Abstract
Objectives
Efficacy and safety of corticosteroids in patients with 2019-nCoV (novel coronavirus 2019) infection still are debated. Because large randomized clinical trials (RCTs) and a well-conducted meta-analysis on the use of corticosteroids, focused on patients with coronavirus disease (COVID-19) in intensive care units, recently were published, a meta-analysis of RCTs on corticosteroids therapy in patients with different disease severity was performed to evaluate the effect on survival.
Design
A meta-analyses of RCTs was performed.
Setting
Patients admitted to hospital.
Participants
Patients with coronavirus disease.
Interventions
Administration of corticosteroids.
Measurements and Main Results
A search was performed for RCTs of adult patients with acute hypoxemic failure related to 2019-nCoV infection who received corticosteroids versus any comparator. The primary endpoint was mortality rate. Five RCTs involving 7,692 patients were included. Overall mortality of patients treated with corticosteroids was slightly but significantly lower than mortality of controls (26% v 28%, relative risk {RR} = 0.89 [95% confidence interval {CI} 0.82-0.96], p = 0.003). The same beneficial effect was found in the subgroup of patients requiring mechanical ventilation (RR = 0.85 [95% CI 0.72-1.00], p = 0.05 number needed to treat {NNT} = 19). Remarkably, corticosteroids increased mortality in the subgroup of patients not requiring oxygen (17% v 13%, RR = 1.23 [95% CI 1.00-1.62], p = 0.05 number needed to harm {NNH} = 29). Tests for comparison between mechanically ventilated subgroups and those not requiring oxygen confirmed that treatment with corticosteroids had a statistically significant different effect on survival. Patients treated with corticosteroids had a significantly lower risk of need for mechanical ventilation.
Conclusions
Corticosteroids may be considered in severe critically ill patients with COVID-19 but must be discouraged in patients not requiring oxygen therapy. Urgently, further trials are warranted before implementing this treatment worldwide.
Key Words: corticosteroids, mortality, COVID-19, 2019-nCoV, mechanical ventilation, meta-analyses
SINCE THE INFLUENZA OUTBREAK of 1918, the coronavirus disease 2019 (COVID-19) pandemic probably represents the biggest global crisis faced by public health worldwide. Different drugs previously used to treat other coronavirus infections, such as severe acute respiratory syndrome and Middle East respiratory syndrome, were considered as the first potential candidates to treat COVID-19. Among them, in addition to other therapeutics, corticosteroids were used widely during severe acute respiratory syndrome and Middle East respiratory syndrome outbreaks and recently were adopted in patients with 2019-nCoV (novel coronavirus 2019) infection.
It is well-known that acute respiratory distress syndrome is caused partly by host immune responses.1 2019-nCoV virus, once entered into humans, targets a key angiotensin-converting enzyme 2 (ACE2) receptor and replicates within cells causing cellular injury or death with release of pro-inflammatory alarmins.2 Moreover, viral particles can stimulate innate immune response, leading to the activation of alveolar macrophages and the complement system. The resultant massive inflammatory response causes alveolar and vascular damage, microvascular thromboses, and a progressive worsening of ventilation–perfusion mismatch.3, 4, 5 In the late stages of the disease, the systemic inflammatory reaction may involve other organs, causing multiorgan failure and death. Theoretically, corticosteroid treatment could have a role in suppressing lung inflammation and inhibiting immune responses and pathogen clearance. Nonetheless, a recent meta-analysis on pharmacologic agents for adults with acute respiratory distress syndrome found insufficient evidence to determine with certainty whether corticosteroids may reduce early all-cause mortality or the duration of mechanical ventilation.6 Even less evidence exists in the literature to indicate whether corticosteroids are effective in treating coronavirus disease infection. 7
A recent large randomized clinical trial (RCT) showed that the use of dexamethasone resulted in lower 28-day mortality among patients with COVID-19 who were receiving either random invasive mechanical ventilation or oxygen alone, but not among patients receiving no respiratory support.8 This study had resonated worldwide because nowadays dexamethasone is the first strategy proven to reduce mortality in COVID-19 patients. The updated living World Health Organization guideline on drugs for COVID-19 suggests not to use corticosteroids in the treatment of patients with nonsevere COVID-19 but with a weak or conditional recommendation.9
Because other high-quality RCTs10, 11, 12, 13 and a well-performed meta-analysis on the effect of corticosteroids on patients in the intensive care unit (ICU) recently were published,14 the authors decided to perform a meta-analysis of RCTs on corticosteroid therapy to evaluate the effect on survival of subgroups of patients with COVID-19 who require different respiratory support.
Methods
Search Strategy
Pertinent studies were searched independently in BioMedCentral, PubMed, Embase, medRxiv, bioRxiv and the Cochrane Central Register of Controlled Trials by two investigators (L.P., G.L.). The full PubMed search strategy aimed to include any RCTs ever performed with corticosteroids in patients with COVID-19 and is presented in the Supplementary Material. In addition, the authors employed backward snowballing (ie, scanning of references of retrieved articles and pertinent reviews) and contacted international experts for further studies. No language restriction was imposed.
Study Selection
References first were examined independently at a title or abstract level by two investigators (L.P., G.L.), with divergences resolved by consensus and then, if potentially pertinent, retrieved as complete articles. The following inclusion criteria were used for potentially relevant studies: random allocation to treatment (corticosteroids v any comparator with no restrictions on dose or time of administration) and studies involving patients with acute hypoxemic failure or pneumonia related to 2019-nCoV infection. The exclusion criteria were duplicate publications (in this case the authors referred to the first article published and retrieved data from the article with the longest follow-up available), nonadult patients, and lack of data on all outcomes of interest. Two investigators (L.P., G.L.) independently assessed compliance to selection criteria and selected studies for the final analysis, with divergences resolved by consensus.
Data Abstraction and Study
Baseline, procedural, and outcome data were abstracted independently by two investigators (L.P., G.L.; Table 1 ). At least two separate attempts to contact original authors were made in cases of missing data. The primary endpoint of the present review was mortality rate at the longest available follow-up. Co-primary endpoints were mortality rate of mechanically ventilated patients and patients who did not receive oxygen therapy. The secondary endpoint was need for mechanical ventilation.
Table 1.
Description of the Studies Included in the Meta-analysis
| First Author | Year | Setting | Inclusion Criteria | Primary Outcome | Corticosteroids Patients | Control Patients | Corticosteroid Type and Dosage | Duration of Study Treatment | Comparator | Antiviral Therapies | Reported Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Jeronimo CMP | 2020 | Ordinary ward | Clinical and/or radiological suspicion of COVID-19 (history of fever and any respiratory symptom (eg, cough or dyspnea and/or ground glass opacity or pulmonary consolidation on CT scan), aged 18 years or older with SpO2 ≤ 94% at room air or in use of supplementary oxygen or under IMV | 28-d mortality | 209 | 207 | Intravenous metyhlprednisolone (0.5 mg/kg) twice daily | 5 d | Placebo + usual care | None | 28 d |
| RECOVERY Trial | 2020 | Ordinary ward | Clinically suspected or laboratory confirmed SARS-CoV-2 infection and no medical history that might, in the opinion of the attending clinician, put patients at substantial risk if they were to participate in the trial. | 28-d mortality | 2,104 | 4,321 | Oral o intravenous dexamethasone 6 mg once daily | Up to 10 d (or until hospital discharge if sooner) | Usual care | 0% to 3% of patients received hydroxychloroquine, lopinavir–ritonavir, or interleukin-6 antagonists. Remdesivir was administered to 3 patients in the dexamethasone group and 2 patients in the usual care group. | 28 d |
| REMAP-CAP Trial | 2020 | ICU | Adult patients with presumed or confirmed SARS-CoV-2 infection who were admitted to an ICU for provision of respiratory or cardiovascular organ support | Organ support-free days within 21 d | 295 | 108 | Intravenous hydrocortisone 50 mg, every 6 h; intravenous hydrocortisone, 50 mg, every 6 h while in shock | For 7 d or for up to 28 d while in shock | Usual care | Patients were eligible for randomized assignment to alternative interventions | 28 d |
| CoDEX Trial | 2020 | ICU | Adult patients with confirmed or suspected COVID-19 infection, receiving MV within 48 h of meeting criteria for moderate to severe ARDS with PaO2:FIO2 ratio of 200 or less | Ventilator-free days during the first 28 d | 151 | 148 | Intravenous Dexamethasone 20mg intravenously once daily for 5 d, followed by 10 mg IV once daily for additional 5 d or until ICU discharge | 10 d or up to ICU discharge | Usual care | None | 28 d |
| Dequin PF | 2020 | ICU | Adult ICU patients with biologically confirmed or suspected COVID-19 and severe acute respiratory syndrome | Treatment failure on day 21 | 76 | 73 | Hydrocortisone 200mg/d until day 7 and then decreased to 100 mg/d for 4 d and 50 mg/d for 3 d | 14 d or ICU discharge | Placebo | Adjunctive antiviral treatments could be administered at the discretion of the patients’ primary physicians | 21 d |
Abbreviations: ARDS, Acute Respiratory Distress Syndrome, CoDEX, COVID Dexamethasone; COVID-19, coronavirus disease 2019; CT, computed tomography; ICU, intensive care unit; IMV, Invasive Mechanical Ventilation; RECOVERY, Randomised Evaluation of COVID-19 Therapy; REMAP-CAP, Randomized, embedded, multifactorial adaptive platform trial for community-acquired pneumonia; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Internal Validity and Risk of Bias Assessment
The internal validity and risk of bias of included trials were appraised by two independent reviewers according to the latest version of the Risk of Bias Assessment Tool developed by The Cochrane collaboration,15 and divergences were resolved by consensus. Publication bias was assessed by visually inspecting funnel plots.
Data Analysis and Synthesis
Computations were performed with Review Manager (RevMan) [Computer program]. Version 5.4. The Cochrane Collaboration, 2020. The hypothesis of statistical heterogeneity was tested by means of Cochran Q test, with statistical significance set at the two-tailed 0.10 level, whereas extent of statistical consistency was measured with I2, defined as 100% x (Q-df)/Q, where Q is Cochran's heterogeneity statistic and df the degrees of freedom.
Binary outcomes were analyzed to compute the individual and pooled risk ratio (RR) and pertinent 95% confidence interval (CI) by means of the same models as previously described. Binary outcomes from individual studies were analyzed to compute individual and pooled RR and pertinent 95% CI by means of inverse variance method, with a fixed-effect model in case of low statistical inconsistency (I2 ≤ 25%), or with random-effect model (which better accommodates clinical and statistical variations) in case of moderate or high statistical inconsistency (I2 > 25%). Sensitivity analyses were performed by sequentially removing each study and reanalyzing the remaining dataset (producing a new analysis for each study removed) by analyzing only data from studies with low risk of bias and by analyzing, with a random effect analysis, studies with low heterogeneity (I2 ≤ 25%). Statistical significance was set at the two-tailed 0.05 level for hypothesis testing. Unadjusted p values were reported throughout. A prespecified trial sequential analysis was performed on mortality outcome. The authors estimated the required information size on the calculated minimal intervention effect considering a type I error of 5% and a power of 80%. This post hoc conservative approach allowed the authors to assess whether the data were convincing enough to prove the effect.
To compare different groups (mechanically ventilated patients and patients who did not require oxygen therapy), tests for subgroup differences were performed based on random-effects models. In case of p values = 0.05, the authors repeated sensitivity analyses with Review Manager and Stata.
This study was registered on PROSPERO (CRD 42020197509) and performed in compliance with The Cochrane Collaboration and Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.16 , 17
Results
Study Characteristics
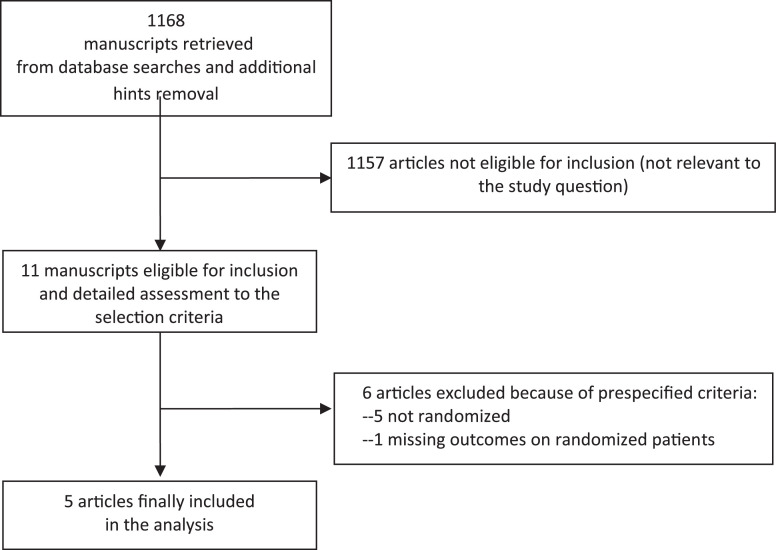
Database searches, snowballing, and contacts with experts yielded a total of 1,168 articles. Excluding 1,157 nonpertinent titles or abstracts, the authors retrieved (in complete form) and assessed 11 studies according to the selection criteria (Fig 1 ). Six studies were further excluded because of the prespecified exclusion criteria: five because they were not randomized,18, 19, 20, 21, 22 and one because it did not report outcomes of randomized patients.23
Fig 1.
Flowchart of article selection.
The five RCTs finally included in the meta-analysis involved 7,692 patients (2,835 received corticosteroids and 4,837 received standard treatment8 , 10, 11, 12, 13; Table 1). Characteristics of included studies are presented in Table 1. Clinical heterogeneity mostly was due to inclusion criteria, initiation of oxygen therapy or mechanical ventilation, type of corticosteroid, dosage and duration of administration, concomitant antiviral or anti-inflammatory drugs, and length of follow-up (Table 1). Overall risk of bias of the included studies was moderate (Supplementary Material).
Quantitative Data Synthesis
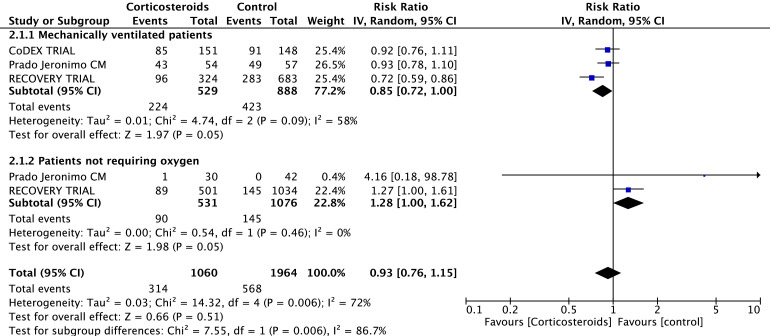
Overall mortality of patients treated with corticosteroids was slightly but significantly lower than mortality of patients in the control group (727 of 2,835 [26%]; in the corticosteroids group v 1,336 of 4,857 [28%] in the control group, RR = 0.89 [95% CI 0.82-0.96], p for effect 0.003, I2 = 0%, with five trials included; see Table 2 and Supplemental Material), with results confirmed at sensitivity analyses. Reduction in mortality also was observed in the subgroup of patients who required mechanical ventilation (224 of 529 [42%] in the corticosteroids group v 423 of 888 [48%] in the control group, RR = 0.85 [95% CI 0.72-1.00], p for effect 0.05, I2 = 58%; see Table 2 and Supplementary Material). Notably, the use of corticosteroids increased mortality in the subgroup of patients not requiring oxygen (90 of 531 [17%] in the corticosteroids group v 145 of 1,076 [13%] in the control group, RR = 1.23 [95% CI 1.00-1.62], p for effect 0.05, I2 = 0%; see Table 2 and Fig 2 ). No difference in mortality was found in the subgroups of patients who did not required intubation. (Table 2) All results were confirmed at sensitivity analyses. Trial sequential analysis suggested that additional trials are needed to confirm the findings (Supplementary Material).
Table 2.
Outcomes
| Outcome | Number of Included Studies | Corticosteroids Patients | Control Patients | RR | 95% CI | p for Effect | I2 (%) |
|---|---|---|---|---|---|---|---|
| Overall studies | 2,835 | 4,857 | |||||
| Mortality | 5 | 727 of 2,835 (26%) | 1,336 of 4,857 (28%) | 0.89 | 0.82-0.96 | 0.003 | 0 |
| MV patients | 3 | 224 of 529 (42%) | 423 of 888 (48%) | 0.85 | 0.72-1.00 | 0.05 | 58 |
| Non-MV patients | 2 | 403 of 1,876 (21%) | 859 of 3,741 (23%) | 0.95 | 0.86-1.06 | 0.35 | 0 |
| Patients who did not require oxygen therapy | 2* | 90 of 531 (17%) | 145 of 1,076 (13%) | 1.28 | 1.00-1.62 | 0.05 | 0 |
| Need for MV | 3 | 126 of 2329 (5%) | 311 of 4,544 (7%) | 0.75 | 0.60-0.94 | 0.007 | 18 |
Abbreviations: RR, relative risk; CI, confidence interval; MV, mechanical ventilation
Additional data provided by corresponding author (Prado-Jeronimo)
Fig 2.
Tests for comparison between mechanically ventilated subgroups and those who did not require oxygen therapy based on random-effects models.
Patients treated with corticosteroids had a significantly lower risk of need for mechanical ventilation than controls (126 of 2,329 [5%] in the corticosteroids group v 311 of 4,544 [7%] in the control group, RR = 0.74 [95% CI 0.59-0.92], p for effect = 0.007, I2 = 18%, three trials included; see Table 2 and Supplementary Material). Nonetheless, this result was not confirmed at sensitivity analyses. Given the low number of included trials, funnel plots were not analyzed.
Tests for comparison between mechanically ventilated subgroups and those who did not require oxygen therapy, based on random-effects models, revealed that treatment with corticosteroids had a statistically significant different effect on survival in patients with COVID-19 with different disease severity: χ2 = 7.55, p for effect = 0.006; I2 = 86.7% (Fig 2).
Discussion
This was the first meta-analysis of RCTs that showed that the effect of corticosteroids on patient survival depended on the disease severity. In fact, although a recent meta-analyses published in JAMA by the REACT (Real-time Assessment of Community Transmission) COVID group concluded that administration of systemic corticosteroids, compared with usual care or placebo, was associated with lower 28-day all-cause mortality in patients admitted to ICU, the present study showed that the use of corticosteroids has detrimental effects on survival of patients not requiring oxygen, with a number needed to harm of 29, and that a significant difference existed between the two patients categories. Unlike analyzing the whole ICU population, as the REACT COVID group did, the authors focused the present study on different disease severity and achieved very informative results. This finding is of paramount importance because, fortunately, the majority of patients with COVID-19 experience a mild or moderate illness and do not require hospital admission. Only a small minority of patients with more severe illness are admitted to the hospital, where they often need oxygen therapy or respiratory mechanical support. Therefore, patients with COVID-19 who might benefit from corticosteroids therapy are a small minority. In addition, the authors found that spontaneously breathing patients treated with corticosteroids had less requirement for intubation and mechanical ventilation, but higher mortality rate than patients who did not receive corticosteroids. These important findings, although apparently incongruous, stress the hypothesis that the use of corticosteroids might improve respiratory function (in patients who are already on oxygen), while probably increasing the risk of death (in patients who are not yet requiring oxygen).
The efficacy and safety of corticosteroids in COVID-19 still is debated. A recent large randomized clinical trial showed that the use of dexamethasone resulted in reduced 28-day mortality among patients who were receiving either invasive MV or oxygen alone, but not among patients who were receiving no respiratory support.8 Actually, this was the first strategy proven to reduce mortality in patients with COVID-19 and had a great scientific impact worldwide. Nonetheless, it showed a potential detrimental effect of dexamethasone in patients who were not receiving oxygen (17.8% mortality in dexamethasone group v 14.0% in placebo group) and who represent the majority of patients worldwide. On the contrary, Jeronimo et al. found a reduced mortality rate only in older patients (>60 years) treated with corticosteroids.10
Moreover, recently published systematic reviews on this topic showed contrasting results. Pei et al., in their study on the use of antiviral agents, glucocorticoids, antibiotics and immunoglobulin usage in patients with COVID-19, showed a probable survival benefit of antiviral agent usage and a harmful effect of glucocorticoids.24 On the contrary, Hasan et al., in accordance with the results of the latest meta-analysis published on this topic,14 found that low-dose corticosteroid therapy or pulse corticosteroid therapy appeared to have a beneficial role in the management of severely ill patients with COVID-19.25 Similarly, corticosteroids have an overall beneficial effect in the majority of critically ill patients, including those with pulmonary disease, as suggested by a meta-analysis of RCTs.26
The authors acknowledge that the study presented some limitations. Although it included randomized clinical trials, the number of included studies was very low. Moreover, these trials did not reach the required sample size to verify the small difference in absolute mortality reduction found between groups (2%). This small effect probably already is reduced, as the observed in-hospital mortality appears to rapidly be decreasing.27 Nonetheless, because there were millions of COVID-19 cases throughout the world, even an absolute mortality reduction of 6% in mechanically ventilated patients (number needed to treat = 19) and an absolute mortality increase of 4% in patients not on oxygen (number needed to harm = 29) can save thousands of lives, especially of patients not requiring oxygen, who are the vast majority of patients with COVID-19. In addition, initiation of oxygen therapy or mechanical ventilation, type of corticosteroid, dosage and duration of administration, and concomitant antiviral or anti-inflammatory drugs were significantly different between studies. Moreover, studies carried out both in ICUs and ordinary wards were included, which increased overall heterogeneity. Nonetheless, the aim of the present study was to investigate the effect of corticosteroids therapy on survival of subgroups of patients with COVID-19 who required different respiratory support. Therefore, it was necessary to include patients admitted to different clinical settings, as it is unlikely to find patients who do not require oxygen in the ICU. In addition, the role of confounding variables, such as age, severity of disease, presence of pulmonary disease, and so on, remains to be explored.
In conclusion, the present study clearly showed that the use of corticosteroids may be considered in severe critically ill patients with COVID-19 but must be discouraged in all patients who do not require oxygen support. Given the small effect on survival of critically ill patients, they must be compared with other anti-inflammatory drugs, as other therapies (eg, anakinra) with better safety profile recently were tested with success.28 Larger, high-quality randomized clinical trials on this topic urgently are warranted before implementing this treatment worldwide, in particular in less critical, younger patients who do not require oxygen therapy or hospitalization.
Conflict of Interest
None.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1053/j.jvca.2020.11.057.
Appendix. Supplementary materials
References
- 1.Wong JJM, Leong JY, Lee JH. Insights into the immuno-pathogenesis of acute respiratory distress syndrome. Ann Transl Med. 2019;7:504. doi: 10.21037/atm.2019.09.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hamming I, Timens W, Bulthuis MLC. Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol. 2004;203(2):631–637. doi: 10.1002/path.1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ciceri F, Beretta L, Scandroglio AM. Microvascular COVID-19 lung vessels obstructive thromboinflammatory syndrome (MicroCLOTS): An atypical acute respiratory distress syndrome working hypothesis. Crit Care Resusc. 2020 doi: 10.51893/2020.2.pov2. Doi: xxxx. Accessed xxxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan CW, Low JGH, Wong WH. Critically ill COVID-19 infected patients exhibit increased clot waveform analysis parameters consistent with hypercoagulability. Am J Hematol. 2020;95(7):E156–E158. doi: 10.1002/ajh.25822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nardelli P, Landoni G. COVID-19-related thromboinflammatory status: Microclots and beyond (editorial) Obshchaya Reanimatologiya. 2020;16:14–15. [Google Scholar]
- 6.Lewis SR, Pritchard MW, Thomas CM, Smith AF. Pharmacological agents for adults with acute respiratory distress syndrome. Cochrane Database Syst Rev. 2019;7 doi: 10.1002/14651858.CD004477.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Villar J, Confalonieri M, Pastores SM, Meduri GU. Rationale for prolonged corticosteroid treatment in the acute respiratory distress syndrome caused by coronavirus disease 2019. Crit Care Explor. 2020;2:e0111. doi: 10.1097/CCE.0000000000000111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.RECOVERY Collaborative Group. Lim WS, Emberson JR. Dexamethasone in Hospitalized patients with covid-19 – Preliminary report [E-pub ahead of print] N Engl J Med. 2020 doi: 10.1056/NEJMoa2021436. Accessed 2020 Jul 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lamontagne F, Agoritsas Thomas, Macdonald Helen. A living WHO guideline on drugs for covid-19. BMJ. 2020;370:m3379. doi: 10.1136/bmj.m3379. [DOI] [PubMed] [Google Scholar]
- 10.Jeronimo CMP, Farias MEL, Val FFA. Methylprednisolone as adjunctive therapy for patients hospitalized with COVID-19 (metcovid): A randomised, double-blind, phase IIb, placebo-controlled trial. Clin Infect Dis. 2020 doi: 10.1093/cid/ciaa1177. Accessed 2020 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dequin PF, Heming N, Meziani F. Effect of hydrocortisone on 21-day mortality or respiratory support among critically ill patients with COVID-19: A randomized clinical trial. JAMA. 2020;324:1298–1306. doi: 10.1001/jama.2020.16761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Angus DC, Derde L, Al-Beidh F. Effect of hydrocortisone on mortality and organ support in patients with severe COVID-19: The REMAP-CAP COVID-19 corticosteroid domain randomized clinical trial. JAMA. 2020;324:1317–1329. doi: 10.1001/jama.2020.17022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tomazini BM, Maia IS, Cavalcanti AB. Effect of Dexamethasone on Days Alive and Ventilator-Free in Patients With Moderate or Severe Acute Respiratory Distress Syndrome and COVID-19: The CoDEX Randomized Clinical Trial. JAMA. 2020;324:1307–1316. doi: 10.1001/jama.2020.17021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.WHO Rapid Evidence Appraisal for COVID-19 Therapies (REACT) Working Group. Sterne JAC, Murthy S. Association between administration of systemic corticosteroids and mortality among critically ill patients with COVID-19: A meta-analysis. JAMA. 2020;324:1330–1341. doi: 10.1001/jama.2020.17023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sterne JAC, Savović J, Page MJ. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi: 10.1136/bmj.l4898. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thomas J, Chandler J. John Wiley & Sons, Inc; Hoboken, NJ: 2019. Cochrane handbook for systematic reviews of interventions. [Google Scholar]
- 17.Liberati A, Altman DG, Tetzlaff J. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: Explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fernández Cruz A, Ruiz-Antoran B, Munoz-Gomez A. Impact of glucocorticoid treatment in SARS-COV-2 infection mortality: A retrospective controlled cohort study. Antimicrob Agents Chemother. 2020;64 doi: 10.1128/AAC.01168-20. e01168-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zha L, Li S, Pan L. Corticosteroid treatment of patients with coronavirus disease 2019 (COVID-19) Med J Aust. 2020;212:416–420. doi: 10.5694/mja2.50577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J, Huang J, Zhu G. Systemic corticosteroids and mortality in severe and critical COVID-19 patients in Wuhan, China. J. Clin. Endocrinol. Metab. 2020 doi: 10.1210/clinem/dgaa627. Accessed 2020 Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fadel R, Morrison AR, Vahia A. Early Short Course Corticosteroids in Hospitalized Patients with COVID-19. Clin Infect Dis. 2020;71(16):2114–2120. doi: 10.1093/cid/ciaa601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubio-Rivas M, Ronda M, Padulles A. Beneficial Effect of Corticosteroids in Preventing Mortality in Patients Receiving Tocilizumab to Treat Severe COVID-19 Illness AUTHORS. Int J Infect Dis. 2020;101:290–297. doi: 10.1101/2020.08.31.20182428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corral L, Bahamonde A, Arnaiz delas Revillas F. GLUCOCOVID: A controlled trial of methylprednisolone in adults hospitalized with COVID-19 pneumonia. medRxiv. 2020 doi: 10.1101/2020.06.17.20133579. Accessed 2020 Aug 12. [DOI] [Google Scholar]
- 24.Pei L, Zhang S, Huang L. Antiviral agents, glucocorticoids, antibiotics, and intravenous immunoglobulin usage in 1142 patients with coronavirus disease 2019: A systematic review and meta-analysis. Polish Arch Intern Med. 2020;130:726–733. doi: 10.20452/pamw.15543. Accessed 2020 Aug 12. [DOI] [PubMed] [Google Scholar]
- 25.Hasan SS, Capstick T, Ahmed R. Mortality in COVID-19 patients with acute respiratory distress syndrome and corticosteroids use: A systematic review and meta-analysis. Expert Rev Respir Med. 2020;14:1149–1163. doi: 10.1080/17476348.2020.1804365. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Martino EA, Baiardo Redaelli M, Sardo S. Steroids and Survival in Critically Ill Adult Patients: A Meta-analysis of 135 Randomized Trials. J Cardiothorac Vasc Anesth. 2018;32:2252–6060. doi: 10.1053/j.jvca.2018.04.017. [DOI] [PubMed] [Google Scholar]
- 27.Ciceri F, Ruggeri R, Lembo R. Decreased in-hospital mortality in patients with COVID-19 pneumonia. Pathog Glob Health. 2020;114:281–282. doi: 10.1080/20477724.2020.1785782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cavalli G, De Luca G, Campochiaro C. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020;2:e325–e331. doi: 10.1016/S2665-9913(20)30127-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.