Abstract
Originated in Wuhan, China, the coronavirus 19 disease (COVID-19) has quickly spread worldwide, reaching countries that already faced other endemics and epidemics. In Brazil, such a concerning situation includes arboviruses, among which the dengue virus stands out. Here, we determined the rate of SARS-CoV-2/dengue virus co-infection in a total of 178 patients with COVID-19 symtoms admitted into a large public hospital of the Federal District of Brazil. Furthermore, we evaluated whether prior or active dengue virus infection influenced hematological, biochemical, and clinical parameters of such patients. One hundred and twelve (63%) individuals tested positive for COVID-19, of which 43 (38.4%) were co-infected with dengue virus, and 50 (44.6%) had antibodies indicative of previous dengue infection. Co-infected patients showed lower numbers of circulating lymphocytes and monocytes, higher glucose rates, and a worse pulmonary condition. Of note, prior infections with dengue virus did not influence clinical parameters, but active dengue fever resulted in higher hospitalization rate. In conclusion, amid the current complex epidemiological scenario in Brazil, our data support the notion that SARS-CoV-2 and dengue co-infection affects an important percentage of COVID-19 patients and leads to worse clinical parameters, requiring greater attention from health authorities.
Keywords: SARS-CoV-2, Dengue fever, Co-infection, Syndemic
Graphical abstract
1. Introduction
The severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), the causative agent of COVID-19, was first identified in December 2019 in patients from Wuhan, China (Gorbalenya et al., 2020). After that, the virus quickly spread to several countries, infecting millions of people worldwide. In March 2020, the World Health Organization (WHO) officially declared the COVID-19 outbreak as a global pandemic (WHO, 2020a). Thereafter, the number of infected individuals from all continents continued to grow daily, reaching the expressive figure of 17,106,007 cases and 668,910 deaths as of July 31, 2020 (WHO, 2020b).
Most SARS-CoV-2-infected individuals are asymptomatic or present non-specific, flu-like symptoms, such as fever, headache, fatigue, and dry cough (Wiersinga et al., 2020). However, clinical conditions can rapidly progress to severe pneumonia and, ultimately, death, mainly in patients who present comorbidities, such as obesity, type 2 diabetes and cardiovascular diseases (Pascarella et al., 2020). Although research is happening at overwhelming speed, to date no effective drugs or vaccines have been developed to treat or prevent COVID-19 (Hosoki et al., 2020).
The impact of this disease on the world has been staggering, both from the public health and economic perspectives (Autrán-Gomez et al., 2020; de Oliveira Andrade, 2020; Lima et al., 2020). A worse scenario has been witnessed in South America, especially in Brazil, Ecuador, Argentina, Chile, and Peru, which recently reported the highest number of cases of COVID-19 in the region (Saavedra-Velasco, 2020). In this sense, it has been hypothesized that such countries possibly experienced an aggravated COVID-19 pandemic due to socioeconomic (de Sousa et al., 2020; Martins-FiIho et al., 2020) and health factors, such as other ongoing epidemics like dengue fever. Indeed, COVID-19/dengue co-infection has been reported in endemic and even in non-endemic countries, the consequences of such co-infection being mostly unknown at this point (Butt et al., 2020; Estofolete et al., 2020; Lazzarini et al., 2020).
Dengue fever is an endemic disease sustained by continuous vector transmission in several tropical areas around the world. Thus, the COVID-19 pandemic brought additional uncertainty to countries that already needed to deal with dengue fever endemics (Olive et al., 2020; Wu et al., 2020a). The circulation of both viruses represents a major challenge for hospitals that will have to face difficulties in determining the diagnosis due to overlapping symptoms between dengue fever and COVID-19 (Saddique et al., 2020). Misdiagnosis of COVID-19 as dengue has been reported even after use of rapid dengue tests (Yan et al., 2020). The consequences of COVID-19 and dengue misdiagnosis are relevant, and may include ineffective patient management, possibly leading to preventable patient death, as well as unsuccessful prevention strategies, including rapid patient isolation (in the case of COVID-19) and vector control (in the case of dengue fever) (Wilder-Smith et al., 2020). Furthermore, the clinical consequences of SARS-CoV-2 and dengue virus co-infection are still unknown (Nacher et al., 2020), justifying investigation in the theme and close vigilance by health authorities.
In the present study, we determined the SARS-CoV-2/dengue virus co-infection rates in patients assisted in a large public hospital from the Federal District of Brazil, in order to shed light on the frequency and uncertain prognosis of COVID-19/dengue virus co-infection. Data regarding patients' hematological and biochemical parameters, as well as clinical outcomes, were collected and analyzed. We detected that a significant percentage of COVID-19 patients presented dengue virus co-infection. Also, even though prior dengue fever did not significantly influence clinical and laboratorial parameters, active SARS-CoV-2/dengue virus co-infection was associated with more frequent detectable lung alterations and hospitalization. Taken together, our data may provide important information to health authorities to improve the identification and the management of these patients.
2. Materials and methods
2.1. Study area and Patients
This study was performed with a convenience sample of patients assisted in Planaltina Regional Hospital, Federal District, Brazil, from June 20 to July 30, 2020. The study was approved by the Ethics Committee of the Federal District's Institute of Healthcare Strategic Management (IGESDF) (CAEE:32186420.0.0000.8153). A total of 178 patients aged 18+ years who were admitted to the hospital emergency room presenting COVID-19 symptoms and who signed informed consent were included.
2.2. Clinical Evaluation
The medical team carefully examined the patients and then filled their medical record with data regarding age, history of fever, cough, dyspnea, fatigue, headache, sore throat, chest pain, diarrhea, vomiting, anosmia, ageusia, and inappetence. Patient clinical follow-up was assessed on medical records and used to determine major clinical outcomes, such as hospitalization.
2.3. COVID-19 and dengue fever diagnosis
COVID-19 diagnosis was confirmed by reverse transcription-polymerase chain reaction (qRT-PCR) assay of nasopharyngeal swab samples. Viral RNA was extracted using the High Pure Viral Nucleic Acid Version 18 Kit (Roche Diagnostics®, Germany). qRT-PCR was performed using the Molecular SARS-CoV-2 kit, following manufacturer's instructions (Biomanguinhos, Rio de Janeiro, Brazil), on a Step One Plus Real-Time PCR Systems (Applied Biosystems®, USA). The protocol was based on Corman et al. Euro Surveill, 2020- Charité, Berlin, Germany, and the protocol CDC USA (N1, N2 and N3).
Dengue fever diagnosis was determined using a rapid test for NS1Ag/IgG/IgM (ABBOTT-Alere® S.A., Brazil), following the manufacturer's instructions. The test is a qualitative immunoassay and simultaneously detects NS1 antigen, IgM and IgG antibodies against dengue virus in serum.
Serology testing was carried out using the Anti-DENV IgM kit (Euroimmun®, Germany). The method is based on indirect ELISA technique, in which microplates are coated with a mixture of purified viral antigens and probed using the patient's serum.
2.4. Hematology Tests and Computed Tomography
Blood samples were used to analyze hematological parameters by flow cytometry on the CELL-DYN® Ruby Abbott Diagnostics equipment (Santa Clara, USA). Serum samples were characterized according to aspartate aminotransferase (AST), alanine aminotransferase (ALT), urea, creatinine, creatinine kinase (CK), lactic dehydrogenase (LDH), and glucose using the CMD 800i equipment and kit (Wiener Lab Group®, Argentina). High-resolution computed tomography (HRCT) was used to assess patient lung condition.
2.5. Statistical Analysis
All statistical analyses were performed using SAS® (v.9.4, Cary, North Carolina). Qualitative data were submitted to analysis of frequency using PROC FREQ procedure with subsequent chi-square analysis at 95% confidence interval to assess whether clinical parameters would significantly varied between COVID-19, co-infection (COVID-19 and dengue fever), and COVID-19/past dengue fever patients. Quantitative data were subjected to the Shapiro-Wilk normality test using PROC UNIVARIATE and then submitted to a Mann-Whitney U Test (at 95% confidence interval using PROC NPAR1WAY) to detect differences between COVID-19, co-infection, and COVID-19/past dengue fever groups.
3. Results
From the 178 patients analyzed, 112 (63%) were diagnosed with COVID-19 after qRT-PCR confirmation. Most patients infected with the SARS-COV-2 virus were male (53.6%), with an average age of 44.55 ± 15.62 years old. The most frequently reported symptoms were: fever (78.6%), cough (76.8%), dyspnoea (50.0%), sore throat (36.6%), and anosmia (41.1%). Forty-three out 112 (38.4%) COVID-19-positive patients were also infected with dengue virus (SARS-CoV-2 +/ anti-dengue virus IgM +), and 50 out 112 (44.6%) COVID-19-positive patients presented anti-dengue IgG antibodies, indicating prior dengue virus infection (SARS-CoV-2 +/ anti-dengue virus IgG +).
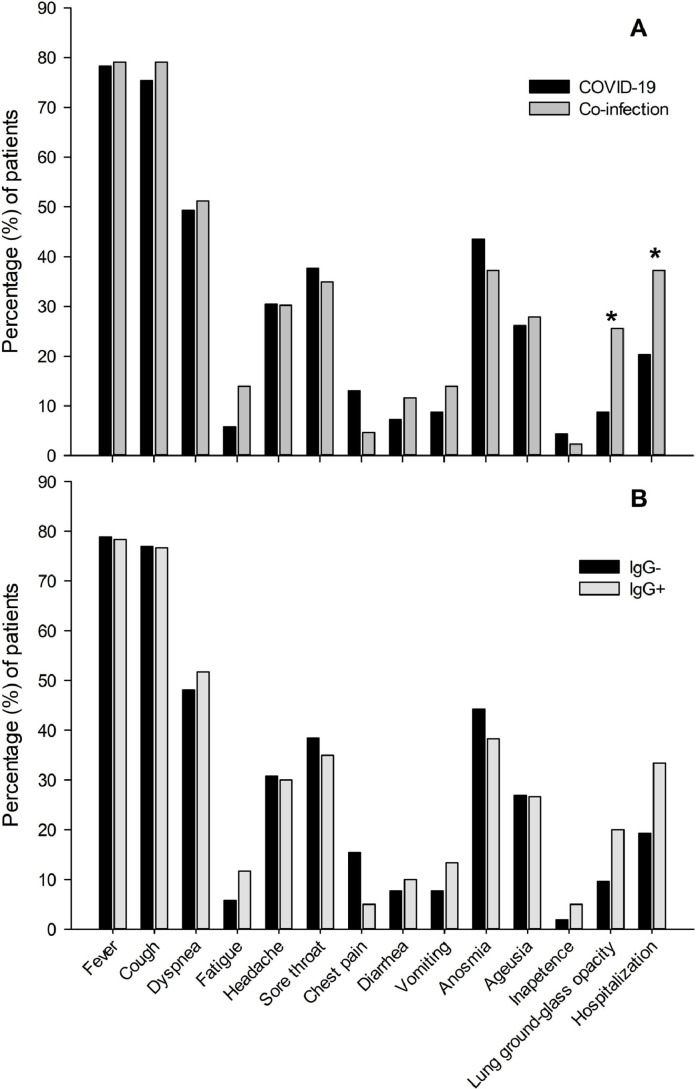
Interestingly, COVID-19 and dengue virus co-infection did not influence reported symptoms but was significantly associated with higher rates of pulmonary impairment (p=0.01) detected by HRCT, and hospitalization-rate (p=0.04) (Fig. 1 A). Of note, COVID-19 and COVID-19/past dengue fever groups presented similar clinical parameters (p>0.05 for all clinical parameters analyzed) (Fig. 1B).
Fig. 1.
Impact of active and prior dengue fever on COVID-19 symptomatology. A) Symptom frequency in COVID-19 versus COVID-19/dengue fever co-infection (SARS-CoV-2 +/ anti-dengue virus IgM +) patients. B) Symptom frequency in COVID-19 (SARS-CoV-2 +/ anti-dengue virus IgG -) versus COVID-19/past dengue fever (SARS-CoV-2 +/ anti-dengue virus IgG +) patients. *p<0.05.
Hematological and biochemical tests showed that ALT, AST, LDH, and CK levels were altered in both COVID 19 and SARS-CoV-2/dengue virus co-infection groups, even though no differences were found between them. Interestingly, patients with active co-infection presented lower levels of blood count lymphocytes (p=0.03), and monocytes (p=0.05) than patients exclusively infected with SARS-CoV-2 (Table 1 ). Furthermore, the glucose levels of co-infected patients were significantly higher than those of patients who tested positive for COVID-19 only (p=0.0006). It was also observed that anti-dengue virus IgG antibodies influenced lymphocyte counts and glucose levels (Table 2 ).
Table 1.
Laboratory parameters of COVID-19 and COVID-19/dengue fever patients.
| Blood Parameter | COVID | COINFECTION | P value | Reference Value2 | ||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Leucocytes (%) | 6.19 | 2.98 | 6.74 | 2.88 | ns1 | 4.0 - 11.0 |
| Neutrophils (1 × 10³/µL) | 3.9 | 2.6 | 4.5 | 2.9 | ns | 1.6-8.1 |
| Neutrophils (%) | 59.61 | 13.81 | 63.41 | 18.19 | ns | 40-74 |
| Lymphocytes (1 × 10³/µL) | 1.7 | 6.7 | 1.5 | 6.6 | ns | 1.0-4.5 |
| Lymphocytes (%) | 30.52 | 12.02 | 26.37 | 14.23 | 0.028 | 20-50 |
| Monocytes (1 × 10³/µL) | 0.45 | 2.6 | 0.42 | 2,00 | ns | 0.0 - 1.0 |
| Monocytes (%) | 7.76 | 3.21 | 6.99 | 3.42 | 0.051 | 2 - 10 |
| Hemoglobin (g/100 mL) | 14,00 | 1.46 | 14.36 | 1.53 | ns | 13-17 |
| Platelets (1 × 10³/µL) | 218.81 | 81.17 | 224.76 | 73.61 | ns | 150 - 450 |
| AST (UI/L) | 42.56 | 33.28 | 43.81 | 25.57 | ns | 0 - 38 |
| ALT (UI/L) | 45.07 | 43.21 | 54.62 | 54.26 | ns | 0 - 41 |
| Urea (mg/100 mL) | 32.08 | 14.11 | 37.63 | 22.74 | ns | 10-50 |
| Creatinine (mg/100 mL) | 0.96 | 0.27 | 1.84 | 5.65 | ns | 0.70 - 1.40 |
| CK (UI/L) | 215.2 | 750.31 | 139.55 | 119.56 | ns | 230 - 460 |
| LDH (U/L) | 479.36 | 281.55 | 545.02 | 308.59 | ns | 24 - 195 |
| Glucose (mg/100 mL) | 110.24 | 60.35 | 172.51 | 124.44 | 0.0006 | 70 - 99 |
ns = not significant
SD = Standard deviation
Table 2.
Laboratory parameters of COVID-19 and COVID-19/past dengue fever patients.
| Blood Parameter | COVID AND PREVIOUS DENGUE | P value | Reference Value2 | |||
|---|---|---|---|---|---|---|
| POSITIVE IgG | NEGATIVE IgG | |||||
| Mean | SD | Mean | SD | |||
| Leucocytes (%) | 6.29 | 2.75 | 6.53 | 3.16 | ns | 4.0 - 11.0 |
| Neutrophils (1 × 10³/µL) | 4.23 | 2.72 | 4.05 | 2.85 | ns | 1.6 - 8.1 |
| Neutrophils (%) | 63.19 | 15.92 | 58.61 | 15.16 | ns | 40 - 74 |
| Lymphocytes (1 × 10³/µL) | 1.52 | 0.67 | 1.76 | 0.65 | 0.01 | 1.0 - 4.5 |
| Lymphocytes (%) | 27.68 | 13.82 | 30.37 | 11.98 | ns | 20 - 50 |
| Monocytes (1 × 10³/µL) | 4.18 | 1.76 | 4.7 | 2.99 | ns | 0.0 - 1.0 |
| Monocytes (%) | 7.29 | 2.99 | 7.67 | 3.65 | ns | 2 - 10 |
| Hemoglobin (g/100 mL) | 14.08 | 1.51 | 14.2 | 1.49 | ns | 13 - 17 |
| Platelets (1 × 10³/µL) | 220.83 | 69.68 | 221.4 | 87.46 | ns | 150 - 450 |
| AST (UI/L) | 43.61 | 30.16 | 42.38 | 31.03 | ns | 0 - 38 |
| ALT (UI/L) | 54.73 | 55.62 | 41.82 | 35.94 | ns | 0 - 41 |
| Urea (mg/100 mL) | 35.78 | 21.77 | 32.4 | 12.38 | ns | 10 - 50 |
| Creatinine (mg/100 mL) | 1.58 | 4.79 | 0.97 | 0.25 | ns | 0.70 - 1.40 |
| CK (UI/L) | 131.03 | 119.3 | 249.77 | 860.91 | ns | 230 - 460 |
| LDH (U/L) | 489.75 | 265.5 | 521.67 | 322.87 | ns | 24 - 195 |
| Glucose (mg/100 mL) | 153.01 | 109 | 112.38 | 70.54 | 0.002 | 70 - 99 |
1ns = not significant
SD = Standard deviation
3. Discussion
The COVID-19 pandemic has taken aback health systems worldwide, exerting immense pressure on the frequently overwhelmed health services of low- and middle-income countries (Wilder-Smith et al., 2020). As mentioned by Wilder-Smith et al., the 100 million annual cases of dengue already demand a high percentage of the health system capacity and exerts an especially heavy toll on Southeast Asia and Latin America, the COVID-19 pandemics aggravating the scenario. The overlapping incidence of COVID-19 and dengue fever poses difficulties in timely patient diagnosis, treatment and disease prevention. Nevertheless, the direct clinical consequences of SARS-CoV-2 and dengue virus co-infection have not been fully characterized at this point.
The Federal District is the smallest federative unit of Brazil, located in the South-West region of the country. The district was created in 1960 and holds the capital of Brazil. Today, the district has a population of approximately 3 million people and Planaltina has 164,939 people (ibge.gov.br). Up to 08/01/2020, 43,857 probable cases of dengue were reported in the Federal District with 2,266 cases in Planaltina (SES-DF). The Federal District has been one of the first regions in which COVID-19 was registered in Brazil, with over 200,000 confirmed cases and 3,000 deaths until november 2020 (SES-DF).
In the current study, 63% of the patients admitted to the hospital emergency room due to suggestive symptoms of COVID-19 were actually infected with the virus. Forty-seven percent of the patients tested negative for COVID-19, supporting the notion that overlapping symptoms of COVID-19 and other diseases compromise clinical diagnosis (Azeredo et al., 2020).
Almost half (44.6%) of COVID-19 patients had previously been infected with dengue virus (anti-dengue virus IgG +) and over a third of patients with confirmed COVID-19 also presented active dengue virus infection (38.4%). Despite the number of patients and our cohort's convenience sample nature, our observation points to the significant proportion of COVID-19 patients in the Federal District which are actually undergoing a SARS-CoV-2/dengue virus co-infection. Also, the percentage of COVID-19 patients with dengue fever history disagrees with a recently published mathematical model that demonstrated a negative correlation between COVID-19 and dengue fever infection in Brazilian patients (Nicolelis et al., 2020). Different from Nicolelis et al. (2020), we did not observe any indication that the infection with dengue virus could protect patients against SARS-CoV-2. In our hands, COVID-19/past dengue fever patients represented a significant fraction of our sample and presented similar clinical parameters to COVID-19 patients.
Conversely, COVID-19 patients with active dengue virus infection presented lower circulating lymphocyte and monocyte counts, lymphocytopenia being described before in SARS-CoV-2 and dengue virus co-infection patients (Saddique et al., 2020). Leukopenia is a usual finding in patients with classic dengue (Chaloemwong et al., 2018; Ali et al.,2007; Oliveira et al., 2009) but not in patients with COVID-19. Furthermore, dengue fever and COVID-19 patients usually present increased numbers of activated monocytes (Azeredo et al., 2020; Zhou et al., 2020). Thus, the association between SARS-CoV-2 and dengue viruses may impair innate and adaptive immune responses. Another important finding of this study was that the dual infection was significantly associated with increased blood glucose levels, a condition known to favor SARS‐CoV‐2 infection (Codo et al., 2020). Of note, it has been described that dengue infection may impair the host's energetic metabolism (Fontaine et al., 2015). Given that the dengue virus depends on glucose availability to efficiently replicate itself, it does induce glycolysis metabolism in infected cells. Unfortunately, some of patients' medical records did not provide information on other comorbidities, such as diabetes and high blood pressure (Pascarella et al., 2020; Werneck et al., 2018), which could contribute to a deeper comprehension of both dengue and COVID-19 clinic evolution.
Altogether, the hematological and biochemical alterations found among SARS-CoV-2/dengue virus co-infection patients was associated with declined lung function and higher frequency of hospitalization. Importantly, the development of severe acute respiratory syndrome is a clinical condition observed in approximately 15% of COVID-19 patients and usually requires intensive support care (Wu et al., 2020b). Furthermore, we show that over a third of COVID-19 patients in this cohort were also suffering from dengue fever. Therefore, the present data brings important information regarding the high rate of SARS-CoV-2 and dengue virus co-infection in this region of Brazil, as well as the aggravated outcomes associated. So, the dengue virus's endemic circulation seems to add more pressure on already overburdened health systems during COVID-19 pandemics Our observations should be expanded and replicated by others and used by both governments and health managers to improve COVID-19 and dengue control, as well as expedite appropriate patient diagnosis and management.. The current syndemic scenario experienced by different countries is not limited to COVID-19 and dengue fever and urges governments and health professionals to intensify research, in order to support control measures and policies to fight dengue and other epidemics while implementing preventive measures against COVID-19 pandemics.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
We thanks to the civil servants of the Hospital of Planaltina: Maria do Socorro Aguiar and Raphael Marques for the support provided.
References
- Ali N., Usman M., Syed N., Khurshid M. Haemorrhagic manifestations and utility of haematological parameters in dengue fever: a tertiary care Centre experience at Karachi. Scand. J. Infect. Dis. 2007;39:1025–1028. doi: 10.1080/00365540701411492. [DOI] [PubMed] [Google Scholar]
- Autrán-Gómez A.M., Favorito L.A. The Social, Economic and Sanitary Impact of COVID-19 Pandemic. Int. Braz. J. Urol. 2020;46:3–5. doi: 10.1590/S1677-5538.IBJU.2020.S1ED2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azeredo E.L., Neves-Souza P.C., Alvarenga A.R., Reis S.R.N.I., Torrentes-Carvalho A., Zagne S.M.O., Nogueira R.M.R., Oliveira-Pinto L.M., Kubelka C.F. Differential regulation of toll-like receptor-2, toll-like receptor-4, CD16 and human leukocyte antigen-DR on peripheral blood monocytes during mild and severe dengue fever. Immunology. 2020;130:202–216. doi: 10.1111/j.1365-2567.2009.03224.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butt M.H., Ahmad A., Misbah S., Tauqueer H.M., Khan Y.H. Dengue Fever and COVID‐19 Co‐Infection; A Threat to Public Health for Co‐epidemic in Pakistan. Journal of Medical Virology. 2020;2020:1–2. doi: 10.1002/jmv.26464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaloemwong J., Tantiworawit A., Rattanathammethee T., Hantrakool S., Chai-Adisaksopha C., Rattarittamrong E., Norasetthada L. Useful clinical features and hematological parameters for the diagnosis of dengue infection in patients with acute febrile illness: A retrospective study. BMC Hematology. 2018;18:1–10. doi: 10.1186/s12878-018-0116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codo A.C., Davanzo G.G., Monteiro L.B.M., Nakaya H.I., Farias A.S., Moraes-Vieira P.M. Elevated Glucose Levels Favor SARS-CoV-2 Infection and Monocyte Response through a HIF-1α/Glycolysis-Dependent. Axis. Cell Metabolism. 2020;32:437–446. doi: 10.1016/j.cmet.2020.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Oliveira Andrade R. Covid-19 is causing the collapse of Brazil's national health service. BMJ. 2020;370:m3032. doi: 10.1136/bmj.m3032. [DOI] [PubMed] [Google Scholar]
- de Souza C.D.F., do Carmo R.F., Machado M.F. The burden of COVID-19 in Brazil is greater in areas with high social deprivation. J. Travel. Med. 2020;31:taaa145. doi: 10.1093/jtm/taaa145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estofolete C.F., Machado F.L., Zini N., Luckemeyer G.D., Moraes M.M., dos Santos T.N.I.L., dos Santos B.F., Ruiz L.G.P., Vasilakis N., Lobo S.M.A., Nogueira M.L. Fatal stroke as presentation of SARS‐CoV‐2 and dengue virus coinfection. Journal of Medical Virology. 2020;2020:1–21. doi: 10.1002/jmv.26476. [DOI] [PubMed] [Google Scholar]
- Fontaine K.A., Sanchez E.L., Camarda R., Lagunoff M. Dengue Virus Induces and Requires Glycolysis for Optimal Replication. J.Virol. 2015;89:2358–2366. doi: 10.1128/JVI.02309-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorbalenya A.E., Baker S.C., Baric R.S., Groot R.J., Drosten C., Gulyaeva A.A., Haagmans B.I., Lauber C., Leontovich A.M., Neuman B.W., Penzar D., Perlman S., Poon L.L.M., Samborskiy D.V., Sidorov I.A., Sola I., Ziebuhr J. species severe acute respiratory syndrome related coronavirus: classifying 2019-nCoV and naming it SARS-CoV-2. Nat. Microbiol. 2020;5:536–544. doi: 10.1038/s41564-020-0695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoki K., Chakraborty A., SUR S. Molecular mechanisms and epidemiology of COVID-19 from an allergist's perspective. Journal of Allergy and Clinical Immunology. 2020;146:285–299. doi: 10.1016/j.jaci.2020.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazzarini L., Barzon L., Foglia F., Manfrin V., Pacenti M., Pavan F., Rassu M., Capelli G., Montarsi F., Martini S., Zanella F., Padovan M.T., Russo F., Gobbi F. First autochthonous dengue outbreak in Italy, August 2020. Euro Surveill. 2020;25:8–11. doi: 10.2807/1560-7917.ES.2020.25.36.2001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima L.D., Pereira A.M.M., Machado C.V. Crisis, conditioning factors, and challenges in the coordination of Brazil's federative State in the context of COVID-19. Cad Saude Publica. 2020;36 doi: 10.1590/0102-311x00185220. [DOI] [PubMed] [Google Scholar]
- Martins-Filho P.R., de Souza Araújo A.A., Quintans-Júnior L.J., Santos V.S. COVID-19 fatality rates related to social inequality in Northeast Brazil: a neighborhood-level analysis. J. Travel. Med. 2020;6:taaa128. doi: 10.1093/jtm/taaa128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nacher M., Douine M., Gaillet M., Flamand C., Rousset D., Rousseau C., Mahdaoui C., Carroll S., Valdes A., Passard N., Carles G., Djossou F., Demar M., Epelboin L. Simultaneous dengue and COVID-19 epidemics: Difficult days ahead? PLOS Neglected Tropical Diseases. 2020;14:1–8. doi: 10.1371/journal.pntd.0008426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolelis M.A.L., Raimundo R.L.G., Peixoto P.S., Andreazzi C.S. How Super-Spreader Cities, Highways, Hospital Bed Availability, and Dengue Fever Influenced the Covid-19 Epidemic in Brazil. MedRxiv. 2020;2020:1–50. doi: 10.1101/2020.09.19.20197749. [DOI] [Google Scholar]
- Olive M.M., Baldet T., Devillers J., Fite J., Paty M.C., Paupy C., Que'nel P., Quillery E., Raude J., Stahl J.P., Thiann-Bo-Morel M., Roiz D. The COVID-19 pandemic should not jeopardize dengue control. PLoS neglected tropical diseases. 2020;14 doi: 10.1371/journal.pntd.0008716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira E.C., Pontes E.R., Cunha R.V., Froes I.B., Nascimento D. Hematological abnormalities in patients with dengue. Rev Soc Bras Med Trop. 2009;42:682–685. doi: 10.1590/s0037-86822009000600014. [DOI] [PubMed] [Google Scholar]
- Pascarella G., Strumia A., Piliego C., Bruno F., Del Buono R., Costa F., Scarlata S., Agrò F.E. COVID-19 diagnosis and management: a comprehensive review. Journal of Internal Medicine. 2020;288:192–206. doi: 10.1111/joim.13091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saavedra-velasco M., Chiara-Chilet C., Pichardo-Rodriguez R., Grandez-Urbina A., Inga-Berrospi F. Coinfección entre dengue y COVID- 19: Necesidad de abordaje en zonas endémicas. [Coinfection between dengue and covid-19: need for approach in endemic zones.] Rev. Fac. Cien. Med. Univ. Nac. Córdoba. 2020;77:52–54. doi: 10.31053/1853.0605.v77.n1.280. [DOI] [PubMed] [Google Scholar]
- Saddique A., Rana M.S., Alam M.M., Ikram A., Usman M., Salman M., Faryal R., Massab U., Bokhari H., Mian M.S., Israr A., Safiullah “Emergence of co-infection of COVID-19 and dengue: A serious public health threat. Journal of Infection. 2020;18:1–3. doi: 10.1016/j.jinf.2020.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werneck G.L., Macias A.E., Mascarenas C., Coudeville L., Morley D., Recamier V., Guergova-Kuras M., Puentes-Rosas E., Baurin N., Toh M. Comorbidities increase in-hospital mortality in dengue patients in Brazil. Memórias do Instituto Oswaldo Cruz. 2018;113 doi: 10.1590/0074-02760180082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO Coronavirus disease 2019 (COVID-19) situation report-51. 2020. World Health Organization. 2020 https://apps.who.int/iris/bitstream/handle/10665/331475/nCoVsitrep11Mar2020-eng.pdf?sequence=1&isAllowed=y Available at. Last accessed 11.09.2020. [Google Scholar]
- WHO Novel Coronavirus(2019-nCoV) Situation Report-193. 2020. World Health Organization. 2020 https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200731-covid-19-sitrep-193.pdf?sfvrsn=42a0221d_4 Available at. Last accessed 11.09.2020. [Google Scholar]
- Wiersinga W.J., Rhodes A., Cheng A.C., Peacock S.J., Prescott H.C. Pathophysiology, Transmission, Diagnosis, and Treatment of Coronavirus Disease 2019 (COVID-19): A Review. JAMA. 2020;324:782–793. doi: 10.1001/jama.2020.12839. [DOI] [PubMed] [Google Scholar]
- Wu D., Lu J., Liu Q., Ma X., He W. To alert co-infection of SARS-COV-2 and dengue virus in developing countries in the dengue-endemic area. Infect Control Hosp Epidemiol. 2020;2020 doi: 10.1017/ice.2020.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Z., McGoogan J.M. Characteristics of and important lessons from the coronavirus disease 2019(COVID-19) outbreak in China. Jama. 2020;323:1239–1242. doi: 10.1001/jama.2020.2648. [DOI] [PubMed] [Google Scholar]
- Zhou Y., Fu B., Zheng X., Wang D., Zhao C., Qi Y., Sun R., Tian Z., Xu X., Wei H. Pathogenic T-cells and inflammatory monocytes incite inflammatory storms in severe COVID-19 patients. National Science Review. 2020;7:998–1002. doi: 10.1093/nsr/nwaa041. [DOI] [PMC free article] [PubMed] [Google Scholar]