Abstract
Background
Administration of Hydroxychloroquine and Azithromycin in patients with coronavirus disease 2019 (COVID-19) prolongs QTc corrected interval (QTc). The effect and safety of Lopinavir/Ritonavir in combination with these therapies have seldom been studied.
Objectives
Our aim was to evaluate changes in QTc in patients receiving double (Hydroxychloroquine + Azithromycin) and triple therapy (Hydroxychloroquine + Azithromycin + Lopinavir/Ritonavir) to treat COVID-19. Secondary outcome was the incidence of in-hospital all-cause mortality.
Methods
Patients under treatment with double (DT) and triple therapy (TT) for COVID-19 were consecutively included in this prospective observational study. Serial in-hospital electrocardiograms were performed to measure QTc at baseline and during therapy.
Results
168 patients (±66.2 years old) were included: 32.1% received DT and 67.9% received TT. The mean baseline QTc was 410.33 ms. Patients under DT and TT prolonged QTc interval respect baseline values (p < 0.001), without significant differences between both therapy groups (p = 0.748). Overall, 33 patients (19.6%) had a peak QTc and/or an increase QTc 60 ms from baseline, with a higher prevalence among those with hypokalemia (p = 0.003). All-cause mortality was similar between both strategy groups (p = 0.093) and high risk QTc prolongation was no related to clinical events in this series.
Conclusions
DT and TT prolong the QTc in patients with COVID-19. Addition of Lopinavir/Ritonavir on top of Hydroxychloroquine and Azithromycin did not increase QTc compared to DT.
Keywords: COVID-19, Hydroxychloroquine, Azithromycin, Lopinavir/ritonavir, QT corrected interval
Abbreviations: QTc, corrected QT interval; COVID-19, Coronavirus disease 2019; DT, Double therapy; TT, Triple therapy; VA, ventricular arrhythmias; SCD, sudden cardiac death
Introduction
Since its emergence in China in the late 2019, severe acute respiratory coronavirus-2, the virus responsible for the coronavirus disease 2019 (COVID-19) has spread around the globe. Some studies have shown an increased rate of cardiac ventricular arrhythmias (VA) and sudden cardiac death (SCD) among these patients [1,2]. The efficacy of current pharmacological therapies to treat this infection has not been yet demonstrated in large-scale studies [3,4]. Despite the lack of evidence, Lopinavir/ritonavir, Hydroxychloroquine and Azithromycin have been commonly used at a first line empiric therapy in most centers. All these drugs have been associated with a prolongation of QT corrected interval (QTc) as a non-uncommon side effect, which has raised concern about the risk of VA, specifically torsade de pointes, and SCD [5,6]. Recent studies performed during the pandemic period have analyzed the effect on the QTc of Azithromycin, Hydroxychloroquine and the combination of both drugs [7,8]. Nevertheless, there is scarce data regarding the effect of Lopinavir/Ritonavir and its combination with Hydroxychloroquine and Azithromycin on the QTc and the risk of VA. The aim of this study was to evaluate the risk of prolonged QTc and VA in COVID-19 patients receiving triple therapy with Lopinavir/Ritonavir on top of Hydroxychloroquine and Azithromycin compared to those treated with Hydroxychloroquine and Azithromycin alone.
Methods
Study design
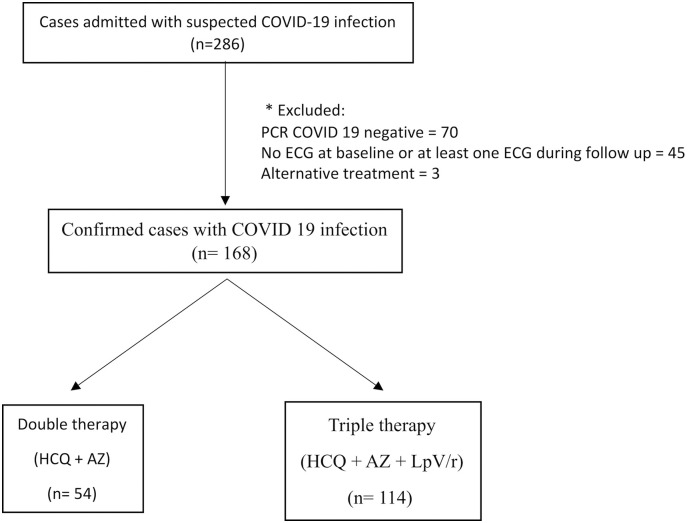
This single center observational prospective study included all consecutive patients admitted in our institution with diagnosis of COVID-19 between March 31st and May 8th. Patients with non-confirmed test for COVID-19, those with no electrocardiogram (ECG) at baseline and at least one ECG during follow up and those who did not receive at least one dose of either prespecified combined therapy were excluded. The flowchart of the study is presented in Fig. 1 .
Fig. 1.
Study flowchart.
Study procedures and definitions
The choice of treatment in each patient was based on physician's criteria and local guidelines. COVID-19 diagnosis was confirmed with polymerase chain reaction (PCR) test in all patients. Serum samples were obtained at admission to analyze biochemical parameters, including acute phase reactants. Lowest serum potassium documented during hospitalization was collected. CURB 65 score (confusion, urea >7 mmol/L, respiratory rate ≥ 30 bpm, blood pressure < 90/60 mmHg, age > 65 years) was calculated at admission [9]. The standard prescription of the 3 analyzed drugs were: 400 mg of Hydroxychloroquine twice on day 1 and 200 mg twice a day on days 2 to 5; 500 mg of Azithromycin once on day 1 and 250 mg daily for 4 days more; and 200/50 mg of Lopinavir/Ritonavir twice a day during two weeks. Patients were classified in two treatment groups: Double therapy (DT) with Hydroxychloroquine and Azithromycin, or Triple therapy (TT) adding Lopinavir/Ritonavir on top of these two drugs. A 12‑lead ECG was performed prior to the initiation of medical therapy and at 48 and 96 h [10]. In patients with baseline QTc > 500 ms, DT or TT were only considered if severe COVID-19 pneumonia without other therapeutic alternatives. In these cases, medications were progressively started and closer ECG monitorization was performed as priorly suggested [10]. ECGs were blindly evaluated by at least one of two experienced cardiologists (C. M. C. and J.E.M) to calculate QTc using Bazzet's formula on lead II and V5 [11]. When ECG complex was not optimal in these leads, I and aVL were used as an alternative. For patients with a wide QRS from either ventricular pacing or left/right bundle branch block the excess correction method was used [12]. In patients with atrial fibrillation (AF), QTc was estimated as the mean of 3–5 beats. Prolonged QTc was defined as ≥450 ms in males and ≥ 470 ms in females [13]. The longest QTc measured after COVID-19 drugs were started was considered as peak QTc during admission. Patients with a peak QTc ≥ 500 ms and those with an increase in the QTc ≥ 60 ms from baseline values were considered at high risk for VA [14]. Discontinuation of any of analyzed COVID-19 therapies was decided by referral physician taking into account the presence of high risk QTc pattern, the severity of underlying pneumonia and the potential alternatives drugs.
Study outcomes
The primary aim of the study was to evaluate changes in QTc in patients receiving DT vs TT to treat COVID-19. Secondary outcome was the incidence of in-hospital all-cause mortality.
Data collection
All data were collected using standardized report forms including demographic features, medical history, baseline clinical characteristics, biochemical and ECG findings, medical treatment during admission and in-hospital clinical outcomes. The study was approved by the local Ethics Committee and adhered to the principles outlined in the Declaration of Helsinki. Individual informed consent was obtained from participants.
Statistical analysis
Continuous variables were summarized as mean ± standard deviation (SD) or as medians and interquartile range (IQR) and were compared using paired or unpaired Student t-tests or the non-parametric Wilcoxon rank sum tests if the normal distribution the variables could not be demonstrated. Derangement from the normal distribution was assessed with the Shapiro-Wilk test. Categorical variables were described as percentages and compared using Chi-square or Fisher exact tests accordingly to expected frequency over or below 5, respectively. Univariate and multivariate logistic regression was used to test factors related to high risk prolonged QTc. Survival curves for time-to-event variables were constructed on the basis of all available follow-up data using Kaplan-Meier estimates and comparisons were performed using the log-rank test. Statistical analyses were performed using STATA software version 14.2.
Results
Patient population
A total of 168 (± 66.2 years old) patients with a diagnosis of COVID-19 infection were enrolled. Baseline characteristics of the included cohort are displayed in Table 1 grouped by the administration of DT (32.1%) or TT (67.9%). Patients under DT had more frequently chronic kidney disease (p = 0.007), with higher levels of serum creatinine at admission (p = 0.046) and were more commonly on other drugs associated with QTc prolongation (p = 0.021). Serum levels of lactate dehydrogenase were higher in the group under TT (p = 0.014).
Table 1.
Baseline characteristics of included cohort according to the medical therapy received.
| All patients (n = 168) |
Double therapy (n = 54) |
Triple therapy (n = 114) |
p-Value | |
|---|---|---|---|---|
| Age (years) | 66.2 ± 14.9 | 68.7 ± 18.4 | 65.0 ±12.8 | 0.130 |
| Male (%) | 98.0 (59) | 32.0 (59.3) | 66.0 (59.5) | 0.980 |
| Hypertension (%) | 78.0 (47.6) | 29.0 (53.7) | 49.0 (44.6) | 0.270 |
| Ischemic heart disease (%) | 13.0 (7.9) | 3.0 (5.6) | 10.0. (9.1) | 0.548 |
| Chronic kidney disease (%) | 32.0 (19.5) | 17.0 (31.5) | 15.0 (13.6) | 0.007 |
| Heart rate (bpm) | 84.9 ±20.5 | 85.1±24.0 | 84.8±18.8 | 0.926 |
| LDH (IU/L) | 362.6 ± 170.8 | 314.5 ± 152.0 | 385.3 ± 175.1 | 0.014 |
| Ferritin (ng/ml) | 1199.0 ± 1303 | 914.0 ± 1332 | 1331.0 ± 1275 | 0.080 |
| CRP (mg/dl) | 112.0 ± 82 | 100.0 ± 80 | 117.0 ± 82 | 0.217 |
| D-dimer (μg/ml) | 6220.0 ± 16,549 | 4474.0 ± 10,495 | 6979.0± 18,833 | 0.374 |
| Serum creatinine (mg/dl) | 1.3 ±1.39 | 1.6 ± 2.26 | 1.2 ± 0.58 | 0.046 |
| Lowest serum potassium (mg/dL) | 3.9 ± 0.45 | 3.8 ± 0.43 | 3.86 ± 0.46 | 0.790 |
| Loop diuretic (%) | 27.0 (16.1) | 12.0 (22.2) | 15.0 (13.2) | 0.135 |
| Other QTc prolonging therapy (%) | 37.0 (22.6) | 18.0 (33.3) | 19.0 (17.3) | 0.021 |
| Tisdale score | 8.3 ±2.23 | 8.0 ±2.27 | 8.4 ± 2.21 | 0.305 |
LDH: Lactate dehydrogenase, CRP: C-reactive protein, QTc: corrected QT interval, BPM: beats per minute, Other QTc prolonging therapy: drugs with effect on QTc not related to COVID 19 treatment (Levofloxacin, Amiodarone, Quetiapine, Olanzapine, Haloperidol…).
Electrocardiographic findings
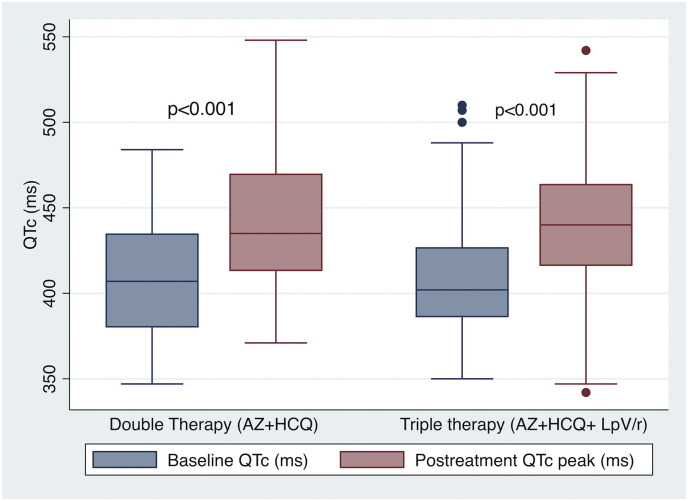
Most patients were in sinus rhythm at admission and AF was present in 15 (8.9%) patients. The mean baseline QTc was 410.3 ± 33.9 ms and 10.7% patients presented prolonged QTc before COVID-19 therapy was started (Table 2 ). Three (1,8%) patients had a QTc ≥ 500 ms at baseline. Indication of combined therapy in these cases was done due to severe clinical status with no reliable therapeutic alternatives. No differences in baseline ECG features were found among patients who received either DT or TT. During in-hospital ECG monitoring, QTc did significantly increased in both therapy arms compared to baseline (DT: 408.0 ± 34.0 ms vs 439.2 ± 38.2 ms, p < 0.001; TT: 411.3 ± 34.0 ms vs 441.2 ± 35.5 ms, p < 0.001; Fig. 2 ), with no difference in peak QTc (439.2 ± 38.2 ms vs 441.2 ± 35.5 ms, p = 0.748) or ΔQTc (31.230.6 vs 29.8 36.5, p = 0.813) associated to the addition of Lopinavir/Ritonavir. Likewise, the proportion of patients fulfilling criteria of prolonged QTc during COVID-19 treatment did also significantly rised (10.7% vs 31.6%, p < 0.001), with no differences between both therapy groups (p = 0.292). Overall, 33 (19.6%) cases were considered at high risk of VA due to an increase of QTc 60 ms or a QTc. In 6 (3.6%) patients' medication was discontinued due to QTc prolongation (Fig. 3 ).
Table 2.
Electrocardiographic in-hospital findings among patients with double or triple therapy for COVID-19.
| All patients (n = 168) |
Double therapy (n = 54) |
Triple therapy (n = 114) |
p-Value | |
|---|---|---|---|---|
| Sinus rhythm (%) | 153.0 (91.1) | 46.0 (85.2) | 107.0 (93.9) | 0.083 |
| Bundle branch block (%) | 18.0 (10.7) | 5.0 (9.3) | 13.0 (11.4) | 0.675 |
| Baseline QTc (ms) | 410.3 ± 33.9 | 408.0 ± 34.0 | 411.3 ± 34.0 | 0.558 |
| Prolonged baseline QTc (%) | 18.0 (10.7) | 5.0 (9.3) | 13.0 (11.4) | 0.675 |
| QTc peak (ms) | 440.6 ± 36.3 | 439.2 ± 38.2 | 441.2 ± 35.5 | 0.748 |
| Δ QTc (ms) | 30.3 ± 34.6 | 31.2 ± 30.6 | 29.8 ± 36.5 | 0.813 |
| Prolonged QTc peak (%) | 53.0 (31.6) | 20.0 (37.0) | 33.0 (29.0) | 0.292 |
| QTc prolongation ≥ 60 ms (%) | 27.0 (16.1) | 6.0 (11.1) | 21.0. (18.4) | 0.228 |
| QTc peak ≥ 500 ms (%) | 9.0 (5.4) | 2.0 (3.7) | 7.0 (6.1) | 0.720 |
| High risk QTc peak (%) | 33.0 (19.6) | 7.0 (13.0) | 26.0 (22.8) | 0.134 |
QTc: corrected QT interval, BPM: beats per minute, MS: milliseconds.
Fig. 2.
Changes in QTc comparing baseline ECG findings before initiation of medical therapy for COVID-19 and peak QTc observed during in-hospital serial ECG monitoring according to received medical treatment.
AZ: azithromycin; HCQ: hydroxychloroquine; LpV/r: Lopinavir/Ritonavir.
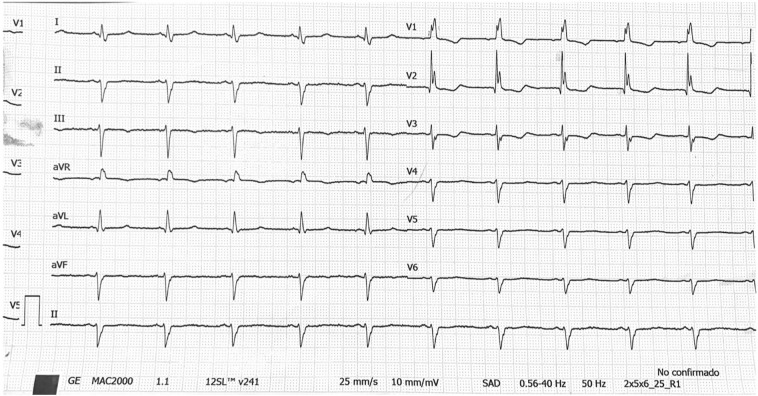
Fig. 3.
ECG recording from a patient with COVID 19 under double therapy. After three days of treatment, QTc was 492 ms and medication was discontinued due to an increase >60 ms compared to baseline.
Table 3 shows univariate and adjusted logistic regression analysis for predictors of high risk QTc prolongation. The administration of loop diuretics (OR 3.02 [1.23–7.42], p = 0.016), low levels of serum potassium (OR 3.02 [1.23–7.42], p = 0.016) and sepsis (3.67 [1.43–9.96], p = 0.013) were related to a higher prevalence of high risk QTc prolongation during follow up. After multivariate adjustment, only low serum potassium below 3.5 mEq/L remained statistically significant (OR 4.0 [1.61–9.95], p = 0.003).
Table 3.
Independent predictors of high risk QTc prolongation (peak QTc ≥ 500 ms and/or ΔQTc ≥60ms).
| Odds ratio (CI 95%) |
p-value | Adjusted Odds ratio (CI 95%) |
p-value | |
|---|---|---|---|---|
| Female | 1.14 (0.53–2.47) | 0.739 | ||
| Triple therapy | 1.98 (0.80–4.91) | 0.139 | ||
| Other QTc interfering drugs* | 1.53 (0.51–4.60) | 0.449 | ||
| Loop diuretic | 3.02 (1.23–7.42) | 0.016 | 1.79 (0.62–5.21) | 0.284 |
| Hypokalemia (K+ ≤ 3.5 mEq/L) | 3.78 (1.65–8.66) | 0.002 | 4.0 (1.61–9.95) | 0.003 |
| Heart failure | 1.48 (0.61–3.57) | 0.389 | ||
| Baseline QTc ≥450 ms | 1.36 (0.49–3.72) | 0.553 | ||
| Sepsis | 3.67 (1.32–10.2) | 0.013 | 2.65 (0.88–7.99) | 0.083 |
QTc: corrected QT interval, ms: milliseconds. *: Levofloxacin, Amiodarone, Haloperidol, Venlafaxine, Famotidine.
In-hospital clinical outcomes
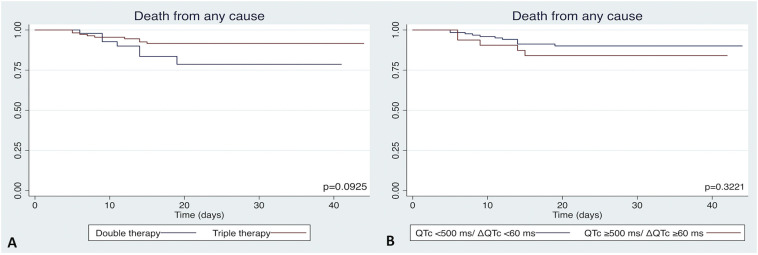
During a median follow up of 23 [13.5–30.0] days, 16 (9.6%) patients died, with no differences between both treatment arms (13.2% vs 8.0%, p = 0.296). In all these patients, worsening of COVID-19 pneumonia was considered the cause of death. Survival free of death from any cause was similar in both therapy groups (p = 0.093, Fig. 4A) and among patients with or without high risk QTc prolongation (p = 0.322, Fig. 4B).
Fig. 4.
Death from any cause in double and triple therapy groups (A), and in patients with or without high risk QTc prolongation (B).
Discussion
The main findings of our study were: 1) the combination of HCA and Azithromycin with or without Lopinavir/Ritonavir increased QTc; 2) the addition of Lopinavir/Ritonavir to Hydroxychloroquine and Azithromycin was not related to a significant increase of the peak QTc compared to stand-alone DT; 3) low levels of serum potassium were independently related to the incidence of high risk prolonged QTc peak; 4) the prolongation of QTc was not associated to all-cause mortality in our series.
Given the lack of data regarding effectiveness of dedicated drugs to treat COVID-19, empiric combination of diverse medications has been widely used in these cases. Among them, Hydroxychloroquine, Azithromycin, and Lopinavir/Ritonavir are commonly administered in subjects requiring hospitalization due to COVID-19 pneumonia [3,4]. Although recent observational reports have shown neutral or even negative outcomes with some of these combined therapies, diverse randomized controlled trials are currently being carried out in order to get a definite answer to their effectiveness.
Hydroxychloroquine is believed to act on the entry and post-entry stages of COVID-19 and blocks the hERG potassium channel, therefore potentially prolonging QTc [15]. Azithromycin is a macrolide frequently prescribed to treat a wide spectrum of infections. The association between Azithromycin and prolongation of QTc has been also published, despite it appears to have a lower interaction with the hERG channel [16]. Lopinavir/Ritonavir inhibits the Human Immunodeficiency Virus (HIV) protease and has shown to be effective in patients with COVID-19 in vitro and in animal models [[17], [18], [19]]. This antiretroviral drug also inhibits the hERG although reported interaction with QTc shows conflicting results [20]. Therefore, these three drugs inhibit the hERG channel, although the grade of affinity of each of them to this receptor has not been already fully reported. Beyond this mechanism, other pharmacological interactions with intracellular ionic currents may play a role in QTc prolongation in this scenario. In this regard, Azithromycin potentiates late INa, which increases intracellular sodium and promotes dysregulation of intracellular calcium. Likewise, Azithromycin and Lopinavir/Ritonavir my interact with IKr, while Hydroxychloroquine affects IK 1 [[21], [22], [23]].
Drug-induced QTc prolongation is an important substrate for VA, although the incidence of potentially life-threatening polymorphic VA in daily practice seems to be low [24]. Bessière et al. found a significant increase in QTc in patients treated with Hydroxychloroquine that was statistically higher in those with DT compared to monotherapy. In this small series of patients admitted in the critical care unit, no polymorphic VA or SCD was documented [25]. Similar results were reported in two larger cohorts of hospitalized patients with COVID-19 pneumonia [7,8]. Given reported very low incidence of VA and the risk of contagious in the setting of COVID-19, serial ECG could be considered if continuous ECG monitoring is not available [10].
In our cohort, patients under DT and TT prolonged QTc compared to baseline values. Nevertheless, patients with TT did not show a greater increase in the QTc compared to those on DT. The interaction between Lopinavir/Ritonavir and QTc has not been completely clarify yet. Although fairly reported in literature, the incidence of prolonged QTc seems to be very low in patients on monotherapy [3]. In this regard, Charbit et al. analyzed the effect of antiretroviral drugs on QTc in nearly 1000 HIV-infected patients [20]. They concluded that despite these medications do inhibit hERG potassium channel, this effect was not independently associated with QTc prolongation. Given these uncertainties in the effect of Lopinavir/Ritonavir on QTc, Giudicessi et al. included this drug as a possible risk factor for torsades de pointes in a recent review about QTc-prolonging in COVID-19 disease, while Hydroxychloroquine and Azithromycin were classified as known triggers. Besides, in our series, those patients who received DT were more likely to receive others QTc prolongation therapy compared to those on TT, which might have influenced the lack of differences between both therapy groups.
From a clinical perspective, although the use of these medications resulted in significant QTc prolongation, the need to withdraw any of these drugs was very infrequent. Nevertheless, given the exceptional circumstances of COVID-19, including oversaturated healthcare resources, wide heterogeneity of physician specialists taking care of these patients, the severe morbidity and mortality of hospitalized patients and the lack of evidence-supported dedicated therapies, this rate might be higher in a more “normalized” setting. No differences in all-cause mortality were reported, similarly to previous studies.
As priorly suggested by Giudicessi et al., we considered patients with a peak QTc ≥ 500 ms and/or an increase of QTc ≥ 60 ms from baseline to be at high risk of VA or SCD [10]. Overall, 19.6% patients fulfilled these criteria of high risk in our cohort. Nevertheless, this pattern was not related to clinical outcomes in our series. In multivariate analysis, hypokalemia was significantly associated to a 4-fold likehood to present this high risk prolonged QTc. Similar criteria were recently proposed by Haseeb et al., who also highlighted some clinical high-risk factors such as history of SCD, VA or long QT syndrome. In this review, they also recommend ECG monitoring and deep control of medication in patients at intermediate risk, such as those with preexisting heart disease, multiple comorbidities and those receiving concomitant drugs that may cause QT prolongation.
Limitations
This study has several limitations inherent to its observational nature. First, sample size is small, and patients were enrolled in one single center so that our results should be interpreted with caution. Second, given non-randomized controlled design both therapy arms presented some significant differences in baseline features which could have influenced these findings. Third, the absence of a control cohort of patients with COVID-19 that were not treated with any of these medications should be taking into account when considering the findings of this report. Four, due to unavailability of continuous ECG monitoring in conventional wards, accurate incidence of VA and arrhythmic SCD could not be documented.
Conclusions
Patients who received DT experienced QTc prolongation and, according to results in this series, Lopinavir/Ritonavir could be safely added to DT in patients with COVID-19 without a significant increase in QTc compared to DT. There were no differences in mortality between both strategies. Despite the increase in QTc in most patients, discontinuation of treatment was seldomly performed.
Funding
The authors did not receive any funding for this work.
Disclosures
The authors have no conflict of interest to declare in relation to the present manuscript.
References
- 1.Guo T., Fan Y., Chen M., Wu X., Zhang L., He T. Cardiovascular implications of fatal outcomes of patients with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shi S., Qin M., Shen B., Cai Y., Liu T., Yang F. Association of cardiac injury with mortality in hospitalized patients with COVID-19 in Wuhan. China JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.0950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir-ritonavir in adults hospitalized with severe covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gautret P., Lagier J.-C., Parola P., Hoang V.T., Meddeb L., Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;105949 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rock E.P., Finkle J., Fingert H.J., Booth B.P., Garnett C.E., Grant S. Assessing proarrhythmic potential of drugs when optimal studies are infeasible. Am Heart J. 2009;157 doi: 10.1016/j.ahj.2009.02.020. 827–836.e1. [DOI] [PubMed] [Google Scholar]
- 6.Drew B.J., Ackerman M.J., Funk M., Gibler W.B., Kligfield P., Menon V. Prevention of torsade de pointes in hospital settings: a scientific statement from the American heart association and the American college of cardiology foundation. Circulation. 2010;121:1047–1060. doi: 10.1161/CIRCULATIONAHA.109.192704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mercuro N.J., Yen C.F., Shim D.J., Maher T.R., McCoy C.M., Zimetbaum P.J. Risk of QT interval prolongation associated with use of Hydroxychloroquine with or without concomitant azithromycin among hospitalized patients testing positive for coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saleh M., Gabriels J., Chang D., Kim B.S., Mansoor A., Mahmood E. The effect of chloroquine, hydroxychloroquine and azithromycin on the corrected QT interval in patients with SARS-CoV-2 infection. Circ Arrhythm Electrophysiol. 2020 doi: 10.1161/CIRCEP.120.008662. CIRCEP.120.008662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lim W.S., Van Der Eerden M.M., Laing R., Boersma W.G., Karalus N., Town G.I. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Giudicessi J.R., Noseworthy P.A., Friedman P.A., Ackerman M.J. Urgent guidance for navigating and circumventing the QTc-prolonging and torsadogenic potential of possible pharmacotherapies for coronavirus disease 19 (COVID-19) Mayo Clin Proc. 2020;0 doi: 10.1016/j.mayocp.2020.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sagie A., Larson M.G., Goldberg R.J., Bengtson J.R., Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham heart study) Am J Cardiol. 1992;70:797–801. doi: 10.1016/0002-9149(92)90562-D. [DOI] [PubMed] [Google Scholar]
- 12.Lanjewar P., Pathak V., Lokhandwala Y. Issues in QT interval measurement. Indian Pacing Electrophysiol J. 2004;4:156–161. [PMC free article] [PubMed] [Google Scholar]
- 13.Goldenberg I., Moss A.J., Zareba W. QT interval: how to measure it and what is “normal.”. J Cardiovasc Electrophysiol. 2006;17:333–336. doi: 10.1111/j.1540-8167.2006.00408.x. [DOI] [PubMed] [Google Scholar]
- 14.Haugaa K.H., Bos J.M., Tarrell R.F., Morlan B.W., Caraballo P.J., Ackerman M.J. Institution-wide QT alert system identifies patients with a high risk of mortality. Mayo Clin Proc. 2013;88:315–325. doi: 10.1016/j.mayocp.2013.01.013. [DOI] [PubMed] [Google Scholar]
- 15.Yao X., Ye F., Zhang M., Cui C., Huang B., Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choi Y., Lim H.S., Chung D., Choi J.G., Yoon D. Risk evaluation of azithromycin-induced QT prolongation in real-world practice. Biomed Res Int. 2018;2018 doi: 10.1155/2018/1574806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan J.F.W., Yao Y., Yeung M.L., Deng W., Bao L., Jia L. Treatment with lopinavir/ritonavir or interferon-β1b improves outcome of MERSCoV infection in a nonhuman primate model of common marmoset. J Infect Dis. 2015;212:1904–1913. doi: 10.1093/infdis/jiv392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park S.Y., Lee J.S., Son J.S., Ko J.H., Peck K.R., Jung Y. Post-exposure prophylaxis for Middle East respiratory syndrome in healthcare workers. J Hosp Infect. 2019;101:42–46. doi: 10.1016/j.jhin.2018.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arabi Y.M., Alothman A., Balkhy H.H., Al-Dawood A., AlJohani S., Al Harbi S. Treatment of Middle East respiratory syndrome with a combination of lopinavir-ritonavir and interferon-β1b (MIRACLE trial): study protocol for a randomized controlled trial. Trials. 2018;19:1–13. doi: 10.1186/s13063-017-2427-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Charbit B., Rosier A., Bollens D., Boccara F., Boelle P.Y., Koubaa A. Relationship between HIV protease inhibitors and QTc interval duration in HIV-infected patients: a cross-sectional study. Br J Clin Pharmacol. 2009;67:76–82. doi: 10.1111/j.1365-2125.2008.03332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Svensson E.M., Dooley K.E., Karlsson M.O. Impact of lopinavir-ritonavir or nevirapine on bedaquiline exposures and potential implications for patients with tuberculosis-HIV coinfection. Antimicrob Agents Chemother. 2014;58:6406–6412. doi: 10.1128/AAC.03246-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang Z., Prinsen J.K., Bersell K.R., Shen W., Yermalitskaya L., Sidorova T. Azithromycin causes a novel proarrhythmic syndrome. Circ Arrhythm Electrophysiol. 2017;10 doi: 10.1161/CIRCEP.115.003560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang J., Bourne G.W., Wang Z., Villemaire C., Talajic M., Nattel S. Comparative mechanisms of antiarrhythmic drug action in experimental atrial fibrillation: importance of use-dependent effects on refractoriness. Circulation. 1993;88:1030–1044. doi: 10.1161/01.CIR.88.3.1030. [DOI] [PubMed] [Google Scholar]
- 24.Roden D.M. Drug-induced prolongation of the QT interval. N Engl J Med. 2004;350:1013–1022. doi: 10.1056/NEJMra032426. [DOI] [PubMed] [Google Scholar]
- 25.Bessière F., Roccia H., Delinière A., Charrière R., Chevalier P., Argaud L. Assessment of QT intervals in a case series of patients with coronavirus disease 2019 (COVID-19) infection treated with hydroxychloroquine alone or in combination with azithromycin in an intensive care unit. JAMA Cardiol. 2020 doi: 10.1001/jamacardio.2020.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]