Abstract
Significance: Selenenyl sulfides (RSeSRs) and thioseleninic acids (RSeSHs) are the monoselenium (Se) analogs of disulfides and persulfides that contain Se-S bonds. These bonds are found in several antioxidant-regenerating enzymes as derivatives of selenocysteine, making them an important player in redox biology as it pertains to sulfur redox regulation.
Recent Advances: Mechanistic studies of redox-regulating selenoenzymes such as thioredoxin reductase and glutathione peroxidase suggest crucial Se-S bonds in the active sites. Peptide models and small-molecule mimics of these active sites have been prepared to study their fundamental chemistry. These advances help pave the road to better understand the functions of the Se-S bond in the body.
Critical Issues: The Se-S bond is unstable at atmospheric temperatures and pressures. Therefore, studying their properties proposes a major challenge. Currently, there are no trapping reagents specific to RSeSRs or RSeSHs, making their presence, identity, and fates in biological environments difficult to track.
Future Directions: Further understanding of the fundamental chemistry/biochemistry of RSeSRs and RSeSHs is needed to understand what their intracellular targets are and to what extent they impact signaling. Besides antioxidant regeneration and peroxide radical reduction, the roles of RSeSR and RSeSHs in other systems need to be further explored.
Keywords: selenenyl sulfide, thioseleninic acid, reactive sulfur species, selenocysteine, chemical reaction
Introduction
Many reactive sulfur species (RSS) have been found to effect redox regulation in the human body. These include persulfides (RSSHs), polysulfides (RSSnH and H2Sn, n ≥ 2), hydrogen sulfide (H2S), glutathione (GSH), etc. All these RSS are derived from sulfur (S)-containing amino acids especially cysteine (Cys) by certain enzymes such as cystathionine β-synthetase, cystathionine γ-lyase, 3-mercaptopyruvate sulfurtransferase (3-MST), and cysteinyl-tRNA synthetase (CARS).
The RSS that are particularly of interest are disulfides (RSSRs) for their roles in redox regulation of protein activity and RSSHs for their roles in redox signaling (14, 55). On the other hand, selenocysteine (Sec), a Se analog to Cys, was recognized as the 21st proteinogenic amino acid (74). This discovery characterized selenium (Se) as an important micronutrient in animals, humans, and even plants (64). Since then, many selenoenzymes such as thioredoxin reductase (ThxR) and glutathione peroxidase (GPx) have been identified and found to play important redox regulatory roles in the body (75). Mechanistic investigations into the active sites of these proteins show an important Se-S bond either between Sec and Cys residues (ThxR) or between Sec and GSH (GPx) (8, 45). Interestingly, small molecules containing the Se-S bond also have redox properties in vivo, solidifying its importance (60). The existence of this bond expands the RSS family and increases the complexity of redox regulation, warranting further research.
Recently, the mono-Se analogs of RSSRs, selenenyl sulfides (RSeSRs), show promise as GPx mimics (13, 17, 18, 33, 58, 59, 73), precursors for selenoinsulins (10), anticancer agents (28), and Sec donors for human serum albumin (32). However, mono-Se analogs of RSSHs, for example, thioseleninic acids (RSeSHs), are largely unexplored. RSeSHs are hypothesized to react under similar environments to RSSHs because of their structural similarity. Their chemical reactivity, however, may be different because of the inherent differences between Se and S. It is expected that Sec can readily react with certain RSS to form RSeSH, analogous to RSSH formation, although this has not been validated. A number of other questions about RSeSH remain unanswered, such as what their intracellular targets are and to what extent such reactions can impact signaling.
This review summarizes several key aspects about RSeSRs and RSeSHs, which include (1) pathways for their synthesis in organic chemistry; (2) chemical utility of the Se-S bond; and (3) exploration of selenoproteins and their mimics.
Preparation of the Se-S Bond
The Se-S linkage has gained much attention throughout the years because it plays an important role in antioxidant-regenerating enzymes such as ThxR. However, studying the characteristics of these bonds and how they are made in the body poses its own sets of challenges. Thus, methods to synthesize Se-S bonds in the laboratory are important to study their fundamental chemistry and to understand the conditions in which these bonds can be made.
Theoretically, they can be made similarly to RSSRs through regular substitution chemistry between a thiol and a diselenide (RSeSeR). However, kinetic studies show that this exchange reaction in the forward direction is two orders of magnitude slower than in the backward direction, shifting the equilibrium toward the reactants (Scheme 1) (76). Further studies show that rate constants for selenates as nucleophiles are two to three orders of magnitude higher than those of thiolates. In biological systems, similar reactivities can be observed because of different pKa values between Cys and Sec (8.5 and 5.2, respectively). These pKa differences cause Cys to be protonated and Sec to be deprotonated under physiological pH, making Sec a better nucleophile than Cys. Interestingly, rate constants of Se (in both RSeSeR and RSeSR) as an electrophile are four orders of magnitude higher than those of their S analogs.
The ability of Se to be both a better nucleophile and electrophile than S indicates that Se-S bond formation through an exchange reaction is not favored. However, the sulfide and selenide are comparable as leaving groups (76), therefore exchange reactions between them should still be acknowledged when studying their chemistry. Due to this unfavorable reaction, there must be a chemical treatment of either Se or S to form the Se-S bond in good yield.
Thiol activation for RSeSR formation
One chemical treatment is to utilize Lewis and Bronsted acids to push the equilibrium of the exchange reaction toward the RSeSR. The exchange reaction between a thiol and RSeSeR is not favored because the selenate by-product is more nucleophilic than the thiol. However, when selenol (RSeH) is stabilized by a Lewis acid, its nucleophilicity decreases, causing the exchange reaction to be more favored. For example, Saravanan et al. reacted diphenyl diselenide (1) with silver(I) trifluoromethanethiolate (AgSCF3) (2) to form PhSeSCF3 (3) in >80% yield (Scheme 2a) (66). This exchange was found to be in equilibrium favoring the RSeSR product even with an electron-deficient thiolate because Ag+ stabilizes the selenate by-product, weakening its nucleophilicity.
Bronsted acids cannot be used to stabilize the RSeH because its pKa is lower than that of thiols (5 vs. 8, respectively). However, a Bronsted acid can be used if a sulfenamine (PhNHSCF3, 4) is reacted with an RSeH (PhSeH, 5). For example, Jereb et al. reacted phenyl RSeH with 4 in the presence of triflic acid (TfOH) to form RSeSR 3 in >50% yield (Scheme 2b) (37). TfOH protonates the PhNH- to form an anillin intermediate (5), making SCF3 a strong enough electrophile to be attacked by RSeH 6, releasing 7 and forming RSeSR 3. A similar rendition can be used for this type of activation with iodine instead of TfOH and a selenonitrile instead of an RSeH (27). To our knowledge, Lewis/Bronsted acid effects for making Se-S bonds are limited to these two methods and therefore not extensively studied.
Redox reactions for RSeSR formation
Redox manipulations of S and Se, on the other hand, are well studied because Se and S generally share similar physical and chemical properties in terms of oxidation states and functional group types (80). However, the chemical differences between S and Se result in going from a smaller to a larger element. For example, the bond strengths between Se-X bonds are weaker than the corresponding S-X bonds because of a larger atomic radius resulting in easier bond-breaking reactions. Theoretically, the σ* orbital of an Se-X bond is lower in energy than that of an S-X bond, meaning that Se is more of an electron acceptor than S. This also indicates that Se is a better electrophile than S in all oxidation states, therefore forming the Se-S bond should be simple between an oxidized Se species and a thiol.
The most common method to make Se-S bonds is reacting nucleophilic thiols with in situ generated selenenyl halide under oxidative halogenation conditions (51). An example is shown in Scheme 3 using sulfuryl chloride (SO2Cl2) as the oxidative chlorinating agent. SO2Cl2 and RSeSeR 8 react to form the selenenyl chloride intermediate (9) and subsequent addition of a thiol yields RSeSR (10). This method has been used to make fluorescent sensors and small-molecule delivery templates that contain the Se-S bond (20, 30). Other oxidation conditions using Br2 or oxygen to make the Se-S bond can also be used (43, 83).
It comes as no surprise that selenenyl halides react readily with nucleophilic thiols, but these Se species are not likely to be found in biological systems. A more biologically relevant oxidized Se species are the seleninic acids, such as compound 11 (Scheme 4), which may be important in Se metabolism. Kice et al. studied the reactivity of seleninic acids with thiols and found that RSeSR was the major product (43). Mechanistic studies identified RSeSH (12) and selenenic acid (RSeOH) (13) as the intermediates before RSeSR (14) was produced. Steps 1 and 2 of this reaction were found to be first order in thiol, with the latter being the rate-determining step. These observations are consistent for pHs ranging from 0.2 to 10, with the overall reaction proceeding faster at higher more basic pHs because of the greater degree of deprotonation of thiol to thiolate. Lower pHs yield RSeSR as well, suggesting that Se-S formation is favored across all pH values when only the seleninic acid and thiols are present. In biological systems, this reactivity has been applied to develop seleninic acid-based inhibitors for protein tyrosine phosphatase, which forms an RSeSR bond with the Cys residue in the enzyme active site (1). In organic chemistry, this reaction provides a method to make biohybrid -Se-S- compounds in high yields under very mild conditions (2). The reaction proceeds at room temperature in water and many organic solvents. Computational studies show that this reaction is energetically favored (15).
In addition to seleninic acids, selenosulfates (RSeSO3−s) are also found to be biologically relevant Se-S bond-forming reagents because they have been shown to be potent inhibitors for thiol-dependent enzymes (69). Patarasakulchai et al. studied the reactivity of RSeSO3− compounds with Cys and found that in aqueous solutions at pH 4.6, RSeSO3−s react immediately to form insoluble RSeSR and RSeSeR (56). At pH 7, the only product observed was the insoluble RSeSeR. This insolubility made detecting reactivity differences between RSeSO3−s and thiols in water challenging. Thus, another solvent (dimethylformamide [DMF]) was used so that all reactants and products were soluble. Interestingly, the formation of RSeSR and RSeH (RSeH) was observed and confirmed by methylation to yield RSeMe. The RSeSR was found to be the major product at 5°C, but RSeSeR was found to be the major product at higher temperatures, indicating that RSeSR reacts further through a disproportionation mechanism. Based on this information, a reaction scheme between RSeSO3− and thiols was validated (Scheme 5).
Based on this work, RSeSRs can theoretically be made by RSeSO3− in aqueous solutions, but care should be taken because the desired product will crash out of the solution as an insoluble mixture with RSeSeR. In organic solvents, the desired product can also be made at lower temperatures, but it reacts further to form RSeSeR at room temperature. Overall, this is not an ideal method to synthesize RSeSRs, but provides information as to how RSeSO3−s can interact with an active site Cys in various enzymes to form new Se-S bonds. This reactivity was also observed with selenenylthiosulfates (RSeS2O3−).
Electrochemical formation of RSeSR
Although fully oxidized Se species can form RSeSRs, electrochemically reduced Se species when reacted with elemental S can as well. Ahrika et al. (5) studied the spectrophotometric and electrochemical characteristics of RSeSeRs and found that the addition of elemental S to electrochemically reduced RSeSeRs forms RSeSR ions. For example, an aromatic RSeSeR, (2-NO2C6H4Se)2 (15), was electrochemically reduced to 2 equivalents of 2-NO2C6H4Se– (16) and subsequently reacted with elemental S to spontaneously produce 2-NO2C6H4SeS– (17) (Scheme 6). A 1:1 ratio of elemental S to RSeSeR produced the best yield of 85%. Interestingly, subsequent addition of S resulted in little oxidation of 16 and 17 to 15 and S82−. During this reaction, the maximum ultraviolet–visible spectroscopy (UV-vis) absorbance values of 16 (520 and 315 nm) decreased and two new maxima for 17 appeared (728 and 340 nm). However, the increase in absorbance to 728 nm was also found for the -Se-Se− analog, indicating that this absorbance was not unique to the Se-S− bond, but indicated a dichalcogen interaction. Electrochemical characteristics suggest that 17 can act as a nucleophile because of the positive correlation between voltammetry and α-effects. Experimentally, the reaction between 17 and BnBr yielded the expected product 2-NO2C6H4SeSBn (18) in 60% yield.
Photochemical formation of RSeSR
An alternative to electrochemical manipulations is photochemical manipulations. RSeSeRs and RSSRs are considered dynamic covalent bonds, meaning they have the ability to be cleaved and reformed under certain environments (39, 50). Therefore, wavelength-controlled dynamic metathesis is a method to efficiently make Se-S bonds under very mild conditions. Xu et al. found that RSeSRs could be formed in >50% yield when RSeSeRs and RSSRs were subjected to 254 nm light (24). For example, in Scheme 7, RSeSeR 19 and diphenyl disulfide (20) were coupled to make RSeSR 21 under 254 nm light in organic solvents. Compounds 19 and 20 were both recovered in 25% yield, indicating a clean reaction with good mass balance. Interestingly, the reverse reaction occurred when visible light (wavelengths greater than 410 nm) was applied to the product (Scheme 7).
When RSeSR reacted with different RSeSeRs at wavelengths >500 nm, exchange reaction products were observed by the formation of a new Se-S bond with no reverse reaction to the parent RSSRs or RSeSeRs. These reactions presumably undergo radical mechanisms based on similar RSeSeR metathesis features (38). Electron spin resonance measurements were conducted with radical scavenger 5,5-dimethyl-1-pyrroline N-oxide (DMPO) and confirmed the formation of both selenyl and thiyl radicals. Radical scavenger 2,2,6,6-tetramethylpiperidinyloxy (TEMPO) was found to greatly hinder the productivity of this reaction as well. This chemistry was applied to polymer materials, and wavelength-controlled cleavage of the polymers containing many Se-S bonds was realized with irradiation. Overall, this chemistry provides a powerful route to Se-S bond formation, with light as the only input instead of traditionally used chemicals.
In situ activated Se for RSeSR formation
An important chemical property of organoselenium compounds is the anchimeric assistance effect of the Se atom. S has been known to exhibit strong anchimeric assistance effects, but Se shows a stronger effect because of its higher nucleophilicity (3). Amosova et al. developed a method that utilizes this effect to make RSeSR bonds regio- and stereoselectively (7). For example, in Scheme 8, 2-bromomethyl-1,3-thiaselenole (22) reversibly forms the seleniranium cation through the anchimeric effect and reacts spontaneously with nitrogen, oxygen, and S nucleophiles to form 23, 24, or 25. The reaction of thiaselenole 22 with these nucleophiles proceeds mainly at room temperature in organic solvents, but shows different reactivities depending on the identity of the nucleophile.
When thiols are used, only the RSeSR 25 is produced in good yield even though the C2 position is also a suitable electrophile. The attack of thiol at the Se atom of the seleniranium intermediate leads to a ring opening with RSeSR formation and a vinylsulfonyl group. This method is consistent with a wide range of thiols and exclusively forms the Z-isomer of the RSeSRs, indicating strong regio- and stereo-selectivity. These vinyl RSeSRs can also rearrange to thermodynamically more stable heterocycles, such as compound 26, under acidic conditions similar to that of allylic RSeSR rearrangements (22). On the other hand, when other nucleophiles are used such as alcohols and amines, the C2 position is attacked exclusively, forming compounds such as 23 and 24. This method provides routes for interesting chemical transformations through the Se anchimeric assistance effect. More importantly, these reactions proceeded under mild conditions with high selectivity at room temperature at an equimolar ratio of the reagents.
Glycosyl RSeSR' formation
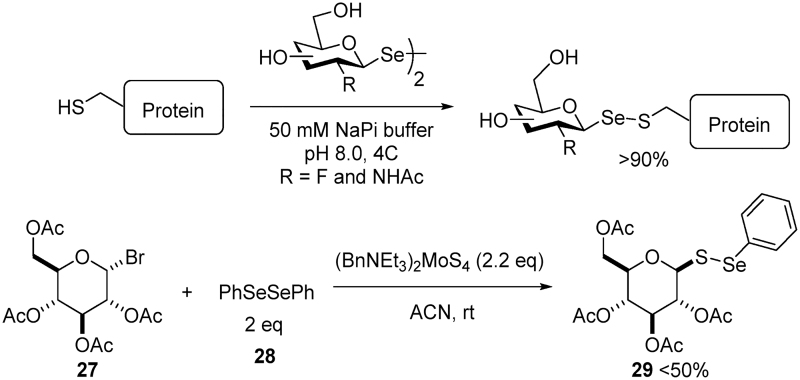
The first glycosyl RSeSR was observed as a side product by Johnston and Pinto and similar products were found to play a role in Se metabolism (19, 40). Since then, methods for synthesizing such glycosyl RSeSRs were reported (Scheme 9) (19, 78). For example, Boutureira et al. (19) reported glycosyl RSeSR formation via an exchange reaction between diglycosyl RSeSeRs and protein thiols in buffered solutions. The substrate scope and efficiency of this Se-S bond-forming method incorporated fluorine and -NAc sugars in excellent yields. Another method by Venkateswarlu et al. (78) used benzyltriethylammonium tetrathiomolybdate ((BnNEt3)2MoS4) as the S transfer reagent. The reaction of glucosyl bromide (27) with RSeSeR (28, 2 equivalents) in the presence of slight excess of (BnNEt3)2MoS4 yielded the mixed glucosyl RSeSR (29) in moderate yield (≤50%). The scope of this reaction was unknown because only diphenyl RSeSeR was used as the substrate. However, different halogenated sugar derivatives with Br on the C1 and C6 positions resulted in the expected products.
These methods produce seleno derivatives of their close analogs, mixed glycosyl RSSRs, which have gained attention because of their applications as glycosyl donors (4). Similar methods for making glycosyl RSeSRs using isoselenuronium salts were also developed (35). The major application for compounds such as 29 is site-selective protein glycoconjugation to form glycoproteins in excellent yields (26). Although the reactivity between mixed glycosyl RSSRs and mixed glycosyl RSeSRs is expected to be similar, the fundamental differences between S and Se warrant more chemical exploration of mixed glycosyl RSeSRs.
Cyclic RSeSR peptide formation
Synthesizing cyclic RSeSR peptides are useful in understanding the active site of ThxR and how it regenerates thioredoxin (Thx) (65, 72). These peptides are generally synthesized using solid-phase peptide synthesis, but benzyl-type protecting groups are required for the RSeH side chain of Sec (81). Deprotection of these groups is problematic because they require harsh conditions such as hydrofluoric acid, silyl-Lewis acids, or thiophilic heavy metals. Harris et al. reported a new extremely mild method for removing benzyl protecting groups such as p-methoxybenzyl (Mob) from Cys and Sec using 2,2′-dithiobis(5-nitropyridine) (DTNP) or 2,2′-dithiodipyridine (DTP) with trifluoroacetic acid (TFA) (Scheme 10) (34).
One equivalent of DTNP was found to deprotect a Mob-protected Sec and Mob-protected Cys with TFA and catalytic amount of thioanisole to yield an RSeSR bond within 3 h when the Sec and Cys residues are in close proximity. High-performance liquid chromatography showed that all Mob groups were deprotected with a near-complete conversion to the cyclic product. When the two protected residues are not in proximity, the DTNP deprotecting conditions will selectively deprotect the Sec residue over the Cys residue without thioanisole. This deprotection and Se-S bond formation aspect can occur without the need for an intermediary step. Thus, this chemistry provides a key improvement for on-resin RSeSR formation of the ThxR active site with ease (25).
Cyclic RSeSR formation
Many acyclic RSeSRs have been made throughout the years, but cyclic RSeSRs have yet to be explored largely because of the lack of a general method of synthesis. Heterocyclic chalcogen-containing compounds are fascinating areas of research because of their versatile applications in pharmaceutical and fundamental research (23, 62). Rafiqul et al. explored the generation of cyclic RSeSRs by reacting 6H-1,3,5-oxachalcogenazines with elemental Se or S in the presence of heat (Scheme 11) (61).
The reaction is hypothesized to undergo a retro Diels–Alder mechanism followed by a [4 + 1] addition of 1,3-chalcogenaza-1,3-butadiene with the elemental chalcogen. Cyclic RSeSRs, RSSRs, and RSeSeRs were made through this reaction in moderate to excellent yields in toluene. Although the resulting heterocycles are interesting, their chemical and biological utilities are still underdeveloped.
Chemical Reactivity in Organic Chemistry and Chemical Biology of RSeSR
Selenoproteins
Four major classes of selenoproteins have been discovered to date: ThxR, GPx, iodothyronine deiodinases, and selenoprotein P (75). Of these four, only ThxR and GPx have an Se-S bond that participates in redox regulation (Scheme 12). ThxR is a flavoprotein known to catalyze the reduction of Thx (a potent antioxidant) by using nicotinamide adenine dinucleotide phosphate (NADPH) and redox-active disulfide and RSeSR bonds (Scheme 12a). The first step is the reduction of both the disulfide and RSeSR by NADPH in acidic conditions to give two thiols, one thiolate, and one selenate ion in the active site. The selenate ion then does a nucleophilic attack on the disulfide of oxidized Thx and the proton exchange regenerates the parent RSeSR bond in ThxR. Further reaction with NADPH completes the catalytic cycle. This activity allows the Thx/ThxR system to protect against oxidative damage. The GPx family is another class of selenoproteins that protects against oxidative damage by utilizing the Se-S bond (Scheme 12b). The first step in this system is the oxidation of a free RSeH into RSeOH by peroxides. The RSeOH then reacts with GSH to form an RSeSR bond between the Sec residue and GSH, releasing H2O. Another equivalent of GSH reacts with the Se-S bond to form glutathione disulfide and free RSeH, completing the catalytic cycle. GSSG can then be reduced back into 2 equivalents of GSH by NADPH.
Selenoprotein mimics
Ebselen is a synthetic organoselenium compound, which mimics the catalytic activity of GPx and can serve as a potential antioxidant for many diseases (68). However, the catalytic efficiency of ebselen depends on the identity of thiol cofactors (16). For example, with aryl or benzylic thiols (phenyl mercaptan [PhSH] or benzyl mercaptan [BnSH]), ebselen is a weak catalyst for reduction of hydroperoxides. The formation of the -Se-S- linkage plays an essential role in its catalytic mechanism. As shown in Scheme 13, the initial step for ebselen to exhibit GPx activity is to react with thiol (using PhSH as the example) to form RSeSR 30. It was found that the neighboring amide plays an important role for the reactivity/stability of 30.
There is a strong -Se-O- interaction with the carbonyl group, which facilitates the attack of thiol at the Se atom rather than at the S atom. As such, the formation of catalytically active selenol (-SeH) as in the case of GPx is not favored. The disproportionation of 30 produces RSeSeR 31 and dihenyl disulfide, likely via a radical process. This is hypothesized to be second order in the RSeSR intermediate, but has yet to be validated in cellular concentrations. However, this step is the rate-determining step of the catalytic cycle and the reaction rate depends on the nature of thiols. For example, disproportionation of RSeSR derived from GSH is considerably faster than that of PhSH. RSeSeR 31 further reacts with hydrogen peroxide (H2O2) to form RSeOH 32 and seleninic acid 33. RSeOH 32 can reform ebselen via self-cyclization and elimination of H2O or be converted to RSeSR 30 by PhSH. Seleninic acid 33 can be reduced to 32 by PhSH. Other selenoprotein mimics such as selenosubtilisin show identical chemical reactivity to ebselen (15).
A computational study by Bachrach et al. predicted that the nucleophilic attack by thiols at Se is both kinetically faster and thermodynamically more favorable than at S (11). Steinmann et al. studied and experimentally compared the reactivity of Se and S in exchange reactions to better understand the difference in reactivity between Se and S in biological systems (76). The kinetics of reduction of RSeSeRs (-Se-Se-) by thiols (-SH) were measured by stopped-flow spectrophotometry. The reduction proceeds via a two-step process, with the formation of RSeSR as the intermediate. For example, the rate constants of the cysteamine/selenocystamine system are shown in Scheme 14. Reaction rates of Se as a nucleophile and as an electrophile are 2–4 orders of magnitude higher than those of S. Sulfides and selenides are comparable as leaving groups. The electrode potentials of selenylsulfides (-Se-S-) are about 70 mV lower than the potentials of corresponding RSSRs. Therefore, RSeSRs are considerably more difficult to reduce and can be utilized in situations where reduction of RSSRs is undesired (31). Additionally, computational studies suggest that the reaction mechanism is a two-step addition–elimination mechanism rather than a one-step SN2 mechanism (11).
These unproductive exchange reactions are hypothesized to be the reason for the inefficiency of ebselen as a GPx mimic. However, when there are S-N/O interactions on the thiol cosubstrate (similar to that of Se), a nucleophilic attack on S is favored over Se (Scheme 15). This is analogous to the active sites of GPx and ThxR where a heteroatom from a neighboring threonine (Thr), histidine (His), or glutamine (Glu) will cause the S atom in the RSeSR intermediate to be more electrophilic than Se (9, 67). Thus, similar chelating effects that make Se a stronger electrophile also make S a stronger electrophile. This study provides the first experimental evidence that heteroatoms are capable of enhancing the electrophilic character of the S in RSeSRs. This interaction would theoretically enhance the antioxidant potency of organo-Se compounds.
Organodiselenides can also show ebselen-like activity, which can be used to repair disulfide bonds in scrambled proteins through a similar Se-S intermediate (71). To explore this reactivity, Rathore et al. developed different organodiselenides that catalyzed the oxidation of thiols into RSSRs by using aerial oxygen and peroxide (Scheme 16) (63). RSeSeR 34 was synthesized in good yield and was found to be the best catalyst with 1 mol% loading to produce >90% of corresponding RSSRs. Mono-Se derivatives showed no reaction for disulfide formation. RSeSeR 34 also showed a broad substrate scope by converting thiophenols containing various functional groups and alkyl thiols into their respective RSSRs.
Biologically relevant thiols such as Cys, homocysteine, and glutathione were also transformed into their respective RSSRs in acetonitrile/H2O (1:1). RSeSeR 34 reacts with 1 equivalent of thiol to produce RSeSR 35 and RSeH 36. RSeSR 35 further reacts with an additional molecule of thiol to produce RSeH 36. The oxidation of 36 by O2 would afford the interesting intermediate selone 37 and H2O2 by electron transfer. This catalytic cycle is hypothesized to undergo a radical reaction mechanism. Thus, RSeSR 35 could also react with oxygen, leading to selone, hydroperoxyl (HO2•), and phenyl thiyl (PhS•) radicals, and dimerization would give RSSR. Finally, addition of another molecule of thiol to the Se of selone 37 followed by a proton transfer would provide RSeSR 35, completing the catalytic cycle. Another possible route for reduction of hydroperoxides and disulfide formation is similar to that of ebselen with oxidation of RSeH 36 to produce RSeOH 38 and subsequent thiol transfers to reform 36.
Other GPx mimics such as cyclic seleninate esters show ebselen-like activity because they produce RSSRs and consume H2O2 (12). However, these particular compounds are not catalytic because RSeSRs are the major products at higher concentrations of thiols. These RSeSRs can theoretically regenerate the seleninate ester catalyst, but oxidation is relatively slow and thermodynamically unfavorable according to some computational studies, thereby decreasing the efficiency of this GPx mimic and providing experimental evidence for the oxidation resistance of RSeSR species (15).
RSeSR'-based fluorescent probes for H2S detection
H2S is a gasotransmitter that modulates diverse cellular functions. Although many fluorescent probes for H2S have been reported in the past several years, few can be applied to real biological detection because of chemoselectivity issues with other biological thiols (47, 48, 84). Additionally, the maximum emission wavelengths of most probes are below 600 nm with small Stokes shifts, which are less than ideal conditions for fluorescent probes. To solve these problems, our laboratory implemented a reactive Se-S bond in the design of fluorescent probes (Scheme 17) (20, 79). This structural template takes the advantage of the unique reaction between the Se-S bond and thiols.
It is known that a nucleophilic attack by RSH at the Se atom is thermodynamically favorable and kinetically faster (76). Moreover, this template should form a strong Se-O interaction with the C = O carbonyl group, which also favors the attack of -SH at the Se atom rather than at the S atom (16). Therefore, the Se-S bond of the probe is highly reactive to both H2S and cellular thiols. With H2S, the Se-S linkage should be rapidly converted to -Se-SH, which then undergoes cyclization to release the fluorophore and turn on fluorescence. When thiols are presented, the original -Se-S- bond will become another -Se-S- linkage in equilibrium and this will not turn on fluorescence. It also will not consume the probe. Only when H2S is present, the equilibrium will be broken to turn on the fluorescence. This design makes the probes, such as SeSP-NIR and SeSP1, highly specific and sensitive for H2S.
Synthesis of (Z)-vinylic selenosulfides using RSeSR'
The preparation of tri- and tetrasubstituted alkenes is important because they are present in the main frameworks for many natural products and drugs (77, 82). Organochalcogen compounds have been well studied as versatile intermediates to form these alkenes (29, 53). A recent example is the formation of vinylic selenosulfides by irradiating RSSRs and RSeSeRs with >300 nm light in the presence of alkynes (Scheme 18a) (54). However, this method has selectivity problems, such as the uncontrollable formation of E- and Z-isomers, which are difficult to purify. Zeni et al. reported an efficient route to only Z-vinylic selenosulfides by thioselenation of propargylic alcohols with unsymmetrical RSeSRs in the presence of 3.0 equivalents of n-butyllithium (Scheme 18b) (70).
Although this method increased the efficiency for Z-vinylic selenosulfide formation, it requires strict anhydrous and harsh conditions. A variation of this method using catalytic cesium hydroxide was found to drastically increase the efficiency of Z-vinylic selenosulfide formation under milder conditions (Scheme 18c) (57). The Z-isomer was obtained as the only product and was confirmed by hydrogen nuclear magnetic resonance (1HNMR) analysis of the crude product. After systematic study, the optimal reaction conditions were found to be equimolar ratios of alkyne and RSeSR, and 20 mol% of CsOH-H2O in DMF at room temperature under nitrogen or argon for 15 h. Z-Vinylic selenosulfides were reported in good to excellent yields. The reaction works best when electron-rich alkynes are used, but it reveals that the reaction is not sensitive to the nature of functional groups presenting at the ortho- and para-positions of the aryl RSeSRs. Thus, a broad range of alkynes and unsymmetrical RSeSRs were tolerated in this method.
2-Thiol-5-nitropyridine-protected Sec
Sec or U, the 21st proteinogenic amino acid, is a structural analog to Cys with an RSeH in place of the thiol. Methods with a direct, robust, and selective route for functionalization of Sec that overcomes limitations, such as in situ RSeH generation, are important for site selective modification of unprotected selenopeptides. 2-Thiol-5-nitropyridine (TNP)-protected Sec is an RSeSR moiety that in the past has been used for in situ RSeH generation after deprotection with ascorbic acid (49). A more interesting use for this particular RSeSR is the use of an umpolung strategy for the bioconjugation of Sec in unprotected peptides (Scheme 19) (21). This approach utilizes the electrophilic character of RSeSRs together with copper, a bidentate ligand, and a nucleophilic aryl boronic acid to produce an arylated Sec residue.
The reaction mechanism is similar to that of the Suzuki reaction with an oxidative insertion of copper into the Se-S bond, followed by a transmetalation with boronic acid, and finally reductive elimination. This method shows biological utility because it does not require oxygen-free conditions and takes place in water-rich media. Control reactions showed that this copper-mediated arylation was selective to Sec over Cys and Serine (Ser). This selectivity is important for this to be used in Sec labeling. This method also shows good functional group versatility with electron-poor/rich aryl and biologically relevant boronic acids to form the corresponding aryl-selenide in good to excellent yields. Although this strategy is effective in peptide-based conjugation, protein application still requires further study.
Preparation of RSeSHs and Their Reactivity
Organolithium-mediated RSeSH formation
In addition to RSeSR', RSeSHs are also hypothesized to readily undergo redox reactions in organic chemistry and chemical biology (42). In the 1980s, elemental Se and S were used to form deprotonated RSeSHs in the presence of organolithium reagents (Scheme 20) (44). Kollemann et al. reported how to make various organyl-polychalcogenolates such as RSeSH to enhance the incorporation of chalcogens in organic compounds. Interestingly, organyl-polychalcogenolates with different chalcogens are only formed if the second chalcogen added is equal to or higher in electronegativity than the previous one. For example, RSe− can react with elemental Se or S, but cannot react with elemental tellurium.
Selenium nuclear magnetic resonance (77SeNMR) was used to characterize these compounds based on relative ppm shifts. The ppm of PhSeSe− (171) is 123 ppm units lower than that of PhSeS− (294), indicating that the Se in PhSeS− is relatively deshielded compared with PhSeSe− because of the electronegativity of S. These compounds are also found to readily disproportionate to the corresponding dimers when exposed to air. This decomposition is the major roadblock when studying RSeSR' and RSeSH chemistry and should be considered. Although this method for making RSeS− is effective and an acid workup should theoretically produce RSeSH, the harsh organolithium reagents needed to make these compounds and rapid disproportionation into the corresponding RSeSeR and RSSH make the RSeSH moiety difficult to study.
Acyl deprotection for RSeSH formation
In situ generation methods to study the chemical reactivities of RSeSHs are required because of their high instability. One method for generating RSeSHs is through acyl deprotection of a selenenyl thioester (RSeSAc). Ishii et al. used this method to isolate and characterize a sterically hindered RSeSH (Scheme 21) (36). This RSeSH was synthesized by reacting TripSeSeTrip with meta-chloroperoxybenzoic acid and thioacetic acid to make TripSeSAc. Then, a subsequent deprotection of the acetyl group with perchloric acid (HClO4) gave TripSeSH in 63% yield. This particular RSeSH is hypothesized to be stable because of steric hindrance of the Trip substituent. To prove that a thioselenenic acid is formed, it was reacted with triphenyl phosphine (PPh3) to give the RSeH and S = PPh3 quantitatively.
The structure of TripSeSH was further determined by X-ray diffraction analysis. The Se-S bond length is 2.1796 A and the C-Se-S bond angle is 103.85°, similar to computational studies (52). The -SeSH proton was found at δ 2.64 in the 1H NMR spectrum and SeS-H stretching in the infrared spectrum was observed at 2520 cm−1. TripSeSH is stable under acidic conditions, but is readily reactive in the presence of a base. When treated with triethyl amine, the corresponding diselenyl sulfide and RSeSeR were observed as the major products. When this same reaction was treated with triphenyltin chloride, stannyl thioselenenate, stannyl selenide, and RSeSeR were observed, indicating that the Se-S-H bonds can readily undergo radical reaction mechanisms.
Previous work showed that Cys-SeSH had a UV-vis absorption maximum of 375 nm. However, TripSeSH did not show such maximum, indicating that the contrasting characteristics of the substituents cause this result and not the -SeSH functional group. TripSeSH also shows that the stability of the -SeSH moiety is dependent on the size of the substituent on the Se. This observation leads to an interesting hypothesis that biologically relevant RSeSHs in the active sites of selenoenzymes may be stable.
Although the sterically hindered RSeSHs seem to be stable enough to isolate and characterize, small RSeSHs are still difficult to study, limiting their biological application. Recently, a series of small acyl-protected RSeSHs have been synthesized and their chemical reactivities and stabilities were studied (30, 41). When these compounds were incubated in a phosphate buffer system at various pHs, hydrolysis of RSeSAc was observed and found to be faster at pHs >7 and slower at pHs <7. The decomposition products were RSeSeR and hydrogen persulfide (H2S2), indicating that hydrolysis indeed released RSeSH. When these compounds are in the presence of nucleophiles such as thiols or amines, interesting reactivities take place (Scheme 22). Acyclic RSeSAc, like compound 39, readily reacted with excess Cys to produce N-acetyl cysteine, diphenyl RSeSeR, cystine, and the gasotransmitter H2S. Mechanistic studies showed that Cys acted as a thiol transfer agent, producing RSSH. RSSH then further reacts with Cys to produce H2S and cystine. The acyl transfer from RSeSAc to NH2- on Cys shows indirect evidence that RSeSH is being released. The same cyclic RSeSAcs also readily react with BnNH2 to produce N-acetylbenzyle amine, diphenyl diselenide, and H2S2. The presence of RSeSeR and H2S2 shows the disproportionation products of RSeSH. These species were found to be good intermediates for generation of H2S and H2S2, which are two important signaling molecules in Sec and Cys-related redox regulation. Furthermore, acyclic acyl RSeSRs produce H2S rapidly and in 80% yield, while the cyclic acyl RSeSRs showed no reaction toward thiols and produce negligible amounts of H2S. Acyclic acyl RSeSRs therefore are a useful tool to better understand the chemical biology of RSeSH as it pertains to redox regulation. The interesting aspect of cyclic acyl RSeSRs is that they produce diselenylsulfides such as compound 40. Compound 40 belongs to the sulfane sulfur family and can act as a sulfur pool for H2S production.
Nevertheless, if Sec-derived thioseleninic acids (SecSeSH) are formed endogenously, the corresponding diselenylsulfides (Sec-Se-Sn-Se-Sec) might be a major product. Both RSeSH intermediates from the acyclic and cyclic acyl RSeSAcs were unable to be trapped by conventional trapping reagents such as iodoacetamide because of their rather high instability. However, indirect evidence was observed when RSeSH reacted with a thiol-blocking reagent (methylsulfonyl benzothiazole [MSBT]).
Hypothetical Endogenous RSeSH Formation
Although it has not been validated, it is expected that the reduced form of Sec (-SeH) can readily react with certain reactive sulfane sulfur species to form endogenous RSeSH in a similar process to that of RSSHs. The major enzymes responsible for RSSH formation are believed to be CARS and 3-3-MST (6, 46). These enzymes produce RSSHs by different means. The CARS system first persulfidates a free Cys into cysteine persulfide, which is then incorporated during protein translation. The 3-MST system uses 3-mercaptopyruvate as a sulfur pool and persulfidates a Cys residue in the active site. This protein RSSH then transfers that extra sulfur onto thiol residues to form the corresponding RSSHs. It is unclear if free Sec serves as a substrate for these enzymatic reactions.
If so, the corresponding RSeSH may be formed. Another possible RSeSH formation is the exchange reaction between oxidized Sec (such as RSeSRs found in ThxR and GPx, or RSeOH/RSeO2H) and H2S. However, this exchange may not be as important because basal H2S concentration is lower than that of other biological thiols such as Cys and GSH. Nevertheless, these possibilities are purely speculative from a chemistry point of view and need to be validated in biological settings.
Conclusions
The discovery of Sec and selenoproteins in living organisms broadened the scope of redox signaling and regulation through RSeSRs and RSeSHs. These species contain important Se-S bonds that are involved in RSS chemistry. So far, only active site Se-S bonds in selenoenzymes are found in biological systems and smaller molecules containing these bonds have yet to be discovered. Because of this, very limited amount of biological information is known. However, exogenous RSeSRs strongly affect sulfur redox reactions, indicating that these moieties have some potential for medical use. This review presents the current understanding of RSeSRs and RSeSHs in organic chemistry and chemical biology. The major limitations for studying these species are the rapid self-disproportionation reactions to produce RSSRs and RSeSeRs. Current studies are mainly focused on selenoenzyme mimics and other utilities include RSS identification, glycoprotein formation, and Sec labeling. The chemistry of the Se-S bond is still relatively unexplored. More research is needed to understand the broader roles of these bonds in biological systems. There is little doubt that the general area of investigation of RSeSRs and RSeSHs will be a topic of research interest in the coming years.
Abbreviations Used
- 3-MST
3-mercaptopyruvate sulfurtransferase
- AgSCF3
silver(I) trifluoromethanethiolate
- (BnNEt3)2MoS4
benzyltriethylammonium tetrathiomolybdate
- BnSH
benzyl mercaptan
- CARS
cysteinyl-tRNA synthetase
- Cys
cysteine
- DMF
dimethylformamide
- DTNP
2,2′-dithiobis(5-nitropyridine)
- DTP
2,2′-dithiodipyridine
- GPx
glutathione peroxidase
- GSH
glutathione
- H2O2
hydrogen peroxide
- H2S
hydrogen sulfide
- H2S2
hydrogen persulfide
- Mob
p-methoxybenzyl
- NADPH
nicotinamide adenine dinucleotide phosphate
- PhSH
phenyl mercaptan
- PPh3
triphenyl phosphine
- RSeH
selenol
- RSeOH
selenenic acid
- RSeSAc
selenenyl thioester
- RSeSeR
diselenide
- RSeSH
thioseleninic acid
- RSeSO3
selenosulfate
- RSeSR
selenenyl sulfide
- RSS
reactive sulfur species
- RSSHs
persulfides
- RSSRs
disulfides
- S
sulfur
- Se
selenium
- Sec
selenocysteine
- SN2
substitution
- SO2Cl2
sulfuryl chloride
- TEMPO
2,2,6,6-tetramethylpiperidinyloxy
- TFA
trifluoroacetic acid
- TfOH
triflic acid
- Thx
thioredoxin
- ThxR
thioredoxin reductase
- TNP
2-thiol-5-nitropyridine
- UV-vis
ultraviolet–visible spectroscopy
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Institutes of Health (R01GM125968).
References
- 1. Abdo M, Liu S, Zhou B, Walls CD, Wu L, Knapp S, and Zhang ZY. Seleninate in place of phosphate: irreversible inhibition of protein tyrosine phosphatases. J Am Chem Soc 130: 13196–13197, 2008 [DOI] [PubMed] [Google Scholar]
- 2. Abdo M, Sun Z, and Knapp S. Biohybrid -Se-S- coupling reactions of an amino acid derived seleninate. Molecules 18: 1963–1972, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Accurso AA, Cho SH, Amin A, Potapov VA, Amosova SV, and Finn MG. Thia-, Aza-, and Selena[3.3.1]bicyclononane dichlorides: rates vs internal nucleophile in anchimeric assistance. J Org Chem 76: 4392–4395, 2011 [DOI] [PubMed] [Google Scholar]
- 4. Adinolfi M, d'Ischia M, Iadonisi A, Leone L, Pezzella A, and Valerio S. Glycosylated eumelanin building blocks by thioglycosylation of 5,6-diacetoxyindole with an expedient selenium-based dynamic-mixture methodology. Eur J Org Chem 23: 4333–4338, 2012 [Google Scholar]
- 5. Ahrika A, Auger J, and Paris J. Stabilisation of 2-nitrophenyl-selenosulfide, -diselenide and -thioselenide ions in N,N-dimethylacetamide. New J Chem 23: 679–681, 1999 [Google Scholar]
- 6. Akaike T, Ida T, Wei FY, Nishida M, Kumagai Y, Alam M, Ihara H, Sawa T, Matsunaga T, Kasamatsu S, Nishimura A, Morita M, Tomizawa K, Nishimura A, Watanabe S, Inaba K, Shima H, Tanuma N, Jung M, Fujii S, Watanabe Y, Ohmuraya M, Nagy P, Feelisch M, Fukuto JM, and Motohashi H. Cysteinyl-tRNA synthetase governs cysteine polysulfidation and mitochondrial bioenergetics. Nat Commun 8: 1177, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Amosova SV, Filippov AS, Potapov VA, Penzik MV, Makhaeva NA, and Albanov AI. Regio- and stereoselective synthesis of a novel family of unsaturated compounds with the S–Se bond and their cyclization to 2,3-dihydro-1,4-thiaselenines. Synthesis 51: 1832–1840, 2019 [Google Scholar]
- 8. Antony S and Bayse CA. Modeling the mechanism of the glutathione peroxidase mimic ebselen. Inorg Chem 50: 12075–12084, 2011 [DOI] [PubMed] [Google Scholar]
- 9. Arai K, Matsunaga T, Ueno H, Akahoshi N, Sato Y, Chakrabarty G, Mugesh G, and Iwaoka M. Modeling thioredoxin reductase-like activity with cyclic selenenyl sulfides: participation of an NH···Se hydrogen bond through stabilization of the mixed Se-S intermediate. Chem Eur J 25: 12751–12760, 2019 [DOI] [PubMed] [Google Scholar]
- 10. Arai K, Takei T, Okumura M, Watanabe S, Amagai Y, Asahina Y, Moroder L, Hojo H, Inaba K, and Iwaoka M. Preparation of selenoinsulin as a long-lasting insulin analogue. Angew Chem Int Ed 56: 5522–5526, 2017 [DOI] [PubMed] [Google Scholar]
- 11. Bachrach SM, Demoin DW, Luk M, and Miller JV Jr. Nucleophilic attack at selenium in diselenides and selenosulfides: a computational study. J Phys Chem 108: 4040–4046, 2004 [Google Scholar]
- 12. Back TG, Kuzma D, and Parvez M. Aromatic derivatives and tellurium analogues of cyclic seleninate esters and spirodioxyselenuranes that act as glutathione peroxidase mimetics. J Org Chem 70: 9230–9236, 2005 [DOI] [PubMed] [Google Scholar]
- 13. Balkrishna SJ, Kumar S, Azad GK, Bhakuni BS, Panini P, Ahalawat N, Tomar RS, Detty MR, and Kumar S. An ebselen like catalyst with enhanced GPx activity via a selenol intermediate. Org Biomol Chem 12: 1215–1219, 2014 [DOI] [PubMed] [Google Scholar]
- 14. Bechtel TJ and Weerapana E. From structure to redox: the diverse functional roles of disulfides and implications in disease. Proteomics 17: 1600391, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Benkova Z, Kóňa J, Gann G, and Fabian WMF. Redox chemistry of organoselenium compounds: Ab initio and density functional theory calculations on model systems for transition states and intermediates of the redox cycle of selenoenzymes. Int J Quantum Chem 90: 555–565, 2002 [Google Scholar]
- 16. Bhabak KP and Mugesh G. Functional mimics of glutathione peroxidase: bioinspired synthetic antioxidants. Acc Chem Res 43: 1408–1419, 2010 [DOI] [PubMed] [Google Scholar]
- 17. Bhowmick D and Mugesh G. Introduction of a catalytic triad increases the glutathione peroxidase-like activity of diaryl diselenides. Org Biomol Chem 13: 9072–9082, 2015 [DOI] [PubMed] [Google Scholar]
- 18. Bhowmick D and Mugesh G. Tertiary amine-based glutathione peroxidase mimics: some insights into the role of steric and electronic effects on antioxidant activity. Tetrahedron 68: 10550–10560, 2012 [Google Scholar]
- 19. Boutureira O, Bernardes GJL, Fernμndez-Gonzμlez M, Anthony DC, and Davis BG. Selenenylsulfide-linked homogeneous glycopeptides andglycoproteins: synthesis of human “hepatic se metabolite a.” Angew Chem Int Ed 51: 1432–1436, 2012 [DOI] [PubMed] [Google Scholar]
- 20. Chen W, Xu S, Day JJ, Wang D, and Xian M. A general strategy for development of near-infrared fluorescent probes for bioimaging. Angew Chem Int Ed 56: 16611–16615, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cohen DT, Zhang C, Pentelute BL, and Buchwald SL. An umpolung approach for the chemoselective arylation of selenocysteine in unprotected peptides. J Am Chem Soc 137: 9784–9787, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Crich D, Krishnamurthy V, Brebion F, Karatholuvhu M, Subramanian V, and Hutton TK. Dechalcogenative allylic selenosulfide and disulfide rearrangements: complementary methods for the formation of allylic sulfides in the absence of electrophiles. Scope, limitations, and application to the functionalization of unprotected peptides in aqueous media. J Am Chem Soc 129: 10282–10294, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dolbier WR Jr, Burkholder C, Abboud KA, and Loehle D. Synthesis of new tetrafluorobenzo heteroaromatic compounds. J Org Chem 59: 7688–7694, 1994 [Google Scholar]
- 24. Fan F, Ji S, Sun C, Liu C, Yu Y, Fu Y, and Xu H. Wavelength controlled dynamic metathesis: a light-driven exchange reaction between disulfide and diselenide bonds. Angew Chem Int Ed 57: 16426–16430, 2018 [DOI] [PubMed] [Google Scholar]
- 25. Flemer S Jr, Lacey BM, and Hondal RJ. Synthesis of peptide substrates for mammalian thioredoxin reductase. J Pept Sci 14: 637–647, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gamblin DP, Garnier P, Kasteren SV, Oldham NJ, Fairbanks AJ, and Davis BG. Glyco-SeS: selenenylsulfide-mediated proteinglycoconjugation—a new strategy in post-translational modification. Angew Chem Int Ed 43: 828–833, 2004 [DOI] [PubMed] [Google Scholar]
- 27. Glenadel Q, Ayad C, D'Elia MA, Billard T, and Toulgoat F. Nucleophilic trifluoromethylthiolation of organoselenocyanates with trifluoromethanesulfenamide reagent: access to CF3SSe-containing compounds. J Fluor Chem 210: 112–116, 2018 [Google Scholar]
- 28. Gowda R, Madhunapantula SV, Desai D, Amin S, and Robertson GP. Simultaneous targeting of COX-2 and AKT using selenocoxib-1-GSH to inhibit melanoma. Mol Cancer Ther 12: 3–15, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gridnev ID, Miyaura N, and Suzuki A. Convenient one-pot synthesis of vinylic sulfides from thioalkynes via a catalytic hydroboration-coupling sequence. J Org Chem 58: 5351–5354, 1993 [Google Scholar]
- 30. Hamsath A, Wang Y, Yang CT, Xu S, Cañedo D, Chen W, and Xian M. Acyl selenyl sulfides as the precursors for reactive sulfur species (hydrogen sulfide, polysulfide, and selenyl sulfide). Org Lett 21: 5685–5688, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hanold LE, Watkins CP, Ton NT, Liaw P, Beedle AM, and Kennedy EJ. Design of a selenylsulfide-bridged EGFR dimerization arm mimic. Bioorg Med Chem 23: 2761–2766, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Haratake M, Sakano T, Fuchigami T, and Nakayama M. Thiol-targeted introduction of selenocysteine to polypeptides for synthesis of glutathione peroxidase mimics. Metallomics 3: 702–709, 2011 [DOI] [PubMed] [Google Scholar]
- 33. Haratake M, Tachibana Y, Emaya Y, Yoshida S, Fuchigami T, and Nakayama M. Synthesis of nanovesicular glutathione peroxidase mimics with a selenenylsulfide-bearing lipid. ACS Omega 1: 58–65, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harris KM, Flemer S Jr, and Hondal RJ. Studies on deprotection of cysteine and selenocysteineside-chain protecting groups. J Pept Sci 13: 81–93, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Illyés TZ, Balla S, Bényei A, Kumar AA, Timári I, Kövér KE, and Szilágyi L. Exploring the syntheses of novel glycomimetics. Carbohydrate derivatives with Se-S or Se-Se glycosidic linkages. Chem Select 1: 2383–2388, 2016 [Google Scholar]
- 36. Ishii A, Takahashi T, Tawata A, Furukawa A, Oshida H, and Nakayama J. First synthesis and characterization of isolable thioselenenic acid, triptycene-9-thioselenenic acid. Chem Commun 23: 2810–2811, 2002 [DOI] [PubMed] [Google Scholar]
- 37. Jereb M and Dolenc D. Electrophilic trifluoromethylthiolation of thiols with trifluoromethanesulfenamide. RSC Adv 5: 58292–58306, 2015 [Google Scholar]
- 38. Ji S, Cao W, Yu Y, and Xu H. Dynamic diselenide bonds: exchange reaction induced by visible light without catalysis. Angew Chem Int Ed 53: 6781–6785, 2014 [DOI] [PubMed] [Google Scholar]
- 39. Ji S, Xia J, and Xu H. Dynamic chemistry of selenium: Se–N and Se–Se dynamic covalent bonds in polymeric systems. ACS Macro Lett 5: 78–82, 2016 [DOI] [PubMed] [Google Scholar]
- 40. Johnston BD and Pinto BM. Synthesis of thio-linked disaccharides by 1→2 intramolecular thioglycosyl migration: oxacarbenium versus episulfonium ion intermediates. J Org Chem 65: 4607–4617, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Kang J, Ferrell AJ, Chen W, Wang D, and Xian M. Cyclic acyl disulfides and acyl selenylsulfides as the precursors for persulfides (RSSH), selenylsulfides (RSeSH), and hydrogen sulfide (H2S). Org Lett 20: 852–855, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kharma A, Grman M, Misak A, Domínguez-Álvarez E, Nasim MJ, Ondrias K, Chovanec M, and Jacob C. Inorganic polysulfides and related reactive sulfur–selenium species from the perspective of chemistry. Molecules 24: 1359–1374, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Kice JL and Lee TWS. Oxidation-reduction reactions of organoselenium compounds. 1. Mechanism of the reaction between seleninic acids and thiols. J Am Chem Soc 100: 5094–5102, 1978 [Google Scholar]
- 44. Kollemann C, Obendorf D, and Sladky F. Lithium organyl polychalcogenolates. Phosphorus Sulfur Relat Elem 38: 69–77, 1988 [Google Scholar]
- 45. Lacey BM, Eckenroth BE, Flemer S Jr, and Hondal RJ. Selenium in thioredoxin reductase: a mechanistic perspective. Biochemistry 47: 12810–12821, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lec JC, Boutserin S, Mazon H, Mulliert G, Boschi-Muller S, and Talfournier F. Unraveling the mechanism of cysteine persulfide formation catalyzed by 3-mercaptopyruvate sulfurtransferases. ACS Catal 8: 2049–2059, 2018 [Google Scholar]
- 47. Li X, Gao X, Shi W, and Ma H. Design strategies for water-soluble small molecular chromogenic and fluorogenic probes. Chem Rev 114: 590–659, 2014 [DOI] [PubMed] [Google Scholar]
- 48. Lin VS, Chen W, Xian M, and Chang CJ. Chemical probes for molecular imaging and detection of hydrogen sulfide and reactive sulfur species in biological systems. Chem Soc Rev 44: 4596–4618, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Marie EJS, Ruggles EL, and Hondal RJ. Removal of the 5-nitro-2-pyridine-sulfenylprotecting group from selenocysteine and cysteine by ascorbolysis. J Pept Sci 22: 571–576, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Matysiak BM, Nowak P, Cvrtila I, Pappas CG, Liu B, Komaromy D, and Otto S. Antiparallel dynamic covalent chemistries. J Am Chem Soc 139: 6744–6751, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Mugesh G, Du-Mont WW, Wismach C, and Jones PG. Biomimetic studies on iodothyronine deiodinase intermediates: modeling the reduction of selenenyl iodide by thiols. ChemBioChem 3: 440–447, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Nikoo S, Meister PJ, Hayward JJ, and Gauld JW. An assessment of computational methods for calculating accurate structures and energies of bio-relevant polysulfur/selenium-containing compounds. Molecules 23: 3323–3340, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ogawa A, Ikeda T, Kimura K, and Hirao T. Highly regio- and stereocontrolled synthesis of vinyl sulfides via transition-metal-catalyzed hydrothiolation of alkynes with thiols. J Am Chem Soc 121: 5108–5114, 1999 [Google Scholar]
- 54. Ogawa A, Obayashi R, Ine H, Tsuboi Y, Sonoda N, and Hirao T. Highly regioselective thioselenation of acetylenes by using a (PhS)2-(PhSe)2 binary system. J Org Chem 63: 881–884, 1998 [DOI] [PubMed] [Google Scholar]
- 55. Fukuto JM, Ignarro LJ, Nagy P, Wink DA, Kevil CG, Feelisch M, Cortese-Krott MM, Bianco CL, Kumagai Y, Hobbs AJ, Lin J, Ida T, and Akaike T. Biological hydropersulfides and related polysulfides—a new concept and perspective in redox biology. FEBS Lett 592: 2140–2152, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Patarasakulchai N and Southwell-Keely PT. Comparative reactivities of selenosulfates and selenenylthiosulfates with thiols. Phosphorus Sulfur Relat Elem 22: 277–282, 1985 [Google Scholar]
- 57. Peng L, Li R, Tang Z, Chen J, Yi R, and Xu X. Cesium-catalyzed highly regioselective synthesis of (Z)-vinylic selenosulfides via thioselenation of alkynes with unsymmetrical diorganoyl dichalcogenides. Tetrahedron 73: 3099–3105, 2017 [Google Scholar]
- 58. Prabhu P, Singh BG, Noguchi M, Phadnis PP, Jain VK, Iwaoka M, and Priyadarsini KI. Stable selones in glutathione-peroxidase-like catalytic cycle of selenonicotinamide derivative. Org Biomol Chem 12: 2404–2412, 2014 [DOI] [PubMed] [Google Scholar]
- 59. Prasad PR, Singh HB, and Butcher RJ. Synthesis, structure and antioxidant activity of cyclohexene-fused selenuranes and related derivatives. Molecules 20: 12670–12685, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Prigol M, Wilhelm EA, Schneider CC, and Nogueira CW. Protective effect of unsymmetrical dichalcogenide, a novel antioxidant agent, in vitro and an in vivo model of brain oxidative damage. Chem-Biol Interact 176: 129–136, 2008 [DOI] [PubMed] [Google Scholar]
- 61. Rafiqul IM, Shimada K, Aoyagi S, Fujisawa Y, and Takikawa Y. Synthesis of 1,2,4-dichalcogenazoles by the reaction of 6H-1,3,5-oxachalcogenazines with elemental chalcogen. Tetrahedron Lett 45: 6187–6190, 2004 [Google Scholar]
- 62. Rakitin OA and Zibarev AV. Synthesis and applications of 5-membered chalcogen-nitrogen π-heterocycles with three heteroatoms. Asian J Org Chem 7: 2397–2416, 2018 [Google Scholar]
- 63. Rathore V, Upadhyay A, and Kumar S. An organodiselenide with dual mimic function of sulfhydryl oxidases and glutathione peroxidases: aerial oxidation of organothiols to organodisulfides. Org Lett 20: 6274–6278, 2018 [DOI] [PubMed] [Google Scholar]
- 64. Reich HJ and Hondal RJ. Why nature chose selenium. ACS Chem Biol 11: 821–841, 2016 [DOI] [PubMed] [Google Scholar]
- 65. Ruggles EL, Deker PB, and Hondal RJ. Conformational analysis of oxidizedpeptide fragments of the C-terminalredox center in thioredoxin reductasesby NMR spectroscopy. J Pept Sci 20: 349–360, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Saravanan P and Anbarasan P. Trifluoromethylthiolative 1,2-difunctionalization of alkenes with diselenides and AgSCF3. Chem Commun 55: 4639–4642, 2019 [DOI] [PubMed] [Google Scholar]
- 67. Sarma BK and Mugesh G. Glutathione Peroxidase (GPx)-like antioxidant activity of the organoselenium drug ebselen: unexpected complications with thiol exchange reactions. J Am Chem Soc 127: 11477–11485, 2005 [DOI] [PubMed] [Google Scholar]
- 68. Satheeshkumar K and Mugesh G. Synthesis and antioxidant activity of peptide-based ebselen analogues. Chem Eur J 17: 4849–4857, 2011 [DOI] [PubMed] [Google Scholar]
- 69. Scarf AR, Cole ER, and Southwell-Keely PT. Alkyl selenosulphates (seleno Bunte salts). A new class of thiol-blocking reagents. Biochem J 201: 305–309, 1982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schneider CC, Godoi B, Prigol M, Nogueira CW, and Zeni G. Highly stereoselective one-pot procedure to prepare unsymmetrical bis- and tris-chalcogenide alkenes via addition of chalcogens to alkynes. Organometallics 26: 4252–4256, 2007 [Google Scholar]
- 71. Shimodaira S, Asano Y, Arai K, and Iwaoka M. Selenoglutathione diselenide: unique redox reactions in the gpx like catalytic cycle and repairing of disulfide bonds in scrambled protein. Biochemistry 56: 5644–5653, 2017 [DOI] [PubMed] [Google Scholar]
- 72. Shimodaira S, Takei T, Hojo H, and Iwaoka M. Synthesis of selenocysteine-containing cyclic peptides via tandem N-to-S acyl migration and intramolecular selenocysteine-mediated native chemical ligation. Chem Commun 54: 11737–11740, 2018 [DOI] [PubMed] [Google Scholar]
- 73. Singh VP, Poon J, Butcher RJ, and Engman L. Pyridoxine-derived organoselenium compounds with glutathione peroxidase-like and chain-breaking antioxidant activity. Chem Eur J 20: 12563–12571, 2014 [DOI] [PubMed] [Google Scholar]
- 74. Stadtman TC. Selenium biochemistry. Science 183: 915–922, 1974 [DOI] [PubMed] [Google Scholar]
- 75. Steinbrenner H, Speckmann B, and Klotz LO. Selenoproteins: antioxidant selenoenzymes and beyond. Arch Biochem Biophys 595: 113–119, 2016 [DOI] [PubMed] [Google Scholar]
- 76. Steinmann D, Nauser T, and Koppenol WH. Selenium and sulfur in exchange reactions: a comparative study. J Org Chem 75: 6696–6699, 2010 [DOI] [PubMed] [Google Scholar]
- 77. Takahashi A, Kirio Y, Sodeoka M, Sasai H, and Shibasaki M. Highly stereoselective synthesis of exocyclic tetrasubstituted enol ethers and olefins. A synthesis of Nileprost. J Am Chem Soc 111: 643–647, 1989 [Google Scholar]
- 78. Venkateswarlu C, Gautam V, and Chandrasekaran S. Synthesis of mixed glycosyl disulfides/selenenylsulfides using benzyltriethylammonium tetrathiomolybdate as a sulfur transfer reagent. Carbohydr Res 402: 200–207, 2015 [DOI] [PubMed] [Google Scholar]
- 79. Wang Y, Yang CT, Xu S, Chen W, and Xian M. Hydrogen sulfide mediated tandem reaction of selenenyl sulfides and its application in fluorescent probe development. Org Lett 21: 7573–7576, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wessjohann LA, Schneider A, Abbas M, and Brandt W. Selenium in chemistry and biochemistry in comparison to sulfur. Biol Chem 388: 997–1006, 2007 [DOI] [PubMed] [Google Scholar]
- 81. Wessjohann LA, Schneider A, Kaluđerović GN, and Brandt W. Solid-phase synthesis of reduced selenocysteine tetrapeptides and their oxidized analogs containing selenenylsulfide eight-membered rings. Mol Divers 17: 537–545, 2013 [DOI] [PubMed] [Google Scholar]
- 82. Williams DR, Ihle DC, and Plummer SV. Total synthesis of (−)-Ratjadone. Org Lett 3: 1383–1386, 2001 [DOI] [PubMed] [Google Scholar]
- 83. Xu Y, Shi X, and Wu L. tBuOK-triggered bond formation reactions. RSC Adv 9: 24025–24029, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Xuan W, Sheng C, Cao Y, He W, and Wang W. Fluorescent probes for the detection of hydrogen sulfide in biological systems. Angew Chem Int Ed 51: 2282–2284, 2012 [DOI] [PubMed] [Google Scholar]