Abstract
In patients coinfected with SARS-CoV-2 and HBV, liver injury was common. However, the interactions between SARS-CoV-2 and HBV coinfection remained unknown. Sixty-seven COVID-19 patients from the previous cohort were enrolled and classified into 2 groups (7 with HBsAg+ and 60 with HBsAg-). The association of HBV- and SARS-CoV-2-related markers were analyzed. During the acute course of SARS-CoV-2 infection, markers of HBV replication did not extensively fluctuate during SARS-CoV-2 infection. Coinfection with HBV did not extend the viral shedding cycle or incubation periods of SARS-CoV-2. Effects of SARS-CoV-2 on the dynamics of chronic HBV infection seemed not apparent. SARS-CoV-2 infection would not be the source of HBV reactivation in these individuals.
Keywords: SARS-CoV-2, COVID-19, HBV, Chronic hepatitis B, Liver injury
1. Introduction
Elevated aminotransferases and bilirubin were common in COVID-19 patients (Hao et al., 2020; Fu et al., 2020) and typical characteristics of patients with chronic hepatitis B(CHB). In patients coinfected with SARS-CoV-2 and HBV, Zou et al. reported a proportion of 27.62% with elevated liver function tests (Zou et al., 2020), which were similar to SARS-CoV-2 infection alone (Huang et al., 2020). However, these reports failed to focus on the direct interactions between SARS-CoV-2 and HBV coinfection. Especially, to what degree the viral kinetics, immune response, or natural history of the two viruses and their impact on disease progression would be influenced remained unknown.
2. Methods
Sixty-seven COVID-19 patients from our previous prospective cohort (Tan et al., 2020) were enrolled in this study. According to the status of HBsAg, they were classified as HBsAg + group (n = 7) and HBsAg- group (n = 60). COVID-19 was confirmed by real-time PCR assay. Clinical and laboratory data were collected on day 1, 4, 7, 14, 18, 21, 28, if available, after admission and at discharge. All 7 HBsAg + COVID-19 patients had reported chronic HBV infection (anti-virus treatment-free) for more than 20 years with normal liver function tests always, except that patient 37 developed HBV-related decompensation liver cirrhosis 3 years ago.
3. Results
The basic characteristics were described in Table S1. Briefly, there were 3 males and 4 females enrolled, including 1 critical case, 3 severe cases, and 3 mild/moderate cases, with the median age of 57 yrs (39–70). In the HBsAg + group, 6 of them were HBeAg-negative chronic infection based on EASL recommendation (6) and 1 of them were HBV-related cirrhosis There were no significant differences in demographic and epidemiological characteristics between the two groups, except median CRP levels. But there was no difference in the rate of patients with CRP elevation. Besides, there were no significant differences in the clinical severities, duration of hospitalization, incubation periods, and treatment response, either. At admission, levels of alanine transaminase (ALT) in 17 (25.4%) cases were over 50IU/L, while 28.4% had a TBil level over 17.1 μmol/L. However, no significant difference was detected between patients with HBsAg positive and negative (Table S1). At discharge, levels of ALT and TBil decreased to 32.3 ± 21.9U/L and 13.3 ± 6.7 μmol/L, but there were still about 15% of patients with abnormal liver indices. Likewise, there was no difference in clinical indices between the two groups at discharge (Table S2). Extreme values (peak or trough values) of each index were also compared, but no significant differences were detected (Table S3).
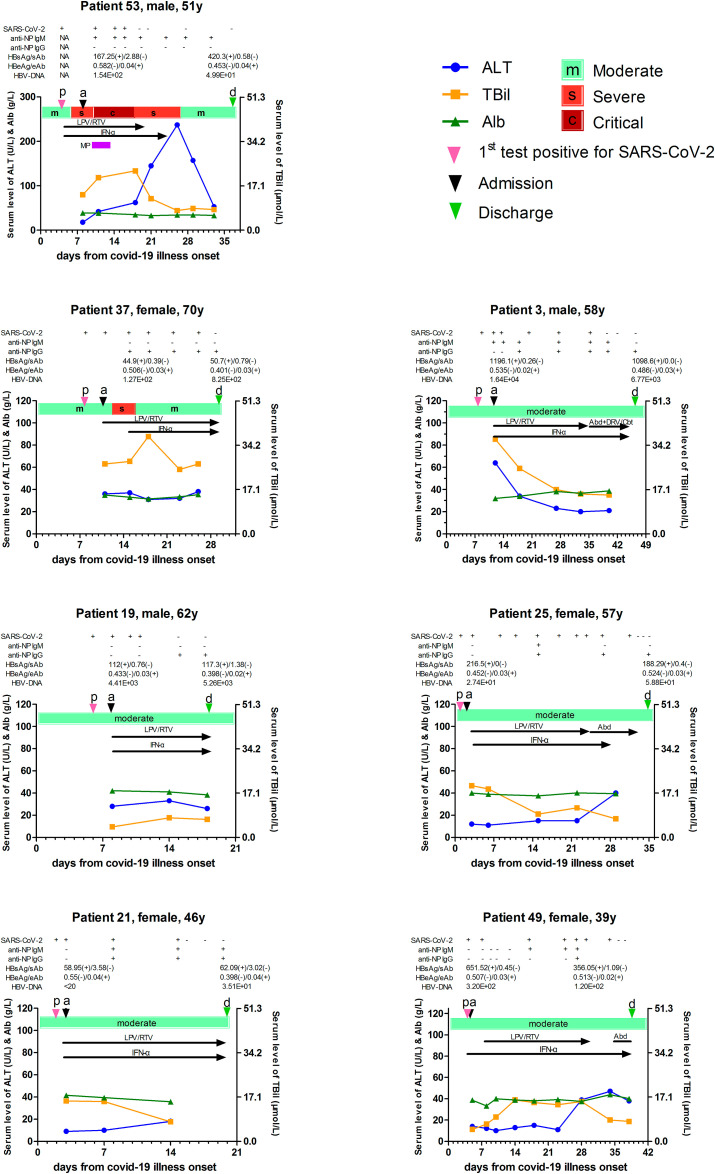
HBV-related markers were measured at both admission and discharge, but there were no significant differences in all markers, including HBsAg, HBsAb, HBeAg, HBeAb, HBcAb, and HBV-DNA (Table S4) at the beginning of and after recovery from COVID-19 for chronic hepatitis B (CHB) cases. Apart from that, sequential serum levels of HBV- and SARS-CoV-2-related parameters along with hepatic enzyme markers were analyzed in each of the 7 patients (Fig. 1 ). Firstly, there were no obvious changes in the serum level of HBsAg/Ab, HBeAg/Ab, or HBV-DNA viral load been observed during the course of acute SARS-CoV-2 infection and/or the anti-nucleocapsid protein (NP) antibody development in these 7 patients. Secondly, serum levels of ALT and TBil in CHB patients did not change before and after hospitalization either. Particularly, patient 53 presented a liver injury since day 18, which was considered just as the side-effect of lopinavir/ritonavir (LPV/RTV), and ALT decreased to normal after LPV/RTV withdrawal. Patient 37 had an abnormal serum TBil level, which we thought was attributed to HBV-related decompensated liver cirrhosis for more than 3 years. Thirdly, our results showed that the administration of antiviral drugs, like LPV/RTV, arbidol, and interferon-α 1b, which were prescribed by clinicians attempting to inhibit SARS-CoV-2 in the initial stage, seemed to have no affection on the replication of HBV. The other way round, the pre-existing HBV in the host body seemed to have no effect on COVID-19 progression, anti-NP antibody development, the intensity of anti-NP response, and even the liver injury after acute SARS-CoV-2 infection (Table 1 ). All 67 patients were discharged from the hospital with recovery.
Fig. 1.
Sequential SARS-CoV-2- and HBV-related parameters along with serum hepatic enzyme markers for 7 chronic hepatitis B patients after acute new coronavirus infectious. The color bar shows the severity of pneumonia from moderate, severe, to critical. Black arrows indicate the administration of antiviral drugs against SARS-CoV-2. HBV DNA was determined by COBAS Amplicor monitor test (Roche Molecular Systems, Branchburg, NJ), meanwhile, the HBsAg, anti-HBs, HBeAg, and anti-HBe were detected quantitatively by chemiluminescent immunoassay (Abbott Laboratory, Chicago, IL). ALT, alanine transaminase; TBil, total bilirubin; Alb, Albumin; NP, nucleocapsid protein; LPV/RTV, lopinavir/ritonavir; IFN-α 1b, interferon-α 1b; MP, methylprednisolone; Abd, arbidol; DRV/Cbt, darunavir/cobicistat.
Table 1.
The COVID-19 progression, the intensity of anti-NP response, and liver injury after acute SARS-CoV-2 infection in patients with or without chronic hepatitis B.
| All patients (n = 67) | COVID-19 patients with HBsAg positive (n = 7) | COVID-19 patients without HBV (n = 60) | p-value | |
|---|---|---|---|---|
| HBsAg positive | 7 (10.4) | 7 (100.0) | 0 | – |
| HBeAg positive | 0 | 0 | 0 | – |
| Comorbidity besides Hepatitis B | 21/67 (31.3) | 2/7 (28.6) | 19/60 (31.7) | >0.999 |
| Pulmonary disease † | 3 (4.5) | 1 (14.3) | 2 (3.3) | |
| Diabetes and/or Hypertension | 15 (22.3) | 1 (14.3) | 14 (23.3) | |
| Hyperlipemia and/or CHD | 3 (4.5) | 0 | 3 (5.0) | |
| Liver injury at admission # | 5/67 (7.5) | 0 | 5/60 (8.3) | >0.999 |
| Liver injury admission & during hospitalization | 19/67 (28.4) | 3/7 (42.9) | 16/60 (26.7) | 0.395 |
| Liver injury type # | 0.432 | |||
| Hepatocellular | 3/67 (4.5) | 1/7 (14.3) | 2/60 (3.3) | |
| Ductular | 11/67 (16.4) | 2/7 (28.6) | 9/60 (15.0) | |
| Mix | 5/67 (7.5) | 0 | 5/60 (8.3) | |
| COVID-19 progression after admission | 14/67 (20.9) | 2/7 (28.6) | 12/60 (20.0) | 0.63 |
| COVID-19 stable after admission | 53/67 (79.1) | 5/7 (71.4) | 48/60 (80.0) | 0.63 |
| Shedding time of SARS-CoV-2, days* | 25.0 ± 9.4 | 27.1 ± 9.0 | 24.7 ± 9.5 | 0.52 |
| anti-NP-IgM development | 28/58 (48.3) | 4/7 (57.1) | 24/51 (47.1) | 0.701 |
| weak-response | 10/58 (17.2) | 1/7 (14.3) | 9/51 (17.6) | >0.999 |
| strong-response | 18/58 (31.0) | 3/7 (42.9) | 15/51 (29.4) | 0.665 |
| Days of anti-NP-IgM first detectable § | 12.3 ± 4.4 | 12.5 ± 3.7 | 12.2 ± 4.6 | 0.909 |
| anti-NP-IgG development | 45/54 (83.3) | 6/7 (85.7) | 39/47 (83.0) | >0.999 |
| weak-response | 33/54 (61.1) | 4/7 (57.1) | 29/47 (61.7) | >0.999 |
| strong-response | 12/54 (22.2) | 2/7 (28.6) | 10/47 (21.3) | 0.645 |
| Days of anti-NP-IgG first detectable § | 14.3 ± 4.9 | 15.8 ± 6.2 | 14.0 ± 4.7 | 0.405 |
| Duration of Hospitalization, days | 21.0 ± 9.1 | 25.0 ± 10.7 | 20.6 ± 8.9 | 0.228 |
| COVID-19 course, days | 27.6 ± 9.2 | 32.0 ± 10.4 | 27.0 ± 9.0 | 0.178 |
| Discharge | 67/67 (100.0) | 7/7 (100.0) | 60/60 (100.0) | >0.999 |
† Includes one patient with bronchiectasis and chronic bronchitis, one patient with pulmonary tuberculosis, and one patient with asthma. CHD, coronary heart disease. # In this study, we defined ALT and/or AST more than 3 times of the upper limit units (ULN), GGT, and/or TBIL more than 2 × ULN as liver injury. ALT and/or AST over 3 × ULN were classified as hepatocellular type, GGT and/or TBIL over 2 × ULN as ductular type, while combined with ALT and/or AST over 3 × ULN and GGT and/or TBIL over 2 × ULN as mix type. * Virus shedding in any type of sample, including nasopharyngeal swab, sputum, and stool. § Days from symptom onset. Data are mean ± standard deviation or n/N (%), where N is the total number of patients with available data.
4. Discussion
In this study, we reported the sequential levels of HBV-related markers during the acute infection of SARS-CoV-2. Previous studies on patients coinfected with SARS-CoV-2 and HBV have shown that the existence of coinfection could not aggravate the liver injury or extend the duration of hospitalization compared with HBV infection alone (Chen et al., 2020; Liu et al., 2020). However, these studies failed to depict levels of all HBV-related markers during the infection and clearance of SARS-CoV-2 infection. In this study, although just 7 cases were enrolled, we described the intact dynamics of these markers, indicating that SARS-CoV-2 infection had a limited influence on HBV-DNA replication or the natural history of HBV infection. Firstly, the quantitative levels of HBsAg/Ab, HBeAg/Ab, and HBV-DNA did not extensively fluctuate during the infection or clearance of SARS-CoV-2, suggesting that SARS-CoV-2 had no impact on HBV kinetics. Secondly, the coinfection of SARS-CoV-2 did not trigger the reactivation or the seroconversion of chronic hepatitis B, which is also reported in Rodríguez-Tajes S et al.’s study (Rodríguez-Tajes Sergio et al., 2020). Consequently, SARS-CoV-2 infection would not be the source of HBV reactivation in these individuals. On the other hand, the existence of HBV did not influence SARS-CoV-2, either. Virologically, coinfection with HBV did not extend the viral shedding cycle or incubation periods of SARS-CoV-2 infection. Clinically, the coinfection of HBV did not increase the severity of diseases or duration of hospitalization. At discharge, no significant differences could be detected between the two groups in disease recovery, either. Besides, it has been reported that HBV antiviral therapy had a limited effect on COVID-19 incidence and outcomes (Sabela et al., 2020). From both sides, we discussed the interactions between HBV and SARS-CoV-2 infection.
Apparently, hepatic impairment could be detected in some COVID-19 patients. But we thought it was more likely to be the consequence of illness severity, rather than the direct cytotoxic effect of SARS-CoV-2 on the liver. Consistent with our conclusions, previous larger sample studies also found that there was no significant difference in liver enzymes between coinfection and HBV infection alone (7,8). The elevations of the liver enzyme in COVID-19 patients could also happen in many respiratory viruses infection, which might be related to immune interactions involving systematic cytokine storms (Adams and Hubscher, 2006). Apart from that, sarcolysis during acute viral infection (Wang and Medzhitov, 2019) and drug-induced liver injury (Marta et al., 2020) could also explain the elevation of liver enzymes.
However, it's undeniable that this is a preliminary study enrolling just 7 patients coinfected with SARS-CoV-2 and HBV in a single center. Although detailed patients' information was collected, retrospective design restricted the further analysis. For example, only 1 out of 7 patients had a history of liver cirrhosis, and COVID-19 is reported to be associated with liver function deterioration and elevated mortality in patients with cirrhosis and COVID-19 (Massimo et al., 2020). As a result, we could hardly know whether coinfection had a different story in patients with liver cirrhosis in our study. Consequently, further large-scale studies should be conducted to reveal the exact interactions between SARS-CoV-2 and HBV coinfection.
Above all, our study suggested that the effects of SARS-CoV-2 on the dynamics of chronic HBV infection seemed not apparent. SARS-CoV-2 infection would not be the source of HBV reactivation in these individuals. More attention should be paid to viral control, immune modulation, and reasonable medication in the therapy of patients coinfected with HBV and SARS-CoV-2.
Specific author contributions
GD, WT, YC; Acquisition of data: ST, YD, YL, JZ, ZT, XH, XX, YZ, YG, WT, YC; Statistical analysis: WT; Interpretation of data: RY, WT, GD; Drafting and critical revision of manuscript: RY, WT, GD. All authors checked and approved the final draft of the letter.
Guarantor of the article
Wenting Tan, Ph.D., Yaokai Chen, Ph.D., and Guohong Deng, Ph.D.
Financial support
This work is supported by PLA Youth Talent Project 17QNP010, Chongqing Health Commission COVID-19 Project 2020NCPZX01, TMMU project 2020-2017-033, and the Chinese State Key Project Specialized for Infectious Diseases (2018ZX10302206). And we appreciate the supports of the Academy of Medical Sciences Newton International Fellowship (Tan W).
Declaration of competing interest
Potential competing interest: None to report.
Footnotes
Supplementary data related to this article can be found at https://doi.org/10.1016/j.virol.2020.11.012.
Appendix A. Supplementary data
The following is the supplementary data related to this article:
References
- Adams D.H., Hubscher S.G. Systemic viral infections and collateral damage in the liver. Am. J. Pathol. 2006;168:1057–1059. doi: 10.2353/ajpath.2006.051296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Liping, Huang Shaoping, Yang Jingmao, et al. Clinical characteristics in patients with SARS-CoV-2/HBV co-infection. J. Viral Hepat. 2020:1–4. doi: 10.1111/jvh.13362. 00. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu Y., Zhu R., Bai T. Clinical features of COVID-19-infected patients with elevated liver biochemistries: a multicenter, retrospective study. Hepatology. 2020 doi: 10.1002/hep.31446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao S.R., Zhang S.Y., Lian J.S., et al. Liver enzyme elevation in coronavirus disease 2019: a multicenter, retrospective, cross-sectional study. Am. J. Gastroenterol. 2020;115:1075–1083. doi: 10.14309/ajg.0000000000000717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Jiaye, Wang Tingyan, Cai Qingxian, et al. Longitudinal changes of liver function and hepatitis B reactivation in COVID-19 patients with pre-existing chronic hepatitis B virus infection. Hepatol. Res. 2020 doi: 10.1111/hepr.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marta Colaneri, Pietro Valsecchi, Luciano Perotti, et al. Running out of bullets: the challenging management of acute hepatitis and SARS-COV-2 from the SMatteo COvid19 Registry (SMACORE) Liver Int. 2020;40:2655–2659. doi: 10.1111/liv.14609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massimo Iavarone, Roberta D'Ambrosio, Soria Alessandro, et al. High rates of 30-day mortality in patients with cirrhosis and COVID-19. J. Hepatol. 2020;73:1063–1071. doi: 10.1016/j.jhep.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Tajes Sergio, Anna Miralpeix, Costa Josep, et al. Low risk of hepatitis B reactivation in patients with severe COVID-19 who receive immunosuppressive therapy. J. Viral Hepat. 2020 doi: 10.1111/jvh.13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabela Lens, Mireia Miquel, Beatriz Mateos-Muñoz, et al. SARS-CoV-2 in patients on antiviral HBV and HCV therapy in Spain. J. Hepatol. 2020;73:1262–1263. doi: 10.1016/j.jhep.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan W., Lu Y., Zhang J., et al. Viral kinetics and antibody responses in patients with COVID-19. MedRxiv. 2020 doi: 10.1101/2020.03.24.20042382. [DOI] [Google Scholar]
- Wang A., Medzhitov R. Not the usual suspect: type I interferon-responsive T cells drive infection-induced cachexia. Nat. Immunol. 2019;20:666–667. doi: 10.1038/s41590-019-0374-5. [DOI] [PubMed] [Google Scholar]
- Zou X., Fang M., Li S., et al. Characteristics of liver function in patients with SARS-CoV-2 and chronic HBV co-infection. Clin. Gastroenterol. Hepatol. 2020 doi: 10.1016/j.cgh.2020.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.