Abstract
Colistin is an important antibiotic currently used to manage infections caused by multidrug-resistant pathogens in both humans and livestock animals. A new mobile colistin-resistance (mcr-9) gene was recently discovered; this discovery highlighted the need for rigorous monitoring of bacterial resistance against colistin. Salmonella is one of the major pathogens responsible for foodborne illnesses; however, there is minimal information regarding the presence of mcr genes in foodborne Salmonella strains. The aim of this study was to investigate the presence of mcr genes among 178 Salmonella strains isolated from chicken meat in Korea. Antimicrobial susceptibility was measured using the broth microdilution method. Bioinformatics characterization of colistin-resistant strains and genetic environment of the mcr-9 gene were analyzed using next-generation sequencing. Transferability of the mcr-9 carrying colistin-resistant Salmonella strain was tested using broth-mating conjugation. Thirteen of the 178 Salmonella isolates showed colistin resistance, but only one strain, Salmonella Dessau ST14 (KUFSE-SAL043) from a traditional chicken market in Korea, carried an mcr family gene, mcr-9. This strain also carried other acquired antimicrobial resistance genes such as blaTEM-1B, qnrS1, and aac(6′)-Iaa. Only the IncX1 plasmid replicon type was detected in this strain. In the strain KUFSE-SAL043, the mcr-9 gene was located between two insertion sequences, IS903B and IS26, followed by the downstream regulatory genes qseB-like and qseC-like, which were located between IS1R and ΔIS1R. Conjugation tests revealed that the mcr-9 gene was successfully transferred to Escherichia coli J53 at a mean frequency of 2.03 × 10−7. This is the first report of a transferable mcr-9 gene in Salmonella isolated from chicken meat in Korea, highlighting the possibility of transfer of colistin resistance. Therefore, the wide use of colistin should be reconsidered, and a One Health perspective should be adopted to monitor the antimicrobial resistance of Enterobacteriaceae strains in humans, livestock, and the environment.
Keywords: mcr-9, Salmonella, chicken meat, colistin
Introduction
Colistin is the last therapeutic resort to treat infections by multidrug-resistant Enterobacteriaceae (Carroll et al., 2019). Colistin has been widely used in raising livestock, especially for poultry production as a treatment or prevention of enteric diseases and growth-promoting purposes (Rau et al., 2019). However, the emergence, transmission, and spread of mobile colistin-resistance (mcr) genes have raised concern over the continued use of colistin and its significance in spread of resistance. mcr encodes phosphoethanolamine transferase and induces colistin resistance by modifying the lipid A moiety of lipopolysaccharides (Kieffer et al., 2019). To date, nine mcr genes have been identified, with the most recent mcr gene (mcr-9) identified in a colistin-susceptible Salmonella strain, highlighting the need for stringent monitoring of the potential spread of this new gene (Carroll et al., 2019). The expression patterns, transferability, resistance characteristics, and molecular dynamics of the mcr-9 gene have been described in Enterobacter spp. and Escherichia coli strains isolated in clinical settings (Börjesson et al., 2019; Chavda et al., 2019; Kieffer et al., 2019; Yuan et al., 2019); however, at present, there are no published studies that have characterized the mcr-9 gene in foodborne strains.
Salmonella is a zoonotic bacterium and an important cause of foodborne illnesses. It represents the second most prevalent bacterial cause of foodborne illnesses in Korea from 2002 to 2020 (www.foodsafetykorea.go.kr). However, mcr genes are rarely reported in Salmonella compared with other foodborne Enterobacteriaceae (Lima et al., 2019). Furthermore, there are no reports of Salmonella isolates from Korea carrying an mcr gene; thus, their distribution is unclear so far. Therefore, we investigated colistin resistance in Salmonella spp. isolated from chicken meat sold in traditional markets or hypermarkets in Korea. For bioinformatics characterization, next-generation sequencing (NGS) was performed on colistin-resistant strains. The genetic environment of the mcr-9 gene was also investigated. Moreover, broth-mating conjugation experiments were performed to examine the potential of transferability of the mcr-9 gene from Salmonella. This is the first study to verify the presence of the mcr-9 gene in colistin-resistant Salmonella in Korea.
Materials and Methods
Bacterial strains and colistin resistance screening
Colistin resistance was examined in 178 strains of Salmonella collected from chicken meat sold in traditional markets and hypermarkets during a Korean nationwide surveillance study involving 33 markets in 22 cities between 2012 and 2017. Colistin resistance was measured in all isolates using the Trekstar Sensititre KNIHCOL custom panel (colistin test range: 0.25–128 μg/mL; Trek Diagnostic Systems, Westlake, OH) according to the manufacturer's instructions. For colistin-resistant strains, the Trekstar Sensititre KRCDCF custom panel (Trek Diagnostic Systems) was used to measure the minimum inhibitory concentration (MIC) of the following antimicrobials: ciprofloxacin (0.12–16 μg/mL), amoxicillin/clavulanate (2:1 ratio, 2–64 μg/mL amoxicillin, 1–32 μg/mL clavulanate), tetracycline (2–128 μg/mL), ampicillin (2–64 μg/mL), gentamicin (1–64 μg/mL), streptomycin (2–128 μ/mL), nalidixic acid (2–128 μg/mL), cefoxitin (2–64 μg/mL), cephalothin (2–64 μg/mL), ceftriaxone (1–32 μg/mL), cefotaxime (1–4 μg/mL), chloramphenicol (2–32 μg/mL), ampicillin/sulbactam (2:1 ratio, 2–32 μg/mL ampicillin, 1–16 μg/mL sulbactam), amikacin (4–64 μg/mL), trimethoprim/sulfamethoxazole (1–16 μg/mL trimethoprim, 19–304 μg/mL sulfamethoxazole), and imipenem (2–16 μg/mL). Escherichia coli ATCC 25922 (American Type Culture Collection, Manassas, VA) was used as the quality control strain. Breakpoints for colistin (MIC >2 μg/mL) were determined according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) guidelines (2019), and all other antimicrobial breakpoints followed the Clinical Laboratory Standards Institute (CLSI) guidelines (2017). Layouts of the two custom panels used in this study are provided in Supplementary Figures S1 and S2.
NGS and bioinformatics analyses
NGS was used to characterize Salmonella isolates and identify specific serotypes. The NGS analysis was performed using the Illumina MiSeq platform (Illumina, San Diego, CA), and the genome sequences of colistin-resistant strains were obtained. Identification of the acquired antimicrobial-resistance gene, plasmid replicon typing, serotyping, and multilocus sequence typing (MLST) were performed in silico using ResFinder 3.2, PlasmidFinder 2.1, SeqSero 1.2, and MLST 2.0 webserver (https://cge.cbs.dtu.dk, accessed December 20, 2019), respectively (Larsen et al., 2012; Zankari et al., 2012; Carattoli et al., 2014; Zhang et al., 2015). For the mcr-9 gene-carrying isolate KUFSE-SAL043, further analysis was performed as described hereunder. To carry out the genetic analysis of the mcr-9 gene, hybrid genome assembly was performed with additional long read sequence data obtained using PacBio Sequel (Pacific Biosciences of California, Menlo Park, CA). The genome was annotated using Rapid Annotation using Subsystem Technology (http://rast.theseed.org) to analyze the genetic environment of the mcr-9 gene (Aziz et al., 2008). Basic Local Alignment Search Tool (BLAST) was used to align the genetic sequences flanking the mcr-9 gene, and the results were visualized using Easyfig version 2.2.3 (Sullivan et al., 2011).
Conjugation assay
To test the transferability of the mcr-9 gene, broth-mating conjugation experiments were performed using the mcr-9-containing strain isolated from the chicken meat as the donor (KUFSE-SAL043) and the sodium azide-resistant strain Escherichia coli J53 as the recipient (Tamang et al., 2007). Transconjugants were selected on Mueller–Hinton agar (Oxoid, Basingstoke, United Kingdom) plates containing 150 μg/mL sodium azide (Sigma-Aldrich, St. Louis, MO), and 1 μg/mL colistin (Sigma-Aldrich). Additional identification of the transconjugants was performed using the VITEK-MS system (bioMerieux, Marcy l'Etoile, France). The transfer of the acquired resistance genes in the transconjugants was tested using conventional polymerase chain reaction (PCR), and the PCR products were sequenced using Sanger sequencing. Previously reported primers were used for amplification of blaTEM-1B and qnrS1 genes (Kim et al., 2009, 2011), and the primers for amplification of mcr-9 and aac(6′)-Iaa genes were designed using the National Center for Biotechnology Information (NCBI) Primer BLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast, accessed August 30, 2019) (Ye et al., 2012) (Supplementary Table S1). PCR was performed using Accupower PCR premix (Bioneer, Daejeon, Korea) in accordance with the manufacturer's instructions. The PCR products were subjected to Sanger sequencing (Macrogen, Daejeon, Korea). To validate the PCR primers, KUFSE-SAL043 was used as the positive control and Escherichia coli J53 was used as the negative control. To confirm the transfer of colistin, ampicillin, streptomycin, and nalidixic acid resistance, susceptibility tests were performed with KUFSE-SAL043 and the obtained transconjugants using the KRNV5F and KNIHCOL custom panels (Trek Diagnostic Systems) as described in bacterial strains and colistin resistance screening section of Materials and Methods.
Results
Antimicrobial resistance of colistin-resistant strains
Colistin resistance was tested in 178 Salmonella strains, and colistin did not have an MIC ≤0.5 μg/mL against any of the strains. MIC of colistin was 1 and 2 μg/mL against 106 (59.6%) and 59 (33.1%) strains, respectively. Thirteen strains (7.3%) were identified as colistin resistant, with a resistance of 4 μg/mL against 10 (5.6%) strains and 8 μg/mL against three strains (1.7%). All the colistin-resistant isolates were resistant to nalidixic acid, and most of them were resistant to ampicillin (12/13), cefotaxime (10/13), tetracycline (10/13), and gentamicin (8/13). All 13 strains exhibited multidrug resistance, which was defined as resistance to at least 3 subclasses of antimicrobials. However, none of the isolates were resistant to imipenem, chloramphenicol, or amikacin (Table 1).
Table 1.
Characteristics of 13 Colistin-Resistant Salmonella Isolates from Chicken Meat in Korea from 2012 to 2017
| Isolates | Year of isolation | Origin | Serotype | MLST | Plasmid replicon typing | Antimicrobial resistance genes | COL MIC (μg/mL) | Antimicrobial phenotypic resistanceb |
|---|---|---|---|---|---|---|---|---|
| KUFSE-SAL022 | 2012 | Traditional market | Enteritidis | ST11 | IncFIB(S), IncFII(S), IncX1 | aac(6′)-Iaa, aph(3″)-Ib, aph(6)-Id, blaTEM-1B, catA2, sul2 | 4 | COL, AMP, NAL, STR [128<]c |
| KUFSE-SAL027 | 2012 | Traditional market | 4:-:1,2 | ST19 | IncFIB(S), IncFII(S) | aac(6′)-Iaa, aph(6)-Id | 4 | COL, TET, GEN, NAL |
| KUFSE-SAL034 | 2012 | Traditional market | Enteritidis | ST11 | IncFIB(S), IncFII(S), IncHI2, IncHI2A, IncQ1 | aac(6′)-Iaa, aph(3″)-Ib, aph(3′)-Ia, aph(6)-Id, blaCTX-M-15, sul2, tet(A) | 4 | COL, TET, AMP, AXO, NAL, FOT, CEP [64<], STR [128<] |
| KUFSE-SAL043a | 2012 | Traditional market | Dessau | ST14 | IncX1 | mcr-9, aac(6′)-Iaa, blaTEM-1B, qnrS1 | 4 | COL, AMP, AXO, NAL, CEP [64<], STR [128] |
| KUFSE-SAL082 | 2012 | Traditional market | Istanbul | ST33 | IncFII(S), IncHI2A | aac(6′)-Iaa, aph(3″)-Ib, aph(6)-Id, tet(A) | 8 | COL, TET, AMP, AXO, GEN, NAL, FOT, CEP [64<], STR [128] |
| KUFSE-SAL123 | 2013 | Traditional market | Enteritidis | ST11 | IncFIB(S), IncFII(S) | aac(6′)-Iaa | 4 | COL, AMP, AXO, GEN, NAL, FOT, CEP [64<], STR [128<] |
| KUFSE-SAL132 | 2013 | Traditional market | Enteritidis | ST11 | IncFIB(S), IncFII(S), IncX1 | aac(6′)-Iaa, aph(3″)-Ib, aph(6)-Id, blaTEM-1B, sul2 | 4 | COL, TET, AMP, AXO, A/S2, NAL, FOT, STR [128<] |
| KUFSE-SAL134 | 2013 | Traditional market | Enteritidis | ST11 | IncFIB(S), IncFII(S), IncQ1 | aac(3)-IId, aac(6′)-Iaa, aph(3″)-Ib, aph(3′)-Ia, aph(6)-Id, blaCTX-M-15, sul2, tet(A) | 4 | COL, TET, AMP, AXO, GEN, NAL, FOT, CEP [64<], STR [128<] |
| KUFSE-SAL149 | 2013 | Traditional market | Enteritidis | ST11 | IncFIB(S), IncFII(S), IncQ1 | aac(3)-IId, aac(6′)-Iaa, aph(3″)-Ib, aph(3′)-Ia, aph(6)-Id, blaCTX-M-15, sul2, tet(A) | 4 | COL, TET, AMP, AXO, GEN, NAL, FOT, CEP [64<], STR [128<] |
| KUFSE-SAL160 | 2014 | Hypermarket | Enteritidis | ST11 | IncFIB(S), IncFII(S), IncQ1 | aac(3)-IId, aac(6′)-Iaa, aph(3″)-Ib, aph(3′)-Ia, aph(6)-Id, blaCTX-M-15, sul2, tet(A) | 8 | COL, TET, AMP, AXO, GEN, NAL, FOT, CEP [64<], STR [128<] |
| KUFSE-SAL161 | 2014 | Hypermarket | Enteritidis | ST11 | IncFIB(S), IncFII(S), IncQ1 | aac(3)-IId, aac(6′)-Iaa, aph(3″)-Ib, aph(6)-Id, blaCTX-M-15, sul2, tet(A) | 8 | COL, TET, AMP, AXO, GEN, NAL, FOT, CEP, STR [128<] |
| KUFSE-SAL180 | 2017 | Hypermarket | Heidelberg | ST15 | ColpVC, IncC, IncI1, IncX1 | aac(6′)-Iaa, aph(3″)-Ib, blaCMY-2, fosA7, sul2, tet(A) | 4 | COL, AUG2, TET, AMP, AXO, NAL, FOX, FOT, CEP [64<] |
| KUFSE-SAL187 | 2015 | Hypermarket | Newport | ST166 | No hit found | aac(6′)-Iaa, floR | 4 | COL, CIP, TET, AMP, AXO, GEN, NAL, SXT, FOT, CEP [64<], STR [128<] |
mcr-9 gene was detected only in strain KUFSE-SAL043.
None of the isolates was resistant to IMI, CHL, or AMI.
There are no Clinical and Laboratory Standards Institute breakpoints for CEP and STR.
MIC value (μg/mL) of these antimicrobials were indicated in brackets.
A/S2, ampicillin/sulbactam 2:1 ratio; AMI, amikacin; AMP, ampicillin; AUG2, amoxicillin/clavulanate 2:1 ratio; AXO, ceftriaxone; CEP, cephalothin; CHL, chloramphenicol; CIP, ciprofloxacin; COL, colistin; FOT, cefotaxime; FOX, cefoxitin; GEN, gentamicin; IMI, imipenem; MIC, minimum inhibitory concentration; MLST, multilocus sequence typing; NAL, nalidixic acid; STR, streptomycin; SXT, trimethoprim/sulfamethoxazole; TET, tetracycline.
Detection of mcr-9 and in silico molecular characteristics
Table 1 shows the Resfinder, Plasmidfinder, SeqSero, and MLST analysis results for the colistin-resistant strains. Among the 13 colistin-resistant strains, only one Salmonella strain was found to harbor the mcr-9 gene and was designated KUFSE-SAL043 (NCBI GenBank Accession no. JAALJB000000000). This new strain was isolated from commercial chicken meat bought from a Korean traditional market in 2012, and contained other acquired resistance genes, such as blaTEM-1B, aac(6′)-Iaa, and qnrS1. The serotype of this strain was identified as Dessau, and MLST determined its sequence type to be ST14.
Genetic context of mcr-9 in KUFSE-SAL043
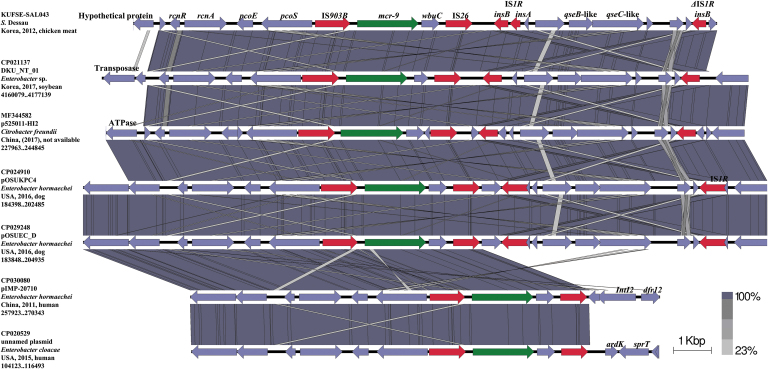
Figure 1 provides the overall genetic environment of mcr-9 in the genome sequence of KUFSE-SAL043. Sequence alignment was performed at a linear interval (∼11 kb) from the IS903B element upstream of the mcr-9 gene to the ΔIS1R element downstream of the mcr-9 gene. Sequences with GenBank accession nos. CP029248 (Enterobacter hormaechei, dog, United States), CP024910 (E. hormaechei, dog, United States), MF344582 (Citrobacter freundii, China), CP021137 (Enterobacter sp., soybean, Korea), CP030080 (E. hormaechei, human, China), and CP020529 (E. cloacae, human, United States) were identified as sequences with a query coverage of 100% and sequence identity <99.94%, and were further used for sequence comparison. In KUFSE-SAL043, IS903B was located upstream of mcr-9, whereas wbuC, IS26, IS1R, qseB-like, qseC-like, and ΔIS1R were located downstream. Four of the Enterobacteriaceae sequences (CP029248, CP024910, MF344582, and CP021137) revealed similar genetic environments, with qseB-like and qseC-like sequences found downstream of IS26 and between two IS1Rs, whereas the other two genomes (CP030080 and CP020529) did not contain downstream qseB-like and qseC-like sequences.
FIG. 1.
Genetic environment of the mcr-9 gene in KUFSE-SAL043 (JAALJB000000000) compared with mcr-9-containing regions of different plasmid reservoirs and chromosomes of Enterobacteriaceae strains. Arrows indicate the position and direction of the genes and Δ indicates truncated genes. mcr-9 genes are indicated in green, and IS or truncated IS are highlighted in red. Gray shading denotes shared regions with a high degree of similarity. Sequence comparison was performed using BLAST and Easyfig version 2.2.3. BLAST, Basic Local Alignment Search Tool.
Transferability of mcr-9
Conjugation experiments demonstrated the transfer of the mcr-9 gene from KUFSE-SAL043 to Escherichia coli J53 at a mean frequency of 2.03 × 10–7 (±5.42 × 10–8). The NCBI BLAST results showed that the nucleotide sequence of the mcr-9 PCR product of transconjugant TrECJ53-KUFSE-SAL043 was 100% identical with those of the previously deposited mcr-9 gene in GenBank. The antimicrobial resistance genes blaTEM-1B, qnrS1, and aac(6′)-Iaa were amplified from TrECJ53-KUFSE-SAL043 and also confirmed. MIC of colistin against TrECJ53-KUFSE-SAL043 was 2 μg/mL, which was lower than that of the donor strain KUFSE-SAL043. The transconjugant also showed resistance to ampicillin, streptomycin, and nalidixic acid, which was consistent with the results of conventional PCR and Sanger sequencing (Table 2).
Table 2.
Conjugation Transfer Efficiency of the mcr-9 Gene and Antimicrobial Resistance of Transconjugant TrECJ53-KUFSE-SAL043
| Colistin MIC | Phenotypic resistance | Resistance genes | Concentration (CFU/mL) | Conjugation frequencya | |
|---|---|---|---|---|---|
| KUFSE-SAL043 (Donor) | 4 (μg/mL) | COL, AMP, STR, NAL | mcr-9, aac(6′)-Iaa, blaTEM-1B, qnrS1 | 4.60 × 109 | 2.03 × 10−7 ± 5.42 × 10−8 |
| TrECJ53-KUFSE-SAL043 (Transconjugant) | 2 (μg/mL) | COL, AMP, STR, NAL | mcr-9, aac(6′)-Iaa, blaTEM-1B, qnrS1 | 9.33 × 102 | |
| Escherichia coli J53 AZR (Recipient) | — | — | — | 1.37 × 109 |
Conjugation frequency is presented as mean ± standard deviation.
AMP, ampicillin; CFU, colony-forming unit; COL, colistin; MIC, minimum inhibitory concentration; NAL, nalidixic acid. STR, streptomycin.
Discussion
Salmonella is a major cause of foodborne illnesses, with 95 million Salmonella enterocolitis cases, leading to 50,771 deaths, even in 2017 (Stanaway et al., 2019). Antimicrobial resistance of foodborne pathogens is a growing global health concern, complicating the treatment of these infections and contributing to this mortality rate. Colistin is a last resort antimicrobial, but its efficacy is decreasing because of the emergence of drug-resistant strains, leaving patients vulnerable. Continuous use of antimicrobials including colistin in food producing animals has increased the resistance rate of Salmonella isolates found in retail chicken. In this study, we investigated the colistin resistance of Salmonella isolated from retail chicken meat found in Korean markets. Through antimicrobial susceptibility testing, we found that the colistin resistance rate of Salmonella in this study (7.3%) was similar to that reported in previous studies (3–5%) in Korea (Shang et al., 2018; Seo et al., 2019). This resistance rate is lower than a recent study from the Sichuan province in China (13.9%) (Ma et al., 2017). The difference in resistance rates in both studies suggests that the increased use of colistin in livestock industry could promote antibiotic resistance in bacteria.
mcr is a family of genes found to promote colistin resistance in bacteria, such as E. coli. Recently, the increasing prevalence of mcr-1 was reported in E. coli isolated from fresh vegetables and livestock in Korea, which was also associated with an increase in multidrug resistance (Oh et al., 2020). However, no mcr genes have been reported among colistin-resistant Salmonella strains isolated from chicken farms in Korea (Shang et al., 2018; Seo et al., 2019). In this study, we have identified a Salmonella strain harboring a member of the mcr gene family, and to the best of our knowledge, this is the first study in Korea to have reported this finding. This strain, KUFSE-SAL043, demonstrated an MIC to 4 μg/mL of colistin, which was higher than that previously described in a mcr-9-containing Salmonella Typhimurium strain, where the MIC of colistin was 2 μg/mL (Carroll et al., 2019).
KUFSE-SAL043 was further identified to belong to the serotype Dessau and sequencing type ST14, both of which are rarely reported in Salmonella isolates from Korea. Only one strain of Salmonella Typhimurium isolated from pigs in 1995 has been confirmed to be ST14 so far (Yang et al., 2002). In 2009, a study surveyed chicken meat sold in Korean grocery stores for the presence and antimicrobial susceptibility of Salmonella serovars and reported three strains of Salmonella Dessau (Hyeon et al., 2011). Despite the rare incidence of Salmonella Dessau isolation in clinical samples, further studies should include genetic investigation of KUFSE-SAL043 because of inherent pathogenicity of Salmonella strains. Furthermore, as the remaining 12 colistin-resistant strains identified in this study did not carry mcr genes, there is a need for additional research to identify the underlying mcr-independent mechanisms promoting the development of colistin resistance.
We further described the genetic environment of mcr-9 in KUFSE-SAL043 and identified the presence of IS26 and IS1R in the genome, which revealed a distinct genetic environment compared with previous studies (Chavda et al., 2019; Kieffer et al., 2019; Yuan et al., 2019). Because mcr-9 was located between IS903B and IS26, these flanking sequences can also be potentially transferred to other bacteria along with mcr-9. In addition, qseB-like and qseC-like sequences, which are known to influence mcr-9 gene function (Kieffer et al., 2019), were located downstream of IS26 and between two IS1Rs, together with ΔIS1R in four of the Enterobacteriaceae sequences (CP029248, CP024910, MF344582, and CP021137). Because of this proximity, it is possible that this entire region could also be transferred with the mcr-9 gene. However, qseB-like and qseC-like sequences were not present downstream of the mcr-9 gene in the other two Enterobacteriaceae genomes (CP030080 and CP020529), indicating that this sequence between IS903B and IS26 and between the two IS1Rs may not universally exhibit the same behavior during gene transfer or loss. The origin of mcr-9-wbuC is not entirely known; however, a previous study suggested that these genes originated from Buttiauxella spp., although the qseB-qseC tandem was obtained from another source (Kieffer et al., 2019). Our observation that IS26 and IS1R are located between wbuC and qseB-like sequences strongly supports this assumption. However, further studies are required to fully understand the details of the transfer route of mcr-9-wbuC and qseB-qseC.
We also determined the transferability of mcr-9 and other resistance genes from KUFSE-SAL043 to E. coli. Previously, mcr-9 gene dissemination was found to be mediated by the IncHI2- or IncHI2A-type plasmids in human and horse isolates (Börjesson et al., 2019; Carroll et al., 2019; Chavda et al., 2019; Kieffer et al., 2019; Yuan et al., 2019). However, our study detected only the IncX1-type plasmid in the KUFSE-SAL043 strain, suggesting a novel route of mcr-9 transference. Results obtained from the conjugation assay also elucidated the functional characteristics of mcr-9. The MIC of colistin for the conjugated TrECJ53-KUFSE-SAL043 strain was 2 μg/mL, which was lower than that of the donor (4 μg/mL). This suggests that mcr-9 is not the only factor driving colistin resistance. This is supported by a previous finding that colistin resistance is also affected by the expression rates of qseB and qseC, which may be transferred together with the mcr-9 gene (Kieffer et al., 2019). The findings in our study highlight the transferability of the mcr-9 gene and the need for the reassessment of increased use of colistin in livestock industry.
Conclusions
This study confirmed the presence of the mcr-9 gene in Salmonella isolated from chicken meat sold in retail markets in Korea. This is the first report of a transferable mcr-9 gene in colistin-resistant Salmonella isolated from chicken meat in Korea, further suggesting the possibility of transfer of the flanking sequences IS903B upstream and IS26 and IS1R downstream as an entire region. This finding also suggests the possibility of emergence of other colistin-resistant bacteria in chicken farms or retail chicken. The distribution of the mcr-9 gene and its characteristics are reported in different environments, such as in clinical settings and livestock. Therefore, further studies are required to investigate the distribution, resistance mechanisms, and transmission routes of the newly described colistin-resistance gene, mcr-9. Future research should also take into consideration a wide range of food and clinical sources. Our results also support the growing concern that the indiscriminate use of colistin should be carefully reassessed. Therefore, it is necessary to implement a One Health perspective to better monitor the antimicrobial resistance of Enterobacteriaceae in humans, animals, and the environment.
Sequence Data
Sequences were deposited to GenBank under accession numbers JAALIP000000000, JAALIQ000000000, JAALIR000000000, JAALIS000000000, JAALIT000000000, JAALIU000000000, JAALIV000000000, JAALIW000000000, JAALIX000000000, JAALIY000000000, JAALIZ000000000, JAALJA000000000, and JAALJB000000000.
Supplementary Material
Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the Research Program funded by the Korea National Institute of Health (Grant Nos. 2017-NI41004 and 2017ER540601) and by a grant from the Korea Rural Development Administration (Grant No. PJ010500).
Supplementary Material
References
- Aziz RK, Bartels D, Best AA, DeJongh M, Disz T, Edwards RA, Formsma K, Gerdes S, Glass EM, Kubal M, Meyer F, Olsen GJ, Olson R, Osterman AL, Overbeek RA, McNeil LK, Paarmann D, Paczian T, Parrello B, Pusch GD, Reich C, Stevens R, Vassieva O, Vonstein V, Wilke A, Zagnitko O. The RAST Server: Rapid annotations using subsystems technology. BMC Genomics 2008;9:75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Börjesson S, Greko C, Myrenås M, Landén A, Nilsson O, Pedersen K. A link between the newly described colistin resistance gene mcr-9 and clinical Enterobacteriaceae isolates carrying blaSHV-12 from horses in Sweden. J Global Antimicrob Resist 2019;5:285–289 [DOI] [PubMed] [Google Scholar]
- Carattoli A, Zankari E, Garcìa-Fernandez A, Larsen MV, Lund O, Villa L, Aarestrup FM, Hasman H. PlasmidFinder and pMLST: In silico detection and typing of plasmids. Antimicrob Agents Chemother 2014;58:3895–3903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll LM, Gaballa A, Guldimann C, Sullivan G, Henderson LO, Wiedmann M. Identification of novel mobilized colistin resistance gene mcr-9 in a multidrug-resistant, colistin-susceptible Salmonella enterica serotype Typhimurium isolate. mBio 2019;10:e00853-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavda KD, Westblade LF, Satlin MJ, Hemmert AC, Castanheira M, Jenkins SG, Chen L, Kreiswirth BN. First report of blaVIM-4-and mcr-9-coharboring Enterobacter species isolated from a pediatric patient. mSphere 2019;4:e00629-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing. CLSI Document M100. Wayne, PA: CLSI, 2017 [Google Scholar]
- EUCAST (The European Committee on Antimicrobial Susceptibility Testing). Breakpoint Tables for Interpretation of MICs and Zone Diameters. 2019. Version 9.0. Available at: http://www.eucast.org accessed December25, 2019
- Hyeon J-Y, Chon J-W, Hwang I-G, Kwak H-S, Kim M-S, Kim S-K, Choi I-S, Song C-S, Park C, Seo K-H. Prevalence, antibiotic resistance, and molecular characterization of Salmonella serovars in retail meat products. J Food Prot 2011;74:161–166 [DOI] [PubMed] [Google Scholar]
- Kieffer N, Royer G, Decousser J-W, Bourrel AS, Palmieri M, Ortiz De La Rosa JM, Jacquier H, Denamur E, Nordmann P, Poirel L. mcr-9, an inducible gene encoding an acquired phosphoethanolamine transferase in Escherichia coli, and its origin. Antimicrob Agents Chemother 2019;63:e00965-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Jeon S, Rhie H, Lee B, Park M, Lee H, Lee J, Kim S. Rapid detection of extended spectrum β-lactamase (ESBL) for Enterobacteriaceae by use of a multiplex PCR-based method. Infect Chemother 2009;41:181–184 [Google Scholar]
- Kim K-Y, Park J-H, Kwak H-S, Woo G-J. Characterization of the quinolone resistance mechanism in foodborne Salmonella isolates with high nalidixic acid resistance. Int J Food Microbiol 2011;146:52–56 [DOI] [PubMed] [Google Scholar]
- Larsen MV, Cosentino S, Rasmussen S, Friis C, Hasman H, Marvig RL, Jelsbak L, Sicheritz-Pontén T, Ussery DW, Aarestrup FM. Multilocus sequence typing of total-genome-sequenced bacteria. J Clin Microbiol 2012;50:1355–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima T, Domingues S, Da Silva GJ. Plasmid-mediated colistin resistance in Salmonella enterica: A review. Microorganisms 2019;7:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma S, Lei C, Kong L, Jiang W, Liu B, Men S, Yang Y, Cheng G, Chen Y, Wang H. Prevalence, antimicrobial resistance, and relatedness of Salmonella isolated from chickens and pigs on farms, abattoirs, and markets in Sichuan province, China. Foodborne Pathog Dis 2017;14:667–677 [DOI] [PubMed] [Google Scholar]
- Oh S-S, Song J, Kim J, Shin J. Increasing prevalence of multidrug-resistant mcr-1-positive Escherichia coli isolates from fresh vegetables and healthy food animals in South Korea. Int J Infect Dis 2020;92:53–55 [DOI] [PubMed] [Google Scholar]
- Rau RB, de Lima-Morales D, Wink PL, Ribeiro AR, Barth AL. Salmonella enterica mcr-1 positive from food in Brazil: Detection and characterization. Foodborne Pathog Dis 2019;17:202–208 [DOI] [PubMed] [Google Scholar]
- Seo KW, Kim JJ, Mo IP, Lee YJ. Molecular characteristic of antimicrobial resistance of Salmonella gallinarum isolates from chickens in Korea, 2014 to 2018. Poultry Sci 2019;98:5416–5423 [DOI] [PubMed] [Google Scholar]
- Shang K, Wei B, Kang M. Distribution and dissemination of antimicrobial-resistant Salmonella in broiler farms with or without enrofloxacin use. BMC Vet Res 2018;14:257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanaway JD, Parisi A, Sarkar K, Blacker BF, Reiner RC, Hay SI, Nixon MR, Dolecek C, James SL, Mokdad AH, Abebe G, Ahmadian E, Alahdab F, Alemnew BTT, Alipour V, Allah Bakeshei F, Animut MD, Ansari F, Arabloo J, Asfaw ET, Bagherzadeh M, Bassat Q, Belayneh YMM, Carvalho F, Daryani A., Demeke FM, Demis ABB, Dubey M, Duken EE, Dunachie SJ, Eftekhari A, Fernandes E, Fouladi Fard R, Gedefaw GA, Geta B, Gibney KB, Hasanzadeh A, Hoang CL, Kasaeian A, Khater A, Kidanemariam ZT, Lakew AM, Malekzadeh R, Melese A, Mengistu DT, Mestrovic T, Miazgowski B, Mohammad KA, Mohammadian M, Mohammadian-Hafshejani A, Nguyen CT, Nguyen LH, Nguyen SH, Nirayo YL, Olagunju AT, Olagunju TO, Pourjafar H, Qorbani M, Rabiee M, Rabiee N, Rafay A, Rezapour A, Samy AM, Sepanlou SG, Shaikh MA, Sharif M, Shigematsu M, Tessema B, Tran BX, Ullah I, Yimer EM, Zaidi Z, Murray CJL, Crump JA.. The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect Dis 2019;19:1312–1324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MJ, Petty NK, Beatson SA. Easyfig: A genome comparison visualizer. Bioinformatics 2011;27:1009–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamang MD, Oh JY, Seol SY, Kang HY, Lee JC, Lee YC, Cho DT, Kim J. Emergence of multidrug-resistant Salmonella enterica serovar Typhi associated with a class 1 integron carrying the dfrA7 gene cassette in Nepal. Int J Antimicrob Agents 2007;30:330–335 [DOI] [PubMed] [Google Scholar]
- Yang SJ, Park KY, Kim SH, No KM, Besser TE, Yoo HS, Kim SH, Lee BK, Park YH. Antimicrobial resistance in Salmonella enterica serovars Enteritidis and Typhimurium isolated from animals in Korea: Comparison of phenotypic and genotypic resistance characterization. Vet Microbiol 2002;86:295–301 [DOI] [PubMed] [Google Scholar]
- Ye J, Coulouris G, Zaretskaya I, Cutcutache I, Rozen S, Madden T. Primer-BLAST: A tool to design target-specific primers for polymerase chain reaction. BMC Bioinform 2012;13:134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Li Y, Wang G, Li C, Xiang L, She J, Yang Y, Zhong F, Zhang L. Coproduction of MCR-9 and NDM-1 by colistin-resistant Enterobacter hormaechei isolated from bloodstream infection. Infect Drug Resist 2019;12:2979–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zankari E, Hasman H, Cosentino S, Vestergaard M, Rasmussen S, Lund O, Aarestrup FM, Larsen MV. Identification of acquired antimicrobial resistance genes. J Antimicrob Chemother 2012;67:2640–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Yin Y, Jones MB, Zhang Z, Kaiser BL, Dinsmore BA, Fitzgerald C, Fields PI, Deng X. Salmonella serotype determination utilizing high-throughput genome sequencing data. J Clin Microbiol 2015;53:1685–1692 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.