Abstract
As COVID-19 (coronavirus disease 2019) continues to rapidly spread throughout the world, the incidence varies greatly among different countries. These differences raise the question whether nations with a lower incidence share any medical commonalities that could be used not only to explain that lower incidence but also to provide guidance for potential treatments elsewhere. Such a treatment would be particularly valuable if it could be used as a prophylactic against SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2) transmission, thereby effectively slowing the spread of the disease while we await the wide availability of safe and effective vaccines. Here, we show that countries with routine mass drug administration of prophylactic chemotherapy including ivermectin have a significantly lower incidence of COVID-19. Prophylactic use of ivermectin against parasitic infections is most common in Africa and we hence show that the reported correlation is highly significant both when compared among African nations as well as in a worldwide context. We surmise that this may be connected to ivermectin's ability to inhibit SARS-CoV-2 replication, which likely leads to lower infection rates. However, other pathways must exist to explain the persistence of such an inhibitory effect after serum levels of ivermectin have declined. It is suggested that ivermectin be evaluated for potential off-label prophylactic use in certain cases to help bridge the time until a safe and effective vaccine becomes available.
Keywords: SARS-CoV-2, COVID-19, Prophylaxis, Ivermectin, Mass drug administration, Prophylactic chemotherapy
1. Introduction
Coronavirus disease 2019 (COVID-19) is caused by SARS-CoV-2 (severe acute respiratory syndrome coronavirus 2), a novel coronavirus that emerged in Wuhan, Hubei Province, China, in December 2019 [1] and quickly spread throughout the entire world [2]. As of 20 October 2020, approximately 41 million people have been diagnosed and more than one million have died from the disease worldwide [3]. Many nations have responded by implementing strict social distancing guidelines [4] that are beginning to show promising results in some countries [5]. While this is in itself a positive development, a decrease in new cases is also likely to decrease adherence to protective measures or to cause authorities to lift restrictions aimed at containing the spread of the virus as they consider competing economic interests. This, paired with the continued resistance to initial control measures in some nations such as the USA [4], carries the risk of further accelerating global disease spread and, consequently, fatalities. On 18 April 2020, a total of 11 265 patients died from COVID-19 in a single day worldwide [3]. While several vaccine candidates are entering clinical trial phases, it is unlikely that a safe and effective vaccine will be available to the public within the next few months [6]. In order to effectively reduce the spread of SARS-CoV-2 and especially the associated fatalities, a highly effective treatment option is needed.
As time is of the essence and the approval process for new drugs can be lengthy [7], there have been many attempts at repurposing existing and approved drugs for the treatment of SARS-CoV-2 infection [8], including malaria drugs such as hydroxychloroquine and chloroquine, which have recently been shown to be less effective than originally thought while carrying considerable risk of sometimes fatal complications and interactions [9]. Other approaches focus on repurposing existing antiviral drugs such as remdesivir, which has been shown to significantly reduce the recovery time in hospitalised patients [10]. However, there is currently no accepted treatment for patients who are not yet hospitalised. Treating patients before they need to be admitted—perhaps even prophylactically—could greatly reduce the load on hospitals, protect healthcare professionals and reduce the spread of SARS-CoV-2.
One avenue to slow viral transmission would be to inhibit replication of the virus, thus reducing the viral load in infected individuals. Interestingly, the relatively old antiparasitic drug ivermectin has recently been reported to inhibit SARS-CoV-2 replication in vitro [11], although the authors rightfully caution that additional studies will be needed to determine dosing for potential use in COVID-19 patients. This is particularly important as the serum levels used in their study far exceeded those that would be achieved with commonly administered safe doses. While they certainly do not suggest prophylactic use of ivermectin for SARS-CoV-2, the drug is actually widely used prophylactically in mass drug administration (MDA) campaigns both against filariasis [12] and onchocerciasis [13]. Throughout the past few months, interest in ivermectin as a treatment for SARS-CoV-2-infected patients has grown [14] and the drug has been shown to reduce mortality among hospitalised patients [15]. In contrast to most other recently explored treatments, ivermectin has been reported as especially promising in early and mild cases of COVID-19 [16]. This strong precedent, paired with ivermectin's well understood safety profile [17], naturally raises the question whether it could also be used prophylactically against SARS-CoV-2.
2. Methods
To answer this intriguing question, we collected data from countries that routinely deploy prophylactic chemotherapy (PCT) using various drugs including ivermectin [18]. Based on the varying MDA designs, we grouped these countries into two different categories—those that include ivermectin in their PCT and those that do not. We then proceeded to compare COVID-19 proliferation between these two groups and further contrasted them against a third group of countries that do not use PCT at all [3].
2.1. Data collection
The data used in this study were obtained from two publicly available databases. Information about PCT was extracted from the PCT Databank administered by the World Health Organization (WHO), which provides current and historic data regarding MDA campaigns [18]. Current data on COVID-19 cases were obtained from Worldometer, a public data aggregation site used—among others—by the COVID-19 portal published by Johns Hopkins University [3]. All data are current as of 20 October 2020. As in all cases involving more than one data source, there was a certain amount of missing data, which we addressed by omitting any country that did not have sufficient coverage in both sources. We then extracted and aggregated the data in a standard spreadsheet format provided in the Supplementary material.
2.2. Statistical analysis
Statistical analysis was performed using IBM SPSS Statistics v.23.0 (IBM Corp., Armonk, NY, USA). We grouped countries into three different bins: countries that do not use any PCT; countries that use some PCT that does not include ivermectin; and countries that use PCT with ivermectin. As a dependent variable, we selected the incidence of COVID-19 measured in confirmed cases per 100 000 population.
Standard analysis of variance (ANOVA) was not an option for data analysis since the data were not normally distributed as determined by the Shapiro–Wilk test. This is primarily due to large variability within the ‘No PCT’ group as well as the starkly different sizes of the three groups. We therefore analysed the assembled data using a one-way non-parametric Kruskal–Wallis ANOVA on ranks with incidence as the dependent variable and the three treatments as factor (PCT with ivermectin, PCT without ivermectin and no PCT). Post-hoc comparisons were run using Dunn's test. Significances were adjusted using the Bonferroni method.
3. Results and discussion
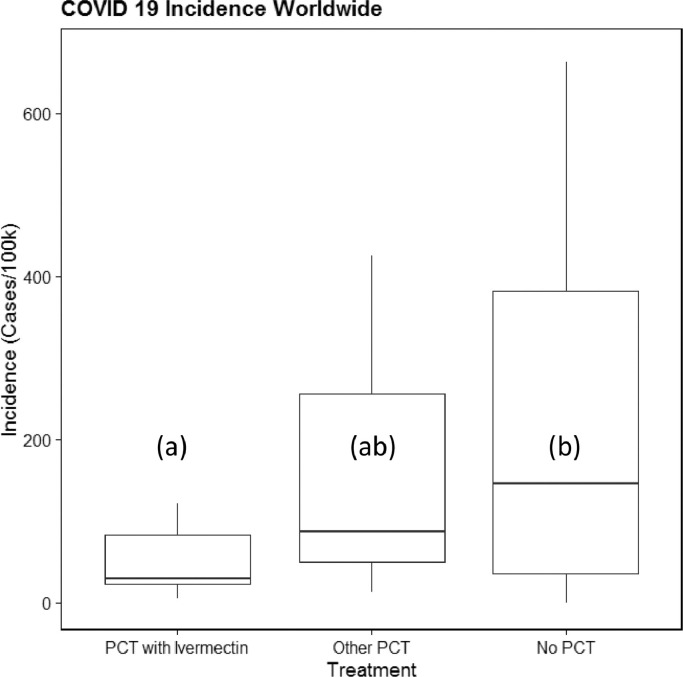
Our study compared the incidence of COVID-19 among countries with different PCT campaigns and those countries in which PCT is non-existent. It is perhaps obvious that the latter group is by far the largest. It should also not be surprising that this set of samples had a rather large variability (Fig. 1 ). However, in spite of this, the difference between nations that deploy PCT using ivermectin and those that do not use any PCT turned out to be highly significant (adjusted significance P < 0.01). These initial results were obtained on 15 April 2020 and because at that time SARS-CoV-2 was still being detected in new countries on an almost daily basis, we chose to monitor the situation and observe whether this correlation would over time become less significant. We updated our calculations and added additional newly affected countries several times throughout the month of May 2020 and noticed that the observed association between ivermectin MDA and lower COVID-19 incidence actually grew strictly stronger over time. By 5 June 2020, the adjusted significance had improved to P < 0.001, actually reported by IBM SPSS Statistics as 0.000. It has remained at that level since.
Fig. 1.
Country-specific COVID-19 (coronavirus disease 2019) incidence in groups with different types of prophylactic chemotherapy (PCT) for parasitic infections. The letters (a,b) denote statistically significant groups (P ≤ 0.05). Outliers above the 95th percentile were removed for visual clarification. Whiskers represent 10th and 90th percentiles.
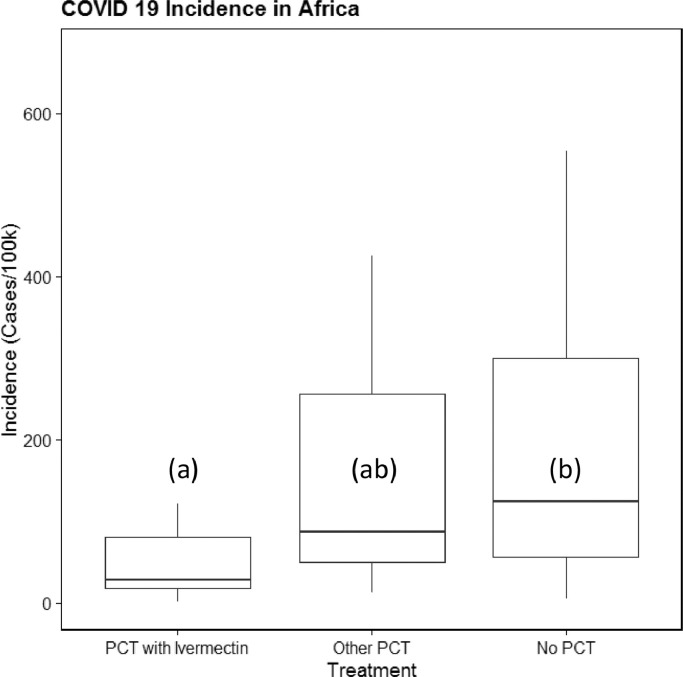
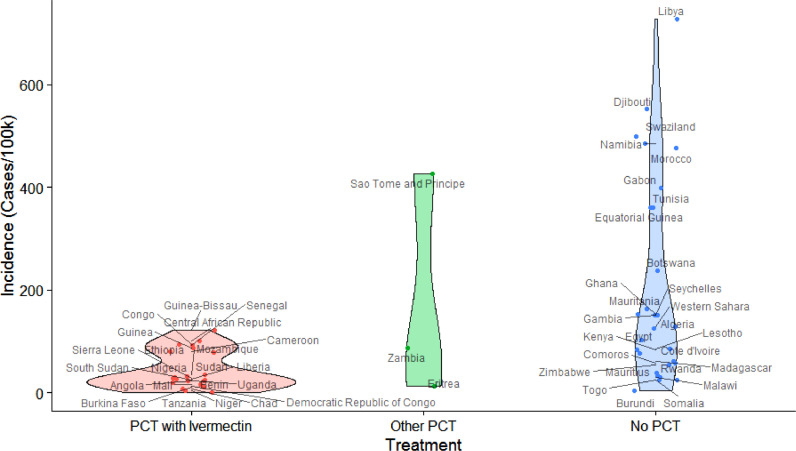
As we have stated, the sizes of the three samples (PCT with ivermectin, other PCT and no PCT) vary greatly. Another important aspect to consider is the fact that many of the ivermectin campaigns are unsurprisingly administered in African countries as the underlying parasitic infections are particularly common in these nations. As such, it is important to look at the subset of African countries separately as well. Fig. 2 shows a boxplot similar to Fig. 1 but containing only the African countries in the data set. It should not be surprising that the largest difference between the two analyses can be seen in the ‘No PCT’ group as this group contained the most non-African countries. The much smaller number of nations on the African continent and associated island groups allows us to enumerate the individual countries and to visualise them in a violin plot. Fig. 3 quite clearly visualises the strong correlation between PCT with ivermectin and lower incidence of COVID-19. This relationship is statistically significant (P = 0.017), making it only slightly less significant in Africa than among the worldwide data set.
Fig. 2.
Incidence of COVID-19 (coronavirus disease 2019) as a function of prophylactic chemotherapy (PCT) with ivermectin in African countries. The letters (a,b) denote statistically significant groups (P ≤ 0.05). Outliers above the 95th percentile were removed for visual clarification. Whiskers represent 10th and 90th percentiles.
Fig. 3.
Incidence of COVID-19 (coronavirus disease 2019) as a function of prophylactic chemotherapy (PCT) with ivermectin in African countries as violin plot.
As COVID-19 is such a new disease, none of the existing MDA campaigns are targeted at controlling its spread. Nor is there any documented prophylactic use of the deployed drugs against SARS-CoV-2 infection. However, there is a very strong negative correlation between the use of PCT—especially involving ivermectin—and COVID-19 proliferation. This, paired with ivermectin's proven inhibitory effect on SARS-CoV-2 replication in vitro, leads us to the hypothesis that the drug may have a—likely indirect—prophylactic effect and thereby reduce the spread of the disease.
It might be interesting to note that the percentage of the overall population that received PCT using ivermectin mostly ranged from 30–90%, yet there was no significant difference in the resulting incidence of COVID-19. Even the lower treatment coverages achieved the same reductions resulting from MDA reaching nearly the entire population. The reasons for this fact are so far unexplained. There was also no detectable advantage to any one administration timeframe or interval. While individual dosages generally varied between 150 μg and 200 μg per kilogram of body weight, there seemed to be no notable difference in COVID-19 incidence among recipients of different dosages either. It must therefore be assumed that any pathway connecting ivermectin administration and lower COVID-19 incidence is achieved by administration of the drug in relatively low doses far below potentially dangerous levels considered elsewhere as potentially effective for COVID-19 treatment [19]. This becomes less surprising once we consider the relatively short half-life of ivermectin [20], meaning that the added effect of any higher dose would not be prolonged. Instead, we hypothesise that there is an as of yet unknown pathway that can be triggered with lower, proven safe doses.
The fact that PCT without ivermectin also showed a strong negative—albeit not statistically significant—correlation with COVID-19 incidence suggests that other drugs used in MDA campaigns might include additional candidates for the treatment and/or prevention of COVID-19. It is, however, important to note that many of the analysed countries that only administered these other drugs in 2018 actually have used ivermectin in previous or following years. Hence, a residual effect of an ivermectin-induced pathway cannot be ruled out, although the exact nature of such a pathway would still need to be discovered. This speculation would gain further strength if experimental analysis could prove that SARS-CoV-2 replication remains inhibited after serum levels of ivermectin decline.
It is important to note that the hypothesis that ivermectin might have a prophylactic effect against SARS-CoV-2 is merely based on a rather strong correlation. On the other hand, this correlation has grown increasingly stronger in the worldwide data set earlier this year and then been independently replicated within the African data set later in the summer. Both remain highly significant, suggesting that there may be a causal connection, which is also suggested by other recent findings reported in literature. We therefore hope that this communication may serve as an invitation to further investigate and consider ivermectin as a potential prophylactic against COVID-19. In addition to the obvious advantages of a potential prophylactic, more refined results could hopefully also deter the public from further dangerous self-medication with ivermectin that has sometimes included veterinary-grade products that contain additional ingredients [21]. In this sense, even negative results might be very valuable to the health community and to society at large.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ijantimicag.2020.106248.
Appendix. Supplementary materials
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Munster VJ, Koopmans M, van Doremalen N, van Riel D, de Wit E. A novel coronavirus emerging in China—key questions for impact assessment. N Engl J Med. 2020;382:692–694. doi: 10.1056/NEJMp2000929. [DOI] [PubMed] [Google Scholar]
- 3.Worldometer . April 2020. COVID-19 coronavirus pandemic. https://www.worldometers.info/coronavirus [accessed 18. [Google Scholar]
- 4.Allcott H, Boxell L, Conway J, Gentzkow M, Thaler M, Yang DY. Polarization and public health: partisan differences in social distancing during the coronavirus pandemic. Working Paper 26946. National Bureau of Economic Research. 2020 doi: 10.3386/w26946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Singer HM. The COVID-19 pandemic: growth patterns, power law scaling, and saturation. Phys Biol. 2020;17 doi: 10.1088/1478-3975/ab9bf5. [DOI] [PubMed] [Google Scholar]
- 6.Cohen J. Vaccine designers take first shots at COVID-19. Science. 2020;368:14–16. doi: 10.1126/science.368.6486.14. [DOI] [PubMed] [Google Scholar]
- 7.Van Norman GA Drugs, devices, and the FDA: Part 1: an overview of approval processes for drugs. JACC Basic Transl Sci. 2016;1:170–179. doi: 10.1016/j.jacbts.2016.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou Y, Hou Y, Shen J, Huang Y, Martin W, Cheng F. Network-based drug repurposing for novel coronavirus 2019-nCoV/SARS-CoV-2. Cell Discov. 2020;6:14. doi: 10.1038/s41421-020-0153-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colson P, Rolain JM, Lagier JC, Brouqui P, Raoult D. Chloroquine and hydroxychloroquine as available weapons to fight COVID-19. Int J Antimicrob Agents. 2020;55 doi: 10.1016/j.ijantimicag.2020.105932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Tawfiq JA, Al-Homoud AH, Memish ZA. Remdesivir as a possible therapeutic option for the COVID-19. Travel Med Infect Dis. 2020;34 doi: 10.1016/j.tmaid.2020.101615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caly L, Druce JD, Catton MG, Jans DA, Wagstaff KM. The FDA-approved drug ivermectin inhibits the replication of SARS-CoV-2 in vitro. Antiviral Res. 2020;178 doi: 10.1016/j.antiviral.2020.104787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Richard-Lenoble D, Chandenier J, Gaxotte P. Ivermectin and filariasis. Fundam Clin Pharmacol. 2003;17:199–203. doi: 10.1046/j.1472-8206.2003.00170.x. [DOI] [PubMed] [Google Scholar]
- 13.Greene BM, Taylor HR, Cupp EW, Murphy RP, White AT, Aziz MA. Comparison of ivermectin and diethylcarbamazine in the treatment of onchocerciasis. N Engl J Med. 1985;313:133–138. doi: 10.1056/NEJM198507183130301. [DOI] [PubMed] [Google Scholar]
- 14.Scheim D. Ivermectin for COVID-19 treatment: clinical response at quasi-threshold doses via hypothesized alleviation of CD147-mediated vascular occlusion. SSRN. 2020 Jun 26 doi: 10.2139/ssrn.3636557. [DOI] [Google Scholar]
- 15.Rajter JC, Sherman MS, Fatteh N, Vogel F, Sacks J, Rajter JJ. ICON (Ivermectin in COvid Nineteen) Study: use of ivermectin is associated with lower mortality in hospitalized patients with COVID-19. SSRN. 2020 Jun 16 doi: 10.2139/ssrn.3631261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heidary F, Ivermectin Gharebaghi R. a systematic review from antiviral effects to COVID-19 complementary regimen. J Antibiot (Tokyo) 2020;73:593–602. doi: 10.1038/s41429-020-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Muñoz J, Ballester MR, Antonijoan RM, Gich I, Rodríguez M, Colli E. Safety and pharmacokinetic profile of fixed-dose ivermectin with an innovative 18mg tablet in healthy adult volunteers. PLoS Negl Trop Dis. 2018;12 doi: 10.1371/journal.pntd.0006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.World Health Organization (WHO) WHO; 2020. PCT Databank. https://www.who.int/neglected_diseases/preventive_chemotherapy/lf/en/ [accessed 18 April 2020]. [Google Scholar]
- 19.Schmith VD, Zhou J, Lohmer LRL. The approved dose of ivermectin alone is not the ideal dose for the treatment of COVID-19. Clin Pharmacol Ther. 2020;108:762–765. doi: 10.1002/cpt.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fink DW, Porras AG. Pharmacokinetics of ivermectin in animals and humans. In: Campbell WC, editor. Ivermectin and abamectin. Springer; New York, NY: 1989. pp. 113–130. [DOI] [Google Scholar]
- 21.US Food and Drug Administration (FDA) FDA; 2020. COVID-19 and ivermectin intended for animals. https://www.fda.gov/animal-veterinary/product-safety-information/faq-covid-19-and-ivermectin-intended-animals [accessed 19 April 2020]. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.