Abstract
Staphylococcus xylosus is found in the microbiota of traditional cheeses, particularly in the rind of soft smeared cheeses. Despite its frequency, the molecular mechanisms allowing the growth and adaptation of S. xylosus in dairy products are still poorly understood. A transcriptomic approach was used to determine how the gene expression profile is modified during the fermentation step in a solid dairy matrix. S. xylosus developed an aerobic metabolism perfectly suited to the cheese rind. It overexpressed genes involved in the aerobic catabolism of two carbon sources in the dairy matrix, lactose and citrate. Interestingly, S. xylosus must cope with nutritional shortage such as amino acids, peptides, and nucleotides, consequently, an extensive up-regulation of genes involved in their biosynthesis was observed. As expected, the gene sigB was overexpressed in relation with general stress and entry into the stationary phase and several genes under its regulation, such as those involved in transport of anions, cations and in pigmentation were up-regulated. Up-regulation of genes encoding antioxidant enzymes and glycine betaine transport and synthesis systems showed that S. xylosus has to cope with oxidative and osmotic stresses. S. xylosus expressed an original system potentially involved in iron acquisition from lactoferrin.
Keywords: Staphylococcus, cheese model, physiology, nutrient shortage, osmotic stress, oxidative stress
1. Introduction
Cheeses are one of the oldest fermented products, representing more than 1400 varieties of traditional cheeses around the world and nearly 1000 varieties in France [1]. Their microbial communities, composed of “house microbiota” and starters, are shaped by the technological processes and contribute to the development of the sensorial properties of cheeses. Studies of this microbial diversity combining phenotypic and genomic approaches have revealed two ecosystems, the core and the rind. Lactic acid bacteria (LAB) largely dominate the inner part of the cheese [1,2,3]. The study of rinds from 137 different cheeses from 10 countries and 12 different French cheeses revealed a dominant core of 14 bacterial and 10 fungal genera [1,4]. The genus Staphylococcus is one of the genera of the bacterial core in milk and cheese [1,4,5,6] and is often identified in different types of cheese [7,8,9,10]. Within this genus, Staphylococcus xylosus has been identified in soft red smeared cheeses, Raclette, Saint Nectaire [2], and Livarot [11]. S. xylosus was enumerated at 105 to 109 CFU/g in Camembert and blue-veined cheeses [12]. S. xylosus is also commercially available as an adjunct culture to enhance the aroma and texture of cheeses, as well as the color of the surface of smeared cheeses [13].
The transcriptomic or more recently the metatranscriptomic responses of LAB have been well established in milk and cheese [14,15,16,17,18,19]. The potential of surface microbiota has been less investigated. Several studies have characterized the role of yeasts, in co-culture with LAB, and/or in association with other ripening bacteria [20,21,22,23]. Very few studies have considered coagulase-negative staphylococci, despite their frequency in cheese. The investigation of interaction between S. xylosus, Lactococcus lactis, and Yarrowia lipolytica in culture media revealed that the expression of some genes of S. xylosus such as the lactate dehydrogenase gene (ldh) and genes involved in amino acid transport, significantly decreased in mixed cultures [24]. Three studies have characterized the transcriptomic response in milk or cheese of Staphylococcus aureus in mixed cultures with different species of lactic acid bacteria, revealing in particular the increased expression of genes associated with the acid and redox stress response, the repression of certain enterotoxin genes and of the agr system, a major virulence regulator [25,26,27].
The adaptation mechanisms of S. xylosus in cheese are poorly documented despite its importance for cheese flavor development and typicity. To better understand S. xylosus behavior in a dairy matrix at the molecular level, we analyzed the transcriptome of S. xylosus in a solid dairy matrix incubated for 48 h in conditions that mimic the fermentation step. The in situ response of S. xylosus was analyzed at 24 and 48 h vs 6 h.
2. Materials and Methods
2.1. Dairy Matrix and Inoculation
The dairy matrix was made as described previously [28]. In brief, retentate was prepared from 5.5-fold concentrated ultra-filtered cow’s milk supplemented with salt (0.6%) and UHT cream (5.2%). Its composition was: dry matter, 258.5 g/kg; fat, 52.3 g/kg; lactose, 40.9 g/kg; total nitrogen, 140.15 g/kg; noncaseinic nitrogen, 26.9 g/kg; nonprotein nitrogen, 1.61 g/kg; and NaCl, 6.2 g/kg. The pH was 6.54.
The S. xylosus C2a strain, whose complete genome is available (LN554884), was used in this study. It was grown for 15 h at 30 °C with shaking (150 rpm) in brain-heart infusion (BHI) broth (Becton, Dickinson and Compagny, Le Pont de Claix, France). Then, the culture was centrifuged and inoculated at a concentration equivalent to 106 CFU/mL in the retentate heated at 25 °C before addition of 0.3 µL/mL of rennet (DSM Food Specialties; Delft, Netherlands). The dairy matrix (per 30 g) was incubated for 6 h at 30 °C and then the solid dairy matrix was transferred at 12 °C into an incubation chamber. Three independent experiments were done.
The bacterial cells were enumerated immediately following inoculation and after 6 h, 24 h, and 48 h of incubation. The numbers of CFU were determined after serial dilutions on plates of BHI agar incubated at 30 °C for 24 h.
At 6, 24, and 48 h of incubation, 200 mg samples were taken and immediately frozen in liquid nitrogen to stabilize the bacterial RNA.
2.2. RNA Extraction and Purification
RNA was extracted from the bacterial cells separated from the solid dairy matrix according to the protocol described previously [28]. Briefly, the samples were thawed and homogenized in trisodium citrate solution (2% w/v) at 4 °C using a mechanical Waring blender. The cells were then recovered by centrifugation for 5 min at 6000× g at 4 °C and washed with TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 8.0). the cell pellets were resuspended in Tris-EDTA buffer (20 mM Tris-HCl, 2 mM EDTA, pH 8.0) and RLT buffer (RNeasy Mini Kit, Qiagen, Courtaboeuf, France) at a ratio of 30/70 (v/v). After addition of chloroform, the samples were vigorously shaken in a bead beater (Biospec Products Inc., Bartlesville, OK, USA) and centrifuged for 20 min at 12,000× g at 4 °C. The RNA was isolated from the aqueous phase using RNeasy Mini Kit according to the manufacturer’s instructions. DNase treatment of RNA was performed with Turbo DNAse (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. The absence of S. xylosus genomic DNA contamination was verified by PCR. DNase treatment of RNA was performed with Turbo DNAse (Ambion, Austin, TX, USA) according to the manufacturer’s instructions. The absence of S. xylosus genomic DNA contamination was verified by PCR. Total RNA isolated was quantified using a Nanodrop 1000 (Thermo Fisher Scientific, Wilmington, DE, USA) and RNA quality was analyzed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Santa Clara, CA, USA) according to the manufacturer’s instructions. The RNA was stored at −80 °C.
2.3. RNA Labeling and Microarray Analyses and Validation
The RNA labeling and hybridization on the Agilent microarray were carried out as described previously [29]. A complete description of the array developed for S. xylosus C2a is available at the NCBI Gene Expression Omnibus (GEO) database under platform accession number GPL19201. The microarrays were analyzed as described previously [29]. Significant differences in the probe set intensities between the conditions were identified using a linear model with an empirical Bayes method using all information probes to moderate the standard errors of the estimated log-fold changes [30]. The probabilities were corrected by the Benjamini–Hochberg procedure in order to control the false-discovery rate (FDR) with a p value cut-off of 0.05. All the probes with an FDR ≤ 0.05 were considered to be differentially expressed. Finally, a gene was considered to be differentially expressed if at least 50% of the corresponding probes were differentially expressed and if the ratio of expression was ≥2 or ≤0.5.
The microarray data were validated as described previously [29]. The targeted genes for qPCR and primer sequences are listed in Supplementary Table S1. The analyses were performed on the same samples of RNA as used for the microarray experiments. The relative fold change of gene expression, using measured tuf housekeeping gene expression, was determined by the 2−ΔΔCt method [31].
2.4. Chemical Analysis of the Solid Dairy Matrix
The methods for determining sugar, organic acid, and free amino-acid content were carried out as described previously [14].
3. Results and Discussion
3.1. Growth of S. xylosus in the Solid Dairy Matrix and Transcriptome Profile
S. xylosus was inoculated in the milk retentate at 5.6 log CFU/g. The dairy matrix was immediately solidified by addition of rennet. S. xylosus grew and reached 7.4 log CFU/g after 6 h of incubation at 30 °C. At 6h-post inoculation, the temperature of incubation was reduced to 12 °C, the strain went up growing with a reduced slope and reached 8.7 log CFU/g, 24 h post-inoculation. Then, the population remained almost at this level (8.9 log CFU/g) until the end of the experiment (48 h). The initial pH was 6.54 and did not vary during the incubation. Similarly, Staphylococcus aureus grown in the same solid dairy matrix did not acidify the medium [25].
The in situ S. xylosus response revealed a change in gene expression at 24 and 48 h of incubation in the solid dairy matrix in comparison with the 6 h time point of sampling used as reference in our study. There were 1175 genes differentially expressed at 24 and 48 h, with 779 genes in common (421 up-, 358 down-regulated), indicating that transcriptional changes had occurred at 24 h and lasted up to 48 h (Supplementary Table S2). These differentially expressed genes were classified into different functional categories: the most represented being metabolism (29%), information storage and processing (14%), and cellular processes (9%).
To validate the microarray analysis independently, the relative expression of 62 differentially expressed genes representing about 8% of the genes at both 24 and 48 h was measured by qPCR (Supplementary Table S1). The microarray and qPCR results for the tested genes were correlated for the two times of incubation (24 h: r² = 0.911, slope y = 1.1178x; 48 h: r² = 0.928, slope y = 1.3302x) and the expected trend in the expression pattern was confirmed.
The transcriptomic profile illustrated a general slowdown of S. xylosus activity at 24 and 48 h. Not only were the genes encoding the translation machinery, synthesis, and modifications of ribosomal proteins (14 genes rps, 14 genes rpl, 6 genes rpm) underexpressed but so were the genes involved in DNA replication, recombination, and repair (Supplementary Table S2). This underexpression of ribosomal proteins attested to the decrease in growth rate of S. xylosus observed by cultural approach. Such down-regulation of genes involved in replication and translation machineries has already been observed for Lactococcus lactis cultured in the same cheese model after 24 h of growth in the same conditions [14]. This decrease in S. xylosus growth was accompanied by the induction of the rnr gene involved in ribosome degradation and uvrAB genes in DNA excision repair (Table 1). Genes involved in cell division, peptidoglycan synthesis (divIB, murD, mraY, pbp1, ftsL), and cell lysis (phage) were underexpressed for S. xylosus grown in the dairy matrix (Supplementary Table S2), while all these genes were overexpressed in S. xylosus grown in a meat model [32]. This could be due to the decrease in temperature (from 30 to 12 °C) applied to the dairy matrix to mimic cheese making process. However, the transcriptomic data did not reveal any induction of genes involved in adaptation to cold, e.g., cspA encoding a cold shock protein was underexpressed (Table 1). In relation with this slowdown activity, only the cluster hslUV, a member of the Hsp100 (Clp) family of ATPases, was overexpressed (Table 1). This ATP protease is necessary for cellular protein homeostasis and protein quality control [33].
Table 1.
Genes of Staphylococcus xylosus differentially expressed at 24 h and/or 48 h compared to 6 h in the dairy matrix model.
| Gene ID | Gene Name | Description | Mean Ratio of Expression | |
|---|---|---|---|---|
| 24 h/6 h | 48 h/6 h | |||
| Cellular Processes | ||||
| SXYL_02062 | rnr | Ribonuclease R | 2.7 | 2.1 |
| SXYL_02088-89 | uvrAB | UvrABC system protein A and B | 3.1 * | 2.5 * |
| SXYL_01472 | cspA | Cold shock protein CspA | 0.3 | 0.5 |
| SXYL_01630-31 | hslUV | ATP-dependent protease ATPase | 2.4 * | 2.1 * |
| Carbohydrate Metabolism | ||||
| Transporters | ||||
| SXYL_00773-76 | mtlDFA | Mannitol transport system | 9.9 * | 7.1 * |
| SXYL_00268-70 | ulaA | PTS ascorbate transporter | 3.9 * | 3.2 |
| SXYL_00255-60 | PTS sugar transport system | 12.1 * | 7.6 * | |
| Regulator | ||||
| SXYL_01129 | ccpA | Catabolite control protein A | 2.1 | |
| Galactose/lactose | ||||
| SXYL_00082 | lacR | Lactose operon transcription activator | 0.5 | 0.5 |
| SXYL_00672-74 | galKET | Galactokinase, UDP-glucose 4-epimerase and galactose-1-phosphate uridylyltransferase | 4.0 * | |
| SXYL_01351 | malA | Alpha-D-1,4-glucosidase | 2.3 | 2.2 |
| Pentose phosphate pathway/pentose and glucuronate interconversions | ||||
| SXYL_01353 | zwf | Glucose-6-phosphate 1-dehydrogenase | 2.3 | 2.2 |
| SXYL_02290 | 6-phospho-3-hexuloisomerase | 13.7 | 9.3 | |
| SXYL_02291 | 3-hexulose-6-phosphate synthase | 17.5 | 12.5 | |
| SXYL_00568 | rpi | Ribose-5-phosphate isomerase | 3.0 | 3.4 |
| SXYL_02430 | prs | Ribose-phosphate pyrophosphokinase | 2.4 | 2.4 |
| SXYL_02582 | 6-phosphogluconate dehydrogenase | 2.9 | 4.2 | |
| SXYL_02337-39 | uxuAC2 | D-mannonate oxidoreductase, Mannonate dehydratase and Glucuronate isomerase | 2.9 * | 2.0 * |
| SXYL_00132 | xylE | Xylose transporter | 2.0 | |
| SXYL_00133-34 | xylBA | D-xylulose kinase and xylose isomerase | 3.7 * | 4.5 * |
| SXYL_02250 | Xylose isomerase | 3.7 | 3.3 | |
| SXYL_00607 | araB1 | Ribulokinase | 2.6 | 2.0 |
| SXYL_02343 | 2-keto-3-deoxygluconate kinase | 2.0 | ||
| Glycolysis | ||||
| SXYL_00221 | fda | Fructose-bisphosphate aldolase class 1 | 2.2 | 2.7 |
| SXYL_01169 | pfkA | 6-phosphofructokinase | 2.7 | |
| SXYL_02071 | gapR | Glycolytic operon regulator | 3.6 | 3.4 |
| Pyruvate metabolism | ||||
| SXYL_00276 | ldhB | L-lactate dehydrogenase | 2.6 | 4.8 |
| SXYL_00170 | lqo | L-lactate-quinone oxidoreductase | 2.8 | 2.2 |
| SXYL_00366-67 | cidBC | Holin-like protein CidB and pyruvate oxidase | 12.0 * | 8.2 * |
| SXYL_01023-24 | pflAB | Formate acetyltransferase | 3.0 * | |
| SXYL_00431 | budA | Alpha-acetolactate decarboxylase | 2.6 | 2.3 |
| TCA cycle and respiratory chain | ||||
| SXYL_01592-93 | 2-oxoglutarate ferredoxin oxidoreductase | 4.6 * | 5.2 * | |
| SXYL_00481 | Citrate transporter | 3.3 | 3.5 | |
| SXYL_02340-42 | C4-dicarboxylate transport system | 2.6 * | ||
| SXYL_01012 | fumC | Fumarate hydratase class II | 2.2 | 2.7 |
| SXYL_01172 | Citrate synthase | 2.1 | ||
| SXYL_01173 | icd | Isocitrate dehydrogenase [NADP] | 2.1 | |
| SXYL_01821 | pyc | Pyruvate carboxylase | 0.5 | 0.3 |
| SXYL_01534 | acnA | Aconitate hydratase | 3.1 | 2.5 |
| SXYL_00218 | gabD | Succinate-semialdehyde dehydrogenase | 2.2 | |
| SXYL_01849-50 | cydBA | Cytochrome bd-type quinol oxidase | 3.3 * | 2.9 * |
| SXYL_00470 | ppk | Polyphosphate kinase | 6.7 | 4.9 |
| SXYL_01851 | Glutaredoxin | 3.1 | 4.2 | |
| Amino Acids Synthesis | ||||
| Transporters | ||||
| SXYL_00265-66 | ABC-type amino acid transport system | 2.2 * | ||
| SXYL_00661 | ABC-type amino acid transport system permease | 2.0 | ||
| SXYL_02264 | Amino acid permease | 6.5 | 8.3 | |
| SXYL_02687 | cstA | Carbon starvation protein CstA | 3.1 | 2.9 |
| Regulators | ||||
| SXYL_01629 | codY | GTP-sensing transcriptional pleiotropic repressor | 2.2 | 3.1 |
| Histidine | ||||
| SXYL_00460-64 | hisIFAHB | Histidine biosynthesis | 2.4 * | |
| SXYL_00465-68 | hisCDGZ | Histidine biosynthesis | 3.1 * | 2.4 * |
| SXYL_02126 | hisC2 | Histidinol-phosphate aminotransferase | 2.2 | 2.4 |
| SXYL_00008 | hutH | Histidine ammonia-lyase (Histidase) | 2.0 | |
| SXYL_00614 | hutG | Formimidoylglutamase | 7.6 | 7.7 |
| SXYL_00617 | hutU | Urocanate hydratase | 2.2 | |
| SXYL_00618 | hutI | Imidazolonepropionase | 3.8 | 3.7 |
| Glutamate, glutamine | ||||
| SXYL_00106-08 | glnA2 | Short-chain dehydrogenase, glutamine synthetase and aldehyde dehydrogenase | 25.5 * | 15.1 * |
| SXYL_00476 | Glutamate synthase | 5.1 | 3.7 | |
| SXYL_02393 | gltX | Glutamate--tRNA ligase | 3.7 | 4.1 |
| SXYL_00347 | rocA | 1-pyrroline-5-carboxylate dehydrogenase | 2.4 | 2.2 |
| SXYL_01964 | gluD1 | Glutamate dehydrogenase | 2.1 | |
| Arginine | ||||
| SXYL_01961-62 | argGH | Argininosuccinate synthase and lyase | 3.0 * | 6.1 * |
| SXYL_01965 | rocD2 | Ornithine aminotransferase 2 | 2.4 | |
| SXYL_00252 | arcB | Ornithine carbamoyltransferase | 4.9 | |
| SXYL_00239-41 | argCJB | Initial steps of the arginine biosynthetic pathway | 3.5 * | 4.4 * |
| SXYL_00769 | arg | Arginase | 2.3 | |
| SXYL_01355 | proC | Pyrroline-5-carboxylate reductase | 3.2 | 4.6 |
| Urea catabolism | ||||
| SXYL_00291-96 | ureGFECBA | Urease | 3.0 * | 2.1 * |
| SXYL_00297 | Urea transporter | 2.2 | 2.0 | |
| Lysine | ||||
| SXYL_01476-79 | dapHBAasd | Lysine biosynthesis | 2.7 * | 2.6 * |
| SXYL_01480 | Aspartokinase | 2.1 | ||
| SXYL_00377 | dapE | Succinyl-diaminopimelate desuccinylase | 2.0 | |
| Leucine, valine, isoleucine | ||||
| SXYL_00867-69 | ilvAleuDCB | Leucine, valine, isoleucine biosynthesis | 4.7 * | 2.9 * |
| SXYL_00870 | leuB | 3-isopropylmalate dehydrogenase | 2.5 | |
| SXYL_02469 | ilvD2 | Dihydroxy-acid dehydratase | 3.0 | 3.0 |
| Glycine | ||||
| SXYL_01317-19 | gcvTPAPB | Glycine metabolism | 4.7 * | 3.1 * |
| Tryptophan | ||||
| SXYL_01497 | trpA | Tryptophan synthase alpha chain | 5.4 | |
| SXYL_01498-503 | trpBFCDGE | Tryptophan biosynthesis | 6.3 * | 6.2 * |
| Cysteine, methionine | ||||
| SXYL_02630-38 | cysCsatcobAcysIJH | Cysteine biosynthesis | 41.6 * | 39.0 * |
| SXYL_02417 | cysK | Cysteine synthase | 2.7 | 4.2 |
| SXYL_02391-93 | cysSEgltX | Cysteine--tRNA ligase, serine acetyltransferase and glutamate--tRNA ligase | 4.5 * | 3.8 * |
| SXYL_00283-85 | cysDMmetB | Cysteine biosynthesis | 5.9 * | 6.2 * |
| SXYL_02641-42 | Cystathionine gamma-synthase | 23.0 * | 23.1 * | |
| SXYL_02643-45 | Methionine biosynthesis | 105.2 * | 92.5 * | |
| SXYL_01238 | Cysteine desulfurase | 4.3 | 3.2 | |
| SXYL_00118 | Dihydrofolate reductase family protein | 6.8 | 5.4 | |
| Nucleic Acid Bases, Vitamins, Cofactors Syntheses | ||||
| Pyrimidine | ||||
| SXYL_01686 | carB | Carbamoyl-phosphate synthase large chain | 2.2 | |
| SXYL_01687-91 | carApyrCBPR | UMP biosynthesis | 7.2 * | 6.6 * |
| SXYL_01249 | udk | Uridine kinase | 2.3 | |
| Purine | ||||
| SXYL_01861-64 | purDHNM | IMP biosynthesis | 3.0 * | |
| SXYL_01865-71 | purFLQCKE | IMP biosynthesis | 12.9 * | 7.0 * |
| SXYL_00928 | purB | Adenylosuccinate lyase (ASL) | 3.0 | 3.4 |
| Thiamine | ||||
| SXYL_02587 | thiC | Phosphomethylpyrimidine synthase | 2.1 | |
| SXYL_00839 | thiD | Hydroxy-phosphomethylpyrimidine kinase | 2.4 | |
| SXYL_00840 | thiM | Hydroxyethylthiazole kinase | 2.4 | |
| Panthotenate | ||||
| SXYL_00168 | panB | 3-methyl-2-oxobutanoate hydroxymethyltransferase | 3.2 | 2.4 |
| SXYL_00169 | panC | Pantothenate synthetase | 3.1 | 2.3 |
| SXYL_01178 | coaE | Dephospho-CoA kinase | 2.7 | 2.2 |
| SXYL_00273 | 2-dehydropantoate 2-reductase | 9.3 | 7.0 | |
| Nicotinate | ||||
| SXYL_00924 | Nicotinate phosphoribosyltransferase | 3.2 | 2.1 | |
| SXYL_00925 | nadE | NH(3)-dependent NAD(+) synthetase | 2.9 | 2.3 |
| SXYL_01458 | Dihydrofolate reductase family protein | 2.0 | ||
| SXYL_02532 | nrlA | NADPH-dependent oxidoreductase | 18.9 | 13.1 |
| Lipids, Glycerolipids Metabolism | ||||
| SXYL_02652 | Acetyl-CoA acetyltransferase | 4.9 | 2.7 | |
| SXYL_02653 | NAD binding 3-hydroxyacyl-CoA dehydrogenase | 4.7 | 2.1 | |
| SXYL_02654 | Acyl-CoA dehydrogenase | 2.8 | ||
| SXYL_01253-54 | Acetyl-CoA carboxylase, biotin carboxyl carrier protein and biotin carboxylase | 3.8 * | 3.2 * | |
| SXYL_00566-67 | dhaK2L2 | Dihydroxyacetone kinase, K and L subunits | 4.5 * | 4.0 * |
| SXYL_02198-200 | dhaMLK | Dihydroxyacetone kinase, M, L and K subunits | 3.5 * | 2.5 * |
| SXYL_01576 | glpD | Aerobic glycerol-3-phosphate dehydrogenase | 2.2 | 2.5 |
| SXYL_02208 | tagD | Glycerol-3-phosphate cytidylyltransferase | 2.1 | |
| SXYL_01997 | lipA | Lipoyl synthase | 2.2 | 2.0 |
| SXYL_01915 | ugtP | Processive diacylglycerol beta-glucosyltransferase | 2.5 | 2.2 |
| SXYL_01916 | ltaA | Probable glycolipid permease LtaA | 2.0 | |
| Iron Transport | ||||
| SXYL_00561-63 | Oxidoreductase, monooxygenase and transporter | 24.4 * | 36.5 * | |
| SXYL_00749 | sfaB | Siderophore biosynthesis protein, IucA/IucC family | 2.1 | 2.5 |
| SXYL_00459 | ABC-type cobalamin Fe3+-siderophores transporter | 2.2 | 2.0 | |
| Response to Stress | ||||
| General stress | ||||
| SXYL_00859 | rsbU | Serine phosphatase RsbU regulator of sigma subunit | 0.4 | 0.3 |
| SXYL_00860-62 | rsbVW, sigB | Anti-sigma-B factor antagonist, Serine-protein kinase RsbW and RNA polymerase sigma factor | 3.8 * | 3.8 * |
| SXYL_00743 | opuD2 | Glycine betaine transporter | 5.0 | 3.7 |
| SXYL_00744 | amaP | Alkaline shock response membrane anchor protein | 13.2 | 11.3 |
| SXYL_00745 | DUF2273 domain-containing protein | 11.6 | 11.2 | |
| SXYL_00746 | asp23 | Alkaline shock protein 23 | 9.2 | 12.8 |
| SXYL_00309 | Universal stress protein | 2.2 | 2.2 | |
| SXYL_00196 | General stress protein | 11.0 | 8.6 | |
| SXYL_00548 | Heat shock protein Hsp20 | 2.0 | ||
| Cation transport | ||||
| SXYL_00416 | Zinc ABC transporter substrate-binding protein | 2.2 | 2.2 | |
| SXYL_00559 | Na/Pi cotransporter family protein | 2,3 | ||
| SXYL_01831 | mntH | Divalent metal cation transporter MntH | 2.2 | 2.6 |
| SXYL_01859 | ABC-type cobalt transport system ATPase | 2.4 | ||
| SXYL_02658-60 | mtsC | Metal ABC transporter | 3.9 * | 4.4 * |
| SXYL_00784 | czrA | Zinc and cobalt transport repressor CzrA | 5.5 | 6.1 |
| Phosphate | ||||
| SXYL_01484-87 | pstSCAB | Phosphate transporter | 2.5 * | 3.3 * |
| SXYL_02189 | Inorganic phosphate transporter | 2.3 | ||
| SXYL_00470-71 | ppk, ppx | Polyphosphate kinase and Exopolyphosphatase | 8.2 * | 5.4 * |
| Pigmentation | ||||
| SXYL_00050 | crtO | Glycosyl-4,4 -diaponeurosporenoate acyltransferase | 4.7 | 2.8 |
| SXYL_00051-53 | crtPQM | Diapolycopene oxygenase, 4,4 -diaponeurosporenoate glycosyltransferase and dehydrosqualene synthase | 2.5 * | |
| Oxidative stress | ||||
| SXYL_00923 | nos | Nitric oxide synthase oxygenase | 3.2 | 2.3 |
| SXYL_02533 | katC | Catalase C | 20.8 | 12.9 |
| SXYL_02505 | katA | Catalase A | 0.2 | 0.1 |
| SXYL_01303 | sodA | Superoxide dismutase [Mn/Fe] | 2.8 | 4.1 |
| SXYL_02639 | hmpA | Flavohemoprotein | 2.5 | 2.5 |
| SXYL_02418 | hslO | 33 kDa chaperonin | 2.5 | 2.2 |
| Osmotic stress | ||||
| SXYL_02127 | Glycine/betaine/choline ABC transporter | 2.4 | 2.0 | |
| SXYL_02128 | Glycine/betaine/choline ABC transporter ATPase | 2.1 | ||
| SXYL_00486 | lcdH | L-carnitine dehydrogenase | 4.9 | 3.9 |
| SXYL_00407 | Sodium:solute symporter | 3.0 | ||
| SXYL_00232 | MFS transporter | 2.6 | 3.1 | |
| SXYL_00286 | MFS transporter | 4.2 | 3.8 | |
| SXYL_00379-82 | capBCAE | Polyglutamate synthesis | 2.2 * | 2.5 * |
| SXYL_00114-15 | saeRS | Response regulator and Histidine protein kinase | 0.4 * | 0.2 * |
| SXYL_00323 | isaA | Transglycosylase IsaA | 3.7 | 5.1 |
| SXYL_00116 | sceD2 | Transglycosylase SceD 2 | 20.2 | 27.8 |
| SXYL_00117 | sceD1 | Transglycosylase SceD1 | 116.5 | 124.1 |
| SXYL_02188 | Secretory antigen SsaA-like protein | 27.9 | 36.1 | |
* Means of the expression of the clustered genes differentially expressed.
3.2. Aerobic Carbohydrate Catabolism
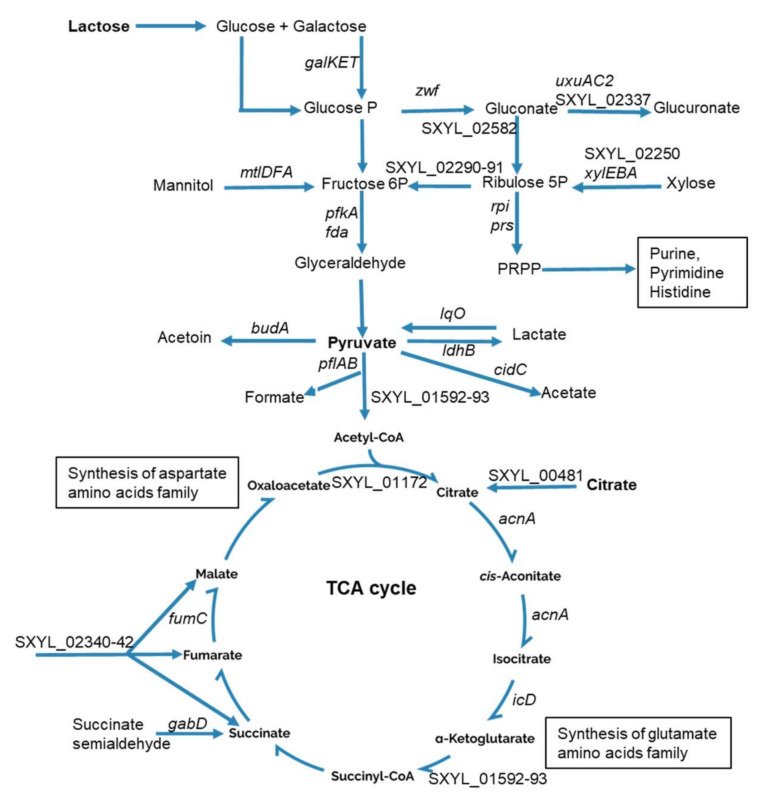
The main carbohydrate in the dairy matrix is lactose. Its initial concentration was 113.4 mM and it slightly decreased during incubation. Only 7.5 mM of lactose was consumed by S. xylosus after 48 h of incubation; lactate and acetate were not detected, and pH did not vary. Interestingly, similar results were observed for S. aureus grown in milk or in cheese, with very low or no consumption of lactose and no detection of lactate and acetate [25,27]. Like S. aureus, S. xylosus, however, has the genetic potential to assimilate lactose via a system composed of two genes, lacP and lacH, which, respectively, encode a lactose permease and a β-galactosidase, which hydrolyzes lactose to glucose and galactose [34,35]. A lacR regulatory gene is positioned upstream of the operon and oriented opposite to the lacPH operon [34]. In our conditions, lacR was underexpressed (Table 1), which might have resulted in low and constitutive lacPH expression in S. xylosus as previously observed [34]. The lacPH expression was also subject to carbon catabolite repression mediated by CcpA encoded by ccpA overexpressed in our study at 48 h (Table 1) which might explain the low expression of lacPH in our conditions. Apart from lactose related genes, we found several genes encoding phosphoenolpyruvate-dependent sugar phosphotransferase systems (PTS) that were highly overexpressed with the cluster SXYL_00773-76 coding for the mannitol transport system and another uncharacterized cluster SXYL_00255-260 (Table 1). The operon galKET was overexpressed at 24 h of incubation. It encodes enzymes involved in the degradation of galactose to glucose 1-P. Then, in our conditions, glucose 1-P could be further metabolized via the pentose phosphate (PP) and pentose/glucoronate interconversion pathways as shown by 15 genes overexpressed in these pathways (Table 1, Figure 1).
Figure 1.
Summary of carbohydrate catabolism by Staphylococcus xylosus in solid dairy matrix showing the overexpressed genes (the level of expression of these genes and the name of the corresponding enzymes are given Table 1).
Two genes (fda, pfkA) of the Embden–Meyerhof–Parnas (EMP) pathway related to fructose catabolism were overexpressed, as was the cluster mtl, which could lead to fructose. The glycolytic operon regulator gapR was up-regulated. In S. aureus, gapR down regulates the glycolytic operon (gap, pgk, tpi, pgm, eno) [36], which was underexpressed in our conditions (Supplementary Table S2).
Pyruvate is a nexus between several metabolic pathways, and it can be catabolized into several metabolites (Figure 1). These include catabolism into lactate by lactate dehydrogenase encoded by ldhB in S. xylosus. Here, we found ldhB to be overexpressed, as was lqo, which encodes the reverse reaction (Table 1, Figure 1), this bidirectional flow could explain we did not measure lactate. Pyruvate can also lead to acetate, the cluster cidBC being highly overexpressed in the dairy matrix. The cidC gene encodes a pyruvate oxidase, which converts the excess of pyruvate into acetate. Interestingly, this enzyme has been shown to be coupled to aerobic respiration due to its ability to shuttle electrons to quinone intermediates [37]. The acetate can then be oxidized in the TCA cycle to generate energy [38]; this could explain why we did not measure acetate under our conditions. Pyruvate can also be catabolized into formate and acetyl CoA and the pflAB operon encoding the pyruvate formate lyase was overexpressed at 24 h. Likewise, pyruvate formate lyase was shown to be the major up-regulated protein in Streptococcus thermophilus grown in milk [39]. Pyruvate can also be catabolized to acetyl CoA by 2-oxoglutarate ferredoxin oxidoreductase encoded by SXYL_01592-93 overexpressed. In both cases, acetyl-CoA fuels the TCA cycle. Finally, pyruvate can lead to the synthesis of acetoin, a compound involved in butter aroma, by the acetolactate decarboxylase encoded by budA overexpressed in S. xylosus (Table 1, Figure 1).
Citrate was assayed in the dairy matrix. Its initial concentration was about 3 mM, and 0.46 mM was consumed by S. xylosus after 48 h of incubation. In line with these observations, the gene SXYL_00481 encoding a citrate transporter was overexpressed (Table 1, Figure 1). Furthermore, the cluster SXYL_02340-42 encoding a transport system for C4-dicarboxylates such as malate, fumarate, and succinate was also overexpressed. All these transporters can feed the TCA cycle at one step or another. Eight genes encoding enzymes of the TCA cycle were found overexpressed in this study (Table 1, Figure 1). These genes encode enzymes leading to the production of α-ketoglutarate and oxaloacetate involved in amino acid synthesis. Consistent with what we observed in S. xylosus, L. lactis grown in skimmed milk or in a cheese model consumes citrate, with concomitant positive regulation of the cit operon [14,40]. However, consumption of citrate by L. lactis was complete (from 12 mmol.kg−1 to 0) after 24 h of incubation whereas it was rather slow or incomplete in S. xylosus with only 15% of citrate consumed after 48 h.
Finally, the operon cydBA and the gene ppk involved in oxidative phosphorylation and SXYL_01851 encoding a glutaredoxin functioning as electron carriers were up-regulated (Table 1) and could contribute to the energy used to produce adenosine triphosphate (ATP).
These data, in particular the up-regulation of the genes of the TCA cycle together with the absence of acidification corroborated by absence of lactate and acetate suggested that S. xylosus adopted an aerobic lifestyle in the dairy matrix.
3.3. Amino Acid Synthesis
The dairy matrix was rich in protein essentially casein but deficient in free amino acids, their concentration being below the detection limit of 25 µM. As a result of these amino acid deficiencies, only four genes encoding amino acid transport systems were overexpressed by S. xylosus (Table 1). A gene potentially involved in peptide transport, ctsA, was also overexpressed (Table 1). In Escherichia coli and Campylobacter jejuni, CstA was shown to be involved in peptide transport and cstA was overexpressed when cells entered the stationary phase [41,42].
L. lactis had an efficient proteolytic system to cope with this low concentration of free amino acids [14]. S. xylosus did not hydrolyze casein, but it is prototrophic and can grow on a medium containing ammonium as the sole nitrogen source [43]. In support of this, bioinformatic analyses of the S. xylosus genome revealed a complete repertoire of biosynthetic operons needed to synthesize all 20 amino acids [35]. Consequently, S. xylosus overexpressed genes involved in several pathways of amino acids synthesis in the dairy matrix (Table 1). Intriguingly, the transcriptional pleiotropic repressor codY was also up-regulated. In S. aureus, Cod Y acts as a direct repressor of transcription of genes encoding amino acid biosynthesis, and amino acid and peptide transport, but carbon or nitrogen limitation relieved CodY regulation [44]. Thus, in our conditions, S. xylosus must have perceived a nitrogen limitation that relieved codY repression. Consistent with that, a cluster of 9 his genes and the hisC2 gene involved in histidine synthesis were overexpressed (Table 1, Figure 2). Then, histidine can be degraded by a four-step pathway to glutamate, the 4 hut genes encoding these enzymes being overexpressed (Table 1, Figure 2). Glutamate can also be synthesized from α-ketoglutarate by a glutamate synthase (encoded by SXYL_00476) or a glutamate dehydrogenase (encoded by gluD1). Glutamate can be further catabolized in different pathways. It can lead to glutamine via glutamine synthetase encoded by glnA2, which was highly overexpressed; then, glutamine can fuel different pathways such as purine and pyrimidine syntheses. Glutamate can also be catabolized to glutamate 5-semialdehyde and then contributed to proline synthesis (Figure 2). Arginine and proline can be synthesized via the glutamate pathway as described for S. aureus [45] and illustrated in Figure 2 for S. xylosus. Finally, arginine through arginase (arg) released ornithine and urea further catabolized in NH3 by urease encoded by a cluster of 6 genes (ureGFECBA) overexpressed in our conditions (Table 1). Furthermore S. xylosus can import urea (SXYL_00297) suggesting that it can consume urea present in the dairy matrix as it has been already shown for S. aureus in a similar matrix [25]. This ammonia will serve as nitrogen source for S. xylosus growth. It has been shown that urease is induced in the case of nitrogen starvation in Bacillus subtilis and Corynebacterium glutamicum [46,47].
Figure 2.
Summary of synthesis pathways for amino acids of the glutamate and aspartate families and for histidine by Staphylococcus xylosus in solid dairy matrix showing the overexpressed genes (the level of expression of these genes and the name of the corresponding enzymes are given Table 1).
The pathway of lysine and branched-chain amino acids synthesis from aspartate is shown in Figure 2. Six genes encoding enzymes involved in the synthesis of lysine were overexpressed. The first two, asd and SXYL_01480, were involved in the catabolism of aspartate to aspartate semialdehyde, and the four others were involved in the formation of diaminopimelate, which will further lead to lysine (Table 1, Figure 2). A cluster of 4 genes (ilvA, leuDCB) and the gene ilvD2 encoded enzymes involved in valine, leucine, and isoleucine formation from threonine, were all up-regulated (Table 1, Figure 2).
Glycine is produced through the interconversion of serine and glycine by L-serine hydroxymethyltransferase providing 5,10-methylene-THF [48]. In our conditions, a cluster of three genes (gcvTPAPB) involved in the catabolism of this metabolite was up-regulated leading to the synthesis of lipoylproteins (Table 1).
The synthesis of aromatic amino acids depends on chorismate as common precursor [49]. From this precursor, the tryptophan synthesis involves seven enzymes encoded by the cluster trpABFCDGE, which was up-regulated in our conditions (Table 1).
The synthesis of cysteine and methionine required a thiol group that can be furnished via the assimilatory sulfate reduction pathway as a cluster of nine genes involved in this pathway was strongly up-regulated (SXYL_02630-38, about 40-fold) (Table 1, Figure 3). This cluster presented high similarity with the one described in B. subtilis [48]. The sulfide will serve as a thiol group to replace the hydroxyl group of serine for the synthesis of cysteine; this synthesis occurred in two steps in S. xylosus encoded by cysE and cysK (Table 1, Figure 3). The synthesis of methionine could derive from O-acetylhomoserine according to two pathways in S. xylosus, as illustrated in Figure 3. In the transsulfuration pathway, O-acetylhomoserine and cysteine can lead to homocysteine and then to methionine by enzymes encoded by genes highly up-regulated (SXYL_02641-42, about 20-fold, SXYL_02643, 60-fold, metE, 120-fold) (Table 1, Figure 3). In the alternate pathway, sulfhydration of O-acetylhomoserine to homocysteine occurred via the enzyme encoded by cysD, up-regulated 4-fold. It is noteworthy that, the genes cysM and metB encoding enzymes involved in the formation of cysteine from homocysteine were also overexpressed, but at a level lower than the reverse reaction (Table 1, Figure 3).
Figure 3.
Scheme of synthesis pathways of cysteine and methionine by Staphylococcus xylosus in solid dairy matrix showing the overexpressed genes (the level of expression of these genes and the name of the corresponding enzymes are given Table 1).
3.4. Nucleic Acid Base, Vitamin, and Cofactor Syntheses
Nucleic acid bases are limiting in milk, consequently, S. xylosus overexpressed genes encoding enzymes involved in de novo pyrimidine and purine nucleotide biosynthesis. The precursor is the 5-phosphoribosyl-1-pyrophosphate (PRPP) catalyzed by the enzyme encoded by prs, which was overexpressed in our conditions (Table 1). The UMP can be synthetized by several pathways in S. xylosus [35]. In our conditions, it can be synthetized from: (i) glutamine by enzymes encoded by the operon (carABpyrBCPR) overexpressed, (ii) extracellular uracil involving a uracil permease encoded by pyrP and a bifunctional protein, encoded by pyrR, which has uracil phosphoribosyltransferase activity and regulates expression of the operon, and (iii) the phosphorylation of uridine by a uridine kinase encoded by udk, which was overexpressed in our conditions (Table 1).
The pathway for purine first involves the synthesis of IMP in 11 steps as well-described in B. subtilis [50]. In S. xylosus, the genes required for this synthesis were overexpressed in our conditions (Table 1). Two metabolites of this pathway, the 5-phosphoribosyl-4-carboxamide-5-aminoimidazole and the 5-phosphoribosyl-5-aminomidazole, may be further catabolized to thiamine by enzymes encoded by the overexpressed thiC, thiD, thiM genes (Table 1).
Four genes encoding enzymes involved in the synthesis of pantothenate and coenzyme A and three genes involved in nicotinate synthesis were also up-regulated (Table 1). Moreover, the gene nrlA encoding an NADPH-dependent oxidoreductase involved in cofactor and vitamin synthesis was highly overexpressed.
3.5. Lipid and Glycerolipid Metabolism
The dairy matrix contained fat (52.3 g/kg). S. xylosus can catabolize fatty acids in acetyl-CoA by three enzymes, encoded by SXYL_02652-54, which was overexpressed in this environment (Table 1). Acetyl-CoA can also arise from the activity of the enzymes encoded by the cluster SXYL_01253-54, which was overexpressed (Table 1). Acety-CoA will then fuel the TCA cycle. Ten genes encoding enzymes involved in glycerolipid and glycerophospholipid syntheses, which play a key role in membrane biogenesis, were also overexpressed (Table 1).
3.6. Iron Homeostasis
Milk and dairy products are considered as poor sources of iron [51]. Iron is associated with casein, citrate, and lactoferrin. Lactoferrin, a member of transferrin family, is a glycoprotein able to bind and transfer iron (Fe3+ ions) [52]. Lactoferrin inhibits the growth of iron-dependent bacteria and in certain cases may serve as an iron donor and support the growth of some beneficial bacteria with low iron demands, such as Lactobacillus and Bifidobacterium species [52,53,54]. Lactoferrin reduces the growth of S. aureus and modulates the expression of iron-regulated surface Isd proteins [55]. S. xylosus has developed a plethora of mechanisms to acquire iron, including the elaboration of siderophores (sfa, hts), the utilization of exogenous siderophores (sst, fhu), the acquisition of iron from ferritin (SXYL_00561-63) and the uptake of inorganic free iron (sit) [35,56]. In the dairy matrix, the operon SXYL_00561-63 encoding an oxidoreductase, a monooxygenase and a transporter involved in the acquisition of iron from ferritin [56] was highly overexpressed at 24 h (24-fold) and 48 h (36-fold) (Table 1). This assumes that lactoferrin could be the preferred source of iron for S. xylosus in the dairy matrix.
3.7. Response to Stresses
The S. aureus alternative transcription factor SigB is activated by environmental stress and following entry into the stationary phase of cell growth [57]. The S. aureus gene sigB is in an operon composed of four genes rsbUVWsigB [57], and a similar organization was identified in S. xylosus. In the conditions used here, three genes of the cluster were found overexpressed in S. xylosus (Table 1). The S. aureus SigB controls a large regulon of 251 genes, with 198 positively controlled and 53 repressed, including genes involved in cellular processes, intermediary metabolism, and signaling pathways [58]. The asp23 gene is exclusively regulated by SigB and is a marker of the SigB activity in S. aureus [59]. It is the last gene of a cluster of 4 genes, which encodes, in addition to Asp23, an osmoprotectant transporter and two transmembrane proteins, which are well-conserved in staphylococci [60]. These authors [60] suggested a function for Asp23 related to the protection of the cell envelope of non-growing cells. In our study, this cluster (SYL_00743-00746) was also overexpressed (Table 1). Two genes (SXYL_00309, SXYL_00196) encoding proteins involved in universal or general stress were up-regulated. Such general stress proteins (Gsps) were shown to be under the SigB control in B. subtilis [61].
Genes encoding proteins involved in transport of cations or anions are positively regulated by SigB in S. aureus [57,58]. In S. xylosus, the transcription of 8 genes encoding cation transporters and 6 genes encoding phosphate transporters were up-regulated in our conditions (Table 1). In addition to phosphate transporter genes, the genes ppk, encoding a polyphosphate kinase, and ppx, an exopolyphosphatase, were highly overexpressed (Table 1). These genes could be involved in the synthesis of polyphosphate by using phosphate present in the dairy matrix, either as colloidal particles integrated in the casein micelles or covalently bound to casein molecules as serine-phosphate [62]. Polyphosphates are polymers that contribute to responses to many stresses. In E. coli, they are required for stationary-phase survival in response to deficiencies in amino acids or nitrogen [63,64]. The polyphosphates could then contribute to the survival of S. xylosus in the dairy matrix deficient in amino acids.
SigB is also shown to affect pigmentation, with crtMN up-regulated in S. aureus [58]. In this study, S. xylosus overexpressed the cluster crtOPQM involved in the carotenoid pigment synthesis pathway and the corollary was that the surface of dairy matrix had an orange color at the end of incubation. This pigment can protect against oxidative stress by scavenging free radicals as it has already been shown for S. aureus [65]. S. xylosus overexpressed five additional genes in response to this stress (Table 1). Among them, the gene nos encoding nitric oxide synthase (NOS) promotes resistance to hydrogen peroxide in S. xylosus [66]. These authors demonstrated, that in a nos deleted mutant, the expression of genes encoding catalases was modulated, with the up-regulation of katA and the down-regulation of katB and katC. In our study, katC was strongly overexpressed, while katA was underexpressed (Table 1). In addition, the sodA gene encoding superoxide dismutase was overexpressed by S. xylosus, as already observed in S. aureus for confronting oxidative stress [67,68]. Furthermore, as NO is a highly reactive free radical gas, which at low concentration acts as a signaling molecule and at high concentration promotes nitrosative stress, it can be detoxified by a flavohemoprotein encoded by hpm [69], this gene being up-regulated in our conditions (Table 1). Finally, hslO was also up-regulated (Table 1). It encoded a redox regulated molecular chaperone, which could protect oxidatively damaged proteins from irreversible aggregation.
S. xylosus had to adapt to NaCl in the dairy matrix. Five genes encoding enzymes involved in transport or synthesis of glycine betaine, a powerful osmoprotectant, were up-regulated (Table 1). The presence of salt also induced three genes encoding sodium:solute symporter and MFS transporters, which could contribute to extrusion of Na [70,71]. The cap locus was up-regulated in S. xylosus grown in the salted dairy matrix (Table 1). This locus encodes enzymes involved in the synthesis of poly-γ-DL-glutamic acid (PGA) in Staphylococcus epidermidis, this polymer protected S. epidermidis against a high salt concentration, a key feature of its natural environment, the human skin [72]. Three genes (isaA, sceD2, sceD1) encoding transglycolases were overexpressed in S. xylosus grown in the dairy matrix. In S. aureus, the two proteins, IsaA and SceD, have an autolytic activity and the potential to affect cell separation. The expression of sceD is greatly up-regulated by the presence of NaCl [73]. The expression of sceD is also positively regulated by SigB, but the greatest effect was that of SaeR, a negative regulator [73]. It is noteworthy that, in our study, the cluster saeRS was down-regulated. In S. aureus, isaA inactivation resulted not only in up-regulation of sceD but also of ssaA, which encodes staphylococcal secretory antigen, which has peptidoglycan hydrolase activity [73]. The gene SXYL_02188 encoding an SsaA-like protein was highly overexpressed in S. xylosus (Table 1).
4. Conclusions
This study provides an extensive view of S. xylosus transcriptome when grown in a solid dairy matrix. Our transcriptome approach, combined with chemical analysis of the dairy matrix, has revealed some physiological adaptation to this peculiar medium, some of which significantly differ from lactic acid bacteria physiology in similar conditions. S. xylosus uses lactose and citrate as substrates but contrary to lactic acid bacteria, it adopts respiratory metabolism rather than fermentation. S. xylosus overcomes the amino acid deficiency of this environment by using urea as a source of nitrogen and overexpresses amino acid synthesis pathways. S. xylosus must cope with several stresses, some of which are regulated by the SigB regulator, and may use an original system for iron acquisition from lactoferrin. As S. xylosus is part of the microbial cheese ripening community, this study provides knowledge that can be used to analyze the transcriptomic data of this community of several species or to analyze the metatranscriptomic data of the whole microbial community of cheeses.
Acknowledgments
The authors are grateful to David Marsh for correcting the English writing.
Supplementary Materials
Supplementary materials can be found at https://www.mdpi.com/2076-2607/8/11/1807/s1, Table S1: Targeted genes of Staphylococcus xylosus for the validation of microarray data by qPCR: expression at T24 h and T48 h in solid dairy matrix compared with T6 h. Table S2: Total genes of Staphylococcus xylosus differentially expressed at 24 h and/or 48 h of incubation compared to 6 h in solid dairy matrix.
Author Contributions
Conceptualization, S.L. and R.T.; Data curation, P.M. and A.d.L.F.; Formal analysis, S.L., P.M., A.d.L.F. and V.L.; Funding acquisition, S.L. and R.T.; Methodology, S.E., P.M. and V.L.; Supervision, S.L.; Writing—original draft, S.L. and R.T.; Writing—review & editing, S.L., S.E., Y.L.L. and R.T. All authors read and approved the final version. All authors have read and agreed to the published version of the manuscript.
Funding
The work was financially supported by the French National Research Agency (ANR) project NABAB (ANR-08-ALIA-11) and INRAE.
Conflicts of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dugat-Bony E., Garnier L., Denonfoux J., Ferreira S., Sarthou A.-S., Bonnarme P., Irlinger F. Highlighting the microbial diversity of 12 French cheese varieties. Int. J. Food Microbiol. 2016;238:265–273. doi: 10.1016/j.ijfoodmicro.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 2.Montel M.-C., Buchin S., Mallet A., Delbes-Paus C., Vuitton D.A., Desmasures N., Berthier F. Traditional cheeses: Rich and diverse microbiota with associated benefits. Int. J. Food Microbiol. 2014;177:136–154. doi: 10.1016/j.ijfoodmicro.2014.02.019. [DOI] [PubMed] [Google Scholar]
- 3.Kergourlay G., Taminiau B., Daube G., Champomier-Vergès M.-C. Metagenomic insights into the dynamics of microbial communities in food. Int. J. Food Microbiol. 2015;213:31–39. doi: 10.1016/j.ijfoodmicro.2015.09.010. [DOI] [PubMed] [Google Scholar]
- 4.Wolfe B.E., Button J.E., Santarelli M., Dutton R.J. Cheese Rind Communities Provide Tractable Systems for In Situ and In Vitro Studies of Microbial Diversity. Cell. 2014;158:422–433. doi: 10.1016/j.cell.2014.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niccum B.A., Kastman E.K., Kfoury N., Robbat A., Wolfe B.E. Strain-Level Diversity Impacts Cheese Rind Microbiome Assembly and Function. mSystems. 2020;5:00149–20. doi: 10.1128/mSystems.00149-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Oikonomou G., Addis M.F., Chassard C., Nader-Macias M.E.F., Grant I., Delbès C., Bogni C.I., Le Loir Y., Even S. Milk Microbiota: What Are We Exactly Talking About? Front. Microbiol. 2020;11:60. doi: 10.3389/fmicb.2020.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Delbes C., Ali-Mandjee L., Montel M.-C. Monitoring Bacterial Communities in Raw Milk and Cheese by Culture-Dependent and -Independent 16S rRNA Gene-Based Analyses. Appl. Environ. Microbiol. 2007;73:1882–1891. doi: 10.1128/AEM.01716-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Aldrete-Tapia A., Escobar-Ramírez M.C., Tamplin M.L., Hernández-Iturriaga M. High-throughput sequencing of microbial communities in Poro cheese, an artisanal Mexican cheese. Food Microbiol. 2014;44:136–141. doi: 10.1016/j.fm.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 9.Riquelme C., Câmara S., Dapkevicius M.D.L.N.E., Vinuesa P., Da Silva C.C.G., Malcata F.X., Rego O.A. Characterization of the bacterial biodiversity in Pico cheese (an artisanal Azorean food) Int. J. Food Microbiol. 2015;192:86–94. doi: 10.1016/j.ijfoodmicro.2014.09.031. [DOI] [PubMed] [Google Scholar]
- 10.Ryssel M.B., Jespersen L., Abu Al-Soud W., Sørensen S., Arneborg N., Jespersen L. Microbial diversity and dynamics throughout manufacturing and ripening of surface ripened semi-hard Danish Danbo cheeses investigated by culture-independent techniques. Int. J. Food Microbiol. 2015;215:124–130. doi: 10.1016/j.ijfoodmicro.2015.09.012. [DOI] [PubMed] [Google Scholar]
- 11.Larpin-Laborde S., Imran M., Bonaïti C., Bora N., Gelsomino R., Goerges S., Irlinger F., Goodfellow M., Ward A.C., Vancanneyt M., et al. Surface microbial consortia from Livarot, a French smear-ripened cheese. Can. J. Microbiol. 2011;57:651–660. doi: 10.1139/w11-050. [DOI] [PubMed] [Google Scholar]
- 12.Addis E., Fleet G., Cox J., Kolak D., Leung T. The growth, properties and interactions of yeasts and bacteria associated with the maturation of Camembert and blue-veined cheeses. Int. J. Food Microbiol. 2001;69:25–36. doi: 10.1016/S0168-1605(01)00569-4. [DOI] [PubMed] [Google Scholar]
- 13.Bockelmann W., Willems K., Neve H., Heller K. Cultures for the ripening of smear cheeses. Int. Dairy J. 2005;15:719–732. doi: 10.1016/j.idairyj.2004.08.022. [DOI] [Google Scholar]
- 14.Cretenet M., Laroute V., Ulvé V., Jeanson S., Nouaille S., Even S., Piot M., Girbal L., Le Loir Y., Loubière P., et al. Dynamic Analysis of the Lactococcus lactis Transcriptome in Cheeses Made from Milk Concentrated by Ultrafiltration Reveals Multiple Strategies of Adaptation to Stresses. Appl. Environ. Microbiol. 2010;77:247–257. doi: 10.1128/AEM.01174-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Taïbi A., Dabour N., Lamoureux M., Roy D., Lapointe G. Comparative transcriptome analysis of Lactococcus lactis subsp. cremoris strains under conditions simulating Cheddar cheese manufacture. Int. J. Food Microbiol. 2011;146:263–275. doi: 10.1016/j.ijfoodmicro.2011.02.034. [DOI] [PubMed] [Google Scholar]
- 16.De Filippis F., Genovese A., Ferranti P., Gilbert J.A., Ercolini D. Metatranscriptomics reveals temperature-driven functional changes in microbiome impacting cheese maturation rate. Sci. Rep. 2016;6:21871. doi: 10.1038/srep21871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Porcellato D., Skeie S.B. Bacterial dynamics and functional analysis of microbial metagenomes during ripening of Dutch-type cheese. Int. Dairy J. 2016;61:182–188. doi: 10.1016/j.idairyj.2016.05.005. [DOI] [Google Scholar]
- 18.Levante A., De Filippis F., La Storia A., Gatti M., Neviani E., Ercolini D., Lazzi C. Metabolic gene-targeted monitoring of non-starter lactic acid bacteria during cheese ripening. Int. J. Food Microbiol. 2017;257:276–284. doi: 10.1016/j.ijfoodmicro.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Duru I.C., Laine P.K., Andreevskaya M., Paulin L., Kananen S., Tynkkynen S., Auvinen P., Smolander O.-P. Metagenomic and metatranscriptomic analysis of the microbial community in Swiss-type Maasdam cheese during ripening. Int. J. Food Microbiol. 2018;281:10–22. doi: 10.1016/j.ijfoodmicro.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 20.Arfi K., Leclercq-Perlat M.-N., Baucher A., Tache R., Delettre J., Bonnarme P. Contribution of several cheese-ripening microbial associations to aroma compound production. Le Lait. 2004;84:435–447. doi: 10.1051/lait:2004016. [DOI] [Google Scholar]
- 21.Sørensen L.M., Gori K., Petersen M.A., Jespersen L., Arneborg N. Flavour compound production by Yarrowia lipolytica, Saccharomyces cerevisiae and Debaryomyces hansenii in a cheese-surface model. Int. Dairy J. 2011;21:970–978. doi: 10.1016/j.idairyj.2011.06.005. [DOI] [Google Scholar]
- 22.Monnet C., Dugat-Bony E., Swennen D., Beckerich J.-M., Irlinger F., Fraud S., Bonnarme P. Investigation of the Activity of the Microorganisms in a Reblochon-Style Cheese by Metatranscriptomic Analysis. Front. Microbiol. 2016;7:536. doi: 10.3389/fmicb.2016.00536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pham N.-P., Landaud S., Lieben P., Bonnarme P., Monnet C. Transcription Profiling Reveals Cooperative Metabolic Interactions in a Microbial Cheese-Ripening Community Composed of Debaryomyces hansenii, Brevibacterium aurantiacum, and Hafnia alvei. Front. Microbiol. 2019;10:1901. doi: 10.3389/fmicb.2019.01901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mansour S., Bailly J., Landaud S., Monnet C., Sarthou A.S., Cocaign-Bousquet M., Leroy S., Irlinger F., Bonnarme P. Investigation of Associations of Yarrowia lipolytica, Staphylococcus xylosus, and Lactococcus lactis in Culture as a First Step in Microbial Interaction Analysis. Appl. Environ. Microbiol. 2009;75:6422–6430. doi: 10.1128/AEM.00228-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cretenet M., Nouaille S., Thouin J., Rault L., Stenz L., François P., Hennekinne J.-A., Piot M., Maillard M.B., Fauquant J., et al. Staphylococcus aureus virulence and metabolism are dramatically affected by Lactococcus lactis in cheese matrix. Environ. Microbiol. Rep. 2011;3:340–351. doi: 10.1111/j.1758-2229.2010.00230.x. [DOI] [PubMed] [Google Scholar]
- 26.Zdenkova K., Alibayov B., Karamonova L., Purkrtova S., Karpiskova R., Demnerova K. Transcriptomic and metabolic responses of Staphylococcus aureus in mixed culture with Lactobacillus plantarum, Streptococcus thermophilus and Enterococcus durans in milk. J. Ind. Microbiol. Biotechnol. 2016;43:1237–1247. doi: 10.1007/s10295-016-1794-y. [DOI] [PubMed] [Google Scholar]
- 27.Viçosa G.N., Botta C., Ferrocino I., Bertolino M., Ventura M., Nero L.A., Cocolin L. Staphylococcus aureus undergoes major transcriptional reorganization during growth with Enterococcus faecalis in milk. Food Microbiol. 2018;73:17–28. doi: 10.1016/j.fm.2018.01.007. [DOI] [PubMed] [Google Scholar]
- 28.Ulve V., Monnet C., Valence-Bertel F., Fauquant J., Falentin H., Lortal S. RNA extraction from cheese for analysis of in situ gene expression of Lactococcus lactis. J. Appl. Microbiol. 2008;105:1327–1333. doi: 10.1111/j.1365-2672.2008.03869.x. [DOI] [PubMed] [Google Scholar]
- 29.Evermassen A., La Foye A.E., Eloux V., Talon R., Leroy S. Transcriptomic analysis of Staphylococcus xylosus in the presence of nitrate and nitrite in meat reveals its response to nitrosative stress. Front. Microbiol. 2014;5:691. doi: 10.3389/fmicb.2014.00691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smyth G.K. Linear Models and Empirical Bayes Methods for Assessing Differential Expression in Microarray Experiments. Stat. Appl. Genet. Mol. Biol. 2004;3:1–25. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 31.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 32.Vermassen A., Dordet-Frisoni E., De La Foye A., Micheau P., Laroute V., Leroy S., Talon R. Adaptation of Staphylococcus xylosus to Nutrients and Osmotic Stress in a Salted Meat Model. Front. Microbiol. 2016;7:87. doi: 10.3389/fmicb.2016.00087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Turgay K. Bacterial Stress Responses. 2nd ed. ASM Press; Washington, DC, USA: 2010. Role of Proteolysis and Chaperones in Stress Response and Regulation; pp. 75–90. [Google Scholar]
- 34.Brückner R., Rosenstein R. Carbohydrate catabolism: Pathways and Regulation. In: Fischetti V., Novick R., Ferretti J., Portnoy D., Rood J., editors. Gram-Positive Pathogens. 2nd ed. ASM Press; Washington, DC, USA: 2006. pp. 427–433. [Google Scholar]
- 35.Leroy S., Vermassen A., Ras G., Talon R. Insight into the Genome of Staphylococcus xylosus, a Ubiquitous Species Well Adapted to Meat Products. Microorganisms. 2017;5:52. doi: 10.3390/microorganisms5030052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Purves J., Cockayne A., Moody P.C.E., Morrissey J.A. Comparison of the Regulation, Metabolic Functions, and Roles in Virulence of the Glyceraldehyde-3-Phosphate Dehydrogenase Homologues gapA and gapB in Staphylococcus aureus. Infect. Immun. 2010;78:5223–5232. doi: 10.1128/IAI.00762-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chaudhari S.S., Thomas V.C., Sadykov M.R., Bose J.L., Ahn D.J., Zimmerman M.C., Bayles K.W. The LysR-type transcriptional regulator, CidR, regulates stationary phase cell death in Staphylococcus aureus. Mol. Microbiol. 2016;101:942–953. doi: 10.1111/mmi.13433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas V.C., Sadykov M.R., Chaudhari S.S., Jones J., Endres J.L., Widhelm T.J., Ahn J.-S., Jawa R.S., Zimmerman M.C., Bayles K.W. A Central Role for Carbon-Overflow Pathways in the Modulation of Bacterial Cell Death. PLoS Pathog. 2014;10:e1004205. doi: 10.1371/journal.ppat.1004205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Derzelle S., Bolotin A., Mistou M.-Y., Rul F. Proteome Analysis of Streptococcus thermophilus Grown in Milk Reveals Pyruvate Formate-Lyase as the Major Upregulated Protein. Appl. Environ. Microbiol. 2005;71:8597–8605. doi: 10.1128/AEM.71.12.8597-8605.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raynaud S., Perrin R., Cocaign-Bousquet M., Loubière P. Metabolic and Transcriptomic Adaptation of Lactococcus lactis subsp. lactis Biovar diacetylactis in Response to Autoacidification and Temperature Downshift in Skim Milk. Appl. Environ. Microbiol. 2005;71:8016–8023. doi: 10.1128/aem.71.12.8016-8023.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dubey A.K., Baker C.S., Suzuki K., Jones A.D., Pandit P., Romeo T., Babitzke P. CsrA Regulates Translation of the Escherichia coli Carbon Starvation Gene, cstA, by Blocking Ribosome Access to the cstA Transcript. J. Bacteriol. 2003;185:4450–4460. doi: 10.1128/JB.185.15.4450-4460.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rasmussen J.J., Vegge C.S., Frøkiær H., Howlett R.M., Krogfelt K.A., Kelly D.J., Ingmer H. Campylobacter jejuni carbon starvation protein A (CstA) is involved in peptide utilization, motility and agglutination, and has a role in stimulation of dendritic cells. J. Med. Microbiol. 2013;62:1135–1143. doi: 10.1099/jmm.0.059345-0. [DOI] [PubMed] [Google Scholar]
- 43.Fiegler H., Brückner R. Identification of the serine acetyltransferase gene of Staphylococcus xylosus. FEMS Microbiol. Lett. 2006;148:181–187. doi: 10.1111/j.1574-6968.1997.tb10286.x. [DOI] [PubMed] [Google Scholar]
- 44.Majerczyk C.D., Dunman P.M., Luong T.T., Lee C.Y., Sadykov M.R., Somerville G.A., Bodi K., Sonenshein A.L. Direct Targets of CodY in Staphylococcus aureus. J. Bacteriol. 2010;192:2861–2877. doi: 10.1128/JB.00220-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nuxoll A.S., Halouska S.M., Sadykov M.R., Hanke M.L., Bayles K.W., Kielian T., Powers R., Fey P.D. CcpA Regulates Arginine Biosynthesis in Staphylococcus aureus through Repression of Proline Catabolism. PLoS Pathog. 2012;8:e1003033. doi: 10.1371/journal.ppat.1003033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schmid R., Uhlemann E.-M., Nolden L., Wersch G., Hecker R., Hermann T., Marx A., Burkovski A. Response to nitrogen starvation in Corynebacterium glutamicum. FEMS Microbiol. Lett. 2000;187:83–88. doi: 10.1111/j.1574-6968.2000.tb09141.x. [DOI] [PubMed] [Google Scholar]
- 47.Brandenburg J.L., Wray J.L.V., Beier L., Jarmer H., Saxild H.H., Fisher S.H. Roles of PucR, GlnR, and TnrA in Regulating Expression of the Bacillus subtilis ure P3 Promoter. J. Bacteriol. 2002;184:6060–6064. doi: 10.1128/JB.184.21.6060-6064.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grundy F.J., Henkin T.M. Synthesis of Serine, Glycine, Cysteine and Methionine. In: Sonenshein A.L., Hoch J.A., Losick R., editors. Bacillus subtilis and Its Closest Relatives: From Genes to Cells. ASM Press; Washington, DC, USA: 2002. pp. 245–253. [Google Scholar]
- 49.Gollnick P., Babitzke P., Merino E., Yanofsky C. Aromatic Amino Acid Metabolism in Bacillus subtilis. In: Sonenshein A.L., Hoch J.A., Losick R., editors. Bacillus subtilis and Its Closest Relatives: From Genes to Cells. ASM Press; Washington, DC, USA: 2002. pp. 233–244. [Google Scholar]
- 50.Switzer R.L., Zalkin H., Saxild H.H. Purine, Pyrimidine, and Pyridine Nucleotide Metabolism. In: Sonenshein A.L., Hoch J.A., Losick R., editors. Bacillus subtilis and Its Closest Relatives: From Genes to Cells. ASM Press; Washington, DC, USA: 2002. pp. 255–269. [Google Scholar]
- 51.Monnet C., Back A., Irlinger F. Growth of Aerobic Ripening Bacteria at the Cheese Surface Is Limited by the Availability of Iron. Appl. Environ. Microbiol. 2012;78:3185–3192. doi: 10.1128/AEM.00085-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Oda H., Wakabayashi H., Yamauchi K., Abe F. Lactoferrin and bifidobacteria. BioMetals. 2014;27:915–922. doi: 10.1007/s10534-014-9741-8. [DOI] [PubMed] [Google Scholar]
- 53.Jenssen H., Hancock R.E. Antimicrobial properties of lactoferrin. Biochimie. 2009;91:19–29. doi: 10.1016/j.biochi.2008.05.015. [DOI] [PubMed] [Google Scholar]
- 54.Duhutrel P., Bordat C., Wu T.-D., Zagorec M., Guerquin-Kern J.-L., Champomier-Vergès M.-C. Iron Sources Used by the Nonpathogenic Lactic Acid Bacterium Lactobacillus sakei as Revealed by Electron Energy Loss Spectroscopy and Secondary-Ion Mass Spectrometry. Appl. Environ. Microbiol. 2009;76:560–565. doi: 10.1128/AEM.02205-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vella G.S., Reznikov E.A., Monaco M., Donovan S. Regulation cell growth and virulence gene expression in Staphylococcus aureus by the iron-binding proteins lactoferrin and hemin. i-ACES. 2015;1:123–133. [Google Scholar]
- 56.Vermassen A., Talon R., Leroy S. Ferritin, an iron source in meat for Staphylococcus xylosus? Int. J. Food Microbiol. 2016;225:20–26. doi: 10.1016/j.ijfoodmicro.2016.03.005. [DOI] [PubMed] [Google Scholar]
- 57.Pané-Farré J., Jonas B., Förstner K., Engelmann S., Hecker M. The σB regulon in Staphylococcus aureus and its regulation. Int. J. Med Microbiol. 2006;296:237–258. doi: 10.1016/j.ijmm.2005.11.011. [DOI] [PubMed] [Google Scholar]
- 58.Bischoff M., Dunman P., Kormanec J., Macapagal D., Murphy E., Mounts W., Berger-Bächi B., Projan S. Microarray-based analysis of the Staphylococcus aureus Sigma B regulon. J. Bact. 2004;186:4085–4099. doi: 10.1128/JB.186.13.4085-4099.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Stenz L., François P., Fischer A., Huyghe A., Tangomo M., Hernandez D., Cassat J., Linder P., Schrenzel J. Impact of oleic acid (cis-9-octadecenoic acid) on bacterial viability and biofilm production in Staphylococcus aureus. FEMS Microbiol. Lett. 2008;287:149–155. doi: 10.1111/j.1574-6968.2008.01316.x. [DOI] [PubMed] [Google Scholar]
- 60.Müller M., Reiß S., Schlüter R., Mäder U., Beyer A., Reiß W., Marles-Wright J., Lewis R.J., Pförtner H., Völker U., et al. Deletion of membrane-associated Asp23 leads to upregulation of cell wall stress genes in Staphylococcus aureus. Mol. Microbiol. 2014;93:1259–1268. doi: 10.1111/mmi.12733. [DOI] [PubMed] [Google Scholar]
- 61.Völker U., Engelmann S., Maul B., Riethdorf S., Völker A., Schmid R., Mach H., Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140:741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
- 62.Nielsen E.W. Principles of Cheese Production. In: Hui Y.H., Meunier-Goddik L., Hansen Å.S., Josephsen J., Nip W.-K., Stanfield P.S., Toldrà F., editors. Handbook of Food and Beverage Fermentation Technology. Marcel Dekker; New York, NY, USA: 2004. pp. 219–239. [Google Scholar]
- 63.Bolesch D.G., Keasling J.D. Polyphosphate Binding and Chain Length Recognition of Escherichia coli Exopolyphosphatase. J. Biol. Chem. 2000;275:33814–33819. doi: 10.1074/jbc.M002039200. [DOI] [PubMed] [Google Scholar]
- 64.Rangarajan E.S., Nadeau G., Li Y., Wagner J., Hung M.-N., Schrag J.D., Cygler M., Matte A. The Structure of the Exopolyphosphatase (PPX) from Escherichia coli O157:H7 Suggests a Binding Mode for Long Polyphosphate Chains. J. Mol. Biol. 2006;359:1249–1260. doi: 10.1016/j.jmb.2006.04.031. [DOI] [PubMed] [Google Scholar]
- 65.Clauditz A., Resch A., Wieland K.-P., Peschel A., Götz F. Staphyloxanthin Plays a Role in the Fitness of Staphylococcus aureus and Its Ability to Cope with Oxidative Stress. Infect. Immun. 2006;74:4950–4953. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ras G., Zuliani V., Derkx P., Seibert T.M., Leroy S., Talon R. Evidence for Nitric Oxide Synthase Activity in Staphylococcus xylosus Mediating Nitrosoheme Formation. Front. Microbiology. 2017;8:598. doi: 10.3389/fmicb.2017.00598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gusarov I., Nudler E. NO-mediated cytoprotection: Instant adaptation to oxidative stress in bacteria. Proc. Natl. Acad. Sci. USA. 2005;102:13855–13860. doi: 10.1073/pnas.0504307102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vaish M., Singh V.K. Antioxidant Functions of Nitric Oxide Synthase in a Methicillin Sensitive Staphylococcus aureus. Int. J. Microbiol. 2013;2013:1–6. doi: 10.1155/2013/312146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ras G., Leroy S., Talon R. Nitric oxide synthase: What is its potential role in the physiology of staphylococci in meat products? Int. J. Food Microbiol. 2018;282:28–34. doi: 10.1016/j.ijfoodmicro.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 70.Reizer J., Reizer A., Saier M.H. A functional superfamily of sodium/solute symporters. Biochim. Et Biophys. Acta (BBA)-Rev. Biomembr. 1994;1197:133–166. doi: 10.1016/0304-4157(94)90003-5. [DOI] [PubMed] [Google Scholar]
- 71.Pao S.S., Paulsen I.T., Saier M.H. Major Facilitator Superfamily. Microbiol. Mol. Biol. Rev. 1998;62:1–34. doi: 10.1128/MMBR.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kocianova S., Vuong C., Yao Y., Voyich J.M., Fischer E.R., DeLeo F.R., Otto M. Key role of poly-γ-dl-glutamic acid in immune evasion and virulence of Staphylococcus epidermidis. J. Clin. Investig. 2005;115:688–694. doi: 10.1172/jci200523523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stapleton M.R., Horsburgh M.J., Hayhurst E.J., Wright L., Jonsson I.-M., Tarkowski A., Kokai-Kun J.F., Mond J.J., Foster S.J. Characterization of IsaA and SceD, Two Putative Lytic Transglycosylases of Staphylococcus aureus. J. Bacteriol. 2007;189:7316–7325. doi: 10.1128/JB.00734-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.