-J., Peng J. Oregano essential oil decreased susceptibility to oxidative stress-induced dysfunction of intestinal epithelial barrier in rats. J. Funct. Foods. 2015;18:1191–1199. doi: 10.1016/j.jff.2015.02.035. [DOI] [Google Scholar]
- 48.Zou Y., Wang J., Peng J., Wei H. Oregano Essential Oil Induces SOD1 and GSH Expression through Nrf2 Activation and Alleviates Hydrogen Peroxide-Induced Oxidative Damage in IPEC-J2 Cells. Oxidative Med. Cell. Longev. 2016;2016:1–13. doi: 10.1155/2016/5987183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Liu Q., Duan R.J., Zhou Y.F., Wei H.K., Peng J., Li J.L. Supplementing oregano essential oil to boar diet with strengthened fish oil: Effects on semen antioxidant status and semen quality parameters. Andrologia. 2017;49:e12764. doi: 10.1111/and.12764. [DOI] [PubMed] [Google Scholar]
- 50.Cheng C., Zou Y., Peng J. Oregano Essential Oil Attenuates RAW264.7 Cells from Lipopolysaccharide-Induced Inflammatory Response through Regulating NADPH Oxidase Activation-Driven Oxidative Stress. Molecules. 2018;23:1857. doi: 10.3390/molecules23081857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Peng J., Liu Z., Zhou Y., Wei H., Zhang X., Xiaming Z., Deng Z., Zou Y., Jiang S., Peng J. Effect of oregano essential oil supplementation to a reduced-protein, amino acid-supplemented diet on meat quality, fatty acid composition, and oxidative stability of Longissimus thoracis muscle in growing-finishing pigs. Meat Sci. 2017;133:103–109. doi: 10.1016/j.meatsci.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 52.Peng J., Xia M., Zhang X., Wang C., Jiang S., Peng J. Supplementing Oregano Essential Oil in a Reduced-Protein Diet Improves Growth Performance and Nutrient Digestibility by Modulating Intestinal Bacteria, Intestinal Morphology, and Antioxidative Capacity of Growing-Finishing Pigs. Animals. 2018;8:159. doi: 10.3390/ani8090159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Rubio C.P., Hernández-Ruiz J., Martinez-Subiela S., Tvarijonaviciute A., Ceron J.J. Spectrophotometric assays for total antioxidant capacity (TAC) in dog serum: An update. BMC Veter Res. 2016;12:1–7. doi: 10.1186/s12917-016-0792-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.National Research Council . Nutrient Requirements of Swine. The National Academies Press; Washington, DC, USA: 2012. pp. 1–399. [Google Scholar]
- 55.European Commission Council Directive 98/58/EC of 20 July 1998 concerning the protection of animals kept for farming purposes. Off. J. 1998 Jul 20;L 221:23–27. [Google Scholar]
- 56.European Commission Council Directive 2008/120/EC of 18 December 2008 laying down minimum standards for the protection of pigs. [(accessed on 20 February 2020)];Off. J. 2008 L 47:5–13. Available online: http://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1487548100586&uri=CELEX:32008L0120. [Google Scholar]
- 57.Jocelyn P.C. Spectrophotometric assay of thiols. Methods Enzymol. 1987;143:44–67. doi: 10.1016/0076-6879(87)43013-9. [DOI] [PubMed] [Google Scholar]
- 58.Da Costa C.M., Dos Santos R.C.C., Lima E.S. A simple automated procedure for thiol measurement in human serum samples. J. Bras. Patol. Med. Lab. 2006;42:345–350. doi: 10.1590/S1676-24442006000500006. [DOI] [Google Scholar]
- 59.Slaughter M.R., O’Brien P.J. Fully-automated spectrophotometric method for measurement of antioxidant activity of catalase. Clin. Biochem. 2000;33:525–534. doi: 10.1016/S0009-9120(00)00158-2. [DOI] [PubMed] [Google Scholar]
- 60.Arnao M., Caño A., Hernández-Ruiz J., García-Cánovas F., Acosta M. Inhibition byl-Ascorbic Acid and Other Antioxidants of the 2,2′-Azino-bis(3-ethylbenzthiazoline-6-sulfonic Acid) Oxidation Catalyzed by Peroxidase: A New Approach for Determining Total Antioxidant Status of Foods. Anal. Biochem. 1996;236:255–261. doi: 10.1006/abio.1996.0164. [DOI] [PubMed] [Google Scholar]
- 61.Campos C., Guzmán R., López-Fernández E., Casado Á. Evaluation of the copper(II) reduction assay using bathocuproinedisulfonic acid disodium salt for the total antioxidant capacity assessment: The CUPRAC–BCS assay. Anal. Biochem. 2009;392:37–44. doi: 10.1016/j.ab.2009.05.024. [DOI] [PubMed] [Google Scholar]
- 62.Benzie I.F.F., Strain J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 63.Erel O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005;38:1103–1111. doi: 10.1016/j.clinbiochem.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 64.Witko-Sarsat V., Friedlander M., Capeillère-Blandin C., Nguyen-Khoa T., Nguyen A.T., Zingraff J., Jungers P., Descamps-Latscha B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996;49:1304–1313. doi: 10.1038/ki.1996.186. [DOI] [PubMed] [Google Scholar]
- 65.Rhee S.G., Chang T.-S., Jeong W., Kang D. Methods for detection and measurement of hydrogen peroxide inside and outside of cells. Mol. Cells. 2010;29:539–549. doi: 10.1007/s10059-010-0082-3. [DOI] [PubMed] [Google Scholar]
- 66.Escribano D., Fuentes-Rubio M., Cerón J.J. Validation of an automated chemiluminescent immunoassay for salivary cortisol measurements in pigs. J. Veter Diagn. Investig. 2012;24:918–923. doi: 10.1177/1040638712455171. [DOI] [PubMed] [Google Scholar]
- 67.Hernández-Caravaca I., Gourgues S.F., Rodríguez V., Estrada E.D., Cerón J.J., Escribano D., Gourgues S.F., Rodriguez-Vega V. Serum acute phase response induced by different vaccination protocols against circovirus type 2 and Mycoplasma hyopneumoniae in piglets. Res. Veter Sci. 2017;114:69–73. doi: 10.1016/j.rvsc.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 68.Sauerwein H., Schmitz S., Hiss S. The acute phase protein haptoglobin and its relation to oxidative status in piglets undergoing weaning-induced stress. Redox Rep. 2005;10:295–302. doi: 10.1179/135100005X83725. [DOI] [PubMed] [Google Scholar]
- 69.Li Y., Zhao X., Jiang X., Chen L., Hong L., Zhuo Y., Lin Y., Fang Z., Che L., Feng B., et al. Effects of dietary supplementation with exogenous catalase on growth performance, oxidative stress, and hepatic apoptosis in weaned piglets challenged with lipopolysaccharide. J. Anim. Sci. 2020;98 doi: 10.1093/jas/skaa067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hemken R., Bremel D. Possible Role of Beta-Carotene in Improving Fertility in Dairy Cattle. J. Dairy Sci. 1982;65:1069–1073. doi: 10.3168/jds.S0022-0302(82)82314-X. [DOI] [PubMed] [Google Scholar]
- 71.Lauridsen C., Theil P.K., Jensen S.K. Composition of α-tocopherol and fatty acids in porcine tissues after dietary supplementation with vitamin E and different fat sources. Anim. Feed. Sci. Technol. 2013;179:93–102. doi: 10.1016/j.anifeedsci.2012.10.007. [DOI] [Google Scholar]
- 72.Shaish A., Daugherty A., O’Sullivan F., Schonfeld G., Heinecke J.W. Beta-carotene inhibits atherosclerosis in hypercholesterolemic rabbits. J. Clin. Investig. 1995;96:2075–2082. doi: 10.1172/JCI118256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Munday R., Munday J.S., Munday C.M. Comparative effects of mono-, di-, tri-, and tetrasulfides derived from plants of the Allium family: Redox cycling in vitro and hemolytic activity and Phase 2 enzyme induction in vivo. Free Radic. Biol. Med. 2003;34:1200–1211. doi: 10.1016/S0891-5849(03)00144-8. [DOI] [PubMed] [Google Scholar]
- 74.Vega Montalvo R., Carrillo Domínguez C. Efecto sobre la motilidad intestinal y toxicidad aguda oral del extracto fluido de Ocimum gratissimum L. (orégano cimarrón) Rev. Cuba Plantas Med. 1997;2:14–18. [Google Scholar]
- 75.Zou Y., Xiang Q., Wang J., Peng J., Wei H. Oregano Essential Oil Improves Intestinal Morphology and Expression of Tight Junction Proteins Associated with Modulation of Selected Intestinal Bacteria and Immune Status in a Pig Model. BioMed Res. Int. 2016;2016:1–11. doi: 10.1155/2016/5436738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Yan L., Kim I.H. Effects of dietary supplementation of fermented garlic powder on growth performance, apparent total tract digestibility, blood characteristics and faecal microbial concentration in weanling pigs. J. Anim. Physiol. Anim. Nutr. 2012;97:457–464. doi: 10.1111/j.1439-0396.2012.01286.x. [DOI] [PubMed] [Google Scholar]
- 77.Cho J., Liu S., Kim I.H. Effects of dietary Korean aged garlic extract by Leuconostoc mesenteroides KCCM35046 on growth performance, digestibility, blood profiles, gas emissions and microbiotain weanling pigs. Can. J. Anim. Sci. 2020 doi: 10.1139/cjas-2019-0111. [DOI] [Google Scholar]
- 78.Liu Y., Song M., Che T.M., Lee J.J., Bravo D., Maddox C.W., Pettigrew J.E. Dietary plant extracts modulate gene expression profiles in ileal mucosa of weaned pigs after an Escherichia coli infection1. J. Anim. Sci. 2014;92:2050–2062. doi: 10.2527/jas.2013-6422. [DOI] [PubMed] [Google Scholar]