Abstract
Background and objectives: Oxidative stress (OS) participates in the pathophysiology of septic shock, which leads to multiple organ failure (MOF), ischemia-reperfusion injury, and acute respiratory distress syndrome. Therefore, antioxidants have been proposed as therapy. Here, we evaluated the effect of antioxidant treatments in patients with septic shock with MOF and determined levels OS before and after treatment. This study was a randomized, controlled, triple-masked, and with parallel assignment clinical trial with a control group without treatment. Materials and Methods: It included 97 patients of either sex with septic shock. 5 treatments were used each in an independent group of 18 patients. Group 1 received vitamin C (Vit C), group 2 vitamin E (Vit E), group 3 n-acetylcysteine (NAC), group 4 melatonin (MT), and group 5 served as control. All antioxidants were administered orally or through a nasogastric tube for five days as an adjuvant to the standard therapy. Results: The results showed that all patients presented MOF due to sepsis upon admission and that the treatment decreased it (p = 0.007). The antioxidant treatment with NAC increased the total antioxidant capacity (p < 0.05). The patients that received Vit C had decreased levels of the nitrate and nitrite ratio (p < 0.01) and C-reactive protein levels (p = 0.04). Procalcitonin levels were reduced by Vit E (p = 0.04), NAC (p = 0.001), and MT (p = 0.04). Lipid-peroxidation was reduced in patients that received MT (p = 0.04). Conclusions: In conclusion, antioxidant therapy associated with standard therapy reduces MOF, OS, and inflammation in patients with septic shock.
Keywords: shock septic, antioxidant therapy, oxidative stress, multiple organ failure
1. Introduction
Damage caused by oxidative stress (OS) participates in the pathophysiology of serious diseases including multiple organ failure (MOF) due to sepsis. Sepsis is caused by bacteria, fungi, and viruses, or by a combination of them [1]. Sepsis and septic shock are the largest cause of mortality worldwide in intensive care units (ICU) [2] and MOF constitutes a high cost to health systems [3].
Studies in animal models and in patients with septic shock have shown an imbalance between the production of reactive oxygen (ROS) and nitrogen (RNS) species and antioxidant defenses [4]. ROS are generated by phagocytic cells, by the increased activity of enzymes such as NAD(P)H oxidase, xanthine oxidase and inducible nitric oxide (iNOS) and by increased inflammatory mediators through the activation of nuclear factor κB (NFκB) [5]. Mitochondrial damage caused by OS is a component of the pathophysiology of MOF secondary to sepsis [6].
Antioxidants such as N-acetylcysteine (NAC), melatonin (MT), vitamins (A, C and E), enzyme cofactors (selenium and zinc), and endogenous compounds (ubiquinone, α lipoic acid, bilirubin, albumin, ferritin, and quercetin) may inhibit ROS and RNS, counteracting their effects [7]. NAC has anti-inflammatory and antioxidant properties [8]. Its antioxidant capacity is due to the replenishment of glutathione (GSH) deposits and to the sequestration of ROS [9]. NAC improves hemodynamic variables, cardiac indexes, oxygenation and compliance of lung statics [10], hepatosplenic flow, and liver function in septic shock. Thus, NAC could decrease MOF [11] and reduce the levels of IL-8, soluble α receptor tumor necrosis factor p55 [12], IL-6, and ICAM-1 [13]. It reduces mechanical ventilation length, number of days in the ICU, and mortality [14].
Vitamin C (Vit C) can reduce the production of nitric oxide by the iNOS pathway and it may decrease vasoconstriction and loss of vascular permeability [15]. Decreased Vit C levels are related to the severity of MOF and mortality [16]. In some clinical studies, therapy with Vit C decreased sequential organ failure assessment (SOFA) scores, procalcitonin (PCT), C-reactive protein (CRP), and thrombomodulin, leading to a lower mortality rate [17]. Several studies have shown that vitamin E (Vit E) is an important lipophilic antioxidant in cell membranes, protecting them from lipid peroxidation (LPO) [18]. It has also been reported that the administration of Vit E in combination with simvastatin inactivates NAD(P)H oxidase, a source of ROS, in patients with sepsis that have decreased levels of Vit E and O2− overproduction [19].
Melatonin (MT) lowers OS both in plasma and intracellular membranes due to its hydrophilic and lipophilic properties. MT possesses ROS sequestration properties, thus protecting cell membrane lipids, cytosol proteins, and nuclear and mitochondrial DNA [20].
There is a marked increase in ROS and a decrease in endogenous antioxidant defenses in critically ill patients with sepsis [21]. However, the usefulness of different antioxidants has not yet been evaluated through clinical randomized trials. Thus, the aim of this study was to evaluate the antioxidant effect of Vit C, Vit E, NAC, and MT in patients with septic shock determining the SOFA score and measuring antioxidant markers in plasma.
2. Material and Methods
2.1. Study Population
This was a controlled, randomized, and triple masked clinical trial that included 97 patients of either sex with septic shock. It was run in 2 ICUs in Mexico City. Patients were admitted to the ICU with a primary diagnosis of septic shock. Diagnostic criteria for septic shock were based on the Sepsis-3 consensus [22], and patients had to fulfill the criteria within a maximum of 24 h prior to enrollment. Data were collected upon admission to the ICU. In addition, other patients met selection criteria during their stay in intensive care, and they were then randomized. Patients had to have an acute increase of at least 2 points in the SOFA score [23], lactate level greater than 2 mmol/L, and they had to be dependent on a vasopressor for at least 2 h before the time of enrollment. Exclusion occurred when patients were younger than 18 years, when they were not able to grant an informed consent or refused to be included, if they were pregnant or breastfeeding or if they were under chronic use (last 6th months) or recent use of steroids, statins or antioxidants. Patients were also excluded if there was any contraindication for the use of Vit C, Vit E, NAC, or MT.
Ethical approval was obtained from the local ethics committee 24 the April of the 2018 (INcar PT-18-076; ABC-18-19). A written informed consent for enrollment or consent to continue and use patient data was obtained from each patient or their legal surrogate. The protocol was registered (TRIAL REGISTRATION: ClinicalTrials.gov Identifier: NCT 03557229).
2.2. Randomization, Masking, and Drug Administration
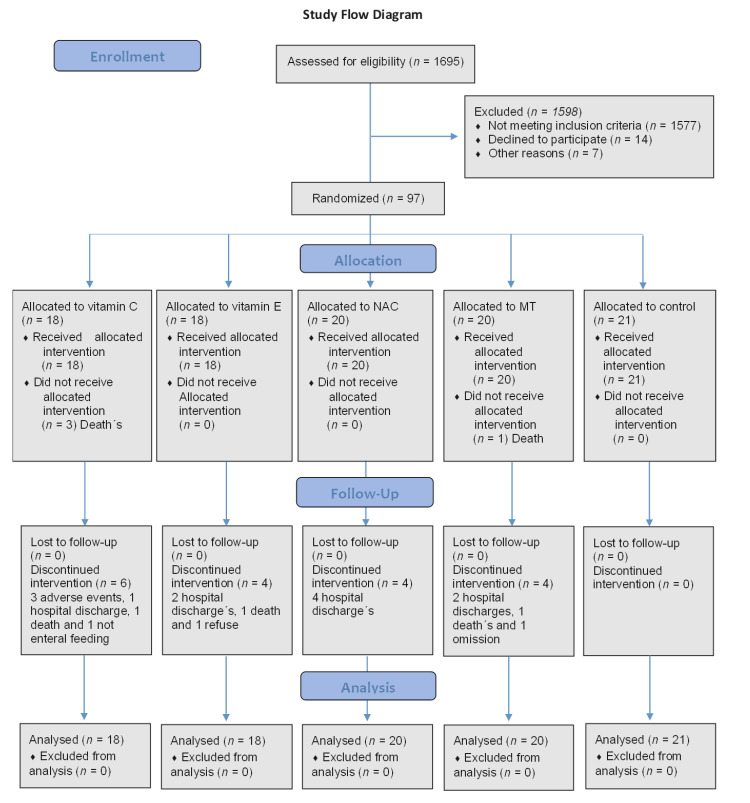
A total of five treatments were used each in an independent group of 18 patients. Group 1 received Vit C, group 2 Vit E, group 3 NAC, group 4 MT and group 5 control (this group did not receive any type of antioxidant therapy).The control group did not receive treatment since the treating physician did not agree for the patient to receive any antioxidant. However, the patients agreed that samples could be processed. All antioxidants were administered orally or through a nasogastric tube during 5 days in addition to the standard therapy. The random allocation sequence for the administration of the antioxidants was generated at the coordinating center, using a computer-generated random program (Figure 1). Blinding was maintained by the investigational pharmacy at each institution. Researchers were also blinded from the onset of the study until the analysis of the outcomes.
Figure 1.
Flow diagram of the study. Abbreviations: Vit C = vitamin C, Vit E = vitamin E, NAC = n-acetylcysteine, MT = melatonin.
All antioxidants were orally administered or applied through a nasogastric tube for 5 days. Tablets of 600 mg every 12 h of NAC were used. Further, 50 mg of MT in capsules of 5 mg were given to patients once a day, and 1 mg Vit C tablets were administered every 6 h. Vit E capsules of 400 UI were given every 8 h. The doses of antioxidants were chosen according to what has been reported in the literature [24,25,26,27]. All data entry was monitored at the coordinating center, with site visits for source data verification. Also, patients were equally distributed, and all patients were analyzed. For patients receiving Vit C (n = 18), there were 3 deaths. For patients receiving Vit E (n = 18) there were 3 deaths. For patients receiving NAC (n = 20), there were 2 deaths, and for those receiving MT (n = 20), there were 4 deaths. Finally, in the control group (n = 21), there were 5 deaths.
2.3. Standard Therapy at the ICU
Patients were treated according to the recommendation of the International Guidelines for Management of Sepsis and Septic Shock. For the evaluation of the outcome, the SOFA scores for 5 days were the primary result. Additionally, 14 pre-specified laboratory results were determined, including plasma OS markers such as nitrate/nitrite (NO3−/NO2−) ratio, LPO, glutathione (GSH) levels, total antioxidant capacity (TAC), carbonylation and Vit C levels at 48 h. Other secondary outcomes were measured on day 28 including mortality due to any cause, ventilator-free days, ICU-free days, and hospital-free days. Ventilator-free days were defined as the number of days a patient was extubated from mechanical ventilation, after ICU admission. When reintubation was required the days without intubation were subtracted from the total days. If the patient died in the hospital, a value of zero was assigned to post-extubation. ICU-free days began the moment the patient was transferred out of the ICU to day 28. Hospital- and ICU-free days were calculated similarly.
2.4. Study Measurements and Procedures
To evaluate the organ dysfunction, the SOFA score (neurologic, respiratory, hemodynamic, hepatic, and hematologic) was calculated on admission and during the days of treatment. The CRP and the PCT determinations were performed on admission, before the beginning of the antioxidant therapy, and during the next 7 days.
2.5. Sampling for the Determination of Oxidative Stress and Antioxidant State
The measurement of OS markers was done before the beginning of the antioxidant therapy and 48 h after its initiation.
2.6. Sample Obtainment and Storage
Blood samples were obtained from each patient that entered the draw, before initiation of the treatment and 48 h after its administration. The blood samples were centrifuged for 20 min at 936× g and 4 °C. The plasma of the samples was placed in 3 or 4 aliquots and stored at −30 °C.
2.7. Oxidative Stress Markers in Plasma
2.7.1. NO3−/NO2− Ratio
The NO3− was reduced to NO2− by the nitrate reductase enzyme reaction. 100 μL of plasma previously deproteinization with 0.5 N, NaOH and 10%, ZnSO4 were mixed and the supernatant was incubated for 30 min at 37 °C in presence of nitrate reductase (5 units). At the end of the incubation period, 200 µL of sulfanilamide 1% and 200 µL of N-naphthyl-ethyldiamine 0.1% were added and the total volume was adjusted to 1 mL. The absorbance was measured at 540 nm [28].
2.7.2. LPO Levels
Briefly, 50 µL CH3-OH with 4% butylated hydroxytoluene plus phosphate buffer pH 7.4 was added to 100 µL of plasma. It was incubated and centrifuged at 4000 rpm in room temperature for 2 min. Then, the n-butanol phase was extracted, and absorbance was measured at 532 nm [28].
2.7.3. GSH Concentration
Briefly, 800 μL of phosphate buffer 50 mM, pH 7.3, plus 100 μL of Ellman reactive (5,5′ dithiobis 2-nitrobenzoic) 1M were added to 100 μL of plasma prior to deproteinization with 20% trichloroacetic acid (v/v). It was incubated at room temperature and absorbance was read at 412 nm [28].
2.7.4. Evaluation of TAC
Briefly, 100 μL of plasma were suspended in 1.5 mL of a reaction mixture prepared as follows: 300 mM acetate buffer pH 3.6, 20 mM hexahydrate of ferric chloride, and 10 mM of 2,4,6-Tris-2- pyridil-s-triazine dissolved in 40 mM HCl. These reactive were added in a relation of 10:1:1 v/v, respectively. After mixing, samples were incubated at 37 °C for 15 min in the dark. The absorbance was measured at 593 nm [28].
2.7.5. Carbonylation Protein Concentration
Briefly, 100 μL of plasma were added to 500 μL of HCl 2.5 N in parallel with another sample with 500 μL of 2, 4-dinitrophenylhydrazine (DNPH) and incubated. At the end of the incubation period, they were centrifuged at 15,000× g for 5 min. The supernatant was discarded. Two washings were performed. The mixture was incubated again at 37 °C for 30 min. Absorbance was read in a spectrophotometer at 370 nm, using bi-distilled water as blank and a molar absorption coefficient of 22,000 M−1 cm−1 [28].
2.7.6. Vitamin C Levels
Briefly, 100 μL of 20% trichloroacetic acid were added to 100 μL of plasma and centrifuged at 5000 rpm for 5 min. Then, 200 μL of Folin-Ciocalteu reagent 0.20 mM was added to the supernatant. The mixture was incubated for 10 min. The absorbance was measured at 760 nm [28].
2.8. Statistical Analysis
Based on a SD of 2.9 of the SOFA score, the study was estimated to require 55 patients (11 per group) to have 84% power (2-sided with an α = 0.05) and 160 (32 per group) for 100% power. In accordance with these calculations, our study enrolled 97 patients to allow for a 10% of dropouts, providing a statistical power of 99%, with an α = 0.05. Testing was 2-sided. Effects are reported with a point estimate and 95% CIs in addition to p values.
Group comparisons were made using χ2 tests for equal proportions, t tests for normally distributed data, Kruskal–Wallis and Wilcoxon rank sum tests otherwise, with results presented as frequencies with percentages, means with SDs, and medians with minimum and maximum, respectively.
The primary end point of the SOFA score and the secondary end points CRP and PCT were analyzed with a mixed linear model and fit to repeated-measures analysis of variance. The model included 1 between-participant factor (group (Vit C, Vit E, NAC, MT, no treatment [control])), 1 within-participant factor (time (0, 1, 2, 3, 4, and 5 days)), and the interaction between group and time, testing the hypothesis that differences between treatment groups are the same over time. Because of a potential for type I error caused by multiple comparisons, findings for analyses of secondary end points should be interpreted as exploratory. Statistical analysis was performed with Stata version 15.1.
3. Results
3.1. Characteristics of the Patients
From July 2018 to November 2019 a total of 1695 eligible patients were identified, of whom 1598 were excluded (reasons listed in Figure 1). Ninety-seven patients were randomized, with 18 assigned to each antioxidant and 21 to the control group. Of all patients included, none was lost in the follow up. Baseline demographic data (age, gender, etc.) were similar between the groups (Table 1).
Table 1.
General characteristics of the patients during hospital stay.
| Characteristics | Vit C (n = 18) |
Vit E (n = 18) |
NAC (n = 20) |
MT (n = 20) |
C (n = 21) |
|---|---|---|---|---|---|
| Age (median, min–max) | 62 (22–95) | 65.5 (22–91) | 67.5 (18–95) | 62.5 (46–95) | 76 (51–89) |
| Weight kg (median, min–max) | 71 (33–112) | 71.5 (40–120) | 69.5 (39–95) | 67 (50–106) | 68 (50–105) |
| BM weight/height2 (median, min–max) | 25.4 (14.7–40.4) | 25 (15.1–41.4) | 22.45 (16.5–0.3) | 25.35 (17.3–52) | 25.4 (19.6–58) |
| Gender (%) | |||||
| Men | 6 (6.19) | 12 (12.37) | 11 (11.34) | 10 (10.31) | 10 (10.31) |
| Women | 12 (12.37) | 6 (6.19) | 9 (9.28) | 10 (10.31) | 11 (11.34) |
| Chronic health condition (%) | |||||
| Diabetes Mellitus | 4 (4.12) | 4 (4.12) | 3 (3.09) | 5 (5.15) | 6 (6.19) |
| Hypertension | 6 (6.19) | 8 (8.25) | 9 (9.28) | 7 (7.22) | 11 (11.34) |
| Cancer | 5 (5.15) | 9 (9.28) | 7 (7.22) | 7 (7.22) | 11 (11.34) |
| Chronic renal failure | 1 (1.03) | 2 (2.03) | 4 (4.12) | 3 (3.09) | 2 (2.06) |
| Admission source (%) | |||||
| Emergency department | 9 (9.28) | 12 (13.37) | 10 (10.31) | 14 (14.43) | 9 (9.28) |
| Operating room | 4 (4.12) | 2 (2.06) | 3 (3.09) | 2 (2.06) | 4 (4.12) |
| Inpatient ward transfer | 3 (3.09) | 4 (4.12) | 7 (7.22) | 4 (4.12) | 7 (7.22) |
| Other | 2 (2.06) | 0 | 0 | 0 | 1 (1.03) |
| Primary site of infection (%) | |||||
| Pulmonary | 7 (7.37) | 9 (9.97) | 9 (9.97) | 8 (8.42) | 6 (6.32) |
| Gastrointestinal | 7 (7.37) | 3 (3.16) | 4 (4.21) | 3 (3.16) | 9 (9.97) |
| Urinary | 2 (2.11) | 2 (2.11) | 5 (5.26) | 5 (5.26) | 3 (3.16) |
| CNS | 0 | 2 (2.11) | 0 | 0 | 1 (1.05) |
| Blood | 0 | 1 (1.05) | 0 | 2 (2.11) | 0 |
| Physiological variables 24 h | |||||
| before randomization | |||||
| (median, min–max) | |||||
| White blood cell count × 103/μL | 11 (5.1–39.9) | 10.8 (0.4–25.4) | 8.6 (0–32.5) | 11.7 (5.2–29.6) | 12 (0.9–49.8) |
| Platelet count × 103/μL | 256 (7–409) | 158 (10–363) | 155 (22–470) | 187.5 (29–543) | 225 (24–436) |
| Lactate (mmol/L) | 1.65 (0–4.8) | 2.1 (0.82–10.5) | 1.74 (0.99–7.8) | 2.27 (1–17) | 2.52 (1.1–12.4) |
| Serum creatinine (mg/dL) | 0.9 (0.5–5.5) | 1.35 (0.5–3.8) | 0.92 (0.5–6.6) | 1.27 (0.57–6.6) | 1.2 (0.5–5.2) |
| Bilirubin (mg/dL) | 0.75 (0.23–3.5) | 1.05 (0.35–4.4) | 0.80 (0.2–4) | 1.03 (0.17–3.7) | 1.15 (0.2–13.6) |
| PaO2/FiO2 (mmHg) | 168.5 (61–408) | 215 (39–271) | 146 (71–367) | 197 (57–261) | 197 (131–560) |
| C reactive protein (mg/dL) | 18.33 (1.9–1.4) | 20.12 (0.5–47) | 13.34 (0.02–6.7) | 21.75 (1.35–6.7) | 20.25 (1.36–5.3) |
| Procalcitonin (ng/dL) | 1.46 (0.16–321) | 2.92 (0.08–109) | 2.35 (0.06–95.5) | 2.32 (0.22–38.7) | 8.25 (0.08–100) |
| Intervention before randomization (%) | |||||
| Mechanical ventilation | 11 (11.58) | 9 (9.47) | 14 (14.47) | 12 (12.63) | 16 (16.84) |
| Vasopressors | 9 (9.38) | 7 (7.29) | 12 (12.50) | 9 (9.38) | 11 (11.46) |
| Norepinephrine | 0 | 1 (1.04) | 0 | 0 | 0 |
| Vasopressin | 8 (8.33) | 10 (10.42) | 8 (8.33) | 11 (11.46) | 10 (10.42) |
| Norepinephrine plus vasopressin | |||||
| Inotropes | |||||
| Dobutamine | 0 | 0 | 0 | 0 | 1 (1.04) |
| Levosimendan | 0 | 5 (5.21) | 1 (1.04) | 3 (3.13) | 5 (5.21) |
| Dopamine | 1 (1.04) | 0 | 0 | 1 (1.04) | 0 |
| Renal replacement Therapy | 1 (1.04) | 2 (2.08) | 2 (2.08) | 1 (1.04) | 3 (3.13) |
| Corticosteroid use before | |||||
| randomization during the study (%) | 6 (6.19) | 11 (11.34) | 9 (9.28) | 8 (8.25) | 10 (10.31) |
| SAPS II (median, min–max) | 38 (16–62) | 40 (24–73) | 38.5 (12–97) | 41.5 (13–73) | 40 (18–79) |
| APACHE III (median, min–max) | 13.5 (5–47) | 19 (11–33) | 14.5 (5–46) | 17 (6–39) | 15 (5–38) |
| SOFA score (median, min–max) | 8.5 (3–16) | 8.5 (5–14) | 8.5 (1–17) | 8 (3–14) | 8 (1–16) |
| Time from ICU admission to randomization hours (median, min–max) |
5 (1.5–70) | 6 (1–17) | 3 (1–140) | 9 (3–48) | - |
The data presented in this table are on admission to intensive care. Several patients met the inclusion criteria several hours or days after admission to the intensive care unit and all patients used vasopressors since it was an inclusion criterion. Abbreviations: Vit C: vitamin C; Vit E: vitamin E; NAC: n-acetylcysteine; MT: melatonin; (min-mx): minimum–maximum; BMI: body mass index; CNS: central nervous system; SAPS II: Simplified Acute Physiology Score; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; ICU: intensive care unit.
3.2. Treatments
Treatments were given for a median of five days. The median of adherence in the 4 different groups of treatment was 100%. There was no difference between groups in the time from meeting eligibility criteria to the first dose, the time receiving the treatment, and the adherence.
Primary Outcome
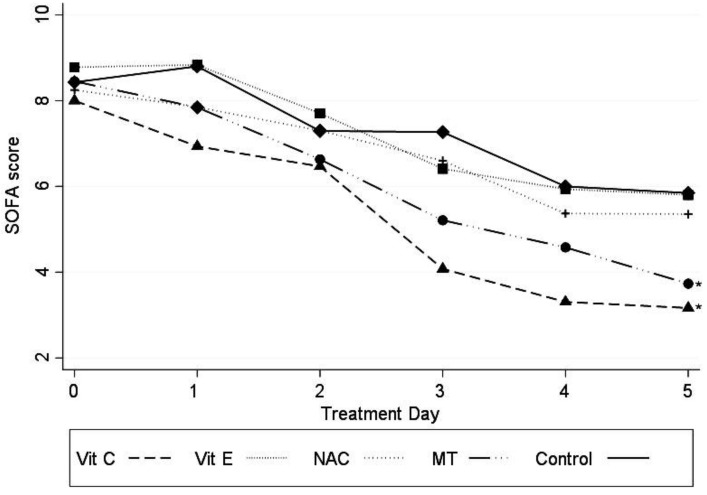
Patients receiving MT and Vit C showed a significant decrease in SOFA score (−1.27 (95% CI −2.21 to −0.34); p = 0.007 for MT and −1.94 (95% CI −2.95 to −0.93); p < 0.001 for Vit C) (Figure 2).
Figure 2.
SOFA score variations in patients with the different antioxidant treatments. Abbreviations: SOFA=sequential organ failure assessment, Vit C = vitamin C, Vit E = vitamin E, NAC = n-acetylcysteíne, MT = melatonin, Const = constant. Marginal approximation model considering the control group as a base: Vit C −1.94 (−2.95 to −0.94; p < 0.001); Vit E −0.14 (−1.10 to 0.81; p = 0.77); NAC −0.62 (−1.55 to 0.30; p = 0.18); MT −1.27 (−2.21 to −0.34; p = 0.007); Const 7.46 (6.78 to 8.13). * p ≤ 0.007.
The LPO levels were significantly reduced in patients treated with MT (p = 0.04) and there was a significant decrease in NO3−/NO2− levels in patients with lung infection treated with Vit C (p < 0.01), Table 2.
Table 2.
Oxidative stress markers before and after 48 h of antioxidant therapy.
| Lipid Peroxidation (nM MDA/mL of Plasma) | |||
|---|---|---|---|
| Pre | Post | p | |
| Vit C (n = 18) | 3.44 (0.52–19.62) | 2.81 (0.23–8.70) | 0.14 |
| Vit E (n = 18) | 4.33 (1.25–15.25) | 3.24 (0.38–12.07) | 0.17 |
| NAC (n = 20) | 3.46 (0.23–9.49) | 3.46 (0.38–11.01) | 0.77 |
| MT (n = 20) | 2.13 (0.23–11.68) | 2.42 (0.23–7.11) | 0.04 |
| Control (n = 21) | 3.44 (0.52–9.49) | 3.90 (0.23–9.10) | 0.75 |
|
NO3 + NO2
(µM/mL of plasma) | |||
| Pre | Post | p | |
| Vit C (n = 18) | 2.10 (0.98–2.73) | 1.49 (0.03–2.57) | <0.01 |
| Vit E (n = 18) | 1.79 (0.53–3.81) | 2.00 (0.76–5.65) | 0.36 |
| NAC (n = 20) | 2.43 (0.80–7.02) | 2.15 (0.01–8.16) | 0.81 |
| MT (n = 20) | 1.72 (0.67–4.77) | 1.32 (0.03–7.42) | 0.19 |
| Control (n = 21) | 2.25 (0.28–2.76) | 2.24 (0.01–7.22) | 0.97 |
|
Total antioxidant capacity
(nM/mL of plasma) | |||
| Pre | Post | p | |
| Vit C (n = 18) | 2226.2 (747.6–3053.4) | 2050.9 (966.6–2551.8) | 0.11 |
| Vit E (n = 18) | 2148.4 (886.3–3287.6) | 2223.1 (618.3–3841.9) | 0.90 |
| NAC (n = 20) | 1453.6 (621.5–2351.4) | 1951 (812.6–3528.7) | 0.05 |
| MT (n = 20) | 1999 (561.3–2519.2) | 1747.5 (456.5–2745.6) | 0.59 |
| Control (n = 21) | 2451.6 (1600–3467.1) | 2064.7 (312.4–3501) | 0.42 |
|
Carbonylation
(ng/mL of plasma) | |||
| Pre | Post | p | |
| Vit C (n = 18) | 48.85 (10.90–114.53) | 44.76 (12.72–98.17) | 0.59 |
| Vit E (n = 18) | 52.26 (27.27–137.25) | 42.723 (21.36–89.53) | 0.07 |
| NAC (n = 20) | 40.22 (22.27–89.99) | 41.13 (22.72–93.17) | 0.47 |
| MT (n = 20) | 74.76 (8.63–181.34) | 62.721 (29.99–142.25) | 0.40 |
| Control (n = 21) | 46.359 (9.99–106.80) | 44.08 (26.36–111.80) | 0.28 |
|
GSH concentration
(nM/mL of plasma) | |||
| Pre | Post | p | |
| Vit C (n = 18) | 0.10 (0.01–0.24) | 0.08 (0.01–0.20) | 0.50 |
| Vit E (n = 18) | 0.05 (0.00–0.30) | 0.07 (0.00–0.32) | 0.38 |
| NAC (n = 20) | 0.08 (0.00–0.54) | 0.10 (0.009–0.57) | 0.14 |
| MT (n = 20) | 0.07 (0.00–0.32) | 0.07 (0.010–0.51) | 0.64 |
| Control (n = 21) | 0.06 (0.03–0.20) | 0.05 (0.01–0.16) | 0.15 |
|
Vit C
(µM/mL of plasma) | |||
| Pre | Post | p | |
| Vit C (n = 18) | 0.17 (0.04–0.87) | 0.27 (0.06–0.99) | <0.01 |
| Vit E (n = 18) | 0.27 (0.08–0.99) | 0.26 (0.12–0.79) | 0.58 |
| NAC (n = 20) | 0.21 (0.09–0.61) | 0.18 (0.00–0.96) | 1.00 |
| MT (n = 20) | 0.21 (0.04–0.56) | 0.21 (0.04–0.43) | 0.83 |
| Control (n = 21) | 0.22 (0.08–0.77) | 0.19 (0.07–0.64) | 0.02 |
Abbreviations: Pre: pre-treatment; Post: post-treatment; Vit C: vitamin C; Vit E: vitamin E; NAC: n-acetylcysteine; MT: melatonin. All values are expressed as median (minimum-maximum). Wilcoxon matched pairs signed rank tests. The bold in the table is to highlight the results with statistical change.
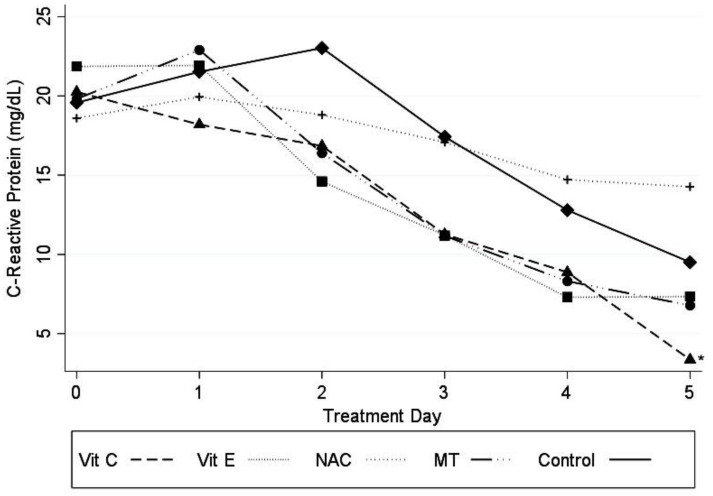
Patients receiving Vit C had a significant decrease in CRP levels on the different days of treatment (p ≤ 0.05), as shown in Figure 3.
Figure 3.
Variations in CRP levels in plasma of patients in receiving the different antioxidant treatments. Abbreviations: CRP = C-reactive protein, Vit C = vitamin C, Vit E = vitamin E, NAC: n-acetylcysteíne, MT = melatonin, Cons = constant. Marginal approximation model considering the control group as a base: Vit C −3.82 (−7.49 to −0.15; p ≤ 0.05); Vit E −2.97 (−6.54 to 6.01; p = 0.103); NAC −2.41 (−3.74 to 3.25; p = 0.892); MT −2.30 (−5.88 to 1.27; p = 0.207); Cons 17.9 (15.45 to 20.36) * p ≤ 0.05.
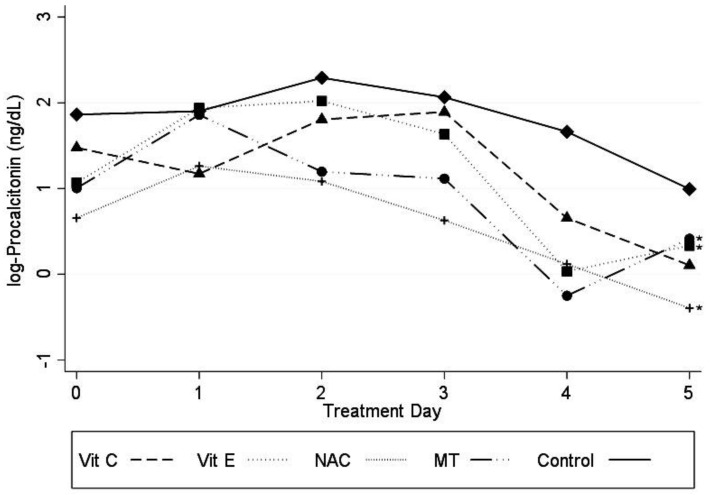
PCT levels were significantly decreased in patients receiving Vit E, NAC, and MT (p < 0.05), as shown in Figure 4. Carbonylation levels tended to be reduced before treatment and Vit E tended to decrease its level after treatment (p = 0.07) without there being a statistically significant difference.
Figure 4.
Log PCT concentration in the plasma from patients of the experimental groups with the different antioxidant treatments. Abbreviations: Log PCT = logarithm of procalcitonin, Vit C = vitamin C, Vit E = vitamin E, NAC = n-acetylcysteíne, MT = melatonin. Marginal approximation model considering the control group as a base: Vit C −0.43 (−1.03 to 0.15; p = 0.149); Vit E −0.59 (−1.18 a −0.006; p ≤ 0.05); NAC −0.92 (−1.48 to −0.35; p = 0.001); MT −0.57 (−1.15 to 0.006; p = 0.05); Cons 1.46 (1.05 to 1.86); * p ≤ 0.05.
Regarding the secondary outcomes, 13 patients (13.68%) required renal replacement therapy, 63 (65.63%) needed mechanical ventilation and 17 (17.89%) died. There was no statistically significant difference in days free of renal replacement therapy, mechanical ventilation, ICU stay length, or hospitalization at 28 days. There was also no statistically significant difference in intrahospital mortality.
3.3. Undesired Side Effects
A patient receiving Vit C presented abdominal pain and another patient underwent a skin rash. Only one patient who received MT reported drowsiness. No adverse events were reported in patients with NAC or Vit E.
4. Discussion
Treatment with antioxidants as an adjuvant in the standard management of patients with sepsis and/or septic shock has been suggested [29,30]. We studied critically ill patients with septic shock, regardless of the etiology and site of infection. All patients had initial low levels of Vit C. This was related with the severity of organ failure and mortality [17]. The decrease in Vit C levels confirms the reported hypovitaminosis (<0.23 µM ascorbic ac/mL) in septic shock [31]. This condition may be due to augmented metabolic demand since intestinal absorption was not compromised in the patients in our study [32]. Vit C restored the normal values of this vitamin, and organ function was improved. The best result was found in subjects with pneumonia which showed a statistically significant difference. This finding is in agreement with previous results [33,34]. The combined use of Vit C, thiamine, and steroids has recently been suggested. It is still necessary to compare if the use of Vit C alone has worse effects than the combinations [35]. In patients with septic shock, the administration of Vit C and MT improved the organ dysfunction assessed by the SOFA score. This finding could be associated to a decrease in the NO3−/NO2− ratio and LPO levels.
The Vitamin C infusion for treatment in sepsis induced acute lung injury (CITRIS-ALI) study in patients with acute respiratory distress syndrome, and organ failure showed no improvement with Vit C [36]. The median time before starting treatment with Vit C was of 5 h in this study, and markers such as CRP were significantly decreased, as in another previous study [37]. The possible difference between the findings of this study and our results could be related to the fact that, in the CITRIS-ALI study, they started the therapy with Vit C later than we did.
The VITAMINS trial showed no significant difference in the SOFA score, or in days without ventilation. However, the use of Vit C lowered mortality [38]. In that same study, CRP levels were not decreased, which was probably due to the late administration of Vit C in advanced stages of sepsis before developing acute respiratory distress syndrome (ARDS) [37]. In contrast, we found a decrease in the levels of NO3−/NO2− which is relevant, since Vit C inhibits the production of superoxide and peroxynitrite, thus preventing abundant NO synthesis, inhibiting mRNA expression and decreasing pathological vasoconstriction [16]. These effects might underlie the clinical benefits of the treatment. A shorter time of use of vasopressors and decreased intrahospital mortality was found in patient receiving Vit C [39].
This is the first study in which the use of MT has been tested in humans with septic shock. Recently MT has been applied in subjects with COVID 19 and it had a high safety profile limiting the disease. Experimental and clinical studies are required to confirm this hypothesis [40]. MT possesses free radical scavenging properties thus protecting cell membrane lipids, cytosol proteins, and nuclear and mitochondrial DNA [29]. In our findings, LPO was significantly decreased in the group of patients who received MT. This result resembled the findings in Galley’s study [29]. MT has beneficial effects in experimental cells, plants, and animals. However, its mechanisms of action remain unknown. The effects of MT might be related to its detoxifying ability, thus protecting molecules from the destructive effects of OS in ischemia/reperfusion (stroke, heart attack), ionizing radiation and drug toxicity. In sepsis, the protective effects of MT are associated with the inhibition of the apoptotic processes and the reduction of OS [41].
Production of ROS was increased in an animal model of septic shock [42]. This coincides with a lowering of the TAC and a reduction of the activity of superoxide dismutase and GSH peroxidase [43]. MT reversed morphological damage and increased the activities of antioxidant enzymes [44,45,46]. Therefore, research through blinded clinical trials and multicenter studies with adequate amounts of MT are needed to determine the potential of MT as an antioxidant treatment [47]. In this clinical trial, we found a reduction of LPO and a potentially benefic effect of MT in organ dysfunction. Its use as an adjuvant in septic shock reduces inflammation and oxidation in animal models with respiratory damage induced by infection. MT has positive physiological actions and could be effective and safe for patients with septic shock of any etiology, including those infected with SARS-CoV-2 [7].
The use of NAC improved the antioxidant capacity and tended to increase GSH, although the difference was not statistically significant. This confirms its antioxidant effect through the replacement of GSH deposits [12]. NAC decreased organ failure, confirming previous findings [14]. Other antioxidants such as polyphenols, β-glucan, and antioxidants targeting mitochondria, selenium salts, and selenium organ compounds are effective for improving OS in sepsis. The study of their pathophysiological implications justifies the combined therapy with antioxidants and standard treatments.
Vit E tended to decrease LPO and carbonylation. This vitamin protects cell membranes from LPO, ending the chain reaction. It is also an O2− and OH sequestrant [48].
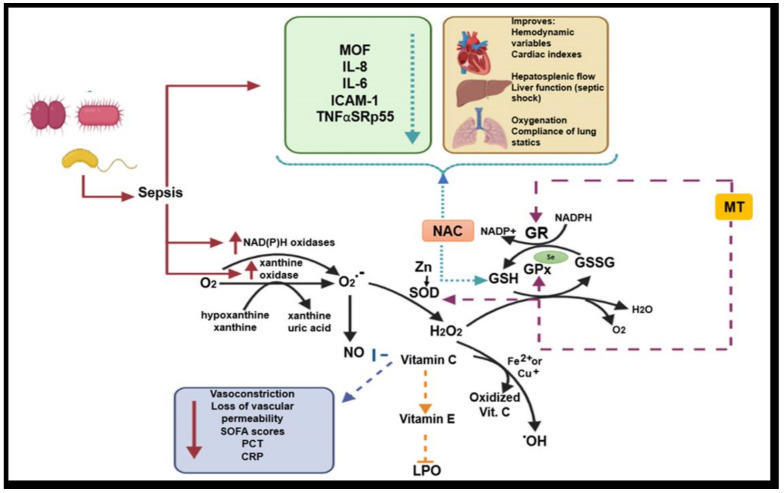
In summary, antioxidants benefit subjects with septic shock. Septic shock is triggered by bacterial stimuli, fungi, or viruses. In this medical condition, it is necessary to regulate inflammation and other mechanisms that lead to OS [48]. In Figure 5, we show the mechanisms involved in the oxidative stress mismatch, the role they play during the induction of damage, and we describe the role of antioxidant systems and enzyme cofactors in the management of sepsis.
Figure 5.
Role of antioxidant systems and enzyme cofactors in the management sepsis and ROS formation. Abbreviations: O2− = superoxide anion, NO = nitric oxide, Zn = zinc, SOD = superoxide dismutase, H2O2 = hydrogen peroxide, OH = hydroxyl radical, NAC = N-acetylcysteine, MT = melatonin, GSH = glutathione, GSSG = oxidized glutathione, GR = glutathione reductase, GPx = glutathione peroxidase, TNFαSRp55 = soluble α receptor tumor necrosis factor p55, MOF = multiple organ failure, LPO = lipoperoxidation, CRP = C reactive protein, SOFA = sequential organ failure assessment.
5. Conclusions
Adding antioxidants to standard therapy regulates inflammation in patients with septic shock. In pulmonary sepsis, replacement therapy with Vit C increases its serum levels, and decreases the levels of CRP, PCT, and NO3−/NO2−. MT decreases LPO and the SOFA score. NAC reduces LPO and improves the antioxidant capacity. Vit E tends to decrease LPO. Each antioxidant has beneficial effect. Thus, they might be combined in clinical trials in patients with septic shock.
6. Limitations
The absorption may be altered by the enteral route of administration. However, we found increases of Vit C levels in serum. The present trial is underpowered to detect differences in mortality and in outcomes between groups because the sample size was calculated for differences in OS.
Acknowledgments
We thank the Pharmacist Omar Gonzalez Marcos and the nursing staff and the central laboratory and mixing laboratories of the ABC hospital for their unconditional support to this project.
Author Contributions
A.A.-A., I.P.-T., and M.E.S. designed the study and wrote the manuscript. V.G.-L. revised the manuscript; A.A.-A., G.C.-A., J.F.-G., and E.A.M.-R. treated and recruited the patients in the intensive care unit and collected all of the results, including the pretreatment and post treatment dates; I.P.-T., A.A.-A., L.M.P., V.G.-L., and R.G.Á. made the laboratory determination; L.M.P. designed the graphical abstract and A.A.-A. designed the tables and figures; A.A.-A. and M.E.S. performed and planned the statistical analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research received funding of American British Cowdray (ABC) Medical Center, I.A.P.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Timsit J.F., Perner A., Bakker J., Bassetti M., Benoit D., Cecconi M., Curtis J.R., Doig G.S., Herridge M., Jaber S., et al. Year in review in intensive care Medicine 2014: III. Severe infections, septic shock, healthcare-associated infections, highly resistant bacteria, invasive fungal infections, severe viral infections, Ebola virus disease and paediatrics. Intensive. Care Med. 2015;41:575–588. doi: 10.1007/s00134-015-3755-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marshall J.C., Vincent J.L., Guyatt G., Angus D.C., Abraham E., Bernard G., Bombardier C., Calandra T., Jørgensen H.S., Sylvester R., et al. Outcome measures for clinical research in sepsis: A report of the 2nd Cambridge Colloquium of the International Sepsis Forum. Crit. Care Med. 2005;33:1708–1716. doi: 10.1097/01.CCM.0000174478.70338.03. [DOI] [PubMed] [Google Scholar]
- 3.Angus D.C., Linde-Zwirble W.T., Lidicker J., Clermont G., Carcillo J., Pinsky M.R. Epidemiology of severe sepsis in the United States: Analysis of incidence, outcome, and associated costs of care. Crit. Care Med. 2001;29:1303–1310. doi: 10.1097/00003246-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 4.Pisoschi A.M., Pop A. The role of antioxidants in the chemistry of oxidative stress: A review. Eur. J. Med. Chem. 2015;97:55–74. doi: 10.1016/j.ejmech.2015.04.040. [DOI] [PubMed] [Google Scholar]
- 5.Galley H.F. Oxidative stress and mitochondrial dysfunction in sepsis. Br. J. Anaesth. 2011;107:57–64. doi: 10.1093/bja/aer093. [DOI] [PubMed] [Google Scholar]
- 6.Crouser E.D. Mitochondrial dysfunction in septic shock and multiple organ dysfunction syndrome. Mitochondrion. 2004;4:729–741. doi: 10.1016/j.mito.2004.07.023. [DOI] [PubMed] [Google Scholar]
- 7.Soto M.E., Guarner-Lans V., Soria-Castro E., Manzano-Pech L., Pérez-Torres I. Is Antioxidant Therapy a Useful Complementary Measure for Covid-19 Treatment? An Algorithm for Its Application. Medicina. 2020;56:386. doi: 10.3390/medicina56080386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chertoff J. N-Acetylcysteine’s role in sepsis and potential benefit in patients with microcirculatory derangements. J. Intensive Care Med. 2018;33:87–96. doi: 10.1177/0885066617696850. [DOI] [PubMed] [Google Scholar]
- 9.Rushworth G.F., Megson I.L. Existing and potential therapeutic uses for N-acetylcysteine: The need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol. Ther. 2014;141:150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Rank N., Michel C., Haertel C., Lenhart A., Welte M., Meier-Hellmann A., Spies C. N-acetylcysteine increases liver blood flow and improves liver function in septic shock patients: Results of a prospective, randomized, double-blind study. Crit. Care Med. 2000;28:3799–3807. doi: 10.1097/00003246-200012000-00006. [DOI] [PubMed] [Google Scholar]
- 11.Spapen H.D., Diltoer M.W., Nguyen D.N., Hendrickx I., Huyghens L.P. Effects of N-acetylcysteine on microalbuminuria and organ failure in acute severe sepsis: Results of a pilot study. Chest. 2005;127:1413–1419. doi: 10.1378/chest.127.4.1413. [DOI] [PubMed] [Google Scholar]
- 12.Cazzola M., Calzetta L., Facciolo F., Rogliani P., Matera M.G. Pharmacological investigation on the anti-oxidant and anti-inflammatory activity of N-acetylcysteine in an ex vivo model of COPD exacerbation. Respir. Res. 2017;18:26. doi: 10.1186/s12931-016-0500-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Paterson R.L., Galley H.F., Webster N.R. The effect of N-acetylcysteine on nuclear factor-kappa B activation, interleukin-6, interleukin-8, and intercellular adhesion molecule-1 expression in patients with sepsis. Crit. Care Med. 2003;31:2574–2578. doi: 10.1097/01.CCM.0000089945.69588.18. [DOI] [PubMed] [Google Scholar]
- 14.Kim J.C., Hong S.W., Shim J.K., Yoo K.J., Chun D.H., Kwak Y.L. Effect of N-acetylcysteine on pulmonary function in patients undergoing off-pump coronary artery bypass surgery. Acta Anaesthesiol. Scand. 2011;55:452–459. doi: 10.1111/j.1399-6576.2011.02407.x. [DOI] [PubMed] [Google Scholar]
- 15.Berger M.M., Oudemans-van S.H.M. Vitamin C supplementation in the critically ill patient. Curr. Opin. Clin. Nutr. Metab. Care. 2015;18:193–201. doi: 10.1097/MCO.0000000000000148. [DOI] [PubMed] [Google Scholar]
- 16.Spoelstra-de Man A.M.E., De Grooth H.J., Elbers P.W.G., Oudemans-van Straaten H.M. Response to Adjuvant vitamin C in cardiac arrest patients undergoing renal replacement therapy: An appeal for a higher high-dose. Crit. Care. 2018;22:350. doi: 10.1186/s13054-018-2200-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fowler A.A., Syed A.A., Knowlson S., Sculthorpe R., Farthing D., DeWilde C., Farthing C.A., Larus T.L., Martin E., Brophy D.F., et al. Phase I safety trial of intravenous ascorbic acid in patients with severe sepsis. J. Transl. Med. 2014;12:32. doi: 10.1186/1479-5876-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Traber M.G., Atkinson J. Vitamin E, antioxidant and nothing more. Free. Radic. Biol. Med. 2007;43:4–15. doi: 10.1016/j.freeradbiomed.2007.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Durant R., Klouche K., Delbosc S., Morena M., Amigues L., Beraud J.J., Canaud B., Cristol J.P. Superoxide anion overproduction in sepsis: Effects of vitamin E and simvastatin. Shock. 2004;22:34–39. doi: 10.1097/01.shk.0000129197.46212.7e. [DOI] [PubMed] [Google Scholar]
- 20.Galley H.F., Lowes D.A., Allen L., Cameron G., Aucott L.S., Webster N.R. Melatonin as a potential therapy for sepsis: A phase I dose escalation study and an ex vivo whole blood model under conditions of sepsis. J. Pineal Res. 2014;56:427–438. doi: 10.1111/jpi.12134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Berger M.M., Chioléro R.L. Antioxidant supplementation in sepsis and systemic inflammatory response syndrome. Crit. Care Med. 2007;35:S584–S590. doi: 10.1097/01.CCM.0000279189.81529.C4. [DOI] [PubMed] [Google Scholar]
- 22.Shankar-Hari M., Phillips G.S., Levy M.L., Seymour C.W., Liu V.X., Deutschman C.S., Angus D.C., Rubenfeld G.D., Singer M. Sepsis definitions task force. Developing a new definition and assessing new clinical criteria for septic shock: For the third international consensus definitions for sepsis and septic shock (Sepsis-3) JAMA. 2016;315:775–787. doi: 10.1001/jama.2016.0289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lambden S., Laterre P.F., Levy M.M., Francois B. The SOFA score-development, utility, and challenges of accurate assessment in clinical trials. Crit. Care. 2019;23:374. doi: 10.1186/s13054-019-2663-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohamed Z.U., Prasannan P., Moni M., Edathadathil F., Prasanna P., Menon A., Nair S., Greeshma C.R., Sathyapalan D.T., Menon V., et al. Vitamin C therapy for routine care in septic shock (ViCTOR) trial: Effect of intravenous vitamin C, thiamine, and hydrocortisone administration on inpatient mortality among patients with septic shock. Indian. J. Crit. Care. Med. 2020;8:653–661. doi: 10.5005/jp-journals-10071-23517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fowler A.A., Truwit J.D., Hite R.D., Morris P.E., DeWilde C., Priday A., Fisher B., Thacker L.R., Natarajan R., Brophy D.F., et al. Effect of vitamin c infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: The CITRIS-ALI randomized clinical trial. JAMA. 2019;322:1261–1270. doi: 10.1001/jama.2019.11825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lowes D.A., Almawash A.M., Webster N.R., Reid V.L., Galley H.F. Melatonin and structurally similar compounds have differing effects on inflammation and mitochondrial function in endothelial cells under conditions mimicking sepsis. Br. J. Anaesth. 2011;107:193–201. doi: 10.1093/bja/aer149. [DOI] [PubMed] [Google Scholar]
- 27.Howe K.P., Clochesy J.M., Goldstein L.S., Owen H. Mechanical ventilation antioxidant trial. Am. J. Crit. Care. 2015;24:440–445. doi: 10.4037/ajcc2015335. [DOI] [PubMed] [Google Scholar]
- 28.Soto M.E., Manzano-Pech L.G., Guarner-Lans V., Díaz-Galindo J.A., Vásquez X., Castrejón-Tellez V., Gamboa R., Huesca C., Fuentevilla-Alvárez G., Pérez-Torres I. Oxidant/antioxidant profile in the thoracic aneurysm of patients with the Loeys-Dietz syndrome. Oxidative Med. Cell. Longev. 2020;2020:5392454. doi: 10.1155/2020/5392454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang R., Wang X., Ni L., Di X., Ma B., Niu S., Liu C., Reiter R.J. COVID-19: Melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. doi: 10.1016/j.lfs.2020.117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Marik P.E. Hydrocortisone, ascorbic acid and thiamine (HAT Therapy) for the treatment of sepsis. Focus on ascorbic acid. Nutrients. 2018;10:1762. doi: 10.3390/nu10111762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Carr A.C., Rosengrave P.C., Bayer S., Chambers S., Mehrtens J., Shaw G.M. Hypovitaminosis C and vitamin C deficiency in critically ill patients despite recommended enteral and parenteral intakes. Crit. Care. 2017;21:300. doi: 10.1186/s13054-017-1891-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evans-Olders R., Eintracht S., Hoffer L.J. Metabolic origin of hypovitaminosis C in acutely hospitalized patients. Nutrition. 2010;26:1070–1074. doi: 10.1016/j.nut.2009.08.015. [DOI] [PubMed] [Google Scholar]
- 33.Ferreira F.L., Bota D.P., Bross A., Mélot C., Vincent J.L. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286:1754–1758. doi: 10.1001/jama.286.14.1754. [DOI] [PubMed] [Google Scholar]
- 34.Joo Y.M., Chae M.K., Hwang S.Y., Jin S.C., Lee T.R., Cha W.C., Jo I.J., Sim M.S., Song K.J., Jeong Y.K., et al. Impact of timely antibiotic administration on outcomes in patients with severe sepsis and septic shock in the emergency department. Clin. Exp. Emerg. Med. 2014;1:35–40. doi: 10.15441/ceem.14.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hwang S.Y., Park J.E., Jo I.J., Kim S., Chung S.P., Kong T., Shin J., Lee H.J., You K.M., Jo Y.H., et al. Combination therapy of vitamin C and thiamine for septic shock in a multicentre, double-blind, randomized, controlled study (ATESS): Study protocol for a randomized controlled trial. Trials. 2019;20:420. doi: 10.1186/s13063-019-3542-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mitchell A.B., Ryan T.E., Gillion A.R., Wells L.D., Muthiah M.P. Vitamin C and thiamine for sepsis and septic shock. Am. J. Med. 2020;133:635–638. doi: 10.1016/j.amjmed.2019.07.054. [DOI] [PubMed] [Google Scholar]
- 37.Boretti A., Banik B.K. Intravenous vitamin C for reduction of cytokines storm in acute respiratory distress syndrome. PharmaNutrition. 2020;12:100190. doi: 10.1016/j.phanu.2020.100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fujii T., Luethi N., Young P.J., Frei D.R., Eastwood G.M., French C.J., Deane A.M., Shehabi Y., Hajjar L.A., Oliveira G., et al. Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: The Vitamins Randomized Clinical Trial. JAMA. 2020;323:423–431. doi: 10.1001/jama.2019.22176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Marik P.E., Khangoora V., Rivera R. Hydrocortisone, vitamin C and thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest. 2017;151:1229–1238. doi: 10.1016/j.chest.2016.11.036. [DOI] [PubMed] [Google Scholar]
- 40.Anderson G., Reiter R.J. Melatonin: Roles in influenza, Covid-19, and other viral infections. Rev. Med. Virol. 2020;30:e2109. doi: 10.1002/rmv.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Carrillo-Vico A., Lardone P.J., Naji L., Fernández-Santos J.M., Martín-Lacave I., Guerrero J.M., Calvo J.R. Beneficial pleiotropic actions of melatonin in an experimental model of septic shock in mice: Regulation of pro-/anti-inflammatory cytokine network, protection against oxidative damage and anti-apoptotic effects. J. Pineal Res. 2005;39:400–408. doi: 10.1111/j.1600-079X.2005.00265.x. [DOI] [PubMed] [Google Scholar]
- 42.Wu C.C., Chiao C.W., Hsiao G., Chen A., Yen M.H. Melatonin prevents endotoxin-induced circulatory failure in rats. J. Pineal Res. 2001;30:147–156. doi: 10.1034/j.1600-079X.2001.300303.x. [DOI] [PubMed] [Google Scholar]
- 43.D’Amato L.A., Mistraletti G., Longhi D., Piva I.R., Marrazzo F., Villa C., Tozzi M., Paroni R., Finati E., Lapichino G. Melatonin blood values and total antioxidant capacity in critically ill patients. Crit. Care. 2014;18:P436. [Google Scholar]
- 44.Wang H., Wei W., Shen Y.X., Dong C., Zhang L.L., Wang N.P., Yue L., Xu S.-Y. Protective effect of melatonin against liver injury in mice induced by Bacillus Calmette-Guerin plus lipopolysaccharide. World J. Gastroenterol. 2004;10:2690–2696. doi: 10.3748/wjg.v10.i18.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li V.G., Musumeci T., Pignatello R., Murabito P., Barbagallo I., Carbone C., Gullo A., Puglisi G. Antioxidant potential of different melatonin-loaded nanomedicines in an experimental model of sepsis. Exp. Biol. Med. 2012;237:670–677. doi: 10.1258/ebm.2012.011425. [DOI] [PubMed] [Google Scholar]
- 46.Sánchez-Barceló E.J., Mediavilla M.D., Tan D.X., Reiter R.J. Clinical uses of melatonin: Evaluation of human trials. Curr. Med. Chem. 2010;17:2070–2095. doi: 10.2174/092986710791233689. [DOI] [PubMed] [Google Scholar]
- 47.Lassnigg A., Punz A., Barker R., Keznickl P., Manhart N., Roth E., Hiesmayr M. Influence of intravenous vitamin E supplementation in cardiac surgery on oxidative stress: A double-blinded, randomized, controlled study. Br. J. Anaesth. 2003;90:148–154. doi: 10.1093/bja/aeg042. [DOI] [PubMed] [Google Scholar]
- 48.Prauchner C.A. Oxidative stress in sepsis: Pathophysiological implications justifying antioxidant co-therapy. Burns. 2017;43:471–485. doi: 10.1016/j.burns.2016.09.023. [DOI] [PubMed] [Google Scholar]