Abstract
Objectives
The objective of the study is the identification of racial differences in characteristics and comorbidities in patients hospitalized for COVID-19 and the impact on outcomes.
Study design
The study design is a retrospective observational study.
Methods
Data for all patients admitted to seven community hospitals in Michigan, United States, with polymerase chain reaction confirmed diagnosis of COVID-19 from March 10 to April 15, 2020 were analyzed. The primary outcomes of racial disparity in inpatient mortality and intubation were analyzed using descriptive statistics and multivariate regression models.
Results
The study included 336 Black and 408 White patients. Black patients were younger (62.9 ± 15.0 years vs 71.8 ± 16.4, P < .001), had a higher mean body mass index (32.4 ± 8.6 kg/m2 vs 28.8 ± 7.5, P < .001), had higher prevalence of diabetes (136/336 vs 130/408, P = .02), and presented later (6.6 ± 5.3 days after symptom onset vs. 5.4 ± 5.4, P = .006) compared with White patients. Younger Black patients had a higher prevalence of obesity (age <65 years, 69.9%) than older Black patients (age >65 years, 39.2%) and younger White patients (age < 65, 55.1%). Intubation did not reach statistical significance for racial difference (Black patients 61/335 vs. 54/406, P = .08). Mortality was not higher in Black patients (65/335 vs. 142/406 in White patients, odds ratio 0.61, 95% confidence interval: 0.37 to 0.99, 2-sided P = .05) in multivariate analysis, accounting for other risk factors associated with mortality.
Conclusions
Higher prevalence of obesity and diabetes in young Black populations may be the critical factor driving disproportionate COVID-19 hospitalizations in Black populations. Hospitalized Black patients do not have worse outcomes compared with White patients.
Keywords: COVID-19, Racial disparities, Hospitalization, Outcomes, Diabetes, Obesity
Introduction
Coronavirus disease 2019 (COVID-19) caused by the severe acute respiratory syndrome coronavirus-2 affects Black populations disproportionately.1 The Centers for Disease Control (CDC) reported over-representation of Black patients with COVID-19 (18% Black population accounting for 33% of admissions).2 Yancy1 opined that the reason for COVID-19's disproportionate impact on Black populations was socio-economic disparities, and higher rates of obesity, diabetes, cardiovascular disease, and hypertension. Historically Black patients have had the highest age-specific mortality in respiratory infections such as pneumonia and influenza.3 A study has shown that although United States (US) Black men are twice as likely to die of prostate cancer than White men, Black men have similar outcomes when provided equal access to care.4 The outcomes for Black and White renal transplant patients in Canada are similar, unlike the US, probably due to more equitable health-care delivery.5 In this study, we looked at the risk factors that may cause disproportionate hospitalizations, and we compared outcomes of Black patients to White patients when provided with similar care for COVID-19.
Methods
Hospital service area population data selection
Hospital service areas (ZIP codes from which most Medicare residents were hospitalized to a particular hospital), as defined by Dartmouth Atlas, were used. The corresponding ZIP code tabulation areas (ZCTAs) and the demographic details of the population in the 2010 census were obtained from data.census.gov (2010 DEC summary file 1 Table 12 and its subparts B, D, H, and I). Population data for Michigan for the year 2010 and the estimates for 2018 were obtained from the American Community Survey from data.census.gov (Supplementary Data Table 1).
The percent change in population for each race was calculated from 2000 to 2018. We assumed that the rate of change remained the same from 2018 to 2020 and calculated the net change by race from 2010 to 2020 to be: White population −1.31%, Black population −0.75%, other races +27.06%. We assumed the change was similar across Michigan, across all ages and for both genders and adjusted each ZCTA population by the percentages mentioned previously to obtain the current estimated demographic mix for each hospital service area (Supplementary Data Table 2).
Study population data collection
We reviewed electronic health records (EHRs) for all patients above the age of 15 admitted to one of the affiliated (two western Michigan (WEMI) and five southeastern Michigan [SEMI]) hospitals with polymerase chain reaction confirmed diagnosis of COVID-19 from March 10, 2020 to April 15, 2020. All hospitals share the same software for EHRs and an overall data collection tool were approved by the Institutional Review Board. Data were collected through manual chart review by the medical students and residents involved in the project and revised by the senior authors on this manuscript, based on the study predetermined patient inclusion and exclusion criteria and clinical definitions.
Seven hundred ninety-nine records (63 from WEMI and 736 from SEMI) of patients who died or were discharged by April 22, 2020 were analyzed, with 408 (51.1%) White, 336 (42.1%) Black, and 55 (6.8%) of other races.
Study outcomes
The primary outcomes were racial disparity in inpatient mortality and intubation. Comorbidities previously reported to influence mortality in patients with COVID-19 patients were studied. These included body mass index (BMI),6 smoking,7 , 8 congestive heart failure (CHF),9 hypertension (HTN),10 chronic obstructive pulmonary disease (COPD),8 diabetes,11 glomerular filtration rate (GFR),12 neutrophil count and lymphocyte to neutrophil ratio.13 A patient was considered to have one of these comorbidities if it was documented in the problem list or past medical history of the patient's chart. Smoking (current or quit within six months) or alcohol (intake of more than six drinks per week) in the chart was considered a ‘Yes’ for that condition. GFR, neutrophil, and lymphocyte counts within 72 h of admission were used as admission laboratory values. Documentation of medication on the home medication list in the EHRs was considered a ‘Yes’ for aspirin and hydroxychloroquine. When no mention was made of symptom onset, the value was considered missing.
Data analysis
After the time from symptom onset to various chronological events (admission, intubation) were calculated in Excel, the data were uploaded to SPSS, version 25.0, where descriptive statistics and univariate analyses (chi-square, Fisher's exact test, Student's t-test, Pearson's correlation coefficient, and analysis of variance) were performed.
Factors presumed clinically relevant (e.g. age, gender, race, specific comorbid conditions, and so on) and additional factors reaching the level of statistical significance in univariate analysis were included in exploratory multivariate logistic regression models using stepwise regression. Based on this, the multivariate regression models for mortality and intubation include different covariates.
All 95% confidence intervals were narrow. Two-sided P values < .05 were considered statistically significant. The analyses were conducted on complete data sets.
Results
Comparing the demographics of population and patients
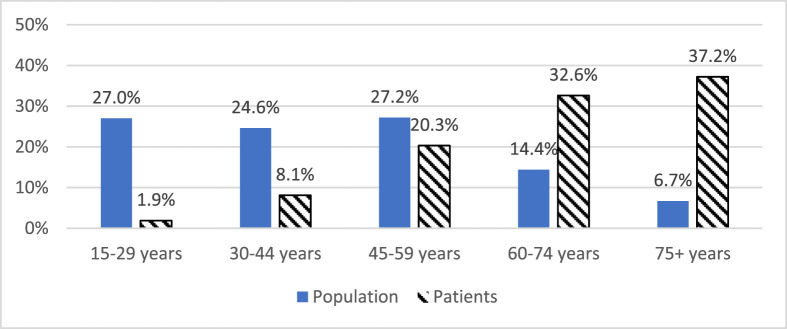
Younger patients were under-represented in the admissions compared with the hospital service area population, and older patients were over-represented in SEMI, WEMI, and all hospitals (P < .005 for all) (Fig. 1 ).
Fig. 1.
Age distribution for hospital service area population and admitted COVID-19 patients.
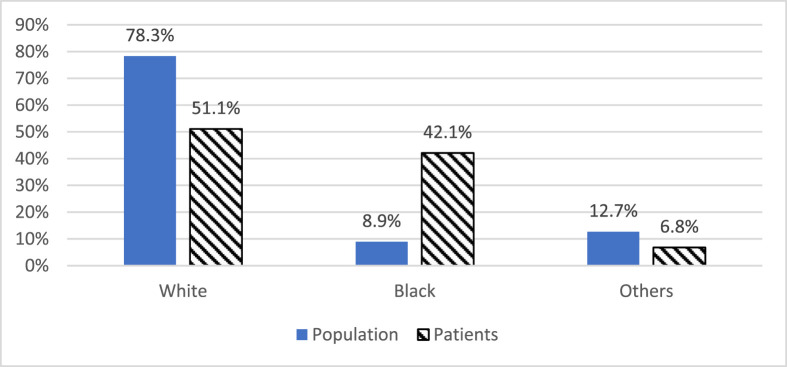
Population gender distribution was similar to patient gender distribution in SEMI (P = .77), WEMI (P = .80), and all hospitals (P = .70). The White race was under-represented, the Black race was over-represented, and other races were similar in SEMI hospitals, WEMI hospitals, and total hospitals (P < .001 for all) (Fig. 2 ). WEMI, western Michigan; SEMI, southeastern Michigan.
Fig. 2.
Race distribution for hospital service area population and admitted COVID-19 patients.
Racial disparities
Univariate analyses of the baseline characteristics for the two main racial groups (White – 50.8% vs. Black – 41.9%) are presented in Table 1 . White patients were on average older as compared with Black patients (71.8 vs. 62.9 years, P < .032) and presented to the hospital a day earlier (5.4 vs. 6.6 days, P < .006). There were also statistically significant differences between the two groups in regard to BMI, smoking status, and diabetes as comorbid condition.
Table 1.
Univariate analyses of main racial groups and risk factors.
| Variable | Black patients N = 336 (42.1%) |
White patients N = 408 (51.1%) |
P value |
|---|---|---|---|
| Age (years) – mean ± standard deviation (SD) | 62.9 ± 15.0 | 71.8 ± 16.4 | <.001∗ |
| Female gender – no. (%) | 174 (51.9) | 211 (52.0) | 1.00 |
| Body mass index (kg/m2) – mean ± standard deviation (SD) | 32.4 ± 8.6 | 28.8 ± 7.5 | <.001∗ |
| Alcohol – no. (%) | 8 (3.3) | 14 (4.6) | .52 |
| Smoking (current) – no. (%) | 21 (7.5) | 12 (3.6) | .03∗ |
| Congestive heart failure – no. (%) | 44 (13.1) | 59 (14.5) | .60 |
| Hypertension – no. (%) | 228 (68.1) | 262 (64.5) | .35 |
| Chronic obstructive pulmonary disease – no. (%) | 38 (11.3) | 64 (15.8) | .09 |
| Diabetes – no. (%) | 136 (40.6) | 130 (32.0) | .02∗ |
| Glomerular filtration rate (mL/min) – mean ± standard deviation (SD) | 60.04 ± 32.2 | 60.34 ± 31.4 | .90 |
| Neutrophil count per mm3 – mean ± standard deviation (SD) | 5.8 ± 3.5 | 6.0 ± 3.4 | .53 |
| Lymphocyte to neutrophil ratio multiplied by 100 – mean ± standard deviation (SD) | 29.2 ± 65.6 | 26.1 ± 73.5 | .57 |
| Aspirin – no. (%) | 97 (29.0) | 134 (33.0) | .27 |
| Hydroxychloroquine – no. (%) | 4 (1.2) | 2 (0.5) | .42 |
| Time from symptoms onset to admission (days) – mean ± standard deviation (SD) | 6.6 ± 5.3 | 5.4 ± 5.4 | .006∗ |
∗2-sided P < .05 statistically significant.
Risk factors associated with mortality
Univariate analyses of variables associated with mortality are presented in Supplementary Data Table 3. In the mortality model, Black patients were 40% less likely to die, and those with diabetes were 77% more likely to die. For each unit increase in GFR, patients were 2% less likely to die, and for each unit increase in neutrophils, patients were 16% more likely to die (Table 2 ).
Table 2.
Multivariate model predicting mortality.
| Variable | OR (95% CI) |
|---|---|
| Age (years) | 1.06 (1.04–1.08) |
| Black racea | 0.61 (0.37–0.99) |
| Other racea | 1.42 (0.58–3.49) |
| Body mass index (kg/m2) | 1.00 (0.97–1.04) |
| Congestive heart failure | 1.43 (0.75–2.74) |
| Hypertension | 0.70 (0.40–1.20) |
| Chronic obstructive pulmonary disease | 1.37 (0.76–2.47) |
| Diabetes | 1.77 (1.10–2.83) |
| Glomerular filtration rate (mL/min) | 0.98 (0.97–0.99) |
| Neutrophil count per mm3 | 1.16 (1.09–1.24) |
| Aspirin | 0.85 (0.53–1.35) |
| Time from symptoms onset to admission (days) | 0.97 (0.92–1.01) |
CI, confidence interval; OR, odds ratio.
The reference group is White race.
Risk factors associated with intubation
Univariate analyses of variables associated with intubation are presented in Supplementary Data Table 4. Females were 45% less likely to be intubated, those with diabetes were 57% more likely to be intubated, and for each unit increase in neutrophils, patients were 16% more likely to be intubated (Table 3 ).
Table 3.
Multivariate model predicting patient intubation.
| Variable | OR (95% CI) |
|---|---|
| Age (years) | 0.99 (0.98–1.01) |
| Female gendera | 0.55 (0.37–0.83) |
| Diabetes | 1.57 (1.04–2.37) |
| Neutrophil count per mm3 | 1.16 (1.10–1.22) |
CI, confidence interval; OR, odds ratio.
The reference group is male gender.
Racial disparities in obesity and diabetes
The rate of obesity (defined as BMI >30 kg/m2) was higher in younger patients under the age of 65 compared with older patients over the age of 65 in both White patients (65/118 = 55.1% vs. 76/273 = 27.8%, P < .001) and Black patients (121/173 = 69.9% vs. 60/153 = 39.2%, P < .001). The rate of obesity was lower in White patients under the age of 65 (55.1%) compared with Black patients under the age of 65 (69.9%), P = .013.
There was no association between the presence of diabetes in those under the age of 65 compared with those 65 and above in White patients (27% vs. 34.3%, P = .2). In contrast, Black patients under 65 had a lower rate of diabetes than Black patients 65 and above (34.9% vs. 46.9%, P = .03). White patients with diabetes had a higher mortality rate (43.8%) as compared to Black patients with diabetes (25.0%, P = .001). White patients with diabetes were older (73.0 ± 14.2 years) than Black patients with diabetes (65.6 ± 14.3 years) with P < .001.
Discussion
Black patients were hospitalized more than seven-fold compared with White patients (210 hospitalizations per 100,000 Black population to 29 per 100,000 for White population). Overall, 42.1% of hospitalized patients were Black, but the Black population was only 8.9% of the total population. Our findings from community hospitals are similar to those reported by Price-Haywood et al.14 in a metropolitan health system that showed Black patients are over-represented in hospitalizations. Similar to prior reports, those aged greater than 75 years constituted a significantly higher proportion of hospitalized patients than their presence in the service area.2 Older age was not a factor in disproportionate hospitalization in Black populations as Black patients (mean age: 62.9 years) requiring hospitalization were significantly younger than White patients (mean age: 71.8 years), confirming higher morbidity in Black populations.
Obesity has been identified as a risk factor for severe COVID-19 illness by CDC, with younger patients hospitalized for COVID-19 more likely to be obese.6 Obesity prevalence is higher in younger (age <64) populations compared with older populations and Black populations compared with White populations in Michigan and the US.15 , 16 A study on hospitalized patients in New Jersey reported a prevalence of obesity of 41.8% in Black patients and 29.3% in White patients which was slightly higher than the prevalence of obesity in the New Jersey Black population (37.6%) and White population (27.0%).17 , 18 In Michigan, the prevalence of obesity in the Black population was 39.9% and in the White population was 31.8%.15 In our COVID-19 hospitalized patients, the prevalence of obesity in Black patients was 55.5% and in the White population was 36.1%. Furthermore, younger Black patients had a higher prevalence of obesity (age <65, 69.9%) than older Black patients (age >65, 39.2%) and younger White patients (age <65, 55.1%). A higher prevalence of obesity in younger Black populations may be responsible for more younger Black patients being admitted to the hospital.
Although Black populations have a higher prevalence of HTN, CHF, diabetes, kidney disease, and a lower prevalence of COPD, we did not detect a significant difference in CHF, HTN, GFR, and COPD between Black and White patients.19, 20, 21, 22
CHF, HTN, kidney disease, and COPD may not play a role in the disparate increased hospitalization of Black patients with COVID-19. Hospitalized Black patients had a significantly higher prevalence of diabetes (40.6%) when compared with White patients (32.0%, P < .02) similar to that reported in other studies.14 The prevalence of diabetes in the Black population is 1.4 times higher than the White population in Michigan adults (8.7% in the White population vs. 12.6% in the Black population).23 Thus diabetes may be the other factor responsible for increased COVID-19 hospitalizations in Black communities.
Although a racial difference has not been reported in the general population in the use of tobacco, smoking prevalence was higher in hospitalized Black patients (7.5%) compared with White patients (3.6%, P = .03).24 Smoking has been reported to be a risk factor for disease progression in COVID-19.7 Black populations who smoke may be more vulnerable to COVID-19 than White populations who smoke.
Black patients (19.4%) had lower mortality than White patients (35.0%) in univariate (P < .001) as well as multivariate analysis (OR 0.61, 95% confidence interval [CI]: 0.37 to 0.99, 2-sided P = .05). Univariate predictors of mortality, namely CHF, HTN, COPD, GFR, total neutrophil count, and aspirin use at home, were not different in the Black patients compared with the White patients.8 , 10 Older age was a predictor of mortality in univariate and multivariate analyses similar to prior reports.9 Black patients were younger than White patients, and this may have provided a better chance of survival in our patient population. Another study reported similar findings with Black race not being associated with in hospital mortality.14
Patients who died had a significantly lower BMI compared to those who survived, in univariate analysis, but this difference was not statistically significant in multivariate analysis. The ‘obesity paradox,’ where obese patients have better outcomes than non-obese patients, has been reported in several conditions, including community-acquired pneumonia.25, 26, 27, 28, 29 The higher BMI of Black patients (mean 32.4 kg/m2 vs. 28.8 kg/m2 for White patients) may have provided survival benefit.
Diabetes was statistically significant in multivariate analysis (independent of obesity) as a predictor of mortality (77% more likely to die), similar to prior reports.11 Although Black patients had a higher prevalence of diabetes (40.6% vs. 32.0%, P = .02), the mortality was not higher in Black diabetic patients (25.0%) compared with White diabetic patients (43.8%, P = .001). The higher mortality observed in White diabetic patients compared with Black diabetic patients was likely due to White diabetic patients being older than Black diabetic patients.
Shorter time from onset of symptoms to admission was associated with mortality on univariate analysis. However, it did not reach significance in multivariate analysis, likely indicating a rapid progression of the disease. White patients had a significantly shorter duration from symptom onset to admission compared to Black patients (mean 5.4 vs. 6.6 days), which could either reflect a faster progression of disease in the White population due to older age or later presentation to the hospital in the younger Black population. The late presentation could be due to a lower level of health insurance coverage and poorer socio-economic status, leading to hesitancy to seek care.30
There were no racial disparities in length of stay and time to intubation. The decision to intubate was undertaken on a case by case basis at each hospital by the intensivist managing that case. At the time of the study period, non-invasive positive pressure ventilation was being avoided due to fear of aerosol generation and patients were intubated early if they were hypoxic on 15 L per minute of oxygen.
The univariate mortality analysis for intubated patients failed to reach statistical significance for Black patients (mortality 62.3%) compared with White patients (79.6%). Black patients had a longer length of intubation compared with White patients (mean five days vs. seven days in survivors), but this difference was not significant. Although BMI did not predict intubation, a higher BMI was significantly correlated with longer length of intubation in patients who survived (r = 0.396, P = .014). Our Black patients had significantly higher BMI and required longer periods of intubation with lower mortality in intubated patients when compared with White patients (although the difference failed to reach a significant level) similar to that reported in a prior meta-analysis of non-COVID-19 mechanically ventilated adults in intensive care units which reported that higher BMI is associated with a longer duration of mechanical ventilation but lower mortality.31
Some incidental findings that did not have a role in racial disparities merit further discussion. Although there was no gender difference in hospitalizations or mortality, females were 45% less likely to be intubated (95% CI: 0.37 to 0.83, 2-sided P = .005), similar to that reported in Wuhan, where only 35% of intubated patients were females.32 A study of cancer patients with COVID-19 disease found that prostate cancer patients treated with androgen deprivation therapy were four-fold less likely to be infected with COVID-19 compared with prostate cancer not treated with androgen deprivation.33 Thus androgens might be associated with a higher incidence of severe disease (intubation) in males, and further study of this disparity may reveal strategies to decrease intubation.
A lower GFR was significantly associated with mortality in the multivariate analysis, similar to prior reports associating elevated serum creatinine with in-hospital mortality.12 Our study is limited in distinguishing between acute kidney injury and chronic kidney disease. A higher neutrophil count was associated with a higher risk for death and intubation in univariate and multivariate analyses similar to prior reports.13 The ability of neutrophils to form neutrophil extracellular traps has been proposed as a mechanism for organ damage and mortality in COVID-19.34 As previous studies have shown, GFR and total neutrophil count can be used as markers for disease severity,12 , 13 , 34 , 35 and therefore we included them in our analyses. A meta-analysis by Fend et al.35 has shown that multiple inflammatory markers such as White blood cell, lymphocyte, procalcitonin, C-reaction protein, neutrophil count and neutrophil-lymphocyte ratio were used to assess COVID-19 disease progression in different studies, and particularly the neutrophil-lymphocyte ratio could be used to identify at an earlier stage high-risk patients diagnosed with COVID-19.
Taking aspirin at home was associated with higher mortality in univariate but not multivariate analysis. Cardiovascular and cerebrovascular disease combined have been reported to increase mortality in COVID-19.9 , 36 The World Health Organization has not found any harm with non-steroidal anti-inflammatory drugs.37 Increased mortality observed in our patients is likely due to aspirin being an innocent bystander, associated with the presence of atherosclerotic cardiovascular disease. The increased mortality associated with aspirin is less likely to be due to the activation of 5′adenosine monophosphate-activated protein kinase (AMPK), which is anti-inflammatory and immunosuppressive.38 Only six patients of 799 were taking hydroxychloroquine as a home medication, and the sample size was not large enough to meaningfully analyze for mortality or intubation. We did not have the data on the total number of patients taking hydroxychloroquine at home in our service areas to investigate the severity of COVID-19 infection in patients on chronic hydroxychloroquine.
The limitations of our study include a small sample size, which could have resulted in some of the factors not reaching statistical significance. The hospital service area population was estimated, as the 2020 census data is not available yet. The authors believe that the variation will be small enough to keep the baseline population data valid. Covariates with a potential confounding or mediator effect such as insurance status, having a primary care physician, area-level access to health-care facilities and social history data were not recorded for many patients, and therefore they were excluded from analyses. Our patient population had a higher burden of all risk factors associated with mortality (older age and comorbidities) compared with other populations reported earlier and hence experienced a higher rate of mortality. All the charts were reviewed manually, and some risk factors (coronary artery disease) were not collected. As multiple hospitals were involved, the laboratory tests sent at admission were different, and only comprehensive metabolic panel and complete blood count with differential were available consistently, limiting our ability to study other inflammatory markers.
We found that Black patients are disproportionately hospitalized with COVID-19, and hospitalized Black patients had higher prevalence of diabetes and obesity compared with White patients. In Black populations, younger patients are being affected and presenting later, likely due to socio-economic disparities in access to care. Aggressive public health preventive measures are needed to prevent the spread of COVID-19 in Black communities to decrease the rate of COVID-19 infection and hospitalization. Our study showed that when given an equal standard of care, Black patients did not suffer worse COVID-19 outcomes, compared with White patients.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.puhe.2020.11.021.
Author statements
Acknowledgments
We thank Fabian Fregoli, MD for his staunch support of academic medicine.
We thank Karen Hagglund, MS for the statistical analyses.
Ethical approval
This study received ethical approval from St Joseph Mercy Oakland Hospital, Pontiac, Michigan, United States and was approved by the Institutional Review Board.
Funding
None.
Competing interests
None declared.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Yancy C.W. COVID-19 and African Americans. JAMA. 2020;323(19):1891–1892. doi: 10.1001/jama.2020.6548. [DOI] [PubMed] [Google Scholar]
- 2.Garg S., Kim L., Whitaker M., O'Halloran A., Cummings C., Holstein R. Hospitalization rates and characteristics of patients hospitalized with laboratory-confirmed coronavirus disease 2019 - COVID-NET, 14 States. CDC. Weekly. 2020;69(15):458–464. doi: 10.15585/mmwr.mm6915e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hutchins S.S., Fiscella K., Levine R.S., Ompad D.C., McDonald M. Protection of racial/ethnic minority populations during an influenza pandemic. Am J Publ Health. 2009;99(Suppl 2):S261–S270. doi: 10.2105/AJPH.2009.161505. Suppl 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Riviere P., Luterstein E., Kumar A., Vitzthum L.K., Deka R., Sarkar R.R. Survival of African American and non-Hispanic white men with prostate cancer in an equal-access health care system. Cancer. 2020;126(8):1683–1690. doi: 10.1002/cncr.32666. [DOI] [PubMed] [Google Scholar]
- 5.Yeates K., Wiebe N., Gill J., Sima C., Schaubel D., Holland D. Similar outcomes among black and white renal allograft recipients. J Am Soc Nephrol. 2009;20(1):172–179. doi: 10.1681/ASN.2007070820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kass D.A., Duggal P., Cingolani O. Obesity could shift severe COVID-19 disease to younger ages. Lancet. 2020;395(10236):1544–1545. doi: 10.1016/S0140-6736(20)31024-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu W., Tao Z.W., Wang L., Yuan M.L., Liu K., Zhou L. Analysis of factors associated with disease outcomes in hospitalized patients with 2019 novel coronavirus disease. Chin Med J. 2020;133(9):1032–1038. doi: 10.1097/CM9.0000000000000775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alqahtani J.S., Oyelade T., Aldhahir A.M., Alghamdi S.M., Almebmadi M., Alqahtami A.S. Prevalence, severity and mortality associated with COPD and smoking in patients with COVID-19: a rapid systematic review and meta-analysis. PloS One. 2020;15(5) doi: 10.1371/journal.pone.0233147. e0233147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du R.H., Liang L.R., Yang C.Q., Wang W., Cao T.Z., Li M. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55(5):2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lippi G., Wong J., Henry B.M. Hypertension in patients with coronavirus disease 2019 (COVID-19): a pooled analysis. Pol Arch Intern Med. 2020;130(4):304–309. doi: 10.20452/pamw.15272. [DOI] [PubMed] [Google Scholar]
- 11.Bode B., Garrett V., Messler J., McFarland R., Crowe J., Booth R., Klonoff D.C. Glycemic characteristics and clinical outcomes of COVID-19 patients hospitalized in the United States. J Diabetes Sci Technol. 2020;14(4):813–821. doi: 10.1177/1932296820924469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cheng Y., Luo R., Wang K., Zhang M., Wang Z., Dong L. Kidney disease is associated with in-hospital death of patients with COVID-19. Kidney Int. 2020;97(5):829–838. doi: 10.1016/j.kint.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang D., Hu B., Hu C., Zhu F., Liu X., Zhang J. Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus-infected pneumonia in Wuhan, China. JAMA. 2020;323(11):1061–1069. doi: 10.1001/jama.2020.1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Price-Haywood E.G., Burton J., Fort D., Seoane L. Hospitalization and mortality among black patients and white patients with Covid-19. NEJM. 2020;382(26):2534–2543. doi: 10.1056/NEJMsa2011686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.The state of obesity in Michigan: adult obesity new data. 2020. https://stateofchildhoodobesity.org/states/mi/ [Google Scholar]
- 16.Differences in prevalence of obesity among black, white, and hispanic adults --- United States, 2006–2008. 2020. https://www.cdc.gov/mmwr/preview/mmwrhtml/mm5827a2.htm [PubMed] [Google Scholar]
- 17.Health indicator report of obesity among adults. 2020. https://www-doh.state.nj.us/doh-shad/indicator/view/Obese.race.html [Google Scholar]
- 18.Hossain M.A., Amin A., Paul A., Qaisar H., Akula M., Amirpour A. Recognizing obesity in adult hospitalized patients: a retrospective cohort study assessing rates of documentation and prevalence of obesity. J Clin Med. 2018;7(8):203. doi: 10.3390/jcm7080203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Graham G. Disparities in cardiovascular disease risk in the United States. Curr Cardiol Rev. 2015;11(3):238–245. doi: 10.2174/1573403X11666141122220003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cowie C.C., Harris M.I., Silverman R.E., Johnson E.W., Rust K.F. Effect of multiple risk factors on differences between blacks and whites in the prevalence of non-insulin-dependent diabetes mellitus in the United States. Am J Epidemiol. 1993;137(7):719–732. doi: 10.1093/oxfordjournals.aje.a116732. [DOI] [PubMed] [Google Scholar]
- 21.Assari S. Racial disparities in chronic kidney diseases in the United States; a pressing public health challenge with social, behavioral and medical causes. J Nephropharmacol. 2016;5(1):4–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Mamary A.J., Stewart J.I., Kinney G.L. Race and gender disparities are evident in COPD underdiagnoses across all severities of measured airflow obstruction. Chronic Obstr Pulm Dis. 2018;5(3):177–184. doi: 10.15326/jcopdf.5.3.2017.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Diabetes in Michigan updated June 2019. 2020. https://www.michigan.gov/documents/mdhhs/diabetes-in-Michigan-update-2019_658300_7.pdf [Google Scholar]
- 24.Current cigarette smoking among U.S. adults aged 18 years and older. 2020. https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm [Google Scholar]
- 25.Hainer V., Aldhoon-Hainerová I. Obesity paradox does exist. Diabetes Care. 2013;36(Suppl 2):S276–S281. doi: 10.2337/dcS13-2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gruberg L., Weissman N.J., Waksman R., Fuchs S., Deible R., Pinnow E.F. The impact of obesity on the short-term and long-term outcomes after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol. 2002;39(4):578–584. doi: 10.1016/s0735-1097(01)01802-2. [DOI] [PubMed] [Google Scholar]
- 27.Corrales-Medina V.F., Valayam J., Serpa J.A., Rueda A.M., Musher D.M. The obesity paradox in community-acquired bacterial pneumonia. Int J Infect Dis. 2011;15(1):e54–e57. doi: 10.1016/j.ijid.2010.09.011. [DOI] [PubMed] [Google Scholar]
- 28.King P., Mortensen E.M., Bollinger M., Restrepo M.I., Copeland L.A., Pugh M.J.V. Impact of obesity on outcomes for patients hospitalised with pneumonia. Eur Respir J. 2013;41(4):929–934. doi: 10.1183/09031936.00185211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Singanayagam A., Chalmers J.D. Obesity is associated with improved survival in community-acquired pneumonia. Eur Respir J. 2013;42(1):180–187. doi: 10.1183/09031936.00115312. [DOI] [PubMed] [Google Scholar]
- 30.National Research Council Panel on Race EaHiLL . The National Academies collection: reports funded by National Institutes of health. In: Bulatao R.A., Anderson N.B., editors. Understanding racial and ethnic differences in health in late life: a research agenda. National Academies Press (US)Copyright © 2004, National Academies; 2004. [PubMed] [Google Scholar]
- 31.Zhao Y., Li Z., Yang T., Wang M., Xi X. Is body mass index associated with outcomes of mechanically ventilated adult patients in intensive critical units? A systematic review and meta-analysis. PloS One. 2018;13(6) doi: 10.1371/journal.pone.0198669. e0198669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao W., Wang T., Jiang B., Gao F., Wang L., Zheng H. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth. 2020;125(1):e28–e37. doi: 10.1016/j.bja.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Montopoli M., Zumerle S., Vettor R., Rugge M., Zorzi M., Catapano C.V. Androgen-deprivation therapies for prostate cancer and risk of infection by SARS-CoV-2: a population-based study (N = 4532) Ann Oncol. 2020;31(8):1040–1045. doi: 10.1016/j.annonc.2020.04.479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Barnes B.J., Adrover J.M., Baxter-Stoltzfus A., Borczuk A., Cools-Lartique J., Crawford J.M. Targeting potential drivers of COVID-19: neutrophil extracellular traps. J Exp Med. 2020;217(6) doi: 10.1084/jem.20200652. e20200652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Feng X., Li S., Sun Q., Zhu J., Chen B., Xiong M. Immune-inflammatory parameters in COVID-19 cases: a systematic review and meta-analysis. Front Med. 2020;7:301. doi: 10.3389/fmed.2020.00301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lewis D.K.L. Harvard Health Publishing; 2020. How does cardiovascular disease increase the risk of severe illness and death from COVID-19?https://www.health.harvard.edu/blog/how-does-cardiovascular-disease-increase-the-risk-of-severe-illness-and-death-from-covid-19-2020040219401 [Google Scholar]
- 37.The use of non-steroidal anti-inflammatory drugs (NSAIDs) in patients with COVID-19. World Health Organization; 2020. https://www.who.int/news-room/commentaries/detail/the-use-of-non-steroidal-anti-inflammatory-drugs-(nsaids)-in-patients-with-covid-19 [Google Scholar]
- 38.Kim J., Yang G., Kim Y., Ha J. AMPK activators: mechanisms of action and physiological activities. Exp Mol Med. 2016;48(4):e224. doi: 10.1038/emm.2016.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.