Abstract
Background: We evaluated the feasibility and acceptability of a pilot behavioral intervention delivered to parents of adolescents with type 1 diabetes (T1D) via mobile-friendly web app. The Type 1 Doing Well app aimed to promote supportive family diabetes management by helping parents recognize and reinforce teens' positive diabetes-related behaviors (“strengths”).
Methods: Parents (n = 80, 74% recruitment) of adolescents (age range = 12–17 years, M = 15.3 ± 1.5 years, 59% female, 56% insulin pump, M hemoglobin A1c (HbA1c) = 9.0% ± 2.1%) were randomized 2:1 to intervention or control (i.e., usual medical care with or without app) for 3–4 months between diabetes appointments. The app prompted parents daily to track adolescents' strengths and generated weekly summaries of their teen's top strengths. Parents could access a library of text messages to praise their teens. Exploratory pre/post data included questionnaires (98% completed) and HbA1c.
Results: Parents used the app for M = 106.1 ± 37.1 days, logging in ≥once/day on 80% of days. Ninety-one percent of parents used the app ≥2 days/week on average. Parents viewed M = 5.6 ± 4.7 weekly summaries and “favorited” 15 praise texts in the library. App acceptability ratings (7-point scale) were high: Satisfaction 5.0 ± 1.5, Usefulness 4.8 ± 1.5, Ease of Use 6.2 ± 0.8, and Ease of Learning 6.5 ± 0.8. Parents (n = 48) and adolescents (n = 47) gave positive feedback and suggestions via qualitative interviews. There were no significant between-group differences for change in exploratory outcomes (HbA1c, questionnaires).
Conclusions: Type 1 Doing Well was feasible to deliver and highly acceptable and engaging for parents of adolescents with T1D. It may have a larger impact on behavioral or clinical outcomes as part of a multicomponent intervention protocol. Trial Registration: ClinicalTrials.gov NCT02877680.
Keywords: Diabetes mellitus, Type 1, Parents, Adolescents, Health behavior, Mobile health, Pilot study, Resilience
Introduction
The developmental, behavioral, and physiological features of adolescence make it a particularly challenging period for type 1 diabetes (T1D) management. As expectations for self-management tend to increase during adolescence,1,2 declines in treatment adherence and above target hemoglobin A1c (HbA1c) are typical.3,4 While glycemic and quality-of-life outcomes improve with teamwork between parents and adolescents,5 diabetes-related conflict is common and can make collaborative family diabetes management difficult.6,7 Parents often worry about the long-term consequences of adolescents' self-management patterns and elevated HbA1c, and this can translate into ineffective communication and adolescents feeling criticized or unsupported.8
Efficacious interventions to improve family relationships, diabetes management, and glycemic outcomes exist,9 predominantly using multicomponent formats with multiple in-person intervention sessions to teach families skills to solve diabetes-related problems. However, their effects have been modest and reach has been limited, due, in part, to the significant time and resource requirements for families and the expensive training of behavioral interventionists.10 Mobile health (mHealth) technology offers a promising avenue to make behavioral interventions for family diabetes management more accessible.11,12 Ecological momentary intervention approaches using mHealth technologies permit the delivery of brief behavior change strategies to people in the context of their daily lives without time-intensive attendance at in-person intervention sessions delivered in a health care setting. For example, prior mHealth interventions in T1D have used text messaging and smartphone applications to provide diabetes education and reinforce engagement in self-management activities to adolescents, with positive reception and promising results.13,14 Using an ecological momentary intervention approach, we designed the Type 1 Doing Well intervention to provide a brief, mHealth-based behavioral intervention to parents of adolescents with T1D via a web-friendly mobile application.15
To address parents' tendency to focus on their concerns with adolescents' diabetes self-management, the Type 1 Doing Well smartphone application used a strengths-based approach with foundations in the Diabetes Resilience Model.16 This model posits that building protective processes can reduce the impact of risk factors and amplify the impact of one's strengths on medical, behavioral, and psychosocial outcomes, leading to positive outcomes even in challenging situations.16 Research in T1D has demonstrated associations among youth strengths, supportive family relationships, and optimal clinical outcomes.17–22 Emerging data from intervention studies targeting resilience in adolescents with T1D have shown promising results.23–26 While previous resilience interventions in this population have largely targeted adolescents, the Type 1 Doing Well intervention focused on parents. We applied principles of positive psychology interventions27 to help parents attend to, recognize, and reinforce adolescents' positive behaviors related to diabetes, called “diabetes strengths.”15 Delivering this strengths-based intervention in a minimally burdensome mobile app format was intended to maximize parental engagement with the intervention and potentially improve adolescent and family outcomes related to diabetes.
Therefore, the primary aim of this pilot study was to evaluate the feasibility and acceptability of Type 1 Doing Well, a new, strengths-based mHealth app for parents of adolescents with T1D. We hypothesized that the intervention would demonstrate feasibility via study consent rates of at least 70% and high app engagement by parents (at least two uses/week) in at least 75% of the intervention group. Engagement with specific app features was explored as additional indices of feasibility. We hypothesized that the intervention would demonstrate acceptability via high satisfaction ratings from parents (questionnaire and interview) and adolescents (interview). A secondary, exploratory aim was to examine any markers of possible impact of the intervention compared with usual care using a randomized-controlled trial design.
Methods
Participants and procedures
Study staff identified potential participants by reviewing diabetes clinic schedules at Texas Children's Hospital and screening the electronic medical chart data of scheduled patients for inclusion and exclusion criteria. Inclusion criteria were adolescent age 12–17 years at enrollment, T1D duration of at least 6 months, receiving care at Texas Children's Hospital, and fluency in English (because validated assessment measures and the app were not available in other languages). Families were excluded from participation if parent or adolescent had a serious medical, cognitive, or mental health comorbidity that would preclude their ability to provide informed consent or participate in data collection or intervention. Families were not eligible to participate in the trial if parent did not have a smartphone with Internet access/data plan; three families were ineligible based on this criterion. People who participated in the earlier intervention design phase of the study15 were not eligible to participate in the pilot trial.
Staff mailed informational letters to potentially eligible participants to introduce the study (including instructions to contact study staff to opt-out of being recruited if desired) and followed up by telephone to assess interest and plan to meet at the adolescent's next diabetes clinic visit. Study staff met with families to confirm eligibility, describe the study protocol, answer questions, and obtain written informed consent (parent) and assent (adolescent).
Following consent, participating parents and adolescents completed separate questionnaire batteries via a secure, Health Insurance Portability and Accountability Act-compliant, web-based survey portal maintained by the Baylor College of Medicine Clinical Trials Management System. Study staff also downloaded data from adolescents' blood glucose meters (Continuous Glucose Monitor data not collected) and conducted chart reviews to extract clinical data, as detailed below. Once questionnaires were completed, families were randomized using a 2:1 ratio to the intervention or control condition, and the intervention group began using the app immediately. Families in both groups completed the questionnaire battery again at their next diabetes clinic appointment, ∼3–4 months later. To assess for any potential intervention process impact on family relationships, participants in both groups also answered a brief survey (detailed in the Measures section) every 2 weeks between baseline and follow-up. Staff administered these questions separately to parent and adolescent via e-mail, text message, or telephone (based on their preference). Study staff monitored diabetes clinic schedules and scheduled study visits to occur at the time of diabetes clinic appointments. We sent all participants reminders for the study visits taking place at clinic appointments via e-mail, text message, or telephone.
Participants in both groups received modest financial incentives following each data collection instance in appreciation of their time and contributions to research. After completing the questionnaire battery at baseline and follow-up, families received $25/person plus $13 for parking/transportation costs. Adolescents received an additional $5/visit for bringing all actively used glucose meters for download, and all participants could earn $5 for each completion of the biweekly relationship questions (maximum $80). At enrollment, all participants also received a small silicone adhesive cell phone wallet branded with the study logo. To offset data use expenses related to app use and intervention-related text messaging, participants in the intervention arm received an additional $10/month during the 3–4 months when they had access to the app.
The Institutional Review Board of Baylor College of Medicine approved this study. Recruitment began on July 31, 2017 and ended November 6, 2018. Study activities paused due to Hurricane Harvey's impact on the Houston area from mid-August through mid-September 2017, and there were delays in scheduling diabetes clinic visits and contacting families for several months related to the storm's aftermath.
Intervention
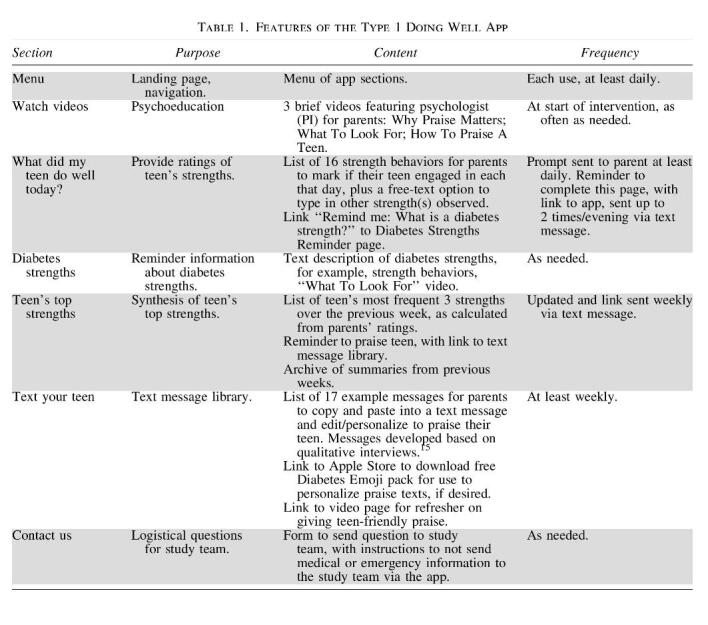
We previously designed the Type 1 Doing Well app intervention with input from adolescents with T1D, their parents, and pediatric diabetes care providers.15 For the current pilot trial, after randomization, study staff assisted families randomized to the intervention arm in installing the web app on the participating parent's mobile phone and oriented them to the features of the app. Table 1 outlines the app features, and details of the app development and functions were reported previously.15 Briefly, parents used the app to record positive T1D-related actions (i.e., diabetes strengths) their teen engaged in each day. The app summarized their ratings each week and presented a personalized weekly strengths summary for each family. Parents had access to videos in the app to learn about recognizing strengths and how to give effective, developmentally appropriate praise. The app also included a library of text messages that parents could use to praise their teen. Intervention group participants had access to the app for 3–4 months, between two quarterly diabetes care appointments. There was no change to intervention group participants' usual medical care for diabetes. The control group received usual medical care for diabetes, but did not have access to the app.
Measures
Participants completed questionnaire batteries at baseline and follow-up. The questionnaires were the same for participants in both intervention arms and at both time points, and included all measures described below, except as otherwise noted.
Participant Characteristics: At baseline, parents reported demographic information, including parent and adolescent race/ethnicity, number of parents/caregivers in the home, adolescent health insurance status (public, private, both, or none), and highest level of parental education. Study staff reviewed electronic medical records to obtain date of birth to calculate adolescent age, date of diagnosis to calculate duration of diabetes, and current diabetes treatment regimen (i.e., pump therapy, basal-bolus/flexible injections, fixed-dose injections).
Psychosocial Outcomes: adolescents rated their diabetes strengths via the diabetes strengths and resilience measure for adolescents.28 They reported how often they typically engaged in 12 adaptive diabetes-related behaviors or attitudes, using a 5-point scale from Never to Almost Always. Internal reliability was good: α = 0.77. Youth rated their diabetes-specific health-related quality of life using the Monitoring Individual Needs in Diabetes Youth Questionnaire (MY-Q).29 They reported the degree to which they experienced 33 positive and negative aspects of living with diabetes. Internal reliability was excellent, α = 0.88. Parents and adolescents reported on diabetes distress using the problem areas in diabetes measures for parents (PAID-PR, 18-item)30 and adolescents (PAID-T, 26-item).31 Using a 6-point scale from Not a Problem to Serious Problem, participants rated the degree to which each aspect of living with diabetes bothered them over the previous month. Internal reliability was excellent: α = 0.93 (parent), 0.96 (adolescent).
Behavioral Outcomes: To assess diabetes treatment adherence, parents completed the 24-item diabetes self-management profile self-report,32 using the version appropriate to their teen's insulin regimen. They selected responses that represented how frequently their teen engaged in diabetes self-management behaviors over the last 3 months. Internal reliability was good: α = 0.82. Adolescents completed the 15-item Self-Care Inventory-Revised,33 a 15-item measure of the frequency with which adolescents engaged in diabetes management behaviors on a 5-point scale from Never to Always. Internal reliability was good, α = 0.80.
Family Outcomes: Parents reported on the impact of diabetes on the family with two measures. They completed the PedsQL Family Impact Module (PedsQL-FI),34 a 38-item measure of the impact of parenting a child with a chronic medical condition on family activities. Each item was rated on a 5-point scale from Never to Always. Internal reliability was excellent: α = 0.96. They also completed the Diabetes Family Impact Scale,35 a 15-item measure of how diabetes specifically impacted family members' activities and relationships. They used a 4-point scale from Almost Never to Almost Always. Internal reliability was good: α = 0.82. To assess family conflict related to diabetes, parents and adolescents completed the 19-item Diabetes Family Conflict Scale-Revised.36 Each item was rated on a three-point scale from Never to Almost Always. Internal reliability was good: α = 0.87 (parent), 0.90 (adolescent). Parents and adolescents also reported on perceptions of parents' miscarried helping via the 15-item Helping for Health Inventory.37 Respondents used a 5-point scale from Rarely to Always to rate how often parents engaged in behaviors intended to be helpful that can have unintended negative effects. Internal consistency was good, α = 0.85 (adolescent), 0.83 (parent).
Intervention Process: To evaluate potential short-term impact of the intervention on parent/adolescent relationship, every 2 weeks from baseline to follow-up, participants in both arms rated their relationship quality using a brief set of questions adapted from the Parent-Youth Relationship Index from the National Longitudinal Study of Youth.38 We administered two items to parents and five items to adolescents. Items assessed how much parents and teens think highly of one another and enjoy spending time with one another, and how often teens felt parents praised them for doing well, helped them, or criticized them.
Clinical Outcome: At baseline and follow-up, glycemic control was evaluated using HbA1c. HbA1c values were obtained as part of routine clinical care via fingerstick capillary blood assay, analyzed using a DCA 2000+ HbA1c Analyzer (Siemens-Bayer, Inc.) point-of-care machine, and extracted from the electronic medical record.
Intervention Satisfaction and Feedback: At follow-up, parents in the intervention arm completed the Usefulness, Satisfaction, and Ease of Use (USE) Questionnaire,39 a measure of their perceptions of the mHealth app. The USE Questionnaire has 30 items across 4 domains (usefulness, ease of use, ease of learning, satisfaction), and respondents rate each item on a 7-point scale from Strongly Disagree to Strongly Agree. Internal reliability was excellent: α = 0.98. In addition, parents and youth participants in the intervention arm also completed semistructured qualitative interviews about their perspectives of the app's functionality and features and the intervention impact on their family. They were also asked for suggestions to refine or improve the intervention.
Data analysis
The primary outcomes for this pilot study were feasibility and acceptability. To assess feasibility, we tabulated recruitment, retention, and app engagement rates. We calculated participant recruitment rates by tracking the number of potentially eligible participants who we prescreened in the medical record, sent informational letters/introduced the study to, and consented for the study. We also tracked the number of people who opted out of learning about the study, who the study team was never able to reach for recruitment, who declined to participate, and their reasons for declining. To calculate retention, we tracked the number of participants who completed baseline questionnaires, were randomized, and completed follow-up questionnaires. We also tracked the number of participants who were lost to follow-up at each phase of the study and the reasons. To calculate app engagement rates among the intervention group, we accessed app use data via a dashboard accessible only to the research team. Table 2 presents the app engagement metrics we calculated. We specifically evaluated intervention feasibility by the upper bound of the two-sided 95% exact, binomial confidence interval (CI) of ≥95% for downloading the app and ≥70% for using the app at least twice per week.

To assess acceptability, we tabulated descriptive statistics (mean ± standard deviation) for participant responses on the USE Questionnaire. We specifically evaluated acceptability by reporting the mean score on the Satisfaction subscale of the USE Questionnaire, as well as the upper bound of the 95% exact, binomial CI of ≥80% for a specific question about intervention satisfaction. We also analyzed the qualitative intervention feedback data via review of the transcribed audio-recorded interview; study staff summarized the data to identify participant preferences about the intervention and suggested changes. We report example excerpts from the interviews to illustrate themes of participant feedback about the intervention.
To describe the sample, we summarized participant demographic and clinical characteristics using descriptive statistics. To conduct exploratory comparisons of any potential impacts of the pilot intervention on parent and adolescent outcomes, we compared mean change (post minus pre) in HbA1c and questionnaire data between groups using an independent, two-sample t test. We assumed equal variances. We used quantile-quantile plots and Shapiro/Wilks tests, stratified by group, to test for approximate normality. We assessed statistical significance at the two-sided α = 0.05 level. Standardized effect sizes (i.e., Cohen's d) were calculated as the mean difference between groups divided by the standard deviation of the estimate. We used a separate analysis for each outcome and did not adjust for multiple hypothesis testing, given these were exploratory analyses for a pilot study designed to primarily assess feasibility and acceptability. All analyses assumed intention to treat. We did not impute missing data, and calculated total scores based on each measure's scoring instructions (including instructions for handling missing item-level responses). We used a general linear mixed model for analysis of the intervention process measures. The model included fixed effects for study time (since first clinic visit), arm, and the time/arm interaction term, as well as random intercept and slope for time. Model contrasts were specified to estimate the mean change per year by treatment arm and to compare the mean change between treatment arms.
Results
Feasibility
Recruitment and retention data demonstrate feasibility of the study design. We mailed informational letters to 180 potential participants based on prescreening data from the electronic medical record. We successfully contacted and confirmed eligibility of 108 families (unable to contact 45 families, 17 were determined to be ineligible, 10 opted out of hearing about the research). Of those, 84 families (78% recruitment rate) provided informed consent. Sixteen families declined due to time demands (n = 8), disinterest in research (n = 6), and feeling the study was not relevant for their child's age (n = 2). Eight families confirmed eligibility and received information about the study but did not reach a decision before recruitment closed. After randomization but before completing baseline questionnaires, two families were withdrawn due to moving/no longer receiving care at the Texas Children's Hospital Diabetes Clinic (n = 1) and time demands (n = 1). We were unable to make follow-up contact with two additional families after consent and they did not complete baseline data, resulting in n = 80 families (74% participation rate) who consented, completed baseline, and were randomized. As per the 2:1 randomization scheme, 55 families were randomized to the intervention arm and 25 families were randomized to usual care (control). Across both study arms, 78 families (98% retention rate of those randomized, n = 54 intervention arm, n = 24 control arm) completed follow-up data collection. The other two families did not schedule a follow-up diabetes care visit within the study period and were not responsive to staff efforts to obtain follow-up data via e-mailed link (1 completed some parent and youth questionnaires but did not finish the battery). We completed postintervention interviews with 47 of the 55 families randomized to the intervention arm (85%). Figure 1 provides CONSORT details, and Table 3 provides participant characteristics.
FIG. 1.
Participant flow diagram.
Table 3.
Participant Characteristics
| Demographic characteristics | M ± SD | n (%) |
|---|---|---|
| Youth sex, female | 47 (59) | |
| Parent role, mother | 64/78 (80) | |
| Youth age at consent | 15.3 ± 1.5 | |
| Youth race/ethnicity | ||
| Non-Hispanic white | 49 (61) | |
| Non-Hispanic black | 10 (13) | |
| Hispanic | 15 (19) | |
| Other or more than one | 6 (7) | |
| Insurance private | 59 (74) | |
| Public or none | 21 (26) | |
| Clinical characteristics | ||
| Insulin regimen | ||
| Pump | 45 (56) | |
| Flexible MDI (basal-bolus) | 32 (40) | |
| Conventional injections | 3 (4) | |
| HbA1c | 9.0% ± 2.1% (n = 79) | |
| Diabetes duration, years | 5.7 ± 3.4 | |
n = 80 except where otherwise specified.
HbA1c, hemoglobin A1c; M, mean; SD, standard deviation.
App use data among those randomized to the intervention arm demonstrate feasibility of the intervention. All intervention arm participants (100%, 95% CI = 94–100) downloaded the app and logged in at least one time, as study staff assisted participants in downloading and logging into the app at randomization. Nearly all participants (n = 53, 96%) logged in at least one additional time. Intervention participants used the app frequently and over a relatively long period. They had access to the app for ∼3–4 months between diabetes clinic visits, with a mean access period of 114.4 ± 31.6 days (range = 61–220 days), or 16.4 ± 4.5 weeks. During this period, participants used the app over a period of 106.1 ± 37.1 days (range = 0–219 days), calculated from first to last time stamped log in, meaning their mean duration of app use covered 92.8% ± 20.9% of the access period (range = 0%–100%). Participants' mean frequency of app use, calculated as the number of days with at least one log in to the app (capped at 1/day), was 79.7% ± 30.6% of the days it was available to them (range = 2.2%–100%). Participants had a mean of 102.3 ± 49.5 log ins to the app over that period (range = 0–228 log ins). Twenty-three participants (42%, 95% CI: 29–56) logged into the app, on average, more than once per day, and 50 (91%, 95% CI: 80–97) logged into the app, on average, two or more days/week.
Nearly all participants (n = 53, 96%) used the strengths tracking section of the app at least once. Participants used all 16 strengths listed in the app. The most frequently selected strength was “Checked BG without being asked” (n = 53 selected at least once) and the least frequently selected strength was “Gave back to someone else with diabetes” (n = 24 selected at least once). In addition, 25 participants (45%) created 90 new entries that they observed in their adolescents (range = 1–14 parent-added entries/participant). Of those, 80 (89%) were positively worded strengths, for example, “Made sure she had all her supplies with her and refilled them on her own,” “Realizing she was low and addressing it properly multiple times today,” “Educated me on how her body feels,” “Joked in a positive way about diabetes:-),” and “Had good communication at the Endo.” Neutral entries (5, 6%) mostly reflected the teen being sick or the parent having little interaction with the teen that day, such as “She's been with her dad most of the day, only brief chats or visits.” Negative entries (6, 7%) reflected the teen or family having a challenging day, such as “Rough day. High BG and affected her attitude about diabetes.”
Most participants (90%) viewed the strength summaries at least once after orientation, and they viewed the weekly strength summaries a mean of 5.6 ± 4.7 times (range = 0–19). The mean access period was 16.4 ± 4.5 weeks, suggesting that on average participants viewed the weekly strength summary on 34% of the weeks they had access to it. Participants “favorited” 15 praise texts for future use. The most commonly favored text was, “I'm so proud of how responsible you've been with your diabetes care this week!” (favored by 12 participants).
Acceptability
Intervention participant responses (n = 50) on the USE Questionnaire indicated high acceptability of the intervention. Mean ratings (on a 7-point scale) on each dimension were as follows: satisfaction 5.0 ± 1.5, usefulness 4.8 ± 1.5, ease of use 6.2 ± 0.8, and ease of learning 6.5 ± 0.8. For the individual item “I am satisfied with it,” in the Satisfaction scale, 87% (95% CI: 76–95) of the sample selected a score of 4 or above (out of 7), indicating they found the app at least somewhat acceptable.
Feedback from parents (n = 48) in the exit interviews was overwhelmingly positive. Regarding overall satisfaction with the app, parent comments included:
“[The app] forced me to think about a positive situation everyday regardless if it was a good day. She appreciated my recognition of her good behavior.” (Mother of 12-year-old female)
“We've been able to work the difficult days together in a much calmer atmosphere since using the app.” (Mother of 17-year-old female)
“We kind of get used to what he does all the time and take it for granted, [so the app helped] remind me as a parent to continue to praise him for the good work he does.” (Mother of 13-year-old male)
“[The app] helped us communicate a little, but without all that mommy nag stuff. It gave us a nice way of being able to communicate about it on a daily basis.” (Mother of 14-year old male)
Comments from adolescents (n = 47) whose parents used the app included:
“[The app] reminds parents to encourage their kids whenever they are doing because there's really bad days, but there's also really good days.” (17-year-old female)
“[The app] gets parents to recognize what teens are doing well and that they're trying. I think it's a good way [for parents and teens] to get connected and I like it.” (14-year-old male)
“I wear my medical identification bracelet more often now,” attributed this to being in the study and her mother noticing (17-year-old female).
Parents noted that the app was straightforward and easy to use. However, 13 parents noted that the mobile-friendly web app format presented some barriers to use, such as needing to re-enter the password to log in; one parent who got a new phone did not know how to add the app icon to the home screen. Seven parents suggested using the app less frequently, perhaps 2–5 times a week, to minimize burden. Two of those parents specified they preferred to use it less frequently since their teens were demonstrating the same strengths each day. Some felt that receiving text message reminders at the programmed evening time was appropriate, while others felt evenings were too busy. Four parents offered suggestions about the timing of prompts to enter strengths, including having an option to receive reminders in the morning to report about the previous day or to set their preferred time of day for reminders. Three parents did not realize or forgot the app had an option to write in other strengths.
Parents made suggestions for other features of future versions of the app, as well. Two parents suggested adding features for praising teens, such as having the app generate a praise text message based on each day's report of the teen's strengths rather than a weekly summary, and three parents suggested adding a “share” function for the parent to send the teen's strength summaries to the teen or other family members. To personalize the text messages, one parent suggested the app include emoji in the text library instead of linking to an app store to download a separate diabetes emoji pack. Parents also recommended adding new features to increase the scope of the intervention. For example, two participants requested expanding the selection of informational videos for parents to include videos about other aspects of parenting teens with diabetes, and one participant suggested adding videos for teens to view. Two parents and one teen recommended including a social media component to allow app users to connect with other families living with diabetes, especially other families using the app. Six parents commented that while they enjoyed using the app, they thought it might be more useful for younger youth or closer to diagnosis, and seven parents suggested offering an age-specific list of strengths for older or young teens. Finally, several teens indicated that they would like to have had an opportunity to use the app, as well, perhaps to log their own strengths.
Regarding study activities outside the intervention, parents and youth reported that the questionnaires were not too long or burdensome and were of reasonable length for a research study. Some parents described feeling reflective or emotional about their experiences with diabetes while completing the questionnaires.
Exploratory outcomes
Exploratory comparisons of mean change in outcomes between groups from baseline to follow-up indicated that there were no statistically significant differences by group for changes in any outcome (Table 4). On average, HbA1c declined in the intervention group by 0.11 and increased in the control group by 0.06, however, this difference was not statistically significant. For the intervention process measures, adolescent ratings of how much their parents praised them improved by 0.0045 points per year in the control group (P = 0.02) and 0.0012 points per year in the intervention group (P = 0.34). However, there was no significant difference between treatment arms (P = 0.15). No other intervention processes significantly changed over time for either arm.
Table 4.
Participant Scores at Baseline and Follow-Up
| Measure | Group | Baseline |
Follow-up |
Mean change | Standard effect size |
t-Test |
||
|---|---|---|---|---|---|---|---|---|
| n | M ± SD | n | M ± SD | d | P | |||
| HbA1c | Intervention | 55 | 9.1 ± 2.1 | 51 | 8.7 ± 1.7 | −0.11 | −0.15 | 0.57 |
| Control | 24 | 8.7 ± 2.1 | 22 | 8.4 ± 1.4 | 0.06 | |||
| DSTAR (Teen) | Intervention | 53 | 37.6 ± 6.3 | 52 | 37.7 ± 5.7 | 0.22 | −0.02 | 0.94 |
| Control | 25 | 34.8 ± 5.3 | 25 | 35.1 ± 5.6 | 0.32 | |||
| PAID (Teen) | Intervention | 55 | 63.5 ± 25.8 | 53 | 65.0 ± 26.1 | 1.2 | 0.01 | 0.96 |
| Control | 25 | 63.5 ± 29.8 | 24 | 65.2 ± 25.7 | 1.0 | |||
| PAID (Parent) | Intervention | 55 | 73.9 ± 21.9 | 55 | 77.6 ± 28.1 | 3.7 | −0.002 | 0.99 |
| Control | 25 | 68.4 ± 18.9 | 24 | 71.0 ± 28.4 | 3.7 | |||
| DFCS-R (Teen) | Intervention | 55 | 24.5 ± 4.7 | 54 | 23.9 ± 4.5 | −0.68 | 0.16 | 0.52 |
| Control | 25 | 26.5 ± 8.4 | 24 | 25.0 ± 6.2 | −1.54 | |||
| DFCS-R (Parent) | Intervention | 55 | 26.7 ± 6.1 | 54 | 25.4 ± 5.2 | −1.33 | −0.23 | 0.34 |
| Control | 25 | 25.2 ± 3.5 | 24 | 24.5 ± 3.9 | −0.41 | |||
| MY-Q (Teen) | Intervention | 54 | 72.3 ± 12.9 | 53 | 72.2 ± 11.8 | −0.52 | 0.20 | 0.42 |
| Control | 25 | 70.7 ± 15.1 | 24 | 68.4 ± 13.8 | −2.16 | |||
| PedsQL (Teen) | Intervention | 55 | 83.7 ± 13.6 | 54 | 82.6 ± 14.1 | −0.82 | −0.18 | 0.48 |
| Control | 25 | 83.9 ± 13.2 | 24 | 84.5 ± 15.4 | 0.93 | |||
| HHI (Teen) | Intervention | 55 | 40.1 ± 11.6 | 51 | 37.9 ± 10.6 | −2.02 | −0.20 | 0.45 |
| Control | 23 | 41.4 ± 12.0 | 23 | 41.4 ± 13.1 | −0.36 | |||
| HHI (Parent) | Intervention | 51 | 39.6 ± 11.0 | 52 | 40.0 ± 12.5 | 1.27 | 0.03 | 0.91 |
| Control | 24 | 39.0 ± 9.2 | 24 | 40.0 ± 9.9 | 1.04 | |||
| DFIS (Parent) | Intervention | 54 | 20.0 ± 12.9 | 54 | 20.7 ± 15.5 | 0.33 | 0.18 | 0.47 |
| Control | 25 | 20.3 ± 17.7 | 24 | 19.1 ± 16.1 | −1.84 | |||
| SCIR (Teen) | Intervention | 55 | 69.1 ± 13.2 | 54 | 66.6 ± 14.0 | −2.62 | −0.42 | 0.09 |
| Control | 25 | 66.3 ± 15.1 | 25 | 68.4 ± 12.9 | 2.08 | |||
| DSMP (Parent) | Intervention | 55 | 52.1 ± 12.8 | 54 | 53.4 ± 10.8 | 1.50 | 0.20 | 0.42 |
| Control | 25 | 52.3 ± 10.5 | 24 | 53.0 ± 9.6 | −0.16 | |||
Standard effect size d indicates change in intervention group compared with change in control group. Negative d indicates intervention < control, positive d indicates intervention > control.
DSTAR, Diabetes Strengths and Resilience; DFCS-R, Diabetes Family Conflict Scale-Revised; DFIS, Diabetes Family Impact Scale; DSMP, diabetes self-management profile; HHI, Helping for Health Inventory; MY-Q, Monitoring Individual Needs in Diabetes Youth Questionnaire; PAID, problem areas in diabetes; PedsQL, Pediatric Quality of Life Scale; SCI-R, Self-Care Inventory-Revised.
Discussion
The Type 1 Doing Well behavioral mHealth intervention for parents of adolescents with T1D is feasible and acceptable and may be an engaging component of future family-based interventions. This study demonstrated strong feasibility of this novel, web-friendly mobile app-based intervention approach, with consent rates greater than 70% and retention near 100%. Engagement in the intervention was also high, with over 90% of participants in the intervention arm using the app at least twice per week, on average. The study demonstrated that the intervention was acceptable, as demonstrated by positive qualitative feedback from participants and high satisfaction ratings about the app on the USE Questionnaire. Qualitative feedback from youth and parents suggested that app use led to increases in supportive communication around diabetes management. This level of participation was despite the occurrence of Hurricane Harvey near the start of the recruitment period, which had a significant effect on residents of the Houston, TX metropolitan area acutely and for many months following the storm. These positive feasibility and acceptability data provide strong preliminary support for this pilot intervention, representing a successful outcome for this critical first step of rigorous intervention development and testing.40
This pilot study advances the field of behavioral intervention research for parents of adolescents with T1D by demonstrating the potential for a brief, minimally burdensome, strengths-based intervention to engage parents and potentially improve parent/teen diabetes-related communication. While previous family-centered intervention research in diabetes has focused on teaching the families communication and problem-solving skills, the multisession, in-person format with trained behavioral interventionists has limited the potential for implementation in clinical practice. The mHealth format of the Type 1 Doing Well app, in contrast, was accessible for participants and appeared to be enjoyable enough for sustained frequent use over several months.
While the Type 1 Doing Well app intervention was feasible to deliver and highly enjoyed by participants, it did not result in any significant changes in the exploratory glycemic, clinical, or behavioral outcomes. This is consistent with a meta-analysis of previous behavioral intervention research in diabetes, which showed small effect sizes on glycemic outcomes.10 As a pilot study, the main purpose of this study was to evaluate the feasibility and acceptability of the newly developed intervention, and aims related to evaluating outcomes were exploratory in nature. Thus, the sample was not powered to detect small to moderate effects.
A limitation of this study is the reliance on a convenience sample of adolescents treated within one tertiary children's hospital setting. Recruitment took place at three hospital locations across a large metropolitan area, which resulted in a relatively large sample for a pilot study, with high racial and ethnic diversity. Nevertheless, additional research about this intervention's feasibility, acceptability, and impact across different institutions and areas of the country would be informative for potential generalizability. The availability of the app and outcome measures only in English precluded our ability to enroll non-English speaking participants, which excluded a subset of families of youth with T1D, and therefore, results cannot generalize across the full population. In addition, as having a smartphone was an eligibility requirement for parents, this intervention is not useful for families who cannot afford or choose not to have a smartphone. Only 3 families were excluded from participating in this study based on this limitation, which is consistent with national data from the Pew Research Center indicating mobile phone access is above 90% in the general population in the United States.41 However, there are some differences in smartphone ownership specifically between the lowest and highest income brackets (71% among those making <$30,000/year vs. 95% for those making ≥$75,000/year).41 A final limitation of this study was the historical effect of Hurricane Harvey, a natural disaster that impacted the region during the study period. Despite the wide-ranging effects on residence, physical and mental health, and availability of public services,42–44 recruitment and retention rates in this study were high. However, it is possible that recruitment, retention, engagement, app usage rates, and the exploratory outcomes may have been impacted by the storm and its aftermath.
Parents and adolescents enjoyed using the Type 1 Doing Well app and described benefits in their family interactions surrounding diabetes management. However, survey data did not show statistically significant changes in any medical or behavioral outcomes. For this age group and at this dose, the intervention appears to not be sufficient on its own to change youth or parent outcomes—it may be more effective with further improvements to the app or if integrated into other intervention protocols. Given the high feasibility and acceptability of this approach, future research is warranted to determine if the app is more impactful if used for a longer period of time, with another age group, and/or delivered in combination with other intervention approaches in a multicomponent intervention protocol.40 Parents and adolescents offered suggestions to improve the app and increase the intervention's impact, many of which would increase the personalization of the app's features or make the features more apparent and easier to access.
Clinically, the long-term vision is to deliver this intervention as part of routine clinical care. After refining the intervention based on user feedback from this study and establishing evidence of efficacy in a fully powered randomized-controlled trial,40 implementation science methods would be useful to determine the most effective methods for dissemination. For example, pragmatic clinical trial research could inform how best to onboard families to use the app as part of regular diabetes care.45 It is possible that families with specific characteristics may be more interested in using an app such as this and may have better outcomes, which could also be evaluated in the dissemination and implementation research. This intervention does not focus on a particular diabetes technology but rather prioritizes teaching parents the skills to recognize and reinforce their teens' positive diabetes-related behaviors. Therefore, it has strong potential to be durable and flexible when implemented in clinical practice, as teens' diabetes behaviors can evolve and adapt as diabetes technologies progress.
In sum, the results of the pilot Type 1 Doing Well study demonstrate the potential of a strengths-based mHealth intervention for parents of adolescents with T1D. This novel app, developed in partnership with end-users and medical stakeholders, was highly feasible and acceptable, and demonstrated higher levels of engagement than are often seen with apps. Given that the evaluation of glycemic and questionnaire outcomes was exploratory at this phase of the research, it is not surprising that there were no differences in outcomes between groups. Rather, these data demonstrate that a low-cost, low-burden, and straightforward intervention was perceived as beneficial by many families and may be an important building block for future efforts to support this vulnerable population. The successful demonstration of high feasibility and acceptability in this study represents a necessary first step to rigorously testing intervention efficacy.40
Acknowledgments
We acknowledge the contributions of the staff of the Center for Behavioral Intervention Technologies at Northwestern University School of Medicine for creating and maintaining the app. We also acknowledge the contributions of the staff of the Clinical Trials Management System in the Office of Research IT at Baylor College of Medicine for creating and maintaining the data collection system.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
The National Institutes of Diabetes and Digestive and Kidney Diseases funded this study: R21 DK107951 (PI: Hilliard). Drs. Hilliard, Thompson, Karaviti, and Anderson received complementary support from 1K12 DK097696 (PI: Anderson).
References
- 1. Wiebe DJ, Chow CM, Palmer DL, et al. : Developmental processes associated with longitudinal declines in parental responsibility and adherence to type 1 diabetes management across adolescence. J Pediatr Psychol 2014;39:532–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Anderson BJ, Holmbeck G, Iannotti RJ, et al. : Dyadic measures of the parent-child relationship during the transition to adolescence and glycemic control in children with type 1 diabetes. Fam Syst Health 2009;27:141–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rausch JR, Hood KK, Delamater A, et al. : Changes in treatment adherence and glycemic control during the transition to adolescence in type 1 diabetes. Diabetes Care 2012;35:1219–1224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Foster NC, Beck RW, Miller KM, et al. : State of type 1 diabetes management and outcomes from the T1D Exchange in 2016–2018. Diabetes Technol Ther 2019;21:66–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Wysocki T, Nansel TR, Holmbeck GN, et al. : Collaborative involvement of primary and secondary caregivers: associations with youths' diabetes outcomes. J Pediatr Psychol 2009;34:869–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jaser SS: Family interaction in pediatric diabetes. Curr Diab Rep 2011;11:480–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hilliard ME, Holmes CS, Chen R, et al. : Disentangling the roles of parental monitoring and family conflict in adolescents' management of type 1 diabetes. Health Psychol 2013;32:388–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Spencer J, Cooper H, Milton B: Qualitative studies of type 1 diabetes in adolescence: a systematic literature review. Pediatr Diabetes 2010;11:364–375 [DOI] [PubMed] [Google Scholar]
- 9. Hilliard ME, Powell PW, Anderson BJ: Evidence-based behavioral interventions to promote diabetes management in children, adolescents, and families. Am Psychol 2016;71:590–601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Hood KK, Rohan JM, Peterson CM, et al. : Interventions with adherence-promoting components in pediatric type 1diabetes: meta-analysis of their impact on glycemic control. Diabetes Care 2010;33:1658–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Schueller SM, Munoz RF, Mohr DC: Realizing the potential of behavioral intervention technologies. Curr Dir Psychol Sci 2013;22:478–483 [Google Scholar]
- 12. Mulvaney SA, Ritterband LM, Bosslet L: Mobile intervention design in diabetes: review and recommendations. Curr Diab Rep 2011;11:486–493 [DOI] [PubMed] [Google Scholar]
- 13. Markowitz JT, Harrington KR, Laffel LMB: Technology to optimize pediatric diabetes management and outcomes. Curr Diab Rep 2013;13:877–885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Herbert L, Owen V, Pascarella L, et al. : Text message interventions for children and adolescents with type 1 diabetes: a systematic review. Diabetes Technol Ther 2013;15:362–370 [DOI] [PubMed] [Google Scholar]
- 15. Hilliard ME, Eshtehardi SS, Minard CG, et al. : Strengths-based behavioral intervention for parents of adolescents with type 1 diabetes using an mHealth app (Type 1 Doing Well): protocol for a pilot randomized controlled trial. JMIR Res Protoc 2018;7:e77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hilliard ME, Harris MA, Weissberg-Benchell J: Diabetes resilience: a model of risk and protection in type 1 diabetes. Curr Diab Rep 2012;12:739–748 [DOI] [PubMed] [Google Scholar]
- 17. Hilliard ME, Hagger V, Hendrieckx C, et al. : Strengths, risk factors, and resilient outcomes in adolescents with type 1 diabetes: results from Diabetes MILES Youth–Australia. Diabetes Care 2017;40:849–855 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rohan JM, Huang B, Pendley JS, et al. : Predicting health resilience in pediatric type 1 diabetes: a test of the resilience model framework. J Pediatr Psychol 2015;40:956–967 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Yi-Frazier JP, Hilliard M, Cochrane K, et al. : The impact of positive psychology on diabetes outcomes: a review. Psychology 2012;3:1116–1124 [Google Scholar]
- 20. Van Allen J, Steele RG, Nelson MB, et al. : A longitudinal examination of hope and optimism and their role in type 1 diabetes in youths. J Pediatr Psychol 2015;41:741–749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Huston SA, Blount RL, Heidesch T, et al. : Resilience, emotion processing and emotion expression among youth with type 1 diabetes. Pediatr Diabetes 2016;17:623–631 [DOI] [PubMed] [Google Scholar]
- 22. Lukács A, Mayer K, Sasvári P, et al. : Health-related quality of life of adolescents with type 1 diabetes in the context of resilience. Pediatr Diabetes 2018;19:1481–1486 [DOI] [PubMed] [Google Scholar]
- 23. Rosenberg AR, Yi-Frazier JP, Eaton L, et al. : Promoting resilience in stress management: a pilot study of a novel resilience-promoting intervention for adolescents and young adults with serious illness. J Pediatr Psychol 2015;40:992–999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kichler JC, Kaugars AS: Applying positive development principles to group interventions for the promotion of family resilience in pediatric psychology. J Pediatr Psychol 2015;40:978–980 [DOI] [PubMed] [Google Scholar]
- 25. Lord JH, Rumburg TM, Jaser SS: Staying positive: positive affect as a predictor of resilience in adolescents with type 1 diabetes. J Pediatr Psychol 2015;40:968–977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hood KK, Iturralde E, Rausch J, et al. : Preventing diabetes distress in adolescents with type 1 diabetes: results 1 year after participation in the STePS program. Diabetes Care 2018;41:1623–1630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Seligman ME, Steen TA, Park N, et al. : Positive psychology progress: empirical validation of interventions. Am Psychol 2005;60:410–421 [DOI] [PubMed] [Google Scholar]
- 28. Hilliard ME, Iturralde E, Weissberg-Benchell J, et al. : The diabetes strengths and resilience measure for adolescents with type 1 diabetes (DSTAR-Teen): validation of a new self-report measure. J Pediatr Psychol 2017;42:995–1005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. de Wit M, Winterdijk P, Aanstoot H, et al. : Assessing diabetes-related quality of life of youth with type 1 diabetes in routine clinical care: the MIND Youth Questionnaire (MY-Q). Pediatr Diabetes 2012;13:638–646 [DOI] [PubMed] [Google Scholar]
- 30. Markowitz JT, Volkening LK, Butler DA, et al. : Re-examining a measure of diabetes-related burden in parents of young people with type 1 diabetes: the Problem Areas in Diabetes Survey—Parent Revised version (PAID-PR). Diabet Med 2012;29:526–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weissberg-Benchell J, Antisdel-Lomaglio J: Diabetes-specific emotional distress among adolescents: feasibility, reliability, and validity of the problem areas in diabetes-teen version. Pediatr Diabetes 2011;12:341–344 [DOI] [PubMed] [Google Scholar]
- 32. Wysocki T, Buckloh LM, Antal H, et al. : Validation of a self-report version of the diabetes self-management profile. Pediatr Diabetes 2012;13:438–443 [DOI] [PubMed] [Google Scholar]
- 33. Lewin AB, LaGreca AM, Geffken GR, et al. : Validity and reliability of an adolescent and parent rating scale of type 1 diabetes adherence behaviors: the Self-Care Inventory (SCI). J Pediatr Psychol 2009;34:999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Varni JW, Sherman SA, Burwinkle TM, et al. : The PedsQL family impact module: preliminary reliability and validity. Health Qual Life Outcomes 2004;2:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Katz ML, Volkening LK, Dougher CE, et al. : Validation of the Diabetes Family Impact Scale: a new measure of diabetes-specific family impact. Diabet Med 2015;32:1227–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hood KK, Butler DA, Anderson BJ, et al. : Updated and revised Diabetes Family Conflict Scale. Diabetes Care 2007;30:1764–1769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Harris MA, Antal H, Oelbaum R, et al. : Good intentions gone awry: assessing parental “miscarried helping” in diabetes. Fam Syst Health 2008;26:393–403 [Google Scholar]
- 38. Jones-Sanpei HA, Day RD, Holmes EK: Core family process measures in the NLSY97: variation by gender, race, income, and family structure. Marriage Fam Rev 2009;45:140–167 [Google Scholar]
- 39. Lund AM: Measuring usability with the USE questionnaire. Usability Interface 2001;8:3–6 [Google Scholar]
- 40. Czajkowski SM, Powell LH, Adler N, et al. : From ideas to efficacy: the ORBIT Model for developing behavioral treatments for chronic diseases. Health Psychol 2015;34:971–982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pew Research Center: Internet & Technology: Mobile Fact Sheet. 2019 [Updated June 12, 2019]. https://www.pewresearch.org/internet/fact-sheet/mobile/ (accessed December5, 2019)
- 42. Schultz JM, Galea S: Mitigating the mental and physical health consequences of Hurricane Harvey. JAMA 2017;318:1437–1438 [DOI] [PubMed] [Google Scholar]
- 43. Hicks M, Burton M: Hurricane Harvey: preliminary estimates of commercial and public-sector damages on the Houston Metropolitan Area. Ball State University. 2017 [Updated September 8, 2017]. https://projects.cberdata.org/reports/HurricaneHarvey2017.pdf (accessed December5, 2019)
- 44. Schwartz RM, Tuminello S, Kerath SM, et al. : Preliminary assessment of Hurricane Harvey exposures and mental health impact. Int J Environ Res Public Health 2018;15:e974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Price J, Beidas R, Wolk CB, et al. : Implementation science in pediatric psychology: the example of type 1 diabetes. J Pediatr Psychol 2019;44:1068–1073 [DOI] [PMC free article] [PubMed] [Google Scholar]



