Highlights
-
•
Few large series describe the clinical characteristics, outcomes and costs of COVID-19 patients in Western countries.
-
•
We report our experience with the first 1255 cases receiving anti-COVID-19 treatment at a Spanish hospital.
-
•
Prevalence of acute respiratory distress syndrome (ARDS) and mortality was high in the early days of the Spanish epidemic.
-
•
Elderly, diabetes, cardiovascular disease, O2Sat <90%, lymphocytopenia & high CRP were associated with increased risk of death.
-
•
Treatment costs are high (€0.44 million per 1000 hospitalised patients), attributable to use of tocilizumab for ARDS.
Keywords: SARS-CoV-2, COVID-19, Risk factors, Mortality, Costs, Spain
Abstract
Few large series describe the clinical characteristics, outcomes and costs of COVID-19 in Western countries. This cohort reports the first 1255 adult cases receiving anti-COVID-19 treatment at a Spanish hospital (1–24 March 2020). Treatment costs were calculated. A logistic regression model was used to explore risk factors on admission associated with ARDS. A bivariate Cox proportional hazard ratio (HR) model was employed to determine the HR between individual factors and death. We included 1255 patients (median age 65 years; 57.8% male), of which 92.3% required hospitalisation. The prevalence of hypertension, cardiovascular disease and diabetes mellitus (DM) was 45.1%, 31.4% and 19.9%, respectively. Lymphocytopenia (54.8%), elevated alanine aminotransferase (33.0%) and elevated lactate dehydrogenase (58.5%) were frequent. Overall, 36.7% of patients developed ARDS, 10.0% were admitted to an ICU and 21.3% died. The most frequent antiviral combinations were lopinavir/ritonavir plus hydroxychloroquine (44.2%), followed by triple therapy with interferon beta-1b (32.7%). Corticosteroids and tocilizumab were used in 25.3% and 12.9% of patients, respectively. Total cost of anti-COVID-19 agents was €511 825 (€408/patient). By multivariate analysis, risk factors associated with ARDS included older age, obesity, DM, severe hypoxaemia, lymphocytopenia, increased creatine kinase and increased C-reactive protein. In multivariate Cox model, older age (HR 1.07, 95% CI 1.06–1.09), cardiovascular disease (HR 1.34, 95% CI 1.01–1.79), DM (HR 1.45, 95% CI 1.09–1.92), severe hypoxaemia (HR 2.01, 95% CI 1.49–2.72), lymphocytopenia (HR 1.62, 95% CI 1.20–2.20) and increased C-reactive protein (HR 1.04, 95% CI 1.02–1.06) were risk factors for mortality.
Graphical abstract
1. Introduction
Spain is one of the Western countries with the highest incidence of COVID-19 (coronavirus disease 2019) patients and many hospitals have suffered an enormous healthcare overload during the present pandemic. The first case with COVID-19 was admitted in our centre on 1 March 2020. Up to 1 June 2020, more than 2700 patients were admitted.
There are few medical series describing the clinical characteristics and outcomes of hospitalised patients with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infection in Western countries. Three previous cohort studies from Italy, the UK and the New York City area (USA) have reported a mortality rate of up to 26% [1], [2], [3]. In addition, treatments for COVID-19 vary significantly between countries, partly because of the lack of evidence of their effectiveness and shortage problems for some of the experimental drugs.
In this study, we report the clinical characteristics, treatment patterns and outcomes of the first 1255 patients who received antiviral or immunosuppressive treatment for COVID-19 at Gregorio Marañón University General Hospital in Madrid (Spain). Risk factors present on admission associated with developing acute respiratory distress syndrome (ARDS) and mortality were explored.
2. Methods
This study was conducted at Gregorio Marañón University General Hospital, a tertiary-care institution with 1200 beds serving a population of 350 000 inhabitants. At the peak of the pandemic on 29 March 2020, this hospital had 1064 COVID-19 beds, of which 135 were intensive care unit (ICU) beds.
The study sample included all consecutive acute COVID-19 cases in adults confirmed by PCR from 1–24 March 2020 who consequently received specific anti-COVID-19 treatment, either antiviral or immunosuppressive. We excluded patients with mild disease that did not require specific treatment and who were referred to primary care for follow-up.
Patients were treated at the discretion of their attending physician according to local protocol (Appendix) and clinical judgement. Routine blood examinations included complete blood cell count, serum biochemical tests [renal and liver function profile, lactate dehydrogenase (LDH) and creatine kinase (CK)], C-reactive protein (CRP) and coagulation profile. If ARDS, co-infection or cardiac complications were suspected, procalcitonin, serum ferritin, interleukin-6 (IL-6) and myocardial enzymes [N-terminal pro-brain natriuretic peptide (NT-proBNP) and troponin] were performed accordingly. Chest radiography or computed tomography (CT) were also performed when necessary.
The criteria for discharge were absence of fever for ≥3 days, a chest radiograph that demonstrated pneumonia stabilisation and remission of respiratory failure [respiratory rate <22 breaths/min and arterial oxygen saturation (O2Sat) of >94% by pulse oximetry].
Patients were followed up until 10 May 2020. Data collected included patient demographics, co-morbidities, laboratory tests and treatments for COVID-19, including type and duration of antiviral and immunosuppressive combinations, oxygen therapy and mechanical ventilation. Outcomes were also analysed, including the development of ARDS, length of stay, discharge, re-admission and mortality. World Health Organization (WHO) interim guidelines were used to define ARDS [4].
We calculated the cost (€) of antiviral and immunosuppressive therapies for COVID-19 based on the drug acquisition cost and the actual dose administered. In the case of Spain, the costs of drugs are based on the laboratory sale price plus 4% value added tax (VAT) minus the 7.5% reduction required by the Spanish government as one of the extraordinary measures to reduce the public deficit [5].
2.1. Ethical issues
The study protocol was approved by the Research Ethics Committee of the hospital and by the Spanish Agency of Medicines and Medical Devices.
2.2. Statistical analysis
Continuous variables were described as the median and interquartile range (IQR) and categorical variables as frequency (percentage). The association between categorical variables was studied using the Pearson's χ2 test. For numerical variables, Student's T-test or Mann–Whitney U-test were used depending on the normality of the variable.
Survival rate was calculated by the Kaplan–Meier method. Time to death was defined as the time from hospital admission to death. The follow-up date was 10 May 2020. To explore risk factors associated with the development of ARDS, a univariable and multivariable logistic regression model was used. A bivariate Cox proportional hazard ratio (HR) model was used to determine the HR and 95% confidence interval (CI) between individual factors and the progression to death. Considering the total number of ARDS cases (n = 461) and deaths (n = 268) in our study and to avoid overfitting the models, 14 variables were chosen for the multivariable analysis. The variables were selected on the following basis: (i) if there was a significant difference (P < 0.10) between groups in the univariate analysis; (ii) if there was previous evidence that the variable could be a risk factor associated with mortality in patients with COVID-19 [1,6]; and (iii) if they were considered as clinically relevant. Variables from the univariable analysis were excluded if the number of events was too small to calculate odds ratios (ORs) or HRs (<2%). We chose age, sex, presence of obesity and five co-morbidities (hypertension, cardiovascular disease, diabetes mellitus, chronic obstructive pulmonary disease and renal impairment). In addition, the presence of an O2Sat of <90% by pulse oximetry, lymphocyte count, CRP, d-dimer, LDH and CK at admission were also included as potential risk factors for ARDS and mortality.
All analyses were based on existing data. In the multivariate analyses, missing values in qualitative variables were considered a separate category. All tests were two-sided, and a P-value of <0.05 was considered statistically significant. IBM SPSS Statistics for Windows v.25.0 (IBM Corp., Armonk, NY, USA) was used for all calculations.
3. Results
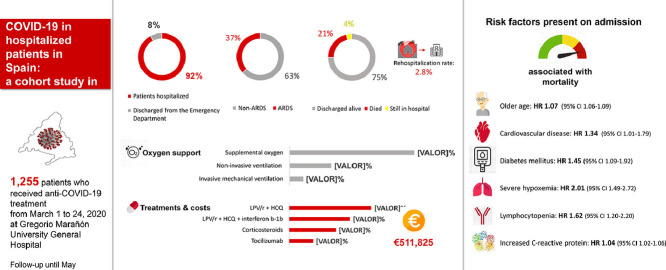
A total of 1255 patients were included in the study, of whom 1158 (92.3%) required hospitalisation whereas 97 (7.7%) were sent home with empirical antiviral treatment after being evaluated and observed in the emergency room for >24 h. We collected and analysed data from these 1255 patients.
The median patient age was 65 years (IQR 51–77 years; range 19–99 years) and 57.8% were male. Hypertension was the most common co-morbidity (45.1%), followed by other cardiovascular diseases (31.4%) and diabetes mellitus (19.9%) (Table 1 ). On admission, 24.1% of patients were febrile, 2.6% had a systolic blood pressure of <90 mmHg, and 50.6% and 19.7% had an O2Sat value <94% and <90%, respectively.
Table 1.
Demographic characteristics and clinical and laboratory findings of COVID-19 patients on admission
| Characteristic | All patients (n = 1255) | All patients (n = 1255) |
Patients discharged alive or who died (n = 1208) |
||||
|---|---|---|---|---|---|---|---|
| Without ARDS (n = 794) | With ARDS (n = 461) | P-value | Discharged alive (n = 940) | Died (n = 268) | P-value | ||
| Age (years) | 65 (51–77) | 60 (47–74) | 74 (59–82) | <0.001 | 60 (47–73) | 80 (74–86) | <0.001 |
| Age ≥65 years | 631 (50.3) | 329 (41.4) | 302 (65.5) | <0.001 | 376 (40.0) | 235 (87.7) | <0.001 |
| Male sex | 725 (57.8) | 420 (52.9) | 305 (66.2) | <0.001 | 514 (54.7) | 175 (65.3) | 0.002 |
| Current smoker | 81 (6.5) | 48 (6.0) | 33 (7.2) | 0.439 | 57 (6.1) | 20 (7.5) | 0.408 |
| Obesity (BMI >30 kg/m2) | 190 (15.1) | 86 (10.8) | 104 (22.6) | <0.001 | 122 (13.0) | 48 (17.9) | 0.041 |
| Co-morbidities | |||||||
| Hypertension | 566 (45.1) | 300 (37.8) | 266 (57.7) | <0.001 | 352 (37.4) | 188 (70.1) | <0.001 |
| Cardiovascular disease | 394 (31.4) | 201 (25.3) | 193 (41.9) | <0.001 | 227 (24.1) | 149 (55.6) | <0.001 |
| Diabetes mellitus | 250 (19.9) | 111 (14.0) | 139 (30.2) | <0.001 | 139 (14.8) | 96 (35.8) | <0.001 |
| COPD | 99 (7.9) | 44 (5.5) | 55 (11.9) | <0.001 | 55 (5.9) | 40 (14.9) | <0.001 |
| Asthma | 98 (7.8) | 64 (8.1) | 34 (7.4) | 0.663 | 77 (8.2) | 16 (6.0) | 0.229 |
| Chronic kidney disease | 148 (11.8) | 67 (8.4) | 81 (17.6) | <0.001 | 78 (8.3) | 59 (22.0) | <0.001 |
| Liver disease | 37 (2.9) | 20 (2.5) | 17 (3.7) | <0.001 | 23 (2.4) | 13 (4.9) | 0.041 |
| Cancer | 107 (8.5) | 63 (7.9) | 44 (9.5) | 0.325 | 61 (6.5) | 39 (14.6) | <0.001 |
| HIV | 12 (1.0) | 11 (1.4) | 1 (0.2) | 0.040 | 9 (1.0) | 3 (1.1) | 0.814 |
| Immunosuppressive therapy | 86 (6.9) | 52 (6.5) | 34 (7.4) | 0.577 | 54 (5.7) | 28 (10.4) | 0.007 |
| ACEi/ARB therapy | 414 (33.0) | 225 (28.3) | 189 (41.0) | <0.001 | 267 (28.4) | 127 (47.4) | <0.001 |
| Triage vitals | |||||||
| Temperature (°C) | 37.2 (36.5–37.9) | 37.1 (36.4–37.8) | 37.4 (36.8–38.0) | <0.001 | 37.2 (36.5–37.9) | 37.2 (36.6–38.0) | <0.403 |
| Temperature >38 °C | 268 (24.1) | 148 (21.2) | 120 (29.05) | 0.003 | 195 (23.2) | 58 (25.0) | 0.565 |
| Pulse ≥125 bpm | 27 (2.4) | 15 (2.2) | 12 (2.9) | 0.478 | 19 (2.3%) | 5 (2.1) | 0.849 |
| Systolic blood pressure <90 mmHg | 24 (2.6) | 13 (2.4) | 11 (2.9) | 0.591 | 12 (1.8) | 12 (5.3) | 0.005 |
| Oxygen saturation | |||||||
| <94% | 544 (50.6) | 256 (38.0) | 288 (71.6) | <0.001 | 345 (42.6) | 174 (76.7) | <0.001 |
| <90% | 212 (19.7) | 55 (8.2) | 157 (39.1) | <0.001 | 90 (11.1) | 106 (46.7) | <0.001 |
| Laboratory findings | |||||||
| Haematological | |||||||
| WBC count (× 109/L) | 5.7 (4.4–7.6) | 5.5 (4.3–7.0) | 6.3 (4.7–8.4) | <0.001 | 5.6 (4.3–7.2) | 6.5 (4.8–8.6) | <0.001 |
| WBC <4 × 109/L | 425 (35.3) | 288 (38.6) | 137 (29.9) | 0.002 | 331 (37.1) | 83 (31.3) | 0.084 |
| WBC > 10 × 109/L | 122 (10.1) | 58 (7.8) | 64 (14.0) | 0.001 | 69 (7.7) | 43 (16.2) | <0.001 |
| Lymphocytes (× 109/L) | 0.9 (0.7–1.2) | 1.0 (0.7–1.3) | 0.8 (0.5–1.0) | <0.001 | 1.0 (0.7–1.3) | 0.7 (0.5–0.9) | <0.001 |
| Lymphocytes <1 × 109/L | 660 (54.8) | 341 (45.7) | 319 (69.7) | <0.001 | 425 (47.6) | 199 (75.1) | <0.001 |
| Neutrophils (× 109/L) | 4.1 (3.0–6.0) | 3.7 (2.7–5.2) | 5.0 (3.3–6.9) | <0.001 | 3.9 (2.8–5.4) | 5.1 (3.5–7.2) | <0.001 |
| Neutrophils <1.5 × 109/L | 31 (2.6) | 22 (2.9) | 9 (2.0) | 0.295 | 24 (2.7) | 6 (2.3) | 0.701 |
| Platelets (× 109/L) | 176 (142–222) | 180 (146–231) | 168 (137–212) | 0.001 | 178 (146–226) | 162 (126–205) | <0.001 |
| Platelets <100 × 109/L | 66 (5.5) | 34 (4.6) | 32 (7.0) | 0.072 | 36 (4.0) | 28 (10.6) | <0.001 |
| Biochemical | |||||||
| Creatinine (mg/dL) | 0.88 (0.72–1.10) | 0.83 (0.68–1.01) | 0.97 (0.79–1.28) | <0.001 | 0.84 (0.69–1.02) | 1.02 (0.83–1.48) | <0.001 |
| Creatinine >1.3 mg/dL | 154 (13.5%) | 58 (7.8) | 96 (21.1) | <0.001 | 71 (8.0) | 75 (28.4) | <0.001 |
| ALT (U/L) | 30 (19–48) | 29 (19–47) | 31 (20–49) | 0.144 | 31 (20–48) | 26 (17–41) | 0.003 |
| ALT > 40 U/L | 375 (33.0) | 226 (31.9) | 149 (34.9) | 0.302 | 293 (34.7) | 64 (25.8) | 0.009 |
| Total bilirubin (mg/dL) | 0.5 (0.4–0.7) | 0.5 (0.4–0.7) | 0.5 (0.4–0.7) | 0.292 | 0.5 (0.4–0.7) | 0.5 (0.4–0.7) | 0.503 |
| Total bilirubin >1.1 mg/dL | 55 (5.3) | 31 (4.9) | 24 (5.9) | 0.473 | 35 (4.6) | 19 (8.1) | 0.036 |
| LDH (U/L) | 265 (211–347) | 243 (202–314) | 310 (243–419) | <0.001 | 254 (205–323) | 316 (238–442) | <0.001 |
| LDH >245 U/L | 362 (58.5) | 193 (49.2) | 169 (74.4) | <0.001 | 249 (53.4) | 94 (75.2) | <0.001 |
| Creatine kinase (U/L) | 94 (59–175) | 85 (55–143) | 116 (69–248) | <0.001 | 90 (57–155) | 111 (60–228) | 0.032 |
| Creatine kinase >300 U/L | 83 (11.4) | 29 (6.4) | 54 (19.7) | <0.001 | 49 (9.1) | 28 (17.0) | 0.005 |
| Infection-related indices | |||||||
| CRP (mg/dL) | 6.0 (2.9–12.9) | 4.6 (2.1–9.2) | 10.7 (5.4–18.6) | <0.001 | 5.0 (2.4–10.0) | 11.4 (6.5–20.4) | <0.001 |
| CRP > 0.5 mg/dL | 1070 (95.0) | 653 (92.8) | 417 (98.8) | <0.001 | 782 (93.4) | 46 (100.0) | <0.001 |
| PCT (μg/L) | 0.08 (0.04–0.16) | 0.05 (0.03–0.10) | 0.14 (0.08–0.36) | <0.001 | 0.06 (0.03–0.10) | 0.18 (0.09–0.47) | <0.001 |
| PCT > 0.5 μg/L | 87 (9.2) | 23 (3.9) | 64 (17.7) | <0.001 | 30 (4.3) | 48 (22.6) | <0.001 |
| Coagulation function | |||||||
| Prothrombin time (s) | 13.1 (12.4–14.1) | 12.9 (12.2–13.8) | 13.5 (12.7–14.7) | <0.001 | 13.0 (12.3–13.9) | 13.5 (12.5–14.9) | <0.001 |
| Prothrombin time >13.5 s | 18 (1.6) | 12 (1.7) | 6 (1.4) | 0.662 | 12 (1.5) | 5 (2.0) | 0.538 |
| d–dimer (ng/mL) | 253 (160–440) | 213 (140–363) | 340 (217–543) | <0.001 | 221 (146–356) | 488 (271–842) | <0.001 |
| d-dimer >1000 ng/mL | 63 (9.0) | 28 (6.3) | 35 (13.4) | 0.001 | 30 (5.5) | 30 (22.6) | <0.001 |
| Myocardial injury | |||||||
| Troponin (pg/mL) | 17.0 (4.0–85.0) | 10.0 (2.5–26.5) | 33.0 (12.2–144.0) | 0.004 | 6.5 (2.0–17.7) | 71.0 (16.5–141.0) | <0.001 |
| Troponin >34 pg/mL | 18 (34.0) | 4 (16.0) | 14 (50.0) | 0.009 | 3 (10.7) | 13 (61.9) | <0.001 |
| NT-proBNP (pg/mL) | 629 (223–2183) | 431 (139–1569) | 924 (323–2629) | <0.001 | 372 (137–1370) | 1435 (461–3519) | <0.001 |
| NT-proBNP >300 pg/mL | 199 (69.1) | 78 (57.4) | 121 (79.6) | <0.001 | 90 (55.2) | 100 (86.2) | <0.001 |
NOTE: Data are presented as the median (interquartile) or n (%). For clinical studies and laboratory testing for which not all patients had values, the percentages of total patients with completed tests are shown.
ACEI, angiotensin-converting enzyme inhibitor; ALT, alanine aminotransferase; ARB, angiotensin II receptor blocker; ARDS, acute respiratory distress syndrome; BMI, body mass index; bpm, beats per min; COPD, chronic obstructive pulmonary disease; COVID-19, coronavirus disease 2019; CRP, C-reactive protein; HIV, human immunodeficiency virus; LDH, lactate dehydrogenase; NT-proBNP, N-terminal pro-brain natriuretic peptide; PCT, procalcitonin; WBC, white blood cell.
Laboratory findings on hospital admission are also shown in Table 1. The most remarkable laboratory abnormalities (according to the local reference ranges) included the following: lymphocytopenia (54.8%); elevated alanine aminotransferase (33.0%); elevated LDH (58.5%); elevated CK (11.4%); and elevated d-dimer (>1000 ng/mL) (9.0%). Most patients presented an elevated CRP (95.0%), whereas only 9.2% of patients presented an elevated procalcitonin.
Throughout the entire follow-up period, 911 patients (72.6%) had findings of bilateral infiltrates on radiographic imaging, whilst 219 (17.5%) had unilateral infiltrates.
3.1. Clinical outcomes
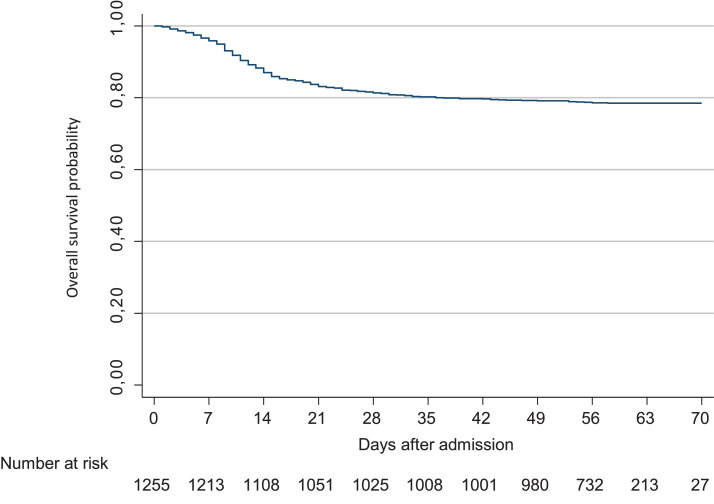
Of 1255 patients included in the study, 1158 (92.3%) required hospitalisation, with a median hospital stay of 11 days (IQR 7–19 days). A total of 461 patients (36.7%) developed ARDS and 126 (10.0%) were admitted to an ICU. The median length of ICU stay was 17 days (IQR 9–31 days). Overall mortality at Day 28 after admission was 18.6% (95% CI 16.6–20.9%). At the end of follow-up on 10 May 2020, a total of 268 patients (21.4%) had died (Fig. 1 ). The mortality rate in hospitalised patients was 22.9% (20.6% in non-ICU units and 41.3% in ICUs). All clinical outcomes are presented in Table 2 .
Fig. 1.
Kaplan–Meier curve of the probability of survival over time in patients with SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) infection hospitalised in Madrid, Spain.
Table 2.
Outcomes of patients discharged alive and those who died and in-hospital at study endpoint
| Outcome | All patients (n = 1255) |
|---|---|
| Patients hospitalised | 1158 (92.3) |
| Patients hospitalised in ICU | 126 (10.0) |
| Patients discharged from the ED | 97 (7.7) |
| ARDS | |
| Non-ARDS | 794 (63.3) |
| ARDS | 461 (36.7) |
| Outcome | |
| Discharged alive | 940 (74.9) |
| Died | 268 (21.4) |
| Still in hospital as of 10 May 2020 | 47 (4.1) |
| Patients hospitalised | 1158 |
| Patients hospitalised in ICU units | 126 (10.9) |
| ICU length of stay (days) | 17 (9–31) |
| Total length of stay (days) | 11 (7–19) |
| Discharged alive | 846 (73.0) |
| Time from illness onset to discharge (days) | 19 (14–26) |
| Time from admission to discharge (days) | 12 (7–19) |
| Died | 265 (22.9) |
| Earlier (≤7 days from admission) | 131 (49.4) |
| Later (>7 days from admission) | 134 (50.6) |
| Time from admission to death (days) | 8 (4–15) |
| Died, of those who received high-flow nasal cannula oxygen therapy or non-invasive mechanical ventilation | 226 (51.2) |
| Died, of those who received invasive mechanical ventilation | 47 (41.6) |
| Died, of those in non-ICU | 213 (20.6) |
| Died, of those in ICU | 52 (41.3) |
| In hospital at study endpoint | 47 (4.1) |
| Re-admission a | |
| ED visit | 52 (6.1) |
| New hospitalisation | 24 (2.8) |
| Patients discharged from the ED | 97 |
| Discharged alive | 94 (96.9) |
| Died | 3 (3.1) |
| Re-admission | |
| New ED visit | 14 (14.4) |
| Hospitalisation | 5 (5.1) |
NOTE: Data are presented as n (%) or median (interquartile range).
ARDS, acute respiratory distress syndrome; ED, emergency department; ICU, intensive care unit.
Among the 846 patients who were hospitalised and discharged at the study endpoint.
3.2. Oxygen support and treatment
Overall, 1025 patients (81.7%) required oxygen support in the hospital, with a median duration of 9 days (IQR 5–15 days). Of these, 345 (27.5%) required non-invasive ventilation (non-invasive positive pressure ventilation or high-flow supplemental oxygen) and 113 (9.0%) required invasive mechanical ventilation (IMV). Only one patient received IMV with extracorporeal membrane oxygenation (ECMO). The median duration of non-invasive oxygen support and IMV were 4 days (IQR 2–8 days) and 16 days respectively.
At admission, most patients received a combination of antivirals (1176; 93.7%) and empirical antibiotic treatment (1077; 85.8%). The median time from onset of symptoms reported by the patients to the start of antivirals was 6 days (IQR 3–7 days). The median duration of antiviral treatment was 9 days (IQR 7–12 days). The most frequent antiviral combination was lopinavir/ritonavir (LPV/r) + hydroxychloroquine (HCQ), which was used in 44.2% of patients (Table 3 ). Triple therapy with interferon beta-1b (IFN-β1b) was prescribed in 32.7% of patients, more frequently in patients with ARDS (47.5%). The combination of LPV/r + HCQ + azithromycin was used only in 7.7% of patients. Overall, 31 patients (2.5%) (all admitted in one of the ICUs) received the experimental antiviral remdesivir (RDV), which was only available on a compassionate-use basis. In all of these cases, patients were treated with an alternative antiviral therapy until RDV was available, with a median delay for its initiation of 16 days (IQR 13–18 days) from the onset of symptoms. Among these 31 patients, 11 patients died (35.5%) and 8 (25.8%) were still hospitalised at the study endpoint.
Table 3.
Treatments among patients with and without acute respiratory distress syndrome (ARDS) and among survivors and non-survivors
| All patients (n = 1255) | All patients (n = 1255) |
Patients discharged alive or died (n = 1208) |
|||||
|---|---|---|---|---|---|---|---|
| Without ARDS (n = 794) | With ARDS (n = 461) | P-value | Discharge alive (n = 940) | Died (n = 268) | P-value | ||
| Oxygen support | |||||||
| Supplemental oxygen | 1025 (81.7) | 564 (71.0) | 461 (100.0) | <0.001 | 720 (76.6) | 261 (97.4) | <0.001 |
| High-flow nasal cannula oxygen therapy/non-invasive mechanical ventilation | 345 (27.5) | 0 (0) | 345 (74.8) | <0.001 | 146 (15.5) | 191 (71.3) | <0.001 |
| Invasive mechanical ventilation | 113 (9.0) | 0 (0) | 113 (24.5) | <0.001 | 31 (3.3) | 47 (17.5) | <0.001 |
| Antiviral treatmenta | |||||||
| LPV/r + HCQ | 555 (44.2) | 426 (53.7) | 129 (28.0) | <0.001 | 459 (48.8) | 84 (31.3) | <0.001 |
| LPV/r + HCQ + IFN-β1b | 411 (32.7) | 192 (24.2) | 219 (47.5) | <0.001 | 261 (27.8) | 132 (49.3) | <0.001 |
| LPV/r + HCQ + AZT b | 97 (7.7) | 55 (6.9) | 42 (9.1) | 0.163 | 72 (7.7) | 15 (5.6) | 0.249 |
| LPV/r + HCQ + IFN-β1b + AZT c | 89 (7.1) | 41 (5.2) | 48 (10.4) | <0.001 | 73 (7.8) | 11 (4.1) | 0.038 |
| LPV/r monotherapy | 53 (4.2) | 45 (5.7) | 8 (1.7) | 0.001 | 41 (4.4) | 12 (4.5) | 0.935 |
| HCQ monotherapy | 23 (1.8) | 18 (2.3) | 5 (1.1) | 0.004 | 16 (1.7) | 5 (1.9) | 0.310 |
| HCQ + AZT | 20 (1.6) | 13 (1.6) | 7 (1.5) | 0.871 | 16 (1.7) | 4 (1.5) | 0.813 |
| RDV d | 31 (2.5) | 0 (0) | 31 (6.7) | <0.001 | 12 (1.3) | 11 (4.1) | 0.003 |
| Corticosteroids | |||||||
| Corticosteroids e | 317 (25.23) | 71 (8.9) | 246 (53.4) | <0.001 | 167 (17.8) | 113 (42.2) | <0.001 |
| Low–intermediate dosages | 225 (17.9) | 42 (5.3) | 183 (39.7) | 0.013 | 106 (11.3) | 91 (33.9) | 0.002 |
| Pulse therapy | 92 (7.3) | 29 (3.6) | 63 (13.7) | 0.013 | 61 (6.5) | 22 (8.2) | 0.002 |
| Immunomodulatory therapy | |||||||
| Tocilizumab | 162 (12.9) | 5 (0.6) | 157 (34.1) | <0.001 | 76 (8.1) | 54 (20.1) | <0.001 |
| 1 dose | 60 (4.8) | 2 (0.2) | 58 (12.6) | <0.001 | 30 (3.2) | 17 (6.3) | 0.019 |
| 2 doses | 45 (3.6) | 2 (0.2) | 43 (9.3) | <0.001 | 25 (2.7) | 17 (6.3) | 0.004 |
| 3 doses | 57 (4.5) | 1 (0.1) | 56 (12.1) | <0.001 | 21 (2.2) | 20 (7.5) | <0.001 |
NOTE: Data are presented as n (%).
AZT, azithromycin; HCQ, hydroxychloroquine; IFN-β1b, interferon beta-1b; LPV/r, lopinavir/ritonavir; RDV, remdesivir.
Combinations that were prescribed in <20 patients are not presented.
69% of patients received this combination of antivirals simultaneously.
61% of patients received this combination of antivirals simultaneously.
All patients were treated with an alternative antiviral therapy until RDV was available.
Corticosteroid treatment was classified as pulse dose if ≥125 mg of methylprednisolone or equivalent was administered every 24 h, or as low–intermediate dosage otherwise.
A total of 317 patients (25.3%) received systemic corticosteroids (Table 3), with a median elapsed time of 11 days (IQR 9–14 days) from the onset of symptoms. The mortality rate in this subpopulation was 35.6%. Corticosteroids were used in 53.4% of patients who developed ARDS. In this subpopulation, the mortality rate was 42.7% compared to 62.3% in those who did not receive corticosteroids (P < 0.001).
Tocilizumab (TCZ) was used in 162 patients (12.9%), of whom 63.0% received two or three doses. More than one-half of the patients (56.8%) were in the ICU at the time of its first administration. An improvement in oxygen support status was achieved in 17.9% and 54.9% of patients at Day 7 and Day 28 post-TCZ administration, respectively. This benefit was clearly higher in patients without IMV (68% vs. 41% at Day 28). At the study endpoint, mortality was 33.3% in patients with ARDS who received TCZ compared with 60.8% in those patients with ARDS who did not receive TCZ (P < 0.001).
Anticoagulation therapy with low-molecular-weight heparin was prescribed at prophylactic or intermediate dosages in 86.7% of hospitalised patients. Only 36 patients (2.9%) received full therapeutic-intensity anticoagulation, in all cases due to high clinical suspicion of thrombosis.
3.3. Treatments costs
The total acquisition cost of antiviral and immunosuppressive agents for the treatment of the 1255 COVID-19 patients was €511 825. The mean treatment cost per patient was €408 (median €80, IQR €51–207). The total cost of antivirals was €137 861 (mean €110/patient; median €74, IQR €47–157). The highest expense in antivirals was due to the consumption of LPV/r (€66 890), followed by IFN-β1b (€64 970), HCQ (€5806) and azithromycin (€195). RDV was purchased at no cost through the Compassionate Use Access Program, which was enabled through the collaboration of the provider (Gilead Sciences, Inc.) and the Spanish Agency of Medicines and Medical Devices (AEMPS).
The total cost of TCZ was €371 784 (mean €2295 per treated patient; median €2096, IQR €1048–3143) and the cost of corticosteroids was €2180 (mean €6.9 per treated patient; median €5.4, IQR €2.7–8.1).
3.4. Risk factors associated with ARDS and mortality
Older age, male sex, presence of obesity, all mentioned co-morbidities (except asthma and cancer), presence of fever and an O2Sat <94% at admission were more common in patients who developed ARDS (Table 1). Regarding blood examinations, the prevalence of lymphocytopenia and elevated values of creatinine, LDH, CK, d-dimer and myocardial enzymes were also significantly higher in this population. Table 4 describes the results of the univariable and multivariable logistic regression models for the risk factors associated with ARDS. In the univariate analysis, all factors included in the model were associated with an increased risk of ARDS. In the multivariable model, however, only older age, presence of obesity, diabetes mellitus, lymphocytopenia, O2Sat <90%, elevated CK and elevated CRP were independently associated with increased odds of ARDS.
Table 4.
Risk factors associated with acute respiratory distress syndrome (ARDS)
| Risk factor | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| OR (95% CI) | P-value | OR (95% CI) | P-value | |
| Age | 1.04 (1.03–1.04) | <0.001 | 1.02 (1.00–1.03) | 0.004 |
| Male sex | 1.74 (1.37–2.21) | <0.001 | 1.31 (0.96–1.78) | 0.086 |
| Obesity | 2.40 (1.75–3.28) | <0.001 | 2.27 (1.52–3.37) | <0.001 |
| Hypertension | 2.25 (1.78–2.84) | <0.001 | 1.00 (0.70–1.42) | 0.996 |
| Cardiovascular disease | 2.13 (1.66–2.71) | <0.001 | 1.04 (0.73–1.48) | 0.801 |
| Diabetes mellitus | 2.66 (2.00–3.52) | <0.001 | 1.93 (1.34–2.78) | <0.001 |
| COPD | 2.31 (1.53–3.50) | <0.001 | 1.05 (0.62–1.78) | 0.863 |
| Renal impairment | 2.31 (1.64–3.27) | <0.001 | 1.39 (0.87–2.21) | 0.163 |
| Oxygen saturation <90% | 7.19 (5.13–10.1) | <0.001 | 3.55 (2.38–5.35) | <0.001 |
| Lymphocytopenia (<1 × 109/L) | 2.72 (2.13–3.48) | <0.001 | 1.96 (1.45–2.65) | <0.001 |
| C-reactive protein | 1.10 (1.08–1.13) | <0.001 | 1.06 (1.04–1.08) | <0.001 |
| Lactate dehydrogenase >245 U/L | 3.00 (2.10–4.30) | <0.001 | 1.40 (0.90–2.18) | 0.130 |
| Creatine kinase >300 U/L | 3.60 (2.23–5.81) | <0.001 | 2.52 (1.40–4.52) | 0.002 |
| d-dimer 250–500 ng/mL | 2.60 (1.81–3.75) | <0.001 | 1.33 (0.86–2.07) | 0.203 |
| d-dimer 500–1000 ng/mL | 3.11 (1.90–5.07) | <0.001 | 1.15 (0.62–2.11) | 0.655 |
| d-dimer >1000 ng/mL | 3.79 (2.18–6.60) | <0.001 | 0.82 (0.40–1.69) | 0.593 |
NOTE: Statistically significant factors (P < 0.05) are in bold.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; OR, odds ratio.
Univariate Cox models showed that all factors related to the development of ARDS, except obesity and elevated CK, were also associated with increased risk of death (Table 5 ). In the multivariate Cox model, older age (HR = 1.07, 95% CI 1.06–1.09), presence of cardiovascular disease (HR = 1.34, 95% CI 1.01–1.79), diabetes mellitus (HR = 1.45, 95% CI 1.09–1.92), O2Sat <90% (HR = 2.01, 95% CI 1.49–2.72), lymphocytopenia (HR = 1.62, 95% CI 1.20–2.20) and increased CRP on admission (HR = 1.04, 95% CI 1.02–1.06) were risk factors independently associated with death.
Table 5.
Cox regression of factors associated with death
| Factor | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| HR (95% CI) | P-value | HR (95% CI) | P-value | |
| Age | 1.08 (1.07–1.09) | <0.001 | 1.07 (1.06–1.09) | <0.001 |
| Male sex | 1.43 (1.11–1.83) | 0.005 | 1.14 (0.85–1.52) | 0.375 |
| Obesity | 1.22 (0.90–1.66) | 0.194 | 1.28 (0.89–1.84) | 0.183 |
| Hypertension | 3.26 (2.51–4.23) | <0.001 | 0.78 (0.57–1.09) | 0.147 |
| Cardiovascular disease | 3.25 (2.56–4.13) | <0.001 | 1.34 (1.01–1.79) | 0.044 |
| Diabetes mellitus | 2.58 (2.01–3.31) | <0.001 | 1.45 (1.09–1.92) | 0.011 |
| COPD | 2.44 (1.73–3.43) | <0.001 | 1.16 (0.79–1.69) | 0.444 |
| Renal impairment | 2.43 (1.82–3.24) | <0.001 | 1.06 (0.76–1.48) | 0.721 |
| Oxygen saturation <90% | 4.65 (3.58–6.02) | <0.001 | 2.01 (1.49–2.72) | <0.001 |
| Lymphocytopenia (<1 × 109/L) | 2.77 (2.10–3.65) | <0.001 | 1.62 (1.20–2.20) | 0.002 |
| C-reactive protein | 1.07 (1.06–1.08) | <0.001 | 1.04 (1.02–1.06) | <0.001 |
| Lactate dehydrogenase >245 U/L | 2.33 (1.56–3.49) | <0.001 | 1.46 (0.94–2.27) | 0.095 |
| Creatine kinase >300 U/L | 1.75 (1.16–2.63) | <0.001 | 1.05 (0.67–1.63) | 0.839 |
| d-dimer 250–500 ng/mL | 2.85 (1.76–4.61) | <0.001 | 1.22 (0.73–2.04) | 0.448 |
| d-dimer 500–1000 ng/mL | 5.75 (3.45–9.56) | <0.001 | 1.60 (0.92–2.80) | 0.095 |
| d-dimer >1000 ng/mL | 8.02 (4.77–13.49) | <0.001 | 1.81 (0.99–3.32) | 0.054 |
NOTE: Statistically significant factors (P < 0.05) are in bold.
CI, confidence interval; COPD, chronic obstructive pulmonary disease; HR, hazard ratio.
4. Discussion
Here we report the clinical characteristics and outcomes of a cohort of over 1200 patients with COVID-19 who were admitted to a public hospital in the Community of Madrid during the rising phase of the SARS-CoV-2 pandemic. The region was one of the most severely affected during the pandemic, with 14 597 COVID-19 cases and 11 153 (76.4%) hospitalisations by the end of this study [7]. To our knowledge, this is the first large cohort describing the use and costs of anti-COVID-19 agents as well as the risk factors present on admission associated with ARDS and mortality in the Spanish population.
One of our main findings is the high proportion (36.7%) of hospitalised COVID-19 patients who developed ARDS, an issue that has not been thoroughly examined in previous studies. Our mortality rate was high (21.3%), although similar to those observed in other Western countries. In particular, a mortality rate of 21% was also found in the Italian region of Lombardy and in the New York City area, and up to 26% in the UK [1], [2], [3].
The proportion of patients admitted to our ICUs (10.0%) was slightly lower than the 14–17% reported in those countries [1], [2], [3], even though the number of ICU beds had been multiplied by 6 (up to 135). Nevertheless, it should be noted that our figures do not include the proportion of patients who received non-invasive mechanical ventilation outside the ICU wards.
As expected, compared with non-ICU units, the mortality rate in ICUs was higher (41.3% vs. 20.6%). A lower mortality rate (26%) was reported in the ICU units of Lombardy region [8], although in this cohort the follow-up period was short, with 58% of patients still in the ICU at the time of analysis. Mortality as high as 58% was observed among patients requiring ICU care and mechanical ventilation in the New York City area (USA) and the Wuhan region in China [2,9]. A recent meta-analysis described a combined ICU mortality of 41.6% (95% CI 34.0–49.7%) in patients with completed ICU admissions [10]. Interestingly, this meta-analysis also found that as the pandemic progresses, the mortality rates have fallen from above 50% to 40%, possibly due to improvements in treatments and less care burden.
Our high rate of hospitalisation and mortality reflect the elevated prevalence of co-morbid conditions in our COVID-19 population. Our patients were predominantly elderly, with hypertension, cardiovascular disease and diabetes mellitus as the main co-morbidities. Multivariate analysis performed using Cox regression modelling confirmed that older age and the presence of diabetes mellitus and cardiovascular disease remain independently associated with mortality. Conversely, we were not able to demonstrate a relationship between obesity and mortality, although this was clearly associated with an increased odds of ARDS (OR = 2.27). This is probably explained by the high prevalence of elderly population admitted and also a possible under-reporting by clinical staff.
Another interesting finding was the relatively short time interval between the onset of symptoms and hospital admission (6 days), although 9% of patients were admitted 10 days after the onset of symptoms. These findings are quite similar to those of the UK [3], where the median time to admission was 4 days (IQR 1–8 days). Despite this, our patients’ respiratory status at admission was generally poor, with one-half of the patients presenting a SatO2 <94%. In addition, laboratory values were indicative of an impaired immune-inflammatory profile characterised by lymphocytopenia and elevated CRP and LDH in more than one-half of the patients. We were able to demonstrate that, at admission, the presence of SatO2 <94%, lymphocytopenia and increased CRP are independently associated with ARDS and mortality.
With respect to treatment, the majority of our patients received a combination of LPV/r + HCQ ± IFN-β1b, according to the protocol of the hospital. Use of HCQ + azithromycin was limited to patients with contraindication to LPV/r, generally because of drug interactions or intolerance in elderly patients with multiple co-morbidities. At the time of this study, there was no evidence about which antivirals were more effective as they were still being tested in clinical trials. On 7 May 2020, a small clinical trial reported that LPV/r was not associated with a reduction in the time to clinical improvement or with mortality rate in patients with severe COVID-19 [11]. However, these patients started LPV/r with a median delay of 13 days from the onset of symptoms, so a benefit in the case of early onset could not be ruled out. Pending the results from other clinical trials, the Pharmacy and Therapeutic Committee of the hospital decided to maintain LPV/r as a feasible alternative, if it could be administered early in the course of the disease. Regarding HCQ, until 5 June 2020 no results from any clinical trial had been reported. However, recent preliminary results from the RECOVERY trial [12] have indicated that HCQ has no benefit on 28-day mortality (26% vs. 23% usual care; HR = 1.11, 95% CI 0.98–1.26). While waiting for the publication of these preliminary results, its off-label use was no longer recommended in our hospital. Finally, RDV was used in only 2.5% of patients owing to the logistical difficulties of obtaining it in Spain. This prevents drawing solid conclusions about its effectiveness, beyond a mortality rate in this small ICU subpopulation which was above 30%.
On the other hand, our study seems to point towards a favourable effect with the use of corticosteroids and TCZ in patients with ARDS. Corticosteroids were used predominantly in this subpopulation because in the earlier weeks of the pandemic their benefit in less critical patients was still to be proven. Analysing only the 461 patients with ARDS, the mortality rate in those treated with corticosteroids was 42.7% compared with 62.3% in those not treated. Similarity, mortality was lower in those patients with ARDS who received TCZ than in those who did not receive it (33.3% vs. 60.8%). However, we cannot draw solid conclusions owing to the retrospective non-randomised design of the study, in which up to 35% of the patients received corticosteroids and TCZ simultaneously. In addition, use of TCZ was limited to patients with a very severe COVID-19 infection because of the lack of evidence of its effectiveness as well as shortage problems in Spain. An earlier use of TCZ may perhaps demonstrate greater benefits. In any case, its actual impact on mortality should be elucidated through the placebo-controlled clinical trials that are currently recruiting patients.
Finally, our study also highlights the elevated cost of COVID-19 treatment, with approximately €0.44 million per 1000 hospitalised patients. Higher expenses were attributable to the treatment of ARDS with TCZ, which accounted for €0.37 million.
This study has some limitations. First, the study population only included patients from Gregorio Marañón University General Hospital, although it should be mentioned that this hospital served up to 13% of the total COVID-19 patients in the Community of Madrid. Second is its observational design that, among other limitations, did not allow us to establish a strong relationship between treatment patterns and outcomes. Third, our registry, though extensive, does not provide data about complications during hospitalisation or drug-related adverse events. Lastly, because this is a real-life study, not all laboratory tests were available for the majority of patients (i.e. serum ferritin, IL-6), therefore their role might be underestimated in predicting in-hospital death.
5. Conclusions
This is one of the largest cohort studies among hospitalised patients with COVID-19 in Western countries, which describes the clinical characteristics, the use and costs of treatments, and the risk factors for ARDS and mortality. We found that older age, the presence of diabetes mellitus, cardiovascular disease, an oxygen saturation <90% at admission, lymphocytopenia and elevated CRP were factors independently associated with ARDS and mortality. Our study also suggests that corticosteroids and TCZ may be beneficial for patients with severe disease. However, double-blinded, placebo-control, randomised clinical trials are still required to determine the most effective treatments for COVID-19.
Acknowledgments
The authors thank AthentoⓇ for their assistance in data mining. The authors also thank J.M. Bellón for assistance with the statistical analyses and Angelica Minero for editing the article.
Funding: This work was partially supported by a grant from MSD [Innovando Juntos initiative, in co-operation with the University Carlos III of Madrid, the University of Seville, the Spanish Society of Infectious Diseases and Clinical Microbiology, the Spanish Society of Hospital Pharmacy, and the Association of Science Parks and Technological of Spain].
Competing interests: None declared.
Ethical approval: The study protocol was approved by the Research Ethics Committee of Gregorio Marañón University General Hospital [FARM-COVID-19 v.1] and by the Spanish Agency of Medicines and Medical Devices [CGR-REM-2020-06].
Editor: Professor Jeffrey Lipman
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2020.106249.
Appendix. Supplementary materials
Appendix A. Supplementary data: Supplementary material related to this article can be found, in the online version.
TS: add in link
References
- 1.Giacomelli A., Ridolfo A.L., Milazzo L., Oreni L., Bernacchia D., Siano M. 30-day mortality in patients hospitalized with COVID-19 during the first wave of the Italian epidemic: a prospective cohort study. Pharmacol Res. 2020;158 doi: 10.1016/j.phrs.2020.104931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Richardson S., Hirsch J., Narasimhan M., Crawford J.M., McGinn T., Davidson K.W. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City area. JAMA. 2020;323:2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Docherty A.B., Harrison E.M., Green C.A., Hardwick H.E., Pius R., Norman L. Features of 20 133 UK patients in hospital with COVID-19 using the ISARIC WHO Clinical Characterisation Protocol: prospective observational cohort study. BMJ. 2020;369:m1985. doi: 10.1136/bmj.m1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.World Health Organization (WHO) WHO; Geneva, Switzerland: 27 May 2020. Clinical management of COVID-19: interim guidance. https://www.who.int/publications/i/item/clinical-management-of-covid-19 [accessed 1 June 2020] [Google Scholar]
- 5.Real Decreto-ley 8/2010, de 20 de mayo, por el que se adoptan medidas extraordinarias para la reducción del déficit público. Boletín Oficial del Estado, n° 126 (24/05/2010).
- 6.Wu C., Chen X., Cai Y., Xia J., Zhou X., Xu S. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 pneumonia in Wuhan, China. JAMA Intern Med. 2020;180:934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ministerio de Sanidad. Gobierno de España: Centro de Coordinación de Alertas y Emergencias Sanitarias. Informe Actualización n° 55. Enfermedad por el coronavirus (COVID-19).https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov/documentos/Actualizacion_55_COVID-19.pdf [accessed 3 December 2020].
- 8.Grasselli G., Zangrillo A., Zanella A., Antonelli M., Cabrini L., Castelli A. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy Region. Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C.Q., He J.X. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Armstrong R.A., Kane A.D., Cook T.M. Outcomes from intensive care in patients with COVID-19: a systematic review and meta-analysis of observational studies. Anaesthesia. 2020;75:1340–1349. doi: 10.1111/anae.15201. [DOI] [PubMed] [Google Scholar]
- 11.Cao B., Wang Y., Wen D., Liu W., Wang J., Fan G. A trial of lopinavir–ritonavir in adults hospitalized with severe Covid-19. N Engl J Med. 2020;382:1787–1799. doi: 10.1056/NEJMoa2001282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Statement from the Chief Investigators of the Randomised Evaluation of COVid-19 thERapY (RECOVERY) Trial on hydroxychloroquine, 5 June 2020. https://www.recoverytrial.net/files/hcq-recovery-statement-050620-final-002.pdf [accessed 3 December 2020].
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix A. Supplementary data: Supplementary material related to this article can be found, in the online version.
TS: add in link