Abstract
Tracheal tissue engineering has become an active area of interest among clinical and scientific communities; however, methods to evaluate success of in vivo tissue-engineered solutions remain primarily qualitative. These evaluation methods have generally relied on the use of photographs to qualitatively demonstrate tracheal patency, endoscopy to image healing over time, and histology to determine the quality of the regenerated extracellular matrix. Although those generally qualitative methods are valuable, they alone may be insufficient. Therefore, to quantitatively assess tracheal regeneration, we recommend the inclusion of microcomputed tomography (μCT) to quantify tracheal patency as a standard outcome analysis. To establish a standard of practice for quantitative μCT assessment for tracheal tissue engineering, we recommend selecting a constant length to quantify airway volume. Dividing airway volumes by a constant length provides an average cross-sectional area for comparing groups. We caution against selecting a length that is unjustifiably large, which may result in artificially inflating the average cross-sectional area and thereby diminishing the ability to detect actual differences between a test group and a healthy control. Therefore, we recommend selecting a length for μCT assessment that corresponds to the length of the defect region. We further recommend quantifying the minimum cross-sectional area, which does not depend on the length, but has functional implications for breathing. We present empirical data to elucidate the rationale for these recommendations. These empirical data may at first glance appear as expected and unsurprising. However, these standard methods for performing μCT and presentation of results do not yet exist in the literature, and are necessary to improve reporting within the field. Quantitative analyses will better enable comparisons between future publications within the tracheal tissue engineering community and empower a more rigorous assessment of results.
Impact statement
The current study argues for the standardization of microcomputed tomography (μCT) as a quantitative method for evaluating tracheal tissue-engineered solutions in vivo or ex vivo. The field of tracheal tissue engineering has generally relied on the use of qualitative methods for determining tracheal patency. A standardized quantitative evaluation method currently does not exist. The standardization of μCT for evaluation of in vivo studies would enable a more robust characterization and allow comparisons between groups within the field. The impact of standardized methods within the tracheal tissue engineering field as presented in the current study would greatly improve the quality of published work.
Keywords: microcomputed tomography, trachea, tracheal lumen, tracheal stenosis, tracheal tissue engineering
Introduction
The tracheal tissue engineering community has actively developed novel strategies for the treatment of tracheal defects culminating in a plethora of creative approaches.1–5 Tracheal stenoses resulting from severe tracheal abnormalities from cancer, intubation, trauma, and idiopathic disorders have driven the need for innovative strategies to treat afflicted patients. Leading clinical treatment paradigms to address short-segment tracheal stenosis have relied on tracheal resection with end-to-end anastomosis and/or laryngotracheal reconstruction, utilizing autograft cartilage tissue harvested from the rib cage.6–8 Long-segment tracheal defects have previously been treated in the clinic using slide-tracheoplasty, a complicated surgical procedure where the trachea is divided, incised lengthwise, then fitted together, thereby shortening the length while expanding the lumen diameter.9–11 Harvesting autograft tissue can be painful for the patient and manipulation of cartilage to fit the defect can be difficult.12 In addition, complicated surgical procedures such as slide-tracheoplasty can limit the number of available surgeons who can perform the procedure. An obvious need exists for new treatment methods for both long- and short-segment defects, empowering the tracheal tissue engineering community to fill the present unmet need.
Various tracheal tissue engineering strategies include the use of decellularized tracheal allografts,13–15 naturally derived acellular materials such as silk fibroin scaffolds,16 shape-specific three-dimensional (3D) bioprinted materials,17–19 electrospun scaffolds,20,21 and cell-based constructs utilizing scaffold-free technology.22–25 Methods to evaluate success criteria have generally relied on the use of photographs to qualitatively demonstrate tracheal patency, endoscopy to image luminal healing over time, and/or histology to demonstrate regenerated extracellular matrix quality. Although the aforementioned methods are valuable and reasonable for assessment of regeneration for a tissue-engineered approach, the limitation is that these methods remain generally qualitative.
The purpose is therefore to present the use of microcomputed tomography (μCT) to quantify tracheal patency as an addition to the standard methods already used. μCT is a nondestructive method to image samples and create 3D models that can quantify tracheal patency. The method can be applied in vivo to evaluate regeneration over time or ex vivo as an endpoint analysis. Previous groups have used computed tomography to visualize the trachea after applying a tissue-engineered intervention; however, patency was not quantified.26,27 Quantifiable methods for evaluating success will allow for better comparisons among various groups within the field. μCT testing can be easily implemented into the current testing paradigm used by many groups as it is a nondestructive imaging method that can be applied to samples designated for histology. Our group has previously used μCT for assessment and quantification of tracheal regeneration in both sheep and rabbit animal models.28–30 The proceeding ex vivo data and associated analyses serve to illustrate methods to perform μCT, how to determine the appropriate volume of interest for analyses, and form the basis for a recommendation for standard practice within the tracheal tissue engineering field.
Materials and Methods
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center (protocol #18-008-SS-A-U).
Explanted rabbit tracheas were fixed in formalin for 48 h followed by incubation in a diluted contrast solution of 40% Optiray 320 and 60% phosphate-buffered saline for 24 h. Incubation in contrast solution enhanced μCT image quality and improved soft tissue contrast. Immediately before μCT imaging, explanted tracheas were removed from contrast solution and patted dry using gauze. The Quantum FX imaging system with a 50 kV X-ray source at 160 μA was used for imaging. A square 60 mm field of view produced a voxel resolution of 118 μm. After μCT imaging, Avizo computational software was used to reconstruct μCT images into a 3D model to quantify tracheal patency. To demonstrate the importance of selecting a representative tracheal length for volumetric quantification, the current study used three lengths of 15, 25, and 35 mm for μCT analysis. The rendered 3D model was then cropped to the desired length of trachea for quantification of lumen volume and minimum cross-sectional area. The empty lumen space could then be partitioned separately from the exterior tissue using a global threshold range. Volumetric quantification of the partitioned lumen volume was then computed using the Avizo software. Other computational software could be used to conduct the analysis described in the current study if desired.
Experiment
Experimental groups
To best illustrate a focus on μCT as an outcome assessment method, we returned to raw data from a rabbit study that we recently published.30 Data from both healthy and stenosed tracheas (n = 6) were obtained for the purpose of comparing the chosen length scale for the volume of interest (VOI). In-depth information regarding patch fabrication was described in the previous publication.30 Stenosed tracheas were induced with a tracheal defect and then patched using an electrospun polycaprolactone (PCL, Mw = 80,000; Wuhan Pensieve Technology Ltd., Wuhan, China) nanofiber patch containing 3D-printed PCL rings. The baseline electrospun PCL patches induced a stenosis after implantation into the proceeding rabbit animal model.
Animal model
The animal experimental protocol was approved by the Institutional Animal Care and Use Committee of the University of Oklahoma Health Sciences Center (protocol #18-008-SS-A-U). The animal model and surgical method are described in detail in our previous publication.30 Briefly, six skeletally mature female New Zealand white rabbits (∼4.5 kg) were purchased from Robinson Services Incorporated (Mocksville, NC). A tracheal defect was induced by excising a diamond-shaped portion of the trachea measuring 15 × 5 mm, where the longer axis was oriented along the length of the trachea. The defects were centered approximately between the carina and cricoid cartilage. For the stenosed group, an electrospun PCL patch with an approximate length of ∼18 mm was sutured to the trachea to cover the defect. After a 12-week surgery, the rabbits were euthanized in accordance with AVMA guidelines. Directly after euthanasia, the rabbit tracheas were harvested and fixed in 10% neutral buffered formalin before μCT analysis. Although tissue contraction from formalin fixation was expected, the preservation of the tracheal tissue counterbalanced the negative effect. Healthy control tracheas received no surgery and were acquired from age-, breed-, and sex-matched rabbits from unrelated studies.
Microcomputed tomography
μCT was used to quantify tracheal patency on harvested tracheas after 12 weeks of recovery (n = 6) as previously described.29,30 Before imaging, explanted tracheas were removed from formalin and then incubated in a diluted solution of 40% Optiray 320 (Mallinckrodt Pharmaceuticals, Staines-upon-Thames, UK) and 60% phosphate-buffered saline for 24 h. Soaking explanted tracheas in the diluted contrast agent was used to better image soft tissue for quantifying tracheal patency. A Quantum FX imaging system (PerkinElmer, Waltham, MA) with a 50 kV X-ray source at 160 μA was used for imaging. A square 60 mm field of view was used that produced a voxel resolution of 118 μm.
Avizo analysis
Avizo computational software (FEI Company, Hillsboro, OR) was used to reconstruct μCT images into a 3D model to quantify tracheal patency. To illustrate the importance of tracheal length that the investigator selects for μCT analysis, three different lengths of 15, 25, and 35 mm were analyzed, each with a corresponding VOI. We emphasize that the 15 mm VOI length corresponds to the 15 mm length of the induced defect. From this VOI, the lumen volume, average cross-sectional area, and minimum cross-sectional area were determined. A global threshold range was used for quantifying the empty space within the trachea. The average cross-sectional area (i.e., normalized volume) was calculated by dividing the lumen volume by the selected VOI length. Tracheal tissue was depicted using a gray scale and the quantified lumen space within the VOI was colored blue for delineation (Fig. 1). Due to potential tissue contraction from formalin fixation, comparisons should only be conducted among samples that have undergone formalin fixation. In addition, species/breed/age/sex-matched comparisons across studies should be careful to avoid in vivo versus ex vivo comparisons due to tissue contraction from formalin fixation.
FIG. 1.
Representative μCT reconstructions of a healthy control trachea and a stenosed trachea. Reconstructions are presented as sagittal cross sections to visualize the inner lumen diameter. The lumen VOI was quantified across investigator-selected lengths of 15, 25, and 35 mm as illustrated by the blue volume within the trachea. Scale bar = 10 mm. n = 6. μCT, microcomputed tomography; VOI, volume of interest.
Statistical methods
All statistical analyses were performed using GraphPad Prism (GraphPad Software, San Diego, CA) statistical software. An unpaired t-test (two-tailed) was used to analyze μCT results between the two groups (i.e., healthy vs. stenosed) for each VOI. Results were considered significant at a level of p < 0.05 and all data were reported as mean ± standard deviation. μCT testing had n = 6 samples per group.
Results
Lumen volume
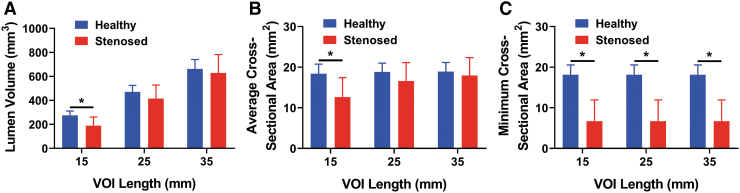
Tracheal lumen volume was compared between the healthy and stenosed tracheas for length scales of 15, 25, and 35 mm (Fig. 2A). The healthy lumen volume (276 ± 36 mm3) for the 15 mm VOI length scale was 1.45 times larger than the stenosed group (p < 0.05). In contrast, in analyzing the exact same specimens using the VOI corresponding to a length of 25 mm, the healthy lumen volume (471 ± 54 mm3) was only 1.13 times larger than the stenosed group, which was not significant (p > 0.05). Likewise, for the VOI corresponding to a length of 35 mm, the healthy lumen volume (662 ± 78 mm3) was 1.05 times larger than the stenosed lumen volume (p > 0.05, not significant).
FIG. 2.
Quantitative analyses from the μCT testing across VOI lengths of 15, 25, and 35 mm. (A) Lumen volume between healthy control tracheas and stenosed tracheas. Note that increasing VOI length sacrificed the detection of a statistically significant difference between the control and stenosed group by artificially inflating the volume of the stenosed group (visualized in Fig.1). (B) Average cross-sectional area determined across varying VOI lengths. Note that the value of the healthy cross-sectional area remains virtually unchanged regardless of the selected length, but that the stenosed values become artificially inflated to approach the healthy value with longer investigator-selected lengths, which likewise sacrifices the detection of a statistically significant difference. (C) Minimum cross-sectional area is independent of the selected length for a given group. Results can therefore be artificially manipulated based on inappropriate (i.e., too long) selection of length for lumen volume and average cross-sectional area, but not for minimum cross-sectional area. *p < 0.05, n = 6.
Average cross-sectional area
Average tracheal cross-sectional area was compared between the healthy and stenosed tracheas at length scales of 15, 25, and 35 mm (Fig. 2B). We emphasize that because a specific length was selected, for example, 15 mm, dividing the volumes of the healthy and stenosed groups by that fixed length resulted in average cross-sectional area differences between the groups that were identical to volume differences reported in the previous section (Fig. 3). Therefore, the healthy average cross-sectional area (18 ± 2 mm2) for the 15 mm VOI length scale was 1.45 times larger than the stenosed group (p < 0.05). For the 25 mm VOI length scale, the healthy average cross-sectional area (19 ± 2 mm2) was 1.13 times larger than the stenosed group (p > 0.05, not significant). Finally, for the 35 mm VOI length scale, the healthy average cross-sectional area (19 ± 2 mm2) was 1.05 times larger than the stenosed group (p > 0.05, not significant).
FIG. 3.
Representation of data to report for future publications. Note that both lumen volume and average cross-sectional area are able to be presented on the same chart, but only because a consistent VOI length (15 mm) is used. The 15 mm VOI length matched the length of the induced defect. *p < 0.05, n = 6.
Minimum cross-sectional area
Minimum tracheal cross-sectional areas were compared between the healthy and stenosed tracheas at length scales of 15, 25, and 35 mm (Fig. 2C). The healthy minimum cross-sectional area (18 ± 2 mm2) for the 15, 25, and 35 mm VOI length scale was 2.70 times larger than the stenosed group (p < 0.05). The minimum cross-sectional area is of course independent of the choice of VOI length scale.
Discussion
Determining the appropriate length for the VOI is vitally important as the volume and average cross-sectional area are both dependent on this choice. Too long a length scale can reduce or diminish the ability to detect actual differences as demonstrated by the lumen volume and average cross-sectional area data reported in Figure 2. To choose an appropriate length scale, we recommend that the decision be based on a standardized length that matches the dimension of the original tracheal defect. The smallest length scale used in the current study (i.e., 15 mm) represents the VOI length scale matching the length of the original defect, allowing the detection of a significant decrease in lumen volume and average cross-sectional area for the stenosed group compared with the healthy control. The ability to detect this difference was sacrificed when larger length scales were used because a greater length of healthy tissue was averaged into the stenosed treatment group, thereby artificially increasing its volume and average cross-sectional area. In other words, increasing length scales (i.e., 25 or 35 mm) demonstrated a “padding effect” that minimized the average difference for the lumen volume and average cross-sectional area between the healthy and stenosed groups. Therefore, the future investigator's selection of an unjustifiably long length may inaccurately imply that a treatment group performed better than it had in reality. To better represent findings, we therefore recommend specifically using a VOI length scale representative of the original defect length.
Generally, the most common method of quantifying tracheal patency has been the use of average cross-sectional diameter or area using an endoscope.31,32 Measurements acquired using an endoscope can be unintentionally skewed as the endoscopic probing can manipulate the shape of the trachea if the regenerated tissue is weak. Use of a rigid endoscope can potentially displace regenerating tracheal tissue. In addition, endoscopic imaging is dependent on the user subjectively determining the point of minimum stenosis. The addition of μCT improves upon current methods because no manipulation is required to achieve full 3D reconstructions. In addition, the total volume can be assessed rather than just a given cross-sectional area. Using a unified length scale among all samples enables both the lumen volume and average cross-sectional area to be presented on the same graph (Fig. 3), allowing for data to be compared with previous and future studies easily.
Separately, as an entirely different outcome metric, we recommend reporting the minimum cross-sectional area in addition to the lumen volume and average cross-sectional area. As demonstrated by the various length scales, the minimum cross-sectional area is independent of the chosen length scale. In addition, the comparison between the lumen volume and minimum cross-sectional area can lend insight to whether the treatment is causing a gradual restenosis across the defect or an acute stenosis (or “pinch point”) at a single point of the defect. Furthermore, there is a functional significance with the minimum cross-sectional area, which may be the most important factor in determining whether the subject will have difficulty breathing. Future studies may additionally normalize the VOI and minimum cross-sectional area to the normal healthy control for comparing groups to enable easier comparisons among future studies.
In conclusion, we recommend the use of μCT to quantify tracheal patency, which enables improved comparisons among treatments within the field. We recommend the use of a VOI corresponding to the defect region and a standardized length scale for evaluating all samples. Increasing the lengths to arbitrarily large values for the VOI was demonstrated to artificially inflate the average cross-sectional area of the stenosed group, thereby decreasing differences between the healthy and stenosed groups and failing to detect statistically significant differences that were evident with the more reasonable 15 mm length corresponding to the true defect region of interest.
In summary, we recommend as standard practice in tracheal tissue engineering reporting the lumen volume and average cross-sectional area together on the same chart (Fig. 3), made possible by selecting a fixed length for all samples that corresponds to the actual length of the defect. Although volumes may differ based on the selected fixed length, the average cross-sectional area for a given healthy species, breed, sex, and age should be fairly reproducible from study to study and thereby offer more rigorous comparisons of treatment groups in studies from different investigators around the world. The corresponding method can equally be applied to a full segmental defect or a defect placed at a different location on the trachea, provided that the proximal and distal anastomoses are included within the VOI. Moreover, we recommend reporting the minimum cross-sectional area as standard practice, given the functional importance with regard to breathing difficulty. Implementation of these quantitative methods will improve the field of tracheal tissue engineering by allowing more standardized, rigorous, and reproducible comparisons across studies.
Acknowledgments
The authors thank Dr. Hong Liu for generously allowing the use of the μCT equipment and Dr. Yuhua Li for assistance with μCT imaging.
Disclosure Statement
No competing financial interests to disclose.
Funding Information
Research reported in this publication was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award number R43 HL140890 (to J.K.J.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1. Haykal S., Salna M., Waddell T.K., and Hofer S.O.. Advances in tracheal reconstruction. Plast Reconstr Surg Glob Open 2, e178, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Law J.X., Liau L.L., Aminuddin B.S., and Ruszymah B.H.. Tissue-engineered trachea: a review. Int J Pediatr Otorhinolaryngol 91, 55, 2016 [DOI] [PubMed] [Google Scholar]
- 3. Ott L.M., Weatherly R.A., and Detamore M.S.. Overview of tracheal tissue engineering: clinical need drives the laboratory approach. Ann Biomed Eng 39, 2091, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Udelsman B., Mathisen D.J., and Ott H.C.. A reassessment of tracheal substitutes-a systematic review. Ann Cardiothorac Surg 7, 175, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Dhasmana A., Singh A., and Rawal S.. Biomedical grafts for tracheal tissue repairing and regeneration “Tracheal tissue engineering: an overview”. J Tissue Eng Regen Med 14, 653, 2020 [DOI] [PubMed] [Google Scholar]
- 6. Cotton R.T., Gray S.D., and Miller R.P.. Update of the Cincinnati experience in pediatric laryngotracheal reconstruction. Laryngoscope 99, 1111, 1989 [DOI] [PubMed] [Google Scholar]
- 7. Gozen E.D., Yener M., Erdur Z.B., and Karaman E.. End-to-end anastomosis in the management of laryngotracheal defects. J Laryngol Otol 131, 447, 2017 [DOI] [PubMed] [Google Scholar]
- 8. Grillo H.C. Tracheal replacement. Ann Thorac Surg 49, 864, 1990 [DOI] [PubMed] [Google Scholar]
- 9. Elliott M., Hartley B.E., Wallis C., and Roebuck D.. Slide tracheoplasty. Curr Opin Otolaryngol Head Neck Surg 16, 75, 2008 [DOI] [PubMed] [Google Scholar]
- 10. Grillo H.C. Tracheal replacement: a critical review. Ann Thorac Surg 73, 1995, 2002 [DOI] [PubMed] [Google Scholar]
- 11. Tsang V., Murday A., Gillbe C., and Goldstraw P.. Slide tracheoplasty for congenital funnel-shaped tracheal stenosis. Ann Thorac Surg 48, 632, 1989 [DOI] [PubMed] [Google Scholar]
- 12. Gilpin D.A., Weidenbecher M.S., and Dennis J.E.. Scaffold-free tissue-engineered cartilage implants for laryngotracheal reconstruction. Laryngoscope 120, 612, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Den Hondt M., Vanaudenaerde B.M., Verbeken E.K., and Vranckx J.J.. Epithelial grafting of a decellularized whole-tracheal segment: an in vivo experimental model. Interact Cardiovasc Thorac Surg 26, 753, 2018 [DOI] [PubMed] [Google Scholar]
- 14. Skolasinski S.D., and Panoskaltsis-Mortari A.. Lung tissue bioengineering for chronic obstructive pulmonary disease: overcoming the need for lung transplantation from human donors. Expert Rev Respir Med 13, 665, 2019 [DOI] [PubMed] [Google Scholar]
- 15. Zhang Y., Xu Y., Liu Y., et al. Porous decellularized trachea scaffold prepared by a laser micropore technique. J Mech Behav Biomed Mater 90, 96, 2019 [DOI] [PubMed] [Google Scholar]
- 16. Galvez Alegria C., Gundogdu G., Yang X., Costa K., and Mauney J.R.. Evaluation of acellular bilayer silk fibroin grafts for onlay tracheoplasty in a rat defect model. Otolaryngol Head Neck Surg 160, 310, 2019 [DOI] [PubMed] [Google Scholar]
- 17. Galliger Z., Vogt C.D., and Panoskaltsis-Mortari A.. 3D bioprinting for lungs and hollow organs. Transl Res 211, 19, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Park H.S., Lee J.S., Jung H., et al. An omentum-cultured 3D-printed artificial trachea: in vivo bioreactor. Artif Cells Nanomed Biotechnol 46, S1131, 2018 [DOI] [PubMed] [Google Scholar]
- 19. Park J.H., Yoon J.K., Lee J.B., et al. Experimental tracheal replacement using 3-dimensional bioprinted artificial trachea with autologous epithelial cells and chondrocytes. Sci Rep 9, 2103, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Dharmadhikari S., Best C.A., King N., et al. Mouse model of tracheal replacement with electrospun nanofiber scaffolds. Ann Otol Rhinol Laryngol 128, 391, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kang Y., Wang C., Qiao Y., et al. Tissue-engineered trachea consisting of electrospun patterned sc-PLA/GO-g-IL fibrous membranes with antibacterial property and 3D-printed skeletons with elasticity. Biomacromolecules 20, 1765, 2019 [DOI] [PubMed] [Google Scholar]
- 22. Li D., Yin Z., Liu Y., et al. Regeneration of trachea graft with cartilage support, vascularization, and epithelization. Acta Biomater 89, 206, 2019 [DOI] [PubMed] [Google Scholar]
- 23. Machino R., Matsumoto K., Taniguchi D., et al. Replacement of rat tracheas by layered, trachea-like, scaffold-free structures of human cells using a bio-3D printing system. Adv Healthc Mater 8, e1800983, 2019 [DOI] [PubMed] [Google Scholar]
- 24. Sueyoshi S., Chitose S.I., Sato K., Fukahori M., Kurita T., and Umeno H.. Stable tracheal regeneration using organotypically cultured tissue composed of autologous chondrocytes and epithelial cells in beagles. Ann Otol Rhinol Laryngol 128, 585, 2019 [DOI] [PubMed] [Google Scholar]
- 25. Dikina A.D., Strobel H.A., Lai B.P., Rolle M.W., and Alsberg E.. Engineered cartilaginous tubes for tracheal tissue replacement via self-assembly and fusion of human mesenchymal stem cell constructs. Biomaterials 52, 452, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bae S.W., Lee K.W., Park J.H., et al. 3D bioprinted artificial trachea with epithelial cells and chondrogenic-differentiated bone marrow-derived mesenchymal stem cells. Int J Mol Sci 19, 1624, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Yamashita M., Kanemaru S., Hirano S., et al. Tracheal regeneration after partial resection: a tissue engineering approach. Laryngoscope 117, 497, 2007 [DOI] [PubMed] [Google Scholar]
- 28. Ott L.M., Vu C.H., Farris A.L., et al. Functional reconstruction of tracheal defects by protein-loaded, cell-seeded, fibrous constructs in rabbits. Tissue Eng Part A 21, 2390, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Townsend J.M., Ott L.M., Salash J.R., et al. Reinforced electrospun polycaprolactone nanofibers for tracheal repair in an in vivo ovine model. Tissue Eng Part A 24, 1301, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Townsend J.M., Hukill M.E., Fung K.M., et al. Biodegradable electrospun patch containing cell adhesion or antimicrobial compounds for trachea repair in vivo. Biomed Mater 15, 025003, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Bergonse Neto N., Jorge L.F., Francisco J.C., et al. Regeneration of tracheal tissue in partial defects using porcine small intestinal submucosa. Stem Cells Int 2018, 5102630, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Xia D., Jin D., Wang Q., et al. Tissue-engineered trachea from a 3D-printed scaffold enhances whole-segment tracheal repair in a goat model. J Tissue Eng Regen Med 13, 694, 2019 [DOI] [PubMed] [Google Scholar]