Abstract
Eventually there may be a broadly acceptable, even perfected, substitute for the human host requirement for direct feeding experiments by arthropods, most notably mosquitoes. However, for now, direct and indirect feeding on human volunteers is an important, if not essential, tool in vector biology research (VBR). This article builds on the foundational publication by Achee et al. (2015) covering considerations for the use of human participants in VBR pursuits. The authors introduced methods involving human participation in VBR, while detailing human-landing collections (catches) as a prime example. Benedict et al. (2018) continued this theme with an overview of human participation and considerations for research that involves release of mosquito vectors into the environment. In this study, we discuss another important aspect of human use in VBR activities: considerations addressing studies that require an arthropod to feed on a live human host. Using mosquito studies as our principal example, in this study, we discuss the tremendous importance and value of this approach to support and allow study of a wide variety of factors and interactions related to our understanding of vector-borne diseases and their control. This includes establishment of laboratory colonies for test populations, characterization of essential nutrients that contribute to mosquito fitness, characterization of blood-feeding (biting) behavior and pathogen transmission, parameterization for modeling transmission dynamics, evaluation of human host attraction and/or agents that repel, and the effectiveness of antivector or parasite therapeutic drug studies.
Keywords: human host, vector-borne diseases, vector biology research, human subjects, biosafety, ethics
Introduction
A wide spectrum of pathogens transmitted by arthropods (insects, ticks, and related animals) causes significant human public health burdens globally. Among the most important are mosquito-borne pathogens causing malaria, arboviral infections such as yellow fever, dengue, chikungunya, West Nile, and Zika viruses, as well as a wide range of tick-borne pathogens such as those responsible for Lyme borreliosis, babesiosis, and viral hemorrhagic fevers. Collectively, these infections are termed vector-borne diseases (VBDs). Research in both laboratory and field environments is essential to understand VBD transmission and combat disease through effective treatment and develop preventative methods (i.e., drug chemoprophylaxis, vaccines).
In this review, we refer to such investigations as vector biology research (VBR). While the importance of this field of study is obvious, until recently there were no guidelines for assisting researchers in the use of humans in VBR either as bloodmeal sources for arthropods, serving as host “attractants,” or as consenting recipients of drugs and/or components of drug therapies that may prevent vector biting or survival. This document intends to provide a framework to aid investigators, Institutional Review Boards (IRBs), and/or relevant ethics committees, as well as funders in judging and weighing the ethics, safety, and scientific merit of VBR requiring the use of human volunteers. This review is the third part of a series of articles focused on VBR, each with objectives to offer considerations and assist VBR stakeholders on the justification, utility, and value of specific VBR topics. The objective of each article is to describe the specific VBR topic, the associated risks, and provide a “living document” framework, from which updates can occur as VBR advances. The framework is based on current United States federally mandated regulations on the use of human subjects as guidance (45 CFR 46 https://www.hhs.gov/ohrp/regulations-and-policy/regulations/45-cfr-46/index.html).
Beginning with Achee et al. (2015), an overview of human participation in VBR and how these practices can be interpreted within the current U.S. regulatory framework was presented. The authors focused specifically on the technique of human landing collections for sampling mosquitoes as an example of a commonly used experimental method. The series continued with Benedict et al. (2018), which provided guidance for evaluating the safety of experimental releases of mosquitoes in the environment, with an emphasis on mark-release-recapture techniques and various considerations for mitigating human risk. The purpose of our review is to provide information to stakeholders (academics, industry, funders) investigating VBD (with methods that involve feeding/exposing mosquitoes (or other biting arthropods) directly on/to humans to meet research objectives) and where there is a lack of substandard alternative testing methods as valid replacements for human use.
Why Is the Use of Humans in VBR for Arthropod Blood Feeding Important?
Many of the most important arthropod species investigated in VBR specialize or feed frequently on human blood. Most of these relationships between arthropod vectors and humans have coevolved over many millennia. In some cases, more recently vectors adapted behaviors resulting in a strong selectivity for human feeding. For example, the dengue mosquito vector, Aedes aegypti (L.), feeds frequently and preferentially on humans (Scott et al. 1993). There is a selective physiological fitness advantage in A. aegypti having a strong proclivity for ingesting human blood. When offered alternative mammalian host blood, their reproduction and survival are compromised (Harrington et al. 2001). Likewise, when mosquitoes are offered human blood indirectly using an artificial membrane feeding system (Fig. 1a) compared to feeding on a human host directly, studies have shown nearly 50% reduction in egg production in female mosquitoes from indirect human blood feeds (Harrington et al. 2001), meaning that direct feeds may be required by investigators, dependent on VBR objectives. While the mechanism for this significant boost in mosquito fitness using human blood is unclear, it is likely due to specific components of human blood chemistry and adaptations of the vector to a specific host species. This same host–vector relationship is believed to drive anthropophagic (human feeding) species of Anopheles (Takken and Verhulst 2013), the mosquito genus responsible for transmitting human malaria.
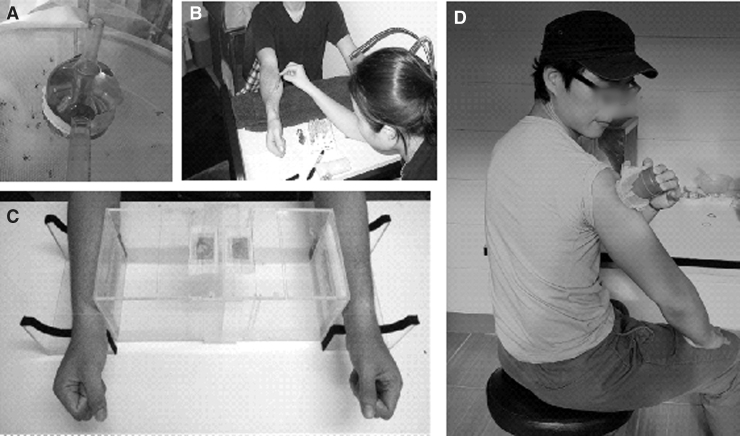
FIG. 1.
Typical human blood-feeding methods in vector biology research. (A) An artificial membrane feeder, (B) live host feeding for blood components/fitness assay, (C) live host attractant used in repellent assay, (D) live host feeding for study of vector-borne disease transmission dynamics (infectivity from host to vector, vector competence).
In addition, human feeding is often critical for establishing some mosquito laboratory colonies, when the initial generations from field-collected mosquitoes are reluctant to feed on alternative live hosts or through artificial membrane feeding systems (Foster 1980, Gerberg et al. 1994). Recent advances in modified mosquito deployment for mosquito and mosquito borne disease control have highlighted challenges when mass rearing mosquitoes on artificial feeding systems. Ross et al. (2019) evaluated the field performance of A. aegypti provided blood naturally and artificially and found significant fitness costs (reduced fertility, longer development time) and behavior (reduced host seeking) for those that had been maintained on artificial feeding systems compared to live human hosts.
Other research that requires direct feeding or direct human exposure includes studies of drug and vaccine efficacy, as well as vector response to repellents. The rationale for continued use of humans for testing drugs or repellent compounds, either in the development phase or as final stage product formulations, is that no current system can mimic the full array of human host attraction cues (host-specific skin emanations, heat, carbon dioxide, pigmentation, etc.) important for assessment purposes. After all, humans are the end user of repellents, and how the product performs under natural conditions and user acceptability are primary considerations for product marketing. However, variation between one human and another in regard to attraction to biting insects, ticks, and mites can confound testing and analysis if not addressed by the study design.
The transmission dynamics of many important pathogens such as malaria and dengue from humans to mosquitoes remains poorly understood, as well as studies of natural mosquito feeding behavior. Artificially provided infectious human bloodmeals (e.g., through membrane feeding system) are generally inferior to feeding mosquitoes directly on infected hosts (Graves et al. 1988, Bonnet et al. 2003, Diallo et al. 2008, Gaye et al. 2015, Ross et al. 2019). In some cases, direct feeding on human hosts has increased our understanding of the contribution and importance of subclinical infections to malaria transmission (Gaye et al. 2015). Similar insights have been gained with dengue viruses utilizing direct human feeding (Tan et al. 2016, Long et al. 2019). Other applications for direct human feeding are with evaluation of modification of mosquito feeding behavior (Moreira et al. 2009, Amuzu et al. 2015). Moreira et al. (2009) evaluated the probing (prefeeding behavior) and blood feeding of Wolbachia infected A. aegypti on human hosts and found significant decreases in the ability to blood feed efficiently compared with wild-type mosquitoes. This information was important to evaluating viability and arbovirus transmission risk for their particular Wolbachia infected mosquito candidate. Additional studies using direct feeding have contributed to our understanding of mosquito host learning (Vinauger et al. 2018). One important component of natural host feeding, as opposed to artificial membrane feeds, is the inclusion of complex and varied vector salivary molecules, which are known to enhance parasite and virus transmission from arthropod vector to hosts by modulating the host immune response and increasing probability of infection (reviewed by Schneider and Higgs 2008). The ability to mimic this in artificial systems is invariably challenging, leading to suboptimal interpretation of experimental results to “real-world” scenarios.
Therefore, research that investigates aspects of vector blood-feeding physiology or behavior ideally requires use of blood directly acquired from a live human host (Table 1). Herein, we describe in more detail these types of studies.
Table 1.
Examples of Direct Feeding Requirements and Studies on Humans
| Supporting vector biology research | Physiological research | Vaccine testing for disease prevention | Epidemiological studies | Drug testing for vector control | Repellent testing |
|---|---|---|---|---|---|
| Laboratory colony establishment | Fitness studies for vectors where there is a fitness deficit with artificial feeding | Determining efficacy of vaccines in the face of challenge under artificial or natural exposure | Determining human infectiousness to reservoir populations | Ivermectin and other endectocides | Laboratory biting assays |
| Xenodiagnosis to detect pathogens that are not detectible in other ways | Understanding vector transmission dynamics and the impact of saliva | Semifield repellency assays |
What Are the Risks for Including Human Feeding in VBR?
Study volunteers are placed at varying levels of risk, either with temporary pain and discomfort due to biting, or potentially more serious consequences due to significant allergic reactions or the possibility of contracting an infection from the bite. Risk depends on the test platform (e.g., laboratory-controlled, semifield, or field conditions) and trial circumstances (test species, biting densities, disease endemicity).
Laboratory and semifield trial designs can mitigate much of the risk using pathogen-free arthropod strains reared in the insectary. In some cases, colony material may not exist or colonization may greatly affect their blood-feeding behavior; therefore, necessitating testing using natural (wild) populations. Doing so in areas with no disease potential is highly preferred. Alternatively, immature stages can be collected from the field and reared to adults, thereby eliminating the potential transmission of most pathogens (e.g., malaria, babesiosis), while other infectious agents (viruses in mosquitoes and viruses, bacteria, and rickettsia in ticks) have varying ability to be vertically transmitted from mother to offspring; thus, a substantial risk remains unless infections are completely cleared from the test arthropods.
Types of Experiments/Studies Requiring Human Blood Feeding
Establishing a vector population colony
Having consistent access to laboratory colonies is a requirement for many types of VBR. Often a researcher will collect arthropods (mosquitoes, for example) from the field environment from which the natural wild-type population is endemic and transfer eggs or adults to insectary facilities to establish a continuously sustained colony. This provides a ready resource to test populations as needed for laboratory experiments, as well as ensures closest resemblance of natural vector characteristics found in the wild for results interpretable to the natural environment where pathogens may be circulating. Depending on the species, many successive generations may be needed to be produced before the colony population adapts to the artificial conditions of the laboratory insectary, even in facilities with sophisticated climate and lighting control regimens that are required to simulate natural field conditions for promoting mating and normal development (Foster 1980, Gerberg et al. 1994). Blood is essential for mosquito reproduction and, thus, essential for establishment of a colony. Sometimes investigators are compelled to provide their own blood directly to early generation populations to initiate colonization, thus ensuring that the nutrients and essential blood proteins for ovarian follicle (egg) development are provided. Gradually, laboratory animal hosts (e.g., rabbits, guinea pigs, mice, chickens) and/or artificial feeding systems can replace direct human feeds. Despite these efforts, some vector species will fail to thrive or are problematic to sustain as a long colonized strain without being provided human blood from a live host.
Measurements of vector fitness
Life table studies are important approaches for assessing vector fitness and survival under varying conditions to compare among groups. Analyses may consider egg production (fecundity), successful egg hatch (fertility), and adult longevity (survival) to calculate R0 (reproductive rate) (Moller-Jacobs et al. 2014, Villarreal et al. 2018). These studies are essential to inform models estimating pathogen transmission and, thus, human risk for VBD subsequently informing potential intervention targets (Villarreal et al. 2018). Life table studies are also conducted to characterize pathogen transmission dynamics from human to vector (Carrington and Simmons 2014). While human blood can be offered using artificial feeding systems, with natural or artificial membranes, the proportion of specimens feeding on the membrane and/or the bloodmeal volume ingested is often lower than when specimens are fed directly on humans (Clements 2000). With Anopheles mosquitoes, direct feeding studies on humans have contributed significantly to our knowledge of reproductive output, biting frequency, and feeding avidity (Straif and Beier 1996, Gary and Foster 2001).
Evaluating new VBD drug and/or vaccine candidates
Feeding pathogen-infected vectors on consenting human subjects is a highly effective method for testing drug and vaccine efficacy. Usually vaccinated/or drug recipient volunteers are “challenged” with exposure to blood feeding infectious vectors and then monitored for signs of infection. This type of VBR has also been used to determine the risk of feeding mosquitoes to become infected from feeding on humans that have received live-attenuated vaccine formulations for arthropod-borne viruses (Bancroft et al. 1982) and/or malaria vaccines (Epstein et al. 2007). Furthermore, there are numerous malaria transmission-blocking vaccine candidates in development that target various antigens on sexual-stage parasites, from gametocytes found in human blood and ingested with the bloodmeal to zygotes that are only found in the mosquito midgut, among others (Kapulu et al. 2015, Burrows et al. 2017). So far, these vaccine candidates have been developed and tested in malaria model animal systems or antibodies have been made and tested for efficacy with standardized or direct membrane feeding assays. At some point in the development and testing of a vaccine it must undergo human testing. Select drug and or vaccine candidates selected to move forward in phase testing to include human clinical trials must eventually demonstrate safety and efficacy through direct feeding on immunized human participants. To our knowledge, there are no substitutes (models or systems) for the use of human volunteers to measure the protective effects (efficacy) of a drug or vaccine intended to protect against a VBD. Mathematical modeling, for instance, can help guide drug and/or vaccine product development and study design but cannot replace human testing whether under artificial or natural infection challenge.
In the case of drugs, the development of ivermectin provides evidence of how both direct and indirect vector feeding assays on humans have been critical in understanding and characterizing the drug's effect. Endectocidal drugs have both antiparasitic effects on endoparasites such as helminths, as well as antiarthropod effects against ectoparasites such as scabies (Sarcoptes spp.) mites, blood-feeding lice and fleas, and various biting dipteran flies, most notably mosquitoes. Ivermectin is the first-in-class endectocidal drug being developed for malaria vector control because it is very well tolerated in humans, and Anopheles mosquitoes have been shown to be particularly sensitive to low concentrations of ivermectin found in human blood following standard doses administered for helminth control efforts.
The initial published experiments testing ivermectin against malaria vectors showed that survival time of several Anopheles species was reduced when they blood fed on treated rabbits (Iakubovich et al. 1989) and dogs (Jones et al. 1992, Gardner et al. 1993). Bockarie et al. (1999) collected wild, human-blood fed Anopheles punctulatus Dönitz and Anopheles koliensis Owen (both human malaria vectors) from inside human-occupied houses in village where ivermectin mass drug administrations (MDA) were occurring for helminth control and in untreated villages in Papua New Guinea. Mosquitoes captured following MDA had significantly reduced survivorship compared to those captured before drug administration and those from untreated villages. Laboratory experiments also demonstrated reduced survivorship of Anopheles farauti Laveran using direct mosquito feeding assays performed on a human volunteer who ingested ivermectin (Foley et al. 2000). Subsequently, others have performed direct and indirect membrane feeding assays to test ivermectin activity against various Anopheles and culicine species (Sylla et al. 2010, Chaccour et al. 2013, Ouedraogo et al. 2015, Sampaio et al. 2016).
The importance of direct human feeding experiments, as opposed to spiking ivermectin into artificial bloodmeals [e.g., Kobylinski et al. (2010)], is that the drug is allowed to proceed through its natural pharmacokinetic and pharmacodynamic pathways that influence the fate and actions of bioactive ingredients in the human before becoming available to the mosquito. As such, researchers have been able to define the time range of lethal activity following oral administration of different doses, a crucial step for predicting the effects of ivermectin dosing regimens on malaria transmission and disease control (Slater et al. 2014). Similarly, natural ivermectin metabolism has been studied in the context of the amount ingested by the mosquito, indicating that bioactive ivermectin metabolites may be acquired when it feeds on subdermal capillaries as opposed to drug found in venous whole blood (Nguyen et al. 2019). This is important because people with a different metabolism or body-mass indices (Ouedraogo et al. 2015) may be more or less lethal to biting Anopheles, thus potentially affecting MDA strategies and the efficacy of ivermectin as a malaria transmission control agent. As ivermectin proceeds into final phase III and IV clinical trials and possible acceptance and registration as a malaria control tool, indirect and direct feeding assays will be essential for gathering further evidence of its epidemiological and entomological effects across different conditions and target vector species. Human feeding assays will also lay the framework for studies to develop the next generation endectocidal/ectocidal drugs with potential to control VBD transmission.
Determining the infectious human reservoir populations in endemic settings
Many studies in VBR aim to understand the human infectious reservoir of a particular disease and understand the force of transmission of the infectious pathogen. These studies rely on direct feeding of laboratory reared and test vectors on infected human hosts. Typically, a small carton or cage of colony-reared mosquitoes is applied to the volunteer's arm. Mosquitoes are allowed to feed on the volunteer through mesh netting. Although some volunteers may not consent to direct human feeding, this method can be preferable to others compared to taking venous blood. Recently, Long et al. 2019 found high acceptability by participants for directly feeding mosquitoes on dengue-infected human patients over intravenous blood draws in Iquitos, Peru.
This approach can reveal the existence and diagnosis of subpatent, chronic, and so-called cryptic infections, which have gained greater interest in the control and potential elimination of VBD. In addition, this approach can lead to a greater understanding of infectious potential of a reservoir host (Mondal et al. 2019), the determination of putative reservoirs and vectors (species incrimination), and studies of competence (pathogen development in vectors and transmissibility). The feeding on “clean,” pathogen-free laboratory-reared arthropods greatly diminishes (for viruses), if not eliminates (for most other pathogens), the risk of inadvertent transmission of a pathogen to a human.
Xenodiagnosis
Xenodiagnosis is a technique that has been utilized for over a century and is still used today (Meiser and Schaub 2011). It is a procedure whereby a pathogen-free, suitable, and susceptible arthropod is allowed to directly blood feed on a human (or other animal) with a suspected infection that cannot, or with greater difficulty and uncertainly, be detected by more standard means (e.g., microscopic examination, immunologically, molecular assays). After a requisite incubation period, the vector is examined for evidence of infection acquired from the patient. Alternatively, indirect xenodiagnoses involving arthropods fed on venous blood in a membrane feeder is an option that avoids direct contact with an arthropod. However, membrane feeding may reduce the sensitivity for detection compared to direct feeding due to handling and technique procedures, as well as a variety of natural factors, related to the host-parasite-vector relationship (Graves et al. 1988, Diallo et al. 2008, Schneider and Higgs 2008, Tan et al. 2016, Long et al. 2019). This procedure is primarily used in the clinical setting, but has found important application in research environment as well. Diagnosis of infection with pathogens such as Plasmodium spp. (malaria), arboviruses, Trypanosoma cruzi (Chagas' disease), Borrelia spp. (Lyme borreliosis), and various forms of Leishmania spp. (leishmaniasis) and others (Bartonella, Onchocerca) has been investigated using this method (Meiser and Schaub 2011). Arthropods that have been/are used in xenodiagnoses include triatomine bugs (Reduviidae), phlebotomine sand flies (Psychodidae), body lice (Pediculus humanus humanus- Phthiraptera), hard ticks (Ixodidae), and mosquitoes (Culicidae). In fact, any laboratory-reared arthropod can be used similarly.
Evaluation of repellents
For repellent testing, human volunteers are essential as the preferred mosquito attractant. The test chemical is applied directly on forearms or other parts of the body and is followed by exposing these skin areas directly to live female mosquitoes. Assessment of repellent actions in the field can also involve human volunteers serving as natural attractive cues (“bait”) for attracting mosquitoes. These experiments can involve protected volunteers, such as those sleeping under bed nets with mechanical traps placed just outside of the net or unprotected workers performing human-landing catches (HLC). The HLC aspect of VBR and IRB considerations is covered in detail in Achee et al. (2015). In other designs, human collectors may periodically aspirate mosquitoes from passive trapping designs (e.g., bed net traps) (Silver 2008). Regardless, human volunteers and field workers, whether provided some form of personal protection or not, are exposed to some element of risk for receiving potential bites and possibly infections during testing.
As with active ingredient and product development, mosquitoes have been the primary target group for repellent assays followed by acarines. Other studies have examined biting flies of disease and nuisance importance (black flies, sand flies, biting gnats, horse/deer flies, stable flies, and others) (Buescher et al. 1987, Debboun et al. 2014). The use of human volunteers for the study of repellent activity against different arthropods has been the overwhelming method of choice and the general “gold standard” in practice. Risk depends on the test platform (e.g., laboratory-controlled, semifield, or field conditions) and trial circumstances (test species, biting densities, disease endemicity). There are several alternative methods that have been used for screening and comparison testing of repellent compounds, including nonhuman animal models (Barnard 2005), in vitro screening systems (Grieco et al. 2005), olfactometers (Barnard 2005), excito-repellency test chamber systems (Chareonviriyaphap et al. 2002), and compounds that mimic human host cues (Klun et al. 2005) that had been found useful without the need of a human host. Although the use of human volunteers is an important, if not essential, part of repellent development, exploration of alternative, nonhuman test systems should continue (Tisgratog et al. 2016). In the interim, all available methods, including the prudent use of humans, should be considered applicable based on objectives while ensuring the utmost protection of humans and animals.
Repellent products are a relatively inexpensive and a practical means of personal protection against nuisance and disease vector arthropods (Curtis et al. 1987, Gupta and Rutledge 1994, Debboun et al. 2014). Appropriate selection of repellents applied to skin or impregnated on clothing can provide significant protection from the majority of biting arthropods (Schreck and McGovern 1989, Drapeau et al. 2009, Lupi et al. 2013). Efforts continue toward discovery of new repellent synthetic and natural-based botanical (essential oils) active ingredients, while improved formulations enhance protection efficacy and persistence (Gupta and Rutledge 1989, Trongtokit et al. 2005, Witting-Bissinger et al. 2008, de Boer et al. 2010, Maia and Moore 2011). To do so, novel compounds are sequentially screened for repellent activity and safety characteristics (Chen-Hussey et al. 2014, Debboun et al. 2014). Overwhelmingly, repellency of mosquitoes, mites, and ticks are the primary target arthropods.
The preferred experimental designs to measure arthropod response to repellents, although varying somewhat in procedure based on the arthropod, analysis, and interpretation, share one general commonality—the prevailing use of human volunteers exposed to live blood-feeding arthropods (Bar-Zeev and Ben-Tamar 1971, Shreck 1977, Gupta and Rutledge 1989, Klun and Debboun 2000, Dautel 2004, Barnard 2005, Debboun et al. 2014). Information on performance test guidelines for insect repellents applied to human skin is available from the United States Environmental Protection Agency (EPA) (OCSPP-EPA 2010). This document covers protection of human subjects in research, informed consent, use of an IRB process, adherence to good laboratory practice standards, standardized terminology, definitions, and other topics. This is one of a series of “harmonized” EPA test guidelines established by the Office of Chemical Safety and Pollution Prevention (OCSPP) for use when testing pesticides and chemical substances to develop data for submission to various federal agencies. For example, across all guidelines, certain vulnerable groups should not be used in repellent testing, such as pregnant and nursing women or children due to potential exposure to repellent active ingredients.
In addition, the World Health Organization (WHO) has provided guidelines on specific procedures, analysis, and criteria for efficacy testing and evaluation of mosquito repellents applied to human skin (WHO 1996, 2009). It applies for laboratory studies, field trials, and the evaluation of active ingredients used in repellent products, including the estimation of effective dosing, “protection time” (end point) analysis, methods to determine application rate, efficacy, effectiveness, and persistence of formulated products. All human testing involves the use of informed and consenting human volunteers for exposing treated or untreated arms to repellent product or alternative. These guidelines were based on references having used human volunteers as the “standard” assessment procedure (Rutledge et al. 1985, 1989, Costantini et al. 2004).
As with mosquitoes, repellents applied to skin and clothing can contribute to a high degree of personal protection against tick and mite attack. Available bioassays have been grouped into three categories (1) use of live hosts, (2) use of a tick attractant associated with hosts, or (3) no attractants (Dautel 2004). The choice between designs must balance between need for test standardization, cost and time required to conduct tests, and ability to extrapolate findings for forecasting the efficacy of a compound or product under practical normal conditions.
Many studies for assessing compounds for repellent activity against tick species have used human volunteers (McMahon et al. 2003, Pretorius et al. 2003, Carroll et al. 2005, Jensenius et al. 2005, Jaenson et al. 2006, Schwantes et al. 2008, Bissinger et al. 2011); fortunately, tick behavior provides some biosafety when conducting direct exposure studies in that ticks often spend a variable amount of time crawling on the host before attempting to bite. This crawling behavior is advantageous for testing repellents. Therefore, testing procedures measure tick movement, when moving away from a repellent-treated surface (e.g., skin or clothing); this is considered a protective outcome. Moreover, the process of biting and the transmission of some pathogenic agents (e.g., Babesia, rickettsiae, borrelia) through salivary secretions (or regurgitation) are generally much slower than seen in biting flies, thus affording a reduction in infection risk if avoiding prolonged attachment of the tick (Eisen 2018). However, other agents such as viruses are transmitted almost instantaneously upon biting (Ebel and Kramer 2004); thereby, extreme caution should be taken when working with potential vectors of these pathogens.
When Does a Human Feeding Study Constitute Human Subjects Research Versus an Occupational Health/Biohazard Risk?
With all the VBR methods described here, careful consideration is necessary for assessing potential health risks to the humans involved. Whether an investigator can participate as a source of human blood in VBR is often regulated at an institutional level rather than by funding agencies. This can be further complicated when collaborations occur between two institutions that have different regulatory policies, requiring the two IRBs to work together and/or enter into a Reliance Agreement. For researchers based in the United States, multiple federal agencies follow the Federal Policy for the Protection of Human Subjects (“Common Rule”). The Office for Human Rights protection (OHRP) provides leadership and guidance for all PHS agencies (https://www.hhs.gov/ohrp/) with detailed information, education, and guidance. For more detailed discussion on whether volunteers are defined as “subjects” of human research, please refer to figure 3 in Achee et al. (2015). Many IRBs in the United States will follow the OHRP and NIH definition of human subjects research https://grants.nih.gov/policy/humansubjects/research.htm As a consequence, they will not consider direct feeding of an arthropod vector on a human as a human subjects research activity that requires IRB approval. This is conditional that no individual data on humans are collected. However, it is recommended that investigators submit protocols having human-feeding components to their respective IRB for determination whether the proposed study should be reviewed as human subjects research, referred to other regulatory committees for review (Institutional Biosafety Committee, Occupational Medicine, or Biosafety), or deemed exempt. In addition, IRBs typically have useful templates and recommendations for informing study participants and obtaining informed consent, if required. In cases of humans participating in VBR as “vector hosts,” all volunteers are considered “subjects.” Documentation of voluntary informed consent must be provided with a full understanding of the goals, objectives, and nature of the study being undertaken and all possible risks associated with participating. All volunteers must be afforded the ability to remove themselves from the study at any time, regardless of reason, and without prejudice or adverse repercussions for doing so.
Foremost, as best as possible, no harm should come to the human volunteers; all risks should be minimized and be manageable at all times. For example, direct contact with biting mosquitoes, ticks, or other biting arthropods (e.g., triatomine “kissing” bugs) can sometimes lead to unusually severe or prolonged allergic reactions (i.e., anaphylaxis) to salivary gland proteins. In some cases, these can result in prolonged or severe reactions (Huang et al. 2018). Furthermore, although every attempt for eliminating infection risks specific to a location should be taken, in some cases, a volunteer may be exposed to a disease agent, whether or not directly related to participating in the study itself. If that occurs, the study must be fully prepared to respond quickly and effectively for the medical benefit of the volunteer. Arthropod colonies used in feeding experiments should be periodically screened using specific molecular-based detection methods to target probable human pathogens and confirmed disease free before experimental use with human volunteers. For pathogens that are not maintained in the arthropod from one generation to another (vertical transmission) and/or between life stages (transstadial transmission), researchers can consider using immature stages reared to adults or first-generation progeny from field-collected individuals. However, those pathogens that can potentially be maintained through vertical and/or transstadial transmission in colony should be screened for infections before use to ensure human volunteer safety. It is essential that researchers work closely with all regulatory offices that reflect investigators institutions, as well as field settings, where VBR activity will be performed. Often the regulatory review process and approvals are obtained from the researcher's institution and other institutions associated with different study investigators, as well as the regulatory body, where the VBR activity is to occur. Decision trees are available at https://www.niaid.nih.gov/grants-contracts/decision-trees-human-subjects and can guide investigators and regulatory bodies when addressing the risks and benefits of using humans in VBR.
What Are the Risks and How to Mitigate Risk?
Ensuring that human volunteers are fully informed of the procedures and potential associated risk is essential for blood-feeding studies. Screened subjects who know that they have significant and prolonged allergic reactions to arthropod bites should be excluded from feeding studies. In addition, vectors should be screened for pathogens before beginning and periodically during the study. This is particularly important for field collected adult mosquitoes, nymphal/adult stage ticks, and other arthropods that may have acquired a pathogen through prior natural blood feeding. Mosquitoes collected as immature stages should be tested for pathogens that might be vertically transmitted (from female to offspring). For drug efficacy testing, careful medical monitoring of subjects for adverse reactions is essential.
Any additional protections for volunteers who are research staff should be followed whenever possible given the potential uneven power structure between principal investigators and staff. IRBs will want to ensure that subjects are not exploited. They may require adding a clause in the consent form stating that “volunteers can refuse to participate at any time without repercussions.”
Conclusions
Use of humans in VBR carries elements of inherent risk, from minor probability to more significant probabilities of occurrence depending on the research activity and innate characteristics of the human volunteer. The primary objective is to minimize or mitigate those risks to the lowest degree possible without compromising the study objectives. Even under very minimal risk scenarios, the total number of volunteers required to ensure statistical integrity of the study should be purposefully kept to a minimum. Depending on the study objectives, acceptable alternatives may be found that do not require the use of direct human exposure to biting arthropods. Reaching “equivalency” between human and surrogate methods for measuring arthropod responses to humans has been a long-term goal (Shreck 1977).
In this review, we have described a number of ways direct feeding on human volunteers is essential for VBR (Table 2). The types of experiments and goals of these studies are varied; however, they have led to unprecedented new information and advancements in our understanding of human risk and control of vector borne diseases. Despite this, considerations on protection, risks, and regulatory assurances must be integrated into VBR that include direct human feeds. Until the day we have a perfected substitute for the human host requirement in VBR, continued investments must be supported to identify equivalent biological models to replace humans although without sacrificing data validity.
Table 2.
Summary Points and Considerations for the Use of Human Blood Feeding and Arthropod Exposure in Vector Biology Research
| Summary points |
|---|
| • Direct and indirect feeding on human volunteers is and remains an important tool in vector-borne disease research. |
| • Several types of studies are important for VBR. |
| • Until recently there were no clear recommendations to guide researchers in the use of human participants as bloodmeal sources, host “attractants,” or antivector molecule recipients in VBR. |
| • Here we explain why there is no viable substitute for human volunteers in some areas of research, including the value of this approach to study the fitness contribution of nutrients in blood, biting behavior and pathogen transmission, as well as benefit to investigations of human host attraction or agents that repel, and antivector or parasite therapeutic drug studies. |
VBR, vector biology research.
Acknowledgments
The authors extend special appreciation to Dr. Adriana Costero-Saint Denis for her continued encouragement for each of the three framework articles in this series. The authors are grateful to the many human volunteers in VBR who have helped make significant scientific progress toward our understanding of vector biology and VBD control and to Dr. Michael Betteken, Cornell University Office of Research Integrity and Assurance, for providing helpful discussion and feedback on an earlier draft of the article.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
Financial support was provided by National Institute of Health/National Institute of Allergy and Infectious Diseases (NIH/NIAID) grant R01AI095491 to Harrington and NIH/NIAID grants R21AI129464 and U01AI138910 to Foy.
References
- Achee NL, Youngblood L, Bangs MJ, Lavery JV, et al. Considerations for the use of human participants in vector biology research: A tool for investigators and regulators. Vector Borne Zoonotic Dis 2015; 15:89–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amuzu HE, Simmons CP, McGraw EA. Effect of repeat human blood feeding on Wolbachia density and dengue virus infection in Aedes aegypti. Parasit Vectors 2015; 8:246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bancroft WH, Scott RM, Brandt WE, McCown JM, et al. Dengue-2 vaccine: Infection of Aedes aegypti mosquitoes by feeding on viremic recipients. Am J Trop Med Hyg 1982; 31:1229–1231 [DOI] [PubMed] [Google Scholar]
- Bar-Zeev M, Ben-Tamar D. Evaluation of mosquito repellents. Mosquito News 1971; 31:56–61 [Google Scholar]
- Barnard DR. Biological assay methods for mosquito repellents. J Am Mosq Control Assoc 2005; 21:12–16 [DOI] [PubMed] [Google Scholar]
- Benedict MQ, Charlwood JD, Harrington LC, Lounibos LP, et al. Guidance for evaluating the safety of experimental releases of mosquitoes, emphasizing mark-release-recapture techniques. Vector Borne Zoonotic Dis 2018; 18:39–48 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissinger BW, Apperson CS, Watson DW, Arellano C, et al. Novel field assays and the comparative repellency of BioUD®, DEET and permethrin against Amblyomma americanum. Med Vet Entomol 2011; 25:217–226 [DOI] [PubMed] [Google Scholar]
- Bockarie MJ, Hii JL, Alexander ND, Bockarie F, et al. Mass treatment with ivermectin for filariasis control in Papua New Guinea: impact on mosquito survival. Med Vet Entomol 1999; 13:120–123 [DOI] [PubMed] [Google Scholar]
- Bonnet S, Gouagna LC, Paul RE, Safeukui I, et al. Estimation of malaria transmission from humans to mosquitoes in two neighbouring villages in South Cameroon: Evaluation and comparison of several indices. Trans R Soc Trop Med Hyg 2003; 97:53–59 [DOI] [PubMed] [Google Scholar]
- Buescher M, Rutledge L, Wirtz R. Studies on the comparative effectiveness of permethrin and deet against bloodsucking arthropods. Pest Sci 1987; 21:165–173 [Google Scholar]
- Burrows JN, Duparc S, Gutteridge WE, van Huijsduijnen RH, et al. Erratum to: New developments in anti-malarial target candidate and product profiles. Malar J 2017; 16:151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrington LB, Simmons CP. Human to mosquito transmission of dengue viruses. Front Immunol 2014; 5:290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JF, Klun JA, Debboun M. Repellency of DEET and SS220 applied to skin involves olfactory sensing by two species of ticks. Med Vet Entomol 2005; 19:101–106 [DOI] [PubMed] [Google Scholar]
- Chaccour CJ, Kobylinski KC, Bassat Q, Bousema T, et al. Ivermectin to reduce malaria transmission: a research agenda for a promising new tool for elimination. Malar J 2013; 12:153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chareonviriyaphap T, Prabaripai A, Sungvornyothrin S. An improved excito-repellency test chamber for mosquito behavioral tests. J Vector Ecol 2002; 27:250–252 [PubMed] [Google Scholar]
- Chen-Hussey V, Behrens R, Logan JG. Assessment of methods used to determine the safety of the topical insect repellent N,N-diethyl-m-toluamide (DEET). Parasit Vectors 2014; 7:173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clements A. The Biology of Mosquitoes. Vol. 1: Development, Nutrition and Reproduction. Cambridge, MA: CABI, 2000 [Google Scholar]
- Costantini C, Badolo A, Ilboudo-Sanogo E. Field evaluation of the efficacy and persistence of insect repellents DEET, IR3535, and KBR 3023 against Anopheles gambiae complex and other Afrotropical vector mosquitoes. Trans R Soc Trop Med Hyg 2004; 98:644–652 [DOI] [PubMed] [Google Scholar]
- Curtis CF, Lines JD, Ijumba J, Callaghan A, et al. The relative efficacy of repellents against mosquito vectors of disease. Med Vet Entomol 1987; 1:109–119 [DOI] [PubMed] [Google Scholar]
- Dautel H. Test systems for tick repellents. Int J Med Microbiol 2004; 293 Suppl 37:182–188 [DOI] [PubMed] [Google Scholar]
- de Boer H, Vongsombath C, Palsson K, Bjork L, et al. Botanical repellents and pesticides traditionally used against hematophagous invertebrates in Lao People's Democratic Republic: A comparative study of plants used in 66 villages. J Med Entomol 2010; 47:400–414 [DOI] [PubMed] [Google Scholar]
- Debboun M, Frances S, Strickman D. Insect Repellents Handbook. Boca Raton: CRC Press; 2014 [Google Scholar]
- Diallo M, Toure AM, Traore SF, Niare O, et al. Evaluation and optimization of membrane feeding compared to direct feeding as an assay for infectivity. Malar J 2008; 7:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapeau J, Fröhler C, Touraud D, Kröckel U, et al. Repellent studies with Aedes aegypti mosquitoes and human olfactory tests on 19 essential oils from Corsica, France. Flav Frag Journal 2009; 24:160–169 [Google Scholar]
- Ebel GD, Kramer LD. Short report: Duration of tick attachment required for transmission of powassan virus by deer ticks. Am J Trop Med Hyg 2004; 71:268–271 [PubMed] [Google Scholar]
- Eisen L. Pathogen transmission in relation to duration of attachment by Ixodes scapularis ticks. Ticks Tick Borne Dis 2018; 9:535–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein JE, Rao S, Williams F, Freilich D, et al. Safety and clinical outcome of experimental challenge of human volunteers with Plasmodium falciparum-infected mosquitoes: An update. J Infect Dis 2007; 196:145–154 [DOI] [PubMed] [Google Scholar]
- Foley DH, Bryan JH, Lawrence GW. The potential of ivermectin to control the malaria vector Anopheles farauti. Trans R Soc Trop Med Hyg 2000; 94:625–628 [DOI] [PubMed] [Google Scholar]
- Foster WA. Colonization and maintenance of mosquitoes in the laboratory. In: Kreier JP, ed. Malaria, Vol. 2 Pathology, Vector studies and Culture NY: Academic Press, 1980:103–151 [Google Scholar]
- Gardner K, Meisch MV, Meek CL, Biven WS. Effects of ivermectin in canine blood on Anopheles quadrimaculatus, Aedes albopictus and Culex salinarius. J Am Mosq Control Assoc 1993; 9:400–402 [PubMed] [Google Scholar]
- Gary RE Jr., Foster WA. Effects of available sugar on the reproductive fitness and vectorial capacity of the malaria vector Anopheles gambiae (Diptera: Culicidae). J Med Entomol 2001; 38:22–28 [DOI] [PubMed] [Google Scholar]
- Gaye A, Bousema T, Libasse G, Ndiath MO, et al. Infectiousness of the human population to Anopheles arabiensis by direct skin feeding in an area hypoendemic for malaria in Senegal. Am J Trop Med Hyg 2015; 92:648–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerberg EJ, Barnard DR, Ward RA. Manual for mosquito rearing and experimental techniques. Lake Charles: American Mosquito Control Association, Inc.; 1994 [Google Scholar]
- Graves PM, Burkot TR, Carter R, Cattani JA, et al. Measurement of malarial infectivity of human populations to mosquitoes in the Madang area, Papua, New Guinea. Parasitology 1988; 96 (Pt 2):251–263 [DOI] [PubMed] [Google Scholar]
- Grieco JP, Achee NL, Sardelis MR, Chauhan KR, et al. A novel high-throughput screening system to evaluate the behavioral response of adult mosquitoes to chemicals. J Am Mosq Control Assoc 2005; 21:404–411 [DOI] [PubMed] [Google Scholar]
- Gupta RK, Rutledge LC. Laboratory evaluation of controlled-release repellent formulations on human volunteers under three climatic regimens. J Am Mosq Control Assoc 1989; 5:52–55 [PubMed] [Google Scholar]
- Gupta RK, Rutledge LC. Role of repellents in vector control and disease prevention. Am J Trop Med Hyg 1994; 50:82–86 [DOI] [PubMed] [Google Scholar]
- Harrington LC, Edman JD, Scott TW. Why do female Aedes aegypti (Diptera: Culicidae) feed preferentially and frequently on human blood? J Med Entomol 2001; 38:411–422 [DOI] [PubMed] [Google Scholar]
- Huang YL, Huang DN, Wu WH, Yang F, et al. Identification and characterization of the causative triatomine bugs of anaphylactic shock in Zhanjiang, China. Infect Dis Poverty 2018; 7:127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iakubovich V, Zakharova NF, Alekseev AN, Alekseev EA. [Evaluation of the action of ivermectin on blood-sucking mosquitoes]. Med Parazitol (Mosk) 1989:60–64 [PubMed] [Google Scholar]
- Jaenson TG, Garboui S, Palsson K. Repellency of oils of lemon eucalyptus, geranium, and lavender and the mosquito repellent MyggA natural to Ixodes ricinus (Acari: Ixodidae) in the laboratory and field. J Med Entomol 2006; 43:731–736 [DOI] [PubMed] [Google Scholar]
- Jensenius M, Pretorius AM, Clarke F, Myrvang B. Repellent efficacy of four commercial DEET lotions against Amblyomma hebraeum (Acari: Ixodidae), the principal vector of Rickettsia africae in southern Africa. Trans R Soc Trop Med Hyg 2005; 99:708–711 [DOI] [PubMed] [Google Scholar]
- Jones JW, Meisch MV, Meek CL, Bivin WS. Lethal effects of ivermectin on Anopheles quadrimaculatus. J Am Mosq Control Assoc 1992; 8:278–280 [PubMed] [Google Scholar]
- Kapulu MC, Da DF, Miura K, Li Y, et al. Comparative assessment of transmission-blocking vaccine candidates against Plasmodium falciparum. Sci Rep 2015; 5:11193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klun J, Debboun M. A new module for quantitative evaluation of repellent efficacy using human subjects. J Med Entomol 2000; 37:177–181 [DOI] [PubMed] [Google Scholar]
- Klun JA, Kramer M, Debboun M. A new in vitro bioassay system for discovery of novel human-use mosquito repellents. J Am Mosq Control Assoc 2005; 21:64–70 [DOI] [PubMed] [Google Scholar]
- Kobylinski KC, Deus KM, Butters MP, Hongyu T, et al. The effect of oral anthelmintics on the survivorship and re-feeding frequency of anthropophilic mosquito disease vectors. Acta Trop 2010; 116:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long KC, Sulca J, Bazan I, Astete H, et al. Feasibility of feeding Aedes aegypti mosquitoes on dengue virus-infected human volunteers for vector competence studies in Iquitos, Peru. PLoS Negl Trop Dis 2019; 13:e000; 7116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lupi E, Hatz C, Schlagenhauf P. The efficacy of repellents against Aedes, Anopheles, Culex and Ixodes spp.—A literature review. Travel Med Infect Dis 2013; 11:374–411 [DOI] [PubMed] [Google Scholar]
- Maia MF, Moore SJ. Plant-based insect repellents: A review of their efficacy, development and testing. Malar J 2011; 10 Suppl 1:S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMahon C, Krober T, Guerin PM. In vitro assays for repellents and deterrents for ticks: Differing effects of products when tested with attractant or arrestment stimuli. Med Vet Entomol 2003; 17:370–378 [DOI] [PubMed] [Google Scholar]
- Meiser CK, Schaub GA. Xenodiagnosis. In: H. M, ed. Nature Helps.… Parasitology Research Monographs. Berlin, Heidelberg: Springer; 2011 [Google Scholar]
- Moller-Jacobs LL, Murdock CC, Thomas MB. Capacity of mosquitoes to transmit malaria depends on larval environment. Parasit Vectors 2014; 7:593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, Bern C, Ghosh D, Rashid M, et al. Quantifying the infectiousness of Post-Kala-Azar dermal Leishmaniasis toward sand flies. Clin Infect Dis 2019; 69:251–258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreira LA, Saig E, Turley AP, Ribeiro JM, et al. Human probing behavior of Aedes aegypti when infected with a life-shortening strain of Wolbachia. PLoS Negl Trop Dis 2009; 3:e568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen C, Gray M, Burton T, Foy S, et al. Evaluation of a novel West Nile virus transmission control strategy that targets Culex tarsalis with endectocide-containing blood meals. PLoS Negl Trop Dis 2019; 13:e0007210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouedraogo AL, Bastiaens GJ, Tiono AB, Guelbeogo WM, et al. Efficacy and safety of the mosquitocidal drug ivermectin to prevent malaria transmission after treatment: a double-blind, randomized, clinical trial. Clin Infect Dis 2015; 60:357–365 [DOI] [PubMed] [Google Scholar]
- Pretorius AM, Jensenius M, Clarke F, Ringertz SH. Repellent efficacy of DEET and KBR 3023 against Amblyomma hebraeum (Acari: Ixodidae). J Med Entomol 2003; 40:245–248 [DOI] [PubMed] [Google Scholar]
- Ross PA, Lau MJ, Hoffmann AA. Does membrane feeding compromise the quality of Aedes aegypti mosquitoes? PLoS One 2019; 14:e0224268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutledge LC, Hooper RL, Wirtz RA, Gupta RK. Efficacy of diethyl methylbenzamide (deet) against Aedes dorsalis and a comparison of two end points for protection time. J Am Mosq Control Assoc 1989; 5:363–368 [PubMed] [Google Scholar]
- Rutledge LC, Wirtz RA, Buescher MD, Mehr ZA. Mathematical models of the effectiveness and persistence of mosquito repellents. J Am Mosq Control Assoc 1985; 1:56–62 [PubMed] [Google Scholar]
- Sampaio VS, Beltran TP, Kobylinski KC, Melo GC, et al. Filling gaps on ivermectin knowledge: effects on the survival and reproduction of Anopheles aquasalis, a Latin American malaria vector. Malar J 2016; 15:491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider BS, Higgs S. The enhancement of arbovirus transmission and disease by mosquito saliva is associated with modulation of the host immune response. Trans R Soc Trop Med Hyg 2008; 102:400–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreck CE, McGovern TP. Repellents and other personal protection strategies against Aedes albopictus. J Am Mosq Control Assoc 1989; 5:247–250 [PubMed] [Google Scholar]
- Schwantes U, Dautel H, Jung G. Prevention of infectious tick-borne diseases in humans: Comparative studies of the repellency of different dodecanoic acid-formulations against Ixodes ricinus ticks (Acari: Ixodidae). Parasit Vectors 2008; 1:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott TW, Clark GG, Lorenz LH, Amerasinghe PH, et al. Detection of multiple blood feeding in Aedes aegypti (Diptera: Culicidae) during a single gonotrophic cycle using a histologic technique. J Med Entomol 1993; 30:94–99 [DOI] [PubMed] [Google Scholar]
- Shreck C. Techniques for the evaluation of insect repellents: A critical review. Ann Rev Entomol 1977; 22:101–119 [DOI] [PubMed] [Google Scholar]
- Silver J. Sampling adults by animal bait catches and by animal-baited traps. In: Service M, ed. Mosquito Ecology: Field Sampling Methods. New York: Springer, 2008; 349–498 [Google Scholar]
- Slater HC, Walker PG, Bousema T, Okell LC, et al. The potential impact of adding ivermectin to a mass treatment intervention to reduce malaria transmission: a modelling study. J Infect Dis 2014; 210:1972–1980 [DOI] [PubMed] [Google Scholar]
- Straif SC, Beier JC. Effects of sugar availability on the blood-feeding behavior of Anopheles gambiae (Diptera:Culicidae). J Med Entomol 1996; 33:608–612 [DOI] [PubMed] [Google Scholar]
- Sylla M, Kobylinski KC, Gray M, Chapman PL, et al. Mass drug administration of ivermectin in south-eastern Senegal reduces the survivorship of wild-caught, blood fed malaria vectors. Malar J 2010; 9:365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takken W, Verhulst NO. Host preferences of blood-feeding mosquitoes. Annu Rev Entomol 2013; 58:433–453 [DOI] [PubMed] [Google Scholar]
- Tan CH, Wong PS, Li MZ, Yang HT, et al. Membrane feeding of dengue patient's blood as a substitute for direct skin feeding in studying Aedes-dengue virus interaction. Parasit Vectors 2016; 9:211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tisgratog R, Kongmee M, Sanguanpong U, Prabaripai A, et al. Evaluation of a noncontact, alternative mosquito repellent assay system. J Am Mosq Control Assoc 2016; 32:177–184 [DOI] [PubMed] [Google Scholar]
- Trongtokit Y, Rongsriyam Y, Komalamisra N, Apiwathnasorn C. Comparative repellency of 38 essential oils against mosquito bites. Phytother Res 2005; 19:303–309 [DOI] [PubMed] [Google Scholar]
- United States Environmental Protection Agency. OPPTS 810.3700: Insect repellents applied to human skin. Product Performance and Test Guidelines, 2010
- Villarreal SM, Pitcher S, Helinski MEH, Johnson L, et al. Male contributions during mating increase female survival in the disease vector mosquito Aedes aegypti. J Insect Physiol 2018; 108:1–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinauger C, Lahondere C, Wolff GH, Locke LT, et al. Modulation of host learning in Aedes aegypti mosquitoes. Curr Biol 2018; 28:333–344.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Report of the WHO informal consultation on the evaluation and testing of insecticides. Control of Tropical Diseases, WHO Pesticide Evaluation Scheme, Informal Consultation 96.1. Geneva: World Health Organization, 1996. [Google Scholar]
- WHO. Guidelines for efficacy testing of mosquito repellents for human skin. Control of Tropical Diseases, WHO Pesticide Evaluation Scheme, Informal Consultation 2009.4. Geneva: World Health Organization, 2009. [Google Scholar]
- Witting-Bissinger BE, Stumpf CF, Donohue KV, Apperson CS, et al. Novel arthropod repellent, BioUD®, is an efficacious alternative to deet. J Med Entomol 2008; 45:891–898 [DOI] [PubMed] [Google Scholar]