Highlights
-
•
HCQ pharmacokinetics in COVID-19 patients cannot be predicted using data from lupus or rheumatoid arthritis patients.
-
•
Bronchoalveolar lavage fluid may be a more instructive matrix than plasma on the degree of HCQ lung exposure.
-
•
Low plasma concentrations should not induce an increase in drug dosage because lung exposure could already be high.
Keywords: COVID-19, Hydroxychloroquine, Plasma drug monitoring, Bronchoalveolar lavage, BAL
Abstract
Different dosage regimens of hydroxychloroquine (HCQ) have been used to manage COVID-19 (coronavirus disease 2019) patients, with no information on lung exposure in this population. The aim of our study was to evaluate HCQ concentrations in the lung epithelial lining fluid (ELF) in patients infected with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), the virus that causes COVID-19. This was a retrospective, observational, multicentre, pharmacokinetic study of HCQ in critically ill COVID-19 patients. No additional interventions or additional samples compared with standard care of these patients were conducted in our teaching hospital. We included all intubated COVID-19 patients treated with crushed HCQ tablets, regardless of the dosage administered by nasogastric tube. Blood and bronchoalveolar lavage samples (n = 28) were collected from 22 COVID-19 patients and total HCQ concentrations in ELF were estimated. Median (interquartile range) HCQ plasma concentrations were 0.09 (0.06–0.14) mg/L and 0.07 (0.05–0.08) mg/L for 400 mg × 1/day and 200 mg × 3/day, respectively. Median HCQ ELF concentrations were 3.74 (1.10–7.26) mg/L and 1.81 (1.20–7.25) for 400 mg × 1/day and 200 mg × 3/day, respectively. The median ratio of ELF/plasma concentrations was 40.0 (7.3–162.7) and 21.2 (18.4–109.5) for 400 mg × 1/day and 200 mg × 3/day, respectively. ELF exposure is likely to be underestimated from HCQ concentrations in plasma. In clinical practice, low plasma concentrations should not induce an increase in drug dosage because lung exposure may already be high.
1. Introduction
Based on in vitro work carried out against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) and preliminary clinical data, hydroxychloroquine (HCQ) is currently being used in the management of patients with COVID-19 (coronavirus disease 2019) [1,2]. HCQ may have an antiviral action through three main mechanisms: (i) prevention of viral entry; (ii) impairment of viral replication; and (iii) a pleiotropic action on the human immune system through immunomodulating activity [3]. Various in vitro studies have shown that the EC50 (half maximal effective concentration) of HCQ ranged from 0.72–4.4 μM (i.e. 0.241–1.4 mg/L) at 48 h and 72 h post-infection, respectively [3], [4], [5]. Pending the results of robust clinical trials and due to the lack of pharmacokinetic/pharmacodynamic information in COVID-19 patients, and in accordance with the National French Team, AC43-ANRS/STP-SFPT, different dosage regimens were applied in Toulouse University Hospital (France) [200 mg × 3/day; 400 mg × 2 on Day 1 then 200 mg × 3/day; 400 mg × 2 on Day 1 then 400 mg/day; and, at least for intensive care unit (ICU) patients, 600 mg × 2 on Day 1 then 400 mg/day] in order to reach pharmacokinetic equilibrium as quickly as possible [6].
On 2 April 2020, the French Ministry of Health imposed a dosage regimen identical to the one used in systemic lupus erythematosus (SLE) (no loading dose and 200 mg × 3/day) for patients treated outside the context of a clinical trial.
Regardless of the dosage regimen, plasma concentrations were monitored in order to evaluate individual drug exposure. It has been proven that the plasma concentrations measured in COVID-19 patients tended to be lower than the values reported in SLE patients, in particular for the standard regimen of ‘200 mg × 3/day’ [7]. These preliminary results suggest that HCQ concentrations are unlikely to be adequately predicted using pharmacokinetic models derived from patients receiving HCQ for SLE or rheumatoid arthritis treatment [8].
As the apparent volume of distribution of HCQ is so large in volunteers as well as in malaria patients (~5000 L for the blood volume of distribution and ~40 000 L for the plasma volume at steady-state) [9,10], it can be suggested that HCQ becomes trapped in red blood cells and granulocytes [9,11,12] and probably in various tissues [10]. It could be assumed that the same occurs for COVID-19 patients. Consequently, it is natural to question the concentration of HCQ at the infectious site (i.e. the lung) [13]. Unfortunately, this information is not available. A lung biopsy is the most informative approach. Even if a biopsy is a mixture of both intracellular and extracellular matrices usually homogenised so as to determine a mean concentration [14], HCQ is trapped in the cells suggesting this drug is more likely present in the cells rather than outside. However, this option would be highly intrusive and an alternative approach would be to evaluate the HCQ concentration in lung epithelial lining fluid (ELF) at the bedside of ICU patients [15,16].
Drug concentrations in ELF can be inferred based on the concentration of HCQ in bronchoalveolar lavage (BAL) fluid and the concentration of urea both in the plasma and the BAL fluid [17]. This method is not new and is usually applied to explore the lung diffusion of antibiotics in ICU patients. Consequently, when available, BAL fluid can be used as a kind of ‘quality control’ used to obtain information on the degree of permeation in the lung for a short period (10–15 days) of treatment.
The aim of our study was to evaluate HCQ in the lung ELF in COVID-19 patients in order to estimate the level of lung exposure.
2. Patients and methods
This was a retrospective, observational, multicentre, pharmacokinetic study of HCQ in critically ill COVID-19 patients. We included all intubated COVID-19 patients treated with crushed HCQ tablets, regardless of the dosage administered by nasogastric tube.
According to the guidelines established by the French National AC43-ANRS/STP-SFPT Team in March/April 2020, blood samples were collected at different time points (from 48 h to 192 h) during clinical management of COVID-19 patients after HCQ initiation and 30 min before drug administration (i.e. trough concentration). In plasma, the steady state is supposed to be reached in 48 h [18].
As part of our standard practice for monitoring patients with acute respiratory distress syndrome (ARDS) who are at high risk for infectious complications, and in the particular context of COVID-19, a mini-bronchoalveolar lavage (BAL) (2 × 20 mL of physiological saline) was systematically performed 7 days (±2 days) after treatment initiation or in the event of a new respiratory deterioration for microbiological monitoring purposes (bacteriology, mycology and viral replication of SARS-CoV-2). A leftover volume of ~500 μL remained after these microbiological investigations, which was used to determine the HCQ concentration. BAL sampling was carried out between two dose administrations, with no specific time imposed. Except for one case, only the BALs for which plasma determinations were performed within 48 h before or after collection were included.
HCQ concentrations in plasma and BAL fluid were determined using a chromatographic analytical method validated as per US Food and Drug Administration (FDA) guidelines. All BAL sample preparations included a protein precipitation and a virus inactivation step in methanol solution. The HCQ dosage method presents a lower limit of quantitation (LLOQ) of 0.05 mg/L in plasma and 0.01 mg/L in BAL fluid, an upper limit of quantitation (ULOQ) of 2 mg/L in plasma and BAL fluid, and an intraday and interday variability of <4% and <10%, respectively. When plasma concentrations were <LLOQ, the value was set to 0.025 mg/L (i.e. half the LLOQ). Plasma samples were stored at +4 °C before analysis for a maximum period of 24 h. BAL fluid samples were stored at –80 °C before analysis for a maximum period of 30 days. Previous studies have shown that HCQ is stable in whole blood under these conditions [19]. As whole blood is a complex matrix, in which xenobiotics tend to be less stable than other biological fluids, we considered that HCQ was also stable in plasma and BAL fluid in these conditions.
Urea is used as an endogenous marker of ELF because urea, a small and relatively non-polar molecule, can freely travel across membranes to reach the outer surface of alveoli. The concentration of urea in ELF (UreaELF) is considered to be the same as in serum (Ureaserum), implying complete distribution. Therefore, the volume of ELF (V ELF) is adjusted for excess exogenous water using the following equation:
Knowing (i) the concentration of HCQ measured in BAL (HCQBAL), (ii) the volume of BAL collected (V BAL) and the estimated ELF volume (V ELF), it is then possible to determine the concentration of HCQ in ELF (HCQELF) using the following formula:
Plasma urea levels were determined using an automated enzymatic method validated as per FDA guidelines. This method presents a LLOQ of 0.5 mmol/L, an ULOQ of 15 mmol/L, and an intraday and interday variability of <2% and <3%, respectively. Safety practices require a greater level of caution when handling respiratory specimens from SARS-CoV-2-positive patients [20]. Thus, urea concentrations in BAL was assayed using a gas chromatography–mass spectrometry method that included a protein precipitation and virus inactivation step in methanol solution. A LLOQ of 0.1 mmol/L and an ULOQ of 20 mmol/L were achieved. Precision assays showed an intraday variability of <9% and an interday variability of <10%. BAL fluid samples were stored at –80 °C before analysis for a maximum period of 60 days. Previous studies have shown that urea is stable in serum in these conditions [21].
Continuous data were expressed as the median and interquartile range (IQR) and categorical variables as number (percentage). The relationship between plasma and ELF concentrations and the other parameters was assessed by simple linear regression. The analysis was performed using MedCalcⓇ15 statistical software (MedCalc Software Ltd., Ostend, Belgium). A P-value of <0.05 was considered statistically significant.
This study is entered in the Toulouse University Hospital register of retrospective studies (registration no. RnIPH 2020-33) and is covered by MR-004 (CNIL no. 2206723 v 0). This study was approved by Toulouse University Hospital and ethical requirements were entirely respected.
3. Results
3.1. Population
A total of 28 HCQ plasma and BAL fluid concentrations from 22 patients were measured (Table 1 ). The median patient age was of 60 years (IQR 53–70 years) and 91% of patients were male. The median body mass index (BMI) was of 28 kg/m2 (IQR 26–31 kg/m2). The median Simplified Acute Physiology Score (SAPS) II and Sepsis-related Organ Failure Assessment (SOFA) score pertaining to the included patients were of 37 (IQR 32–46) and 6 (IQR 3–7), respectively, indicating a critically ill patient population.
Table 1.
Demographic and clinical characteristics of severe COVID-19 patients (n = 22) treated with hydroxychloroquine and for whom bronchoalveolar lavage was performed
| Characteristic | Median (IQR) | Range |
|---|---|---|
| Age (years) | 59.5 (53–70) | 30–81 |
| BMI | 28.3 (26–31.3) | 20.7–37 |
| SAPS II | 37 (32–46) | 8–76 |
| SOFA score | 6 (3–7) | 2–14 |
| Protidaemia (g/L) D7 | 61 (59–68) | 50–77 |
| AST (UI/L) D7 | 65 (69–179) | 28–135 |
| ALT (UI/L) D7 | 99 (69–179) | 18–257 |
| Bilirubin (μmol/L) D0 | 7.6 (5.15–11.2) | 4–29 |
| CKD-EPI D7 (mL/min/1.73m2) | 97 (60.5–105.8) | 9–123 |
| Duration of invasive ventilation (days) | 19.5 (11–28) | 0–22 |
ALT, alanine aminotransferase; AST, aspartate aminotransferase; BMI, body mass index; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; COVID-19, coronavirus disease 2019; D0, Day 0 of hydroxychloroquine initiation, D7, Day 7 after hydroxychloroquine initiation; IQR, interquartile range; SAPS, Simplified Acute Physiology Score; SOFA, Sepsis-related Organ Failure Assessment.
3.2. Hydroxychloroquine trough concentrations
HCQ concentrations in BAL fluid were determined 7–12 days after treatment initiation. For one point, the time from blood collection to BAL was 9.8 days, but the plasma concentration was at steady-state.
The median HCQ plasma concentrations were 0.09 mg/L (IQR 0.06–0.14 mg/L) and 0.07 mg/L (IQR 0.05–0.08 mg/L) for 400 mg × 1/day and 200 mg × 3/day, respectively. The median HCQ ELF concentrations were 3.74 mg/L (IQR 1.10–7.26 mg/L) and 1.81 mg/L (IQR 1.20–7.25 mg/L) for 400 mg × 1/day and 200 mg × 3/day, respectively. The median ratio of ELF/plasma concentrations was 40.0 (IQR 7.3–162.7) and 21.2 (IQR 18.4–109.5) for 400 mg × 1/day and 200 mg × 3/day, respectively (Table 2 ).
Table 2.
Hydroxychloroquine (HCQ) plasma and epithelial lining fluid (ELF) concentrations.
| All dosages | 400 mg × 1/day | 200 mg × 3/day | ||||
|---|---|---|---|---|---|---|
| Median (IQR) | Range | Median (IQR) | Range | Median (IQR) | Range | |
| Plasma HCQ concentrations (mg/L) | 0.09 (0.06–0.14) | 0.03–0.19 | 0.09 (0.06–0.14) | 0.03–0.19 | 0.07 (0.05–0.08) | 0.03–0.09 |
| ELF HCQ concentrations (mg/L) | 3.03 (1.10–6.78) | 0.13–36.75 | 3.74 (1.10–7.26) | 0.13–36.75 | 1.81 (1.20–7.25) | 0.34–10.08 |
| ELF/plasma HCQ concentrations | 38.07 (8.34–138.52) | 2.1–290.4 | 39.96 (7.33–162.66) | 2.1–290.4 | 21.22 (18.41–109.49) | 13.4–168 |
IQR, interquartile range.
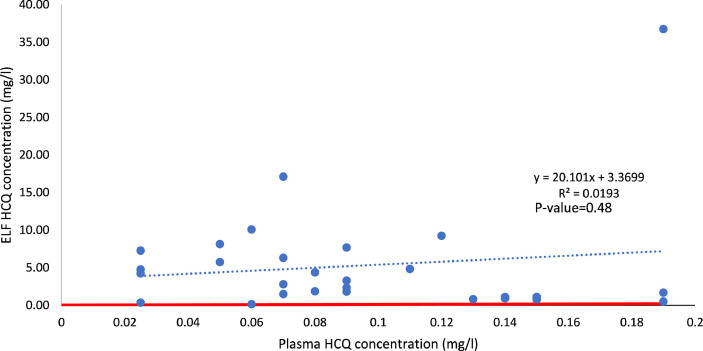
The relationship between ELF and plasma HCQ concentrations is presented in Fig. 1 .
Fig. 1.
Relationship between lung epithelial lining fluid (ELF) and plasma hydroxychloroquine (HCQ) concentrations. The red line is the identity line (i.e. the plasma and ELF concentrations are equal). Dots above the red line indicate that the concentration in ELF is higher than the concentration in plasma [ELF/plasma HCQ concentration ratio, 38.072 (8.338–138.521)]. This figure shows that (i) even with low plasma concentrations, the ELF concentration can be high and (ii) the plasma concentration is a poor predictor of the ELF concentration.
No relationship was observed between the measured HCQ concentrations and the biological parameters characterising renal and hepatic function (Supplementary Table S1).
4. Discussion
As previously reported for many anti-infective drugs used to treat pulmonary infections, ELF concentration gives information on the intracellular and extracellular lung exposure [15,16,22]. However, this approach is essentially reserved for clinical research as therapeutic monitoring of anti-infective drugs in ELF is determined by practical and organisational constraints. First, performing BAL requires that the operator be trained and that the patient be stable enough to tolerate the serum injection in such way that it is only exceptionally carried out on non-intubated patients. Second, drug quantification has to be performed in an unconventional matrix (i.e. BAL) with a very sensitive analytical method (i.e. most often LC-MS/MS). In the special case of COVID-19 patients, the BAL is contaminated by SARS-CoV-2, thus imposing a specific and time-consuming pre-analytical process.
We were able to gather all these conditions in order to assess whether all ELF concentrations are higher than plasma concentrations, despite the variability of ELF values. The significant variability in ELF concentrations may be explained in part by the BAL sampling. In fact, even if the injection volume were standardised (2 × 20 mL), the dwell time and the aspiration pressure cannot be strictly identical [23]. Cells can also be part of the ELF, especially macrophages, and may be lysed when measuring the drug concentration. Depending on their quantity, lysis of these cells may induce an increase in the HCQ concentration [11,17]. As no measurement of the cell burden in the BAL sample was performed owing to insufficient BAL volumes available for pharmacokinetic exploration, this lack of information has to be considered as a limitation of our study. Indeed, the cell burden in the BAL sample is likely associated with the ELF HCQ concentration (i.e. the more cells, the higher the concentration).
Collecting blood and BAL samples at a different moment (day and/or time) and the potential post-dose discrepancy between the blood sample and the collection of BAL could appear as limiting the interpretation of the ELF/plasma concentration ratios. However, HCQ presents a large volume of distribution with deep compartments (i.e. lung, spleen, melanin-containing tissues etc.) [3] leading to different kinetic profiles in plasma and lung tissue [24]. Indeed, half-life elimination is likely to be short in the plasma of COVID-19 patients [7,18] as opposed to deep compartments. Blood samples have always been taken at steady-state, while BALs should be collected always after the plasma concentration peak. Indeed, the staff in charge of carrying out the BALs was warned of this constraint. Furthermore, a flat kinetic profile was expected in the lung tissue [14]. As a consequence, it seems reasonable to suppose that ELF/plasma concentration ratios do not change between administrations, once steady-state has been reached. In fact, the ideal solution would consist in determining the area under the time–concentration curve (AUC) in both plasma and BAL matrices at steady-state and to calculate the AUC ratio. This option is not feasible and is not ethical for critically ill patients presenting ARDS because multiple BALs would alter the gas exchange between alveoli and capillaries. It would worsen PaO2/FiO2 ratios [the ratio of arterial oxygen partial pressure (PaO2 in mmHg) to fractional inspired oxygen (FiO2 expressed as a fraction)]. However, as our data were retrospectively collected from a small population of ICU patients, one limitation of our study is that the interindividual variability of the plasma and ELF HCQ concentrations likely under/overestimates the actual interindividual value.
Our results show that HCQ concentrations in the lung are higher than in the ELF. Passage from the blood compartment to the ELF involves passing through the pulmonary epithelial cells (i.e. prime target for the replication of SARS-CoV-2 [17,25,26]) in which HCQ is most likely accumulated with pharmacokinetic hysteresis. The mechanism of action of HCQ is poorly elucidated but includes, among others, the increase in endolysosomal pH necessary for viral fusion. The initial fusion between the viral and cellular membranes (e.g. lung epithelial cells) requires an interaction between the surface proteins of the two partners, and this interaction can only take place under particular acidic conditions, through the phenomenon of endocytosis. The inability to obtain the ideal pH can block this process and it is probably through this means that HCQ may act. Other properties may be involved: modification in the glycosylation of angiotensin-converting enzyme 2 (ACE2), the receptor that SARS-CoV-2 uses to enter the cells; and/or post-translational modification of some viral proteins [27]. In addition, viral invasion may also trigger a massive margination of phagocytic cells to the infection site, which may deliver increased amounts of HCQ [28]. But, in the absence of clear information on the influence of the inflammatory status reported in COVID-19 patients as to the accumulation of HCQ in lungs, this point should be considered as a limit of our study.
Plasma concentration is not predictive of lung concentration, as shown in Fig. 1. Therefore, drug dosage determinations in studies assuming equilibrium between epithelium and plasma concentrations may lead to overly high dosages [29]. Furthermore, in clinical practice, low plasma concentrations should not induce an increase in the drug dosage because the lung exposure may already be high. The various in vitro studies have shown that the EC50 of HCQ ranged from 0.72–4.4 μM (i.e. 0.241–1.4 mg/L) at 48 h and 72 h post-infection, respectively [3], [4], [5]. However, the EC50 could be determined in Vero cell lines, but not in a human epithelial cell model [4]. This discrepancy may explain why HCQ has not shown efficacy in clinical trials [29]. Thus, whether for a dose of 400 mg × 1/day or 200 mg × 3/day, the median ELF concentration of HCQ is above the maximum EC50 value. Therefore, both regimens lead to a median lung exposure that could be sufficient to eradicate the virus. However, the heterogeneity of EC50 values raises the problem of selecting the ‘right’ threshold used to determine the dosage, in particular through modelling techniques. In conclusion, despite all its imperfections, BAL fluid provides a rough idea of lung exposure.
Acknowledgments
The authors would like to thank Dr Marion Grare and Dr Sophie Cassaing for their help in sampling bronchoalveolar fluid; the technicians of the Pharmacokinetics and Toxicology Laboratory of Toulouse University Hospital; Magali Centelles for her help in data collection; and Della Hoy for her kind help proofreading the English.
Funding: This study was supported by internal funding.
Competing interests: None declared.
Ethical approval: This study was entered in the Toulouse University Hospital register of retrospective studies [registration no. RnIPH 2020-33] and is covered by MR-004 [CNIL no. 2206723 v 0]. This study was approved by Toulouse University Hospital and all ethical requirements were complied with.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2020.106247.
Appendix. Supplementary materials
References
- 1.Gautret P, Lagier JC, Parola P, Hoang VT, Meddeb L, Mailhe M. Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.105949. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Chen J, Liu D, Liu L, Liu P, Xu Q, Xia L. A pilot study of hydroxychloroquine in treatment of patients with moderate COVID-19 [in Chinese] Zhejiang Da Xue Xue Bao Yi Xue Ban. 2020;49:215–219. doi: 10.3785/j.issn.1008-9292.2020.03.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oscanoa TJ, Romero-Ortuno R, Carvajal A, Savarino A. A pharmacological perspective of chloroquine in SARS-CoV-2 infection: an old drug for the fight against a new coronavirus. Int J Antimicrob Agents. 2020;56 doi: 10.1016/j.ijantimicag.2020.106078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maisonnasse P, Guedj J, Contreras V, Behillil S, Solas C, Marlin R. Hydroxychloroquine use against SARS-CoV-2 infection in non-human primates. Nature. 2020;585:854–857. doi: 10.1038/s41586-020-2558-4. [DOI] [PubMed] [Google Scholar]
- 5.Yao X, Ye F, Zhang M, Cui C, Huang B, Niu P. In vitro antiviral activity and projection of optimized dosing design of hydroxychloroquine for the treatment of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Clin Infect Dis. 2020;71:732–739. doi: 10.1093/cid/ciaa237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lê MP, Peiffer-Smadja N, Guedj J, Náant N, Mentrá F, Ader F. Rationale of a loading dose initiation for hydroxychloroquine treatment in COVID-19 infection in the DisCoVeRy trial. J Antimicrob Chemother. 2020;75:2376–2380. doi: 10.1093/jac/dkaa191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Martin-Blondel G, Ruiz S, Murris M, Faguer S, Duhalde V, Eyvrard F. Hydroxychloroquine in COVID-19 patients: what still needs to be known about the kinetics. Clin Infect Dis. 2020 May 11 doi: 10.1093/cid/ciaa558. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perinel S, Launay M, É Botelho-Nevers, É Diconne, Louf-Durier A, Lachand R. Towards optimization of hydroxychloroquine dosing in intensive care unit COVID-19 patients. Clin Infect Dis. 2020;71:2227–2229. doi: 10.1093/cid/ciaa394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tett SE, Cutler DJ, Day RO, Brown KF. A dose-ranging study of the pharmacokinetics of hydroxy-chloroquine following intravenous administration to healthy volunteers. Br J Clin Pharmacol. 1988;26:303–313. doi: 10.1111/j.1365-2125.1988.tb05281.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lim HS, Im JS, Cho JY, Bae KS, Klein TA, Yeom JS. Pharmacokinetics of hydroxychloroquine and its clinical implications in chemoprophylaxis against malaria caused by Plasmodium vivax. Antimicrob Agents Chemother. 2009;53:1468–1475. doi: 10.1128/AAC.00339-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Bari MA. Chloroquine analogues in drug discovery: new directions of uses, mechanisms of actions and toxic manifestations from malaria to multifarious diseases. J Antimicrob Chemother. 2015;70:1608–1621. doi: 10.1093/jac/dkv018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nosál R, Ericsson O, Sjöqvist F, Durisová M. Distribution of chloroquine in human blood fractions. Methods Find Exp Clin Pharmacol. 1988;10:581–587. [PubMed] [Google Scholar]
- 13.Wang Y, Chen L. Lung tissue distribution of drugs as a key factor for COVID-19 treatment. Br J Pharmacol. 2020;177:4995–4996. doi: 10.1111/bph.15102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mouton JW, Theuretzbacher U, Craig WA, Tulkens PM, Derendorf H, Cars O. Tissue concentrations: do we ever learn? J Antimicrob Chemother. 2008;61:235–237. doi: 10.1093/jac/dkm476. [DOI] [PubMed] [Google Scholar]
- 15.Mimoz O, Rolland D, Adoun M, Marchand S, Breilh D, Brumpt I. Steady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12 mg/kg per day in patients with ventilator-associated pneumonia. Intensive Care Med. 2006;32:775–779. doi: 10.1007/s00134-006-0136-3. [DOI] [PubMed] [Google Scholar]
- 16.Boselli E, Breilh D, Saux MC, Gordien JB, Allaouchiche B. Pharmacokinetics and lung concentrations of ertapenem in patients with ventilator-associated pneumonia. Intensive Care Med. 2006;32:2059–2062. doi: 10.1007/s00134-006-0401-5. [DOI] [PubMed] [Google Scholar]
- 17.Kiem S, Schentag JJ. Interpretation of antibiotic concentration ratios measured in epithelial lining fluid. Antimicrob Agents Chemother. 2008;52:24–36. doi: 10.1128/AAC.00133-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morita S, Takahashi T, Yoshida Y, Yokota N. Population pharmacokinetics of hydroxychloroquine in Japanese patients with cutaneous or systemic lupus erythematosus. Ther Drug Monit. 2016;38:259–267. doi: 10.1097/FTD.0000000000000261. [DOI] [PubMed] [Google Scholar]
- 19.Chhonker YS, Sleightholm RL, Li J, Oupický D, Murry DJ. Simultaneous quantitation of hydroxychloroquine and its metabolites in mouse blood and tissues using LC-ESI–MS/MS: an application for pharmacokinetic studies. J Chromatogr B Analyt Technol Biomed Life Sci. 2018;1072:320–327. doi: 10.1016/j.jchromb.2017.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Iwen PC, Stiles KL, Pentella MA. Safety considerations in the laboratory testing of specimens suspected or known to contain the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Am J Clin Pathol. 2020;153:567–570. doi: 10.1093/ajcp/aqaa047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brinc D, Chan MK, Venner AA, Pasic MD, Colantonio D, Kyriakopolou L. Long-term stability of biochemical markers in pediatric serum specimens stored at –80 °C: a CALIPER Substudy. Clin Biochem. 2012;45:816–826. doi: 10.1016/j.clinbiochem.2012.03.029. [DOI] [PubMed] [Google Scholar]
- 22.Boselli E, Breilh D, Cannesson M, Xuereb F, Rimmele T, Chassard D. Steady-state plasma and intrapulmonary concentrations of piperacillin/tazobactam 4 g/0.5 g administered to critically ill patients with severe nosocomial pneumonia. Intensive Care Med. 2004;30:976–979. doi: 10.1007/s00134-004-2222-8. [DOI] [PubMed] [Google Scholar]
- 23.Rodvold KA, George JM, Yoo L. Penetration of anti-infective agents into pulmonary epithelial lining fluid: focus on antibacterial agents. Clin Pharmacokinet. 2011;50:637–664. doi: 10.2165/11594090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 24.Toutain PL, Bousquet-Melou A. Plasma terminal half-life. J Vet Pharmacol Ther. 2004;27:427–439. doi: 10.1111/j.1365-2885.2004.00600.x. [DOI] [PubMed] [Google Scholar]
- 25.Bao L, Deng W, Huang B, Gao H, Liu J, Ren L. The pathogenicity of SARS-CoV-2 in hACE2 transgenic mice. Nature. 2020;583:830–833. doi: 10.1038/s41586-020-2312-y. [DOI] [PubMed] [Google Scholar]
- 26.Inciardi RM, Lupi L, Zaccone G, Italia L, Raffo M, Tomasoni D. Cardiac involvement in a patient with coronavirus disease 2019 (COVID-19) JAMA Cardiol. 2020;5:819–824. doi: 10.1001/jamacardio.2020.1096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Devaux CA, Rolain JM, Colson P, Raoult D. New insights on the antiviral effects of chloroquine against coronavirus: what to expect for COVID-19? Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gladue RP, Bright GM, Isaacson RE, Newborg MF. In vitro and in vivo uptake of azithromycin (CP-62,993) by phagocytic cells: possible mechanism of delivery and release at sites of infection. Antimicrob Agents Chemother. 1989;33:277–282. doi: 10.1128/aac.33.3.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Horby P, Mafham M, Linsell L, Bell JL, Staplin N. Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med. 2020;383:2030–2040. doi: 10.1056/NEJMoa2022926. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.