Abstract
Simple Summary
This study investigated the association between survival outcome and the new grading system among advanced stage lung adenocarcinoma (LADC) (stages IIIA, IIIB and IV) patients who were diagnosed as LADC with a pathologic report according to a new grading system by the International Association for the Study of Lung Cancer (IASLC) pathology committee. The results indicate that the poorly differentiated group had a poorer prognosis in PFS, as did patients with wild-type EGFR who were treated with chemotherapy. No survival difference could be found among EGFR mutation patients. Older age and a lower body mass index also led to worse survival. Patients with poorly differentiated adenocarcinoma likewise had worse survival, especially compared to those with moderately differentiated adenocarcinoma. Our findings highlight that the therapeutic regimen should be adjusted for wild-type EGFR patients with poorly differentiated adenocarcinoma treated with chemotherapy to provide better outcomes. No survival difference could be seen among EGFR mutation patients.
Abstract
The impact of the new International Association for the Study of Lung Cancer pathology committee grading system for advanced lung adenocarcinoma (LADC) on survival is unclear, especially in Asian populations. In this study, we reviewed the prognostic outcomes of patients with late-stage disease according to the new grading system. We reviewed 136 LADC cases who underwent a small biopsy from 2007 to 2018. Tumors were classified according to the new grading system for LADC. Baseline characteristics (age, sex, smoking status, body mass index, and driver gene mutations) were analyzed. Kaplan–Meier and Cox regression analyses were used to determine correlations with the new grading system and prognosis. Patients with poorly differentiated adenocarcinoma were significantly correlated with a poor progression-free survival (PFS) (p = 0.013) but not overall survival (OS) (p = 0.154). Subgroup analysis showed that wild-type EGFR patients with poorly differentiated adenocarcinoma treated with chemotherapy had significantly worse PFS (p = 0.011). There was no significant difference in survival among the patients with epidermal growth factor receptor mutations who were treated with tyrosine kinase inhibitors. Patients aged >70 years and those with a BMI ≤ 25 kg/m2 and wild-type patients had significantly worse OS in both univariate (HR = 1.822, p = 0.006; HR = 2.250, p = 0.004; HR = 1.537, p = 0.046, respectively) and multivariate analyses (HR = 1.984, p = 0.002; HR = 2.383, p = 0.002; HR = 1.632, p = 0.028, respectively). Despite therapy, patients with poorly differentiated tumors still fared worse than those with better differentiated tumors. No differences were found among the EGFR mutations treated with TKI. Our findings highlight that the therapeutic regimen should be adjusted for EGFR Wild-type patients with poorly differentiated adenocarcinoma treated with chemotherapy to provide better outcomes.
Keywords: late stage, lung adenocarcinoma, histology, subtype, EGFR mutation, smoker, sex
1. Introduction
Lung cancer is a leading cause of life-threatening malignancy worldwide. According to the revised classification criteria released by the International Association for the Study of Lung Cancer (IASLC), the American Thoracic Society (ATS), and the European Respiratory Society (ERS) in 2011, lung adenocarcinoma (LADC) can be categorized into five subtypes (lepidic, acinar, solid, papillary, and micropapillary) [1]. Most previous cohort studies were based on the IASLC/ATS/ERS classification, which is used to predict outcomes in the early stages of the disease [2,3,4,5,6,7]. However, Moreira et al. proposed a new grading system for invasive pulmonary adenocarcinoma taking into account the heterogeneity of pulmonary adenocarcinomas that involves useful prognostic groupings based on the predominant and high-grade histologic subtypes [8].
To date, several studies have investigated the impact of the IASLC/ATS/ERS classification system introduced in 2011 to predict the prognosis in patients with advanced LADC, although tumor heterogeneity in late-stage LADC is widely known to be complex [9,10,11,12]. Campos-Parra et al. reported that high-grade LADC (micropapillary, papillary, and solid-predominant) was associated with better outcomes compared to intermediate-grade LADC (lepidic- and acinar-predominant), especially when treated with standard platinum-based doublet chemotherapy [12]. Da Cruz et al. concluded that predominant subtyping is reliable in the prediction stage for IV LADC, especially in solid subtype [10], whereas Clay et al. indicated that the major solid histologic subtype of metastatic LADC is associated with inferior survival outcomes under systemic treatment [9]. Small biopsies remain the primary method for diagnosis, classification, and molecular analysis, accounting for 70% in advanced disease [13]. As the histologic subtype is an important predictive factor, core biopsy results are reliable representations of the original tumor entity and valuable for predicting prognosis [14,15,16,17,18]. In addition, it is vital to assess the relevance of driver gene mutations, such as the epidermal growth factor receptor (EGFR), anaplastic lymphoma kinase (ALK), Kirsten rat sarcoma (KRAS), and proto-oncogene B-Raf (BRAF), in different subtypes when predicting a patient’s prognosis [19,20,21,22].
To evaluate the prognostic relevance of the new grading system [8], we aimed to apply this system to patients with advanced LADC harboring (or not) driver gene mutations and analyze the potential prognostic differences. In this study, we examined the clinical relevance for Asian patients with late-stage disease using small biopsy samples.
2. Results
2.1. Clinicopathological Factors
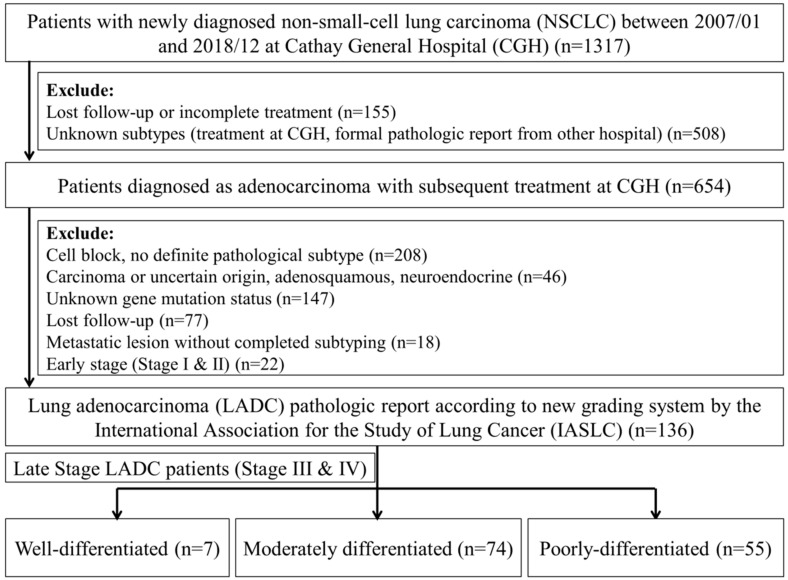
As classified by the IASLC pathology committee’s new grading system, 136 LADC cases out of a total of 1317 met the inclusion criteria and were analyzed (Figure 1). Of the 136 patients, 7 (5.1%) had well differentiated adenocarcinoma, 74 (54.4%) had moderately differentiated adenocarcinoma, and 55 (40.4%) had poorly differentiated adenocarcinoma (Figure 1 and Figure 2 and Table 1). There were 71 (52.2%) females and 65 (47.8%) males in the sample, ranging in age from 31 to 91 years (mean = 65.3 years), and 87 (63.5%) patients were aged <70 years. In total, 92 patients (67.6%) were never smokers, and 93 patients (72.1%) had a BMI ≤ 25 kg/m2. Fifty-eight (42.6%) patients had wild-type EGFR, and 78 (57.4%) had driver gene mutations. Seventy-six (69.7%) patients received a first-line treatment with TKIs, and 33 (30.3%) received platinum-based chemotherapy. Sex, BMI, smoking status, stage, and treatment were all independent of the grade (p > 0.05, chi-square test) (Table 2).
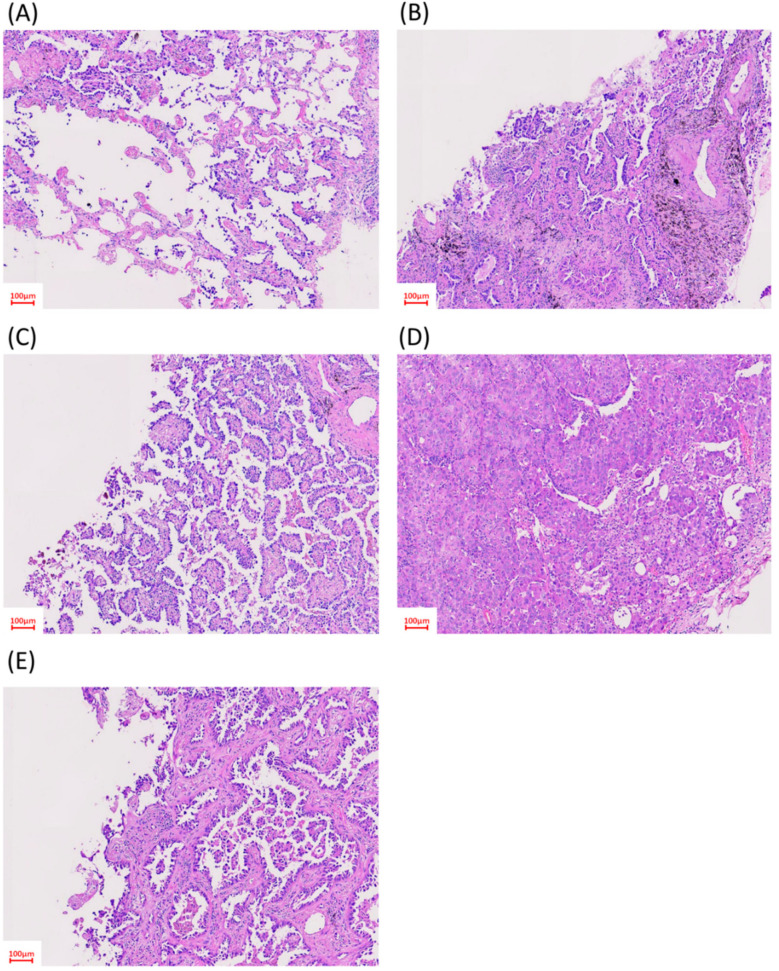
Figure 1.
Histological patterns of adenocarcinoma. Representative hematoxylin and eosin-stained biopsy specimens: (A) lepidic-predominant pattern; (B) acinar-predominant pattern; (C) papillary-predominant pattern; (D) solid-predominant pattern; and (E) micropapillary-predominant pattern.
Figure 2.
Selection criteria for the subjects.
Table 1.
Adenocarcinoma subtyping according to the New IASLC grading system.
| Grade | Differentiation | N (%) |
|---|---|---|
| Grade 1 | Well differentiated | 7 (5.1%) |
| Grade 2 | Moderately differentiated | 74 (54.4%) |
| Grade 3 | Poorly differentiated | 55 (40.5%) |
| Total number of included patients: 136 | ||
Table 2.
Relationships among differentiation and clinicopathological variables in advanced lung adenocarcinoma patients.
| Variables | N (%) | Well Differentiated | Moderately Differentiated | Poorly Differentiated | p Value |
|---|---|---|---|---|---|
| 7 (5.1%) | 74 (54.4%) | 55 (40.4%) | |||
| Sex | |||||
| Male | 65 (47.8%) | 3 (2.2%) | 31 (22.8%) | 31 (22.8%) | 0.257 |
| Female | 71 (52.2%) | 4 (2.9%) | 43 (31.6%) | 24 (17.6%) | |
| Age groups (years) | |||||
| ≤70 | 87 (64.0%) | 7 (5.1%) | 50 (36.8%) | 30 (22.1%) | 0.039 |
| >70 | 49 (36.0%) | 0 (0.0%) | 24 (17.6%) | 25 (18.4%) | |
| BMI (kg/m2) | |||||
| ≤25 | 93 (72.1%) | 5 (3.9%) | 50 (38.8%) | 38 (29.5%) | 0.979 |
| >25 | 36 (27.9%) | 2 (1.6%) | 20 (15.5%) | 14 (10.9%) | |
| Missing | 7 | 0 | 4 | 3 | |
| Smoking Status | |||||
| Non-smoker | 92 (67.6%) | 6 (4.4%) | 49 (36.0%) | 37 (27.2%) | 0.572 |
| Ever smoker | 44 (32.4%) | 1 (0.7%) | 25 (18.4%) | 18 (13.2%) | |
| Stage | |||||
| IIIA & IIIB | 17 (12.5%) | 1 (0.7%) | 9 (6.6%) | 7 (5.1%) | 0.985 |
| IV | 119 (87.5%) | 6 (4.4%) | 65 (47.8%) | 48 (35.3%) | |
| Driver gene mutation | |||||
| EGFR Wild-type | 58 (42.6%) | 3 (2.2%) | 29 (21.3%) | 26 (19.1%) | 0.656 |
| EGFR Mutation | 78 (57.4%) | 4 (2.9%) | 45 (33.1%) | 29 (21.3%) | |
| Exon 18 mutation | 7 (5.1%) | 1 (0.7%) | 5 (3.7%) | 1 (0.7%) | |
| Exon 19 deletion | 33 (24.3%) | 2 (1.5%) | 16 (11.8%) | 15 (11.0%) | |
| Exon 20 insertion mutation | 2 (1.5%) | 0 (0.0%) | 2 (1.5%) | 0 (0.0%) | |
| Exon 21 point mutation | 34 (25.0%) | 1 (0.7%) | 21 (15.4%) | 12 (8.8%) | |
| EML4-ALK | 2 (1.5%) | 0 (0.0%) | 1 (0.7%) | 1 (0.7%) | |
| Treatments | |||||
| TKIs | 76 (69.7%) | 5 (4.6%) | 44 (40.4%) | 27 (24.8%) | 0.650 |
| Chemotherapy | 33 (30.3%) | 1 (0.9%) | 18 (16.5%) | 14 (12.78%) | |
| Not systemic treatment | 27 | 1 | 12 | 14 |
Tyrosine kinase inhibitor (TKI), gefitinib (Iressa), erlotinib (Tarceva), afatinib (Giotrif), and crizotinib; chemotherapy, pemetrexed (Alimta), gemcitabine (Gemzar), and vinorelbine (Navelbine); p-values from Pearson’s chi-squared test. BMI: body mass index; EGFR: epidermal growth factor receptor; EML4: echinoderm microtubule-associated protein-like 4; ALK: anaplastic lymphoma kinase;
2.2. EGFR Wild-Type and EGFR Mutations and First-Line Treatment
EGFR mutations were also independent of grade (p > 0.05, chi-square test). There were 58 wild-type EGFR patients (42.6%), and 78 (57.4%) had EGFR mutations. Among those with EGFR mutations, there were 7 exon 18 mutations (5.1%), 33 exon 19 deletions (24.3%), 2 exon 20 insertions (1.5%), and 34 exon 21 point mutations (25.0%). Only two patients had EML4-ALK fusions (1.5%). In the poorly differentiated group, 29 (21.3%) patients had EGFR mutations, compared to 45 (33.1%) in the moderately differentiated group and 4 (2.9%) in the well differentiated group. Most of the mutations were exon 19 deletions and exon 21 point mutations. The frequency of gene mutations was slightly higher in the moderately differentiated group, which was also detected in those receiving first-line TKIs (44, 40.4%) (Table 2).
2.3. Survival Analysis
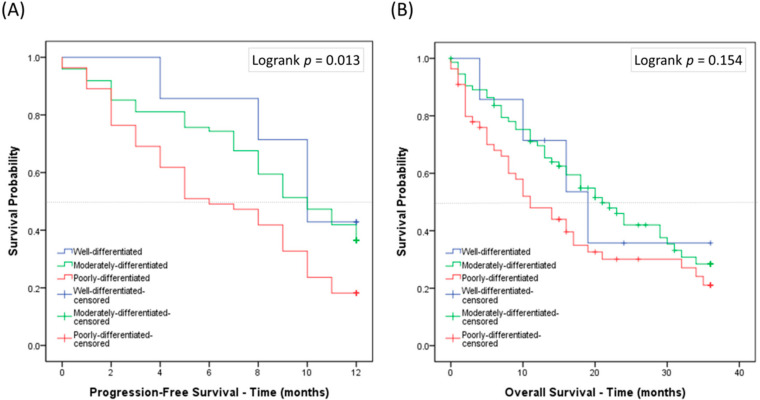
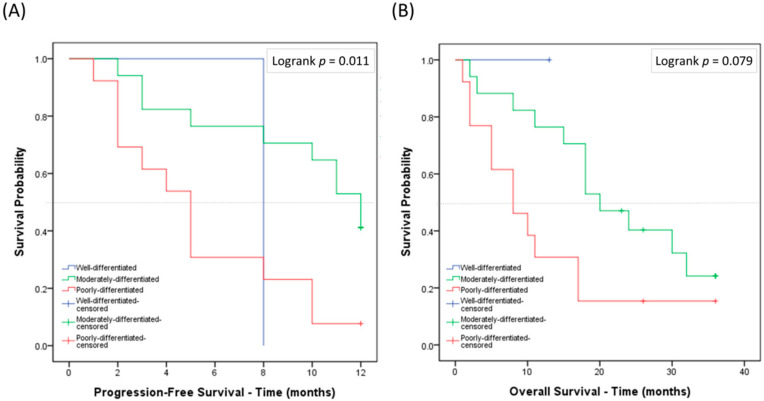
The survival outcomes of the poorly differentiated, moderately differentiated, and well differentiated groups diagnosed between January 2007 and December 2018 were analyzed. Each group had a statistically significant difference in PFS (p = 0.013) but not in OS (p = 0.154) (Figure 3A,B). Those without systemic treatment (n = 27, Table 2) had a predominantly inferior outcome in both PFS (p < 0.001) and OS (p < 0.001) (Figure S2). No difference in PFS or OS was seen between each group (Figure S3). When the survival analysis examined only wild-type EGFR patients, the poorly differentiated group had the worst PFS (p = 0.011) when treated with chemotherapy (Figure 4A,B). This was not observed in those with EGFR mutations when treated with TKIs (Figure S1A,B).
Figure 3.
The Kaplan–Meier survival curves for each group of the new grading system: (A) progression-free survival; and (B) overall survival.
Figure 4.
Kaplan–Meier survival curves for each group with wild-type EGFR and those treated with chemotherapy under new grading system: (A) progression-free survival; and (B) overall survival. Patient numbers in the respective groups are listed in Table 1.
We found no differences in the prognostic outcomes with regards to age, sex, or smoking status. Poorer overall survival was detected in the patients with a BMI ≤ 25 kg/m2 and age >70 years in late-stage disease (Figure S4A,B). In the univariate analysis, those aged >70 years, those with a BMI ≤ 25, and wild-type EGFR patients were significantly associated with a worse OS (HR = 1.822, p = 0.006; HR = 2.250, p = 0.004; HR = 1.537, p = 0.046, Table 3). Similar results were also detected in the multivariate analysis (HR = 1.984, p = 0.002; HR = 2.383, p = 0.002; HR = 1.632, p = 0.028, Table 3). In particular, the poorly differentiated group had a worse PFS compared to the moderately differentiated group (Table 4). The Kaplan–Meier analysis provided the same results for older age, lower BMI, and wild-type EGFR patients (Table 5).
Table 3.
Univariate and multivariate analyses of the overall survival in advanced lung adenocarcinoma patients.
| Variables | OS | |||||
|---|---|---|---|---|---|---|
| Univariate | Multivariate | |||||
| HR | 95% CI | p Value | HR | 95% CI | p Value | |
| Groups | ||||||
| Well differentiated | 1 | |||||
| Moderately differentiated | 1.030 | 0.370–2.866 | 0.955 | |||
| Poorly differentiated | 1.548 | 0.552–4.340 | 0.406 | |||
| Age groups (years) | ||||||
| ≤70 | 1 | 1 | ||||
| >70 | 1.822 | 1.183–2.807 | 0.006 ** | 1.984 | 1.273–3.093 | 0.002 ** |
| Sex | ||||||
| Female | 1 | |||||
| Male | 1.039 | 0.841–1.285 | 0.721 | |||
| Smoking Status | ||||||
| Non-smoker | 1 | |||||
| Ever-Smoker | 0.956 | 0.607–1.506 | 0.846 | |||
| BMI group (kg/m2) | ||||||
| >25 | 1 | 1 | ||||
| ≤25 | 2.250 | 1.297–3.906 | 0.004 ** | 2.383 | 1.372–4.139 | 0.002 ** |
| EGFR | ||||||
| Mutation | 1 | 1 | ||||
| Wild-type | 1.537 | 1.008–2.344 | 0.046 * | 1.632 | 1.055–2.523 | 0.028 * |
| Treatments | ||||||
| TKI | 1 | |||||
| Chemotherapy | 1.538 | 0.939–2.520 | 0.087 | |||
OS, overall survival. Note: Missing values are excluded. TKI: gefitinib (Iressa), erlotinib (Tarceva), afatinib (Giotrif), and crizotinib. Chemotherapy: pemetrexed (Alimta), gemcitabine (Gemzar), and vinorelbine (Navelbine). HR: hazard ratio, ** p < 0.01, * p < 0.05.
Table 4.
Factors associated with progression-free survival (PFS) under chemotherapy.
| Variables (N) | PFS Months | Univariate | ||
|---|---|---|---|---|
| HR | 95% CI | p Value | ||
| Well differentiated (3) | 10 (6.8–13.2) | 1 | ||
| Moderately differentiated (29) | 11 (8.4–13.6) | 0.952 | 0.219–4.142 | 0.948 |
| Well differentiated (3) | 10 (6.8–13.2) | 1 | ||
| Poorly differentiated (26) | 4 (2.5–5.5) | 2.897 | 0.676–12.415 | 0.152 |
| Moderately differentiated (29) | 11 (8.4–13.6) | 1 | ||
| Poorly differentiated (26) | 4 (2.5–5.5) | 2.800 | 1.495–5.352 | 0.002 ** |
Data are presented as the median (95% CI). HR, hazard ratio. ** p < 0.01.
Table 5.
Progression-free survival and overall survival according to baseline characteristics.
| Variables | Number | Median PFS (Months) | p Value a | Median OS (Months) | p Value a |
|---|---|---|---|---|---|
| Groups | |||||
| Well differentiated | 7 | 10 (7.434–12.566) | 0.013 ** | 19 (9.224–28.776) | 0.154 |
| Moderately differentiated | 74 | 10 (8.057–11.943) | 21 (15.527–26.473) | ||
| Poorly differentiated | 55 | 6 (3.358–8.642) | 11 (6.106–15.894) | ||
| Age groups (years) | |||||
| ≤70 | 87 | 10 (8.945–11.055) | 0.118 | 21 (15.912–26.088) | 0.005 ** |
| >70 | 49 | 7 (4.441–9.559) | 11 (6.003–15.997) | ||
| Sex | |||||
| Male | 65 | 8 (5.637–10.363) | 0.144 | 13 (8.755–17.245) | 0.716 |
| Female | 71 | 9 (7.731–10.269) | 19 (14.818–23.182) | ||
| BMI group (kg/m2) | |||||
| ≤25 | 93 | 9 (7.903–10.097) | 0.222 | 16 (12.009–19.991) | 0.003 ** |
| >25 | 36 | 10 (7.652–12.348) | 34 | ||
| Smoking Status | |||||
| Non-smoker | 92 | 9 (7.958–10.042) | 0.384 | 19 (13.620–24.380) | 0.843 |
| Ever-Smoker | 44 | 8 (6.927–9.073) | 17 (12.180–21.820) | ||
| EGFR | |||||
| Wild-type | 58 | 6 (3.512–8.488) | 0.131 | 13 (8.285–17.715) | 0.041 * |
| Mutation | 78 | 9 (7.848–10.152) | 21 (10.759–31.241) | ||
| EGFR-m b | |||||
| Sex | |||||
| Male | 34 | 9 (6.718–11.282) | 0.723 | 29 (9.052–48.948) | 0.696 |
| Female | 44 | 9 (7.701–10.299) | 21 (17.448–24.552) | ||
| EGFR-m & Non-smoker | |||||
| Sex | |||||
| Male | 20 | 9 (6.820–11.180) | 0.481 | 14 (9.866–18.134) | 0.913 |
| Female | 41 | 10 (8.754–11.246) | 21 (17.429–24.571) | ||
| Treatments | |||||
| TKI c | 76 | 10 (8.938–11.062) | 0.140 | 22 (16.760–27.240) | 0.080 |
| Chemotherapy d | 33 | 8 (3.981–12.019) | 17 (10.728–23.272) |
a Log rank test; b EGFR-m, EGFR mutation; c TKI, gefitinib (Iressa), erlotinib (Tarceva), afatinib (Giotrif), and crizotinib; d chemotherapy, pemetrexed (Alimta), gemcitabine (Gemzar), and vinorelbine (Navelbine); PFS, progression-free survival; OS, overall survival. Note: Missing values are excluded. ** p < 0.01, * p < 0.05.
In brief, poorly differentiated patients had the worst PFS in this study. In the subgroup analysis, the poorly differentiated group with wild-type EGFR also had a worse PFS when treated with chemotherapy.
3. Discussion
The classification of patients with LADC has been addressed in previous studies, and valuable prognostic features have been identified according to the revised IASLC/ATS/ERS system released in 2011. The importance in early stage is well-documented [1,2,4,6,23,24,25,26]. The predominant type of LADC can be used to predict survival after adjuvant chemoradiotherapy, which represents OS in low-risk groups with a reported OS of 78.5 months for lepidic-predominant LADC, 67.3 months for intermediate-grade (acinar-predominant) LADC, and 57.2 months for high-grade (papillary, micropapillary, and solid-predominant) LADC. In this study, the patients with papillary-predominant LADC had an equivalent survival rate to the patients with micropapillary- and solid-predominant LADC [27]. Similar results have also been reported in other cohorts. It is generally accepted that patients with solid and/or micropapillary patterns have a worse prognosis whether or not the subtype is predominant [28,29,30].
The previous limited resected sample of LADC was able to predict poor prognostic outcomes in early-stage disease. The new grade system proposed by Moreira et al. provided a more complete scale for redefining the predominant subtyping into different categories for predicting the prognosis [8]. Although the clinical relevance of major histologic patterns in late-stage disease has been addressed, the specimens in these studies were mainly acquired by surgical resection or open biopsy [9,10]. Whether a small biopsy is representative of the actual predominant subtypes needs verification using a larger cohort [15]. Since most patients were diagnosed using small biopsy specimens in our study, a validation and correlation of grading between biopsies and resection is warranted. The new grading system in our research is applicable for two reasons. First, small biopsies and cytology specimens from primary or metastatic sites were verified by Sørensen et al. according to the 1981 WHO classification [31]. Second, despite the heterogenous nature of advanced LADC [13,25,32] (also detected in small biopsy specimens [14,15,16,17,18], a cutoff of 20% solid or micropapillary patterns was proposed to determine metastatic potential [3,8]. Clay et al. also noted that a solid pattern is the most frequent pattern in metastatic LADC, and 48% patients in this study were diagnosed by either open biopsy or core biopsy [9]. Therefore, we surmise that biopsy in late-stage LADC and a critical cutoff of 20% solid or micropapillary patterns could provide a strong indicator of prognosis.
The previous limited resected sample sizes of LADC were able to predict poor prognostic outcomes in early-stage disease, but the role of each subtype in predicting the prognosis during the late stage remains unclear [2,9,14,15,29]. In the current study, we showed that the use of the new grading system in patients with advanced stage disease is reproducible and applicable in clinical practice. More than 70% of our patients were diagnosed with advanced-stage lung cancer, which is usually proven via small biopsies or cytology in late-stage disease. The finding that most of the tumors were of a predominant pattern represents the heterogenous nature of tumors in advanced LADC [13,19,25,32]. On the other hand, Moreira et al. and Sica et al. proposed that a cutoff of 20% solid or micropapillary patterns can indicate metastatic potential [3,8] in early-stage disease. Thus, poorly differentiated adenocarcinoma with more than a 20% solid or micropapillary dominant patterns could provide a strong indicator of poor prognosis for patients compared with other groups (moderately differentiated and well differentiated).
Another important issue is that driver gene mutations are highly expressed in certain subtypes [33,34,35]. In patients with advanced-stage LADC harboring EGFR mutations, TKIs are efficient and widely used as the first-line therapeutic choice. Nevertheless, the duration of PFS and OS ranges widely according to the individuals. Cancer stem cell formation with tumor heterogeneity, DNA and epigenetic changes, transcriptome or signal pathway alterations, gene copy number variations, and chromosomal instability may all contribute to drug resistance and eventually cause treatment failure [20].
In this study, the poorly differentiated group had the worst PFS, and this result was also seen in the subgroup analysis of those with the wild-type EGFR receiving chemotherapy. There were no significant differences in sex and smoking status. Those aged >70 years and those with a BMI ≤ 25 kg/m2 had a worse OS. This highlights that those with poorly differentiated adenocarcinoma might fare worse than the other groups with systemic chemotherapy, which is similar to previous research showing that major solid patterns indicate inferior OS after systemic treatment [9]. Nevertheless, this result seems to conflict with other studies’ conclusions [36,37,38,39]. It is possible that different regimens may influence survival, especially as the method of chemotherapy can vary over time [29]. Therefore, we still provided possible predictors of preterm treatment failure in patients receiving first-line chemotherapy based on the histologic predominant subtype. Earlier treatment strategies should be tailored to improve the prognosis of patients with certain characteristics. For example, chemotherapy plus anti-angiogenic drugs or immunotherapy might be choices for active disease control and improving drug efficacy. Further studies are also needed to examine the efficacy of combination therapy. No significant differences were found among patients with EGFR mutations who received first-line EGFR TKIs in this study, which highlights the important role of molecular staging and cancer genomics in future precision medicine [20,40]. To date, relatively little progress has been made in identifying patients who are at risk of relapse after surgical resection or the metastatic process based on the characteristics of advanced-stage lung cancer. Understanding the surveillance of immunotherapy and the microenvironment targeting neoantigens may provide new insight into relevant treatment strategies. Our results indicate that pathologic subtyping in patients with LADC can provide guidance in wild-type EGFR patients, whereas a genomic survey is crucial for targeted therapy.
There are several limitations to the current study. First, the relatively small sample size may have affected the interpretation of the results of the survey. This could also have generated selection bias when performing the statistical analysis, so the results must be validated in a larger cohort in the future. Second, the tumor samples were acquired from small biopsies or surgical resection, and this may have affected the accuracy of the pathological interpretation; however, this remains the standard method for diagnosis in late-stage disease. The tumor heterogeneity in late-stage disease highlights the complexity of the tumor, and different approaches for sample acquisition could eliminate sampling errors. Third, around 30.3% of the wild-type EGFR patients received first-line chemotherapy. This relatively low treatment rate could also have led to a potential bias during analysis, even though our results were statistically significant.
4. Materials and Methods
4.1. Participants
From January 2007 to December 2018, patients with late-stage (stage IIIA, IIIB and IV) LADC who received treatment at Cathay General Hospital (CGH), Taipei, Taiwan were identified. The inclusion criteria for the current study were as follows: (i) patients who received a lung tumor biopsy at CGH (FFPE: formalin-fixed paraffin-embedded tissues obtained through computed tomography (CT)-guided needle biopsy, echo-guided core needle biopsy, or transbronchial biopsy); and (ii) pathological confirmation of LADC using the new grading system [8]. The exclusion criteria were patients: (i) who were lost to follow-up; (ii) who received incomplete treatment; (iii) who received a formal pathology report from another hospital; (iv) who were diagnosed pathologically through a pleural effusion cell block; (v) for whom no definite pathological subtype was identified; (vi) for whom the carcinoma was of uncertain origin, adenosquamous, or neuroendocrine; and (vii) with unknown gene mutation status. This study was approved by Cathay General Hospital (CGH), Taipei, Taiwan (NO.: CGH-P108001). The informed consent form was waived in this study.
All biopsy specimen interpretations under the new grading system based on the IASLC pathology committee classified the adenocarcinomas as being well differentiated, moderately differentiated, or poorly differentiated. The grading scheme in this model comprised lepidic predominant patterns with a 20% or lower cutoff of high-grade patterns categorized as the well differentiated group, while acinar or papillary predominant patterns with or less than 20% of high-grade patterns comprised the moderately differentiated group. Finally, any tumor with 20% or more high-grade patterns was defined as poorly differentiated [8].
We also evaluated other parameters including sex, age at diagnosis, smoking history, body mass index (BMI), gene mutation status, and staging according to the eighth edition of the lung cancer staging system [41]. The first-line treatment regimen used, progression-free survival (PFS), and overall survival (OS) were recorded based on the follow-up medical records. The current study was approved by the Institutional Review Board of Cathay General Hospital (Approval No. CGH-P108001).
4.2. Mutational Analysis
In total, 137 FFPE lung malignant tissue samples were collected by CT-guided or echo-guided biopsy from the Department of Pathology, CGH. Mutations of EGFR, KRAS, and BRAF were determined following standard protocols (EGFR Mutation Test V2, KRAS Mutation Test V2, and BRAF/ NRAS Mutation Test (LSR); Roche, Mannheim, Germany). ALK fusions (D5F3; Roche, Mannheim, Germany) were confirmed by immunohistochemistry.
4.3. Statistical Analysis
The statistical analysis was performed using SPSS version 20.0 (IBM Corp, Armonk, NY, USA). Correlations between two categorical variables were examined using Pearson’s chi-squared test. PFS and OS were calculated at 12 and 36 months after the initial diagnosis, respectively. The Kaplan–Meier method was used to analyze the distributions of PFS and OS, and log-rank tests were performed to compare the differences between two categories. Univariate analysis and multivariate survival analysis were conducted using Cox proportional hazard regression to obtain the hazard ratios (HRs) with 95% confidence intervals (CIs) and identify independent prognostic factors for PFS and OS. Statistical significance was set at α = 0.05.
5. Conclusions
In conclusion, this is the first study to apply the new grading system of the IASLC for predicting the survival prognosis of Asian patients with advanced LADC. Negative impacts were shown on PFS and OS in certain patient subgroups, including poorly differentiated patients, those with a lower BMI, and the elderly. Despite therapy, patients with poorly differentiated tumors still fared worse than those with better differentiated tumors. Conventional first-line chemotherapy treatment with combination therapy using anti-angiogenic agents or immunotherapy might be helpful for improving treatment outcomes. Further large-scale studies are needed to establish whether this treatment strategy is valid.
Acknowledgments
This study was supported by the Cathay General Hospital, which provided the database. The contents of the article do not represent any official views of Cathay General Hospital. The authors had full access to all of the data used in the study and take full responsibility for the integrity of the data and the accuracy of the data analysis.
Supplementary Materials
The following are available online at https://www.mdpi.com/2072-6694/12/11/3426/s1, Figure S1: Kaplan–Meier survival curves for (A) progression-free survival and (B) overall survival according to subanalysis of EGFR mutation pattern groups with TKI treatment. Patient numbers in the respective groups are listed in Table 1. Figure S2, Kaplan–Meier survival curves for (A) progression-free survival and (B) overall survival according to a subanalysis of patients with (n = 109) and without (n = 27) systemic treatments. Figure S3: Kaplan–Meier survival curves for (A) progression-free survival and (B) overall survival according to a subanalysis of different groups without systemic treatments; Figure S4: Kaplan–Meier survival curves for (A) overall-survival in those with a BMI > 25 or ≤ 25 kg/m2 and (B) overall survival in those aged >70 or ≤70 years. Patient numbers in the respective groups are listed in Table 1.
Author Contributions
Conceptualization, C.-F.W., H.H.-C.L., and T.-Y.L.; methodology, C.-F.W. and C.-J.H.; validation, S.-H.H., C.-F.W., and Y.-C.S.; formal analysis, M.-H.W.; investigation, C.-F.W.; resources, S.-H.H.; data curation, C.-F.W., and M.-H.W.; writing—original draft preparation, C.-F.W.; writing—review and editing, A.H.T.; visualization, M.-H.W.; supervision, H.H.-C.L. and T.-Y.L.; and project administration, C.-F.W. and C.-J.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Russell P.A., Wainer Z., Wright G.M., Daniels M., Conron M., Williams R.A. Does lung adenocarcinoma subtype predict patient survival? A clinicopathologic study based on the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society International Multidisciplinary lung adenocarcinoma classification. J. Thorac. Oncol. 2011;6:1496–1504. doi: 10.1097/JTO.0b013e318221f701. [DOI] [PubMed] [Google Scholar]
- 2.Hung J.-J., Yeh Y.-C., Jeng W.-J., Wu K.-J., Huang B.-S., Wu Y.-C., Chou T.-Y., Hsu W.-H., Jeng W.-J., Gung C., et al. Predictive value of the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification of lung adenocarcinoma in tumor recurrence and patient survival. J. Clin. Oncol. 2014;32:2357–2364. doi: 10.1200/JCO.2013.50.1049. [DOI] [PubMed] [Google Scholar]
- 3.Sica G., Yoshizawa A., Sima C.S., Azzoli C.G., Downey R.J., Rusch V.W., Travis W.D., Moreira A.L. A grading system of lung adenocarcinomas based on histologic pattern is predictive of disease recurrence in stage I tumors. Am. J. Surg. Pathol. 2010;34:1155–1162. doi: 10.1097/PAS.0b013e3181e4ee32. [DOI] [PubMed] [Google Scholar]
- 4.Sumiyoshi S., Yoshizawa A., Sonobe M., Kobayashi M., Fujimoto M., Tsuruyama T., Date H., Haga H. Pulmonary adenocarcinomas with micropapillary component significantly correlate with recurrence, but can be well controlled with EGFR tyrosine kinase inhibitors in the early stages. Lung Cancer. 2013;81:53–59. doi: 10.1016/j.lungcan.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Yeh Y.-C., Wu Y.-C., Chen C.-Y., Wang L.-S., Hsu W.-H., Chou T.-Y. Stromal invasion and micropapillary pattern in 212 consecutive surgically resected stage I lung adenocarcinomas: Histopathological categories for prognosis prediction. J. Clin. Pathol. 2012;65:910–918. doi: 10.1136/jclinpath-2012-200882. [DOI] [PubMed] [Google Scholar]
- 6.Zhang J., Wu J., Tan Q., Zhu L., Gao W. Why do pathological stage IA lung adenocarcinomas vary from prognosis? A clinicopathologic study of 176 patients with pathological stage IA lung adenocarcinoma based on the IASLC/ATS/ERS classification. J. Thorac. Oncol. 2013;8:1196–1202. doi: 10.1097/JTO.0b013e31829f09a7. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y., Sun Y., Xiang J., Zhang Y., Hu H., Chen H. A clinicopathologic prediction model for postoperative recurrence in stage Ia non–small cell lung cancer. J. Thorac. Cardiovasc. Surg. 2014;148:1193–1199. doi: 10.1016/j.jtcvs.2014.02.064. [DOI] [PubMed] [Google Scholar]
- 8.Moreira A.L., Ocampo P.S.S., Xia Y., Zhong H., Russell P.A., Minami Y., Cooper W.A., Yoshida A., Bubendorf L., Papotti M., et al. A Grading System for Invasive Pulmonary Adenocarcinoma: A Proposal From the International Association for the Study of Lung Cancer Pathology Committee. J. Thorac. Oncol. 2020;15:1599–1610. doi: 10.1016/j.jtho.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Clay T.D., Do H., Sundararajan V., Moore M.M., Conron M., Wright G.M., McLachlan S.-A., Dobrovic A., Russell P.A. The clinical relevance of pathologic subtypes in metastatic lung adenocarcinoma. J. Thorac. Oncol. 2014;9:654–663. doi: 10.1097/JTO.0000000000000150. [DOI] [PubMed] [Google Scholar]
- 10.Da Cruz V., Yvorel V., Casteillo F., Tissot C., Luchez A., Bayle-Bleuez S., Fournel P., Tiffet O., Péoc’h M., Forest F., et al. Histopathological subtyping is a prognostic factor in stage IV lung adenocarcinoma. Lung Cancer. 2020;147:77–82. doi: 10.1016/j.lungcan.2020.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Campos-Parra A.D., Aviles A., Contreras-Reyes S., Rojas-Marin C.E., Sanchez-Reyes R., Borbolla-Escoboza R.J., Arrieta O. Relevance of the novel IASLC/ATS/ERS classification of lung adenocarcinoma in advanced disease. Eur. Respir. J. 2014;43:1439–1447. doi: 10.1183/09031936.00138813. [DOI] [PubMed] [Google Scholar]
- 12.Casteillo F., Guy J.B., Dal-Col P., Karpathiou G., Pommier B., Bayle-Bleuez S., Fournel P., Vassal F., Forest F. Pathologic Subtypes of Lung Adenocarcinoma Brain Metastasis Is a Strong Predictor of Survival After Resection. Am. J. Surg. Pathol. 2018;42:1701–1707. doi: 10.1097/PAS.0000000000001161. [DOI] [PubMed] [Google Scholar]
- 13.Travis W.D., Brambilla E., Noguchi M., Nicholson A.G., Geisinger K., Yatabe Y., Ishikawa Y., Wistuba I., Flieder D.B., Franklin W., et al. Diagnosis of lung cancer in small biopsies and cytology: Implications of the 2011 International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification. Arch. Pathol. Lab. Med. 2013;137:668–684. doi: 10.5858/arpa.2012-0263-RA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gao S., Stein S., Petre E.N., Shady W., Durack J.C., Ridge C., Adusumilli P., Rekhtman N., Solomon S.B., Ziv E., et al. Micropapillary and/or Solid Histologic Subtype Based on Pre-Treatment Biopsy Predicts Local Recurrence After Thermal Ablation of Lung Adenocarcinoma. Cardiovasc. Intervent. Radiol. 2018;41:253–259. doi: 10.1007/s00270-017-1760-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Riely G.J., Travis W.D. Can IASLC/ATS/ERS subtype help predict response to chemotherapy in small biopsies of advanced lung adenocarcinoma? Eur. Respir. J. 2014;43:1240–1242. doi: 10.1183/09031936.00048814. [DOI] [PubMed] [Google Scholar]
- 16.Kim T.H., Buonocore D., Petre E.N., Durack J.C., Maybody M., Johnston R.P., Travis W.D., Adusumilli P.S., Solomon S.B., Ziv E., et al. Utility of Core Biopsy Specimen to Identify Histologic Subtype and Predict Outcome for Lung Adenocarcinoma. Ann. Thorac. Surg. 2019;108:392–398. doi: 10.1016/j.athoracsur.2019.03.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leeman J.E., Rimner A., Montecalvo J., Hsu M., Zhang Z., von Reibnitz D., Panchoo K., Yorke E., Adusumilli P.S., Travis W., et al. Histologic Subtype in Core Lung Biopsies of Early-Stage Lung Adenocarcinoma is a Prognostic Factor for Treatment Response and Failure Patterns After Stereotactic Body Radiation Therapy. Int. J. Radiat. Oncol. Biol. Phys. 2017;97:138–145. doi: 10.1016/j.ijrobp.2016.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuzawa R., Kirita K., Kuwata T., Umemura S., Matsumoto S., Fujii S., Yoh K., Kojima M., Niho S., Ohmatsu H., et al. Factors influencing the concordance of histological subtype diagnosis from biopsy and resected specimens of lung adenocarcinoma. Lung Cancer. 2016;94:1–6. doi: 10.1016/j.lungcan.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 19.Chao L., Yi-Sheng H., Yu C., Li-Xu Y., Xin-Lan L., Dong-Lan L., Jie C., Yi-Lon W., Hui L.Y. Relevance of EGFR mutation with micropapillary pattern according to the novel IASLC/ATS/ERS lung adenocarcinoma classification and correlation with prognosis in Chinese patients. Lung Cancer. 2014;86:164–169. doi: 10.1016/j.lungcan.2014.08.018. [DOI] [PubMed] [Google Scholar]
- 20.Swanton C., Govindan R. Clinical Implications of Genomic Discoveries in Lung Cancer. N. Engl. J. Med. 2016;374:1864–1873. doi: 10.1056/NEJMra1504688. [DOI] [PubMed] [Google Scholar]
- 21.Warth A., Penzel R., Lindenmaier H., Brandt R., Stenzinger A., Herpel E., Goeppert B., Thomas M., Herth F.J., Dienemann H., et al. EGFR, KRAS, BRAF and ALK gene alterations in lung adenocarcinomas: Patient outcome, interplay with morphology and immunophenotype. Eur. Respir. J. 2014;43:872–883. doi: 10.1183/09031936.00018013. [DOI] [PubMed] [Google Scholar]
- 22.Yanagawa N., Shiono S., Abiko M., Ogata S.Y., Sato T., Tamura G. The correlation of the International Association for the Study of Lung Cancer (IASLC)/American Thoracic Society (ATS)/European Respiratory Society (ERS) classification with prognosis and EGFR mutation in lung adenocarcinoma. Ann. Thorac. Surg. 2014;98:453–458. doi: 10.1016/j.athoracsur.2014.04.108. [DOI] [PubMed] [Google Scholar]
- 23.Hoshi R., Tsuzuku M., Horai T., Ishikawa Y., Satoh Y. Micropapillary clusters in early-stage lung adenocarcinomas: A distinct cytologic sign of significantly poor prognosis. Cancer Cytopathol. Interdiscip. Int. J. Am. Cancer Soc. 2004;102:81–86. doi: 10.1002/cncr.20125. [DOI] [PubMed] [Google Scholar]
- 24.Hung J.-J., Jeng W.-J., Chou T.-Y., Hsu W.-H., Wu K.-J., Huang B.-S., Wu Y.-C. Prognostic value of the new International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification on death and recurrence in completely resected stage I lung adenocarcinoma. Ann. Surg. 2013;258:1079–1086. doi: 10.1097/SLA.0b013e31828920c0. [DOI] [PubMed] [Google Scholar]
- 25.Travis W.D., Brambilla E., Riely G.J. New pathologic classification of lung cancer: Relevance for clinical practice and clinical trials. J. Clin. Oncol. 2013;31:992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- 26.Yoshizawa A., Motoi N., Riely G.J., Sima C.S., Gerald W.L., Kris M.G., Park B.J., Rusch V.W., Travis W.D. Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: Prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod. Pathol. 2011;24:653–664. doi: 10.1038/modpathol.2010.232. [DOI] [PubMed] [Google Scholar]
- 27.Warth A., Muley T., Meister M., Stenzinger A., Thomas M., Schirmacher P., Schnabel P.A., Budczies J., Hoffmann H., Weichert W., et al. The novel histologic International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society classification system of lung adenocarcinoma is a stage-independent predictor of survival. J. Clin. Oncol. 2012;30:1438–1446. doi: 10.1200/JCO.2011.37.2185. [DOI] [PubMed] [Google Scholar]
- 28.Cha M.J., Lee H.Y., Lee K.S., Jeong J.Y., Han J., Shim Y.M., Hwang H.S. Micropapillary and solid subtypes of invasive lung adenocarcinoma: Clinical predictors of histopathology and outcome. J. Thorac. Cardiovasc. Surg. 2014;147:921–928. doi: 10.1016/j.jtcvs.2013.09.045. [DOI] [PubMed] [Google Scholar]
- 29.Yanagawa N. The Clinical Impact of Solid and Micropapillary Patterns in Resected Lung Adenocarcinoma: Author’s Reply. J. Thorac. Oncol. 2017;12:e25–e26. doi: 10.1016/j.jtho.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 30.Yanagawa N., Shiono S., Abiko M., Katahira M., Osakabe M., Ogata S.Y. The Clinical Impact of Solid and Micropapillary Patterns in Resected Lung Adenocarcinoma. J. Thorac. Oncol. 2016;11:1976–1983. doi: 10.1016/j.jtho.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 31.Sorensen J.B., Hirsch F.R., Olsen J. The prognostic implication of histopathologic subtyping of pulmonary adenocarcinoma according to the classification of the World Health Organization. An analysis of 259 consecutive patients with advanced disease. Cancer. 1988;62:361–367. doi: 10.1002/1097-0142(19880715)62:2<361::AID-CNCR2820620222>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 32.Yokose T., Suzuki K., Nagai K., Nishiwaki Y., Sasaki S., Ochiai A. Favorable and unfavorable morphological prognostic factors in peripheral adenocarcinoma of the lung 3 cm or less in diameter. Lung Cancer. 2000;29:179–188. doi: 10.1016/S0169-5002(00)00103-3. [DOI] [PubMed] [Google Scholar]
- 33.Song Z., Zhu H., Guo Z., Wu W., Sun W., Zhang Y. Correlation of EGFR mutation and predominant histologic subtype according to the new lung adenocarcinoma classification in Chinese patients. Med. Oncol. 2013;30:645. doi: 10.1007/s12032-013-0645-1. [DOI] [PubMed] [Google Scholar]
- 34.Yoshizawa A., Sumiyoshi S., Sonobe M., Kobayashi M., Fujimoto M., Kawakami F., Tsuruyama T., Travis W.D., Date H., Haga H., et al. Validation of the IASLC/ATS/ERS lung adenocarcinoma classification for prognosis and association with EGFR and KRAS gene mutations: Analysis of 440 Japanese patients. J. Thorac. Oncol. 2013;8:52–61. doi: 10.1097/JTO.0b013e3182769aa8. [DOI] [PubMed] [Google Scholar]
- 35.Shim H.S., Lee D.H., Park E.J., Kim S.H. Histopathologic characteristics of lung adenocarcinomas with epidermal growth factor receptor mutations in the International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society lung adenocarcinoma classification. Arch. Pathol. Lab. Med. 2011;135:1329–1334. doi: 10.5858/arpa.2010-0493-OA. [DOI] [PubMed] [Google Scholar]
- 36.Qian F., Yang W., Wang R., Xu J., Wang S., Zhang Y., Jin B., Yu K., Han B. Prognostic significance and adjuvant chemotherapy survival benefits of a solid or micropapillary pattern in patients with resected stage IB lung adenocarcinoma. J. Thorac. Cardiovasc. Surg. 2018;155:1227–1235. doi: 10.1016/j.jtcvs.2017.09.143. [DOI] [PubMed] [Google Scholar]
- 37.Tsao M.S., Marguet S., Le Teuff G., Lantuejoul S., Shepherd F.A., Seymour L., Kratzke R., Graziano S.L., Popper H.H., Rosell R., et al. Subtype Classification of Lung Adenocarcinoma Predicts Benefit From Adjuvant Chemotherapy in Patients Undergoing Complete Resection. J. Clin. Oncol. 2015;33:3439–3446. doi: 10.1200/JCO.2014.58.8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hung J.J., Wu Y.C., Chou T.Y., Jeng W.J., Yeh Y.C., Hsu W.H. Adjuvant Chemotherapy Improves the Probability of Freedom From Recurrence in Patients With Resected Stage IB Lung Adenocarcinoma. Ann. Thorac. Surg. 2016;101:1346–1353. doi: 10.1016/j.athoracsur.2015.10.075. [DOI] [PubMed] [Google Scholar]
- 39.Ma M., She Y., Ren Y., Dai C., Zhang L., Xie H., Wu C., Yang M., Xie D., Chen C. Micropapillary or solid pattern predicts recurrence free survival benefit from adjuvant chemotherapy in patients with stage IB lung adenocarcinoma. J. Thorac. Dis. 2018;10:5384–5393. doi: 10.21037/jtd.2018.08.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nogrady B. How cancer genomics is transforming diagnosis and treatment. Nature. 2020;579:S10–S11. doi: 10.1038/d41586-020-00845-4. [DOI] [PubMed] [Google Scholar]
- 41.Asamura H., Chansky K., Crowley J., Goldstraw P., Rusch V.W., Vansteenkiste J.F., Watanabe H., Wu Y.-L., Zielinski M., Ball D. The International Association for the Study of Lung Cancer Lung Cancer Staging Project: Proposals for the Revision of the N Descriptors in the Forthcoming 8th Edition of the TNM Classification for Lung Cancer. J. Thorac. Oncol. 2015;10:1675–1684. doi: 10.1097/JTO.0000000000000678. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.