Abstract
Background:
Primary immune thrombocytopenia (ITP) decreases platelet count as well as increases the risk of bleeding due to platelet destruction in an autoimmune disorder. For many years, prednisone (PDN) has been the standard first-line treatment in ITP practical guidelines. The current randomized clinical trial compared the efficacy of treatments between three-pulse high-dose dexamethasone (HD-DXM) and the traditional PDN regimen among untreated patients with ITP in accordance with platelet count responses and adverse events.
Materials and Methods:
We randomly assigned eligible patients with ITP to receive PDN or a three-pulse regimen of HD-DXM. In the HD-DXM group, 40 mg of DXM was administered intravenously for 4 consecutive days and was repeated in 14-day intervals for three pulses of treatment. Patients in the PDN group received 1.0 mg/kg of PDN orally per day for 4 consecutive weeks. The Mann–Whitney test was used for comparing the median of platelet count between the two groups, and logistic regression was used to evaluate odds ratio (OR) in the response rate of platelet count between the two groups. Blindness was not applied for both patients and physicians.
Results:
The initial response rate of platelet count in the HD-DXM group was significantly higher than the PDN group (P < 0.05). According to the results of logistic regression, the initial and sustained response (SR) rate of platelet count in the HD-DXM group was significantly higher than the PDN group (OR: 5.68 and 4.17, respectively, P < 0.05). In fact, in the HD-DXM group, more patients reached SR after the 8-month follow-up (88.9% vs. 66.6%, P < 0.05).
Conclusion:
In patients with ITP disease who have not received any kind of treatment, HD-DXM was more effective than conventional PDN therapy.
Keywords: Autoimmune diseases, dexamethasone, immune thrombocytopenia
INTRODUCTION
Primary immune thrombocytopenia (ITP) leads to a decrease in platelet count and also increases the bleeding risk by platelet destruction as an autoimmune disorder. ITP pathogenesis is identified with different mechanisms such as autoantibody-mediated platelet destruction, cytotoxic T-lymphocyte platelet lysis, maturation, and production of impaired platelets. ITP is diagnosed by exclusion of other causes of thrombocytopenia such as infection, malignancy, and immunologic disorders.[1,2]
The preliminary therapy for ITP has not been altered for several years and comprises corticosteroids, intravenous (IV) immunoglobulin, and anti-D antibody. Corticosteroids are inexpensive and increase platelet count in nearly 75% of patients within one to 2 days. However, long-term responses are observed in only 25% of patients. The side effects of these medications are common and predictable, including hypertension, fatigue, hyperglycemia, and adrenal insufficiency.[3]
Prednisone (PDN) is the regular corticosteroid in the ITP practical guidelines generally prescribed in a dose of 1 mg/kg daily for 4 weeks and then tapered.[4,5] Dexamethasone was primarily applied in the treatment of relapsing and refractory ITP and revealed different benefits in treated patients. Recently, high-dose dexamethasone (HD-DXM) was used to treat ITP, and there are many controversies about its efficacy and duration of responses based on different courses and protocols of treatments. According to the guidelines, HD-DXM is recommended as an alternative first-line therapy in children and adults with ITP.[4,6,7,8]
The current randomized clinical trial compared the effectiveness of a three-pulse HD-DXM regimen with traditional therapy among untreated patients with ITP regarding platelet count response and adverse events.
MATERIALS AND METHODS
Study design
The current single-center randomized clinical trial was approved by the Ethics Committee of the Isfahan University of Medical Sciences, Isfahan, Iran. Furthermore, this study was registered in ClinicalTrials.gov (approval code: NCT02914054). Data were collected from adults referred to Seyed-al-Shohada Hospital (a hematology referral center in Isfahan Province of Iran) with a definite diagnosis of ITP from September 2016 to November 2017. In order to compare platelet count response at a 95% confidence interval and the test power of 80%, the sample size was calculated.[9] Written informed consent was obtained from all the patients before enrollment.
Participants
Inclusion criteria
The inclusion criteria were patients with 18 years old or above, along with a new primary ITP formulated a diagnosis based on the International Working Group guidelines.[8] Other inclusion criteria represented by the naïve ITP for 3 months from diagnosis, and a count of platelet <30 × 109/L or more than 30 × 109/L with the signs of bleeding existence based on bleeding grading scores [Table 1].[10]
Table 1.
Grade and score of bleeding symptoms[8]
| Grade | Bleeding symptom |
|---|---|
| 0 | Absent |
| 1 | Petechiae |
| 2 | Ecchymoses with minor blood loss |
| 3 | Major mucous hemorrhage without sequelae |
| 4 | Major blood loss with sequelae or death |
Exclusion criteria
The exclusion criteria were hypertension, cardiovascular disorders, failure of liver or kidney, autoimmune hemolytic anemia, psychosis, connective tissue disorders, active infections, HIV, hepatitis B or C detection or any recent viral infections, diabetes, osteoporosis having pregnancy or lactation, and malignancy. Patients with a history of receiving corticosteroid or immunosuppressive medicines 3 months before diagnosis and any preceding ITP-specific treatments were excluded from the study.
To exclude any pathologic alterations in the lymphoid and myeloid series, bone marrow aspiration was performed in all patients. Autoimmune markers (antinuclear, antimitochondrial, and anticardiolipin antibodies) and the direct antiglobulin test were checked to rule out any autoimmunity, and patients who were positive were excluded from the study.
Intervention
In the HD-DXM group, 40 mg of DXM was administered in 500 mL normal saline (0.9% saline) intravenously throughout 1 h for 4 successive days and afterward halted. This period was continued in 14-day intervals to obtain three treatment cycles. Cell count evaluation was also performed every 2 months on the last day of the 2nd month after completion of 8 months of therapy.[10,11]
Patients in the PDN group received 1.0 mg/kg of PDN orally on a daily basis for 4 successive weeks. After responses, the medication was tapered to <15 mg on each day or aborted over 4-6 weeks to maintain a count of platelet over 30 × 109/L. The count of cell blood was assessed every 2 months up to 8 months or until the loss of response to treatment.[9,12]
Randomization
The qualified patients were randomly assigned 1:1 to receive either conventional PDN therapy or a three-pulse regimen of HD-DXM. Randomization was conducted at Seyed-al-Shohada Hospital using precoded concealed envelopes generated by permuted-block randomization with a block size of 4. No blinding of physicians or patients was used.
Outcome criteria
The primary endpoints included a response (R), sustained response (SR), and complete response (CR). CR was characterized as a count of platelet ≥100 × 109/L and bleeding absence. The response was recognized as a count of platelet ≥30 × 109/L and as a minimum two-fold increase in the count of baseline and bleeding absence. Lack of response was characterized as a count of platelet <30 × 109/L or less than a two-fold increase in the count of baseline or any bleeding presence. The SR was characterized as a response for at least 6 consecutive months. Relapse was characterized by platelet count <30 × 109/L or bleeding symptom presence after response achievement.
The secondary endpoints were bleeding scores and adverse events. Table 1 summarizes the parameters used for the assessment of the bleeding scores before and after each course of medication.[10] Adverse events were assessed based on the Common Terminology Criteria for Adverse Events version 4.0 by the US National Cancer Institute. A comprehensive checklist, including demographic and clinical data, was completed after 1 month of treatment from all the participants. Adverse events were evaluated and recorded monthly up to the 8th month of follow-up.
Statistical analysis
Finally, the collected data were entered into SPSS (version 22; SPSS Inc., Chicago, IL, USA) and were represented by frequency (percentage), mean ± standard deviation, or median (minimum-maximum). At the level of inferential statistics, an independent samples t-test and the Chi-square test were used to compare the means of age and to compare the frequency distribution of qualitative data between the two groups, respectively. Furthermore, according to the results of the Kolmogorov-Smirnov test indicating the abnormality of the platelet count distribution, the Mann-Whitney test was used for comparing the median of platelet count between the two groups and the Friedman test to compare the changes of platelet count by passing the time from baseline to 8 months after intervention in each group. Moreover, logistic regression was used to evaluate odds ratio (OR) in the response rate of platelet count between the two groups by adjusted of other parameters such as age, gender, and bleeding score. A significance level of <0.05 was considered in all analyses.
RESULTS
Baseline characteristics
Between September 2016 and September 2017, ninety patients were registered in the study. Ten of them were excluded, and eighty patients were randomly assigned between the two arms of the study. In the HD-DXM group, three patients did not take the assigned intervention due to the consent withdrawal. Five cases were lost to follow-up including one in the HD-DXM arm and four in the PDN arm. Based on the sample size formula, ninety patients were registered, and among them, 36 patients in each group completed the follow-up and others were lost [Figure 1].
Figure 1.
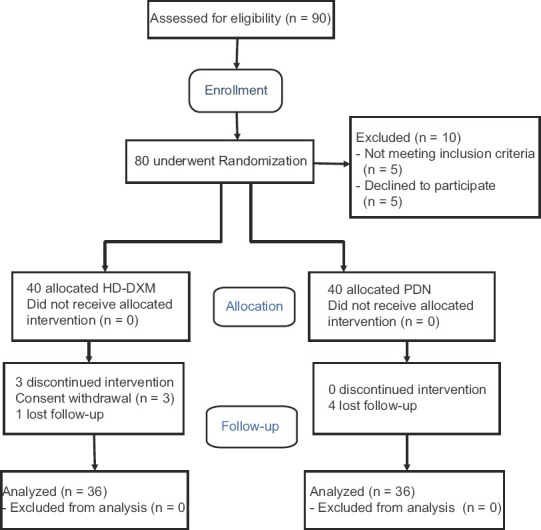
Flow diagram of the study
The detailed baseline features of the patients in each group are shown in Table 2. There was no significant difference in baseline characteristics. Symptoms of bleeding were commonly mild to moderate in both the groups, and the skin and mucus membranes were the most common bleeding sites (30.6% in the HD-DXM and 33.4% in the PDN groups) with no significant differences (P > 0.05).
Table 2.
Comparison of baseline characteristics between the three-pulse high-dose dexamethasone and traditional prednisone regimen groups
| Characteristics | PDN (n=36), n (%) | HD-DXM (n=36), n (%) | P |
|---|---|---|---|
| Sex | |||
| Male | 12 (33.3) | 18 (50) | 0.151† |
| Female | 24 (66.7) | 18 (50) | |
| Age; years | 39.80±17.12 | 39.36±11.65 | 0.898†† |
| Bleeding score | |||
| 0 | 24 (66.7) | 25 (69.4) | 0.838† |
| 1 | 10 (27.8) | 10 (27.8) | |
| 2 | 2 (5.6) | 1 (2.8) |
Data shown mean±SD or n (%). †Used of Chi-square test; ††Used of independent samples t-test. SD=Standard deviation; PDN=Prednisone; HD-DXM=High-dose dexamethasone
Outcomes
In the baseline, the median platelet count did not differ significantly between the two groups (P > 0.05). However, from the 2nd month to the 8th month after the intervention, the platelet count in the HD-DXM group was significantly higher than the PDN group (P < 0.05). In addition, over the 8 months after the intervention, both the groups have seen an increase in platelet counts. However, this increase has been more pronounced in the HD-DXM group than in the PDN group (P < 0.05) [Table 3].
Table 3.
Comparison of median platelet count at 8-month follow-up between the three-pulse high-dose dexamethasone and traditional prednisone regimen groups
| Platelet count, ×109/L | PDN (n=36) | HD-DXM (n=36) | P† |
|---|---|---|---|
| Baseline | 10.00 (2.0–74.0) | 11.00 (3.0–26.0) | 0.804 |
| After 2 months | 20.50 (5.0–121.0) | 36.00 (6.0–230.0) | 0.001 |
| After 4 months | 29.50 (3.0–140.0) | 61.50 (12.0–217.0) | 0.002 |
| After 6 months | 35.00 (3.0–157.0) | 100.00 (12.0–243.0) | 0.001 |
| After 8 months | 36.00 (4.0–226.0) | 104.00 (20.0–210.0) | 0.009 |
| P†† | 0.001 | <0.001 |
Data shown median (minimum–maximum). †Used of Mann–Whitney test for comparison of median platelet count between the two groups; ††Used of Friedman test for comparison of median platelet count by passing time (baseline to after 8 months) in each group. PDN=Prednisone; HD-DXM=High-dose dexamethasone
According to the results of logistic regression, the initial and SR rate of platelet count in the HD-DXM group was significantly higher than the PDN group (OR: 5.68 and 4.17, respectively, P < 0.05). In fact, in the HD-DXM group, more patients reached SR after the 8-month follow-up (88.9% vs. 66.6%, P < 0.05) [Table 4].
Table 4.
Comparison of response rate between the three-pulse high-dose dexamethasone and traditional prednisone regimen groups
| Response | PDN (n=36), n (%) | HD-DXM (n=36), n (%) | OR (95% CI) | P† |
|---|---|---|---|---|
| Initial response* | 11 (30.6) | 25 (69.4) | 5.68 (2.05–15.76) | 0.001 |
| Complete response** | 3 (8.3) | 8 (22.2) | 3.05 (0.74–12.62) | 0.124 |
| Sustained response*** | 24 (66.7) | 32 (88.9) | 4.17 (1.19–14.60) | 0.025 |
*Initial response=Platelet count over 30×109/l after 1 month from treatment; **Complete response=Platelet count over 100×109/l after 1 month from treatment; ***Sustained response=Platelet count over 30×109/l for at least 6 months from treatment; †Significance level calculated from logistic regression with adjusted of other parameters such as age, gender, and bleeding score. PDN=Prednisone; HD-DXM=High-dose dexamethasone; OR=Odds ratio; CI=Confidence interval
As shown in Table 5, after three courses of HD-DXM, the most common adverse events reported include hypertension and hyperglycemia. Type and incidence of the disease were not significantly different between the groups (P > 0.05). There were no reports of bleeding complications in either group during the 8-month follow-up after intervention, as bleeding symptoms initially were generally mild to moderate. Other adverse events such as insomnia and mood disorder were reported by fewer patients and did not indicate any difference in accordance with either group of the study or the type of treatment. Most of the adverse events were mild and usually resolved spontaneously after treatment was completed. No patients left the study because of adverse events of therapy.
Table 5.
Comparison of adverse events between the three-pulse high-dose dexamethasone and traditional prednisone regimen groups
| PDN (n=36), n (%) | HD-DXM (n=36), n (%) | P† | |
|---|---|---|---|
| Hypertension | 5 (13.9) | 3 (8.3) | 0.710 |
| Hyperglycemia | 2 (5.6) | 1 (2.8) | 0.555 |
| Insomnia | 0 | 4 (11.1) | 0.115 |
| Mood disorders | 0 | 2 (5.6) | 0.493 |
| Weight gain | 1 (2.8) | 0 | 0.314 |
| Cushingoid appearance | 3 (8.3) | 0 | 0.239 |
†Significance level of Chi-square test. PDN=Prednisone; HD-DXM=High-dose dexamethasone
DISCUSSION
The main goal of the initial ITP treatment is to overcome the risk of bleeding and achieve a persistent response, especially after discontinuing treatment, with no further treatments, and so prevent long-term adverse events such as metabolic or infectious alterations. Glucocorticoids are the cornerstone of ITP treatment.[4,7] Based on recent guidelines, most of the patients are currently treated with PDN (1.0 mg/kg/day for 2-4 weeks orally) as the first-line treatment. An initial response rate of 50%-60% is estimated using this approach, but the long-term response rate with no other therapies is very low (10%-25%).[4]
Although there is no consensus on the optimal dosage of glucocorticoids and the type and duration of administration, the major concern is represented by the potential side effects of corticosteroids. Glucocorticoid toxicity is generally related to both the average dosage and the cumulative duration of administration, however, no toxicity threshold dose and duration are established.[13] Controversial exists over the relative safety of low-dose glucocorticoid use (≤10 mg/day of PDN or equivalent) in chronic conditions. Several large retrospective reviews showed that long-term glucocorticoid use, even in low doses, is a significant independent predictor of numerous serious adverse effects and that risk is both dose and time dependent.[14,15]
The administration of immunosuppressive agents for patients with ITP often increases the risk of infection. A study on 152 patients with ITP, conducted by Portielje et al., showed that the administration of immunosuppressive treatment significantly increased the mortality and morbidity rates in patients.[16] Low doses of PDN (5-10 mg/day) or high doses of corticosteroids are used in short-term courses to sustain an adequate platelet count in refractory patients with ITP. Experiences with this study showed more toxicity.[17]
PDN protocol has been the standard of treatment in naïve ITP adult patients.[4,7,18,19] However, current studies suggested pulses of HD-DXM regimen (40 mg/day for four days) as an alternative treatment.[10,11,12,20,21,22,23] Other studies suggested that repeated pulses of HD-DXM regimen may be better to induce long-term remission than a single-pulse regimen.[9,20,24,25] In a study conducted by Din et al., 61 patients with ITP received 40 mg/day of DXM in three 4-day pulses with 14-day intervals. It was revealed that treatment with a three-pulse HD-DXM regimen with low-dose DXM maintenance was an effective strategy for previously untreated ITP patients.[24] In our trial, the administration route of dexamethasone was IV with no maintenance dose in order to compare the effects and adverse events of IV administration with previous studies.[9,10]
The Gruppo Italiano Malattie Ematologiche dell'Adulto (GIMEMA) ITP Working Party planned the first pilot monocentric study to evaluate the efficiency, protection, and sustainability of HD-DXM as a first-line treatment in naïve ITP patients, using a six-pulse regimen. The preliminary response level was so hopeful (about 90%), and the long-term response was about 68% (25 / 37, with 20 cases of CR) in all evaluated patients (median follow-up: 26 months). After that, the GIMEMA ITP Working Party planned a multicenter pilot study in order to obtain better compliance, feasibility, and satisfactory efficacy through modifying the regimen used in the first study by a number of courses reducing four instead of six and their intervals (14 days rather 28 days). The GIMEMA experience demonstrated a persistent response after four treatment courses in about 67% of adult patients and suggested that three courses of HD-DXM may be the optimal regimen and should be assessed in the next trials.[10]
Mithoowani et al. did a systematic review and meta-analysis of randomized trials to compare HD-DXM pulses and PDN regarding platelet responses.[26] From five trials excluding Din et al.,[24] other trials compared 1-2 cycles of HD-DXM with PDN. The study found no differences in overall platelet count responses at 6 months as opposed to the findings of our study similar to Din e t a l. trial, showed more durable platelet responses with three cycles of HD-DXM pulses. The study showed similar findings in fewer toxicities and higher initial responses with HD-DXM.
The results of the current study recommend that a three-pulse HD-DXM regimen was more effective than conventional treatment with PDN. It resulted in both a higher overall response and a more SR during the 8-month follow-up. In contrast with other studies,[9,24] the CR was not significantly higher in the HD-DXM group than in the PDN group. However, the findings suggest that a three-pulse HD-DXM regimen could be better to achieve the SR and overall response without the burden of long-term corticosteroid consumption.
Study strengths and limitations
In the current study, both the groups were matched by the baseline characteristics including age, gender, and bleeding score, which were the strength of this study. The limitation of the current study was that the size of each group did not allow comparing the results of different pulses of HD-DXM treatment with a single course of treatment. Follow-up for more than 1 year could also increase the efficacy of comparison between the two groups. In our study, bleeding symptoms of patients were mild to moderate, and patients with bleeding score of 3 or 4 were not included. Another study enrolled patients with more severe bleeding symptoms found that bleeding was more effectively controlled with HD-DXM and accompanied by fewer bleeding events.[9]
CONCLUSION
The current randomized, clinical trial showed that a three-pulse HD-DXM regimen was more effective and resulted in more SRs than the conventional PDN therapy, without an increase in the rate of adverse events. As a result of the current study, a three-pulse HD-DXM regimen could turn into the favored choice for the treatment of ITP. More studies are required to compare the efficiency and security of different courses of HD-DXM.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
The authors acknowledge all members of the Internal Medicine Department of IUMS contributed to data collection for this article (ethical approval no: 395821).
REFERENCES
- 1.McKenzie CG, Guo L, Freedman J, Semple JW. Cellular immune dysfunction in immune thrombocytopenia (ITP) Br J Haematol. 2013;163:10–23. doi: 10.1111/bjh.12480. [DOI] [PubMed] [Google Scholar]
- 2.Cuker A, Prak ET, Cines DB. Can immune thrombocytopenia be cured with medical therapy? Semin Thromb Hemost. 2015;41:395–404. doi: 10.1055/s-0034-1544001. [DOI] [PubMed] [Google Scholar]
- 3.Provan D, Newland AC. Current management of primary immune thrombocytopenia. Adv Ther. 2015;32:875–87. doi: 10.1007/s12325-015-0251-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khan AM, Mydra H, Nevarez A. Clinical practice updates in the management of immune thrombocytopenia. P T. 2017;42:756–63. [PMC free article] [PubMed] [Google Scholar]
- 5.Neunert C, Lim W, Crowther M, Cohen A, Solberg L, Jr, Crowther MA, et al. The American Society of Hematology 2011 evidence-based practice guideline for immune thrombocytopenia. Blood. 2011;117:4190–207. doi: 10.1182/blood-2010-08-302984. [DOI] [PubMed] [Google Scholar]
- 6.Generali JA, Cada DJ. Dexamethasone: Idiopathic thrombocytopenic purpura in children and adolescents. Hosp Pharm. 2013;48:108–10. doi: 10.1310/hpj4802-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lambert MP, Gernsheimer TB. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129:2829–35. doi: 10.1182/blood-2017-03-754119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodeghiero F, Stasi R, Gernsheimer T, Michel M, Provan D, Arnold DM, et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood. 2009;113:2386–93. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 9.Wei Y, Ji XB, Wang YW, Wang JX, Yang EQ, Wang ZC, et al. High-dose dexamethasone vs. prednisone for treatment of adult immune thrombocytopenia: A prospective multicenter randomized trial. Blood. 2016;127:296–302. doi: 10.1182/blood-2015-07-659656. [DOI] [PubMed] [Google Scholar]
- 10.Mazzucconi MG, Fazi P, Bernasconi S, de Rossi G, Leone G, Gugliotta L, et al. Therapy with high-dose dexamethasone (HD-DXM) in previously untreated patients affected by idiopathic thrombocytopenic purpura: A GIMEMA experience. Blood. 2007;109:1401–7. doi: 10.1182/blood-2005-12-015222. [DOI] [PubMed] [Google Scholar]
- 11.Cheng Y, Wong RS, Soo YO, Chui CH, Lau FY, Chan NP, et al. Initial treatment of immune thrombocytopenic purpura with high-dose dexamethasone. N Engl J Med. 2003;349:831–6. doi: 10.1056/NEJMoa030254. [DOI] [PubMed] [Google Scholar]
- 12.Sakamoto K, Nakasone H, Tsurumi S, Sasaki K, Mitani K, Kida M, et al. Prednisone versus high-dose dexamethasone for untreated primary immune thrombocytopenia. A retrospective study of the Japan Hematology & Oncology Clinical Study Group. J Thromb Thrombolysis. 2014;37:279–86. doi: 10.1007/s11239-013-0939-3. [DOI] [PubMed] [Google Scholar]
- 13.Schäcke H, Berger M, Rehwinkel H, Asadullah K. Selective glucocorticoid receptor agonists (SEGRAs): Novel ligands with an improved therapeutic index. Mol Cell Endocrinol. 2007;275:109–17. doi: 10.1016/j.mce.2007.05.014. [DOI] [PubMed] [Google Scholar]
- 14.Da Silva JA, Jacobs JW, Kirwan JR, Boers M, Saag KG, Inês LB, et al. Safety of low dose glucocorticoid treatment in rheumatoid arthritis: Published evidence and prospective trial data. Ann Rheum Dis. 2006;65:285–93. doi: 10.1136/ard.2005.038638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Saag KG, Koehnke R, Caldwell JR, Brasington R, Burmeister LF, Zimmerman B, et al. Low dose long-term corticosteroid therapy in rheumatoid arthritis: An analysis of serious adverse events. Am J Med. 1994;96:115–23. doi: 10.1016/0002-9343(94)90131-7. [DOI] [PubMed] [Google Scholar]
- 16.Portielje JE, Westendorp RG, Kluin-Nelemans HC, Brand A. Morbidity and mortality in adults with idiopathic thrombocytopenic purpura. Blood. 2001;97:2549–54. doi: 10.1182/blood.v97.9.2549. [DOI] [PubMed] [Google Scholar]
- 17.van Staa TP, Leufkens HG, Cooper C. The epidemiology of corticosteroid-induced osteoporosis: A meta-analysis. Osteoporos Int. 2002;13:777–87. doi: 10.1007/s001980200108. [DOI] [PubMed] [Google Scholar]
- 18.Cuker A, Cines DB, Neunert CE. Controversies in the treatment of immune thrombocytopenia. Curr Opin Hematol. 2016;23:479–85. doi: 10.1097/MOH.0000000000000270. [DOI] [PubMed] [Google Scholar]
- 19.Rodeghiero F. First-line therapies for immune thrombocytopenic purpura: Re-evaluating the need to treat. Eur J Haematol Suppl. 2008;69:19–26. doi: 10.1111/j.1600-0609.2007.01000.x. [DOI] [PubMed] [Google Scholar]
- 20.Matschke J, Muller-Beissenhirtz H, Novotny J, Vester I, Hertenstein B, Eisele L, et al. A randomized trial of daily prednisone versus pulsed dexamethasone in treatment-naive adult patients with immune thrombocytopenia: EIS 2002 Study. Acta Haematol. 2016;136:101–7. doi: 10.1159/000445420. [DOI] [PubMed] [Google Scholar]
- 21.Andersen JC. Response of resistant idiopathic thrombocytopenic purpura to pulsed high-dose dexamethasone therapy. N Engl J Med. 1994;330:1560–4. doi: 10.1056/NEJM199406023302203. [DOI] [PubMed] [Google Scholar]
- 22.Godeau B. High-dose dexamethasone or oral prednisone for immune thrombocytopenia? Lancet Haematol. 2016;3:e453–4. doi: 10.1016/S2352-3026(16)30124-7. [DOI] [PubMed] [Google Scholar]
- 23.Mashhadi MA, Kaykhaei MA, Sepehri Z, Miri-Moghaddam E. Single course of high dose dexamethasone is more effective than conventional prednisolone therapy in the treatment of primary newly diagnosed immune thrombocytopenia. Daru. 2012;20:7. doi: 10.1186/2008-2231-20-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Din B, Wang X, Shi Y, Li Y. Long-term effect of high-dose dexamethasone with or without low-dose dexamethasone maintenance in untreated immune thrombocytopenia. Acta Haematol. 2015;133:124–8. doi: 10.1159/000362529. [DOI] [PubMed] [Google Scholar]
- 25.Nakazaki K, Hosoi M, Hangaishi A, Ichikawa M, Nannya Y, Kurokawa M. Comparison between pulsed high-dose dexamethasone and daily corticosteroid therapy for adult primary immune thrombocytopenia: A retrospective study. Intern Med. 2012;51:859–63. doi: 10.2169/internalmedicine.51.7005. [DOI] [PubMed] [Google Scholar]
- 26.Mithoowani S, Gregory-Miller K, Goy J, Miller MC, Wang G, Noroozi N, et al. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: A systematic review and meta-analysis. Lancet Haematol. 2016;3:e489–96. doi: 10.1016/S2352-3026(16)30109-0. [DOI] [PubMed] [Google Scholar]


