Abstract
Extracorporeal membrane oxygenation (ECMO) is extracorporeal life support for life-threatening cardiopulmonary failure. Since its introduction, ECMO use has expanded to patients with more complex co-morbidities without change in patient mortality rates. Although many patients survive, significant neurologic complications like seizures, ischemic strokes and intracranial hemorrhage can occur during ECMO care. The risks of these complications often add to the complexity of decision making surrounding ECMO support. In this review, we discuss the pathophysiology and incidence of neurological complications in children supported on ECMO, factors influencing the incidence of these complications, commonly used neurologic monitoring modalities, and outcomes for this complex patient population. We discuss the current literature on the use of electroencephalography (EEG) for both seizure detection and monitoring of background EEG changes, in addition to the use of less commonly used imaging modalities like transcranial doppler (TCD). We present the knowledge gap and the lack of clinical concensus guidelines in managing these potentially life changing neurological complications. Additionally, we present future directions to further understand the pathophysiology of ECMO-related neurological complications.
Keywords: Extracorporeal membrane oxygenation, seizures, stroke, intracranial hemorrhage, electroencephalography
Introduction:
Extracorporeal membrane oxygenation (ECMO) is an advanced life support technique employed in intensive care units to support patients with cardiopulmonary failure. Venous blood is drained from the body into the extracorporeal circuit and pumped through an oxygenator for gas exchange. Oxygenated blood is then returned to the patient either through the venous circulation in venovenous ECMO (VV ECMO) or the arterial circulation in venoarterial ECMO (VA ECMO). ECMO has grown since its inception to become an invaluable tool in supporting children and adults with severe cardiac and pulmonary failure refractory to conventional management1. ECMO utilization has quadrupled in children and increased ten-fold in adults in the last two decades2,3. However, patients supported on ECMO continue to be at significant risk for neurologic injury, including hypoxic ischemic injury in the peri-cannulation period, as well as thromboembolic stroke and intracranial hemorrhage while on ECMO support. Neurologic injury on ECMO is associated with increased risk of mortality and neurologic disability among survivors4. In this review we briefly discuss the history of ECMO support in children, neurologic complications associated with extracorporeal life support, and future directions in neuromonitoring of ECMO patients, focused on minimizing neurologic complications and their sequelae in this vulnerable population.
Pediatric ECMO and pathophysiology of neurologic injury during ECMO
The concept of artificial oxygenation and perfusion was first used successfully for cardiac surgery in 19535, and the first successful long-term support for severe respiratory failure in an adult patient with post-traumatic acute respiratory failure was reported in 19726. The first report of successful use of ECMO in a newborn with severe respiratory failure by Bartlett in 19767 led to rapid expansion of extracorporeal support in children, particularly newborn infants, over the following two decades8.
The cannulation strategy and circuit configuration depend on the type of support needed: cardiovascular (VA ECMO), respiratory (VV ECMO) or both (VA ECMO), as well as the clinical context (e.g., emergent extracorporeal cardiopulmonary resuscitation [ECPR] with percutaneous bedside cannulation, ECMO initiation for cardiogenic shock post-cardiac surgery with central cannulation via sternotomy, etc.). ECMO involves cannulation of the heart or of one or two large vessels, typically the right common carotid artery and the right internal jugular vein in infants and small children, and the femoral vessels in older children and adults 9,10,11. Some ECMO centers place cephalad jugular catheters in infants with a jugular venous ECMO cannula, in an effort to augment venous return, reduce atrial recirculation, and decompress the cerebral venous circulation. The most recent evaluations of this practice showed similar outcomes, including survival and neurologic complications, when comparing ECMO patients with vs without cephalad jugular catheter placement 12.
Rates of neurologic complications are higher in infants and children supported on VA ECMO compared to those supported on VV ECMO 13. While VV vs VA cannulation decisions are made driven primarily by type of support needed, the added physiologic impact of a carotid or aortic cannulation are nontrivial. With an intact circle of Willis, blood supply can reach the right hemisphere normally perfused by the ligated carotid artery. However, blood flow to the right hemisphere may have decreased velocity, particularly with concomitant decreased venous outflow from the ligated right internal jugular vein 14–16. ECMO also appears to alter cerebral blood flow within smaller vessels. Animal studies demonstrate impaired cerebrovascular autoregulation at lower perfusion pressures 17, likely due to lack of flow pulsatility or exhaustion of compensatory dilation with lower flow states 18. The effects of carotid artery cannulation on long-term neurologic outcomes remain unclear, especially as there is variability in practice in terms of the carotid artery repair at the time of decannulation, varying from 6.6% of all patients to 10.7% of patients older than 5 years 19–22.
The alteration of cerebral blood flow and cerebrovascular autoregulation during ECMO, combined with the underlying pathophysiology of critical illness and the use of sedative medications, contribute to decreased cerebral metabolism during ECMO. This is reflected in reduced power and amplitude on electroencephalography (EEG), and a global decrease in power on quantitative EEG during ECMO, which can be restored after decannulation from ECMO 23.
Lastly, exposure to the foreign surfaces of the ECMO circuit triggers inflammatory and pro-thrombotic responses 24–27 that are additive to the patients’ critical illness processes such as disseminated intravascular coagulopathy [DIC] 28, sepsis, autoimmune diseases, pregnancy or trauma 29. To avoid clot formation in the circuit, ECMO patients are typically anticoagulated using a continuous infusion of unfractionated heparin or a direct thrombin inhibitor. Patients are therefore at risk for both thromboembolic events due to clots dislodged from the circuit, and hemorrhagic events associated with long-term anticoagulation in the presence of DIC, surgical wounds, etc. Bleeding (intracranial hemorrhage [ICH]) and thrombotic (stroke) neurologic complications can occur at any time during the days, weeks or months of ECMO support 26.
Outcomes of critically ill children supported on ECMO
Severe refractory cardiopulmonary failure or cardiac arrest requiring ECMO support in children continues to be associated with high mortality. In general, survival trends have been stable over the last decade. In a recent report of Extracorporeal Life Support Organization (ELSO) registry data, neonatal respiratory, pediatric respiratory and pediatric cardiac ECMO survival rates were around 61%, while neonatal cardiac, and neonatal and pediatric ECPR survival rates were within three percentage points of 42% 3.
A systematic review of the literature of neurologic outcomes following ECMO support in infants and children summarized data from 60 articles published between 2000 and 2016 30. At hospital discharge, 48% of survivors (cumulative n=673) had favorable outcome defined as Pediatric Cerebral Performance Category (PCPC) <3 or unchanged from baseline. Long-term follow-up showed that 10% to 50% of children scored more than 2SD below the population mean on cognitive testing, 16% to 46% displayed behavioral problems, and 12% had evidence of severe motor impairment 30. The wide ranges in disability rates reported were due to heterogeneity of underlying diagnoses, of outcomes measures employed, and varying age at follow-up 30. Overall, neurologic outcomes in congenital diaphragmatic hernia (CDH), cardiac disease, and cardiac arrest populations tended to be worse than those in respiratory or mixed ECMO populations 30.
New data are emerging from more reports published since 2016, with mixed results. In a mixed neonatal and pediatric ECMO population, results from a multicenter U.S. cohort study showed that, at hospital discharge, 32% of 282 survivors had good, 40% mildly abnormal, 24% moderately abnormal, and 5% severely or very severely abnormal function based on the Functional Status Scale 31. A standardized 1-year follow-up clinic after respiratory ECMO identified neurodevelopmental problems in 30% survivors who attended the clinic (n=98) 32. In a two-center U.S. cohort study conducted in a mixed neonatal and pediatric ECMO population, structured 6-month and 1-year post-ECMO neurologic and neuropsychological evaluations showed that ECMO survivors had scores for adaptive behavior, cognitive, neurologic and quality of life that were all below the general population means, but mostly considered minor within each domain 4. The median Vineland Adaptive Behavior Scales-II (VABS-II) score was 91 (interquartile range [IQR], 81-98) (general population mean, 100; SD, 15), with 65% of survivors who attended the study follow-up visits having a VABS-II score ≥85 (n=40) [4]. Lastly, in a secondary analysis of the Therapeutic Hypothermia After Pediatric Cardiac Arrest (THAPCA) trials, 30.5% of study participants who underwent ECPR survived to 12 months with VABS-II ≥70 33. While the heterogeneity of patient populations and outcomes measures used precludes aggregate analyses, in these recent cohorts, approximately one third of surviors to hospital discharge or long-term follow-up display moderate to severe neurodevelopmental, behavioral, or functional deficits.
Neurologic complications in pediatric ECMO patients
Neurologic injury associated with ECMO may be sustained prior to, during, or after ECMO support 34. Despite the increase in ECMO use over the last decade, the incidence of reported neurological complications has not changed significantly3. Table 1 summarizes some of the reported ECMO related risk factors associated with neurological complications 21,31,35–41.
Table 1.
Reported risk factors associated with neurologic complications and post-discharge functional, neurobehavioral or cognitive deficits in children requiring ECMO.
| Factor potentially increasing risk | Neurologic complication | Reference | ||||
|---|---|---|---|---|---|---|
| Seizure | Stroke | Any CNS complication | Brain Death | Post-discharge deficits | ||
| ECMO Indication | ||||||
| ECPR | ⅹ | Ⅹ | 31,35–37,41 | |||
| ECMO modality and cannulation site | ||||||
| VA | Ⅹ | 31,35,37 | ||||
| Carotid | Ⅹ | Ⅹ | 21,36,38 | |||
| Femoral | Ⅹ | 36 | ||||
| Patient factors | ||||||
| Younger age | Ⅹ | ⅹ | Ⅹ | 37,39–41 | ||
| Renal Failure | ⅹ | Ⅹ | 31,35 | |||
| Hepatic dysfunction | Ⅹ | 31 | ||||
| Sepsis | ⅹ | 41 | ||||
| Pre-ECMO chronic conditions | Ⅹ | 31,33 | ||||
| Longer hospitalization | Ⅹ | 31 | ||||
| Lactate | Ⅹ | 33 | ||||
| Creatinine >3.0 mg/dL | Ⅹ | 35 | ||||
| pH <7.2 pH <7.1 |
Ⅹ | ⅹ | Ⅹ Ⅹ |
35,40,41 37 |
||
| Inotropic support | ⅹ | Ⅹ | 35,41 | |||
| Thrombocytopenia <30 cells/µL | Ⅹ | 40 | ||||
ECMO = extracorporeal membrane oxygenation, ECPR = extracorporeal cardiopulmonary resuscitation, VA = venoarterial, RBC = red blood cells
Commonly reported neurological complications include seizures, ischemic strokes and intracranial hemorrhage:
Seizures
Several studies have shown that seizures occur in approximately 20% (18%−23%) of neonates and children supported on ECMO 40,42,43 (Table 2).
Table 2.
Reported incidence of seizures in children on ECMO support monitored by continuous EEG
| Year | Author | n | Agea | % ES | % NCS | % SE |
|---|---|---|---|---|---|---|
| 2013 | Piantino et al. | 19 | 2.7 (0.5-6.8) years | 21% | 16% | 10% |
| 2017 | Lin et al. | 99 | 20 (3-120)b vs 13 (3-364)c days | 18% | 15% | 11% |
| 2018 | Okochi et al. | 70 | 0.6 (0.2-3.6)b vs 6.7 (1.1-62.3)c months | 23% | 12% | 5% |
n, number of patients; ES, electrographic-confirmed seizures; NCS, non-convulsive seizures; SE, status epilepticus
Age reported as median (interquartile range);
Seizure group,
No seizure group
The incidence of electrographic seizures without clinical correlates ranged between 12% and 16% of all monitored patients, with evidence of status epilepticus in over half of these patients. The majority of the identified seizures occurred in the first 24 hours of monitoring following initiation of ECMO support 40,42,43. The incidence of seizures correlated with the presence of intracranial pathology on further head imaging and with higher mortality and unfavorable neurodevelopmental outcomes 44, findings consistent with others evaluating the impact of seizures on long term functional outcomes in children 45,46.
Similar rates of seizures, convulsive and non-convulsive, have been reported in ECMO patients monitored with intermittent EEG 47. The July 2019 ELSO registry international report showed EEG-confirmed seizure rates ranging between 1.9% in pediatric patients on respiratory ECMO support to 11.4% in neonates who underwent ECPR (data for 2018 ELSO registry entries) (Table 3) 48. The noticeably lower seizure rates in the ELSO registry are likely an underestimate of the true prevalence of seizures during ECMO, as detected by single center quality improvement or research studies deploying active EEG monitoring.
Table 3.
Neurological complication rates by age group and mode of ECMO support for 2018 as reported in the July 2019 ELSO registry international report48
| Neonatal | Pediatric | |
|---|---|---|
| Seizures confirmed by EEG | ||
| Respiratory ECMO | 4.8% | 1.9% |
| Cardiac ECMO | 5.4% | 3% |
| ECPR | 11.4% | 8.3% |
| Central nervous system bleeding confirmed by ultrasound, CT or MRI | ||
| Respiratory ECMO | 10.1% | 7.8% |
| Cardiac ECMO | 11.1% | 5.1% |
| ECPR | 17.7% | 8.1% |
| Central nervous system infarction confirmed by ultrasound, CT or MRI | ||
| Respiratory ECMO | 3.4% | 5% |
| Cardiac ECMO | 2% | 3.1% |
| ECPR | 4.4% | 7.2% |
The etiology of seizures in neonates and children supported with ECMO is multifactorial. Risk factors include hypoxic ischemic injury following cardiac arrest, and presence of intracranial hemorrhage or ischemic stroke45. Earlier attempts to link the pathophysiology of seizures to alterations in cerebral blood flow during ECMO by examining patients with subclavian steal phenomenon in the setting of carotid artery cannulation were not fruitful 49.
Seizures increase a child’s risk for unfavorable short-term neurologic outcomes 50, death 51–53, and increased rates of cerebral palsy and cognitive deficits at long-term follow-up44,54. The frequency and potential long-term impact of seizures emphasize the need for vigilant EEG monitoring, with prompt detection and treatment of seizures for all patients supported on ECMO.
Ischemic stroke
Ischemic strokes occur in up to 33% of pediatric ECMO patients 38,55,56, with much lower rates reported in the ELSO registry (2-7.2%) (Table 3). In children, strokes have a variety of presentations, including a mix of both focal and diffuse signs (e.g., altered mental status) in half of cases, and seizures in up to a third of all cases 57. Standard medical care for ECMO, including the use of sedation, potential need for neuromuscular blockade and post-cannulation patient positioning may render detection of early stroke signs challenging. Risk factors for stroke during ECMO include circuit thrombosis with dislodgement of emboli, and acute alterations in cerebral arterial circulation with carotid artery cannulation, although studies are mixed as to whether carotid cannulation increases ischemic stroke risk 21 or not 58.
Intracranial hemorrhage (ICH)
Despite advances in monitoring of hemostasis and anticoagulation management on ECMO, bleeding events complicate up to 66%-80% of ECMO runs 48,59. Incidence of ICH in the literature ranges between 3.6% in combined adult and pediatric registry studies and up to 16% in pediatric-only cohorts 59–61. This rather wide range may be explained by underdetection and underreporting in registry data and the lack of standardized screening and uniform definitions for ICH. Reported ICH diagnoses include large intraparenchymal hemorrhages (IPH), petechial IPH, subarachnoid hemorrhage, subdural hemorrhage and combinations thereof 62,63. Subtle abnormalities such as cerebral microbleeds have also been detected on post-ECMO MRI, with unclear clinical significance at this time and phenotypes ranging from asymptomatic to persistent coma 64–68. The incidence of ICH appears to be highest within the first 4 to 5 days of the ECMO course 59,69. ICH has been shown to correlate with both increased mortality and worse functional neurologic outcomes 59,70.
In adults, risk factors for ICH on ECMO include disease-related factors such as pre-ECMO cardiac arrest 71, sepsis 41,60, , influenza71, renal failure 63, use of renal replacement therapy, pre-ECMO duration of mechanical ventilation and low admission fibrinogen 63. ECMO-related risk factors include thrombocytopenia 63, hemolysis 71, inotropic support 41 and acute rapid increase in PaO2 and decrease in PaCO2 with the initiation of VV ECMO 68. Cerebral venous congestion due to obstruction of venous outflow from cannulation of the right internal jugular vein may also increase the risk of ICH 72. Interestingly, in a single center study, patient coagulation profiles were not predictive for acute ICH or infarct on ECMO 73.
Neurologic monitoring of ECMO patients
The combination of critical illness with hypoxia, acidosis, and multiple organ failure, cannulation and ligation of major neck vessels, impaired cerebrovascular autoregulation, and concomitant risk for thrombus dislodgment from the ECMO circuit and bleeding due to exposure to systemic anticoagulation, render ECMO patients at high risk for neurologic complications. Clinical monitoring by bedside examination is often limited by sedatives and possibly neuromuscular blockade. Additional neuromonitoring methods therefore enhance neurologic assessment during ECMO and assist with early detection of neurologic injury 74. Table 4 summarizes the recommended guidelines for pediatric and neonatal neuromonitoring during ECMO support 75,76.
Table 4.
Neuromonitoring recommendations for pediatric and neonatal patients on ECMO support
| Age group | ECMO indication | Recommendation |
|---|---|---|
| Neonatal | Respiratory failure | • Serial cranial ultrasound while on ECMO • Brain MRI prior to discharge • Continuous EEG monitoring for at least 24 hours |
| Pediatric | Respiratory failure | • Head CT or brain MRI prior to discharge • Continuous EEG monitoring for at least 24 hours |
| Neonatal Pediatric | Cardiac failure | • Screening cranial ultrasound and more definitive neuroimaging (head CT) as needed during ECMO • Brain MRI prior to discharge • Continuous EEG monitoring for at least 24 hours |
ECMO = extracorporeal membrane oxygenation, MRI = magnetic resonance imaging, CT = computed tomography, EEG = electroencephalography
A. Modalities to monitor brain physiology that can impact ECMO management
Electroencephalography (EEG)
EEG is used to assess for the presence of seizures and to evaluate the brain’s background electrical activity and organization, including state change, asymmetry, and focal abnormalities such as slowing or epileptiform discharges (Figure 1). EEG background abnormalities are associated with mortality and unfavorable neurodevelopmental outcomes in neonatal and pediatric patients 51,77,78,79. EEG monitoring requires experience in both set-up and interpretation but has become the recommended modality of monitoring for critically ill children at risk for seizures and acute neurologic injury, including those supported on ECMO 75. The American Clinical Neurophysiology Society (ACNS) Consensus Statement on continuous EEG (cEEG) in Critically Ill Adults and Children designated children on ECMO requiring sedation and paralysis as a high-risk group that should be monitored by cEEG 75.
Figure 1: Examples of EEG tracings for patients supported on ECMO.
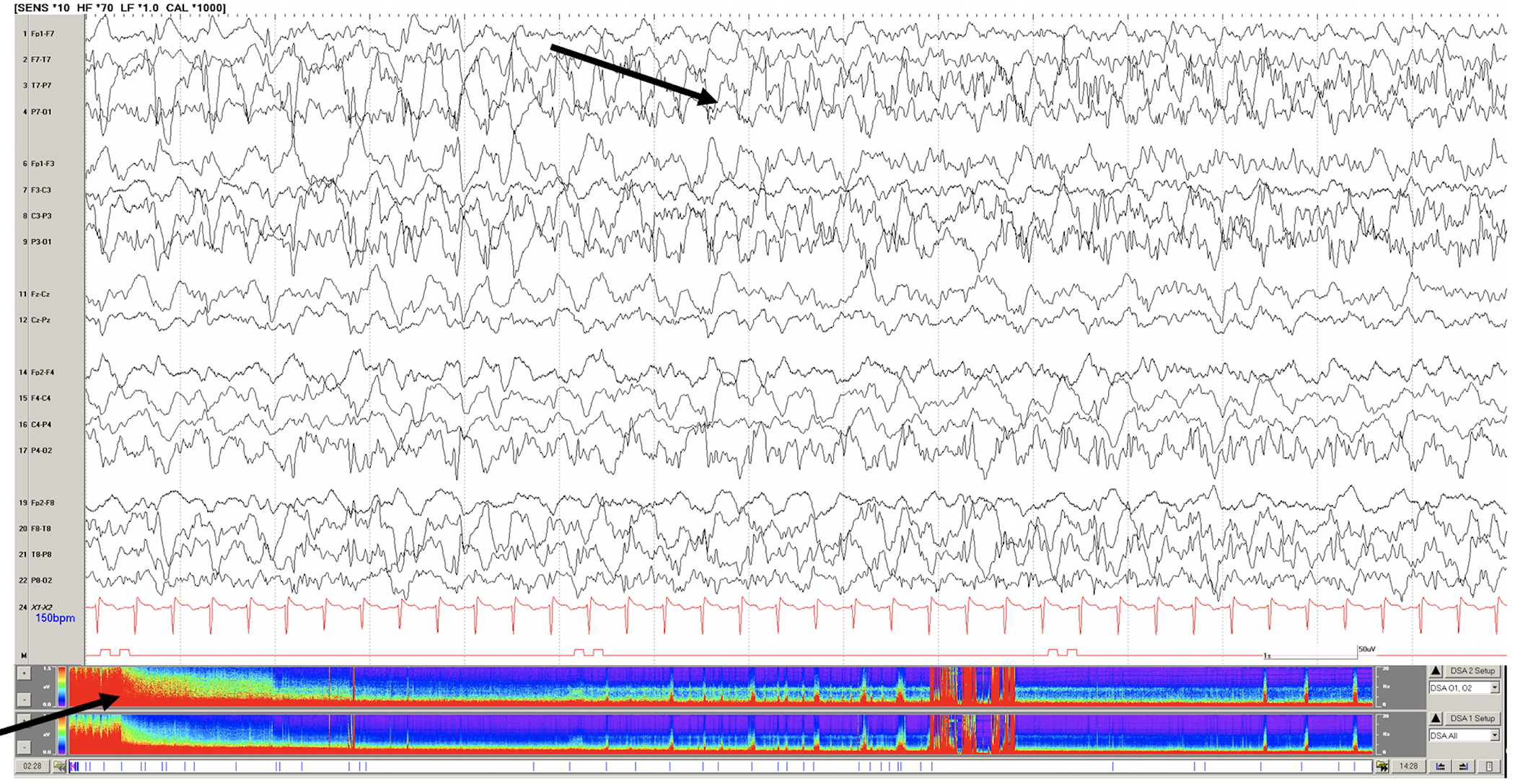
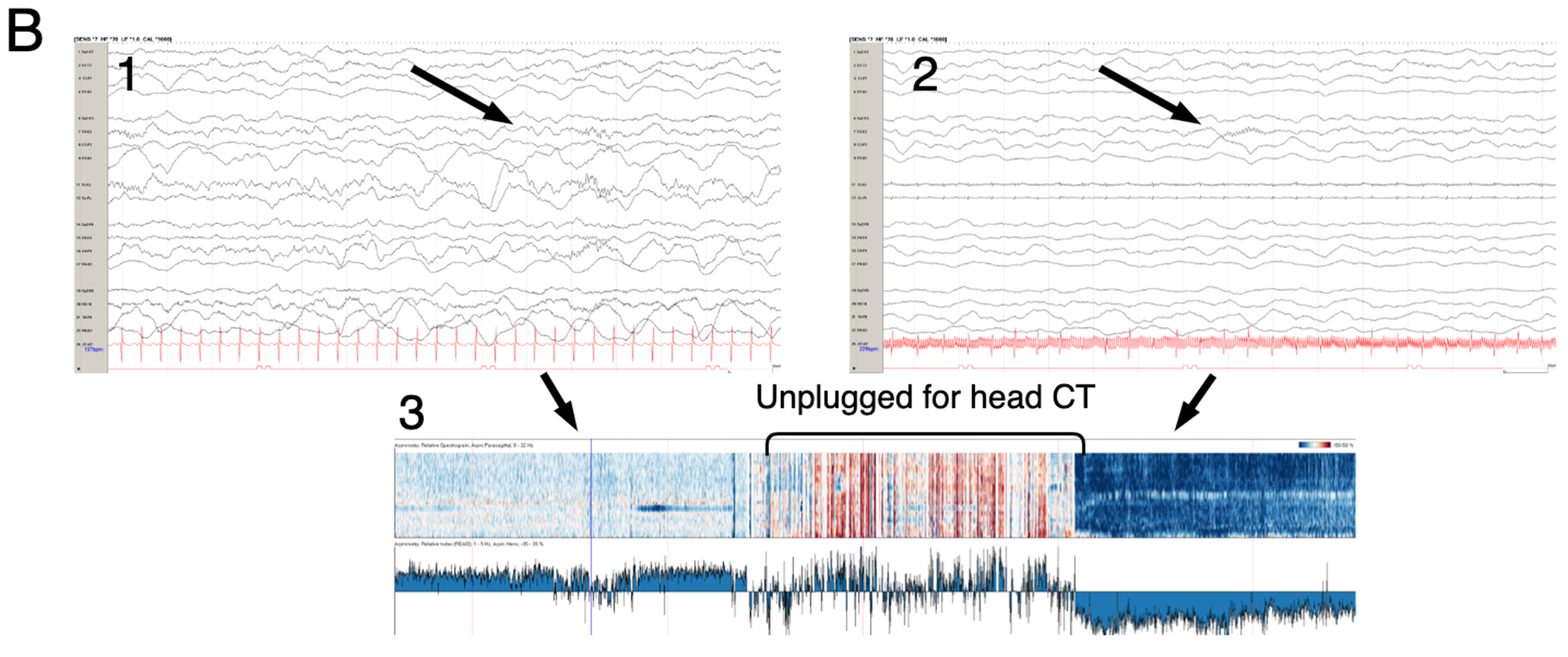
A. Raw EEG tracing of a 7 year old with respiratory failure requiring VA ECMO support. Continuous video EEG monitoring initiated soon after cannulation revealed status epilepticus (black arrows on raw EEG tracing and density spectral array) and subsequent seizures during recording. B. Raw EEG (1 and 2) and asymmetry index from quantitative EEG (3) of a 1 year-old child on VA ECMO support after near-drowning. While unplugged from EEG recording for head CT the patient suffered a right hemispheric stroke. (1) Raw EEG tracing with symmetric sleep spindles corresponding to normal asymmetry index. Upon return from CT suite, the patient demonstrated asymmetry of sleep spindles (2) and dramatically increased relative power or activity (dark blue in 3) on the left and not right hemisphere.
There are recognized challenges of EEG monitoring. Placement of EEG electrodes for extended periods of time poses the risk of compromising scalp integrity, especially in neonates. Electrical artifact from the ECMO circuit can mimic pathologic EEG findings, particularly in younger children 74. However, these challenges are easily mitigate with experience, expertise, and careful skin monitoring. There is growing interest in the utiity of bedside real time compressed EEG data as Digital Spectral Array (DSA) to aid clinicians in early seizure detection 80,81. Prompt recognition and quantification of seizures is important as there is growing evidence of the correlation between seizure burden and worse brain injury and outcomes 82.
Imaging
Ten to 60% of pediatric ECMO survivors have been found to have abnormal neuroimaging studies 83–85. Most ECMO centers have institutional clinical protocols for the type and timing of neuroimaging studies employed during and following ECMO (cranial ultrasound, brain CT or brain MRI) explaining the wide range of these reported incidences.
Cranial Ultrasound
Ultrasonography of the brain is a non-invasive, portable imaging option for infants with an open fontanelle. Ultrasound has become part of the routine clinical care of infants supported on ECMO. ELSO guidelines recommend obtaining a pre-ECMO ultrasound followed by daily head ultrasounds for the first week on ECMO, then every-other-day screening or more frequent if indicated 76. Raets et al proposed a schematic classification of neuroimaging abnormalities detected by ultrasound in neonatal ECMO consisting of vessel occlusion (i.e., arterial ischemic stroke, venous sinus thrombosis), primary hemorrhage (i.e., extracerebral, choroid plexus, parenchymal) and other abnormalities (e.g., postasphyxial injury, preterm white matter injury, etc) 61. Abnormal ultrasound findings have not been shown to predict neurodevelopmental outcomes 86,87, although the type and location of certain lesions detected by ultrasound may correlate with neurologic outcomes. Cranial ultrasound is limited by the size of an open anterior fontanelle, limited field of view, and diagnostic variability 88. In a single center study of 50 neonates with a brain MRI after ECMO, 50% of infants with normal cranial ultrasounds during ECMO had an abnormal MRI83. For these reasons, cranial ultrasound remains a screening tool, with brain CT and MRI recommended as confirmatory studies76.
Measurement of resistive indeces (RI) using cranial ultrasounds represents a potential neurologic monitoring method for infants on ECMO 89. The variability of the RI on Doppler sonography measured at baseline and after gentle fontanel compression has been proposed as a test to detect early disturbances in cerebrovascular regulation. In a single center study, RI variability in the anterior cerebral artery of 10% or greater on any ECMO day was associated with increased risk for cerebrovascular complications in neonates supported on ECMO89.
Transcranial Doppler (TCD)
TCD is the application of ultrasound technology to sonate blood flow through major arteries. Unlike cranial imaging ultrasounds, TCD is not limited to children with open fontanelles, and allows for real-time monitoring of cerebral blood flow patterns in the broader pediatric critical care population 90. Flow patterns for children supported on ECMO are altered compared to age-matched normal controls 91. Higher than normal cerebral blood flow velocities on ECMO may signal cerebral hemorrhage days prior to clinical recognition 92. Increased pulsatility index may also correlate with ischemic injury 93. As with cranial ultrasound, wide use of TCD is rendered difficult by limited fields of view and operator dependence 88.
Computed Tomography (CT)
Older infants and children cannot be monitored with serial cranial ultrasounds while on ECMO, thus neuroimaging studies during ECMO in this patient population are limited to head CT. Head CT is mainly performed when there is clinical suspicion of acute neurologic injury (Figure 2). Its use is limited by the need for transport (unless portable CT is available)94, exposure to radiation and relative insensitivity to early ischemic injury. Recent studies have shown higher detection rates of ischemic stroke and ICH with more active CT use, reflecting the possible underreporting in centers with less frequent imaging 55,69. Advantages of CT include a full field of view of the brain, operator independence and excellent detection of ICH.
Figure 2.
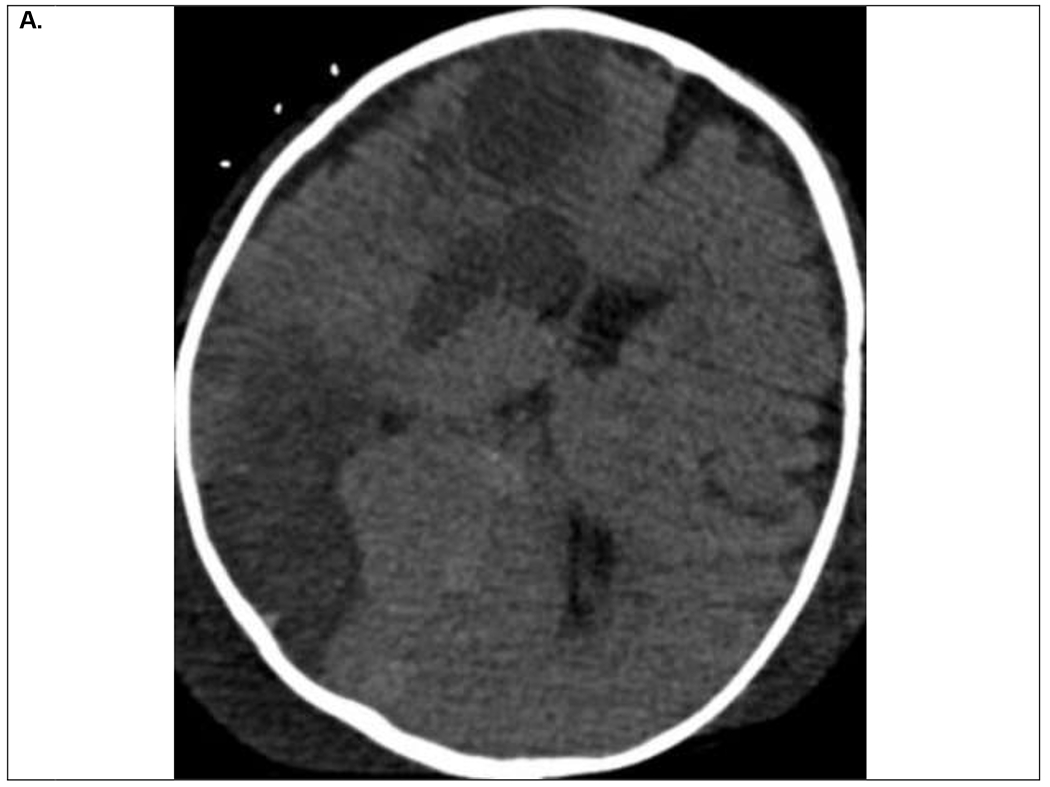
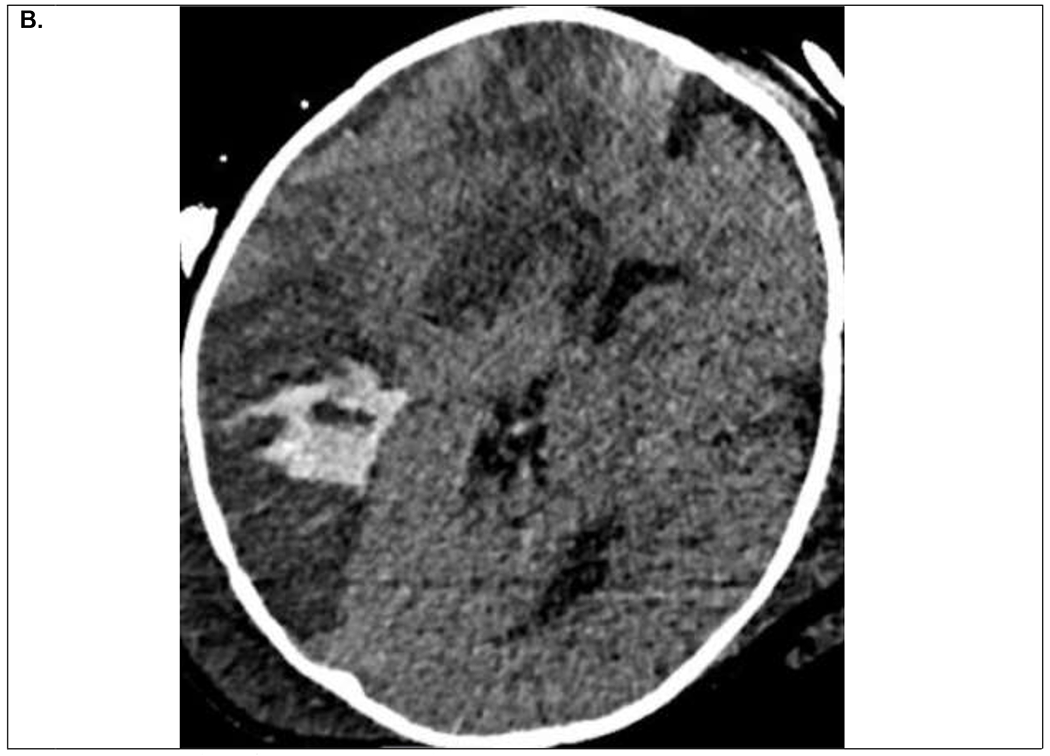
9 month old infant on VA ECMO support for refractory septic shock and multiorgan dysfunction. Within first 24 hours of ECMO course, the patient developed clinical signs of right sided facial venous congestion and asymmetric slowing on EEG, initially thought to be secondary to superior vena cava obstruction by the ECMO venous drainage cannula. A. Head CT on ECMO day 3 demonstrated multifocal ischemic infarcts within the right hemisphere for which his systemic anticoagulation was temporarily held. B. Follow up head CT on ECMO day 5 demonstrated evolution of the ischemic infarcts and hemorrhagic conversion within the posterior ischemic infarct. He was decannulated on ECMO day 9 and discharged home on hospital day 68 with residual left hemiparesis.
Detection of neuroimaging abnormalities, ICH or ischemic stroke, may affect anticoagulation strategies and the ability to continue extracorporeal support and systemic anticoagulation, depending on the severity of the recognized complication.
Near Infrared Spectroscopy (NIRS)
NIRS technology utilizes light in the near-infrared spectrum to distinguish between oxygenated and de-oxygenated red blood cells, reflecting changes in tissue oxygenation and blood volume 95. This technology has been present for the past two decades, but its clinical applications are still expanding. Absolute or relative (e.g., decline by >20% from baseline) reduction in regional cerebral oximetry has been shown to be associated with neurological injury confirmed by imaging in critically ill children 96–98 and adults 99 supported on ECMO.
B. Monitoring modalities for outcome prognostication
Plasma brain injury biomarkers
Recent years have seen an increasing number of studies investigating plasma brain injury biomarkers as both neuromonitoring and prognostication tools in neurocritical care conditions, spearheaded by initial efforts in the field of traumatic brain injury 100. The complexity of brain injury in ECMO patients is unlikely to be captured by a single biomarker, but panels of biomarkers may prove useful in distinguishing different pathologic-anatomic processes. High concentrations of brain-specific proteins circulating in blood have been shown to be associated with abnormal neuroimaging findings during or immediately following ECMO support, with survival after critical illness requiring ECMO support, or with neurofunctional status at hospital discharge in former ECMO patients101–106. These proteins reflect neuronal injury (e.g., neuron specific enolase [NSE]), astrocytic injury (e.g., glial fibrillary acidic protein [GFAP], S100b), and neuroinflammation (e.g., monocyte chemoattractant protein 1 [MCP1], intercellular adhesion molecule-5 [ICAM-5])101–106.
Magnetic resonance imaging (MRI)
ECMO equipment is not MRI compatible and brain MRI studies are therefore limited to the post-decannulation period. As such, MRI is not technically a neuromonitoring modality while on ECMO. MRI is more sensitive and specific for intracranial pathology compared to cranial ultrasound and head CT and does not involve expose to radiation 83,85. However, there is institutional variability in indications for and timing of post-ECMO MRI 74. The current ELSO guidelines recommend neuroimaging in the form of head CT or MRI prior to discharge in neonatal and pediatric patients supported on ECMO for any indication 76.
Future directions
The contemporary ICU allows for multimodal neuromonitoring of critically ill patients at risk for acute neurologic injury. Increasingly, patients are monitored and systems are developed for concomitant cerebral oximetry, continuous EEG, non-invasive cerebrovascular autoregulation monitoring and serial TCD monitoring 74,107–109. Quantitative EEG or cerebrovascular autoregulation software allowing for rapid interpretation of otherwise complicated and difficult-to-interpret continuous data are being evaluated in at-risk ICU populations 110–114. Emerging plasma biomarkers of brain injury could provide a real-time, non-invasive measure of acute neurologic injury and recovery 101,103,115, with promising progress towards the development of point-of-care devices to measure such biomarkers 116. There is also growing appreciation of the importance of post-ECMO functional outcome assessments.
Studies of long-term outcomes of ECMO survivors are limited by the heterogeneity in pathologies, outcome measures used, and age at follow-up 30. Professional society and research networks are developing working groups aiming to collaborate in drafting recommendations for the use of standardized outcomes measures that are easy to implement and validated in several languages. Timing of follow-up would also need to be harmonized with age-specific recommendations by groups focusing on specific pathologies (e.g., congenital heart disease)117. Development of a core set of outcome measures to be used in future ECMO studies would aid in further understanding the risk factors and prognostic markers for unfavorable outcomes, and in identifying opportunities for intervention to improve patient outcomes 30.
Recent advances in ECMO technology have attempted to minimize the pro-inflammatory and pro-thrombotic effects of exposure to the ECMO circuit through development of hollow fiber oxygenators, biocompatible coating for tubing and oxygenators, reduced surface area of ECMO circuits, and application of high blood flow rates that minimize blood stasis within the circuit 118. Emerging circuit surface modifications have the potential to decrease the risk of thrombus formation and thus of embolization, and to safely allow for reduced or complete elimination of systemic anticoagulation eventually, thus theoretically decreasing the risk for both ischemic embolic stroke and intracranial hemorrhage 119. Surface modifications include biomimetic surfaces (e.g., heparin, nitric oxide, direct thrombin inhibitors), biopassive surfaces (e.g., phosphorylcholine, albumin, etc) and endothelialization of blood contacting surfaces 119.
In summary, the burden of acute neurologic injury surrounding neonatal and pediatric ECMO support remains high, as do the rates of mortality and of unfavorable outcomes in survivors. Improving current neurologic monitoring methods and developing novel, real-time monitoring modalities during the high-risk periods leading to ECMO and during ECMO support are paramount for early detection of acute neurologic injury. Standardizing neurologic outcome assessments among survivors is an important next step for evaluating the true success of ECMO monitoring, management and long-term follow up. This in turn would promote early neuroprotective interventions with the ultimate goal to improve neurodevelopmental outcomes in this vulnerable population.
Acknowledgement:
This work was supported by K23NS099472 (KPG) and R01NS106292 (MMB).
References:
- 1.Makdisi G, Wang IW. Extra Corporeal Membrane Oxygenation (ECMO) review of a lifesaving technology. J Thorac Dis. 2015;7(7):E166–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Thiagarajan RR, Barbaro RP, Rycus PT, et al. Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63(1):60–67. [DOI] [PubMed] [Google Scholar]
- 3.Barbaro RP, Paden ML, Guner YS, et al. Pediatric Extracorporeal Life Support Organization Registry International Report 2016. ASAIO J. 2017;63(4):456–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bembea MM, Felling RJ, Caprarola SD, et al. Neurologic Outcomes in a Two-Center Cohort of Neonatal and Pediatric Patients Supported on Extracorporeal Membrane Oxygenation. ASAIO J. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gibbon JH Jr. Application of a mechanical heart and lung apparatus to cardiac surgery. Minn Med. 1954;37(3):171–185; passim. [PubMed] [Google Scholar]
- 6.Hill JD, O’Brien TG, Murray JJ, et al. Prolonged extracorporeal oxygenation for acute post-traumatic respiratory failure (shock-lung syndrome). Use of the Bramson membrane lung. N Engl J Med. 1972;286(12):629–634. [DOI] [PubMed] [Google Scholar]
- 7.Bartlett RH, Gazzaniga AB, Jefferies MR, Huxtable RF, Haiduc NJ, Fong SW. Extracorporeal membrane oxygenation (ECMO) cardiopulmonary support in infancy. Trans Am Soc Artif Intern Organs. 1976;22:80–93. [PubMed] [Google Scholar]
- 8.Bartlett RH. Esperanza: The First Neonatal ECMO Patient. ASAIO J. 2017;63(6):832–843. [DOI] [PubMed] [Google Scholar]
- 9.Pavlushkov E, Berman M, Valchanov K. Cannulation techniques for extracorporeal life support. Ann Transl Med. 2017;5(4):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stulak JM, Dearani JA, Burkhart HM, Barnes RD, Scott PD, Schears GJ. ECMO cannulation controversies and complications. Semin Cardiothorac Vasc Anesth. 2009;13(3):176–182. [DOI] [PubMed] [Google Scholar]
- 11.Harvey C Cannulation for Neonatal and Pediatric Extracorporeal Membrane Oxygenation for Cardiac Support. Front Pediatr. 2018;6:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Skarsgard ED, Salt DR, Lee SK, Extracorporeal Life Support Organization R. Venovenous extracorporeal membrane oxygenation in neonatal respiratory failure: does routine, cephalad jugular drainage improve outcome? J Pediatr Surg. 2004;39(5):672–676. [DOI] [PubMed] [Google Scholar]
- 13.Rollins MD, Hubbard A, Zabrocki L, Barnhart DC, Bratton SL. Extracorporeal membrane oxygenation cannulation trends for pediatric respiratory failure and central nervous system injury. J Pediatr Surg. 2012;47(1):68–75. [DOI] [PubMed] [Google Scholar]
- 14.Raju TN, Kim SY, Meller JL, Srinivasan G, Ghai V, Reyes H. Circle of Willis blood velocity and flow direction after common carotid artery ligation for neonatal extracorporeal membrane oxygenation. Pediatrics. 1989;83(3):343–347. [PubMed] [Google Scholar]
- 15.Matsumoto JS, Babcock DS, Brody AS, Weiss RG, Ryckman FG, Hiyama D. Right common carotid artery ligation for extracorporeal membrane oxygenation: cerebral blood flow velocity measurement with Doppler duplex US. Radiology. 1990;175(3):757–760. [DOI] [PubMed] [Google Scholar]
- 16.Weber TR, Kountzman B. The effects of venous occlusion on cerebral blood flow characteristics during ECMO. J Pediatr Surg. 1996;31(8):1124–1127. [DOI] [PubMed] [Google Scholar]
- 17.Short BL, Walker LK, Bender KS, Traystman RJ. Impairment of cerebral autoregulation during extracorporeal membrane oxygenation in newborn lambs. Pediatr Res. 1993;33(3):289–294. [DOI] [PubMed] [Google Scholar]
- 18.Kazmi SO, Sivakumar S, Karakitsos D, Alharthy A, Lazaridis C. Cerebral Pathophysiology in Extracorporeal Membrane Oxygenation: Pitfalls in Daily Clinical Management. Crit Care Res Pract. 2018;2018:3237810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.H IJ, Hunfeld M, Schiller RM, et al. Improving Long-Term Outcomes After Extracorporeal Membrane Oxygenation: From Observational Follow-Up Programs Toward Risk Stratification. Front Pediatr. 2018;6:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia AV, Jeyaraju M, Ladd MR, et al. Survey of the American Pediatric Surgical Association on cannulation practices in pediatric ECMO. J Pediatr Surg. 2018;53(9):1843–1848. [DOI] [PubMed] [Google Scholar]
- 21.Di Gennaro JL, Chan T, Farris RWD, Weiss NS, McMullan DM. Increased Stroke Risk in Children and Young Adults on Extracorporeal Life Support with Carotid Cannulation. ASAIO J. 2019. [DOI] [PubMed] [Google Scholar]
- 22.Sutter R, Tisljar K, Marsch S. Acute Neurologic Complications During Extracorporeal Membrane Oxygenation: A Systematic Review. Crit Care Med. 2018;46(9):1506–1513. [DOI] [PubMed] [Google Scholar]
- 23.Trittenwein G, Plenk S, Mach E, et al. Quantitative electroencephalography values of neonates during and after venoarterial extracorporeal membrane oxygenation and permanent ligation of right common carotid artery. Artif Organs. 2006;30(6):447–451. [DOI] [PubMed] [Google Scholar]
- 24.Besser MW, Klein AA. The coagulopathy of cardiopulmonary bypass. Crit Rev Clin Lab Sci. 2010;47(5–6):197–212. [DOI] [PubMed] [Google Scholar]
- 25.Lo B, Fijnheer R, Castigliego D, Borst C, Kalkman CJ, Nierich AP. Activation of hemostasis after coronary artery bypass grafting with or without cardiopulmonary bypass. Anesth Analg. 2004;99(3):634–640, table of contents. [DOI] [PubMed] [Google Scholar]
- 26.Millar JE, Fanning JP, McDonald CI, McAuley DF, Fraser JF. The inflammatory response to extracorporeal membrane oxygenation (ECMO): a review of the pathophysiology. Crit Care. 2016;20(1):387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Da Q, Teruya M, Guchhait P, Teruya J, Olson JS, Cruz MA. Free hemoglobin increases von Willebrand factor-mediated platelet adhesion in vitro: implications for circulatory devices. Blood. 2015;126(20):2338–2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wada H, Matsumoto T, Yamashita Y. Diagnosis and treatment of disseminated intravascular coagulation (DIC) according to four DIC guidelines. J Intensive Care. 2014;2(1):15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Raffini L Anticoagulation with VADs and ECMO: walking the tightrope. Hematology Am Soc Hematol Educ Program. 2017;2017(1):674–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyle K, Felling R, Yiu A, et al. Neurologic Outcomes After Extracorporeal Membrane Oxygenation: A Systematic Review. Pediatr Crit Care Med. 2018;19(8):760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cashen K, Reeder R, Dalton HJ, et al. Functional Status of Neonatal and Pediatric Patients After Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med. 2017;18(6):561–570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kakat S, O’Callaghan M, Smith L, et al. The 1-Year Follow-Up Clinic for Neonates and Children After Respiratory Extracorporeal Membrane Oxygenation Support: A 10-Year Single Institution Experience. Pediatr Crit Care Med. 2017;18(11):1047–1054. [DOI] [PubMed] [Google Scholar]
- 33.Meert KL, Guerguerian AM, Barbaro R, et al. Extracorporeal Cardiopulmonary Resuscitation: One-Year Survival and Neurobehavioral Outcome Among Infants and Children With In-Hospital Cardiac Arrest. Crit Care Med. 2019;47(3):393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta A, Ibsen LM. Neurologic complications and neurodevelopmental outcome with extracorporeal life support. World J Crit Care Med. 2013;2(4):40–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cengiz P, Seidel K, Rycus PT, Brogan TV, Roberts JS. Central nervous system complications during pediatric extracorporeal life support: incidence and risk factors. Crit Care Med. 2005;33(12):2817–2824. [DOI] [PubMed] [Google Scholar]
- 36.Teele SA, Salvin JW, Barrett CS, et al. The association of carotid artery cannulation and neurologic injury in pediatric patients supported with venoarterial extracorporeal membrane oxygenation*. Pediatr Crit Care Med. 2014;15(4):355–361. [DOI] [PubMed] [Google Scholar]
- 37.Polito A, Barrett CS, Wypij D, et al. Neurologic complications in neonates supported with extracorporeal membrane oxygenation. An analysis of ELSO registry data. Intensive Care Med. 2013;39(9):1594–1601. [DOI] [PubMed] [Google Scholar]
- 38.Pinto VL, Pruthi S, Westrick AC, Shannon CN, Bridges BC, Le TM. Brain Magnetic Resonance Imaging Findings in Pediatric Patients Post Extracorporeal Membrane Oxygenation. ASAIO J. 2017;63(6):810–814. [DOI] [PubMed] [Google Scholar]
- 39.Abend NS, Arndt DH, Carpenter JL, et al. Electrographic seizures in pediatric ICU patients: cohort study of risk factors and mortality. Neurology. 2013;81(4):383–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Okochi S, Shakoor A, Barton S, et al. Prevalence of Seizures in Pediatric Extracorporeal Membrane Oxygenation Patients as Measured by Continuous Electroencephalography. Pediatr Crit Care Med. 2018;19(12):1162–1167. [DOI] [PubMed] [Google Scholar]
- 41.Hardart GE, Fackler JC. Predictors of intracranial hemorrhage during neonatal extracorporeal membrane oxygenation. J Pediatr. 1999;134(2):156–159. [DOI] [PubMed] [Google Scholar]
- 42.Piantino JA, Wainwright MS, Grimason M, et al. Nonconvulsive seizures are common in children treated with extracorporeal cardiac life support. Pediatr Crit Care Med. 2013;14(6):601–609. [DOI] [PubMed] [Google Scholar]
- 43.Lin JJ, Banwell BL, Berg RA, et al. Electrographic Seizures in Children and Neonates Undergoing Extracorporeal Membrane Oxygenation. Pediatr Crit Care Med. 2017;18(3):249–257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Parish AP, Bunyapen C, Cohen MJ, Garrison T, Bhatia J. Seizures as a predictor of long-term neurodevelopmental outcome in survivors of neonatal extracorporeal membrane oxygenation (ECMO). J Child Neurol. 2004;19(12):930–934. [DOI] [PubMed] [Google Scholar]
- 45.Abend NS, Dlugos DJ, Clancy RR. A review of long-term EEG monitoring in critically ill children with hypoxic-ischemic encephalopathy, congenital heart disease, ECMO, and stroke. J Clin Neurophysiol. 2013;30(2):134–142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Topjian AA, Gutierrez-Colina AM, Sanchez SM, et al. Electrographic status epilepticus is associated with mortality and worse short-term outcome in critically ill children. Crit Care Med. 2013;41(1):215–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Horan M, Azzopardi D, Edwards AD, Firmin RK, Field D. Lack of influence of mild hypothermia on amplitude integrated-electroencephalography in neonates receiving extracorporeal membrane oxygenation. Early Hum Dev. 2007;83(2):69–75. [DOI] [PubMed] [Google Scholar]
- 48.Organization ELS. International Complication Trend Report. Ann Arbor, MI: Extracorporeal Life Support Organization; July 2019 2019. [Google Scholar]
- 49.Graziani LJ, Streletz LJ, Mitchell DG, et al. Electroencephalographic, neuroradiologic, and neurodevelopmental studies in infants with subclavian steal during ECMO. Pediatr Neurol. 1994;10(2):97–103. [DOI] [PubMed] [Google Scholar]
- 50.Streletz LJ, Bej MD, Graziani LJ, et al. Utility of serial EEGs in neonates during extracorporeal membrane oxygenation. Pediatr Neurol. 1992;8(3):190–196. [DOI] [PubMed] [Google Scholar]
- 51.Hahn JS, Vaucher Y, Bejar R, Coen RW. Electroencephalographic and neuroimaging findings in neonates undergoing extracorporeal membrane oxygenation. Neuropediatrics. 1993;24(1):19–24. [DOI] [PubMed] [Google Scholar]
- 52.Pappas A, Shankaran S, Stockmann PT, Bara R. Changes in amplitude-integrated electroencephalography in neonates treated with extracorporeal membrane oxygenation: a pilot study. J Pediatr. 2006;148(1):125–127. [DOI] [PubMed] [Google Scholar]
- 53.Tharp BR, Laboyrie PM. The incidence of EEG abnormalities and outcome of infants paralyzed with neuromuscular blocking agents. Crit Care Med. 1983;11(12):926–929. [DOI] [PubMed] [Google Scholar]
- 54.Bennett CC, Johnson A, Field DJ, Group UKCET. A comparison of clinical variables that predict adverse outcome in term infants with severe respiratory failure randomised to a policy of extracorporeal membrane oxygenation or to conventional neonatal intensive care. J Perinat Med. 2002;30(3):225–230. [DOI] [PubMed] [Google Scholar]
- 55.LaRovere KL, Vonberg FW, Prabhu SP, et al. Patterns of Head Computed Tomography Abnormalities During Pediatric Extracorporeal Membrane Oxygenation and Association With Outcomes. Pediatr Neurol. 2017;73:64–70. [DOI] [PubMed] [Google Scholar]
- 56.Werho DK, Pasquali SK, Yu S, et al. Epidemiology of Stroke in Pediatric Cardiac Surgical Patients Supported With Extracorporeal Membrane Oxygenation. Ann Thorac Surg. 2015;100(5):1751–1757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.deVeber GA, Kirton A, Booth FA, et al. Epidemiology and Outcomes of Arterial Ischemic Stroke in Children: The Canadian Pediatric Ischemic Stroke Registry. Pediatr Neurol. 2017;69:58–70. [DOI] [PubMed] [Google Scholar]
- 58.Johnson K, Jarboe MD, Mychaliska GB, et al. Is there a best approach for extracorporeal life support cannulation: a review of the extracorporeal life support organization. J Pediatr Surg. 2018;53(7):1301–1304. [DOI] [PubMed] [Google Scholar]
- 59.Dalton HJ, Reeder R, Garcia-Filion P, et al. Factors Associated with Bleeding and Thrombosis in Children Receiving Extracorporeal Membrane Oxygenation. Am J Respir Crit Care Med. 2017;196(6):762–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Nasr DM, Rabinstein AA. Neurologic Complications of Extracorporeal Membrane Oxygenation. J Clin Neurol. 2015;11(4):383–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Raets MM, Dudink J, Ijsselstijn H, et al. Brain injury associated with neonatal extracorporeal membrane oxygenation in the Netherlands: a nationwide evaluation spanning two decades. Pediatr Crit Care Med. 2013;14(9):884–892. [DOI] [PubMed] [Google Scholar]
- 62.Fletcher Sandersjoo A, Bartek J Jr., Thelin EP, et al. Predictors of intracranial hemorrhage in adult patients on extracorporeal membrane oxygenation: an observational cohort study. J Intensive Care. 2017;5:27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lockie CJA, Gillon SA, Barrett NA, et al. Severe Respiratory Failure, Extracorporeal Membrane Oxygenation, and Intracranial Hemorrhage. Crit Care Med. 2017;45(10):1642–1649. [DOI] [PubMed] [Google Scholar]
- 64.Le Guennec L, Bertrand A, Laurent C, et al. Diffuse cerebral microbleeds after extracorporeal membrane oxygenation support. Am J Respir Crit Care Med. 2015;191(5):594–596. [DOI] [PubMed] [Google Scholar]
- 65.Yates PA, Villemagne VL, Ellis KA, Desmond PM, Masters CL, Rowe CC. Cerebral microbleeds: a review of clinical, genetic, and neuroimaging associations. Front Neurol. 2014;4:205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Liebeskind DS, Sanossian N, Sapo ML, Saver JL. Cerebral microbleeds after use of extracorporeal membrane oxygenation in children. J Neuroimaging. 2013;23(1):75–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Riech S, Kallenberg K, Moerer O, et al. The Pattern of Brain Microhemorrhages After Severe Lung Failure Resembles the One Seen in High-Altitude Cerebral Edema. Crit Care Med. 2015;43(9):e386–389. [DOI] [PubMed] [Google Scholar]
- 68.Luyt CE, Brechot N, Demondion P, et al. Brain injury during venovenous extracorporeal membrane oxygenation. Intensive Care Med. 2016;42(5):897–907. [DOI] [PubMed] [Google Scholar]
- 69.Lidegran MK, Mosskin M, Ringertz HG, Frenckner BP, Linden VB. Cranial CT for diagnosis of intracranial complications in adult and pediatric patients during ECMO: Clinical benefits in diagnosis and treatment. Acad Radiol. 2007;14(1):62–71. [DOI] [PubMed] [Google Scholar]
- 70.Le Guennec L, Cholet C, Huang F, et al. Ischemic and hemorrhagic brain injury during venoarterial-extracorporeal membrane oxygenation. Ann Intensive Care. 2018;8(1):129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lorusso R, Barili F, Mauro MD, et al. In-Hospital Neurologic Complications in Adult Patients Undergoing Venoarterial Extracorporeal Membrane Oxygenation: Results From the Extracorporeal Life Support Organization Registry. Crit Care Med. 2016;44(10):e964–972. [DOI] [PubMed] [Google Scholar]
- 72.Mazzeffi M, Kon Z, Menaker J, et al. Large Dual-Lumen Extracorporeal Membrane Oxygenation Cannulas Are Associated with More Intracranial Hemorrhage. ASAIO J. 2018. [DOI] [PubMed] [Google Scholar]
- 73.Anton-Martin P, Journeycake J, Modem V, et al. Coagulation Profile Is Not a Predictor of Acute Cerebrovascular Events in Pediatric Extracorporeal Membrane Oxygenation Patients. ASAIO J. 2017;63(6):793–801. [DOI] [PubMed] [Google Scholar]
- 74.Lin N, Flibotte J, Licht DJ. Neuromonitoring in the neonatal ECMO patient. Semin Perinatol. 2018;42(2):111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Herman ST, Abend NS, Bleck TP, et al. Consensus statement on continuous EEG in critically ill adults and children, part I: indications. J Clin Neurophysiol. 2015;32(2):87–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Organization ELS. ELSO guidelines: Patient Care Practice Guidelines. 2015; https://www.elso.org/Resources/Guidelines.aspx.
- 77.Murray DM, Boylan GB, Ryan CA, Connolly S. Early EEG findings in hypoxic-ischemic encephalopathy predict outcomes at 2 years. Pediatrics. 2009;124(3):e459–467. [DOI] [PubMed] [Google Scholar]
- 78.Jeong I, Woo Y, Kim D, Kim N, Cho H, Ma J. Efficacy of Electroencephalographic Monitoring for the Evaluation of Intracranial Injury during Extracorporeal Membrane Oxygenation Support in Neonates and Infants. The Korean Journal of Critical Care Medicine:. 2014;29(2):70–76. [Google Scholar]
- 79.Shope R, Harris M, Kralik S, Ho C, Mietzsch U. Sleep-wake-cycle as a Tool to Predict Neurodevelopmental Outcome in Neonates Treated with Ecmo. 2018. [Google Scholar]
- 80.Du Pont-Thibodeau G, Sanchez SM, Jawad AF, et al. Seizure Detection by Critical Care Providers Using Amplitude-Integrated Electroencephalography and Color Density Spectral Array in Pediatric Cardiac Arrest Patients. Pediatr Crit Care Med. 2017;18(4):363–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Lalgudi Ganesan S, Stewart CP, Atenafu EG, et al. Seizure Identification by Critical Care Providers Using Quantitative Electroencephalography. Crit Care Med. 2018;46(12):e1105–e1111. [DOI] [PubMed] [Google Scholar]
- 82.Payne ET, Zhao XY, Frndova H, et al. Seizure burden is independently associated with short term outcome in critically ill children. Brain. 2014;137(Pt 5):1429–1438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rollins MD, Yoder BA, Moore KR, et al. Utility of neuroradiographic imaging in predicting outcomes after neonatal extracorporeal membrane oxygenation. J Pediatr Surg. 2012;47(1):76–80. [DOI] [PubMed] [Google Scholar]
- 84.van Heijst AF, de Mol AC, Ijsselstijn H. ECMO in neonates: neuroimaging findings and outcome. Semin Perinatol. 2014;38(2):104–113. [DOI] [PubMed] [Google Scholar]
- 85.Wien MA, Whitehead MT, Bulas D, et al. Patterns of Brain Injury in Newborns Treated with Extracorporeal Membrane Oxygenation. AJNR Am J Neuroradiol. 2017;38(4):820–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Glass P, Bulas DI, Wagner AE, et al. Severity of brain injury following neonatal extracorporeal membrane oxygenation and outcome at age 5 years. Dev Med Child Neurol. 1997;39(7):441–448. [DOI] [PubMed] [Google Scholar]
- 87.Lazar EL, Abramson SJ, Weinstein S, Stolar CJ. Neuroimaging of brain injury in neonates treated with extracorporeal membrane oxygenation: lessons learned from serial examinations. J Pediatr Surg. 1994;29(2):186–190; discussion 190–181. [DOI] [PubMed] [Google Scholar]
- 88.Pinto J, Paneth N, Kazam E, et al. Interobserver variability in neonatal cranial ultrasonography. Paediatr Perinat Epidemiol. 1988;2(1):43–58. [DOI] [PubMed] [Google Scholar]
- 89.Zamora CA, Oshmyansky A, Bembea M, et al. Resistive Index Variability in Anterior Cerebral Artery Measurements During Daily Transcranial Duplex Sonography: A Predictor of Cerebrovascular Complications in Infants Undergoing Extracorporeal Membrane Oxygenation? J Ultrasound Med. 2016;35(11):2459–2465. [DOI] [PubMed] [Google Scholar]
- 90.LaRovere KL, O’Brien NF. Transcranial Doppler Sonography in Pediatric Neurocritical Care: A Review of Clinical Applications and Case Illustrations in the Pediatric Intensive Care Unit. J Ultrasound Med. 2015;34(12):2121–2132. [DOI] [PubMed] [Google Scholar]
- 91.Rilinger JF, Smith CM, deRegnier RAO, et al. Transcranial Doppler Identification of Neurologic Injury during Pediatric Extracorporeal Membrane Oxygenation Therapy. J Stroke Cerebrovasc Dis. 2017;26(10):2336–2345. [DOI] [PubMed] [Google Scholar]
- 92.O’Brien NF, Hall MW. Extracorporeal membrane oxygenation and cerebral blood flow velocity in children. Pediatr Crit Care Med. 2013;14(3):e126–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.O’Brien NF, Buttram SDW, Maa T, et al. Cerebrovascular Physiology During Pediatric Extracorporeal Membrane Oxygenation: A Multicenter Study Using Transcranial Doppler Ultrasonography. Pediatr Crit Care Med. 2019;20(2):178–186. [DOI] [PubMed] [Google Scholar]
- 94.LaRovere KL, Brett MS, Tasker RC, Strauss KJ, Burns JP, Pediatric Critical Nervous System P. Head computed tomography scanning during pediatric neurocritical care: diagnostic yield and the utility of portable studies. Neurocrit Care. 2012;16(2):251–257. [DOI] [PubMed] [Google Scholar]
- 95.Goff DA, Buckley EM, Durduran T, Wang J, Licht DJ. Noninvasive cerebral perfusion imaging in high-risk neonates. Semin Perinatol. 2010;34(1):46–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Clair MP, Rambaud J, Flahault A, et al. Prognostic value of cerebral tissue oxygen saturation during neonatal extracorporeal membrane oxygenation. PLoS One. 2017;12(3):e0172991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ejike JC, Schenkman KA, Seidel K, Ramamoorthy C, Roberts JS. Cerebral oxygenation in neonatal and pediatric patients during veno-arterial extracorporeal life support. Pediatr Crit Care Med. 2006;7(2):154–158. [DOI] [PubMed] [Google Scholar]
- 98.Rais-Bahrami K, Rivera O, Short BL. Validation of a noninvasive neonatal optical cerebral oximeter in veno-venous ECMO patients with a cephalad catheter. J Perinatol. 2006;26(10):628–635. [DOI] [PubMed] [Google Scholar]
- 99.Khan I, Rehan M, Parikh G, et al. Regional Cerebral Oximetry as an Indicator of Acute Brain Injury in Adults Undergoing Veno-Arterial Extracorporeal Membrane Oxygenation-A Prospective Pilot Study. Front Neurol. 2018;9:993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kochanek PM, Berger RP, Fink EL, et al. The potential for bio-mediators and biomarkers in pediatric traumatic brain injury and neurocritical care. Front Neurol. 2013;4:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Bembea MM, Rizkalla N, Freedy J, et al. Plasma Biomarkers of Brain Injury as Diagnostic Tools and Outcome Predictors After Extracorporeal Membrane Oxygenation. Crit Care Med. 2015;43(10):2202–2211. [DOI] [PubMed] [Google Scholar]
- 102.Bembea MM, Savage W, Strouse JJ, et al. Glial fibrillary acidic protein as a brain injury biomarker in children undergoing extracorporeal membrane oxygenation. Pediatr Crit Care Med. 2011;12(5):572–579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fletcher-Sandersjoo A, Lindblad C, Thelin EP, et al. Serial S100B Sampling Detects Intracranial Lesion Development in Patients on Extracorporeal Membrane Oxygenation. Front Neurol. 2019;10:512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gazzolo D, Masetti P, Meli M, Grutzfeld D, Michetti F. Elevated S100B protein as an early indicator of intracranial haemorrhage in infants subjected to extracorporeal membrane oxygenation. Acta Paediatr. 2002;91(2):218–221. [DOI] [PubMed] [Google Scholar]
- 105.Nguyen DN, Huyghens L, Wellens F, Schiettecatte J, Smitz J, Vincent JL. Serum S100B protein could help to detect cerebral complications associated with extracorporeal membrane oxygenation (ECMO). Neurocrit Care. 2014;20(3):367–374. [DOI] [PubMed] [Google Scholar]
- 106.Floerchinger B, Philipp A, Foltan M, et al. Neuron-specific enolase serum levels predict severe neuronal injury after extracorporeal life support in resuscitation. Eur J Cardiothorac Surg. 2014;45(3):496–501. [DOI] [PubMed] [Google Scholar]
- 107.Sekhon MS, Gooderham P, Menon DK, et al. The Burden of Brain Hypoxia and Optimal Mean Arterial Pressure in Patients With Hypoxic Ischemic Brain Injury After Cardiac Arrest. Crit Care Med. 2019;47(7):960–969. [DOI] [PubMed] [Google Scholar]
- 108.Cho SM, Ziai W, Mayasi Y, et al. Noninvasive Neurological Monitoring in Extracorporeal Membrane Oxygenation. ASAIO J. 2019. [DOI] [PubMed] [Google Scholar]
- 109.L R, FS T, B M T, M B, et al. Brain monitoring in adult and pediatric ECMO patients: the importance of early and late assessments. Minerva Anestesiol. 2017;83(10):1061–1074. [DOI] [PubMed] [Google Scholar]
- 110.Doerrfuss JI, Kilic T, Ahmadi M, Holtkamp M, Weber JE. Quantitative and Qualitative EEG as a Prediction Tool for Outcome and Complications in Acute Stroke Patients. Clin EEG Neurosci. 2019:1550059419875916. [DOI] [PubMed] [Google Scholar]
- 111.Asgari S, Moshirvaziri H, Scalzo F, Ramezan-Arab N. Quantitative measures of EEG for prediction of outcome in cardiac arrest subjects treated with hypothermia: a literature review. J Clin Monit Comput. 2018;32(6):977–992. [DOI] [PubMed] [Google Scholar]
- 112.Amorim E, van der Stoel M, Nagaraj SB, et al. Quantitative EEG reactivity and machine learning for prognostication in hypoxic-ischemic brain injury. Clin Neurophysiol. 2019;130(10):1908–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rivera-Lara L, Geocadin R, Zorrilla-Vaca A, et al. Optimizing Mean Arterial Pressure in Acutely Comatose Patients Using Cerebral Autoregulation Multimodal Monitoring With Near-Infrared Spectroscopy. Crit Care Med. 2019;47(10):1409–1415. [DOI] [PubMed] [Google Scholar]
- 114.Asgari S, Adams H, Kasprowicz M, Czosnyka M, Smielewski P, Ercole A. Feasibility of Hidden Markov Models for the Description of Time-Varying Physiologic State After Severe Traumatic Brain Injury. Crit Care Med. 2019. [DOI] [PubMed] [Google Scholar]
- 115.Floerchinger B, Philipp A, Camboni D, et al. NSE serum levels in extracorporeal life support patients-Relevance for neurological outcome? Resuscitation. 2017;121:166–171. [DOI] [PubMed] [Google Scholar]
- 116.Song J, Dailey J, Li H, et al. Extended Solution Gate OFET-based Biosensor for Label-free Glial Fibrillary Acidic Protein Detection with Polyethylene Glycol-Containing Bioreceptor Layer. Adv Funct Mater. 2017;27(20). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Marino BS, Lipkin PH, Newburger JW, et al. Neurodevelopmental outcomes in children with congenital heart disease: evaluation and management: a scientific statement from the American Heart Association. Circulation. 2012;126(9):1143–1172. [DOI] [PubMed] [Google Scholar]
- 118.Lafc G, Budak AB, Yener AU, Cicek OF. Use of extracorporeal membrane oxygenation in adults. Heart Lung Circ. 2014;23(1):10–23. [DOI] [PubMed] [Google Scholar]
- 119.Ontaneda A, Annich GM. Novel Surfaces in Extracorporeal Membrane Oxygenation Circuits. Front Med (Lausanne). 2018;5:321. [DOI] [PMC free article] [PubMed] [Google Scholar]


