Abstract
Traumatic brain injury (TBI) affects millions of individuals each year and is a leading cause of death and disability worldwide. TBI is heterogeneous and outcome is influenced by a combination of factors that include injury location, severity, genetics, and environmental factors. More recently, sex as a biological variable has been incorporated into TBI research, although there is conflicting literature regarding clinical outcomes in males versus females after TBI. We review the current clinical literature investigating sex differences after TBI. We focus our discussion on differences within contemporary gender categories to suggest that binary categories of male and female are not sufficient to guide clinical decisions for neurotrauma. Some studies have considered physiological variables that influence sex such as hormone cycles and stages in males and females pre- and post-TBI. These data suggest that there are phasic differences within male populations and within female populations that influence an individual's outcome after TBI. Finally, we discuss the impact of gender identity and expression on outcome after TBI and highlight the lack of neurotrauma research that includes non-binary individuals. Social constructs regarding gender impact an individual's vulnerability to violence and consequent TBI, including the successful reintegration to society after TBI. We call for incorporation of gender beyond the binary in TBI education, research, and clinical care. Precision medicine necessarily must progress beyond the binary to treat individuals after TBI.
Keywords: gender, hormones, non-binary, sex, traumatic brain injury
Introduction
Everyone is susceptible to traumatic brain injury (TBI) and at least 2.8 million cases of TBI are reported in the United States each year,1 which is likely an underestimate as many TBIs go unreported and undiagnosed. TBI is defined as an external force imposed on the head or skull that interrupts normal brain function.2 Forces of injury to the skull/brain can be penetrating, linear, rotational, or blast wave, which cause direct damage and/or acceleration/deceleration of the brain within the skull. With respect to the application of injury forces, the resultant TBI is categorized as focal or diffuse depending on the extent to which pathology extends across brain regions. Each type of injury is further categorized by severity based on the magnitude of injury force, clinical imaging, and symptomatology. Common mechanisms of TBI include falls, motor vehicle collisions, violence, assault, contact sports, and combat, for which all individuals can be at risk.3 Diffuse TBI accounts for approximately 80–90% of all TBI incidences.4
Injury severity, genetics, and environmental factors all influence clinical outcome from TBI, which spans from acute deficits to chronic morbidities. Our understanding of TBI must be interpreted with caution, as the majority of TBI data come from male case subjects. Further, the designation of male is stratified only by biological sex. In 2001, the National Institutes of Health (NIH) issued a mandate to include women and minority individuals as subjects in NIH-funded clinical research.5 In 2015, the NIH again amended its guidelines to include sex as a biological variable in NIH-funded pre-clinical research.6,7 Biological sex, defined by chromosomes, reproductive organs, and hormone profiles, is binary in such studies that categorize patients as male or female. Further, the term “gender” is often used interchangeably with sex in research reports. Gender, however, is a social construct and incorporates behavior, culture, identity, and expression.8 Gender can be influenced by biological sex, but should not be used interchangeably with biological sex. In 2009, Stanford University created the Gendered Innovations in Science, Health & Medicine, Engineering, and Environment tool to provide investigators with methodology, steps, literature search terms, and checklists to successfully incorporate sex and gender into studies.9 The purpose of our review is to discuss the current use of sex and gender in the field of neurotrauma.
The majority of literature reports present outcomes from TBI stratified in the binary between male and female. In a recent comprehensive review of sex differences after TBI, Gupte and colleagues outlined 156 clinical studies comparing outcomes between male and female after TBI.10 As a point of necessity, we review some of that literature, before considering additional variables that further expand the two classical sexes and therefore broaden our concept of an individual with TBI. Finally, we go beyond physiological variables to discuss the impact of gender on outcome after TBI and call for better understanding of sex and gender in TBI research and clinical care. The current approach is not considered a meta-analysis, because of scarce existing literature. Publications were identified through public database and Internet searches that included combinations of the following search terms: “traumatic brain injury,” “concussion,” “sex,” “gender,” “male,” “female,” “menstrual cycle,” “estrogen,” “progesterone,” “testosterone,” “gender identity,” “gender expression,” “transgender,” and “non-binary.” Search engine suggestions of similar articles were included. Almost uniformly, the search results were small, and individual articles were selected for presentation and discussion based on relevance to the topic. The paucity of relevant literature further justifies the need to go beyond binary gender in neurotrauma research.
Sex differences in TBI
The incidence of TBI is higher in males than females in the general population across all age groups (0–65+),11,12 but some research suggests that females report more symptoms post-TBI, regardless of age, and are more likely to seek medical attention.13 Symptoms commonly reported by females include headache, anxiety, and depression, with symptoms that persist longer than those reported by males,14–18 whereas, chronic symptoms commonly reported by males include sleep disturbances, aggression, and substance abuse.14–16 When comparing functional outcomes after TBI between males and females, however, literature reports are conflicting. Of the included clinical studies in Gupte and colleagues' comprehensive review, 73 studies demonstrated females having worse outcomes compared with males, 41 studies demonstrated females having better outcomes compared with males, 28 studies found no sex differences when comparing outcomes, and 14 studies found mixed results in outcomes after TBI in males and females.10
In aggregate, these data reveal discrepancies regarding outcome of males and females after TBI. For example, despite persistent symptoms in females, sex did not predict cognitive or motor functional outcome after in-patient rehabilitation at 1 year post-injury.19 Mechanism of injury, however, predicted outcome only in females and comorbidities predicted outcome only in males.19 Some studies found that females had worse cognitive outcomes compared with males after TBI,20,21 whereas others found better cognitive recovery in females compared with males.22,23 Still, other studies found no sex differences in cognitive outcome between males and females after TBI.24,25 On one hand, males and females often report different categories of symptoms that may interact with outcome measures. On the other, the non-descript designation of sex/gender may artificially divide a heterogeneous sample population.
Sex differences have also been reported for the TBI incidence among high-risk populations, including athletes, soldiers, and victims of domestic violence (abuse by any household member including child and elder abuse) and intimate partner violence (IPV, abuse by a current or previous partner in a committed relationship). Non-professional (high school and collegiate) female athletes reported higher rates of TBI and had longer recovery times than male athletes,26,27 with women's soccer having the highest TBI incidence overall.28 Similar to findings in the general population, findings in female athletes included more post-concussive symptoms (difficulty in concentration, dizziness, and fatigue) compared with male athletes.29
Males make up the majority of active military and veteran populations and not surprisingly, experience higher rates of TBI in military-related activity.30 Females, however, reported more post-concussive symptoms following TBI in military-related activity.31 Of all individuals, victims of IPV have perhaps the highest risk of and are most vulnerable to TBI, and yet are the most under-represented population in published TBI research, education, or advocacy. Due to limited research and unreported cases, the exact incidence of TBI in victims of IPV is unknown, but it is estimated that TBI occurs in 50–90% of reported IPV cases.32,33 Both males and females are victims of IPV, but females make up the majority of the population and particularly those subjected to assault. Females are at exorbitant risk for TBI as a result of IPV.32 In addition, IPV during pregnancy, which includes physical violence toward the head, face, and abdomen, can have adverse effects on offspring.34 Among vulnerable populations, differences between male and female populations exist in the risk, incidence, and reporting of TBI.
When data are stratified by biological sex, the expected outcome is a main effect of sex in response to TBI; however, the literature contains conflicting reports on TBI outcome between males and females. When considering only biological sex, physiological variables that are associated with sex may obscure the true effect on outcome after TBI. The push toward personalized medicine has moved investigators to consider additional physiological variables, such as hormone cycles, for individuals in TBI research.
Female hormone cycle affects TBI outcome
The female reproductive life begins with the first menarche during adolescence and on average lasts through the mid- to late-40s, terminating with menopause.35 The fluctuation of endogenous sex hormones defines the luteal and follicular phases that make up the human female menstrual cycle.36 During the follicular phase, the levels of estradiol, luteinizing hormone (LH), and follicular stimulating hormone (FSH) increase and reach their peak before ovulation (day 14 of the cycle).37 During the luteal phase, the levels of progesterone increase and the levels of LH and FSH decrease.38 Hormonal fluctuation during the menstrual cycle phase can influence outcome after TBI.39 Females in the luteal phase of the menstrual cycle (elevated progesterone) at the time of injury had worse post-concussive symptoms at 1-month post-injury when compared with females injured during the follicular phase according to the Rivermead Post-Concussion Questionnaire, EuroQoL Index Score, and EuroQoL General Health Ratings.40 Another study reported no difference in post-concussion symptom severity and postural stability in college students according to menstrual cycle phase.41 Thus, pre-injury differences within female subjects could influence outcome after TBI but require additional studies. As we strive toward personalized medicine, the last menstrual period data collected during patient history could increase confidence in predicting outcome or informing treatment strategies in females after TBI.
Hormonal contraceptives are the most common contraceptive method in females ages 15–44 years in the United States.42 Females that self-reported synthetic progestin use had better outcomes 1 month after mild TBI compared with females injured during the luteal phase of the menstrual cycle with no self-reported synthetic progestin use.40 In studies reporting symptom severity, college-age students on hormonal or oral contraceptives reported less severe symptoms after TBI compared with students not on oral contraceptives or on non-hormonal contraceptives; male students reported greater symptom severity than both female groups.41,43 Together, these data suggest that hormonal contraceptives at the time of TBI may be beneficial with regard to clinical outcome.
Limited data exist on the effects of hormone levels after menopause on outcome after TBI or hormone levels during pregnancy on outcome after TBI. One study compared neuropsychological and cognitive function in pre-menopausal (age 25–44 years) versus post-menopausal (age 55–64 years) females after TBI measured by written and oral tests.44 Post-menopausal females performed worse than pre-menopausal females but performed better than age-matched males (age 55–64 years) after TBI.44 When comparing outcome after moderate to severe TBI in pregnant versus non-pregnant women, no statistical differences were found in mortality rates.45 Although estrogen and progesterone are elevated in pregnant women, this study and other epidemiological studies often do not collect hormonal levels at the time of injury, which leaves doubt regarding menstrual phase or stage of pregnancy when drawing conclusions from the data.46
Menstrual cycle phase can affect outcome after TBI, but inversely, TBI can affect the menstrual cycle. Females aged 18–45 years and menstruating at the time of injury were surveyed about health-related quality of life after completing in-patient rehabilitation. Menstrual disturbances such as amenorrhea and painful menses were increased post-injury compared with pre-injury.47 TBI severity predicted the duration of amenorrhea and not surprisingly, shorter duration of amenorrhea predicted better outcomes in patients.47 In another retrospective study, 68% of females with moderate to severe TBI reported cycle irregularities and 46% reported amenorrhea duration up to 60 months post-injury.48 Ranganathan and associates investigated the association between stress and sex hormones in pre- and post-menopausal females after TBI and reported that serum cortisol levels were elevated and gonadotrophins were suppressed during post-injury amenorrhea; hence, TBI-induced amenorrhea could be due to disruption in hypothalamic-pituitary-gonadal axis function.39 Changes in endogenous female hormone levels have been reported 2 years post-TBI.49 Therefore, when patients are dichotomized into male and female, physiological variables (female cycle phase, hormonal contraceptive use, pre-puberty, pregnancy, and post-menopause) could confound the results and interpretation.
The effects of cycling hormones in males on outcome after TBI
Testosterone is the primary endogenous sex hormone in males. Testosterone levels fluctuate daily in a circadian cycle, with increased testosterone levels between 2:00 AM and 10:00 AM.50 In healthy young men (mean age 25 years, range 18–40 years), the minimum testosterone concentration is approximately 511 ng/dL at 8:00 PM and the maximum concentration approximately 634 ng/dL at 6:00 AM.51–53 Testosterone levels have been shown to affect outcome after TBI in males. Normal to high levels of testosterone reduced the risk of unconscious state in males after TBI.54,55 TBI can also affect hormonal levels in males.56 In a cross-sectional study of former professional American-style male football players, players with higher concussion symptom severity and loss of consciousness during their football playing career experienced lower testosterone levels and higher rates of erectile dysfunction, even after adjustments for potential confounding medication use.55 Additionally, after moderate to severe TBI, males who were not professional athletes had lower levels of testosterone compared with healthy controls, which was associated with hypogonadism and predicted poor outcome and longer recovery time.57,58 These data indicate the need to separate gender categories and we recommend the collection of the time of day of the injury and testosterone levels in males pre-and post-TBI to reduce variance within male subjects.
Beyond physiological variables and TBI outcome
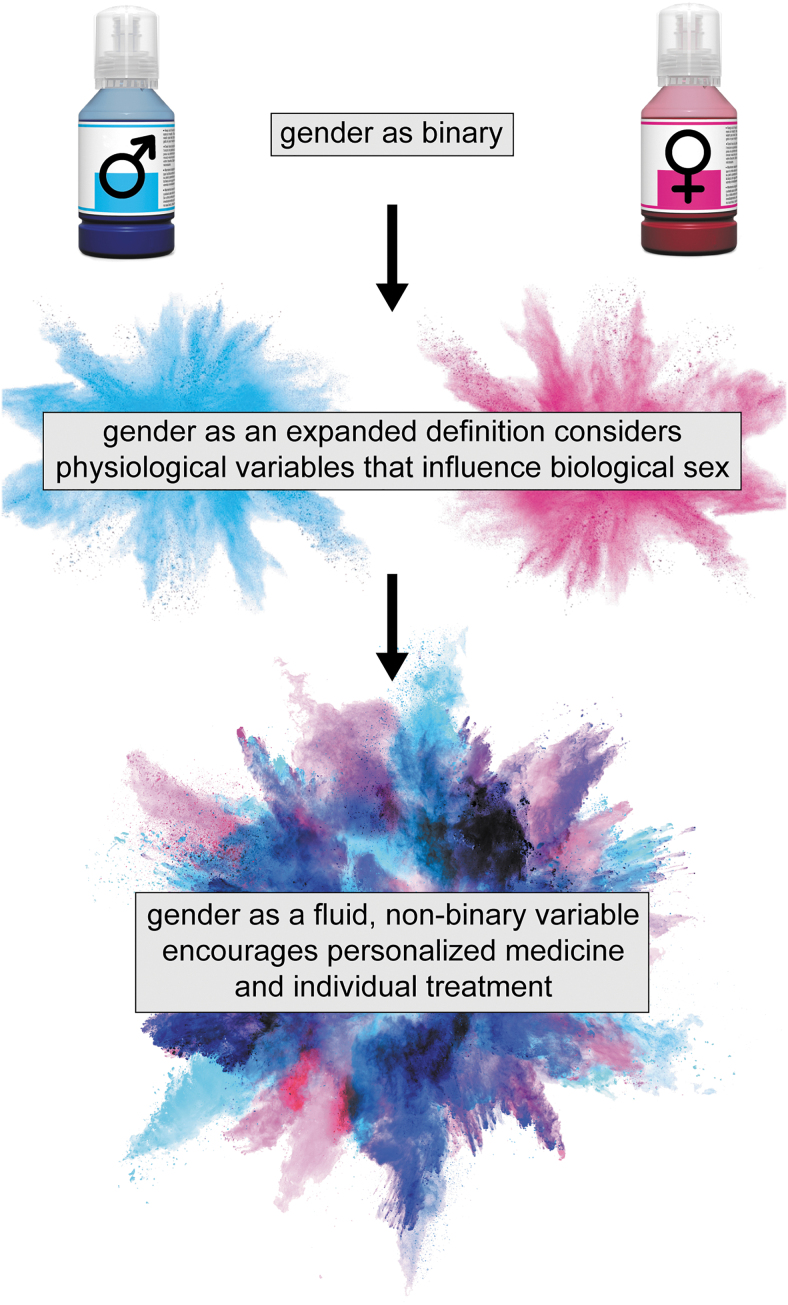
Up to this point, we have discussed physiological variables associated with traditional binary categories of sex and their effects on outcome after TBI. In some cases, the discussion has extended to the impact of TBI on physiology. In these contexts, sex has been discussed as biological, whereas gender is a sociocultural concept. Gender encompasses both identity and expression. Gender identity refers to an individual's internal feelings about their gender; gender expression refers to how an individual outwardly expresses their gender.59 Gender is non-binary, fluid, and can both affect outcome after TBI and be affected by TBI (Fig. 1).
FIG. 1.
In contemporary usage for traumatic brain injury (TBI) research and health care, gender is recorded as a binary variable, with values of male and female. Some electronic medical records permit expanded definitions of gender that often do not advance into data analysis and research reports. The constraints on gender fail to consider variables within individuals (e.g., sex hormones) and precise populations (e.g., hormonal contraceptive) that may influence recovery and outcome after TBI, in addition to social and cultural influences. Even further, consideration of fluid, non-binary gender (gender identity, gender expression, sexual orientation) requires inclusion of all individuals as we strive toward personalized medicine to diagnose and treat symptoms of TBI.
The long-term consequences of TBI can impact an individual's notion of self, social relationships such as familial/spouse relationships, and integration back into society.60 In one study conducted by Gutman and Napier-Klemic, four individuals aged 18–30 years were interviewed about their perceived masculinity and femininity and adherence to stereotypic gender roles at least 1 year post-injury.61 Female participants (n = 2) reported feeling secure in their femininity, whereas male participants (n = 2) cited feelings of inadequacy and insecurity in their gender identity.61 Social constructs of the traditional values of masculinity and femininity may influence an individual's decision to seek necessary treatment and complicate reintegration into society after injury.
Security in gender identity, influenced by TBI, also affects an individual's social relationships and reintegration into society after injury. Moore and colleagues explored the use of coping methods in the families of male soldiers who had suffered TBI.60 Younger families with greater financial burdens reported using fewer coping methods, such as seeking social and spiritual support, which resulted in strained marital relationships compared with older, more financially stable families.60 Patients found difficulty in disclosing information about their security in gender identity, placing stress on their ability to navigate and manage relationships. Moore and colleagues emphasized the importance of coping strategies suggested by social and spiritual support networks to regain a sense of identity and maintain relationships post-injury. Gender identity is individualized and requires personalized resources to support both real and perceived conflict.62 Health care professionals should consider and incorporate counseling for partners, significant others, and loved ones into the health care plan for individuals with TBI.
Mollayeva and associates discussed the intersection of sex and gender in TBI research, noting the lack of investigations on the effects of both sex and gender.63 And yet, the stratification of groups by social convention excludes specific populations with disproportionate risk for TBI.63 Transgender and gender non-conforming individuals are affected by violence at higher rates compared with cisgender individuals due to anti-transgender stigma and discrimination in society.64 The 2015 U.S. Transgender Survey reported that of 28,000 survey participants, 9% experienced physical violence in the last year due to their gender, 33% experienced at least one negative interaction with a health care provider due to their gender, and 54% were victims of IPV at some point in their life.65 Of IPV victims in this population, 24% experienced severe physical violence, which likely involved assaults to the head, neck, and face.65
Despite higher rates of violence, this vulnerable population remains vastly understudied and underserved in the TBI field, which creates health care disparities and limits the ability to deliver appropriate health care to transgender individuals with TBI.66 We identified two self-reporting TBI studies with 2700–2800 subjects that included transgender as a gender identification, in addition to cisgender male and female subjects.67,68 As reported by Juengst and coworkers, the transgender groups were too small to be included in analyses or to draw conclusions about incidence and outcome in transgender individuals after TBI.68 The inclusion of transgender participants in TBI research is a vital first step. It is critical, however, when going beyond binary gender to not stop at trinary gender categories (cis male, cis female, transgender/other) in our studies and analyses.
Despite the incidence of TBI in gender non-conforming populations, health care delivery and treatment have not been personalized for these patients. One case study of a 17-year-old Mexican-American transgender male patient with TBI, consequent post-traumatic stress disorder (PTSD), and chronic back pain raised awareness about the dearth of research to guide evidence-based clinical decisions for transgender patients.69 The author discussed the importance of considering the patient's female-to-male transition, ethnicity, and mental health when assembling a treatment plan. In fact, in reconsidering the treatment plan, the author proposed hormone therapy to treat chronic pain, but inconclusive and few studies about sex steroids and nociception in transgender individuals prevented an informed decision.69 Considerations of every individual's gender-specific needs are critical for effective treatment for symptoms of TBI especially in gender and ethnic minorities. Currently, diagnosis, prognosis, and treatment are confounded by disregarded hormonal responses and the lack of precision research, which prevent health care professionals from providing evidence-based care.
Conclusions
The health care field relies on evidence-based research. A lack of research on individuals who do not fit the mold of the traditional Caucasian, heterosexual, cisgender male TBI patient found in the literature limits the health care provided to all TBI patients. Nonetheless, significant strides are being made toward incorporating sex and gender into scientific literature. Stanford's Gendered Innovations in Science, Health & Medicine, Engineering, and Environment tool provides scientists and clinicians alike with a user-friendly website designed to spread awareness about (1) defining “sex” and “gender” in research, (2) incorporating sex and gender into scientific questions at both a basic and applied science level, and (3) stressing the innovations mastered because of incorporating the terms “sex” and “gender” into scientific literature.9
As the field moves forward, it is important that larger institutions be involved in gender inclusion. Change is only solidified with appropriate inclusive policy. The World Health Organization drafted an action plan to “mainstream” gender into their future programs. The NIH continues to mandate sex be reported and explicitly stated in scientific studies. The European Commission designed the term “gender dimension” to refer to the integration of sex and gender analysis into research, calling for sex and gender to be involved in planning stages of research and not just added as an afterthought.9 Canada Institutes of Health (CIH) gathered leading sex and gender TBI experts to (1) understand the perspective of all individuals across the gender spectrum who have suffered from mild TBI regarding their experience with their clinicians, (2) create an educational program for clinicians/scientists, and (3) train clinicians and scientists to follow feasible protocols in the conduct and report of findings related to the gender dimension.70
The onus lies with biomedical research and health care delivery to understand the patient and where they come from in order to treat them. This includes consideration of non-binary sex and non-binary gender. We call for scientists to be trained so they can understand sex and gender influences on their experiments, for health care professionals to be actively mindful about the role TBI can play in relation to sex and gender and vice versa, and finally for improved rehabilitation methods that take sex and gender into account.71,72 Sex and gender are just two variables that contribute to an individual's identity. As we continue the push toward personalized medicine, we must consider multiple marginalized identities (e.g., sexuality, education, class, ability, culture, age, and race) in treating individual patients with TBI so quality of life can be maximized.
Funding Information
KG was supported by NINDS of the National Institutes of Health under award number F31NS113408 and the Brain Injury Association of America's Brain Injury Research Fund (www.biausa.org/research). LMRV was supported by a Fulbright Scholarship from the Administrative Department for Science, Technology and Innovation (COLCIENCIAS) of the government of Colombia, Fulbright Commission-Colombia.
The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of the funding agencies.
Author Disclosure Statement
No competing financial interests exist.
References
- 1. Peterson A.B., Xu L., Daugherty J., and Breiding M.J. (2019). Surveillance report of traumatic brain injury-related emergency department visits, hospitalizations, and deaths, United States, 2014. Centers for Disease Control and Prevention. https://www.cdc.gov/traumaticbraininjury/pdf/TBI-Surveillance-Report-FINAL_508.pdf (Last accessed July2, 2020)
- 2. McCrory P., Meeuwisse W., Dvorak J., Aubry M., Bailes J., Broglio S., Cantu R.C., Cassidy D., Echemendia R.J., and Castellani R.J. (2017). Consensus statement on concussion in sport—the 5th International Conference on Concussion in Sport held in Berlin, October 2016. Br. J. Sports Med. 51, 838–847 [DOI] [PubMed] [Google Scholar]
- 3. Faul M., and Coronado V. (2015). Epidemiology of traumatic brain injury. Handb. Clin. Neurol. 127, 3–13 [DOI] [PubMed] [Google Scholar]
- 4. Blennow K., Brody D.L., Kochanek P.M., Levin H., McKee A., Ribbers G.M., Yaffe K., and Zetterberg H. (2016). Traumatic brain injuries. Nat. Rev. Dis. Primers 2, 16084. [DOI] [PubMed] [Google Scholar]
- 5. Baird K.L. (1999). The new NIH and FDA medical research policies: targeting gender, promoting justice. J. Health Polit. Policy Law 24, 531–565 [DOI] [PubMed] [Google Scholar]
- 6. Miller L.R., Marks C., Becker J.B., Hurn P.D., Chen W.J., Woodruff T., McCarthy M.M., Sohrabji F., Schiebinger L., Wetherington C.L., Makris S., Arnold A.P., Einstein G., Miller V.M., Sandberg K., Maier S., Cornelison T.L., and Clayton J.A. (2017). Considering sex as a biological variable in preclinical research. FASEB J 31, 29–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Clayton J.A., and Collins F.S. (2014). Policy: NIH to balance sex in cell and animal studies. Nature News 509, 282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gender Spectrum (2019). Understanding gender. https://www.genderspectrum.org/quick-links/understanding-gender (Last accessed January22, 2020)
- 9. Schiebinger L. (2014). Scientific research must take gender into account. Nature 507, 9–9 [DOI] [PubMed] [Google Scholar]
- 10. Gupte R., Brooks W., Vukas R., Pierce J., and Harris J. (2019). Sex differences in traumatic brain injury: what we know and what we should know. J. Neurotrauma 36, 3063–3091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Faul M., Xu L., Wald M.M., Coronado V., and Dellinger A.M. (2010). Traumatic brain injury in the United States: national estimates of prevalence and incidence, 2002–2006. Inj. Prev. 16, A268 [Google Scholar]
- 12. Gardner A.J., and Zafonte R. (2016). Neuroepidemiology of traumatic brain injury. Handb. Clin. Neurol. 138, 207–223 [DOI] [PubMed] [Google Scholar]
- 13. Stergiou-Kita M., Mansfield E., Sokoloff S., and Colantonio A. (2016). Gender influences on return to work after mild traumatic brain injury. Arch. Phys. Med. Rehabil. 97, Suppl. 2, S40–S45 [DOI] [PubMed] [Google Scholar]
- 14. Colantonio A., Harris J.E., Ratcliff G., Chase S., and Ellis K. (2010). Gender differences in self reported long term outcomes following moderate to severe traumatic brain injury. BMC Neurol. 10, 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Scott C., McKinlay A., McLellan T., Britt E., Grace R., and MacFarlane M. (2015). A comparison of adult outcomes for males compared to females following pediatric traumatic brain injury. Neuropsychology 29, 501–508 [DOI] [PubMed] [Google Scholar]
- 16. McGlade E., Rogowska J., and Yurgelun-Todd D. (2015). Sex differences in orbitofrontal connectivity in male and female veterans with TBI. Brain Imaging Behav. 9, 535–549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Styrke J., Sojka P., Bjornstig U., Bylund P.O., and Stalnacke B.M. (2013). Sex-differences in symptoms, disability, and life satisfaction three years after mild traumatic brain injury: a population-based cohort study. J. Rehabil. Med. 45, 749–757 [DOI] [PubMed] [Google Scholar]
- 18. Iverson G.L., Gardner A.J., Terry D.P., Ponsford J.L., Sills A.K., Broshek D.K., and Solomon G.S. (2017). Predictors of clinical recovery from concussion: a systematic review. Br. J. Sports Med. 51, 941–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Chan V., Mollayeva T., Ottenbacher K.J., and Colantonio A. (2016). Sex-specific predictors of inpatient rehabilitation outcomes after traumatic brain injury. Arch. Phys. Med. Rehabil. 97, 772–780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sandel N.K., Schatz P., Goldberg K.B., and Lazar M. (2017). Sex-based differences in cognitive deficits and symptom reporting among acutely concussed adolescent lacrosse and soccer players. Am. J. Sports Med. 45, 937–944 [DOI] [PubMed] [Google Scholar]
- 21. Covassin T., Elbin R., Bleecker A., Lipchik A., and Kontos A.P. (2013). Are there differences in neurocognitive function and symptoms between male and female soccer players after concussions? Am. J. Sports Med. 41, 2890–2895 [DOI] [PubMed] [Google Scholar]
- 22. Ratcliff J.J., Greenspan A.I., Goldstein F.C., Stringer A.Y., Bushnik T., Hammond F.M., Novack T.A., Whyte J., and Wright D.W. (2007). Gender and traumatic brain injury: do the sexes fare differently? Brain Inj. 21, 1023–1030 [DOI] [PubMed] [Google Scholar]
- 23. Donders J., and Hoffman N.M. (2002). Gender differences in learning and memory after pediatric traumatic brain injury. Neuropsychology 16, 491–499 [DOI] [PubMed] [Google Scholar]
- 24. Hsu H.-L., Chen D.Y.-T., Tseng Y.-C., Kuo Y.-S., Huang Y.-L., Chiu W.-T., Yan F.-X., Wang W.-S., and Chen C.-J. (2015). Sex differences in working memory after mild traumatic brain injury: a functional MR imaging study. Radiology 276, 828–835 [DOI] [PubMed] [Google Scholar]
- 25. Brooks B.L., Mrazik M., Barlow K.M., McKay C.D., Meeuwisse W.H., and Emery C.A. (2014). Absence of differences between male and female adolescents with prior sport concussion. J. Head Trauma Rehabil. 29, 257–264 [DOI] [PubMed] [Google Scholar]
- 26. Covassin T., Moran R., and Elbin R.J. (2016). Sex differences in reported concussion injury rates and time loss from participation: an update of the National Collegiate Athletic Association injury surveillance program from 2004–2005 through 2008–2009. J. Athl. Train. 51, 189–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Arambula S.E., Reinl E.L., El Demerdash N., McCarthy M.M., and Robertson C.L. (2019). Sex differences in pediatric traumatic brain injury. Exp. Neurol. 317, 168–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schallmo M.S., Weiner J.A., and Hsu W.K. (2017). Sport and sex-specific reporting trends in the epidemiology of concussions sustained by high school athletes. J. Bone Joint Surg. Am. 99, 1314–1320 [DOI] [PubMed] [Google Scholar]
- 29. Broshek D.K., Kaushik T., Freeman J.R., Erlanger D., Webbe F., and Barth J.T. (2005). Sex differences in outcome following sports-related concussion. J. Neurosurg. 102, 856–863 [DOI] [PubMed] [Google Scholar]
- 30. Amoroso T., and Iverson K.M. (2017). Acknowledging the risk for traumatic brain injury in women veterans. J. Nerv. Ment. Dis. 205, 318–323 [DOI] [PubMed] [Google Scholar]
- 31. Brickell T.A., Lippa S.M., French L.M., Kennedy J.E., Bailie J.M., and Lange R.T. (2017). Female service members and symptom reporting after combat and non-combat-related mild traumatic brain injury. J. Neurotrauma 34, 300–312 [DOI] [PubMed] [Google Scholar]
- 32. Baxter K., and Hellewell S.C. (2019). Traumatic brain injury within domestic relationships: complications, consequences and contributing factors. Journal of Aggression Maltreatment & Trauma 28, 660–676 [Google Scholar]
- 33. St. Ivany A., and Schminkey D. (2019). Rethinking traumatic brain injury from intimate partner violence: a theoretical model of the cycle of transmission. J. Aggression Maltreatment Trauma 28, 785–806 [Google Scholar]
- 34. Abel K.M., Heuvelman H., Rai D., Timpson N.J., Sarginson J., Shallcross R., Mitchell H., Hope H., and Emsley R. (2019). Intelligence in offspring born to women exposed to intimate partner violence: a population-based cohort study. Wellcome Open Res. 4, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bogin B., and Smith B.H. (1996). Evolution of the human life cycle. Am. J. Hum. Biol. 8, 703–716 [DOI] [PubMed] [Google Scholar]
- 36. Mihm M., Gangooly S., and Muttukrishna S. (2011). The normal menstrual cycle in women. Anim. Reprod. Sci. 124, 229–236 [DOI] [PubMed] [Google Scholar]
- 37. Alvergne A., and Hogqvist Tabor V. (2018). Is female health cyclical? Evolutionary perspectives on menstruation. Trends Ecol. Evol. 33, 399–414 [DOI] [PubMed] [Google Scholar]
- 38. Draper C., Duisters K., Weger B., Chakrabarti A., Harms A., Brennan L., Hankemeier T., Goulet L., Konz T., and Martin F. (2018). Menstrual cycle rhythmicity: metabolic patterns in healthy women. Sci. Rep. 8, 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Ranganathan P., Kumar R.G., Davis K., McCullough E.H., Berga S.L., and Wagner A.K. (2016). Longitudinal sex and stress hormone profiles among reproductive age and post-menopausal women after severe TBI: a case series analysis. Brain Inj. 30, 452–461 [DOI] [PubMed] [Google Scholar]
- 40. Wunderle K., Hoeger K.M., Wasserman E., and Bazarian J.J. (2014). Menstrual phase as predictor of outcome after mild traumatic brain injury in women. J. Head Trauma Rehabil. 29, E1–E8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mihalik J.P., Ondrak K.S., Guskiewicz K.M., and McMurray R.G. (2009). The effects of menstrual cycle phase on clinical measures of concussion in healthy college-aged females. J. Sci. Med. Sport 12, 383–387 [DOI] [PubMed] [Google Scholar]
- 42. Kavanaugh M.L., and Jerman J. (2018). Contraceptive method use in the United States: trends and characteristics between 2008, 2012 and 2014. Contraception 97, 14–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Gallagher V., Kramer N., Abbott K., Alexander J., Breiter H., Herrold A., Lindley T., Mjaanes J., and Reilly J. (2018). The effects of sex differences and hormonal contraception on outcomes after collegiate sports-related concussion. J. Neurotrauma 35, 1242–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Niemeier J.P., Marwitz J.H., Walker W.C., Davis L.C., Bushnik T., Ripley D.L., and Ketchum J.M. (2013). Are there cognitive and neurobehavioural correlates of hormonal neuroprotection for women after TBI? Neuropsychol. Rehabil. 23, 363–382 [DOI] [PubMed] [Google Scholar]
- 45. Berry C., Ley E.J., Mirocha J., Margulies D.R., Tillou A., and Salim A. (2011). Do pregnant women have improved outcomes after traumatic brain injury? Am. J. Surg. 201, 429–432 [DOI] [PubMed] [Google Scholar]
- 46. Wright D.W., Stein D.G., Sayeed I., Hua F., Atif F., Yousf S., Ishrat T., and Wali B. (2012). Response to: Do pregnant women have improved outcomes after traumatic brain injury? Am. J. Surg. 204, 803–804 [DOI] [PubMed] [Google Scholar]
- 47. Ripley D.L., Harrison-Felix C., Sendroy-Terrill M., Cusick C.P., Dannels-McClure A., and Morey C. (2008). The impact of female reproductive function on outcomes after traumatic brain injury. Arch. Phys. Med. Rehabil. 89, 1090–1096 [DOI] [PubMed] [Google Scholar]
- 48. Colantonio A., Mar W., Escobar M., Yoshida K., Velikonja D., Rizoli S., Cusimano M., and Cullen N. (2010). Women's health outcomes after traumatic brain injury. J. Womens Health 19, 1109–1116 [DOI] [PubMed] [Google Scholar]
- 49. Ruggeri R.M., Smedile G., Granata F., Longo M., Cannaio S., Sarlis N.J., Trimarchi F., and Benvenga S. (2010). Spontaneous recovery from isolated post-traumatic central hypogonadism in a woman. Hormones (Athens) 9, 332–337 [DOI] [PubMed] [Google Scholar]
- 50. Liu Z., Liu J., Shi X., Wang L., Yang Y., and Tao M. (2015). Dynamic alteration of serum testosterone with aging: a cross-sectional study from Shanghai, China. Reprod. Biol. Endocrinol. 13, 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Gupta S.K., Lindemulder E.A., and Sathyan G. (2000). Modeling of circadian testosterone in healthy men and hypogonadal men. J. Clin. Pharmacol. 40, 731–738 [DOI] [PubMed] [Google Scholar]
- 52. Mermall H., Sothern R.B., Kanabrocki E.L., Quadri S.F., Bremner F.W., Nemchausky B.A., and Scheving L.E. (1995). Temporal (circadian) and functional relationship between prostate-specific antigen and testosterone in healthy men. Urology 46, 45–53 [DOI] [PubMed] [Google Scholar]
- 53. Tenover J.S., Matsumoto A.M., Clifton D.K., and Bremner W.J. (1988). Age-related alterations in the circadian rhythms of pulsatile luteinizing hormone and testosterone secretion in healthy men. J. Gerontol. 43, M163–M169 [DOI] [PubMed] [Google Scholar]
- 54. Zhong Y.H., Wu H.Y., He R.H., Zheng B.E., and Fan J.Z. (2019). Sex differences in sex hormone profiles and prediction of consciousness recovery after severe traumatic brain injury. Front. Endocrinol. 10, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Grashow R., Weisskopf M.G., Miller K.K., Nathan D.M., Zafonte R., Speizer F.E., Courtney T.K., Baggish A., Taylor H.A., Pascual-Leone A., Nadler L.M., and Roberts A.L. (2019). Association of concussion symptoms with testosterone levels and erectile dysfunction in former professional US-style football players. JAMA Neurol. 76, 1428–1438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Hohl A., Zanela F.A., Ghisi G., Ronsoni M.F., Diaz A.P., Schwarzbold M.L., Dafre A.L., Reddi B., Lin K., Pizzol F.D., and Walz R. (2018). Luteinizing hormone and testosterone levels during acute phase of severe traumatic brain injury: prognostic implications for adult male patients. Front. Endocrinol. 9, 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Barton D.J., Kumar R.G., McCullough E.H., Galang G., Arenth P.M., Berga S.L., and Wagner A.K. (2016). Persistent hypogonadotropic hypogonadism in men after severe traumatic brain injury: temporal hormone profiles and outcome prediction. J. Head Trauma Rehabil. 31, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Vijapur S.M., Yang Z., Barton D.J., Vaughan L., Awan N., Kumar R.G., Oh B.M., Berga S.L., Wang K.K., and Wagner A.K. (2020). Anti-pituitary and anti-hypothalamus autoantibody associations with inflammation and persistent hypogonadotropic hypogonadism in men with traumatic brain injury. J. Neurotrauma 37, 1609–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Ontario Human Rights Commission. (2014). Policy on preventing discrimination because of gender identity and gender expression. http://www.ohrc.on.ca/en/policy-preventing-discrimination-because-gender-identity-and-gender-expression (Last accessed February28, 2020)
- 60. Moore A.D., Stambrook M., Peters L.C., and Lubusko A. (1991). Family coping and marital adjustment after traumatic brain injury. J. Head Trauma Rehabil. 6, 83–89 [Google Scholar]
- 61. Gutman S.A., and Napier-Klemic J. (1996). The experience of head injury on the impairment of gender identity and gender role. Am. J. Occup. Ther. 50, 535–544 [DOI] [PubMed] [Google Scholar]
- 62. Thomas E.J., Levack W.M., and Taylor W.J. (2014). Self-reflective meaning making in troubled times: change in self-identity after traumatic brain injury. Qual. Health Res. 24, 1033–1047 [DOI] [PubMed] [Google Scholar]
- 63. Mollayeva T., Mollayeva S., and Colantonio A. (2018). Traumatic brain injury: sex, gender and intersecting vulnerabilities. Nat. Rev. Neurol. 14, 711–722 [DOI] [PubMed] [Google Scholar]
- 64. Jauk D. (2013). Gender violence revisited: lessons from violent victimization of transgender identified individuals. Sexualities 16, 807–825 [Google Scholar]
- 65. James S., Herman J., Rankin S., Keisling M., Mottet L., and Anafi M.A. (2016). The report of the 2015 U.S. Transgender Survey. Washington DC: National Center for Transgender Equality. https://www.transequality.org/sites/default/files/docs/USTS-Full-Report-FINAL.PDF (Last accessed December19, 2019)
- 66. Safer J.D., Coleman E., Feldman J., Garofalo R., Hembree W., Radix A., and Sevelius J. (2016). Barriers to health care for transgender individuals. Curr. Opin. Endocrinol. Diabetes Obes. 23, 168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Mackelprang J.L., Harpin S.B., Grubenhoff J.A., and Rivara F.P. (2014). Adverse outcomes among homeless adolescents and young adults who report a history of traumatic brain injury. Am. J. Public Health 104, 1986–1992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Juengst S.B., Nabasny A., and Terhorst L. (2019). Neurobehavioral symptoms in community-dwelling adults with and without chronic traumatic brain injury: differences by age, gender, education, and health condition. Front. Neurol. 10, 1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Anaya V.K. (2018). A transgender adolescent with chronic pain, depression, and PTSD. J. Curr.Psychiatry 17, e1–e3 [Google Scholar]
- 70. Mollayeva T., Amodio V., Mollayeva S., D'Souza A., Colquhoun H., Quilico E., Haag H.L., and Colantonio A. (2019). A gender-transformative approach to improve outcomes and equity among persons with traumatic brain injury. BMJ Open 9, e024674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Husu L., Pollitzer E., and Hearn J. (2010). Recommendations for action on the gender dimension in science EU FP7 report to the European Commission: authors: Pollitzer E., Crane E., Dale H., Blaszczuk A., Hearn J., Husu L., Kikis-Papadakis K., Margetousaki A., Urban C., Reimer R., and Strähle M. https://gender-summit.com/images/genSET_Recommendations_for_Action_on_the_Gender_Dimension_in_Science.pdf (Last accessed March6, 2020)
- 72. Tannenbaum C., Greaves L., and Graham I.D. (2016). Why sex and gender matter in implementation research. BMC Med. Res. Methodol. 16, 145. [DOI] [PMC free article] [PubMed] [Google Scholar]